

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Serine protease 1 (Bos taurus (bovine)) | BDBM50076227 ((1S,7S)-7-Amino-7-benzyl-hexahydro-pyrazolo[1,2-a]...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0270 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Molecumetics Ltd. Curated by ChEMBL | Assay Description Inhibitory concentration of the compound against trypsin. | J Med Chem 42: 1367-75 (1999) Article DOI: 10.1021/jm980354p BindingDB Entry DOI: 10.7270/Q20Z72G1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50076227 ((1S,7S)-7-Amino-7-benzyl-hexahydro-pyrazolo[1,2-a]...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0710 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Molecumetics Ltd. Curated by ChEMBL | Assay Description Inhibitory concentration of the compound against thrombin. | J Med Chem 42: 1367-75 (1999) Article DOI: 10.1021/jm980354p BindingDB Entry DOI: 10.7270/Q20Z72G1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50076224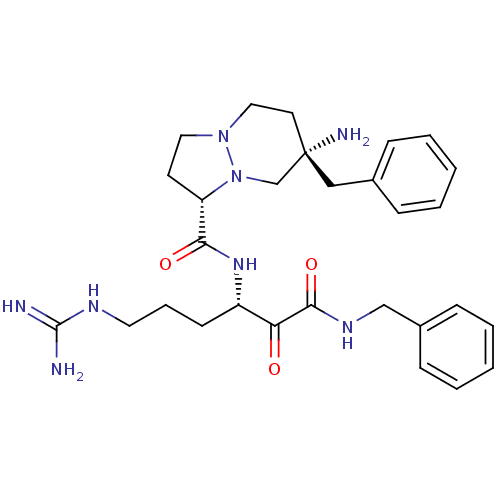 ((1S,7S)-7-Amino-7-benzyl-hexahydro-pyrazolo[1,2-a]...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0730 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Molecumetics Ltd. Curated by ChEMBL | Assay Description Inhibitory concentration of the compound against thrombin. | J Med Chem 42: 1367-75 (1999) Article DOI: 10.1021/jm980354p BindingDB Entry DOI: 10.7270/Q20Z72G1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine protease 1 (Bos taurus (bovine)) | BDBM50076224 ((1S,7S)-7-Amino-7-benzyl-hexahydro-pyrazolo[1,2-a]...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0730 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Molecumetics Ltd. Curated by ChEMBL | Assay Description Inhibitory concentration of the compound against trypsin. | J Med Chem 42: 1367-75 (1999) Article DOI: 10.1021/jm980354p BindingDB Entry DOI: 10.7270/Q20Z72G1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M2 (Homo sapiens (Human)) | BDBM85817 (NNC 11-1585) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | PubMed | 0.0790 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Melbourne Curated by PDSP Ki Database | J Pharmacol Exp Ther 298: 1260-8 (2001) BindingDB Entry DOI: 10.7270/Q26M35D8 | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M1 (Homo sapiens (Human)) | BDBM85817 (NNC 11-1585) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | PubMed | 0.129 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Melbourne Curated by PDSP Ki Database | J Pharmacol Exp Ther 298: 1260-8 (2001) BindingDB Entry DOI: 10.7270/Q26M35D8 | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine protease 1 (Bos taurus (bovine)) | BDBM50076219 ((3S,6R)-6-Amino-6-benzyl-octahydro-indolizine-3-ca...