

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Proprotein convertase subtilisin/kexin type 9 (Homo sapiens (Human)) | BDBM50581548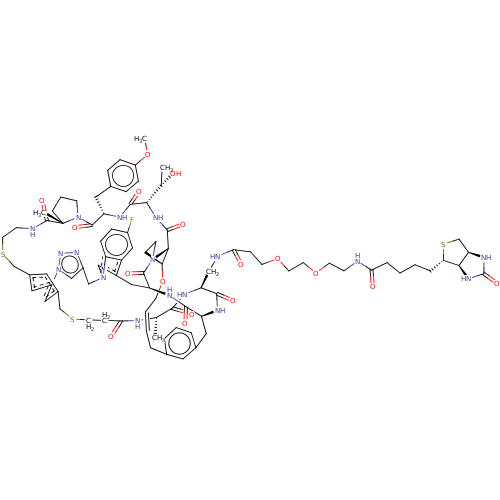 (CHEMBL5085124) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.000600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human PCSK9 using Alexa Fluor 674 as substrate incubated for 2 hrs by FRET ultra assay | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c01599 BindingDB Entry DOI: 10.7270/Q2TM7G0Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proprotein convertase subtilisin/kexin type 9 (Homo sapiens (Human)) | BDBM50581547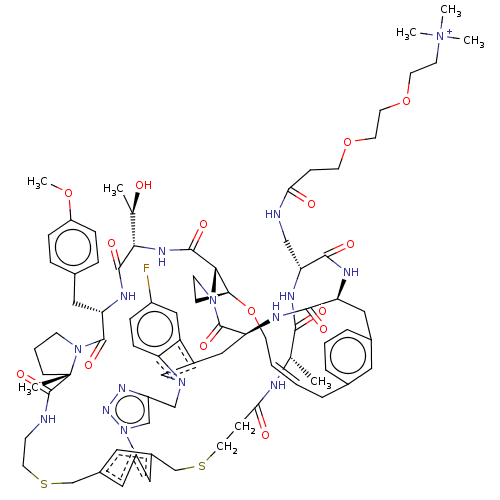 (CHEMBL5081349) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.000930 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human PCSK9 using Alexa Fluor 674 as substrate incubated for 2 hrs by FRET ultra assay | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c01599 BindingDB Entry DOI: 10.7270/Q2TM7G0Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Purine nucleoside phosphorylase (Homo sapiens (Human)) | BDBM50293089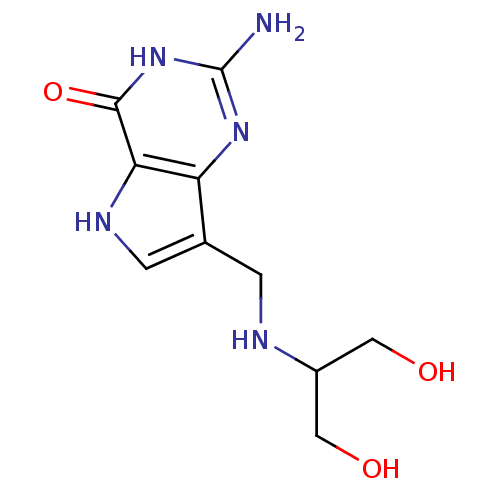 (2-Amino-7-{[(1,3-dihydroxypropan-2-yl)amino]methyl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.00200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Industrial Research Limited Curated by ChEMBL | Assay Description Inhibition of human PNP by xanthine-oxidase coupled assay | J Med Chem 52: 1126-43 (2009) Article DOI: 10.1021/jm801421q BindingDB Entry DOI: 10.7270/Q2QR4Z18 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proprotein convertase subtilisin/kexin type 9 (Homo sapiens (Human)) | BDBM50581546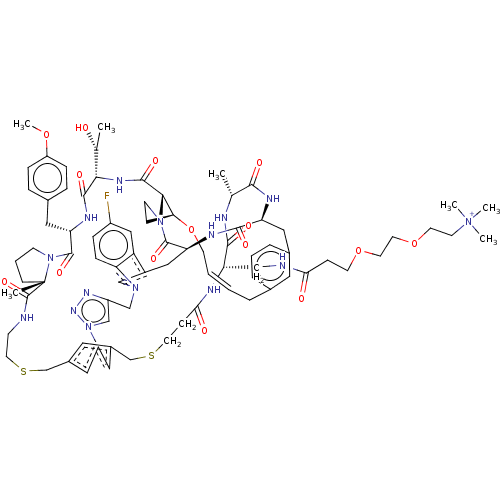 (CHEMBL5084416) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | 0.00239 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human PCSK9 using Alexa Fluor 674 as substrate incubated for 2 hrs by FRET ultra assay | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c01599 BindingDB Entry DOI: 10.7270/Q2TM7G0Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Purine nucleoside phosphorylase (Homo sapiens (Human)) | BDBM50293090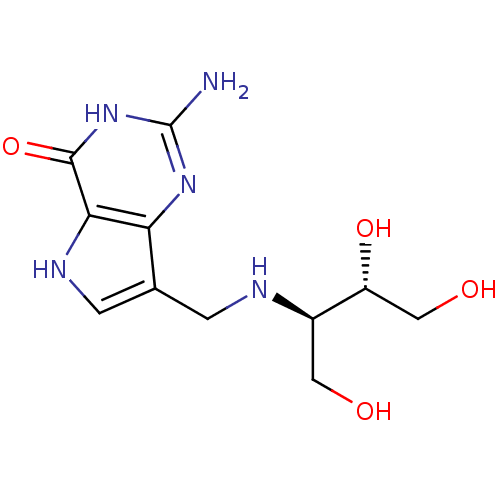 (2-Amino-7-({[(2R,3S)-1,3,4-trihydroxybutan-2-yl]am...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.00300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Industrial Research Limited Curated by ChEMBL | Assay Description Inhibition of human PNP by xanthine-oxidase coupled assay | J Med Chem 52: 1126-43 (2009) Article DOI: 10.1021/jm801421q BindingDB Entry DOI: 10.7270/Q2QR4Z18 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Purine nucleoside phosphorylase (Homo sapiens (Human)) | BDBM50246593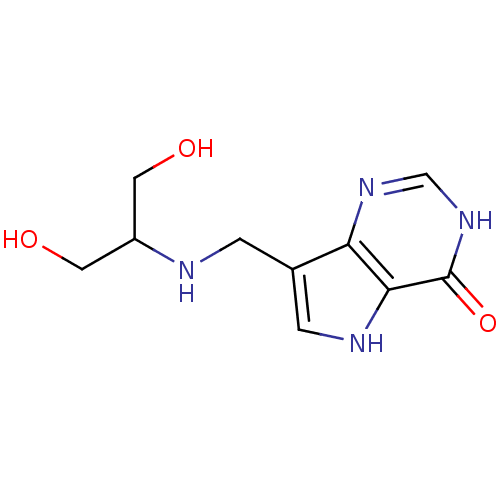 (7-((1,3-dihydroxypropan-2-ylamino)methyl)-3H-pyrro...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Similars | PDB Article PubMed | 0.00500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Industrial Research Limited Curated by ChEMBL | Assay Description Inhibition of human PNP by xanthine-oxidase coupled assay | J Med Chem 52: 1126-43 (2009) Article DOI: 10.1021/jm801421q BindingDB Entry DOI: 10.7270/Q2QR4Z18 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Purine nucleoside phosphorylase (Homo sapiens (Human)) | BDBM50246593 (7-((1,3-dihydroxypropan-2-ylamino)methyl)-3H-pyrro...