 Found 14513 hits with Last Name = 'wan' and Initial = 'q'
Found 14513 hits with Last Name = 'wan' and Initial = 'q' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Dihydrofolate reductase
(Homo sapiens (Human)) | BDBM50457437
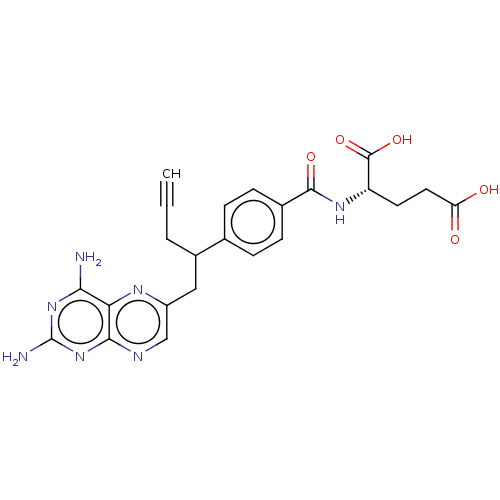
(CHEBI:71223 | Folotyn | PDX | Pralatrexate)Show SMILES [H][C@@](CCC(O)=O)(NC(=O)c1ccc(cc1)C(CC#C)Cc1cnc2nc(N)nc(N)c2n1)C(O)=O |r| Show InChI InChI=1S/C23H23N7O5/c1-2-3-14(10-15-11-26-20-18(27-15)19(24)29-23(25)30-20)12-4-6-13(7-5-12)21(33)28-16(22(34)35)8-9-17(31)32/h1,4-7,11,14,16H,3,8-10H2,(H,28,33)(H,31,32)(H,34,35)(H4,24,25,26,29,30)/t14?,16-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| 0.0130 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2021.113218
BindingDB Entry DOI: 10.7270/Q2PN99PG |
More data for this
Ligand-Target Pair | |
Transient receptor potential cation channel subfamily V member 1
(Rattus norvegicus (rat)) | BDBM50366620