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.230 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Molecumetics Ltd. Curated by ChEMBL | Assay Description Inhibitory concentration of the compound against trypsin. | J Med Chem 42: 1367-75 (1999) Article DOI: 10.1021/jm980354p BindingDB Entry DOI: 10.7270/Q20Z72G1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A3 (Homo sapiens (Human)) | BDBM50252829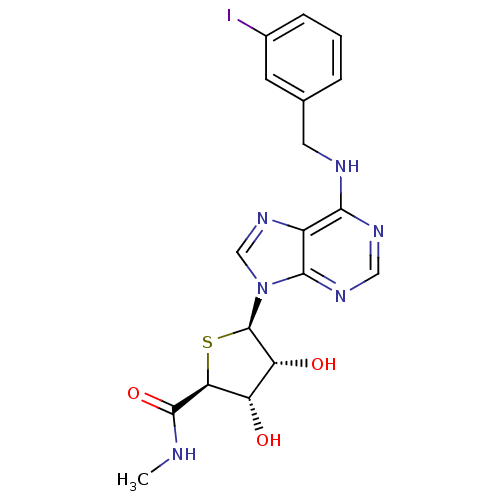 ((2S,3S,4R,5R)-5-(6-(3-iodobenzylamino)-9H-purin-9-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.25 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ewha Womans University Curated by ChEMBL | Assay Description Displacement of [125I]I-AB-MECA from human adenosine A3 receptor expressed in CHO cells | J Med Chem 51: 6609-13 (2008) Article DOI: 10.1021/jm8008647 BindingDB Entry DOI: 10.7270/Q2XG9QZG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A3 (Homo sapiens (Human)) | BDBM50252829 ((2S,3S,4R,5R)-5-(6-(3-iodobenzylamino)-9H-purin-9-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.25 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ewha Womans University Curated by ChEMBL | Assay Description Displacement of [125I]AB-MECA from human adenosine A3 receptor expressed in CHO cells | Bioorg Med Chem 17: 8003-11 (2009) Article DOI: 10.1016/j.bmc.2009.10.011 BindingDB Entry DOI: 10.7270/Q29K4B9Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A3 (Homo sapiens (Human)) | BDBM50180206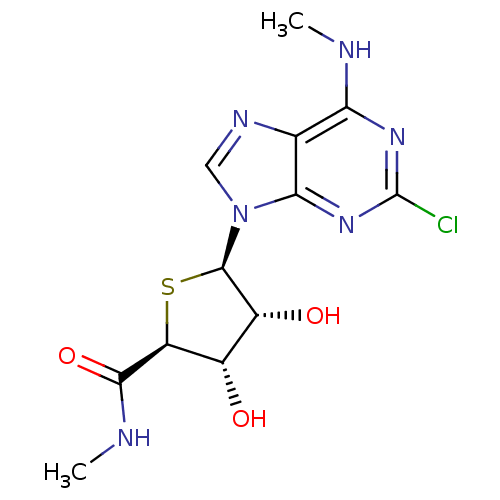 ((2S,3S,4R,5R)-5-(2-chloro-6-(methylamino)-9H-purin...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.280 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ewha Womans University Curated by ChEMBL | Assay Description Binding affinity to human adenosine A3 receptor | Bioorg Med Chem 17: 8003-11 (2009) Article DOI: 10.1016/j.bmc.2009.10.011 BindingDB Entry DOI: 10.7270/Q29K4B9Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A3 (Homo sapiens (Human)) | BDBM50180206 ((2S,3S,4R,5R)-5-(2-chloro-6-(methylamino)-9H-purin...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.280 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ewha Womans University Curated by ChEMBL | Assay Description Displacement of [125I]AB-MECA from human adenosine A3 receptor transfected in CHO cells | J Med Chem 49: 273-81 (2006) Article DOI: 10.1021/jm050595e BindingDB Entry DOI: 10.7270/Q2736QG2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A3 (Homo sapiens (Human)) | BDBM50180206 ((2S,3S,4R,5R)-5-(2-chloro-6-(methylamino)-9H-purin...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.280 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ewha Womans University Curated by ChEMBL | Assay Description Displacement of [125I]AB-MECA from human adenosine A3 receptor expressed in CHO cells | Bioorg Med Chem 17: 8003-11 (2009) Article DOI: 10.1016/j.bmc.2009.10.011 BindingDB Entry DOI: 10.7270/Q29K4B9Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50076223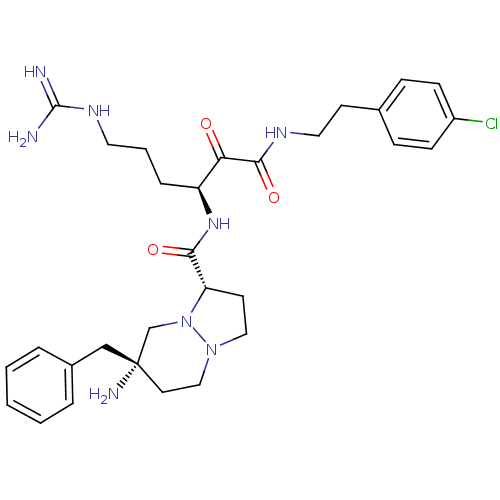 ((1S,7S)-7-Amino-7-benzyl-hexahydro-pyrazolo[1,2-a]...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.310 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Molecumetics Ltd. Curated by ChEMBL | Assay Description Inhibitory concentration of the compound against thrombin. | J Med Chem 42: 1367-75 (1999) Article DOI: 10.1021/jm980354p BindingDB Entry DOI: 10.7270/Q20Z72G1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A3 (Rattus norvegicus) | BDBM21221 ((2S,3S,4R,5R)-5-(2-chloro-6-{[(3-iodophenyl)methyl...