

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Histone-lysine N-methyltransferase EZH2 (Homo sapiens (Human)) | BDBM50246967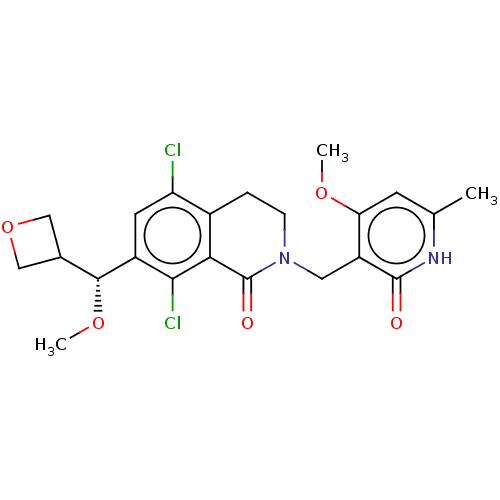 (CHEMBL4080228 | US10570121, Example 81) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | MCE PC cid PC sid PDB UniChem | PDB Article PubMed | <0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
WuXi AppTec Curated by ChEMBL | Assay Description Binding affinity to EZH2 (unknown origin) | J Med Chem 61: 650-665 (2018) Article DOI: 10.1021/acs.jmedchem.7b01375 BindingDB Entry DOI: 10.7270/Q2X069G8 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Metabotropic glutamate receptor 5 (Rattus norvegicus (Rat)) | BDBM50084137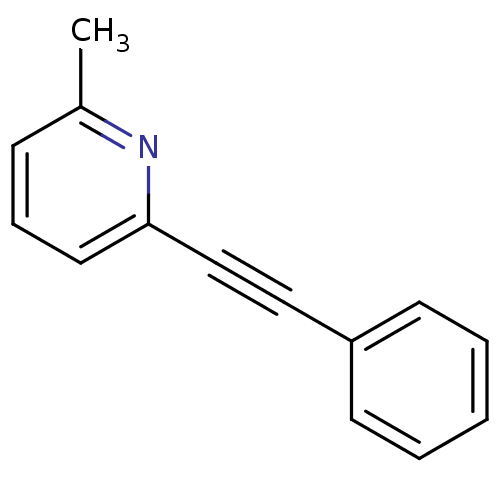 (2-Methyl-6-(phenylethynyl)pyridine | 2-Methyl-6-ph...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by PDSP Ki Database | Life Sci 73: 371-9 (2003) Article DOI: 10.1016/s0024-3205(03)00272-8 BindingDB Entry DOI: 10.7270/Q2KD1WHW | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Rattus norvegicus (rat)) | BDBM27213 (4-[3-(4-cyclopropanecarbonylphenoxy)propyl]-1H-imi...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | 0.490 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Freie Universität Berlin Curated by ChEMBL | Assay Description In vitro binding affinity against rat histamine H3 receptor | J Med Chem 47: 2678-87 (2004) Article DOI: 10.1021/jm031065q BindingDB Entry DOI: 10.7270/Q2SQ8ZTC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Rattus norvegicus (rat)) | BDBM27213 (4-[3-(4-cyclopropanecarbonylphenoxy)propyl]-1H-imi...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | PubMed | 0.490 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Freie Universit£t Berlin Curated by ChEMBL | Assay Description Effect on histamine H3 receptors in vitro, on synaptosomes of rat cerebral cortex for the release of [3H]histamine | J Med Chem 43: 3987-94 (2000) BindingDB Entry DOI: 10.7270/Q2833SRN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 3-phosphoinositide-dependent protein kinase 1 (Homo sapiens (Human)) | BDBM50361648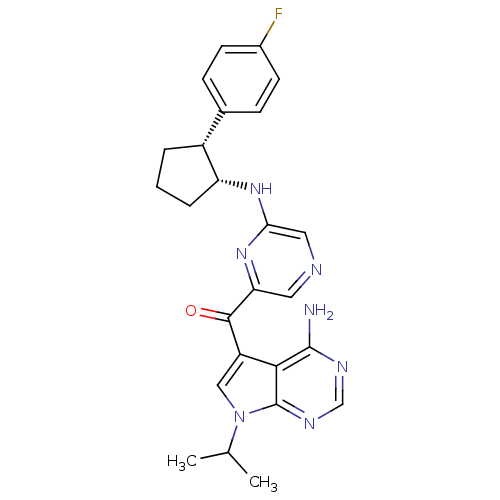 (CHEMBL1940246) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Inhibition of PDK1-mediated AKT1 phosphorylation at T308 in human H460 cells after 2 hrs by ELISA | J Med Chem 54: 8490-500 (2011) Article DOI: 10.1021/jm201019k BindingDB Entry DOI: 10.7270/Q23N23TV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 3-phosphoinositide-dependent protein kinase 1 (Homo sapiens (Human)) | BDBM50361649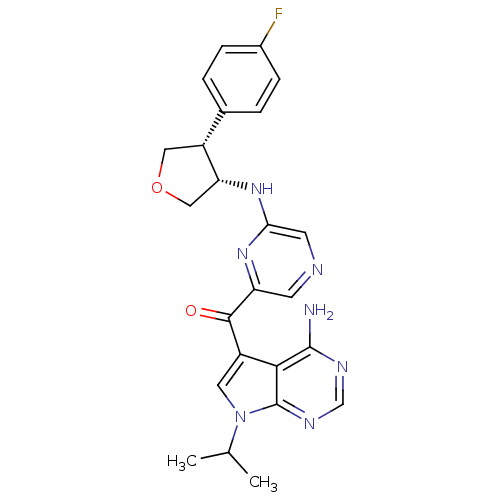 (CHEMBL1938415) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid PDB UniChem Similars | PDB Article PubMed | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Inhibition of PDK1-mediated AKT1 phosphorylation at T308 in human H460 cells after 2 hrs by ELISA | J Med Chem 54: 8490-500 (2011) Article DOI: 10.1021/jm201019k BindingDB Entry DOI: 10.7270/Q23N23TV | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Histamine H3 receptor (Rattus norvegicus (rat)) | BDBM22541 (Clobenpropit | N''-[(4-chlorophenyl)methyl]{[3-(1H...