

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Estrogen receptor (Homo sapiens (Human)) | BDBM50238727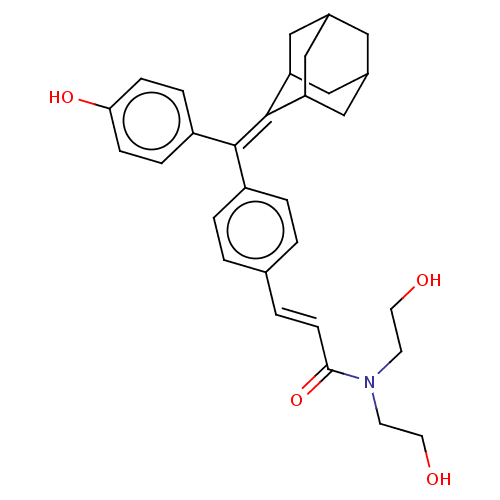 (CHEMBL4065838) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.130 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute Curated by ChEMBL | Assay Description Displacement of [3H]estradiol from full-length human ERalpha receptor by scintillation counting | J Med Chem 60: 6321-6336 (2017) Article DOI: 10.1021/acs.jmedchem.7b00585 BindingDB Entry DOI: 10.7270/Q2RB76V7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen receptor (Homo sapiens (Human)) | BDBM50238724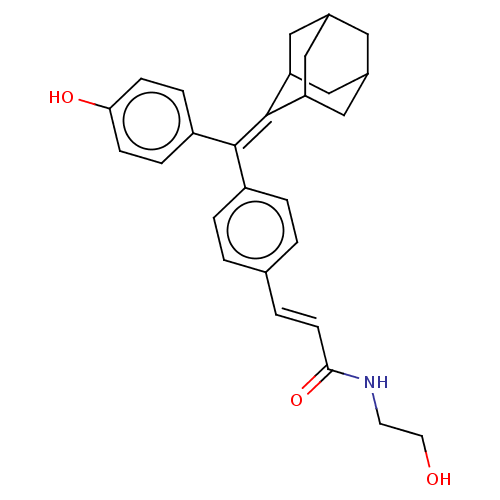 (CHEMBL4060067) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Patents Similars | Article PubMed | 0.180 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute Curated by ChEMBL | Assay Description Displacement of [3H]estradiol from full-length human ERalpha receptor by scintillation counting | J Med Chem 60: 6321-6336 (2017) Article DOI: 10.1021/acs.jmedchem.7b00585 BindingDB Entry DOI: 10.7270/Q2RB76V7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen receptor (Homo sapiens (Human)) | BDBM50238716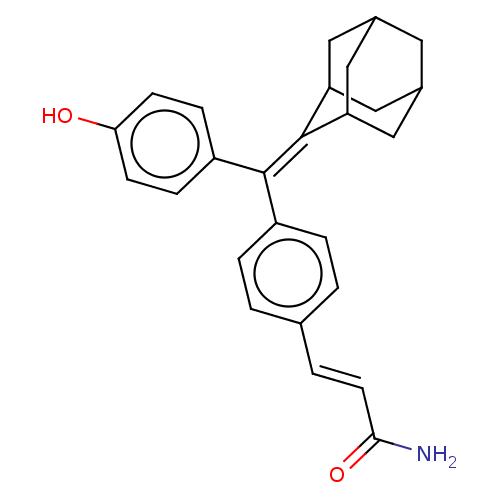 (CHEMBL4079530) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.270 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute Curated by ChEMBL | Assay Description Displacement of [3H]estradiol from full-length human ERalpha receptor by scintillation counting | J Med Chem 60: 6321-6336 (2017) Article DOI: 10.1021/acs.jmedchem.7b00585 BindingDB Entry DOI: 10.7270/Q2RB76V7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen receptor (Homo sapiens (Human)) | BDBM50238726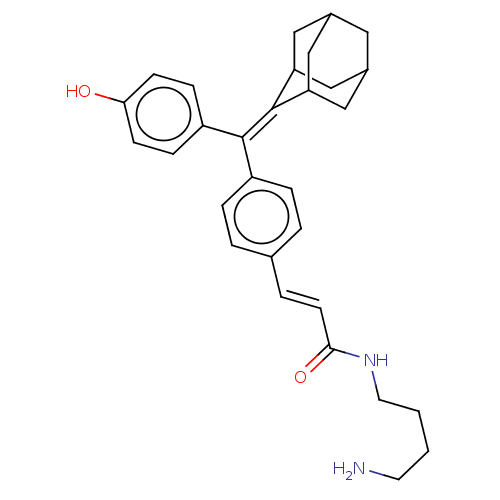 (CHEMBL4083780) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.320 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute Curated by ChEMBL | Assay Description Displacement of [3H]estradiol from full-length human ERalpha receptor by scintillation counting | J Med Chem 60: 6321-6336 (2017) Article DOI: 10.1021/acs.jmedchem.7b00585 BindingDB Entry DOI: 10.7270/Q2RB76V7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen receptor (Homo sapiens (Human)) | BDBM50238743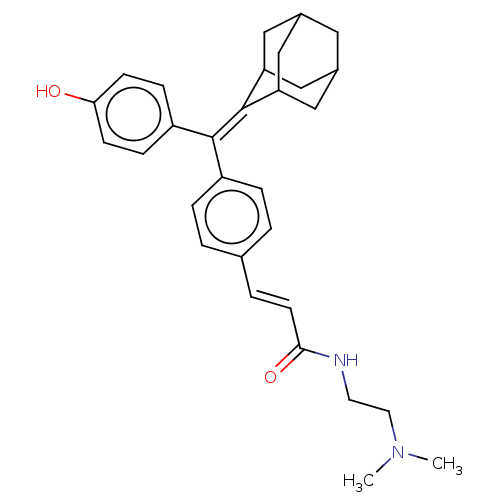 (CHEMBL4070669) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.350 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute Curated by ChEMBL | Assay Description Displacement of [3H]estradiol from full-length human ERalpha receptor by scintillation counting | J Med Chem 60: 6321-6336 (2017) Article DOI: 10.1021/acs.jmedchem.7b00585 BindingDB Entry DOI: 10.7270/Q2RB76V7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen receptor (Homo sapiens (Human)) | BDBM50238720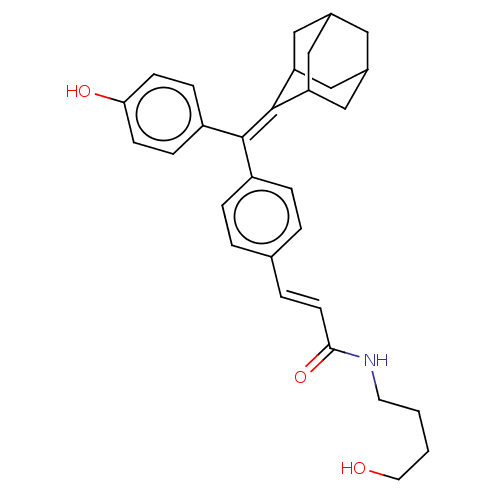 (CHEMBL4102857) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.360 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute Curated by ChEMBL | Assay Description Displacement of [3H]estradiol from full-length human ERalpha receptor by scintillation counting | J Med Chem 60: 6321-6336 (2017) Article DOI: 10.1021/acs.jmedchem.7b00585 BindingDB Entry DOI: 10.7270/Q2RB76V7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen receptor (Homo sapiens (Human)) | BDBM50238742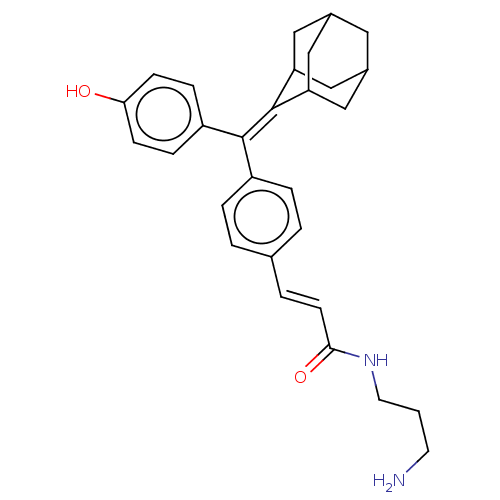 (CHEMBL4062045) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.370 