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Similars | PDB Article PubMed | 0.00520 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Albert Einstein College of Medicine of Yeshiva University Curated by ChEMBL | Assay Description Equilibrium binding affinity to wild type human PNP | Bioorg Med Chem Lett 18: 5900-3 (2008) Article DOI: 10.1016/j.bmcl.2008.08.047 BindingDB Entry DOI: 10.7270/Q2SX6F4H | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Serine/threonine-protein kinase pim-1 (Homo sapiens (Human)) | BDBM106870 (US8592455, 70) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem Similars | Article PubMed | 0.00600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research Curated by ChEMBL | Assay Description Inhibition of PIM1 kinase (unknown origin) using Biotin-AGAGRSRHSSYPAGT-OH as substrate after 2 hrs by alphascreen assay | J Med Chem 58: 8373-86 (2015) Article DOI: 10.1021/acs.jmedchem.5b01275 BindingDB Entry DOI: 10.7270/Q2H41VGN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Purine nucleoside phosphorylase (Homo sapiens (Human)) | BDBM50293087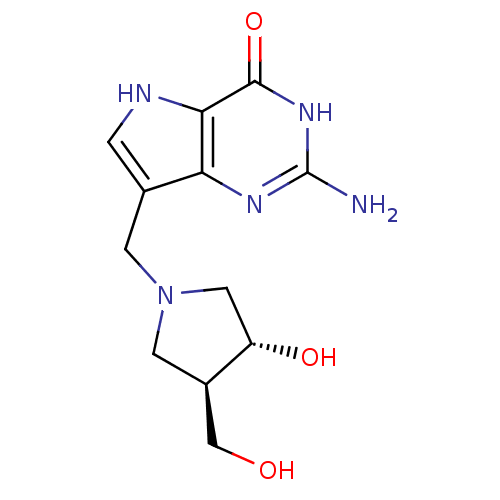 (2-amino-7-(((3R,4R)-3-hydroxy-4-(hydroxymethyl)pyr...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid PDB UniChem Similars | PDB Article PubMed | 0.00700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Industrial Research Limited Curated by ChEMBL | Assay Description Inhibition of human PNP by xanthine-oxidase coupled assay | J Med Chem 52: 1126-43 (2009) Article DOI: 10.1021/jm801421q BindingDB Entry DOI: 10.7270/Q2QR4Z18 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Proprotein convertase subtilisin/kexin type 9 (Homo sapiens (Human)) | BDBM50581547 (CHEMBL5081349) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.00736 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human PCSK9 using Alexa Fluor 674 as substrate incubated for 2 hrs by FRET plus assay | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c01599 BindingDB Entry DOI: 10.7270/Q2TM7G0Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proprotein convertase subtilisin/kexin type 9 (Homo sapiens (Human)) | BDBM50581545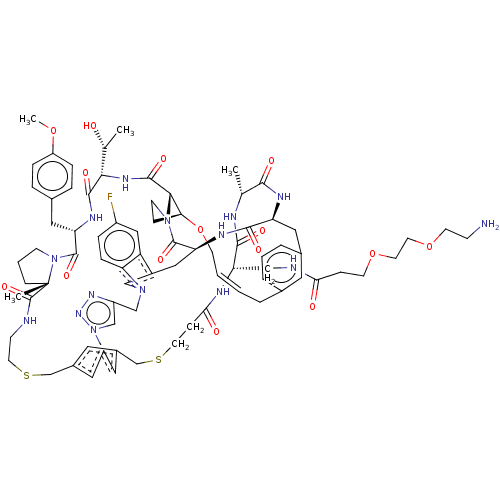 (CHEMBL5084902) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.00813 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human PCSK9 using Alexa Fluor 674 as substrate incubated for 2 hrs by FRET ultra assay | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c01599 BindingDB Entry DOI: 10.7270/Q2TM7G0Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proprotein convertase subtilisin/kexin type 9 (Homo sapiens (Human)) | BDBM50581544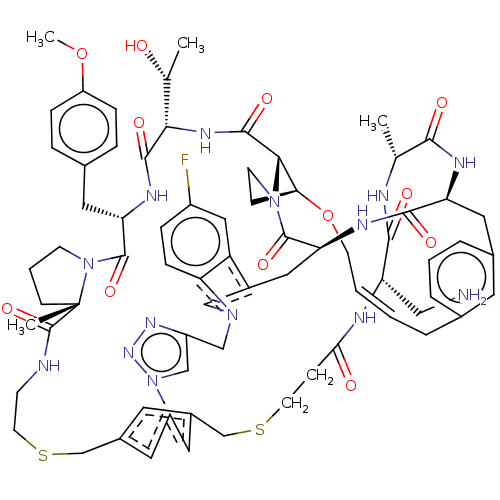 (CHEMBL5086475) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.00826 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human PCSK9 using Alexa Fluor 674 as substrate incubated for 2 hrs by FRET plus assay | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c01599 BindingDB Entry DOI: 10.7270/Q2TM7G0Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase pim-3 (Homo sapiens (Human)) | BDBM106870 (US8592455, 70) | KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem Similars | Article PubMed | 0.00900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research Curated by ChEMBL | Assay Description Inhibition of PIM3 kinase (unknown origin) using Biotin-AGAGRSRHSSYPAGT-OH as substrate after 2 hrs by alphascreen assay | J Med Chem 58: 8373-86 (2015) Article DOI: 10.1021/acs.jmedchem.5b01275 BindingDB Entry DOI: 10.7270/Q2H41VGN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Purine nucleoside phosphorylase (Homo sapiens (Human)) | BDBM50293091 (7-({[(1R,2S)-2,3-DIHYDROXY-1-(HYDROXYMETHYL)PROPYL...