

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Leukotriene B4 receptor 1 (Homo sapiens (Human)) | BDBM50001609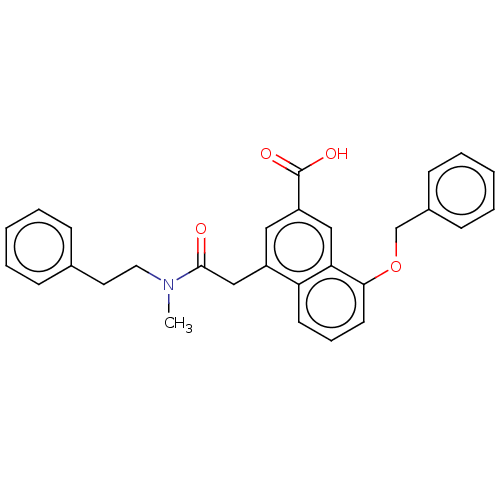 (8-Benzyloxy-4-[(methyl-phenethyl-carbamoyl)-methyl...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL MCE PC cid PC sid UniChem Patents Similars | PubMed | 0.140 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Rhone-Poulenc Rorer Central Research Curated by ChEMBL | Assay Description Antagonistic activity of the compound against LTB4 receptor using guinea pig (GP) spleen cell membrane | J Med Chem 35: 4253-5 (1992) BindingDB Entry DOI: 10.7270/Q208648W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leukotriene B4 receptor 1 (Homo sapiens (Human)) | BDBM50001609 (8-Benzyloxy-4-[(methyl-phenethyl-carbamoyl)-methyl...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL MCE PC cid PC sid UniChem Patents Similars | PubMed | 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Rhone-Poulenc Rorer Central Research Curated by ChEMBL | Assay Description Antagonistic activity of the compound against monkey neutrophil LTB4 receptor 2 min after an iv dose of 3 mg/kg . | J Med Chem 35: 4253-5 (1992) BindingDB Entry DOI: 10.7270/Q208648W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 4 (Homo sapiens (Human)) | BDBM11123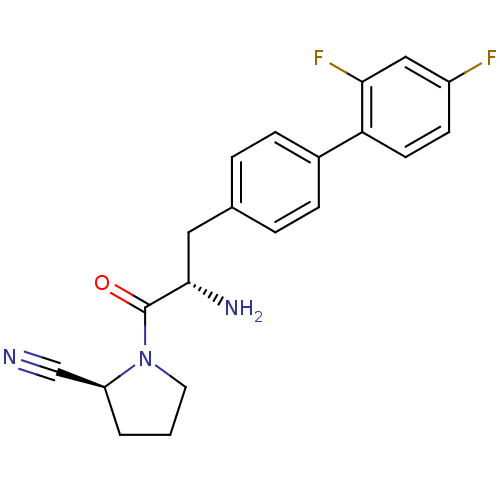 ((2S)-1-[(2S)-2-amino-3-[4-(2,4-difluorophenyl)phen...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 2.20 | -49.4 | n/a | n/a | n/a | n/a | n/a | 7.4 | 25 |
Johnson & Johnson Pharmaceutical | Assay Description The progress of DPPIV inhibition by compounds was measured by recording the liberation of free pNA at 405 nm. IC50 was determined from the slope of r... | Bioorg Med Chem Lett 16: 123-8 (2006) Article DOI: 10.1016/j.bmcl.2005.09.037 BindingDB Entry DOI: 10.7270/Q2S180QJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 4 (Homo sapiens (Human)) | BDBM11121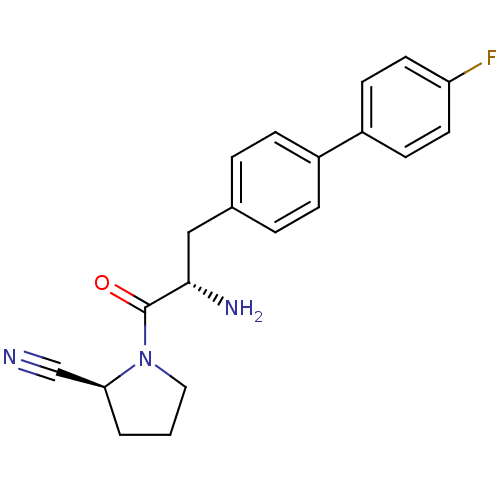 ((2S)-1-[(2S)-2-amino-3-[4-(4-fluorophenyl)phenyl]p...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 3.10 | -48.6 | n/a | n/a | n/a | n/a | n/a | 7.4 | 25 |
Johnson & Johnson Pharmaceutical | Assay Description The progress of DPPIV inhibition by compounds was measured by recording the liberation of free pNA at 405 nm. IC50 was determined from the slope of r... | Bioorg Med Chem Lett 16: 123-8 (2006) Article DOI: 10.1016/j.bmcl.2005.09.037 BindingDB Entry DOI: 10.7270/Q2S180QJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 4 (Homo sapiens (Human)) | BDBM11122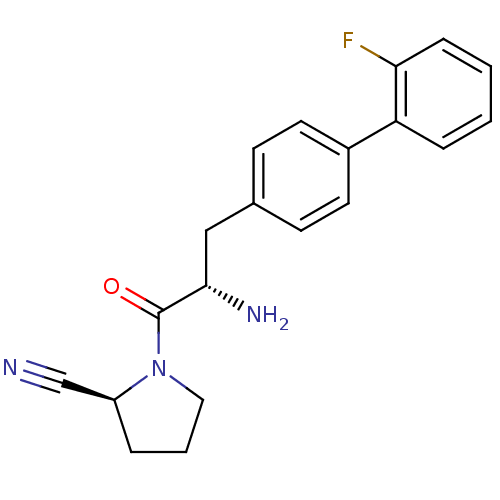 ((2S)-1-[(2S)-2-amino-3-[4-(2-fluorophenyl)phenyl]p...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 5.30 | -47.2 | n/a | n/a | n/a | n/a | n/a | 7.4 | 25 |
Johnson & Johnson Pharmaceutical | Assay Description The progress of DPPIV inhibition by compounds was measured by recording the liberation of free pNA at 405 nm. IC50 was determined from the slope of r... | Bioorg Med Chem Lett 16: 123-8 (2006) Article DOI: 10.1016/j.bmcl.2005.09.037 BindingDB Entry DOI: 10.7270/Q2S180QJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 4 (Homo sapiens (Human)) | BDBM11118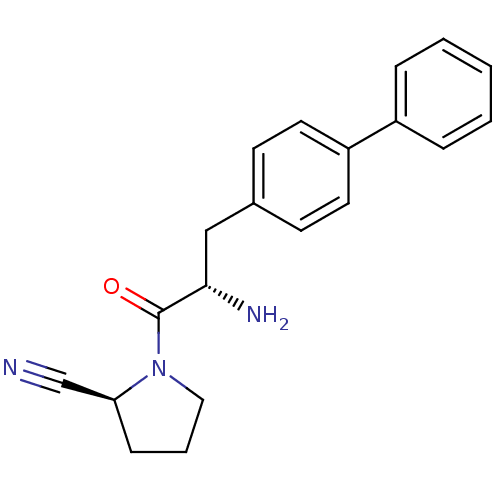 ((2S)-1-[(2S)-2-amino-3-(4-phenylphenyl)propanoyl]p...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 13 | -45.0 | n/a | n/a | n/a | n/a | n/a | 7.4 | 25 |
Johnson & Johnson Pharmaceutical | Assay Description The progress of DPPIV inhibition by compounds was measured by recording the liberation of free pNA at 405 nm. IC50 was determined from the slope of r... | Bioorg Med Chem Lett 16: 123-8 (2006) Article DOI: 10.1016/j.bmcl.2005.09.037 BindingDB Entry DOI: 10.7270/Q2S180QJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 4 (Homo sapiens (Human)) | BDBM11119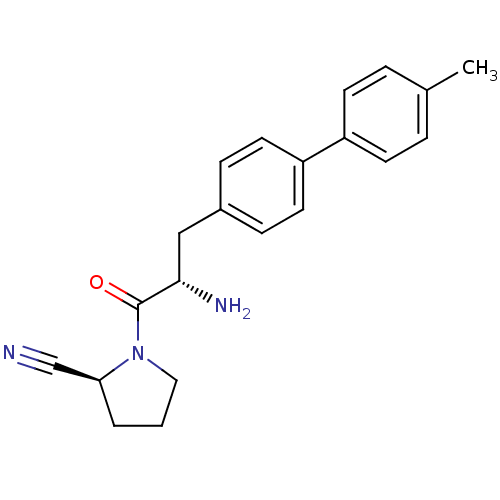 ((2S)-1-[(2S)-2-amino-3-[4-(4-methylphenyl)phenyl]p...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 20 | -43.9 | n/a | n/a | n/a | n/a | n/a | 7.4 | 25 |
