

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Protease (Human immunodeficiency virus 1 (HIV-1)) | BDBM50469417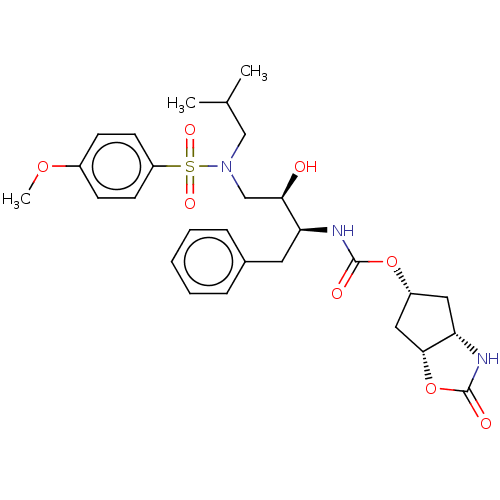 (CHEMBL4293023) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid PDB UniChem | Article PubMed | 0.00120 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University Curated by ChEMBL | Assay Description Inhibition of HIV1 wild type NL4-3 protease expressed in Escherichia coli Rosetta (DE3) pLysS using Abz-Thr-Ile-Nle-Phe-(pNO2)-Gln-Arg-NH2 as substra... | J Med Chem 61: 9722-9737 (2018) Article DOI: 10.1021/acs.jmedchem.8b01227 BindingDB Entry DOI: 10.7270/Q25T3P6J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protease (Human immunodeficiency virus 1 (HIV-1)) | BDBM50469407 (CHEMBL4286714) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.0160 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University Curated by ChEMBL | Assay Description Inhibition of HIV1 wild type NL4-3 protease expressed in Escherichia coli Rosetta (DE3) pLysS using Abz-Thr-Ile-Nle-Phe-(pNO2)-Gln-Arg-NH2 as substra... | J Med Chem 61: 9722-9737 (2018) Article DOI: 10.1021/acs.jmedchem.8b01227 BindingDB Entry DOI: 10.7270/Q25T3P6J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protease (Human immunodeficiency virus 1 (HIV-1)) | BDBM8125 ((3R,3aS,6aR)-hexahydrofuro[2,3-b]furan-3-yl N-[(2S...) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | 0.0160 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University Curated by ChEMBL | Assay Description Inhibition of HIV1 wild type NL4-3 protease expressed in Escherichia coli Rosetta (DE3) pLysS using Abz-Thr-Ile-Nle-Phe-(pNO2)-Gln-Arg-NH2 as substra... | J Med Chem 61: 9722-9737 (2018) Article DOI: 10.1021/acs.jmedchem.8b01227 BindingDB Entry DOI: 10.7270/Q25T3P6J | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Protease (Human immunodeficiency virus 1 (HIV-1)) | BDBM8125 ((3R,3aS,6aR)-hexahydrofuro[2,3-b]furan-3-yl N-[(2S...) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | 0.0160 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University Curated by ChEMBL | Assay Description Inhibition of HIV1 protease using Ac-Thr-Ile-Nle-Nle-Gln-Arg-NH2 substrate by continuous fluorometric assay | Bioorg Med Chem Lett 29: 2565-2570 (2019) Article DOI: 10.1016/j.bmcl.2019.08.006 BindingDB Entry DOI: 10.7270/Q2JM2F1C | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Protease (Human immunodeficiency virus 1 (HIV-1)) | BDBM50469413 (CHEMBL4286231) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.0160 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University Curated by ChEMBL | Assay Description Inhibition of HIV1 wild type NL4-3 protease expressed in Escherichia coli Rosetta (DE3) pLysS using Abz-Thr-Ile-Nle-Phe-(pNO2)-Gln-Arg-NH2 as substra... | J Med Chem 61: 9722-9737 (2018) Article DOI: 10.1021/acs.jmedchem.8b01227 BindingDB Entry DOI: 10.7270/Q25T3P6J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protease (Human immunodeficiency virus 1 (HIV-1)) | BDBM50469410 (CHEMBL4277886) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.0300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University Curated by ChEMBL | Assay Description Inhibition