 Found 472 hits with Last Name = 'vidal' and Initial = 'l'
Found 472 hits with Last Name = 'vidal' and Initial = 'l' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Mitogen-activated protein kinase 14
(Homo sapiens (Human)) | BDBM50357914
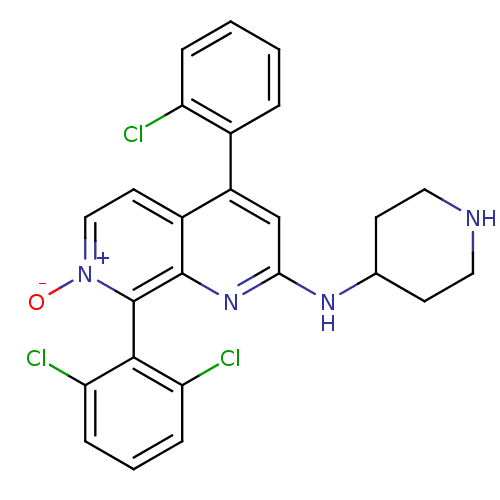
(CHEMBL1916528)Show SMILES [O-][n+]1ccc2c(cc(NC3CCNCC3)nc2c1-c1c(Cl)cccc1Cl)-c1ccccc1Cl |(20.25,-36.83,;21.59,-36.06,;21.59,-34.52,;22.92,-33.75,;24.25,-34.51,;25.59,-33.73,;26.93,-34.51,;26.93,-36.06,;28.26,-36.83,;29.59,-36.07,;30.92,-36.85,;32.25,-36.09,;32.27,-34.55,;30.93,-33.77,;29.59,-34.53,;25.58,-36.83,;24.25,-36.06,;22.92,-36.83,;22.93,-38.37,;21.59,-39.14,;20.26,-38.37,;21.59,-40.68,;22.93,-41.45,;24.27,-40.66,;24.26,-39.13,;25.59,-38.35,;25.59,-32.2,;26.93,-31.43,;26.93,-29.89,;25.59,-29.12,;24.25,-29.9,;24.26,-31.44,;22.93,-32.21,)| Show InChI InChI=1S/C25H21Cl3N4O/c26-19-5-2-1-4-16(19)18-14-22(30-15-8-11-29-12-9-15)31-24-17(18)10-13-32(33)25(24)23-20(27)6-3-7-21(23)28/h1-7,10,13-15,29H,8-9,11-12H2,(H,30,31) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.130 | n/a | n/a | n/a | n/a | n/a | n/a |
Rhône-Poulenc Rorer
Curated by ChEMBL
| Assay Description
Inhibition of human p38alpha MAP kinase after 1 hr by FRET analysis |
J Med Chem 54: 7899-910 (2011)
Article DOI: 10.1021/jm200975u
BindingDB Entry DOI: 10.7270/Q2MW2HKR |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 14
(Homo sapiens (Human)) | BDBM50357913
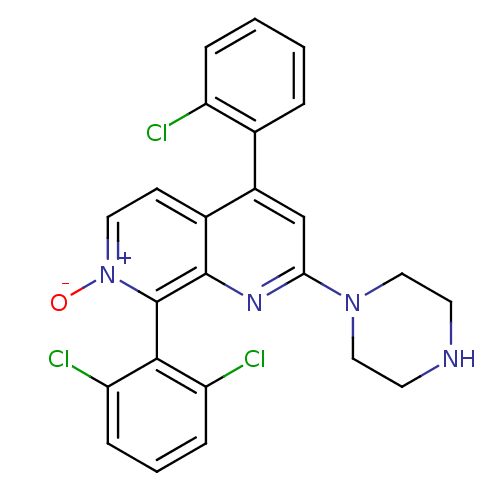
(CHEMBL1916527)Show SMILES [O-][n+]1ccc2c(cc(nc2c1-c1c(Cl)cccc1Cl)N1CCNCC1)-c1ccccc1Cl |(6.31,-36.87,;7.64,-36.1,;7.64,-34.56,;8.97,-33.79,;10.31,-34.55,;11.64,-33.77,;12.98,-34.55,;12.98,-36.1,;11.64,-36.87,;10.3,-36.1,;8.98,-36.87,;8.98,-38.4,;7.64,-39.18,;6.31,-38.41,;7.65,-40.71,;8.98,-41.48,;10.32,-40.7,;10.31,-39.17,;11.64,-38.39,;14.3,-36.87,;14.29,-38.41,;15.62,-39.18,;16.96,-38.42,;16.97,-36.88,;15.63,-36.1,;11.64,-32.24,;12.98,-31.47,;12.98,-29.93,;11.64,-29.16,;10.31,-29.94,;10.31,-31.48,;8.98,-32.25,)| Show InChI InChI=1S/C24H19Cl3N4O/c25-18-5-2-1-4-15(18)17-14-21(30-12-9-28-10-13-30)29-23-16(17)8-11-31(32)24(23)22-19(26)6-3-7-20(22)27/h1-8,11,14,28H,9-10,12-13H2 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.260 | n/a | n/a | n/a | n/a | n/a | n/a |
Rhône-Poulenc Rorer
Curated by ChEMBL
| Assay Description
Inhibition of human p38alpha MAP kinase after 1 hr by FRET analysis |
J Med Chem 54: 7899-910 (2011)
Article DOI: 10.1021/jm200975u
BindingDB Entry DOI: 10.7270/Q2MW2HKR |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 14
(Homo sapiens (Human)) | BDBM50357909
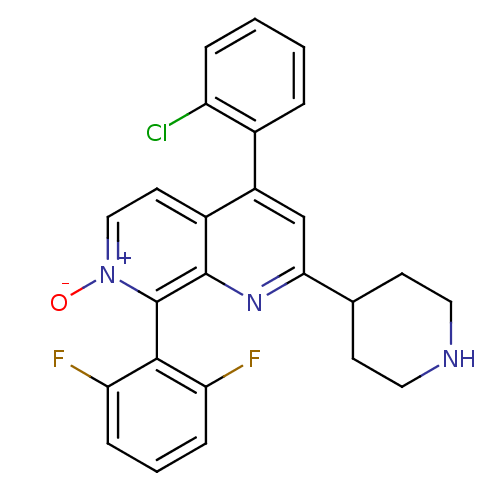
(CHEMBL1916523)Show SMILES [O-][n+]1ccc2c(cc(nc2c1-c1c(F)cccc1F)C1CCNCC1)-c1ccccc1Cl |(-8.74,-20.09,;-7.4,-19.32,;-7.4,-17.78,;-6.07,-17.01,;-4.74,-17.77,;-3.4,-16.99,;-2.06,-17.77,;-2.07,-19.32,;-3.41,-20.09,;-4.74,-19.32,;-6.07,-20.09,;-6.06,-21.63,;-7.4,-22.4,;-8.73,-21.63,;-7.4,-23.94,;-6.06,-24.71,;-4.72,-23.92,;-4.73,-22.39,;-3.4,-21.61,;-.73,-20.09,;-.74,-21.63,;.59,-22.4,;1.93,-21.64,;1.93,-20.1,;.6,-19.32,;-3.4,-15.46,;-2.07,-14.69,;-2.06,-13.15,;-3.4,-12.38,;-4.74,-13.16,;-4.73,-14.7,;-6.06,-15.47,)| Show InChI InChI=1S/C25H20ClF2N3O/c26-19-5-2-1-4-16(19)18-14-22(15-8-11-29-12-9-15)30-24-17(18)10-13-31(32)25(24)23-20(27)6-3-7-21(23)28/h1-7,10,13-15,29H,8-9,11-12H2 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.270 | n/a | n/a | n/a | n/a | n/a | n/a |
Rhône-Poulenc Rorer
Curated by ChEMBL
| Assay Description
Inhibition of human p38alpha MAP kinase after 1 hr by FRET analysis |
J Med Chem 54: 7899-910 (2011)
Article DOI: 10.1021/jm200975u
BindingDB Entry DOI: 10.7270/Q2MW2HKR |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 14
(Homo sapiens (Human)) | BDBM50357911
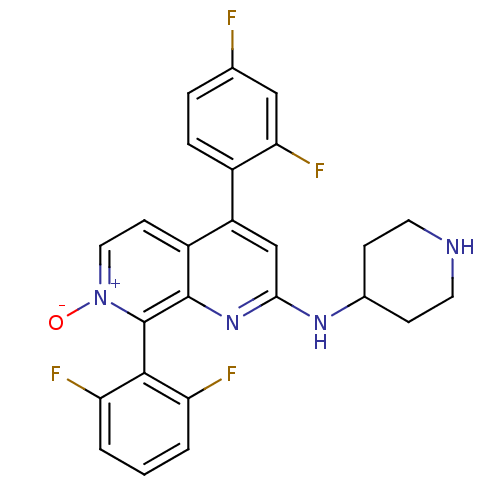
(CHEMBL1916525)Show SMILES [O-][n+]1ccc2c(cc(NC3CCNCC3)nc2c1-c1c(F)cccc1F)-c1ccc(F)cc1F |(23.08,-19.61,;24.41,-18.84,;24.41,-17.3,;25.74,-16.53,;27.08,-17.29,;28.41,-16.51,;29.75,-17.29,;29.75,-18.84,;31.08,-19.62,;32.42,-18.85,;33.75,-19.63,;35.08,-18.87,;35.09,-17.33,;33.76,-16.55,;32.41,-17.31,;28.41,-19.61,;27.08,-18.84,;25.75,-19.61,;25.75,-21.15,;27.08,-21.91,;28.41,-21.13,;27.09,-23.45,;25.76,-24.23,;24.42,-23.46,;24.42,-21.92,;23.08,-21.15,;28.41,-14.98,;29.75,-14.21,;29.75,-12.67,;28.42,-11.9,;28.41,-10.36,;27.08,-12.68,;27.08,-14.22,;25.75,-14.99,)| Show InChI InChI=1S/C25H20F4N4O/c26-14-4-5-16(21(29)12-14)18-13-22(31-15-6-9-30-10-7-15)32-24-17(18)8-11-33(34)25(24)23-19(27)2-1-3-20(23)28/h1-5,8,11-13,15,30H,6-7,9-10H2,(H,31,32) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.280 | n/a | n/a | n/a | n/a | n/a | n/a |
Rhône-Poulenc Rorer
Curated by ChEMBL
| Assay Description
Inhibition of human p38alpha MAP kinase after 1 hr by FRET analysis |
J Med Chem 54: 7899-910 (2011)
Article DOI: 10.1021/jm200975u
BindingDB Entry DOI: 10.7270/Q2MW2HKR |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 14
(Homo sapiens (Human)) | BDBM50357916
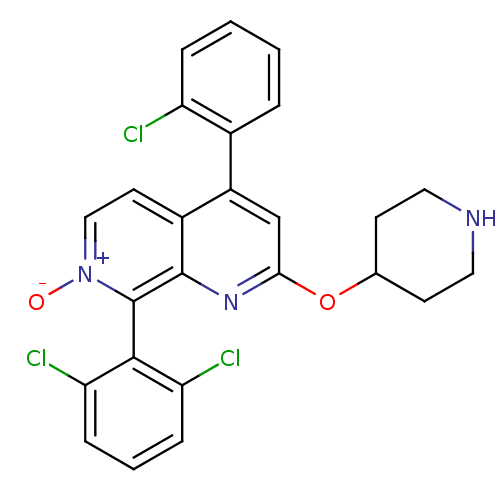
(CHEMBL1916530)Show SMILES [O-][n+]1ccc2c(cc(OC3CCNCC3)nc2c1-c1c(Cl)cccc1Cl)-c1ccccc1Cl |(8.24,-4.99,;9.58,-4.22,;9.58,-2.68,;10.91,-1.9,;12.24,-2.67,;13.58,-1.89,;14.92,-2.67,;14.91,-4.22,;16.25,-4.99,;17.58,-4.23,;18.91,-5.01,;20.25,-4.25,;20.26,-2.71,;18.93,-1.93,;17.58,-2.69,;13.57,-4.99,;12.24,-4.22,;10.91,-4.99,;10.92,-6.52,;9.58,-7.3,;8.25,-6.53,;9.58,-8.83,;10.92,-9.6,;12.26,-8.82,;12.25,-7.29,;13.58,-6.51,;13.58,-.36,;14.91,.41,;14.92,1.95,;13.58,2.72,;12.24,1.94,;12.25,.4,;10.92,-.37,)| Show InChI InChI=1S/C25H20Cl3N3O2/c26-19-5-2-1-4-16(19)18-14-22(33-15-8-11-29-12-9-15)30-24-17(18)10-13-31(32)25(24)23-20(27)6-3-7-21(23)28/h1-7,10,13-15,29H,8-9,11-12H2 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.460 | n/a | n/a | n/a | n/a | n/a | n/a |
Rhône-Poulenc Rorer
Curated by ChEMBL
| Assay Description
Inhibition of human p38alpha MAP kinase after 1 hr by FRET analysis |
J Med Chem 54: 7899-910 (2011)
Article DOI: 10.1021/jm200975u
BindingDB Entry DOI: 10.7270/Q2MW2HKR |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 14
(Homo sapiens (Human)) | BDBM50357903
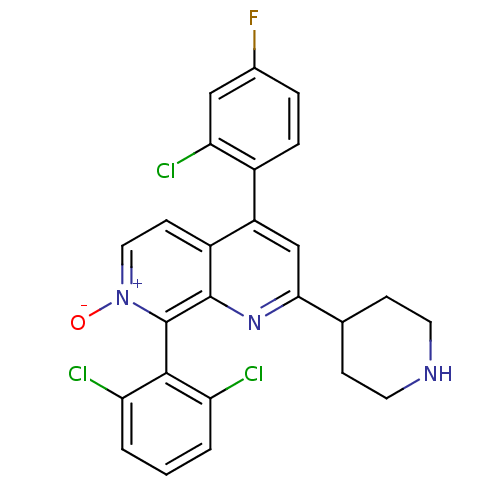
(CHEMBL1916360)Show SMILES [O-][n+]1ccc2c(cc(nc2c1-c1c(Cl)cccc1Cl)C1CCNCC1)-c1ccc(F)cc1Cl |(-9.19,-39.37,;-7.86,-38.61,;-7.86,-37.06,;-6.53,-36.29,;-5.19,-37.05,;-3.86,-36.28,;-2.52,-37.06,;-2.52,-38.6,;-3.87,-39.37,;-5.2,-38.6,;-6.53,-39.38,;-6.52,-40.91,;-7.86,-41.68,;-9.19,-40.91,;-7.85,-43.22,;-6.52,-43.99,;-5.18,-43.21,;-5.19,-41.67,;-3.86,-40.89,;-1.19,-39.37,;-1.2,-40.91,;.13,-41.69,;1.47,-40.93,;1.47,-39.38,;.14,-38.6,;-3.86,-34.75,;-2.52,-33.98,;-2.52,-32.44,;-3.86,-31.67,;-3.86,-30.13,;-5.2,-32.45,;-5.19,-33.98,;-6.52,-34.76,)| Show InChI InChI=1S/C25H19Cl3FN3O/c26-19-2-1-3-20(27)23(19)25-24-17(8-11-32(25)33)18(16-5-4-15(29)12-21(16)28)13-22(31-24)14-6-9-30-10-7-14/h1-5,8,11-14,30H,6-7,9-10H2 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.490 | n/a | n/a | n/a | n/a | n/a | n/a |
Rhône-Poulenc Rorer
Curated by ChEMBL
| Assay Description
Inhibition of human p38alpha MAP kinase after 1 hr by FRET analysis |
J Med Chem 54: 7899-910 (2011)
Article DOI: 10.1021/jm200975u
BindingDB Entry DOI: 10.7270/Q2MW2HKR |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 14
(Homo sapiens (Human)) | BDBM50357915
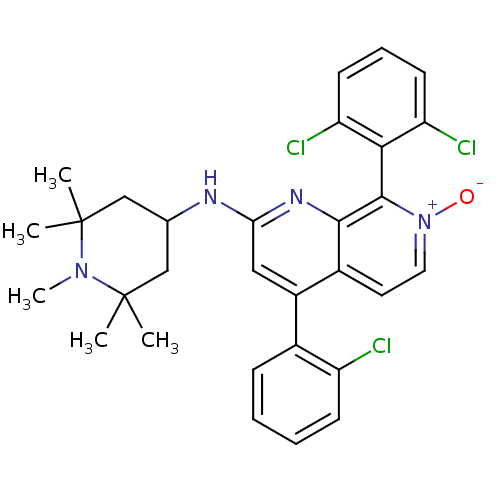
(CHEMBL1916529)Show SMILES CN1C(C)(C)CC(CC1(C)C)Nc1cc(-c2ccccc2Cl)c2cc[n+]([O-])c(-c3c(Cl)cccc3Cl)c2n1 |(4.35,-2.11,;3.01,-2.87,;2.99,-4.41,;4.53,-4.41,;3.76,-5.74,;1.66,-5.17,;.33,-4.39,;.33,-2.86,;1.67,-2.09,;.89,-.75,;2.44,-.75,;-1.01,-5.16,;-2.34,-4.38,;-2.34,-2.84,;-3.67,-2.06,;-3.67,-.52,;-2.34,.24,;-2.34,1.78,;-3.67,2.55,;-5.01,1.78,;-5,.24,;-6.33,-.53,;-5.01,-2.83,;-6.34,-2.07,;-7.67,-2.84,;-7.67,-4.38,;-9.01,-5.15,;-6.34,-5.15,;-6.33,-6.69,;-7.67,-7.46,;-9,-6.69,;-7.67,-9,;-6.33,-9.77,;-4.99,-8.98,;-5,-7.45,;-3.67,-6.67,;-5.01,-4.38,;-3.68,-5.15,)| Show InChI InChI=1S/C30H31Cl3N4O/c1-29(2)16-18(17-30(3,4)36(29)5)34-25-15-21(19-9-6-7-10-22(19)31)20-13-14-37(38)28(27(20)35-25)26-23(32)11-8-12-24(26)33/h6-15,18H,16-17H2,1-5H3,(H,34,35) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Rhône-Poulenc Rorer
Curated by ChEMBL
| Assay Description
Inhibition of human p38alpha MAP kinase after 1 hr by FRET analysis |
J Med Chem 54: 7899-910 (2011)
Article DOI: 10.1021/jm200975u
BindingDB Entry DOI: 10.7270/Q2MW2HKR |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 14
(Homo sapiens (Human)) | BDBM50357906
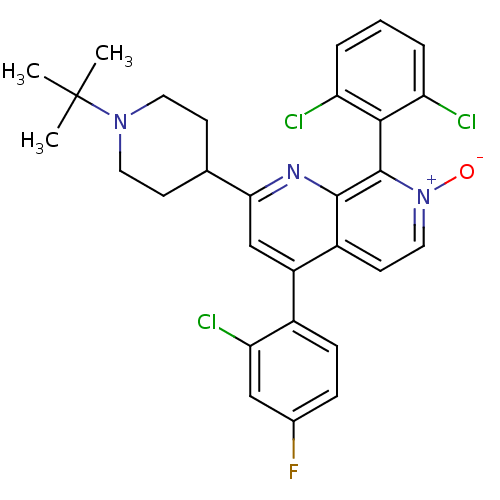
(CHEMBL1916520)Show SMILES CC(C)(C)N1CCC(CC1)c1cc(-c2ccc(F)cc2Cl)c2cc[n+]([O-])c(-c3c(Cl)cccc3Cl)c2n1 |(2.33,-7.59,;2.34,-6.04,;3.68,-5.28,;3.67,-6.81,;1.02,-5.27,;-.33,-6.03,;-1.65,-5.25,;-1.64,-3.71,;-.31,-2.94,;1.02,-3.72,;-2.98,-2.94,;-2.97,-1.4,;-4.31,-.62,;-4.31,.91,;-2.98,1.68,;-2.97,3.22,;-4.31,3.99,;-4.31,5.53,;-5.65,3.22,;-5.64,1.68,;-6.97,.91,;-5.65,-1.39,;-6.98,-.63,;-8.31,-1.4,;-8.31,-2.94,;-9.65,-3.71,;-6.98,-3.72,;-6.97,-5.25,;-8.31,-6.02,;-9.64,-5.25,;-8.31,-7.56,;-6.97,-8.33,;-5.63,-7.55,;-5.64,-6.01,;-4.31,-5.23,;-5.65,-2.94,;-4.32,-3.71,)| Show InChI InChI=1S/C29H27Cl3FN3O/c1-29(2,3)35-12-9-17(10-13-35)25-16-21(19-8-7-18(33)15-24(19)32)20-11-14-36(37)28(27(20)34-25)26-22(30)5-4-6-23(26)31/h4-8,11,14-17H,9-10,12-13H2,1-3H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.530 | n/a | n/a | n/a | n/a | n/a | n/a |