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Patents Similars | Article PubMed | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Freie Universität Berlin Curated by ChEMBL | Assay Description In vitro antagonist potency at Histamine H3 receptor measured as K+-evoked [3H]-histamine release from synaptosomes of rat cerebral cortex. | J Med Chem 41: 4171-6 (1998) Article DOI: 10.1021/jm9802970 BindingDB Entry DOI: 10.7270/Q2MK6C0W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Rattus norvegicus (rat)) | BDBM22541 (Clobenpropit | N''-[(4-chlorophenyl)methyl]{[3-(1H...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Patents Similars | PubMed | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Freie Universit£t Berlin Curated by ChEMBL | Assay Description Effect on histamine H3 receptors in vitro, on synaptosomes of rat cerebral cortex for the release of [3H]histamine | J Med Chem 43: 3987-94 (2000) BindingDB Entry DOI: 10.7270/Q2833SRN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 3-phosphoinositide-dependent protein kinase 1 (Homo sapiens (Human)) | BDBM50361642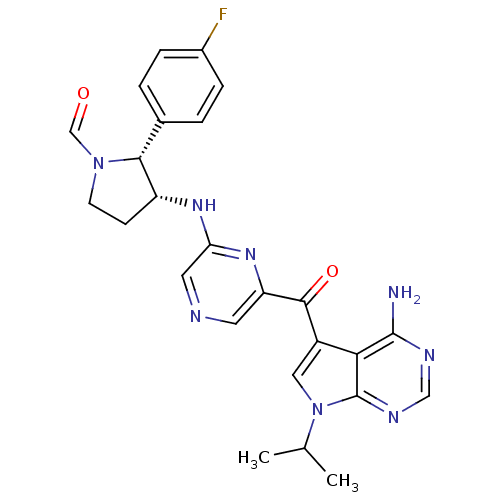 (CHEMBL1940251) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Inhibition of recombinant human His-tagged PDK1 catalytic domain using Ac-Sox-PKTFCGTPEYLAPEVRREPRILSEEEQEMFRDFDYIAD-NH2 as substrate by fluorescence... | J Med Chem 54: 8490-500 (2011) Article DOI: 10.1021/jm201019k BindingDB Entry DOI: 10.7270/Q23N23TV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Rattus norvegicus (rat)) | BDBM50092853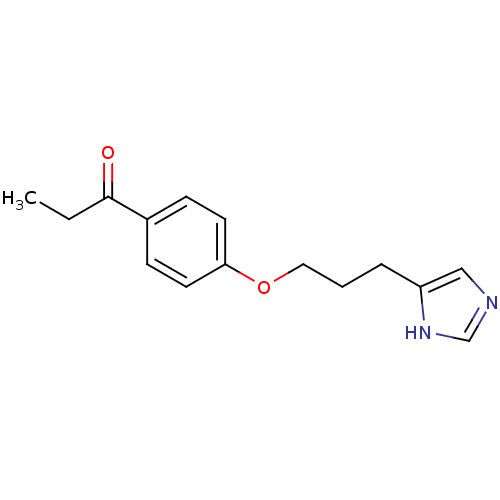 (1-{4-[3-(1H-Imidazol-4-yl)-propoxy]-phenyl}-propan...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.740 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Freie Universit£t Berlin Curated by ChEMBL | Assay Description Effect on histamine H3 receptors in vitro, on synaptosomes of rat cerebral cortex for the release of [3H]histamine | J Med Chem 43: 3987-94 (2000) BindingDB Entry DOI: 10.7270/Q2833SRN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (GUINEA PIG) | BDBM50283867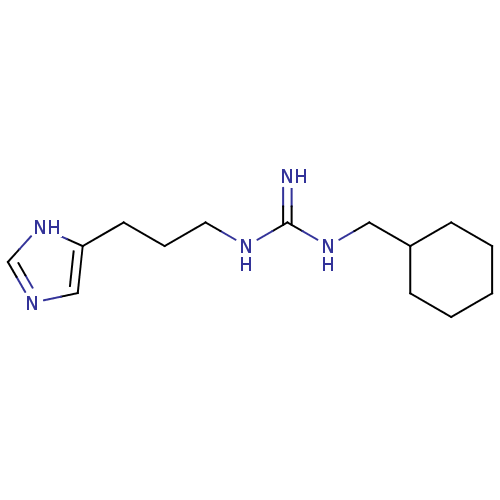 (CHEMBL98165 | N-Cyclohexylmethyl-N'-[3-(1H-imidazo...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article | 0.740 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Antagonistic activity for Histamine H3 receptor binding in guinea pig | Bioorg Med Chem Lett 4: 2907-2912 (1994) Article DOI: 10.1016/S0960-894X(01)80838-6 BindingDB Entry DOI: 10.7270/Q20K28H8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (GUINEA PIG) | BDBM50283867 (CHEMBL98165 | N-Cyclohexylmethyl-N'-[3-(1H-imidazo...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article | 0.794 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Antagonistic activity for Histamine H3 receptor on electrically evoked guinea-pig ileum contraction | Bioorg Med Chem Lett 4: 2907-2912 (1994) Article DOI: 10.1016/S0960-894X(01)80838-6 BindingDB Entry DOI: 10.7270/Q20K28H8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Rattus norvegicus (rat)) | BDBM50091386 (1-{4-[3-(1H-Imidazol-4-yl)-propoxy]-phenyl}-ethano...