 Found 164 hits with Last Name = 'ishibashi' and Initial = 'k'
Found 164 hits with Last Name = 'ishibashi' and Initial = 'k' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Amyloid-beta precursor protein
(Homo sapiens (Human)) | BDBM50176892
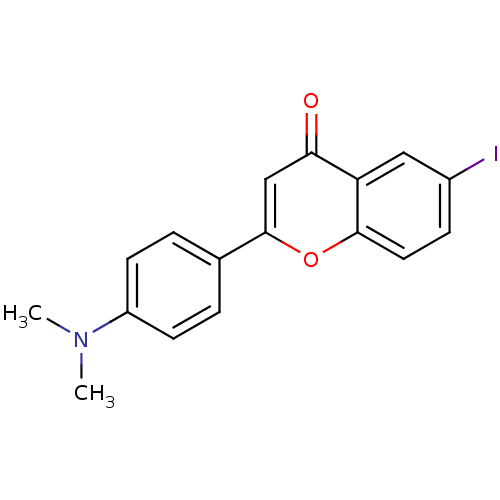
(6-iodo-4'-dimethyaminoflavone | CHEMBL375934)Show InChI InChI=1S/C17H14INO2/c1-19(2)13-6-3-11(4-7-13)17-10-15(20)14-9-12(18)5-8-16(14)21-17/h3-10H,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 13.2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Nagasaki University
Curated by ChEMBL
| Assay Description
Displacement of [125I]6-iodo-4'-dimethyaminofl avone from beta amyloid (1-40) protein aggregates |
J Med Chem 48: 7253-60 (2005)
Article DOI: 10.1021/jm050635e
BindingDB Entry DOI: 10.7270/Q2H70FC0 |
More data for this
Ligand-Target Pair | |
Amyloid-beta precursor protein
(Homo sapiens (Human)) | BDBM50176892

(6-iodo-4'-dimethyaminoflavone | CHEMBL375934)Show InChI InChI=1S/C17H14INO2/c1-19(2)13-6-3-11(4-7-13)17-10-15(20)14-9-12(18)5-8-16(14)21-17/h3-10H,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 15.6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Nagasaki University
Curated by ChEMBL
| Assay Description
Displacement of [125I]6-iodo-4'-dimethyaminofl avone from beta amyloid (1-42) protein aggregates |
J Med Chem 48: 7253-60 (2005)
Article DOI: 10.1021/jm050635e
BindingDB Entry DOI: 10.7270/Q2H70FC0 |
More data for this
Ligand-Target Pair | |
Amyloid-beta precursor protein
(Homo sapiens (Human)) | BDBM50176896
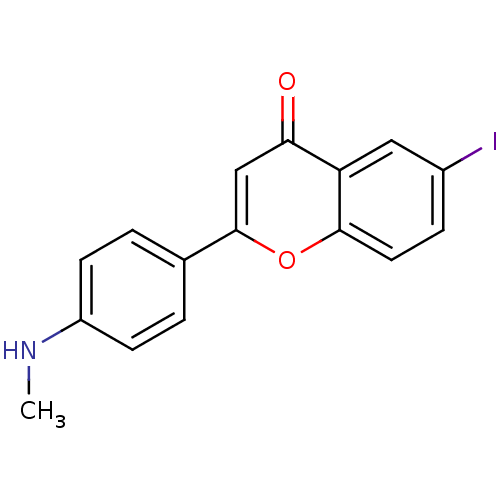
(6-iodo-4'-methylaminoflavone | CHEMBL224643)Show InChI InChI=1S/C16H12INO2/c1-18-12-5-2-10(3-6-12)16-9-14(19)13-8-11(17)4-7-15(13)20-16/h2-9,18H,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 22.6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Nagasaki University
Curated by ChEMBL
| Assay Description
Displacement of [125I]6-iodo-4'-dimethyaminofl avone from beta amyloid (1-40) protein aggregates |
J Med Chem 48: 7253-60 (2005)
Article DOI: 10.1021/jm050635e
BindingDB Entry DOI: 10.7270/Q2H70FC0 |
More data for this
Ligand-Target Pair | |
Amyloid-beta precursor protein
(Homo sapiens (Human)) | BDBM50176894
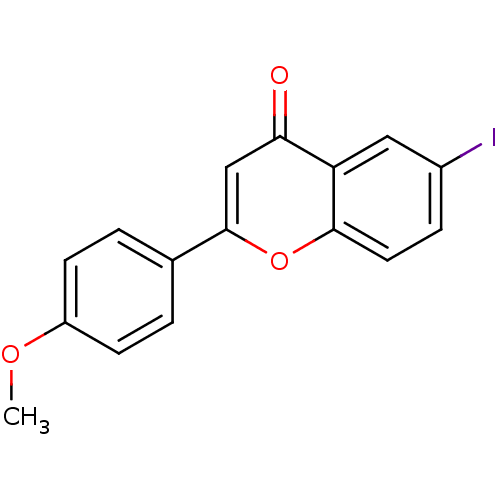
(6-iodo-4'-methoxyflavone | CHEMBL224064)Show InChI InChI=1S/C16H11IO3/c1-19-12-5-2-10(3-6-12)16-9-14(18)13-8-11(17)4-7-15(13)20-16/h2-9H,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 29 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Nagasaki University
Curated by ChEMBL
| Assay Description
Displacement of [125I]6-iodo-4'-dimethyaminofl avone from beta amyloid (1-40) protein aggregates |
J Med Chem 48: 7253-60 (2005)
Article DOI: 10.1021/jm050635e
BindingDB Entry DOI: 10.7270/Q2H70FC0 |
More data for this
Ligand-Target Pair | |
Amyloid-beta precursor protein
(Homo sapiens (Human)) | BDBM50176896

(6-iodo-4'-methylaminoflavone | CHEMBL224643)Show InChI InChI=1S/C16H12INO2/c1-18-12-5-2-10(3-6-12)16-9-14(19)13-8-11(17)4-7-15(13)20-16/h2-9,18H,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Nagasaki University
Curated by ChEMBL
| Assay Description
Displacement of [125I]6-iodo-4'-dimethyaminofl avone from beta amyloid (1-42) protein aggregates |
J Med Chem 48: 7253-60 (2005)
Article DOI: 10.1021/jm050635e
BindingDB Entry DOI: 10.7270/Q2H70FC0 |
More data for this
Ligand-Target Pair | |
Amyloid-beta precursor protein
(Homo sapiens (Human)) | BDBM50176894

(6-iodo-4'-methoxyflavone | CHEMBL224064)Show InChI InChI=1S/C16H11IO3/c1-19-12-5-2-10(3-6-12)16-9-14(18)13-8-11(17)4-7-15(13)20-16/h2-9H,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 38.3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Nagasaki University
Curated by ChEMBL
| Assay Description
Displacement of [125I]6-iodo-4'-dimethyaminofl avone from beta amyloid (1-42) protein aggregates |
J Med Chem 48: 7253-60 (2005)
Article DOI: 10.1021/jm050635e
BindingDB Entry DOI: 10.7270/Q2H70FC0 |
More data for this
Ligand-Target Pair | |
Amyloid-beta precursor protein
(Homo sapiens (Human)) | BDBM50176895
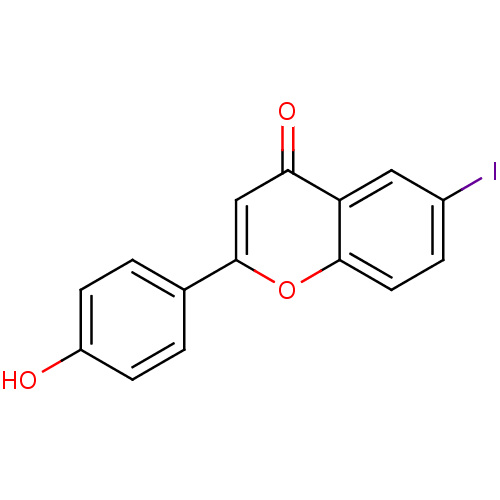
(6-iodo-4'-hydroxyflavone | CHEMBL388844)Show InChI InChI=1S/C15H9IO3/c16-10-3-6-14-12(7-10)13(18)8-15(19-14)9-1-4-11(17)5-2-9/h1-8,17H | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 72.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Nagasaki University
Curated by ChEMBL
| Assay Description
Displacement of [125I]6-iodo-4'-dimethyaminofl avone from beta amyloid (1-40) protein aggregates |
J Med Chem 48: 7253-60 (2005)
Article DOI: 10.1021/jm050635e
BindingDB Entry DOI: 10.7270/Q2H70FC0 |
More data for this
Ligand-Target Pair | |
Amyloid-beta precursor protein
(Homo sapiens (Human)) | BDBM50176895

(6-iodo-4'-hydroxyflavone | CHEMBL388844)Show InChI InChI=1S/C15H9IO3/c16-10-3-6-14-12(7-10)13(18)8-15(19-14)9-1-4-11(17)5-2-9/h1-8,17H | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 77.2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Nagasaki University
Curated by ChEMBL
| Assay Description
Displacement of [125I]6-iodo-4'-dimethyaminofl avone from beta amyloid (1-42) protein aggregates |
J Med Chem 48: 7253-60 (2005)
Article DOI: 10.1021/jm050635e
BindingDB Entry DOI: 10.7270/Q2H70FC0 |
More data for this
Ligand-Target Pair | |
Amyloid-beta precursor protein
(Homo sapiens (Human)) | BDBM50079267
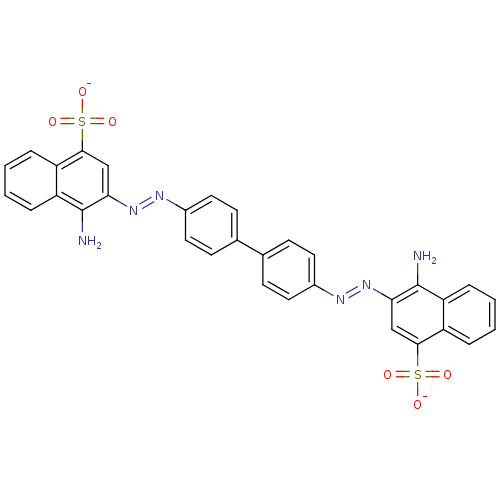
