 Found 341 hits with Last Name = 'müller' and Initial = 'm'
Found 341 hits with Last Name = 'müller' and Initial = 'm' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM50040929

(4,5-dianilinophthalimide | 5,6-bis(phenylamino)-1H...)Show InChI InChI=1S/C20H15N3O2/c24-19-15-11-17(21-13-7-3-1-4-8-13)18(12-16(15)20(25)23-19)22-14-9-5-2-6-10-14/h1-12,21-22H,(H,23,24,25) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 160 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ciba-Geigy Limited
Curated by ChEMBL
| Assay Description
Competitive inhibition of ATP binding to EGF-R |
J Med Chem 37: 1015-27 (1994)
BindingDB Entry DOI: 10.7270/Q2M32TTP |
More data for this
Ligand-Target Pair | |
Renin
(Homo sapiens (Human)) | BDBM50077669

((S)-2-Benzyl-N-[(S)-1-((1S,2R,3S)-1-cyclohexylmeth...)Show SMILES CC(C)(C)S(=O)(=O)C[C@@H](Cc1ccccc1)C(=O)N[C@@H](Cc1cnc[nH]1)C(=O)N[C@@H](CC1CCCCC1)[C@@H](O)[C@@H](O)C1CC1 Show InChI InChI=1S/C33H50N4O6S/c1-33(2,3)44(42,43)20-25(16-22-10-6-4-7-11-22)31(40)37-28(18-26-19-34-21-35-26)32(41)36-27(17-23-12-8-5-9-13-23)30(39)29(38)24-14-15-24/h4,6-7,10-11,19,21,23-25,27-30,38-39H,5,8-9,12-18,20H2,1-3H3,(H,34,35)(H,36,41)(H,37,40)/t25-,27+,28+,29+,30-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| MMDB
PDB
PubMed
| n/a | n/a | 0.0250 | n/a | n/a | n/a | n/a | n/a | n/a |
F. Hoffmann-La Roche Ltd
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity against purified recombinant human renin |
Bioorg Med Chem Lett 9: 1397-402 (1999)
BindingDB Entry DOI: 10.7270/Q24F1PWN |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Renin
(Homo sapiens (Human)) | BDBM50077678

((3R,4R)-3-(1,4-Dimethoxy-naphthalen-2-ylmethoxy)-4...)Show SMILES COc1ccccc1COCCCOc1ccc(cc1)[C@H]1CCNC[C@@H]1OCc1cc(OC)c2ccccc2c1OC Show InChI InChI=1S/C35H41NO6/c1-37-32-12-7-4-9-26(32)23-40-19-8-20-41-28-15-13-25(14-16-28)29-17-18-36-22-34(29)42-24-27-21-33(38-2)30-10-5-6-11-31(30)35(27)39-3/h4-7,9-16,21,29,34,36H,8,17-20,22-24H2,1-3H3/t29-,34+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 0.0600 | n/a | n/a | n/a | n/a | n/a | n/a |
F. Hoffmann-La Roche Ltd
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity against purified recombinant human renin |
Bioorg Med Chem Lett 9: 1397-402 (1999)
BindingDB Entry DOI: 10.7270/Q24F1PWN |
More data for this
Ligand-Target Pair | |
Renin
(Homo sapiens (Human)) | BDBM50077678

((3R,4R)-3-(1,4-Dimethoxy-naphthalen-2-ylmethoxy)-4...)Show SMILES COc1ccccc1COCCCOc1ccc(cc1)[C@H]1CCNC[C@@H]1OCc1cc(OC)c2ccccc2c1OC Show InChI InChI=1S/C35H41NO6/c1-37-32-12-7-4-9-26(32)23-40-19-8-20-41-28-15-13-25(14-16-28)29-17-18-36-22-34(29)42-24-27-21-33(38-2)30-10-5-6-11-31(30)35(27)39-3/h4-7,9-16,21,29,34,36H,8,17-20,22-24H2,1-3H3/t29-,34+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 0.0870 | n/a | n/a | n/a | n/a | n/a | n/a |
F. Hoffmann-La Roche Ltd.
Curated by ChEMBL
| Assay Description
Inhibitory activity against purified recombinant human renin |
Bioorg Med Chem Lett 9: 1403-8 (1999)
BindingDB Entry DOI: 10.7270/Q20P0Z6S |
More data for this
Ligand-Target Pair | |
Integrin alpha-IIb/beta-3
(Homo sapiens (Human)) | BDBM50003852
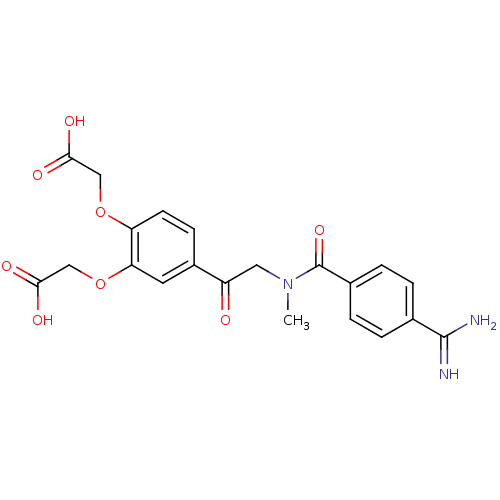
((4-{2-[(4-Carbamimidoyl-benzoyl)-methyl-amino]-ace...)Show SMILES CN(CC(=O)c1ccc(OCC(O)=O)c(OCC(O)=O)c1)C(=O)c1ccc(cc1)C(N)=N Show InChI InChI=1S/C21H21N3O8/c1-24(21(30)13-4-2-12(3-5-13)20(22)23)9-15(25)14-6-7-16(31-10-18(26)27)17(8-14)32-11-19(28)29/h2-8H,9-11H2,1H3,(H3,22,23)(H,26,27)(H,28,29) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
F. Hoffmann-La Roche Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of FG binding to alpha IIb beta3 integrin |
J Med Chem 39: 3139-47 (1996)
Article DOI: 10.1021/jm9509298
BindingDB Entry DOI: 10.7270/Q2GT5M7Q |
More data for this
Ligand-Target Pair | |
Integrin alpha-IIb/beta-3
(Homo sapiens (Human)) | BDBM50003852

((4-{2-[(4-Carbamimidoyl-benzoyl)-methyl-amino]-ace...)Show SMILES CN(CC(=O)c1ccc(OCC(O)=O)c(OCC(O)=O)c1)C(=O)c1ccc(cc1)C(N)=N Show InChI InChI=1S/C21H21N3O8/c1-24(21(30)13-4-2-12(3-5-13)20(22)23)9-15(25)14-6-7-16(31-10-18(26)27)17(8-14)32-11-19(28)29/h2-8H,9-11H2,1H3,(H3,22,23)(H,26,27)(H,28,29) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
F. Hoffmann-La Roche Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of FG binding to alpha IIb beta3 integrin |
J Med Chem 39: 3139-47 (1996)
Article DOI: 10.1021/jm9509298
BindingDB Entry DOI: 10.7270/Q2GT5M7Q |
More data for this
Ligand-Target Pair | |
Renin
(Homo sapiens (Human)) | BDBM50077702