(RESINIFERATOXIN)Show SMILES COc1cc(CC(=O)OCC2=C[C@H]3[C@H]4OC5(Cc6ccccc6)O[C@@]4(C[C@@H](C)[C@]3(O5)[C@@H]3C=C(C)C(=O)[C@@]3(O)C2)C(C)=C)ccc1O |r,t:10,35,TLB:23:15:12:24.25.26,THB:16:15:12:24.25.26| Show InChI InChI=1S/C37H40O9/c1-21(2)35-17-23(4)37-27(33(35)44-36(45-35,46-37)19-24-9-7-6-8-10-24)14-26(18-34(41)30(37)13-22(3)32(34)40)20-43-31(39)16-25-11-12-28(38)29(15-25)42-5/h6-15,23,27,30,33,38,41H,1,16-20H2,2-5H3/t23-,27+,30-,33-,34-,35+,36?,37-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| PubMed
| 0.0230 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Seoul National University
Curated by ChEMBL
| Assay Description
In vitro binding affinity towards vanilloid receptor by [3H]RTX displacement. |
Bioorg Med Chem Lett 9: 2909-14 (1999)
BindingDB Entry DOI: 10.7270/Q2HT2PTB |
More data for this
Ligand-Target Pair | |
Protein kinase C alpha type
(Homo sapiens (Human)) | BDBM86434
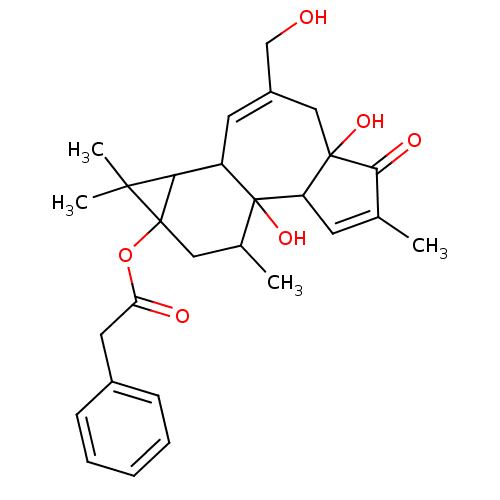
(12-Deoxyphorbol 13-phenylacetate | CAS_105100 | NS...)Show SMILES CC1CC2(OC(=O)Cc3ccccc3)C(C3C=C(CO)CC4(O)C(C=C(C)C4=O)C13O)C2(C)C |t:17,25| Show InChI InChI=1S/C28H34O6/c1-16-10-21-26(32,24(16)31)14-19(15-29)11-20-23-25(3,4)27(23,13-17(2)28(20,21)33)34-22(30)12-18-8-6-5-7-9-18/h5-11,17,20-21,23,29,32-33H,12-15H2,1-4H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.140 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Pittsburgh
Curated by PDSP Ki Database
| |
Mol Pharmacol 64: 1342-8 (2003)
Article DOI: 10.1124/mol.64.6.1342
BindingDB Entry DOI: 10.7270/Q270800T |
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Homo sapiens (Human)) | BDBM50079413
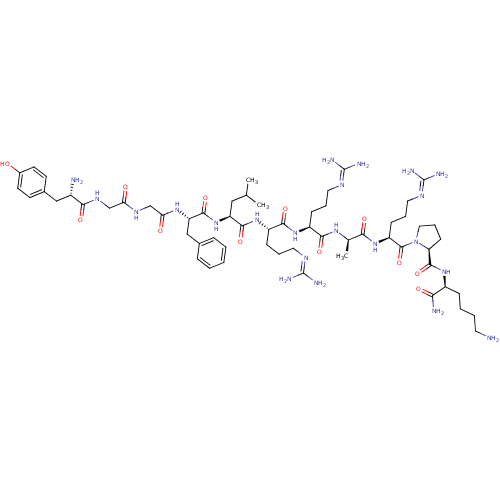
(CHEMBL407084 | Opioid Peptide [d-Ala(8)]Dynorphin ...)Show SMILES CC(C)C[C@H](NC(=O)[C@H](Cc1ccccc1)NC(=O)CNC(=O)CNC(=O)[C@@H](N)Cc1ccc(O)cc1)C(=O)N[C@@H](CCCN=C(N)N)C(=O)N[C@@H](CCCN=C(N)N)C(=O)N[C@H](C)C(=O)N[C@@H](CCCN=C(N)N)C(=O)N1CCC[C@H]1C(=O)N[C@@H](CCCCN)C(N)=O Show InChI InChI=1S/C60H98N22O12/c1-34(2)29-44(81-55(92)45(31-36-13-5-4-6-14-36)76-48(85)33-73-47(84)32-74-51(88)39(62)30-37-20-22-38(83)23-21-37)54(91)79-42(17-10-26-71-59(66)67)53(90)78-41(16-9-25-70-58(64)65)52(89)75-35(3)50(87)80-43(18-11-27-72-60(68)69)57(94)82-28-12-19-46(82)56(93)77-40(49(63)86)15-7-8-24-61/h4-6,13-14,20-23,34-35,39-46,83H,7-12,15-19,24-33,61-62H2,1-3H3,(H2,63,86)(H,73,84)(H,74,88)(H,75,89)(H,76,85)(H,77,93)(H,78,90)(H,79,91)(H,80,87)(H,81,92)(H4,64,65,70)(H4,66,67,71)(H4,68,69,72)/t35-,39+,40+,41+,42+,43+,44+,45+,46+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.190 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
The compound was tested for binding affinity against Opioid receptor kappa 1 by using [3H]Diprenorphine as a radioligand |
J Med Chem 42: 3011-3 (1999)
Article DOI: 10.1021/jm9901071
BindingDB Entry DOI: 10.7270/Q2N58KKX |
More data for this
Ligand-Target Pair | |
Protein kinase C alpha type
(Homo sapiens (Human)) | BDBM86429
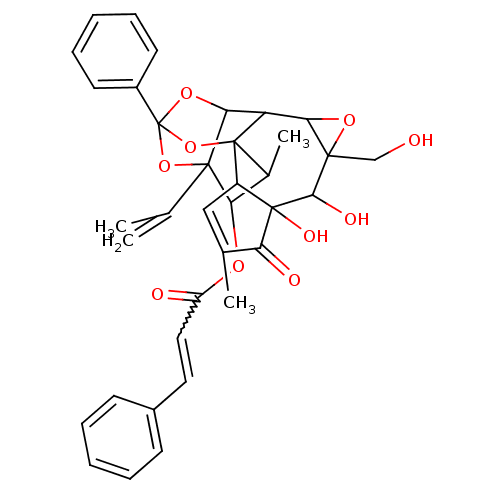
(CAS_6437389 | NSC_6437389 | Thymeleatoxin | Thymel...)Show SMILES CC1C(OC(=O)C=Cc2ccccc2)C2(OC3(OC2C2C4OC4(CO)C(O)C4(O)C(C=C(C)C4=O)C12O3)c1ccccc1)C(C)=C |w:6.5,t:33,TLB:1:35:17:14.15,THB:2:14:19.35.36:17,20:19:17:14.15,20:19:1.2.14:36.16.17| Show InChI InChI=1S/C36H36O10/c1-19(2)34-28(42-25(38)16-15-22-11-7-5-8-12-22)21(4)35-24-17-20(3)27(39)33(24,41)31(40)32(18-37)29(43-32)26(35)30(34)44-36(45-34,46-35)23-13-9-6-10-14-23/h5-17,21,24,26,28-31,37,40-41H,1,18H2,2-4H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.220 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Pittsburgh
Curated by PDSP Ki Database
| |
Mol Pharmacol 64: 1342-8 (2003)
Article DOI: 10.1124/mol.64.6.1342
BindingDB Entry DOI: 10.7270/Q270800T |
More data for this
Ligand-Target Pair | |
Protein kinase C alpha type
(Homo sapiens (Human)) | BDBM86431
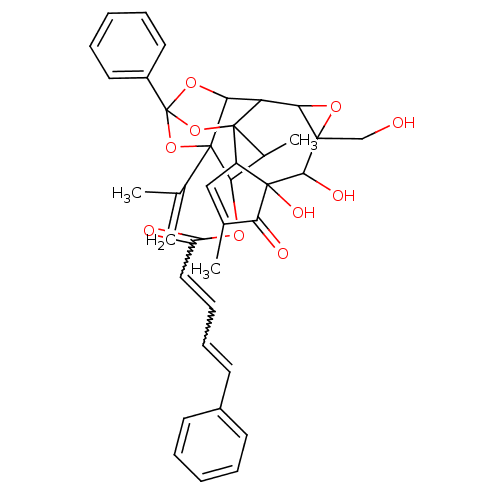
(CAS_4179 | Mezerein | NSC_4179)Show SMILES CC1C(OC(=O)C=CC=Cc2ccccc2)C2(OC3(OC2C2C4OC4(CO)C(O)C4(O)C(C=C(C)C4=O)C12O3)c1ccccc1)C(C)=C |w:6.5,8.7,t:35,TLB:1:37:19:16.17,THB:2:16:21.37.38:19,22:21:19:16.17,22:21:1.2.16:38.18.19| Show InChI InChI=1S/C38H38O10/c1-21(2)36-30(44-27(40)18-12-11-15-24-13-7-5-8-14-24)23(4)37-26-19-22(3)29(41)35(26,43)33(42)34(20-39)31(45-34)28(37)32(36)46-38(47-36,48-37)25-16-9-6-10-17-25/h5-19,23,26,28,30-33,39,42-43H,1,20H2,2-4H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.270 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Pittsburgh
Curated by PDSP Ki Database
| |
Mol Pharmacol 64: 1342-8 (2003)
Article DOI: 10.1124/mol.64.6.1342
BindingDB Entry DOI: 10.7270/Q270800T |
More data for this
Ligand-Target Pair | |
Protein kinase C alpha type
(Homo sapiens (Human)) | BDBM86429

