 Found 2752 hits with Last Name = 'jung' and Initial = 'me'
Found 2752 hits with Last Name = 'jung' and Initial = 'me' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Serine/threonine-protein kinase 17B
(Homo sapiens (Human)) | BDBM50166121
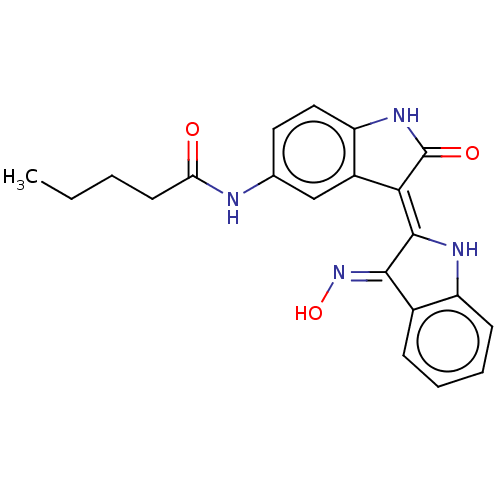
(CHEMBL3797480)Show SMILES CCCCC(=O)Nc1ccc2NC(=O)\C(=C3/Nc4ccccc4/C/3=N\O)c2c1 Show InChI InChI=1S/C21H20N4O3/c1-2-3-8-17(26)22-12-9-10-16-14(11-12)18(21(27)24-16)20-19(25-28)13-6-4-5-7-15(13)23-20/h4-7,9-11,23,28H,2-3,8H2,1H3,(H,22,26)(H,24,27)/b20-18-,25-19+ | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.260 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Research Institute of Chemical Technology
Curated by ChEMBL
| Assay Description
Competitive inhibition of DRAK2 (unknown origin) using MRLC3 peptide as substrate incubated for 2 hrs by Lineweaver-Burk plot analysis in presence of... |
Bioorg Med Chem Lett 26: 2719-23 (2016)
Article DOI: 10.1016/j.bmcl.2016.03.111
BindingDB Entry DOI: 10.7270/Q2N29ZTZ |
More data for this
Ligand-Target Pair | |
Deoxycytidine kinase
(Homo sapiens (Human)) | BDBM50440172
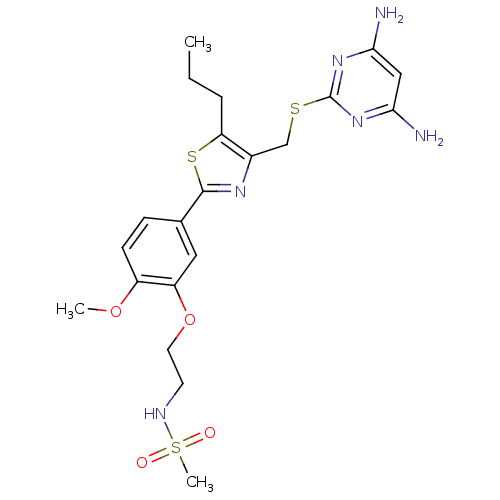
(CHEMBL2426574)Show SMILES CCCc1sc(nc1CSc1nc(N)cc(N)n1)-c1ccc(OC)c(OCCNS(C)(=O)=O)c1 Show InChI InChI=1S/C21H28N6O4S3/c1-4-5-17-14(12-32-21-26-18(22)11-19(23)27-21)25-20(33-17)13-6-7-15(30-2)16(10-13)31-9-8-24-34(3,28)29/h6-7,10-11,24H,4-5,8-9,12H2,1-3H3,(H4,22,23,26,27) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Illinois at Chicago
Curated by ChEMBL
| Assay Description
Apparent inhibition of human dCK by steady-state kinetic assay |
J Med Chem 57: 9480-94 (2014)
Article DOI: 10.1021/jm501124j
BindingDB Entry DOI: 10.7270/Q29025DM |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Deoxycytidine kinase
(Homo sapiens (Human)) | BDBM50440172

(CHEMBL2426574)Show SMILES CCCc1sc(nc1CSc1nc(N)cc(N)n1)-c1ccc(OC)c(OCCNS(C)(=O)=O)c1 Show InChI InChI=1S/C21H28N6O4S3/c1-4-5-17-14(12-32-21-26-18(22)11-19(23)27-21)25-20(33-17)13-6-7-15(30-2)16(10-13)31-9-8-24-34(3,28)29/h6-7,10-11,24H,4-5,8-9,12H2,1-3H3,(H4,22,23,26,27) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
California NanoSystems Institute
Curated by ChEMBL
| Assay Description
Inhibition of dCK (unknown origin) by steady-state kinetic assay |
J Med Chem 56: 6696-708 (2013)
Article DOI: 10.1021/jm400457y
BindingDB Entry DOI: 10.7270/Q2JS9RWP |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Deoxycytidine kinase
(Homo sapiens (Human)) | BDBM50440176
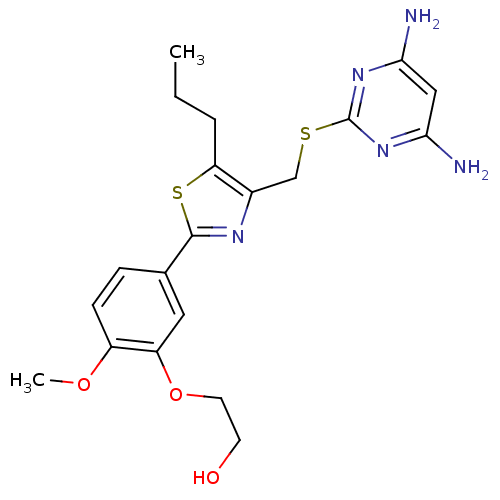
(CHEMBL2426570)Show SMILES CCCc1sc(nc1CSc1nc(N)cc(N)n1)-c1ccc(OC)c(OCCO)c1 Show InChI InChI=1S/C20H25N5O3S2/c1-3-4-16-13(11-29-20-24-17(21)10-18(22)25-20)23-19(30-16)12-5-6-14(27-2)15(9-12)28-8-7-26/h5-6,9-10,26H,3-4,7-8,11H2,1-2H3,(H4,21,22,24,25) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Illinois at Chicago
Curated by ChEMBL
| Assay Description
Apparent inhibition of human dCK by steady-state kinetic assay |
J Med Chem 57: 9480-94 (2014)
Article DOI: 10.1021/jm501124j
BindingDB Entry DOI: 10.7270/Q29025DM |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Deoxycytidine kinase
(Homo sapiens (Human)) | BDBM50440173
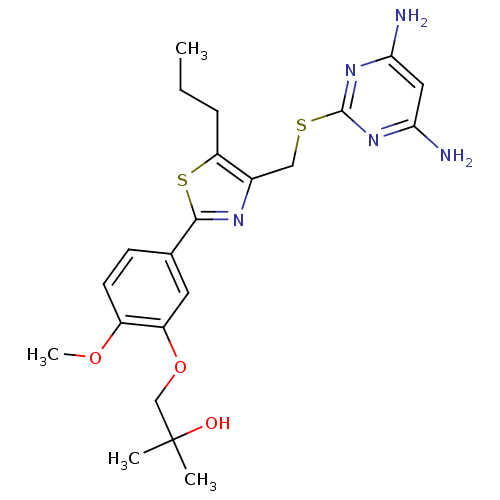
(CHEMBL2426573)Show SMILES CCCc1sc(nc1CSc1nc(N)cc(N)n1)-c1ccc(OC)c(OCC(C)(C)O)c1 Show InChI InChI=1S/C22H29N5O3S2/c1-5-6-17-14(11-31-21-26-18(23)10-19(24)27-21)25-20(32-17)13-7-8-15(29-4)16(9-13)30-12-22(2,3)28/h7-10,28H,5-6,11-12H2,1-4H3,(H4,23,24,26,27) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| 0.800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
California NanoSystems Institute
Curated by ChEMBL
| Assay Description
Inhibition of dCK (unknown origin) by steady-state kinetic assay |
J Med Chem 56: 6696-708 (2013)
Article DOI: 10.1021/jm400457y
BindingDB Entry DOI: 10.7270/Q2JS9RWP |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
5-hydroxytryptamine receptor 2A
(Homo sapiens (Human)) | BDBM50130293

