 Found 228 hits with Last Name = 'hogan' and Initial = 'm'
Found 228 hits with Last Name = 'hogan' and Initial = 'm' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Matrix metalloproteinase-9
(Homo sapiens (Human)) | BDBM50126626
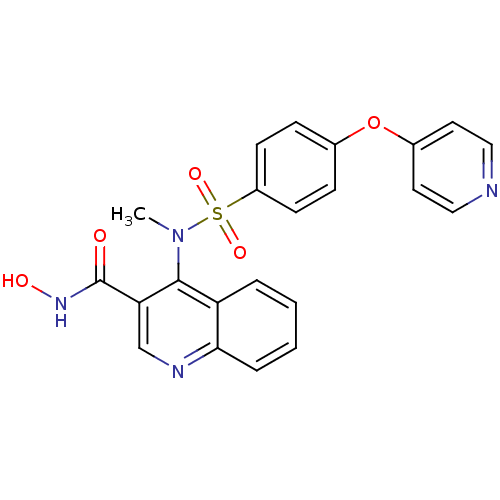
(4-{Methyl-[4-(pyridin-4-yloxy)-benzenesulfonyl]-am...)Show SMILES CN(c1c(cnc2ccccc12)C(=O)NO)S(=O)(=O)c1ccc(Oc2ccncc2)cc1 Show InChI InChI=1S/C22H18N4O5S/c1-26(21-18-4-2-3-5-20(18)24-14-19(21)22(27)25-28)32(29,30)17-8-6-15(7-9-17)31-16-10-12-23-13-11-16/h2-14,28H,1H3,(H,25,27) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth-Ayerst Research
Curated by ChEMBL
| Assay Description
Inhibitory activity against matrix metalloproteinase 9 (MMP-9). |
Bioorg Med Chem Lett 13: 1487-90 (2003)
BindingDB Entry DOI: 10.7270/Q29W0DW0 |
More data for this
Ligand-Target Pair | |
Matrix metalloproteinase-9
(Homo sapiens (Human)) | BDBM50126612
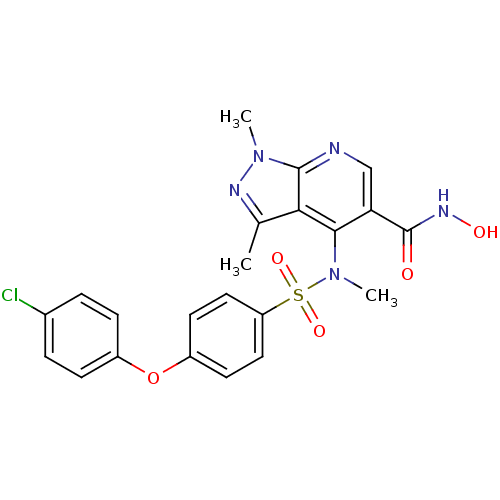
(4-{[4-(4-Chloro-phenoxy)-benzenesulfonyl]-methyl-a...)Show SMILES CN(c1c(cnc2n(C)nc(C)c12)C(=O)NO)S(=O)(=O)c1ccc(Oc2ccc(Cl)cc2)cc1 Show InChI InChI=1S/C22H20ClN5O5S/c1-13-19-20(18(22(29)26-30)12-24-21(19)27(2)25-13)28(3)34(31,32)17-10-8-16(9-11-17)33-15-6-4-14(23)5-7-15/h4-12,30H,1-3H3,(H,26,29) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth-Ayerst Research
Curated by ChEMBL
| Assay Description
Inhibitory activity against matrix metalloproteinase 9 (MMP-9). |
Bioorg Med Chem Lett 13: 1487-90 (2003)
BindingDB Entry DOI: 10.7270/Q29W0DW0 |
More data for this
Ligand-Target Pair | |
Collagenase 3
(Homo sapiens (Human)) | BDBM50126600
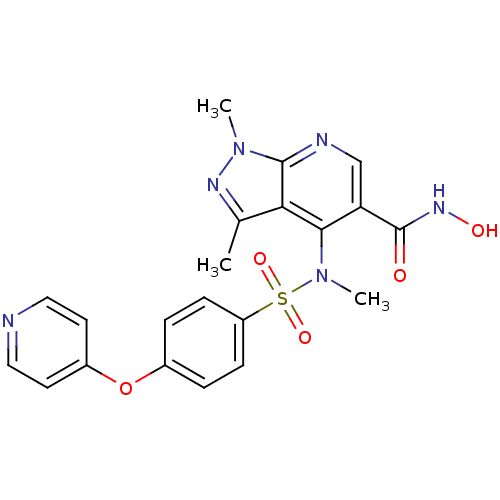
(1,3-Dimethyl-4-{methyl-[4-(pyridin-4-yloxy)-benzen...)Show SMILES CN(c1c(cnc2n(C)nc(C)c12)C(=O)NO)S(=O)(=O)c1ccc(Oc2ccncc2)cc1 Show InChI InChI=1S/C21H20N6O5S/c1-13-18-19(17(21(28)25-29)12-23-20(18)26(2)24-13)27(3)33(30,31)16-6-4-14(5-7-16)32-15-8-10-22-11-9-15/h4-12,29H,1-3H3,(H,25,28) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth-Ayerst Research
Curated by ChEMBL
| Assay Description
Inhibitory activity against matrix metalloproteinase 13 (MMP-13). |
Bioorg Med Chem Lett 13: 1487-90 (2003)
BindingDB Entry DOI: 10.7270/Q29W0DW0 |
More data for this
Ligand-Target Pair | |
Collagenase 3
(Homo sapiens (Human)) | BDBM50126624
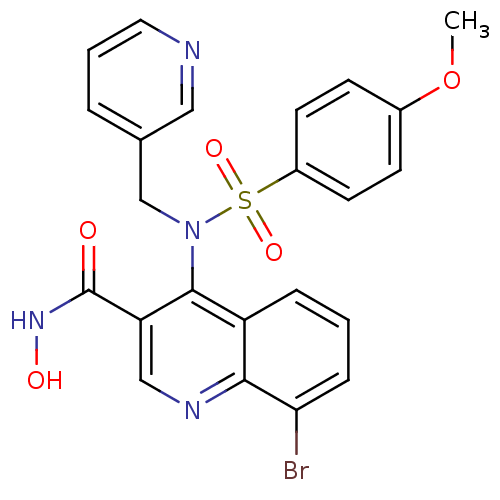
(8-Bromo-4-[(4-methoxy-benzenesulfonyl)-pyridin-3-y...)Show SMILES COc1ccc(cc1)S(=O)(=O)N(Cc1cccnc1)c1c(cnc2c(Br)cccc12)C(=O)NO Show InChI InChI=1S/C23H19BrN4O5S/c1-33-16-7-9-17(10-8-16)34(31,32)28(14-15-4-3-11-25-12-15)22-18-5-2-6-20(24)21(18)26-13-19(22)23(29)27-30/h2-13,30H,14H2,1H3,(H,27,29) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth-Ayerst Research
Curated by ChEMBL
| Assay Description
Inhibitory activity against matrix metalloproteinase 13 (MMP-13). |
Bioorg Med Chem Lett 13: 1487-90 (2003)
BindingDB Entry DOI: 10.7270/Q29W0DW0 |
More data for this
Ligand-Target Pair | |
Collagenase 3
(Homo sapiens (Human)) | BDBM50126620
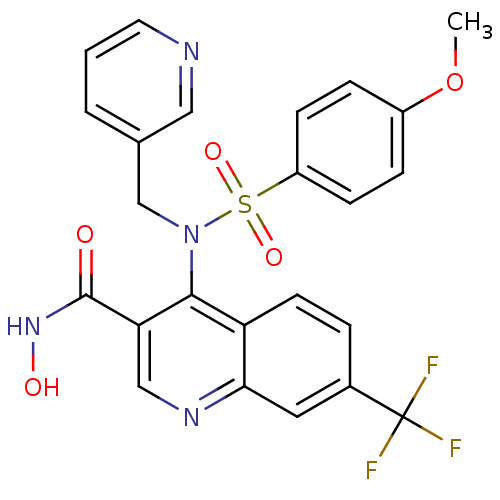
(4-[(4-Methoxy-benzenesulfonyl)-pyridin-3-ylmethyl-...)Show SMILES COc1ccc(cc1)S(=O)(=O)N(Cc1cccnc1)c1c(cnc2cc(ccc12)C(F)(F)F)C(=O)NO Show InChI InChI=1S/C24H19F3N4O5S/c1-36-17-5-7-18(8-6-17)37(34,35)31(14-15-3-2-10-28-12-15)22-19-9-4-16(24(25,26)27)11-21(19)29-13-20(22)23(32)30-33/h2-13,33H,14H2,1H3,(H,30,32) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth-Ayerst Research
Curated by ChEMBL
| Assay Description
Inhibitory activity against matrix metalloproteinase 13 (MMP-13). |
Bioorg Med Chem Lett 13: 1487-90 (2003)
BindingDB Entry DOI: 10.7270/Q29W0DW0 |
More data for this
Ligand-Target Pair | |
Collagenase 3
(Homo sapiens (Human)) | BDBM50126623
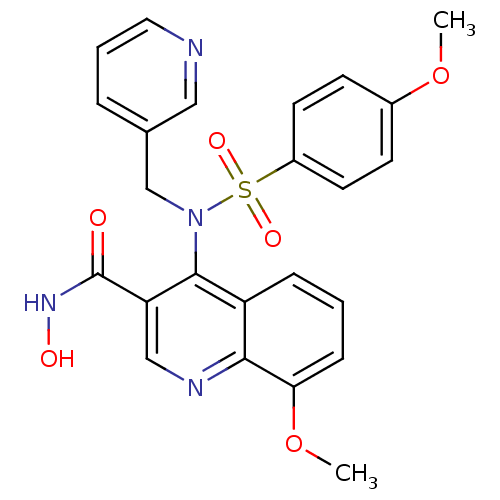
(8-Methoxy-4-[(4-methoxy-benzenesulfonyl)-pyridin-3...)Show SMILES COc1ccc(cc1)S(=O)(=O)N(Cc1cccnc1)c1c(cnc2c(OC)cccc12)C(=O)NO Show InChI InChI=1S/C24H22N4O6S/c1-33-17-8-10-18(11-9-17)35(31,32)28(15-16-5-4-12-25-13-16)23-19-6-3-7-21(34-2)22(19)26-14-20(23)24(29)27-30/h3-14,30H,15H2,1-2H3,(H,27,29) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth-Ayerst Research
Curated by ChEMBL
| Assay Description
Inhibitory activity against matrix metalloproteinase 13 (MMP-13). |
Bioorg Med Chem Lett 13: 1487-90 (2003)
BindingDB Entry DOI: 10.7270/Q29W0DW0 |
More data for this
Ligand-Target Pair | |
Collagenase 3
(Homo sapiens (Human)) | BDBM50126626

