

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Hrh3 protein (RAT) | BDBM22911 (2-(3H-imidazol-4-yl)ethylsulfanylmethanimidamide |...) | KEGG UniProtKB/TrEMBL GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Patents Similars | PubMed | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
R. W. Johnson Pharmaceutical Research Institute Curated by PDSP Ki Database | J Pharmacol Exp Ther 293: 771-8 (2000) BindingDB Entry DOI: 10.7270/Q2348HX8 | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2A (Homo sapiens (Human)) | BDBM50205787 ((E)-5-fluoro-2-(2-(5-(2-fluorophenylsulfonyl)pyrid...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.190 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp and Dohme Curated by ChEMBL | Assay Description Displacement of [3H]ketanserin from human 5HT2A receptor expressed in CHO cells | Bioorg Med Chem Lett 17: 2643-8 (2007) Article DOI: 10.1016/j.bmcl.2007.01.098 BindingDB Entry DOI: 10.7270/Q2F47PZH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 6 (Homo sapiens (Human)) | BDBM50090529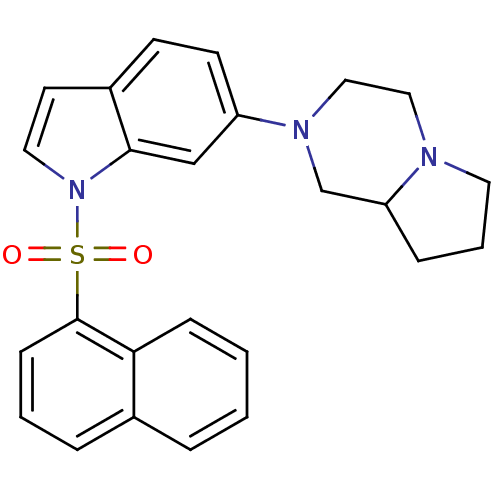 (2-[1-(Naphthalene-1-sulfonyl)-1H-indol-6-yl]-octah...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | PubMed | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
NPS Allelix Corp. Curated by ChEMBL | Assay Description Displacement of [3H]-LSD from human 5-hydroxytryptamine 6 receptor expressed in HEK 293 cells | Bioorg Med Chem Lett 10: 1719-21 (2000) BindingDB Entry DOI: 10.7270/Q2BP021W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phosphodiesterase (Macaca mulatta (Rhesus macaque)) | BDBM331979 ((R)- or (S)-2- (3,4-dimethoxy- benzyl)-6-(1-(1- (2...) | PDB UniProtKB/TrEMBL GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp. US Patent | Assay Description The activity of the compounds in accordance with the present invention as PDE2 inhibitors may be readily determined using a fluorescence polarization... | US Patent US10195201 (2019) BindingDB Entry DOI: 10.7270/Q20G3N7K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50209809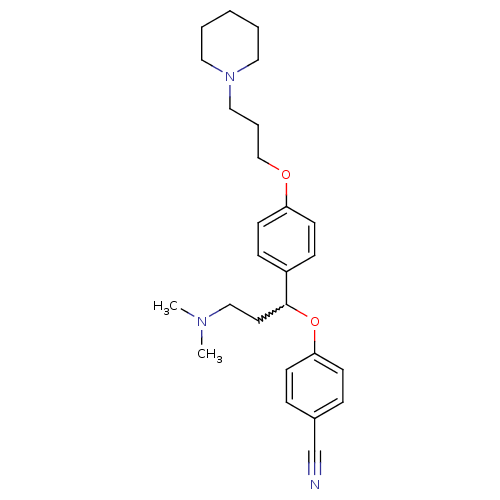 (4-(3-(dimethylamino)-1-(4-(3-(piperidin-1-yl)propo...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research & Development Curated by ChEMBL | Assay Description Binding affinity at human histamine H3 receptor | Bioorg Med Chem Lett 17: 3130-5 (2007) Article DOI: 10.1016/j.bmcl.2007.03.034 BindingDB Entry DOI: 10.7270/Q2H131QK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hrh3 protein (RAT) | BDBM22911 (2-(3H-imidazol-4-yl)ethylsulfanylmethanimidamide |...) | KEGG UniProtKB/TrEMBL GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Patents Similars | PubMed | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