of HIV1 wild type NL4-3 protease expressed in Escherichia coli Rosetta (DE3) pLysS using Abz-Thr-Ile-Nle-Phe-(pNO2)-Gln-Arg-NH2 as substra... | J Med Chem 61: 9722-9737 (2018) Article DOI: 10.1021/acs.jmedchem.8b01227 BindingDB Entry DOI: 10.7270/Q25T3P6J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-1A adrenergic receptor (Homo sapiens (Human)) | BDBM50220746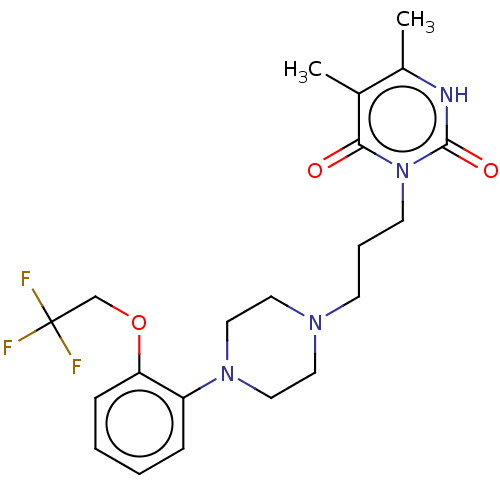 (CHEMBL292189) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.0398 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Bioscience Curated by ChEMBL | Assay Description Inhibition of [3H]prazosin binding to CHO-K1 whole cells expressing human cloned Alpha-1A adrenergic receptor | Bioorg Med Chem Lett 13: 1873-8 (2003) BindingDB Entry DOI: 10.7270/Q2S46V50 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protease (Human immunodeficiency virus 1 (HIV-1)) | BDBM50469405 (CHEMBL4292006) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.0400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University Curated by ChEMBL | Assay Description Inhibition of HIV1 wild type NL4-3 protease expressed in Escherichia coli Rosetta (DE3) pLysS using Abz-Thr-Ile-Nle-Phe-(pNO2)-Gln-Arg-NH2 as substra... | J Med Chem 61: 9722-9737 (2018) Article DOI: 10.1021/acs.jmedchem.8b01227 BindingDB Entry DOI: 10.7270/Q25T3P6J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM50061031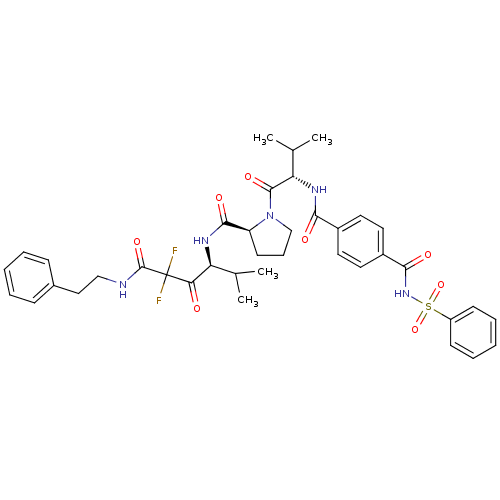 ((S)-1-[(S)-2-(4-Benzenesulfonylaminocarbonyl-benzo...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
ZENECA Pharmaceuticals Curated by ChEMBL | Assay Description Binding affinity for human leukocyte elastase | J Med Chem 40: 3173-81 (1997) Article DOI: 10.1021/jm970250z BindingDB Entry DOI: 10.7270/Q2GT5M85 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM50031196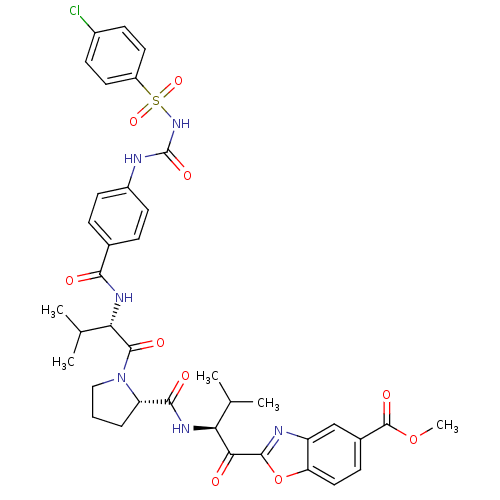 (2-[3-Methyl-2-({1-[3-methyl-2-(4-chlorophenyl-sulf...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.0600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
ZENECA Pharmaceuticals Curated by ChEMBL | Assay Description Inhibition of human neutrophil elastase-catalyzed hydrolysis of the synthetic substrate MeO-Suc-Ala-Ala-Pro-Val-pNa | J Med Chem 38: 3972-82 (1995) BindingDB Entry DOI: 10.7270/Q2T152NN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-1A adrenergic receptor (Homo sapiens (Human)) | BDBM50220747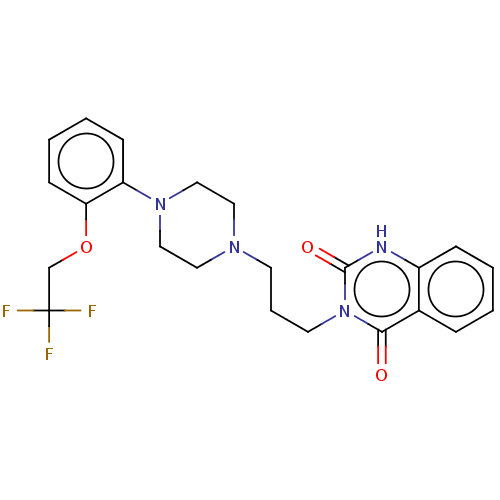 (CHEMBL56863) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.0631 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Bioscience Curated by ChEMBL | Assay Description Inhibition of [3H]prazosin binding to CHO-K1 whole cells expressing human cloned Alpha-1A adrenergic receptor | Bioorg Med Chem Lett 13: 1873-8 (2003) BindingDB Entry DOI: 10.7270/Q2S46V50 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-1A adrenergic receptor (CALF) | BDBM50408198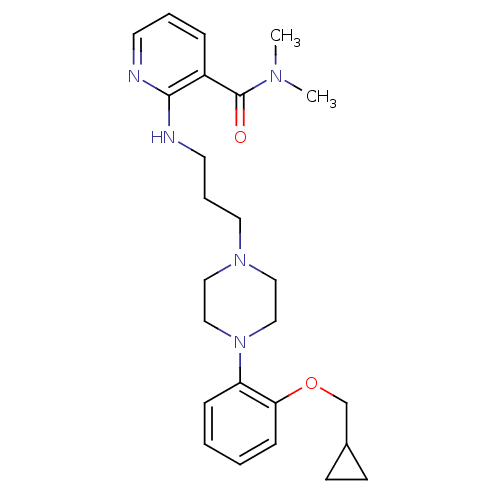 (CHEMBL91278) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0794 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Bioscience Curated by ChEMBL | Assay Description In vitro for the displacement of [3H]prazosin binding to bovine Alpha-1A adrenergic receptor | J Med Chem 40: 2674-87 (1997) Checked by Author Article DOI: 10.1021/jm970166j BindingDB Entry DOI: 10.7270/Q2R78GD2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protease (Human immunodeficiency virus 1 (HIV-1)) | BDBM50469412 (CHEMBL4277970) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.0800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University Curated by ChEMBL | Assay Description Inhibition of HIV1 wild type NL4-3 protease expressed in Escherichia coli Rosetta (DE3) pLysS using Abz-Thr-Ile-Nle-Phe-(pNO2)-Gln-Arg-NH2 as substra... | J Med Chem 61: 9722-9737 (2018) Article DOI: 10.1021/acs.jmedchem.8b01227 BindingDB Entry DOI: 10.7270/Q25T3P6J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM50031185 (2-{(S)-2-[((S)-1-{(S)-2-[4-(4-Chloro-benzenesulfon...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
ZENECA Pharmaceuticals Curated by ChEMBL | Assay Description Inhibition of human neutrophil elastase-catalyzed hydrolysis of the synthetic substrate MeO-Suc-Ala-Ala-Pro-Val-pNa | J Med Chem 38: 3972-82 (1995) BindingDB Entry DOI: 10.7270/Q2T152NN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neurotensin receptor type 1 (MOUSE) | BDBM50240339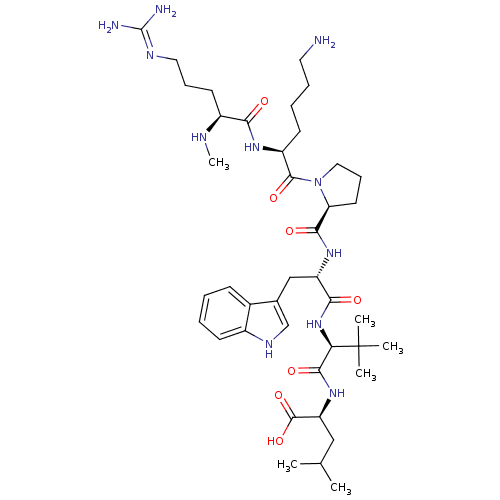 ((S)-2-((S)-2-((S)-2-((S)-1-((S)-6-amino-2-((S)-5-g...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.110 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by PDSP Ki Database | J Pharmacol Exp Ther 300: 305-13 (2002) Article DOI: 10.1124/jpet.300.1.305 BindingDB Entry DOI: 10.7270/Q2X34W09 | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-1A adrenergic receptor (CALF) | BDBM50408201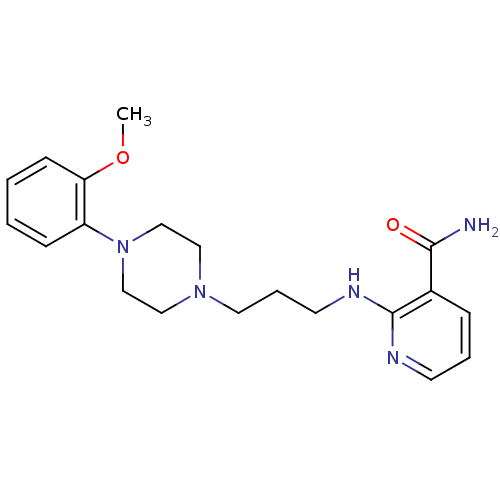 (CHEMBL88512) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.126 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Bioscience Curated by ChEMBL | Assay Description In vitro for the displacement of [3H]prazosin binding to bovine Alpha-1A adrenergic receptor | J Med Chem 40: 2674-87 (1997) Checked by Author Article DOI: 10.1021/jm970166j BindingDB Entry DOI: 10.7270/Q2R78GD2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-1A adrenergic receptor (CALF) | BDBM29568 (CHEMBL2 | PRAZOSIN | PRAZOSIN HYDROCHLORIDE | [3H]...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 0.126 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Bioscience Curated by ChEMBL | Assay Description In vitro for the displacement of [3H]prazosin binding to bovine Alpha-1A adrenergic receptor | J Med Chem 40: 2674-87 (1997) Checked by Author Article DOI: 10.1021/jm970166j BindingDB Entry DOI: 10.7270/Q2R78GD2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-1A adrenergic receptor (CALF) | BDBM50060964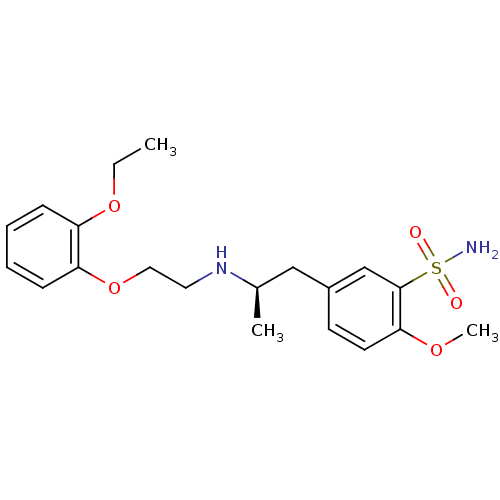 ((R)-5-(2-((2-(2-ethoxyphenoxy)ethyl)amino)propyl)-...