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid PDB UniChem Patents Similars | MMDB PDB Article PubMed | 0.00900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Industrial Research Limited Curated by ChEMBL | Assay Description Inhibition of human PNP by xanthine-oxidase coupled assay | J Med Chem 52: 1126-43 (2009) Article DOI: 10.1021/jm801421q BindingDB Entry DOI: 10.7270/Q2QR4Z18 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Purine nucleoside phosphorylase (Homo sapiens (Human)) | BDBM22109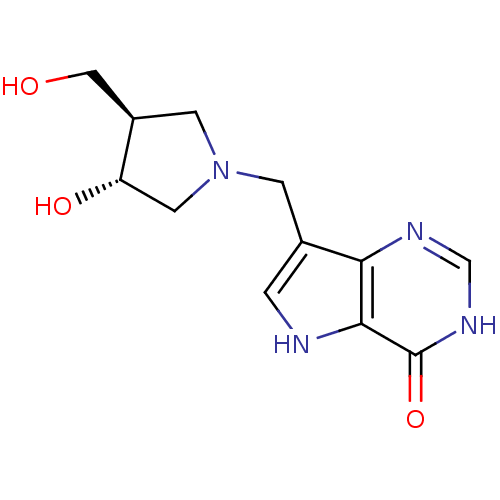 (7-{[(3R,4R)-3-hydroxy-4-(hydroxymethyl)pyrrolidin-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid PDB UniChem Similars | MMDB PDB Article PubMed | 0.00900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Industrial Research Limited Curated by ChEMBL | Assay Description Inhibition of human PNP by xanthine-oxidase coupled assay | J Med Chem 52: 1126-43 (2009) Article DOI: 10.1021/jm801421q BindingDB Entry DOI: 10.7270/Q2QR4Z18 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Proprotein convertase subtilisin/kexin type 9 (Homo sapiens (Human)) | BDBM50581544 (CHEMBL5086475) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.00940 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human PCSK9 using Alexa Fluor 674 as substrate incubated for 2 hrs by FRET ultra assay | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c01599 BindingDB Entry DOI: 10.7270/Q2TM7G0Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Purine nucleoside phosphorylase (Homo sapiens (Human)) | BDBM50246590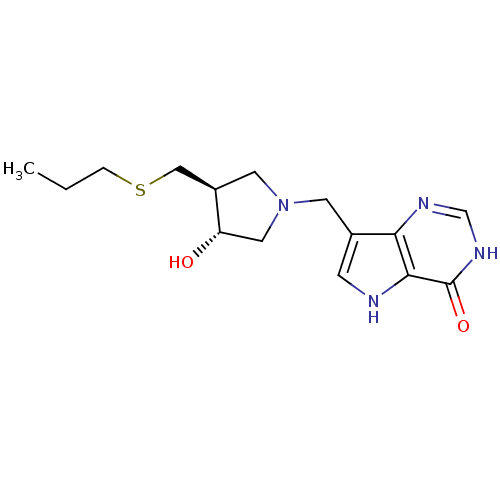 (7-(((3R,4S)-3-hydroxy-4-(propylthiomethyl)pyrrolid...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.00980 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Albert Einstein College of Medicine of Yeshiva University Curated by ChEMBL | Assay Description Equilibrium binding affinity to wild type human PNP | Bioorg Med Chem Lett 18: 5900-3 (2008) Article DOI: 10.1016/j.bmcl.2008.08.047 BindingDB Entry DOI: 10.7270/Q2SX6F4H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proprotein convertase subtilisin/kexin type 9 (Homo sapiens (Human)) | BDBM50581548 (CHEMBL5085124) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | <0.0100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human PCSK9 using Alexa Fluor 674 as substrate incubated for 2 hrs by FRET plus assay | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c01599 BindingDB Entry DOI: 10.7270/Q2TM7G0Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Purine nucleoside phosphorylase (Homo sapiens (Human)) | BDBM22109 (7-{[(3R,4R)-3-hydroxy-4-(hydroxymethyl)pyrrolidin-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid PDB UniChem Similars | MMDB PDB Article PubMed | 0.0107 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Albert Einstein College of Medicine of Yeshiva University Curated by ChEMBL | Assay Description Equilibrium binding affinity to wild type human PNP | Bioorg Med Chem Lett 18: 5900-3 (2008) Article DOI: 10.1016/j.bmcl.2008.08.047 BindingDB Entry DOI: 10.7270/Q2SX6F4H | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Proprotein convertase subtilisin/kexin type 9 (Homo sapiens (Human)) | BDBM50581546 (CHEMBL5084416) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | 0.0114 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human PCSK9 using