R. W. Johnson Pharmaceutical Research Institute Curated by PDSP Ki Database | J Pharmacol Exp Ther 293: 771-8 (2000) BindingDB Entry DOI: 10.7270/Q2348HX8 | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50346209 (5-(3-cyclobutyl-2,3,4,5-tetrahydro-1H-benzo[d]azep...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | Article PubMed | 0.210 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research and Development Curated by ChEMBL | Assay Description Displacement of [3H]N-alpha-methylhistamine from human histamine H3 receptor expressed in SK-N-MC cells | Eur J Med Chem 44: 4413-25 (2009) Article DOI: 10.1016/j.ejmech.2009.06.007 BindingDB Entry DOI: 10.7270/Q28S4Q7C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Salicyl-AMP ligase / salicyl-S-ArCP synthetase (Mycobacterium tuberculosis) | BDBM50244934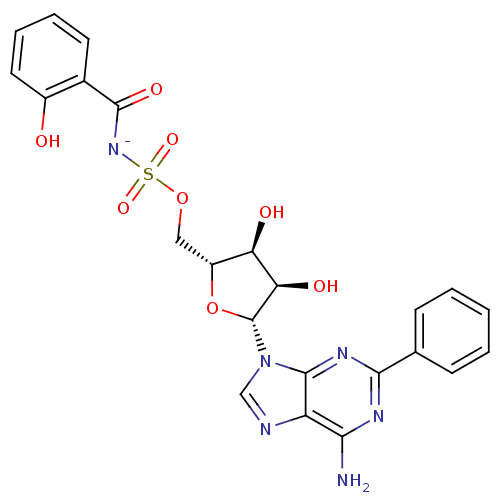 (5'-O-[N-(2-Hydroxybenzoyl)sulfamoyl]-2-phenyladeno...) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.270 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Minnesota Curated by ChEMBL | Assay Description Inhibition of Mycobacterium tuberculosis recombinant MbtA expressed in Escherichia coli by ATP-PPi exchange assay | J Med Chem 51: 5349-70 (2008) Article DOI: 10.1021/jm800567v BindingDB Entry DOI: 10.7270/Q2BZ66X7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Salicyl-AMP ligase / salicyl-S-ArCP synthetase (Mycobacterium tuberculosis) | BDBM50412833 (CHEMBL611772) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.290 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Minnesota Curated by ChEMBL | Assay Description Inhibition of Mycobacterium tuberculosis MbtA expressed in Escherichia coli assessed as ATP-[32P]Ppi exchange | J Med Chem 51: 7495-507 (2009) Article DOI: 10.1021/jm8008037 BindingDB Entry DOI: 10.7270/Q2HH6M96 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E2 receptor EP1 subtype (Homo sapiens (Human)) | BDBM50160917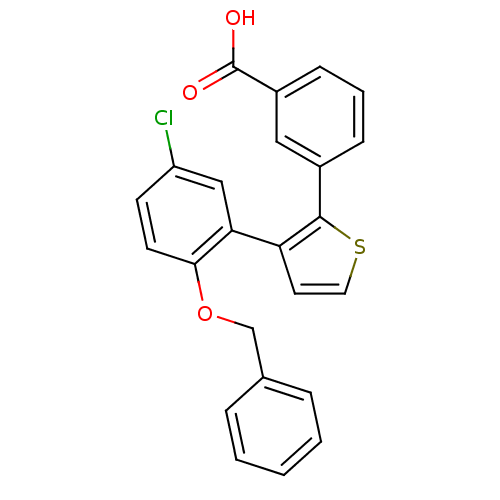 (3-(3-(2-(benzyloxy)-5-chlorophenyl)thiophen-2-yl)b...) | KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Antagonist activity against PGE2 activated EP1 receptor assessed as ability to inhibit intracellular calcium mobilisation by FLIPR | Bioorg Med Chem Lett 16: 2666-71 (2006) Article DOI: 10.1016/j.bmcl.2006.02.014 BindingDB Entry DOI: 10.7270/Q2J102RM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent serotonin transporter (Rattus norvegicus (rat)) | BDBM50217586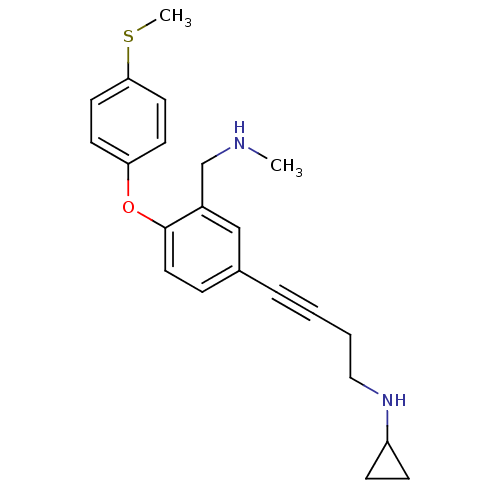 (CHEMBL442080 | N-(4-(3-((methylamino)methyl)-4-(4-...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research & Development LLC Curated by ChEMBL | Assay Description Binding affinity to rat SERT | Bioorg Med Chem Lett 17: 4799-803 (2007) Article DOI: 10.1016/j.bmcl.2007.06.061 BindingDB Entry DOI: 10.7270/Q2F18ZFF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM22911 (2-(3H-imidazol-4-yl)ethylsulfanylmethanimidamide |...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The R. W. Johnson Pharmaceutical Research Institute Curated by PDSP Ki Database | Mol Pharmacol 59: 420-6 (2001) Article DOI: 10.1124/mol.59.3.420 BindingDB Entry DOI: 10.7270/Q26Q1VSR | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Salicyl-AMP ligase / salicyl-S-ArCP synthetase (Mycobacterium tuberculosis) | BDBM50412824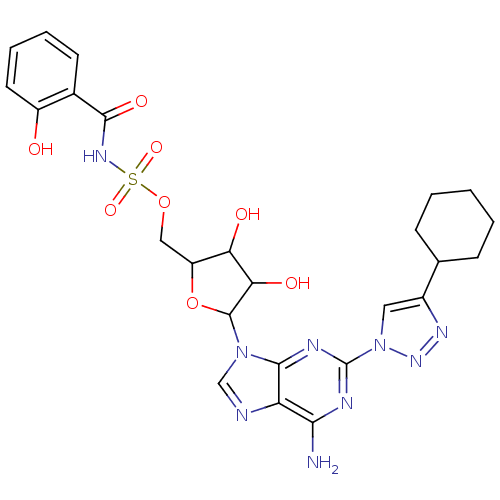 (CHEMBL611766) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.320 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Minnesota Curated by ChEMBL | Assay Description Inhibition of Mycobacterium tuberculosis MbtA expressed in Escherichia coli assessed as ATP-[32P]Ppi exchange | J Med Chem 51: 7495-507 (2009) Article DOI: 10.1021/jm8008037 BindingDB Entry DOI: 10.7270/Q2HH6M96 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2A (Homo sapiens (Human)) | BDBM50169842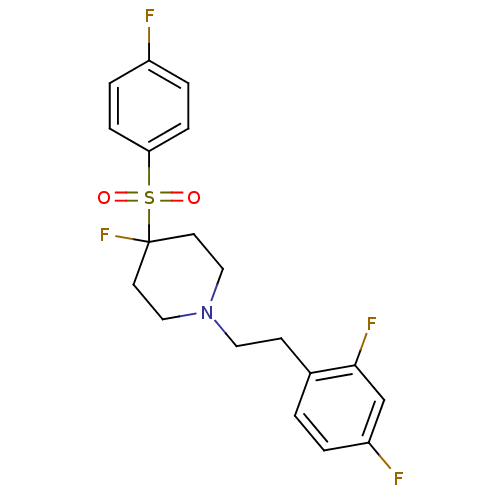 (1-(2,4-difluorophenethyl)-4-fluoro-4-(4-fluorophen...