(4-[(3S,4R,5R)-4-[4-(3-Benzyloxy-propoxy)-phenyl]-5...)Show SMILES C(COCc1ccccc1)COc1ccc(cc1)[C@H]1[C@H](CSc2ccncc2)CNC[C@@H]1OCc1ccc2ccccc2c1 Show InChI InChI=1S/C38H40N2O3S/c1-2-7-29(8-3-1)26-41-21-6-22-42-35-15-13-32(14-16-35)38-34(28-44-36-17-19-39-20-18-36)24-40-25-37(38)43-27-30-11-12-31-9-4-5-10-33(31)23-30/h1-5,7-20,23,34,37-38,40H,6,21-22,24-28H2/t34-,37-,38-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| PubMed
| n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
F. Hoffmann-La Roche Ltd.
Curated by ChEMBL
| Assay Description
Inhibitory activity against purified recombinant human renin |
Bioorg Med Chem Lett 9: 1403-8 (1999)
BindingDB Entry DOI: 10.7270/Q20P0Z6S |
More data for this
Ligand-Target Pair | |
Renin
(Homo sapiens (Human)) | BDBM50077693
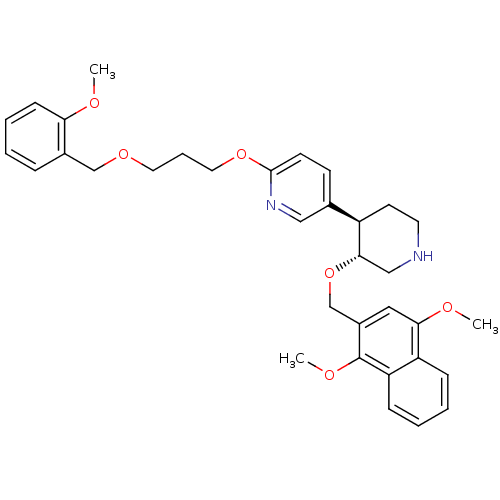
((3'R,4'R)-3'-(1,4-Dimethoxy-naphthalen-2-ylmethoxy...)Show SMILES COc1ccccc1COCCCOc1ccc(cn1)[C@H]1CCNC[C@@H]1OCc1cc(OC)c2ccccc2c1OC Show InChI InChI=1S/C34H40N2O6/c1-37-30-12-7-4-9-25(30)22-40-17-8-18-41-33-14-13-24(20-36-33)27-15-16-35-21-32(27)42-23-26-19-31(38-2)28-10-5-6-11-29(28)34(26)39-3/h4-7,9-14,19-20,27,32,35H,8,15-18,21-23H2,1-3H3/t27-,32+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
F. Hoffmann-La Roche Ltd.
Curated by ChEMBL
| Assay Description
Inhibitory activity against purified recombinant human renin |
Bioorg Med Chem Lett 9: 1403-8 (1999)
BindingDB Entry DOI: 10.7270/Q20P0Z6S |
More data for this
Ligand-Target Pair | |
Renin
(Homo sapiens (Human)) | BDBM50077699
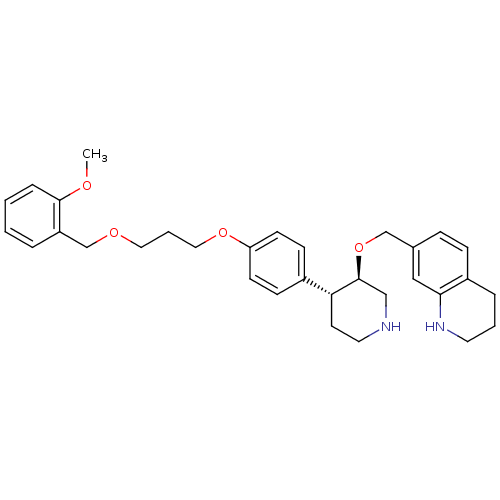
(7-((3R,4R)-4-{4-[3-(2-Methoxy-benzyloxy)-propoxy]-...)Show SMILES COc1ccccc1COCCCOc1ccc(cc1)[C@H]1CCNC[C@@H]1OCc1ccc2CCCNc2c1 Show InChI InChI=1S/C32H40N2O4/c1-35-31-8-3-2-6-27(31)23-36-18-5-19-37-28-13-11-25(12-14-28)29-15-17-33-21-32(29)38-22-24-9-10-26-7-4-16-34-30(26)20-24/h2-3,6,8-14,20,29,32-34H,4-5,7,15-19,21-23H2,1H3/t29-,32+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 0.670 | n/a | n/a | n/a | n/a | n/a | n/a |
F. Hoffmann-La Roche Ltd.
Curated by ChEMBL
| Assay Description
Inhibitory activity against human plasma renin |
Bioorg Med Chem Lett 9: 1403-8 (1999)
BindingDB Entry DOI: 10.7270/Q20P0Z6S |
More data for this
Ligand-Target Pair | |
Renin
(Homo sapiens (Human)) | BDBM50077692
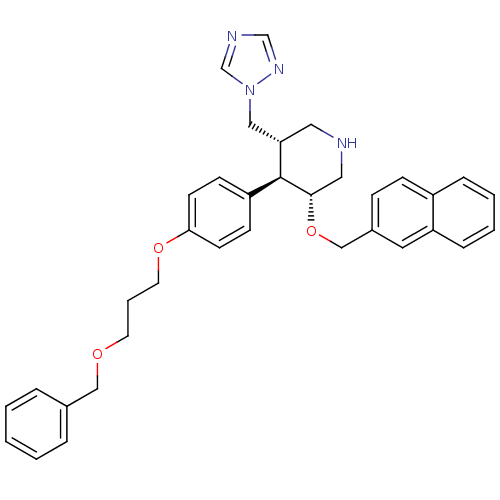
((3R,4R,5S)-4-[4-(3-Benzyloxy-propoxy)-phenyl]-3-(n...)Show SMILES C(COCc1ccccc1)COc1ccc(cc1)[C@H]1[C@H](Cn2cncn2)CNC[C@@H]1OCc1ccc2ccccc2c1 Show InChI InChI=1S/C35H38N4O3/c1-2-7-27(8-3-1)23-40-17-6-18-41-33-15-13-30(14-16-33)35-32(22-39-26-37-25-38-39)20-36-21-34(35)42-24-28-11-12-29-9-4-5-10-31(29)19-28/h1-5,7-16,19,25-26,32,34-36H,6,17-18,20-24H2/t32-,34-,35-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| PubMed
| n/a | n/a | 0.920 | n/a | n/a | n/a | n/a | n/a | n/a |
F. Hoffmann-La Roche Ltd.
Curated by ChEMBL
| Assay Description
Inhibitory activity against purified recombinant human renin |
Bioorg Med Chem Lett 9: 1403-8 (1999)
BindingDB Entry DOI: 10.7270/Q20P0Z6S |
More data for this
Ligand-Target Pair | |
Integrin alpha-IIb/beta-3
(Homo sapiens (Human)) | BDBM50003874
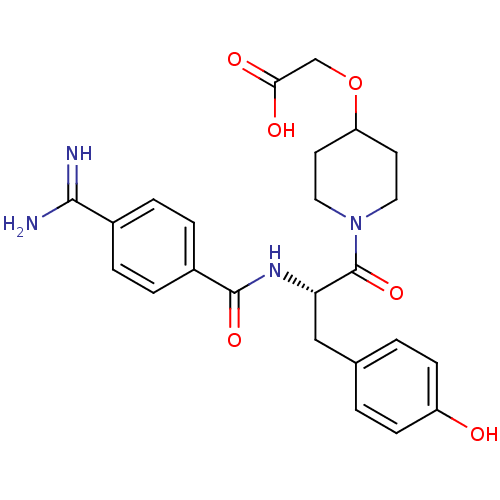
(CHEMBL108111 | LAMIFIBAN | Ro-44-9883 | {1-[(S)-2-...)Show SMILES NC(=N)c1ccc(cc1)C(=O)N[C@@H](Cc1ccc(O)cc1)C(=O)N1CCC(CC1)OCC(O)=O Show InChI InChI=1S/C24H28N4O6/c25-22(26)16-3-5-17(6-4-16)23(32)27-20(13-15-1-7-18(29)8-2-15)24(33)28-11-9-19(10-12-28)34-14-21(30)31/h1-8,19-20,29H,9-14H2,(H3,25,26)(H,27,32)(H,30,31)/t20-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a |
F. Hoffmann-La Roche Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of FG binding to alpha IIb beta3 integrin |
J Med Chem 39: 3139-47 (1996)
Article DOI: 10.1021/jm9509298
BindingDB Entry DOI: 10.7270/Q2GT5M7Q |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Integrin alpha-IIb/beta-3
(Homo sapiens (Human)) | BDBM50003874

(CHEMBL108111 | LAMIFIBAN | Ro-44-9883 | {1-[(S)-2-...)Show SMILES NC(=N)c1ccc(cc1)C(=O)N[C@@H](Cc1ccc(O)cc1)C(=O)N1CCC(CC1)OCC(O)=O Show InChI InChI=1S/C24H28N4O6/c25-22(26)16-3-5-17(6-4-16)23(32)27-20(13-15-1-7-18(29)8-2-15)24(33)28-11-9-19(10-12-28)34-14-21(30)31/h1-8,19-20,29H,9-14H2,(H3,25,26)(H,27,32)(H,30,31)/t20-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a |
F. Hoffmann-La Roche Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of FG binding to alpha IIb beta3 integrin |
J Med Chem 39: 3139-47 (1996)
Article DOI: 10.1021/jm9509298
BindingDB Entry DOI: 10.7270/Q2GT5M7Q |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Renin
(Homo sapiens (Human)) | BDBM50077688

((3R,4R)-4-{4-[3-(2-Methoxy-benzyloxy)-propoxy]-phe...)Show SMILES COc1ccccc1COCCCOc1ccc(cc1)[C@H]1CCNC[C@@H]1OCc1ccc2ccccc2c1 Show InChI InChI=1S/C33H37NO4/c1-35-32-10-5-4-9-29(32)24-36-19-6-20-37-30-15-13-27(14-16-30)31-17-18-34-22-33(31)38-23-25-11-12-26-7-2-3-8-28(26)21-25/h2-5,7-16,21,31,33-34H,6,17-20,22-24H2,1H3/t31-,33+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a |
F. Hoffmann-La Roche Ltd
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity against purified recombinant human renin |
Bioorg Med Chem Lett 9: 1397-402 (1999)
BindingDB Entry DOI: 10.7270/Q24F1PWN |
More data for this
Ligand-Target Pair | |
Renin
(Homo sapiens (Human)) | BDBM50077703
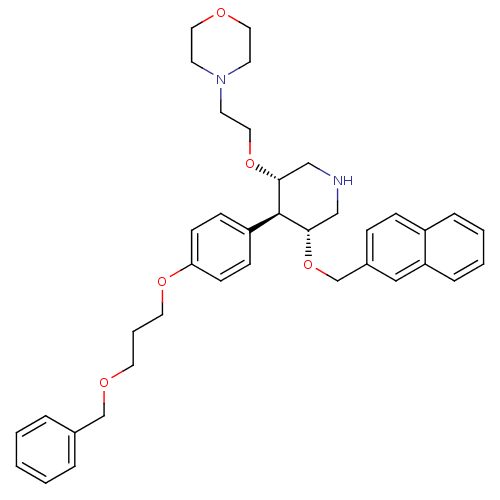
(4-{2-[(3S,4R,5R)-4-[4-(3-Benzyloxy-propoxy)-phenyl...)Show SMILES C(COCc1ccccc1)COc1ccc(cc1)[C@H]1[C@@H](CNC[C@@H]1OCc1ccc2ccccc2c1)OCCN1CCOCC1 Show InChI InChI=1S/C38H46N2O5/c1-2-7-30(8-3-1)28-42-20-6-21-43-35-15-13-33(14-16-35)38-36(44-24-19-40-17-22-41-23-18-40)26-39-27-37(38)45-29-31-11-12-32-9-4-5-10-34(32)25-31/h1-5,7-16,25,36-39H,6,17-24,26-29H2/t36-,37+,38+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 1.80 | n/a | n/a | n/a | n/a | n/a | n/a |
F. Hoffmann-La Roche Ltd.
Curated by ChEMBL
| Assay Description
Inhibitory activity against purified recombinant human renin |
Bioorg Med Chem Lett 9: 1403-8 (1999)
BindingDB Entry DOI: 10.7270/Q20P0Z6S |
More data for this
Ligand-Target Pair | |
Integrin alpha-IIb/beta-3
(Homo sapiens (Human)) | BDBM50003867
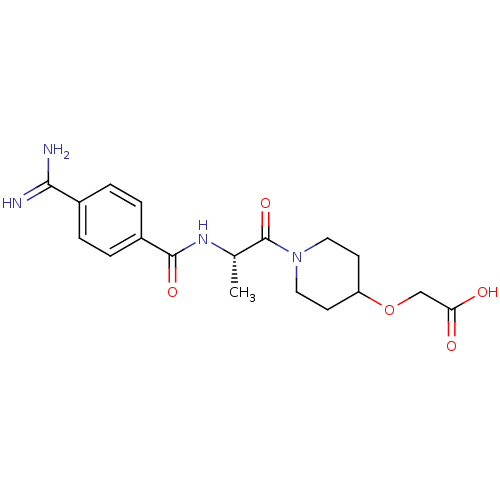
(CHEMBL65267 | Ro-44-3888 | {1-[(S)-2-(4-Carbamimid...)Show SMILES C[C@H](NC(=O)c1ccc(cc1)C(N)=N)C(=O)N1CCC(CC1)OCC(O)=O Show InChI InChI=1S/C18H24N4O5/c1-11(21-17(25)13-4-2-12(3-5-13)16(19)20)18(26)22-8-6-14(7-9-22)27-10-15(23)24/h2-5,11,14H,6-10H2,1H3,(H3,19,20)(H,21,25)(H,23,24)/t11-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 1.90 | n/a | n/a | n/a | n/a | n/a | n/a |
F. Hoffmann-La Roche Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of FG binding to alpha IIb beta3 integrin |
J Med Chem 39: 3139-47 (1996)
Article DOI: 10.1021/jm9509298
BindingDB Entry DOI: 10.7270/Q2GT5M7Q |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Integrin alpha-IIb/beta-3
(Homo sapiens (Human)) | BDBM50003867