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute Curated by ChEMBL | Assay Description Displacement of [3H]estradiol from full-length human ERalpha receptor by scintillation counting | J Med Chem 60: 6321-6336 (2017) Article DOI: 10.1021/acs.jmedchem.7b00585 BindingDB Entry DOI: 10.7270/Q2RB76V7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen receptor (Homo sapiens (Human)) | BDBM50238733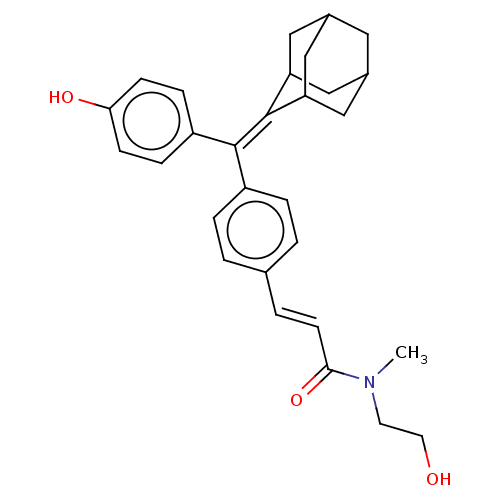 (CHEMBL4099302) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.370 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute Curated by ChEMBL | Assay Description Displacement of [3H]estradiol from full-length human ERalpha receptor by scintillation counting | J Med Chem 60: 6321-6336 (2017) Article DOI: 10.1021/acs.jmedchem.7b00585 BindingDB Entry DOI: 10.7270/Q2RB76V7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen receptor (Homo sapiens (Human)) | BDBM50238739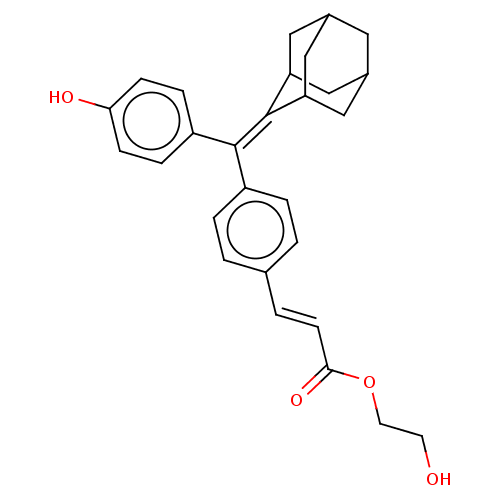 (CHEMBL4079644) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.370 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute Curated by ChEMBL | Assay Description Inhibition constant was evaluated against Histamine N-methyl-transferase | J Med Chem 60: 6321-6336 (2017) Article DOI: 10.1021/acs.jmedchem.7b00585 BindingDB Entry DOI: 10.7270/Q2RB76V7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen receptor (Homo sapiens (Human)) | BDBM50238717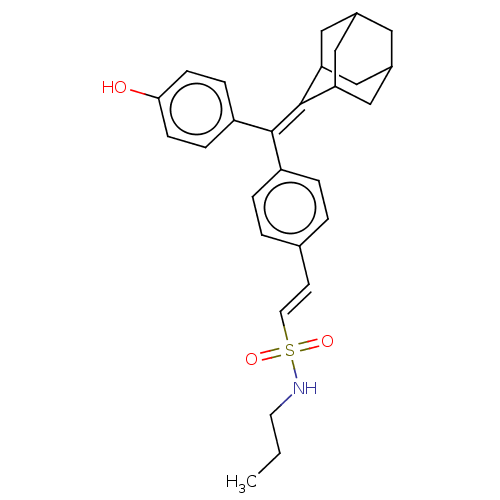 (CHEMBL4097562) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.380 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute Curated by ChEMBL | Assay Description Displacement of [3H]estradiol from full-length human ERalpha receptor by scintillation counting | J Med Chem 60: 6321-6336 (2017) Article DOI: 10.1021/acs.jmedchem.7b00585 BindingDB Entry DOI: 10.7270/Q2RB76V7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen receptor (Homo sapiens (Human)) | BDBM50238737 (CHEMBL4083658) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute Curated by ChEMBL | Assay Description Displacement of [3H]estradiol from full-length human ERalpha receptor by scintillation counting | J Med Chem 60: 6321-6336 (2017) Article DOI: 10.1021/acs.jmedchem.7b00585 BindingDB Entry DOI: 10.7270/Q2RB76V7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen receptor (Homo sapiens (Human)) | BDBM50238740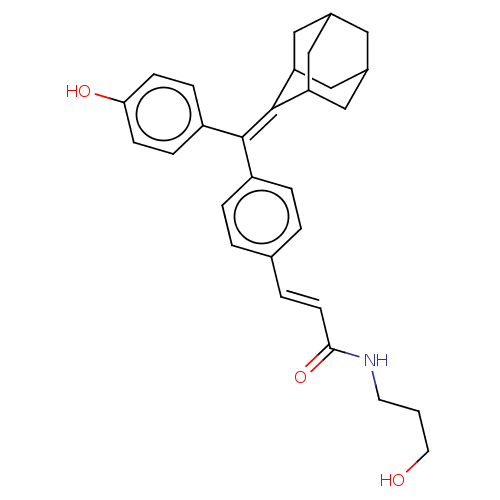 (CHEMBL4078577) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.430 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute Curated by ChEMBL | Assay Description Displacement of [3H]estradiol from full-length human ERalpha receptor by scintillation counting | J Med Chem 60: 6321-6336 (2017) Article DOI: 10.1021/acs.jmedchem.7b00585 BindingDB Entry DOI: 10.7270/Q2RB76V7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen receptor (Homo sapiens (Human)) | BDBM50238738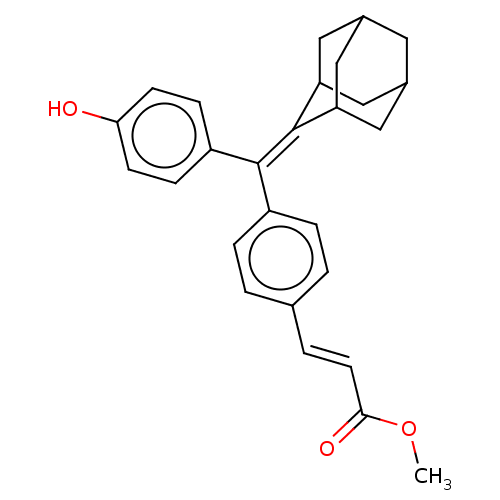 (CHEMBL4060817) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.430 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute Curated by ChEMBL | Assay Description Displacement of [3H]estradiol from full-length human ERalpha receptor by scintillation counting | J Med Chem 60: 6321-6336 (2017) Article DOI: 10.1021/acs.jmedchem.7b00585 BindingDB Entry DOI: 10.7270/Q2RB76V7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen receptor (Homo sapiens (Human)) | BDBM50238728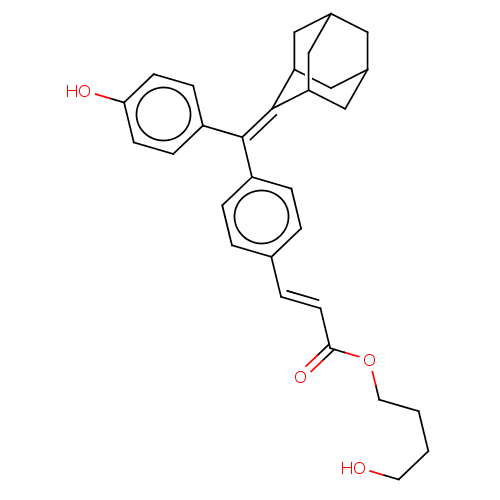 (CHEMBL4089824) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.450 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute Curated by ChEMBL | Assay Description Displacement of [3H]estradiol from full-length human ERalpha receptor by scintillation counting | J Med Chem 60: 6321-6336 (2017) Article DOI: 10.1021/acs.jmedchem.7b00585 BindingDB Entry DOI: 10.7270/Q2RB76V7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen receptor (Homo sapiens (Human)) | BDBM50238719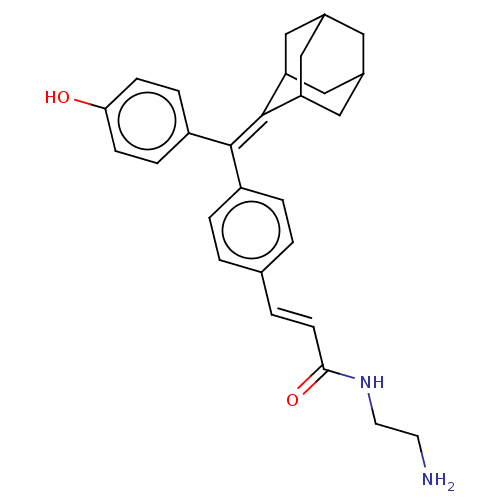 (CHEMBL4073395) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.570 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute Curated by ChEMBL | Assay Description Displacement of [3H]estradiol from full-length human ERalpha receptor by scintillation counting | J Med Chem 60: 6321-6336 (2017) Article DOI: 10.1021/acs.jmedchem.7b00585 BindingDB Entry DOI: 10.7270/Q2RB76V7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen receptor (Homo sapiens (Human)) | BDBM50238735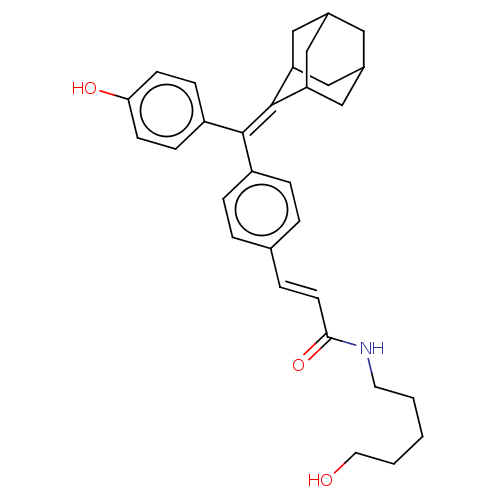 (CHEMBL4100247) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.650 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute Curated by ChEMBL | Assay Description Displacement of [3H]estradiol from full-length human ERalpha receptor by scintillation counting | J Med Chem 60: 6321-6336 (2017) Article DOI: 10.1021/acs.jmedchem.7b00585 BindingDB Entry DOI: 10.7270/Q2RB76V7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen receptor (Homo sapiens (Human)) | BDBM50238715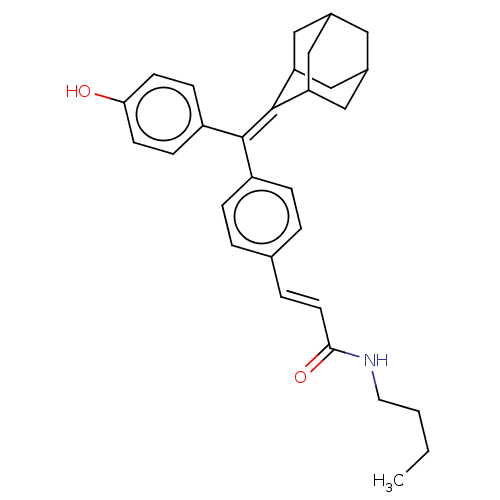 (CHEMBL4098470) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.710 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute Curated by ChEMBL | Assay Description Displacement of [3H]estradiol from full-length human ERalpha receptor by scintillation counting | J Med Chem 60: 6321-6336 (2017) Article DOI: 10.1021/acs.jmedchem.7b00585 BindingDB Entry DOI: 10.7270/Q2RB76V7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen receptor (Homo sapiens (Human)) | BDBM50238729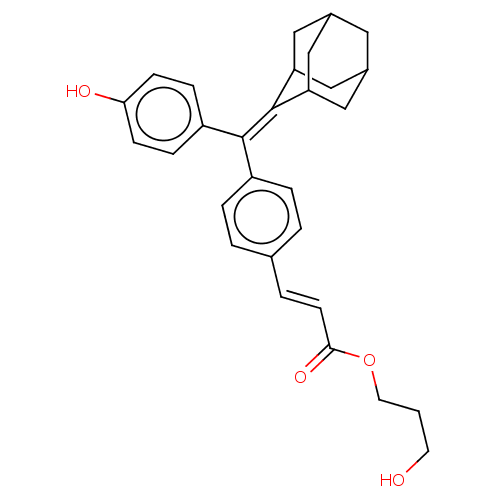 (CHEMBL4098232) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.710 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute Curated by ChEMBL | Assay Description Displacement of [3H]estradiol from full-length human ERalpha receptor by scintillation counting | J Med Chem 60: 6321-6336 (2017) Article DOI: 10.1021/acs.jmedchem.7b00585 BindingDB Entry DOI: 10.7270/Q2RB76V7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen receptor (Homo sapiens (Human)) | BDBM50238725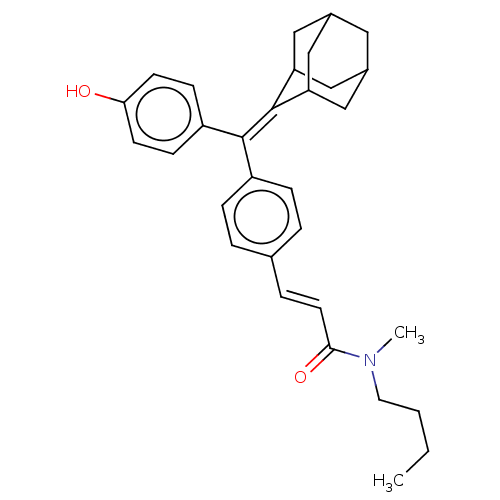 (CHEMBL4081283) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.710 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute Curated by ChEMBL | Assay Description Displacement of [3H]estradiol from full-length human ERalpha receptor by scintillation counting | J Med Chem 60: 6321-6336 (2017) Article DOI: 10.1021/acs.jmedchem.7b00585 BindingDB Entry DOI: 10.7270/Q2RB76V7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen receptor (Homo sapiens (Human)) | BDBM50238714 (CHEMBL4081969) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.740 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute Curated by ChEMBL | Assay Description Displacement of [3H]estradiol from full-length human ERalpha receptor by scintillation counting | J Med Chem 60: 6321-6336 (2017) Article DOI: 