

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Type-1 angiotensin II receptor (RABBIT) | BDBM50041969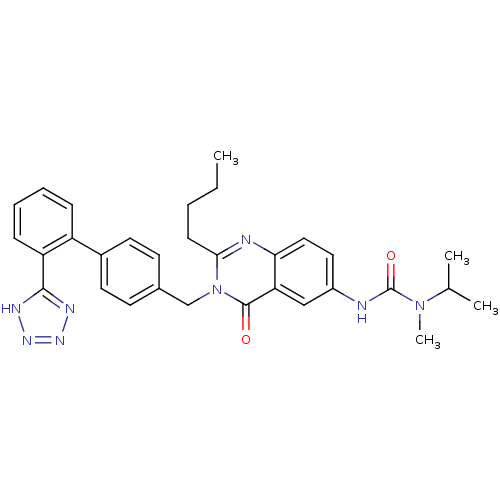 (3-{2-Butyl-4-oxo-3-[2'-(1H-tetrazol-5-yl)-biphenyl...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Binding affinity against AT-1 receptor from rabbit aorta using [125I]-Sar1-Ile8-Ang II without BSA | J Med Chem 36: 3207-10 (1993) BindingDB Entry DOI: 10.7270/Q2QR4W6P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor (RABBIT) | BDBM50041969 (3-{2-Butyl-4-oxo-3-[2'-(1H-tetrazol-5-yl)-biphenyl...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Displacement of [125I]-Sar1-Ile8-AII (without BSA) from type 1 Angiotensin II receptor of rabbit aorta | Bioorg Med Chem Lett 3: 1299-1304 (1993) Article DOI: 10.1016/S0960-894X(00)80335-2 BindingDB Entry DOI: 10.7270/Q29P31KG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor (RABBIT) | BDBM50035445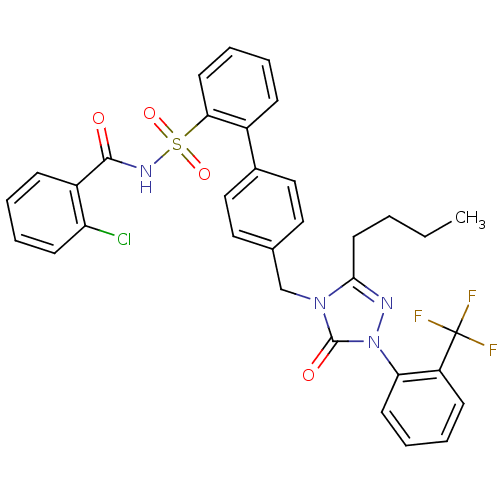 (4'-[3-Butyl-5-oxo-1-(2-trifluoromethyl-phenyl)-1,5...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.110 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description In vitro inhibitory activity against angiotensin II rabbit aorta AT1 receptor using radioligand [125I]-Sar Ile-AII | J Med Chem 37: 2808-24 (1994) BindingDB Entry DOI: 10.7270/Q2M907QM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor A/B (RAT) | BDBM50283305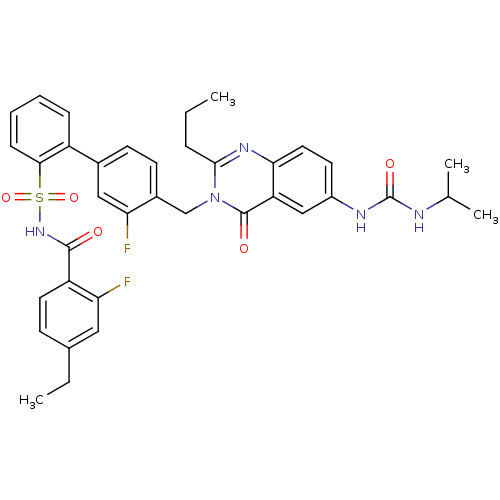 (3'-Fluoro-4'-[6-(3-isopropyl-ureido)-4-oxo-2-propy...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | 0.110 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description In vivo inhibitory concentration against Angiotensin II receptor, type 1 of rat adrenal membrane | Bioorg Med Chem Lett 4: 2337-2342 (1994) Article DOI: 10.1016/0960-894X(94)85036-4 BindingDB Entry DOI: 10.7270/Q2X066Z9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor (RABBIT) | BDBM50282470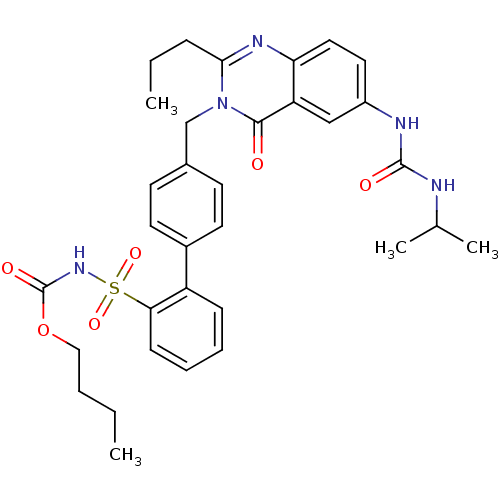 (CHEMBL353037 | Quinazolinone Analog) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | 0.120 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Tested in vitro for binding affinity against Angiotensin II receptor, type 1 in rabbit aortic membrane | Bioorg Med Chem Lett 4: 81-86 (1994) Article DOI: 10.1016/S0960-894X(01)81126-4 BindingDB Entry DOI: 10.7270/Q2D79BBS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor (RABBIT) | BDBM50283319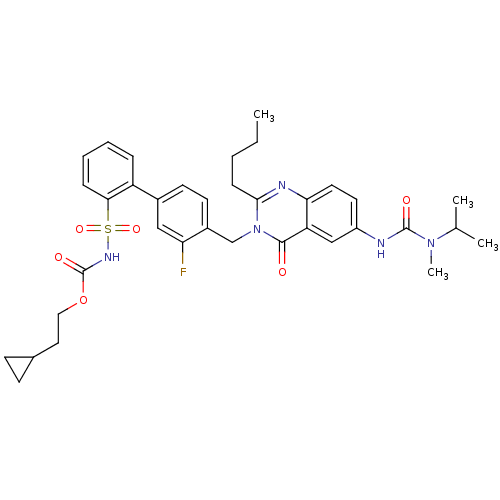 (2-Butyl-3-(3-fluoro-2'-(2-cyclopropylethyloxycarbo...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | 0.130 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description In vivo inhibitory concentration of the compound against Angiotensin II receptor, type 1 of rabbit aorta membrane | Bioorg Med Chem Lett 4: 2337-2342 (1994) Article DOI: 10.1016/0960-894X(94)85036-4 BindingDB Entry DOI: 10.7270/Q2X066Z9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor A/B (RAT) | BDBM50283317 (2-Propyl-3-(3-fluoro-2'-(3-methylbutyloxycarbonyla...