(CHEMBL65267 | Ro-44-3888 | {1-[(S)-2-(4-Carbamimid...)Show SMILES C[C@H](NC(=O)c1ccc(cc1)C(N)=N)C(=O)N1CCC(CC1)OCC(O)=O Show InChI InChI=1S/C18H24N4O5/c1-11(21-17(25)13-4-2-12(3-5-13)16(19)20)18(26)22-8-6-14(7-9-22)27-10-15(23)24/h2-5,11,14H,6-10H2,1H3,(H3,19,20)(H,21,25)(H,23,24)/t11-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 1.90 | n/a | n/a | n/a | n/a | n/a | n/a |
F. Hoffmann-La Roche Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of FG binding to alpha IIb beta3 integrin |
J Med Chem 39: 3139-47 (1996)
Article DOI: 10.1021/jm9509298
BindingDB Entry DOI: 10.7270/Q2GT5M7Q |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Renin
(Homo sapiens (Human)) | BDBM50077704

(CHEMBL31880 | Sulfuric acid mono-[(3S,4R,5R)-4-[4-...)Show SMILES OS(=O)(=O)OC[C@@H]1CNC[C@H](OCc2ccc3ccccc3c2)[C@H]1c1ccc(OCCCOCc2ccccc2)cc1 Show InChI InChI=1S/C33H37NO7S/c35-42(36,37)41-24-30-20-34-21-32(40-23-26-11-12-27-9-4-5-10-29(27)19-26)33(30)28-13-15-31(16-14-28)39-18-6-17-38-22-25-7-2-1-3-8-25/h1-5,7-16,19,30,32-34H,6,17-18,20-24H2,(H,35,36,37)/t30-,32-,33-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
| PubMed
| n/a | n/a | 2.30 | n/a | n/a | n/a | n/a | n/a | n/a |
F. Hoffmann-La Roche Ltd.
Curated by ChEMBL
| Assay Description
Inhibitory activity against human plasma renin |
Bioorg Med Chem Lett 9: 1403-8 (1999)
BindingDB Entry DOI: 10.7270/Q20P0Z6S |
More data for this
Ligand-Target Pair | |
Renin
(Homo sapiens (Human)) | BDBM50077709
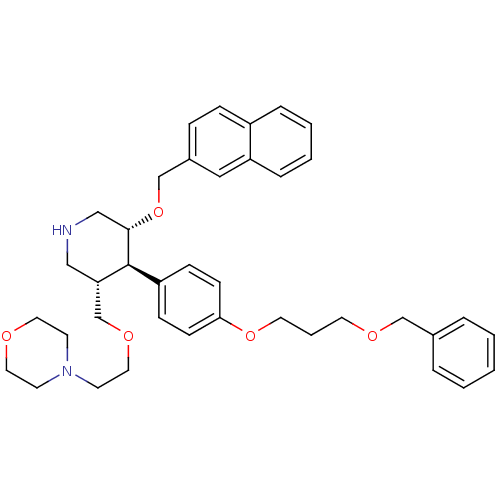
(4-{2-[(3S,4R,5R)-4-[4-(3-Benzyloxy-propoxy)-phenyl...)Show SMILES C(COCc1ccccc1)COc1ccc(cc1)[C@H]1[C@H](COCCN2CCOCC2)CNC[C@@H]1OCc1ccc2ccccc2c1 Show InChI InChI=1S/C39H48N2O5/c1-2-7-31(8-3-1)28-43-20-6-21-45-37-15-13-34(14-16-37)39-36(30-44-24-19-41-17-22-42-23-18-41)26-40-27-38(39)46-29-32-11-12-33-9-4-5-10-35(33)25-32/h1-5,7-16,25,36,38-40H,6,17-24,26-30H2/t36-,38-,39-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 3.30 | n/a | n/a | n/a | n/a | n/a | n/a |
F. Hoffmann-La Roche Ltd.
Curated by ChEMBL
| Assay Description
Inhibitory activity against purified recombinant human renin |
Bioorg Med Chem Lett 9: 1403-8 (1999)
BindingDB Entry DOI: 10.7270/Q20P0Z6S |
More data for this
Ligand-Target Pair | |
Renin
(Homo sapiens (Human)) | BDBM50077695
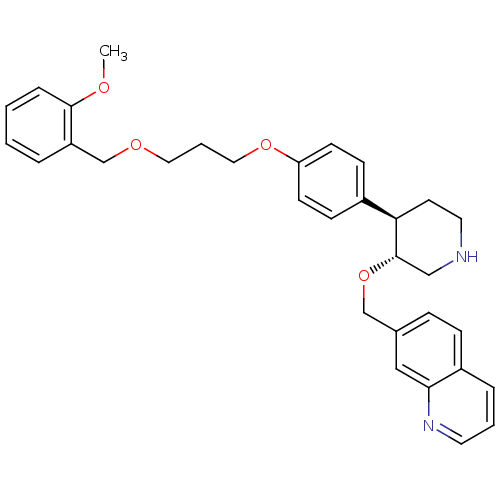
(7-((3R,4R)-4-{4-[3-(2-Methoxy-benzyloxy)-propoxy]-...)Show SMILES COc1ccccc1COCCCOc1ccc(cc1)[C@H]1CCNC[C@@H]1OCc1ccc2cccnc2c1 Show InChI InChI=1S/C32H36N2O4/c1-35-31-8-3-2-6-27(31)23-36-18-5-19-37-28-13-11-25(12-14-28)29-15-17-33-21-32(29)38-22-24-9-10-26-7-4-16-34-30(26)20-24/h2-4,6-14,16,20,29,32-33H,5,15,17-19,21-23H2,1H3/t29-,32+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
F. Hoffmann-La Roche Ltd.
Curated by ChEMBL
| Assay Description
Inhibitory activity against purified recombinant human renin |
Bioorg Med Chem Lett 9: 1403-8 (1999)
BindingDB Entry DOI: 10.7270/Q20P0Z6S |
More data for this
Ligand-Target Pair | |
Renin
(Homo sapiens (Human)) | BDBM50077689
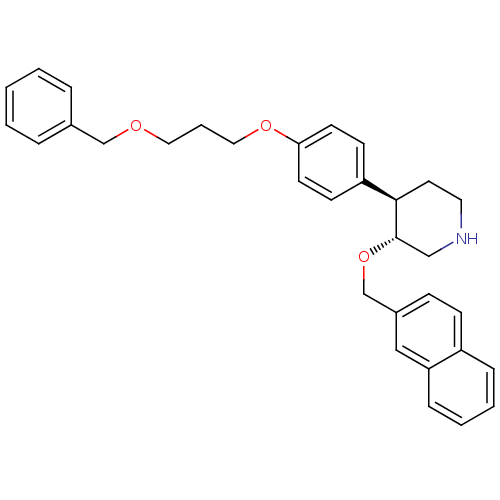
((3R,4R)-4-[4-(3-Benzyloxy-propoxy)-phenyl]-3-(naph...)Show SMILES C(COCc1ccccc1)COc1ccc(cc1)[C@H]1CCNC[C@@H]1OCc1ccc2ccccc2c1 Show InChI InChI=1S/C32H35NO3/c1-2-7-25(8-3-1)23-34-19-6-20-35-30-15-13-28(14-16-30)31-17-18-33-22-32(31)36-24-26-11-12-27-9-4-5-10-29(27)21-26/h1-5,7-16,21,31-33H,6,17-20,22-24H2/t31-,32+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
F. Hoffmann-La Roche Ltd.
Curated by ChEMBL
| Assay Description
Inhibitory activity against purified recombinant human renin |
Bioorg Med Chem Lett 9: 1403-8 (1999)
BindingDB Entry DOI: 10.7270/Q20P0Z6S |
More data for this
Ligand-Target Pair | |
Renin
(Homo sapiens (Human)) | BDBM50077694