Johnson & Johnson Pharmaceutical | Assay Description The progress of DPPIV inhibition by compounds was measured by recording the liberation of free pNA at 405 nm. IC50 was determined from the slope of r... | Bioorg Med Chem Lett 16: 123-8 (2006) Article DOI: 10.1016/j.bmcl.2005.09.037 BindingDB Entry DOI: 10.7270/Q2S180QJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 4 (Homo sapiens (Human)) | BDBM11120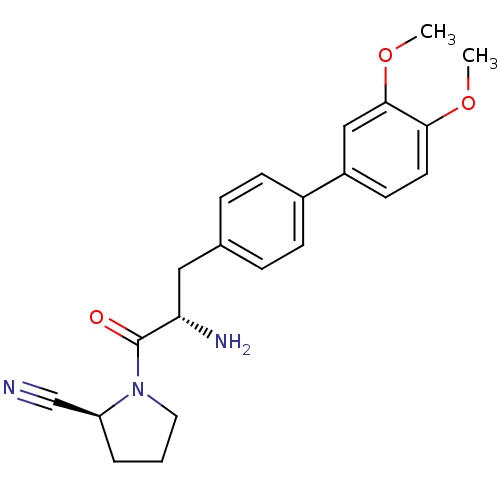 ((2S)-1-[(2S)-2-amino-3-[4-(3,4-dimethoxyphenyl)phe...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 26 | -43.3 | n/a | n/a | n/a | n/a | n/a | 7.4 | 25 |
Johnson & Johnson Pharmaceutical | Assay Description The progress of DPPIV inhibition by compounds was measured by recording the liberation of free pNA at 405 nm. IC50 was determined from the slope of r... | Bioorg Med Chem Lett 16: 123-8 (2006) Article DOI: 10.1016/j.bmcl.2005.09.037 BindingDB Entry DOI: 10.7270/Q2S180QJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 4 (Homo sapiens (Human)) | BDBM11113 (6-{[2-({2-[(2S)-2-cyanopyrrolidin-1-yl]-2-oxoethyl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | 27 | -43.2 | n/a | n/a | n/a | n/a | n/a | 7.4 | 25 |
Johnson & Johnson Pharmaceutical | Assay Description The progress of DPPIV inhibition by compounds was measured by recording the liberation of free pNA at 405 nm. IC50 was determined from the slope of r... | Bioorg Med Chem Lett 16: 123-8 (2006) Article DOI: 10.1016/j.bmcl.2005.09.037 BindingDB Entry DOI: 10.7270/Q2S180QJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 4 (Homo sapiens (Human)) | BDBM11116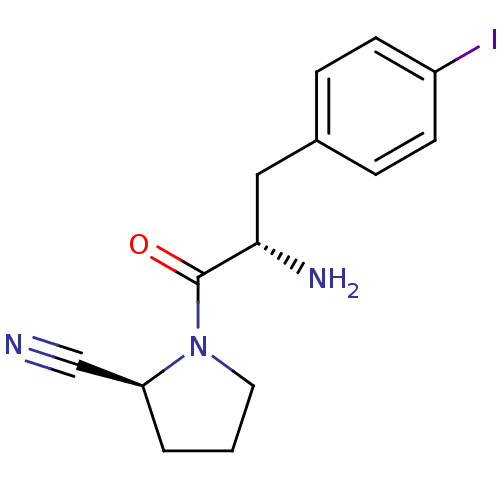 ((2S)-1-[(2S)-2-amino-3-(4-iodophenyl)propanoyl]pyr...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 34 | -42.6 | n/a | n/a | n/a | n/a | n/a | 7.4 | 25 |
Johnson & Johnson Pharmaceutical | Assay Description The progress of DPPIV inhibition by compounds was measured by recording the liberation of free pNA at 405 nm. IC50 was determined from the slope of r... | Bioorg Med Chem Lett 16: 123-8 (2006) Article DOI: 10.1016/j.bmcl.2005.09.037 BindingDB Entry DOI: 10.7270/Q2S180QJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 4 (Homo sapiens (Human)) | BDBM11124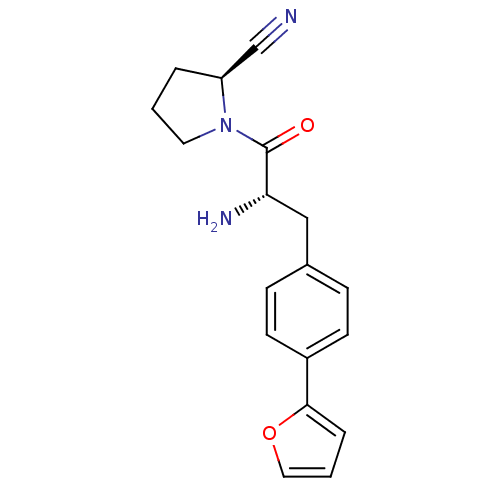 ((2S)-1-[(2S)-2-amino-3-[4-(furan-2-yl)phenyl]propa...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 36 | -42.5 | n/a | n/a | n/a | n/a | n/a | 7.4 | 25 |
Johnson & Johnson Pharmaceutical | Assay Description The progress of DPPIV inhibition by compounds was measured by recording the liberation of free pNA at 405 nm. IC50 was determined from the slope of r... | Bioorg Med Chem Lett 16: 123-8 (2006) Article DOI: 10.1016/j.bmcl.2005.09.037 BindingDB Entry DOI: 10.7270/Q2S180QJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 4 (Homo sapiens (Human)) | BDBM11115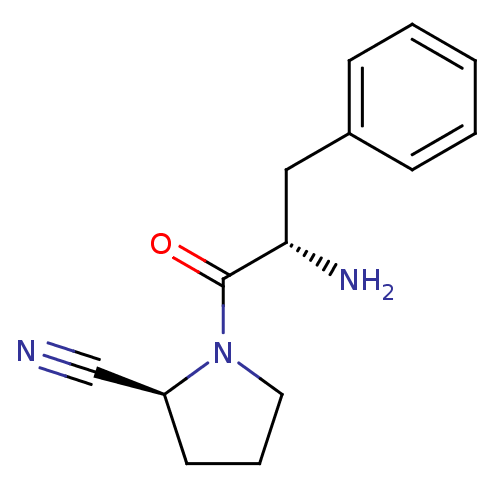 ((2S)-1-[(2S)-2-amino-3-phenylpropanoyl]pyrrolidine...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 63 | -41.1 | n/a | n/a | n/a | n/a | n/a | 7.4 | 25 |
Johnson & Johnson Pharmaceutical | Assay Description The progress of DPPIV inhibition by compounds was measured by recording the liberation of free pNA at 405 nm. IC50 was determined from the slope of r... | Bioorg Med Chem Lett 16: 123-8 (2006) Article DOI: 10.1016/j.bmcl.2005.09.037 BindingDB Entry DOI: 10.7270/Q2S180QJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 4 (Homo sapiens (Human)) | BDBM11136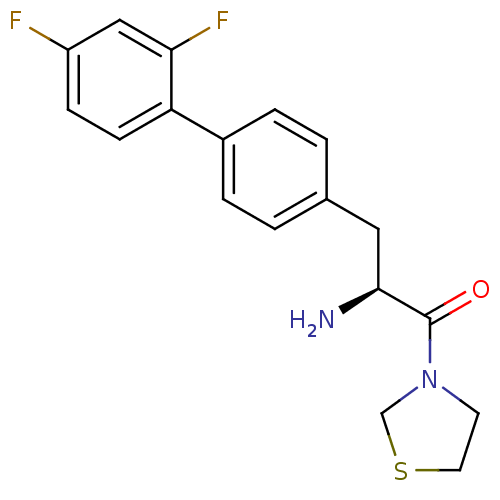 ((2S)-2-amino-3-[4-(2,4-difluorophenyl)phenyl]-1-(1...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 96 | -40.1 | n/a | n/a | n/a | n/a | n/a | 7.4 | 25 |