10.1021/acs.jmedchem.7b00585 BindingDB Entry DOI: 10.7270/Q2RB76V7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen receptor (Homo sapiens (Human)) | BDBM50238736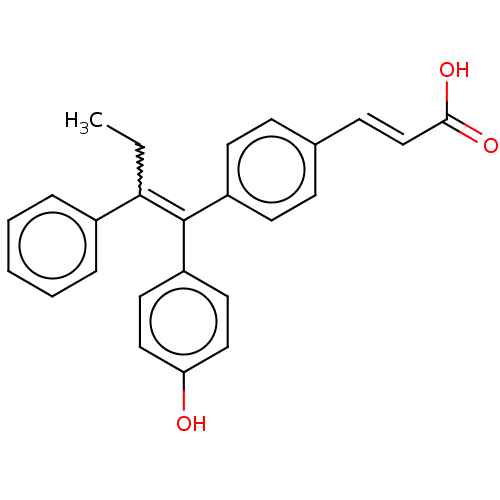 (CHEMBL1091535 | GW7604) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute Curated by ChEMBL | Assay Description Displacement of [3H]estradiol from full-length human ERalpha receptor by scintillation counting | J Med Chem 60: 6321-6336 (2017) Article DOI: 10.1021/acs.jmedchem.7b00585 BindingDB Entry DOI: 10.7270/Q2RB76V7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen receptor (Homo sapiens (Human)) | BDBM50238721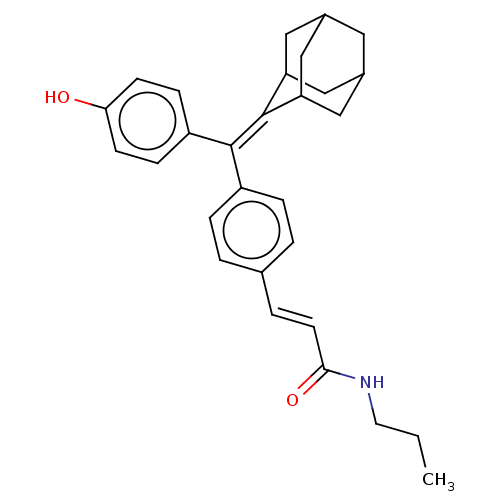 (CHEMBL4071436) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute Curated by ChEMBL | Assay Description Displacement of [3H]estradiol from full-length human ERalpha receptor by scintillation counting | J Med Chem 60: 6321-6336 (2017) Article DOI: 10.1021/acs.jmedchem.7b00585 BindingDB Entry DOI: 10.7270/Q2RB76V7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen receptor (Homo sapiens (Human)) | BDBM50238731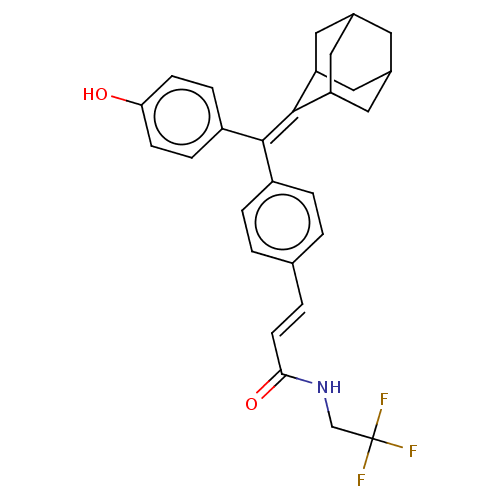 (CHEMBL4091563) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute Curated by ChEMBL | Assay Description Displacement of [3H]estradiol from full-length human ERalpha receptor by scintillation counting | J Med Chem 60: 6321-6336 (2017) Article DOI: 10.1021/acs.jmedchem.7b00585 BindingDB Entry DOI: 10.7270/Q2RB76V7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen receptor (Homo sapiens (Human)) | BDBM50238722 (CHEMBL4068722) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute Curated by ChEMBL | Assay Description Displacement of [3H]estradiol from full-length human ERalpha receptor by scintillation counting | J Med Chem 60: 6321-6336 (2017) Article DOI: 10.1021/acs.jmedchem.7b00585 BindingDB Entry DOI: 10.7270/Q2RB76V7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen receptor (Homo sapiens (Human)) | BDBM50238734 (CHEMBL4101656) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute Curated by ChEMBL | Assay Description Displacement of [3H]estradiol from full-length human ERalpha receptor by scintillation counting | J Med Chem 60: 6321-6336 (2017) Article DOI: 10.1021/acs.jmedchem.7b00585 BindingDB Entry DOI: 10.7270/Q2RB76V7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen receptor (Homo sapiens (Human)) | BDBM50238730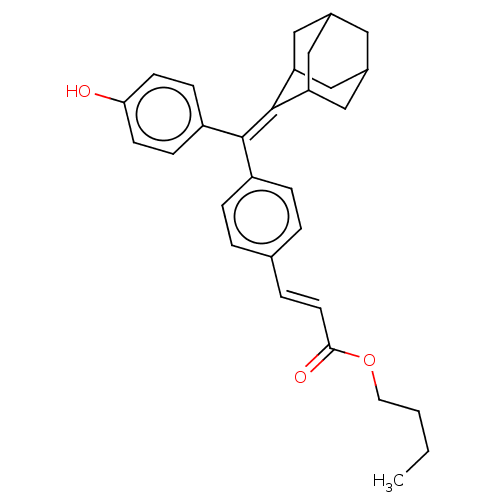 (CHEMBL4099166) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute Curated by ChEMBL | Assay Description Displacement of [3H]estradiol from full-length human ERalpha receptor by scintillation counting | J Med Chem 60: 6321-6336 (2017) Article DOI: 10.1021/acs.jmedchem.7b00585 BindingDB Entry DOI: 10.7270/Q2RB76V7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen receptor (Homo sapiens (Human)) | BDBM50238732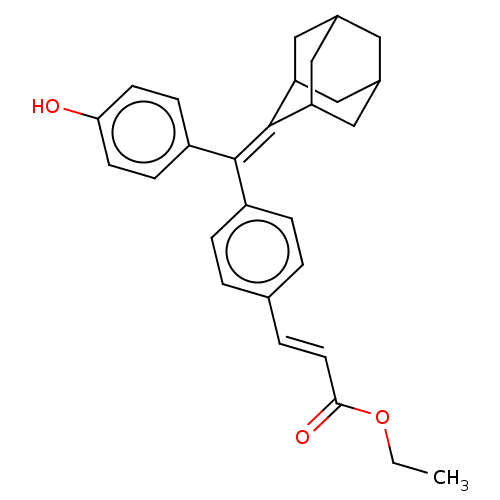 (CHEMBL4082698) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 3.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute Curated by ChEMBL | Assay Description Displacement of [3H]estradiol from full-length human ERalpha receptor by scintillation counting | J Med Chem 60: 6321-6336 (2017) Article DOI: 10.1021/acs.jmedchem.7b00585 BindingDB Entry DOI: 10.7270/Q2RB76V7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional epoxide hydrolase 2 (Homo sapiens (Human)) | BDBM50556773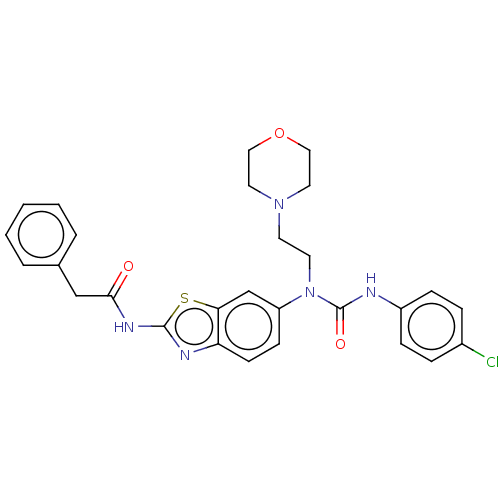 (CHEMBL4745610) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 4.