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | PubMed | 0.330 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Diabetes Curated by ChEMBL | Assay Description Tested for the binding affinity of A3 receptor by displacing N6-[[125I]-4-amino-3-iodobenzyl]-adenosine-5''-N-methyluronamide from membranes of CHO ... | J Med Chem 37: 3614-21 (1994) BindingDB Entry DOI: 10.7270/Q2348M1V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A3 (Rattus norvegicus) | BDBM50453675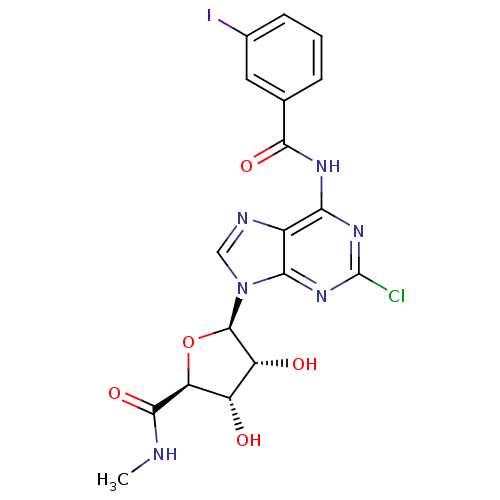 (CHEMBL2113400) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.330 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Diabetes Curated by ChEMBL | Assay Description Binding affinity against adenosine A3 receptor from rat brain. | J Med Chem 38: 1720-35 (1995) BindingDB Entry DOI: 10.7270/Q21Z453H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A3 (Rattus norvegicus) | BDBM21221 ((2S,3S,4R,5R)-5-(2-chloro-6-{[(3-iodophenyl)methyl...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | PubMed | 0.330 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Seoul National University Curated by ChEMBL | Assay Description Binding affinity for rat Adenosine A3 receptor | Bioorg Med Chem Lett 13: 817-20 (2003) BindingDB Entry DOI: 10.7270/Q2M32WB7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A3 (Rattus norvegicus) | BDBM21221 ((2S,3S,4R,5R)-5-(2-chloro-6-{[(3-iodophenyl)methyl...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | 0.330 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ewha Womans University Curated by ChEMBL | Assay Description Displacement of [125I]I-AB-MECA from rat adenosine A3 receptor expressed in CHO cells | J Med Chem 51: 6609-13 (2008) Article DOI: 10.1021/jm8008647 BindingDB Entry DOI: 10.7270/Q2XG9QZG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuronal acetylcholine receptor subunit alpha-4/beta-2 (Rattus norvegicus (Rat)) | BDBM50474902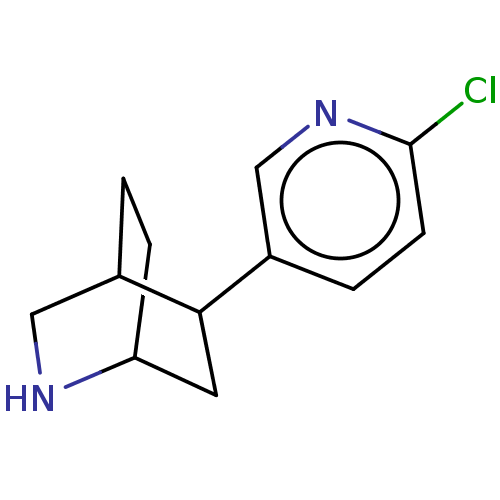 (CHEMBL187309) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.340 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibition of [3H]- cytisine binding to Nicotinic acetylcholine receptor alpha4-beta2 of rat cortical membranes | Bioorg Med Chem Lett 14: 5573-7 (2004) Article DOI: 10.1016/j.bmcl.2004.08.058 BindingDB Entry DOI: 10.7270/Q2XG9TWT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A3 (Homo sapiens (Human)) | BDBM50180197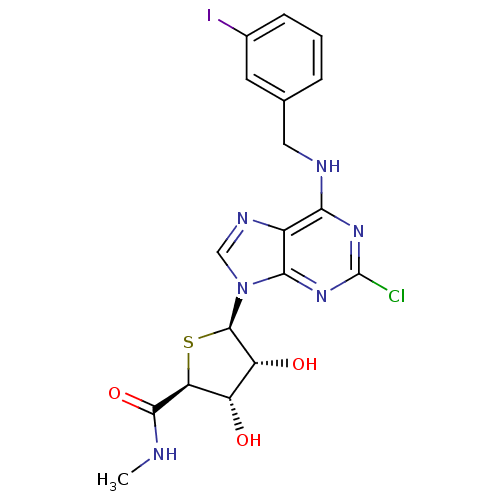 ((2S,3S,4R,5R)-5-(2-chloro-6-(3-iodobenzylamino)-9H...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.380 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ewha Womans University Curated by ChEMBL | Assay Description Displacement of [3H]ABMECA from human adenosine A3 receptor expressed in CHO cells | Bioorg Med Chem Lett 18: 1612-6 (2008) Article DOI: 10.1016/j.bmcl.2008.01.070 BindingDB Entry DOI: 10.7270/Q2RN38QV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A3 (Homo sapiens (Human)) | BDBM50180197 ((2S,3S,4R,5R)-5-(2-chloro-6-(3-iodobenzylamino)-9H...