

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Neutrophil elastase (Homo sapiens (Human)) | BDBM50095523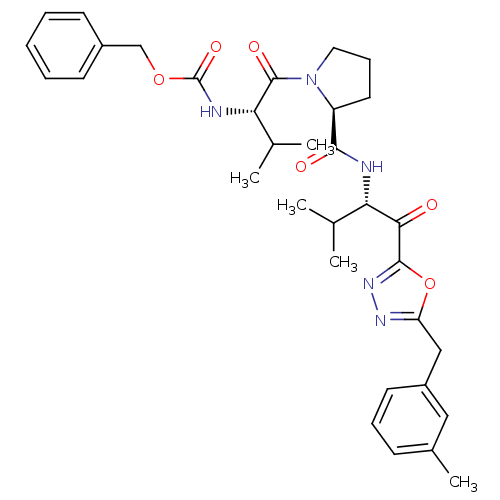 (CHEMBL285231 | [(S)-2-methyl-1-((S)-2-{(S)-2-methy...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | PubMed | 0.0250 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Cortech, Inc. Curated by ChEMBL | Assay Description Binding constant derived from inhibition of elastase catalyzed hydrolysis of synthetic substrate | J Med Chem 44: 1268-85 (2001) BindingDB Entry DOI: 10.7270/Q2B858VW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM50095523 (CHEMBL285231 | [(S)-2-methyl-1-((S)-2-{(S)-2-methy...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | PubMed | 0.0250 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Colorado State University Curated by ChEMBL | Assay Description Inhibition of human neutrophil elastase HNE, hydrolysis of MeO-Suc-Ala-Ala-Pro-Val-pNa | J Med Chem 43: 4927-9 (2001) BindingDB Entry DOI: 10.7270/Q25D8R3S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM50095526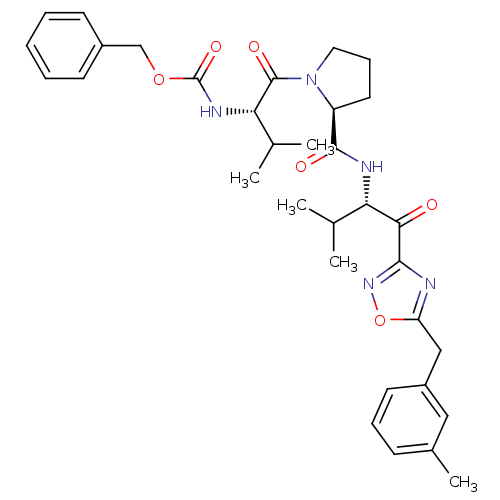 (CHEMBL24058 | [(S)-2-methyl-1-((S)-2-{(S)-2-methyl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | PubMed | 0.490 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Colorado State University Curated by ChEMBL | Assay Description Inhibition of human neutrophil elastase HNE, hydrolysis of MeO-Suc-Ala-Ala-Pro-Val-pNa | J Med Chem 43: 4927-9 (2001) BindingDB Entry DOI: 10.7270/Q25D8R3S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM50095526 (CHEMBL24058 | [(S)-2-methyl-1-((S)-2-{(S)-2-methyl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | PubMed | 0.490 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Cortech, Inc. Curated by ChEMBL | Assay Description Binding constant derived from inhibition of elastase catalyzed hydrolysis of synthetic substrate | J Med Chem 44: 1268-85 (2001) BindingDB Entry DOI: 10.7270/Q2B858VW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM50095530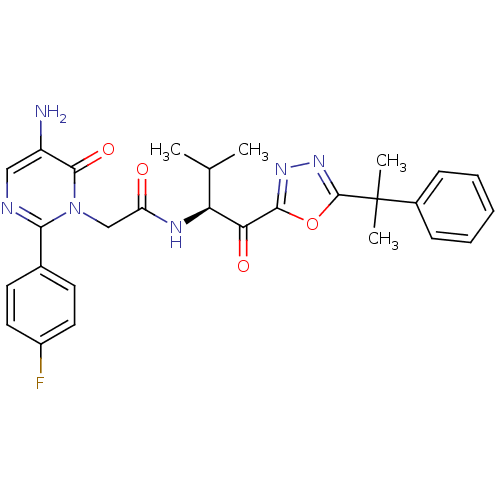 ((S)-2-(5-amino-2-(4-fluorophenyl)-6-oxopyrimidin-1...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 0.520 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Cortech, Inc. Curated by ChEMBL | Assay Description Binding constant derived from inhibition of elastase catalyzed hydrolysis of synthetic substrate | J Med Chem 44: 1268-85 (2001) BindingDB Entry DOI: 10.7270/Q2B858VW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM50095530 ((S)-2-(5-amino-2-(4-fluorophenyl)-6-oxopyrimidin-1...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 0.520 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Colorado State University Curated by ChEMBL | Assay Description Inhibition of human neutrophil elastase HNE, hydrolysis of MeO-Suc-Ala-Ala-Pro-Val-pNa | J Med Chem 43: 4927-9 (2001) BindingDB Entry DOI: 10.7270/Q25D8R3S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM50095521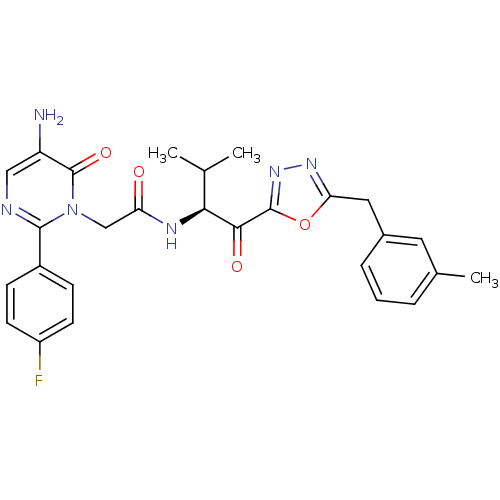 ((S)-2-[5-Amino-2-(4-fluoro-phenyl)-6-oxo-6H-pyrimi...