Johnson & Johnson Pharmaceutical | Assay Description The progress of DPPIV inhibition by compounds was measured by recording the liberation of free pNA at 405 nm. IC50 was determined from the slope of r... | Bioorg Med Chem Lett 16: 123-8 (2006) Article DOI: 10.1016/j.bmcl.2005.09.037 BindingDB Entry DOI: 10.7270/Q2S180QJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 4 (Homo sapiens (Human)) | BDBM11134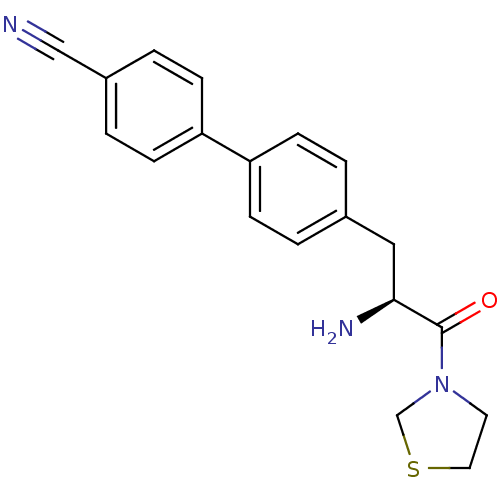 (4-{4-[(2S)-2-amino-3-oxo-3-(1,3-thiazolidin-3-yl)p...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 160 | -38.8 | n/a | n/a | n/a | n/a | n/a | 7.4 | 25 |
Johnson & Johnson Pharmaceutical | Assay Description The progress of DPPIV inhibition by compounds was measured by recording the liberation of free pNA at 405 nm. IC50 was determined from the slope of r... | Bioorg Med Chem Lett 16: 123-8 (2006) Article DOI: 10.1016/j.bmcl.2005.09.037 BindingDB Entry DOI: 10.7270/Q2S180QJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 4 (Homo sapiens (Human)) | BDBM11133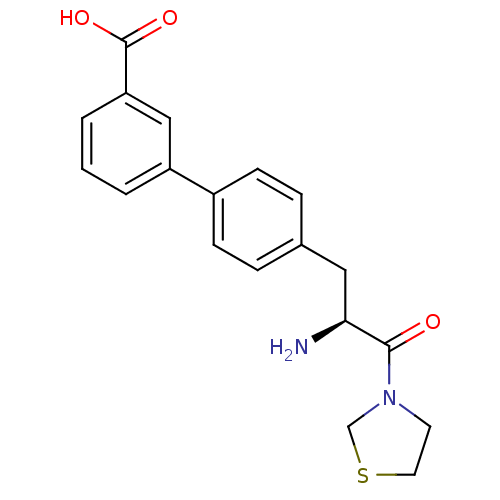 (3-{4-[(2S)-2-amino-3-oxo-3-(1,3-thiazolidin-3-yl)p...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 166 | -38.7 | n/a | n/a | n/a | n/a | n/a | 7.4 | 25 |
Johnson & Johnson Pharmaceutical | Assay Description The progress of DPPIV inhibition by compounds was measured by recording the liberation of free pNA at 405 nm. IC50 was determined from the slope of r... | Bioorg Med Chem Lett 16: 123-8 (2006) Article DOI: 10.1016/j.bmcl.2005.09.037 BindingDB Entry DOI: 10.7270/Q2S180QJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 4 (Homo sapiens (Human)) | BDBM11135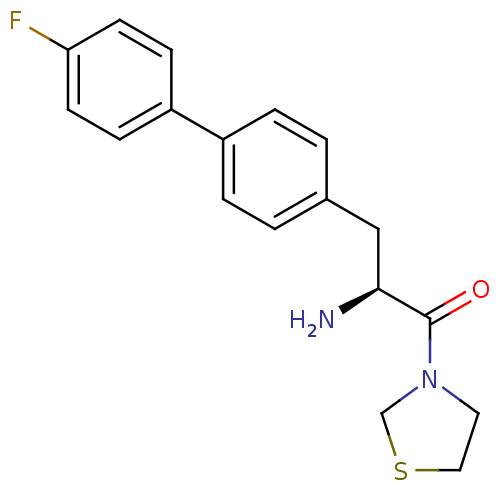 ((2S)-2-amino-3-[4-(4-fluorophenyl)phenyl]-1-(1,3-t...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 170 | -38.6 | n/a | n/a | n/a | n/a | n/a | 7.4 | 25 |
Johnson & Johnson Pharmaceutical | Assay Description The progress of DPPIV inhibition by compounds was measured by recording the liberation of free pNA at 405 nm. IC50 was determined from the slope of r... | Bioorg Med Chem Lett 16: 123-8 (2006) Article DOI: 10.1016/j.bmcl.2005.09.037 BindingDB Entry DOI: 10.7270/Q2S180QJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 4 (Homo sapiens (Human)) | BDBM11132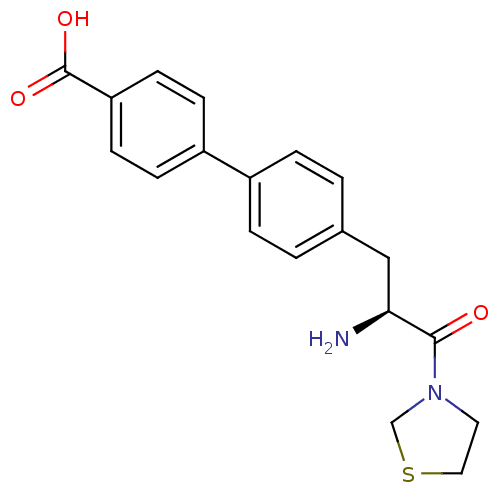 (4-{4-[(2S)-2-amino-3-oxo-3-(1,3-thiazolidin-3-yl)p...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 310 | -37.1 | n/a | n/a | n/a | n/a | n/a | 7.4 | 25 |
Johnson & Johnson Pharmaceutical | Assay Description The progress of DPPIV inhibition by compounds was measured by recording the liberation of free pNA at 405 nm. IC50 was determined from the slope of r... | Bioorg Med Chem Lett 16: 123-8 (2006) Article DOI: 10.1016/j.bmcl.2005.09.037 BindingDB Entry DOI: 10.7270/Q2S180QJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 4 (Homo sapiens (Human)) | BDBM11131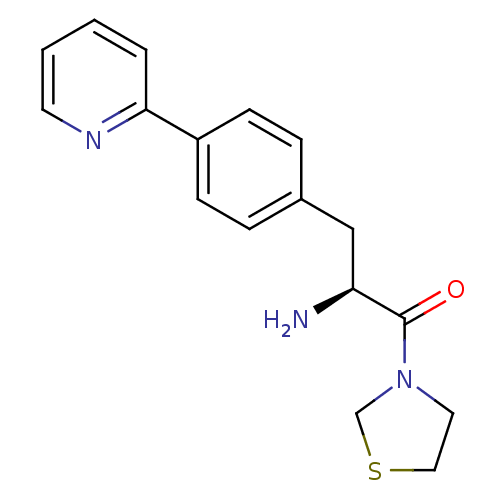 ((2S)-2-amino-3-[4-(pyridin-2-yl)phenyl]-1-(1,3-thi...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 355 | -36.8 | n/a | n/a | n/a | n/a | n/a | 7.4 | 25 |
Johnson & Johnson Pharmaceutical | Assay Description The progress of DPPIV inhibition by compounds was measured by recording the liberation of free pNA at 405 nm. IC50 was determined from the slope of r... | Bioorg Med Chem Lett 16: 123-8 (2006) Article DOI: 10.1016/j.bmcl.2005.09.037 BindingDB Entry DOI: 10.7270/Q2S180QJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 4 (Homo sapiens (Human)) | BDBM11125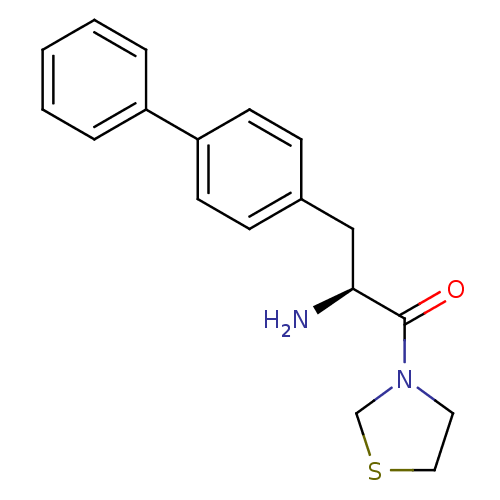 ((2S)-2-amino-3-(4-phenylphenyl)-1-(1,3-thiazolidin...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 360 | -36.8 | n/a | n/a | n/a | n/a | n/a | 7.4 | 25 |