Alexa Fluor 674 as substrate incubated for 2 hrs by FRET plus assay | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c01599 BindingDB Entry DOI: 10.7270/Q2TM7G0Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase pim-1 (Homo sapiens (Human)) | BDBM50387298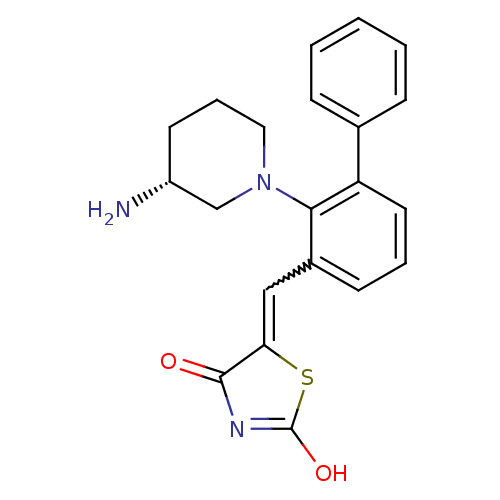 (CHEMBL2048872) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0170 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research Curated by ChEMBL | Assay Description Inhibition of PIM1 kinase (unknown origin) using Biotin-AGAGRSRHSSYPAGT-OH as substrate after 2 hrs by alphascreen assay | J Med Chem 58: 8373-86 (2015) Article DOI: 10.1021/acs.jmedchem.5b01275 BindingDB Entry DOI: 10.7270/Q2H41VGN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase pim-2 (Homo sapiens (Human)) | BDBM106870 (US8592455, 70) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem Similars | Article PubMed | 0.0180 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research Curated by ChEMBL | Assay Description Inhibition of PIM2 kinase (unknown origin) using Biotin-AGAGRSRHSSYPAGT-OH as substrate after 2 hrs by alphascreen assay | J Med Chem 58: 8373-86 (2015) Article DOI: 10.1021/acs.jmedchem.5b01275 BindingDB Entry DOI: 10.7270/Q2H41VGN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Purine nucleoside phosphorylase (Homo sapiens (Human)) | BDBM50247151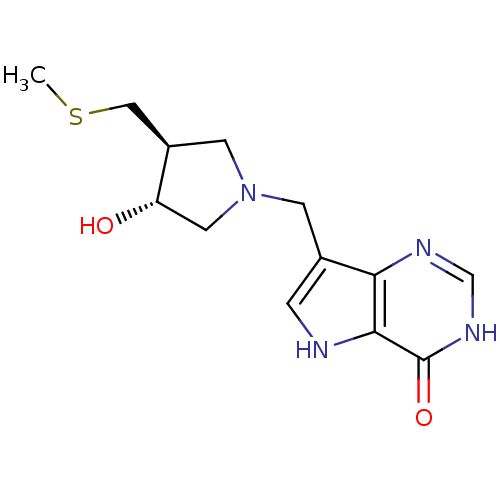 (7-(((3R,4S)-3-hydroxy-4-(methylthiomethyl)pyrrolid...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0196 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Albert Einstein College of Medicine of Yeshiva University Curated by ChEMBL | Assay Description Equilibrium binding affinity to wild type human PNP | Bioorg Med Chem Lett 18: 5900-3 (2008) Article DOI: 10.1016/j.bmcl.2008.08.047 BindingDB Entry DOI: 10.7270/Q2SX6F4H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Platelet-activating factor acetylhydrolase (Homo sapiens (Human)) | BDBM50117772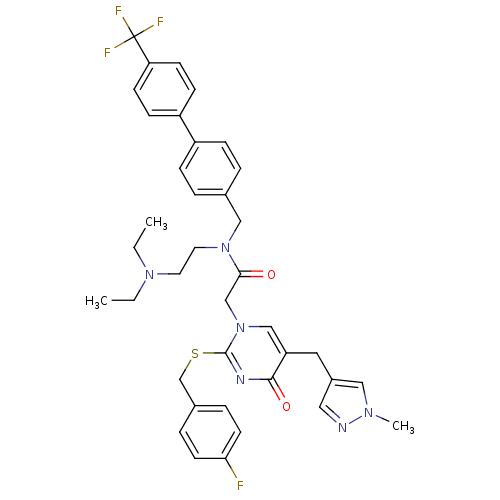 (CHEMBL10921 | N-(2-Diethylamino-ethyl)-2-[2-(4-flu...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | PubMed | 0.0300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Steady state and transient kinetics to a freely reversible, non-covalently bound, human recombinant Phospholipase A2 (rhLp-PLA2) was determined | Bioorg Med Chem Lett 12: 2603-6 (2002) BindingDB Entry DOI: 10.7270/Q2G44PNN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| S-methyl-5'-thioinosine phosphorylase (Pseudomonas aeruginosa) | BDBM50247150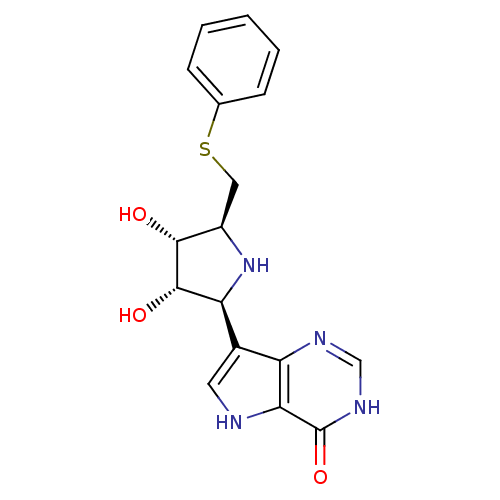 (5'-phenylthio-ImmH | CHEMBL474328 | US9290501, (B)) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | US Patent | 0.0350 | -59.7 | n/a | n/a | n/a | n/a | n/a | 7.4 | 25 |
Albert Einstein College of Medicine, Inc.; Victoria Link Limited US Patent | Assay Description Assays for slow-onset inhibitors were carried out by adding 1 nM PaMTIP into reaction mixtures at 25 °C. containing 100 mM Hepes, pH 7.4, 100 mM ... | US Patent US9290501 (2016) BindingDB Entry DOI: 10.7270/Q2639NKD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| S-methyl-5'-thioinosine phosphorylase (Pseudomonas aeruginosa) | BDBM218675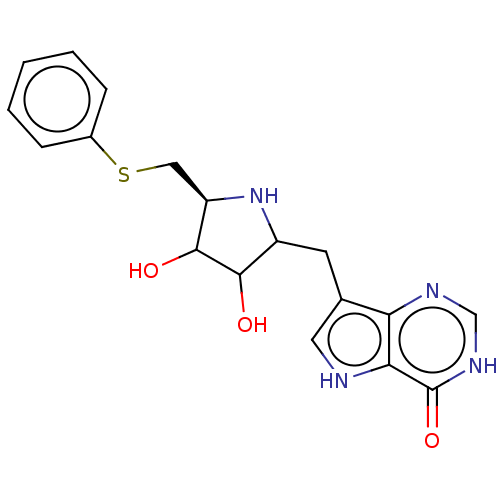 (US9290501, PT-ImmH) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.0350 | -59.7 | n/a | n/a | n/a | n/a | n/a | 7.4 | 25 |