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 0.640 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Colorado State University Curated by ChEMBL | Assay Description Inhibition of human neutrophil elastase HNE, hydrolysis of MeO-Suc-Ala-Ala-Pro-Val-pNa | J Med Chem 43: 4927-9 (2001) BindingDB Entry DOI: 10.7270/Q25D8R3S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM50095521 ((S)-2-[5-Amino-2-(4-fluoro-phenyl)-6-oxo-6H-pyrimi...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 0.640 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Cortech, Inc. Curated by ChEMBL | Assay Description Binding constant derived from inhibition of elastase catalyzed hydrolysis of synthetic substrate | J Med Chem 44: 1268-85 (2001) BindingDB Entry DOI: 10.7270/Q2B858VW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM50095519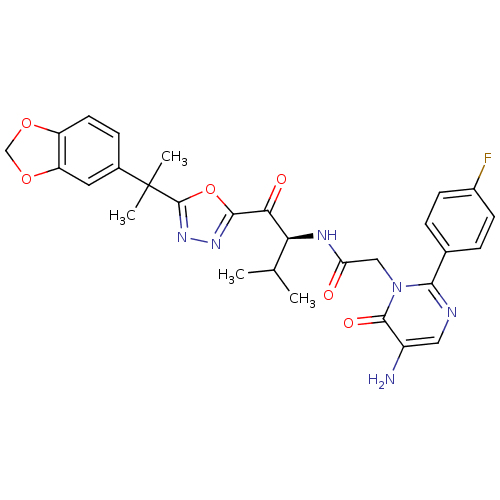 ((S)-2-[5-Amino-2-(4-fluoro-phenyl)-6-oxo-6H-pyrimi...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 1.18 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Cortech, Inc. Curated by ChEMBL | Assay Description Binding constant derived from inhibition of elastase catalyzed hydrolysis of synthetic substrate | J Med Chem 44: 1268-85 (2001) BindingDB Entry DOI: 10.7270/Q2B858VW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM50095519 ((S)-2-[5-Amino-2-(4-fluoro-phenyl)-6-oxo-6H-pyrimi...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 1.18 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Colorado State University Curated by ChEMBL | Assay Description Inhibition of human neutrophil elastase HNE, hydrolysis of MeO-Suc-Ala-Ala-Pro-Val-pNa | J Med Chem 43: 4927-9 (2001) BindingDB Entry DOI: 10.7270/Q25D8R3S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM50095525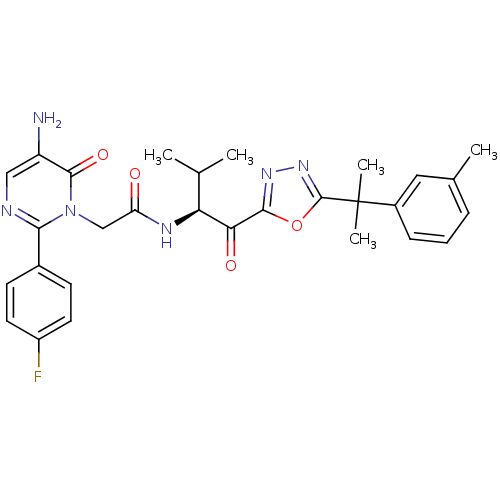 ((S)-2-(5-amino-2-(4-fluorophenyl)-6-oxopyrimidin-1...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 1.37 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Colorado State University Curated by ChEMBL | Assay Description Inhibition of human neutrophil elastase HNE, hydrolysis of MeO-Suc-Ala-Ala-Pro-Val-pNa | J Med Chem 43: 4927-9 (2001) BindingDB Entry DOI: 10.7270/Q25D8R3S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM50095525 ((S)-2-(5-amino-2-(4-fluorophenyl)-6-oxopyrimidin-1...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 1.37 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Cortech, Inc. Curated by ChEMBL | Assay Description Binding constant derived from inhibition of elastase catalyzed hydrolysis of synthetic substrate | J Med Chem 44: 1268-85 (2001) BindingDB Entry DOI: 10.7270/Q2B858VW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM50098818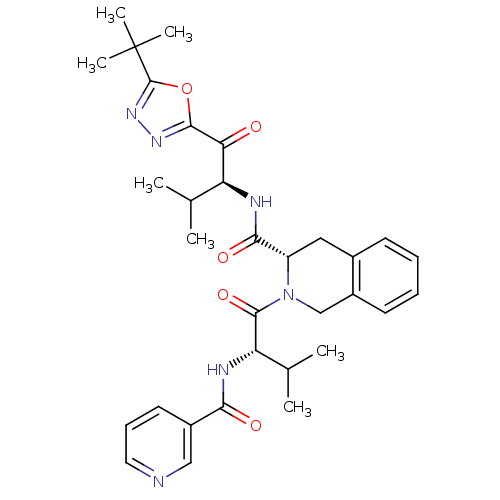 (2-{3-Methyl-2-[(pyridine-3-carbonyl)-amino]-butyry...