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Freie Universit£t Berlin Curated by ChEMBL | Assay Description Effect on histamine H3 receptors in vitro, on synaptosomes of rat cerebral cortex for the release of [3H]histamine | J Med Chem 43: 3987-94 (2000) BindingDB Entry DOI: 10.7270/Q2833SRN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Rattus norvegicus (rat)) | BDBM50092841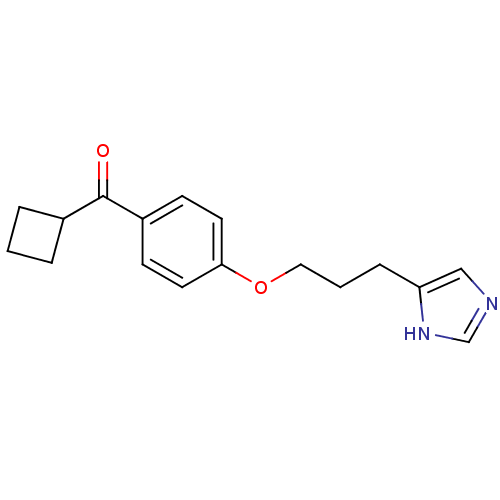 (CHEMBL128690 | Cyclobutyl-{4-[3-(1H-imidazol-4-yl)...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.890 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Freie Universit£t Berlin Curated by ChEMBL | Assay Description Effect on histamine H3 receptors in vitro, on synaptosomes of rat cerebral cortex for the release of [3H]histamine | J Med Chem 43: 3987-94 (2000) BindingDB Entry DOI: 10.7270/Q2833SRN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 3-phosphoinositide-dependent protein kinase 1 (Homo sapiens (Human)) | BDBM50361641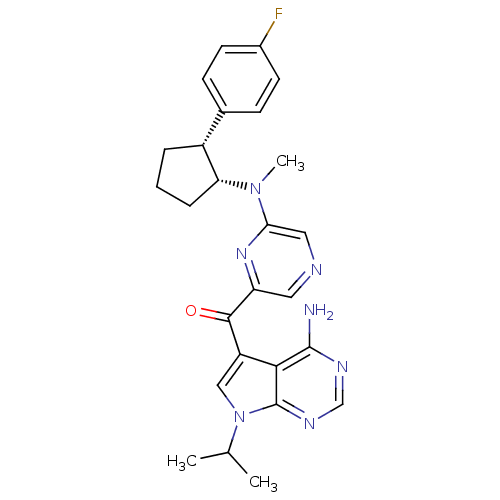 (CHEMBL1940247) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Inhibition of PDK1-mediated AKT1 phosphorylation at T308 in human H460 cells after 2 hrs by ELISA | J Med Chem 54: 8490-500 (2011) Article DOI: 10.1021/jm201019k BindingDB Entry DOI: 10.7270/Q23N23TV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone-lysine N-methyltransferase EZH2 (Homo sapiens (Human)) | BDBM50193709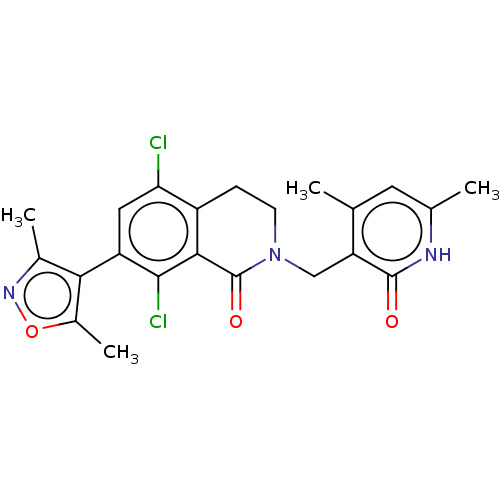 (CHEMBL3911017) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Similars | PDB Article PubMed | 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of wild type EZH2 (unknown origin) | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c02112 BindingDB Entry DOI: 10.7270/Q23J3HPC | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| 3-phosphoinositide-dependent protein kinase 1 (Homo sapiens (Human)) | BDBM50361652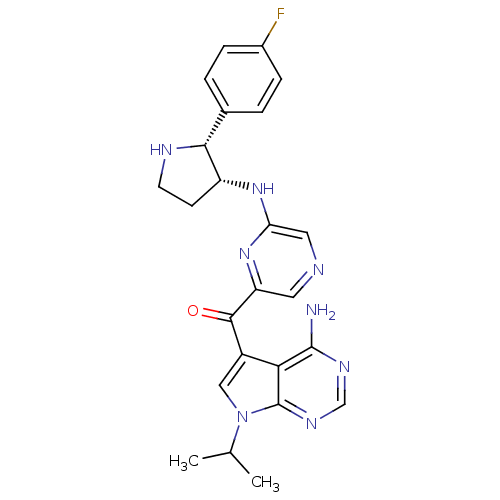 (CHEMBL1940250) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Inhibition of PDK1-mediated AKT1 phosphorylation at T308 in human H460 cells after 2 hrs by ELISA | J Med Chem 54: 8490-500 (2011) Article DOI: 10.1021/jm201019k BindingDB Entry DOI: 10.7270/Q23N23TV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Rattus norvegicus (rat)) | BDBM50092846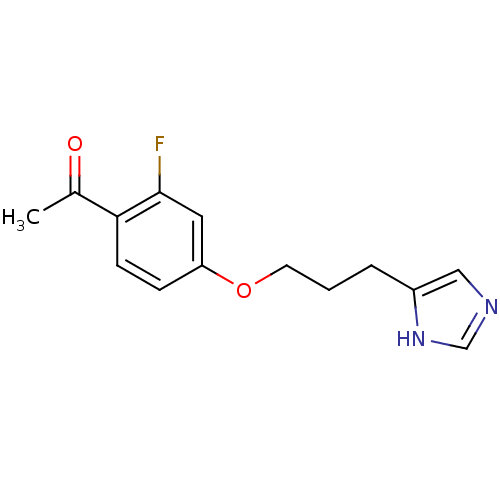 (1-{2-Fluoro-4-[3-(1H-imidazol-4-yl)-propoxy]-pheny...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Freie Universit£t Berlin Curated by ChEMBL | Assay Description Effect on histamine H3 receptors in vitro, on synaptosomes of rat cerebral cortex for the release of [3H]histamine | J Med Chem 43: 3987-94 (2000) BindingDB Entry DOI: 10.7270/Q2833SRN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Rattus norvegicus (rat)) | BDBM50092848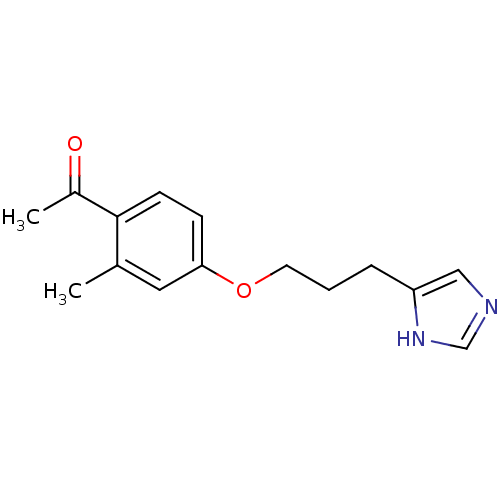 (1-{4-[3-(1H-Imidazol-4-yl)-propoxy]-2-methyl-pheny...