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | 0.130 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description In vivo inhibitory concentration of the compound against Angiotensin II, type 2 of rat midbrain membrane | Bioorg Med Chem Lett 4: 2337-2342 (1994) Article DOI: 10.1016/0960-894X(94)85036-4 BindingDB Entry DOI: 10.7270/Q2X066Z9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor (RABBIT) | BDBM50039862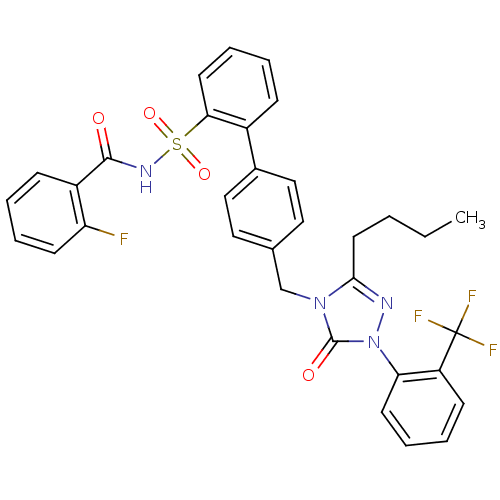 (4'-[3-Butyl-5-oxo-1-(2-trifluoromethyl-phenyl)-1,5...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 0.130 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description In vitro inhibitory activity against angiotensin II rabbit aorta AT1 receptor using radioligand [125I]-Sar Ile-AII | J Med Chem 37: 2808-24 (1994) BindingDB Entry DOI: 10.7270/Q2M907QM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor A/B (RAT) | BDBM50283300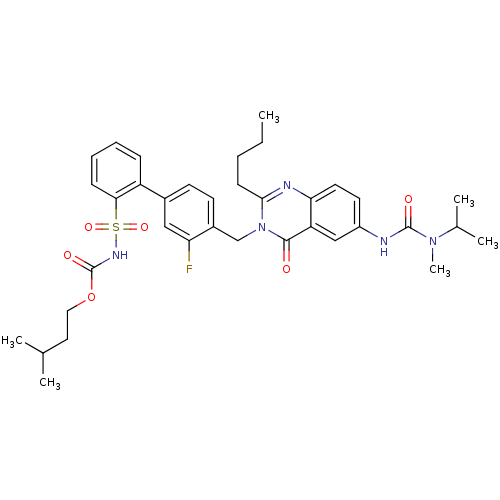 (2-Butyl-3-(3-fluoro-2'-(3-methylbutyloxycarbonylam...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | 0.140 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description In vivo inhibitory concentration of the compound against Angiotensin II, type 2 of rat midbrain membrane | Bioorg Med Chem Lett 4: 2337-2342 (1994) Article DOI: 10.1016/0960-894X(94)85036-4 BindingDB Entry DOI: 10.7270/Q2X066Z9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor A/B (RAT) | BDBM50283310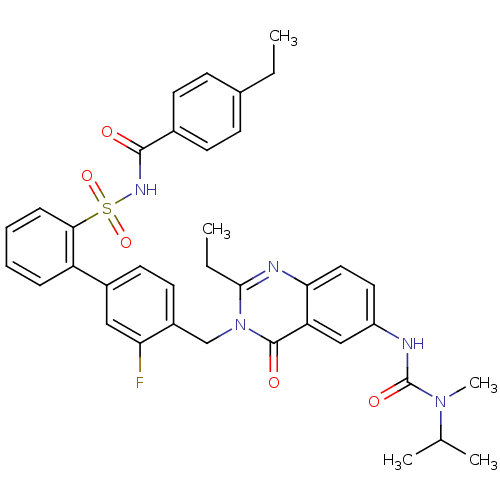 (4'-[2-Ethyl-6-(3-isopropyl-3-methyl-ureido)-4-oxo-...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | 0.140 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description In vivo inhibitory concentration of the compound against Angiotensin II, type 2 of rat midbrain membrane | Bioorg Med Chem Lett 4: 2337-2342 (1994) Article DOI: 10.1016/0960-894X(94)85036-4 BindingDB Entry DOI: 10.7270/Q2X066Z9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor A/B (RAT) | BDBM50283312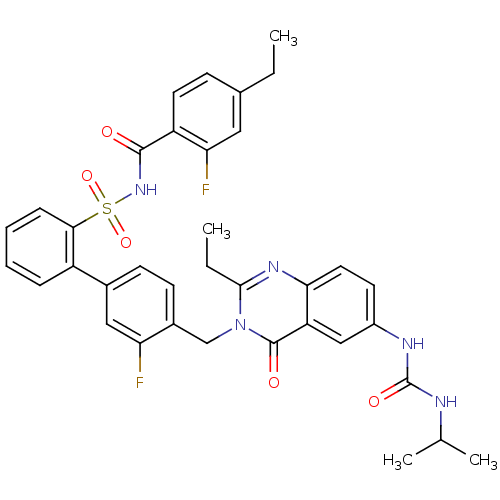 (4'-[2-Ethyl-6-(3-isopropyl-ureido)-4-oxo-4H-quinaz...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | 0.140 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description In vivo inhibitory concentration of the compound against AT2 receptor of rat adrenal membrane | Bioorg Med Chem Lett 4: 2337-2342 (1994) Article DOI: 10.1016/0960-894X(94)85036-4 BindingDB Entry DOI: 10.7270/Q2X066Z9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor (RABBIT) | BDBM50282468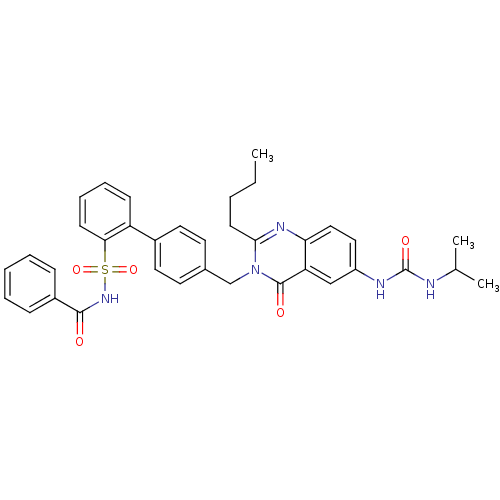 (4'-[2-Butyl-6-(3-isopropyl-ureido)-4-oxo-4H-quinaz...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | 0.150 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Tested in vitro for binding affinity against Angiotensin II receptor, type 1 in rabbit aortic membrane | Bioorg Med Chem Lett 4: 81-86 (1994) Article DOI: 10.1016/S0960-894X(01)81126-4 BindingDB Entry DOI: 10.7270/Q2D79BBS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor (RABBIT) | BDBM50039880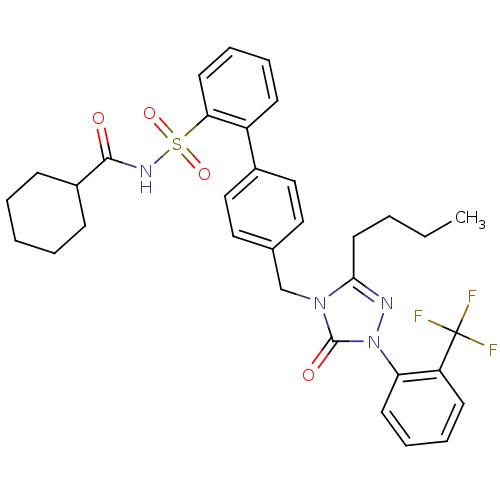 (4'-[3-Butyl-5-oxo-1-(2-trifluoromethyl-phenyl)-1,5...