Johnson & Johnson Pharmaceutical | Assay Description The progress of DPPIV inhibition by compounds was measured by recording the liberation of free pNA at 405 nm. IC50 was determined from the slope of r... | Bioorg Med Chem Lett 16: 123-8 (2006) Article DOI: 10.1016/j.bmcl.2005.09.037 BindingDB Entry DOI: 10.7270/Q2S180QJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 4 (Homo sapiens (Human)) | BDBM11117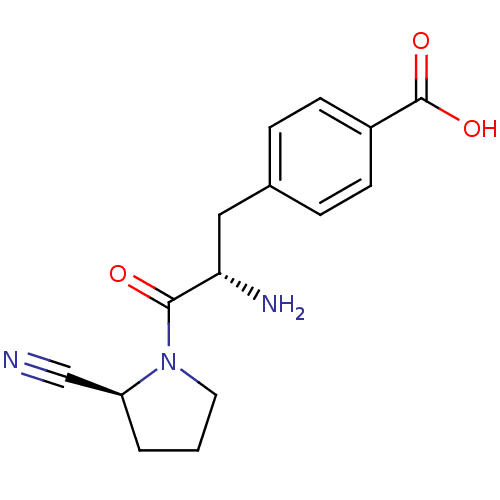 (4-[(2S)-2-amino-3-[(2S)-2-cyanopyrrolidin-1-yl]-3-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 470 | -36.1 | n/a | n/a | n/a | n/a | n/a | 7.4 | 25 |
Johnson & Johnson Pharmaceutical | Assay Description The progress of DPPIV inhibition by compounds was measured by recording the liberation of free pNA at 405 nm. IC50 was determined from the slope of r... | Bioorg Med Chem Lett 16: 123-8 (2006) Article DOI: 10.1016/j.bmcl.2005.09.037 BindingDB Entry DOI: 10.7270/Q2S180QJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 4 (Homo sapiens (Human)) | BDBM11130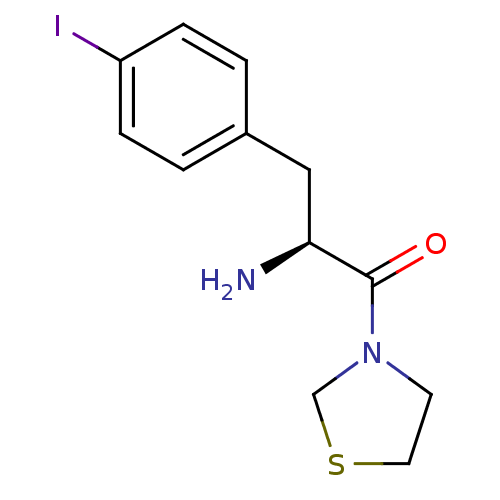 ((2S)-2-amino-3-(4-iodophenyl)-1-(1,3-thiazolidin-3...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 980 | -34.3 | n/a | n/a | n/a | n/a | n/a | 7.4 | 25 |
Johnson & Johnson Pharmaceutical | Assay Description The progress of DPPIV inhibition by compounds was measured by recording the liberation of free pNA at 405 nm. IC50 was determined from the slope of r... | Bioorg Med Chem Lett 16: 123-8 (2006) Article DOI: 10.1016/j.bmcl.2005.09.037 BindingDB Entry DOI: 10.7270/Q2S180QJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 4 (Homo sapiens (Human)) | BDBM11126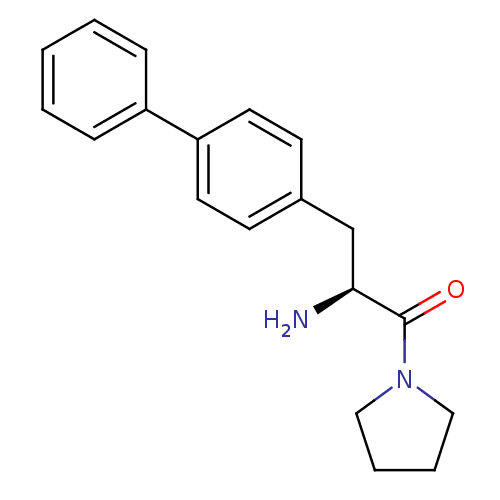 ((2S)-2-amino-3-(4-phenylphenyl)-1-(pyrrolidin-1-yl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 1.16E+3 | -33.9 | n/a | n/a | n/a | n/a | n/a | 7.4 | 25 |
Johnson & Johnson Pharmaceutical | Assay Description The progress of DPPIV inhibition by compounds was measured by recording the liberation of free pNA at 405 nm. IC50 was determined from the slope of r... | Bioorg Med Chem Lett 16: 123-8 (2006) Article DOI: 10.1016/j.bmcl.2005.09.037 BindingDB Entry DOI: 10.7270/Q2S180QJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 4 (Homo sapiens (Human)) | BDBM11137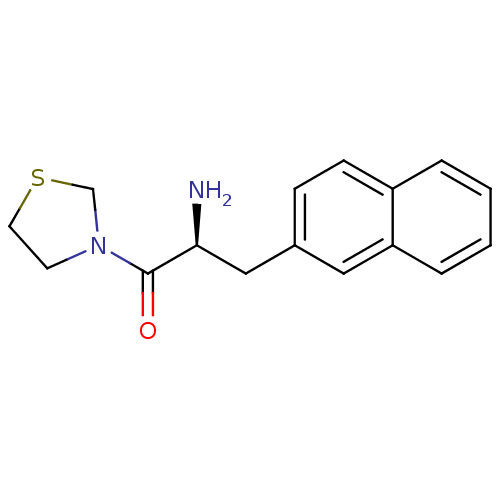 ((2S)-2-amino-3-(naphthalen-2-yl)-1-(1,3-thiazolidi...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 8.90E+3 | -28.8 | n/a | n/a | n/a | n/a | n/a | 7.4 | 25 |
Johnson & Johnson Pharmaceutical | Assay Description The progress of DPPIV inhibition by compounds was measured by recording the liberation of free pNA at 405 nm. IC50 was determined from the slope of r... | Bioorg Med Chem Lett 16: 123-8 (2006) Article DOI: 10.1016/j.bmcl.2005.09.037 BindingDB Entry DOI: 10.7270/Q2S180QJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 4 (Homo sapiens (Human)) | BDBM11129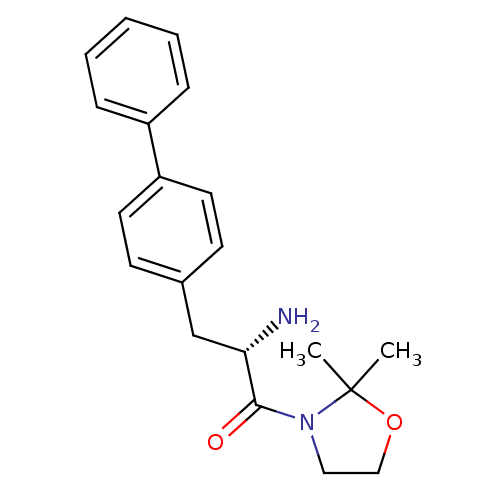 ((2S)-2-amino-1-(2,2-dimethyl-1,3-oxazolidin-3-yl)-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | >1.28E+4 | >-27.9 | n/a | n/a | n/a | n/a | n/a | 7.4 | 25 |
Johnson & Johnson Pharmaceutical | Assay Description The progress of DPPIV inhibition by compounds was measured by recording the liberation of free pNA at 405 nm. IC50 was determined from the slope of r... | Bioorg Med Chem Lett 16: 123-8 (2006) Article DOI: 10.1016/j.bmcl.2005.09.037 BindingDB Entry DOI: 10.7270/Q2S180QJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 4 (Homo sapiens (Human)) | BDBM11128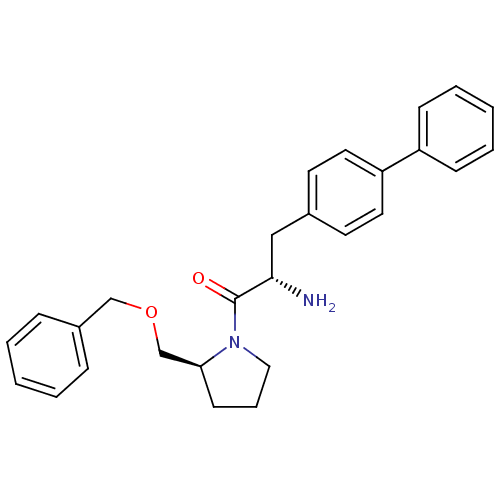 ((2S)-2-amino-1-[(2S)-2-[(benzyloxy)methyl]pyrrolid...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | >1.28E+4 | >-27.9 | n/a | n/a | n/a | n/a | n/a | 7.4 | 25 |
