

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50037121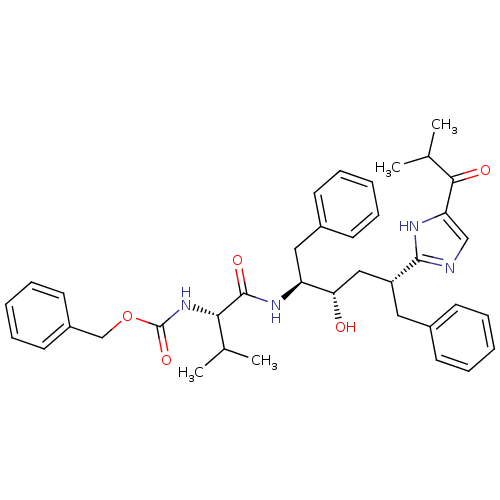 (2-[(1R,3S,4S)-1-BENZYL-4-[N-(BENZYLOXYCARBONYL)-L-...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid PDB UniChem Patents Similars | MMDB PDB PubMed | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals Curated by ChEMBL | Assay Description Apparent inhibition constant against recombinant HIV-1 protease | J Med Chem 37: 3100-7 (1994) BindingDB Entry DOI: 10.7270/Q2T72GG9 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50403349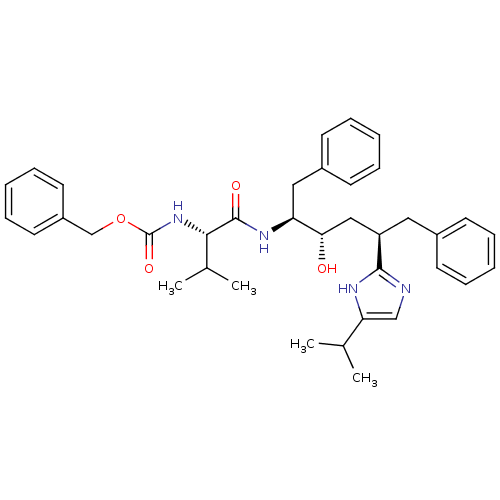 (CHEMBL407551) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibitory activity against HIV-1 protease | Bioorg Med Chem Lett 4: 2441-2446 (1994) Article DOI: 10.1016/S0960-894X(01)80406-6 BindingDB Entry DOI: 10.7270/Q2X63P48 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50037124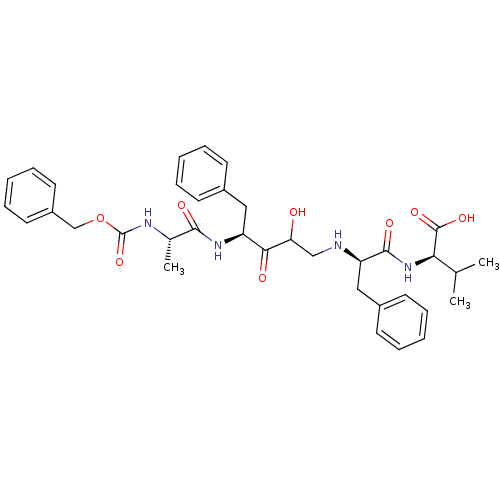 ((R)-2-{(R)-2-[(S)-4-((S)-2-Benzyloxycarbonylamino-...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals Curated by ChEMBL | Assay Description Apparent inhibition constant against recombinant HIV-1 protease | J Med Chem 37: 3100-7 (1994) BindingDB Entry DOI: 10.7270/Q2T72GG9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50403357 (CHEMBL419286) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibitory activity against HIV-1 protease | Bioorg Med Chem Lett 4: 2441-2446 (1994) Article DOI: 10.1016/S0960-894X(01)80406-6 BindingDB Entry DOI: 10.7270/Q2X63P48 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50403360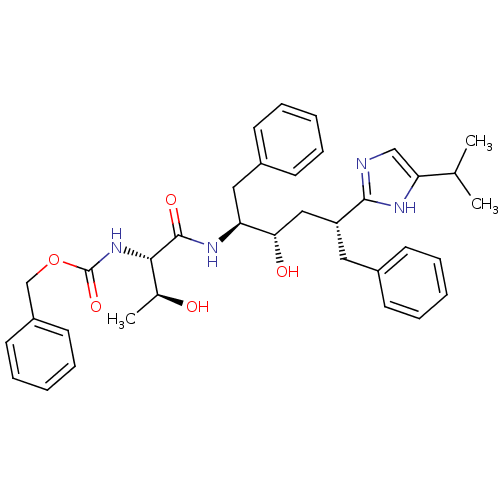 (CHEMBL1790592) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibitory activity against HIV-1 protease | Bioorg Med Chem Lett 4: 2441-2446 (1994) Article DOI: 10.1016/S0960-894X(01)80406-6 BindingDB Entry DOI: 10.7270/Q2X63P48 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50403351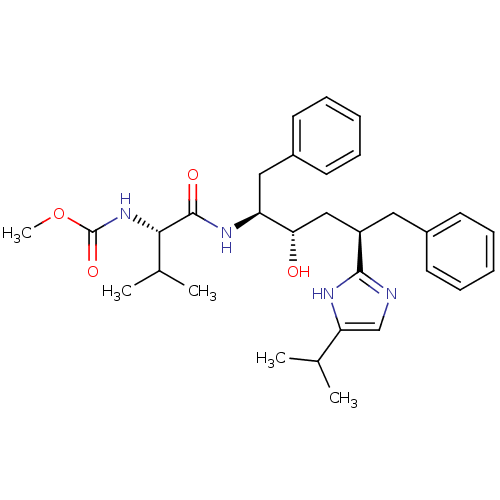 (CHEMBL79698) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | 2.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibitory activity against HIV-1 protease | Bioorg Med Chem Lett 4: 2441-2446 (1994) Article DOI: 10.1016/S0960-894X(01)80406-6 BindingDB Entry DOI: 10.7270/Q2X63P48 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50403358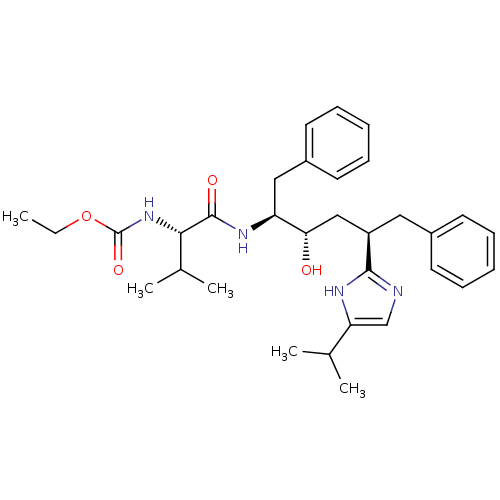 (CHEMBL78531) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | 2.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibitory activity against HIV-1 protease | Bioorg Med Chem Lett 4: 2441-2446 (1994) Article DOI: 10.1016/S0960-894X(01)80406-6 BindingDB Entry DOI: 10.7270/Q2X63P48 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50403352 (CHEMBL81517) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article | 4.