

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Adenosine deaminase (Homo sapiens (Human)) | BDBM50407749 (CHEMBL2112110) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.00250 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of California Curated by ChEMBL | Assay Description Binding affinity towards Adenosine deaminase | J Med Chem 39: 277-84 (1996) Article DOI: 10.1021/jm9505674 BindingDB Entry DOI: 10.7270/Q2ZS2X6V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine deaminase (Homo sapiens (Human)) | BDBM50369126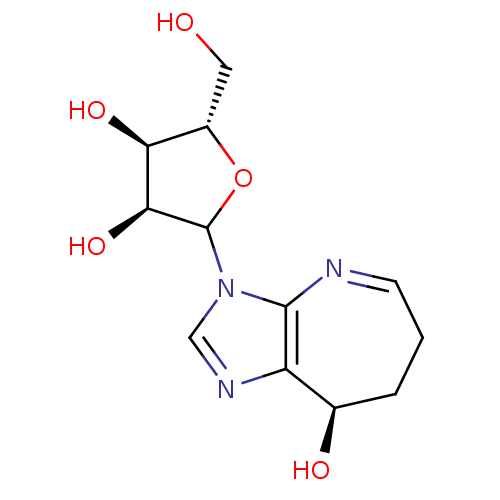 (CONFORMYCIN) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.0100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of California Curated by ChEMBL | Assay Description Binding affinity towards Adenosine deaminase | J Med Chem 39: 277-84 (1996) Article DOI: 10.1021/jm9505674 BindingDB Entry DOI: 10.7270/Q2ZS2X6V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| dTDP-4-dehydrorhamnose 3,5-epimerase (Mycobacterium tuberculosis H37Rv) | BDBM50481568 (CHEMBL592712) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase PC cid PC sid UniChem | Article PubMed | 62 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Pennsylvania Curated by ChEMBL | Assay Description Inhibition of Mycobacterium tuberculosis RmlC expressed in Escherichia coli by spectrophotometry | Bioorg Med Chem 18: 896-908 (2010) Article DOI: 10.1016/j.bmc.2009.11.033 BindingDB Entry DOI: 10.7270/Q2N300SV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| dTDP-4-dehydrorhamnose 3,5-epimerase (Mycobacterium tuberculosis H37Rv) | BDBM51599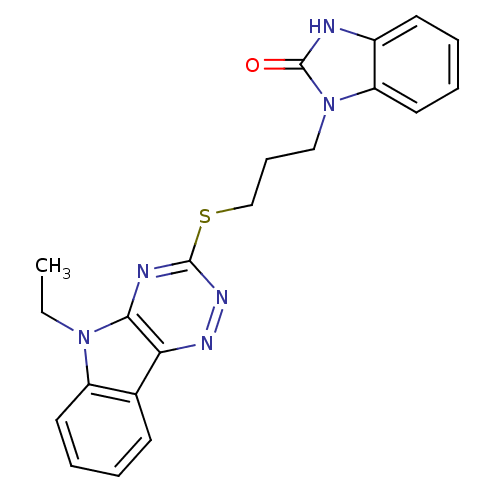 (3-[3-[(5-ethyl-[1,2,4]triazin[5,6-b]indol-3-yl)thi...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | Article PubMed | 100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Pennsylvania Curated by ChEMBL | Assay Description Inhibition of Mycobacterium tuberculosis RmlC expressed in Escherichia coli by spectrophotometry | Bioorg Med Chem 18: 896-908 (2010) Article DOI: 10.1016/j.bmc.2009.11.033 BindingDB Entry DOI: 10.7270/Q2N300SV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 4-hydroxy-3-methylbut-2-enyl diphosphate reductase (Pseudomonas aeruginosa) | BDBM228817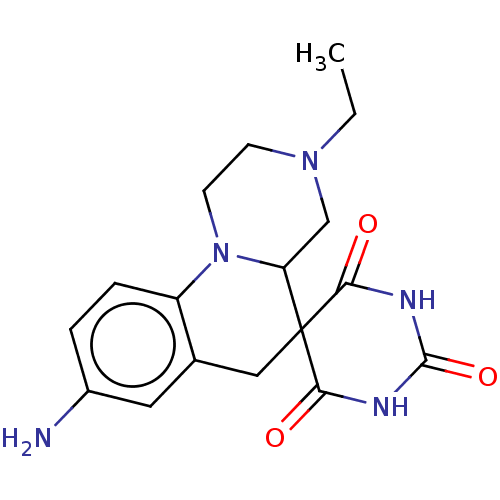 (IspH inhibitor, 8) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Illinois | Assay Description In order to find new inhibitors, we carried out in silico screening of AaIspH and EcIspH using ZINC and National Cancer Institute (NCI) libraries and... | Chembiochem 18: 914-920 (2017) Article DOI: 10.1002/cbic.201700052 BindingDB Entry DOI: 10.7270/Q27S7MNB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 4-hydroxy-3-methylbut-2-enyl diphosphate reductase (Aquifex aeolicus) | BDBM228818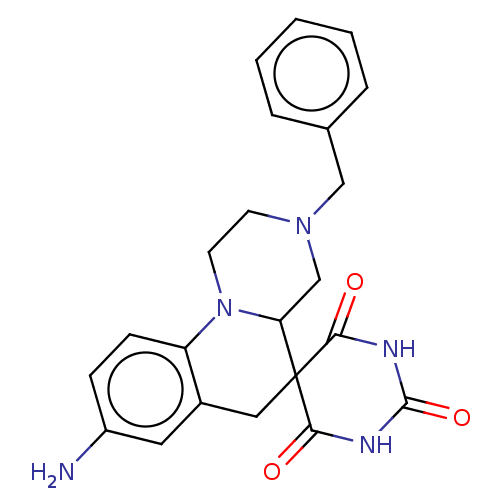 (8-Amino-3-benzyl-2,3,4,4 a-tetrahydro-1,2'H,6H...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase PC cid PC sid UniChem | Article PubMed | 700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Illinois | Assay Description In order to find new inhibitors, we carried out in silico screening of AaIspH and EcIspH using ZINC and National Cancer Institute (NCI) libraries and... | Chembiochem 18: 914-920 (2017) Article DOI: 10.1002/cbic.201700052 BindingDB Entry DOI: 10.7270/Q27S7MNB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 4-hydroxy-3-methylbut-2-enyl diphosphate reductase (Escherichia coli (Enterobacteria)) | BDBM228818 (8-Amino-3-benzyl-2,3,4,4 a-tetrahydro-1,2'H,6H...