(4-{Methyl-[4-(pyridin-4-yloxy)-benzenesulfonyl]-am...)Show SMILES CN(c1c(cnc2ccccc12)C(=O)NO)S(=O)(=O)c1ccc(Oc2ccncc2)cc1 Show InChI InChI=1S/C22H18N4O5S/c1-26(21-18-4-2-3-5-20(18)24-14-19(21)22(27)25-28)32(29,30)17-8-6-15(7-9-17)31-16-10-12-23-13-11-16/h2-14,28H,1H3,(H,25,27) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth-Ayerst Research
Curated by ChEMBL
| Assay Description
Inhibitory activity against matrix metalloproteinase 13 (MMP-13). |
Bioorg Med Chem Lett 13: 1487-90 (2003)
BindingDB Entry DOI: 10.7270/Q29W0DW0 |
More data for this
Ligand-Target Pair | |
Collagenase 3
(Homo sapiens (Human)) | BDBM50126622
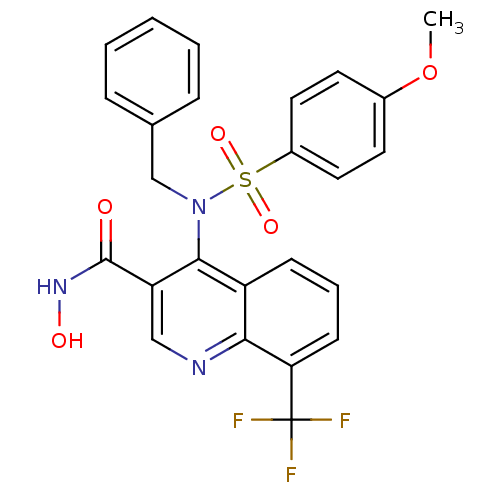
(4-(N-benzyl-4-methoxyphenylsulfonamido)-N-hydroxy-...)Show SMILES COc1ccc(cc1)S(=O)(=O)N(Cc1ccccc1)c1c(cnc2c(cccc12)C(F)(F)F)C(=O)NO Show InChI InChI=1S/C25H20F3N3O5S/c1-36-17-10-12-18(13-11-17)37(34,35)31(15-16-6-3-2-4-7-16)23-19-8-5-9-21(25(26,27)28)22(19)29-14-20(23)24(32)30-33/h2-14,33H,15H2,1H3,(H,30,32) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth-Ayerst Research
Curated by ChEMBL
| Assay Description
Inhibitory activity against matrix metalloproteinase 13 (MMP-13). |
Bioorg Med Chem Lett 13: 1487-90 (2003)
BindingDB Entry DOI: 10.7270/Q29W0DW0 |
More data for this
Ligand-Target Pair | |
Collagenase 3
(Homo sapiens (Human)) | BDBM50126612

(4-{[4-(4-Chloro-phenoxy)-benzenesulfonyl]-methyl-a...)Show SMILES CN(c1c(cnc2n(C)nc(C)c12)C(=O)NO)S(=O)(=O)c1ccc(Oc2ccc(Cl)cc2)cc1 Show InChI InChI=1S/C22H20ClN5O5S/c1-13-19-20(18(22(29)26-30)12-24-21(19)27(2)25-13)28(3)34(31,32)17-10-8-16(9-11-17)33-15-6-4-14(23)5-7-15/h4-12,30H,1-3H3,(H,26,29) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth-Ayerst Research
Curated by ChEMBL
| Assay Description
Inhibitory activity against matrix metalloproteinase 13 (MMP-13). |
Bioorg Med Chem Lett 13: 1487-90 (2003)
BindingDB Entry DOI: 10.7270/Q29W0DW0 |
More data for this
Ligand-Target Pair | |
Collagenase 3
(Homo sapiens (Human)) | BDBM50103161

(3-Furan-2-yl-N-hydroxy-2-[(4-methoxy-benzenesulfon...)Show SMILES COc1ccc(cc1)S(=O)(=O)N(Cc1cccnc1)c1c(cc(C)cc1-c1ccco1)C(=O)NO Show InChI InChI=1S/C25H23N3O6S/c1-17-13-21(23-6-4-12-34-23)24(22(14-17)25(29)27-30)28(16-18-5-3-11-26-15-18)35(31,32)20-9-7-19(33-2)8-10-20/h3-15,30H,16H2,1-2H3,(H,27,29) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth-Ayerst Research
Curated by ChEMBL
| Assay Description
Inhibition of matrix metalloprotease-13 |
Bioorg Med Chem Lett 11: 2189-92 (2001)
BindingDB Entry DOI: 10.7270/Q2154HK0 |
More data for this
Ligand-Target Pair | |
Matrix metalloproteinase-9
(Homo sapiens (Human)) | BDBM50103171