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibitory activity against HIV-1 protease | Bioorg Med Chem Lett 4: 2441-2446 (1994) Article DOI: 10.1016/S0960-894X(01)80406-6 BindingDB Entry DOI: 10.7270/Q2X63P48 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cysteinyl leukotriene receptor 1 (GUINEA PIG) | BDBM50009070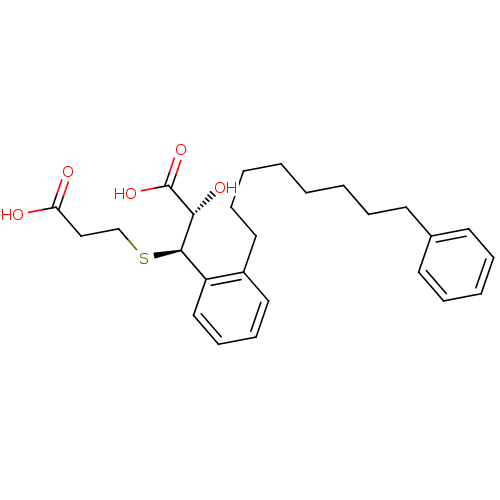 ((2S,3R)-3-(2-Carboxy-ethylsulfanyl)-2-hydroxy-3-[2...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Patents Similars | PubMed | 5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Binding affinity against Cysteinyl leukotriene D4 receptor in guinea pig lung membranes using [3H]-LTD4 as the radioligand. | J Med Chem 30: 959-61 (1987) BindingDB Entry DOI: 10.7270/Q2D50KZN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50403348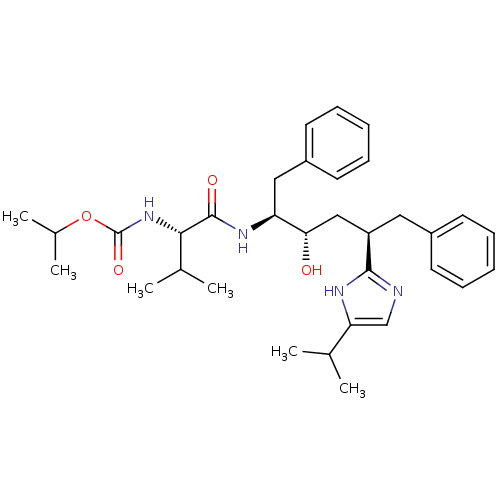 (CHEMBL421709) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | 7.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibitory activity against HIV-1 protease | Bioorg Med Chem Lett 4: 2441-2446 (1994) Article DOI: 10.1016/S0960-894X(01)80406-6 BindingDB Entry DOI: 10.7270/Q2X63P48 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cysteinyl leukotriene receptor 1 (Homo sapiens (Human)) | BDBM50009070 ((2S,3R)-3-(2-Carboxy-ethylsulfanyl)-2-hydroxy-3-[2...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Patents Similars | PubMed | 10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Binding affinity against Cysteinyl leukotriene D4 receptor in guinea pig lung membranes using [3H]-LTD4 as the radioligand. | J Med Chem 30: 959-61 (1987) BindingDB Entry DOI: 10.7270/Q2D50KZN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50403345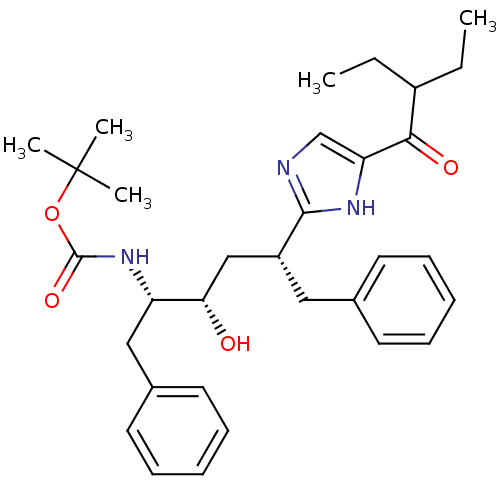 (CHEMBL83739) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | 46 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibitory activity against HIV-1 protease | Bioorg Med Chem Lett 4: 2441-2446 (1994) Article DOI: 10.1016/S0960-894X(01)80406-6 BindingDB Entry DOI: 10.7270/Q2X63P48 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50403350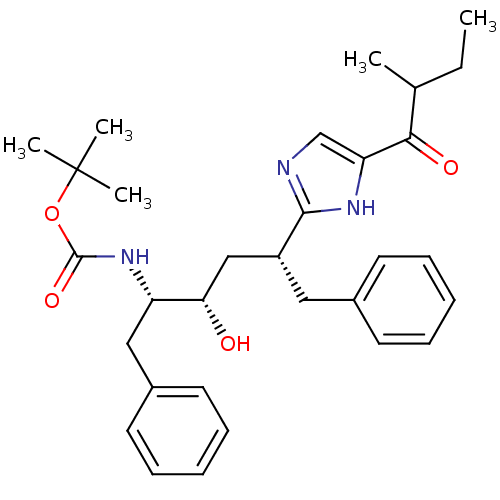 (CHEMBL81190) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | 53 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibitory activity against HIV-1 protease | Bioorg Med Chem Lett 4: 2441-2446 (1994) Article DOI: 10.1016/S0960-894X(01)80406-6 BindingDB Entry DOI: 10.7270/Q2X63P48 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cysteinyl leukotriene receptor 1 (GUINEA PIG) | BDBM50021780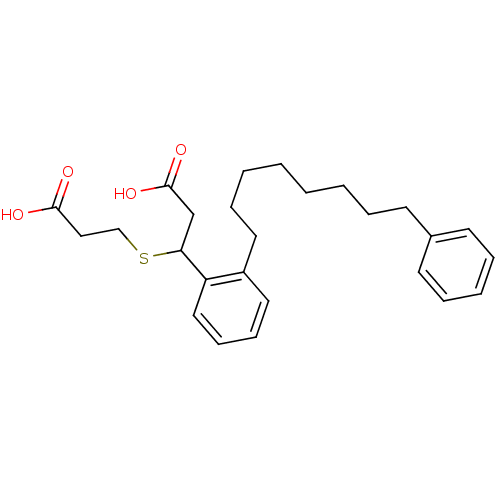 (3-(2-Carboxy-ethylsulfanyl)-3-[2-(8-phenyl-octyl)-...