(7-{4-[4-(2,3-dichlorophenyl)piperazin-1-yl]butoxy}...)Show SMILES Clc1cccc(N2CCN(CCCCOc3ccc4CCC(=O)Nc4c3)CC2)c1Cl Show InChI InChI=1S/C23H27Cl2N3O2/c24-19-4-3-5-21(23(19)25)28-13-11-27(12-14-28)10-1-2-15-30-18-8-6-17-7-9-22(29)26-20(17)16-18/h3-6,8,16H,1-2,7,9-15H2,(H,26,29) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 0.800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Green Cross Corporation
Curated by ChEMBL
| Assay Description
Binding affinity to 5-HT2A |
J Med Chem 54: 6305-18 (2011)
Article DOI: 10.1021/jm200682b
BindingDB Entry DOI: 10.7270/Q2SX6DMC |
More data for this
Ligand-Target Pair | |
Deoxycytidine kinase
(Homo sapiens (Human)) | BDBM50440173

(CHEMBL2426573)Show SMILES CCCc1sc(nc1CSc1nc(N)cc(N)n1)-c1ccc(OC)c(OCC(C)(C)O)c1 Show InChI InChI=1S/C22H29N5O3S2/c1-5-6-17-14(11-31-21-26-18(23)10-19(24)27-21)25-20(32-17)13-7-8-15(29-4)16(9-13)30-12-22(2,3)28/h7-10,28H,5-6,11-12H2,1-4H3,(H4,23,24,26,27) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| 0.800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Illinois at Chicago
Curated by ChEMBL
| Assay Description
Apparent inhibition of human dCK by steady-state kinetic assay |
J Med Chem 57: 9480-94 (2014)
Article DOI: 10.1021/jm501124j
BindingDB Entry DOI: 10.7270/Q29025DM |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
5-hydroxytryptamine receptor 2A
(Homo sapiens (Human)) | BDBM50313283

(1-{2-[4-(6-Fluoro-1H-indol-3-yl)-3,6-dihydro-2H-py...)Show SMILES Fc1ccc2c(c[nH]c2c1)C1=CCN(CCN2c3cccc4CCCN(c34)S2(=O)=O)CC1 |t:12| Show InChI InChI=1S/C24H25FN4O2S/c25-19-6-7-20-21(16-26-22(20)15-19)17-8-11-27(12-9-17)13-14-28-23-5-1-3-18-4-2-10-29(24(18)23)32(28,30)31/h1,3,5-8,15-16,26H,2,4,9-14H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| 0.810 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Green Cross Corporation
Curated by ChEMBL
| Assay Description
Binding affinity to 5-HT2A |
J Med Chem 54: 6305-18 (2011)
Article DOI: 10.1021/jm200682b
BindingDB Entry DOI: 10.7270/Q2SX6DMC |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2A
(Homo sapiens (Human)) | BDBM50313283

(1-{2-[4-(6-Fluoro-1H-indol-3-yl)-3,6-dihydro-2H-py...)Show SMILES Fc1ccc2c(c[nH]c2c1)C1=CCN(CCN2c3cccc4CCCN(c34)S2(=O)=O)CC1 |t:12| Show InChI InChI=1S/C24H25FN4O2S/c25-19-6-7-20-21(16-26-22(20)15-19)17-8-11-27(12-9-17)13-14-28-23-5-1-3-18-4-2-10-29(24(18)23)32(28,30)31/h1,3,5-8,15-16,26H,2,4,9-14H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| 0.810 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Green Cross Corporation
Curated by ChEMBL
| Assay Description
Inhibition of 5-HT2A receptor |
Bioorg Med Chem Lett 20: 1705-11 (2010)
Article DOI: 10.1016/j.bmcl.2010.01.093
BindingDB Entry DOI: 10.7270/Q2SN093C |
More data for this
Ligand-Target Pair | |
Deoxycytidine kinase
(Homo sapiens (Human)) | BDBM50440151
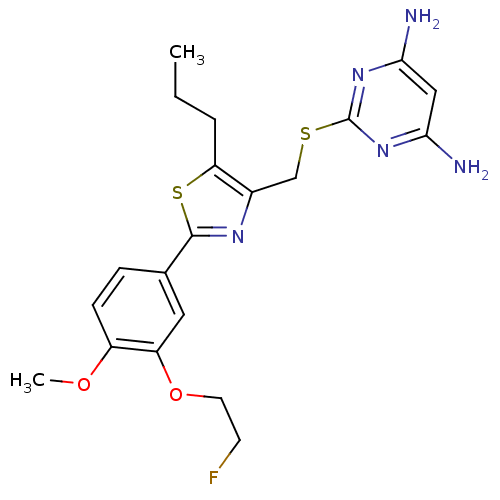
(CHEMBL2426558)Show SMILES CCCc1sc(nc1CSc1nc(N)cc(N)n1)-c1ccc(OC)c(OCCF)c1 Show InChI InChI=1S/C20H24FN5O2S2/c1-3-4-16-13(11-29-20-25-17(22)10-18(23)26-20)24-19(30-16)12-5-6-14(27-2)15(9-12)28-8-7-21/h5-6,9-10H,3-4,7-8,11H2,1-2H3,(H4,22,23,25,26) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| 1.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
California NanoSystems Institute
Curated by ChEMBL
| Assay Description
Inhibition of dCK (unknown origin) by steady-state kinetic assay |
J Med Chem 56: 6696-708 (2013)
Article DOI: 10.1021/jm400457y
BindingDB Entry DOI: 10.7270/Q2JS9RWP |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Deoxycytidine kinase
(Homo sapiens (Human)) | BDBM50031352
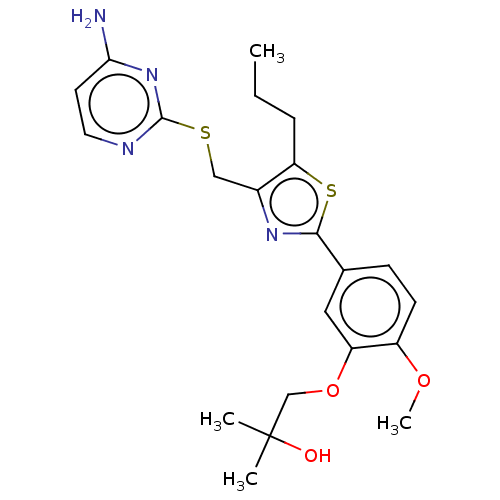
(CHEMBL3358091)Show SMILES CCCc1sc(nc1CSc1nccc(N)n1)-c1ccc(OC)c(OCC(C)(C)O)c1 Show InChI InChI=1S/C22H28N4O3S2/c1-5-6-18-15(12-30-21-24-10-9-19(23)26-21)25-20(31-18)14-7-8-16(28-4)17(11-14)29-13-22(2,3)27/h7-11,27H,5-6,12-13H2,1-4H3,(H2,23,24,26) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Illinois at Chicago
Curated by ChEMBL
| Assay Description
Apparent inhibition of human dCK by steady-state kinetic assay |
J Med Chem 57: 9480-94 (2014)
Article DOI: 10.1021/jm501124j
BindingDB Entry DOI: 10.7270/Q29025DM |
More data for this
Ligand-Target Pair | |
Deoxycytidine kinase
(Homo sapiens (Human)) | BDBM50440181
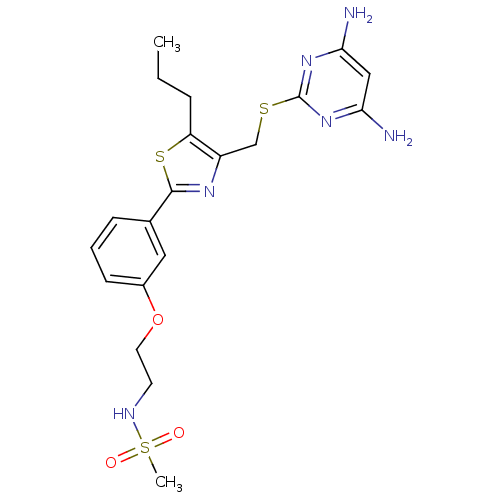
(CHEMBL2426565)Show SMILES CCCc1sc(nc1CSc1nc(N)cc(N)n1)-c1cccc(OCCNS(C)(=O)=O)c1 Show InChI InChI=1S/C20H26N6O3S3/c1-3-5-16-15(12-30-20-25-17(21)11-18(22)26-20)24-19(31-16)13-6-4-7-14(10-13)29-9-8-23-32(2,27)28/h4,6-7,10-11,23H,3,5,8-9,12H2,1-2H3,(H4,21,22,25,26) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| 1.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Illinois at Chicago
Curated by ChEMBL
| Assay Description
Apparent inhibition of human dCK by steady-state kinetic assay |
J Med Chem 57: 9480-94 (2014)
Article DOI: 10.1021/jm501124j
BindingDB Entry DOI: 10.7270/Q29025DM |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Deoxycytidine kinase
(Homo sapiens (Human)) | BDBM50440179
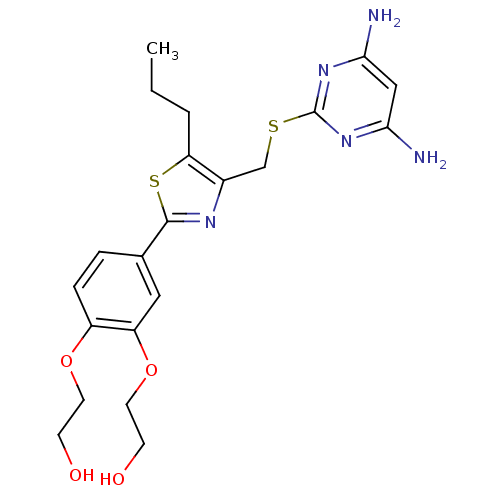
(CHEMBL2426567)Show SMILES CCCc1sc(nc1CSc1nc(N)cc(N)n1)-c1ccc(OCCO)c(OCCO)c1 Show InChI InChI=1S/C21H27N5O4S2/c1-2-3-17-14(12-31-21-25-18(22)11-19(23)26-21)24-20(32-17)13-4-5-15(29-8-6-27)16(10-13)30-9-7-28/h4-5,10-11,27-28H,2-3,6-9,12H2,1H3,(H4,22,23,25,26) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Illinois at Chicago
Curated by ChEMBL
| Assay Description
Apparent inhibition of human dCK by steady-state kinetic assay |
J Med Chem 57: 9480-94 (2014)
Article DOI: 10.1021/jm501124j
BindingDB Entry DOI: 10.7270/Q29025DM |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Sodium-dependent serotonin transporter
(Homo sapiens (Human)) | BDBM50313283