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase PC cid PC sid UniChem | Article PubMed | 3.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Illinois | Assay Description In order to find new inhibitors, we carried out in silico screening of AaIspH and EcIspH using ZINC and National Cancer Institute (NCI) libraries and... | Chembiochem 18: 914-920 (2017) Article DOI: 10.1002/cbic.201700052 BindingDB Entry DOI: 10.7270/Q27S7MNB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 2 (Homo sapiens (Human)) | BDBM50057418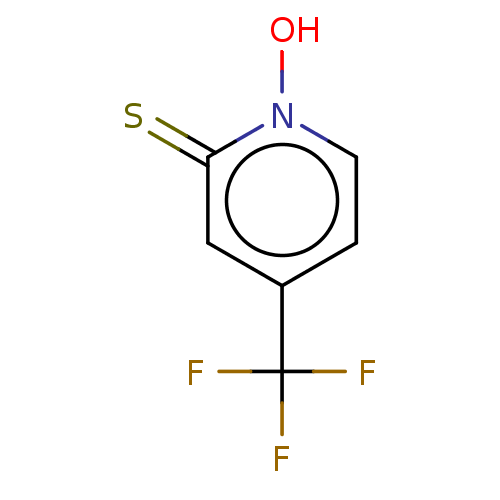 (CHEMBL3326435) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Similars | Article PubMed | 1.40E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of California Curated by ChEMBL | Assay Description Inhibition of human carbonic anhydrase using p-nitrophenylacetate as substrate preincubated for 10 mins before substrate addition by spectrophotometr... | J Med Chem 57: 7126-35 (2014) Article DOI: 10.1021/jm500984b BindingDB Entry DOI: 10.7270/Q20V8FD1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 2 (Homo sapiens (Human)) | BDBM50057411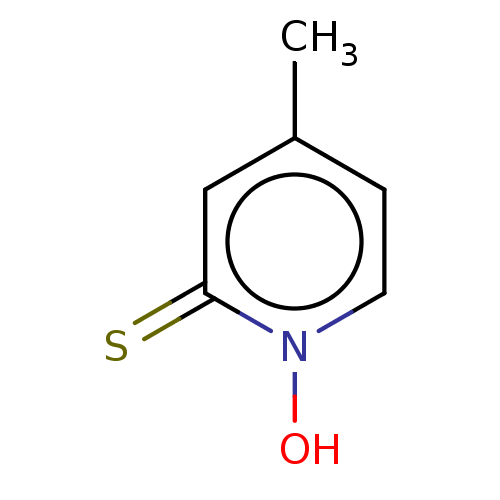 (CHEMBL3326430) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Similars | Article PubMed | 3.80E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of California Curated by ChEMBL | Assay Description Inhibition of human carbonic anhydrase using p-nitrophenylacetate as substrate preincubated for 10 mins before substrate addition by spectrophotometr... | J Med Chem 57: 7126-35 (2014) Article DOI: 10.1021/jm500984b BindingDB Entry DOI: 10.7270/Q20V8FD1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 2 (Homo sapiens (Human)) | BDBM50057412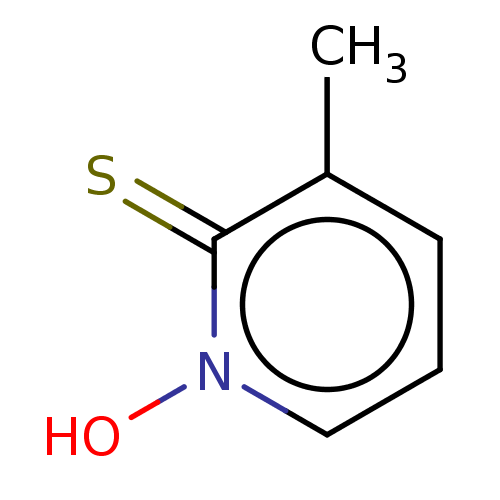 (CHEMBL3326429) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Similars | Article PubMed | 4.80E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of California Curated by ChEMBL | Assay Description Inhibition of human carbonic anhydrase using p-nitrophenylacetate as substrate preincubated for 10 mins before substrate addition by spectrophotometr... | J Med Chem 57: 7126-35 (2014) Article DOI: 10.1021/jm500984b BindingDB Entry DOI: 10.7270/Q20V8FD1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 2 (Homo sapiens (Human)) | BDBM50057406 (CHEMBL3326434) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Similars | Article PubMed | 6.40E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of California Curated by ChEMBL | Assay Description Inhibition of human carbonic anhydrase using p-nitrophenylacetate as substrate preincubated for 10 mins before substrate addition by spectrophotometr... | J Med Chem 57: 7126-35 (2014) Article DOI: 10.1021/jm500984b BindingDB Entry DOI: 10.7270/Q20V8FD1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 2 (Homo sapiens (Human)) | BDBM50057410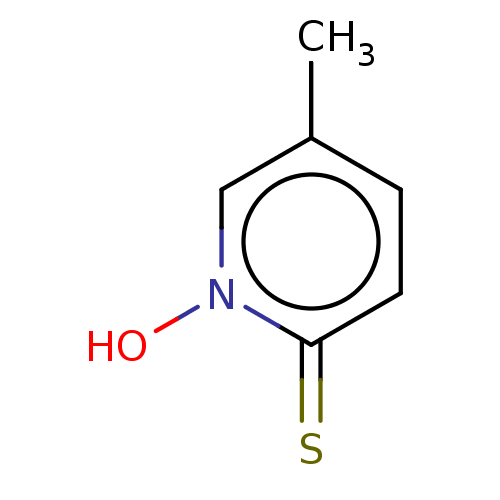 (CHEMBL3326431) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Similars | Article PubMed | 2.60E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of California Curated by ChEMBL | Assay Description Inhibition of human carbonic anhydrase using p-nitrophenylacetate as substrate preincubated for 10 mins before substrate addition by spectrophotometr... | J Med Chem 57: 7126-35 (2014) Article DOI: 10.1021/jm500984b BindingDB Entry DOI: 