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Binding affinity against Cysteinyl leukotriene D4 receptor in guinea pig lung membranes using [3H]-LTD4 as the radioligand. | J Med Chem 30: 959-61 (1987) BindingDB Entry DOI: 10.7270/Q2D50KZN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cysteinyl leukotriene receptor 1/2 (Homo sapiens (Human)) | BDBM50226391 (CHEMBL35611) | PDB UniProtKB/SwissProt antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 78 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Compound is evaluated for its ability to inhibit [3H]LTD4 binding to Cysteinyl leukotriene D4 receptor in guinea pig lung membranes | J Med Chem 29: 1442-52 (1986) BindingDB Entry DOI: 10.7270/Q2S75JHD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50037122 (CHEMBL108016 | [(1S,2S,4R)-1-Benzyl-2-hydroxy-4-(5...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 83 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals Curated by ChEMBL | Assay Description Apparent inhibition constant against recombinant HIV-1 protease | J Med Chem 37: 3100-7 (1994) BindingDB Entry DOI: 10.7270/Q2T72GG9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50037126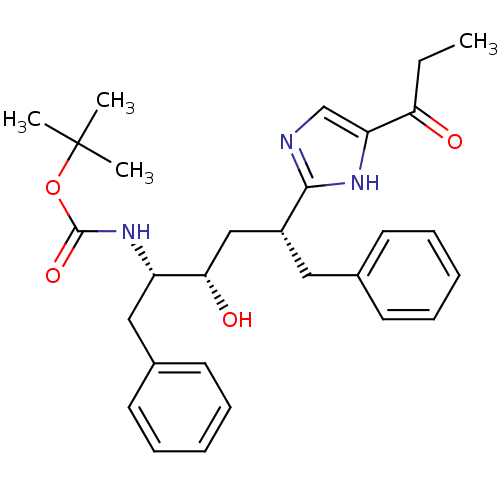 (CHEMBL80098 | [(1S,2S,4R)-1-Benzyl-2-hydroxy-5-phe...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 92 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals Curated by ChEMBL | Assay Description Apparent inhibition constant against recombinant HIV-1 protease | J Med Chem 37: 3100-7 (1994) BindingDB Entry DOI: 10.7270/Q2T72GG9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50037126 (CHEMBL80098 | [(1S,2S,4R)-1-Benzyl-2-hydroxy-5-phe...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article | 92 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibitory activity against HIV-1 protease | Bioorg Med Chem Lett 4: 2441-2446 (1994) Article DOI: 10.1016/S0960-894X(01)80406-6 BindingDB Entry DOI: 10.7270/Q2X63P48 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50037125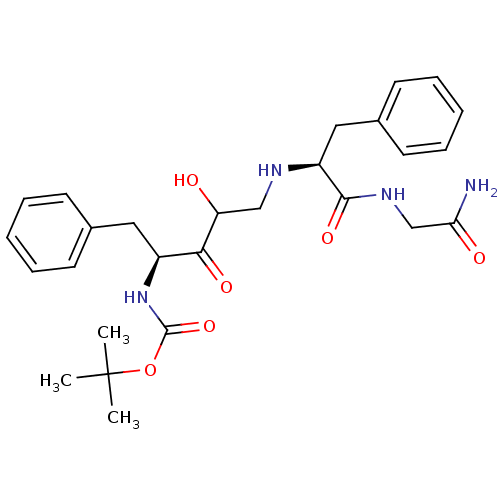 (CHEMBL107849 | {(S)-1-Benzyl-4-[(S)-1-(carbamoylme...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | 120 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals Curated by ChEMBL | Assay Description Apparent inhibition constant against recombinant HIV-1 protease | J Med Chem 37: 3100-7 (1994) BindingDB Entry DOI: 10.7270/Q2T72GG9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cysteinyl leukotriene receptor 1/2 (Homo sapiens (Human)) | BDBM50226392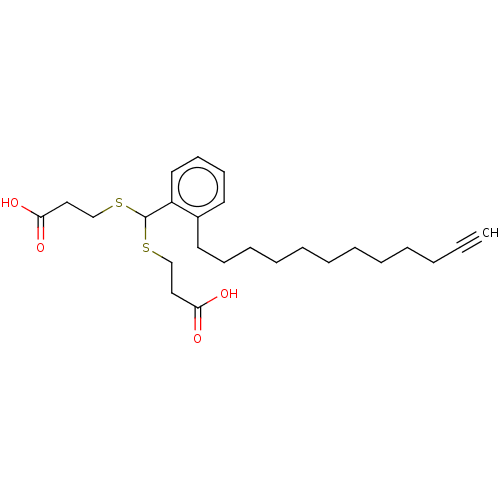 (CHEMBL285073) | PDB UniProtKB/SwissProt antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 141 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Compound is evaluated for its ability to inhibit [3H]LTD4 binding to Cysteinyl leukotriene D4 receptor in guinea pig lung membranes | J Med Chem 29: 1442-52 (1986) BindingDB Entry DOI: 10.7270/Q2S75JHD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cysteinyl leukotriene receptor 1/2 (Homo sapiens (Human)) | BDBM50226367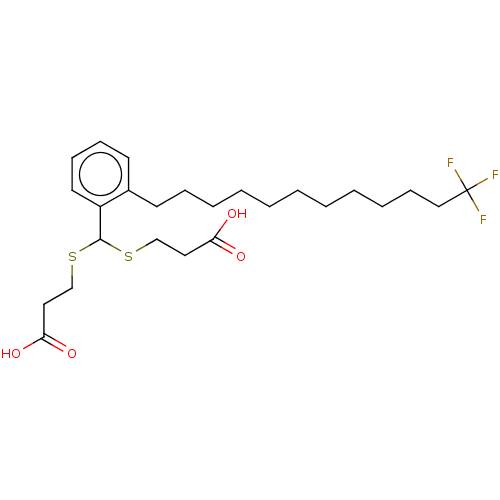 (CHEMBL287354) | PDB UniProtKB/SwissProt antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 147 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Compound