(1-{2-[4-(6-Fluoro-1H-indol-3-yl)-3,6-dihydro-2H-py...)Show SMILES Fc1ccc2c(c[nH]c2c1)C1=CCN(CCN2c3cccc4CCCN(c34)S2(=O)=O)CC1 |t:12| Show InChI InChI=1S/C24H25FN4O2S/c25-19-6-7-20-21(16-26-22(20)15-19)17-8-11-27(12-9-17)13-14-28-23-5-1-3-18-4-2-10-29(24(18)23)32(28,30)31/h1,3,5-8,15-16,26H,2,4,9-14H2 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| 2.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Green Cross Corporation
Curated by ChEMBL
| Assay Description
Binding affinity to SERT |
J Med Chem 54: 6305-18 (2011)
Article DOI: 10.1021/jm200682b
BindingDB Entry DOI: 10.7270/Q2SX6DMC |
More data for this
Ligand-Target Pair | |
Sodium-dependent serotonin transporter
(Homo sapiens (Human)) | BDBM50313283

(1-{2-[4-(6-Fluoro-1H-indol-3-yl)-3,6-dihydro-2H-py...)Show SMILES Fc1ccc2c(c[nH]c2c1)C1=CCN(CCN2c3cccc4CCCN(c34)S2(=O)=O)CC1 |t:12| Show InChI InChI=1S/C24H25FN4O2S/c25-19-6-7-20-21(16-26-22(20)15-19)17-8-11-27(12-9-17)13-14-28-23-5-1-3-18-4-2-10-29(24(18)23)32(28,30)31/h1,3,5-8,15-16,26H,2,4,9-14H2 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| 2.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Green Cross Corporation
Curated by ChEMBL
| Assay Description
Inhibition of SERT |
Bioorg Med Chem Lett 20: 1705-11 (2010)
Article DOI: 10.1016/j.bmcl.2010.01.093
BindingDB Entry DOI: 10.7270/Q2SN093C |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2A
(Homo sapiens (Human)) | BDBM50069447
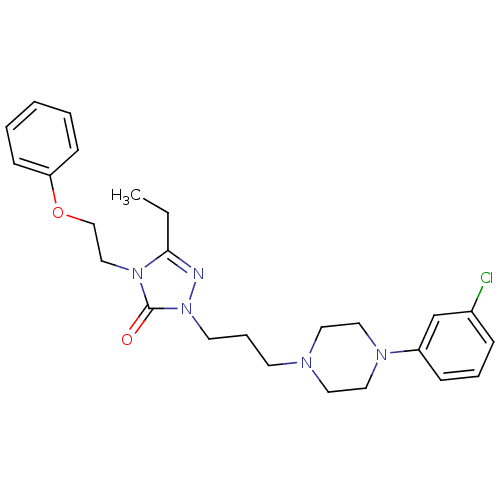
(1-(3-(4-(m-Chlorophenyl)-1-piperazinyl)propyl)-3-e...)Show SMILES CCc1nn(CCCN2CCN(CC2)c2cccc(Cl)c2)c(=O)n1CCOc1ccccc1 Show InChI InChI=1S/C25H32ClN5O2/c1-2-24-27-31(25(32)30(24)18-19-33-23-10-4-3-5-11-23)13-7-12-28-14-16-29(17-15-28)22-9-6-8-21(26)20-22/h3-6,8-11,20H,2,7,12-19H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 5.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Green Cross Corporation
Curated by ChEMBL
| Assay Description
Binding affinity to 5-HT2A |
J Med Chem 54: 6305-18 (2011)
Article DOI: 10.1021/jm200682b
BindingDB Entry DOI: 10.7270/Q2SX6DMC |
More data for this
Ligand-Target Pair | |
Deoxycytidine kinase
(Homo sapiens (Human)) | BDBM50031349
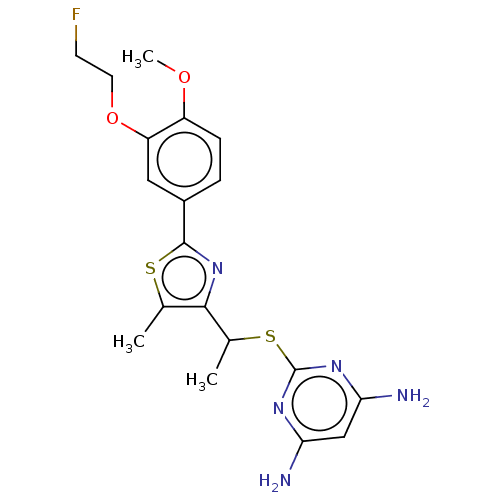
(CHEMBL3358094)Show SMILES COc1ccc(cc1OCCF)-c1nc(C(C)Sc2nc(N)cc(N)n2)c(C)s1 Show InChI InChI=1S/C19H22FN5O2S2/c1-10-17(11(2)29-19-23-15(21)9-16(22)24-19)25-18(28-10)12-4-5-13(26-3)14(8-12)27-7-6-20/h4-5,8-9,11H,6-7H2,1-3H3,(H4,21,22,23,24) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| 6.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Illinois at Chicago
Curated by ChEMBL
| Assay Description
Apparent inhibition of human dCK by steady-state kinetic assay |
J Med Chem 57: 9480-94 (2014)
Article DOI: 10.1021/jm501124j
BindingDB Entry DOI: 10.7270/Q29025DM |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Deoxycytidine kinase
(Homo sapiens (Human)) | BDBM50440178
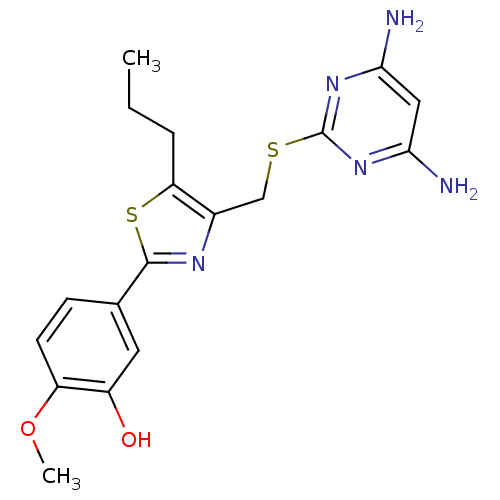
(CHEMBL2426568)Show SMILES CCCc1sc(nc1CSc1nc(N)cc(N)n1)-c1ccc(OC)c(O)c1 Show InChI InChI=1S/C18H21N5O2S2/c1-3-4-14-11(9-26-18-22-15(19)8-16(20)23-18)21-17(27-14)10-5-6-13(25-2)12(24)7-10/h5-8,24H,3-4,9H2,1-2H3,(H4,19,20,22,23) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| 8.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Illinois at Chicago
Curated by ChEMBL
| Assay Description