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 1.66 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Cortech, Inc. Curated by ChEMBL | Assay Description Binding constant derived from inhibition of elastase catalyzed hydrolysis of synthetic substrate | J Med Chem 44: 1268-85 (2001) BindingDB Entry DOI: 10.7270/Q2B858VW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM50098833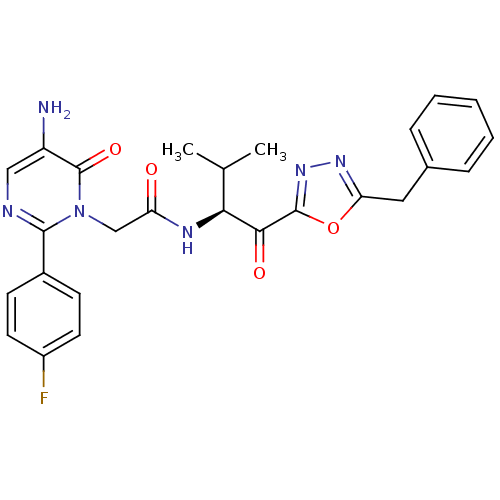 (2-[5-Amino-2-(4-fluoro-phenyl)-6-oxo-6H-pyrimidin-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 2.25 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Cortech, Inc. Curated by ChEMBL | Assay Description Binding constant derived from inhibition of elastase catalyzed hydrolysis of synthetic substrate | J Med Chem 44: 1268-85 (2001) BindingDB Entry DOI: 10.7270/Q2B858VW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM50098827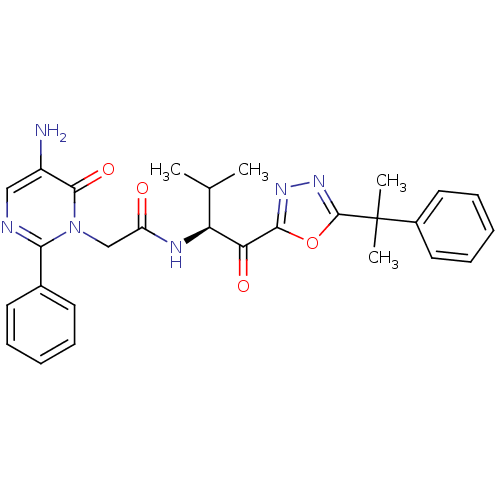 (2-(5-Amino-6-oxo-2-phenyl-6H-pyrimidin-1-yl)-N-{2-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 2.55 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Cortech, Inc. Curated by ChEMBL | Assay Description Binding constant derived from inhibition of elastase catalyzed hydrolysis of synthetic substrate | J Med Chem 44: 1268-85 (2001) BindingDB Entry DOI: 10.7270/Q2B858VW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM50098817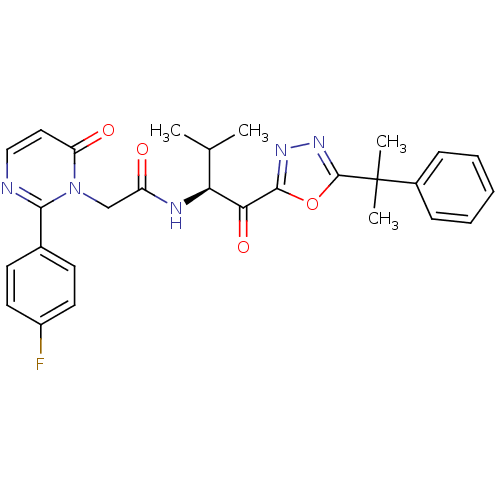 (2-[2-(4-Fluoro-phenyl)-6-oxo-6H-pyrimidin-1-yl]-N-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 3.35 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Cortech, Inc. Curated by ChEMBL | Assay Description Binding constant derived from inhibition of elastase catalyzed hydrolysis of synthetic substrate | J Med Chem 44: 1268-85 (2001) BindingDB Entry DOI: 10.7270/Q2B858VW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM50095527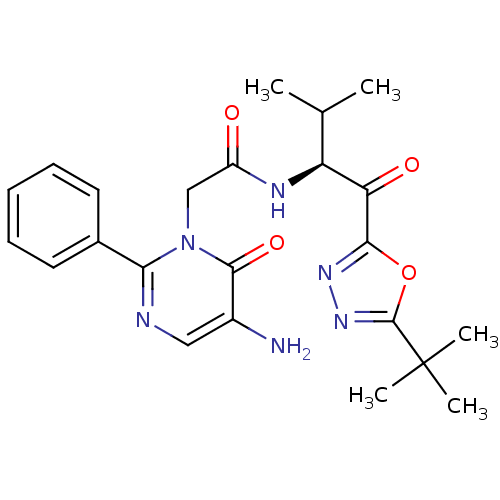 ((S)-2-(5-Amino-6-oxo-2-phenyl-6H-pyrimidin-1-yl)-N...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | PubMed | 3.59 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Cortech, Inc. Curated by ChEMBL | Assay Description Binding constant derived from inhibition of elastase catalyzed hydrolysis of synthetic substrate | J Med Chem 44: 1268-85 (2001) BindingDB Entry DOI: 10.7270/Q2B858VW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM50095527 ((S)-2-(5-Amino-6-oxo-2-phenyl-6H-pyrimidin-1-yl)-N...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | PubMed | 3.59 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Colorado State University Curated by ChEMBL | Assay Description Inhibition of human neutrophil elastase HNE, hydrolysis of MeO-Suc-Ala-Ala-Pro-Val-pNa | J Med Chem 43: 4927-9 (2001) BindingDB Entry DOI: 10.7270/Q25D8R3S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM50098830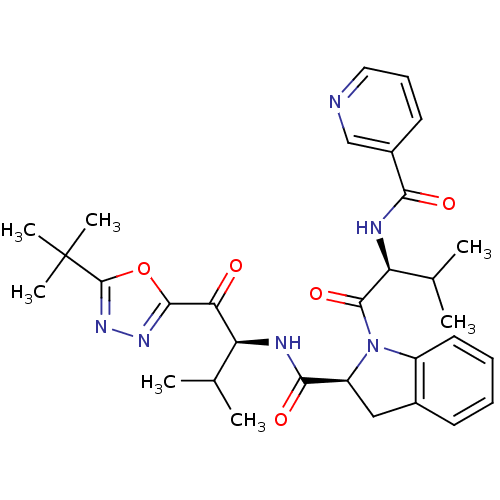 (1-{3-Methyl-2-[(pyridine-3-carbonyl)-amino]-butyry...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 5.