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.150 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description In vitro inhibitory activity against angiotensin II rabbit aorta AT1 receptor using radioligand [125I]-Sar Ile-AII | J Med Chem 37: 2808-24 (1994) BindingDB Entry DOI: 10.7270/Q2M907QM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor (RABBIT) | BDBM50282453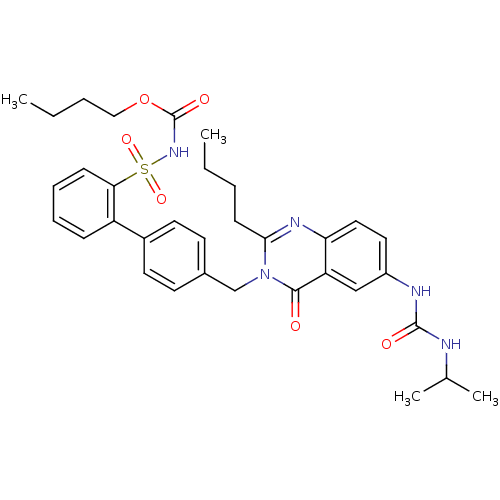 (CHEMBL353800 | Quinazolinone Analog) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | 0.150 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Tested in vitro for binding affinity against Angiotensin II receptor, type 1 in rabbit aortic membrane | Bioorg Med Chem Lett 4: 81-86 (1994) Article DOI: 10.1016/S0960-894X(01)81126-4 BindingDB Entry DOI: 10.7270/Q2D79BBS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor (RABBIT) | BDBM50039929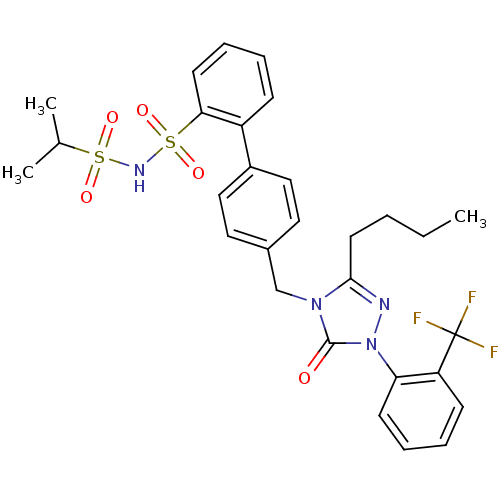 (2-{4-[3-butyl-5-oxo-1-(2-trifluoromethylphenyl)-4,...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 0.160 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description In vitro inhibitory activity against angiotensin II rabbit aorta AT1 receptor using radioligand [125I]-Sar Ile-AII | J Med Chem 37: 2808-24 (1994) BindingDB Entry DOI: 10.7270/Q2M907QM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor A/B (RAT) | BDBM50283316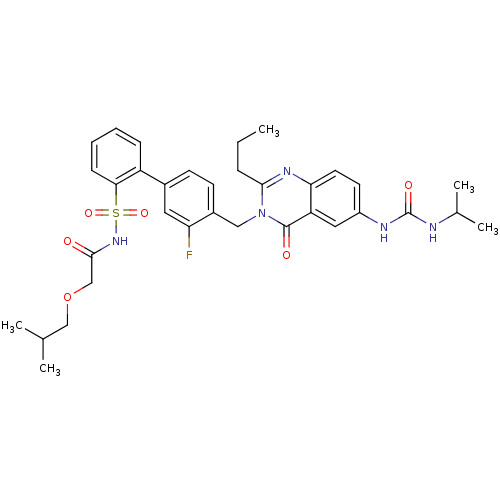 (3'-Fluoro-4'-[6-(3-isopropyl-ureido)-4-oxo-2-propy...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | 0.170 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description In vivo inhibitory concentration of the compound against Angiotensin II, type 2 of rat midbrain membrane | Bioorg Med Chem Lett 4: 2337-2342 (1994) Article DOI: 10.1016/0960-894X(94)85036-4 BindingDB Entry DOI: 10.7270/Q2X066Z9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor (RABBIT) | BDBM50282466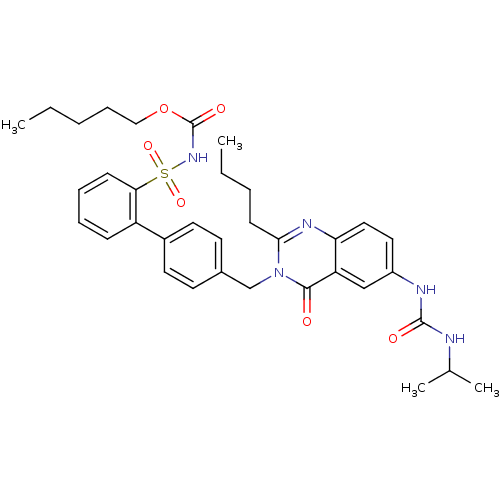 (CHEMBL354779 | Quinazolinone Analog) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | 0.170 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Tested in vitro for binding affinity against Angiotensin II receptor, type 1 in rabbit aortic membrane | Bioorg Med Chem Lett 4: 81-86 (1994) Article DOI: 10.1016/S0960-894X(01)81126-4 BindingDB Entry DOI: 10.7270/Q2D79BBS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor (RABBIT) | BDBM50039882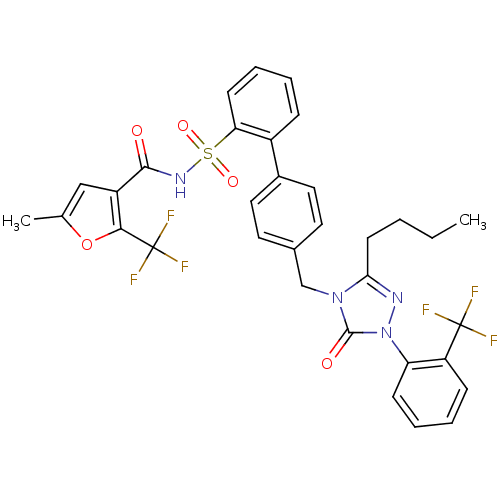 (4'-[3-Butyl-5-oxo-1-(2-trifluoromethyl-phenyl)-1,5...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.170 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description In vitro inhibitory activity against angiotensin II rabbit aorta AT1 receptor using radioligand [125I]-Sar Ile-AII | J Med Chem 37: 2808-24 (1994) BindingDB Entry DOI: 10.7270/Q2M907QM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor (RABBIT) | BDBM50039887 (4'-[3-Butyl-5-oxo-1-(2-trifluoromethyl-phenyl)-1,5...