(4-[(4-Methoxy-benzenesulfonyl)-pyridin-3-ylmethyl-...)Show SMILES COc1ccc(cc1)S(=O)(=O)N(Cc1cccnc1)c1c(C)cc(cc1C(=O)NO)-c1cccc(c1)C(F)(F)F Show InChI InChI=1S/C28H24F3N3O5S/c1-18-13-21(20-6-3-7-22(14-20)28(29,30)31)15-25(27(35)33-36)26(18)34(17-19-5-4-12-32-16-19)40(37,38)24-10-8-23(39-2)9-11-24/h3-16,36H,17H2,1-2H3,(H,33,35) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth-Ayerst Research
Curated by ChEMBL
| Assay Description
In vitro ability to inhibit matrix metalloprotease-9. |
Bioorg Med Chem Lett 11: 2189-92 (2001)
BindingDB Entry DOI: 10.7270/Q2154HK0 |
More data for this
Ligand-Target Pair | |
Collagenase 3
(Homo sapiens (Human)) | BDBM50103162
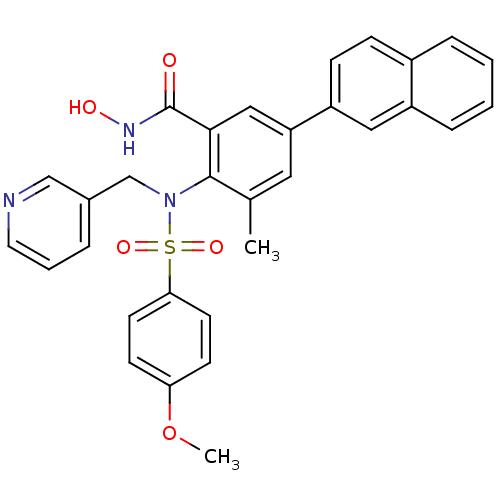
(CHEMBL68542 | N-Hydroxy-2-[(4-methoxy-benzenesulfo...)Show SMILES COc1ccc(cc1)S(=O)(=O)N(Cc1cccnc1)c1c(C)cc(cc1C(=O)NO)-c1ccc2ccccc2c1 Show InChI InChI=1S/C31H27N3O5S/c1-21-16-26(25-10-9-23-7-3-4-8-24(23)17-25)18-29(31(35)33-36)30(21)34(20-22-6-5-15-32-19-22)40(37,38)28-13-11-27(39-2)12-14-28/h3-19,36H,20H2,1-2H3,(H,33,35) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth-Ayerst Research
Curated by ChEMBL
| Assay Description
Inhibition of matrix metalloprotease-13 |
Bioorg Med Chem Lett 11: 2189-92 (2001)
BindingDB Entry DOI: 10.7270/Q2154HK0 |
More data for this
Ligand-Target Pair | |
Matrix metalloproteinase-9
(Homo sapiens (Human)) | BDBM50126623

(8-Methoxy-4-[(4-methoxy-benzenesulfonyl)-pyridin-3...)Show SMILES COc1ccc(cc1)S(=O)(=O)N(Cc1cccnc1)c1c(cnc2c(OC)cccc12)C(=O)NO Show InChI InChI=1S/C24H22N4O6S/c1-33-17-8-10-18(11-9-17)35(31,32)28(15-16-5-4-12-25-13-16)23-19-6-3-7-21(34-2)22(19)26-14-20(23)24(29)27-30/h3-14,30H,15H2,1-2H3,(H,27,29) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth-Ayerst Research
Curated by ChEMBL
| Assay Description
Inhibitory activity against matrix metalloproteinase 9 (MMP-9). |
Bioorg Med Chem Lett 13: 1487-90 (2003)
BindingDB Entry DOI: 10.7270/Q29W0DW0 |
More data for this
Ligand-Target Pair | |
Matrix metalloproteinase-9
(Homo sapiens (Human)) | BDBM50126630
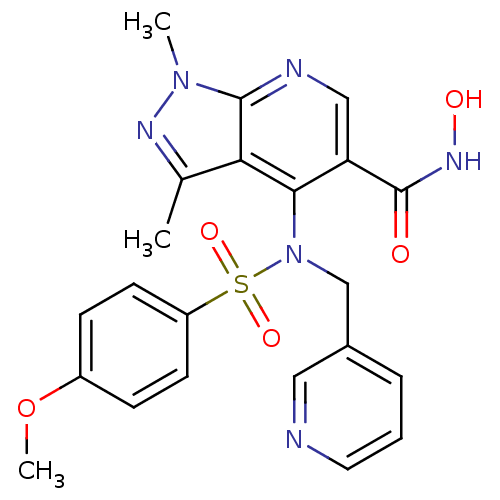
(4-[(4-Methoxy-benzenesulfonyl)-pyridin-3-ylmethyl-...)Show SMILES COc1ccc(cc1)S(=O)(=O)N(Cc1cccnc1)c1c(cnc2n(C)nc(C)c12)C(=O)NO Show InChI InChI=1S/C22H22N6O5S/c1-14-19-20(18(22(29)26-30)12-24-21(19)27(2)25-14)28(13-15-5-4-10-23-11-15)34(31,32)17-8-6-16(33-3)7-9-17/h4-12,30H,13H2,1-3H3,(H,26,29) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth-Ayerst Research
Curated by ChEMBL
| Assay Description
Inhibitory activity against matrix metalloproteinase 9 (MMP-9). |
Bioorg Med Chem Lett 13: 1487-90 (2003)
BindingDB Entry DOI: 10.7270/Q29W0DW0 |
More data for this
Ligand-Target Pair | |
Matrix metalloproteinase-9
(Homo sapiens (Human)) | BDBM50126604
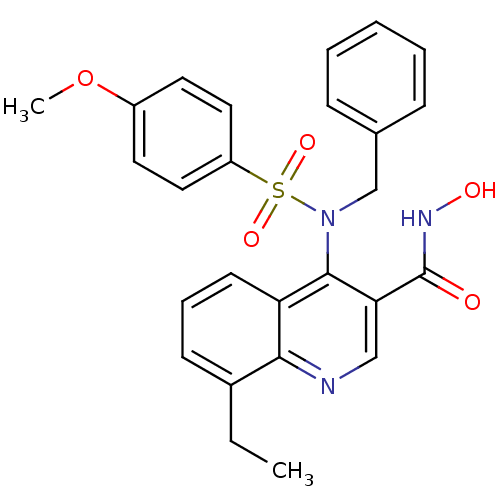
(4-(N-benzyl-4-methoxyphenylsulfonamido)-8-ethyl-N-...)Show SMILES CCc1cccc2c(N(Cc3ccccc3)S(=O)(=O)c3ccc(OC)cc3)c(cnc12)C(=O)NO Show InChI InChI=1S/C26H25N3O5S/c1-3-19-10-7-11-22-24(19)27-16-23(26(30)28-31)25(22)29(17-18-8-5-4-6-9-18)35(32,33)21-14-12-20(34-2)13-15-21/h4-16,31H,3,17H2,1-2H3,(H,28,30) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth-Ayerst Research
Curated by ChEMBL
| Assay Description
Inhibitory activity against matrix metalloproteinase 9 (MMP-9). |
Bioorg Med Chem Lett 13: 1487-90 (2003)
BindingDB Entry DOI: 10.7270/Q29W0DW0 |
More data for this
Ligand-Target Pair | |
Matrix metalloproteinase-9
(Homo sapiens (Human)) | BDBM50126624