Albert Einstein College of Medicine, Inc.; Victoria Link Limited US Patent | Assay Description Assays for slow-onset inhibitors were carried out by adding 1 nM PaMTIP into reaction mixtures at 25 °C. containing 100 mM Hepes, pH 7.4, 100 mM ... | US Patent US9290501 (2016) BindingDB Entry DOI: 10.7270/Q2639NKD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proprotein convertase subtilisin/kexin type 9 (Homo sapiens (Human)) | BDBM50581549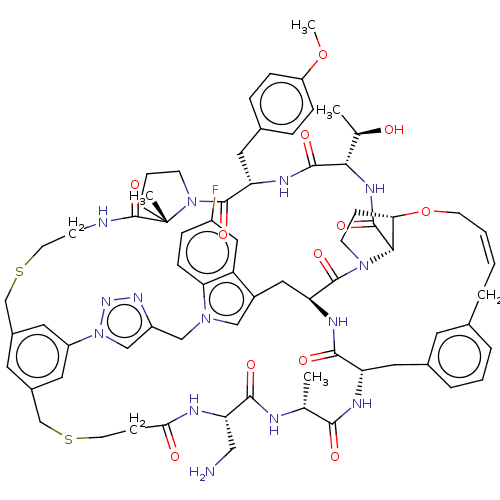 (CHEMBL5082483) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.0370 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human PCSK9 using Alexa Fluor 674 as substrate incubated for 2 hrs by FRET plus assay | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c01599 BindingDB Entry DOI: 10.7270/Q2TM7G0Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Purine nucleoside phosphorylase (Homo sapiens (Human)) | BDBM50293086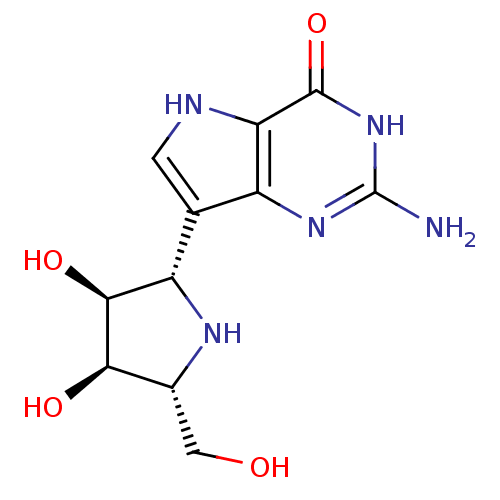 ((1S)-1,4-dideoxy-1,4-imino-1-(9-deazaguanin-9-yl)-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid PDB UniChem Patents Similars | MMDB Article PubMed | 0.0420 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Industrial Research Limited Curated by ChEMBL | Assay Description Inhibition of human PNP by xanthine-oxidase coupled assay | J Med Chem 52: 1126-43 (2009) Article DOI: 10.1021/jm801421q BindingDB Entry DOI: 10.7270/Q2QR4Z18 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Apelin receptor (Rattus norvegicus) | BDBM50009575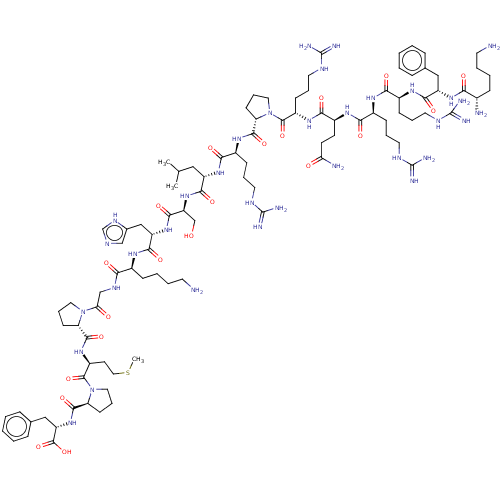 (CHEMBL3234446) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | 0.0530 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Department of Chemistry, University of Alberta , 11227 Saskatchewan Drive NW, Edmonton, Alberta T6G 2G2, Canada. Curated by ChEMBL | Assay Description Displacement of [125I]-pyr-1-apelin-13 from rat C-terminal EGFP-tagged APJ receptor expressed in CHO cell membranes after 3 hrs by Wallac gamma count... | J Med Chem 60: 6408-6427 (2017) Article DOI: 10.1021/acs.jmedchem.7b00723 BindingDB Entry DOI: 10.7270/Q2WS8WPC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Purine nucleoside phosphorylase (Homo sapiens (Human)) | BDBM50195587 (1,4-DIDEOXY-4-AZA-1-(S)-(9-DEAZAHYPOXANTHIN-9-YL)-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MMDB PC cid PC sid PDB UniChem Patents Similars | MMDB PDB Article PubMed | 0.0560 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Industrial Research Limited Curated by ChEMBL | Assay Description Inhibition of human PNP by xanthine-oxidase coupled assay | J Med Chem 52: 1126-43 (2009) Article DOI: 10.1021/jm801421q BindingDB Entry DOI: 10.7270/Q2QR4Z18 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Purine nucleoside phosphorylase (Homo sapiens (Human)) | BDBM50195587 (1,4-DIDEOXY-4-AZA-1-(S)-(9-DEAZAHYPOXANTHIN-9-YL)-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MMDB PC cid PC sid PDB UniChem Patents Similars | MMDB PDB Article PubMed | 0.0579 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Albert Einstein College of Medicine of Yeshiva University Curated by ChEMBL | Assay Description Equilibrium binding affinity to wild type human PNP | Bioorg Med Chem Lett 18: 5900-3 (2008) Article DOI: 10.1016/j.bmcl.2008.08.047 BindingDB Entry DOI: 10.7270/Q2SX6F4H | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Neuronal acetylcholine receptor subunit alpha-4 (Rattus norvegicus (Rat)) | BDBM50049757 (()-2-(6-Chloro-pyridin-3-yl)-7-aza-bicyclo[2.2.1]h...) | PDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 0.0700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Targacept, Inc. Curated by PDSP Ki Database | J Pharmacol Exp Ther 312: 619-26 (2005) Article DOI: 10.1124/jpet.104.075069 BindingDB Entry DOI: 10.7270/Q2BK19XS | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| S-methyl-5'-thioinosine phosphorylase (Pseudomonas aeruginosa) | BDBM218673 (US9290501, PrT-DADMe-ImmH) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.0720 | -57.9 | n/a | n/a | n/a | n/a | n/a | 7.4 | 25 |