(CAS_6437389 | NSC_6437389 | Thymeleatoxin | Thymel...)Show SMILES CC1C(OC(=O)C=Cc2ccccc2)C2(OC3(OC2C2C4OC4(CO)C(O)C4(O)C(C=C(C)C4=O)C12O3)c1ccccc1)C(C)=C |w:6.5,t:33,TLB:1:35:17:14.15,THB:2:14:19.35.36:17,20:19:17:14.15,20:19:1.2.14:36.16.17| Show InChI InChI=1S/C36H36O10/c1-19(2)34-28(42-25(38)16-15-22-11-7-5-8-12-22)21(4)35-24-17-20(3)27(39)33(24,41)31(40)32(18-37)29(43-32)26(35)30(34)44-36(45-34,46-35)23-13-9-6-10-14-23/h5-17,21,24,26,28-31,37,40-41H,1,18H2,2-4H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.290 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Pittsburgh
Curated by PDSP Ki Database
| |
Mol Pharmacol 64: 1342-8 (2003)
Article DOI: 10.1124/mol.64.6.1342
BindingDB Entry DOI: 10.7270/Q270800T |
More data for this
Ligand-Target Pair | |
Polycystin-1
(Homo sapiens (Human)) | BDBM86434

(12-Deoxyphorbol 13-phenylacetate | CAS_105100 | NS...)Show SMILES CC1CC2(OC(=O)Cc3ccccc3)C(C3C=C(CO)CC4(O)C(C=C(C)C4=O)C13O)C2(C)C |t:17,25| Show InChI InChI=1S/C28H34O6/c1-16-10-21-26(32,24(16)31)14-19(15-29)11-20-23-25(3,4)27(23,13-17(2)28(20,21)33)34-22(30)12-18-8-6-5-7-9-18/h5-11,17,20-21,23,29,32-33H,12-15H2,1-4H3 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.350 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Pittsburgh
Curated by PDSP Ki Database
| |
Mol Pharmacol 64: 1342-8 (2003)
Article DOI: 10.1124/mol.64.6.1342
BindingDB Entry DOI: 10.7270/Q270800T |
More data for this
Ligand-Target Pair | |
Maternal embryonic leucine zipper kinase
(Homo sapiens (Human)) | BDBM50248937
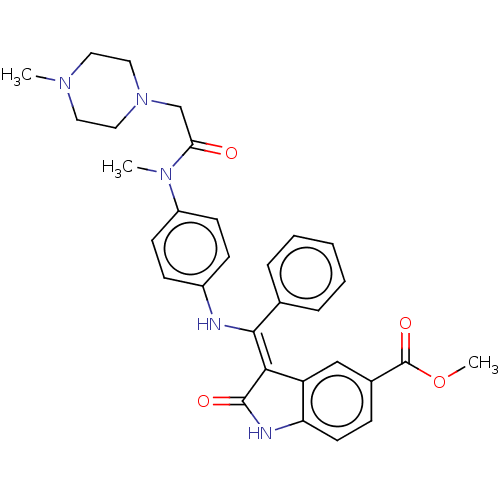
(CHEMBL4062168 | US10981896, Compound 15)Show SMILES COC(=O)c1ccc2NC(=O)\C(=C(/Nc3ccc(cc3)N(C)C(=O)CN3CCN(C)CC3)c3ccccc3)c2c1 Show InChI InChI=1S/C31H33N5O4/c1-34-15-17-36(18-16-34)20-27(37)35(2)24-12-10-23(11-13-24)32-29(21-7-5-4-6-8-21)28-25-19-22(31(39)40-3)9-14-26(25)33-30(28)38/h4-14,19,32H,15-18,20H2,1-3H3,(H,33,38)/b29-28- | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.390 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Texas at Austin
Curated by ChEMBL
| Assay Description
Inhibition of His6-tagged MELK catalytic domain (1 to 340 residues) (unknown origin) expressed in Escherichia coli BL21 (DE3) cells using Bcl-GL as s... |
Bioorg Med Chem 25: 2609-2616 (2017)
Article DOI: 10.1016/j.bmc.2017.03.018
BindingDB Entry DOI: 10.7270/Q2833VFF |
More data for this
Ligand-Target Pair | |
Polycystin-1
(Homo sapiens (Human)) | BDBM86431

(CAS_4179 | Mezerein | NSC_4179)Show SMILES CC1C(OC(=O)C=CC=Cc2ccccc2)C2(OC3(OC2C2C4OC4(CO)C(O)C4(O)C(C=C(C)C4=O)C12O3)c1ccccc1)C(C)=C |w:6.5,8.7,t:35,TLB:1:37:19:16.17,THB:2:16:21.37.38:19,22:21:19:16.17,22:21:1.2.16:38.18.19| Show InChI InChI=1S/C38H38O10/c1-21(2)36-30(44-27(40)18-12-11-15-24-13-7-5-8-14-24)23(4)37-26-19-22(3)29(41)35(26,43)33(42)34(20-39)31(45-34)28(37)32(36)46-38(47-36,48-37)25-16-9-6-10-17-25/h5-19,23,26,28,30-33,39,42-43H,1,20H2,2-4H3 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.430 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Pittsburgh
Curated by PDSP Ki Database
| |
Mol Pharmacol 64: 1342-8 (2003)
Article DOI: 10.1124/mol.64.6.1342
BindingDB Entry DOI: 10.7270/Q270800T |
More data for this
Ligand-Target Pair | |
Polycystin-1
(Homo sapiens (Human)) | BDBM86432
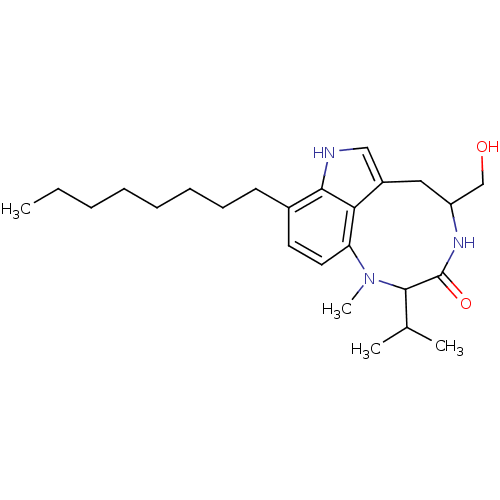
((-)-Octylindolactam V | CAS_159320 | NSC_159320)Show SMILES CCCCCCCCc1ccc2N(C)C(C(C)C)C(=O)NC(CO)Cc3c[nH]c1c23 Show InChI InChI=1S/C25H39N3O2/c1-5-6-7-8-9-10-11-18-12-13-21-22-19(15-26-23(18)22)14-20(16-29)27-25(30)24(17(2)3)28(21)4/h12-13,15,17,20,24,26,29H,5-11,14,16H2,1-4H3,(H,27,30) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.450 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Pittsburgh
Curated by PDSP Ki Database
| |
Mol Pharmacol 64: 1342-8 (2003)
Article DOI: 10.1124/mol.64.6.1342
BindingDB Entry DOI: 10.7270/Q270800T |
More data for this
Ligand-Target Pair | |
Maternal embryonic leucine zipper kinase
(Homo sapiens (Human)) | BDBM50248946