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 0.126 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Bioscience Curated by ChEMBL | Assay Description In vitro for the displacement of [3H]prazosin binding to bovine Alpha-1A adrenergic receptor | J Med Chem 40: 2674-87 (1997) Checked by Author Article DOI: 10.1021/jm970166j BindingDB Entry DOI: 10.7270/Q2R78GD2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Collagenase 3 (Homo sapiens (Human)) | BDBM50343081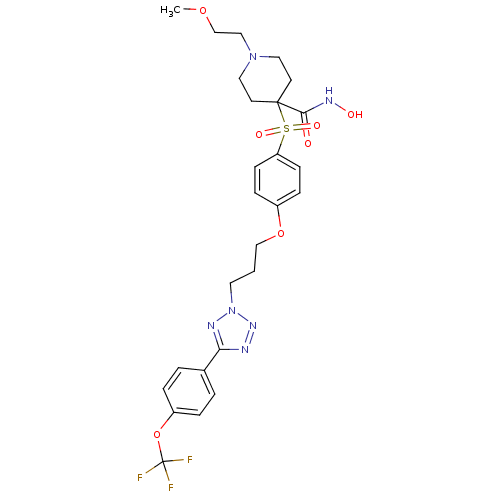 (CHEMBL1771216 | N-hydroxy-1-(2-methoxyethyl)-4-(4-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid PDB UniChem Patents | PDB Article PubMed | 0.130 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Inhibition of human MMP-13 | Bioorg Med Chem Lett 21: 2820-2 (2011) Article DOI: 10.1016/j.bmcl.2011.03.099 BindingDB Entry DOI: 10.7270/Q2416XCK | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Protease (Human immunodeficiency virus 1 (HIV-1)) | BDBM50469418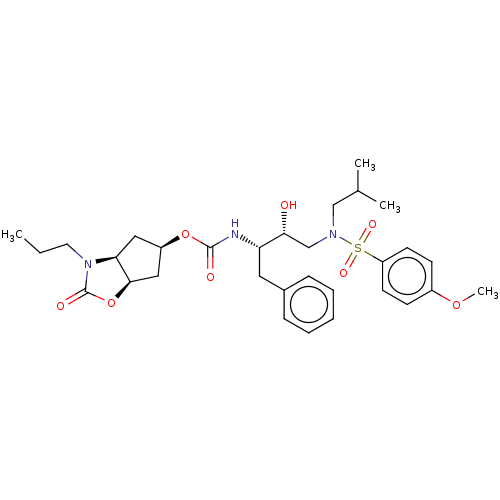 (CHEMBL4283819) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.140 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University Curated by ChEMBL | Assay Description Inhibition of HIV1 wild type NL4-3 protease expressed in Escherichia coli Rosetta (DE3) pLysS using Abz-Thr-Ile-Nle-Phe-(pNO2)-Gln-Arg-NH2 as substra... | J Med Chem 61: 9722-9737 (2018) Article DOI: 10.1021/acs.jmedchem.8b01227 BindingDB Entry DOI: 10.7270/Q25T3P6J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-1A adrenergic receptor (Homo sapiens (Human)) | BDBM50220750 (CHEMBL55695) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.158 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Bioscience Curated by ChEMBL | Assay Description Inhibition of [3H]prazosin binding to CHO-K1 whole cells expressing human cloned Alpha-1A adrenergic receptor | Bioorg Med Chem Lett 13: 1873-8 (2003) BindingDB Entry DOI: 10.7270/Q2S46V50 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protease (Human immunodeficiency virus 1 (HIV-1)) | BDBM50469406 (CHEMBL4277539) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.160 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University Curated by ChEMBL | Assay Description Inhibition of HIV1 wild type NL4-3 protease expressed in Escherichia coli Rosetta (DE3) pLysS using Abz-Thr-Ile-Nle-Phe-(pNO2)-Gln-Arg-NH2 as substra... | J Med Chem 61: 9722-9737 (2018) Article DOI: 10.1021/acs.jmedchem.8b01227 BindingDB Entry DOI: 10.7270/Q25T3P6J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protease (Human immunodeficiency virus 1 (HIV-1)) | BDBM50469409 (CHEMBL4278306) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.160 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University Curated by ChEMBL | Assay Description Inhibition of HIV1 wild type NL4-3 protease expressed in Escherichia coli Rosetta (DE3) pLysS using Abz-Thr-Ile-Nle-Phe-(pNO2)-Gln-Arg-NH2 as substra... | J Med Chem 61: 9722-9737 (2018) Article DOI: 10.1021/acs.jmedchem.8b01227 BindingDB Entry DOI: 10.7270/Q25T3P6J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM50031203 (2-{(S)-2-[((S)-1-{(S)-2-[4-(4-Chloro-benzenesulfon...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.180 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
ZENECA Pharmaceuticals Curated by ChEMBL | Assay Description Inhibition of human neutrophil elastase-catalyzed hydrolysis of the synthetic substrate MeO-Suc-Ala-Ala-Pro-Val-pNa | J Med Chem 38: 3972-82 (1995) BindingDB Entry DOI: 10.7270/Q2T152NN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A2a (Homo sapiens (Human)) | BDBM26236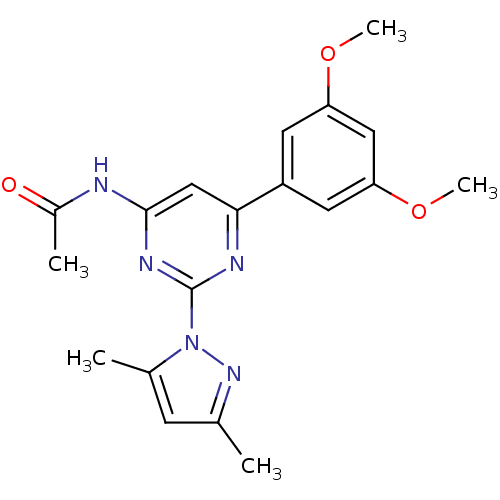 (CHEMBL487569 | N-[6-(3,5-dimethoxyphenyl)-2-(3,5-d...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.190 | -54.9 | n/a | n/a | n/a | n/a | n/a | 7.4 | 22 |