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Cortech, Inc. Curated by ChEMBL | Assay Description Binding constant derived from inhibition of elastase catalyzed hydrolysis of synthetic substrate | J Med Chem 44: 1268-85 (2001) BindingDB Entry DOI: 10.7270/Q2B858VW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM50095520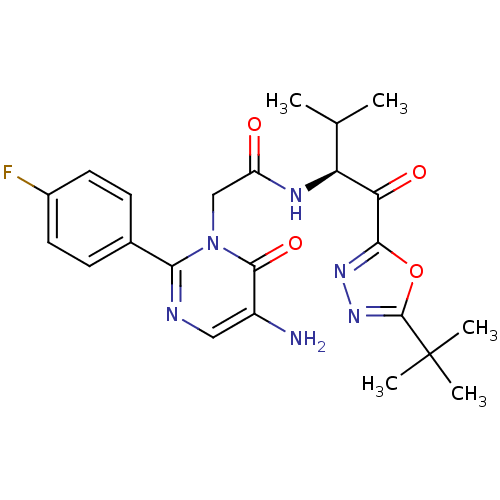 ((S)-2-(5-amino-2-(4-fluorophenyl)-6-oxopyrimidin-1...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 6.38 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Colorado State University Curated by ChEMBL | Assay Description Inhibition of human neutrophil elastase HNE, hydrolysis of MeO-Suc-Ala-Ala-Pro-Val-pNa | J Med Chem 43: 4927-9 (2001) BindingDB Entry DOI: 10.7270/Q25D8R3S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM50095520 ((S)-2-(5-amino-2-(4-fluorophenyl)-6-oxopyrimidin-1...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 6.38 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Cortech, Inc. Curated by ChEMBL | Assay Description Binding constant derived from inhibition of elastase catalyzed hydrolysis of synthetic substrate | J Med Chem 44: 1268-85 (2001) BindingDB Entry DOI: 10.7270/Q2B858VW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM50098823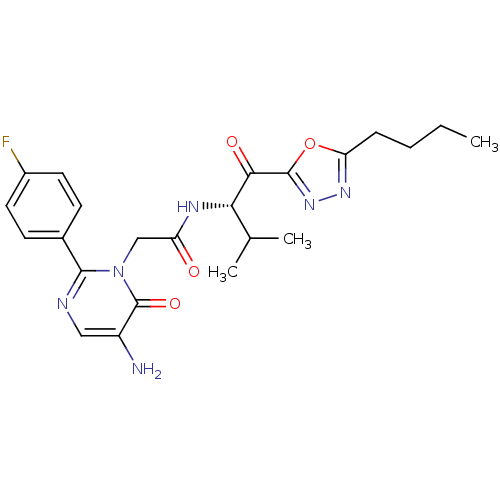 (2-[5-Amino-2-(4-fluoro-phenyl)-6-oxo-6H-pyrimidin-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 6.76 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Cortech, Inc. Curated by ChEMBL | Assay Description Binding constant derived from inhibition of elastase catalyzed hydrolysis of synthetic substrate | J Med Chem 44: 1268-85 (2001) BindingDB Entry DOI: 10.7270/Q2B858VW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM50098826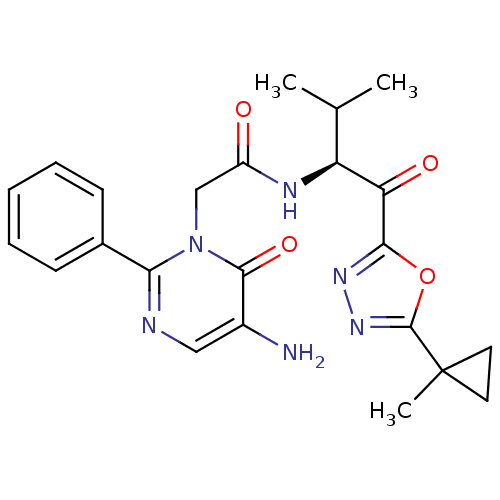 (2-(5-Amino-6-oxo-2-phenyl-6H-pyrimidin-1-yl)-N-{2-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 8.75 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Cortech, Inc. Curated by ChEMBL | Assay Description Binding constant derived from inhibition of elastase catalyzed hydrolysis of synthetic substrate | J Med Chem 44: 1268-85 (2001) BindingDB Entry DOI: 10.7270/Q2B858VW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM50098821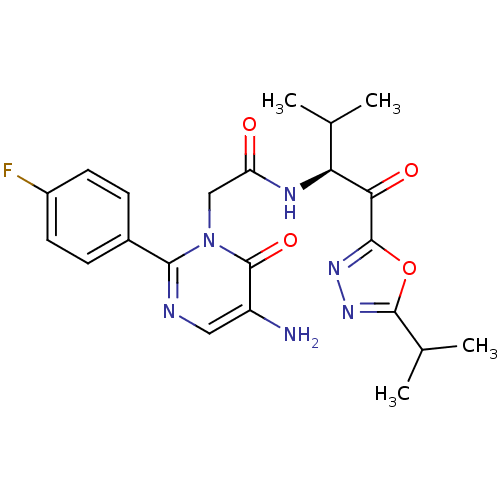 (2-[5-Amino-2-(4-fluoro-phenyl)-6-oxo-6H-pyrimidin-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 10.7 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Cortech, Inc. Curated by ChEMBL | Assay Description Binding constant derived from inhibition of elastase catalyzed hydrolysis of synthetic substrate | J Med Chem 44: 1268-85 (2001) BindingDB Entry DOI: 10.7270/Q2B858VW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM50095529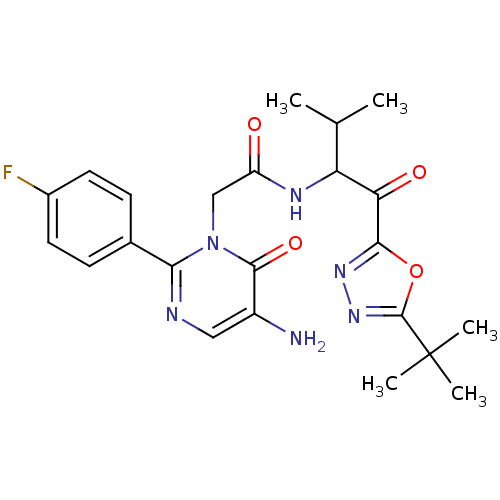 (2-[5-Amino-2-(4-fluoro-phenyl)-6-oxo-6H-pyrimidin-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 11.8 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Colorado State University Curated by ChEMBL | Assay Description Inhibition of human neutrophil elastase HNE, hydrolysis of MeO-Suc-Ala-Ala-Pro-Val-pNa | J Med Chem 43: 4927-9 (2001) BindingDB Entry DOI: 10.7270/Q25D8R3S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM50095529 (2-[5-Amino-2-(4-fluoro-phenyl)-6-oxo-6H-pyrimidin-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 11.8 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Cortech, Inc. Curated by ChEMBL | Assay Description Binding constant derived from inhibition of elastase catalyzed hydrolysis of synthetic substrate | J Med Chem 44: 1268-85 (2001) BindingDB Entry DOI: 10.7270/Q2B858VW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM50098815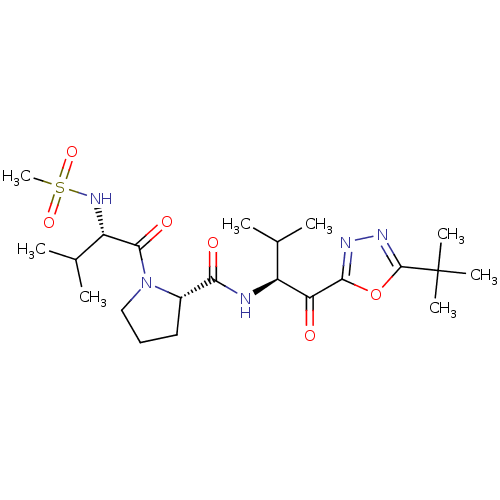 (1-(2-Methanesulfonylamino-3-methyl-butyryl)-pyrrol...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | PubMed | 12 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Cortech, Inc. Curated by ChEMBL | Assay Description Binding constant derived from inhibition of elastase catalyzed hydrolysis of synthetic substrate | J Med Chem 44: 1268-85 (2001) BindingDB Entry DOI: 10.7270/Q2B858VW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM50095522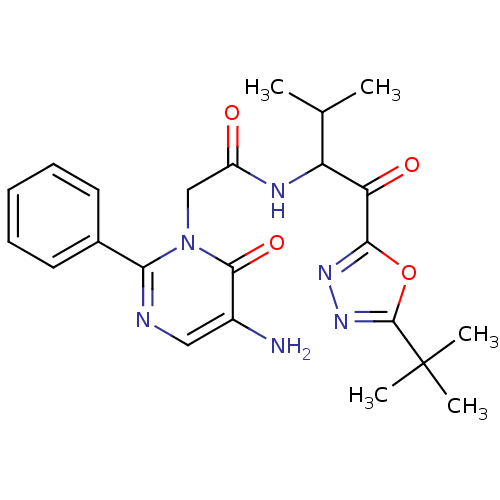 (2-(5-Amino-6-oxo-2-phenyl-6H-pyrimidin-1-yl)-N-[1-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | PubMed | 12.2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Colorado State University Curated by ChEMBL | Assay Description Inhibition of human neutrophil elastase HNE, hydrolysis of MeO-Suc-Ala-Ala-Pro-Val-pNa | J Med Chem 43: 4927-9 (2001) BindingDB Entry DOI: 10.7270/Q25D8R3S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM50095522 (2-(5-Amino-6-oxo-2-phenyl-6H-pyrimidin-1-yl)-N-[1-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | PubMed | 12.2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Cortech, Inc. Curated by ChEMBL | Assay Description Binding constant derived from inhibition of elastase catalyzed hydrolysis of synthetic substrate | J Med Chem 44: 1268-85 (2001) BindingDB Entry DOI: 10.7270/Q2B858VW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM50098824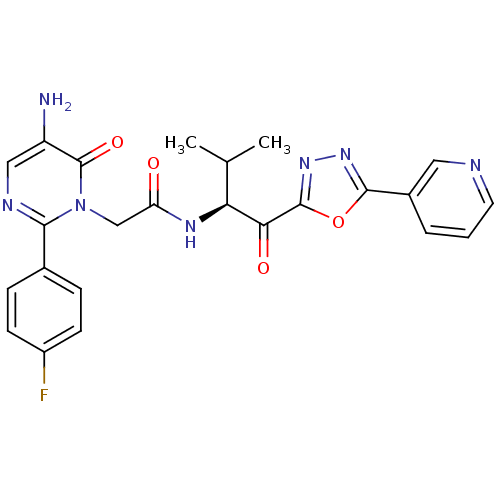 (2-[5-Amino-2-(4-fluoro-phenyl)-6-oxo-6H-pyrimidin-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 13.9 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Cortech, Inc. Curated by ChEMBL | Assay Description Binding constant derived from inhibition of elastase catalyzed hydrolysis of synthetic substrate | J Med Chem 44: 1268-85 (2001) BindingDB Entry DOI: 10.7270/Q2B858VW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM50098819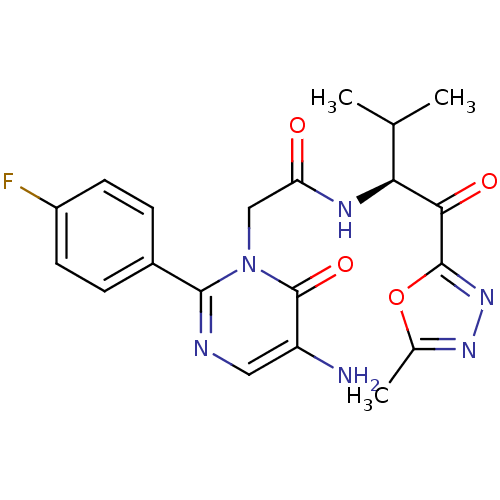 (2-[5-Amino-2-(4-fluoro-phenyl)-6-oxo-6H-pyrimidin-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 14 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Cortech, Inc. Curated by ChEMBL | Assay Description Binding constant derived from inhibition of elastase catalyzed hydrolysis of synthetic substrate | J Med Chem 44: 1268-85 (2001) BindingDB Entry DOI: 10.7270/Q2B858VW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM50095524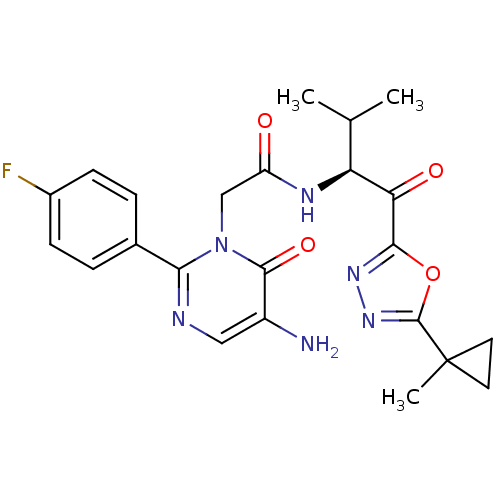 ((S)-2-[5-Amino-2-(4-fluoro-phenyl)-6-oxo-6H-pyrimi...