(CHEMBL1908392 | US10981896, Compound 16)Show SMILES CN(C(=O)CN1CCN(C)CC1)c1ccc(N\C(=C2/C(=O)Nc3ccccc23)c2ccccc2)cc1 Show InChI InChI=1S/C29H31N5O2/c1-32-16-18-34(19-17-32)20-26(35)33(2)23-14-12-22(13-15-23)30-28(21-8-4-3-5-9-21)27-24-10-6-7-11-25(24)31-29(27)36/h3-15,30H,16-20H2,1-2H3,(H,31,36)/b28-27- | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.470 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Texas at Austin
Curated by ChEMBL
| Assay Description
Inhibition of His6-tagged MELK catalytic domain (1 to 340 residues) (unknown origin) expressed in Escherichia coli BL21 (DE3) cells using Bcl-GL as s... |
Bioorg Med Chem 25: 2609-2616 (2017)
Article DOI: 10.1016/j.bmc.2017.03.018
BindingDB Entry DOI: 10.7270/Q2833VFF |
More data for this
Ligand-Target Pair | |
Protein kinase C alpha type
(Homo sapiens (Human)) | BDBM86432

((-)-Octylindolactam V | CAS_159320 | NSC_159320)Show SMILES CCCCCCCCc1ccc2N(C)C(C(C)C)C(=O)NC(CO)Cc3c[nH]c1c23 Show InChI InChI=1S/C25H39N3O2/c1-5-6-7-8-9-10-11-18-12-13-21-22-19(15-26-23(18)22)14-20(16-29)27-25(30)24(17(2)3)28(21)4/h12-13,15,17,20,24,26,29H,5-11,14,16H2,1-4H3,(H,27,30) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.530 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Pittsburgh
Curated by PDSP Ki Database
| |
Mol Pharmacol 64: 1342-8 (2003)
Article DOI: 10.1124/mol.64.6.1342
BindingDB Entry DOI: 10.7270/Q270800T |
More data for this
Ligand-Target Pair | |
Protein kinase C delta type
(Homo sapiens (Human)) | BDBM86431

(CAS_4179 | Mezerein | NSC_4179)Show SMILES CC1C(OC(=O)C=CC=Cc2ccccc2)C2(OC3(OC2C2C4OC4(CO)C(O)C4(O)C(C=C(C)C4=O)C12O3)c1ccccc1)C(C)=C |w:6.5,8.7,t:35,TLB:1:37:19:16.17,THB:2:16:21.37.38:19,22:21:19:16.17,22:21:1.2.16:38.18.19| Show InChI InChI=1S/C38H38O10/c1-21(2)36-30(44-27(40)18-12-11-15-24-13-7-5-8-14-24)23(4)37-26-19-22(3)29(41)35(26,43)33(42)34(20-39)31(45-34)28(37)32(36)46-38(47-36,48-37)25-16-9-6-10-17-25/h5-19,23,26,28,30-33,39,42-43H,1,20H2,2-4H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.550 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Pittsburgh
Curated by PDSP Ki Database
| |
Mol Pharmacol 64: 1342-8 (2003)
Article DOI: 10.1124/mol.64.6.1342
BindingDB Entry DOI: 10.7270/Q270800T |
More data for this
Ligand-Target Pair | |
Protein kinase C delta type
(Homo sapiens (Human)) | BDBM86434

(12-Deoxyphorbol 13-phenylacetate | CAS_105100 | NS...)Show SMILES CC1CC2(OC(=O)Cc3ccccc3)C(C3C=C(CO)CC4(O)C(C=C(C)C4=O)C13O)C2(C)C |t:17,25| Show InChI InChI=1S/C28H34O6/c1-16-10-21-26(32,24(16)31)14-19(15-29)11-20-23-25(3,4)27(23,13-17(2)28(20,21)33)34-22(30)12-18-8-6-5-7-9-18/h5-11,17,20-21,23,29,32-33H,12-15H2,1-4H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.560 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Pittsburgh
Curated by PDSP Ki Database
| |
Mol Pharmacol 64: 1342-8 (2003)
Article DOI: 10.1124/mol.64.6.1342
BindingDB Entry DOI: 10.7270/Q270800T |
More data for this
Ligand-Target Pair | |
Maternal embryonic leucine zipper kinase
(Homo sapiens (Human)) | BDBM50248938