Apparent inhibition of human dCK by steady-state kinetic assay |
J Med Chem 57: 9480-94 (2014)
Article DOI: 10.1021/jm501124j
BindingDB Entry DOI: 10.7270/Q29025DM |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Deoxycytidine kinase
(Homo sapiens (Human)) | BDBM50031337
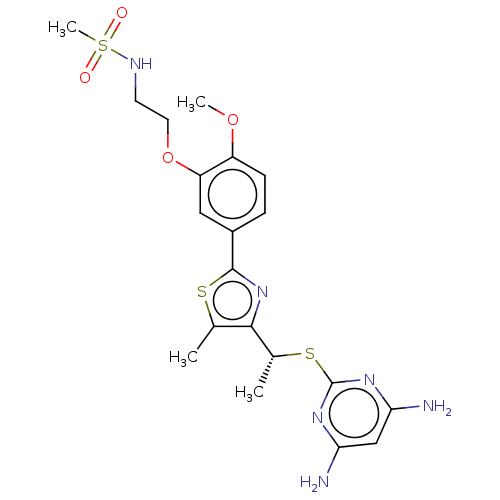
(CHEMBL3358097)Show SMILES COc1ccc(cc1OCCNS(C)(=O)=O)-c1nc([C@@H](C)Sc2nc(N)cc(N)n2)c(C)s1 |r| Show InChI InChI=1S/C20H26N6O4S3/c1-11-18(12(2)32-20-24-16(21)10-17(22)25-20)26-19(31-11)13-5-6-14(29-3)15(9-13)30-8-7-23-33(4,27)28/h5-6,9-10,12,23H,7-8H2,1-4H3,(H4,21,22,24,25)/t12-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| 9.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Illinois at Chicago
Curated by ChEMBL
| Assay Description
Apparent inhibition of human dCK by steady-state kinetic assay |
J Med Chem 57: 9480-94 (2014)
Article DOI: 10.1021/jm501124j
BindingDB Entry DOI: 10.7270/Q29025DM |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Deoxycytidine kinase
(Homo sapiens (Human)) | BDBM50440140
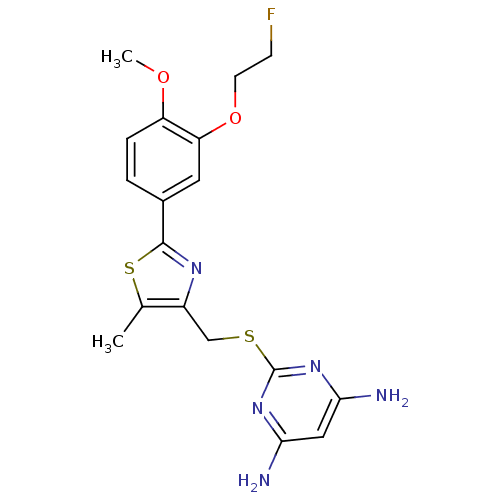
(CHEMBL2426588)Show SMILES COc1ccc(cc1OCCF)-c1nc(CSc2nc(N)cc(N)n2)c(C)s1 Show InChI InChI=1S/C18H20FN5O2S2/c1-10-12(9-27-18-23-15(20)8-16(21)24-18)22-17(28-10)11-3-4-13(25-2)14(7-11)26-6-5-19/h3-4,7-8H,5-6,9H2,1-2H3,(H4,20,21,23,24) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| 9.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
California NanoSystems Institute
Curated by ChEMBL
| Assay Description
Inhibition of dCK (unknown origin) by steady-state kinetic assay |
J Med Chem 56: 6696-708 (2013)
Article DOI: 10.1021/jm400457y
BindingDB Entry DOI: 10.7270/Q2JS9RWP |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Deoxycytidine kinase
(Homo sapiens (Human)) | BDBM50031339
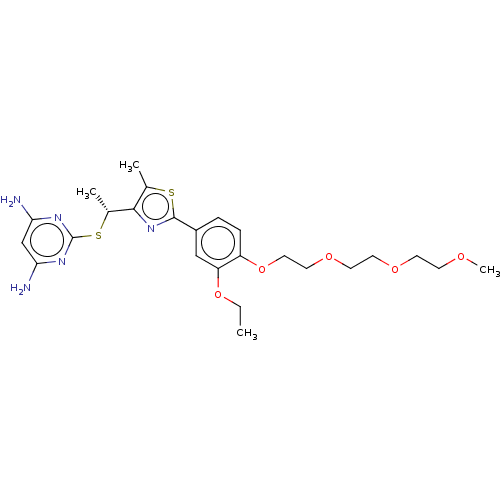
(CHEMBL3358096)Show SMILES CCOc1cc(ccc1OCCOCCOCCOC)-c1nc([C@@H](C)Sc2nc(N)cc(N)n2)c(C)s1 |r| Show InChI InChI=1S/C25H35N5O5S2/c1-5-34-20-14-18(6-7-19(20)35-13-12-33-11-10-32-9-8-31-4)24-30-23(16(2)36-24)17(3)37-25-28-21(26)15-22(27)29-25/h6-7,14-15,17H,5,8-13H2,1-4H3,(H4,26,27,28,29)/t17-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 14 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Illinois at Chicago
Curated by ChEMBL
| Assay Description
Apparent inhibition of human dCK by steady-state kinetic assay |
J Med Chem 57: 9480-94 (2014)
Article DOI: 10.1021/jm501124j
BindingDB Entry DOI: 10.7270/Q29025DM |
More data for this
Ligand-Target Pair | |
Deoxycytidine kinase
(Homo sapiens (Human)) | BDBM50031350
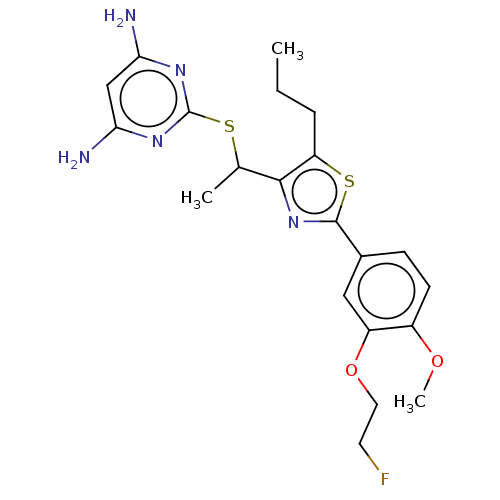
(CHEMBL3358093)Show SMILES CCCc1sc(nc1C(C)Sc1nc(N)cc(N)n1)-c1ccc(OC)c(OCCF)c1 Show InChI InChI=1S/C21H26FN5O2S2/c1-4-5-16-19(12(2)30-21-25-17(23)11-18(24)26-21)27-20(31-16)13-6-7-14(28-3)15(10-13)29-9-8-22/h6-7,10-12H,4-5,8-9H2,1-3H3,(H4,23,24,25,26) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| 17 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Illinois at Chicago
Curated by ChEMBL
| Assay Description
Apparent inhibition of human dCK by steady-state kinetic assay |
J Med Chem 57: 9480-94 (2014)
Article DOI: 10.1021/jm501124j
BindingDB Entry DOI: 10.7270/Q29025DM |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Sodium-dependent serotonin transporter
(Homo sapiens (Human)) | BDBM50313284