Rhône-Poulenc Rorer
Curated by ChEMBL
| Assay Description
Inhibition of human p38alpha MAP kinase after 1 hr by FRET analysis |
J Med Chem 54: 7899-910 (2011)
Article DOI: 10.1021/jm200975u
BindingDB Entry DOI: 10.7270/Q2MW2HKR |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 14
(Homo sapiens (Human)) | BDBM50357905
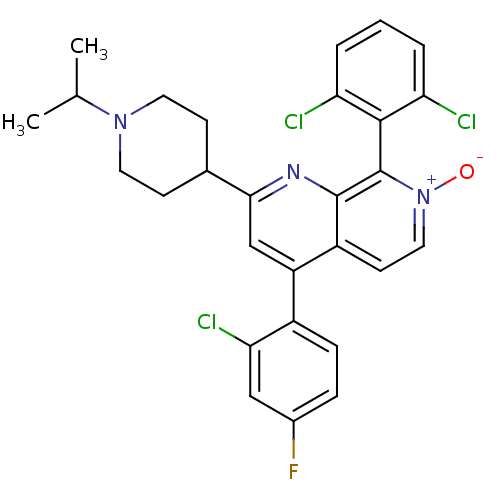
(CHEMBL1916519)Show SMILES CC(C)N1CCC(CC1)c1cc(-c2ccc(F)cc2Cl)c2cc[n+]([O-])c(-c3c(Cl)cccc3Cl)c2n1 |(32.53,-43.15,;32.54,-41.61,;33.88,-40.85,;31.21,-40.83,;29.87,-41.59,;28.54,-40.82,;28.55,-39.28,;29.88,-38.51,;31.22,-39.29,;27.23,-38.51,;27.23,-36.96,;25.89,-36.19,;25.89,-34.65,;27.23,-33.88,;27.23,-32.35,;25.89,-31.57,;25.89,-30.03,;24.55,-32.35,;24.56,-33.89,;23.23,-34.66,;24.56,-36.96,;23.22,-36.2,;21.89,-36.97,;21.89,-38.51,;20.56,-39.28,;23.22,-39.28,;23.23,-40.82,;21.89,-41.59,;20.56,-40.82,;21.9,-43.13,;23.23,-43.89,;24.57,-43.11,;24.56,-41.58,;25.89,-40.8,;24.55,-38.51,;25.88,-39.28,)| Show InChI InChI=1S/C28H25Cl3FN3O/c1-16(2)34-11-8-17(9-12-34)25-15-21(19-7-6-18(32)14-24(19)31)20-10-13-35(36)28(27(20)33-25)26-22(29)4-3-5-23(26)30/h3-7,10,13-17H,8-9,11-12H2,1-2H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.590 | n/a | n/a | n/a | n/a | n/a | n/a |
Rhône-Poulenc Rorer
Curated by ChEMBL
| Assay Description
Inhibition of human p38alpha MAP kinase after 1 hr by FRET analysis |
J Med Chem 54: 7899-910 (2011)
Article DOI: 10.1021/jm200975u
BindingDB Entry DOI: 10.7270/Q2MW2HKR |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 13
(Homo sapiens (Human)) | BDBM50420761
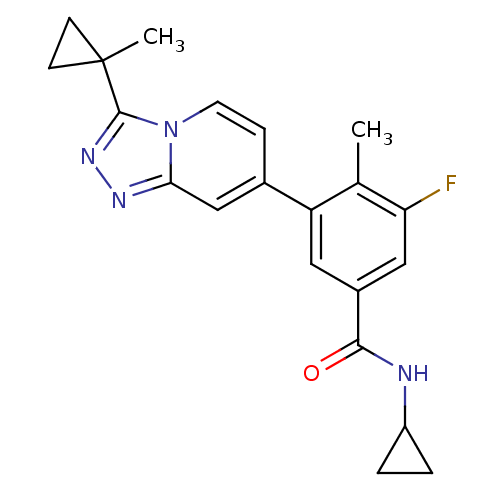
(CHEMBL2087507)Show SMILES Cc1c(F)cc(cc1-c1ccn2c(nnc2c1)C1(C)CC1)C(=O)NC1CC1 Show InChI InChI=1S/C21H21FN4O/c1-12-16(9-14(10-17(12)22)19(27)23-15-3-4-15)13-5-8-26-18(11-13)24-25-20(26)21(2)6-7-21/h5,8-11,15H,3-4,6-7H2,1-2H3,(H,23,27) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.640 | n/a | n/a | n/a | n/a | n/a | n/a |
Rhône-Poulenc Rorer
Curated by ChEMBL
| Assay Description
Inhibition of p38alpha |
Bioorg Med Chem Lett 22: 3431-6 (2012)
Article DOI: 10.1016/j.bmcl.2012.03.099
BindingDB Entry DOI: 10.7270/Q2348MNX |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 14
(Homo sapiens (Human)) | BDBM50357904
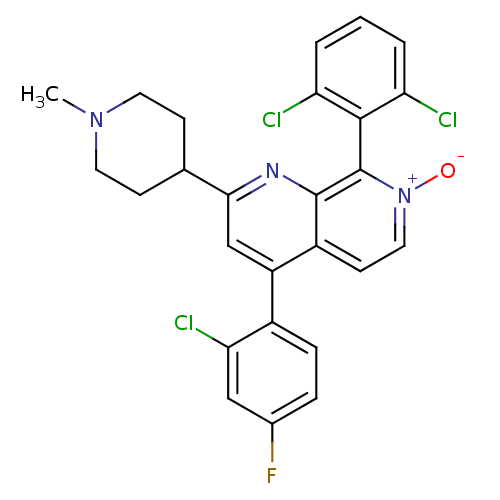
(CHEMBL1916361)Show SMILES CN1CCC(CC1)c1cc(-c2ccc(F)cc2Cl)c2cc[n+]([O-])c(-c3c(Cl)cccc3Cl)c2n1 |(17.77,-41.9,;16.45,-41.12,;15.11,-41.88,;13.78,-41.11,;13.79,-39.56,;15.12,-38.8,;16.45,-39.58,;12.46,-38.8,;12.47,-37.25,;11.13,-36.47,;11.13,-34.94,;12.46,-34.17,;12.47,-32.63,;11.13,-31.86,;11.13,-30.32,;9.79,-32.64,;9.8,-34.18,;8.47,-34.95,;9.79,-37.25,;8.46,-36.48,;7.13,-37.26,;7.13,-38.8,;5.79,-39.57,;8.46,-39.57,;8.47,-41.1,;7.13,-41.88,;5.8,-41.11,;7.13,-43.41,;8.47,-44.18,;9.81,-43.4,;9.8,-41.87,;11.13,-41.09,;9.79,-38.8,;11.12,-39.57,)| Show InChI InChI=1S/C26H21Cl3FN3O/c1-32-10-7-15(8-11-32)23-14-19(17-6-5-16(30)13-22(17)29)18-9-12-33(34)26(25(18)31-23)24-20(27)3-2-4-21(24)28/h2-6,9,12-15H,7-8,10-11H2,1H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.720 | n/a | n/a | n/a | n/a | n/a | n/a |
Rhône-Poulenc Rorer
Curated by ChEMBL
| Assay Description
Inhibition of human p38alpha MAP kinase after 1 hr by FRET analysis |
J Med Chem 54: 7899-910 (2011)
Article DOI: 10.1021/jm200975u
BindingDB Entry DOI: 10.7270/Q2MW2HKR |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 14
(Homo sapiens (Human)) | BDBM15242
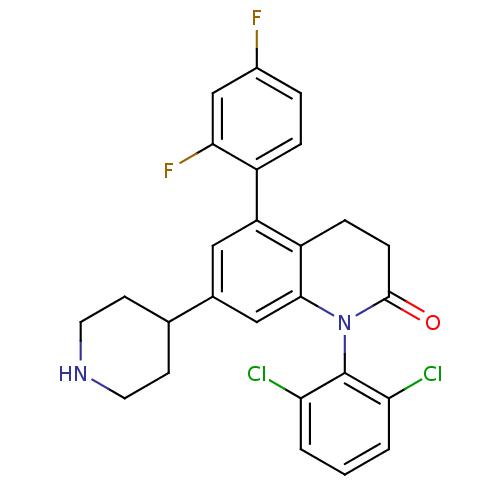
(1-(2,6-dichlorophenyl)-5-(2,4-difluorophenyl)-7-(4...)Show SMILES Fc1ccc(c(F)c1)-c1cc(cc2N(C(=O)CCc12)c1c(Cl)cccc1Cl)C1CCNCC1 Show InChI InChI=1S/C26H22Cl2F2N2O/c27-21-2-1-3-22(28)26(21)32-24-13-16(15-8-10-31-11-9-15)12-20(19(24)6-7-25(32)33)18-5-4-17(29)14-23(18)30/h1-5,12-15,31H,6-11H2 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| DrugBank
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
MMDB
PDB
Article
PubMed
| n/a | n/a | 0.740 | n/a | n/a | n/a | n/a | n/a | n/a |
Rhône-Poulenc Rorer
Curated by ChEMBL
| Assay Description
Inhibition of p38alpha MAP kinase |
J Med Chem 54: 7899-910 (2011)
Article DOI: 10.1021/jm200975u
BindingDB Entry DOI: 10.7270/Q2MW2HKR |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Mitogen-activated protein kinase 14
(Homo sapiens (Human)) | BDBM50357902
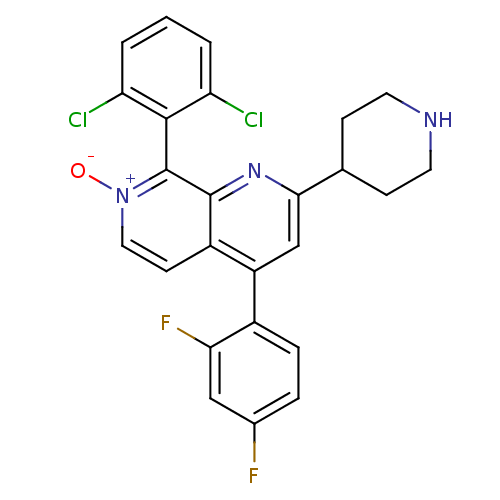
(CHEMBL1738839)Show SMILES [O-][n+]1ccc2c(cc(nc2c1-c1c(Cl)cccc1Cl)C1CCNCC1)-c1ccc(F)cc1F |(3.73,1.38,;2.66,.77,;2.66,-.77,;1.33,-1.54,;,-.77,;-1.33,-1.54,;-2.68,-.77,;-2.68,.77,;-1.33,1.54,;,.77,;1.33,1.54,;1.33,3.08,;-0,3.85,;-1.07,3.23,;-.01,5.39,;1.33,6.16,;2.66,5.39,;2.66,3.85,;3.73,3.24,;-4.01,1.54,;-5.35,.78,;-6.68,1.56,;-6.67,3.1,;-5.33,3.86,;-4,3.08,;-1.33,-3.08,;.01,-3.84,;.01,-5.38,;-1.32,-6.16,;-1.32,-7.39,;-2.65,-5.39,;-2.66,-3.85,;-3.73,-3.24,)| Show InChI InChI=1S/C25H19Cl2F2N3O/c26-19-2-1-3-20(27)23(19)25-24-17(8-11-32(25)33)18(16-5-4-15(28)12-21(16)29)13-22(31-24)14-6-9-30-10-7-14/h1-5,8,11-14,30H,6-7,9-10H2 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | 0.820 | n/a | n/a | n/a | n/a | n/a | n/a |
Rhône-Poulenc Rorer
Curated by ChEMBL
| Assay Description
Inhibition of human p38alpha MAP kinase after 1 hr by FRET analysis |
J Med Chem 54: 7899-910 (2011)
Article DOI: 10.1021/jm200975u
BindingDB Entry DOI: 10.7270/Q2MW2HKR |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Mitogen-activated protein kinase 13
(Homo sapiens (Human)) | BDBM50420769
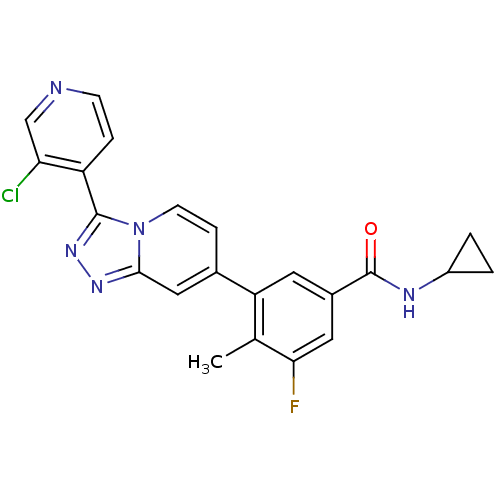
(CHEMBL2087515)Show SMILES Cc1c(F)cc(cc1-c1ccn2c(nnc2c1)-c1ccncc1Cl)C(=O)NC1CC1 Show InChI InChI=1S/C22H17ClFN5O/c1-12-17(8-14(9-19(12)24)22(30)26-15-2-3-15)13-5-7-29-20(10-13)27-28-21(29)16-4-6-25-11-18(16)23/h4-11,15H,2-3H2,1H3,(H,26,30) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.830 | n/a | n/a | n/a | n/a | n/a | n/a |
Rhône-Poulenc Rorer
Curated by ChEMBL
| Assay Description
Inhibition of p38alpha |
Bioorg Med Chem Lett 22: 3431-6 (2012)
Article DOI: 10.1016/j.bmcl.2012.03.099
BindingDB Entry DOI: 10.7270/Q2348MNX |
More data for this
Ligand-Target Pair | |
Prostaglandin D2 receptor 2
(Homo sapiens (Human)) | BDBM50035089
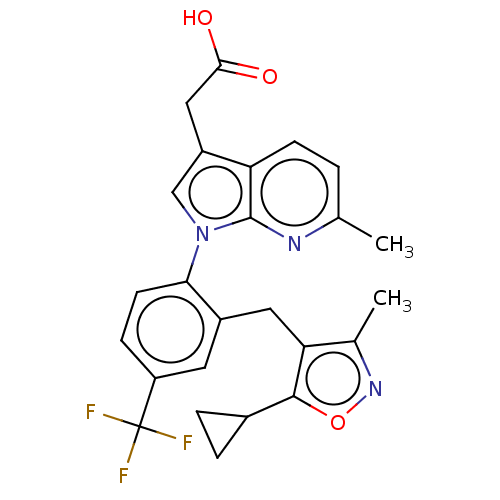
(CHEMBL3338297)Show SMILES Cc1noc(C2CC2)c1Cc1cc(ccc1-n1cc(CC(O)=O)c2ccc(C)nc12)C(F)(F)F Show InChI InChI=1S/C25H22F3N3O3/c1-13-3-7-19-17(11-22(32)33)12-31(24(19)29-13)21-8-6-18(25(26,27)28)9-16(21)10-20-14(2)30-34-23(20)15-4-5-15/h3,6-9,12,15H,4-5,10-11H2,1-2H3,(H,32,33) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Rhône-Poulenc Rorer
Curated by ChEMBL
| Assay Description
Antagonist activity at CRTh2 (unknown origin) stably expressed in PGD2-stimulated CHO.K1 cells preincubated for 1 hr by radio-labelled [35S]-GTPgamma... |
Bioorg Med Chem Lett 24: 5127-33 (2014)
Article DOI: 10.1016/j.bmcl.2014.08.028
BindingDB Entry DOI: 10.7270/Q27P911Z |
More data for this
Ligand-Target Pair | |
Prostaglandin D2 receptor 2
(Homo sapiens (Human)) | BDBM50035088
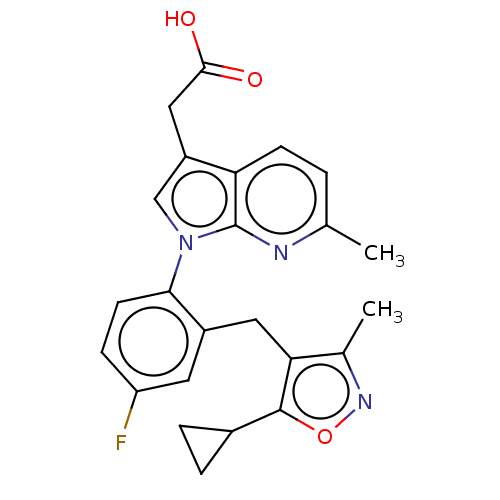
(CHEMBL3338296)Show SMILES Cc1noc(C2CC2)c1Cc1cc(F)ccc1-n1cc(CC(O)=O)c2ccc(C)nc12 Show InChI InChI=1S/C24H22FN3O3/c1-13-3-7-19-17(11-22(29)30)12-28(24(19)26-13)21-8-6-18(25)9-16(21)10-20-14(2)27-31-23(20)15-4-5-15/h3,6-9,12,15H,4-5,10-11H2,1-2H3,(H,29,30) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Rhône-Poulenc Rorer
Curated by ChEMBL
| Assay Description
Antagonist activity at CRTH2 receptor in human whole blood assessed as inhibition of PGD2-induced eosinophil shape change after 10 mins by fluorescen... |
Bioorg Med Chem Lett 24: 5127-33 (2014)
Article DOI: 10.1016/j.bmcl.2014.08.028
BindingDB Entry DOI: 10.7270/Q27P911Z |
More data for this
Ligand-Target Pair | |
Prostaglandin D2 receptor 2
(Homo sapiens (Human)) | BDBM50035039
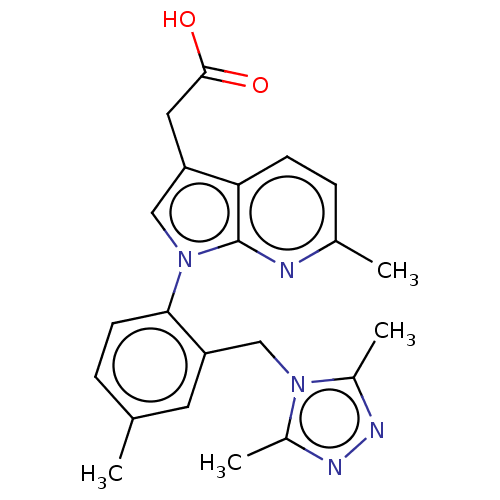
(CHEMBL3338270)Show SMILES Cc1nnc(C)n1Cc1cc(C)ccc1-n1cc(CC(O)=O)c2ccc(C)nc12 Show InChI InChI=1S/C22H23N5O2/c1-13-5-8-20(18(9-13)12-26-15(3)24-25-16(26)4)27-11-17(10-21(28)29)19-7-6-14(2)23-22(19)27/h5-9,11H,10,12H2,1-4H3,(H,28,29) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Rhône-Poulenc Rorer
Curated by ChEMBL
| Assay Description
Antagonist activity at CRTH2 receptor in human whole blood assessed as inhibition of PGD2-induced eosinophil shape change after 10 mins by fluorescen... |
Bioorg Med Chem Lett 24: 5127-33 (2014)
Article DOI: 10.1016/j.bmcl.2014.08.028
BindingDB Entry DOI: 10.7270/Q27P911Z |
More data for this
Ligand-Target Pair | |
Prostaglandin D2 receptor 2
(Homo sapiens (Human)) | BDBM50034956
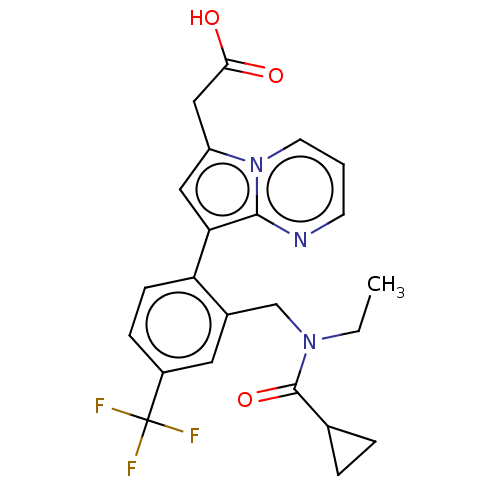
(CHEMBL3338150)Show SMILES CCN(Cc1cc(ccc1-c1cc(CC(O)=O)n2cccnc12)C(F)(F)F)C(=O)C1CC1 Show InChI InChI=1S/C23H22F3N3O3/c1-2-28(22(32)14-4-5-14)13-15-10-16(23(24,25)26)6-7-18(15)19-11-17(12-20(30)31)29-9-3-8-27-21(19)29/h3,6-11,14H,2,4-5,12-13H2,1H3,(H,30,31) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Rhône-Poulenc Rorer
Curated by ChEMBL
| Assay Description
Antagonist activity at CRTh2 receptor in human eosinophil assessed as inhibition of PGD2-induced cell shape change |
Bioorg Med Chem Lett 24: 5118-22 (2014)
Article DOI: 10.1016/j.bmcl.2014.09.005
BindingDB Entry DOI: 10.7270/Q2H70HFQ |
More data for this
Ligand-Target Pair | |
Prostaglandin D2 receptor 2
(Homo sapiens (Human)) | BDBM50034956

