

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50200340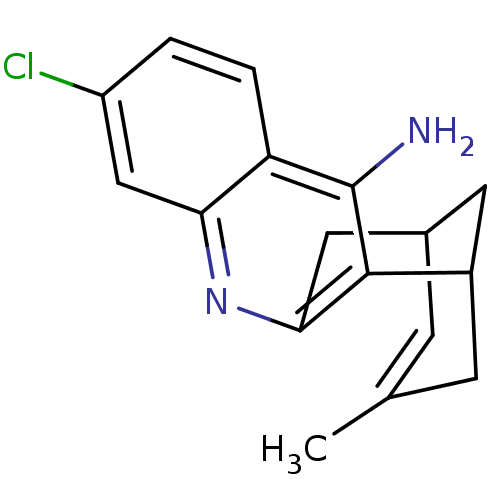 ((+/-)-huprine Y-HCl | (+/-)-huprineY hydrochloride...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0330 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universitat de Barcelona Curated by ChEMBL | Assay Description Binding affinity to human AChE | J Med Chem 49: 6833-40 (2006) Article DOI: 10.1021/jm060945c BindingDB Entry DOI: 10.7270/Q29K4C1S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mitogen-activated protein kinase 14 (Homo sapiens (Human)) | BDBM50376226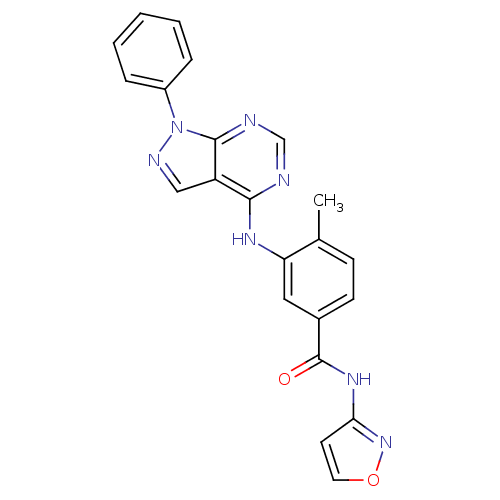 (CHEMBL259922) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development Curated by ChEMBL | Assay Description Inhibition of human recombinant p38alpha-mediated myelin basic protein phosphorylation | Bioorg Med Chem Lett 18: 2652-7 (2008) Article DOI: 10.1016/j.bmcl.2008.03.019 BindingDB Entry DOI: 10.7270/Q2C53MR6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mitogen-activated protein kinase 14 (Homo sapiens (Human)) | BDBM50376226 (CHEMBL259922) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development Curated by ChEMBL | Assay Description Inhibition of human recombinant p38alpha-mediated myelin basic protein phosphorylation | Bioorg Med Chem Lett 18: 2652-7 (2008) Article DOI: 10.1016/j.bmcl.2008.03.019 BindingDB Entry DOI: 10.7270/Q2C53MR6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional dihydrofolate reductase-thymidylate synthase (Plasmodium falciparum (isolate K1 / Thailand)) | BDBM18784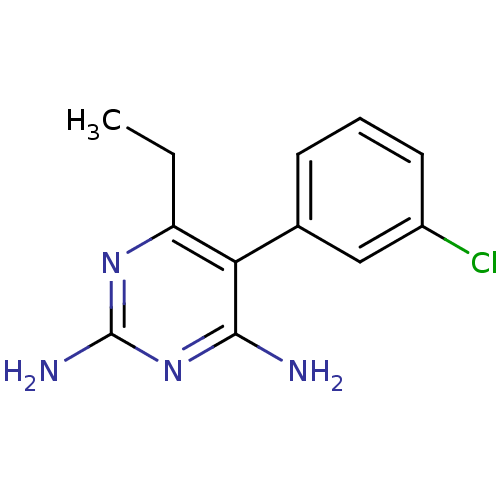 (5-(3-chlorophenyl)-6-ethylpyrimidine-2,4-diamine |...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Manchester Curated by ChEMBL | Assay Description Binding affinity was evaluated as inhibition of recombinant wild type (WT) Plasmodium falciparum DHFR-TS. | J Med Chem 41: 1367-70 (1998) Article DOI: 10.1021/jm970845u BindingDB Entry DOI: 10.7270/Q2ZC83JJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50107460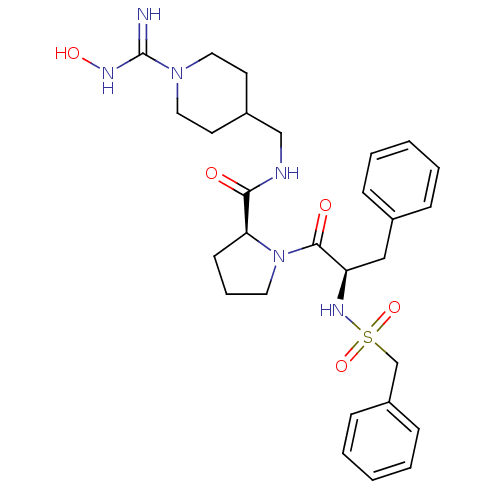 ((S)-1-((R)-3-Phenyl-2-phenylmethanesulfonylamino-p...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.460 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Competitive kinetic for thrombin inhibition Ki was determined | Bioorg Med Chem Lett 12: 45-9 (2001) BindingDB Entry DOI: 10.7270/Q2GX4C3P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase Lck (Homo sapiens (Human)) | BDBM50131114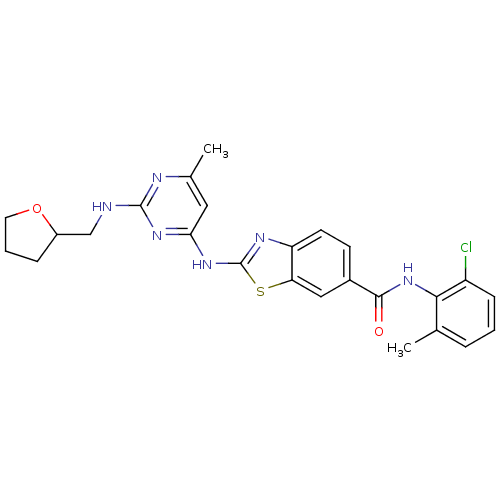 (2-{6-Methyl-2-[(tetrahydro-furan-2-ylmethyl)-amino...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.540 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibition of p56 Lck tyrosine kinase | Bioorg Med Chem Lett 13: 2587-90 (2003) BindingDB Entry DOI: 10.7270/Q2FX78V4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase Lck (Homo sapiens (Human)) | BDBM13357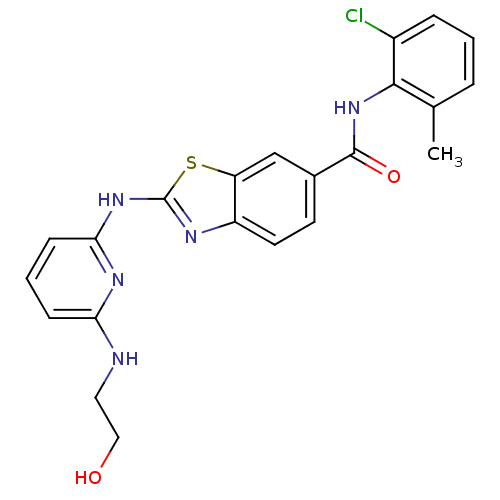 (CHEMBL312933 | N-(2-chloro-6-methylphenyl)-2-({6-[...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibition of p56 Lck tyrosine kinase | Bioorg Med Chem Lett 13: 2587-90 (2003) BindingDB Entry DOI: 10.7270/Q2FX78V4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional dihydrofolate reductase-thymidylate synthase (Plasmodium falciparum (isolate K1 / Thailand)) | BDBM50064020 (6-Ethyl-5-(3-methoxy-phenyl)-pyrimidine-2,4-diamin...