(Congo Red | Direct red 28 | Kongorot | Sodium diph...)Show SMILES Nc1c(cc(c2ccccc12)S([O-])(=O)=O)\N=N\c1ccc(cc1)-c1ccc(cc1)\N=N\c1cc(c2ccccc2c1N)S([O-])(=O)=O Show InChI InChI=1S/C32H24N6O6S2/c33-31-25-7-3-1-5-23(25)29(45(39,40)41)17-27(31)37-35-21-13-9-19(10-14-21)20-11-15-22(16-12-20)36-38-28-18-30(46(42,43)44)24-6-2-4-8-26(24)32(28)34/h1-18H,33-34H2,(H,39,40,41)(H,42,43,44)/p-2/b37-35+,38-36+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
KEGG
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Nagasaki University
Curated by ChEMBL
| Assay Description
Displacement of [125I]6-iodo-4'-dimethyaminofl avone from beta amyloid (1-42) protein aggregates |
J Med Chem 48: 7253-60 (2005)
Article DOI: 10.1021/jm050635e
BindingDB Entry DOI: 10.7270/Q2H70FC0 |
More data for this
Ligand-Target Pair | |
Amyloid-beta precursor protein
(Homo sapiens (Human)) | BDBM50100134

(2-(4-Dimethylamino-phenyl)-3,6-dimethyl-benzothiaz...)Show InChI InChI=1S/C17H19N2S/c1-12-5-10-15-16(11-12)20-17(19(15)4)13-6-8-14(9-7-13)18(2)3/h5-11H,1-4H3/q+1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Nagasaki University
Curated by ChEMBL
| Assay Description
Displacement of [125I]6-iodo-4'-dimethyaminofl avone from beta amyloid (1-42) protein aggregates |
J Med Chem 48: 7253-60 (2005)
Article DOI: 10.1021/jm050635e
BindingDB Entry DOI: 10.7270/Q2H70FC0 |
More data for this
Ligand-Target Pair | |
Amyloid-beta precursor protein
(Homo sapiens (Human)) | BDBM50100134

(2-(4-Dimethylamino-phenyl)-3,6-dimethyl-benzothiaz...)Show InChI InChI=1S/C17H19N2S/c1-12-5-10-15-16(11-12)20-17(19(15)4)13-6-8-14(9-7-13)18(2)3/h5-11H,1-4H3/q+1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Nagasaki University
Curated by ChEMBL
| Assay Description
Displacement of [125I]6-iodo-4'-dimethyaminofl avone from beta amyloid (1-40) protein aggregates |
J Med Chem 48: 7253-60 (2005)
Article DOI: 10.1021/jm050635e
BindingDB Entry DOI: 10.7270/Q2H70FC0 |
More data for this
Ligand-Target Pair | |
Amyloid-beta precursor protein
(Homo sapiens (Human)) | BDBM50079267

(Congo Red | Direct red 28 | Kongorot | Sodium diph...)Show SMILES Nc1c(cc(c2ccccc12)S([O-])(=O)=O)\N=N\c1ccc(cc1)-c1ccc(cc1)\N=N\c1cc(c2ccccc2c1N)S([O-])(=O)=O Show InChI InChI=1S/C32H24N6O6S2/c33-31-25-7-3-1-5-23(25)29(45(39,40)41)17-27(31)37-35-21-13-9-19(10-14-21)20-11-15-22(16-12-20)36-38-28-18-30(46(42,43)44)24-6-2-4-8-26(24)32(28)34/h1-18H,33-34H2,(H,39,40,41)(H,42,43,44)/p-2/b37-35+,38-36+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
KEGG
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Nagasaki University
Curated by ChEMBL
| Assay Description
Displacement of [125I]6-iodo-4'-dimethyaminofl avone from beta amyloid (1-40) protein aggregates |
J Med Chem 48: 7253-60 (2005)
Article DOI: 10.1021/jm050635e
BindingDB Entry DOI: 10.7270/Q2H70FC0 |
More data for this
Ligand-Target Pair | |
cGMP-specific 3',5'-cyclic phosphodiesterase
(Homo sapiens (Human)) | BDBM50089825

(1-[4-(3-Chloro-4-methoxy-benzylamino)-6-nitro-phth...)Show SMILES COc1ccc(CNc2nnc(N3CCC(O)CC3)c3ccc(cc23)[N+]([O-])=O)cc1Cl Show InChI InChI=1S/C21H22ClN5O4/c1-31-19-5-2-13(10-18(19)22)12-23-20-17-11-14(27(29)30)3-4-16(17)21(25-24-20)26-8-6-15(28)7-9-26/h2-5,10-11,15,28H,6-9,12H2,1H3,(H,23,24) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| PubMed
| n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
Eisai Company, Ltd.
Curated by ChEMBL
| Assay Description
50% inhibitory concentration against phosphodiesterase 5 (PDE5) from porcine platelets |
J Med Chem 43: 2523-9 (2000)
BindingDB Entry DOI: 10.7270/Q2T72J4N |
More data for this
Ligand-Target Pair | |
cGMP-specific 3',5'-cyclic phosphodiesterase
(Homo sapiens (Human)) | BDBM50089837
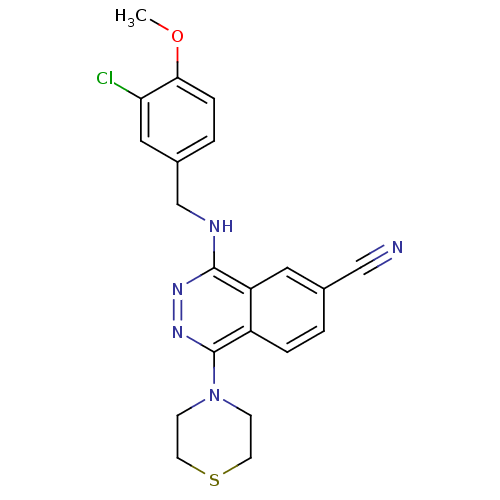
(4-(3-Chloro-4-methoxy-benzylamino)-1-thiomorpholin...)Show SMILES COc1ccc(CNc2nnc(N3CCSCC3)c3ccc(cc23)C#N)cc1Cl Show InChI InChI=1S/C21H20ClN5OS/c1-28-19-5-3-15(11-18(19)22)13-24-20-17-10-14(12-23)2-4-16(17)21(26-25-20)27-6-8-29-9-7-27/h2-5,10-11H,6-9,13H2,1H3,(H,24,25) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 0.320 | n/a | n/a | n/a | n/a | n/a | n/a |
Eisai Company, Ltd.
Curated by ChEMBL
| Assay Description
50% inhibitory concentration against phosphodiesterase 5 (PDE5) from porcine platelets |
J Med Chem 43: 2523-9 (2000)
BindingDB Entry DOI: 10.7270/Q2T72J4N |
More data for this
Ligand-Target Pair | |
cGMP-specific 3',5'-cyclic phosphodiesterase
(Homo sapiens (Human)) | BDBM50089826

(1-[4-(3-Chloro-4-methoxy-benzylamino)-6-cyano-phth...)Show SMILES COc1ccc(CNc2nnc(N3CCC(CC3)C(N)=O)c3ccc(cc23)C#N)cc1Cl Show InChI InChI=1S/C23H23ClN6O2/c1-32-20-5-3-15(11-19(20)24)13-27-22-18-10-14(12-25)2-4-17(18)23(29-28-22)30-8-6-16(7-9-30)21(26)31/h2-5,10-11,16H,6-9,13H2,1H3,(H2,26,31)(H,27,28) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Eisai Company, Ltd.
Curated by ChEMBL
| Assay Description
50% inhibitory concentration against phosphodiesterase 5 (PDE5) from porcine platelets |
J Med Chem 43: 2523-9 (2000)
BindingDB Entry DOI: 10.7270/Q2T72J4N |
More data for this
Ligand-Target Pair | |
cGMP-specific 3',5'-cyclic phosphodiesterase
(Homo sapiens (Human)) | BDBM50089807
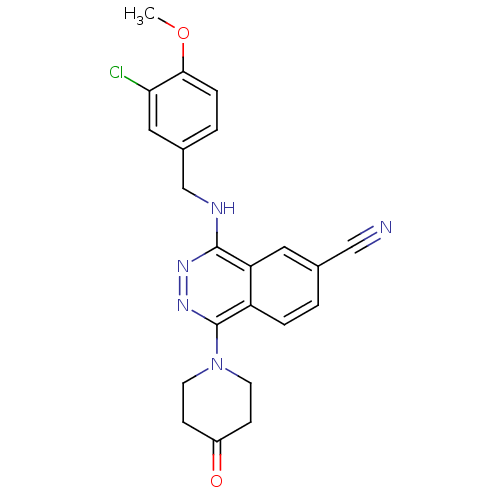
(4-(3-Chloro-4-methoxy-benzylamino)-1-(4-oxo-piperi...)Show SMILES COc1ccc(CNc2nnc(N3CCC(=O)CC3)c3ccc(cc23)C#N)cc1Cl Show InChI InChI=1S/C22H20ClN5O2/c1-30-20-5-3-15(11-19(20)23)13-25-21-18-10-14(12-24)2-4-17(18)22(27-26-21)28-8-6-16(29)7-9-28/h2-5,10-11H,6-9,13H2,1H3,(H,25,26) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 0.530 | n/a | n/a | n/a | n/a | n/a | n/a |
Eisai Company, Ltd.