Albert Einstein College of Medicine, Inc.; Victoria Link Limited US Patent | Assay Description Assays for slow-onset inhibitors were carried out by adding 1 nM PaMTIP into reaction mixtures at 25 °C. containing 100 mM Hepes, pH 7.4, 100 mM ... | US Patent US9290501 (2016) BindingDB Entry DOI: 10.7270/Q2639NKD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| S-methyl-5'-thioinosine phosphorylase (Pseudomonas aeruginosa) | BDBM50247149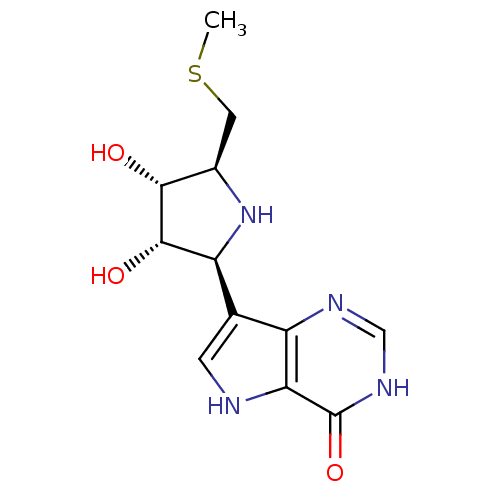 (5'-Methylthio-ImmH | CHEMBL473929 | US9290501, (A)) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid UniChem Similars | US Patent | 0.0760 | -57.8 | n/a | n/a | n/a | n/a | n/a | 7.4 | 25 |
Albert Einstein College of Medicine, Inc.; Victoria Link Limited US Patent | Assay Description Assays for slow-onset inhibitors were carried out by adding 1 nM PaMTIP into reaction mixtures at 25 °C. containing 100 mM Hepes, pH 7.4, 100 mM ... | US Patent US9290501 (2016) BindingDB Entry DOI: 10.7270/Q2639NKD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| S-methyl-5'-thioinosine phosphorylase (Pseudomonas aeruginosa) | BDBM218674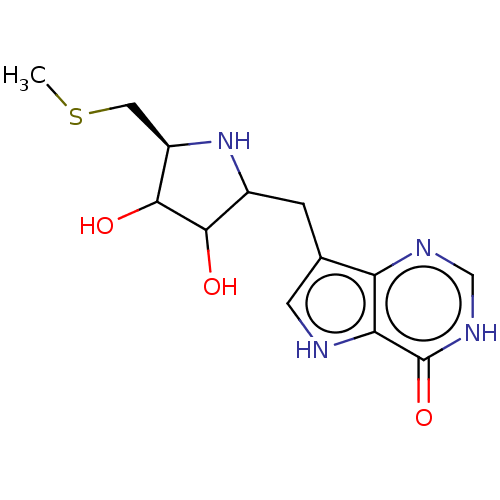 (US9290501, MT-ImmH) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.0760 | -57.8 | n/a | n/a | n/a | n/a | n/a | 7.4 | 25 |
Albert Einstein College of Medicine, Inc.; Victoria Link Limited US Patent | Assay Description Assays for slow-onset inhibitors were carried out by adding 1 nM PaMTIP into reaction mixtures at 25 °C. containing 100 mM Hepes, pH 7.4, 100 mM ... | US Patent US9290501 (2016) BindingDB Entry DOI: 10.7270/Q2639NKD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| S-methyl-5'-thioadenosine phosphorylase (Homo sapiens (Human)) | BDBM22113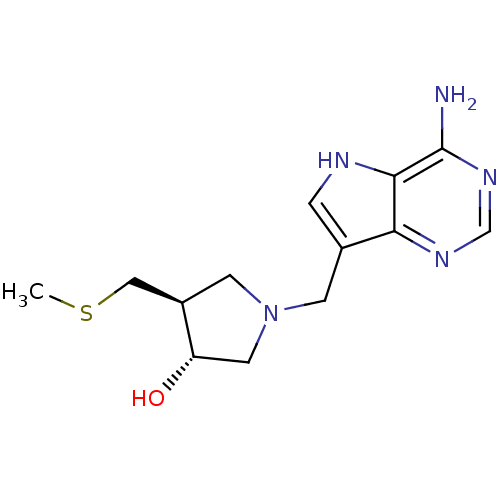 ((3R,4S)-1-({4-amino-5H-pyrrolo[3,2-d]pyrimidin-7-y...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase DrugBank MCE MMDB PC cid PC sid PDB UniChem Similars | PDB Article PubMed | 0.0900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Industrial Research Limited Curated by ChEMBL | Assay Description Inhibition of human MTAP assessed as reduction in methylthioadenosine phosphorolysis/hydrolysis | Bioorg Med Chem 20: 5181-7 (2012) Article DOI: 10.1016/j.bmc.2012.07.006 BindingDB Entry DOI: 10.7270/Q2XG9S6F | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Neuronal acetylcholine receptor subunit alpha-5 (RAT) | BDBM50049757 (()-2-(6-Chloro-pyridin-3-yl)-7-aza-bicyclo[2.2.1]h...) | UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 0.0960 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Targacept, Inc. Curated by PDSP Ki Database | J Pharmacol Exp Ther 312: 619-26 (2005) Article DOI: 10.1124/jpet.104.075069 BindingDB Entry DOI: 10.7270/Q2BK19XS | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| S-methyl-5'-thioinosine phosphorylase (Pseudomonas aeruginosa) | BDBM50246594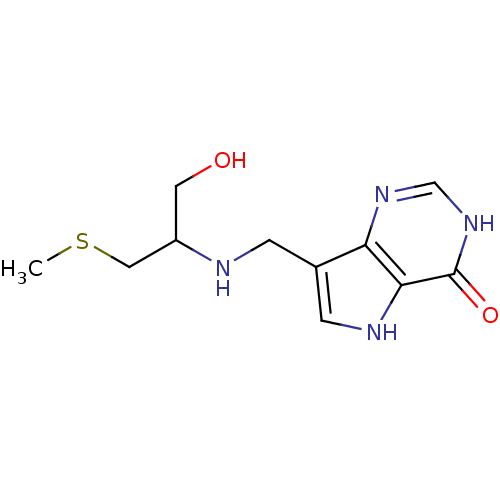 ((+/-)-7-((1-hydroxy-3-(methylthio)propan-2-ylamino...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | US Patent | 0.0960 | -57.2 | n/a | n/a | n/a | n/a | n/a | 7.4 | 25 |