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Manchester Curated by ChEMBL | Assay Description Binding affinity was evaluated as inhibition of recombinant wild type (WT) Plasmodium falciparum DHFR-TS. | J Med Chem 41: 1367-70 (1998) Article DOI: 10.1021/jm970845u BindingDB Entry DOI: 10.7270/Q2ZC83JJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase Fyn (Homo sapiens (Human)) | BDBM50131114 (2-{6-Methyl-2-[(tetrahydro-furan-2-ylmethyl)-amino...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | PubMed | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibition of Fyn protein kinase | Bioorg Med Chem Lett 13: 2587-90 (2003) BindingDB Entry DOI: 10.7270/Q2FX78V4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional dihydrofolate reductase-thymidylate synthase (Plasmodium falciparum (isolate K1 / Thailand)) | BDBM18512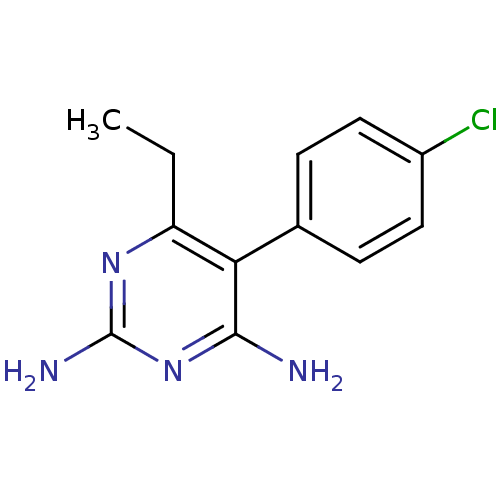 (5-(4-chlorophenyl)-6-ethylpyrimidine-2,4-diamine |...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | DrugBank PDB Article PubMed | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Manchester Curated by ChEMBL | Assay Description Binding affinity was evaluated as inhibition of recombinant wild type (WT) Plasmodium falciparum DHFR-TS. | J Med Chem 41: 1367-70 (1998) Article DOI: 10.1021/jm970845u BindingDB Entry DOI: 10.7270/Q2ZC83JJ | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Retinoic acid receptor RXR-alpha (Homo sapiens (Human)) | BDBM50052588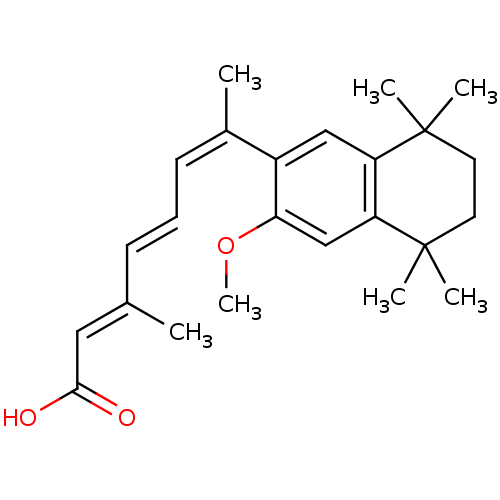 ((2E,4E,6Z)-7-(3-Methoxy-5,5,8,8-tetramethyl-5,6,7,...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ligand Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description Inhibition of [3H]-Targretin binding to Retinoid X receptor RXR alpha | J Med Chem 39: 3229-34 (1996) Article DOI: 10.1021/jm960311d BindingDB Entry DOI: 10.7270/Q2ZW1K14 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Retinoic acid receptor RXR-beta (Homo sapiens (Human)) | BDBM50052588 ((2E,4E,6Z)-7-(3-Methoxy-5,5,8,8-tetramethyl-5,6,7,...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ligand Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description Inhibition of [3H]-Targretin binding to Retinoid X receptor RXR beta | J Med Chem 39: 3229-34 (1996) Article DOI: 10.1021/jm960311d BindingDB Entry DOI: 10.7270/Q2ZW1K14 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional dihydrofolate reductase-thymidylate synthase (Plasmodium falciparum (isolate K1 / Thailand)) | BDBM18784 (5-(3-chlorophenyl)-6-ethylpyrimidine-2,4-diamine |...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Manchester Curated by ChEMBL | Assay Description Binding affinity was evaluated as inhibition of mutant (C59R + S108N) Plasmodium falciparum DHFR-TS. | J Med Chem 41: 1367-70 (1998) Article DOI: 10.1021/jm970845u BindingDB Entry DOI: 10.7270/Q2ZC83JJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase Fyn (Homo sapiens (Human)) | BDBM13357 (CHEMBL312933 | N-(2-chloro-6-methylphenyl)-2-({6-[...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 2.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibition of Fyn protein kinase | Bioorg Med Chem Lett 13: 2587-90 (2003) BindingDB Entry DOI: 10.7270/Q2FX78V4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional dihydrofolate reductase-thymidylate synthase (Plasmodium falciparum (isolate K1 / Thailand)) | BDBM18792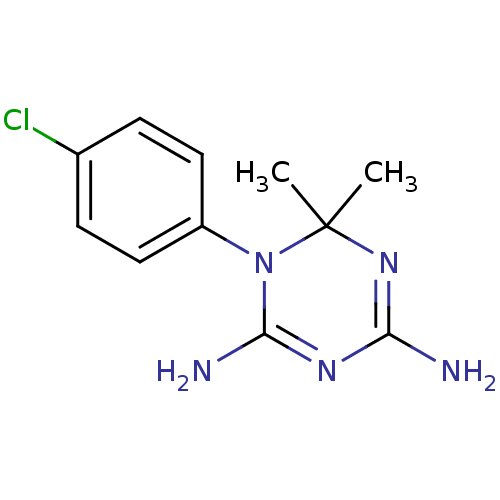 (1-(4-chlorophenyl)-6,6-dimethyl-1,6-dihydro-1,3,5-...