10.7270/Q20V8FD1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 2 (Homo sapiens (Human)) | BDBM50057417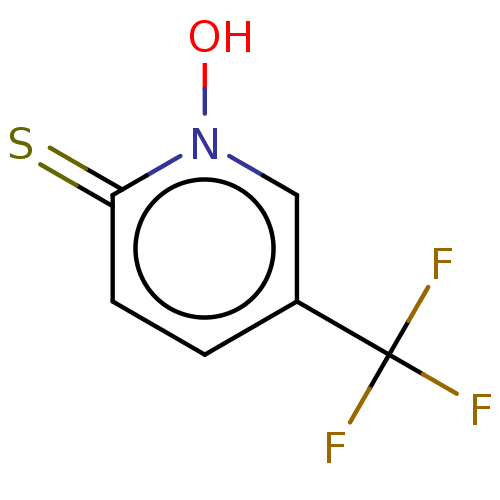 (CHEMBL3326436) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Similars | Article PubMed | 2.70E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of California Curated by ChEMBL | Assay Description Inhibition of human carbonic anhydrase using p-nitrophenylacetate as substrate preincubated for 10 mins before substrate addition by spectrophotometr... | J Med Chem 57: 7126-35 (2014) Article DOI: 10.1021/jm500984b BindingDB Entry DOI: 10.7270/Q20V8FD1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 2 (Homo sapiens (Human)) | BDBM60985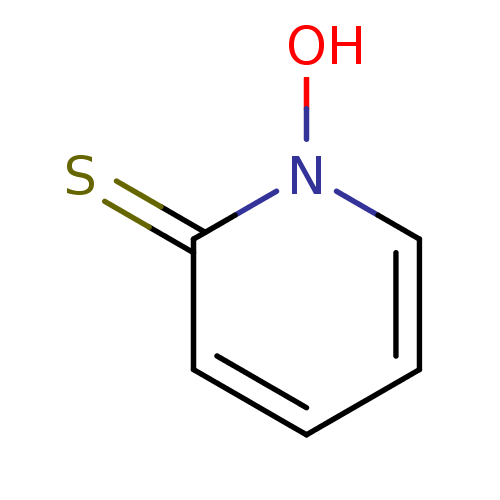 (1-hydroxy-2-pyridinethione | 1-hydroxypyridine-2-t...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | 5.10E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of California Curated by ChEMBL | Assay Description Inhibition of human carbonic anhydrase using p-nitrophenylacetate as substrate preincubated for 10 mins before substrate addition by spectrophotometr... | J Med Chem 57: 7126-35 (2014) Article DOI: 10.1021/jm500984b BindingDB Entry DOI: 10.7270/Q20V8FD1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 2 (Homo sapiens (Human)) | BDBM50057408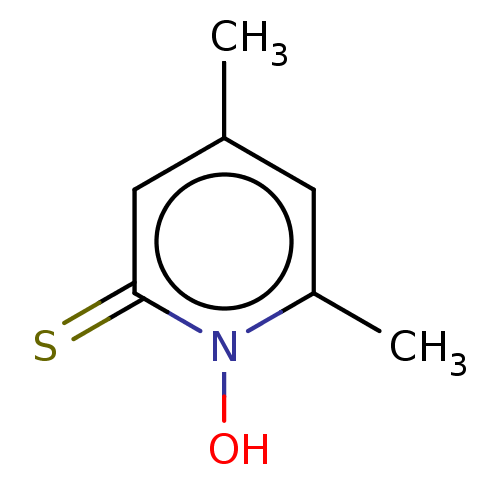 (CHEMBL3326433) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Similars | Article PubMed | 9.50E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of California Curated by ChEMBL | Assay Description Inhibition of human carbonic anhydrase using p-nitrophenylacetate as substrate preincubated for 10 mins before substrate addition by spectrophotometr... | J Med Chem 57: 7126-35 (2014) Article DOI: 10.1021/jm500984b BindingDB Entry DOI: 10.7270/Q20V8FD1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 2 (Homo sapiens (Human)) | BDBM50057414 (CHEMBL3326439) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 9.50E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of California Curated by ChEMBL | Assay Description Inhibition of human carbonic anhydrase using p-nitrophenylacetate as substrate preincubated for 10 mins before substrate addition by spectrophotometr... | J Med Chem 57: 7126-35 (2014) Article DOI: 10.1021/jm500984b BindingDB Entry DOI: 10.7270/Q20V8FD1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 2 (Homo sapiens (Human)) | BDBM50057409 (CHEMBL3326432) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Similars | Article PubMed | 2.30E+6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of California Curated by ChEMBL | Assay Description Inhibition of human carbonic anhydrase using p-nitrophenylacetate as substrate preincubated for 10 mins before substrate addition by spectrophotometr... | J Med Chem 57: 7126-35 (2014) Article DOI: 10.1021/jm500984b BindingDB Entry DOI: 10.7270/Q20V8FD1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 2 (Homo sapiens (Human)) | BDBM50057415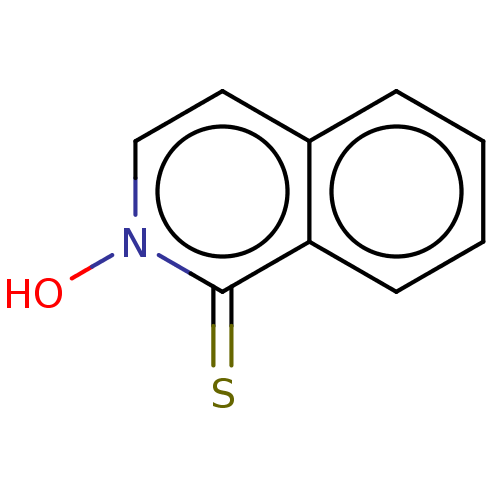 (CHEMBL3326438) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Similars | Article PubMed | 2.30E+6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of California Curated by ChEMBL | Assay Description Inhibition of human carbonic anhydrase using p-nitrophenylacetate as substrate preincubated for 10 mins before substrate addition by spectrophotometr... | J Med Chem 57: 7126-35 (2014) Article DOI: 10.1021/jm500984b BindingDB Entry DOI: 