is evaluated for its ability to inhibit [3H]LTD4 binding to Cysteinyl leukotriene D4 receptor in guinea pig lung membranes | J Med Chem 29: 1442-52 (1986) BindingDB Entry DOI: 10.7270/Q2S75JHD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50403353 (CHEMBL309773) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | 150 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibitory activity against HIV-1 protease | Bioorg Med Chem Lett 4: 2441-2446 (1994) Article DOI: 10.1016/S0960-894X(01)80406-6 BindingDB Entry DOI: 10.7270/Q2X63P48 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50037128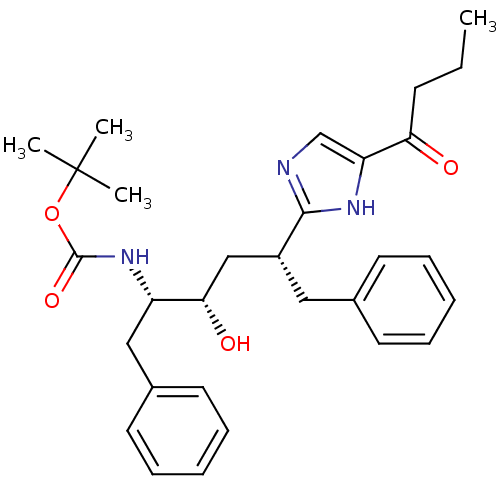 (CHEMBL419921 | [(1S,2S,4R)-1-Benzyl-4-(5-butyryl-1...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 150 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals Curated by ChEMBL | Assay Description Apparent inhibition constant against recombinant HIV-1 protease | J Med Chem 37: 3100-7 (1994) BindingDB Entry DOI: 10.7270/Q2T72GG9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50403362 (CHEMBL312709) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | 150 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibitory activity against HIV-1 protease | Bioorg Med Chem Lett 4: 2441-2446 (1994) Article DOI: 10.1016/S0960-894X(01)80406-6 BindingDB Entry DOI: 10.7270/Q2X63P48 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cysteinyl leukotriene receptor 1/2 (Homo sapiens (Human)) | BDBM50226395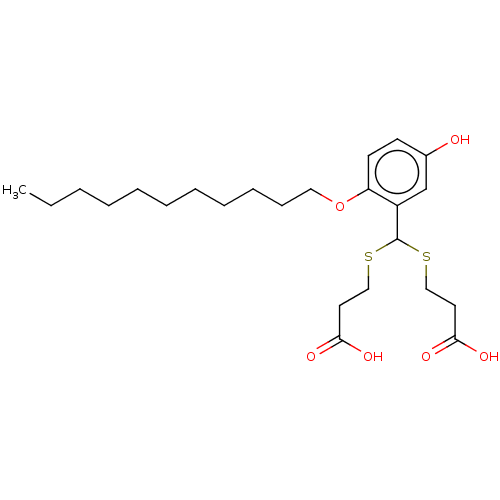 (CHEMBL286074) | PDB UniProtKB/SwissProt antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 156 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Compound is evaluated for its ability to inhibit [3H]LTD4 binding to Cysteinyl leukotriene D4 receptor in guinea pig lung membranes | J Med Chem 29: 1442-52 (1986) BindingDB Entry DOI: 10.7270/Q2S75JHD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cysteinyl leukotriene receptor 1 (GUINEA PIG) | BDBM50021781 (3-(2-Carboxy-ethylsulfanyl)-2-hydroxy-3-[2-(8-phen...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Patents Similars | PubMed | 180 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Binding affinity against Cysteinyl leukotriene D4 receptor in guinea pig lung membranes using [3H]-LTD4 as the radioligand. | J Med Chem 30: 959-61 (1987) BindingDB Entry DOI: 10.7270/Q2D50KZN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cysteinyl leukotriene receptor 1/2 (Homo sapiens (Human)) | BDBM50226362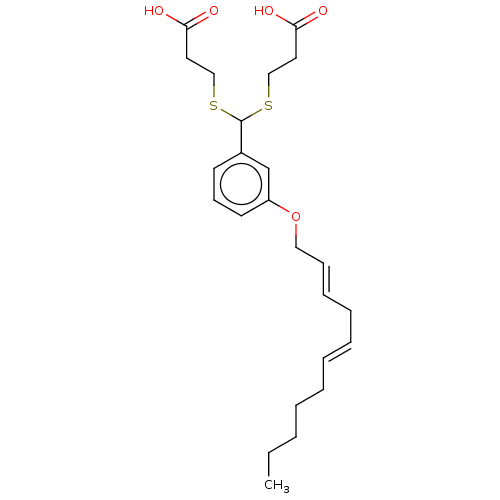 (CHEMBL33712) | PDB UniProtKB/SwissProt antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 187 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Compound is evaluated for its ability to inhibit [3H]LTD4 binding to Cysteinyl leukotriene D4 receptor in guinea pig lung membranes | J Med Chem 29: 1442-52 (1986) BindingDB Entry DOI: 10.7270/Q2S75JHD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leukotriene B4 receptor 1 (Homo sapiens (Human)) | BDBM50001610 (7-[3-(4-Acetyl-3-methoxy-2-propyl-phenoxy)-propoxy...