(7-{(3R,4R)-4-[4-(3-Benzyloxy-propoxy)-phenyl]-pipe...)Show SMILES C(COCc1ccccc1)COc1ccc(cc1)[C@H]1CCNC[C@@H]1OCc1ccc2CCCNc2c1 Show InChI InChI=1S/C31H38N2O3/c1-2-6-24(7-3-1)22-34-18-5-19-35-28-13-11-26(12-14-28)29-15-17-32-21-31(29)36-23-25-9-10-27-8-4-16-33-30(27)20-25/h1-3,6-7,9-14,20,29,31-33H,4-5,8,15-19,21-23H2/t29-,31+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 5.10 | n/a | n/a | n/a | n/a | n/a | n/a |
F. Hoffmann-La Roche Ltd.
Curated by ChEMBL
| Assay Description
Inhibitory activity against purified recombinant human renin |
Bioorg Med Chem Lett 9: 1403-8 (1999)
BindingDB Entry DOI: 10.7270/Q20P0Z6S |
More data for this
Ligand-Target Pair | |
Renin
(Homo sapiens (Human)) | BDBM50077705
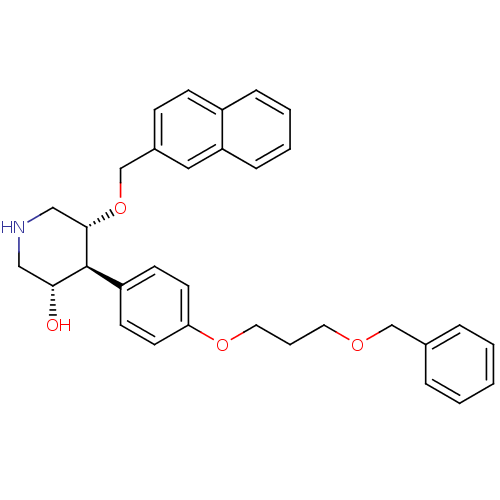
((3S,4S,5R)-4-[4-(3-Benzyloxy-propoxy)-phenyl]-5-(n...)Show SMILES O[C@@H]1CNC[C@H](OCc2ccc3ccccc3c2)[C@H]1c1ccc(OCCCOCc2ccccc2)cc1 Show InChI InChI=1S/C32H35NO4/c34-30-20-33-21-31(37-23-25-11-12-26-9-4-5-10-28(26)19-25)32(30)27-13-15-29(16-14-27)36-18-6-17-35-22-24-7-2-1-3-8-24/h1-5,7-16,19,30-34H,6,17-18,20-23H2/t30-,31+,32+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 6.40 | n/a | n/a | n/a | n/a | n/a | n/a |
F. Hoffmann-La Roche Ltd.
Curated by ChEMBL
| Assay Description
Inhibitory activity against purified recombinant human renin |
Bioorg Med Chem Lett 9: 1403-8 (1999)
BindingDB Entry DOI: 10.7270/Q20P0Z6S |
More data for this
Ligand-Target Pair | |
Renin
(Homo sapiens (Human)) | BDBM50077697

(7-((3R,4R)-4-{4-[3-(2-Methoxy-benzyloxy)-propoxy]-...)Show SMILES COc1ccccc1COCCCOc1ccc(cc1)[C@H]1CCNC[C@@H]1OCc1ccc2ccc(=O)[nH]c2c1 Show InChI InChI=1S/C32H36N2O5/c1-36-30-6-3-2-5-26(30)22-37-17-4-18-38-27-12-9-24(10-13-27)28-15-16-33-20-31(28)39-21-23-7-8-25-11-14-32(35)34-29(25)19-23/h2-3,5-14,19,28,31,33H,4,15-18,20-22H2,1H3,(H,34,35)/t28-,31+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
F. Hoffmann-La Roche Ltd.
Curated by ChEMBL
| Assay Description
Inhibitory activity against purified recombinant human renin |
Bioorg Med Chem Lett 9: 1403-8 (1999)
BindingDB Entry DOI: 10.7270/Q20P0Z6S |
More data for this
Ligand-Target Pair | |
Renin
(Homo sapiens (Human)) | BDBM50077689

((3R,4R)-4-[4-(3-Benzyloxy-propoxy)-phenyl]-3-(naph...)Show SMILES C(COCc1ccccc1)COc1ccc(cc1)[C@H]1CCNC[C@@H]1OCc1ccc2ccccc2c1 Show InChI InChI=1S/C32H35NO3/c1-2-7-25(8-3-1)23-34-19-6-20-35-30-15-13-28(14-16-30)31-17-18-33-22-32(31)36-24-26-11-12-27-9-4-5-10-29(27)21-26/h1-5,7-16,21,31-33H,6,17-20,22-24H2/t31-,32+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
F. Hoffmann-La Roche Ltd
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity against purified recombinant human renin |
Bioorg Med Chem Lett 9: 1397-402 (1999)
BindingDB Entry DOI: 10.7270/Q24F1PWN |
More data for this
Ligand-Target Pair | |
Renin
(Homo sapiens (Human)) | BDBM50077707
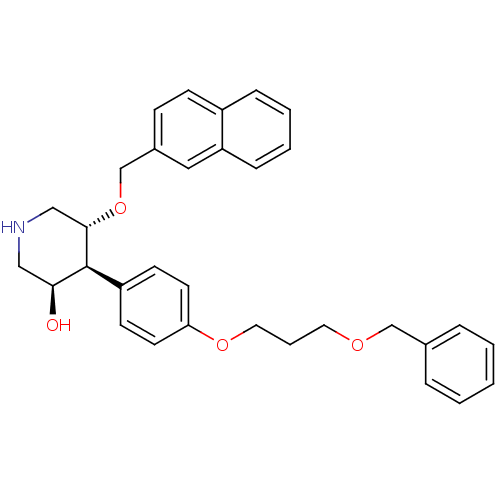
((3R,4S,5R)-4-[4-(3-Benzyloxy-propoxy)-phenyl]-5-(n...)Show SMILES O[C@H]1CNC[C@H](OCc2ccc3ccccc3c2)[C@H]1c1ccc(OCCCOCc2ccccc2)cc1 Show InChI InChI=1S/C32H35NO4/c34-30-20-33-21-31(37-23-25-11-12-26-9-4-5-10-28(26)19-25)32(30)27-13-15-29(16-14-27)36-18-6-17-35-22-24-7-2-1-3-8-24/h1-5,7-16,19,30-34H,6,17-18,20-23H2/t30-,31-,32-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
F. Hoffmann-La Roche Ltd.
Curated by ChEMBL
| Assay Description
Inhibitory activity against purified recombinant human renin |
Bioorg Med Chem Lett 9: 1403-8 (1999)
BindingDB Entry DOI: 10.7270/Q20P0Z6S |
More data for this
Ligand-Target Pair | |
Renin
(Homo sapiens (Human)) | BDBM50077698
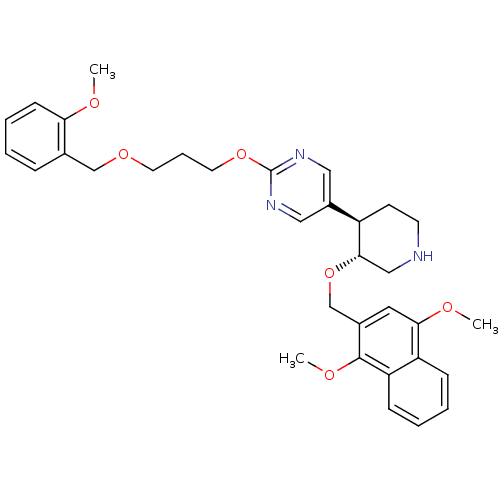
(5-[(3R,4R)-3-(1,4-Dimethoxy-naphthalen-2-ylmethoxy...)Show SMILES COc1ccccc1COCCCOc1ncc(cn1)[C@H]1CCNC[C@@H]1OCc1cc(OC)c2ccccc2c1OC Show InChI InChI=1S/C33H39N3O6/c1-37-29-12-7-4-9-23(29)21-40-15-8-16-41-33-35-18-25(19-36-33)26-13-14-34-20-31(26)42-22-24-17-30(38-2)27-10-5-6-11-28(27)32(24)39-3/h4-7,9-12,17-19,26,31,34H,8,13-16,20-22H2,1-3H3/t26-,31+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
F. Hoffmann-La Roche Ltd.
Curated by ChEMBL
| Assay Description
Inhibitory activity against purified recombinant human renin |
Bioorg Med Chem Lett 9: 1403-8 (1999)
BindingDB Entry DOI: 10.7270/Q20P0Z6S |
More data for this
Ligand-Target Pair | |
Renin
(Homo sapiens (Human)) | BDBM50077678

((3R,4R)-3-(1,4-Dimethoxy-naphthalen-2-ylmethoxy)-4...)Show SMILES COc1ccccc1COCCCOc1ccc(cc1)[C@H]1CCNC[C@@H]1OCc1cc(OC)c2ccccc2c1OC Show InChI InChI=1S/C35H41NO6/c1-37-32-12-7-4-9-26(32)23-40-19-8-20-41-28-15-13-25(14-16-28)29-17-18-36-22-34(29)42-24-27-21-33(38-2)30-10-5-6-11-31(30)35(27)39-3/h4-7,9-16,21,29,34,36H,8,17-20,22-24H2,1-3H3/t29-,34+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
F. Hoffmann-La Roche Ltd.
Curated by ChEMBL
| Assay Description
Inhibitory activity against human plasma renin |
Bioorg Med Chem Lett 9: 1403-8 (1999)
BindingDB Entry DOI: 10.7270/Q20P0Z6S |
More data for this
Ligand-Target Pair | |
Renin
(Homo sapiens (Human)) | BDBM50077706