Curated by ChEMBL
| Assay Description
50% inhibitory concentration against phosphodiesterase 5 (PDE5) from porcine platelets |
J Med Chem 43: 2523-9 (2000)
BindingDB Entry DOI: 10.7270/Q2T72J4N |
More data for this
Ligand-Target Pair | |
cGMP-specific 3',5'-cyclic phosphodiesterase
(Homo sapiens (Human)) | BDBM50089833
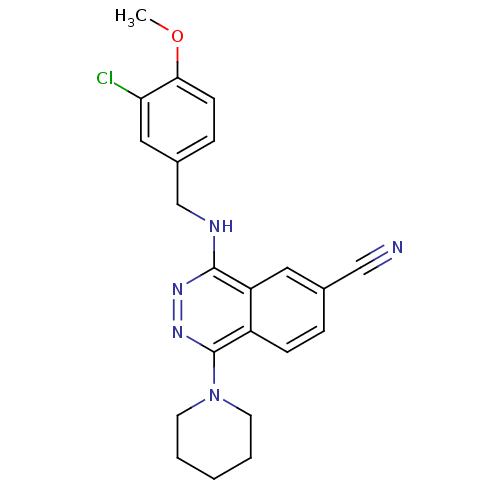
(4-(3-Chloro-4-methoxy-benzylamino)-1-piperidin-1-y...)Show SMILES COc1ccc(CNc2nnc(N3CCCCC3)c3ccc(cc23)C#N)cc1Cl Show InChI InChI=1S/C22H22ClN5O/c1-29-20-8-6-16(12-19(20)23)14-25-21-18-11-15(13-24)5-7-17(18)22(27-26-21)28-9-3-2-4-10-28/h5-8,11-12H,2-4,9-10,14H2,1H3,(H,25,26) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 0.550 | n/a | n/a | n/a | n/a | n/a | n/a |
Eisai Company, Ltd.
Curated by ChEMBL
| Assay Description
50% inhibitory concentration against phosphodiesterase 5 (PDE5) from porcine platelets |
J Med Chem 43: 2523-9 (2000)
BindingDB Entry DOI: 10.7270/Q2T72J4N |
More data for this
Ligand-Target Pair | |
cGMP-specific 3',5'-cyclic phosphodiesterase
(Homo sapiens (Human)) | BDBM50089814
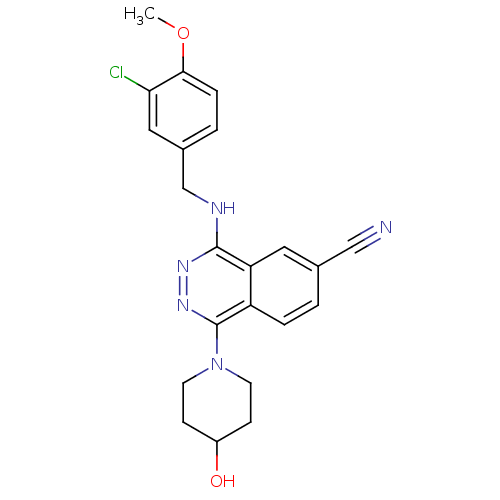
(4-(3-Chloro-4-methoxy-benzylamino)-1-(4-hydroxy-pi...)Show SMILES COc1ccc(CNc2nnc(N3CCC(O)CC3)c3ccc(cc23)C#N)cc1Cl Show InChI InChI=1S/C22H22ClN5O2/c1-30-20-5-3-15(11-19(20)23)13-25-21-18-10-14(12-24)2-4-17(18)22(27-26-21)28-8-6-16(29)7-9-28/h2-5,10-11,16,29H,6-9,13H2,1H3,(H,25,26) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 0.560 | n/a | n/a | n/a | n/a | n/a | n/a |
Eisai Company, Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of phosphodiesterase 5 (PDE5) from porcine platelets, range 0.442-0.710 |
J Med Chem 43: 2523-9 (2000)
BindingDB Entry DOI: 10.7270/Q2T72J4N |
More data for this
Ligand-Target Pair | |
cGMP-specific 3',5'-cyclic phosphodiesterase
(Homo sapiens (Human)) | BDBM50089821
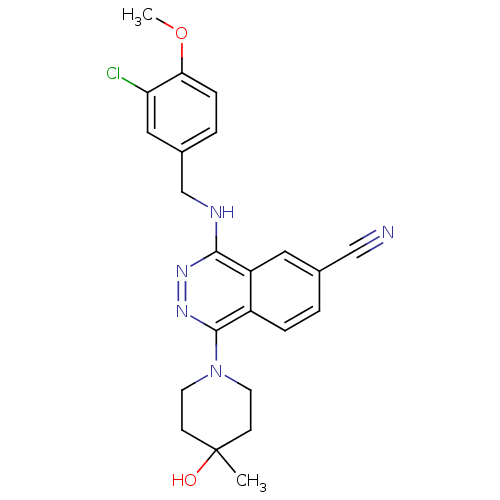
(4-(3-Chloro-4-methoxy-benzylamino)-1-(4-hydroxy-4-...)Show SMILES COc1ccc(CNc2nnc(N3CCC(C)(O)CC3)c3ccc(cc23)C#N)cc1Cl Show InChI InChI=1S/C23H24ClN5O2/c1-23(30)7-9-29(10-8-23)22-17-5-3-15(13-25)11-18(17)21(27-28-22)26-14-16-4-6-20(31-2)19(24)12-16/h3-6,11-12,30H,7-10,14H2,1-2H3,(H,26,27) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a |
Eisai Company, Ltd.
Curated by ChEMBL
| Assay Description
50% inhibitory concentration against phosphodiesterase 5 (PDE5) from porcine platelets |
J Med Chem 43: 2523-9 (2000)
BindingDB Entry DOI: 10.7270/Q2T72J4N |
More data for this
Ligand-Target Pair | |
cGMP-specific 3',5'-cyclic phosphodiesterase
(Homo sapiens (Human)) | BDBM50089827
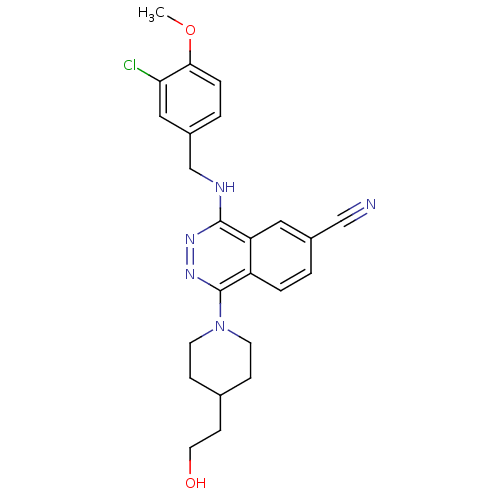
(4-(3-Chloro-4-methoxy-benzylamino)-1-[4-(2-hydroxy...)Show SMILES COc1ccc(CNc2nnc(N3CCC(CCO)CC3)c3ccc(cc23)C#N)cc1Cl Show InChI InChI=1S/C24H26ClN5O2/c1-32-22-5-3-18(13-21(22)25)15-27-23-20-12-17(14-26)2-4-19(20)24(29-28-23)30-9-6-16(7-10-30)8-11-31/h2-5,12-13,16,31H,6-11,15H2,1H3,(H,27,28) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a |
Eisai Company, Ltd.
Curated by ChEMBL
| Assay Description
50% inhibitory concentration against phosphodiesterase 5 (PDE5) from porcine platelets |
J Med Chem 43: 2523-9 (2000)
BindingDB Entry DOI: 10.7270/Q2T72J4N |
More data for this
Ligand-Target Pair | |
cGMP-specific 3',5'-cyclic phosphodiesterase
(Homo sapiens (Human)) | BDBM50369544
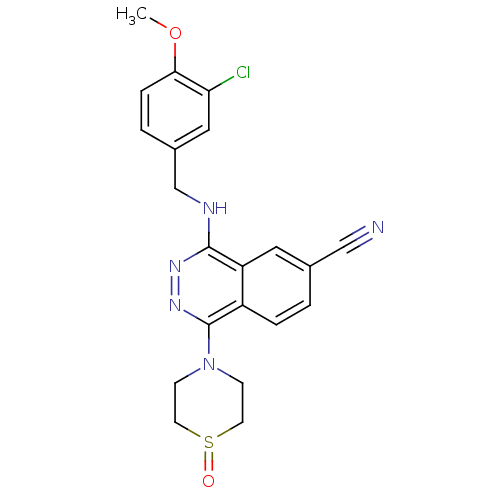
(CHEMBL545012)Show SMILES COc1ccc(CNc2nnc(N3CCS(=O)CC3)c3ccc(cc23)C#N)cc1Cl Show InChI InChI=1S/C21H20ClN5O2S/c1-29-19-5-3-15(11-18(19)22)13-24-20-17-10-14(12-23)2-4-16(17)21(26-25-20)27-6-8-30(28)9-7-27/h2-5,10-11H,6-9,13H2,1H3,(H,24,25) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a |
Eisai Company, Ltd.