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Freie Universit£t Berlin Curated by ChEMBL | Assay Description Effect on histamine H3 receptors in vitro, on synaptosomes of rat cerebral cortex for the release of [3H]histamine | J Med Chem 43: 3987-94 (2000) BindingDB Entry DOI: 10.7270/Q2833SRN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 3-phosphoinositide-dependent protein kinase 1 (Homo sapiens (Human)) | BDBM50361650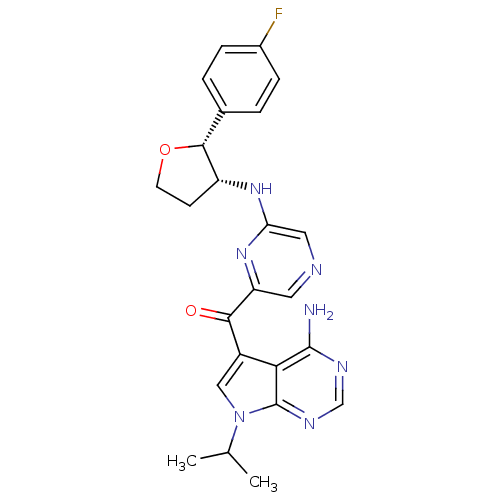 (CHEMBL1940248) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Inhibition of PDK1-mediated AKT1 phosphorylation at T308 in human H460 cells after 2 hrs by ELISA | J Med Chem 54: 8490-500 (2011) Article DOI: 10.1021/jm201019k BindingDB Entry DOI: 10.7270/Q23N23TV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 3-phosphoinositide-dependent protein kinase 1 (Homo sapiens (Human)) | BDBM50361643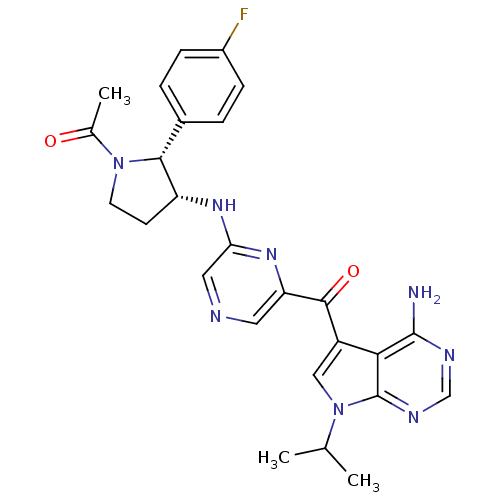 (CHEMBL1940252) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Inhibition of recombinant human His-tagged PDK1 catalytic domain using Ac-Sox-PKTFCGTPEYLAPEVRREPRILSEEEQEMFRDFDYIAD-NH2 as substrate by fluorescence... | J Med Chem 54: 8490-500 (2011) Article DOI: 10.1021/jm201019k BindingDB Entry DOI: 10.7270/Q23N23TV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 3-phosphoinositide-dependent protein kinase 1 (Homo sapiens (Human)) | BDBM50361644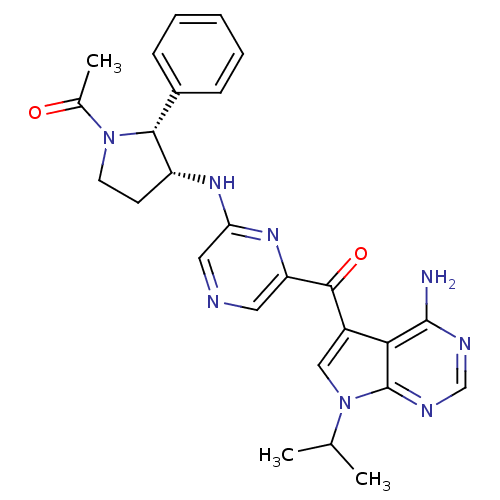 (CHEMBL1940253) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Inhibition of recombinant human His-tagged PDK1 catalytic domain using Ac-Sox-PKTFCGTPEYLAPEVRREPRILSEEEQEMFRDFDYIAD-NH2 as substrate by fluorescence... | J Med Chem 54: 8490-500 (2011) Article DOI: 10.1021/jm201019k BindingDB Entry DOI: 10.7270/Q23N23TV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (GUINEA PIG) | BDBM50283869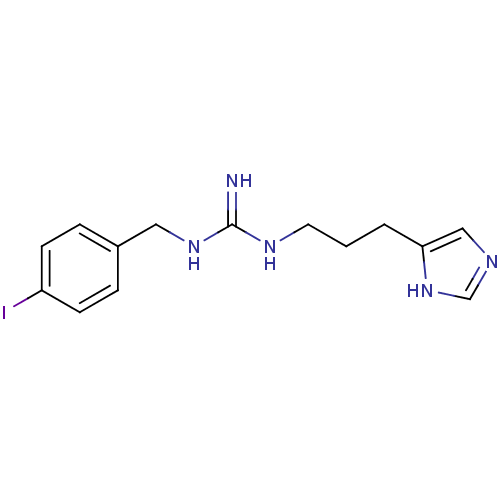 (CHEMBL99830 | N-[3-(1H-Imidazol-4-yl)-propyl]-N'-(...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | 1.58 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Antagonistic activity for Histamine H3 receptor on electrically evoked guinea-pig ileum contraction | Bioorg Med Chem Lett 4: 2907-2912 (1994) Article DOI: 10.1016/S0960-894X(01)80838-6 BindingDB Entry DOI: 10.7270/Q20K28H8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (GUINEA PIG) | BDBM50283869 (CHEMBL99830 | N-[3-(1H-Imidazol-4-yl)-propyl]-N'-(...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Antagonistic activity for Histamine H3 receptor binding in guinea pig | Bioorg Med Chem Lett 4: 2907-2912 (1994) Article DOI: 10.1016/S0960-894X(01)80838-6 BindingDB Entry DOI: 10.7270/Q20K28H8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Rattus norvegicus (rat)) | BDBM50092845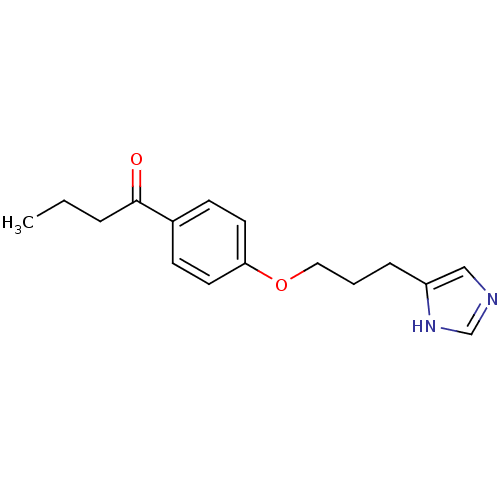 (1-{4-[3-(1H-Imidazol-4-yl)-propoxy]-phenyl}-butan-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 1.