(7-{(3R,4R)-4-[4-(3-Benzyloxy-propoxy)-phenyl]-pipe...)Show SMILES C(COCc1ccccc1)COc1ccc(cc1)[C@H]1CCNC[C@@H]1OCc1ccc2cccnc2c1 Show InChI InChI=1S/C31H34N2O3/c1-2-6-24(7-3-1)22-34-18-5-19-35-28-13-11-26(12-14-28)29-15-17-32-21-31(29)36-23-25-9-10-27-8-4-16-33-30(27)20-25/h1-4,6-14,16,20,29,31-32H,5,15,17-19,21-23H2/t29-,31+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
F. Hoffmann-La Roche Ltd.
Curated by ChEMBL
| Assay Description
Inhibitory activity against purified recombinant human renin |
Bioorg Med Chem Lett 9: 1403-8 (1999)
BindingDB Entry DOI: 10.7270/Q20P0Z6S |
More data for this
Ligand-Target Pair | |
Renin
(Homo sapiens (Human)) | BDBM50077696
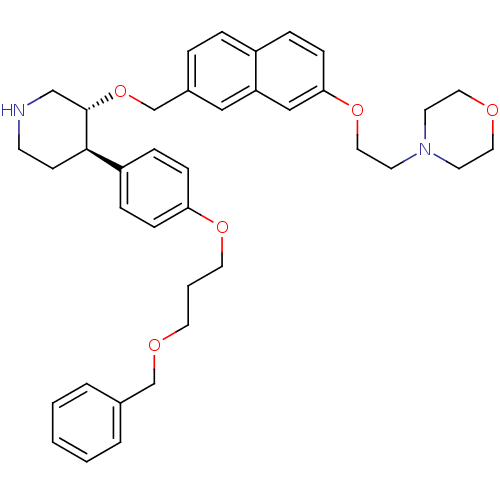
(4-[2-(7-{(3R,4R)-4-[4-(3-Benzyloxy-propoxy)-phenyl...)Show SMILES C(COCc1ccccc1)COc1ccc(cc1)[C@H]1CCNC[C@@H]1OCc1ccc2ccc(OCCN3CCOCC3)cc2c1 Show InChI InChI=1S/C38H46N2O5/c1-2-5-30(6-3-1)28-42-20-4-21-43-35-12-10-33(11-13-35)37-15-16-39-27-38(37)45-29-31-7-8-32-9-14-36(26-34(32)25-31)44-24-19-40-17-22-41-23-18-40/h1-3,5-14,25-26,37-39H,4,15-24,27-29H2/t37-,38+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
F. Hoffmann-La Roche Ltd.
Curated by ChEMBL
| Assay Description
Inhibitory activity against human plasma renin |
Bioorg Med Chem Lett 9: 1403-8 (1999)
BindingDB Entry DOI: 10.7270/Q20P0Z6S |
More data for this
Ligand-Target Pair | |
Acetyl-CoA carboxylase 2
(Homo sapiens (Human)) | BDBM50332563
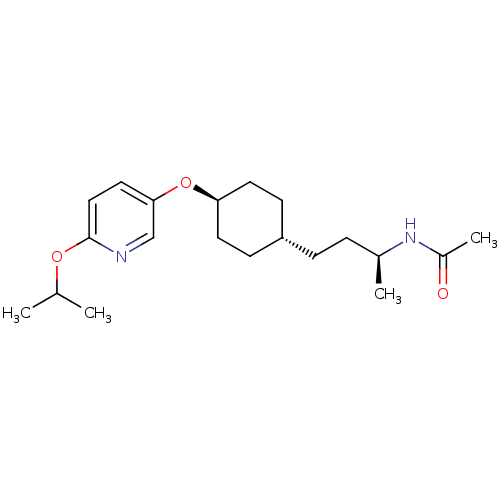
(CHEMBL1630721 | trans-(S)-N-(4-(4-(6-isopropoxypyr...)Show SMILES CC(C)Oc1ccc(O[C@H]2CC[C@H](CC[C@H](C)NC(C)=O)CC2)cn1 |r,wU:15.15,9.8,wD:12.12,(-5.64,-6.46,;-4.31,-5.7,;-4.3,-4.16,;-2.97,-6.47,;-1.64,-5.7,;-.3,-6.47,;1.03,-5.7,;1.03,-4.15,;2.36,-3.37,;3.7,-4.14,;5.02,-3.36,;6.35,-4.11,;6.36,-5.66,;7.7,-6.43,;9.03,-5.65,;10.37,-6.41,;10.35,-7.95,;11.7,-5.63,;13.03,-6.4,;14.36,-5.62,;13.04,-7.94,;5.03,-6.44,;3.7,-5.67,;-.31,-3.38,;-1.64,-4.15,)| Show InChI InChI=1S/C20H32N2O3/c1-14(2)24-20-12-11-19(13-21-20)25-18-9-7-17(8-10-18)6-5-15(3)22-16(4)23/h11-15,17-18H,5-10H2,1-4H3,(H,22,23)/t15-,17-,18-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi-Aventis Deutschland GmbH
Curated by ChEMBL
| Assay Description
Inhibition of N-terminal His-tagged human recombinant ACC2 expressed in high five insect cells by ATP consumption assay |
J Med Chem 53: 8679-87 (2010)
Article DOI: 10.1021/jm101179e
BindingDB Entry DOI: 10.7270/Q28K79BT |
More data for this
Ligand-Target Pair | |
Renin
(Homo sapiens (Human)) | BDBM50077708
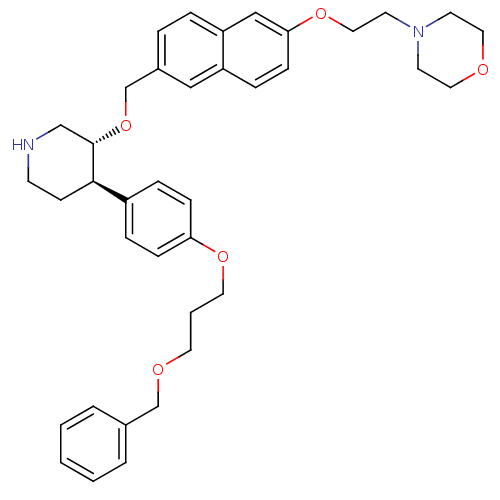
(4-[2-(6-{(3R,4R)-4-[4-(3-Benzyloxy-propoxy)-phenyl...)Show SMILES C(COCc1ccccc1)COc1ccc(cc1)[C@H]1CCNC[C@@H]1OCc1ccc2cc(OCCN3CCOCC3)ccc2c1 Show InChI InChI=1S/C38H46N2O5/c1-2-5-30(6-3-1)28-42-20-4-21-43-35-12-9-32(10-13-35)37-15-16-39-27-38(37)45-29-31-7-8-34-26-36(14-11-33(34)25-31)44-24-19-40-17-22-41-23-18-40/h1-3,5-14,25-26,37-39H,4,15-24,27-29H2/t37-,38+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 22 | n/a | n/a | n/a | n/a | n/a | n/a |
F. Hoffmann-La Roche Ltd.
Curated by ChEMBL
| Assay Description
Inhibitory activity against human plasma renin |
Bioorg Med Chem Lett 9: 1403-8 (1999)
BindingDB Entry DOI: 10.7270/Q20P0Z6S |
More data for this
Ligand-Target Pair | |
Renin
(Homo sapiens (Human)) | BDBM50077682
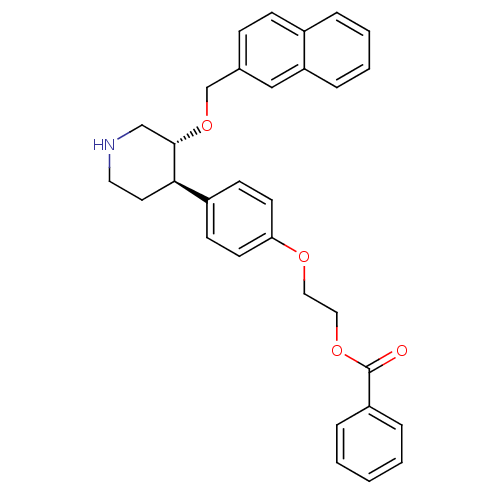
(Benzoic acid 2-{4-[(3R,4R)-3-(naphthalen-2-ylmetho...)Show SMILES O=C(OCCOc1ccc(cc1)[C@H]1CCNC[C@@H]1OCc1ccc2ccccc2c1)c1ccccc1 Show InChI InChI=1S/C31H31NO4/c33-31(26-7-2-1-3-8-26)35-19-18-34-28-14-12-25(13-15-28)29-16-17-32-21-30(29)36-22-23-10-11-24-6-4-5-9-27(24)20-23/h1-15,20,29-30,32H,16-19,21-22H2/t29-,30+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 26 | n/a | n/a | n/a | n/a | n/a | n/a |
F. Hoffmann-La Roche Ltd
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity against purified recombinant human renin |
Bioorg Med Chem Lett 9: 1397-402 (1999)
BindingDB Entry DOI: 10.7270/Q24F1PWN |
More data for this
Ligand-Target Pair | |
Acetyl-CoA carboxylase 2
(Homo sapiens (Human)) | BDBM97610
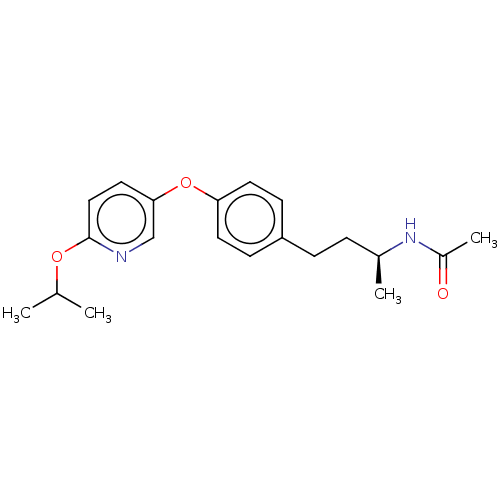
(CHEMBL1630715 | US8470841, 35)Show SMILES CC(C)Oc1ccc(Oc2ccc(CC[C@H](C)NC(C)=O)cc2)cn1 |r| Show InChI InChI=1S/C20H26N2O3/c1-14(2)24-20-12-11-19(13-21-20)25-18-9-7-17(8-10-18)6-5-15(3)22-16(4)23/h7-15H,5-6H2,1-4H3,(H,22,23)/t15-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi-Aventis Deutschland GmbH
Curated by ChEMBL
| Assay Description
Inhibition of N-terminal His-tagged human recombinant ACC2 expressed in high five insect cells by ATP consumption assay |
J Med Chem 53: 8679-87 (2010)
Article DOI: 10.1021/jm101179e
BindingDB Entry DOI: 10.7270/Q28K79BT |
More data for this
Ligand-Target Pair | |
Renin
(Homo sapiens (Human)) | BDBM50077699