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.390 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp and Dohme Curated by ChEMBL | Assay Description Displacement of [3H]ketanserin from human 5HT2A receptor expressed in CHO cells | Bioorg Med Chem Lett 17: 2643-8 (2007) Article DOI: 10.1016/j.bmcl.2007.01.098 BindingDB Entry DOI: 10.7270/Q2F47PZH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50159110 (1-(3-(4-(piperidin-1-ylmethyl)phenoxy)propyl)piper...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research and Development Curated by ChEMBL | Assay Description Displacement of N-[3H]methylhistamine from human histamine H3 receptor expressed in SK-N-MC cells | Bioorg Med Chem Lett 16: 897-900 (2006) Article DOI: 10.1016/j.bmcl.2005.11.003 BindingDB Entry DOI: 10.7270/Q2H131M7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM22542 (4-(1H-imidazol-4-ylmethyl)piperidine | 4-(1H-imida...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Patents Similars | Article PubMed | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The R. W. Johnson Pharmaceutical Research Institute Curated by PDSP Ki Database | Mol Pharmacol 59: 420-6 (2001) Article DOI: 10.1124/mol.59.3.420 BindingDB Entry DOI: 10.7270/Q26Q1VSR | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hrh3 protein (RAT) | BDBM22541 (Clobenpropit | N''-[(4-chlorophenyl)methyl]{[3-(1H...) | KEGG UniProtKB/TrEMBL GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Patents Similars | PubMed | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
R. W. Johnson Pharmaceutical Research Institute Curated by PDSP Ki Database | J Pharmacol Exp Ther 293: 771-8 (2000) BindingDB Entry DOI: 10.7270/Q2348HX8 | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 72 kDa type IV collagenase (Homo sapiens (Human)) | BDBM11551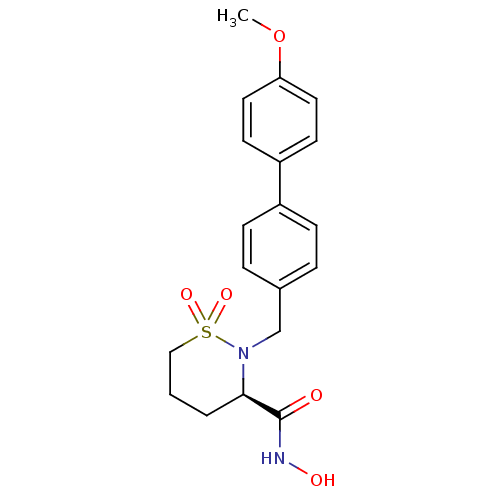 ((3R)-N-hydroxy-2-[(4-methoxy-1,1-biphenyl-4-yl)met...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research and Development Curated by ChEMBL | Assay Description Inhibition of MMP2 | Bioorg Med Chem Lett 18: 1140-5 (2008) Article DOI: 10.1016/j.bmcl.2007.11.129 BindingDB Entry DOI: 10.7270/Q2BP02JM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 72 kDa type IV collagenase (Homo sapiens (Human)) | BDBM50160855 (1-[4-(4-chloro-phenoxy)-phenyl]-1,7,9-triaza-spiro...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research and Development Curated by ChEMBL | Assay Description Inhibition of MMP2 | Bioorg Med Chem Lett 18: 1140-5 (2008) Article DOI: 10.1016/j.bmcl.2007.11.129 BindingDB Entry DOI: 10.7270/Q2BP02JM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent serotonin transporter (Rattus norvegicus (rat)) | BDBM50217575 (4-(4-(3-((methylamino)methyl)-4-(4-(methylthio)phe...