(8-Bromo-4-[(4-methoxy-benzenesulfonyl)-pyridin-3-y...)Show SMILES COc1ccc(cc1)S(=O)(=O)N(Cc1cccnc1)c1c(cnc2c(Br)cccc12)C(=O)NO Show InChI InChI=1S/C23H19BrN4O5S/c1-33-16-7-9-17(10-8-16)34(31,32)28(14-15-4-3-11-25-12-15)22-18-5-2-6-20(24)21(18)26-13-19(22)23(29)27-30/h2-13,30H,14H2,1H3,(H,27,29) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth-Ayerst Research
Curated by ChEMBL
| Assay Description
Inhibitory activity against matrix metalloproteinase 9 (MMP-9). |
Bioorg Med Chem Lett 13: 1487-90 (2003)
BindingDB Entry DOI: 10.7270/Q29W0DW0 |
More data for this
Ligand-Target Pair | |
Matrix metalloproteinase-9
(Homo sapiens (Human)) | BDBM50126606
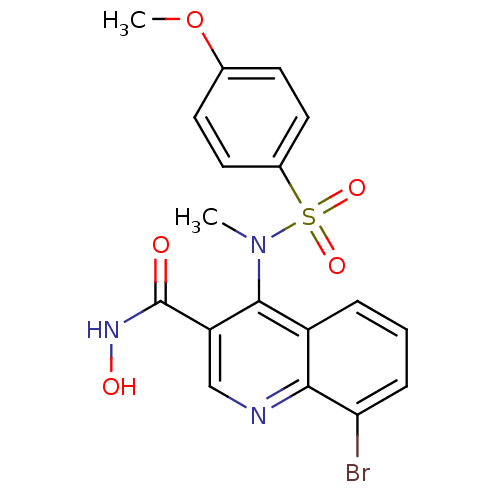
(8-Bromo-4-[(4-methoxy-benzenesulfonyl)-methyl-amin...)Show SMILES COc1ccc(cc1)S(=O)(=O)N(C)c1c(cnc2c(Br)cccc12)C(=O)NO Show InChI InChI=1S/C18H16BrN3O5S/c1-22(28(25,26)12-8-6-11(27-2)7-9-12)17-13-4-3-5-15(19)16(13)20-10-14(17)18(23)21-24/h3-10,24H,1-2H3,(H,21,23) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth-Ayerst Research
Curated by ChEMBL
| Assay Description
Inhibitory activity against matrix metalloproteinase 9 (MMP-9). |
Bioorg Med Chem Lett 13: 1487-90 (2003)
BindingDB Entry DOI: 10.7270/Q29W0DW0 |
More data for this
Ligand-Target Pair | |
Matrix metalloproteinase-9
(Homo sapiens (Human)) | BDBM50126602
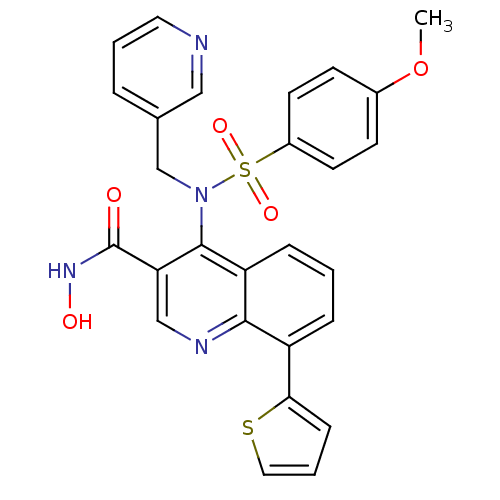
(4-[(4-Methoxy-benzenesulfonyl)-pyridin-3-ylmethyl-...)Show SMILES COc1ccc(cc1)S(=O)(=O)N(Cc1cccnc1)c1c(cnc2c(cccc12)-c1cccs1)C(=O)NO Show InChI InChI=1S/C27H22N4O5S2/c1-36-19-9-11-20(12-10-19)38(34,35)31(17-18-5-3-13-28-15-18)26-22-7-2-6-21(24-8-4-14-37-24)25(22)29-16-23(26)27(32)30-33/h2-16,33H,17H2,1H3,(H,30,32) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth-Ayerst Research
Curated by ChEMBL
| Assay Description
Inhibitory activity against matrix metalloproteinase 9 (MMP-9). |
Bioorg Med Chem Lett 13: 1487-90 (2003)
BindingDB Entry DOI: 10.7270/Q29W0DW0 |
More data for this
Ligand-Target Pair | |
Matrix metalloproteinase-9
(Homo sapiens (Human)) | BDBM50126622

(4-(N-benzyl-4-methoxyphenylsulfonamido)-N-hydroxy-...)Show SMILES COc1ccc(cc1)S(=O)(=O)N(Cc1ccccc1)c1c(cnc2c(cccc12)C(F)(F)F)C(=O)NO Show InChI InChI=1S/C25H20F3N3O5S/c1-36-17-10-12-18(13-11-17)37(34,35)31(15-16-6-3-2-4-7-16)23-19-8-5-9-21(25(26,27)28)22(19)29-14-20(23)24(32)30-33/h2-14,33H,15H2,1H3,(H,30,32) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth-Ayerst Research
Curated by ChEMBL
| Assay Description
Inhibitory activity against matrix metalloproteinase 9 (MMP-9). |
Bioorg Med Chem Lett 13: 1487-90 (2003)
BindingDB Entry DOI: 10.7270/Q29W0DW0 |
More data for this
Ligand-Target Pair | |
Matrix metalloproteinase-9
(Homo sapiens (Human)) | BDBM50126609
(4-[(4-Methoxy-benzenesulfonyl)-pyridin-3-ylmethyl-...)Show SMILES COc1ccc(cc1)S(=O)(=O)N(Cc1cccnc1)c1c(cnc2n(nc(C)c12)-c1ccccc1)C(=O)NO Show InChI InChI=1S/C27H24N6O5S/c1-18-24-25(23(27(34)31-35)16-29-26(24)33(30-18)20-8-4-3-5-9-20)32(17-19-7-6-14-28-15-19)39(36,37)22-12-10-21(38-2)11-13-22/h3-16,35H,17H2,1-2H3,(H,31,34) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth-Ayerst Research
Curated by ChEMBL
| Assay Description
Inhibitory activity against matrix metalloproteinase 9 (MMP-9). |
Bioorg Med Chem Lett 13: 1487-90 (2003)
BindingDB Entry DOI: 10.7270/Q29W0DW0 |
More data for this
Ligand-Target Pair | |
Matrix metalloproteinase-9
(Homo sapiens (Human)) | BDBM50126599
(4-[(4-Methoxy-benzenesulfonyl)-pyridin-3-ylmethyl-...)Show SMILES COc1ccc(cc1)S(=O)(=O)N(Cc1cccnc1)c1c(cnc2snc(C)c12)C(=O)NO Show InChI InChI=1S/C21H19N5O5S2/c1-13-18-19(17(20(27)24-28)11-23-21(18)32-25-13)26(12-14-4-3-9-22-10-14)33(29,30)16-7-5-15(31-2)6-8-16/h3-11,28H,12H2,1-2H3,(H,24,27) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth-Ayerst Research
Curated by ChEMBL
| Assay Description
Inhibitory activity against matrix metalloproteinase 9 (MMP-9). |
Bioorg Med Chem Lett 13: 1487-90 (2003)
BindingDB Entry DOI: 10.7270/Q29W0DW0 |
More data for this
Ligand-Target Pair | |
Matrix metalloproteinase-9
(Homo sapiens (Human)) | BDBM50126600

(1,3-Dimethyl-4-{methyl-[4-(pyridin-4-yloxy)-benzen...)Show SMILES CN(c1c(cnc2n(C)nc(C)c12)C(=O)NO)S(=O)(=O)c1ccc(Oc2ccncc2)cc1 Show InChI InChI=1S/C21H20N6O5S/c1-13-18-19(17(21(28)25-29)12-23-20(18)26(2)24-13)27(3)33(30,31)16-6-4-14(5-7-16)32-15-8-10-22-11-9-15/h4-12,29H,1-3H3,(H,25,28) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth-Ayerst Research
Curated by ChEMBL
| Assay Description
Inhibitory activity against matrix metalloproteinase 9 (MMP-9). |
Bioorg Med Chem Lett 13: 1487-90 (2003)
BindingDB Entry DOI: 10.7270/Q29W0DW0 |
More data for this
Ligand-Target Pair | |
Collagenase 3
(Homo sapiens (Human)) | BDBM50126602

(4-[(4-Methoxy-benzenesulfonyl)-pyridin-3-ylmethyl-...)Show SMILES COc1ccc(cc1)S(=O)(=O)N(Cc1cccnc1)c1c(cnc2c(cccc12)-c1cccs1)C(=O)NO Show InChI InChI=1S/C27H22N4O5S2/c1-36-19-9-11-20(12-10-19)38(34,35)31(17-18-5-3-13-28-15-18)26-22-7-2-6-21(24-8-4-14-37-24)25(22)29-16-23(26)27(32)30-33/h2-16,33H,17H2,1H3,(H,30,32) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth-Ayerst Research
Curated by ChEMBL
| Assay Description
Inhibitory activity against matrix metalloproteinase 13 (MMP-13). |
Bioorg Med Chem Lett 13: 1487-90 (2003)
BindingDB Entry DOI: 10.7270/Q29W0DW0 |
More data for this
Ligand-Target Pair | |
Collagenase 3
(Homo sapiens (Human)) | BDBM50126630