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Freie Universit£t Berlin Curated by ChEMBL | Assay Description Effect on histamine H3 receptors in vitro, on synaptosomes of rat cerebral cortex for the release of [3H]histamine | J Med Chem 43: 3987-94 (2000) BindingDB Entry DOI: 10.7270/Q2833SRN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 3-phosphoinositide-dependent protein kinase 1 (Homo sapiens (Human)) | BDBM50361653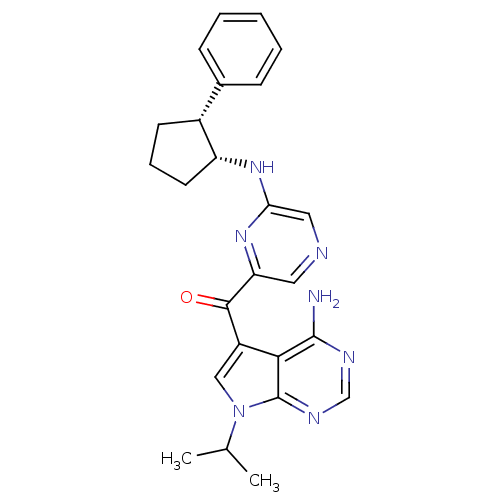 (CHEMBL1940245) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Inhibition of PDK1-mediated AKT1 phosphorylation at T308 in human H460 cells after 2 hrs by ELISA | J Med Chem 54: 8490-500 (2011) Article DOI: 10.1021/jm201019k BindingDB Entry DOI: 10.7270/Q23N23TV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone-lysine N-methyltransferase EZH2 (Homo sapiens (Human)) | BDBM50562994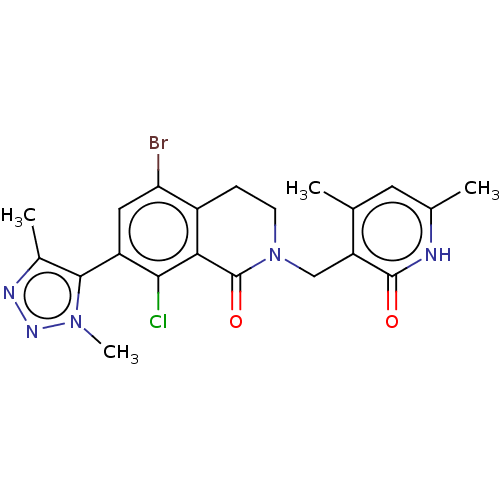 (CHEMBL4740532) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of wild type EZH2 (unknown origin) | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c02112 BindingDB Entry DOI: 10.7270/Q23J3HPC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Rattus norvegicus (rat)) | BDBM50092844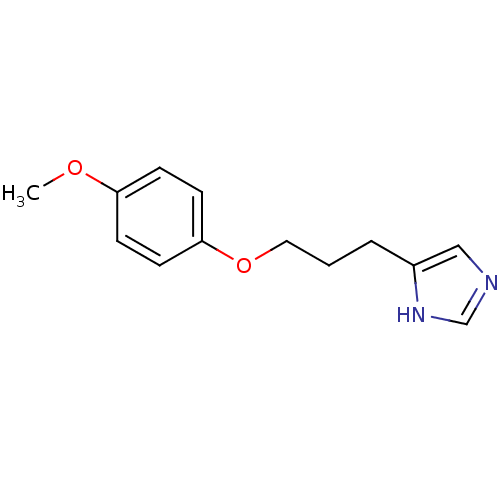 (4-[3-(4-Methoxy-phenoxy)-propyl]-1H-imidazole; com...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 2.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Freie Universit£t Berlin Curated by ChEMBL | Assay Description Effect on histamine H3 receptors in vitro, on synaptosomes of rat cerebral cortex for the release of [3H]histamine | J Med Chem 43: 3987-94 (2000) BindingDB Entry DOI: 10.7270/Q2833SRN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Rattus norvegicus (rat)) | BDBM50067472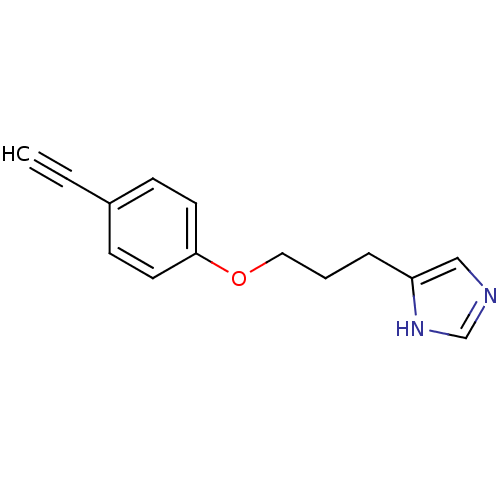 (4-[3-(4-Ethynyl-phenoxy)-propyl]-1H-imidazole | 4-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Freie Universität Berlin Curated by ChEMBL | Assay Description In vitro antagonist potency at Histamine H3 receptor measured as K+-evoked [3H]-histamine release from synaptosomes of rat cerebral cortex. | J Med Chem 41: 4171-6 (1998) Article DOI: 10.1021/jm9802970 BindingDB Entry DOI: 10.7270/Q2MK6C0W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Rattus norvegicus (rat)) | BDBM50067472 (4-[3-(4-Ethynyl-phenoxy)-propyl]-1H-imidazole | 4-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 2.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Freie Universit£t Berlin Curated by ChEMBL | Assay Description Effect on histamine H3 receptors in vitro, on synaptosomes of rat cerebral cortex for the release of [3H]histamine | J Med Chem 43: 3987-94 (2000) BindingDB Entry DOI: 10.7270/Q2833SRN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuromedin-K receptor (Homo sapiens (Human)) | BDBM50277511 (3-[(3aR,4R,5S,7aS)-5-{(1R)-1-[3,5-Bis(trifluoromet...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | 2.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Binding affinity to human NK3 receptor | J Med Chem 52: 3039-46 (2009) Article DOI: 10.1021/jm8016514 BindingDB Entry DOI: 10.7270/Q2FX79BT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-K receptor (Homo sapiens (Human)) | BDBM50277511 (3-[(3aR,4R,5S,7aS)-5-{(1R)-1-[3,5-Bis(trifluoromet...