((S)-2-((7-fluoro-2,3-dihydro-1H-inden-4-yloxy)meth...)Show InChI InChI=1S/C14H18FNO2/c15-13-4-5-14(12-3-1-2-11(12)13)18-9-10-8-16-6-7-17-10/h4-5,10,16H,1-3,6-9H2/t10-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
DrugBank
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 21 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Green Cross Corporation
Curated by ChEMBL
| Assay Description
Inhibition of SERT |
Bioorg Med Chem Lett 20: 1705-11 (2010)
Article DOI: 10.1016/j.bmcl.2010.01.093
BindingDB Entry DOI: 10.7270/Q2SN093C |
More data for this
Ligand-Target Pair | |
Sodium-dependent serotonin transporter
(Homo sapiens (Human)) | BDBM50313284

((S)-2-((7-fluoro-2,3-dihydro-1H-inden-4-yloxy)meth...)Show InChI InChI=1S/C14H18FNO2/c15-13-4-5-14(12-3-1-2-11(12)13)18-9-10-8-16-6-7-17-10/h4-5,10,16H,1-3,6-9H2/t10-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
DrugBank
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 21 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Green Cross Corporation
Curated by ChEMBL
| Assay Description
Binding affinity to SERT |
J Med Chem 54: 6305-18 (2011)
Article DOI: 10.1021/jm200682b
BindingDB Entry DOI: 10.7270/Q2SX6DMC |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2A
(Homo sapiens (Human)) | BDBM50313284

((S)-2-((7-fluoro-2,3-dihydro-1H-inden-4-yloxy)meth...)Show InChI InChI=1S/C14H18FNO2/c15-13-4-5-14(12-3-1-2-11(12)13)18-9-10-8-16-6-7-17-10/h4-5,10,16H,1-3,6-9H2/t10-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
DrugBank
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 86 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Green Cross Corporation
Curated by ChEMBL
| Assay Description
Inhibition of 5-HT2A receptor |
Bioorg Med Chem Lett 20: 1705-11 (2010)
Article DOI: 10.1016/j.bmcl.2010.01.093
BindingDB Entry DOI: 10.7270/Q2SN093C |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2A
(Homo sapiens (Human)) | BDBM50313284

((S)-2-((7-fluoro-2,3-dihydro-1H-inden-4-yloxy)meth...)Show InChI InChI=1S/C14H18FNO2/c15-13-4-5-14(12-3-1-2-11(12)13)18-9-10-8-16-6-7-17-10/h4-5,10,16H,1-3,6-9H2/t10-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
DrugBank
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 86 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Green Cross Corporation
Curated by ChEMBL
| Assay Description
Binding affinity to 5-HT2A |
J Med Chem 54: 6305-18 (2011)
Article DOI: 10.1021/jm200682b
BindingDB Entry DOI: 10.7270/Q2SX6DMC |
More data for this
Ligand-Target Pair | |
Sodium-dependent serotonin transporter
(Homo sapiens (Human)) | BDBM50130293

(7-{4-[4-(2,3-dichlorophenyl)piperazin-1-yl]butoxy}...)Show SMILES Clc1cccc(N2CCN(CCCCOc3ccc4CCC(=O)Nc4c3)CC2)c1Cl Show InChI InChI=1S/C23H27Cl2N3O2/c24-19-4-3-5-21(23(19)25)28-13-11-27(12-14-28)10-1-2-15-30-18-8-6-17-7-9-22(29)26-20(17)16-18/h3-6,8,16H,1-2,7,9-15H2,(H,26,29) | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 98 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Green Cross Corporation
Curated by ChEMBL
| Assay Description
Binding affinity to SERT |
J Med Chem 54: 6305-18 (2011)
Article DOI: 10.1021/jm200682b
BindingDB Entry DOI: 10.7270/Q2SX6DMC |
More data for this
Ligand-Target Pair | |
Sodium-dependent serotonin transporter
(Homo sapiens (Human)) | BDBM50069447

(1-(3-(4-(m-Chlorophenyl)-1-piperazinyl)propyl)-3-e...)Show SMILES CCc1nn(CCCN2CCN(CC2)c2cccc(Cl)c2)c(=O)n1CCOc1ccccc1 Show InChI InChI=1S/C25H32ClN5O2/c1-2-24-27-31(25(32)30(24)18-19-33-23-10-4-3-5-11-23)13-7-12-28-14-16-29(17-15-28)22-9-6-8-21(26)20-22/h3-6,8-11,20H,2,7,12-19H2,1H3 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| DrugBank
Article
PubMed
| 290 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Green Cross Corporation
Curated by ChEMBL
| Assay Description
Binding affinity to SERT |
J Med Chem 54: 6305-18 (2011)
Article DOI: 10.1021/jm200682b
BindingDB Entry DOI: 10.7270/Q2SX6DMC |
More data for this
Ligand-Target Pair | |
Deoxycytidine kinase
(Homo sapiens (Human)) | BDBM50031338
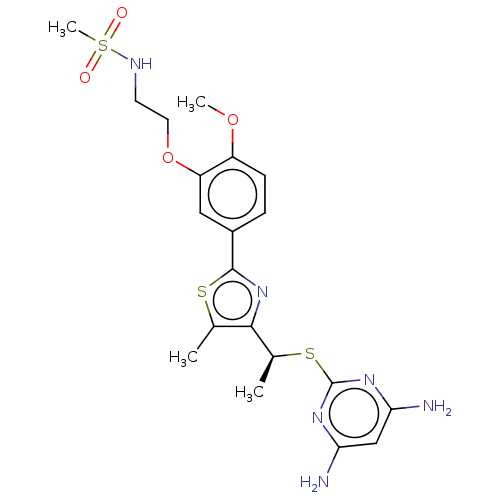
(CHEMBL3358090)Show SMILES COc1ccc(cc1OCCNS(C)(=O)=O)-c1nc([C@H](C)Sc2nc(N)cc(N)n2)c(C)s1 |r| Show InChI InChI=1S/C20H26N6O4S3/c1-11-18(12(2)32-20-24-16(21)10-17(22)25-20)26-19(31-11)13-5-6-14(29-3)15(9-13)30-8-7-23-33(4,27)28/h5-6,9-10,12,23H,7-8H2,1-4H3,(H4,21,22,24,25)/t12-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 585 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Illinois at Chicago
Curated by ChEMBL
| Assay Description
Apparent inhibition of human dCK by steady-state kinetic assay |
J Med Chem 57: 9480-94 (2014)
Article DOI: 10.1021/jm501124j
BindingDB Entry DOI: 10.7270/Q29025DM |
More data for this
Ligand-Target Pair | |
Deoxycytidine kinase
(Homo sapiens (Human)) | BDBM50031351
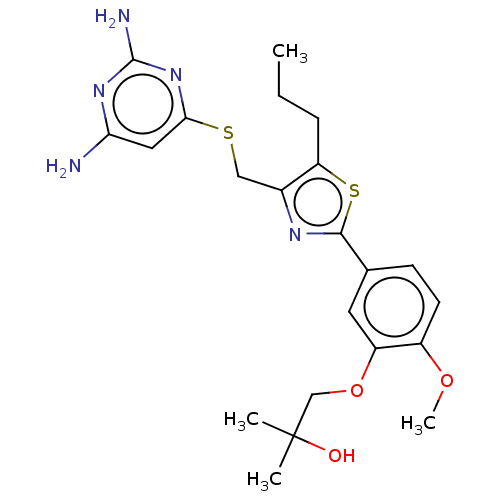
(CHEMBL3358092)Show SMILES CCCc1sc(nc1CSc1cc(N)nc(N)n1)-c1ccc(OC)c(OCC(C)(C)O)c1 Show InChI InChI=1S/C22H29N5O3S2/c1-5-6-17-14(11-31-19-10-18(23)26-21(24)27-19)25-20(32-17)13-7-8-15(29-4)16(9-13)30-12-22(2,3)28/h7-10,28H,5-6,11-12H2,1-4H3,(H4,23,24,26,27) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| 735 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Illinois at Chicago
Curated by ChEMBL
| Assay Description
Apparent inhibition of human dCK by steady-state kinetic assay |
J Med Chem 57: 9480-94 (2014)
Article DOI: 10.1021/jm501124j
BindingDB Entry DOI: 10.7270/Q29025DM |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Deoxycytidine kinase
(Homo sapiens (Human)) | BDBM50031340
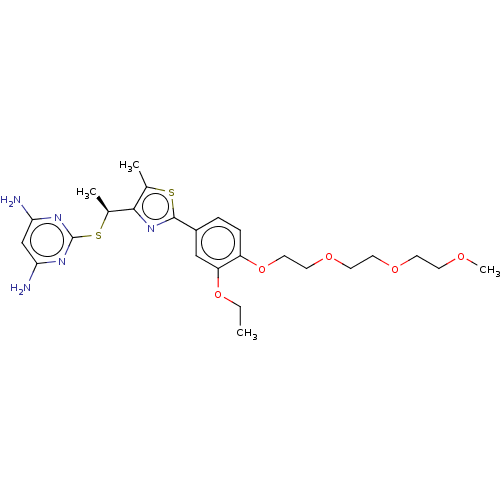
(CHEMBL3358095)Show SMILES CCOc1cc(ccc1OCCOCCOCCOC)-c1nc([C@H](C)Sc2nc(N)cc(N)n2)c(C)s1 |r| Show InChI InChI=1S/C25H35N5O5S2/c1-5-34-20-14-18(6-7-19(20)35-13-12-33-11-10-32-9-8-31-4)24-30-23(16(2)36-24)17(3)37-25-28-21(26)15-22(27)29-25/h6-7,14-15,17H,5,8-13H2,1-4H3,(H4,26,27,28,29)/t17-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 5.61E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Illinois at Chicago
Curated by ChEMBL
| Assay Description
Apparent inhibition of human dCK by steady-state kinetic assay |
J Med Chem 57: 9480-94 (2014)
Article DOI: 10.1021/jm501124j
BindingDB Entry DOI: 10.7270/Q29025DM |
More data for this
Ligand-Target Pair | |
Epidermal growth factor receptor [1-18,20-1210]
(Homo sapiens (Human)) | BDBM538119
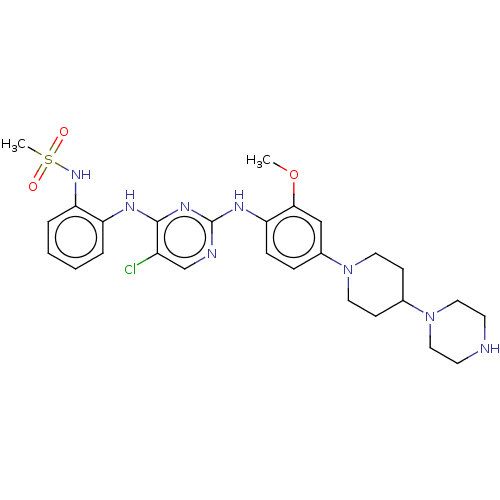
(US11253516, Example 21)Show SMILES COc1cc(ccc1Nc1ncc(Cl)c(Nc2ccccc2NS(C)(=O)=O)n1)N1CCC(CC1)N1CCNCC1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
The composition of the assay buffer used in the activity measurement was 50 mM Tris-HCl pH 7.5, 100 mM NaCl, 7.5 mM MgCl2, 3 mM KCl, 0.01% Tween 20, ... |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2RJ4NNV |
More data for this
Ligand-Target Pair | |
Epidermal growth factor receptor [1-18,20-1210,T790M,C797S]
(Homo sapiens (Human)) | BDBM538135
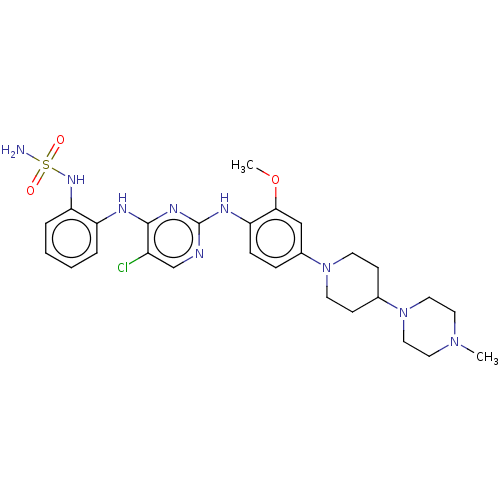
(US11253516, Example 37)Show SMILES COc1cc(ccc1Nc1ncc(Cl)c(Nc2ccccc2NS(N)(=O)=O)n1)N1CCC(CC1)N1CCN(C)CC1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
The composition of the assay buffer used in the activity measurement was 50 mM Tris-HCl pH 7.5, 100 mM NaCl, 7.5 mM MgCl2, 3 mM KCl, 0.01% Tween 20, ... |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2RJ4NNV |
More data for this
Ligand-Target Pair | |
Epidermal growth factor receptor [1-18,20-1210,T790M,C797S]
(Homo sapiens (Human)) | BDBM538139