(4-[(4-Methoxy-benzenesulfonyl)-pyridin-3-ylmethyl-...)Show SMILES COc1ccc(cc1)S(=O)(=O)N(Cc1cccnc1)c1c(cnc2n(C)nc(C)c12)C(=O)NO Show InChI InChI=1S/C22H22N6O5S/c1-14-19-20(18(22(29)26-30)12-24-21(19)27(2)25-14)28(13-15-5-4-10-23-11-15)34(31,32)17-8-6-16(33-3)7-9-17/h4-12,30H,13H2,1-3H3,(H,26,29) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth-Ayerst Research
Curated by ChEMBL
| Assay Description
Inhibitory activity against matrix metalloproteinase 13 (MMP-13). |
Bioorg Med Chem Lett 13: 1487-90 (2003)
BindingDB Entry DOI: 10.7270/Q29W0DW0 |
More data for this
Ligand-Target Pair | |
Collagenase 3
(Homo sapiens (Human)) | BDBM50126606

(8-Bromo-4-[(4-methoxy-benzenesulfonyl)-methyl-amin...)Show SMILES COc1ccc(cc1)S(=O)(=O)N(C)c1c(cnc2c(Br)cccc12)C(=O)NO Show InChI InChI=1S/C18H16BrN3O5S/c1-22(28(25,26)12-8-6-11(27-2)7-9-12)17-13-4-3-5-15(19)16(13)20-10-14(17)18(23)21-24/h3-10,24H,1-2H3,(H,21,23) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth-Ayerst Research
Curated by ChEMBL
| Assay Description
Inhibitory activity against matrix metalloproteinase 13 (MMP-13). |
Bioorg Med Chem Lett 13: 1487-90 (2003)
BindingDB Entry DOI: 10.7270/Q29W0DW0 |
More data for this
Ligand-Target Pair | |
Matrix metalloproteinase-9
(Homo sapiens (Human)) | BDBM50103159
(5-Dimethylamino-N-hydroxy-2-[(4-methoxy-benzenesul...)Show SMILES COc1ccc(cc1)S(=O)(=O)N(Cc1cccnc1)c1c(C)cc(cc1C(=O)NO)N(C)C Show InChI InChI=1S/C23H26N4O5S/c1-16-12-18(26(2)3)13-21(23(28)25-29)22(16)27(15-17-6-5-11-24-14-17)33(30,31)20-9-7-19(32-4)8-10-20/h5-14,29H,15H2,1-4H3,(H,25,28) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth-Ayerst Research
Curated by ChEMBL
| Assay Description
In vitro ability to inhibit matrix metalloprotease-9. |
Bioorg Med Chem Lett 11: 2189-92 (2001)
BindingDB Entry DOI: 10.7270/Q2154HK0 |
More data for this
Ligand-Target Pair | |
Collagenase 3
(Homo sapiens (Human)) | BDBM50103171

(4-[(4-Methoxy-benzenesulfonyl)-pyridin-3-ylmethyl-...)Show SMILES COc1ccc(cc1)S(=O)(=O)N(Cc1cccnc1)c1c(C)cc(cc1C(=O)NO)-c1cccc(c1)C(F)(F)F Show InChI InChI=1S/C28H24F3N3O5S/c1-18-13-21(20-6-3-7-22(14-20)28(29,30)31)15-25(27(35)33-36)26(18)34(17-19-5-4-12-32-16-19)40(37,38)24-10-8-23(39-2)9-11-24/h3-16,36H,17H2,1-2H3,(H,33,35) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth-Ayerst Research
Curated by ChEMBL
| Assay Description
Inhibition of matrix metalloprotease-13 |
Bioorg Med Chem Lett 11: 2189-92 (2001)
BindingDB Entry DOI: 10.7270/Q2154HK0 |
More data for this
Ligand-Target Pair | |
Matrix metalloproteinase-9
(Homo sapiens (Human)) | BDBM50103162

(CHEMBL68542 | N-Hydroxy-2-[(4-methoxy-benzenesulfo...)Show SMILES COc1ccc(cc1)S(=O)(=O)N(Cc1cccnc1)c1c(C)cc(cc1C(=O)NO)-c1ccc2ccccc2c1 Show InChI InChI=1S/C31H27N3O5S/c1-21-16-26(25-10-9-23-7-3-4-8-24(23)17-25)18-29(31(35)33-36)30(21)34(20-22-6-5-15-32-19-22)40(37,38)28-13-11-27(39-2)12-14-28/h3-19,36H,20H2,1-2H3,(H,33,35) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth-Ayerst Research
Curated by ChEMBL
| Assay Description
In vitro ability to inhibit matrix metalloprotease-9. |
Bioorg Med Chem Lett 11: 2189-92 (2001)
BindingDB Entry DOI: 10.7270/Q2154HK0 |
More data for this
Ligand-Target Pair | |
Matrix metalloproteinase-9
(Homo sapiens (Human)) | BDBM50126605
(4-[(4-Methoxy-benzenesulfonyl)-pyridin-3-ylmethyl-...)Show SMILES COc1ccc(cc1)S(=O)(=O)N(Cc1cccnc1)c1c(cnc2c(cccc12)-c1ccccc1)C(=O)NO Show InChI InChI=1S/C29H24N4O5S/c1-38-22-12-14-23(15-13-22)39(36,37)33(19-20-7-6-16-30-17-20)28-25-11-5-10-24(21-8-3-2-4-9-21)27(25)31-18-26(28)29(34)32-35/h2-18,35H,19H2,1H3,(H,32,34) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth-Ayerst Research
Curated by ChEMBL
| Assay Description
Inhibitory activity against matrix metalloproteinase 9 (MMP-9). |
Bioorg Med Chem Lett 13: 1487-90 (2003)
BindingDB Entry DOI: 10.7270/Q29W0DW0 |
More data for this
Ligand-Target Pair | |
Matrix metalloproteinase-9
(Homo sapiens (Human)) | BDBM50126617

(8-Iodo-4-[(4-methoxy-benzenesulfonyl)-pyridin-3-yl...)Show SMILES COc1ccc(cc1)S(=O)(=O)N(Cc1cccnc1)c1c(cnc2c(I)cccc12)C(=O)NO Show InChI InChI=1S/C23H19IN4O5S/c1-33-16-7-9-17(10-8-16)34(31,32)28(14-15-4-3-11-25-12-15)22-18-5-2-6-20(24)21(18)26-13-19(22)23(29)27-30/h2-13,30H,14H2,1H3,(H,27,29) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth-Ayerst Research
Curated by ChEMBL
| Assay Description
Inhibitory activity against matrix metalloproteinase 9 (MMP-9). |
Bioorg Med Chem Lett 13: 1487-90 (2003)
BindingDB Entry DOI: 10.7270/Q29W0DW0 |
More data for this
Ligand-Target Pair | |
Collagenase 3
(Homo sapiens (Human)) | BDBM50126599

(4-[(4-Methoxy-benzenesulfonyl)-pyridin-3-ylmethyl-...)Show SMILES COc1ccc(cc1)S(=O)(=O)N(Cc1cccnc1)c1c(cnc2snc(C)c12)C(=O)NO Show InChI InChI=1S/C21H19N5O5S2/c1-13-18-19(17(20(27)24-28)11-23-21(18)32-25-13)26(12-14-4-3-9-22-10-14)33(29,30)16-7-5-15(31-2)6-8-16/h3-11,28H,12H2,1-2H3,(H,24,27) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth-Ayerst Research
Curated by ChEMBL
| Assay Description
Inhibitory activity against matrix metalloproteinase 13 (MMP-13). |
Bioorg Med Chem Lett 13: 1487-90 (2003)
BindingDB Entry DOI: 10.7270/Q29W0DW0 |
More data for this
Ligand-Target Pair | |
Collagenase 3
(Homo sapiens (Human)) | BDBM50126609