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Mixed-type inhibition of recombinant human sEH using PHOME as substrate measured after 15 mins by fluorescence based Lineweaver-burk plot analysis | Citation and Details Article DOI: 10.1016/j.ejmech.2020.113028 BindingDB Entry DOI: 10.7270/Q208690C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent dopamine transporter (Homo sapiens (Human)) | BDBM22403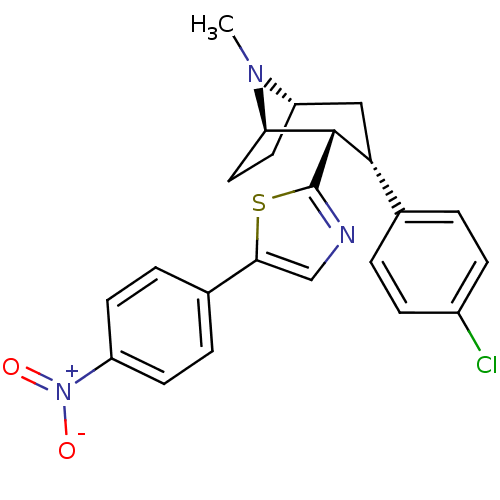 (2-[(1R,2S,3S,5S)-3-(4-chlorophenyl)-8-methyl-8-aza...) | NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 6.10 | n/a | n/a | n/a | 30 | n/a | n/a | n/a | n/a |
Research Triangle Institute | Assay Description Membranes were preincubated with drugs before the addition of [125I]RTI-55. The reaction was terminated by filtration through Wallac Filtermat A filt... | J Med Chem 50: 3686-95 (2007) Article DOI: 10.1021/jm0703035 BindingDB Entry DOI: 10.7270/Q25D8Q4T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen receptor (Homo sapiens (Human)) | BDBM50238723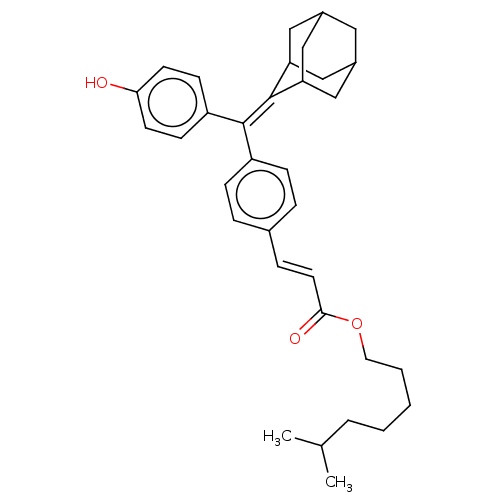 (CHEMBL4072205) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 7.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute Curated by ChEMBL | Assay Description Displacement of [3H]estradiol from full-length human ERalpha receptor by scintillation counting | J Med Chem 60: 6321-6336 (2017) Article DOI: 10.1021/acs.jmedchem.7b00585 BindingDB Entry DOI: 10.7270/Q2RB76V7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent dopamine transporter (Homo sapiens (Human)) | BDBM22404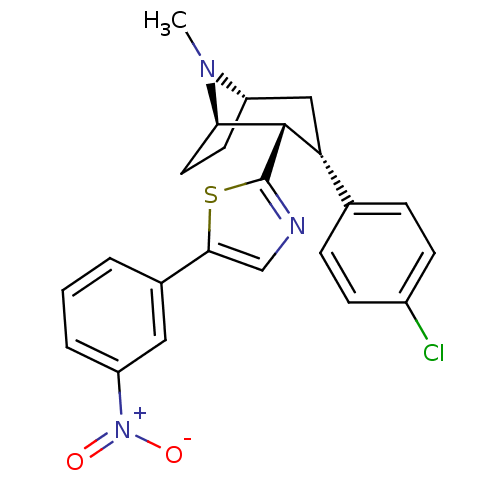 (2-[(1R,2S,3S,5S)-3-(4-chlorophenyl)-8-methyl-8-aza...) | NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 7.70 | n/a | n/a | n/a | 45 | n/a | n/a | n/a | n/a |
Research Triangle Institute | Assay Description Membranes were preincubated with drugs before the addition of [125I]RTI-55. The reaction was terminated by filtration through Wallac Filtermat A filt... | J Med Chem 50: 3686-95 (2007) Article DOI: 10.1021/jm0703035 BindingDB Entry DOI: 10.7270/Q25D8Q4T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DCN1-like protein 1 (Homo sapiens) | BDBM50584167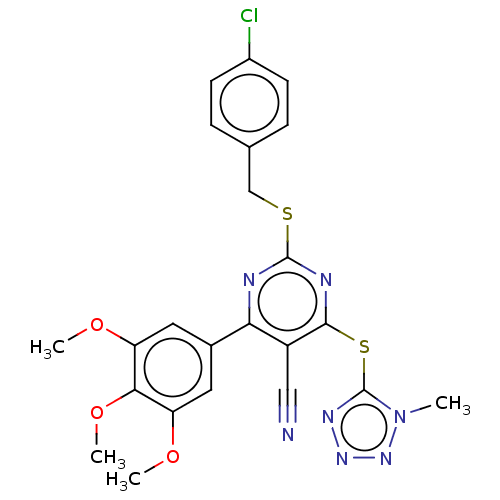 (CHEMBL5085822) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | 8.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Binding affinity to DCN1 (unknown origin) assessed as inhibitory constant | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c01207 BindingDB Entry DOI: 10.7270/Q2JD51NJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent dopamine transporter (Homo sapiens (Human)) | BDBM22405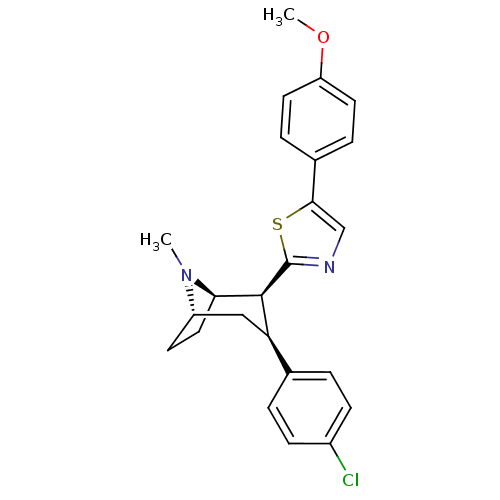 (2-[(1R,2S,3S,5S)-3-(4-chlorophenyl)-8-methyl-8-aza...) | NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 8.60 | n/a | n/a | n/a | 38 | n/a | n/a | n/a | n/a |
Research Triangle Institute | Assay Description Membranes were preincubated with drugs before the addition of [125I]RTI-55. The reaction was terminated by filtration through Wallac Filtermat A filt... | J Med Chem 50: 3686-95 (2007) Article DOI: 10.1021/jm0703035 BindingDB Entry DOI: 10.7270/Q25D8Q4T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent dopamine transporter (Homo sapiens (Human)) | BDBM22410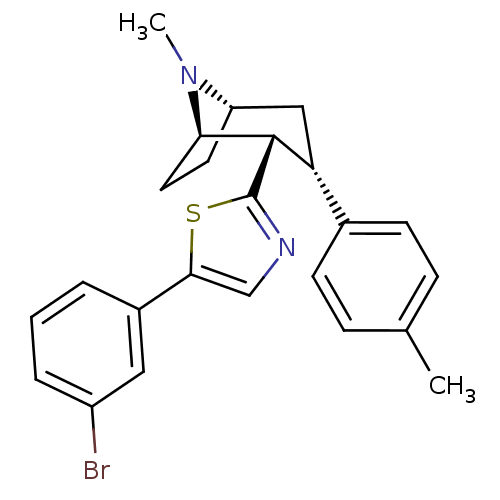 (5-(3-bromophenyl)-2-[(1R,2S,3S,5S)-8-methyl-3-(4-m...) | NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 12 | n/a | n/a | n/a | 98 | n/a | n/a | n/a | n/a |