Johnson & Johnson Pharmaceutical | Assay Description The progress of DPPIV inhibition by compounds was measured by recording the liberation of free pNA at 405 nm. IC50 was determined from the slope of r... | Bioorg Med Chem Lett 16: 123-8 (2006) Article DOI: 10.1016/j.bmcl.2005.09.037 BindingDB Entry DOI: 10.7270/Q2S180QJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 4 (Homo sapiens (Human)) | BDBM11127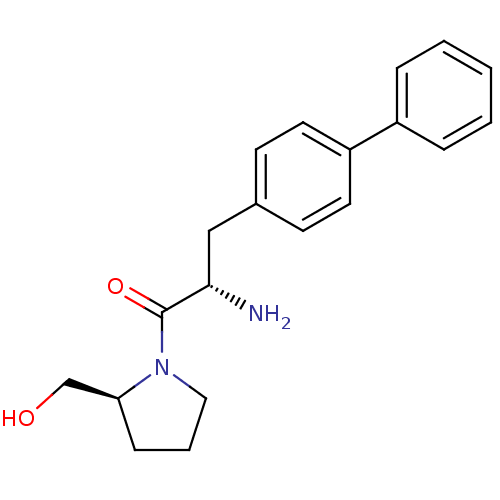 ((2S)-2-amino-1-[(2S)-2-(hydroxymethyl)pyrrolidin-1...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | >1.28E+4 | >-27.9 | n/a | n/a | n/a | n/a | n/a | 7.4 | 25 |
Johnson & Johnson Pharmaceutical | Assay Description The progress of DPPIV inhibition by compounds was measured by recording the liberation of free pNA at 405 nm. IC50 was determined from the slope of r... | Bioorg Med Chem Lett 16: 123-8 (2006) Article DOI: 10.1016/j.bmcl.2005.09.037 BindingDB Entry DOI: 10.7270/Q2S180QJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 4 (Homo sapiens (Human)) | BDBM11138 ((2S)-2-amino-3-(naphthalen-1-yl)-1-(1,3-thiazolidi...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | >1.28E+4 | >-27.9 | n/a | n/a | n/a | n/a | n/a | 7.4 | 25 |
Johnson & Johnson Pharmaceutical | Assay Description The progress of DPPIV inhibition by compounds was measured by recording the liberation of free pNA at 405 nm. IC50 was determined from the slope of r... | Bioorg Med Chem Lett 16: 123-8 (2006) Article DOI: 10.1016/j.bmcl.2005.09.037 BindingDB Entry DOI: 10.7270/Q2S180QJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 4 (Homo sapiens (Human)) | BDBM11139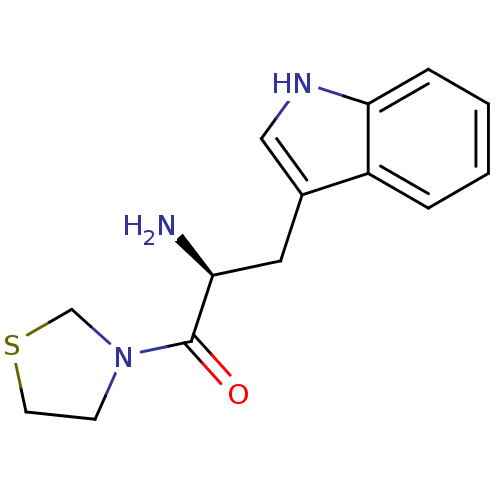 ((2S)-2-amino-3-(1H-indol-3-yl)-1-(1,3-thiazolidin-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | >1.28E+4 | >-27.9 | n/a | n/a | n/a | n/a | n/a | 7.4 | 25 |
Johnson & Johnson Pharmaceutical | Assay Description The progress of DPPIV inhibition by compounds was measured by recording the liberation of free pNA at 405 nm. IC50 was determined from the slope of r... | Bioorg Med Chem Lett 16: 123-8 (2006) Article DOI: 10.1016/j.bmcl.2005.09.037 BindingDB Entry DOI: 10.7270/Q2S180QJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leukotriene B4 receptor 1 (Homo sapiens (Human)) | BDBM50001609 (8-Benzyloxy-4-[(methyl-phenethyl-carbamoyl)-methyl...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL MCE PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 0.360 | n/a | n/a | n/a | n/a | n/a | n/a |
Rhone-Poulenc Rorer Central Research Curated by ChEMBL | Assay Description Affinity of the compound for human PMN LTB-4 receptors. | J Med Chem 35: 4253-5 (1992) BindingDB Entry DOI: 10.7270/Q208648W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aurora kinase A (Mus musculus (mouse)) | BDBM14208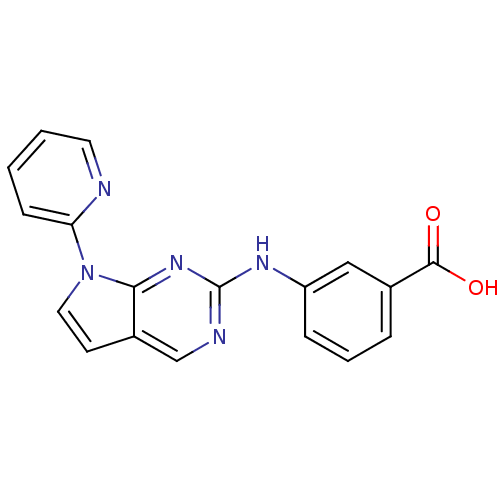 (3-{[7-(pyridin-2-yl)-7H-pyrrolo[2,3-d]pyrimidin-2-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | 7.5 | 22 |
Johnson & Johnson Pharmaceutical | Assay Description The compounds were tested for their ability to inhibit the phosphorylation of serine 10 of histone-H3 by purified recombinant murine Aurora-A enzyme.... | Bioorg Med Chem Lett 16: 5778-83 (2006) Article DOI: 10.1016/j.bmcl.2006.08.080 BindingDB Entry DOI: 10.7270/Q2D21VVZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aurora kinase A (Mus musculus (mouse)) | BDBM13534 (CHEMBL572878 | N-[4-({4-[(3-methyl-1H-pyrazol-5-yl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | 7.5 | 22 |