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | 2.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Binding affinity to human NK2 receptor | J Med Chem 52: 3039-46 (2009) Article DOI: 10.1021/jm8016514 BindingDB Entry DOI: 10.7270/Q2FX79BT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 3-phosphoinositide-dependent protein kinase 1 (Homo sapiens (Human)) | BDBM50361651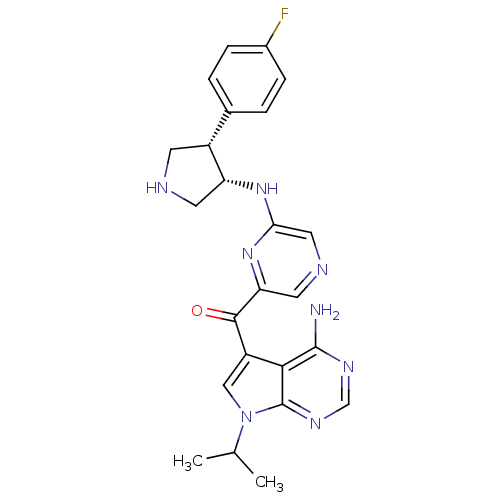 (CHEMBL1940249) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Inhibition of PDK1-mediated AKT1 phosphorylation at T308 in human H460 cells after 2 hrs by ELISA | J Med Chem 54: 8490-500 (2011) Article DOI: 10.1021/jm201019k BindingDB Entry DOI: 10.7270/Q23N23TV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Rattus norvegicus (rat)) | BDBM50092843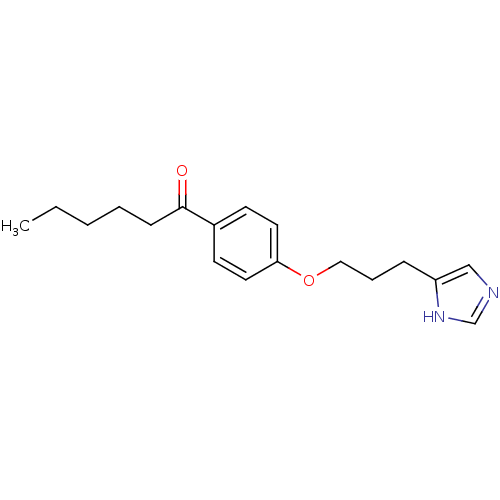 (1-{4-[3-(1H-Imidazol-4-yl)-propoxy]-phenyl}-hexan-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 2.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Freie Universit£t Berlin Curated by ChEMBL | Assay Description Effect on histamine H3 receptors in vitro, on synaptosomes of rat cerebral cortex for the release of [3H]histamine | J Med Chem 43: 3987-94 (2000) BindingDB Entry DOI: 10.7270/Q2833SRN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Rattus norvegicus (rat)) | BDBM50092838 (1-{4-[3-(1H-Imidazol-4-yl)-propoxy]-phenyl}-pentan...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 2.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Freie Universit£t Berlin Curated by ChEMBL | Assay Description Effect on histamine H3 receptors in vitro, on synaptosomes of rat cerebral cortex for the release of [3H]histamine | J Med Chem 43: 3987-94 (2000) BindingDB Entry DOI: 10.7270/Q2833SRN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuronal acetylcholine receptor subunit alpha-7 (Rattus norvegicus (Rat)) | BDBM50377050 (CHEMBL403858 | PH-709829) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Displacement of [3H]MLA from alpha-7 nACh receptor in rat brain | Bioorg Med Chem Lett 18: 3611-5 (2008) Article DOI: 10.1016/j.bmcl.2008.04.070 BindingDB Entry DOI: 10.7270/Q2KD1ZS9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone-lysine N-methyltransferase EZH2 (Homo sapiens (Human)) | BDBM50193709 (CHEMBL3911017) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Similars | PDB Article PubMed | 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of wild type EZH2 Y641N mutant (unknown origin) | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c02112 BindingDB Entry DOI: 10.7270/Q23J3HPC | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Histamine H3 receptor (GUINEA PIG) | BDBM22914 (CHEMBL260374 | N-cyclohexyl-4-(1H-imidazol-5-yl)pi...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE MMDB PC cid PC sid PDB UniChem Patents Similars | Article | 3.98 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Antagonistic activity for Histamine H3 receptor on electrically evoked guinea-pig ileum contraction | Bioorg Med Chem Lett 4: 2907-2912 (1994) Article DOI: 10.1016/S0960-894X(01)80838-6 BindingDB Entry DOI: 10.7270/Q20K28H8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Rattus norvegicus (rat)) | BDBM22914 (CHEMBL260374 | N-cyclohexyl-4-(1H-imidazol-5-yl)pi...