(4-[(4-Methoxy-benzenesulfonyl)-pyridin-3-ylmethyl-...)Show SMILES COc1ccc(cc1)S(=O)(=O)N(Cc1cccnc1)c1c(cnc2n(nc(C)c12)-c1ccccc1)C(=O)NO Show InChI InChI=1S/C27H24N6O5S/c1-18-24-25(23(27(34)31-35)16-29-26(24)33(30-18)20-8-4-3-5-9-20)32(17-19-7-6-14-28-15-19)39(36,37)22-12-10-21(38-2)11-13-22/h3-16,35H,17H2,1-2H3,(H,31,34) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth-Ayerst Research
Curated by ChEMBL
| Assay Description
Inhibitory activity against matrix metalloproteinase 13 (MMP-13). |
Bioorg Med Chem Lett 13: 1487-90 (2003)
BindingDB Entry DOI: 10.7270/Q29W0DW0 |
More data for this
Ligand-Target Pair | |
Collagenase 3
(Homo sapiens (Human)) | BDBM50126621

(4-[(4-Butoxy-benzenesulfonyl)-methyl-amino]-1,3-di...)Show SMILES CCCCOc1ccc(cc1)S(=O)(=O)N(C)c1c(cnc2n(C)nc(C)c12)C(=O)NO Show InChI InChI=1S/C20H25N5O5S/c1-5-6-11-30-14-7-9-15(10-8-14)31(28,29)25(4)18-16(20(26)23-27)12-21-19-17(18)13(2)22-24(19)3/h7-10,12,27H,5-6,11H2,1-4H3,(H,23,26) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth-Ayerst Research
Curated by ChEMBL
| Assay Description
Inhibitory activity against matrix metalloproteinase 13 (MMP-13). |
Bioorg Med Chem Lett 13: 1487-90 (2003)
BindingDB Entry DOI: 10.7270/Q29W0DW0 |
More data for this
Ligand-Target Pair | |
Collagenase 3
(Homo sapiens (Human)) | BDBM50126617

(8-Iodo-4-[(4-methoxy-benzenesulfonyl)-pyridin-3-yl...)Show SMILES COc1ccc(cc1)S(=O)(=O)N(Cc1cccnc1)c1c(cnc2c(I)cccc12)C(=O)NO Show InChI InChI=1S/C23H19IN4O5S/c1-33-16-7-9-17(10-8-16)34(31,32)28(14-15-4-3-11-25-12-15)22-18-5-2-6-20(24)21(18)26-13-19(22)23(29)27-30/h2-13,30H,14H2,1H3,(H,27,29) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth-Ayerst Research
Curated by ChEMBL
| Assay Description
Inhibitory activity against matrix metalloproteinase 13 (MMP-13). |
Bioorg Med Chem Lett 13: 1487-90 (2003)
BindingDB Entry DOI: 10.7270/Q29W0DW0 |
More data for this
Ligand-Target Pair | |
Collagenase 3
(Homo sapiens (Human)) | BDBM50126625

(8-Benzyl-4-[(4-methoxy-benzenesulfonyl)-pyridin-3-...)Show SMILES COc1ccc(cc1)S(=O)(=O)N(Cc1cccnc1)c1c(cnc2c(Cc3ccccc3)cccc12)C(=O)NO Show InChI InChI=1S/C30H26N4O5S/c1-39-24-12-14-25(15-13-24)40(37,38)34(20-22-9-6-16-31-18-22)29-26-11-5-10-23(17-21-7-3-2-4-8-21)28(26)32-19-27(29)30(35)33-36/h2-16,18-19,36H,17,20H2,1H3,(H,33,35) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth-Ayerst Research
Curated by ChEMBL
| Assay Description
Inhibitory activity against matrix metalloproteinase 13 (MMP-13). |
Bioorg Med Chem Lett 13: 1487-90 (2003)
BindingDB Entry DOI: 10.7270/Q29W0DW0 |
More data for this
Ligand-Target Pair | |
Collagenase 3
(Homo sapiens (Human)) | BDBM50103164
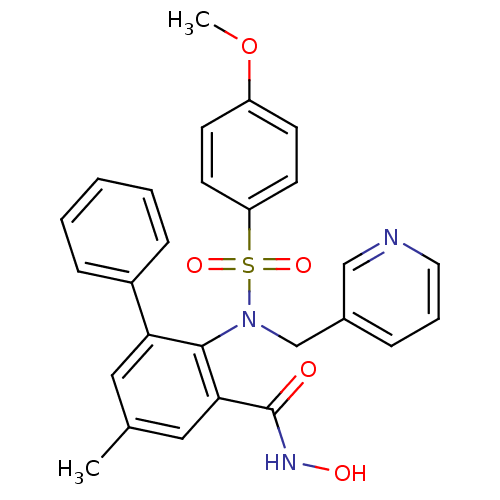
(2-[(4-Methoxy-benzenesulfonyl)-pyridin-3-ylmethyl-...)Show SMILES COc1ccc(cc1)S(=O)(=O)N(Cc1cccnc1)c1c(cc(C)cc1-c1ccccc1)C(=O)NO Show InChI InChI=1S/C27H25N3O5S/c1-19-15-24(21-8-4-3-5-9-21)26(25(16-19)27(31)29-32)30(18-20-7-6-14-28-17-20)36(33,34)23-12-10-22(35-2)11-13-23/h3-17,32H,18H2,1-2H3,(H,29,31) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth-Ayerst Research
Curated by ChEMBL
| Assay Description
Inhibition of matrix metalloprotease-13 |
Bioorg Med Chem Lett 11: 2189-92 (2001)
BindingDB Entry DOI: 10.7270/Q2154HK0 |
More data for this
Ligand-Target Pair | |
Collagenase 3
(Homo sapiens (Human)) | BDBM50103168
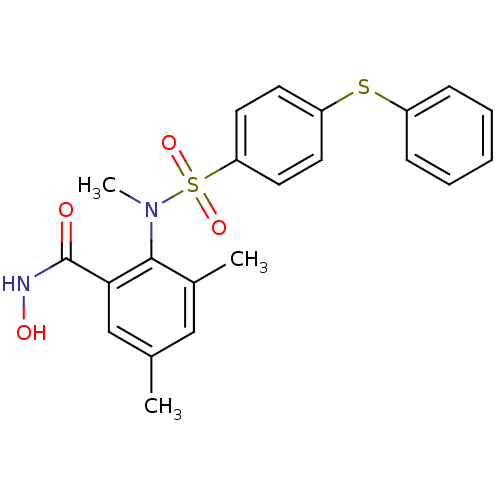
(CHEMBL71686 | N-Hydroxy-3,5-dimethyl-2-[methyl-(4-...)Show SMILES CN(c1c(C)cc(C)cc1C(=O)NO)S(=O)(=O)c1ccc(Sc2ccccc2)cc1 Show InChI InChI=1S/C22H22N2O4S2/c1-15-13-16(2)21(20(14-15)22(25)23-26)24(3)30(27,28)19-11-9-18(10-12-19)29-17-7-5-4-6-8-17/h4-14,26H,1-3H3,(H,23,25) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth-Ayerst Research
Curated by ChEMBL
| Assay Description
Inhibition of matrix metalloprotease-13 |
Bioorg Med Chem Lett 11: 2189-92 (2001)
BindingDB Entry DOI: 10.7270/Q2154HK0 |
More data for this
Ligand-Target Pair | |
Matrix metalloproteinase-9
(Homo sapiens (Human)) | BDBM50103169
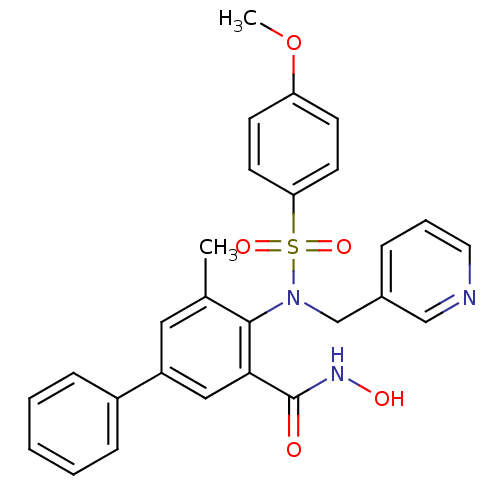
(4-[(4-Methoxy-benzenesulfonyl)-pyridin-3-ylmethyl-...)Show SMILES COc1ccc(cc1)S(=O)(=O)N(Cc1cccnc1)c1c(C)cc(cc1C(=O)NO)-c1ccccc1 Show InChI InChI=1S/C27H25N3O5S/c1-19-15-22(21-8-4-3-5-9-21)16-25(27(31)29-32)26(19)30(18-20-7-6-14-28-17-20)36(33,34)24-12-10-23(35-2)11-13-24/h3-17,32H,18H2,1-2H3,(H,29,31) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth-Ayerst Research
Curated by ChEMBL
| Assay Description
In vitro ability to inhibit matrix metalloprotease-9. |
Bioorg Med Chem Lett 11: 2189-92 (2001)
BindingDB Entry DOI: 10.7270/Q2154HK0 |
More data for this
Ligand-Target Pair | |
Matrix metalloproteinase-9
(Homo sapiens (Human)) | BDBM50126625