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | 2.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Manchester Curated by ChEMBL | Assay Description Binding affinity was evaluated as inhibition of recombinant wild type (WT) Plasmodium falciparum DHFR-TS. | J Med Chem 41: 1367-70 (1998) Article DOI: 10.1021/jm970845u BindingDB Entry DOI: 10.7270/Q2ZC83JJ | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Proto-oncogene tyrosine-protein kinase Src (Homo sapiens (Human)) | BDBM50131114 (2-{6-Methyl-2-[(tetrahydro-furan-2-ylmethyl)-amino...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | PubMed | 3.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibition of Src protein tryrosine kinase | Bioorg Med Chem Lett 13: 2587-90 (2003) BindingDB Entry DOI: 10.7270/Q2FX78V4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50366780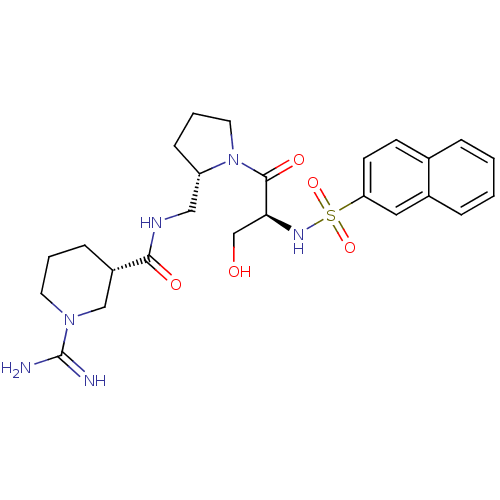 (BMS-189090 | CHEMBL138877) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid PDB UniChem Similars | PubMed | 3.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description In vitro reversible inhibition of thrombin catalytic activity | Bioorg Med Chem Lett 12: 41-4 (2001) BindingDB Entry DOI: 10.7270/Q2MP53T0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Retinoic acid receptor RXR-gamma (Homo sapiens (Human)) | BDBM50052588 ((2E,4E,6Z)-7-(3-Methoxy-5,5,8,8-tetramethyl-5,6,7,...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ligand Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description Inhibition of [3H]-Targretin binding to Retinoid X receptor RXR gamma | J Med Chem 39: 3229-34 (1996) Article DOI: 10.1021/jm960311d BindingDB Entry DOI: 10.7270/Q2ZW1K14 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium/glucose cotransporter 2 (Homo sapiens (Human)) | BDBM20877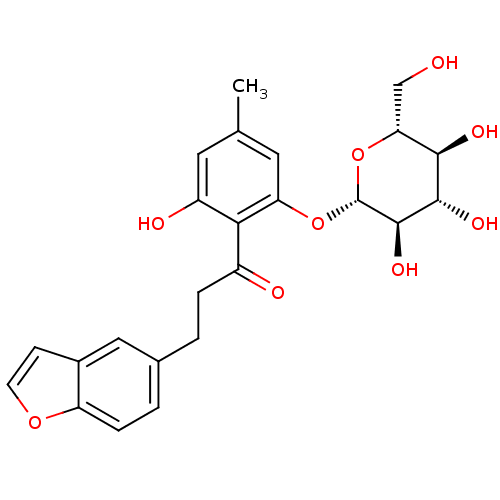 (3-(1-benzofuran-5-yl)-1-(2-hydroxy-4-methyl-6-{[(2...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Patents Similars | Article PubMed | 4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research and Development Curated by ChEMBL | Assay Description In vitro inhibition of Na-dependent [14C]-AMG uptake in CHO-K1 cells expressing human sodium glucose co-transporter 2 | Bioorg Med Chem Lett 14: 5121-5 (2004) Article DOI: 10.1016/j.bmcl.2004.07.082 BindingDB Entry DOI: 10.7270/Q2VD6XX2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Retinoic acid receptor RXR-alpha (Homo sapiens (Human)) | BDBM50052590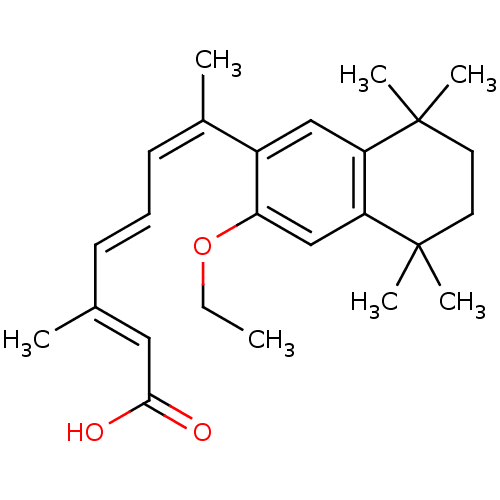 ((2E,4E,6Z)-7-(3-Ethoxy-5,5,8,8-tetramethyl-5,6,7,8...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ligand Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description Inhibition of [3H]-Targretin binding to Retinoid X receptor RXR alpha | J Med Chem 39: 3229-34 (1996) Article DOI: 10.1021/jm960311d BindingDB Entry DOI: 10.7270/Q2ZW1K14 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional dihydrofolate reductase-thymidylate synthase (Plasmodium falciparum (isolate K1 / Thailand)) | BDBM50192451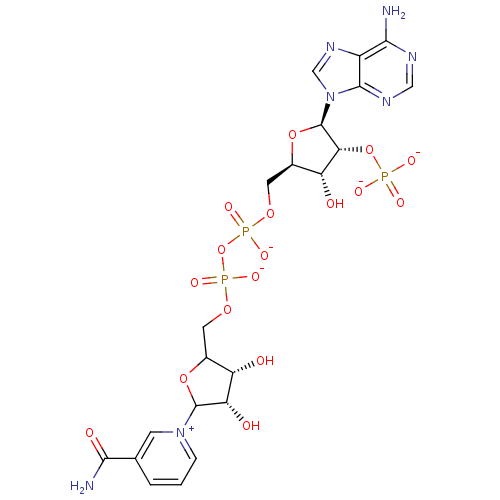 (CHEMBL213053 | NADP+) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 4.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Manchester Curated by ChEMBL | Assay Description Binding affinity was evaluated as inhibition of recombinant wild type (WT) Plasmodium falciparum DHFR-TS. | J Med Chem 41: 1367-70 (1998) Article DOI: 10.1021/jm970845u BindingDB Entry DOI: 10.7270/Q2ZC83JJ | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (docked) | ||||||||||||