Research Triangle Institute | Assay Description Membranes were preincubated with drugs before the addition of [125I]RTI-55. The reaction was terminated by filtration through Wallac Filtermat A filt... | J Med Chem 50: 3686-95 (2007) Article DOI: 10.1021/jm0703035 BindingDB Entry DOI: 10.7270/Q25D8Q4T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent dopamine transporter (Homo sapiens (Human)) | BDBM22399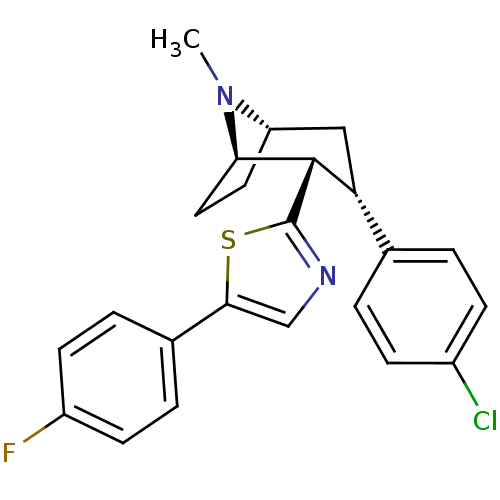 (2-[(1R,2S,3S,5S)-3-(4-chlorophenyl)-8-methyl-8-aza...) | NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 12 | n/a | n/a | n/a | 30 | n/a | n/a | n/a | n/a |
Research Triangle Institute | Assay Description Membranes were preincubated with drugs before the addition of [125I]RTI-55. The reaction was terminated by filtration through Wallac Filtermat A filt... | J Med Chem 50: 3686-95 (2007) Article DOI: 10.1021/jm0703035 BindingDB Entry DOI: 10.7270/Q25D8Q4T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DCN1-like protein 1 (Homo sapiens) | BDBM50525313 (CHEMBL4592844) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 14 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Binding affinity to DCN1 (unknown origin) assessed as inhibitory constant | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c01207 BindingDB Entry DOI: 10.7270/Q2JD51NJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent dopamine transporter (Homo sapiens (Human)) | BDBM22412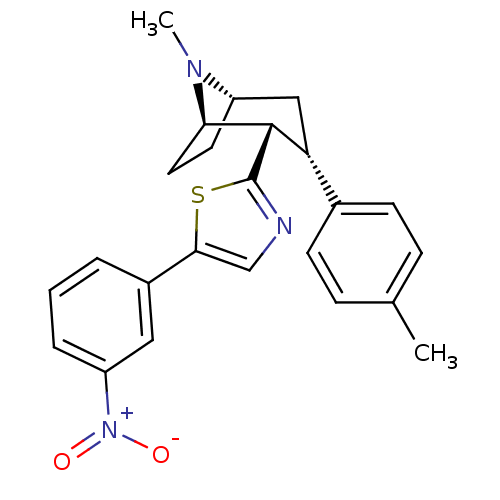 (2-[(1R,2S,3S,5S)-8-methyl-3-(4-methylphenyl)-8-aza...) | NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 14 | n/a | n/a | n/a | 23 | n/a | n/a | n/a | n/a |
Research Triangle Institute | Assay Description Membranes were preincubated with drugs before the addition of [125I]RTI-55. The reaction was terminated by filtration through Wallac Filtermat A filt... | J Med Chem 50: 3686-95 (2007) Article DOI: 10.1021/jm0703035 BindingDB Entry DOI: 10.7270/Q25D8Q4T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent dopamine transporter (Homo sapiens (Human)) | BDBM22398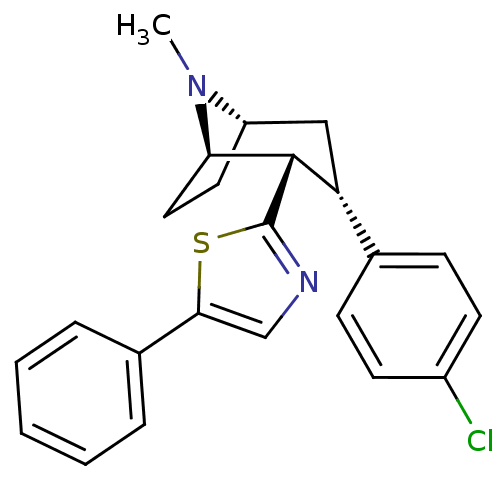 (2-[(1R,2S,3S,5S)-3-(4-chlorophenyl)-8-methyl-8-aza...) | NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 18 | n/a | n/a | n/a | 158 | n/a | n/a | n/a | n/a |
Research Triangle Institute | Assay Description Membranes were preincubated with drugs before the addition of [125I]RTI-55. The reaction was terminated by filtration through Wallac Filtermat A filt... | J Med Chem 50: 3686-95 (2007) Article DOI: 10.1021/jm0703035 BindingDB Entry DOI: 10.7270/Q25D8Q4T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent dopamine transporter (Homo sapiens (Human)) | BDBM22400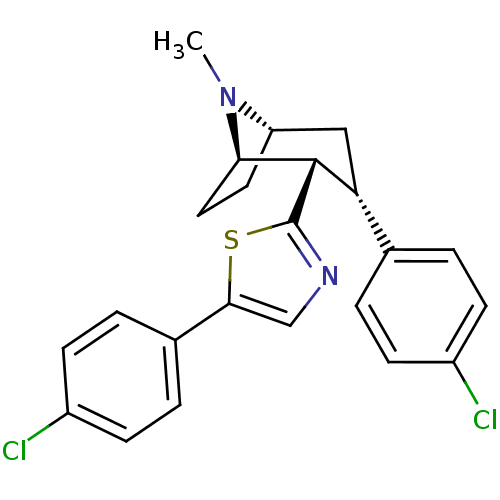 (5-(4-chlorophenyl)-2-[(1R,2S,3S,5S)-3-(4-chlorophe...) | NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 28 | n/a | n/a | n/a | 41 | n/a | n/a | n/a | n/a |
Research Triangle Institute | Assay Description Membranes were preincubated with drugs before the addition of [125I]RTI-55. The reaction was terminated by filtration through Wallac Filtermat A filt... | J Med Chem 50: 3686-95 (2007) Article DOI: 10.1021/jm0703035 BindingDB Entry DOI: 10.7270/Q25D8Q4T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent dopamine transporter (Homo sapiens (Human)) | BDBM22401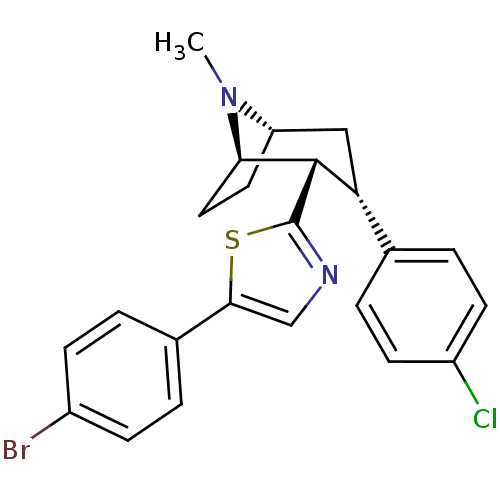 (5-(4-bromophenyl)-2-[(1R,2S,3S,5S)-3-(4-chlorophen...) | NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 35 | n/a | n/a | n/a | 107 | n/a | n/a | n/a | n/a |