(8-Benzyl-4-[(4-methoxy-benzenesulfonyl)-pyridin-3-...)Show SMILES COc1ccc(cc1)S(=O)(=O)N(Cc1cccnc1)c1c(cnc2c(Cc3ccccc3)cccc12)C(=O)NO Show InChI InChI=1S/C30H26N4O5S/c1-39-24-12-14-25(15-13-24)40(37,38)34(20-22-9-6-16-31-18-22)29-26-11-5-10-23(17-21-7-3-2-4-8-21)28(26)32-19-27(29)30(35)33-36/h2-16,18-19,36H,17,20H2,1H3,(H,33,35) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth-Ayerst Research
Curated by ChEMBL
| Assay Description
Inhibitory activity against matrix metalloproteinase 9 (MMP-9). |
Bioorg Med Chem Lett 13: 1487-90 (2003)
BindingDB Entry DOI: 10.7270/Q29W0DW0 |
More data for this
Ligand-Target Pair | |
Matrix metalloproteinase-9
(Homo sapiens (Human)) | BDBM50126620

(4-[(4-Methoxy-benzenesulfonyl)-pyridin-3-ylmethyl-...)Show SMILES COc1ccc(cc1)S(=O)(=O)N(Cc1cccnc1)c1c(cnc2cc(ccc12)C(F)(F)F)C(=O)NO Show InChI InChI=1S/C24H19F3N4O5S/c1-36-17-5-7-18(8-6-17)37(34,35)31(14-15-3-2-10-28-12-15)22-19-9-4-16(24(25,26)27)11-21(19)29-13-20(22)23(32)30-33/h2-13,33H,14H2,1H3,(H,30,32) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth-Ayerst Research
Curated by ChEMBL
| Assay Description
Inhibitory activity against matrix metalloproteinase 9 (MMP-9). |
Bioorg Med Chem Lett 13: 1487-90 (2003)
BindingDB Entry DOI: 10.7270/Q29W0DW0 |
More data for this
Ligand-Target Pair | |
Collagenase 3
(Homo sapiens (Human)) | BDBM50126604

(4-(N-benzyl-4-methoxyphenylsulfonamido)-8-ethyl-N-...)Show SMILES CCc1cccc2c(N(Cc3ccccc3)S(=O)(=O)c3ccc(OC)cc3)c(cnc12)C(=O)NO Show InChI InChI=1S/C26H25N3O5S/c1-3-19-10-7-11-22-24(19)27-16-23(26(30)28-31)25(22)29(17-18-8-5-4-6-9-18)35(32,33)21-14-12-20(34-2)13-15-21/h4-16,31H,3,17H2,1-2H3,(H,28,30) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth-Ayerst Research
Curated by ChEMBL
| Assay Description
Inhibitory activity against matrix metalloproteinase 13 (MMP-13). |
Bioorg Med Chem Lett 13: 1487-90 (2003)
BindingDB Entry DOI: 10.7270/Q29W0DW0 |
More data for this
Ligand-Target Pair | |
Collagenase 3
(Homo sapiens (Human)) | BDBM50126605

(4-[(4-Methoxy-benzenesulfonyl)-pyridin-3-ylmethyl-...)Show SMILES COc1ccc(cc1)S(=O)(=O)N(Cc1cccnc1)c1c(cnc2c(cccc12)-c1ccccc1)C(=O)NO Show InChI InChI=1S/C29H24N4O5S/c1-38-22-12-14-23(15-13-22)39(36,37)33(19-20-7-6-16-30-17-20)28-25-11-5-10-24(21-8-3-2-4-9-21)27(25)31-18-26(28)29(34)32-35/h2-18,35H,19H2,1H3,(H,32,34) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth-Ayerst Research
Curated by ChEMBL
| Assay Description
Inhibitory activity against matrix metalloproteinase 13 (MMP-13). |
Bioorg Med Chem Lett 13: 1487-90 (2003)
BindingDB Entry DOI: 10.7270/Q29W0DW0 |
More data for this
Ligand-Target Pair | |
Matrix metalloproteinase-9
(Homo sapiens (Human)) | BDBM50103156
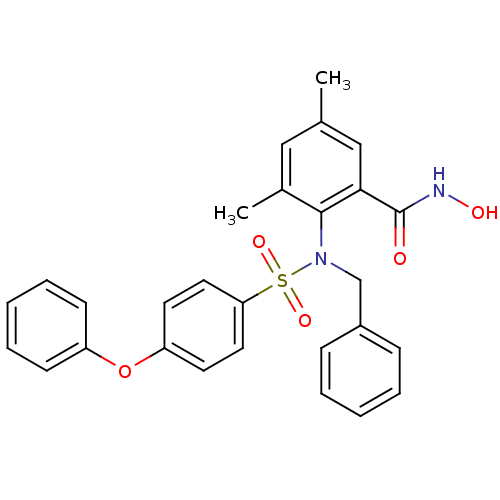
(2-[Benzyl-(4-phenoxy-benzenesulfonyl)-amino]-N-hyd...)Show SMILES Cc1cc(C)c(N(Cc2ccccc2)S(=O)(=O)c2ccc(Oc3ccccc3)cc2)c(c1)C(=O)NO Show InChI InChI=1S/C28H26N2O5S/c1-20-17-21(2)27(26(18-20)28(31)29-32)30(19-22-9-5-3-6-10-22)36(33,34)25-15-13-24(14-16-25)35-23-11-7-4-8-12-23/h3-18,32H,19H2,1-2H3,(H,29,31) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth-Ayerst Research
Curated by ChEMBL
| Assay Description
In vitro ability to inhibit matrix metalloprotease-9. |
Bioorg Med Chem Lett 11: 2189-92 (2001)
BindingDB Entry DOI: 10.7270/Q2154HK0 |
More data for this
Ligand-Target Pair | |
Collagenase 3
(Homo sapiens (Human)) | BDBM50103165
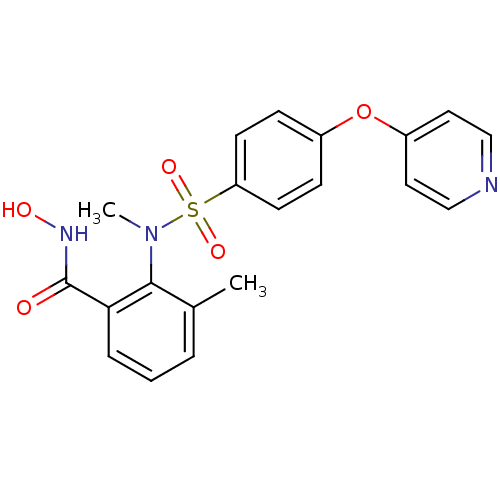
(CHEMBL68212 | N-Hydroxy-3-methyl-2-{methyl-[4-(pyr...)Show SMILES CN(c1c(C)cccc1C(=O)NO)S(=O)(=O)c1ccc(Oc2ccncc2)cc1 Show InChI InChI=1S/C20H19N3O5S/c1-14-4-3-5-18(20(24)22-25)19(14)23(2)29(26,27)17-8-6-15(7-9-17)28-16-10-12-21-13-11-16/h3-13,25H,1-2H3,(H,22,24) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth-Ayerst Research
Curated by ChEMBL
| Assay Description
Inhibition of matrix metalloprotease-13 |
Bioorg Med Chem Lett 11: 2189-92 (2001)
BindingDB Entry DOI: 10.7270/Q2154HK0 |
More data for this
Ligand-Target Pair | |
Collagenase 3
(Homo sapiens (Human)) | BDBM50103169