10.7270/Q20V8FD1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 2 (Homo sapiens (Human)) | BDBM50057416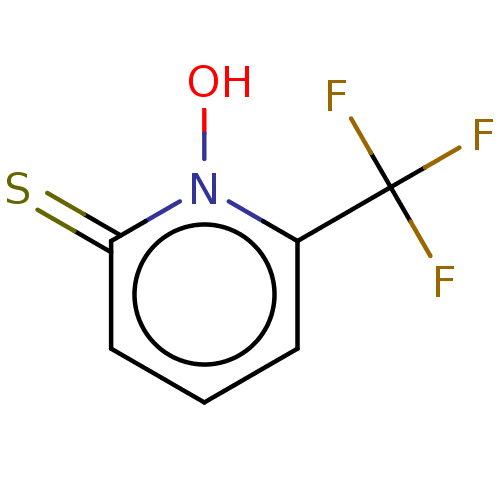 (CHEMBL3326437) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | >1.00E+7 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of California Curated by ChEMBL | Assay Description Inhibition of human carbonic anhydrase using p-nitrophenylacetate as substrate preincubated for 10 mins before substrate addition by spectrophotometr... | J Med Chem 57: 7126-35 (2014) Article DOI: 10.1021/jm500984b BindingDB Entry DOI: 10.7270/Q20V8FD1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein kinase C alpha type (Homo sapiens (Human)) | BDBM3149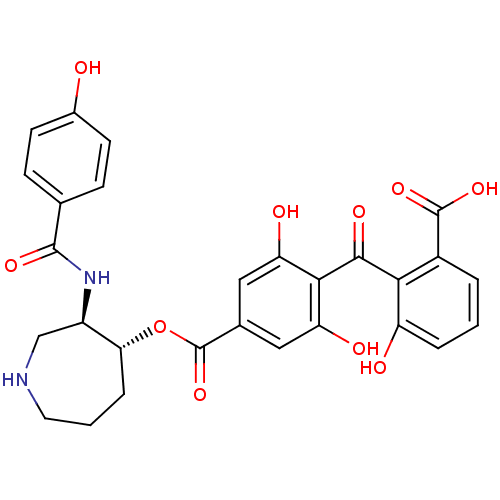 (2-{[2,6-dihydroxy-4-({[(3R,4R)-3-[(4-hydroxybenzen...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE MMDB PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | 0.0740 | n/a | n/a | n/a | n/a | n/a | n/a |
University of California at San Diego Curated by ChEMBL | Assay Description Inhibition of Protein kinase C alpha | J Med Chem 44: 1530-9 (2001) Article DOI: 10.1021/jm000443d BindingDB Entry DOI: 10.7270/Q28D0007 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein kinase C alpha type (Homo sapiens (Human)) | BDBM50473233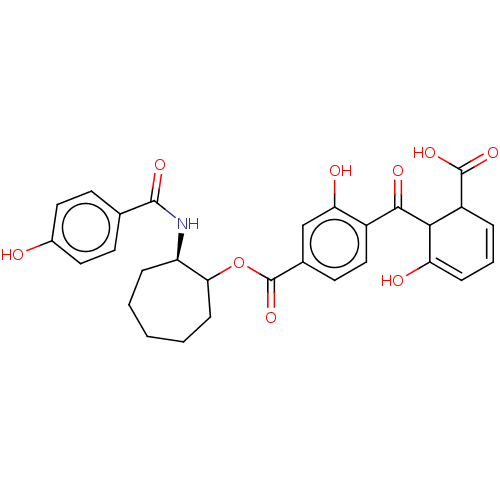 (CHEMBL38655) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.270 | n/a | n/a | n/a | n/a | n/a | n/a |
University of California at San Diego Curated by ChEMBL | Assay Description Inhibition of Protein kinase C alpha | J Med Chem 44: 1530-9 (2001) Article DOI: 10.1021/jm000443d BindingDB Entry DOI: 10.7270/Q28D0007 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein kinase C alpha type (Homo sapiens (Human)) | BDBM50473235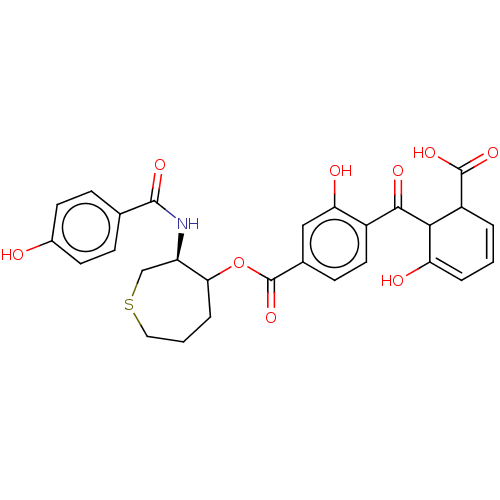 (CHEMBL38726) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 3.30 | n/a | n/a | n/a | n/a | n/a | n/a |
University of California at San Diego Curated by ChEMBL | Assay Description Inhibition of Protein kinase C alpha | J Med Chem 44: 1530-9 (2001) Article DOI: 10.1021/jm000443d BindingDB Entry DOI: 10.7270/Q28D0007 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Procathepsin L (Homo sapiens (Human)) | BDBM50033762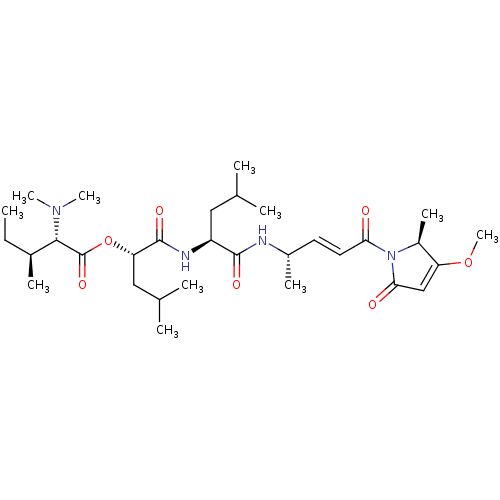 (Gallinamide A) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
University of California Curated by ChEMBL | Assay Description Inhibition of recombinant human Cathepsin L using Z-Phe-Arg-aminomethylcoumarin as substrate preincubated for 30 mins followed by substrate addition | J Nat Prod 77: 92-9 (2014) Article DOI: 10.1021/np400727r BindingDB Entry DOI: 10.7270/Q2Z89GC6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein kinase C alpha type (Homo sapiens (Human)) | BDBM50473234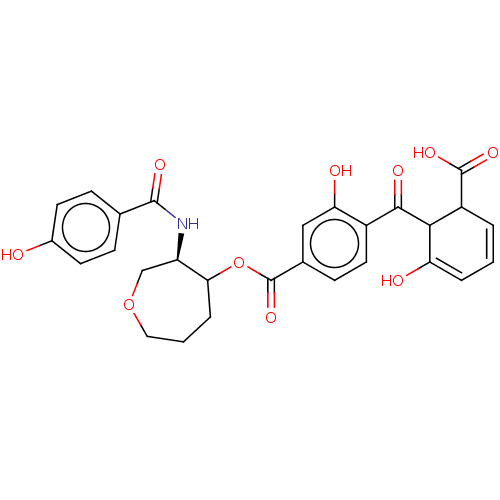 (CHEMBL287775) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 6.70 | n/a | n/a | n/a | n/a | n/a | n/a |
University of California at San Diego Curated by ChEMBL | Assay Description Inhibition of Protein kinase C alpha | J Med Chem 44: 1530-9 (2001) Article DOI: 10.1021/jm000443d BindingDB Entry DOI: 10.7270/Q28D0007 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein kinase C alpha type (Homo sapiens (Human)) | BDBM50473236 (CHEMBL38575) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 9.60 | n/a | n/a | n/a | n/a | n/a | n/a |