(7-((3R,4R)-4-{4-[3-(2-Methoxy-benzyloxy)-propoxy]-...)Show SMILES COc1ccccc1COCCCOc1ccc(cc1)[C@H]1CCNC[C@@H]1OCc1ccc2CCCNc2c1 Show InChI InChI=1S/C32H40N2O4/c1-35-31-8-3-2-6-27(31)23-36-18-5-19-37-28-13-11-25(12-14-28)29-15-17-33-21-32(29)38-22-24-9-10-26-7-4-16-34-30(26)20-24/h2-3,6,8-14,20,29,32-34H,4-5,7,15-19,21-23H2,1H3/t29-,32+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 37 | n/a | n/a | n/a | n/a | n/a | n/a |
F. Hoffmann-La Roche Ltd.
Curated by ChEMBL
| Assay Description
Inhibitory activity against human plasma renin |
Bioorg Med Chem Lett 9: 1403-8 (1999)
BindingDB Entry DOI: 10.7270/Q20P0Z6S |
More data for this
Ligand-Target Pair | |
Renin
(Homo sapiens (Human)) | BDBM50077671
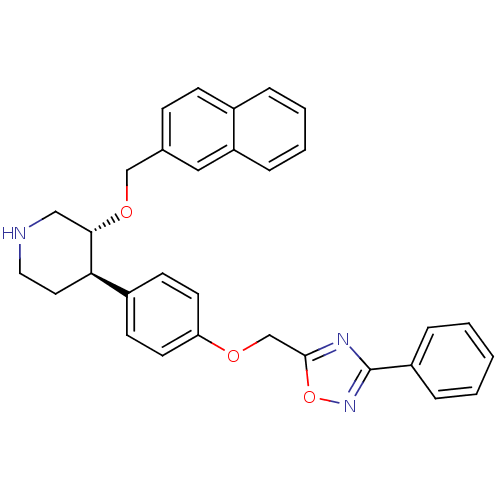
((3R,4R)-3-(Naphthalen-2-ylmethoxy)-4-[4-(3-phenyl-...)Show SMILES C(Oc1ccc(cc1)[C@H]1CCNC[C@@H]1OCc1ccc2ccccc2c1)c1nc(no1)-c1ccccc1 Show InChI InChI=1S/C31H29N3O3/c1-2-7-25(8-3-1)31-33-30(37-34-31)21-35-27-14-12-24(13-15-27)28-16-17-32-19-29(28)36-20-22-10-11-23-6-4-5-9-26(23)18-22/h1-15,18,28-29,32H,16-17,19-21H2/t28-,29+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 41 | n/a | n/a | n/a | n/a | n/a | n/a |
F. Hoffmann-La Roche Ltd
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity against purified recombinant human renin |
Bioorg Med Chem Lett 9: 1397-402 (1999)
BindingDB Entry DOI: 10.7270/Q24F1PWN |
More data for this
Ligand-Target Pair | |
Integrin alpha-IIb/beta-3
(Homo sapiens (Human)) | BDBM50052488

(CHEMBL107279 | {1-[(S)-2-(4-Carbamimidoyl-benzoyla...)Show SMILES CCOC(=O)COC1CCN(CC1)C(=O)[C@H](Cc1ccc(O)cc1)NC(=O)c1ccc(cc1)C(N)=N Show InChI InChI=1S/C26H32N4O6/c1-2-35-23(32)16-36-21-11-13-30(14-12-21)26(34)22(15-17-3-9-20(31)10-4-17)29-25(33)19-7-5-18(6-8-19)24(27)28/h3-10,21-22,31H,2,11-16H2,1H3,(H3,27,28)(H,29,33)/t22-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 51 | n/a | n/a | n/a | n/a | n/a | n/a |
F. Hoffmann-La Roche Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of FG binding to alpha IIb beta3 integrin |
J Med Chem 39: 3139-47 (1996)
Article DOI: 10.1021/jm9509298
BindingDB Entry DOI: 10.7270/Q2GT5M7Q |
More data for this
Ligand-Target Pair | |
Integrin alpha-IIb/beta-3
(Homo sapiens (Human)) | BDBM50052488

(CHEMBL107279 | {1-[(S)-2-(4-Carbamimidoyl-benzoyla...)Show SMILES CCOC(=O)COC1CCN(CC1)C(=O)[C@H](Cc1ccc(O)cc1)NC(=O)c1ccc(cc1)C(N)=N Show InChI InChI=1S/C26H32N4O6/c1-2-35-23(32)16-36-21-11-13-30(14-12-21)26(34)22(15-17-3-9-20(31)10-4-17)29-25(33)19-7-5-18(6-8-19)24(27)28/h3-10,21-22,31H,2,11-16H2,1H3,(H3,27,28)(H,29,33)/t22-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 51 | n/a | n/a | n/a | n/a | n/a | n/a |
F. Hoffmann-La Roche Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of FG binding to alpha IIb beta3 integrin |
J Med Chem 39: 3139-47 (1996)
Article DOI: 10.1021/jm9509298
BindingDB Entry DOI: 10.7270/Q2GT5M7Q |
More data for this
Ligand-Target Pair | |
Renin
(Homo sapiens (Human)) | BDBM50077690
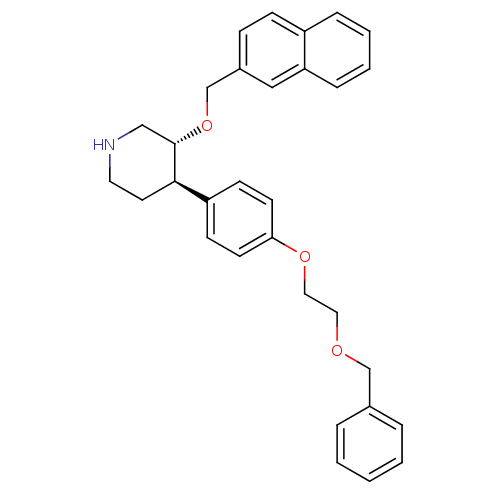
((3R,4R)-4-[4-(2-Benzyloxy-ethoxy)-phenyl]-3-(napht...)Show SMILES C(COc1ccc(cc1)[C@H]1CCNC[C@@H]1OCc1ccc2ccccc2c1)OCc1ccccc1 Show InChI InChI=1S/C31H33NO3/c1-2-6-24(7-3-1)22-33-18-19-34-29-14-12-27(13-15-29)30-16-17-32-21-31(30)35-23-25-10-11-26-8-4-5-9-28(26)20-25/h1-15,20,30-32H,16-19,21-23H2/t30-,31+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 53 | n/a | n/a | n/a | n/a | n/a | n/a |
F. Hoffmann-La Roche Ltd
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity against purified recombinant human renin |
Bioorg Med Chem Lett 9: 1397-402 (1999)
BindingDB Entry DOI: 10.7270/Q24F1PWN |
More data for this
Ligand-Target Pair | |
Renin
(Homo sapiens (Human)) | BDBM50077679
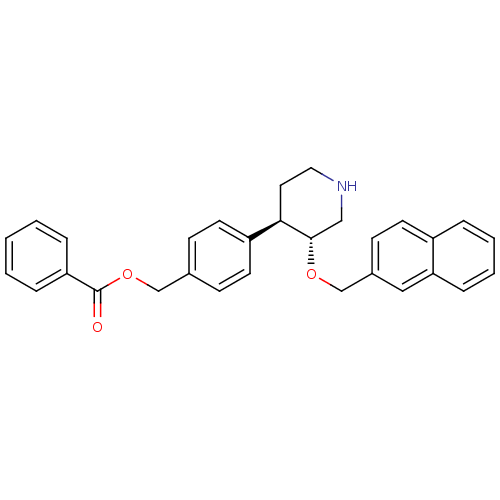
(Benzoic acid 4-[(3R,4R)-3-(naphthalen-2-ylmethoxy)...)Show SMILES O=C(OCc1ccc(cc1)[C@H]1CCNC[C@@H]1OCc1ccc2ccccc2c1)c1ccccc1 Show InChI InChI=1S/C30H29NO3/c32-30(26-7-2-1-3-8-26)34-20-22-10-14-25(15-11-22)28-16-17-31-19-29(28)33-21-23-12-13-24-6-4-5-9-27(24)18-23/h1-15,18,28-29,31H,16-17,19-21H2/t28-,29+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 55 | n/a | n/a | n/a | n/a | n/a | n/a |
F. Hoffmann-La Roche Ltd
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity against purified recombinant human renin |
Bioorg Med Chem Lett 9: 1397-402 (1999)
BindingDB Entry DOI: 10.7270/Q24F1PWN |
More data for this
Ligand-Target Pair | |
Acetyl-CoA carboxylase 2
(Homo sapiens (Human)) | BDBM50332562