Johnson & Johnson Pharmaceutical | Assay Description The compounds were tested for their ability to inhibit the phosphorylation of serine 10 of histone-H3 by purified recombinant murine Aurora-A enzyme.... | Bioorg Med Chem Lett 16: 5778-83 (2006) Article DOI: 10.1016/j.bmcl.2006.08.080 BindingDB Entry DOI: 10.7270/Q2D21VVZ | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Aurora kinase A (Mus musculus (mouse)) | BDBM14192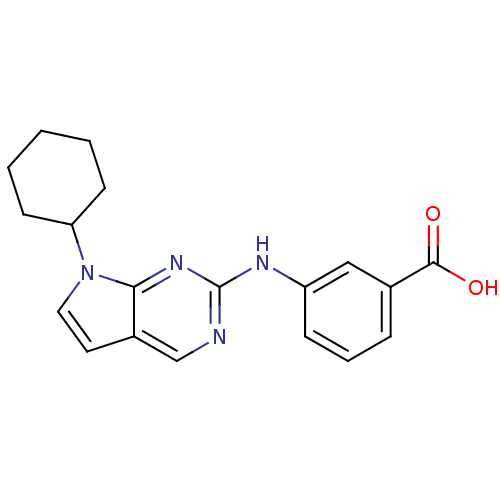 (3-({7-cyclohexyl-7H-pyrrolo[2,3-d]pyrimidin-2-yl}a...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | 7.5 | 22 |
Johnson & Johnson Pharmaceutical | Assay Description The compounds were tested for their ability to inhibit the phosphorylation of serine 10 of histone-H3 by purified recombinant murine Aurora-A enzyme.... | Bioorg Med Chem Lett 16: 5778-83 (2006) Article DOI: 10.1016/j.bmcl.2006.08.080 BindingDB Entry DOI: 10.7270/Q2D21VVZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aurora kinase A (Mus musculus (mouse)) | BDBM14209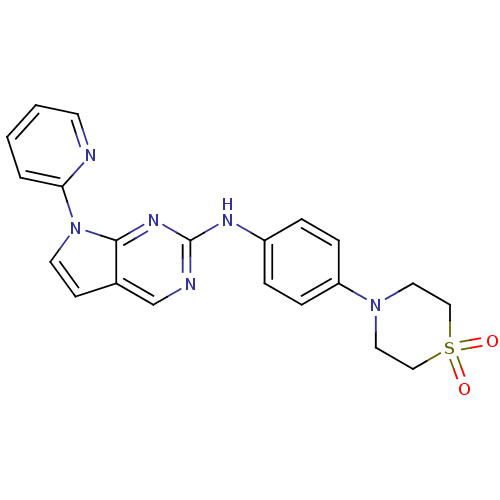 (4-(4-{[7-(pyridin-2-yl)-7H-pyrrolo[2,3-d]pyrimidin...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.800 | n/a | n/a | n/a | n/a | 7.5 | 22 |
Johnson & Johnson Pharmaceutical | Assay Description The compounds were tested for their ability to inhibit the phosphorylation of serine 10 of histone-H3 by purified recombinant murine Aurora-A enzyme.... | Bioorg Med Chem Lett 16: 5778-83 (2006) Article DOI: 10.1016/j.bmcl.2006.08.080 BindingDB Entry DOI: 10.7270/Q2D21VVZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leukotriene B4 receptor 1 (Homo sapiens (Human)) | BDBM50001609 (8-Benzyloxy-4-[(methyl-phenethyl-carbamoyl)-methyl...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL MCE PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a |
Rhone-Poulenc Rorer Central Research Curated by ChEMBL | Assay Description Concentration of the compound inhibiting 1 nM LTB4-induced aggregation in GP polymorphonuclear (PMN) leukocytes. | J Med Chem 35: 4253-5 (1992) BindingDB Entry DOI: 10.7270/Q208648W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Integrin alpha-4/beta-1 [T112H] (Homo sapiens (Human)) | BDBM17922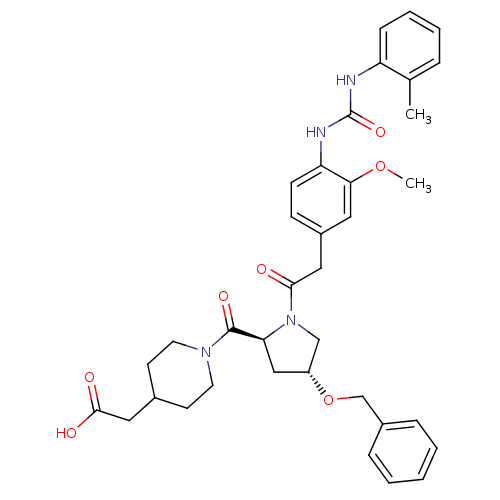 (2-(1-{[(2S,4R)-4-(benzyloxy)-1-[2-(3-methoxy-4-{[(...) | PDB UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.940 | n/a | n/a | n/a | n/a | 7.5 | 22 |
Daiichi Pharmaceutical | Assay Description The 4B4 cells expressing VLA-4 were distributed into each well of a 96-well culture plate for 2 days. Compound and Eu-labeled Human VCAM-1/Fc Chimera... | Bioorg Med Chem 14: 2725-46 (2006) Article DOI: 10.1016/j.bmc.2005.11.058 BindingDB Entry DOI: 10.7270/Q2ZW1J5H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Integrin alpha-4/beta-1 [T112H] (Homo sapiens (Human)) | BDBM17930 (2-(4-{[(2S,3S)-3-hydroxy-1-[2-(3-methoxy-4-{[(2-me...) | PDB UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.960 | n/a | n/a | n/a | n/a | 7.5 | 22 |
Daiichi Pharmaceutical | Assay Description The 4B4 cells expressing VLA-4 were distributed into each well of a 96-well culture plate for 2 days. Compound and Eu-labeled Human VCAM-1/Fc Chimera... | Bioorg Med Chem 14: 2725-46 (2006) Article DOI: 10.1016/j.bmc.2005.11.058 BindingDB Entry DOI: 10.7270/Q2ZW1J5H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Integrin alpha-4/beta-1 [T112H] (Homo sapiens (Human)) | BDBM17937 (2-(4-{[(2S)-1-[2-(3-methoxy-4-{[(2-methylphenyl)ca...) | PDB UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | 7.5 | 22 |