University of California at San Diego Curated by ChEMBL | Assay Description Inhibition of Protein kinase C alpha | J Med Chem 44: 1530-9 (2001) Article DOI: 10.1021/jm000443d BindingDB Entry DOI: 10.7270/Q28D0007 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Stromelysin-1 (Homo sapiens (Human)) | BDBM82186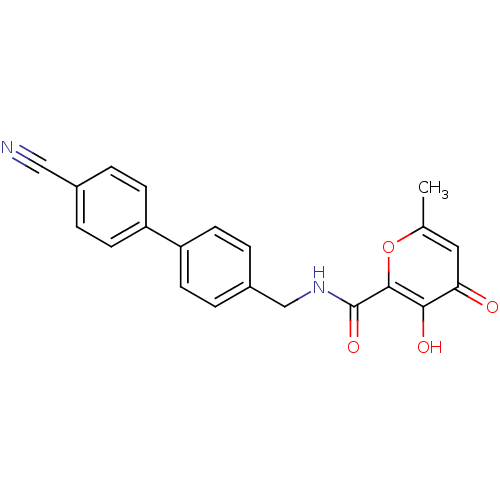 (AM-5) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
University of California at San Diego | Assay Description Inhibition assay using matrix metalloproteinases. | Chem Biol Drug Des 78: 191-8 (2011) Article DOI: 10.1111/j.1747-0285.2011.01148.x BindingDB Entry DOI: 10.7270/Q2KD1WDJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Stromelysin-1 (Homo sapiens (Human)) | BDBM50264772 (3-Hydroxy-6-methyl-4-oxo-4H-pyran-2-carboxylic aci...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 19 | n/a | n/a | n/a | n/a | n/a | n/a |
University of California at San Diego | Assay Description Inhibition assay using matrix metalloproteinases. | Chem Biol Drug Des 78: 191-8 (2011) Article DOI: 10.1111/j.1747-0285.2011.01148.x BindingDB Entry DOI: 10.7270/Q2KD1WDJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cyclin-dependent kinase 2 (Homo sapiens (Human)) | BDBM7501 (4-{(Z)-[4,6-dihydroxy-7-(1-methylpiperidin-4-yl)-3...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a |
University of California Curated by ChEMBL | Assay Description Inhibition of Cyclin-dependent kinase 2 (CDK2) | J Med Chem 46: 3314-25 (2003) Article DOI: 10.1021/jm0205043 BindingDB Entry DOI: 10.7270/Q2RB7406 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Decaprenyl diphosphate synthase (Mycobacterium tuberculosis H37Rv) | BDBM153301 (BPH-1463) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 31 | n/a | n/a | n/a | n/a | 7.5 | n/a |
University of California San Diego | Assay Description We screened an in-house library of 43 compounds using GPP as substrate. Briefly, the condensation of IPP and GPP, FPP, or cis-FPP catalyzed by DPPS w... | Chem Biol Drug Des 85: 756-69 (2015) Article DOI: 10.1111/cbdd.12463 BindingDB Entry DOI: 10.7270/Q2ZG6QZN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Decaprenyl diphosphate synthase (Mycobacterium tuberculosis H37Rv) | BDBM153302 (BPH-948) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 36 | n/a | n/a | n/a | n/a | 7.5 | n/a |
University of California San Diego | Assay Description We screened an in-house library of 43 compounds using GPP as substrate. Briefly, the condensation of IPP and GPP, FPP, or cis-FPP catalyzed by DPPS w... | Chem Biol Drug Des 85: 756-69 (2015) Article DOI: 10.1111/cbdd.12463 BindingDB Entry DOI: 10.7270/Q2ZG6QZN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Decaprenyl diphosphate synthase (Mycobacterium tuberculosis H37Rv) | BDBM153303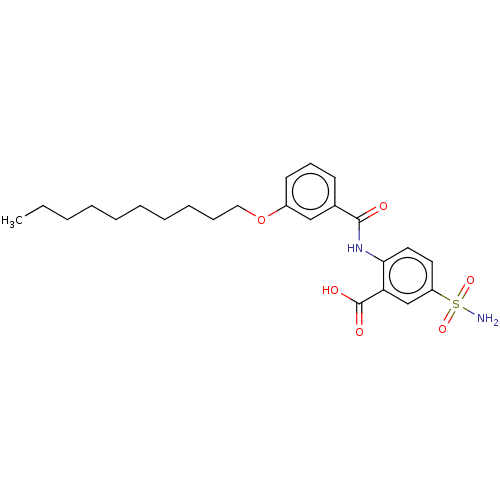 (BPH-1256) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 41 | n/a | n/a | n/a | n/a | 7.5 | n/a |
University of California San Diego | Assay Description We screened an in-house library of 43 compounds using GPP as substrate. Briefly, the condensation of IPP and GPP, FPP, or cis-FPP catalyzed by DPPS w... | Chem Biol Drug Des 85: 756-69 (2015) Article DOI: 10.1111/cbdd.12463 BindingDB Entry DOI: 10.7270/Q2ZG6QZN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Decaprenyl diphosphate synthase (Mycobacterium tuberculosis H37Rv) | BDBM153304 (BPH-1477) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 43 | n/a | n/a | n/a | n/a | 7.5 | n/a |