| Retinoic acid receptor RXR-gamma (Homo sapiens (Human)) | BDBM50074300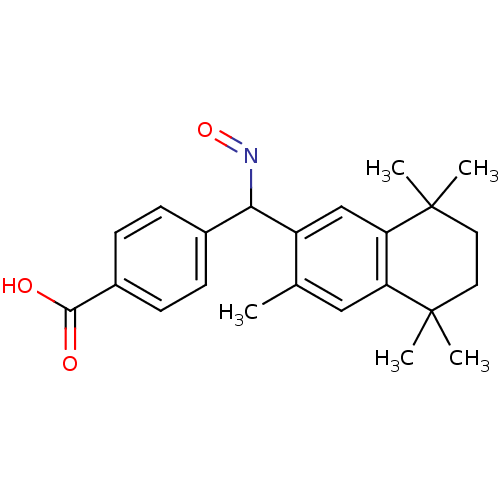 (4-[[(E)-Hydroxyimino]-(3,5,5,8,8-pentamethyl-5,6,7...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ligand Pharmaceuticals, Inc Curated by ChEMBL | Assay Description Binding affinity towards recombinantly expressed Retinoic acid receptor RXR-gamma in baculoviral sysytem, by using 5 nM [3H]-targretin in a competiti... | J Med Chem 42: 742-50 (1999) Article DOI: 10.1021/jm980621r BindingDB Entry DOI: 10.7270/Q2K936Q1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Retinoic acid receptor RXR-beta (Homo sapiens (Human)) | BDBM50074300 (4-[[(E)-Hydroxyimino]-(3,5,5,8,8-pentamethyl-5,6,7...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ligand Pharmaceuticals, Inc Curated by ChEMBL | Assay Description Binding affinity towards recombinantly expressed Retinoic acid receptor RXR-beta in baculoviral sysytem, by using 5 nM [3H]-targretin in a competitiv... | J Med Chem 42: 742-50 (1999) Article DOI: 10.1021/jm980621r BindingDB Entry DOI: 10.7270/Q2K936Q1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase Lck (Homo sapiens (Human)) | BDBM50129307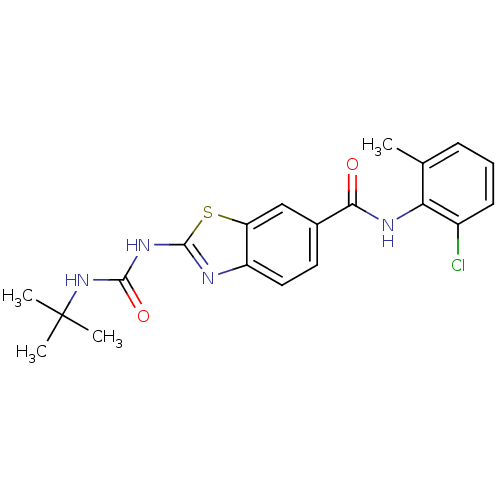 (2-(3-tert-Butyl-ureido)-benzothiazole-6-carboxylic...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 5.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibition of p56 Lck tyrosine kinase | Bioorg Med Chem Lett 13: 2587-90 (2003) BindingDB Entry DOI: 10.7270/Q2FX78V4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Retinoic acid receptor RXR-alpha (Homo sapiens (Human)) | BDBM50074307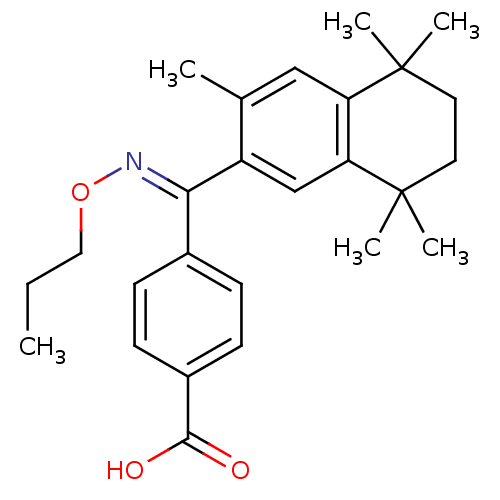 (4-{(3,5,5,8,8-Pentamethyl-5,6,7,8-tetrahydro-napht...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ligand Pharmaceuticals, Inc Curated by ChEMBL | Assay Description Binding affinity towards recombinantly expressed Retinoic acid receptor RXR-alpha in baculoviral sysytem, by using 5 nM [3H]-targretin in a competiti... | J Med Chem 42: 742-50 (1999) Article DOI: 10.1021/jm980621r BindingDB Entry DOI: 10.7270/Q2K936Q1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Retinoic acid receptor RXR-alpha (Homo sapiens (Human)) | BDBM50074300 (4-[[(E)-Hydroxyimino]-(3,5,5,8,8-pentamethyl-5,6,7...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ligand Pharmaceuticals, Inc Curated by ChEMBL | Assay Description Binding affinity towards recombinantly expressed Retinoic acid receptor RXR-alpha in baculoviral sysytem, by using 5 nM [3H]-targretin in a competiti... | J Med Chem 42: 742-50 (1999) Article DOI: 10.1021/jm980621r BindingDB Entry DOI: 10.7270/Q2K936Q1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Retinoic acid receptor RXR-beta (Homo sapiens (Human)) | BDBM50074304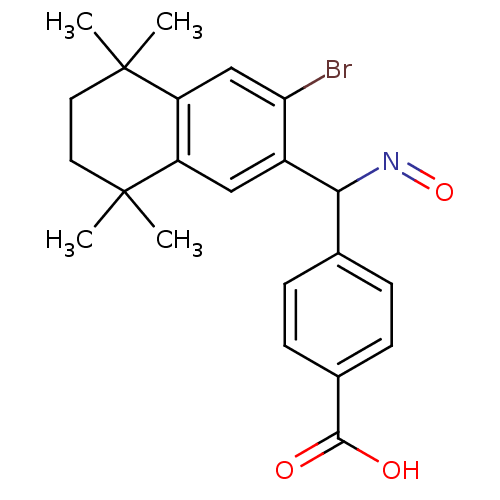 (4-{(3-Bromo-5,5,8,8-tetramethyl-5,6,7,8-tetrahydro...