Research Triangle Institute | Assay Description Membranes were preincubated with drugs before the addition of [125I]RTI-55. The reaction was terminated by filtration through Wallac Filtermat A filt... | J Med Chem 50: 3686-95 (2007) Article DOI: 10.1021/jm0703035 BindingDB Entry DOI: 10.7270/Q25D8Q4T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent dopamine transporter (Homo sapiens (Human)) | BDBM22402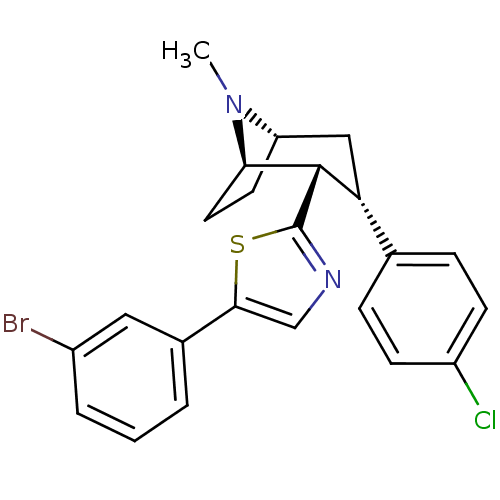 (5-(3-bromophenyl)-2-[(1R,2S,3S,5S)-3-(4-chlorophen...) | NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 39 | n/a | n/a | n/a | 63 | n/a | n/a | n/a | n/a |
Research Triangle Institute | Assay Description Membranes were preincubated with drugs before the addition of [125I]RTI-55. The reaction was terminated by filtration through Wallac Filtermat A filt... | J Med Chem 50: 3686-95 (2007) Article DOI: 10.1021/jm0703035 BindingDB Entry DOI: 10.7270/Q25D8Q4T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent dopamine transporter (Homo sapiens (Human)) | BDBM22413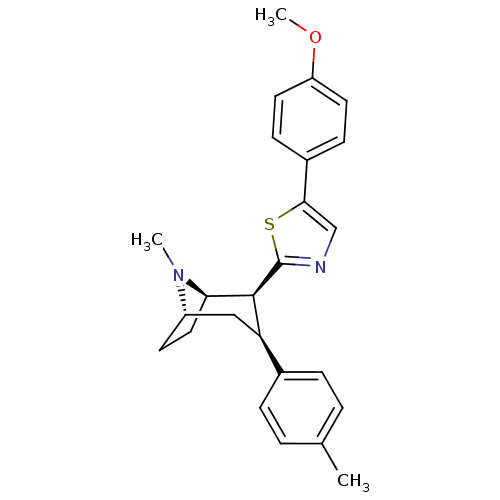 (5-(4-methoxyphenyl)-2-[(1R,2S,3S,5S)-8-methyl-3-(4...) | NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 53 | n/a | n/a | n/a | 226 | n/a | n/a | n/a | n/a |
Research Triangle Institute | Assay Description Membranes were preincubated with drugs before the addition of [125I]RTI-55. The reaction was terminated by filtration through Wallac Filtermat A filt... | J Med Chem 50: 3686-95 (2007) Article DOI: 10.1021/jm0703035 BindingDB Entry DOI: 10.7270/Q25D8Q4T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent dopamine transporter (Homo sapiens (Human)) | BDBM22408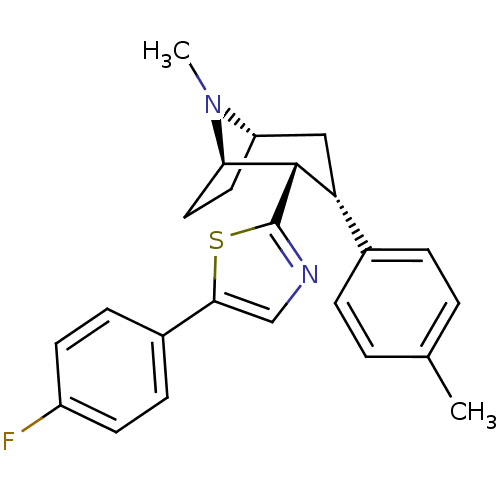 (5-(4-fluorophenyl)-2-[(1R,2S,3S,5S)-8-methyl-3-(4-...) | NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 61 | n/a | n/a | n/a | 181 | n/a | n/a | n/a | n/a |
Research Triangle Institute | Assay Description Membranes were preincubated with drugs before the addition of [125I]RTI-55. The reaction was terminated by filtration through Wallac Filtermat A filt... | J Med Chem 50: 3686-95 (2007) Article DOI: 10.1021/jm0703035 BindingDB Entry DOI: 10.7270/Q25D8Q4T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent dopamine transporter (Homo sapiens (Human)) | BDBM22406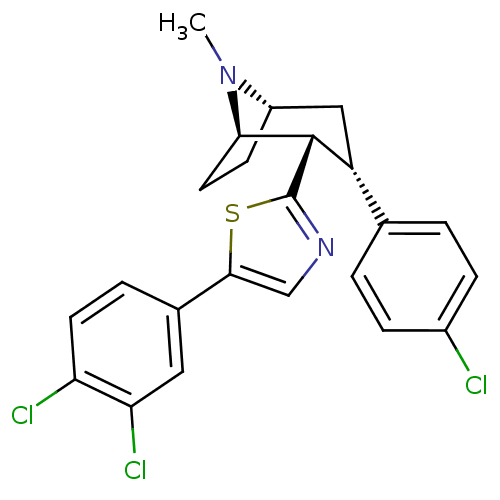 (2-[(1R,2S,3S,5S)-3-(4-chlorophenyl)-8-methyl-8-aza...) | NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 66 | n/a | n/a | n/a | 416 | n/a | n/a | n/a | n/a |
Research Triangle Institute | Assay Description Membranes were preincubated with drugs before the addition of [125I]RTI-55. The reaction was terminated by filtration through Wallac Filtermat A filt... | J Med Chem 50: 3686-95 (2007) Article DOI: 10.1021/jm0703035 BindingDB Entry DOI: 10.7270/Q25D8Q4T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent dopamine transporter (Homo sapiens (Human)) | BDBM22414 (5-(3,4-dichlorophenyl)-2-[(1R,2S,3S,5S)-8-methyl-3...) | NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 105 | n/a | n/a | n/a | 469 | n/a | n/a | n/a | n/a |