University of California San Diego | Assay Description We screened an in-house library of 43 compounds using GPP as substrate. Briefly, the condensation of IPP and GPP, FPP, or cis-FPP catalyzed by DPPS w... | Chem Biol Drug Des 85: 756-69 (2015) Article DOI: 10.1111/cbdd.12463 BindingDB Entry DOI: 10.7270/Q2ZG6QZN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Procathepsin L (Homo sapiens (Human)) | BDBM50033762 (Gallinamide A) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 47 | n/a | n/a | n/a | n/a | n/a | n/a |
University of California Curated by ChEMBL | Assay Description Inhibition of recombinant human Cathepsin L using Z-Phe-Arg-aminomethylcoumarin as substrate | J Nat Prod 77: 92-9 (2014) Article DOI: 10.1021/np400727r BindingDB Entry DOI: 10.7270/Q2Z89GC6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Decaprenyl diphosphate synthase (Mycobacterium tuberculosis H37Rv) | BDBM153305 (BPH-1264) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 68 | n/a | n/a | n/a | n/a | 7.5 | n/a |
University of California San Diego | Assay Description We screened an in-house library of 43 compounds using GPP as substrate. Briefly, the condensation of IPP and GPP, FPP, or cis-FPP catalyzed by DPPS w... | Chem Biol Drug Des 85: 756-69 (2015) Article DOI: 10.1111/cbdd.12463 BindingDB Entry DOI: 10.7270/Q2ZG6QZN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Decaprenyl diphosphate synthase (Mycobacterium tuberculosis H37Rv) | BDBM153306 (BPH-1247) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 119 | n/a | n/a | n/a | n/a | 7.5 | n/a |
University of California San Diego | Assay Description We screened an in-house library of 43 compounds using GPP as substrate. Briefly, the condensation of IPP and GPP, FPP, or cis-FPP catalyzed by DPPS w... | Chem Biol Drug Des 85: 756-69 (2015) Article DOI: 10.1111/cbdd.12463 BindingDB Entry DOI: 10.7270/Q2ZG6QZN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| dTDP-4-dehydrorhamnose 3,5-epimerase (Mycobacterium tuberculosis H37Rv) | BDBM50481568 (CHEMBL592712) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase PC cid PC sid UniChem | Article PubMed | n/a | n/a | 120 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Pennsylvania Curated by ChEMBL | Assay Description Inhibition of Mycobacterium tuberculosis RmlC expressed in Escherichia coli by spectrophotometry | Bioorg Med Chem 18: 896-908 (2010) Article DOI: 10.1016/j.bmc.2009.11.033 BindingDB Entry DOI: 10.7270/Q2N300SV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Integrase (Human immunodeficiency virus 1) | BDBM50073630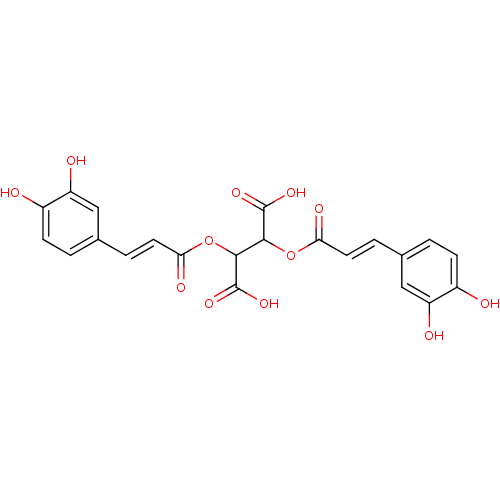 (2,3-Bis-[(E)-3-(3,4-dihydroxy-phenyl)-acryloyloxy]...) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 130 | n/a | n/a | n/a | n/a | n/a | n/a |
University of California Curated by ChEMBL | Assay Description Tested for inhibition of HIV-1 integrase, under 1 uM for the strand transfer | J Med Chem 43: 2100-14 (2000) BindingDB Entry DOI: 10.7270/Q27D2VTS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Decaprenyl diphosphate synthase (Mycobacterium tuberculosis H37Rv) | BDBM153307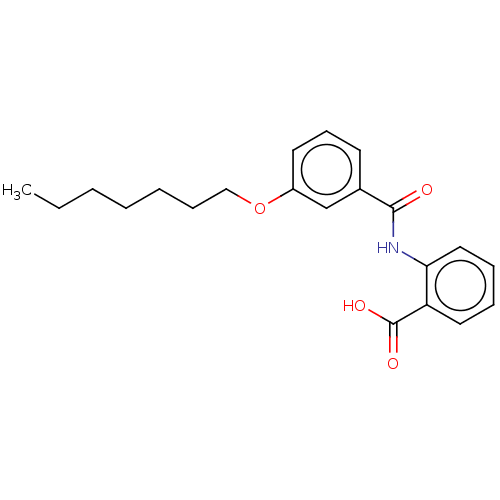 (BPH-1167) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 133 | n/a | n/a | n/a | n/a | 7.5 | n/a |