(US11253516, Example 41)Show SMILES COc1cc(ccc1Nc1ncc(Cl)c(Nc2ccccc2NS(C)(=O)=O)n1)N1CCC(CC1)NCCO | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
The composition of the assay buffer used in the activity measurement was 50 mM Tris-HCl pH 7.5, 100 mM NaCl, 7.5 mM MgCl2, 3 mM KCl, 0.01% Tween 20, ... |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2RJ4NNV |
More data for this
Ligand-Target Pair | |
Epidermal growth factor receptor [1-18,20-1210,T790M,C797S]
(Homo sapiens (Human)) | BDBM538108
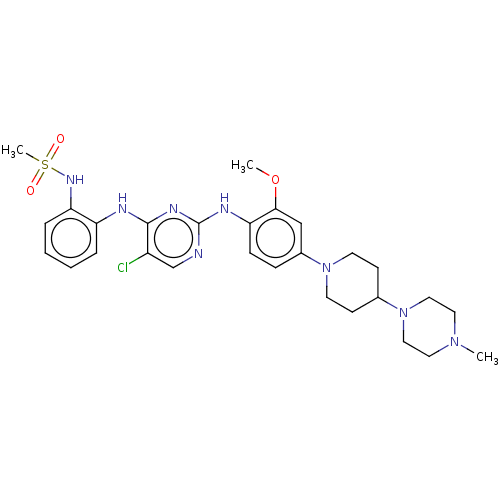
(US11253516, Example 10)Show SMILES COc1cc(ccc1Nc1ncc(Cl)c(Nc2ccccc2NS(C)(=O)=O)n1)N1CCC(CC1)N1CCN(C)CC1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
The composition of the assay buffer used in the activity measurement was 50 mM Tris-HCl pH 7.5, 100 mM NaCl, 7.5 mM MgCl2, 3 mM KCl, 0.01% Tween 20, ... |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2RJ4NNV |
More data for this
Ligand-Target Pair | |
Epidermal growth factor receptor [1-18,20-1210]
(Homo sapiens (Human)) | BDBM538123
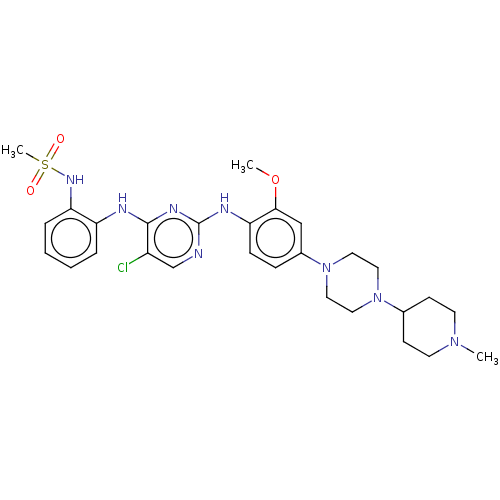
(US11253516, Example 25)Show SMILES COc1cc(ccc1Nc1ncc(Cl)c(Nc2ccccc2NS(C)(=O)=O)n1)N1CCN(CC1)C1CCN(C)CC1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
The composition of the assay buffer used in the activity measurement was 50 mM Tris-HCl pH 7.5, 100 mM NaCl, 7.5 mM MgCl2, 3 mM KCl, 0.01% Tween 20, ... |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2RJ4NNV |
More data for this
Ligand-Target Pair | |
Epidermal growth factor receptor [1-18,20-1210,T790M,C797S]
(Homo sapiens (Human)) | BDBM538134
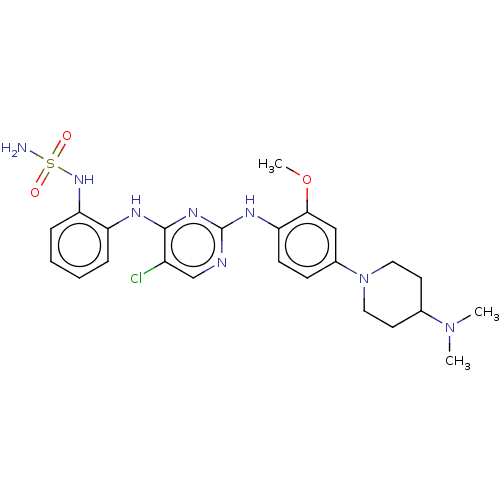
(US11253516, Example 36)Show SMILES COc1cc(ccc1Nc1ncc(Cl)c(Nc2ccccc2NS(N)(=O)=O)n1)N1CCC(CC1)N(C)C | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
The composition of the assay buffer used in the activity measurement was 50 mM Tris-HCl pH 7.5, 100 mM NaCl, 7.5 mM MgCl2, 3 mM KCl, 0.01% Tween 20, ... |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2RJ4NNV |
More data for this
Ligand-Target Pair | |
Epidermal growth factor receptor [1-18,20-1210,T790M,C797S]
(Homo sapiens (Human)) | BDBM538137