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research & Development LLC Curated by ChEMBL | Assay Description Binding affinity to rat SERT | Bioorg Med Chem Lett 17: 4799-803 (2007) Article DOI: 10.1016/j.bmcl.2007.06.061 BindingDB Entry DOI: 10.7270/Q2F18ZFF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Salicyl-AMP ligase / salicyl-S-ArCP synthetase (Mycobacterium tuberculosis) | BDBM50412382 (CHEMBL611777) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Minnesota Curated by ChEMBL | Assay Description Inhibition of Mycobacterium tuberculosis recombinant MbtA expressed in Escherichia coli by ATP-PPi exchange assay | J Med Chem 51: 5349-70 (2008) Article DOI: 10.1021/jm800567v BindingDB Entry DOI: 10.7270/Q2BZ66X7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cGMP-dependent 3',5'-cyclic phosphodiesterase (Homo sapiens (Human)) | BDBM408573 (US10358435, Example 89 | US10358435, Example 90) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp US Patent | Assay Description In a typical experiment the PDE2 inhibitory activity of the compounds of the present invention was determined in accordance with the following experi... | US Patent US10358435 (2019) BindingDB Entry DOI: 10.7270/Q2Q242M7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cGMP-dependent 3',5'-cyclic phosphodiesterase (Homo sapiens (Human)) | BDBM385987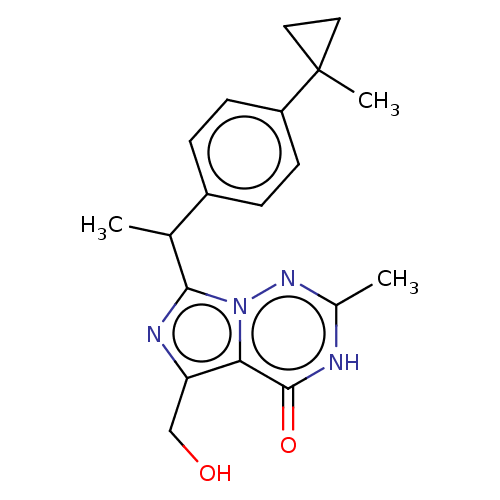 ((R)- or (S)-5- (hydroxymethyl)-2- methyl-7-(1-(4-(...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Wisconsin at Madison | Assay Description In a typical experiment the PDE2 inhibitory activity of the compounds of the present invention was determined in accordance with the following experi... | J Med Chem 51: 7243-52 (2008) BindingDB Entry DOI: 10.7270/Q2G1634M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phosphodiesterase (Macaca mulatta (Rhesus macaque)) | BDBM355246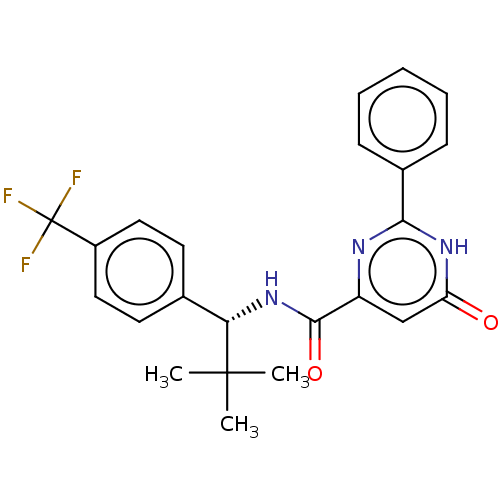 (US9815796, Example 337) | PDB UniProtKB/TrEMBL GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp.; MSD R & D (China) Co. LTD. US Patent | Assay Description In a typical experiment the PDE2 inhibitory activity of the compounds of the present invention was determined in accordance with the following experi... | US Patent US9815796 (2017) BindingDB Entry DOI: 10.7270/Q2V69MQQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cGMP-dependent 3',5'-cyclic phosphodiesterase (Homo sapiens (Human)) | BDBM331979 ((R)- or (S)-2- (3,4-dimethoxy- benzyl)-6-(1-(1- (2...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp. US Patent | Assay Description TBD | US Patent US10195201 (2019) BindingDB Entry DOI: 10.7270/Q20G3N7K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phosphodiesterase (Macaca mulatta (Rhesus macaque)) | BDBM331979 ((R)- or (S)-2- (3,4-dimethoxy- benzyl)-6-(1-(1- (2...) | PDB UniProtKB/TrEMBL GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp. US Patent | Assay Description The activity of the compounds in accordance with the present invention as PDE2 inhibitors may be readily determined using a fluorescence polarization... | US Patent US10195201 (2019) BindingDB Entry DOI: 10.7270/Q20G3N7K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent serotonin transporter (Rattus norvegicus (rat)) | BDBM50217584 ((5-(4-(4-isopropylpiperazin-1-yl)butyl)-2-(4-(meth...