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 15.3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Cortech, Inc. Curated by ChEMBL | Assay Description Binding constant derived from inhibition of elastase catalyzed hydrolysis of synthetic substrate | J Med Chem 44: 1268-85 (2001) BindingDB Entry DOI: 10.7270/Q2B858VW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM50095524 ((S)-2-[5-Amino-2-(4-fluoro-phenyl)-6-oxo-6H-pyrimi...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 15.3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Colorado State University Curated by ChEMBL | Assay Description Inhibition of human neutrophil elastase HNE, hydrolysis of MeO-Suc-Ala-Ala-Pro-Val-pNa | J Med Chem 43: 4927-9 (2001) BindingDB Entry DOI: 10.7270/Q25D8R3S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM50098816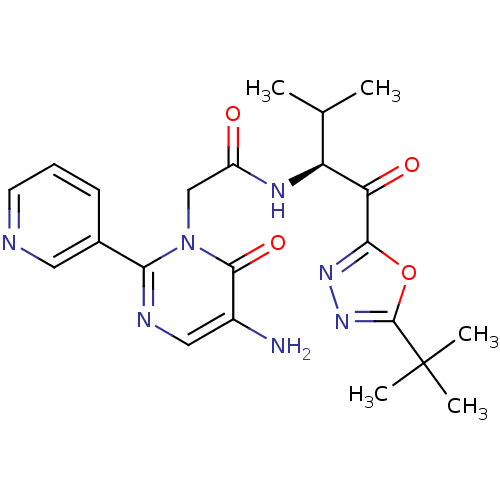 (2-(5-Amino-6-oxo-2-pyridin-3-yl-6H-pyrimidin-1-yl)...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 16.6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Cortech, Inc. Curated by ChEMBL | Assay Description Binding constant derived from inhibition of elastase catalyzed hydrolysis of synthetic substrate | J Med Chem 44: 1268-85 (2001) BindingDB Entry DOI: 10.7270/Q2B858VW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM50098828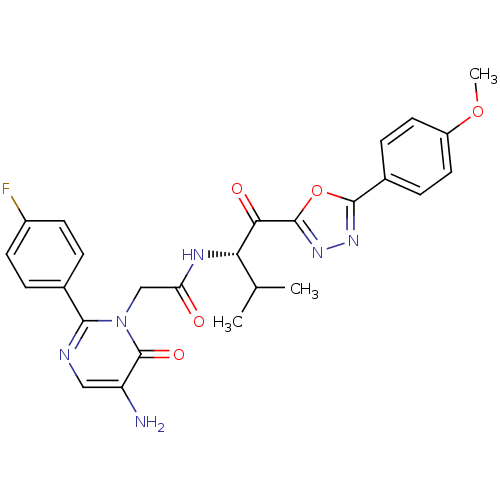 (2-[5-Amino-2-(4-fluoro-phenyl)-6-oxo-6H-pyrimidin-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 21.2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Cortech, Inc. Curated by ChEMBL | Assay Description Binding constant derived from inhibition of elastase catalyzed hydrolysis of synthetic substrate | J Med Chem 44: 1268-85 (2001) BindingDB Entry DOI: 10.7270/Q2B858VW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM50098832 (CHEMBL282947 | N-[1-(5-tert-Butyl-[1,3,4]oxadiazol...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 23.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Cortech, Inc. Curated by ChEMBL | Assay Description Binding constant derived from inhibition of elastase catalyzed hydrolysis of synthetic substrate | J Med Chem 44: 1268-85 (2001) BindingDB Entry DOI: 10.7270/Q2B858VW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM50098822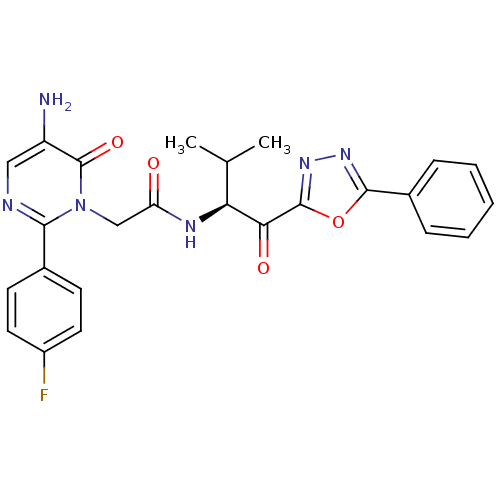 (2-[5-Amino-2-(4-fluoro-phenyl)-6-oxo-6H-pyrimidin-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 24.8 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Cortech, Inc. Curated by ChEMBL | Assay Description Binding constant derived from inhibition of elastase catalyzed hydrolysis of synthetic substrate | J Med Chem 44: 1268-85 (2001) BindingDB Entry DOI: 10.7270/Q2B858VW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM50098814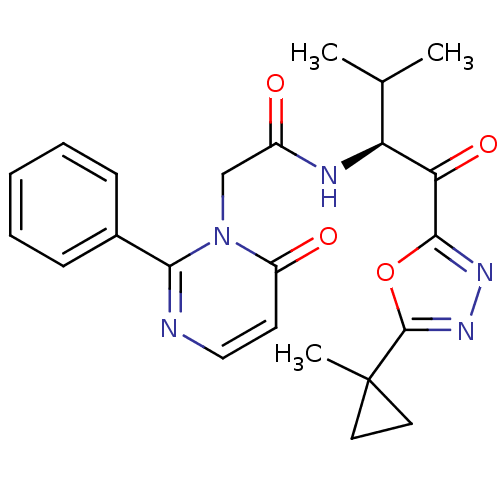 (CHEMBL277194 | N-{2-Methyl-1-[5-(1-methyl-cyclopro...