Albert Einstein College of Medicine, Inc.; Victoria Link Limited US Patent | Assay Description Assays for slow-onset inhibitors were carried out by adding 1 nM PaMTIP into reaction mixtures at 25 °C. containing 100 mM Hepes, pH 7.4, 100 mM ... | US Patent US9290501 (2016) BindingDB Entry DOI: 10.7270/Q2639NKD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| S-methyl-5'-thioinosine phosphorylase (Pseudomonas aeruginosa) | BDBM50246594 ((+/-)-7-((1-hydroxy-3-(methylthio)propan-2-ylamino...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | US Patent | 0.0960 | -57.2 | n/a | n/a | n/a | n/a | n/a | 7.4 | 25 |
Albert Einstein College of Medicine, Inc.; Victoria Link Limited US Patent | Assay Description Assays for slow-onset inhibitors were carried out by adding 1 nM PaMTIP into reaction mixtures at 25 °C. containing 100 mM Hepes, pH 7.4, 100 mM ... | US Patent US9290501 (2016) BindingDB Entry DOI: 10.7270/Q2639NKD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Apelin receptor (Rattus norvegicus) | BDBM50257221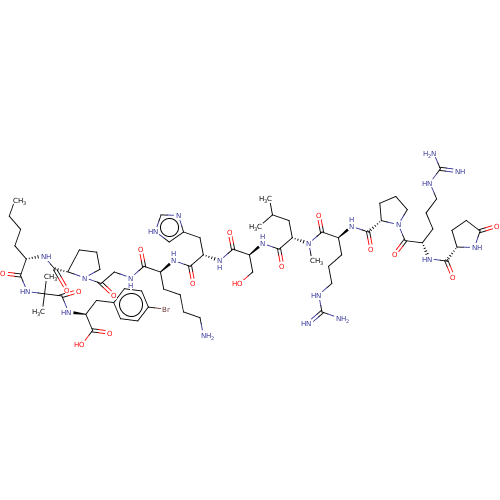 (CHEMBL4059998) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | KEGG PC cid PC sid UniChem | Article PubMed | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Department of Chemistry, University of Alberta , 11227 Saskatchewan Drive NW, Edmonton, Alberta T6G 2G2, Canada. Curated by ChEMBL | Assay Description Displacement of [125I]-pyr-1-apelin-13 from rat C-terminal EGFP-tagged APJ receptor expressed in CHO cell membranes after 3 hrs by Wallac gamma count... | J Med Chem 60: 6408-6427 (2017) Article DOI: 10.1021/acs.jmedchem.7b00723 BindingDB Entry DOI: 10.7270/Q2WS8WPC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nociceptin receptor (Homo sapiens (Human)) | BDBM50100983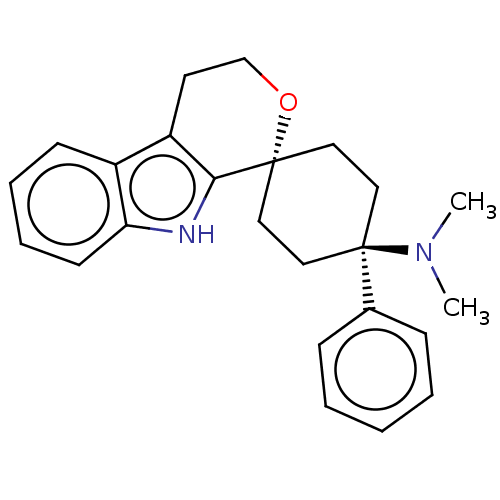 (CHEMBL3326224) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmacokinetics Curated by ChEMBL | Assay Description Displacement of [3H]nociceptin from human NOP receptor expressed in CHO-K1 cells by scintillation proximity assay | ACS Med Chem Lett 5: 857-62 (2014) Article DOI: 10.1021/ml500117c BindingDB Entry DOI: 10.7270/Q25140ZK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Purine nucleoside phosphorylase (Homo sapiens (Human)) | BDBM50247151 (7-(((3R,4S)-3-hydroxy-4-(methylthiomethyl)pyrrolid...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Albert Einstein College of Medicine of Yeshiva University Curated by ChEMBL | Assay Description Initial binding affinity to wild type human PNP | Bioorg Med Chem Lett 18: 5900-3 (2008) Article DOI: 10.1016/j.bmcl.2008.08.047 BindingDB Entry DOI: 10.7270/Q2SX6F4H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Platelet-activating factor acetylhydrolase (Homo sapiens (Human)) | BDBM50125265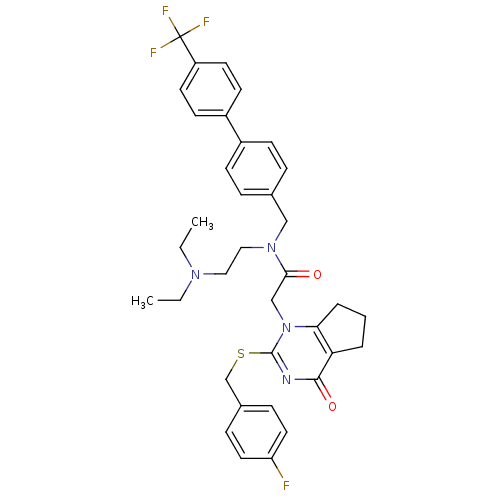 (CHEMBL204021 | N-(2-Diethylamino-ethyl)-2-[2-(4-fl...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Patents Similars | PDB PubMed | 0.110 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibitory activity against recombinant human Lp-PLA2 | Bioorg Med Chem Lett 13: 1067-70 (2003) BindingDB Entry DOI: 10.7270/Q2S75FPD | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Purine nucleoside phosphorylase (Homo sapiens (Human)) | BDBM50246593 (7-((1,3-dihydroxypropan-2-ylamino)methyl)-3H-pyrro...