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research & Development LLC Curated by ChEMBL | Assay Description Binding affinity to rat SERT | Bioorg Med Chem Lett 17: 4799-803 (2007) Article DOI: 10.1016/j.bmcl.2007.06.061 BindingDB Entry DOI: 10.7270/Q2F18ZFF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Salicyl-AMP ligase / salicyl-S-ArCP synthetase (Mycobacterium tuberculosis) | BDBM50412832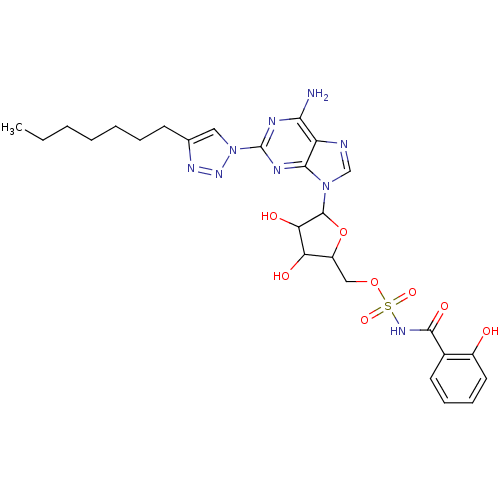 (CHEMBL612049) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.410 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Minnesota Curated by ChEMBL | Assay Description Inhibition of Mycobacterium tuberculosis MbtA expressed in Escherichia coli assessed as ATP-[32P]Ppi exchange | J Med Chem 51: 7495-507 (2009) Article DOI: 10.1021/jm8008037 BindingDB Entry DOI: 10.7270/Q2HH6M96 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent dopamine transporter (Homo sapiens (Human)) | BDBM81946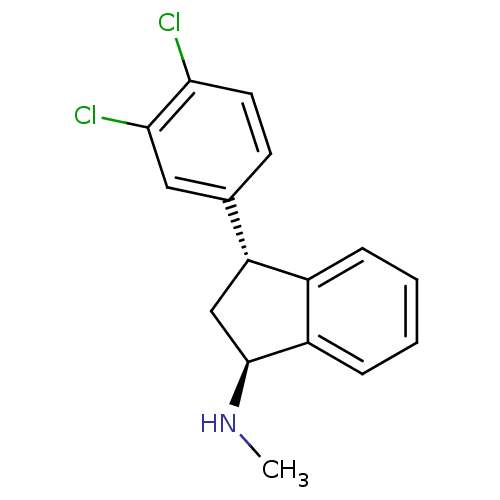 (CAS_96850-13-4 | LU 19,005 | LU 19-005) | NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | PubMed | 0.410 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Toronto Curated by PDSP Ki Database | Mol Pharmacol 45: 125-35 (1994) BindingDB Entry DOI: 10.7270/Q2NG4P5C | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cGMP-dependent 3',5'-cyclic phosphodiesterase (Homo sapiens (Human)) | BDBM408558 (US10358435, Example 74 | US10358435, Example 75) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.420 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp US Patent | Assay Description In a typical experiment the PDE2 inhibitory activity of the compounds of the present invention was determined in accordance with the following experi... | US Patent US10358435 (2019) BindingDB Entry DOI: 10.7270/Q2Q242M7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50410342 (CHEMBL195408) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.447 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research and Development Curated by ChEMBL | Assay Description Mean binding affinity for human H3 receptor | J Med Chem 48: 2229-38 (2005) Article DOI: 10.1021/jm049212n BindingDB Entry DOI: 10.7270/Q2GB258T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cGMP-dependent 3',5'-cyclic phosphodiesterase (Homo sapiens (Human)) | BDBM354912 (1-Methylcyclopropanamine Oxalate | US9815796, Exam...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.450 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp.; MSD R & D (China) Co. LTD. US Patent | Assay Description In a typical experiment the PDE2 inhibitory activity of the compounds of the present invention was determined in accordance with the following experi... | US Patent US9815796 (2017) BindingDB Entry DOI: 10.7270/Q2V69MQQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50139391 ((R)-4-(2-(2-(2-methylpyrrolidin-1-yl)ethyl)benzofu...