Curated by ChEMBL
| Assay Description
50% inhibitory concentration against phosphodiesterase 5 (PDE5) from porcine platelets |
J Med Chem 43: 2523-9 (2000)
BindingDB Entry DOI: 10.7270/Q2T72J4N |
More data for this
Ligand-Target Pair | |
Neuromedin-K receptor
(GUINEA PIG) | BDBM50403793
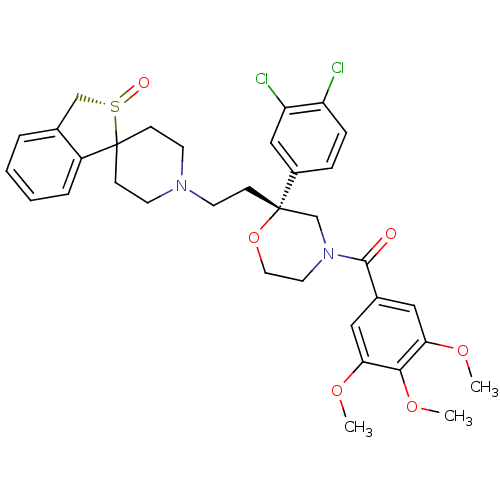
(CHEMBL2115415)Show SMILES COc1cc(cc(OC)c1OC)C(=O)N1CCO[C@@](CCN2CCC3(CC2)c2ccccc2C[S@@]3=O)(C1)c1ccc(Cl)c(Cl)c1 Show InChI InChI=1S/C34H38Cl2N2O6S/c1-41-29-18-24(19-30(42-2)31(29)43-3)32(39)38-16-17-44-33(22-38,25-8-9-27(35)28(36)20-25)10-13-37-14-11-34(12-15-37)26-7-5-4-6-23(26)21-45(34)40/h4-9,18-20H,10-17,21-22H2,1-3H3/t33-,45-/m0/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 0.720 | n/a | n/a | n/a | n/a | n/a | n/a |
Sankyo Co., Ltd.
Curated by ChEMBL
| Assay Description
The compound was evaluated in vitro for its ability to displace [3H]-SP from Tachykinin receptor 3 of male guinea pig lung membrane |
Bioorg Med Chem Lett 10: 1665-8 (2000)
BindingDB Entry DOI: 10.7270/Q2QZ297D |
More data for this
Ligand-Target Pair | |
cGMP-specific 3',5'-cyclic phosphodiesterase
(Homo sapiens (Human)) | BDBM50089815
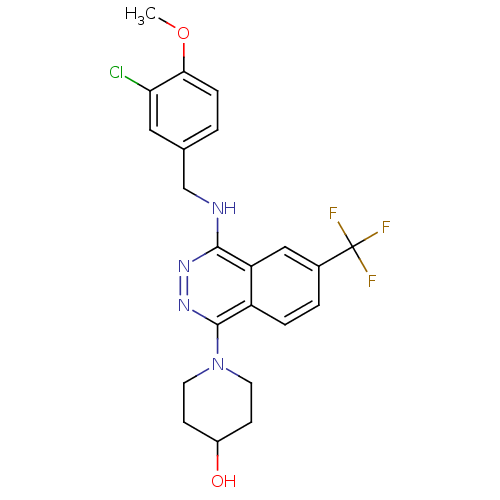
(1-[4-(3-Chloro-4-methoxy-benzylamino)-6-trifluorom...)Show SMILES COc1ccc(CNc2nnc(N3CCC(O)CC3)c3ccc(cc23)C(F)(F)F)cc1Cl Show InChI InChI=1S/C22H22ClF3N4O2/c1-32-19-5-2-13(10-18(19)23)12-27-20-17-11-14(22(24,25)26)3-4-16(17)21(29-28-20)30-8-6-15(31)7-9-30/h2-5,10-11,15,31H,6-9,12H2,1H3,(H,27,28) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 0.730 | n/a | n/a | n/a | n/a | n/a | n/a |
Eisai Company, Ltd.
Curated by ChEMBL
| Assay Description
50% inhibitory concentration against phosphodiesterase 5 (PDE5) from porcine platelets |
J Med Chem 43: 2523-9 (2000)
BindingDB Entry DOI: 10.7270/Q2T72J4N |
More data for this
Ligand-Target Pair | |
cGMP-specific 3',5'-cyclic phosphodiesterase
(Homo sapiens (Human)) | BDBM50089841
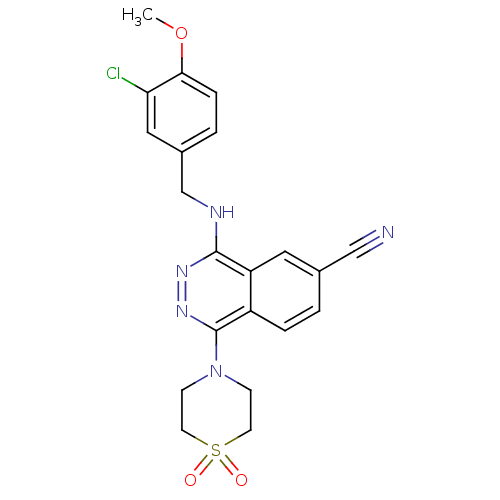
(4-(3-Chloro-4-methoxy-benzylamino)-1-(1,1-dioxo-1l...)Show SMILES COc1ccc(CNc2nnc(N3CCS(=O)(=O)CC3)c3ccc(cc23)C#N)cc1Cl Show InChI InChI=1S/C21H20ClN5O3S/c1-30-19-5-3-15(11-18(19)22)13-24-20-17-10-14(12-23)2-4-16(17)21(26-25-20)27-6-8-31(28,29)9-7-27/h2-5,10-11H,6-9,13H2,1H3,(H,24,25) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 0.790 | n/a | n/a | n/a | n/a | n/a | n/a |
Eisai Company, Ltd.
Curated by ChEMBL
| Assay Description
50% inhibitory concentration against phosphodiesterase 5 (PDE5) from porcine platelets |
J Med Chem 43: 2523-9 (2000)
BindingDB Entry DOI: 10.7270/Q2T72J4N |
More data for this
Ligand-Target Pair | |
cGMP-specific 3',5'-cyclic phosphodiesterase
(Homo sapiens (Human)) | BDBM50089832

(1-[6,7-Dichloro-4-(3-chloro-4-methoxy-benzylamino)...)Show SMILES COc1ccc(CNc2nnc(N3CCC(O)CC3)c3cc(Cl)c(Cl)cc23)cc1Cl Show InChI InChI=1S/C21H21Cl3N4O2/c1-30-19-3-2-12(8-18(19)24)11-25-20-14-9-16(22)17(23)10-15(14)21(27-26-20)28-6-4-13(29)5-7-28/h2-3,8-10,13,29H,4-7,11H2,1H3,(H,25,26) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 0.860 | n/a | n/a | n/a | n/a | n/a | n/a |
Eisai Company, Ltd.
Curated by ChEMBL
| Assay Description
50% inhibitory concentration against phosphodiesterase 5 (PDE5) from porcine platelets |
J Med Chem 43: 2523-9 (2000)
BindingDB Entry DOI: 10.7270/Q2T72J4N |
More data for this
Ligand-Target Pair | |
cGMP-specific 3',5'-cyclic phosphodiesterase
(Homo sapiens (Human)) | BDBM50089820
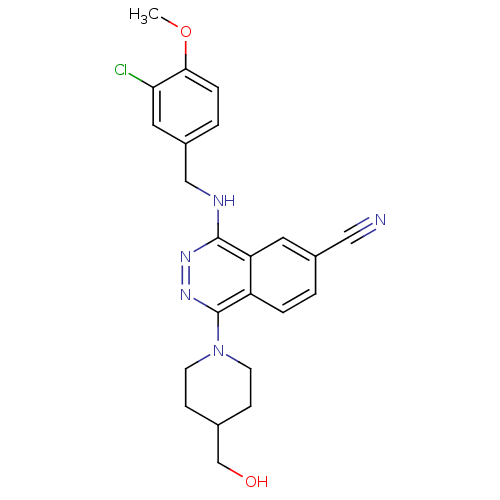
(4-(3-Chloro-4-methoxy-benzylamino)-1-(4-hydroxymet...)Show SMILES COc1ccc(CNc2nnc(N3CCC(CO)CC3)c3ccc(cc23)C#N)cc1Cl Show InChI InChI=1S/C23H24ClN5O2/c1-31-21-5-3-17(11-20(21)24)13-26-22-19-10-16(12-25)2-4-18(19)23(28-27-22)29-8-6-15(14-30)7-9-29/h2-5,10-11,15,30H,6-9,13-14H2,1H3,(H,26,27) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 0.930 | n/a | n/a | n/a | n/a | n/a | n/a |
Eisai Company, Ltd.
Curated by ChEMBL
| Assay Description
50% inhibitory concentration against phosphodiesterase 5 (PDE5) from porcine platelets |
J Med Chem 43: 2523-9 (2000)
BindingDB Entry DOI: 10.7270/Q2T72J4N |
More data for this
Ligand-Target Pair | |
cGMP-specific 3',5'-cyclic phosphodiesterase
(Homo sapiens (Human)) | BDBM50089830
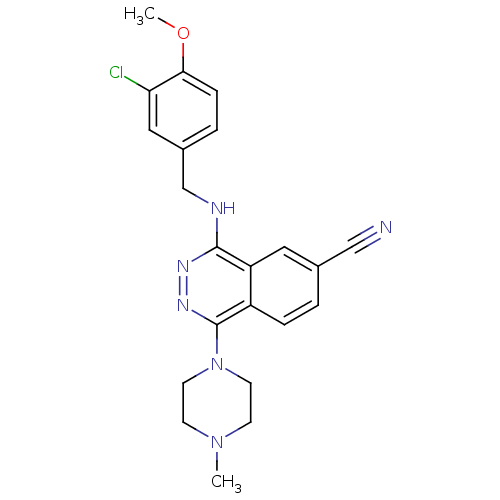
(4-(3-Chloro-4-methoxy-benzylamino)-1-(4-methyl-pip...)Show SMILES COc1ccc(CNc2nnc(N3CCN(C)CC3)c3ccc(cc23)C#N)cc1Cl Show InChI InChI=1S/C22H23ClN6O/c1-28-7-9-29(10-8-28)22-17-5-3-15(13-24)11-18(17)21(26-27-22)25-14-16-4-6-20(30-2)19(23)12-16/h3-6,11-12H,7-10,14H2,1-2H3,(H,25,26) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 0.950 | n/a | n/a | n/a | n/a | n/a | n/a |
Eisai Company, Ltd.