(US11253516, Example 39)Show SMILES COc1cc(ccc1Nc1ncc(Cl)c(Nc2ccccc2NS(C)(=O)=O)n1)N1CCC(CC1)NC(C)=O | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
The composition of the assay buffer used in the activity measurement was 50 mM Tris-HCl pH 7.5, 100 mM NaCl, 7.5 mM MgCl2, 3 mM KCl, 0.01% Tween 20, ... |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2RJ4NNV |
More data for this
Ligand-Target Pair | |
Epidermal growth factor receptor [1-18,20-1210,T790M,C797S]
(Homo sapiens (Human)) | BDBM538140
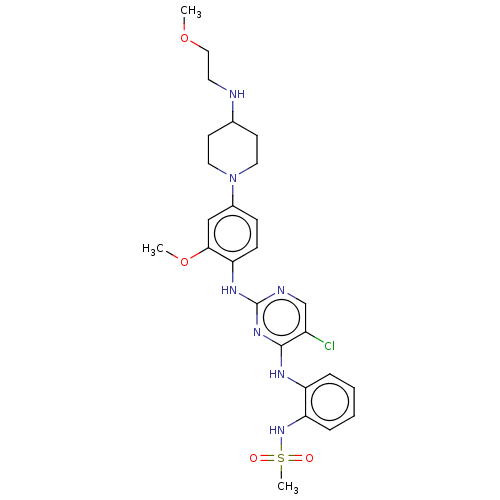
(US11253516, Example 42)Show SMILES COCCNC1CCN(CC1)c1ccc(Nc2ncc(Cl)c(Nc3ccccc3NS(C)(=O)=O)n2)c(OC)c1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
The composition of the assay buffer used in the activity measurement was 50 mM Tris-HCl pH 7.5, 100 mM NaCl, 7.5 mM MgCl2, 3 mM KCl, 0.01% Tween 20, ... |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2RJ4NNV |
More data for this
Ligand-Target Pair | |
Epidermal growth factor receptor [1-18,20-1210]
(Homo sapiens (Human)) | BDBM538125

(US11253516, Example 27)Show SMILES COc1cc(ccc1Nc1ncc(Cl)c(Nc2ccccc2NS(C)(=O)=O)n1)N1CCN(CC1)C1CCNCC1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
The composition of the assay buffer used in the activity measurement was 50 mM Tris-HCl pH 7.5, 100 mM NaCl, 7.5 mM MgCl2, 3 mM KCl, 0.01% Tween 20, ... |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2RJ4NNV |
More data for this
Ligand-Target Pair | |
Sodium/glucose cotransporter 2
(Homo sapiens (Human)) | BDBM159472
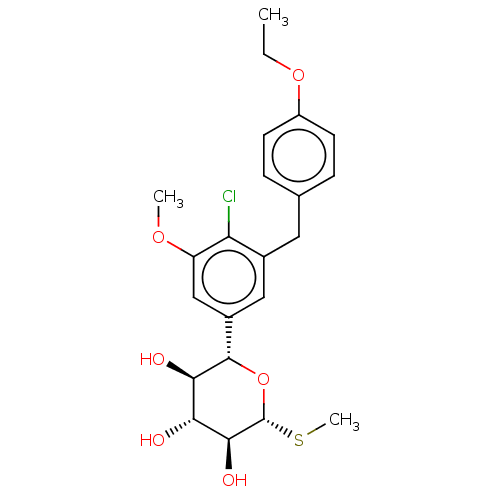
(US9034921, E114)Show SMILES CCOc1ccc(Cc2cc(cc(OC)c2Cl)[C@@H]2O[C@H](SC)[C@@H](O)[C@H](O)[C@H]2O)cc1 |r| Show InChI InChI=1S/C22H27ClO6S/c1-4-28-15-7-5-12(6-8-15)9-13-10-14(11-16(27-2)17(13)23)21-19(25)18(24)20(26)22(29-21)30-3/h5-8,10-11,18-22,24-26H,4,9H2,1-3H3/t18-,19-,20+,21+,22-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 0.308 | n/a | n/a | n/a | n/a | 7.4 | 37 |
GREEN CROSS CORPORATION
US Patent
| Assay Description
For sodium-dependent glucose transport assay, cells expressing hSGLT2 were seeded into a 96-well culture plate at a density of 5x104 cells/well in RP... |
US Patent US9034921 (2015)
BindingDB Entry DOI: 10.7270/Q2QF8RMT |
More data for this
Ligand-Target Pair | |
Sodium/glucose cotransporter 2
(Homo sapiens (Human)) | BDBM159528
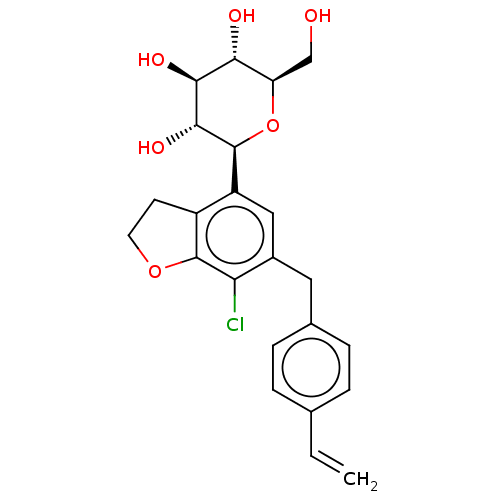
(US9034921, E171 | US9340521, E028)Show SMILES OC[C@H]1O[C@H]([C@H](O)[C@@H](O)[C@@H]1O)c1cc(Cc2ccc(C=C)cc2)c(Cl)c2OCCc12 |r| Show InChI InChI=1S/C23H25ClO6/c1-2-12-3-5-13(6-4-12)9-14-10-16(15-7-8-29-22(15)18(14)24)23-21(28)20(27)19(26)17(11-25)30-23/h2-6,10,17,19-21,23,25-28H,1,7-9,11H2/t17-,19-,20+,21-,23+/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 0.366 | n/a | n/a | n/a | n/a | 7.4 | 37 |
GREEN CROSS CORPORATION
US Patent
| Assay Description
For sodium-dependent glucose transport assay, cells expressing hSGLT2 were seeded into a 96-well culture plate at a density of 5x104 cells/well in RP... |
US Patent US9034921 (2015)
BindingDB Entry DOI: 10.7270/Q2QF8RMT |
More data for this
Ligand-Target Pair | |
Sodium/glucose cotransporter 2
(Homo sapiens (Human)) | BDBM159516

(US9034921, E158 | US9340521, E024)Show SMILES OC[C@H]1O[C@H]([C@H](O)[C@@H](O)[C@@H]1O)c1cc(Cc2ccc(cc2)C2CC2)c(Cl)c2OCOc12 |r| Show InChI InChI=1S/C23H25ClO7/c24-17-14(7-11-1-3-12(4-2-11)13-5-6-13)8-15(22-23(17)30-10-29-22)21-20(28)19(27)18(26)16(9-25)31-21/h1-4,8,13,16,18-21,25-28H,5-7,9-10H2/t16-,18-,19+,20-,21+/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 0.366 | n/a | n/a | n/a | n/a | 7.4 | 37 |
GREEN CROSS CORPORATION
US Patent
| Assay Description
For sodium-dependent glucose transport assay, cells expressing hSGLT2 were seeded into a 96-well culture plate at a density of 5x104 cells/well in RP... |
US Patent US9034921 (2015)
BindingDB Entry DOI: 10.7270/Q2QF8RMT |
More data for this
Ligand-Target Pair | |
Sodium/glucose cotransporter 2
(Homo sapiens (Human)) | BDBM159528