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | Article PubMed | 6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ligand Pharmaceuticals, Inc Curated by ChEMBL | Assay Description Binding affinity towards recombinantly expressed Retinoic acid receptor RXR-beta in baculoviral sysytem, by using 5 nM [3H]-targretin in a competitiv... | J Med Chem 42: 742-50 (1999) Article DOI: 10.1021/jm980621r BindingDB Entry DOI: 10.7270/Q2K936Q1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase Lck (Homo sapiens (Human)) | BDBM50131131 (2-(6-Methylamino-pyrimidin-4-ylamino)-benzothiazol...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 6.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibition of p56 Lck tyrosine kinase | Bioorg Med Chem Lett 13: 2587-90 (2003) BindingDB Entry DOI: 10.7270/Q2FX78V4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Retinoic acid receptor RXR-alpha (Homo sapiens (Human)) | BDBM50074306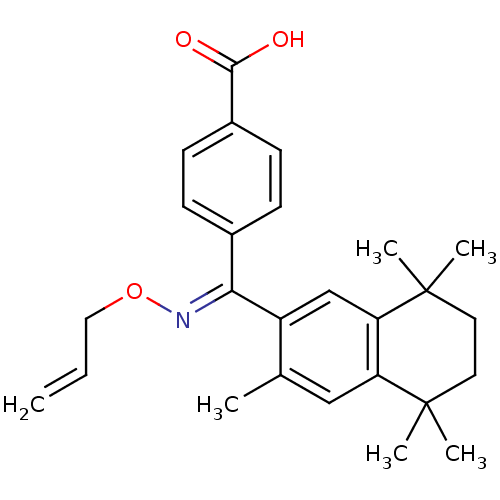 (4-[[(E)-Allyloxyimino]-(3,5,5,8,8-pentamethyl-5,6,...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 7 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ligand Pharmaceuticals, Inc Curated by ChEMBL | Assay Description Binding affinity towards recombinantly expressed Retinoic acid receptor RXR-alpha in baculoviral sysytem, by using 5 nM [3H]-targretin in a competiti... | J Med Chem 42: 742-50 (1999) Article DOI: 10.1021/jm980621r BindingDB Entry DOI: 10.7270/Q2K936Q1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Retinoic acid receptor RXR-gamma (Homo sapiens (Human)) | BDBM50074295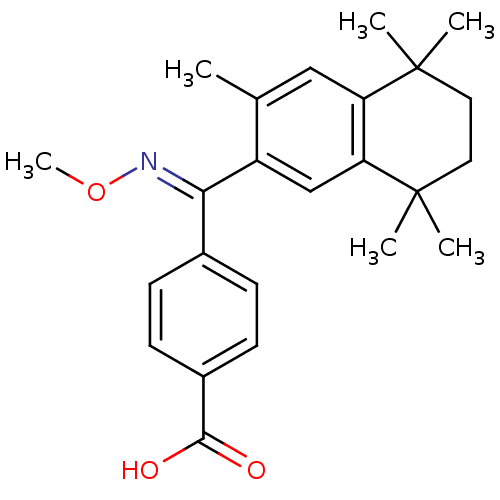 (4-[[(E)-Methoxyimino]-(3,5,5,8,8-pentamethyl-5,6,7...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 8 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ligand Pharmaceuticals, Inc Curated by ChEMBL | Assay Description Binding affinity towards recombinantly expressed Retinoic acid receptor RXR-gamma in baculoviral sysytem, by using 5 nM [3H]-targretin in a competiti... | J Med Chem 42: 742-50 (1999) Article DOI: 10.1021/jm980621r BindingDB Entry DOI: 10.7270/Q2K936Q1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Retinoic acid receptor RXR-beta (Homo sapiens (Human)) | BDBM50074295 (4-[[(E)-Methoxyimino]-(3,5,5,8,8-pentamethyl-5,6,7...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 8 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ligand Pharmaceuticals, Inc Curated by ChEMBL | Assay Description Binding affinity towards recombinantly expressed Retinoic acid receptor RXR-beta in baculoviral sysytem, by using 5 nM [3H]-targretin in a competitiv... | J Med Chem 42: 742-50 (1999) Article DOI: 10.1021/jm980621r BindingDB Entry DOI: 10.7270/Q2K936Q1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Retinoic acid receptor RXR-alpha (Homo sapiens (Human)) | BDBM31892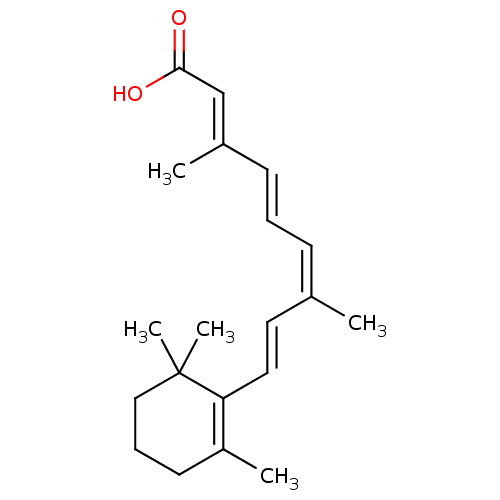 (9-cis retinoic acid | 9C-RA | CHEMBL705 | Panretin...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | DrugBank MMDB PDB Article PubMed | 8 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ligand Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description Inhibition of [3H]-Targretin binding to Retinoid X receptor RXR alpha | J Med Chem 39: 3229-34 (1996) Article DOI: 10.1021/jm960311d BindingDB Entry DOI: 10.7270/Q2ZW1K14 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Retinoic acid receptor RXR-alpha (Homo sapiens (Human)) | BDBM50052589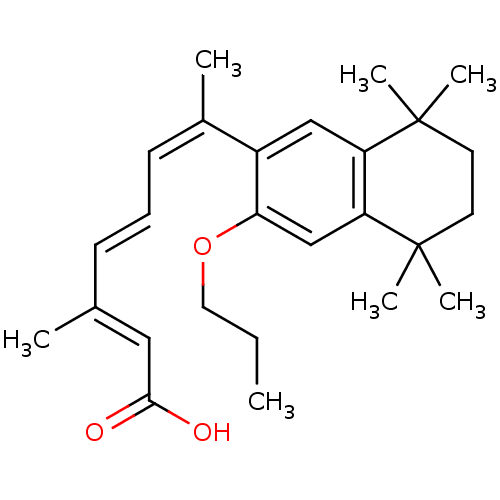 ((2E,4E,6Z)-3-Methyl-7-(5,5,8,8-tetramethyl-3-propo...