Curated by ChEMBL
| Assay Description
50% inhibitory concentration against phosphodiesterase 5 (PDE5) from porcine platelets |
J Med Chem 43: 2523-9 (2000)
BindingDB Entry DOI: 10.7270/Q2T72J4N |
More data for this
Ligand-Target Pair | |
cGMP-specific 3',5'-cyclic phosphodiesterase
(Homo sapiens (Human)) | BDBM50089816
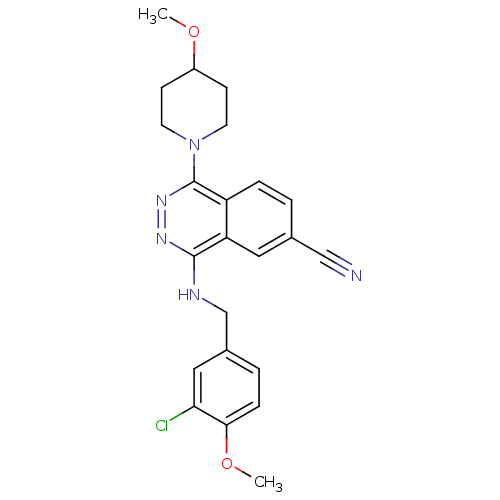
(4-(3-Chloro-4-methoxy-benzylamino)-1-(4-methoxy-pi...)Show SMILES COC1CCN(CC1)c1nnc(NCc2ccc(OC)c(Cl)c2)c2cc(ccc12)C#N Show InChI InChI=1S/C23H24ClN5O2/c1-30-17-7-9-29(10-8-17)23-18-5-3-15(13-25)11-19(18)22(27-28-23)26-14-16-4-6-21(31-2)20(24)12-16/h3-6,11-12,17H,7-10,14H2,1-2H3,(H,26,27) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Eisai Company, Ltd.
Curated by ChEMBL
| Assay Description
50% inhibitory concentration against phosphodiesterase 5 (PDE5) from porcine platelets |
J Med Chem 43: 2523-9 (2000)
BindingDB Entry DOI: 10.7270/Q2T72J4N |
More data for this
Ligand-Target Pair | |
cGMP-specific 3',5'-cyclic phosphodiesterase
(Homo sapiens (Human)) | BDBM50089809
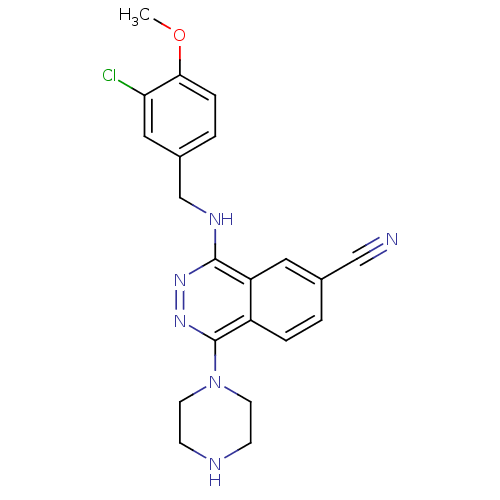
(4-(3-Chloro-4-methoxy-benzylamino)-1-piperazin-1-y...)Show SMILES COc1ccc(CNc2nnc(N3CCNCC3)c3ccc(cc23)C#N)cc1Cl Show InChI InChI=1S/C21H21ClN6O/c1-29-19-5-3-15(11-18(19)22)13-25-20-17-10-14(12-23)2-4-16(17)21(27-26-20)28-8-6-24-7-9-28/h2-5,10-11,24H,6-9,13H2,1H3,(H,25,26) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Eisai Company, Ltd.
Curated by ChEMBL
| Assay Description
50% inhibitory concentration against phosphodiesterase 5 (PDE5) from porcine platelets |
J Med Chem 43: 2523-9 (2000)
BindingDB Entry DOI: 10.7270/Q2T72J4N |
More data for this
Ligand-Target Pair | |
cGMP-specific 3',5'-cyclic phosphodiesterase
(Homo sapiens (Human)) | BDBM50089838
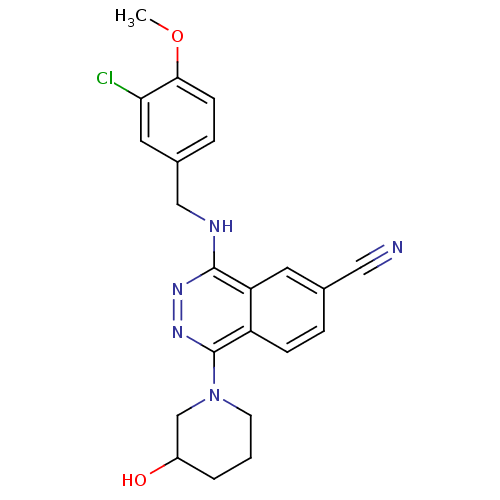
(4-(3-Chloro-4-methoxy-benzylamino)-1-(3-hydroxy-pi...)Show SMILES COc1ccc(CNc2nnc(N3CCCC(O)C3)c3ccc(cc23)C#N)cc1Cl Show InChI InChI=1S/C22H22ClN5O2/c1-30-20-7-5-15(10-19(20)23)12-25-21-18-9-14(11-24)4-6-17(18)22(27-26-21)28-8-2-3-16(29)13-28/h4-7,9-10,16,29H,2-3,8,12-13H2,1H3,(H,25,26) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Eisai Company, Ltd.
Curated by ChEMBL
| Assay Description
50% inhibitory concentration against phosphodiesterase 5 (PDE5) from porcine platelets |
J Med Chem 43: 2523-9 (2000)
BindingDB Entry DOI: 10.7270/Q2T72J4N |
More data for this
Ligand-Target Pair | |
cGMP-specific 3',5'-cyclic phosphodiesterase
(Homo sapiens (Human)) | BDBM50089842
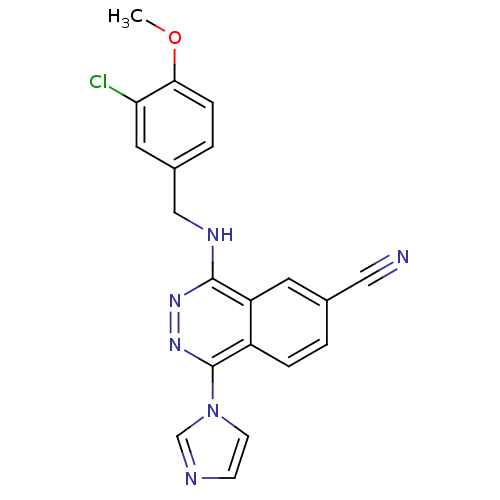
(4-(3-Chloro-4-methoxy-benzylamino)-1-imidazol-1-yl...)Show SMILES COc1ccc(CNc2nnc(-n3ccnc3)c3ccc(cc23)C#N)cc1Cl Show InChI InChI=1S/C20H15ClN6O/c1-28-18-5-3-14(9-17(18)21)11-24-19-16-8-13(10-22)2-4-15(16)20(26-25-19)27-7-6-23-12-27/h2-9,12H,11H2,1H3,(H,24,25) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| PubMed
| n/a | n/a | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Eisai Company, Ltd.
Curated by ChEMBL
| Assay Description
50% inhibitory concentration against phosphodiesterase 5 (PDE5) from porcine platelets |
J Med Chem 43: 2523-9 (2000)
BindingDB Entry DOI: 10.7270/Q2T72J4N |
More data for this
Ligand-Target Pair | |
cGMP-specific 3',5'-cyclic phosphodiesterase
(Homo sapiens (Human)) | BDBM50089818
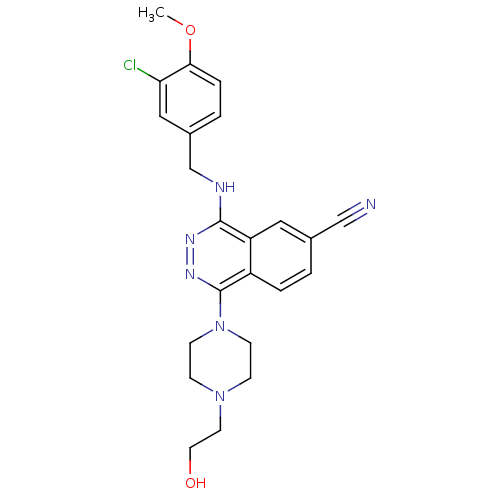
(4-(3-Chloro-4-methoxy-benzylamino)-1-[4-(2-hydroxy...)Show SMILES COc1ccc(CNc2nnc(N3CCN(CCO)CC3)c3ccc(cc23)C#N)cc1Cl Show InChI InChI=1S/C23H25ClN6O2/c1-32-21-5-3-17(13-20(21)24)15-26-22-19-12-16(14-25)2-4-18(19)23(28-27-22)30-8-6-29(7-9-30)10-11-31/h2-5,12-13,31H,6-11,15H2,1H3,(H,26,27) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 1.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Eisai Company, Ltd.