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL MCE PC cid PC sid UniChem Patents Similars | PubMed | 220 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Smithkline Beecham Pharmaceuticals Curated by ChEMBL | Assay Description Binding affinity against Leukotriene B4 receptor on intact differentiated U-937 cells in competitive binding assay with [3H]-LTB4 | J Med Chem 36: 3333-40 (1993) BindingDB Entry DOI: 10.7270/Q2BG2N1M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cysteinyl leukotriene receptor 1 (GUINEA PIG) | BDBM50021023 (3-{(2-Carboxy-ethylsulfanyl)-[2-(8-phenyl-octyl)-p...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 245 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Binding constant was determined as affinity to displace [3H]-LTD4 from Cysteinyl leukotriene D4 receptor on guinea pig lung membrane | J Med Chem 28: 1145-7 (1985) BindingDB Entry DOI: 10.7270/Q26M35V3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cysteinyl leukotriene receptor 1/2 (Homo sapiens (Human)) | BDBM50021023 (3-{(2-Carboxy-ethylsulfanyl)-[2-(8-phenyl-octyl)-p...) | PDB UniProtKB/SwissProt antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 245 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Compound is evaluated for its ability to inhibit [3H]LTD4 binding to Cysteinyl leukotriene D4 receptor in guinea pig lung membranes | J Med Chem 29: 1442-52 (1986) BindingDB Entry DOI: 10.7270/Q2S75JHD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50037123 (CHEMBL108226 | {(1S,2S,4R)-1-Benzyl-4-[5-(2,2-dime...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 270 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals Curated by ChEMBL | Assay Description Apparent inhibition constant against recombinant HIV-1 protease | J Med Chem 37: 3100-7 (1994) BindingDB Entry DOI: 10.7270/Q2T72GG9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cysteinyl leukotriene receptor 1/2 (Homo sapiens (Human)) | BDBM50226377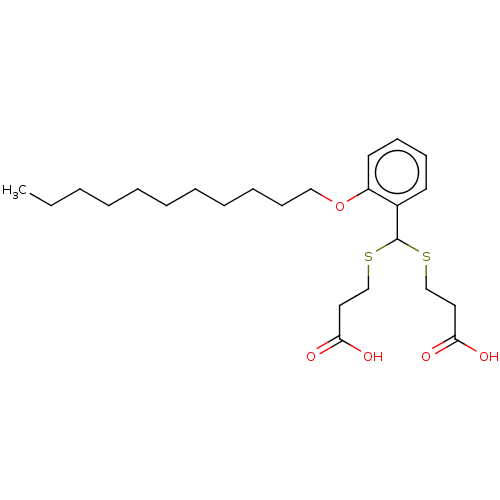 (CHEMBL32489) | PDB UniProtKB/SwissProt antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 287 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Compound is evaluated for its ability to inhibit [3H]LTD4 binding to Cysteinyl leukotriene D4 receptor in guinea pig lung membranes | J Med Chem 29: 1442-52 (1986) BindingDB Entry DOI: 10.7270/Q2S75JHD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50403356 (CHEMBL430437) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | 300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibitory activity against HIV-1 protease | Bioorg Med Chem Lett 4: 2441-2446 (1994) Article DOI: 10.1016/S0960-894X(01)80406-6 BindingDB Entry