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | 8 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ligand Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description Inhibition of [3H]-Targretin binding to Retinoid X receptor RXR alpha | J Med Chem 39: 3229-34 (1996) Article DOI: 10.1021/jm960311d BindingDB Entry DOI: 10.7270/Q2ZW1K14 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50107463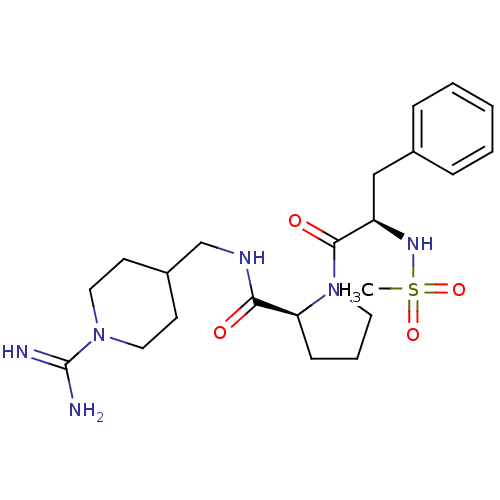 ((S)-1-((R)-2-Methanesulfonylamino-3-phenyl-propion...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid PDB UniChem Patents Similars | PubMed | 8.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Competitive kinetic for human alpha thrombin inhibition Ki was determined | Bioorg Med Chem Lett 12: 45-9 (2001) BindingDB Entry DOI: 10.7270/Q2GX4C3P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase Fyn (Homo sapiens (Human)) | BDBM50131131 (2-(6-Methylamino-pyrimidin-4-ylamino)-benzothiazol...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 8.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibition of Fyn protein kinase | Bioorg Med Chem Lett 13: 2587-90 (2003) BindingDB Entry DOI: 10.7270/Q2FX78V4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium/glucose cotransporter 2 (Homo sapiens (Human)) | BDBM50153302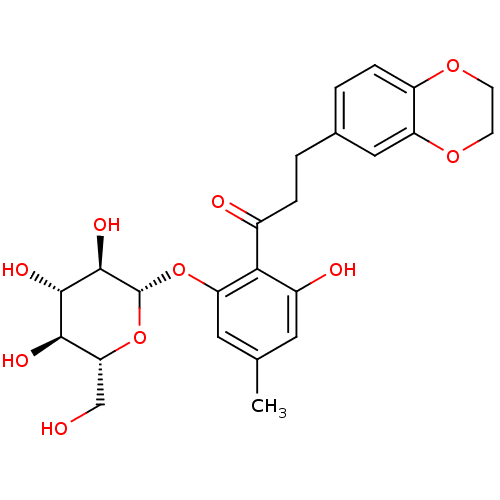 (3-(2,3-Dihydro-benzo[1,4]dioxin-6-yl)-1-[2-hydroxy...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | 9 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research and Development Curated by ChEMBL | Assay Description In vitro inhibition of Na-dependent [14C]-AMG uptake in CHO-K1 cells expressing human sodium glucose co-transporter 2 | Bioorg Med Chem Lett 14: 5121-5 (2004) Article DOI: 10.1016/j.bmcl.2004.07.082 BindingDB Entry DOI: 10.7270/Q2VD6XX2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Retinoic acid receptor RXR-alpha (Homo sapiens (Human)) | BDBM50074295 (4-[[(E)-Methoxyimino]-(3,5,5,8,8-pentamethyl-5,6,7...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 9 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ligand Pharmaceuticals, Inc Curated by ChEMBL | Assay Description Binding affinity towards recombinantly expressed Retinoic acid receptor RXR-alpha in baculoviral sysytem, by using 5 nM [3H]-targretin in a competiti... | J Med Chem 42: 742-50 (1999) Article DOI: 10.1021/jm980621r BindingDB Entry DOI: 10.7270/Q2K936Q1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Retinoic acid receptor RXR-beta (Homo sapiens (Human)) | BDBM31892 (9-cis retinoic acid | 9C-RA | CHEMBL705 | Panretin...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | DrugBank MMDB PDB Article PubMed | 9 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ligand Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description Inhibition of [3H]-Targretin binding to Retinoid X receptor RXR beta | J Med Chem 39: 3229-34 (1996) Article DOI: 10.1021/jm960311d BindingDB Entry DOI: 10.7270/Q2ZW1K14 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Retinoic acid receptor RXR-beta (Homo sapiens (Human)) | BDBM50052589 ((2E,4E,6Z)-3-Methyl-7-(5,5,8,8-tetramethyl-3-propo...