Research Triangle Institute | Assay Description Membranes were preincubated with drugs before the addition of [125I]RTI-55. The reaction was terminated by filtration through Wallac Filtermat A filt... | J Med Chem 50: 3686-95 (2007) Article DOI: 10.1021/jm0703035 BindingDB Entry DOI: 10.7270/Q25D8Q4T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| ATP-dependent translocase ABCB1 (Homo sapiens (Human)) | BDBM50115973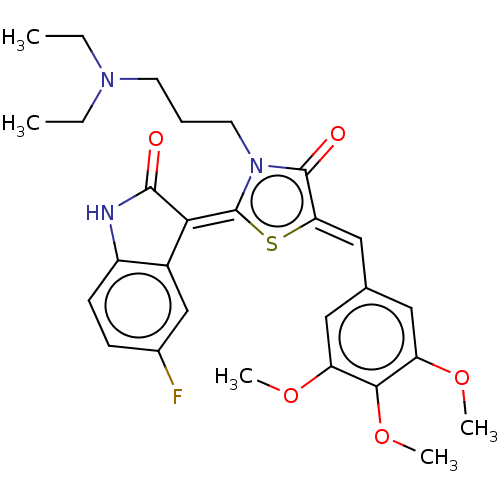 (CHEMBL3612151) | PDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 120 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The First Affiliated Hospital of Dalian Medical University Curated by ChEMBL | Assay Description Inhibition of P-glycoprotein in human drug-resistant K562/ADR cells assessed as reduction in P-gp mediated rhodamine 123 efflux by spectrofluorometry | Eur J Med Chem 101: 126-32 (2015) Article DOI: 10.1016/j.ejmech.2015.06.002 BindingDB Entry DOI: 10.7270/Q2PN97FH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| ATP-dependent translocase ABCB1 (Homo sapiens (Human)) | BDBM50115974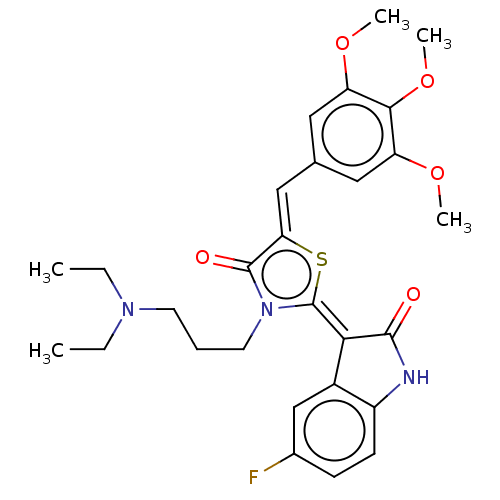 (CHEMBL3612170) | PDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 120 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The First Affiliated Hospital of Dalian Medical University Curated by ChEMBL | Assay Description Inhibition of P-glycoprotein in human drug-resistant K562/ADR cells assessed as reduction in P-gp mediated rhodamine 123 efflux by spectrofluorometry | Eur J Med Chem 101: 126-32 (2015) Article DOI: 10.1016/j.ejmech.2015.06.002 BindingDB Entry DOI: 10.7270/Q2PN97FH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent dopamine transporter (Homo sapiens (Human)) | BDBM22419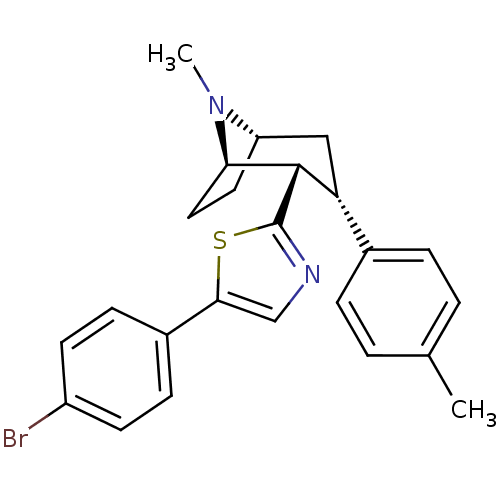 (5-(4-bromophenyl)-2-[(1R,2S,3S,5S)-8-methyl-3-(4-m...) | NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 130 | n/a | n/a | n/a | 810 | n/a | n/a | n/a | n/a |
Research Triangle Institute | Assay Description Membranes were preincubated with drugs before the addition of [125I]RTI-55. The reaction was terminated by filtration through Wallac Filtermat A filt... | J Med Chem 50: 3686-95 (2007) Article DOI: 10.1021/jm0703035 BindingDB Entry DOI: 10.7270/Q25D8Q4T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent dopamine transporter (Homo sapiens (Human)) | BDBM22411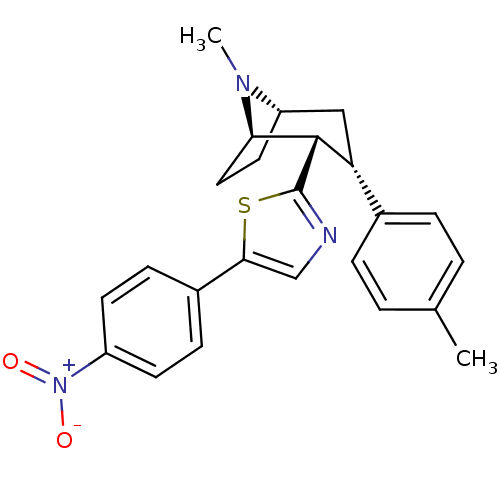 (2-[(1R,2S,3S,5S)-8-methyl-3-(4-methylphenyl)-8-aza...) | NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 140 | n/a | n/a | n/a | 197 | n/a | n/a | n/a | n/a |
Research Triangle Institute | Assay Description Membranes were preincubated with drugs before the addition of [125I]RTI-55. The reaction was terminated by filtration through Wallac Filtermat A filt... | J Med Chem 50: 3686-95 (2007) Article DOI: 10.1021/jm0703035 BindingDB Entry DOI: 10.7270/Q25D8Q4T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent serotonin transporter (Rattus norvegicus (rat)) | BDBM22414 (5-(3,4-dichlorophenyl)-2-[(1R,2S,3S,5S)-8-methyl-3...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 190 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Research Triangle Institute | Assay Description Brains from male Sprague-Dawley rats were removed, dissected, and rapidly frozen. Ligand binding experiments were conducted in assay tubes containing... | J Med Chem 50: 3686-95 (2007) Article DOI: 10.1021/jm0703035 BindingDB Entry DOI: 10.7270/Q25D8Q4T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 1528 total ) | Next | Last >> |