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.380 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ewha Womans University Curated by ChEMBL | Assay Description Displacement of [125I]AB-MECA from human adenosine A3 receptor transfected in CHO cells | J Med Chem 49: 273-81 (2006) Article DOI: 10.1021/jm050595e BindingDB Entry DOI: 10.7270/Q2736QG2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A3 (Homo sapiens (Human)) | BDBM50180197 ((2S,3S,4R,5R)-5-(2-chloro-6-(3-iodobenzylamino)-9H...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.380 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ewha Womans University Curated by ChEMBL | Assay Description Binding affinity to human adenosine A3 receptor | Bioorg Med Chem 17: 8003-11 (2009) Article DOI: 10.1016/j.bmc.2009.10.011 BindingDB Entry DOI: 10.7270/Q29K4B9Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A3 (Homo sapiens (Human)) | BDBM50180197 ((2S,3S,4R,5R)-5-(2-chloro-6-(3-iodobenzylamino)-9H...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.380 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ewha Womans University Curated by ChEMBL | Assay Description Displacement of [125I]I-AB-MECA from human adenosine A3 receptor expressed CHO cells after 60 mins by gamma counting | Bioorg Med Chem 18: 7015-21 (2010) Article DOI: 10.1016/j.bmc.2010.08.018 BindingDB Entry DOI: 10.7270/Q21N81CV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A3 (Homo sapiens (Human)) | BDBM50180197 ((2S,3S,4R,5R)-5-(2-chloro-6-(3-iodobenzylamino)-9H...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.380 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ewha Womans University Curated by ChEMBL | Assay Description Displacement of [125I]AB-MECA from human adenosine A3 receptor expressed in CHO cells | Bioorg Med Chem 17: 8003-11 (2009) Article DOI: 10.1016/j.bmc.2009.10.011 BindingDB Entry DOI: 10.7270/Q29K4B9Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A3 (Homo sapiens (Human)) | BDBM50180197 ((2S,3S,4R,5R)-5-(2-chloro-6-(3-iodobenzylamino)-9H...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.380 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ewha Womans University Curated by ChEMBL | Assay Description Displacement of [3H]I-AB-MECA from human recombinant adenosine A3 receptor expressed in CHO cells by liquid scintillation counting | Bioorg Med Chem 17: 3733-8 (2009) Article DOI: 10.1016/j.bmc.2009.03.034 BindingDB Entry DOI: 10.7270/Q2V69JMJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A3 (Homo sapiens (Human)) | BDBM50180197 ((2S,3S,4R,5R)-5-(2-chloro-6-(3-iodobenzylamino)-9H...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.380 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ewha Womans University Curated by ChEMBL | Assay Description Binding affinity to human adenosine A3 receptor | J Med Chem 55: 342-56 (2012) Article DOI: 10.1021/jm201229j BindingDB Entry DOI: 10.7270/Q2VQ334S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A3 (Homo sapiens (Human)) | BDBM50180197 ((2S,3S,4R,5R)-5-(2-chloro-6-(3-iodobenzylamino)-9H...