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | 0.450 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research and Development Curated by ChEMBL | Assay Description Displacement of [3H]N-alpha-methylhistamine from human histamine H3 receptor expressed in SK-N-MC cells | Eur J Med Chem 44: 4413-25 (2009) Article DOI: 10.1016/j.ejmech.2009.06.007 BindingDB Entry DOI: 10.7270/Q28S4Q7C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Salicyl-AMP ligase / salicyl-S-ArCP synthetase (Mycobacterium tuberculosis) | BDBM50412826 (CHEMBL612048) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.460 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Minnesota Curated by ChEMBL | Assay Description Inhibition of Mycobacterium tuberculosis MbtA expressed in Escherichia coli assessed as ATP-[32P]Ppi exchange | J Med Chem 51: 7495-507 (2009) Article DOI: 10.1021/jm8008037 BindingDB Entry DOI: 10.7270/Q2HH6M96 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Salicyl-AMP ligase / salicyl-S-ArCP synthetase (Mycobacterium tuberculosis) | BDBM50412819 (CHEMBL611785) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.480 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Minnesota Curated by ChEMBL | Assay Description Inhibition of Mycobacterium tuberculosis MbtA expressed in Escherichia coli assessed as ATP-[32P]Ppi exchange | J Med Chem 51: 7495-507 (2009) Article DOI: 10.1021/jm8008037 BindingDB Entry DOI: 10.7270/Q2HH6M96 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cGMP-dependent 3',5'-cyclic phosphodiesterase (Homo sapiens (Human)) | BDBM355376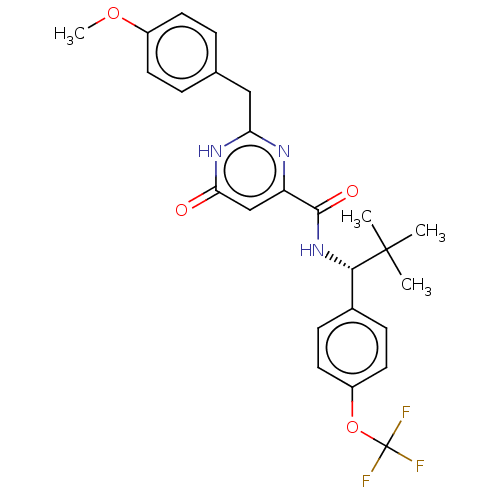 (N-{(1R)-2,2-dimethyl-1-[4- (trifluoromethoxy)pheny...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.490 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp.; MSD R & D (China) Co. LTD. US Patent | Assay Description In a typical experiment the PDE2 inhibitory activity of the compounds of the present invention was determined in accordance with the following experi... | US Patent US9815796 (2017) BindingDB Entry DOI: 10.7270/Q2V69MQQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50177731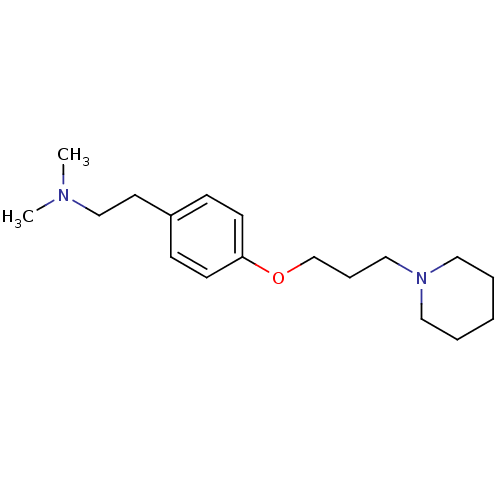 (CHEMBL204872 | dimethyl-{2-[4-(3-piperidin-1-yl-pr...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research and Development Curated by ChEMBL | Assay Description Displacement of N-[3H]methylhistamine from human histamine H3 receptor expressed in SK-N-MC cells | Bioorg Med Chem Lett 16: 897-900 (2006) Article DOI: 10.1016/j.bmcl.2005.11.003 BindingDB Entry DOI: 10.7270/Q2H131M7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM22530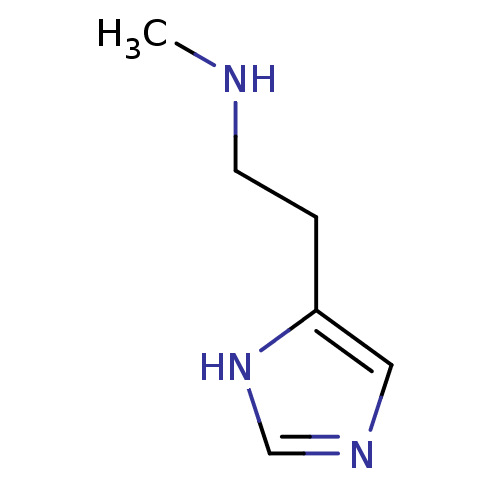 (N(alpha)-Methylhistamine | N-alpha-methylhistamine...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Patents Similars | Article PubMed | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The R. W. Johnson Pharmaceutical Research Institute Curated by PDSP Ki Database | Mol Pharmacol 59: 420-6 (2001) Article DOI: 10.1124/mol.59.3.420 BindingDB Entry DOI: 10.7270/Q26Q1VSR | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hrh3 protein (RAT) | BDBM22541 (Clobenpropit | N''-[(4-chlorophenyl)methyl]{[3-(1H...) | KEGG UniProtKB/TrEMBL GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Patents Similars | PubMed | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