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.170 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description In vitro inhibitory activity against angiotensin II rabbit aorta AT1 receptor using radioligand [125I]-Sar Ile-AII | J Med Chem 37: 2808-24 (1994) BindingDB Entry DOI: 10.7270/Q2M907QM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor (RABBIT) | BDBM50042565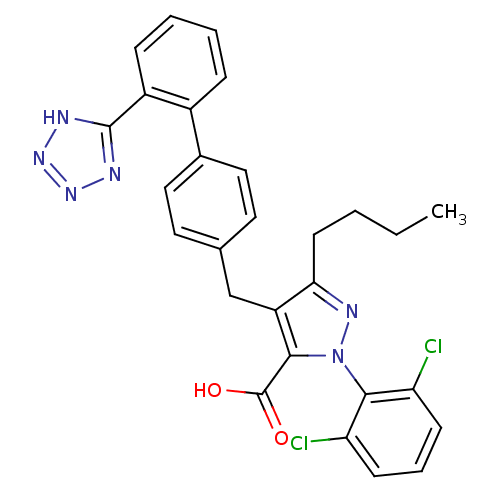 (5-Butyl-2-(2,6-dichloro-phenyl)-4-[2'-(1H-tetrazol...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.180 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Evaluation of Angiotensin II antagonistic activity by displacement of [125I]-Sar Ile-AII at the rabbit aorta Angiotensin II receptor, type 1 | J Med Chem 36: 3595-605 (1994) BindingDB Entry DOI: 10.7270/Q25Q4V5H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor (RABBIT) | BDBM50282472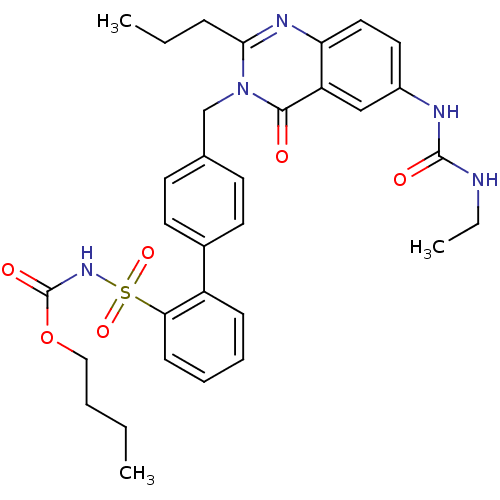 (CHEMBL169221 | Quinazolinone Analog) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | 0.180 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Tested in vitro for binding affinity against Angiotensin II receptor, type 1 in rabbit aortic membrane | Bioorg Med Chem Lett 4: 81-86 (1994) Article DOI: 10.1016/S0960-894X(01)81126-4 BindingDB Entry DOI: 10.7270/Q2D79BBS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor (RABBIT) | BDBM50282464 (CHEMBL169568 | Quinazolinone Analog) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | 0.180 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Tested in vitro for binding affinity against Angiotensin II receptor, type 1 in rabbit aortic membrane | Bioorg Med Chem Lett 4: 81-86 (1994) Article DOI: 10.1016/S0960-894X(01)81126-4 BindingDB Entry DOI: 10.7270/Q2D79BBS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor (RABBIT) | BDBM50283312 (4'-[2-Ethyl-6-(3-isopropyl-ureido)-4-oxo-4H-quinaz...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | 0.190 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description In vivo inhibitory concentration of the compound against Angiotensin II receptor, type 1 of rabbit aorta membrane | Bioorg Med Chem Lett 4: 2337-2342 (1994) Article DOI: 10.1016/0960-894X(94)85036-4 BindingDB Entry DOI: 10.7270/Q2X066Z9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor (RABBIT) | BDBM50282462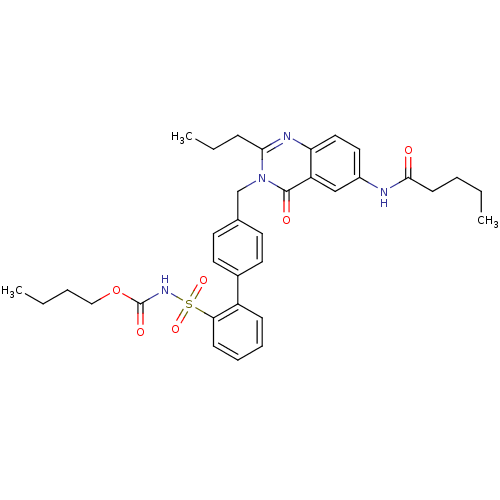 (CHEMBL352503 | Quinazolinone Analog) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | 0.190 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Tested in vitro for binding affinity against Angiotensin II receptor, type 1 in rabbit aortic membrane | Bioorg Med Chem Lett 4: 81-86 (1994) Article DOI: 10.1016/S0960-894X(01)81126-4 BindingDB Entry DOI: 10.7270/Q2D79BBS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor (RABBIT) | BDBM50039930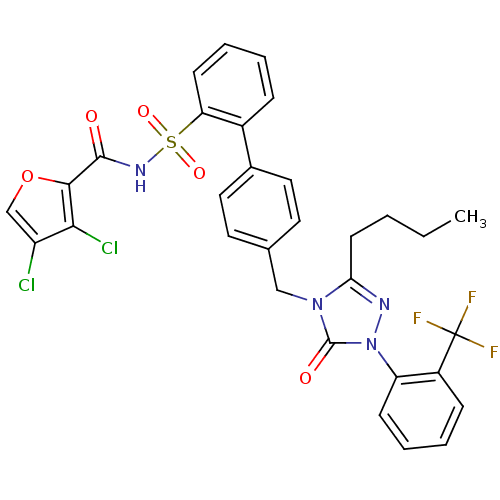 (4'-[3-Butyl-5-oxo-1-(2-trifluoromethyl-phenyl)-1,5...