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.380 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ewha Womans University Curated by ChEMBL | Assay Description Displacement of [125I]I-AB-MECA from human adenosine A3 receptor expressed in CHO cells | J Med Chem 51: 6609-13 (2008) Article DOI: 10.1021/jm8008647 BindingDB Entry DOI: 10.7270/Q2XG9QZG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A3 (Homo sapiens (Human)) | BDBM50180197 ((2S,3S,4R,5R)-5-(2-chloro-6-(3-iodobenzylamino)-9H...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.380 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ewha Womans University Curated by ChEMBL | Assay Description Displacement of [125I]AB-MECA from human adenosine A3 receptor expressed in CHO cells | J Med Chem 50: 3159-62 (2007) Article DOI: 10.1021/jm070259t BindingDB Entry DOI: 10.7270/Q2513XXX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A3 (Homo sapiens (Human)) | BDBM50180219 ((2-chloropurin-9-yl)-3,4-dihydroxytetrahydrothiphe...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ewha Womans University Curated by ChEMBL | Assay Description Displacement of [125I]AB-MECA from human adenosine A3 receptor transfected in CHO cells | J Med Chem 49: 273-81 (2006) Article DOI: 10.1021/jm050595e BindingDB Entry DOI: 10.7270/Q2736QG2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A3 (Homo sapiens (Human)) | BDBM50304635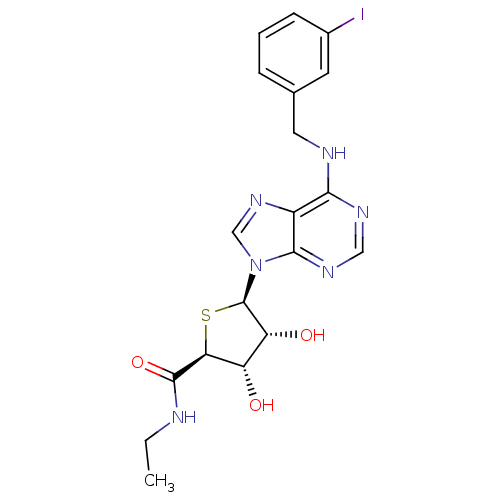 (9-(5'-Ethylaminocarbonyl-4-thio-beta-D-ribofuranos...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.420 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ewha Womans University Curated by ChEMBL | Assay Description Displacement of [125I]AB-MECA from human adenosine A3 receptor expressed in CHO cells | Bioorg Med Chem 17: 8003-11 (2009) Article DOI: 10.1016/j.bmc.2009.10.011 BindingDB Entry DOI: 10.7270/Q29K4B9Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50076225 ((1S,7S)-7-Amino-7-benzyl-hexahydro-pyrazolo[1,2-a]...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.450 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Molecumetics Ltd. Curated by ChEMBL | Assay Description Inhibitory concentration of the compound against thrombin. | J Med Chem 42: 1367-75 (1999) Article DOI: 10.1021/jm980354p BindingDB Entry DOI: 10.7270/Q20Z72G1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A1 (Rattus norvegicus (rat)) | BDBM21173 (1,3-dipropyl-8-cyclopentylxanthine | 8-cyclopentyl...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL KEGG PC cid PC sid UniChem Patents Similars | PubMed | 0.460 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Diabetes Curated by ChEMBL | Assay Description Displacement of [3H]-PIA from adenosine A1 receptors of rat brain membrane | J Med Chem 37: 3373-82 (1994) BindingDB Entry DOI: 10.7270/Q28914WW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A1 (Rattus norvegicus (rat)) | BDBM25400 ((2R,3R,4S,5R)-2-[6-(cyclopentylamino)-9H-purin-9-y...