R. W. Johnson Pharmaceutical Research Institute Curated by PDSP Ki Database | J Pharmacol Exp Ther 293: 771-8 (2000) BindingDB Entry DOI: 10.7270/Q2348HX8 | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cGMP-dependent 3',5'-cyclic phosphodiesterase (Homo sapiens (Human)) | BDBM355552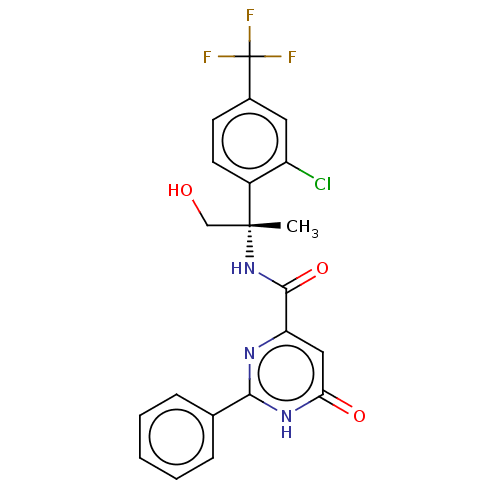 (N-{(1S)-1-[2-chloro-4- (trifluoromethyl)phenyl]-2-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp.; MSD R & D (China) Co. LTD. US Patent | Assay Description In a typical experiment the PDE2 inhibitory activity of the compounds of the present invention was determined in accordance with the following experi... | US Patent US9815796 (2017) BindingDB Entry DOI: 10.7270/Q2V69MQQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50371305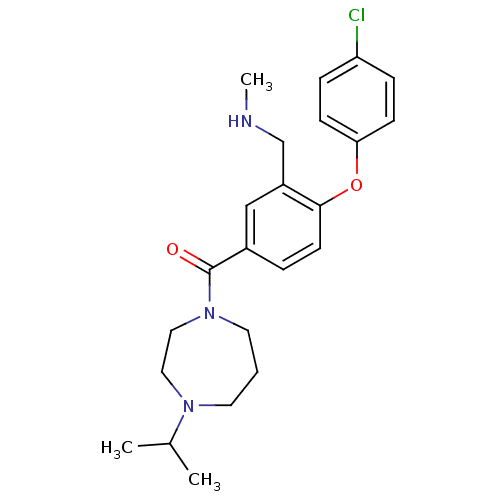 (CHEMBL272077) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development L.L.C. Curated by ChEMBL | Assay Description Inhibition of human histamine H3 receptor | Bioorg Med Chem Lett 18: 39-43 (2008) Article DOI: 10.1016/j.bmcl.2007.11.016 BindingDB Entry DOI: 10.7270/Q2R49RMD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent serotonin transporter (Rattus norvegicus (rat)) | BDBM50217592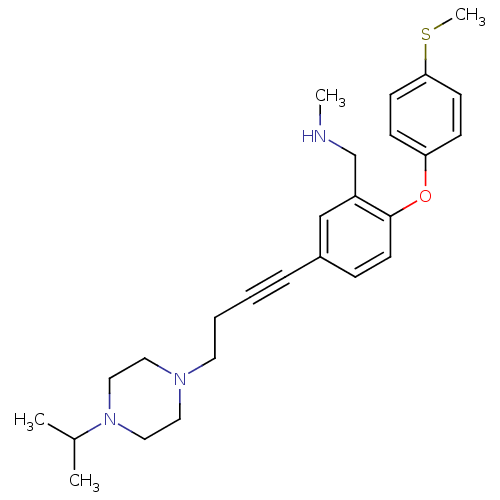 ((5-(4-(4-isopropylpiperazin-1-yl)but-1-ynyl)-2-(4-...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research & Development LLC Curated by ChEMBL | Assay Description Binding affinity to rat SERT | Bioorg Med Chem Lett 17: 4799-803 (2007) Article DOI: 10.1016/j.bmcl.2007.06.061 BindingDB Entry DOI: 10.7270/Q2F18ZFF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent serotonin transporter (Rattus norvegicus (rat)) | BDBM50217593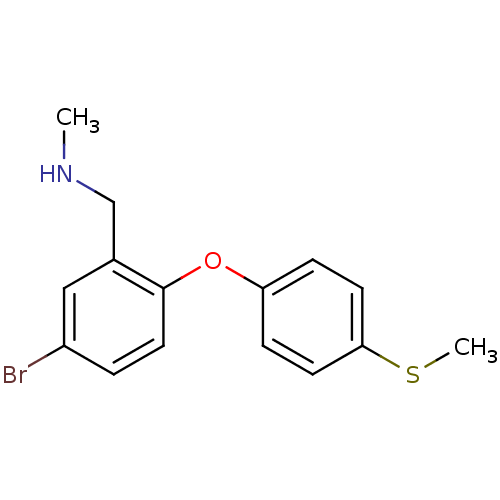 ((5-bromo-2-(4-(methylthio)phenoxy)phenyl)-N-methyl...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research & Development LLC Curated by ChEMBL | Assay Description Binding affinity to rat SERT | Bioorg Med Chem Lett 17: 4799-803 (2007) Article DOI: 10.1016/j.bmcl.2007.06.061 BindingDB Entry DOI: 10.7270/Q2F18ZFF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hrh3 protein (RAT) | BDBM22542 (4-(1H-imidazol-4-ylmethyl)piperidine | 4-(1H-imida...) | KEGG UniProtKB/TrEMBL GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Patents Similars | PubMed | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