(CHEMBL4089284 | US10981896, Compound 21)Show SMILES CN(C(=O)CN1CCN(C)CC1)c1ccc(N\C(=C2/C(=O)Nc3ccc(cc23)C(O)=O)c2ccccc2)cc1 Show InChI InChI=1S/C30H31N5O4/c1-33-14-16-35(17-15-33)19-26(36)34(2)23-11-9-22(10-12-23)31-28(20-6-4-3-5-7-20)27-24-18-21(30(38)39)8-13-25(24)32-29(27)37/h3-13,18,31H,14-17,19H2,1-2H3,(H,32,37)(H,38,39)/b28-27- | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.680 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Texas at Austin
Curated by ChEMBL
| Assay Description
Inhibition of His6-tagged MELK catalytic domain (1 to 340 residues) (unknown origin) expressed in Escherichia coli BL21 (DE3) cells using Bcl-GL as s... |
Bioorg Med Chem 25: 2609-2616 (2017)
Article DOI: 10.1016/j.bmc.2017.03.018
BindingDB Entry DOI: 10.7270/Q2833VFF |
More data for this
Ligand-Target Pair | |
Protein kinase C delta type
(Homo sapiens (Human)) | BDBM86432

((-)-Octylindolactam V | CAS_159320 | NSC_159320)Show SMILES CCCCCCCCc1ccc2N(C)C(C(C)C)C(=O)NC(CO)Cc3c[nH]c1c23 Show InChI InChI=1S/C25H39N3O2/c1-5-6-7-8-9-10-11-18-12-13-21-22-19(15-26-23(18)22)14-20(16-29)27-25(30)24(17(2)3)28(21)4/h12-13,15,17,20,24,26,29H,5-11,14,16H2,1-4H3,(H,27,30) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.770 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Pittsburgh
Curated by PDSP Ki Database
| |
Mol Pharmacol 64: 1342-8 (2003)
Article DOI: 10.1124/mol.64.6.1342
BindingDB Entry DOI: 10.7270/Q270800T |
More data for this
Ligand-Target Pair | |
D(3) dopamine receptor
(Homo sapiens (Human)) | BDBM50339691

(4-(dimethylamino)-N-(4-(4-(2-methoxyphenyl)piperaz...)Show SMILES COc1ccccc1N1CCN(CCCCNC(=O)c2ccc(cc2)N(C)C)CC1 Show InChI InChI=1S/C24H34N4O2/c1-26(2)21-12-10-20(11-13-21)24(29)25-14-6-7-15-27-16-18-28(19-17-27)22-8-4-5-9-23(22)30-3/h4-5,8-13H,6-7,14-19H2,1-3H3,(H,25,29) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Washington University School of Medicine
Curated by ChEMBL
| Assay Description
Displacement of of [125I]-IABN from human D3 receptor expressed in HEK293 cell membranes after 60 mins by filtration binding assay |
Bioorg Med Chem Lett 25: 519-23 (2015)
Article DOI: 10.1016/j.bmcl.2014.12.023
BindingDB Entry DOI: 10.7270/Q27W6DS9 |
More data for this
Ligand-Target Pair | |
Cyclin-dependent kinase 4
(Homo sapiens (Human)) | BDBM12621

(2,4-Diamino-5-ketopyrimidine 39 | 5-[(2,3-difluoro...)Show SMILES COc1ccc(F)c(F)c1C(=O)c1cnc(NC2CCN(CC2)S(C)(=O)=O)nc1N Show InChI InChI=1S/C18H21F2N5O4S/c1-29-13-4-3-12(19)15(20)14(13)16(26)11-9-22-18(24-17(11)21)23-10-5-7-25(8-6-10)30(2,27)28/h3-4,9-10H,5-8H2,1-2H3,(H3,21,22,23,24) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c02190
BindingDB Entry DOI: 10.7270/Q2BV7MPD |
More data for this
Ligand-Target Pair | |
Thymidylate synthase
(Homo sapiens (Human)) | BDBM50027656
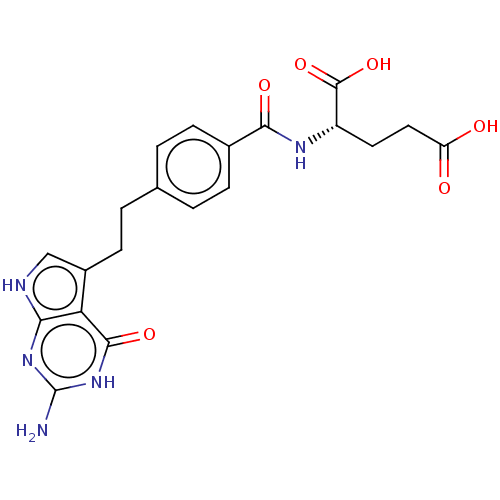
(CHEBI:63616 | LY-2315 | LY-231514 | PEMETREXED | U...)Show SMILES Nc1nc2[nH]cc(CCc3ccc(cc3)C(=O)N[C@@H](CCC(O)=O)C(O)=O)c2c(=O)[nH]1 Show InChI InChI=1S/C20H21N5O6/c21-20-24-16-15(18(29)25-20)12(9-22-16)6-3-10-1-4-11(5-2-10)17(28)23-13(19(30)31)7-8-14(26)27/h1-2,4-5,9,13H,3,6-8H2,(H,23,28)(H,26,27)(H,30,31)(H4,21,22,24,25,29)/t13-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| 1.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2021.113218
BindingDB Entry DOI: 10.7270/Q2PN99PG |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
5-hydroxytryptamine receptor 1A
(Homo sapiens (Human)) | BDBM50042844
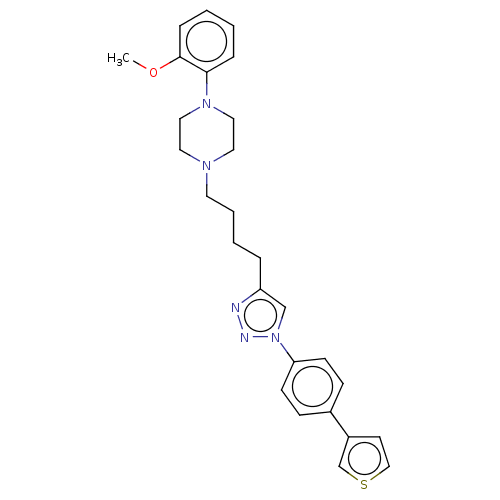
(CHEMBL3353901)Show SMILES COc1ccccc1N1CCN(CCCCc2cn(nn2)-c2ccc(cc2)-c2ccsc2)CC1 Show InChI InChI=1S/C27H31N5OS/c1-33-27-8-3-2-7-26(27)31-17-15-30(16-18-31)14-5-4-6-24-20-32(29-28-24)25-11-9-22(10-12-25)23-13-19-34-21-23/h2-3,7-13,19-21H,4-6,14-18H2,1H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Washington University School of Medicine
Curated by ChEMBL
| Assay Description
Displacement of of [3H]8-OH-DPAT from human 5HT1A receptor after 60 mins by filtration binding assay |
Bioorg Med Chem Lett 25: 519-23 (2015)
Article DOI: 10.1016/j.bmcl.2014.12.023
BindingDB Entry DOI: 10.7270/Q27W6DS9 |
More data for this
Ligand-Target Pair | |
Protein kinase C delta type
(Homo sapiens (Human)) | BDBM86429