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 26.4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Cortech, Inc. Curated by ChEMBL | Assay Description Binding constant derived from inhibition of elastase catalyzed hydrolysis of synthetic substrate | J Med Chem 44: 1268-85 (2001) BindingDB Entry DOI: 10.7270/Q2B858VW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM50095528 (2-(5-Amino-6-oxo-2-phenyl-6H-pyrimidin-1-yl)-N-[1-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | PubMed | 29.1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Colorado State University Curated by ChEMBL | Assay Description Inhibition of human neutrophil elastase HNE, hydrolysis of MeO-Suc-Ala-Ala-Pro-Val-pNa | J Med Chem 43: 4927-9 (2001) BindingDB Entry DOI: 10.7270/Q25D8R3S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM50098812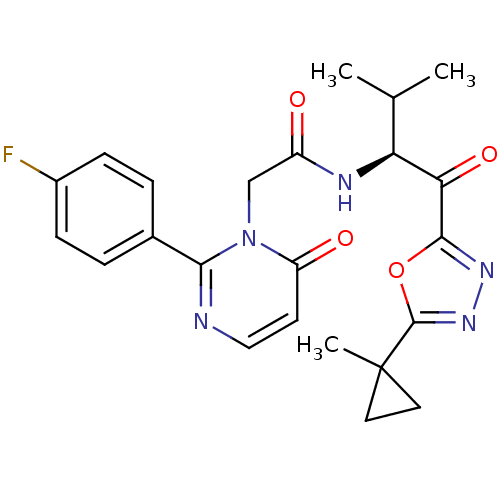 (2-[2-(4-Fluoro-phenyl)-6-oxo-6H-pyrimidin-1-yl]-N-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 43.8 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Cortech, Inc. Curated by ChEMBL | Assay Description Binding constant derived from inhibition of elastase catalyzed hydrolysis of synthetic substrate | J Med Chem 44: 1268-85 (2001) BindingDB Entry DOI: 10.7270/Q2B858VW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM50095518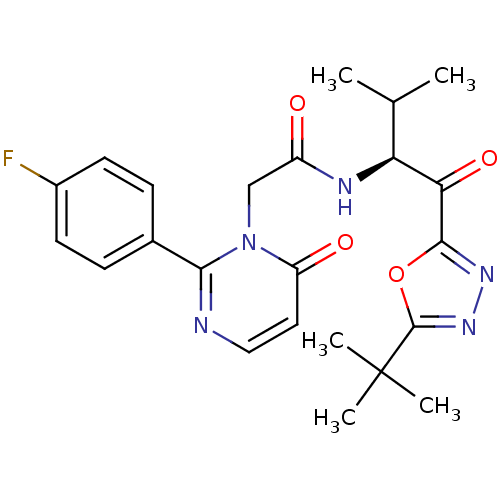 ((S)-N-[1-(5-tert-Butyl-[1,3,4]oxadiazole-2-carbony...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 44.3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Cortech, Inc. Curated by ChEMBL | Assay Description Binding constant derived from inhibition of elastase catalyzed hydrolysis of synthetic substrate | J Med Chem 44: 1268-85 (2001) BindingDB Entry DOI: 10.7270/Q2B858VW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM50095518 ((S)-N-[1-(5-tert-Butyl-[1,3,4]oxadiazole-2-carbony...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 44.3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Colorado State University Curated by ChEMBL | Assay Description Inhibition of human neutrophil elastase HNE, hydrolysis of MeO-Suc-Ala-Ala-Pro-Val-pNa | J Med Chem 43: 4927-9 (2001) BindingDB Entry DOI: 10.7270/Q25D8R3S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM50098831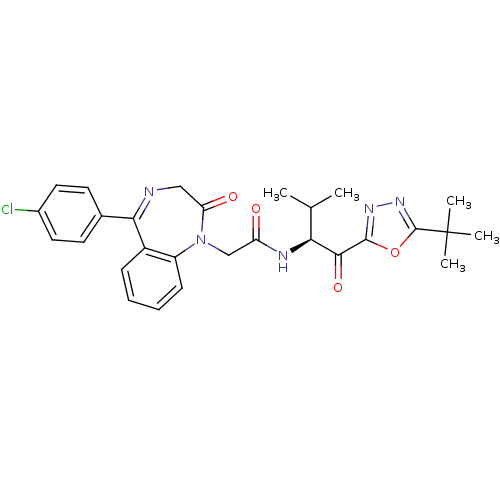 (CHEMBL23952 | N-[1-(5-tert-Butyl-[1,3,4]oxadiazole...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | PubMed | 57.8 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Cortech, Inc. Curated by ChEMBL | Assay Description Binding constant derived from inhibition of elastase catalyzed hydrolysis of synthetic substrate | J Med Chem 44: 1268-85 (2001) BindingDB Entry DOI: 10.7270/Q2B858VW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM50098834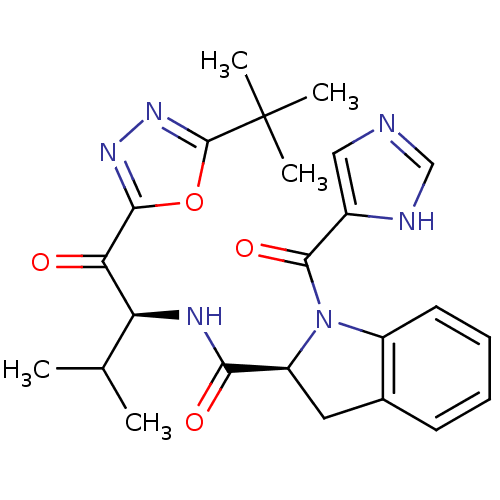 (1-(3H-Imidazole-4-carbonyl)-2,3-dihydro-1H-indole-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 79.7 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Cortech, Inc. Curated by ChEMBL | Assay Description Binding constant derived from inhibition of elastase catalyzed hydrolysis of synthetic substrate | J Med Chem 44: 1268-85 (2001) BindingDB Entry DOI: 10.7270/Q2B858VW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E2 