Curated by ChEMBL
| Assay Description
50% inhibitory concentration against phosphodiesterase 5 (PDE5) from porcine platelets |
J Med Chem 43: 2523-9 (2000)
BindingDB Entry DOI: 10.7270/Q2T72J4N |
More data for this
Ligand-Target Pair | |
Substance-P receptor
(GUINEA PIG) | BDBM50217526
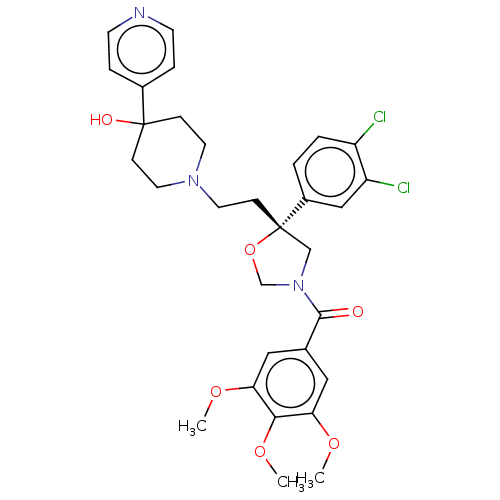
(CHEMBL366972)Show SMILES COc1cc(cc(OC)c1OC)C(=O)N1CO[C@@](CCN2CCC(O)(CC2)c2ccncc2)(C1)c1ccc(Cl)c(Cl)c1 Show InChI InChI=1S/C31H35Cl2N3O6/c1-39-26-16-21(17-27(40-2)28(26)41-3)29(37)36-19-31(42-20-36,23-4-5-24(32)25(33)18-23)10-15-35-13-8-30(38,9-14-35)22-6-11-34-12-7-22/h4-7,11-12,16-18,38H,8-10,13-15,19-20H2,1-3H3/t31-/m0/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 1.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Sankyo Co., Ltd.
Curated by ChEMBL
| Assay Description
Inhibitory activity against Neurokinin 1 (NK1) receptor from guinea pig lung |
Bioorg Med Chem Lett 9: 875-80 (1999)
BindingDB Entry DOI: 10.7270/Q2PN97TF |
More data for this
Ligand-Target Pair | |
cGMP-specific 3',5'-cyclic phosphodiesterase
(Homo sapiens (Human)) | BDBM50089812
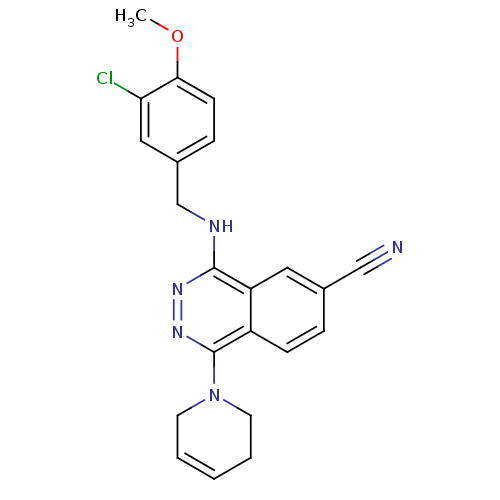
(4-(3-Chloro-4-methoxy-benzylamino)-1-(3,6-dihydro-...)Show SMILES COc1ccc(CNc2nnc(N3CCC=CC3)c3ccc(cc23)C#N)cc1Cl |c:15| Show InChI InChI=1S/C22H20ClN5O/c1-29-20-8-6-16(12-19(20)23)14-25-21-18-11-15(13-24)5-7-17(18)22(27-26-21)28-9-3-2-4-10-28/h2-3,5-8,11-12H,4,9-10,14H2,1H3,(H,25,26) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 1.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Eisai Company, Ltd.
Curated by ChEMBL
| Assay Description
50% inhibitory concentration against phosphodiesterase 5 (PDE5) from porcine platelets |
J Med Chem 43: 2523-9 (2000)
BindingDB Entry DOI: 10.7270/Q2T72J4N |
More data for this
Ligand-Target Pair | |
cGMP-specific 3',5'-cyclic phosphodiesterase
(Homo sapiens (Human)) | BDBM50089836
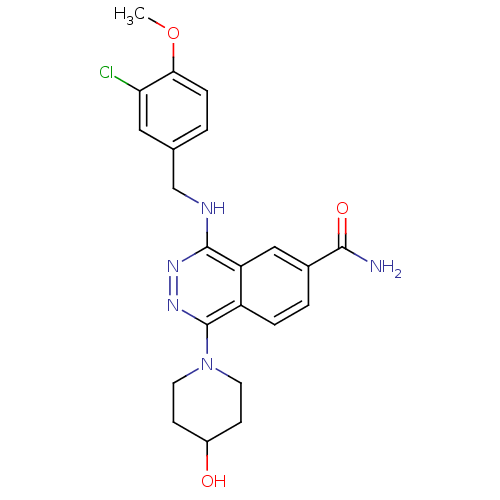
(4-(3-Chloro-4-methoxy-benzylamino)-1-(4-hydroxy-pi...)Show SMILES COc1ccc(CNc2nnc(N3CCC(O)CC3)c3ccc(cc23)C(N)=O)cc1Cl Show InChI InChI=1S/C22H24ClN5O3/c1-31-19-5-2-13(10-18(19)23)12-25-21-17-11-14(20(24)30)3-4-16(17)22(27-26-21)28-8-6-15(29)7-9-28/h2-5,10-11,15,29H,6-9,12H2,1H3,(H2,24,30)(H,25,26) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Eisai Company, Ltd.
Curated by ChEMBL
| Assay Description
50% inhibitory concentration against phosphodiesterase 5 (PDE5) from porcine platelets |
J Med Chem 43: 2523-9 (2000)
BindingDB Entry DOI: 10.7270/Q2T72J4N |
More data for this
Ligand-Target Pair | |
cGMP-specific 3',5'-cyclic phosphodiesterase
(Homo sapiens (Human)) | BDBM14390

(5-[2-ethoxy-5-(4-methyl-1-piperazinylsulfonyl)phen...)Show SMILES CCCc1nn(C)c2c1nc([nH]c2=O)-c1cc(ccc1OCC)S(=O)(=O)N1CCN(C)CC1 Show InChI InChI=1S/C22H30N6O4S/c1-5-7-17-19-20(27(4)25-17)22(29)24-21(23-19)16-14-15(8-9-18(16)32-6-2)33(30,31)28-12-10-26(3)11-13-28/h8-9,14H,5-7,10-13H2,1-4H3,(H,23,24,29) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| MMDB
PDB
PubMed
| n/a | n/a | 2.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Eisai Company, Ltd.
Curated by ChEMBL
| Assay Description
50% inhibitory concentration against phosphodiesterase 5 (PDE5) from porcine platelets |
J Med Chem 43: 2523-9 (2000)
BindingDB Entry DOI: 10.7270/Q2T72J4N |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
cGMP-specific 3',5'-cyclic phosphodiesterase
(Homo sapiens (Human)) | BDBM50089817
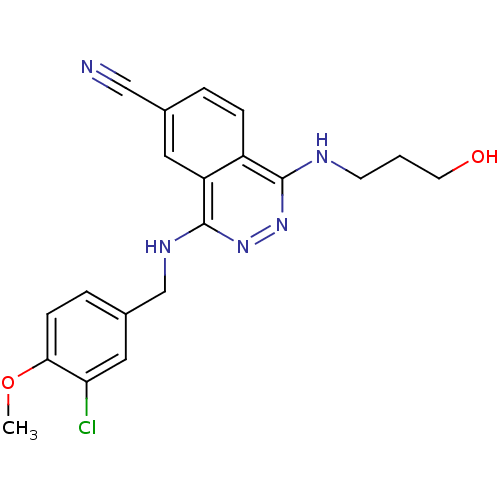
(4-(3-Chloro-4-methoxy-benzylamino)-1-(3-hydroxy-pr...)Show InChI InChI=1S/C20H20ClN5O2/c1-28-18-6-4-14(10-17(18)21)12-24-20-16-9-13(11-22)3-5-15(16)19(25-26-20)23-7-2-8-27/h3-6,9-10,27H,2,7-8,12H2,1H3,(H,23,25)(H,24,26) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 2.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Eisai Company, Ltd.
Curated by ChEMBL
| Assay Description
50% inhibitory concentration against phosphodiesterase 5 (PDE5) from porcine platelets |
J Med Chem 43: 2523-9 (2000)
BindingDB Entry DOI: 10.7270/Q2T72J4N |
More data for this
Ligand-Target Pair | |
Neuromedin-K receptor
(GUINEA PIG) | BDBM50090485
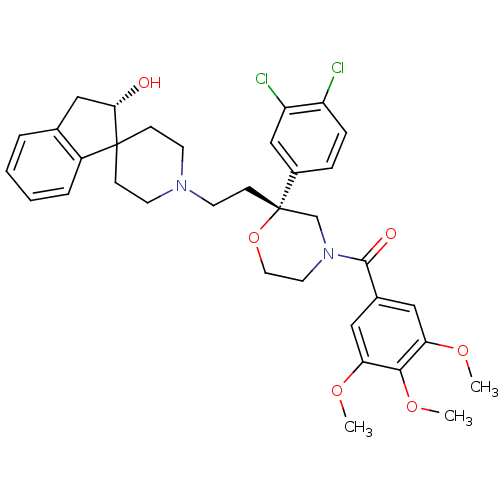
(CHEMBL295615 | spiro[(2-hydroxy)indane-1,40-piperi...)Show SMILES COc1cc(cc(OC)c1OC)C(=O)N1CCO[C@@](CCN2CCC3(CC2)[C@@H](O)Cc2ccccc32)(C1)c1ccc(Cl)c(Cl)c1 Show InChI InChI=1S/C35H40Cl2N2O6/c1-42-29-18-24(19-30(43-2)32(29)44-3)33(41)39-16-17-45-35(22-39,25-8-9-27(36)28(37)21-25)12-15-38-13-10-34(11-14-38)26-7-5-4-6-23(26)20-31(34)40/h4-9,18-19,21,31,40H,10-17,20,22H2,1-3H3/t31-,35-/m0/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 2.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Sankyo Co., Ltd.