(CAS_6437389 | NSC_6437389 | Thymeleatoxin | Thymel...)Show SMILES CC1C(OC(=O)C=Cc2ccccc2)C2(OC3(OC2C2C4OC4(CO)C(O)C4(O)C(C=C(C)C4=O)C12O3)c1ccccc1)C(C)=C |w:6.5,t:33,TLB:1:35:17:14.15,THB:2:14:19.35.36:17,20:19:17:14.15,20:19:1.2.14:36.16.17| Show InChI InChI=1S/C36H36O10/c1-19(2)34-28(42-25(38)16-15-22-11-7-5-8-12-22)21(4)35-24-17-20(3)27(39)33(24,41)31(40)32(18-37)29(43-32)26(35)30(34)44-36(45-34,46-35)23-13-9-6-10-14-23/h5-17,21,24,26,28-31,37,40-41H,1,18H2,2-4H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.31 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Pittsburgh
Curated by PDSP Ki Database
| |
Mol Pharmacol 64: 1342-8 (2003)
Article DOI: 10.1124/mol.64.6.1342
BindingDB Entry DOI: 10.7270/Q270800T |
More data for this
Ligand-Target Pair | |
Poly [ADP-ribose] polymerase 1
(Homo sapiens (Human)) | BDBM50446130

(AG-014699 | AG-14447 | RUCAPARIB CAMSYLATE | Rucap...)Show SMILES CNCc1ccc(cc1)-c1[nH]c2cc(F)cc3C(=O)NCCc1c23 Show InChI InChI=1S/C19H18FN3O/c1-21-10-11-2-4-12(5-3-11)18-14-6-7-22-19(24)15-8-13(20)9-16(23-18)17(14)15/h2-5,8-9,21,23H,6-7,10H2,1H3,(H,22,24) | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
Similars
| PDB
| 1.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Cathepsin B
(Homo sapiens (Human)) | BDBM50137733
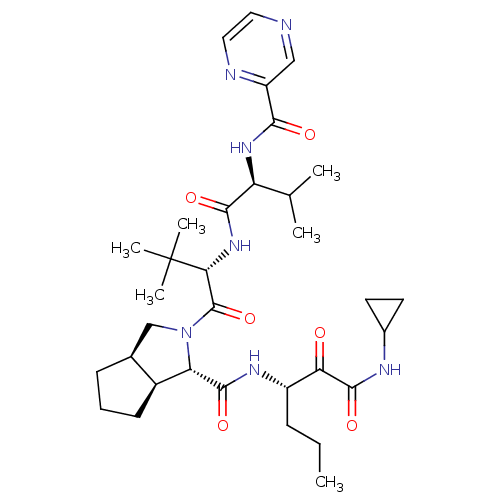
((1S,5S,6R)-2-((S)-3,3-Dimethyl-2-{(S)-3-methyl-2-[...)Show SMILES CCC[C@H](NC(=O)[C@@H]1[C@H]2CCC[C@H]2CN1C(=O)[C@@H](NC(=O)[C@@H](NC(=O)c1cnccn1)C(C)C)C(C)(C)C)C(=O)C(=O)NC1CC1 Show InChI InChI=1S/C33H49N7O6/c1-7-9-22(26(41)31(45)36-20-12-13-20)37-30(44)25-21-11-8-10-19(21)17-40(25)32(46)27(33(4,5)6)39-29(43)24(18(2)3)38-28(42)23-16-34-14-15-35-23/h14-16,18-22,24-25,27H,7-13,17H2,1-6H3,(H,36,45)(H,37,44)(H,38,42)(H,39,43)/t19-,21-,22-,24-,25-,27+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 1.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratory
Curated by ChEMBL
| Assay Description
Inhibitory activity of the compound against cathepsin B |
Bioorg Med Chem Lett 14: 257-61 (2003)
BindingDB Entry DOI: 10.7270/Q20P0ZDG |
More data for this
Ligand-Target Pair | |
NUAK family SNF1-like kinase 1
(Homo sapiens (Human)) | BDBM50248946