(US9034921, E171 | US9340521, E028)Show SMILES OC[C@H]1O[C@H]([C@H](O)[C@@H](O)[C@@H]1O)c1cc(Cc2ccc(C=C)cc2)c(Cl)c2OCCc12 |r| Show InChI InChI=1S/C23H25ClO6/c1-2-12-3-5-13(6-4-12)9-14-10-16(15-7-8-29-22(15)18(14)24)23-21(28)20(27)19(26)17(11-25)30-23/h2-6,10,17,19-21,23,25-28H,1,7-9,11H2/t17-,19-,20+,21-,23+/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.370 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.bmcl.2021.128466
BindingDB Entry DOI: 10.7270/Q24J0K5N |
More data for this
Ligand-Target Pair | |
Sodium/glucose cotransporter 2
(Homo sapiens (Human)) | BDBM159528

(US9034921, E171 | US9340521, E028)Show SMILES OC[C@H]1O[C@H]([C@H](O)[C@@H](O)[C@@H]1O)c1cc(Cc2ccc(C=C)cc2)c(Cl)c2OCCc12 |r| Show InChI InChI=1S/C23H25ClO6/c1-2-12-3-5-13(6-4-12)9-14-10-16(15-7-8-29-22(15)18(14)24)23-21(28)20(27)19(26)17(11-25)30-23/h2-6,10,17,19-21,23,25-28H,1,7-9,11H2/t17-,19-,20+,21-,23+/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 0.370 | n/a | n/a | n/a | n/a | 7.4 | 37 |
GREEN CROSS CORPORATION
US Patent
| Assay Description
For sodium-dependent glucose transport assay, cells expressing hSGLT2 were seeded into a 96-well culture plate at a density of 5×104 cells/well... |
US Patent US9340521 (2016)
BindingDB Entry DOI: 10.7270/Q2TQ60CH |
More data for this
Ligand-Target Pair | |
Sodium/glucose cotransporter 2
(Homo sapiens (Human)) | BDBM159516

(US9034921, E158 | US9340521, E024)Show SMILES OC[C@H]1O[C@H]([C@H](O)[C@@H](O)[C@@H]1O)c1cc(Cc2ccc(cc2)C2CC2)c(Cl)c2OCOc12 |r| Show InChI InChI=1S/C23H25ClO7/c24-17-14(7-11-1-3-12(4-2-11)13-5-6-13)8-15(22-23(17)30-10-29-22)21-20(28)19(27)18(26)16(9-25)31-21/h1-4,8,13,16,18-21,25-28H,5-7,9-10H2/t16-,18-,19+,20-,21+/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 0.370 | n/a | n/a | n/a | n/a | 7.4 | 37 |
GREEN CROSS CORPORATION
US Patent
| Assay Description
For sodium-dependent glucose transport assay, cells expressing hSGLT2 were seeded into a 96-well culture plate at a density of 5×104 cells/well... |
US Patent US9340521 (2016)
BindingDB Entry DOI: 10.7270/Q2TQ60CH |
More data for this
Ligand-Target Pair | |
Sodium/glucose cotransporter 2
(Homo sapiens (Human)) | BDBM159520
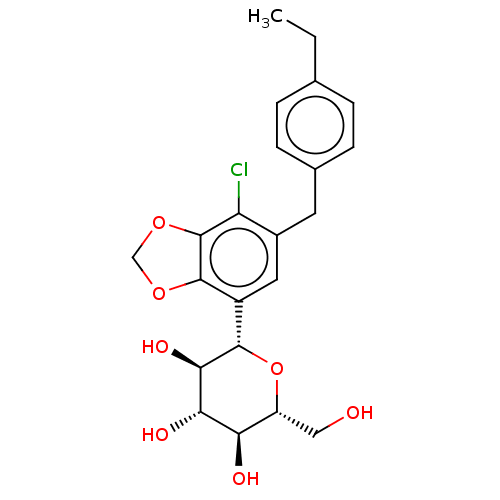
(US9034921, E162 | US9340521, E026)Show SMILES CCc1ccc(Cc2cc([C@@H]3O[C@H](CO)[C@@H](O)[C@H](O)[C@H]3O)c3OCOc3c2Cl)cc1 |r| Show InChI InChI=1S/C22H25ClO7/c1-2-11-3-5-12(6-4-11)7-13-8-14(21-22(16(13)23)29-10-28-21)20-19(27)18(26)17(25)15(9-24)30-20/h3-6,8,15,17-20,24-27H,2,7,9-10H2,1H3/t15-,17-,18+,19-,20+/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | 7.4 | 37 |
GREEN CROSS CORPORATION
US Patent
| Assay Description
For sodium-dependent glucose transport assay, cells expressing hSGLT2 were seeded into a 96-well culture plate at a density of 5×104 cells/well... |
US Patent US9340521 (2016)
BindingDB Entry DOI: 10.7270/Q2TQ60CH |
More data for this
Ligand-Target Pair | |
Epidermal growth factor receptor [1-18,20-1210]
(Homo sapiens (Human)) | BDBM538120
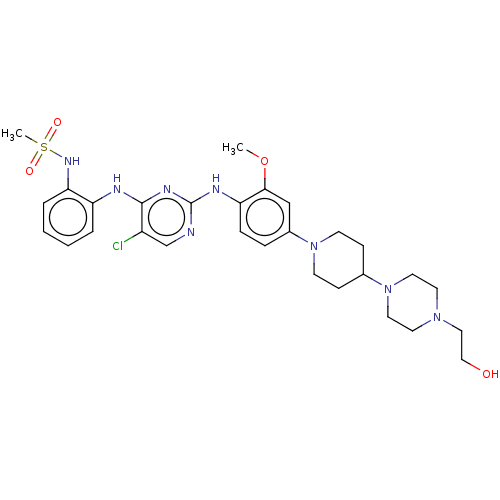
(US11253516, Example 22)Show SMILES COc1cc(ccc1Nc1ncc(Cl)c(Nc2ccccc2NS(C)(=O)=O)n1)N1CCC(CC1)N1CCN(CCO)CC1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
The composition of the assay buffer used in the activity measurement was 50 mM Tris-HCl pH 7.5, 100 mM NaCl, 7.5 mM MgCl2, 3 mM KCl, 0.01% Tween 20, ... |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2RJ4NNV |
More data for this
Ligand-Target Pair | |
Epidermal growth factor receptor [1-18,20-1210,T790M,C797S]
(Homo sapiens (Human)) | BDBM538138

(US11253516, Example 40)Show SMILES CNC1CCN(CC1)c1ccc(Nc2ncc(Cl)c(Nc3ccccc3NS(C)(=O)=O)n2)c(OC)c1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
The composition of the assay buffer used in the activity measurement was 50 mM Tris-HCl pH 7.5, 100 mM NaCl, 7.5 mM MgCl2, 3 mM KCl, 0.01% Tween 20, ... |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2RJ4NNV |
More data for this
Ligand-Target Pair | |
Sodium/glucose cotransporter 2
(Homo sapiens (Human)) | BDBM159520

(US9034921, E162 | US9340521, E026)Show SMILES CCc1ccc(Cc2cc([C@@H]3O[C@H](CO)[C@@H](O)[C@H](O)[C@H]3O)c3OCOc3c2Cl)cc1 |r| Show InChI InChI=1S/C22H25ClO7/c1-2-11-3-5-12(6-4-11)7-13-8-14(21-22(16(13)23)29-10-28-21)20-19(27)18(26)17(25)15(9-24)30-20/h3-6,8,15,17-20,24-27H,2,7,9-10H2,1H3/t15-,17-,18+,19-,20+/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 0.404 | n/a | n/a | n/a | n/a | 7.4 | 37 |
GREEN CROSS CORPORATION
US Patent
| Assay Description
For sodium-dependent glucose transport assay, cells expressing hSGLT2 were seeded into a 96-well culture plate at a density of 5x104 cells/well in RP... |
US Patent US9034921 (2015)
BindingDB Entry DOI: 10.7270/Q2QF8RMT |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data