University of California San Diego | Assay Description We screened an in-house library of 43 compounds using GPP as substrate. Briefly, the condensation of IPP and GPP, FPP, or cis-FPP catalyzed by DPPS w... | Chem Biol Drug Des 85: 756-69 (2015) Article DOI: 10.1111/cbdd.12463 BindingDB Entry DOI: 10.7270/Q2ZG6QZN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Decaprenyl diphosphate synthase (Mycobacterium tuberculosis H37Rv) | BDBM153308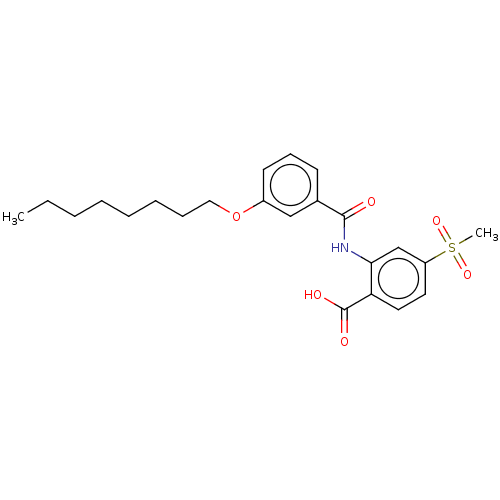 (BPH-1467) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 138 | n/a | n/a | n/a | n/a | 7.5 | n/a |
University of California San Diego | Assay Description We screened an in-house library of 43 compounds using GPP as substrate. Briefly, the condensation of IPP and GPP, FPP, or cis-FPP catalyzed by DPPS w... | Chem Biol Drug Des 85: 756-69 (2015) Article DOI: 10.1111/cbdd.12463 BindingDB Entry DOI: 10.7270/Q2ZG6QZN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin L2 (Homo sapiens (Human)) | BDBM50033762 (Gallinamide A) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 140 | n/a | n/a | n/a | n/a | n/a | n/a |
University of California Curated by ChEMBL | Assay Description Inhibition of human Cathepsin V using Z-Phe-Arg-aminomethylcoumarin as substrate preincubated for 30 mins followed by substrate addition | J Nat Prod 77: 92-9 (2014) Article DOI: 10.1021/np400727r BindingDB Entry DOI: 10.7270/Q2Z89GC6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Integrase (Human immunodeficiency virus 1) | BDBM50073630 (2,3-Bis-[(E)-3-(3,4-dihydroxy-phenyl)-acryloyloxy]...) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 150 | n/a | n/a | n/a | n/a | n/a | n/a |
University of California Curated by ChEMBL | Assay Description Inhibition of HIV-1 integrase, under 1 uM for the 3''-preprocessing | J Med Chem 43: 2100-14 (2000) BindingDB Entry DOI: 10.7270/Q27D2VTS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Decaprenyl diphosphate synthase (Mycobacterium tuberculosis H37Rv) | BDBM153309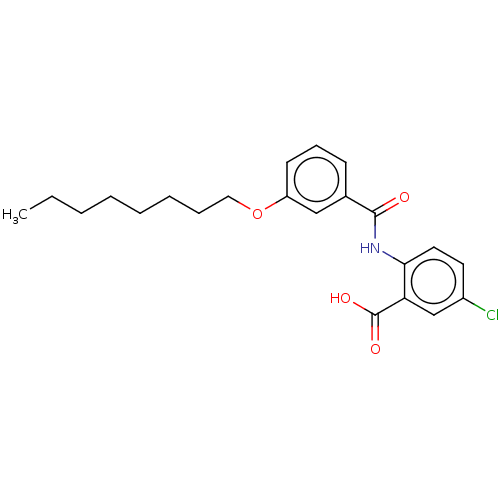 (BPH-1466) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 156 | n/a | n/a | n/a | n/a | 7.5 | n/a |
University of California San Diego | Assay Description We screened an in-house library of 43 compounds using GPP as substrate. Briefly, the condensation of IPP and GPP, FPP, or cis-FPP catalyzed by DPPS w... | Chem Biol Drug Des 85: 756-69 (2015) Article DOI: 10.1111/cbdd.12463 BindingDB Entry DOI: 10.7270/Q2ZG6QZN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Decaprenyl diphosphate synthase (Mycobacterium tuberculosis H37Rv) | BDBM153310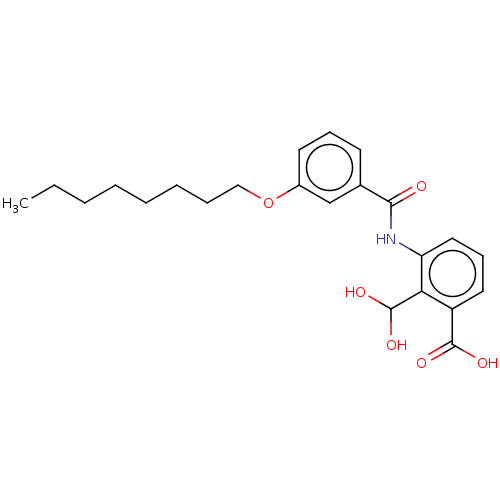 (BPH-1461) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 164 | n/a | n/a | n/a | n/a | 7.5 | n/a |
University of California San Diego | Assay Description We screened an in-house library of 43 compounds using GPP as substrate. Briefly, the condensation of IPP and GPP, FPP, or cis-FPP catalyzed by DPPS w... | Chem Biol Drug Des 85: 756-69 (2015) Article DOI: 10.1111/cbdd.12463 BindingDB Entry DOI: 10.7270/Q2ZG6QZN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| dTDP-4-dehydrorhamnose 3,5-epimerase (Mycobacterium tuberculosis H37Rv) | BDBM51599 (3-[3-[(5-ethyl-[1,2,4]triazin[5,6-b]indol-3-yl)thi...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 200 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Pennsylvania Curated by ChEMBL | Assay Description Inhibition of Mycobacterium tuberculosis RmlC expressed in Escherichia coli by spectrophotometry | Bioorg Med Chem 18: 896-908 (2010) Article DOI: 10.1016/j.bmc.2009.11.033 BindingDB Entry DOI: 10.7270/Q2N300SV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Decaprenyl diphosphate synthase (Mycobacterium tuberculosis H37Rv) | BDBM153311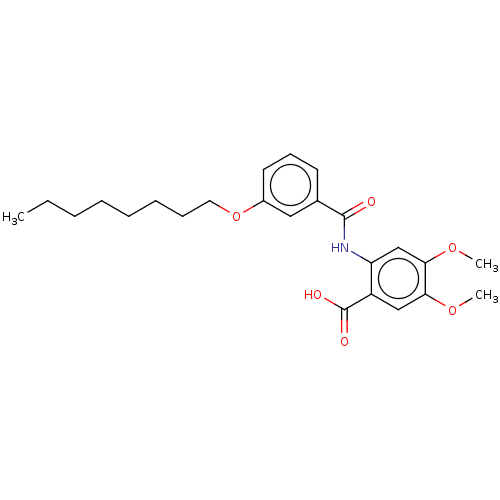 (BPH-1294 | BPH-1296) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 205 | n/a | n/a | n/a | n/a | 7.5 | n/a |