R. W. Johnson Pharmaceutical Research Institute Curated by PDSP Ki Database | J Pharmacol Exp Ther 293: 771-8 (2000) BindingDB Entry DOI: 10.7270/Q2348HX8 | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cGMP-dependent 3',5'-cyclic phosphodiesterase (Homo sapiens (Human)) | BDBM355445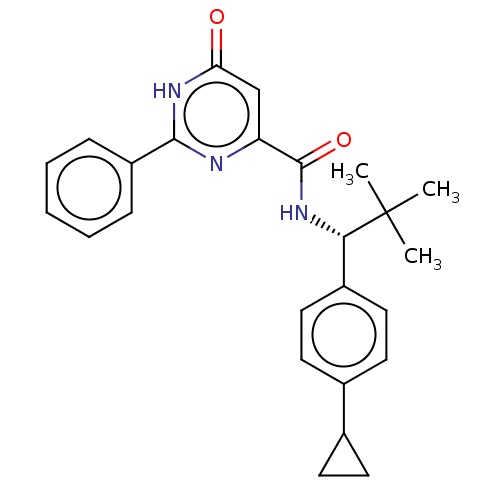 (N-[(1R)-1-(4-cyclopropylphenyl)- 2,2-dimethylpropy...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.510 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp.; MSD R & D (China) Co. LTD. US Patent | Assay Description In a typical experiment the PDE2 inhibitory activity of the compounds of the present invention was determined in accordance with the following experi... | US Patent US9815796 (2017) BindingDB Entry DOI: 10.7270/Q2V69MQQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cGMP-dependent 3',5'-cyclic phosphodiesterase (Homo sapiens (Human)) | BDBM354913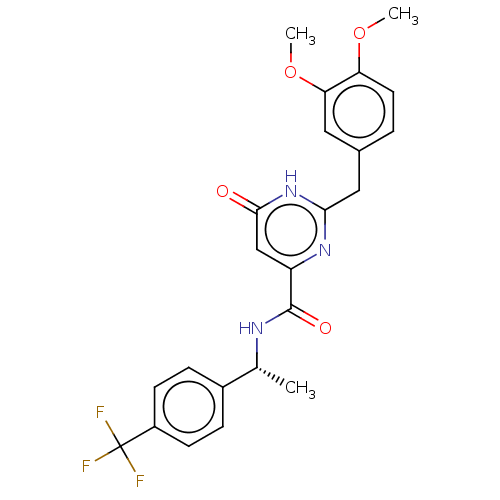 (2-Methyl-2-(1H-pyrazol-1-yl)propanimidamide acetat...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.520 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp.; MSD R & D (China) Co. LTD. US Patent | Assay Description In a typical experiment the PDE2 inhibitory activity of the compounds of the present invention was determined in accordance with the following experi... | US Patent US9815796 (2017) BindingDB Entry DOI: 10.7270/Q2V69MQQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cGMP-dependent 3',5'-cyclic phosphodiesterase (Homo sapiens (Human)) | BDBM408512 (US10358435, Example 29) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.530 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp US Patent | Assay Description In a typical experiment the PDE2 inhibitory activity of the compounds of the present invention was determined in accordance with the following experi... | US Patent US10358435 (2019) BindingDB Entry DOI: 10.7270/Q2Q242M7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Salicyl-AMP ligase / salicyl-S-ArCP synthetase (Mycobacterium tuberculosis) | BDBM50412825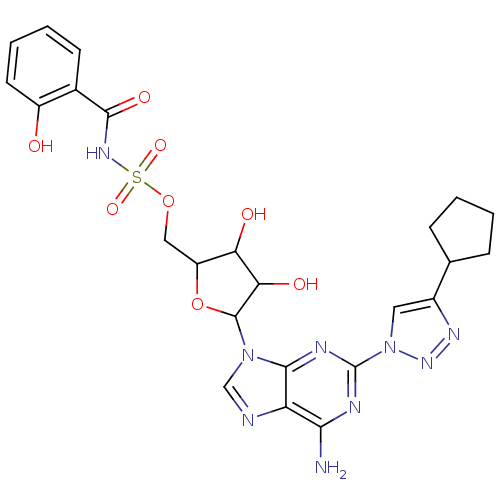 (CHEMBL609105) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.540 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Minnesota Curated by ChEMBL | Assay Description Inhibition of Mycobacterium tuberculosis MbtA expressed in Escherichia coli assessed as ATP-[32P]Ppi exchange | J Med Chem 51: 7495-507 (2009) Article DOI: 10.1021/jm8008037 BindingDB Entry DOI: 10.7270/Q2HH6M96 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phosphodiesterase (Macaca mulatta (Rhesus macaque)) | BDBM354912 (1-Methylcyclopropanamine Oxalate | US9815796, Exam...) | PDB UniProtKB/TrEMBL GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.540 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp.; MSD R & D (China) Co. LTD. US Patent | Assay Description In a typical experiment the PDE2 inhibitory activity of the compounds of the present invention was determined in accordance with the following experi... | US Patent US9815796 (2017) BindingDB Entry DOI: 10.7270/Q2V69MQQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50159110 (1-(3-(4-(piperidin-1-ylmethyl)phenoxy)propyl)piper...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | 0.560 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research and Development, LLC Curated by PDSP Ki Database | Br J Pharmacol 143: 649-61 (2004) Article DOI: 10.1038/sj.bjp.0705964 BindingDB Entry DOI: 10.7270/Q2319TF2 | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 13891 total ) | Next | Last >> |