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.190 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description In vitro inhibitory activity against angiotensin II rabbit aorta AT1 receptor using radioligand [125I]-Sar Ile-AII | J Med Chem 37: 2808-24 (1994) BindingDB Entry DOI: 10.7270/Q2M907QM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor (RABBIT) | BDBM50283318 (2-Butyl-3-(3-fluoro-2'-(butyloxycarbonylaminosulph...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | 0.190 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description In vivo inhibitory concentration of the compound against Angiotensin II receptor, type 1 of rabbit aorta membrane | Bioorg Med Chem Lett 4: 2337-2342 (1994) Article DOI: 10.1016/0960-894X(94)85036-4 BindingDB Entry DOI: 10.7270/Q2X066Z9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor (RABBIT) | BDBM50283305 (3'-Fluoro-4'-[6-(3-isopropyl-ureido)-4-oxo-2-propy...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description In vivo inhibitory concentration of the compound against Angiotensin II receptor, type 1 of rabbit aorta membrane | Bioorg Med Chem Lett 4: 2337-2342 (1994) Article DOI: 10.1016/0960-894X(94)85036-4 BindingDB Entry DOI: 10.7270/Q2X066Z9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1/Type-2 angiotensin II receptor (Homo sapiens (Human)) | BDBM50229575 (CHEMBL2369937) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
Exploratory Chemistry Merck Sharp and Dohme Research Laboratories Curated by ChEMBL | Assay Description Binding affinity towards Angiotensin receptor from rabbit aorta | J Med Chem 34: 2919-22 (1991) BindingDB Entry DOI: 10.7270/Q26Q20H6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor (RABBIT) | BDBM50039895 (2-{4-[3-butyl-5-oxo-1-(2-trifluoromethylphenyl)-4,...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description In vitro inhibitory activity against angiotensin II rabbit aorta AT1 receptor using radioligand [125I]-Sar Ile-AII | J Med Chem 37: 2808-24 (1994) BindingDB Entry DOI: 10.7270/Q2M907QM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor (RABBIT) | BDBM50038189 (4'-(2-Ethyl-5,7-dimethyl-imidazo[4,5-b]pyridin-3-y...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description In vitro binding affinity of the compound for angiotensin II AT1 receptor in rabbit aorta | J Med Chem 37: 4068-72 (1995) BindingDB Entry DOI: 10.7270/Q2DV1HZ3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-2 angiotensin II receptor (RAT) | BDBM50283302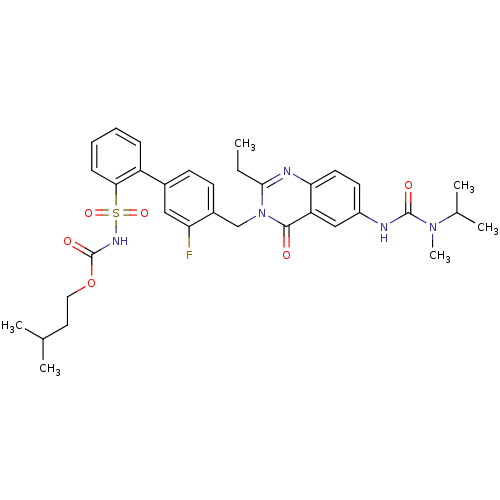 (2-ethyl-3-(3-fluoro-2'-(3-methyl-butyloxycarbonyla...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description In vivo inhibitory concentration of the compound against Angiotensin II, type 2 of rat midbrain membrane | Bioorg Med Chem Lett 4: 2337-2342 (1994) Article DOI: 10.1016/0960-894X(94)85036-4 BindingDB Entry DOI: 10.7270/Q2X066Z9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor (RABBIT) | BDBM50282431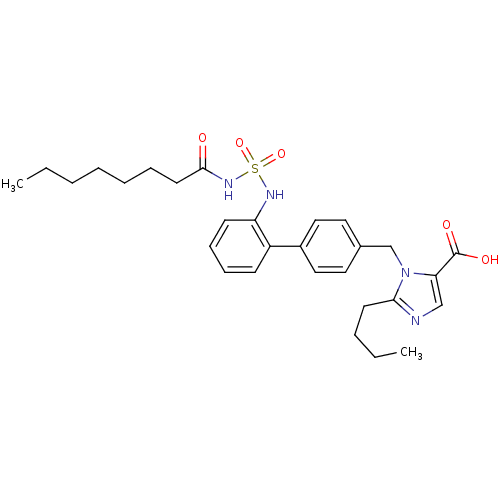 (Acyl sulfonamide derivative | CHEMBL350121) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article | n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description In vitro inhibitory activity against Angiotensin II receptor, type 1 to displace 125I-Sar,Ile8-AII in rabbit aorta | Bioorg Med Chem Lett 4: 69-74 (1994) Article DOI: 10.1016/S0960-894X(01)81124-0 BindingDB Entry DOI: 10.7270/Q2NS0TVK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor (RABBIT) | BDBM50042571 (2-Benzyl-5-butyl-4-[2'-(1H-tetrazol-5-yl)-biphenyl...