Curated by ChEMBL
| Assay Description
The compound was evaluated in vitro for its ability to displace [3H]-SP from Tachykinin receptor 3 of male guinea pig lung membrane |
Bioorg Med Chem Lett 10: 1665-8 (2000)
BindingDB Entry DOI: 10.7270/Q2QZ297D |
More data for this
Ligand-Target Pair | |
cGMP-specific 3',5'-cyclic phosphodiesterase
(Homo sapiens (Human)) | BDBM50089823
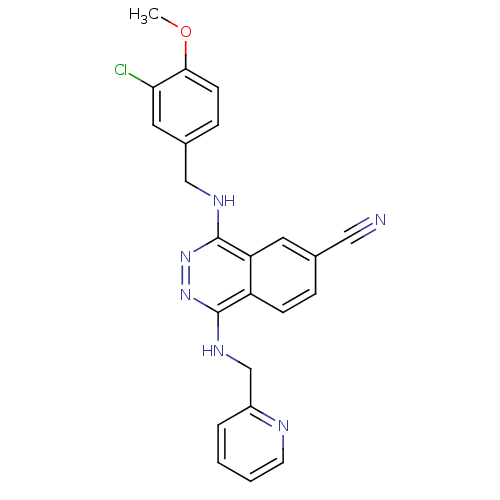
(4-(3-Chloro-4-methoxy-benzylamino)-1-[(pyridin-2-y...)Show SMILES COc1ccc(CNc2nnc(NCc3ccccn3)c3ccc(cc23)C#N)cc1Cl Show InChI InChI=1S/C23H19ClN6O/c1-31-21-8-6-16(11-20(21)24)13-27-23-19-10-15(12-25)5-7-18(19)22(29-30-23)28-14-17-4-2-3-9-26-17/h2-11H,13-14H2,1H3,(H,27,30)(H,28,29) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 2.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Eisai Company, Ltd.
Curated by ChEMBL
| Assay Description
50% inhibitory concentration against phosphodiesterase 5 (PDE5) from porcine platelets |
J Med Chem 43: 2523-9 (2000)
BindingDB Entry DOI: 10.7270/Q2T72J4N |
More data for this
Ligand-Target Pair | |
cGMP-specific 3',5'-cyclic phosphodiesterase
(Homo sapiens (Human)) | BDBM50066450
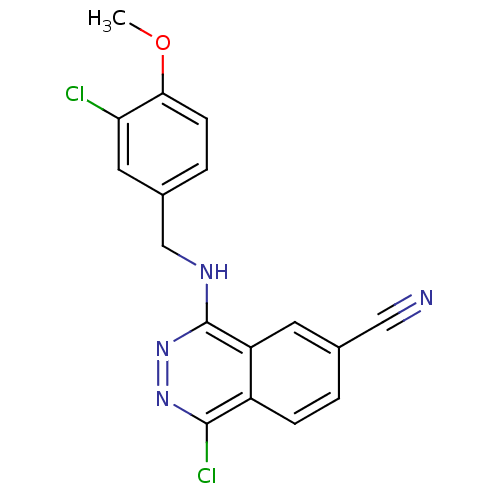
(1-Chloro-4-(3-chloro-4-methoxy-benzylamino)-phthal...)Show InChI InChI=1S/C17H12Cl2N4O/c1-24-15-5-3-11(7-14(15)18)9-21-17-13-6-10(8-20)2-4-12(13)16(19)22-23-17/h2-7H,9H2,1H3,(H,21,23) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Eisai Company, Ltd.
Curated by ChEMBL
| Assay Description
50% inhibitory concentration against phosphodiesterase 5 (PDE5) from porcine platelets |
J Med Chem 43: 2523-9 (2000)
BindingDB Entry DOI: 10.7270/Q2T72J4N |
More data for this
Ligand-Target Pair | |
3-oxo-5-alpha-steroid 4-dehydrogenase 1
(Homo sapiens (Human)) | BDBM50057477
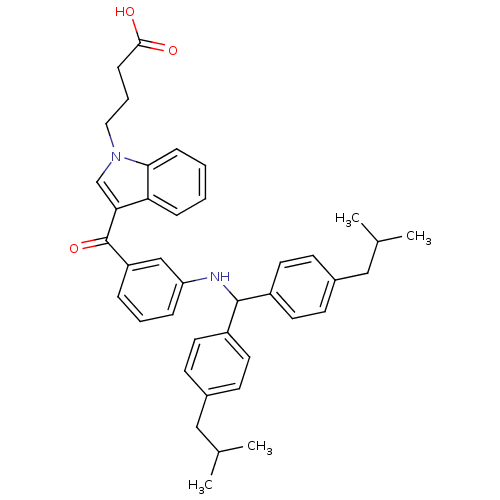
(4-(3-{3-[(4-Isobutyl-benzyl)-(4-isobutyl-phenyl)-a...)Show SMILES CC(C)Cc1ccc(cc1)C(Nc1cccc(c1)C(=O)c1cn(CCCC(O)=O)c2ccccc12)c1ccc(CC(C)C)cc1 Show InChI InChI=1S/C40H44N2O3/c1-27(2)23-29-14-18-31(19-15-29)39(32-20-16-30(17-21-32)24-28(3)4)41-34-10-7-9-33(25-34)40(45)36-26-42(22-8-13-38(43)44)37-12-6-5-11-35(36)37/h5-7,9-12,14-21,25-28,39,41H,8,13,22-24H2,1-4H3,(H,43,44) | UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Sankyo Co., Ltd.
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity against human Steroid 5-alpha-reductase type 1 in COS-1 cells was determined |
Bioorg Med Chem Lett 8: 561-6 (1999)
BindingDB Entry DOI: 10.7270/Q2571B5B |
More data for this
Ligand-Target Pair | |
Substance-K receptor
(GUINEA PIG) | BDBM50217519

(CHEMBL354709)Show SMILES COc1cc(cc(OC)c1OC)C(=O)N1CO[C@@](CCN2CCC3(CC2)NC(=O)Cc2ccccc32)(C1)c1ccc(Cl)c(Cl)c1 Show InChI InChI=1S/C34H37Cl2N3O6/c1-42-28-16-23(17-29(43-2)31(28)44-3)32(41)39-20-34(45-21-39,24-8-9-26(35)27(36)19-24)12-15-38-13-10-33(11-14-38)25-7-5-4-6-22(25)18-30(40)37-33/h4-9,16-17,19H,10-15,18,20-21H2,1-3H3,(H,37,40)/t34-/m0/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Sankyo Co., Ltd.
Curated by ChEMBL
| Assay Description
Inhibitory activity against Neurokinin 2 (NK2) receptor from guinea pig ileum |
Bioorg Med Chem Lett 9: 875-80 (1999)
BindingDB Entry DOI: 10.7270/Q2PN97TF |
More data for this
Ligand-Target Pair | |
Substance-K receptor
(GUINEA PIG) | BDBM50403793

(CHEMBL2115415)Show SMILES COc1cc(cc(OC)c1OC)C(=O)N1CCO[C@@](CCN2CCC3(CC2)c2ccccc2C[S@@]3=O)(C1)c1ccc(Cl)c(Cl)c1 Show InChI InChI=1S/C34H38Cl2N2O6S/c1-41-29-18-24(19-30(42-2)31(29)43-3)32(39)38-16-17-44-33(22-38,25-8-9-27(35)28(36)20-25)10-13-37-14-11-34(12-15-37)26-7-5-4-6-23(26)21-45(34)40/h4-9,18-20H,10-17,21-22H2,1-3H3/t33-,45-/m0/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 3.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Sankyo Co., Ltd.
Curated by ChEMBL
| Assay Description
The compound was evaluated in vitro for its ability to displace [3H]-SP from Tachykinin receptor 2 of male guinea pig lung membrane |
Bioorg Med Chem Lett 10: 1665-8 (2000)
BindingDB Entry DOI: 10.7270/Q2QZ297D |
More data for this
Ligand-Target Pair | |
cGMP-specific 3',5'-cyclic phosphodiesterase
(Homo sapiens (Human)) | BDBM50089839
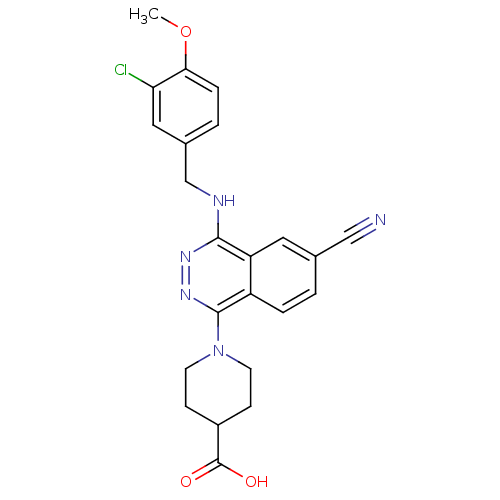
(1-[4-(3-Chloro-4-methoxy-benzylamino)-6-cyano-phth...)Show SMILES COc1ccc(CNc2nnc(N3CCC(CC3)C(O)=O)c3ccc(cc23)C#N)cc1Cl Show InChI InChI=1S/C23H22ClN5O3/c1-32-20-5-3-15(11-19(20)24)13-26-21-18-10-14(12-25)2-4-17(18)22(28-27-21)29-8-6-16(7-9-29)23(30)31/h2-5,10-11,16H,6-9,13H2,1H3,(H,26,27)(H,30,31) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 3.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Eisai Company, Ltd.