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | 9 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ligand Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description Inhibition of [3H]-Targretin binding to Retinoid X receptor RXR beta | J Med Chem 39: 3229-34 (1996) Article DOI: 10.1021/jm960311d BindingDB Entry DOI: 10.7270/Q2ZW1K14 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Sodium/glucose cotransporter 2 (Homo sapiens (Human)) | BDBM50153301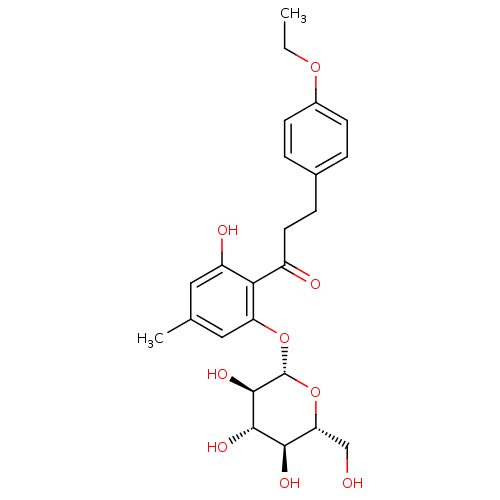 (3-(4-Ethoxy-phenyl)-1-[2-hydroxy-4-methyl-6-(3,4,5...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | 10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research and Development Curated by ChEMBL | Assay Description In vitro inhibition of Na-dependent [14C]-AMG uptake in CHO-K1 cells expressing human sodium glucose co-transporter 2 | Bioorg Med Chem Lett 14: 5121-5 (2004) Article DOI: 10.1016/j.bmcl.2004.07.082 BindingDB Entry DOI: 10.7270/Q2VD6XX2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium/glucose cotransporter 2 (Homo sapiens (Human)) | BDBM50153312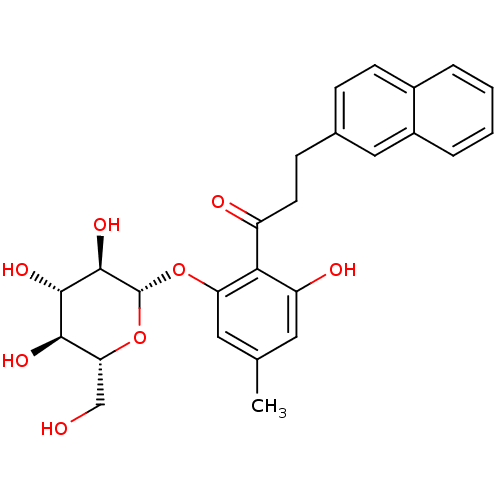 (1-[2-Hydroxy-4-methyl-6-(3,4,5-trihydroxy-6-hydrox...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | 10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research and Development Curated by ChEMBL | Assay Description In vitro inhibition of Na-dependent [14C]-AMG uptake in CHO-K1 cells expressing human sodium glucose co-transporter 2 | Bioorg Med Chem Lett 14: 5121-5 (2004) Article DOI: 10.1016/j.bmcl.2004.07.082 BindingDB Entry DOI: 10.7270/Q2VD6XX2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium/glucose cotransporter 2 (Homo sapiens (Human)) | BDBM50153298 (3-(2,3-Dihydro-benzo[1,4]dioxin-6-yl)-1-[4-ethyl-2...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | 10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research and Development Curated by ChEMBL | Assay Description In vitro inhibition of Na-dependent [14C]-AMG uptake in CHO-K1 cells expressing human sodium glucose co-transporter 2 | Bioorg Med Chem Lett 14: 5121-5 (2004) Article DOI: 10.1016/j.bmcl.2004.07.082 BindingDB Entry DOI: 10.7270/Q2VD6XX2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium/glucose cotransporter 2 (Homo sapiens (Human)) | BDBM50153305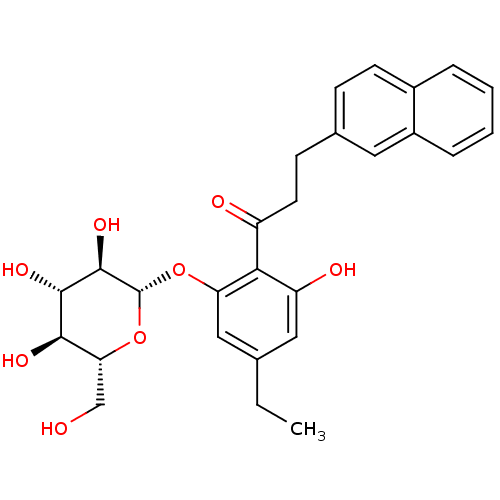 (1-[4-Ethyl-2-hydroxy-6-(3,4,5-trihydroxy-6-hydroxy...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | 10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research and Development Curated by ChEMBL | Assay Description In vitro inhibition of Na-dependent [14C]-AMG uptake in CHO-K1 cells expressing human sodium glucose co-transporter 2 | Bioorg Med Chem Lett 14: 5121-5 (2004) Article DOI: 10.1016/j.bmcl.2004.07.082 BindingDB Entry DOI: 10.7270/Q2VD6XX2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Retinoic acid receptor RXR-alpha (Homo sapiens (Human)) | BDBM50074297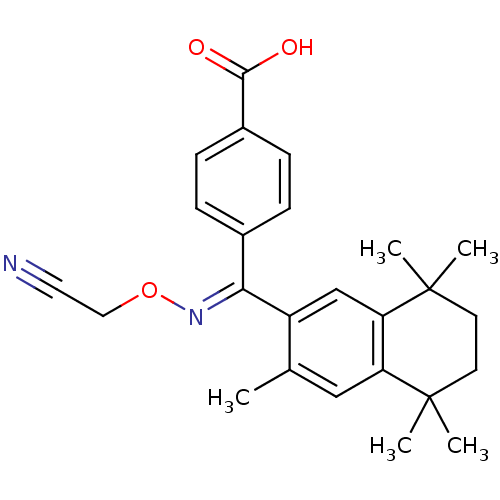 (4-[[(E)-Cyanomethoxyimino]-(3,5,5,8,8-pentamethyl-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 11 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ligand Pharmaceuticals, Inc Curated by ChEMBL | Assay Description Binding affinity towards recombinantly expressed Retinoic acid receptor RXR-alpha in baculoviral sysytem, by using 5 nM [3H]-targretin in a competiti... | J Med Chem 42: 742-50 (1999) Article DOI: 10.1021/jm980621r BindingDB Entry DOI: 10.7270/Q2K936Q1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Retinoic acid receptor RXR-beta (Homo sapiens (Human)) | BDBM50052590 ((2E,4E,6Z)-7-(3-Ethoxy-5,5,8,8-tetramethyl-5,6,7,8...