(CHEMBL3338150)Show SMILES CCN(Cc1cc(ccc1-c1cc(CC(O)=O)n2cccnc12)C(F)(F)F)C(=O)C1CC1 Show InChI InChI=1S/C23H22F3N3O3/c1-2-28(22(32)14-4-5-14)13-15-10-16(23(24,25)26)6-7-18(15)19-11-17(12-20(30)31)29-9-3-8-27-21(19)29/h3,6-11,14H,2,4-5,12-13H2,1H3,(H,30,31) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Rhône-Poulenc Rorer
Curated by ChEMBL
| Assay Description
Antagonist activity at CRTh2 receptor in human whole blood assessed as inhibition of PGD2-induced eosinophil shape change preincubated for 10 mins wi... |
Bioorg Med Chem Lett 24: 5123-6 (2014)
Article DOI: 10.1016/j.bmcl.2014.08.029
BindingDB Entry DOI: 10.7270/Q2CF9RRW |
More data for this
Ligand-Target Pair | |
D(2) dopamine receptor
(Rattus norvegicus (rat)) | BDBM50092455
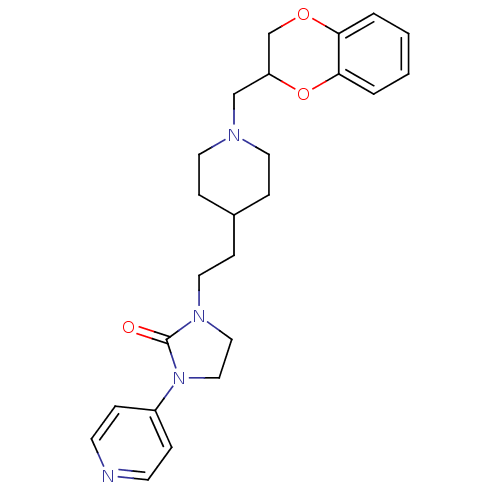
(1-{2-[1-(2,3-Dihydro-benzo[1,4]dioxin-2-ylmethyl)-...)Show SMILES O=C1N(CCC2CCN(CC3COc4ccccc4O3)CC2)CCN1c1ccncc1 Show InChI InChI=1S/C24H30N4O3/c29-24-27(15-16-28(24)20-5-10-25-11-6-20)14-9-19-7-12-26(13-8-19)17-21-18-30-22-3-1-2-4-23(22)31-21/h1-6,10-11,19,21H,7-9,12-18H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Centre de Recherche Pierre Fabre
Curated by ChEMBL
| Assay Description
In vitro inhibition of specific binding of [3H]-YM 09151-2 to Dopamine receptor D2 site in rat striatal tissue. |
J Med Chem 43: 3653-64 (2000)
BindingDB Entry DOI: 10.7270/Q2MW2GD3 |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Rattus norvegicus (rat)) | BDBM50040619