(CHEMBL1630516 | N-{(S)-3-[2-(4-isopropoxy-phenoxy)...)Show SMILES CC(C)Oc1ccc(Oc2ncc(s2)C#C[C@H](C)NC(C)=O)cc1 |r| Show InChI InChI=1S/C18H20N2O3S/c1-12(2)22-15-6-8-16(9-7-15)23-18-19-11-17(24-18)10-5-13(3)20-14(4)21/h6-9,11-13H,1-4H3,(H,20,21)/t13-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 60 | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi-Aventis Deutschland GmbH
Curated by ChEMBL
| Assay Description
Inhibition of N-terminal His-tagged human recombinant ACC2 expressed in high five insect cells by ATP consumption assay |
J Med Chem 53: 8679-87 (2010)
Article DOI: 10.1021/jm101179e
BindingDB Entry DOI: 10.7270/Q28K79BT |
More data for this
Ligand-Target Pair | |
Acetyl-CoA carboxylase 2
(Homo sapiens (Human)) | BDBM50332564
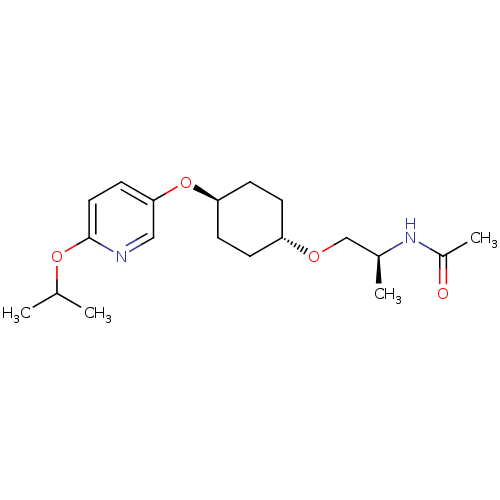
(CHEMBL1630703 | trans-(S)-N-(1-(4-(6-isopropoxypyr...)Show SMILES CC(C)Oc1ccc(O[C@H]2CC[C@@H](CC2)OC[C@H](C)NC(C)=O)cn1 |r,wU:17.18,9.8,wD:12.15,(37.18,-12.08,;38.51,-11.31,;38.51,-9.77,;39.84,-12.08,;41.18,-11.31,;42.51,-12.08,;43.85,-11.31,;43.84,-9.77,;45.17,-8.99,;46.51,-9.75,;47.83,-8.97,;49.17,-9.73,;49.18,-11.28,;47.85,-12.05,;46.51,-11.29,;50.52,-12.04,;51.85,-11.26,;53.18,-12.03,;53.17,-13.56,;54.51,-11.25,;55.85,-12.01,;57.18,-11.24,;55.86,-13.55,;42.51,-9,;41.18,-9.77,)| Show InChI InChI=1S/C19H30N2O4/c1-13(2)24-19-10-9-18(11-20-19)25-17-7-5-16(6-8-17)23-12-14(3)21-15(4)22/h9-11,13-14,16-17H,5-8,12H2,1-4H3,(H,21,22)/t14-,16-,17-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 60 | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi-Aventis Deutschland GmbH
Curated by ChEMBL
| Assay Description
Inhibition of N-terminal His-tagged human recombinant ACC2 expressed in high five insect cells by ATP consumption assay |
J Med Chem 53: 8679-87 (2010)
Article DOI: 10.1021/jm101179e
BindingDB Entry DOI: 10.7270/Q28K79BT |
More data for this
Ligand-Target Pair | |
Acetyl-CoA carboxylase 2
(Homo sapiens (Human)) | BDBM50189617
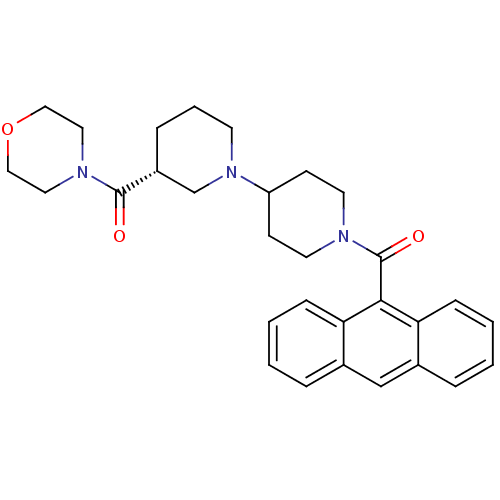
((3R)-1'-(9-anthrylcarbonyl)-3-(morpholin-4-ylcarbo...)Show SMILES O=C([C@@H]1CCCN(C1)C1CCN(CC1)C(=O)c1c2ccccc2cc2ccccc12)N1CCOCC1 |r| Show InChI InChI=1S/C30H35N3O3/c34-29(32-16-18-36-19-17-32)24-8-5-13-33(21-24)25-11-14-31(15-12-25)30(35)28-26-9-3-1-6-22(26)20-23-7-2-4-10-27(23)28/h1-4,6-7,9-10,20,24-25H,5,8,11-19,21H2/t24-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 70 | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi-Aventis Deutschland GmbH
Curated by ChEMBL
| Assay Description
Inhibition of N-terminal His-tagged human recombinant ACC2 expressed in high five insect cells by ATP consumption assay |
J Med Chem 53: 8679-87 (2010)
Article DOI: 10.1021/jm101179e
BindingDB Entry DOI: 10.7270/Q28K79BT |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Acetyl-CoA carboxylase 2
(Homo sapiens (Human)) | BDBM50332554
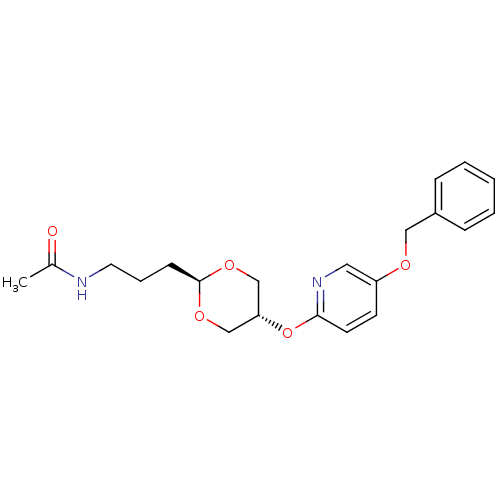
(CHEMBL1630706 | trans-N-(3-(5-(5-(benzyloxy)pyridi...)Show SMILES CC(=O)NCCC[C@H]1OC[C@@H](CO1)Oc1ccc(OCc2ccccc2)cn1 |r,wU:10.13,wD:7.6,(36.32,-14.16,;34.99,-14.93,;34.99,-16.47,;33.65,-14.16,;32.32,-14.93,;30.99,-14.16,;29.65,-14.92,;28.32,-14.16,;28.32,-12.61,;26.98,-11.84,;25.66,-12.62,;25.66,-14.16,;26.99,-14.92,;24.32,-11.84,;22.99,-12.63,;23,-14.18,;21.66,-14.95,;20.33,-14.18,;18.99,-14.95,;17.66,-14.18,;16.33,-14.94,;14.99,-14.18,;13.66,-14.94,;13.66,-16.48,;15,-17.25,;16.33,-16.48,;20.33,-12.63,;21.66,-11.86,)| Show InChI InChI=1S/C21H26N2O5/c1-16(24)22-11-5-8-21-26-14-19(15-27-21)28-20-10-9-18(12-23-20)25-13-17-6-3-2-4-7-17/h2-4,6-7,9-10,12,19,21H,5,8,11,13-15H2,1H3,(H,22,24)/t19-,21- | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 70 | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi-Aventis Deutschland GmbH
Curated by ChEMBL
| Assay Description
Inhibition of N-terminal His-tagged human recombinant ACC2 expressed in high five insect cells by ATP consumption assay |
J Med Chem 53: 8679-87 (2010)
Article DOI: 10.1021/jm101179e
BindingDB Entry DOI: 10.7270/Q28K79BT |
More data for this
Ligand-Target Pair | |
Acetyl-CoA carboxylase 2
(Homo sapiens (Human)) | BDBM97611