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE MMDB PC cid PC sid PDB UniChem Patents Similars | PubMed | 0.590 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Diabetes Curated by ChEMBL | Assay Description Tested for the binding affinity of A1 receptor by displacing the [3H]-PIA in rat brain membranes | J Med Chem 37: 3614-21 (1994) BindingDB Entry DOI: 10.7270/Q2348M1V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A1 (Rattus norvegicus (rat)) | BDBM50085658 ((2R,3R,4S,5R)-2-(2-Chloro-6-cyclopentylamino-purin...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | PubMed | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Diabetes Curated by ChEMBL | Assay Description Tested for the binding affinity of A1 receptor by displacing the [3H]-PIA in rat brain membranes | J Med Chem 37: 3614-21 (1994) BindingDB Entry DOI: 10.7270/Q2348M1V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine protease 1 (Bos taurus (bovine)) | BDBM50076222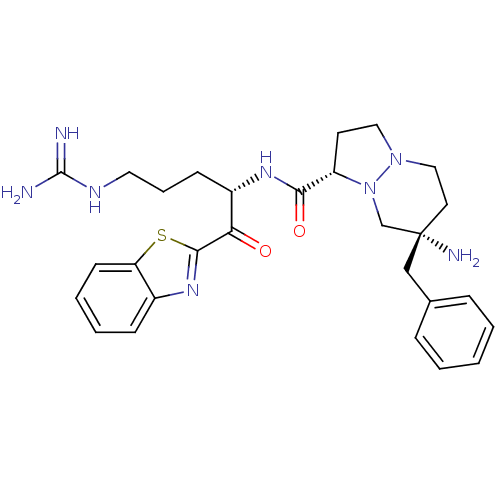 ((1S,7S)-7-Amino-7-benzyl-hexahydro-pyrazolo[1,2-a]...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.640 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Molecumetics Ltd. Curated by ChEMBL | Assay Description Inhibitory concentration of the compound against trypsin. | J Med Chem 42: 1367-75 (1999) Article DOI: 10.1021/jm980354p BindingDB Entry DOI: 10.7270/Q20Z72G1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50076222 ((1S,7S)-7-Amino-7-benzyl-hexahydro-pyrazolo[1,2-a]...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.650 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Molecumetics Ltd. Curated by ChEMBL | Assay Description Inhibitory concentration of the compound against thrombin. | J Med Chem 42: 1367-75 (1999) Article DOI: 10.1021/jm980354p BindingDB Entry DOI: 10.7270/Q20Z72G1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A3 (Homo sapiens (Human)) | BDBM50180204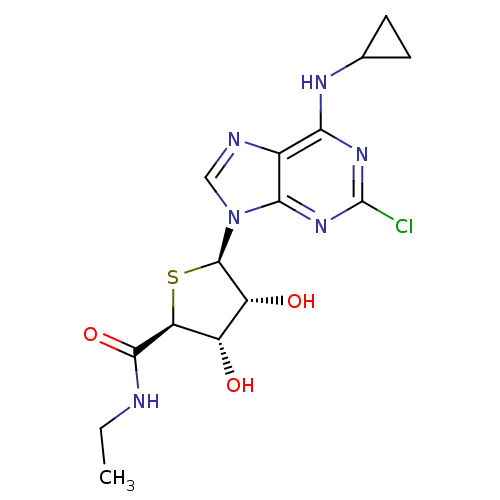 ((2S,3S,4R,5R)-5-(2-chloro-6-(cyclopropylamino)-9H-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.670 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ewha Womans University Curated by ChEMBL | Assay Description Displacement of [125I]AB-MECA from human adenosine A3 receptor transfected in CHO cells | J Med Chem 49: 273-81 (2006) Article DOI: 10.1021/jm050595e BindingDB Entry DOI: 10.7270/Q2736QG2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2Y purinoceptor 1 (Homo sapiens (Human)) | BDBM50373326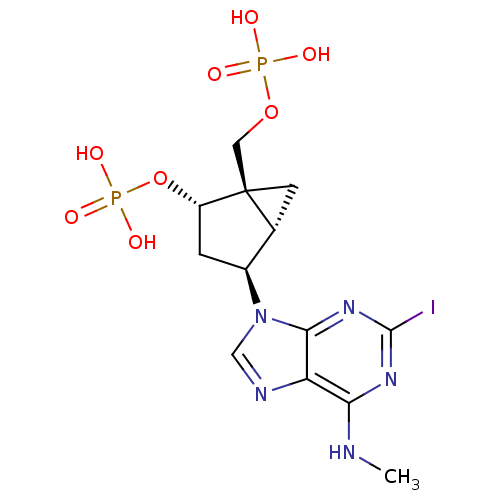 (CHEMBL444278) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid PDB UniChem Similars | PDB Article PubMed | 0.780 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