(CHEMBL1908392 | US10981896, Compound 16)Show SMILES CN(C(=O)CN1CCN(C)CC1)c1ccc(N\C(=C2/C(=O)Nc3ccccc23)c2ccccc2)cc1 Show InChI InChI=1S/C29H31N5O2/c1-32-16-18-34(19-17-32)20-26(35)33(2)23-14-12-22(13-15-23)30-28(21-8-4-3-5-9-21)27-24-10-6-7-11-25(24)31-29(27)36/h3-15,30H,16-20H2,1-2H3,(H,31,36)/b28-27- | KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Texas at Austin
Curated by ChEMBL
| Assay Description
Inhibition of full length recombinant human N-terminal GST-tagged NUAK1 expressed in baculovirus infected Sf9 insect cells using CHK peptide as subst... |
Bioorg Med Chem 25: 2609-2616 (2017)
Article DOI: 10.1016/j.bmc.2017.03.018
BindingDB Entry DOI: 10.7270/Q2833VFF |
More data for this
Ligand-Target Pair | |
D(3) dopamine receptor
(Homo sapiens (Human)) | BDBM50042708
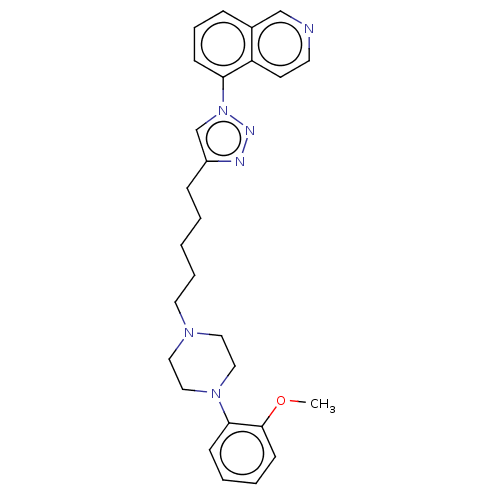
(CHEMBL3353899)Show SMILES COc1ccccc1N1CCN(CCCCCc2cn(nn2)-c2cccc3cnccc23)CC1 Show InChI InChI=1S/C27H32N6O/c1-34-27-12-5-4-10-26(27)32-18-16-31(17-19-32)15-6-2-3-9-23-21-33(30-29-23)25-11-7-8-22-20-28-14-13-24(22)25/h4-5,7-8,10-14,20-21H,2-3,6,9,15-19H2,1H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Washington University School of Medicine
Curated by ChEMBL
| Assay Description
Displacement of of [125I]-IABN from human D3 receptor expressed in HEK293 cell membranes after 60 mins by filtration binding assay |
Bioorg Med Chem Lett 25: 519-23 (2015)
Article DOI: 10.1016/j.bmcl.2014.12.023
BindingDB Entry DOI: 10.7270/Q27W6DS9 |
More data for this
Ligand-Target Pair | |
Endothelin-1 receptor
(Homo sapiens (Human)) | BDBM50117910
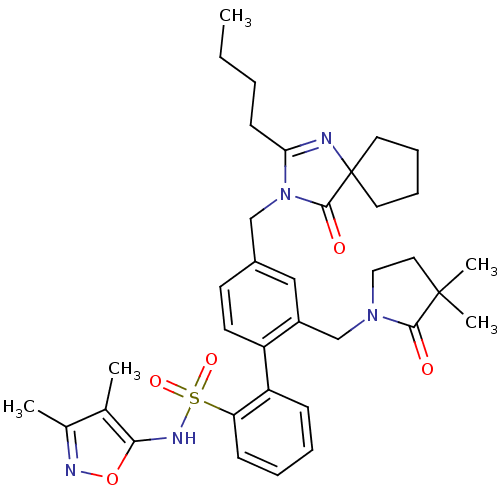
(4''-(2-Butyl-4-oxo-1,3-diaza-spiro[4.4]non-1-en-3-...)Show SMILES CCCCC1=NC2(CCCC2)C(=O)N1Cc1ccc(c(CN2CCC(C)(C)C2=O)c1)-c1ccccc1S(=O)(=O)Nc1onc(C)c1C |t:4| Show InChI InChI=1S/C36H45N5O5S/c1-6-7-14-31-37-36(17-10-11-18-36)34(43)41(31)22-26-15-16-28(27(21-26)23-40-20-19-35(4,5)33(40)42)29-12-8-9-13-30(29)47(44,45)39-32-24(2)25(3)38-46-32/h8-9,12-13,15-16,21,39H,6-7,10-11,14,17-20,22-23H2,1-5H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 1.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
China Pharmaceutical University
Curated by ChEMBL
| Assay Description
Binding affinity at endothelin receptor subtype A |
Bioorg Med Chem 20: 4661-7 (2012)
Article DOI: 10.1016/j.bmc.2012.06.011
BindingDB Entry DOI: 10.7270/Q2G73FS6 |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Homo sapiens (Human)) | BDBM50079413

(CHEMBL407084 | Opioid Peptide [d-Ala(8)]Dynorphin ...)Show SMILES CC(C)C[C@H](NC(=O)[C@H](Cc1ccccc1)NC(=O)CNC(=O)CNC(=O)[C@@H](N)Cc1ccc(O)cc1)C(=O)N[C@@H](CCCN=C(N)N)C(=O)N[C@@H](CCCN=C(N)N)C(=O)N[C@H](C)C(=O)N[C@@H](CCCN=C(N)N)C(=O)N1CCC[C@H]1C(=O)N[C@@H](CCCCN)C(N)=O Show InChI InChI=1S/C60H98N22O12/c1-34(2)29-44(81-55(92)45(31-36-13-5-4-6-14-36)76-48(85)33-73-47(84)32-74-51(88)39(62)30-37-20-22-38(83)23-21-37)54(91)79-42(17-10-26-71-59(66)67)53(90)78-41(16-9-25-70-58(64)65)52(89)75-35(3)50(87)80-43(18-11-27-72-60(68)69)57(94)82-28-12-19-46(82)56(93)77-40(49(63)86)15-7-8-24-61/h4-6,13-14,20-23,34-35,39-46,83H,7-12,15-19,24-33,61-62H2,1-3H3,(H2,63,86)(H,73,84)(H,74,88)(H,75,89)(H,76,85)(H,77,93)(H,78,90)(H,79,91)(H,80,87)(H,81,92)(H4,64,65,70)(H4,66,67,71)(H4,68,69,72)/t35-,39+,40+,41+,42+,43+,44+,45+,46+/m1/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
The compound was tested for binding affinity against Opioid receptor mu 1 by using [3H]DAMGO as a radioligand |
J Med Chem 42: 3011-3 (1999)
Article DOI: 10.1021/jm9901071
BindingDB Entry DOI: 10.7270/Q2N58KKX |
More data for this
Ligand-Target Pair | |
Cyclin-dependent kinase 1
(Homo sapiens (Human)) | BDBM12621