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 12 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ligand Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description Inhibition of [3H]-Targretin binding to Retinoid X receptor RXR beta | J Med Chem 39: 3229-34 (1996) Article DOI: 10.1021/jm960311d BindingDB Entry DOI: 10.7270/Q2ZW1K14 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Retinoic acid receptor RXR-alpha (Homo sapiens (Human)) | BDBM50074304 (4-{(3-Bromo-5,5,8,8-tetramethyl-5,6,7,8-tetrahydro...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | Article PubMed | 12 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ligand Pharmaceuticals, Inc Curated by ChEMBL | Assay Description Binding affinity towards recombinantly expressed Retinoic acid receptor RXR-alpha in baculoviral sysytem, by using 5 nM [3H]-targretin in a competiti... | J Med Chem 42: 742-50 (1999) Article DOI: 10.1021/jm980621r BindingDB Entry DOI: 10.7270/Q2K936Q1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proto-oncogene tyrosine-protein kinase Src (Homo sapiens (Human)) | BDBM13357 (CHEMBL312933 | N-(2-chloro-6-methylphenyl)-2-({6-[...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 13 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibition of Src protein tryrosine kinase | Bioorg Med Chem Lett 13: 2587-90 (2003) BindingDB Entry DOI: 10.7270/Q2FX78V4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Retinoic acid receptor RXR-gamma (Homo sapiens (Human)) | BDBM31892 (9-cis retinoic acid | 9C-RA | CHEMBL705 | Panretin...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | DrugBank MMDB PDB Article PubMed | 14 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ligand Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description Inhibition of [3H]-Targretin binding to Retinoid X receptor RXR gamma | J Med Chem 39: 3229-34 (1996) Article DOI: 10.1021/jm960311d BindingDB Entry DOI: 10.7270/Q2ZW1K14 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Retinoic acid receptor RXR-gamma (Homo sapiens (Human)) | BDBM50074297 (4-[[(E)-Cyanomethoxyimino]-(3,5,5,8,8-pentamethyl-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 14 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ligand Pharmaceuticals, Inc Curated by ChEMBL | Assay Description Binding affinity towards recombinantly expressed Retinoic acid receptor RXR-gamma in baculoviral sysytem, by using 5 nM [3H]-targretin in a competiti... | J Med Chem 42: 742-50 (1999) Article DOI: 10.1021/jm980621r BindingDB Entry DOI: 10.7270/Q2K936Q1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Retinoic acid receptor RXR-gamma (Homo sapiens (Human)) | BDBM50052589 ((2E,4E,6Z)-3-Methyl-7-(5,5,8,8-tetramethyl-3-propo...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | 14 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ligand Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description Inhibition of [3H]-Targretin binding to Retinoid X receptor RXR gamma | J Med Chem 39: 3229-34 (1996) Article DOI: 10.1021/jm960311d BindingDB Entry DOI: 10.7270/Q2ZW1K14 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Displayed 1 to 50 (of 2108 total ) | Next | Last >> |