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Evaluation of Angiotensin II antagonistic activity by displacement of [125I]-Sar Ile-AII at the rabbit aorta Angiotensin II receptor, type 1 | J Med Chem 36: 3595-605 (1994) BindingDB Entry DOI: 10.7270/Q25Q4V5H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-2 angiotensin II receptor (RAT) | BDBM50283305 (3'-Fluoro-4'-[6-(3-isopropyl-ureido)-4-oxo-2-propy...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | 0.210 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description In vivo inhibitory concentration of the compound against AT2 receptor of rat adrenal membrane | Bioorg Med Chem Lett 4: 2337-2342 (1994) Article DOI: 10.1016/0960-894X(94)85036-4 BindingDB Entry DOI: 10.7270/Q2X066Z9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-2 angiotensin II receptor (RAT) | BDBM50283300 (2-Butyl-3-(3-fluoro-2'-(3-methylbutyloxycarbonylam...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | 0.210 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description In vivo inhibitory concentration of the compound against Angiotensin II, type 2 of rat midbrain membrane | Bioorg Med Chem Lett 4: 2337-2342 (1994) Article DOI: 10.1016/0960-894X(94)85036-4 BindingDB Entry DOI: 10.7270/Q2X066Z9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor (RABBIT) | BDBM50039921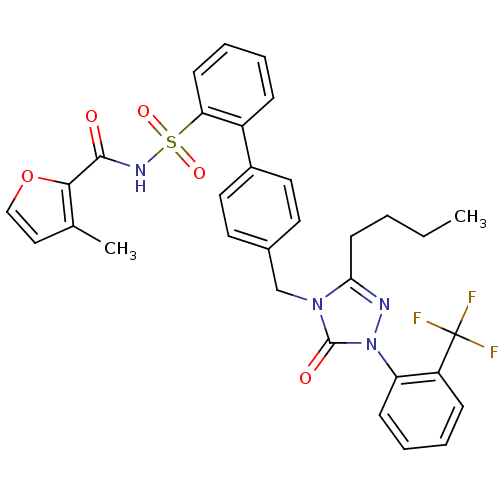 (4'-[3-Butyl-5-oxo-1-(2-trifluoromethyl-phenyl)-1,5...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.210 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description In vitro inhibitory activity against angiotensin II rabbit aorta AT1 receptor using radioligand [125I]-Sar Ile-AII | J Med Chem 37: 2808-24 (1994) BindingDB Entry DOI: 10.7270/Q2M907QM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor (RABBIT) | BDBM50282455 (CHEMBL169967 | Quinazolinone Analog) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | 0.210 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Tested in vitro for binding affinity against Angiotensin II receptor, type 1 in rabbit aortic membrane | Bioorg Med Chem Lett 4: 81-86 (1994) Article DOI: 10.1016/S0960-894X(01)81126-4 BindingDB Entry DOI: 10.7270/Q2D79BBS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor (RABBIT) | BDBM50038190 (4'-(2-Ethyl-5,7-dimethyl-imidazo[4,5-b]pyridin-3-y...