DOI: 10.7270/Q2X63P48 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cysteinyl leukotriene receptor 1/2 (Homo sapiens (Human)) | BDBM50226387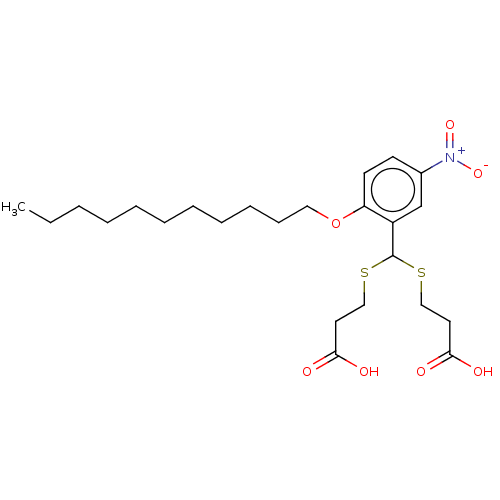 (CHEMBL35497) | PDB UniProtKB/SwissProt antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 303 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Compound is evaluated for its ability to inhibit [3H]LTD4 binding to Cysteinyl leukotriene D4 receptor in guinea pig lung membranes | J Med Chem 29: 1442-52 (1986) BindingDB Entry DOI: 10.7270/Q2S75JHD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leukotriene B4 receptor 1 (Homo sapiens (Human)) | BDBM50001611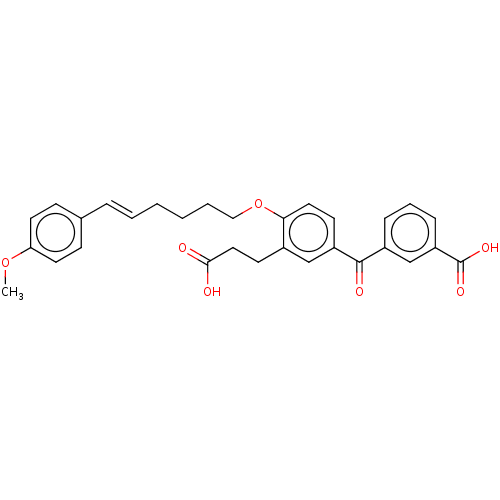 ((E)-3-{3-(2-Carboxy-ethyl)-4-[6-(4-methoxy-phenyl)...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | PubMed | 360 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Smithkline Beecham Pharmaceuticals Curated by ChEMBL | Assay Description Binding affinity against Leukotriene B4 receptor on intact differentiated U-937 cells in competitive binding assay with [3H]-LTB4 | J Med Chem 36: 3333-40 (1993) BindingDB Entry DOI: 10.7270/Q2BG2N1M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50037127 (CHEMBL104285 | [(1S,2S,4R)-4-(5-Acetyl-1H-imidazol...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 370 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals Curated by ChEMBL | Assay Description Apparent inhibition constant against recombinant HIV-1 protease | J Med Chem 37: 3100-7 (1994) BindingDB Entry DOI: 10.7270/Q2T72GG9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cysteinyl leukotriene receptor 1/2 (Homo sapiens (Human)) | BDBM50226363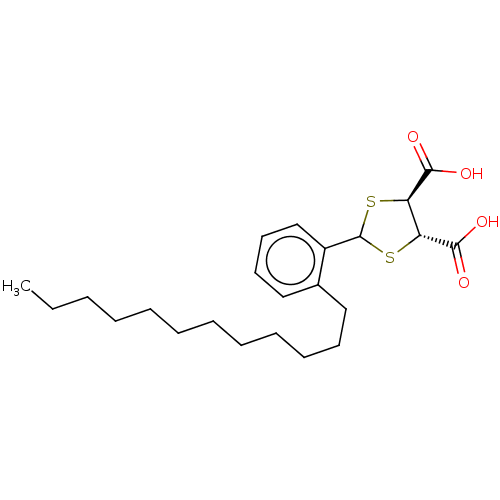 (CHEMBL35892) | PDB UniProtKB/SwissProt antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 401 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Compound is evaluated for its ability to inhibit [3H]LTD4 binding to Cysteinyl leukotriene D4 receptor in guinea pig lung membranes | J Med Chem 29: 1442-52 (1986) BindingDB Entry DOI: 10.7270/Q2S75JHD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cysteinyl leukotriene receptor 1/2 (Homo sapiens (Human)) | BDBM50226360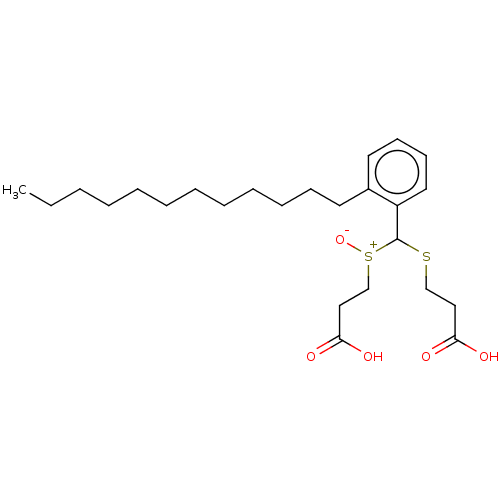 (CHEMBL33764) | PDB UniProtKB/SwissProt antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 423 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Compound is evaluated for its ability to inhibit [3H]LTD4 binding to Cysteinyl leukotriene D4 receptor in guinea pig lung membranes | J Med Chem 29: 1442-52 (1986) BindingDB Entry DOI: 10.7270/Q2S75JHD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cysteinyl leukotriene receptor 1/2 (Homo sapiens (Human)) | BDBM50226365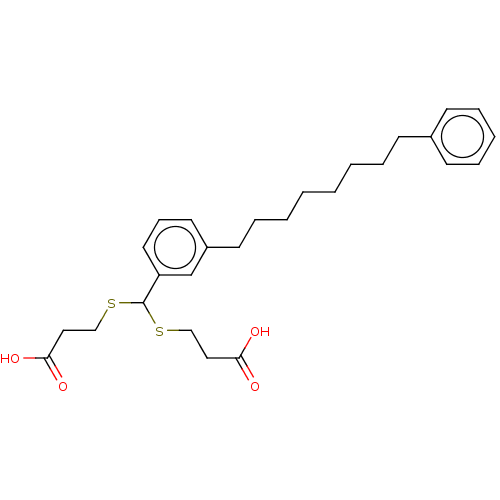 (CHEMBL418049) | PDB UniProtKB/SwissProt antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 430 