(4-[(4-Methoxy-benzenesulfonyl)-pyridin-3-ylmethyl-...)Show SMILES COc1ccc(cc1)S(=O)(=O)N(Cc1cccnc1)c1c(C)cc(cc1C(=O)NO)-c1ccccc1 Show InChI InChI=1S/C27H25N3O5S/c1-19-15-22(21-8-4-3-5-9-21)16-25(27(31)29-32)26(19)30(18-20-7-6-14-28-17-20)36(33,34)24-12-10-23(35-2)11-13-24/h3-17,32H,18H2,1-2H3,(H,29,31) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth-Ayerst Research
Curated by ChEMBL
| Assay Description
Inhibition of matrix metalloprotease-13 |
Bioorg Med Chem Lett 11: 2189-92 (2001)
BindingDB Entry DOI: 10.7270/Q2154HK0 |
More data for this
Ligand-Target Pair | |
Matrix metalloproteinase-9
(Homo sapiens (Human)) | BDBM50126627
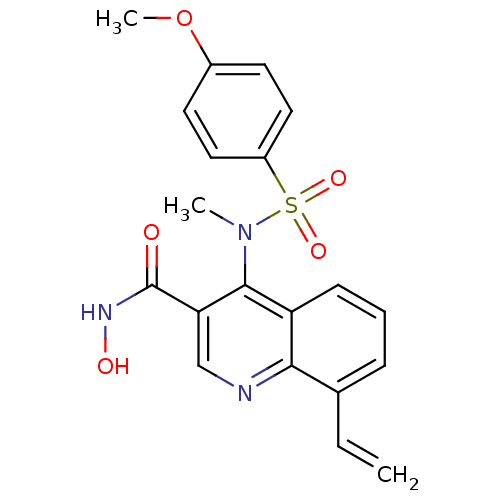
(4-[(4-Methoxy-benzenesulfonyl)-methyl-amino]-8-vin...)Show SMILES COc1ccc(cc1)S(=O)(=O)N(C)c1c(cnc2c(C=C)cccc12)C(=O)NO Show InChI InChI=1S/C20H19N3O5S/c1-4-13-6-5-7-16-18(13)21-12-17(20(24)22-25)19(16)23(2)29(26,27)15-10-8-14(28-3)9-11-15/h4-12,25H,1H2,2-3H3,(H,22,24) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth-Ayerst Research
Curated by ChEMBL
| Assay Description
Inhibitory activity against matrix metalloproteinase 9 (MMP-9). |
Bioorg Med Chem Lett 13: 1487-90 (2003)
BindingDB Entry DOI: 10.7270/Q29W0DW0 |
More data for this
Ligand-Target Pair | |
Collagenase 3
(Homo sapiens (Human)) | BDBM50126627

(4-[(4-Methoxy-benzenesulfonyl)-methyl-amino]-8-vin...)Show SMILES COc1ccc(cc1)S(=O)(=O)N(C)c1c(cnc2c(C=C)cccc12)C(=O)NO Show InChI InChI=1S/C20H19N3O5S/c1-4-13-6-5-7-16-18(13)21-12-17(20(24)22-25)19(16)23(2)29(26,27)15-10-8-14(28-3)9-11-15/h4-12,25H,1H2,2-3H3,(H,22,24) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth-Ayerst Research
Curated by ChEMBL
| Assay Description
Inhibitory activity against matrix metalloproteinase 13 (MMP-13). |
Bioorg Med Chem Lett 13: 1487-90 (2003)
BindingDB Entry DOI: 10.7270/Q29W0DW0 |
More data for this
Ligand-Target Pair | |
Matrix metalloproteinase-9
(Homo sapiens (Human)) | BDBM50103154
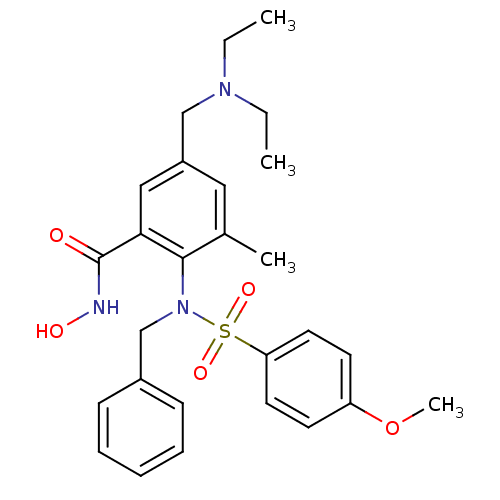
(2-[Benzyl-(4-methoxy-benzenesulfonyl)-amino]-5-die...)Show SMILES CCN(CC)Cc1cc(C)c(N(Cc2ccccc2)S(=O)(=O)c2ccc(OC)cc2)c(c1)C(=O)NO Show InChI InChI=1S/C27H33N3O5S/c1-5-29(6-2)18-22-16-20(3)26(25(17-22)27(31)28-32)30(19-21-10-8-7-9-11-21)36(33,34)24-14-12-23(35-4)13-15-24/h7-17,32H,5-6,18-19H2,1-4H3,(H,28,31) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth-Ayerst Research
Curated by ChEMBL
| Assay Description
In vitro ability to inhibit matrix metalloprotease-9. |
Bioorg Med Chem Lett 11: 2189-92 (2001)
BindingDB Entry DOI: 10.7270/Q2154HK0 |
More data for this
Ligand-Target Pair | |
Matrix metalloproteinase-9
(Homo sapiens (Human)) | BDBM50096463
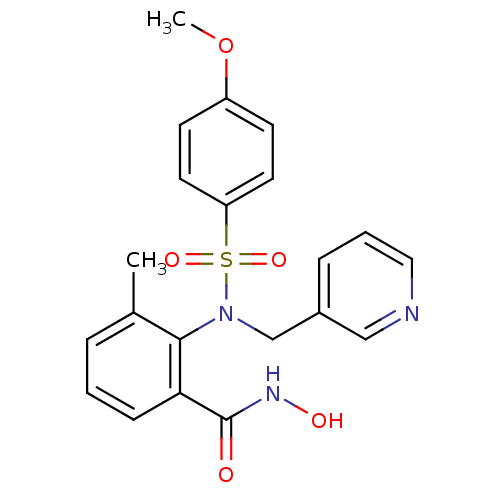
(CHEMBL70176 | N-Hydroxy-2-[(4-methoxy-benzenesulfo...)Show SMILES COc1ccc(cc1)S(=O)(=O)N(Cc1cccnc1)c1c(C)cccc1C(=O)NO Show InChI InChI=1S/C21H21N3O5S/c1-15-5-3-7-19(21(25)23-26)20(15)24(14-16-6-4-12-22-13-16)30(27,28)18-10-8-17(29-2)9-11-18/h3-13,26H,14H2,1-2H3,(H,23,25) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
DrugBank
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth-Ayerst Research
Curated by ChEMBL
| Assay Description
In vitro ability to inhibit matrix metalloprotease-9. |
Bioorg Med Chem Lett 11: 2189-92 (2001)
BindingDB Entry DOI: 10.7270/Q2154HK0 |
More data for this
Ligand-Target Pair | |
Farnesyl pyrophosphate synthase
(Homo sapiens (Human)) | BDBM12576

(Bisphosphonate 1 | CHEMBL923 | JMC515594 Compound ...)Show InChI InChI=1S/C7H11NO7P2/c9-7(16(10,11)12,17(13,14)15)4-6-2-1-3-8-5-6/h1-3,5,9H,4H2,(H2,10,11,12)(H2,13,14,15) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
MMDB
PDB
Article
PubMed
| n/a | n/a | 5.70 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Southern California
Curated by ChEMBL
| Assay Description
Inhibition of human FPPS |
Bioorg Med Chem Lett 18: 2878-82 (2008)
Article DOI: 10.1016/j.bmcl.2008.03.088
BindingDB Entry DOI: 10.7270/Q2WM1F9P |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data