(1-[2-(1-Benzyl-piperidin-4-yl)-ethyl]-3-(3-nitro-b...)Show SMILES [O-][N+](=O)c1cccc(c1)C(=O)[NH+]=C([S-])NCCC1CCN(Cc2ccccc2)CC1 |w:11.11| Show InChI InChI=1S/C22H26N4O3S/c27-21(19-7-4-8-20(15-19)26(28)29)24-22(30)23-12-9-17-10-13-25(14-11-17)16-18-5-2-1-3-6-18/h1-8,15,17H,9-14,16H2,(H2,23,24,27,30) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Centre de Recherche Pierre Fabre Médicament
Curated by ChEMBL
| Assay Description
Inhibition against acetylcholinesterase (AChE) |
J Med Chem 37: 689-95 (1994)
BindingDB Entry DOI: 10.7270/Q2N29W0C |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 13
(Homo sapiens (Human)) | BDBM50420767
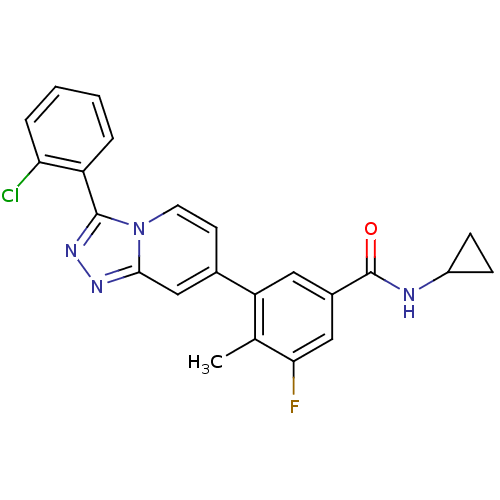
(CHEMBL2087513)Show SMILES Cc1c(F)cc(cc1-c1ccn2c(nnc2c1)-c1ccccc1Cl)C(=O)NC1CC1 Show InChI InChI=1S/C23H18ClFN4O/c1-13-18(10-15(11-20(13)25)23(30)26-16-6-7-16)14-8-9-29-21(12-14)27-28-22(29)17-4-2-3-5-19(17)24/h2-5,8-12,16H,6-7H2,1H3,(H,26,30) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Rhône-Poulenc Rorer
Curated by ChEMBL
| Assay Description
Inhibition of p38alpha |
Bioorg Med Chem Lett 22: 3431-6 (2012)
Article DOI: 10.1016/j.bmcl.2012.03.099
BindingDB Entry DOI: 10.7270/Q2348MNX |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 13
(Homo sapiens (Human)) | BDBM50420746
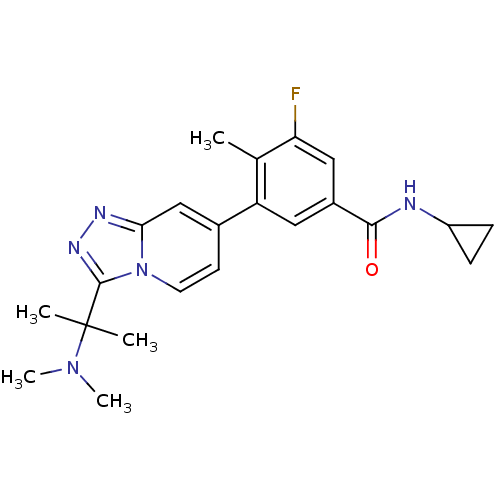
(CHEMBL2087524)Show SMILES CN(C)C(C)(C)c1nnc2cc(ccn12)-c1cc(cc(F)c1C)C(=O)NC1CC1 Show InChI InChI=1S/C22H26FN5O/c1-13-17(10-15(11-18(13)23)20(29)24-16-6-7-16)14-8-9-28-19(12-14)25-26-21(28)22(2,3)27(4)5/h8-12,16H,6-7H2,1-5H3,(H,24,29) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Rhône-Poulenc Rorer
Curated by ChEMBL
| Assay Description
Inhibition of p38alpha |
Bioorg Med Chem Lett 22: 3431-6 (2012)
Article DOI: 10.1016/j.bmcl.2012.03.099
BindingDB Entry DOI: 10.7270/Q2348MNX |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Rattus norvegicus (rat)) | BDBM8960