Daiichi Pharmaceutical | Assay Description The 4B4 cells expressing VLA-4 were distributed into each well of a 96-well culture plate for 2 days. Compound and Eu-labeled Human VCAM-1/Fc Chimera... | Bioorg Med Chem 14: 2725-46 (2006) Article DOI: 10.1016/j.bmc.2005.11.058 BindingDB Entry DOI: 10.7270/Q2ZW1J5H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Integrin alpha-4/beta-1 [T112H] (Homo sapiens (Human)) | BDBM17931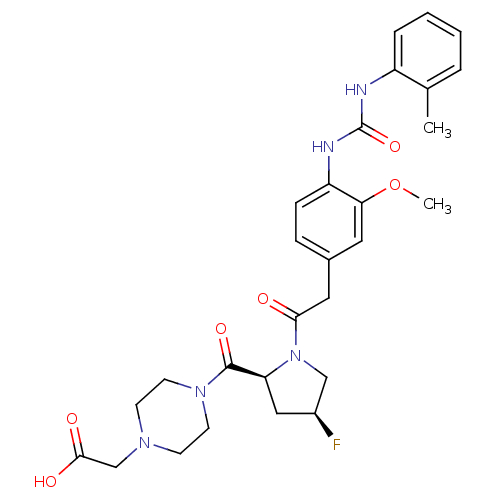 (2-(4-{[(2S,4S)-4-fluoro-1-[2-(3-methoxy-4-{[(2-met...) | PDB UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | 7.5 | 22 |
Daiichi Pharmaceutical | Assay Description The 4B4 cells expressing VLA-4 were distributed into each well of a 96-well culture plate for 2 days. Compound and Eu-labeled Human VCAM-1/Fc Chimera... | Bioorg Med Chem 14: 2725-46 (2006) Article DOI: 10.1016/j.bmc.2005.11.058 BindingDB Entry DOI: 10.7270/Q2ZW1J5H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Homo sapiens (Human)) | BDBM14209 (4-(4-{[7-(pyridin-2-yl)-7H-pyrrolo[2,3-d]pyrimidin...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | <1 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical | Assay Description The compounds were tested for their ability to inhibit the phosphorylation of poly(Glu:Tyr) by purified recombinant human FLT3. The extent of phospho... | Bioorg Med Chem Lett 16: 5778-83 (2006) Article DOI: 10.1016/j.bmcl.2006.08.080 BindingDB Entry DOI: 10.7270/Q2D21VVZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Integrin alpha-4/beta-1 [T112H] (Homo sapiens (Human)) | BDBM17920 (2-(1-{[(2S,4R)-4-methoxy-1-[2-(3-methoxy-4-{[(2-me...) | PDB UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | 7.5 | 22 |
Daiichi Pharmaceutical | Assay Description The 4B4 cells expressing VLA-4 were distributed into each well of a 96-well culture plate for 2 days. Compound and Eu-labeled Human VCAM-1/Fc Chimera... | Bioorg Med Chem 14: 2725-46 (2006) Article DOI: 10.1016/j.bmc.2005.11.058 BindingDB Entry DOI: 10.7270/Q2ZW1J5H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Integrin alpha-4/beta-1 [T112H] (Homo sapiens (Human)) | BDBM17924 (2-(1-{[(2S,4R)-4-hydroxy-1-[2-(3-methoxy-4-{[(2-me...) | PDB UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | 7.5 | 22 |
Daiichi Pharmaceutical | Assay Description The 4B4 cells expressing VLA-4 were distributed into each well of a 96-well culture plate for 2 days. Compound and Eu-labeled Human VCAM-1/Fc Chimera... | Bioorg Med Chem 14: 2725-46 (2006) Article DOI: 10.1016/j.bmc.2005.11.058 BindingDB Entry DOI: 10.7270/Q2ZW1J5H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Integrin alpha-4/beta-1 [T112H] (Homo sapiens (Human)) | BDBM17934 (2-(4-{[(2S,4S)-4-chloro-1-[2-(3-methoxy-4-{[(2-met...) | PDB UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | 7.5 | 22 |
Daiichi Pharmaceutical | Assay Description The 4B4 cells expressing VLA-4 were distributed into each well of a 96-well culture plate for 2 days. Compound and Eu-labeled Human VCAM-1/Fc Chimera... | Bioorg Med Chem 14: 2725-46 (2006) Article DOI: 10.1016/j.bmc.2005.11.058 BindingDB Entry DOI: 10.7270/Q2ZW1J5H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Integrin alpha-4/beta-1 [T112H] (Homo sapiens (Human)) | BDBM17927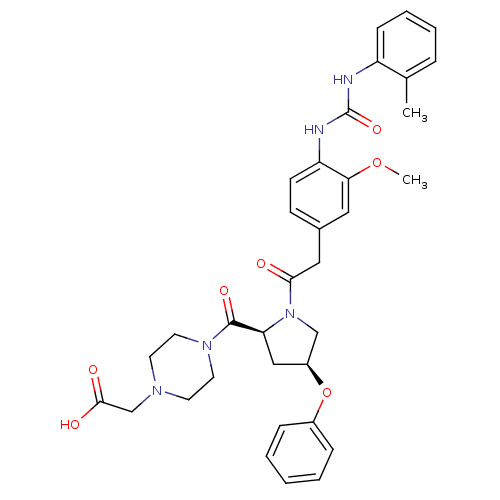 (2-(4-{[(2S,4S)-1-[2-(3-methoxy-4-{[(2-methylphenyl...) | PDB UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | 7.5 | 22 |
Daiichi Pharmaceutical | Assay Description The 4B4 cells expressing VLA-4 were distributed into each well of a 96-well culture plate for 2 days. Compound and Eu-labeled Human VCAM-1/Fc Chimera... | Bioorg Med Chem 14: 2725-46 (2006) Article DOI: 10.1016/j.bmc.2005.11.058 BindingDB Entry DOI: 10.7270/Q2ZW1J5H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Integrin alpha-4/beta-1 [T112H] (Homo sapiens (Human)) | BDBM17929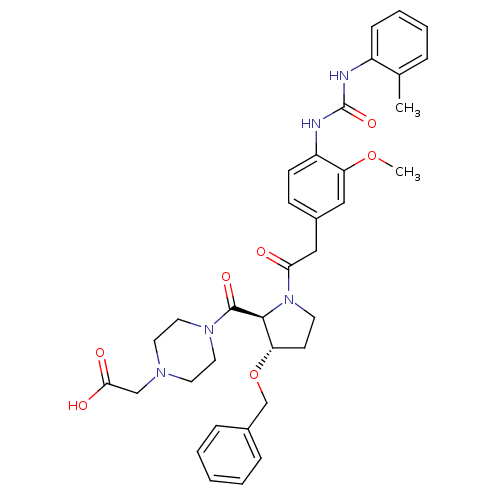 (2-(4-{[(2S,3S)-3-(benzyloxy)-1-[2-(3-methoxy-4-{[(...) | PDB UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.30 | n/a | n/a | n/a | n/a | 7.5 | 22 |