Neurocrine Bioscience | Assay Description The membranes prepared from HEK cells transfected with adenosine receptors were used in binding assays. Nonspecific binding was determined in the pre... | J Med Chem 51: 7099-7110 (2008) Article DOI: 10.1021/jm800851u BindingDB Entry DOI: 10.7270/Q20R9MQG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protease (Human immunodeficiency virus 1 (HIV-1)) | BDBM50469408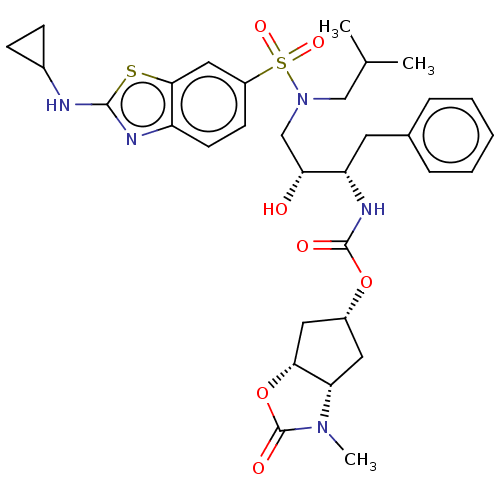 (CHEMBL4278989) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.190 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University Curated by ChEMBL | Assay Description Inhibition of HIV1 wild type NL4-3 protease expressed in Escherichia coli Rosetta (DE3) pLysS using Abz-Thr-Ile-Nle-Phe-(pNO2)-Gln-Arg-NH2 as substra... | J Med Chem 61: 9722-9737 (2018) Article DOI: 10.1021/acs.jmedchem.8b01227 BindingDB Entry DOI: 10.7270/Q25T3P6J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A2a (Homo sapiens (Human)) | BDBM50237067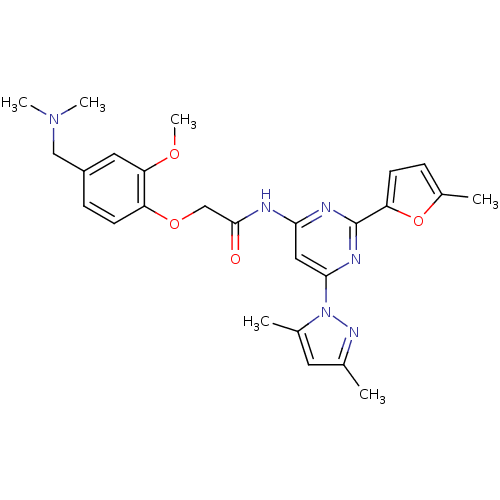 (CHEMBL429125 | N-(6-(3,5-dimethyl-1H-pyrazol-1-yl)...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Neurocrine Biosciences Curated by ChEMBL | Assay Description Displacement of [3H]-ZM241385 from human adenosine A2A receptor expressed in HEK293 cells | Bioorg Med Chem Lett 18: 1778-83 (2008) Article DOI: 10.1016/j.bmcl.2008.02.032 BindingDB Entry DOI: 10.7270/Q2VD6Z62 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM50031187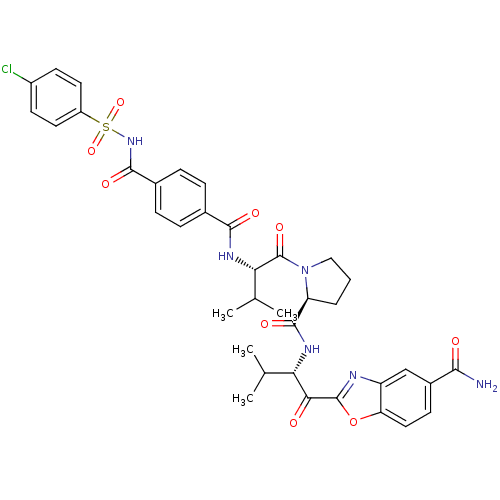 (2-{(S)-2-[((S)-1-{(S)-2-[4-(4-Chloro-benzenesulfon...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
ZENECA Pharmaceuticals Curated by ChEMBL | Assay Description Inhibition of human neutrophil elastase-catalyzed hydrolysis of the synthetic substrate MeO-Suc-Ala-Ala-Pro-Val-pNa | J Med Chem 38: 3972-82 (1995) BindingDB Entry DOI: 10.7270/Q2T152NN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM50031198 (1-(4-(((S)-1-((S)-2-(((S)-1-(benzo[d]oxazol-2-yl)-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
ZENECA Pharmaceuticals Curated by ChEMBL | Assay Description Inhibition of human neutrophil elastase-catalyzed hydrolysis of the synthetic substrate MeO-Suc-Ala-Ala-Pro-Val-pNa | J Med Chem 38: 3972-82 (1995) BindingDB Entry DOI: 10.7270/Q2T152NN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A2a (Homo sapiens (Human)) | BDBM50372562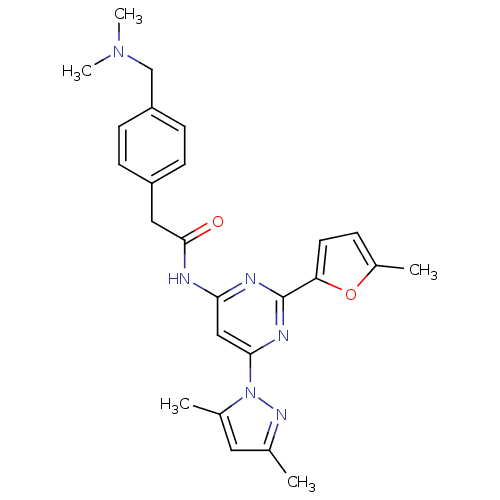 (CHEMBL255739) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Neurocrine Biosciences Curated by ChEMBL | Assay Description Displacement of [3H]ZM241385 from human adenosine A2A receptor expressed in HEK293 cells | Bioorg Med Chem Lett 18: 1269-73 (2008) Article DOI: 10.1016/j.bmcl.2008.01.036 BindingDB Entry DOI: 10.7270/Q2K35VGF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A2a (Homo sapiens (Human)) | BDBM26236 (CHEMBL487569 | N-[6-(3,5-dimethoxyphenyl)-2-(3,5-d...