NIDDK Curated by ChEMBL | Assay Description Inhibition of [3H]-5 binding to P2Y purinoceptor 1 expressed in Sf9 cells | J Med Chem 46: 4974-87 (2003) Article DOI: 10.1021/jm030127+ BindingDB Entry DOI: 10.7270/Q2NS0T9D | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Adenosine receptor A3 (Rattus norvegicus) | BDBM50180197 ((2S,3S,4R,5R)-5-(2-chloro-6-(3-iodobenzylamino)-9H...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.820 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ewha Womans University Curated by ChEMBL | Assay Description Displacement of [125I]I-AB-MECA from rat adenosine A3 receptor expressed in CHO cells | J Med Chem 51: 6609-13 (2008) Article DOI: 10.1021/jm8008647 BindingDB Entry DOI: 10.7270/Q2XG9QZG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50076219 ((3S,6R)-6-Amino-6-benzyl-octahydro-indolizine-3-ca...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.850 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Molecumetics Ltd. Curated by ChEMBL | Assay Description Inhibitory concentration of the compound against thrombin. | J Med Chem 42: 1367-75 (1999) Article DOI: 10.1021/jm980354p BindingDB Entry DOI: 10.7270/Q20Z72G1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50076219 ((3S,6R)-6-Amino-6-benzyl-octahydro-indolizine-3-ca...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.850 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Molecumetics Ltd. Curated by ChEMBL | Assay Description Inhibitory concentration of the compound against thrombin. | J Med Chem 42: 1367-75 (1999) Article DOI: 10.1021/jm980354p BindingDB Entry DOI: 10.7270/Q20Z72G1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A3 (Homo sapiens (Human)) | BDBM50180209 ((2S,3S,4R,5R)-5-(6-(3-iodobenzylamino)-2-chloro-9H...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.890 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ewha Womans University Curated by ChEMBL | Assay Description Displacement of [125I]AB-MECA from human adenosine A3 receptor transfected in CHO cells | J Med Chem 49: 273-81 (2006) Article DOI: 10.1021/jm050595e BindingDB Entry DOI: 10.7270/Q2736QG2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A3 (Homo sapiens (Human)) | BDBM50304631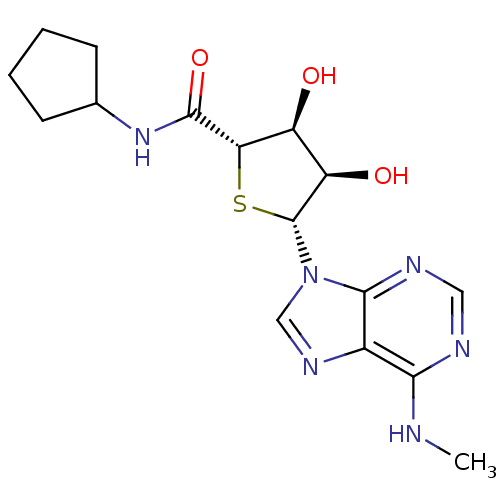 (9-(5'-Cyclopentylaminocarbonyl-4'-thio-beta-D-ribo...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.970 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ewha Womans University Curated by ChEMBL | Assay Description Displacement of [125I]AB-MECA from human adenosine A3 receptor expressed in CHO cells | Bioorg Med Chem 17: 8003-11 (2009) Article DOI: 10.1016/j.bmc.2009.10.011 BindingDB Entry DOI: 10.7270/Q29K4B9Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A3 (Homo sapiens (Human)) | BDBM50304634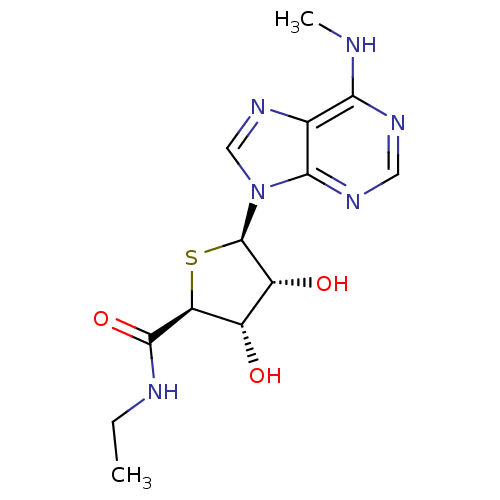 (9-(5'-Ethylaminocarbonyl-4'-thio-beta-D-ribofurano...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.970 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ewha Womans University Curated by ChEMBL | Assay Description Displacement of [125I]AB-MECA from human adenosine A3 receptor expressed in CHO cells | Bioorg Med Chem 17: 8003-11 (2009) Article DOI: 10.1016/j.bmc.2009.10.011 BindingDB Entry DOI: 10.7270/Q29K4B9Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A3 (Homo sapiens (Human)) | BDBM21221 ((2S,3S,4R,5R)-5-(2-chloro-6-{[(3-iodophenyl)methyl...