Daiichi Pharmaceutical | Assay Description The 4B4 cells expressing VLA-4 were distributed into each well of a 96-well culture plate for 2 days. Compound and Eu-labeled Human VCAM-1/Fc Chimera... | Bioorg Med Chem 14: 2725-46 (2006) Article DOI: 10.1016/j.bmc.2005.11.058 BindingDB Entry DOI: 10.7270/Q2ZW1J5H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Integrin alpha-4/beta-1 [T112H] (Homo sapiens (Human)) | BDBM17928 (2-(4-{[(2S,4S)-1-[2-(3-methoxy-4-{[(2-methylphenyl...) | PDB UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.40 | n/a | n/a | n/a | n/a | 7.5 | 22 |
Daiichi Pharmaceutical | Assay Description The 4B4 cells expressing VLA-4 were distributed into each well of a 96-well culture plate for 2 days. Compound and Eu-labeled Human VCAM-1/Fc Chimera... | Bioorg Med Chem 14: 2725-46 (2006) Article DOI: 10.1016/j.bmc.2005.11.058 BindingDB Entry DOI: 10.7270/Q2ZW1J5H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Integrin alpha-4/beta-1 (Homo sapiens (Human)) | BDBM50157050 (CHEMBL361914 | [4-(1-{2-[3-Methoxy-4-(3-o-tolyl-ur...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Daiichi Pharmaceutical Co. Ltd Curated by ChEMBL | Assay Description Inhibition of VLA-4 receptor expressed in CHO cells | Bioorg Med Chem Lett 15: 41-5 (2004) Article DOI: 10.1016/j.bmcl.2004.10.041 BindingDB Entry DOI: 10.7270/Q23J3F7Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Integrin alpha-4/beta-1 [T112H] (Homo sapiens (Human)) | BDBM17926 (2-(4-{[(2S,3R,4S)-3,4-dihydroxy-1-[2-(3-methoxy-4-...) | PDB UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.40 | n/a | n/a | n/a | n/a | 7.5 | 22 |
Daiichi Pharmaceutical | Assay Description The 4B4 cells expressing VLA-4 were distributed into each well of a 96-well culture plate for 2 days. Compound and Eu-labeled Human VCAM-1/Fc Chimera... | Bioorg Med Chem 14: 2725-46 (2006) Article DOI: 10.1016/j.bmc.2005.11.058 BindingDB Entry DOI: 10.7270/Q2ZW1J5H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Integrin alpha-4/beta-1 [T112H] (Homo sapiens (Human)) | BDBM17933 (2-(4-{[(2S)-4,4-difluoro-1-[2-(3-methoxy-4-{[(2-me...) | PDB UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.40 | n/a | n/a | n/a | n/a | 7.5 | 22 |
Daiichi Pharmaceutical | Assay Description The 4B4 cells expressing VLA-4 were distributed into each well of a 96-well culture plate for 2 days. Compound and Eu-labeled Human VCAM-1/Fc Chimera... | Bioorg Med Chem 14: 2725-46 (2006) Article DOI: 10.1016/j.bmc.2005.11.058 BindingDB Entry DOI: 10.7270/Q2ZW1J5H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Integrin alpha-4/beta-1 [T112H] (Homo sapiens (Human)) | BDBM17935 (2-[4-({2-[2-(3-methoxy-4-{[(2-methylphenyl)carbamo...) | PDB UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.5 | n/a | n/a | n/a | n/a | 7.5 | 22 |
Daiichi Pharmaceutical | Assay Description The 4B4 cells expressing VLA-4 were distributed into each well of a 96-well culture plate for 2 days. Compound and Eu-labeled Human VCAM-1/Fc Chimera... | Bioorg Med Chem 14: 2725-46 (2006) Article DOI: 10.1016/j.bmc.2005.11.058 BindingDB Entry DOI: 10.7270/Q2ZW1J5H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Integrin alpha-4/beta-1 (Homo sapiens (Human)) | BDBM50157041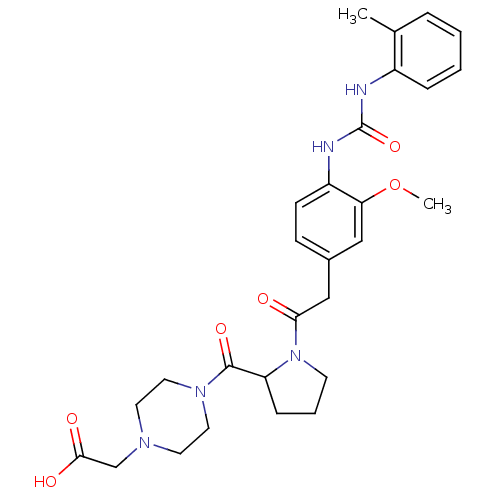 (CHEMBL185571 | [4-(1-{2-[3-Methoxy-4-(3-o-tolyl-ur...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Daiichi Pharmaceutical Co. Ltd Curated by ChEMBL | Assay Description Inhibition of VLA-4 receptor expressed in CHO cells | Bioorg Med Chem Lett 15: 41-5 (2004) Article DOI: 10.1016/j.bmcl.2004.10.041 BindingDB Entry DOI: 10.7270/Q23J3F7Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 308 total ) | Next | Last >> |