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Neurocrine Biosciences Curated by ChEMBL | Assay Description Displacement of [3H]ZM241385 from human adenosine A2A receptor expressed in HEK293 cells | Bioorg Med Chem Lett 18: 5402-5 (2008) Article DOI: 10.1016/j.bmcl.2008.09.048 BindingDB Entry DOI: 10.7270/Q28K78ZG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neurotensin receptor type 1 (MOUSE) | BDBM82078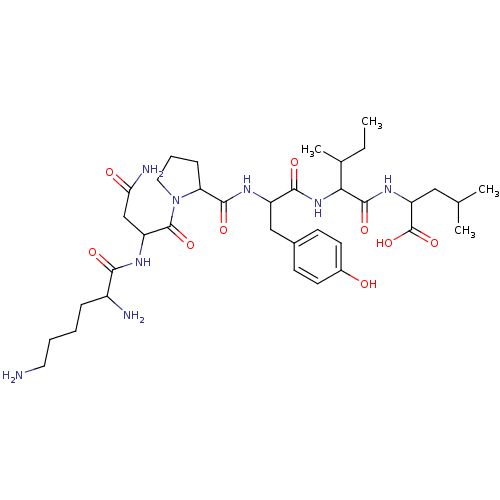 (CAS_55508-42-4 | NSC_128644 | Neurotensin) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.25 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by PDSP Ki Database | J Pharmacol Exp Ther 300: 305-13 (2002) Article DOI: 10.1124/jpet.300.1.305 BindingDB Entry DOI: 10.7270/Q2X34W09 | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-1A adrenergic receptor (Homo sapiens (Human)) | BDBM50220788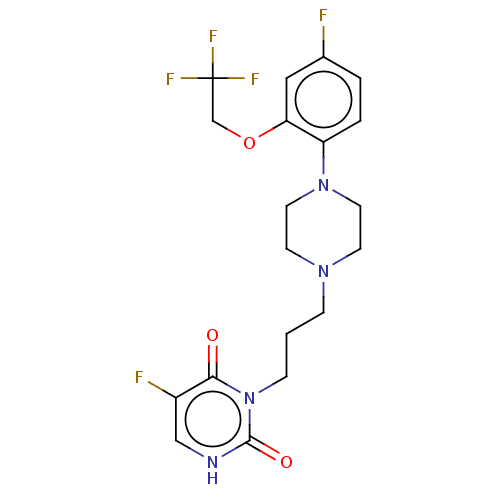 (CHEMBL417954) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.251 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Bioscience Curated by ChEMBL | Assay Description Inhibition of [3H]prazosin binding to CHO-K1 whole cells expressing human cloned Alpha-1A adrenergic receptor | Bioorg Med Chem Lett 13: 1873-8 (2003) BindingDB Entry DOI: 10.7270/Q2S46V50 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-1A adrenergic receptor (Homo sapiens (Human)) | BDBM50160161 (5-Methyl-3-(3-{4-[2-(2,2,2-trifluoro-ethoxy)-pheny...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | PubMed | 0.251 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Bioscience Curated by ChEMBL | Assay Description Inhibition of [3H]prazosin binding to CHO-K1 whole cells expressing human cloned Alpha-1A adrenergic receptor | Bioorg Med Chem Lett 13: 1873-8 (2003) BindingDB Entry DOI: 10.7270/Q2S46V50 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Plasma kallikrein (Homo sapiens (Human)) | BDBM50579244 (Avoralstat | BCX-4161) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem | Article PubMed | 0.260 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of purified human plasma kallikrein assessed as inhibition constant using H-D-Pro-Phe-Arg-pNA.2HCl as substrate measured after 3 mins by m... | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c00511 BindingDB Entry DOI: 10.7270/Q29C7288 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A2a (Homo sapiens (Human)) | BDBM26250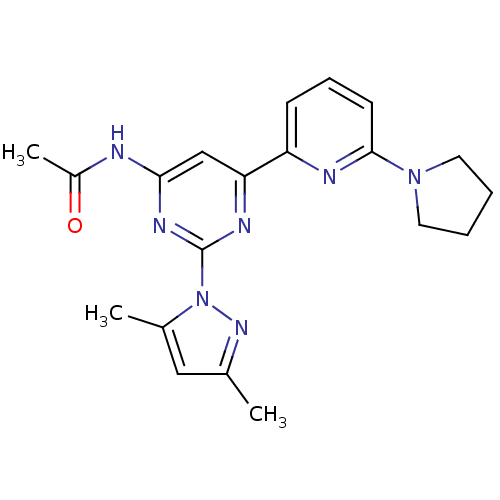 (N-[2-(3,5-dimethyl-1H-pyrazol-1-yl)-6-[6-(pyrrolid...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.260 | -54.2 | n/a | n/a | n/a | n/a | n/a | 7.4 | 22 |
Neurocrine Bioscience | Assay Description The membranes prepared from HEK cells transfected with adenosine receptors were used in binding assays. Nonspecific binding was determined in the pre... | J Med Chem 51: 7099-7110 (2008) Article DOI: 10.1021/jm800851u BindingDB Entry DOI: 10.7270/Q20R9MQG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM50494235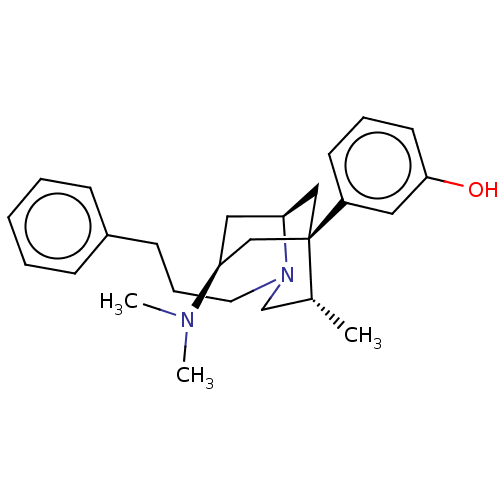 (CHEMBL3216613) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.280 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Research Triangle Institute Curated by ChEMBL | Assay Description Displacement of [3H]DAMGO from human mu opioid receptor expressed in CHO cells | J Med Chem 56: 8826-33 (2013) Article DOI: 10.1021/jm401250s BindingDB Entry DOI: 10.7270/Q2V127SP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A2a (Homo sapiens (Human)) | BDBM50237078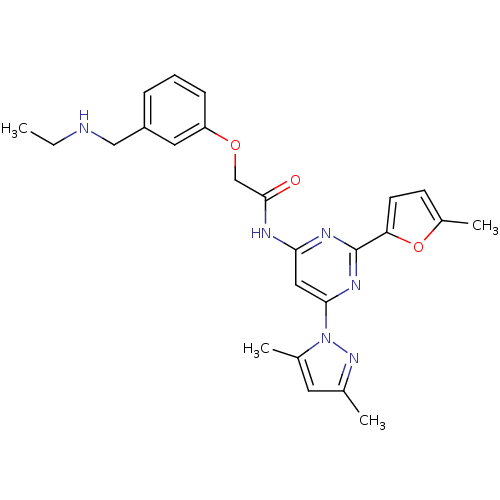 (CHEMBL256123 | N-(6-(3,5-dimethyl-1H-pyrazol-1-yl)...