University of California San Diego | Assay Description We screened an in-house library of 43 compounds using GPP as substrate. Briefly, the condensation of IPP and GPP, FPP, or cis-FPP catalyzed by DPPS w... | Chem Biol Drug Des 85: 756-69 (2015) Article DOI: 10.1111/cbdd.12463 BindingDB Entry DOI: 10.7270/Q2ZG6QZN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Decaprenyl diphosphate synthase (Mycobacterium tuberculosis H37Rv) | BDBM153312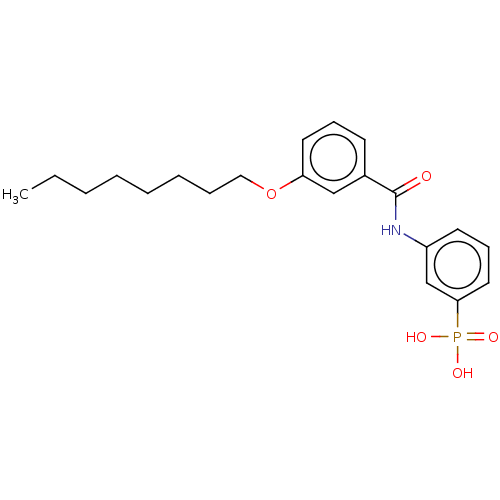 (BPH-1432) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 210 | n/a | n/a | n/a | n/a | 7.5 | n/a |
University of California San Diego | Assay Description We screened an in-house library of 43 compounds using GPP as substrate. Briefly, the condensation of IPP and GPP, FPP, or cis-FPP catalyzed by DPPS w... | Chem Biol Drug Des 85: 756-69 (2015) Article DOI: 10.1111/cbdd.12463 BindingDB Entry DOI: 10.7270/Q2ZG6QZN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tuberculosinyl adenosine transferase (Mycobacterium tuberculosis H37Rv) | BDBM25288 (BPH-629 | [1-hydroxy-2-(3-{8-oxatricyclo[7.4.0.0^{...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | DrugBank MMDB PC cid PC sid PDB UniChem | PDB Article PubMed | n/a | n/a | 210 | n/a | n/a | n/a | n/a | n/a | n/a |
University of California San Diego | Assay Description For inhibition assay of Rv3378c, using tuberculosinyl diphosphate and adenosine as substrates, a mixture of 100 µM TPP, 100 µM adenosine,75 µg/mL Rv3... | Chem Biol Drug Des 85: 756-69 (2015) Article DOI: 10.1111/cbdd.12463 BindingDB Entry DOI: 10.7270/Q2ZG6QZN | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Stromelysin-1 (Homo sapiens (Human)) | BDBM50241504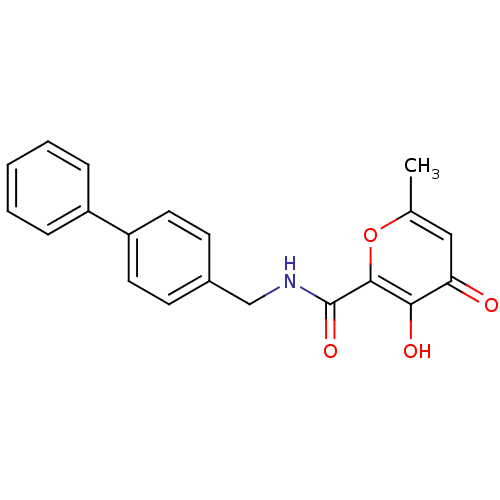 (3-hydroxy-6-methyl-4-oxo-4H-pyran-2-carboxylic aci...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 240 | n/a | n/a | n/a | n/a | n/a | n/a |
University of California at San Diego | Assay Description Inhibition assay using matrix metalloproteinases. | Chem Biol Drug Des 78: 191-8 (2011) Article DOI: 10.1111/j.1747-0285.2011.01148.x BindingDB Entry DOI: 10.7270/Q2KD1WDJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Integrase (Human immunodeficiency virus 1) | BDBM50056908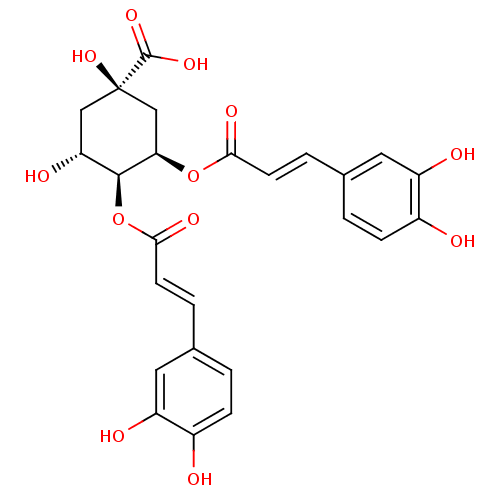 ((1R,3R,4S,5R)-3,4-Bis-[(E)-3-(3,4-dihydroxy-phenyl...) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 250 | n/a | n/a | n/a | n/a | n/a | n/a |
University of California Curated by ChEMBL | Assay Description Inhibition of HIV-1 integrase, under 1 uM for the 3''-preprocessing | J Med Chem 43: 2100-14 (2000) BindingDB Entry DOI: 10.7270/Q27D2VTS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Integrase (Human immunodeficiency virus 1) | BDBM50056908 ((1R,3R,4S,5R)-3,4-Bis-[(E)-3-(3,4-dihydroxy-phenyl...) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 250 | n/a | n/a | n/a | n/a | n/a | n/a |
University of California Curated by ChEMBL | Assay Description Inhibition of HIV-1 integrase, under 1 uM for the 3''-preprocessing | J Med Chem 43: 2100-14 (2000) BindingDB Entry DOI: 10.7270/Q27D2VTS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 304 total ) | Next | Last >> |