receptor EP1 subtype (Homo sapiens (Human)) | BDBM50138045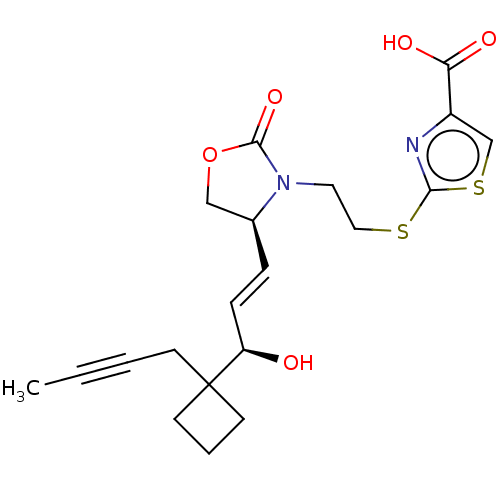 (CHEMBL3753268) | KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 89 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ono Pharmaceutical Co., Ltd Curated by ChEMBL | Assay Description Binding affinity to human EP1 receptor | Bioorg Med Chem Lett 26: 1016-9 (2016) Article DOI: 10.1016/j.bmcl.2015.12.039 BindingDB Entry DOI: 10.7270/Q2JS9S81 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM50036093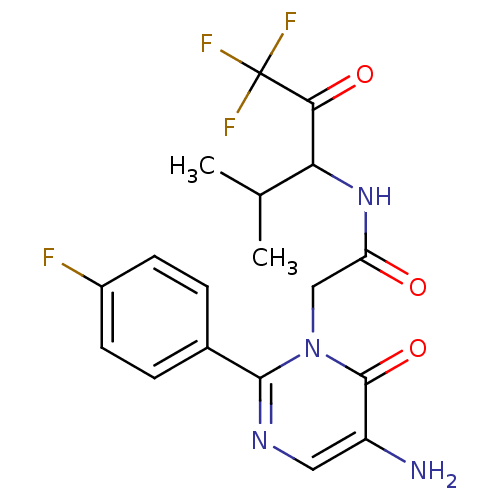 (2-[5-Amino-2-(4-fluoro-phenyl)-6-oxo-6H-pyrimidin-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 101 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Cortech, Inc. Curated by ChEMBL | Assay Description Binding constant derived from inhibition of elastase catalyzed hydrolysis of synthetic substrate | J Med Chem 44: 1268-85 (2001) BindingDB Entry DOI: 10.7270/Q2B858VW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM50036093 (2-[5-Amino-2-(4-fluoro-phenyl)-6-oxo-6H-pyrimidin-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 101 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Colorado State University Curated by ChEMBL | Assay Description Inhibition of human neutrophil elastase HNE, hydrolysis of MeO-Suc-Ala-Ala-Pro-Val-pNa | J Med Chem 43: 4927-9 (2001) BindingDB Entry DOI: 10.7270/Q25D8R3S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM50098820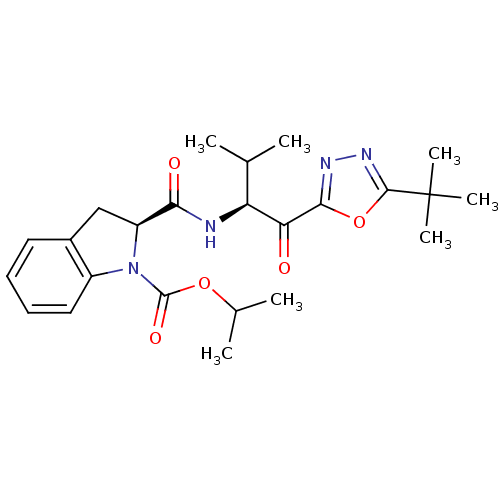 (2-[1-(5-tert-Butyl-[1,3,4]oxadiazole-2-carbonyl)-2...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | PubMed | 114 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Cortech, Inc. Curated by ChEMBL | Assay Description Binding constant derived from inhibition of elastase catalyzed hydrolysis of synthetic substrate | J Med Chem 44: 1268-85 (2001) BindingDB Entry DOI: 10.7270/Q2B858VW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E2 receptor EP1 subtype (Homo sapiens (Human)) | BDBM50138047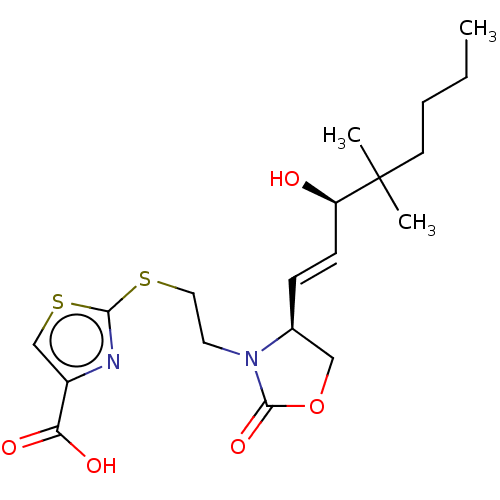 (CHEMBL3753853) | KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 120 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ono Pharmaceutical Co., Ltd Curated by ChEMBL | Assay Description Binding affinity to human EP1 receptor | Bioorg Med Chem Lett 26: 1016-9 (2016) Article DOI: 10.1016/j.bmcl.2015.12.039 BindingDB Entry DOI: 10.7270/Q2JS9S81 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E2 receptor EP1 subtype (Homo sapiens (Human)) | BDBM50138046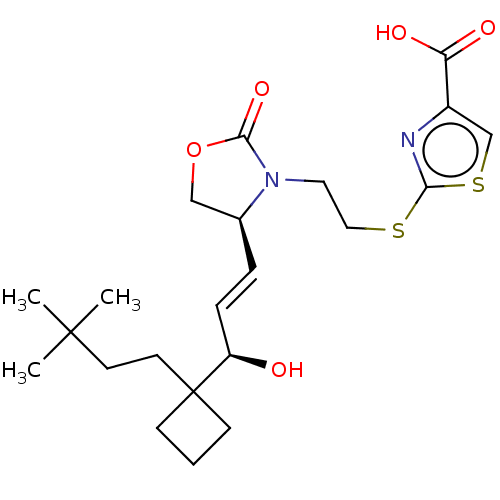 (CHEMBL3752435) | KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 149 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ono Pharmaceutical Co., Ltd Curated by ChEMBL | Assay Description Binding affinity to human EP1 receptor | Bioorg Med Chem Lett 26: 1016-9 (2016) Article DOI: 10.1016/j.bmcl.2015.12.039 BindingDB Entry DOI: 10.7270/Q2JS9S81 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 230 total ) | Next | Last >> |