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Neurocrine Biosciences Curated by ChEMBL | Assay Description Displacement of [3H]-ZM241385 from human adenosine A2A receptor expressed in HEK293 cells | Bioorg Med Chem Lett 18: 1778-83 (2008) Article DOI: 10.1016/j.bmcl.2008.02.032 BindingDB Entry DOI: 10.7270/Q2VD6Z62 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A2a (Homo sapiens (Human)) | BDBM50237075 (CHEMBL253317 | N-(6-(3,5-dimethyl-1H-pyrazol-1-yl)...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Neurocrine Biosciences Curated by ChEMBL | Assay Description Displacement of [3H]-ZM241385 from human adenosine A2A receptor expressed in HEK293 cells | Bioorg Med Chem Lett 18: 1778-83 (2008) Article DOI: 10.1016/j.bmcl.2008.02.032 BindingDB Entry DOI: 10.7270/Q2VD6Z62 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A2a (Homo sapiens (Human)) | BDBM50237083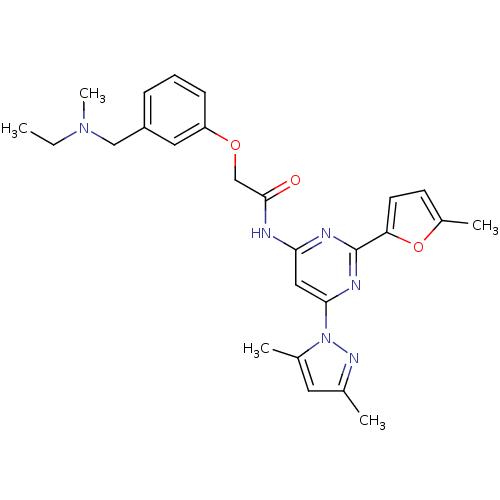 (CHEMBL256124 | N-(6-(3,5-dimethyl-1H-pyrazol-1-yl)...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Neurocrine Biosciences Curated by ChEMBL | Assay Description Binding affinity at adenosine A2A receptor | Bioorg Med Chem Lett 18: 1778-83 (2008) Article DOI: 10.1016/j.bmcl.2008.02.032 BindingDB Entry DOI: 10.7270/Q2VD6Z62 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A2a (Homo sapiens (Human)) | BDBM50237078 (CHEMBL256123 | N-(6-(3,5-dimethyl-1H-pyrazol-1-yl)...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Neurocrine Biosciences Curated by ChEMBL | Assay Description Binding affinity at adenosine A2A receptor | Bioorg Med Chem Lett 18: 1778-83 (2008) Article DOI: 10.1016/j.bmcl.2008.02.032 BindingDB Entry DOI: 10.7270/Q2VD6Z62 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A2a (Homo sapiens (Human)) | BDBM50237065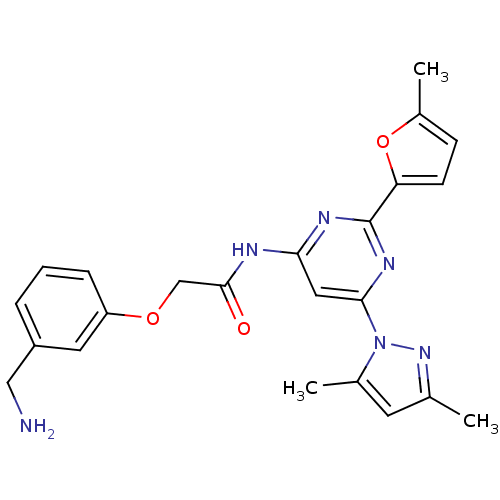 (2-(3-(aminomethyl)phenoxy)-N-(6-(3,5-dimethyl-1H-p...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Neurocrine Biosciences Curated by ChEMBL | Assay Description Binding affinity at adenosine A2A receptor | Bioorg Med Chem Lett 18: 1778-83 (2008) Article DOI: 10.1016/j.bmcl.2008.02.032 BindingDB Entry DOI: 10.7270/Q2VD6Z62 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A2a (Homo sapiens (Human)) | BDBM50237083 (CHEMBL256124 | N-(6-(3,5-dimethyl-1H-pyrazol-1-yl)...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Neurocrine Biosciences Curated by ChEMBL | Assay Description Displacement of [3H]-ZM241385 from human adenosine A2A receptor expressed in HEK293 cells | Bioorg Med Chem Lett 18: 1778-83 (2008) Article DOI: 10.1016/j.bmcl.2008.02.032 BindingDB Entry DOI: 10.7270/Q2VD6Z62 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM50031188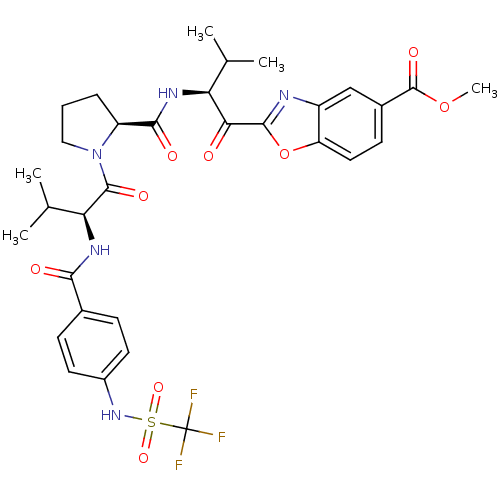 (2-[(S)-3-Methyl-2-({(S)-1-[(S)-3-methyl-2-(4-trifl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