((+/-)-2-[(1-benzylpiperidin-4-yl)methyl]-5,6-dimet...)Show SMILES COc1cc2CC(CC3CCN(Cc4ccccc4)CC3)C(=O)c2cc1OC Show InChI InChI=1S/C24H29NO3/c1-27-22-14-19-13-20(24(26)21(19)15-23(22)28-2)12-17-8-10-25(11-9-17)16-18-6-4-3-5-7-18/h3-7,14-15,17,20H,8-13,16H2,1-2H3 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
PubMed
| n/a | n/a | 1.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Centre de Recherche Pierre Fabre
Curated by ChEMBL
| Assay Description
In vitro inhibitory effect on rat Acetylcholinesterase |
J Med Chem 38: 2969-73 (1995)
BindingDB Entry DOI: 10.7270/Q22B8X2S |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
D(2) dopamine receptor
(Rattus norvegicus (rat)) | BDBM50092453
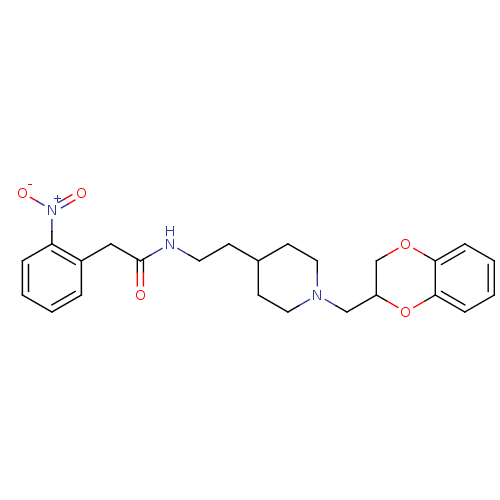
(CHEMBL120989 | N-{2-[1-(2,3-Dihydro-benzo[1,4]diox...)Show SMILES [O-][N+](=O)c1ccccc1CC(=O)NCCC1CCN(CC2COc3ccccc3O2)CC1 Show InChI InChI=1S/C24H29N3O5/c28-24(15-19-5-1-2-6-21(19)27(29)30)25-12-9-18-10-13-26(14-11-18)16-20-17-31-22-7-3-4-8-23(22)32-20/h1-8,18,20H,9-17H2,(H,25,28) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| PubMed
| n/a | n/a | 1.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Centre de Recherche Pierre Fabre
Curated by ChEMBL
| Assay Description
In vitro inhibition of specific binding of [3H]-YM 09151-2 to Dopamine receptor D2 site in rat striatal tissue. |
J Med Chem 43: 3653-64 (2000)
BindingDB Entry DOI: 10.7270/Q2MW2GD3 |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 13
(Homo sapiens (Human)) | BDBM50420763
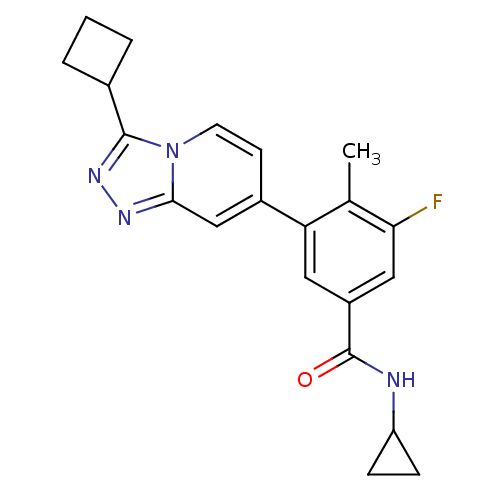
(CHEMBL2087509)Show SMILES Cc1c(F)cc(cc1-c1ccn2c(nnc2c1)C1CCC1)C(=O)NC1CC1 Show InChI InChI=1S/C21H21FN4O/c1-12-17(9-15(10-18(12)22)21(27)23-16-5-6-16)14-7-8-26-19(11-14)24-25-20(26)13-3-2-4-13/h7-11,13,16H,2-6H2,1H3,(H,23,27) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Rhône-Poulenc Rorer
Curated by ChEMBL
| Assay Description
Inhibition of p38alpha |
Bioorg Med Chem Lett 22: 3431-6 (2012)
Article DOI: 10.1016/j.bmcl.2012.03.099
BindingDB Entry DOI: 10.7270/Q2348MNX |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 13
(Homo sapiens (Human)) | BDBM50420756
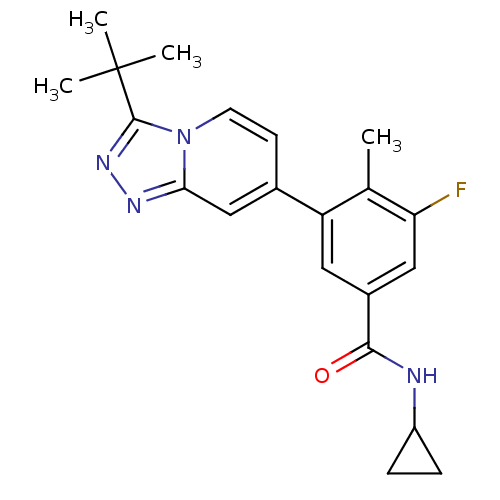
(CHEMBL2087501)Show SMILES Cc1c(F)cc(cc1-c1ccn2c(nnc2c1)C(C)(C)C)C(=O)NC1CC1 Show InChI InChI=1S/C21H23FN4O/c1-12-16(9-14(10-17(12)22)19(27)23-15-5-6-15)13-7-8-26-18(11-13)24-25-20(26)21(2,3)4/h7-11,15H,5-6H2,1-4H3,(H,23,27) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Rhône-Poulenc Rorer
Curated by ChEMBL
| Assay Description
Inhibition of p38alpha |
Bioorg Med Chem Lett 22: 3431-6 (2012)
Article DOI: 10.1016/j.bmcl.2012.03.099
BindingDB Entry DOI: 10.7270/Q2348MNX |
More data for this
Ligand-Target Pair | |
Prostaglandin D2 receptor 2
(Homo sapiens (Human)) | BDBM50154763
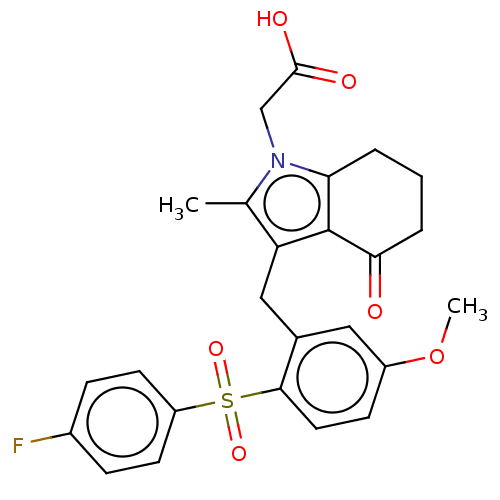
(CHEMBL3774415)Show SMILES COc1ccc(c(Cc2c(C)n(CC(O)=O)c3CCCC(=O)c23)c1)S(=O)(=O)c1ccc(F)cc1 Show InChI InChI=1S/C25H24FNO6S/c1-15-20(25-21(4-3-5-22(25)28)27(15)14-24(29)30)13-16-12-18(33-2)8-11-23(16)34(31,32)19-9-6-17(26)7-10-19/h6-12H,3-5,13-14H2,1-2H3,(H,29,30) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Rhône-Poulenc Rorer
Curated by ChEMBL
| Assay Description
Displacement of [35S]-GTPgammaS from CRTH2 receptor (unknown origin) expressed in CHOK1 cell membrane after 1 hr by liquid scintillation counter |
Eur J Med Chem 113: 102-33 (2016)
Article DOI: 10.1016/j.ejmech.2016.02.023
BindingDB Entry DOI: 10.7270/Q2K64KXM |
More data for this
Ligand-Target Pair | |
Prostaglandin D2 receptor 2
(Homo sapiens (Human)) | BDBM50154805
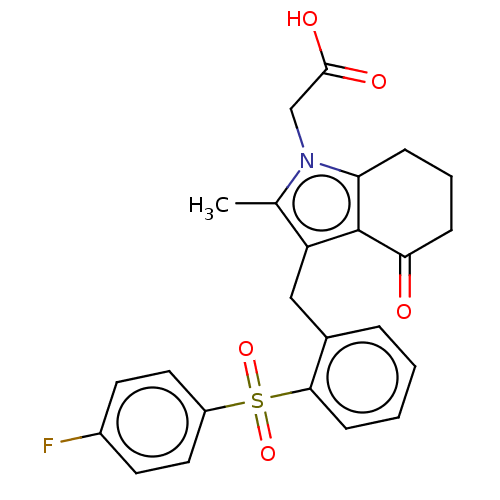
(CHEMBL3775213)Show SMILES Cc1c(Cc2ccccc2S(=O)(=O)c2ccc(F)cc2)c2c(CCCC2=O)n1CC(O)=O Show InChI InChI=1S/C24H22FNO5S/c1-15-19(24-20(6-4-7-21(24)27)26(15)14-23(28)29)13-16-5-2-3-8-22(16)32(30,31)18-11-9-17(25)10-12-18/h2-3,5,8-12H,4,6-7,13-14H2,1H3,(H,28,29) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Rhône-Poulenc Rorer
Curated by ChEMBL
| Assay Description
Displacement of [35S]-GTPgammaS from CRTH2 receptor (unknown origin) expressed in CHOK1 cell membrane after 1 hr by liquid scintillation counter |
Eur J Med Chem 113: 102-33 (2016)
Article DOI: 10.1016/j.ejmech.2016.02.023
BindingDB Entry DOI: 10.7270/Q2K64KXM |
More data for this
Ligand-Target Pair | |
Prostaglandin D2 receptor 2
(Homo sapiens (Human)) | BDBM50035038