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Freie Universität Berlin Curated by ChEMBL | Assay Description In vitro antagonist potency at Histamine H3 receptor measured as K+-evoked [3H]-histamine release from synaptosomes of rat cerebral cortex. | J Med Chem 41: 4171-6 (1998) Article DOI: 10.1021/jm9802970 BindingDB Entry DOI: 10.7270/Q2MK6C0W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Rattus norvegicus (rat)) | BDBM22914 (CHEMBL260374 | N-cyclohexyl-4-(1H-imidazol-5-yl)pi...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE MMDB PC cid PC sid PDB UniChem Patents Similars | PubMed | 4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Freie Universit£t Berlin Curated by ChEMBL | Assay Description Effect on histamine H3 receptors in vitro, on synaptosomes of rat cerebral cortex for the release of [3H]histamine | J Med Chem 43: 3987-94 (2000) BindingDB Entry DOI: 10.7270/Q2833SRN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Rattus norvegicus (rat)) | BDBM22914 (CHEMBL260374 | N-cyclohexyl-4-(1H-imidazol-5-yl)pi...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Freie Universität Berlin Curated by ChEMBL | Assay Description In vitro binding affinity against rat histamine H3 receptor | J Med Chem 47: 2678-87 (2004) Article DOI: 10.1021/jm031065q BindingDB Entry DOI: 10.7270/Q2SQ8ZTC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Rattus norvegicus (rat)) | BDBM50092856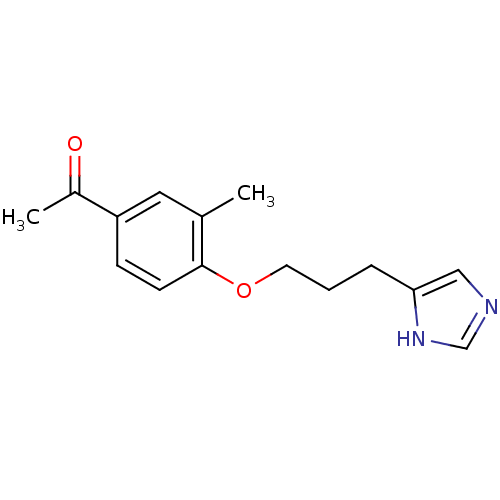 (1-{4-[3-(1H-Imidazol-4-yl)-propoxy]-3-methyl-pheny...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 4.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Freie Universit£t Berlin Curated by ChEMBL | Assay Description Effect on histamine H3 receptors in vitro, on synaptosomes of rat cerebral cortex for the release of [3H]histamine | J Med Chem 43: 3987-94 (2000) BindingDB Entry DOI: 10.7270/Q2833SRN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Rattus norvegicus (rat)) | BDBM50092850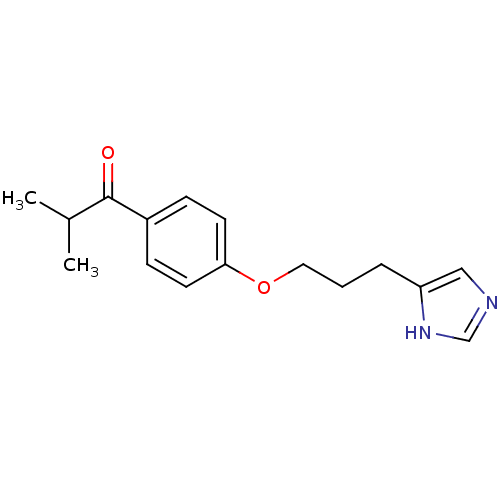 (1-{4-[3-(1H-Imidazol-4-yl)-propoxy]-phenyl}-2-meth...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 4.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Freie Universit£t Berlin Curated by ChEMBL | Assay Description Effect on histamine H3 receptors in vitro, on synaptosomes of rat cerebral cortex for the release of [3H]histamine | J Med Chem 43: 3987-94 (2000) BindingDB Entry DOI: 10.7270/Q2833SRN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (GUINEA PIG) | BDBM50283874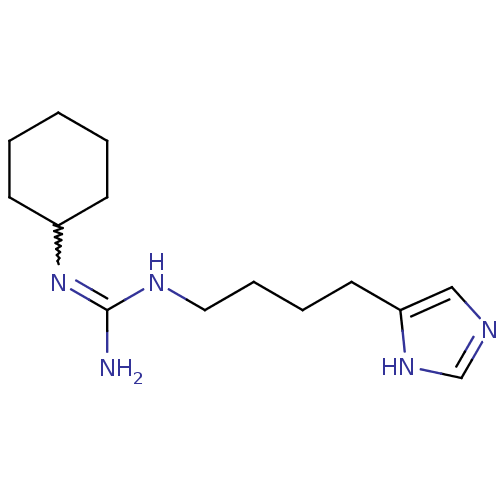 (CHEMBL317325 | N-Cyclohexyl-N'-[4-(1H-imidazol-4-y...