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Compound is evaluated for its ability to inhibit [3H]LTD4 binding to Cysteinyl leukotriene D4 receptor in guinea pig lung membranes | J Med Chem 29: 1442-52 (1986) BindingDB Entry DOI: 10.7270/Q2S75JHD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cysteinyl leukotriene receptor 1/2 (Homo sapiens (Human)) | BDBM50021025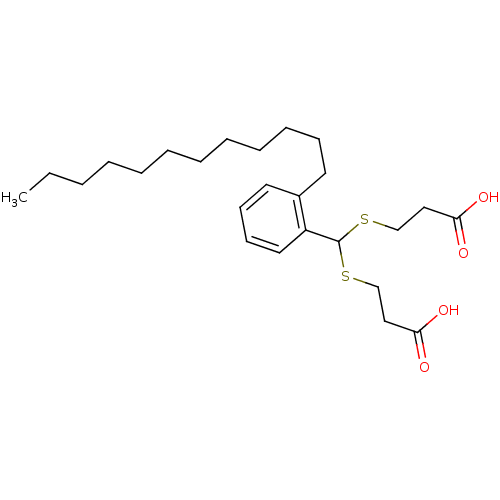 (3-[(2-Carboxy-ethylsulfanyl)-(2-dodecyl-phenyl)-me...) | PDB UniProtKB/SwissProt antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 445 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Compound is evaluated for its ability to inhibit [3H]LTD4 binding to Cysteinyl leukotriene D4 receptor in guinea pig lung membranes | J Med Chem 29: 1442-52 (1986) BindingDB Entry DOI: 10.7270/Q2S75JHD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cysteinyl leukotriene receptor 1 (GUINEA PIG) | BDBM50021025 (3-[(2-Carboxy-ethylsulfanyl)-(2-dodecyl-phenyl)-me...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 445 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Binding constant was determined as affinity to displace [3H]-LTD4 from Cysteinyl leukotriene D4 receptor on guinea pig lung membrane | J Med Chem 28: 1145-7 (1985) BindingDB Entry DOI: 10.7270/Q26M35V3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cysteinyl leukotriene receptor 1/2 (Homo sapiens (Human)) | BDBM50226389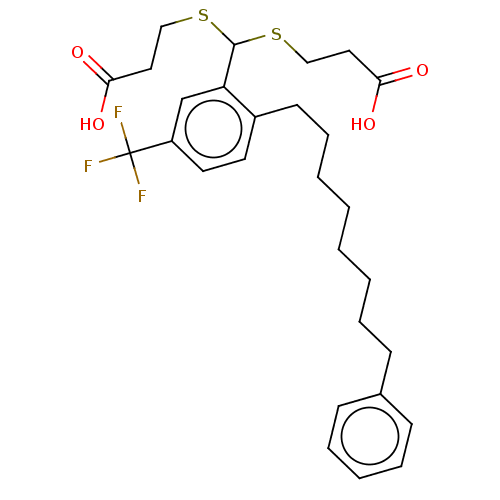 (CHEMBL33020) | PDB UniProtKB/SwissProt antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 476 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Compound is evaluated for its ability to inhibit [3H]LTD4 binding to Cysteinyl leukotriene D4 receptor in guinea pig lung membranes | J Med Chem 29: 1442-52 (1986) BindingDB Entry DOI: 10.7270/Q2S75JHD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cysteinyl leukotriene receptor 1/2 (Homo sapiens (Human)) | BDBM50226366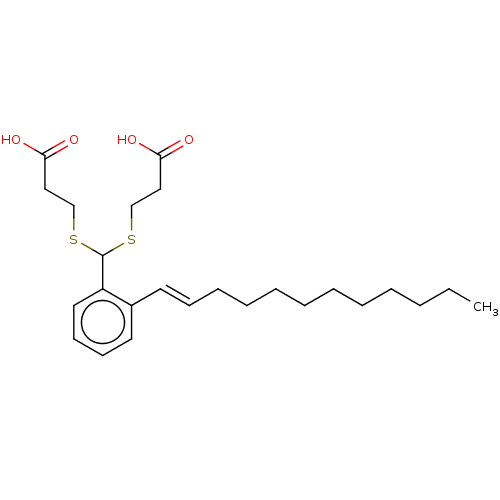 (CHEMBL32376) | PDB UniProtKB/SwissProt antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 491 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Compound is evaluated for its ability to inhibit [3H]LTD4 binding to Cysteinyl leukotriene D4 receptor in guinea pig lung membranes | J Med Chem 29: 1442-52 (1986) BindingDB Entry DOI: 10.7270/Q2S75JHD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cysteinyl leukotriene receptor 1/2 (Homo sapiens (Human)) | BDBM50226355 (CHEMBL34944) | PDB UniProtKB/SwissProt antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 530 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Compound is evaluated for its ability to inhibit [3H]LTD4 binding to Cysteinyl leukotriene D4 receptor in guinea pig lung membranes | J Med Chem 29: 1442-52 (1986) BindingDB Entry DOI: 10.7270/Q2S75JHD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cysteinyl leukotriene