(CHEMBL3338269)Show SMILES Cc1noc(C)c1Cc1cc(F)ccc1-n1cc(CC(O)=O)c2ccc(C)nc12 Show InChI InChI=1S/C22H20FN3O3/c1-12-4-6-18-16(10-21(27)28)11-26(22(18)24-12)20-7-5-17(23)8-15(20)9-19-13(2)25-29-14(19)3/h4-8,11H,9-10H2,1-3H3,(H,27,28) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Rhône-Poulenc Rorer
Curated by ChEMBL
| Assay Description
Antagonist activity at CRTh2 (unknown origin) stably expressed in PGD2-stimulated CHO.K1 cells preincubated for 1 hr by radio-labelled [35S]-GTPgamma... |
Bioorg Med Chem Lett 24: 5127-33 (2014)
Article DOI: 10.1016/j.bmcl.2014.08.028
BindingDB Entry DOI: 10.7270/Q27P911Z |
More data for this
Ligand-Target Pair | |
Prostaglandin D2 receptor 2
(Homo sapiens (Human)) | BDBM50035088

(CHEMBL3338296)Show SMILES Cc1noc(C2CC2)c1Cc1cc(F)ccc1-n1cc(CC(O)=O)c2ccc(C)nc12 Show InChI InChI=1S/C24H22FN3O3/c1-13-3-7-19-17(11-22(29)30)12-28(24(19)26-13)21-8-6-18(25)9-16(21)10-20-14(2)27-31-23(20)15-4-5-15/h3,6-9,12,15H,4-5,10-11H2,1-2H3,(H,29,30) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Rhône-Poulenc Rorer
Curated by ChEMBL
| Assay Description
Antagonist activity at CRTh2 (unknown origin) stably expressed in PGD2-stimulated CHO.K1 cells preincubated for 1 hr by radio-labelled [35S]-GTPgamma... |
Bioorg Med Chem Lett 24: 5127-33 (2014)
Article DOI: 10.1016/j.bmcl.2014.08.028
BindingDB Entry DOI: 10.7270/Q27P911Z |
More data for this
Ligand-Target Pair | |
Prostaglandin D2 receptor 2
(Homo sapiens (Human)) | BDBM50035077
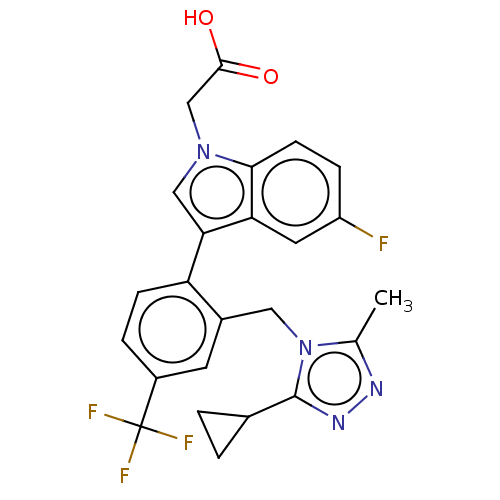
(CHEMBL3338285)Show SMILES Cc1nnc(C2CC2)n1Cc1cc(ccc1-c1cn(CC(O)=O)c2ccc(F)cc12)C(F)(F)F Show InChI InChI=1S/C24H20F4N4O2/c1-13-29-30-23(14-2-3-14)32(13)10-15-8-16(24(26,27)28)4-6-18(15)20-11-31(12-22(33)34)21-7-5-17(25)9-19(20)21/h4-9,11,14H,2-3,10,12H2,1H3,(H,33,34) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Rhône-Poulenc Rorer
Curated by ChEMBL
| Assay Description
Antagonist activity at CRTH2 receptor in human whole blood assessed as inhibition of PGD2-induced eosinophil shape change after 10 mins by fluorescen... |
Bioorg Med Chem Lett 24: 5127-33 (2014)
Article DOI: 10.1016/j.bmcl.2014.08.028
BindingDB Entry DOI: 10.7270/Q27P911Z |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Rattus norvegicus (rat)) | BDBM50040622

(1-[2-(1-Benzyl-piperidin-4-yl)-ethyl]-3-(9,10-diox...)Show SMILES O=C(NC(=S)NCCC1CCN(Cc2ccccc2)CC1)c1ccc2C(=O)c3ccccc3C(=O)c2c1 Show InChI InChI=1S/C30H29N3O3S/c34-27-23-8-4-5-9-24(23)28(35)26-18-22(10-11-25(26)27)29(36)32-30(37)31-15-12-20-13-16-33(17-14-20)19-21-6-2-1-3-7-21/h1-11,18,20H,12-17,19H2,(H2,31,32,36,37) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Centre de Recherche Pierre Fabre Médicament
Curated by ChEMBL
| Assay Description
Inhibitory activity against acetylcholine esterase (AChE) |
J Med Chem 37: 689-95 (1994)
BindingDB Entry DOI: 10.7270/Q2N29W0C |
More data for this
Ligand-Target Pair | |
Prostaglandin D2 receptor 2
(Homo sapiens (Human)) | BDBM50035079
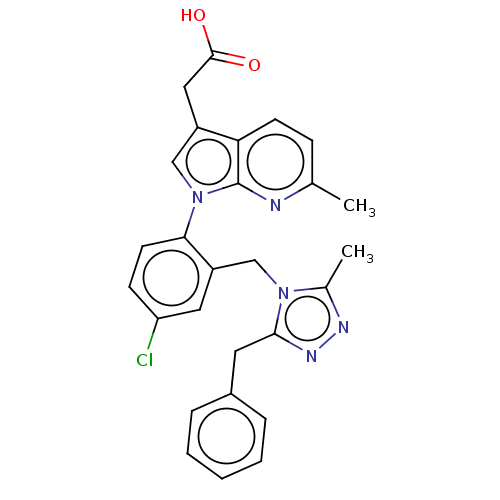
(CHEMBL3338287)Show SMILES Cc1nnc(Cc2ccccc2)n1Cc1cc(Cl)ccc1-n1cc(CC(O)=O)c2ccc(C)nc12 Show InChI InChI=1S/C27H24ClN5O2/c1-17-8-10-23-20(14-26(34)35)15-33(27(23)29-17)24-11-9-22(28)13-21(24)16-32-18(2)30-31-25(32)12-19-6-4-3-5-7-19/h3-11,13,15H,12,14,16H2,1-2H3,(H,34,35) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Rhône-Poulenc Rorer
Curated by ChEMBL
| Assay Description
Antagonist activity at CRTH2 receptor in human whole blood assessed as inhibition of PGD2-induced eosinophil shape change after 10 mins by fluorescen... |
Bioorg Med Chem Lett 24: 5127-33 (2014)
Article DOI: 10.1016/j.bmcl.2014.08.028
BindingDB Entry DOI: 10.7270/Q27P911Z |
More data for this
Ligand-Target Pair | |
D(2) dopamine receptor
(Rattus norvegicus (rat)) | BDBM50092421

(1-(2,6-Dichloro-phenyl)-3-{2-[1-(2,3-dihydro-benzo...)Show SMILES Clc1cccc(Cl)c1N1CCN(CCC2CCN(CC3COc4ccccc4O3)CC2)C1=O Show InChI InChI=1S/C25H29Cl2N3O3/c26-20-4-3-5-21(27)24(20)30-15-14-29(25(30)31)13-10-18-8-11-28(12-9-18)16-19-17-32-22-6-1-2-7-23(22)33-19/h1-7,18-19H,8-17H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 2.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Centre de Recherche Pierre Fabre
Curated by ChEMBL
| Assay Description
In vitro inhibition of specific binding of [3H]-YM 09151-2 to Dopamine receptor D2 site in rat striatal tissue. |
J Med Chem 43: 3653-64 (2000)
BindingDB Entry DOI: 10.7270/Q2MW2GD3 |
More data for this
Ligand-Target Pair | |
D(2) dopamine receptor
(Rattus norvegicus (rat)) | BDBM50092440