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.210 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description In vitro binding affinity of the compound for angiotensin II AT1 receptor in rabbit aorta | J Med Chem 37: 4068-72 (1995) BindingDB Entry DOI: 10.7270/Q2DV1HZ3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-2 angiotensin II receptor (RAT) | BDBM50283307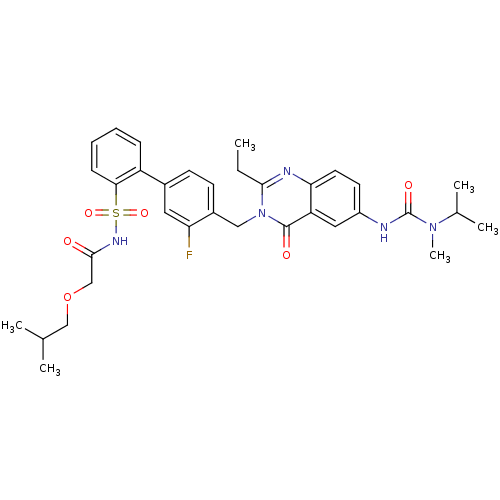 (4'-[2-Ethyl-6-(3-isopropyl-3-methyl-ureido)-4-oxo-...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | 0.210 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description In vivo inhibitory concentration of the compound against Angiotensin II, type 2 of rat midbrain membrane | Bioorg Med Chem Lett 4: 2337-2342 (1994) Article DOI: 10.1016/0960-894X(94)85036-4 BindingDB Entry DOI: 10.7270/Q2X066Z9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-2 angiotensin II receptor (RAT) | BDBM50283300 (2-Butyl-3-(3-fluoro-2'-(3-methylbutyloxycarbonylam...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | 0.220 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description In vivo inhibitory concentration of the compound against AT2 receptor of rat adrenal membrane | Bioorg Med Chem Lett 4: 2337-2342 (1994) Article DOI: 10.1016/0960-894X(94)85036-4 BindingDB Entry DOI: 10.7270/Q2X066Z9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor (RABBIT) | BDBM50039899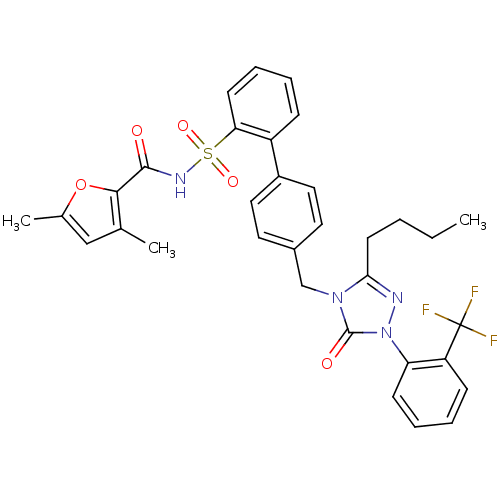 (4'-[3-Butyl-5-oxo-1-(2-trifluoromethyl-phenyl)-1,5...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.220 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description In vitro inhibitory activity against angiotensin II rabbit aorta AT1 receptor using radioligand [125I]-Sar Ile-AII | J Med Chem 37: 2808-24 (1994) BindingDB Entry DOI: 10.7270/Q2M907QM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor (RABBIT) | BDBM50039946 (4'-[3-Butyl-5-oxo-1-(2-trifluoromethyl-phenyl)-1,5...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.220 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description In vitro inhibitory activity against angiotensin II rabbit aorta AT1 receptor using radioligand [125I]-Sar Ile-AII | J Med Chem 37: 2808-24 (1994) BindingDB Entry DOI: 10.7270/Q2M907QM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor A/B (RAT) | BDBM50283319 (2-Butyl-3-(3-fluoro-2'-(2-cyclopropylethyloxycarbo...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | 0.230 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description In vivo inhibitory concentration against Angiotensin II receptor, type 1 of rat adrenal membrane | Bioorg Med Chem Lett 4: 2337-2342 (1994) Article DOI: 10.1016/0960-894X(94)85036-4 BindingDB Entry DOI: 10.7270/Q2X066Z9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor (RABBIT) | BDBM50283300 (2-Butyl-3-(3-fluoro-2'-(3-methylbutyloxycarbonylam...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | 0.230 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description In vivo inhibitory concentration of the compound against Angiotensin II receptor, type 1 of rabbit aorta membrane | Bioorg Med Chem Lett 4: 2337-2342 (1994) Article DOI: 10.1016/0960-894X(94)85036-4 BindingDB Entry DOI: 10.7270/Q2X066Z9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor (RABBIT) | BDBM50283317 (2-Propyl-3-(3-fluoro-2'-(3-methylbutyloxycarbonyla...