receptor 1/2 (Homo sapiens (Human)) | BDBM50226354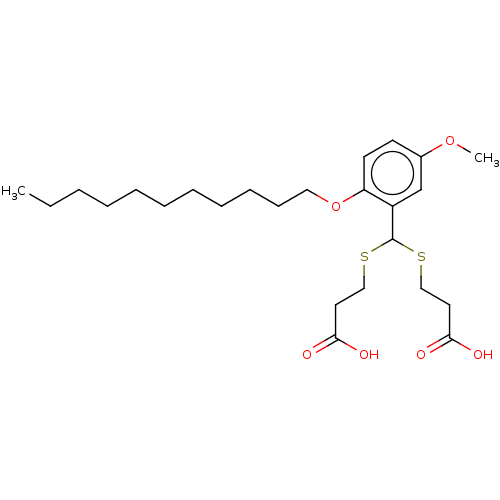 (CHEMBL32269) | PDB UniProtKB/SwissProt antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | 579 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Compound is evaluated for its ability to inhibit [3H]LTD4 binding to Cysteinyl leukotriene D4 receptor in guinea pig lung membranes | J Med Chem 29: 1442-52 (1986) BindingDB Entry DOI: 10.7270/Q2S75JHD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cysteinyl leukotriene receptor 1/2 (Homo sapiens (Human)) | BDBM50226394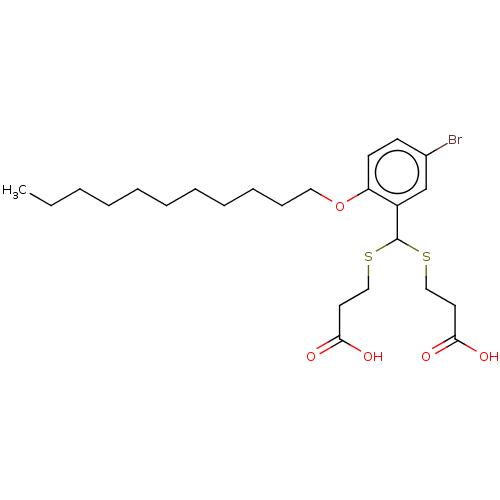 (CHEMBL35070) | PDB UniProtKB/SwissProt antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 624 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Compound is evaluated for its ability to inhibit [3H]LTD4 binding to Cysteinyl leukotriene D4 receptor in guinea pig lung membranes | J Med Chem 29: 1442-52 (1986) BindingDB Entry DOI: 10.7270/Q2S75JHD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cysteinyl leukotriene receptor 1/2 (Homo sapiens (Human)) | BDBM50226370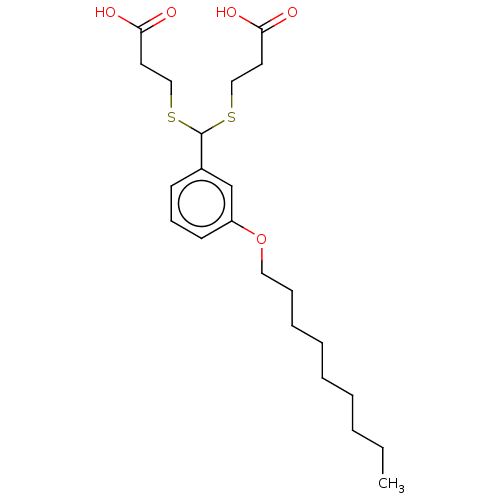 (CHEMBL33713) | PDB UniProtKB/SwissProt antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | 624 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Compound is evaluated for its ability to inhibit [3H]LTD4 binding to Cysteinyl leukotriene D4 receptor in guinea pig lung membranes | J Med Chem 29: 1442-52 (1986) BindingDB Entry DOI: 10.7270/Q2S75JHD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cysteinyl leukotriene receptor 1/2 (Homo sapiens (Human)) | BDBM50226390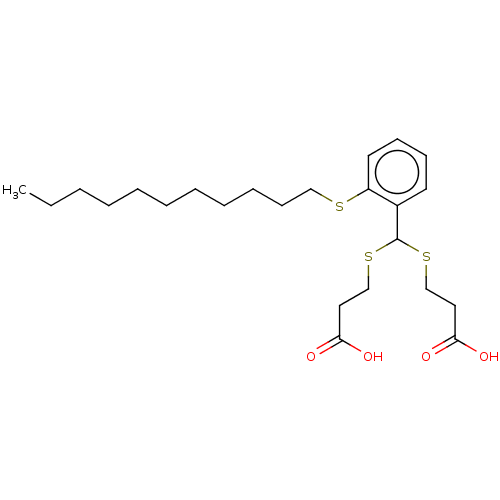 (CHEMBL36176) | PDB UniProtKB/SwissProt antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 646 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Compound is evaluated for its ability to inhibit [3H]LTD4 binding to Cysteinyl leukotriene D4 receptor in guinea pig lung membranes | J Med Chem 29: 1442-52 (1986) BindingDB Entry DOI: 10.7270/Q2S75JHD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50403347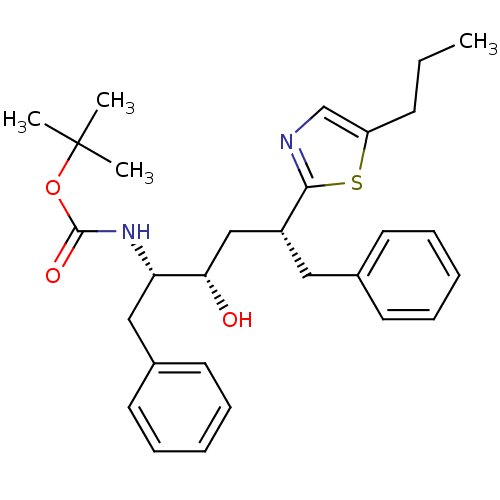 (CHEMBL2115564) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | 700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibitory activity against HIV-1 protease | Bioorg Med Chem Lett 4: 2441-2446 (1994) Article DOI: 10.1016/S0960-894X(01)80406-6 BindingDB Entry DOI: 10.7270/Q2X63P48 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50403355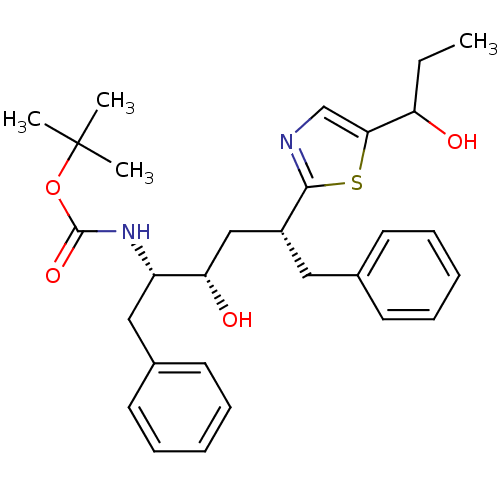 (CHEMBL315930) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | 720 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibitory activity against HIV-1 protease | Bioorg Med Chem Lett 4: 2441-2446 (1994) Article DOI: 10.1016/S0960-894X(01)80406-6 BindingDB Entry DOI: 10.7270/Q2X63P48 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 149 total ) | Next | Last >> |