Curated by ChEMBL
| Assay Description
50% inhibitory concentration against phosphodiesterase 5 (PDE5) from porcine platelets |
J Med Chem 43: 2523-9 (2000)
BindingDB Entry DOI: 10.7270/Q2T72J4N |
More data for this
Ligand-Target Pair | |
Substance-K receptor
(GUINEA PIG) | BDBM50217520
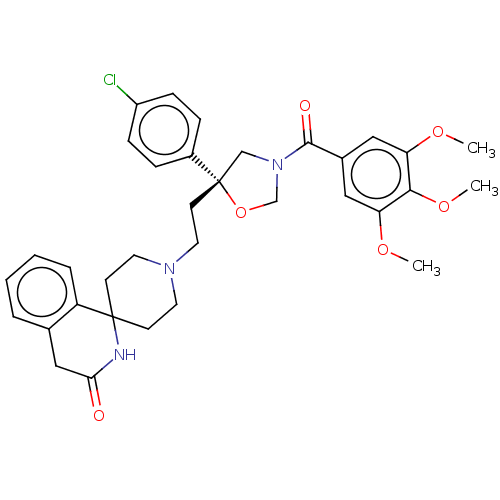
(CHEMBL354645)Show SMILES COc1cc(cc(OC)c1OC)C(=O)N1CO[C@@](CCN2CCC3(CC2)NC(=O)Cc2ccccc32)(C1)c1ccc(Cl)cc1 Show InChI InChI=1S/C34H38ClN3O6/c1-41-28-18-24(19-29(42-2)31(28)43-3)32(40)38-21-34(44-22-38,25-8-10-26(35)11-9-25)14-17-37-15-12-33(13-16-37)27-7-5-4-6-23(27)20-30(39)36-33/h4-11,18-19H,12-17,20-22H2,1-3H3,(H,36,39)/t34-/m0/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 3.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Sankyo Co., Ltd.
Curated by ChEMBL
| Assay Description
Inhibitory activity against Neurokinin 2 (NK2) receptor from guinea pig ileum |
Bioorg Med Chem Lett 9: 875-80 (1999)
BindingDB Entry DOI: 10.7270/Q2PN97TF |
More data for this
Ligand-Target Pair | |
cGMP-specific 3',5'-cyclic phosphodiesterase
(Homo sapiens (Human)) | BDBM50066454
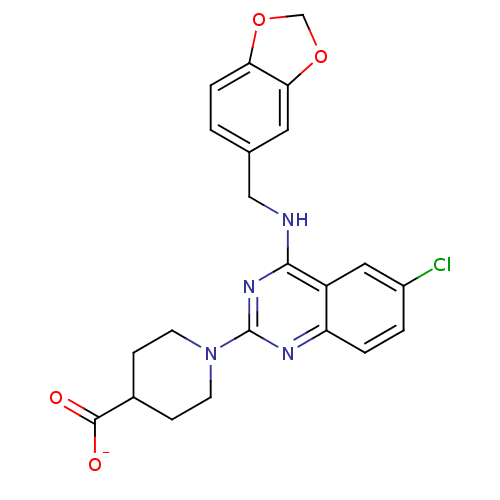
(CHEMBL25616 | Sodium; 1-{4-[(benzo[1,3]dioxol-5-yl...)Show SMILES [O-]C(=O)C1CCN(CC1)c1nc(NCc2ccc3OCOc3c2)c2cc(Cl)ccc2n1 Show InChI InChI=1S/C22H21ClN4O4/c23-15-2-3-17-16(10-15)20(24-11-13-1-4-18-19(9-13)31-12-30-18)26-22(25-17)27-7-5-14(6-8-27)21(28)29/h1-4,9-10,14H,5-8,11-12H2,(H,28,29)(H,24,25,26)/p-1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 3.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Eisai Company, Ltd.
Curated by ChEMBL
| Assay Description
50% inhibitory concentration against phosphodiesterase 5 (PDE5) from porcine platelets |
J Med Chem 43: 2523-9 (2000)
BindingDB Entry DOI: 10.7270/Q2T72J4N |
More data for this
Ligand-Target Pair | |
cGMP-specific 3',5'-cyclic phosphodiesterase
(Homo sapiens (Human)) | BDBM50089808
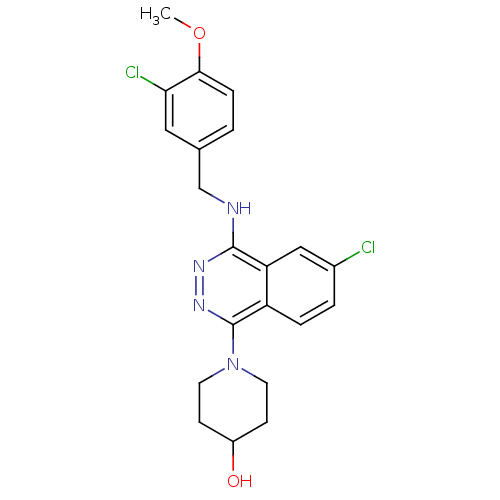
(1-[6-Chloro-4-(3-chloro-4-methoxy-benzylamino)-pht...)Show SMILES COc1ccc(CNc2nnc(N3CCC(O)CC3)c3ccc(Cl)cc23)cc1Cl Show InChI InChI=1S/C21H22Cl2N4O2/c1-29-19-5-2-13(10-18(19)23)12-24-20-17-11-14(22)3-4-16(17)21(26-25-20)27-8-6-15(28)7-9-27/h2-5,10-11,15,28H,6-9,12H2,1H3,(H,24,25) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 4.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Eisai Company, Ltd.
Curated by ChEMBL
| Assay Description
50% inhibitory concentration against phosphodiesterase 5 (PDE5) from porcine platelets |
J Med Chem 43: 2523-9 (2000)
BindingDB Entry DOI: 10.7270/Q2T72J4N |
More data for this
Ligand-Target Pair | |
Substance-K receptor
(GUINEA PIG) | BDBM50217517
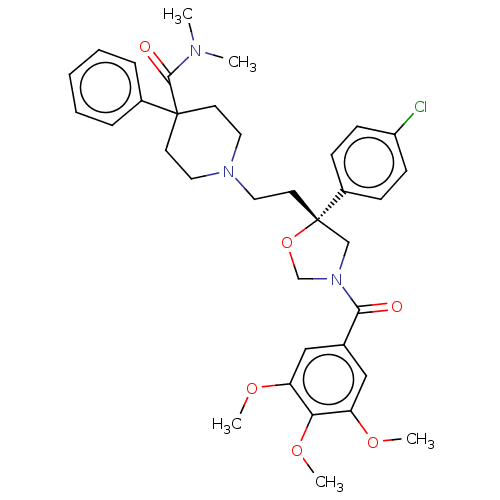
(CHEMBL353158)Show SMILES COc1cc(cc(OC)c1OC)C(=O)N1CO[C@@](CCN2CCC(CC2)(C(=O)N(C)C)c2ccccc2)(C1)c1ccc(Cl)cc1 Show InChI InChI=1S/C35H42ClN3O6/c1-37(2)33(41)34(26-9-7-6-8-10-26)15-18-38(19-16-34)20-17-35(27-11-13-28(36)14-12-27)23-39(24-45-35)32(40)25-21-29(42-3)31(44-5)30(22-25)43-4/h6-14,21-22H,15-20,23-24H2,1-5H3/t35-/m0/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 4.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Sankyo Co., Ltd.
Curated by ChEMBL
| Assay Description
Inhibitory activity against Neurokinin 2 (NK2) receptor from guinea pig ileum |
Bioorg Med Chem Lett 9: 875-80 (1999)
BindingDB Entry DOI: 10.7270/Q2PN97TF |
More data for this
Ligand-Target Pair | |
Substance-P receptor
(GUINEA PIG) | BDBM50217511
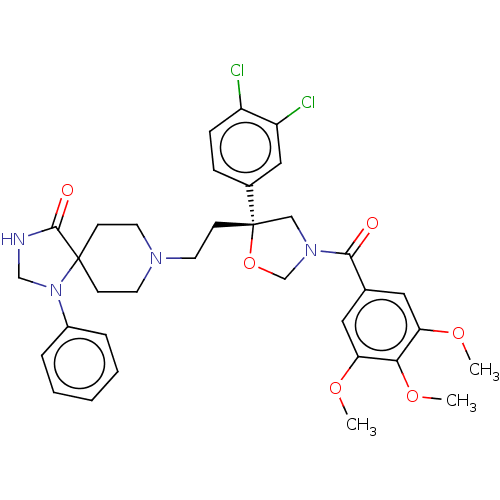
(CHEMBL172342)Show SMILES COc1cc(cc(OC)c1OC)C(=O)N1CO[C@@](CCN2CCC3(CC2)N(CNC3=O)c2ccccc2)(C1)c1ccc(Cl)c(Cl)c1 Show InChI InChI=1S/C34H38Cl2N4O6/c1-43-28-17-23(18-29(44-2)30(28)45-3)31(41)39-20-34(46-22-39,24-9-10-26(35)27(36)19-24)13-16-38-14-11-33(12-15-38)32(42)37-21-40(33)25-7-5-4-6-8-25/h4-10,17-19H,11-16,20-22H2,1-3H3,(H,37,42)/t34-/m0/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Sankyo Co., Ltd.
Curated by ChEMBL
| Assay Description
Inhibitory activity against Neurokinin 1 (NK1) receptor from guinea pig lung |
Bioorg Med Chem Lett 9: 875-80 (1999)
BindingDB Entry DOI: 10.7270/Q2PN97TF |
More data for this
Ligand-Target Pair | |
Substance-K receptor
(GUINEA PIG) | BDBM50217506

(CHEMBL170936)Show SMILES COc1cc(cc(OC)c1OC)C(=O)N1CO[C@@](CCN2CCC(CC2)(C(=O)N(C)C)c2ccccc2)(C1)c1ccc(Cl)c(Cl)c1 Show InChI InChI=1S/C35H41Cl2N3O6/c1-38(2)33(42)34(25-9-7-6-8-10-25)13-16-39(17-14-34)18-15-35(26-11-12-27(36)28(37)21-26)22-40(23-46-35)32(41)24-19-29(43-3)31(45-5)30(20-24)44-4/h6-12,19-21H,13-18,22-23H2,1-5H3/t35-/m0/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 5.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Sankyo Co., Ltd.
Curated by ChEMBL
| Assay Description
Inhibitory activity against Neurokinin 2 (NK2) receptor from guinea pig ileum |
Bioorg Med Chem Lett 9: 875-80 (1999)
BindingDB Entry DOI: 10.7270/Q2PN97TF |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data