(CHEMBL1630712 | US8470841, 36)Show SMILES CC(CCc1ccc(Oc2ccc(OCc3ccccc3)nc2)cc1)NC(C)=O Show InChI InChI=1S/C24H26N2O3/c1-18(26-19(2)27)8-9-20-10-12-22(13-11-20)29-23-14-15-24(25-16-23)28-17-21-6-4-3-5-7-21/h3-7,10-16,18H,8-9,17H2,1-2H3,(H,26,27) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 80 | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi-Aventis Deutschland GmbH
Curated by ChEMBL
| Assay Description
Inhibition of N-terminal His-tagged human recombinant ACC2 expressed in high five insect cells by ATP consumption assay |
J Med Chem 53: 8679-87 (2010)
Article DOI: 10.1021/jm101179e
BindingDB Entry DOI: 10.7270/Q28K79BT |
More data for this
Ligand-Target Pair | |
Acetyl-CoA carboxylase 2
(Homo sapiens (Human)) | BDBM97622
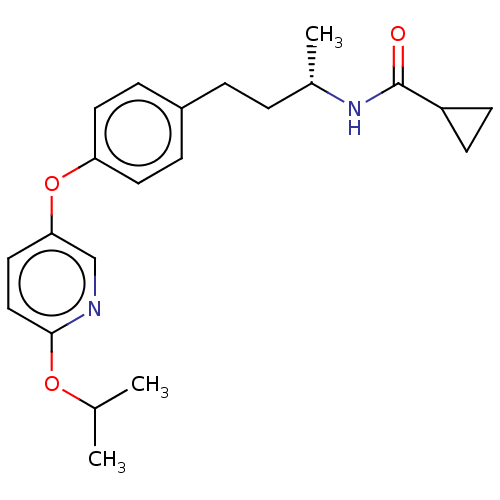
(CHEMBL1630701 | US8470841, 51)Show SMILES CC(C)Oc1ccc(Oc2ccc(CC[C@H](C)NC(=O)C3CC3)cc2)cn1 |r| Show InChI InChI=1S/C22H28N2O3/c1-15(2)26-21-13-12-20(14-23-21)27-19-10-6-17(7-11-19)5-4-16(3)24-22(25)18-8-9-18/h6-7,10-16,18H,4-5,8-9H2,1-3H3,(H,24,25)/t16-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 80 | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi-Aventis Deutschland GmbH
Curated by ChEMBL
| Assay Description
Inhibition of N-terminal His-tagged human recombinant ACC2 expressed in high five insect cells by ATP consumption assay |
J Med Chem 53: 8679-87 (2010)
Article DOI: 10.1021/jm101179e
BindingDB Entry DOI: 10.7270/Q28K79BT |
More data for this
Ligand-Target Pair | |
Renin
(Homo sapiens (Human)) | BDBM50077685
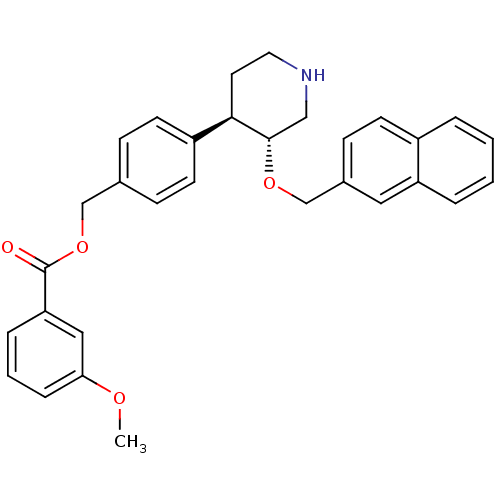
(3-Methoxy-benzoic acid 4-[(3R,4R)-3-(naphthalen-2-...)Show SMILES COc1cccc(c1)C(=O)OCc1ccc(cc1)[C@H]1CCNC[C@@H]1OCc1ccc2ccccc2c1 Show InChI InChI=1S/C31H31NO4/c1-34-28-8-4-7-27(18-28)31(33)36-20-22-9-13-25(14-10-22)29-15-16-32-19-30(29)35-21-23-11-12-24-5-2-3-6-26(24)17-23/h2-14,17-18,29-30,32H,15-16,19-21H2,1H3/t29-,30+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 87 | n/a | n/a | n/a | n/a | n/a | n/a |
F. Hoffmann-La Roche Ltd
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity against purified recombinant human renin |
Bioorg Med Chem Lett 9: 1397-402 (1999)
BindingDB Entry DOI: 10.7270/Q24F1PWN |
More data for this
Ligand-Target Pair | |
Integrin alpha-IIb/beta-3
(Homo sapiens (Human)) | BDBM50052485
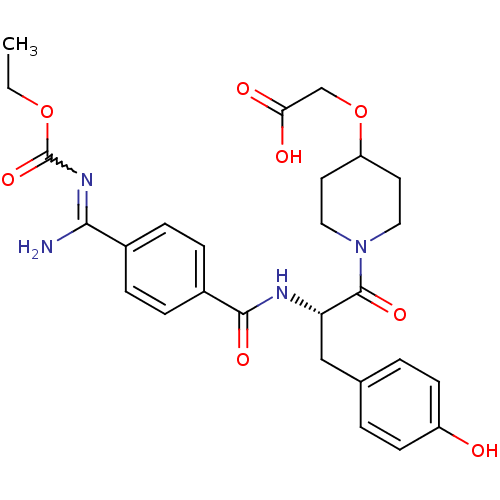
(CHEMBL106349 | {1-[(S)-2-(4-{Amino-[(E)-ethoxycarb...)Show SMILES CCOC(=O)N=C(N)c1ccc(cc1)C(=O)N[C@@H](Cc1ccc(O)cc1)C(=O)N1CCC(CC1)OCC(O)=O |w:5.4| Show InChI InChI=1S/C27H32N4O8/c1-2-38-27(37)30-24(28)18-5-7-19(8-6-18)25(35)29-22(15-17-3-9-20(32)10-4-17)26(36)31-13-11-21(12-14-31)39-16-23(33)34/h3-10,21-22,32H,2,11-16H2,1H3,(H,29,35)(H,33,34)(H2,28,30,37)/t22-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 90 | n/a | n/a | n/a | n/a | n/a | n/a |
F. Hoffmann-La Roche Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of FG binding to alpha IIb beta3 integrin |
J Med Chem 39: 3139-47 (1996)
Article DOI: 10.1021/jm9509298
BindingDB Entry DOI: 10.7270/Q2GT5M7Q |
More data for this
Ligand-Target Pair | |
Integrin alpha-IIb/beta-3
(Homo sapiens (Human)) | BDBM50052485

(CHEMBL106349 | {1-[(S)-2-(4-{Amino-[(E)-ethoxycarb...)Show SMILES CCOC(=O)N=C(N)c1ccc(cc1)C(=O)N[C@@H](Cc1ccc(O)cc1)C(=O)N1CCC(CC1)OCC(O)=O |w:5.4| Show InChI InChI=1S/C27H32N4O8/c1-2-38-27(37)30-24(28)18-5-7-19(8-6-18)25(35)29-22(15-17-3-9-20(32)10-4-17)26(36)31-13-11-21(12-14-31)39-16-23(33)34/h3-10,21-22,32H,2,11-16H2,1H3,(H,29,35)(H,33,34)(H2,28,30,37)/t22-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 90 | n/a | n/a | n/a | n/a | n/a | n/a |
F. Hoffmann-La Roche Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of FG binding to alpha IIb beta3 integrin |
J Med Chem 39: 3139-47 (1996)
Article DOI: 10.1021/jm9509298
BindingDB Entry DOI: 10.7270/Q2GT5M7Q |
More data for this
Ligand-Target Pair | |
Renin
(Homo sapiens (Human)) | BDBM50077691
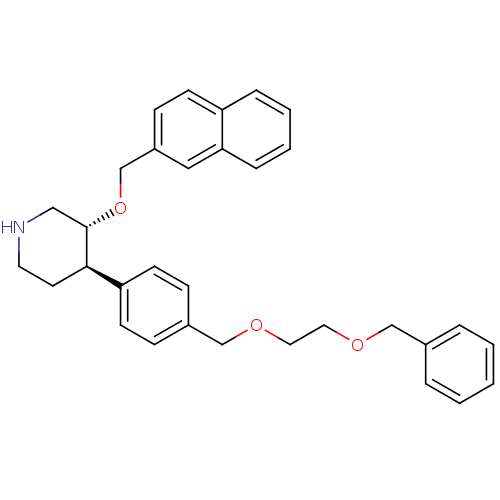
((3R,4R)-4-[4-(2-Benzyloxy-ethoxymethyl)-phenyl]-3-...)Show SMILES C(COCc1ccc(cc1)[C@H]1CCNC[C@@H]1OCc1ccc2ccccc2c1)OCc1ccccc1 Show InChI InChI=1S/C32H35NO3/c1-2-6-25(7-3-1)22-34-18-19-35-23-26-10-14-29(15-11-26)31-16-17-33-21-32(31)36-24-27-12-13-28-8-4-5-9-30(28)20-27/h1-15,20,31-33H,16-19,21-24H2/t31-,32+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 91 | n/a | n/a | n/a | n/a | n/a | n/a |
F. Hoffmann-La Roche Ltd
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity against purified recombinant human renin |
Bioorg Med Chem Lett 9: 1397-402 (1999)
BindingDB Entry DOI: 10.7270/Q24F1PWN |
More data for this
Ligand-Target Pair | |
Renin
(Homo sapiens (Human)) | BDBM50077667
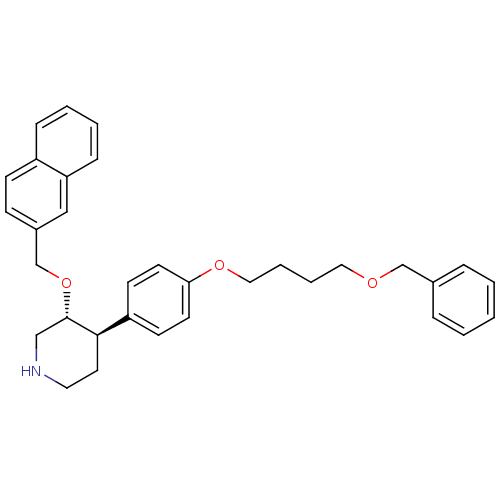
((3R,4R)-4-[4-(4-Benzyloxy-butoxy)-phenyl]-3-(napht...)Show SMILES C(CCOc1ccc(cc1)[C@H]1CCNC[C@@H]1OCc1ccc2ccccc2c1)COCc1ccccc1 Show InChI InChI=1S/C33H37NO3/c1-2-8-26(9-3-1)24-35-20-6-7-21-36-31-16-14-29(15-17-31)32-18-19-34-23-33(32)37-25-27-12-13-28-10-4-5-11-30(28)22-27/h1-5,8-17,22,32-34H,6-7,18-21,23-25H2/t32-,33+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 100 | n/a | n/a | n/a | n/a | n/a | n/a |
F. Hoffmann-La Roche Ltd
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity against purified recombinant human renin |
Bioorg Med Chem Lett 9: 1397-402 (1999)
BindingDB Entry DOI: 10.7270/Q24F1PWN |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data