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ewha Womans University Curated by ChEMBL | Assay Description Binding affinity to human adenosine A3 receptor | Bioorg Med Chem 17: 8003-11 (2009) Article DOI: 10.1016/j.bmc.2009.10.011 BindingDB Entry DOI: 10.7270/Q29K4B9Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A3 (Homo sapiens (Human)) | BDBM50293031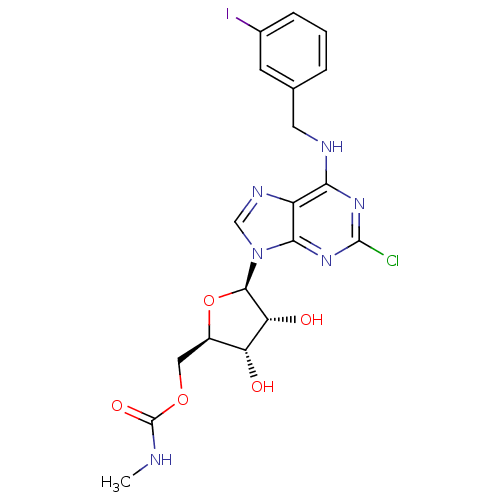 (2-chloro-N6-(3-iodobenzyl)-5'-N-methylcarbamoylade...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ewha Womans University Curated by ChEMBL | Assay Description Binding affinity to human adenosine A3 receptor | J Med Chem 55: 342-56 (2012) Article DOI: 10.1021/jm201229j BindingDB Entry DOI: 10.7270/Q2VQ334S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A3 (Homo sapiens (Human)) | BDBM50118812 ((2S,3S,4R,5R)-3,4-Dihydroxy-5-[6-(3-iodo-benzylami...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ewha Womans University Curated by ChEMBL | Assay Description Displacement of [125I]AB-MECA from human adenosine A3 receptor expressed in CHO cells | Bioorg Med Chem 17: 8003-11 (2009) Article DOI: 10.1016/j.bmc.2009.10.011 BindingDB Entry DOI: 10.7270/Q29K4B9Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A3 (Homo sapiens (Human)) | BDBM50118812 ((2S,3S,4R,5R)-3,4-Dihydroxy-5-[6-(3-iodo-benzylami...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ewha Womans University Curated by ChEMBL | Assay Description Displacement of [125I]I-AB-MECA from human adenosine A3 receptor expressed in CHO cells | J Med Chem 51: 6609-13 (2008) Article DOI: 10.1021/jm8008647 BindingDB Entry DOI: 10.7270/Q2XG9QZG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A3 (Homo sapiens (Human)) | BDBM50304630 (9-(5'-Cyclobutylaminocarbonyl-4'-thio-beta-D-ribof...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.04 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ewha Womans University Curated by ChEMBL | Assay Description Displacement of [125I]AB-MECA from human adenosine A3 receptor expressed in CHO cells | Bioorg Med Chem 17: 8003-11 (2009) Article DOI: 10.1016/j.bmc.2009.10.011 BindingDB Entry DOI: 10.7270/Q29K4B9Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A3 (Rattus norvegicus) | BDBM50118812 ((2S,3S,4R,5R)-3,4-Dihydroxy-5-[6-(3-iodo-benzylami...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ewha Womans University Curated by ChEMBL | Assay Description Displacement of [125I]I-AB-MECA from rat adenosine A3 receptor expressed in CHO cells | J Med Chem 51: 6609-13 (2008) Article DOI: 10.1021/jm8008647 BindingDB Entry DOI: 10.7270/Q2XG9QZG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A3 (Rattus norvegicus) | BDBM50118812 ((2S,3S,4R,5R)-3,4-Dihydroxy-5-[6-(3-iodo-benzylami...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE PC cid PC sid PDB UniChem Patents Similars | PubMed | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Diabetes Curated by ChEMBL | Assay Description Binding affinity against adenosine A3 receptor from rat brain. | J Med Chem 38: 1720-35 (1995) BindingDB Entry DOI: 10.7270/Q21Z453H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 1220 total ) | Next | Last >> |