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article | 5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Antagonistic activity for Histamine H3 receptor binding in guinea pig | Bioorg Med Chem Lett 4: 2907-2912 (1994) Article DOI: 10.1016/S0960-894X(01)80838-6 BindingDB Entry DOI: 10.7270/Q20K28H8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (GUINEA PIG) | BDBM50283874 (CHEMBL317325 | N-Cyclohexyl-N'-[4-(1H-imidazol-4-y...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article | 5.01 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Antagonistic activity for Histamine H3 receptor on electrically evoked guinea-pig ileum contraction | Bioorg Med Chem Lett 4: 2907-2912 (1994) Article DOI: 10.1016/S0960-894X(01)80838-6 BindingDB Entry DOI: 10.7270/Q20K28H8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Rattus norvegicus (rat)) | BDBM50092834 (1-{4-[3-(1H-Imidazol-4-yl)-propoxy]-phenyl}-2-phen...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 5.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Freie Universit£t Berlin Curated by ChEMBL | Assay Description Effect on histamine H3 receptors in vitro, on synaptosomes of rat cerebral cortex for the release of [3H]histamine | J Med Chem 43: 3987-94 (2000) BindingDB Entry DOI: 10.7270/Q2833SRN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuronal acetylcholine receptor subunit alpha-7 (Rattus norvegicus (Rat)) | BDBM50377049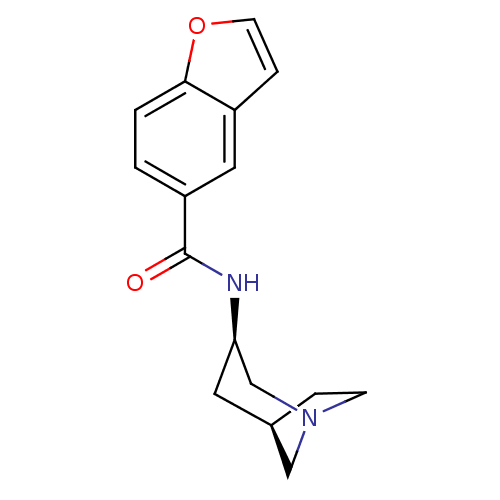 (CHEMBL258031) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 6.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Displacement of [3H]MLA from alpha-7 nACh receptor in rat brain | Bioorg Med Chem Lett 18: 3611-5 (2008) Article DOI: 10.1016/j.bmcl.2008.04.070 BindingDB Entry DOI: 10.7270/Q2KD1ZS9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (GUINEA PIG) | BDBM50283871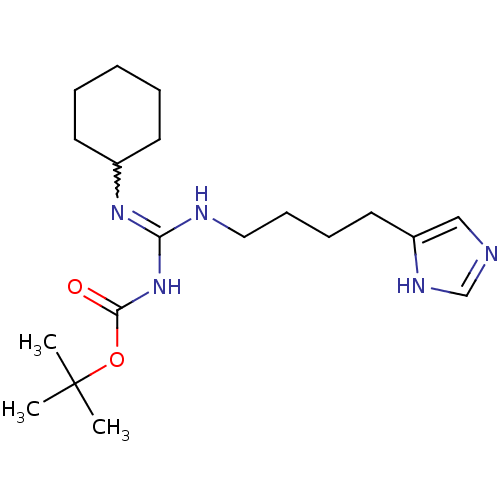 (CHEMBL329275 | N-(tert-Butoxy-carbonyl)-N'-cyclohe...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | 6.31 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Antagonistic activity for Histamine H3 receptor on electrically evoked guinea-pig ileum contraction | Bioorg Med Chem Lett 4: 2907-2912 (1994) Article DOI: 10.1016/S0960-894X(01)80838-6 BindingDB Entry DOI: 10.7270/Q20K28H8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (GUINEA PIG) | BDBM50283871 (CHEMBL329275 | N-(tert-Butoxy-carbonyl)-N'-cyclohe...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | 7 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Antagonistic activity for Histamine H3 receptor binding in guinea pig | Bioorg Med Chem Lett 4: 2907-2912 (1994) Article DOI: 10.1016/S0960-894X(01)80838-6 BindingDB Entry DOI: 10.7270/Q20K28H8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuronal acetylcholine receptor subunit alpha-7 (Rattus norvegicus (Rat)) | BDBM50377048 (CHEMBL403857) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 7 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Displacement of [3H]MLA from alpha-7 nACh receptor in rat brain | Bioorg Med Chem Lett 18: 3611-5 (2008) Article DOI: 10.1016/j.bmcl.2008.04.070 BindingDB Entry DOI: 10.7270/Q2KD1ZS9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 1087 total ) | Next | Last >> |