ZENECA Pharmaceuticals Curated by ChEMBL | Assay Description Inhibition of human neutrophil elastase-catalyzed hydrolysis of the synthetic substrate MeO-Suc-Ala-Ala-Pro-Val-pNa | J Med Chem 38: 3972-82 (1995) BindingDB Entry DOI: 10.7270/Q2T152NN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A2a (Homo sapiens (Human)) | BDBM50237082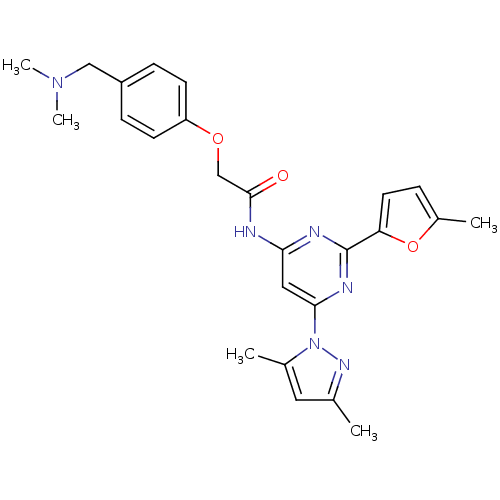 (CHEMBL404863 | N-(6-(3,5-dimethyl-1H-pyrazol-1-yl)...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Neurocrine Biosciences Curated by ChEMBL | Assay Description Displacement of [3H]-ZM241385 from human adenosine A2A receptor expressed in HEK293 cells | Bioorg Med Chem Lett 18: 1778-83 (2008) Article DOI: 10.1016/j.bmcl.2008.02.032 BindingDB Entry DOI: 10.7270/Q2VD6Z62 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-1A adrenergic receptor (Homo sapiens (Human)) | BDBM50220744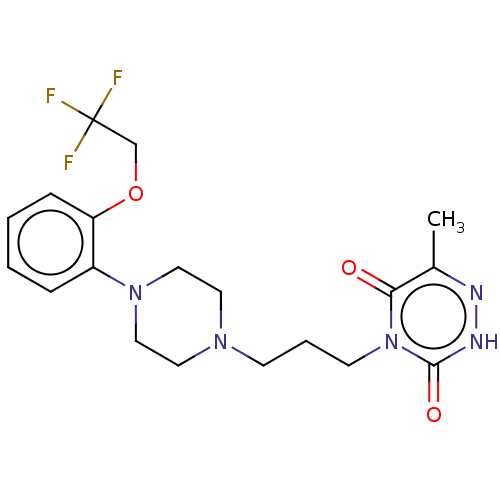 (CHEMBL55290) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.316 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Bioscience Curated by ChEMBL | Assay Description Inhibition of [3H]prazosin binding to CHO-K1 whole cells expressing human cloned Alpha-1A adrenergic receptor | Bioorg Med Chem Lett 13: 1873-8 (2003) BindingDB Entry DOI: 10.7270/Q2S46V50 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protease (Human immunodeficiency virus 1 (HIV-1)) | BDBM50469416 (CHEMBL4286215) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid PDB UniChem | Article PubMed | 0.330 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University Curated by ChEMBL | Assay Description Inhibition of HIV1 wild type NL4-3 protease expressed in Escherichia coli Rosetta (DE3) pLysS using Abz-Thr-Ile-Nle-Phe-(pNO2)-Gln-Arg-NH2 as substra... | J Med Chem 61: 9722-9737 (2018) Article DOI: 10.1021/acs.jmedchem.8b01227 BindingDB Entry DOI: 10.7270/Q25T3P6J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM50031209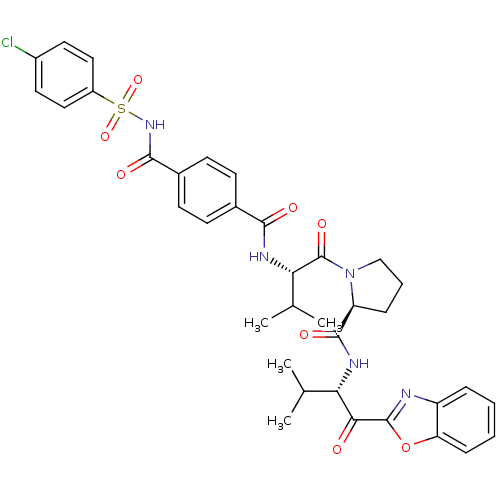 ((S)-1-{(S)-2-[4-(4-Chloro-benzenesulfonylaminocarb...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.330 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
ZENECA Pharmaceuticals Curated by ChEMBL | Assay Description Inhibition of human neutrophil elastase-catalyzed hydrolysis of the synthetic substrate MeO-Suc-Ala-Ala-Pro-Val-pNa | J Med Chem 38: 3972-82 (1995) BindingDB Entry DOI: 10.7270/Q2T152NN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM50031217 (CHEMBL262516 | N-((S)-1-{(S)-2-[(S)-1-(Benzooxazol...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.340 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
ZENECA Pharmaceuticals Curated by ChEMBL | Assay Description Inhibition of human neutrophil elastase-catalyzed hydrolysis of the synthetic substrate MeO-Suc-Ala-Ala-Pro-Val-pNa | J Med Chem 38: 3972-82 (1995) BindingDB Entry DOI: 10.7270/Q2T152NN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM50031197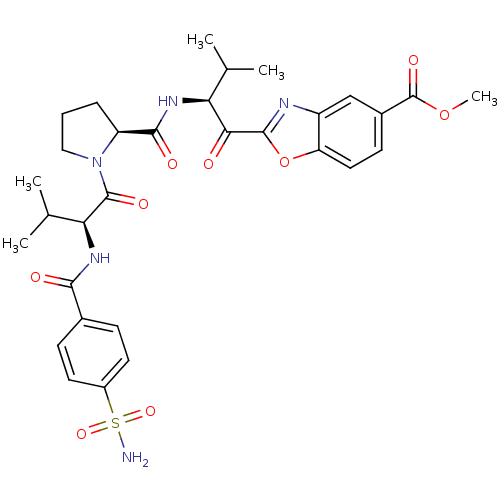 (2-[(S)-3-Methyl-2-({(S)-1-[(S)-3-methyl-2-(4-sulfa...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.350 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
ZENECA Pharmaceuticals Curated by ChEMBL | Assay Description Inhibition of human neutrophil elastase-catalyzed hydrolysis of the synthetic substrate MeO-Suc-Ala-Ala-Pro-Val-pNa | J Med Chem 38: 3972-82 (1995) BindingDB Entry DOI: 10.7270/Q2T152NN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 11194 total ) | Next | Last >> |