(1-Benzoyl-3-{2-[1-(2,3-dihydro-benzo[1,4]dioxin-2-...)Show SMILES O=C(NCCC1CCN(CC2COc3ccccc3O2)CC1)NC(=O)c1ccccc1 Show InChI InChI=1S/C24H29N3O4/c28-23(19-6-2-1-3-7-19)26-24(29)25-13-10-18-11-14-27(15-12-18)16-20-17-30-21-8-4-5-9-22(21)31-20/h1-9,18,20H,10-17H2,(H2,25,26,28,29) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 2.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Centre de Recherche Pierre Fabre
Curated by ChEMBL
| Assay Description
In vitro inhibition of specific binding of [3H]-YM 09151-2 to Dopamine receptor D2 site in rat striatal tissue. |
J Med Chem 43: 3653-64 (2000)
BindingDB Entry DOI: 10.7270/Q2MW2GD3 |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Rattus norvegicus (rat)) | BDBM50040626

(1-[2-(1-Benzyl-piperidin-4-yl)-ethyl]-3-(5-nitro-p...)Show SMILES [O-][N+](=O)c1ccc([N-]C(=[SH+])NCCC2CCN(Cc3ccccc3)CC2)nc1 Show InChI InChI=1S/C20H25N5O2S/c26-25(27)18-6-7-19(22-14-18)23-20(28)21-11-8-16-9-12-24(13-10-16)15-17-4-2-1-3-5-17/h1-7,14,16H,8-13,15H2,(H2,21,22,23,28) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| PubMed
| n/a | n/a | 2.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Centre de Recherche Pierre Fabre Médicament
Curated by ChEMBL
| Assay Description
Inhibition against acetylcholinesterase (AChE) |
J Med Chem 37: 689-95 (1994)
BindingDB Entry DOI: 10.7270/Q2N29W0C |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 13
(Homo sapiens (Human)) | BDBM50420758

(CHEMBL2087503)Show SMILES CC(C)c1nnc2cc(ccn12)-c1cc(cc(F)c1C)C(=O)NC1CC1 Show InChI InChI=1S/C20H21FN4O/c1-11(2)19-24-23-18-10-13(6-7-25(18)19)16-8-14(9-17(21)12(16)3)20(26)22-15-4-5-15/h6-11,15H,4-5H2,1-3H3,(H,22,26) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Rhône-Poulenc Rorer
Curated by ChEMBL
| Assay Description
Inhibition of p38alpha |
Bioorg Med Chem Lett 22: 3431-6 (2012)
Article DOI: 10.1016/j.bmcl.2012.03.099
BindingDB Entry DOI: 10.7270/Q2348MNX |
More data for this
Ligand-Target Pair | |
Prostaglandin D2 receptor 2
(Homo sapiens (Human)) | BDBM50035086

(CHEMBL3338294)Show SMILES Cc1noc(C2CC2)c1Cc1cc(ccc1-c1cc(CC(O)=O)c2ccc(C)nn12)C(F)(F)F Show InChI InChI=1S/C25H22F3N3O3/c1-13-3-8-21-17(12-23(32)33)11-22(31(21)29-13)19-7-6-18(25(26,27)28)9-16(19)10-20-14(2)30-34-24(20)15-4-5-15/h3,6-9,11,15H,4-5,10,12H2,1-2H3,(H,32,33) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Rhône-Poulenc Rorer
Curated by ChEMBL
| Assay Description
Antagonist activity at CRTh2 (unknown origin) stably expressed in PGD2-stimulated CHO.K1 cells preincubated for 1 hr by radio-labelled [35S]-GTPgamma... |
Bioorg Med Chem Lett 24: 5127-33 (2014)
Article DOI: 10.1016/j.bmcl.2014.08.028
BindingDB Entry DOI: 10.7270/Q27P911Z |
More data for this
Ligand-Target Pair | |
Prostaglandin D2 receptor 2
(Homo sapiens (Human)) | BDBM50035093
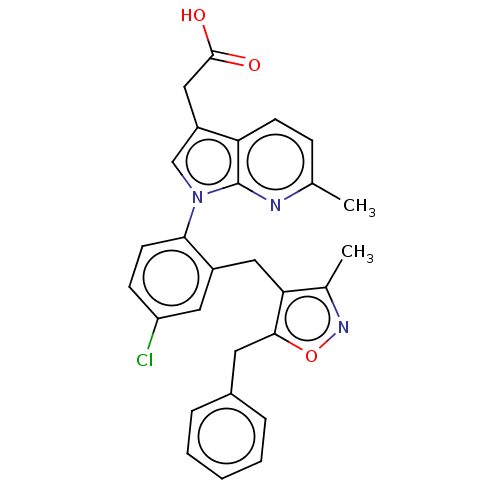
(CHEMBL3338301)Show SMILES Cc1noc(Cc2ccccc2)c1Cc1cc(Cl)ccc1-n1cc(CC(O)=O)c2ccc(C)nc12 Show InChI InChI=1S/C28H24ClN3O3/c1-17-8-10-23-21(15-27(33)34)16-32(28(23)30-17)25-11-9-22(29)13-20(25)14-24-18(2)31-35-26(24)12-19-6-4-3-5-7-19/h3-11,13,16H,12,14-15H2,1-2H3,(H,33,34) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Rhône-Poulenc Rorer
Curated by ChEMBL
| Assay Description
Antagonist activity at CRTh2 (unknown origin) stably expressed in PGD2-stimulated CHO.K1 cells preincubated for 1 hr by radio-labelled [35S]-GTPgamma... |
Bioorg Med Chem Lett 24: 5127-33 (2014)
Article DOI: 10.1016/j.bmcl.2014.08.028
BindingDB Entry DOI: 10.7270/Q27P911Z |
More data for this
Ligand-Target Pair | |
Prostaglandin D2 receptor 2
(Homo sapiens (Human)) | BDBM50035091
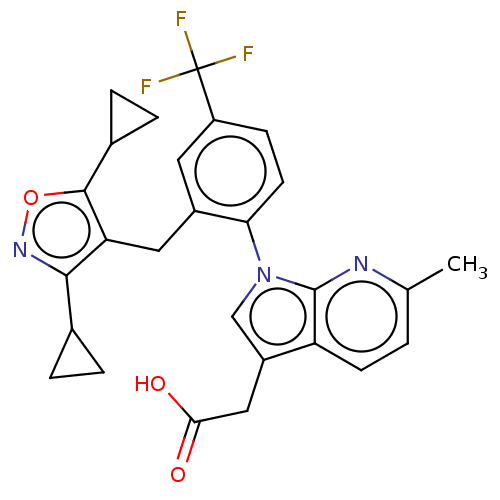
(CHEMBL3338299)Show SMILES Cc1ccc2c(CC(O)=O)cn(-c3ccc(cc3Cc3c(noc3C3CC3)C3CC3)C(F)(F)F)c2n1 Show InChI InChI=1S/C27H24F3N3O3/c1-14-2-8-20-18(12-23(34)35)13-33(26(20)31-14)22-9-7-19(27(28,29)30)10-17(22)11-21-24(15-3-4-15)32-36-25(21)16-5-6-16/h2,7-10,13,15-16H,3-6,11-12H2,1H3,(H,34,35) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Rhône-Poulenc Rorer
Curated by ChEMBL
| Assay Description
Antagonist activity at CRTh2 (unknown origin) stably expressed in PGD2-stimulated CHO.K1 cells preincubated for 1 hr by radio-labelled [35S]-GTPgamma... |
Bioorg Med Chem Lett 24: 5127-33 (2014)
Article DOI: 10.1016/j.bmcl.2014.08.028
BindingDB Entry DOI: 10.7270/Q27P911Z |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 13
(Homo sapiens (Human)) | BDBM50420747
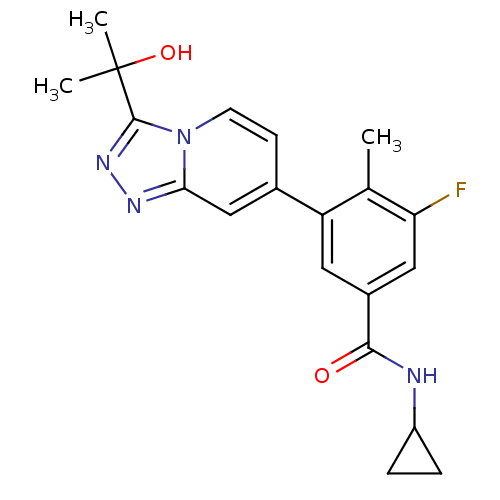
(CHEMBL2087526)Show SMILES Cc1c(F)cc(cc1-c1ccn2c(nnc2c1)C(C)(C)O)C(=O)NC1CC1 Show InChI InChI=1S/C20H21FN4O2/c1-11-15(8-13(9-16(11)21)18(26)22-14-4-5-14)12-6-7-25-17(10-12)23-24-19(25)20(2,3)27/h6-10,14,27H,4-5H2,1-3H3,(H,22,26) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Rhône-Poulenc Rorer
Curated by ChEMBL
| Assay Description
Inhibition of p38alpha |
Bioorg Med Chem Lett 22: 3431-6 (2012)
Article DOI: 10.1016/j.bmcl.2012.03.099
BindingDB Entry DOI: 10.7270/Q2348MNX |
More data for this
Ligand-Target Pair | |
D(2) dopamine receptor
(Rattus norvegicus (rat)) | BDBM50092425

(2-{2-[1-(2,3-Dihydro-benzo[1,4]dioxin-2-ylmethyl)-...)Show SMILES O=C1N(CCC2CCN(CC3COc4ccccc4O3)CC2)Cc2ccccc12 Show InChI InChI=1S/C24H28N2O3/c27-24-21-6-2-1-5-19(21)15-26(24)14-11-18-9-12-25(13-10-18)16-20-17-28-22-7-3-4-8-23(22)29-20/h1-8,18,20H,9-17H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 3.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Centre de Recherche Pierre Fabre
Curated by ChEMBL
| Assay Description
In vitro inhibition of specific binding of [3H]-YM 09151-2 to Dopamine receptor D2 site in rat striatal tissue. |
J Med Chem 43: 3653-64 (2000)
BindingDB Entry DOI: 10.7270/Q2MW2GD3 |
More data for this
Ligand-Target Pair | |
Prostaglandin D2 receptor 2
(Homo sapiens (Human)) | BDBM50035074