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | 0.240 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description In vivo inhibitory concentration of the compound against Angiotensin II receptor, type 1 of rabbit aorta membrane | Bioorg Med Chem Lett 4: 2337-2342 (1994) Article DOI: 10.1016/0960-894X(94)85036-4 BindingDB Entry DOI: 10.7270/Q2X066Z9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor (RABBIT) | BDBM50049181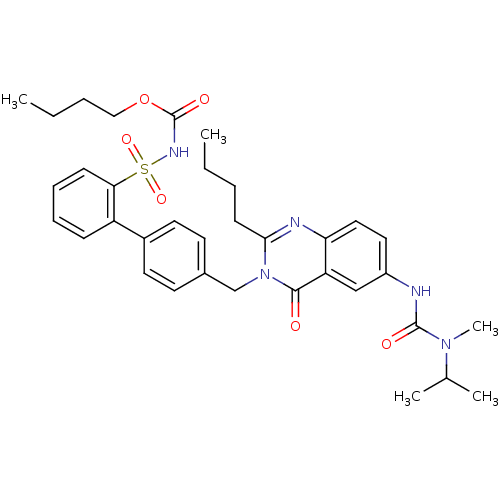 (2-Butyl-3-(2'-(butyloxycarbonylaminosulphonyl)-bip...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | 0.240 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description In vivo inhibitory concentration of the compound against Angiotensin II receptor, type 1 of rabbit aorta membrane | Bioorg Med Chem Lett 4: 2337-2342 (1994) Article DOI: 10.1016/0960-894X(94)85036-4 BindingDB Entry DOI: 10.7270/Q2X066Z9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor (RABBIT) | BDBM50049181 (2-Butyl-3-(2'-(butyloxycarbonylaminosulphonyl)-bip...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | 0.240 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Tested in vitro for binding affinity against Angiotensin II receptor, type 1 in rabbit aortic membrane | Bioorg Med Chem Lett 4: 81-86 (1994) Article DOI: 10.1016/S0960-894X(01)81126-4 BindingDB Entry DOI: 10.7270/Q2D79BBS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor (RABBIT) | BDBM50042578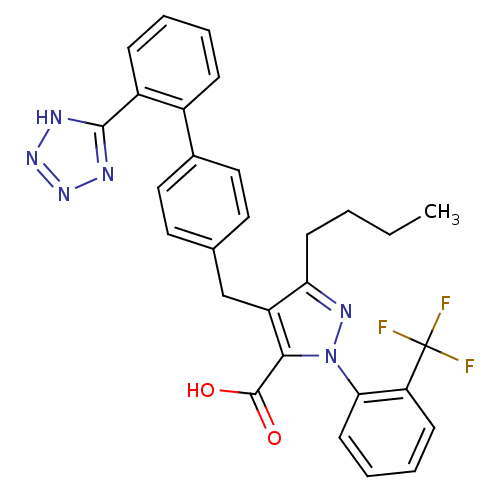 (5-Butyl-4-[2'-(1H-tetrazol-5-yl)-biphenyl-4-ylmeth...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.240 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Evaluation of Angiotensin II antagonistic activity by displacement of [125I]-Sar Ile-AII at the rabbit aorta Angiotensin II receptor, type 1 | J Med Chem 36: 3595-605 (1994) BindingDB Entry DOI: 10.7270/Q25Q4V5H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor (RABBIT) | BDBM50282452 (CHEMBL169488 | Quinazolinone Analog) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | 0.240 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Tested in vitro for binding affinity against Angiotensin II receptor, type 1 in rabbit aortic membrane | Bioorg Med Chem Lett 4: 81-86 (1994) Article DOI: 10.1016/S0960-894X(01)81126-4 BindingDB Entry DOI: 10.7270/Q2D79BBS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor (RABBIT) | BDBM50042570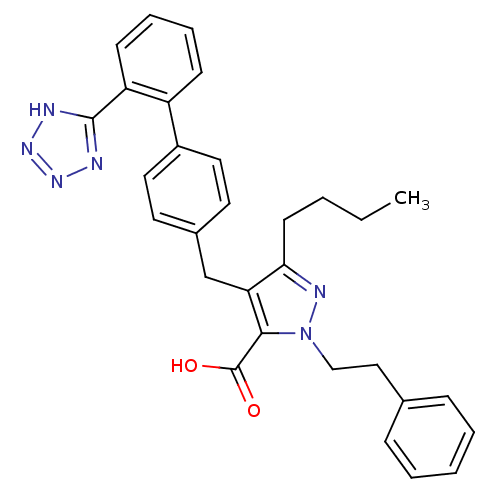 (5-Butyl-2-phenethyl-4-[2'-(1H-tetrazol-5-yl)-biphe...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.240 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Evaluation of Angiotensin II antagonistic activity by displacement of [125I]-Sar Ile-AII at the rabbit aorta Angiotensin II receptor, type 1 | J Med Chem 36: 3595-605 (1994) BindingDB Entry DOI: 10.7270/Q25Q4V5H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 961 total ) | Next | Last >> |