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Similars | PDB Article PubMed | 0.110 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Albert Einstein College of Medicine of Yeshiva University Curated by ChEMBL | Assay Description Initial binding affinity to wild type human PNP | Bioorg Med Chem Lett 18: 5900-3 (2008) Article DOI: 10.1016/j.bmcl.2008.08.047 BindingDB Entry DOI: 10.7270/Q2SX6F4H | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Purine nucleoside phosphorylase (Homo sapiens (Human)) | BDBM50246590 (7-(((3R,4S)-3-hydroxy-4-(propylthiomethyl)pyrrolid...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.117 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Albert Einstein College of Medicine of Yeshiva University Curated by ChEMBL | Assay Description Initial binding affinity to wild type human PNP | Bioorg Med Chem Lett 18: 5900-3 (2008) Article DOI: 10.1016/j.bmcl.2008.08.047 BindingDB Entry DOI: 10.7270/Q2SX6F4H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Apelin receptor (Rattus norvegicus) | BDBM50257220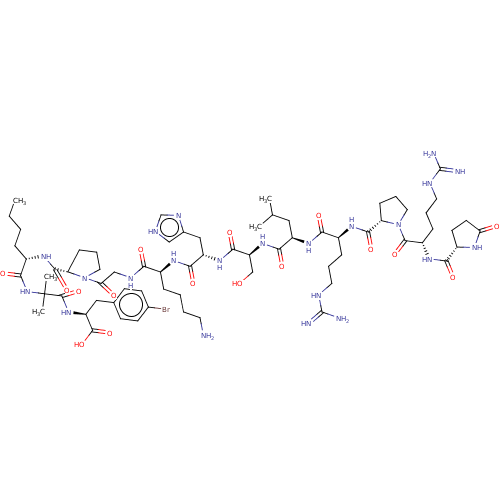 (CHEMBL4075830) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | KEGG PC cid PC sid UniChem | Article PubMed | 0.120 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Department of Chemistry, University of Alberta , 11227 Saskatchewan Drive NW, Edmonton, Alberta T6G 2G2, Canada. Curated by ChEMBL | Assay Description Displacement of [125I]-pyr-1-apelin-13 from rat C-terminal EGFP-tagged APJ receptor expressed in CHO cell membranes after 3 hrs by Wallac gamma count... | J Med Chem 60: 6408-6427 (2017) Article DOI: 10.1021/acs.jmedchem.7b00723 BindingDB Entry DOI: 10.7270/Q2WS8WPC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proprotein convertase subtilisin/kexin type 9 (Homo sapiens (Human)) | BDBM50581548 (CHEMBL5085124) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | >0.129 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human PCSK9 using Alexa Fluor 674 as substrate incubated for 2 hrs by FRET assay | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c01599 BindingDB Entry DOI: 10.7270/Q2TM7G0Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proprotein convertase subtilisin/kexin type 9 (Homo sapiens (Human)) | BDBM50581546 (CHEMBL5084416) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | >0.129 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human PCSK9 using Alexa Fluor 674 as substrate incubated for 2 hrs by FRET assay | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c01599 BindingDB Entry DOI: 10.7270/Q2TM7G0Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proprotein convertase subtilisin/kexin type 9 (Homo sapiens (Human)) | BDBM50581547 (CHEMBL5081349) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | >0.129 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human PCSK9 using Alexa Fluor 674 as substrate incubated for 2 hrs by FRET assay | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c01599 BindingDB Entry DOI: 10.7270/Q2TM7G0Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proprotein convertase subtilisin/kexin type 9 (Homo sapiens (Human)) | BDBM50581544 (CHEMBL5086475) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | >0.129 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human PCSK9 using Alexa Fluor 674 as substrate incubated for 2 hrs by FRET assay | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c01599 BindingDB Entry DOI: 10.7270/Q2TM7G0Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 11238 total ) | Next | Last >> |