(CHEMBL3338282)Show SMILES Cc1cnn(CC2CC2)c1Cc1cc(C)ccc1-n1cc(CC(O)=O)c2ccc(C)nc12 Show InChI InChI=1S/C26H28N4O2/c1-16-4-9-23(20(10-16)11-24-17(2)13-27-30(24)14-19-6-7-19)29-15-21(12-25(31)32)22-8-5-18(3)28-26(22)29/h4-5,8-10,13,15,19H,6-7,11-12,14H2,1-3H3,(H,31,32) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Rhône-Poulenc Rorer
Curated by ChEMBL
| Assay Description
Antagonist activity at CRTH2 receptor in human whole blood assessed as inhibition of PGD2-induced eosinophil shape change after 10 mins by fluorescen... |
Bioorg Med Chem Lett 24: 5127-33 (2014)
Article DOI: 10.1016/j.bmcl.2014.08.028
BindingDB Entry DOI: 10.7270/Q27P911Z |
More data for this
Ligand-Target Pair | |
Prostaglandin D2 receptor 2
(Homo sapiens (Human)) | BDBM50035031
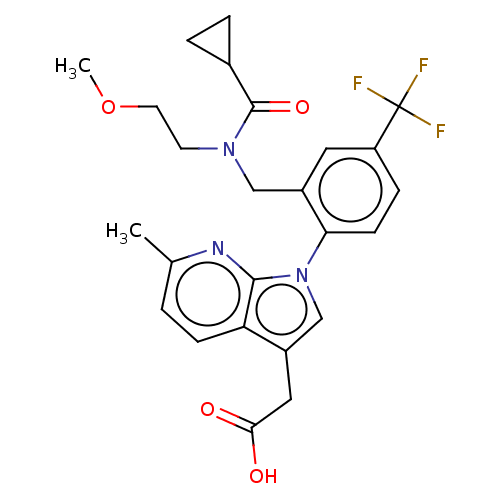
(CHEMBL3343129)Show SMILES COCCN(Cc1cc(ccc1-n1cc(CC(O)=O)c2ccc(C)nc12)C(F)(F)F)C(=O)C1CC1 Show InChI InChI=1S/C25H26F3N3O4/c1-15-3-7-20-17(12-22(32)33)14-31(23(20)29-15)21-8-6-19(25(26,27)28)11-18(21)13-30(9-10-35-2)24(34)16-4-5-16/h3,6-8,11,14,16H,4-5,9-10,12-13H2,1-2H3,(H,32,33) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Rhône-Poulenc Rorer
Curated by ChEMBL
| Assay Description
Antagonist activity at CRTh2 receptor (unknown origin) overexpressed in CHOK1 cell membranes assessed as inhibition of [35S]-GTPgammaS binding measur... |
Bioorg Med Chem Lett 24: 5123-6 (2014)
Article DOI: 10.1016/j.bmcl.2014.08.029
BindingDB Entry DOI: 10.7270/Q2CF9RRW |
More data for this
Ligand-Target Pair | |
Prostaglandin D2 receptor 2
(Homo sapiens (Human)) | BDBM50035030
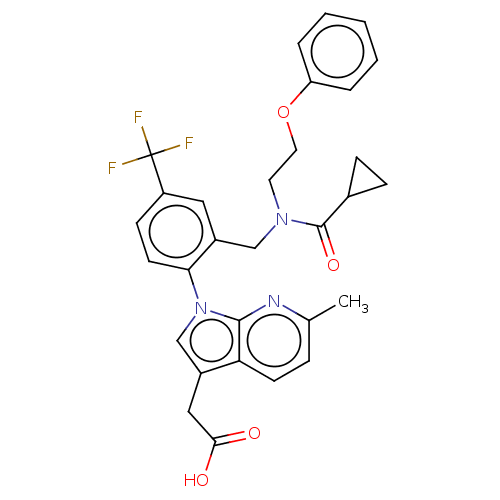
(CHEMBL3343128)Show SMILES Cc1ccc2c(CC(O)=O)cn(-c3ccc(cc3CN(CCOc3ccccc3)C(=O)C3CC3)C(F)(F)F)c2n1 Show InChI InChI=1S/C30H28F3N3O4/c1-19-7-11-25-21(16-27(37)38)18-36(28(25)34-19)26-12-10-23(30(31,32)33)15-22(26)17-35(29(39)20-8-9-20)13-14-40-24-5-3-2-4-6-24/h2-7,10-12,15,18,20H,8-9,13-14,16-17H2,1H3,(H,37,38) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Rhône-Poulenc Rorer
Curated by ChEMBL
| Assay Description
Antagonist activity at CRTh2 receptor (unknown origin) overexpressed in CHOK1 cell membranes assessed as inhibition of [35S]-GTPgammaS binding measur... |
Bioorg Med Chem Lett 24: 5123-6 (2014)
Article DOI: 10.1016/j.bmcl.2014.08.029
BindingDB Entry DOI: 10.7270/Q2CF9RRW |
More data for this
Ligand-Target Pair | |
Prostaglandin D2 receptor 2
(Homo sapiens (Human)) | BDBM50035076

(CHEMBL3338284)Show SMILES Cc1nnc(C2CC2)n1Cc1cc(Cl)ccc1-n1cc(CC(O)=O)c2ccc(C)nc12 Show InChI InChI=1S/C23H22ClN5O2/c1-13-3-7-19-16(10-21(30)31)11-29(23(19)25-13)20-8-6-18(24)9-17(20)12-28-14(2)26-27-22(28)15-4-5-15/h3,6-9,11,15H,4-5,10,12H2,1-2H3,(H,30,31) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Rhône-Poulenc Rorer
Curated by ChEMBL
| Assay Description
Antagonist activity at CRTH2 receptor in human whole blood assessed as inhibition of PGD2-induced eosinophil shape change after 10 mins by fluorescen... |
Bioorg Med Chem Lett 24: 5127-33 (2014)
Article DOI: 10.1016/j.bmcl.2014.08.028
BindingDB Entry DOI: 10.7270/Q27P911Z |
More data for this
Ligand-Target Pair | |
Prostaglandin D2 receptor 2
(Homo sapiens (Human)) | BDBM50035103
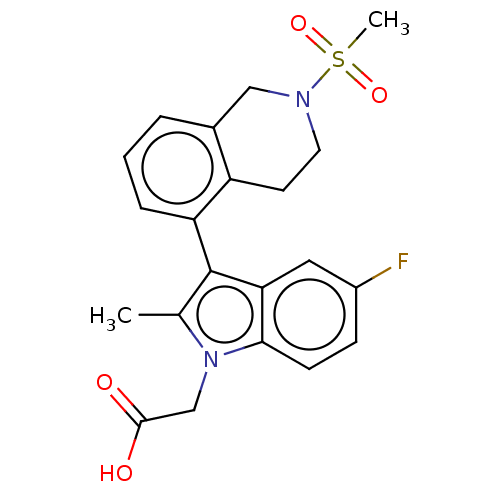
(CHEMBL3337464)Show SMILES Cc1c(-c2cccc3CN(CCc23)S(C)(=O)=O)c2cc(F)ccc2n1CC(O)=O |(5.67,-9.17,;5.99,-10.68,;7.4,-11.31,;8.74,-10.54,;10.07,-11.31,;11.42,-10.54,;11.41,-8.98,;10.07,-8.23,;10.07,-6.7,;8.74,-5.93,;7.42,-6.7,;7.41,-8.23,;8.74,-8.99,;8.74,-4.38,;10.09,-3.62,;7.2,-4.37,;7.98,-3.04,;7.24,-12.84,;8.26,-13.97,;7.79,-15.43,;8.83,-16.57,;6.29,-15.75,;5.26,-14.61,;5.74,-13.15,;4.96,-11.82,;3.42,-11.67,;2.52,-12.92,;.98,-12.77,;3.15,-14.33,)| Show InChI InChI=1S/C21H21FN2O4S/c1-13-21(18-10-15(22)6-7-19(18)24(13)12-20(25)26)17-5-3-4-14-11-23(29(2,27)28)9-8-16(14)17/h3-7,10H,8-9,11-12H2,1-2H3,(H,25,26) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Rhône-Poulenc Rorer
Curated by ChEMBL
| Assay Description
Antagonist activity at CRTh2 (unknown origin) stably expressed in PGD2-stimulated CHO.K1 cells preincubated for 1 hr by radio-labelled [35S]-GTPgamma... |
Bioorg Med Chem Lett 24: 5127-33 (2014)
Article DOI: 10.1016/j.bmcl.2014.08.028
BindingDB Entry DOI: 10.7270/Q27P911Z |
More data for this
Ligand-Target Pair | |
Prostaglandin D2 receptor 2
(Homo sapiens (Human)) | BDBM50154762
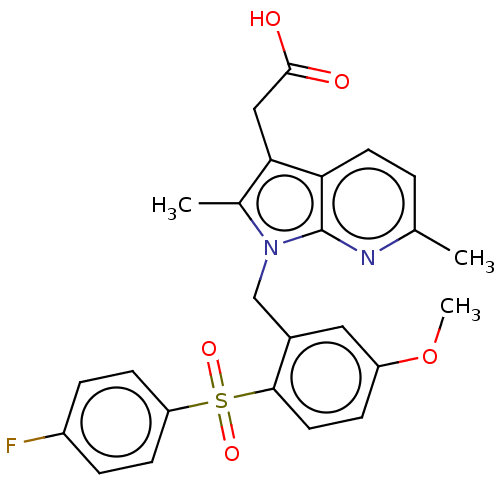
(CHEMBL3774971)Show SMILES COc1ccc(c(Cn2c(C)c(CC(O)=O)c3ccc(C)nc23)c1)S(=O)(=O)c1ccc(F)cc1 Show InChI InChI=1S/C25H23FN2O5S/c1-15-4-10-21-22(13-24(29)30)16(2)28(25(21)27-15)14-17-12-19(33-3)7-11-23(17)34(31,32)20-8-5-18(26)6-9-20/h4-12H,13-14H2,1-3H3,(H,29,30) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Rhône-Poulenc Rorer
Curated by ChEMBL
| Assay Description
Displacement of [35S]-GTPgammaS from CRTH2 receptor (unknown origin) expressed in CHOK1 cell membrane after 1 hr by liquid scintillation counter |
Eur J Med Chem 113: 102-33 (2016)
Article DOI: 10.1016/j.ejmech.2016.02.023
BindingDB Entry DOI: 10.7270/Q2K64KXM |
More data for this
Ligand-Target Pair | |
Prostaglandin D2 receptor 2
(Homo sapiens (Human)) | BDBM50035034

(CHEMBL3338261)Show SMILES Cc1ccc2c(CC(O)=O)cn(-c3ccc(cc3CSC(C)(C)C)C(=O)NCCc3ccccc3)c2n1 Show InChI InChI=1S/C30H33N3O3S/c1-20-10-12-25-23(17-27(34)35)18-33(28(25)32-20)26-13-11-22(16-24(26)19-37-30(2,3)4)29(36)31-15-14-21-8-6-5-7-9-21/h5-13,16,18H,14-15,17,19H2,1-4H3,(H,31,36)(H,34,35) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Rhône-Poulenc Rorer
Curated by ChEMBL
| Assay Description
Antagonist activity at CRTh2 (unknown origin) stably expressed in PGD2-stimulated CHO.K1 cells preincubated for 1 hr by radio-labelled [35S]-GTPgamma... |
Bioorg Med Chem Lett 24: 5127-33 (2014)
Article DOI: 10.1016/j.bmcl.2014.08.028
BindingDB Entry DOI: 10.7270/Q27P911Z |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data