(2,4-Diamino-5-ketopyrimidine 39 | 5-[(2,3-difluoro...)Show SMILES COc1ccc(F)c(F)c1C(=O)c1cnc(NC2CCN(CC2)S(C)(=O)=O)nc1N Show InChI InChI=1S/C18H21F2N5O4S/c1-29-13-4-3-12(19)15(20)14(13)16(26)11-9-22-18(24-17(11)21)23-10-5-7-25(8-6-10)30(2,27)28/h3-4,9-10H,5-8H2,1-2H3,(H3,21,22,23,24) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c02190
BindingDB Entry DOI: 10.7270/Q2BV7MPD |
More data for this
Ligand-Target Pair | |
D(3) dopamine receptor
(Homo sapiens (Human)) | BDBM50042734
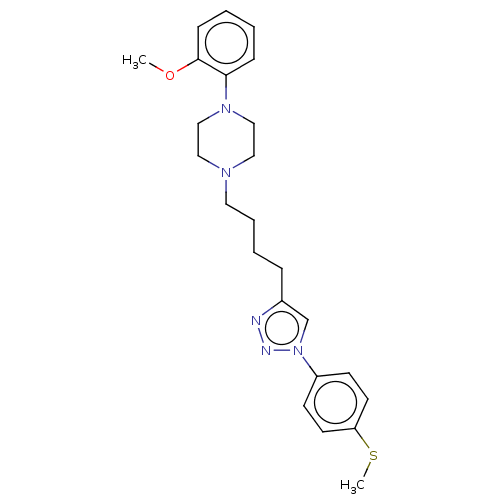
(CHEMBL3353913)Show SMILES COc1ccccc1N1CCN(CCCCc2cn(nn2)-c2ccc(SC)cc2)CC1 Show InChI InChI=1S/C24H31N5OS/c1-30-24-9-4-3-8-23(24)28-17-15-27(16-18-28)14-6-5-7-20-19-29(26-25-20)21-10-12-22(31-2)13-11-21/h3-4,8-13,19H,5-7,14-18H2,1-2H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Washington University School of Medicine
Curated by ChEMBL
| Assay Description
Displacement of of [125I]-IABN from human D3 receptor expressed in HEK293 cell membranes after 60 mins by filtration binding assay |
Bioorg Med Chem Lett 25: 519-23 (2015)
Article DOI: 10.1016/j.bmcl.2014.12.023
BindingDB Entry DOI: 10.7270/Q27W6DS9 |
More data for this
Ligand-Target Pair | |
Metabotropic glutamate receptor 2
(Homo sapiens (Human)) | BDBM50151435
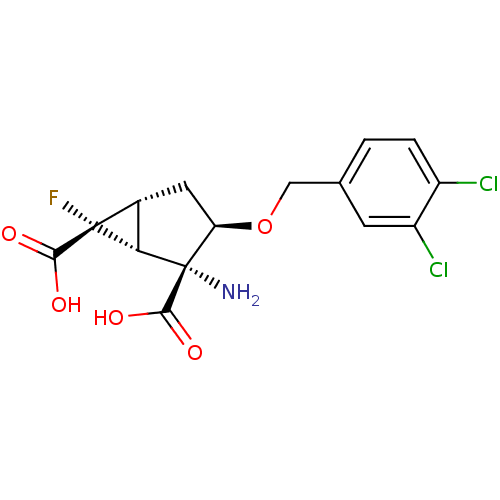
((1R,2R,3R,5R,6R)-2-Amino-3-(3,4-dichloro-benzyloxy...)Show SMILES N[C@@]1([C@H]2[C@@H](C[C@H]1OCc1ccc(Cl)c(Cl)c1)[C@]2(F)C(O)=O)C(O)=O Show InChI InChI=1S/C15H14Cl2FNO5/c16-8-2-1-6(3-9(8)17)5-24-10-4-7-11(14(7,18)12(20)21)15(10,19)13(22)23/h1-3,7,10-11H,4-5,19H2,(H,20,21)(H,22,23)/t7-,10-,11+,14-,15+/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 2.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Massachusetts General Hospital
Curated by ChEMBL
| Assay Description
Binding affinity to mGLUR2 |
Bioorg Med Chem Lett 22: 1958-62 (2012)
Article DOI: 10.1016/j.bmcl.2012.01.039
BindingDB Entry DOI: 10.7270/Q2T72JJ1 |
More data for this
Ligand-Target Pair | |
P2X purinoceptor 7
(Rattus norvegicus (Rat)) | BDBM254203

(US10112937, Example 88 | US10150765, Example 88 | ...)Show SMILES C[C@H]1N(CCc2c1nnn2-c1ncccn1)C(=O)c1cccc(c1Cl)C(F)(F)F |r| Show InChI InChI=1S/C18H14ClF3N6O/c1-10-15-13(28(26-25-15)17-23-7-3-8-24-17)6-9-27(10)16(29)11-4-2-5-12(14(11)19)18(20,21)22/h2-5,7-8,10H,6,9H2,1H3/t10-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 2.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Janssen Research& Development, LLC , 3210 Merryfield Row, San Diego, California 92121, United States.
Curated by ChEMBL
| Assay Description
Antagonist activity at recombinant rat P2X7 expressed in human 1321N1 cells assessed as inhibition of BzATP-induced calcium flux preincubated for 30 ... |
J Med Chem 60: 4559-4572 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00408
BindingDB Entry DOI: 10.7270/Q2QN697T |
More data for this
Ligand-Target Pair | |
Maternal embryonic leucine zipper kinase
(Homo sapiens (Human)) | BDBM50248936

(CHEMBL4083922 | US10981896, Compound 25)Show SMILES CNC(=O)c1ccc2NC(=O)\C(=C(/Nc3ccc(cc3)N(C)C(=O)CN3CCN(C)CC3)c3ccccc3)c2c1 Show InChI InChI=1S/C31H34N6O3/c1-32-30(39)22-9-14-26-25(19-22)28(31(40)34-26)29(21-7-5-4-6-8-21)33-23-10-12-24(13-11-23)36(3)27(38)20-37-17-15-35(2)16-18-37/h4-14,19,33H,15-18,20H2,1-3H3,(H,32,39)(H,34,40)/b29-28- | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Texas at Austin
Curated by ChEMBL
| Assay Description
Inhibition of His6-tagged MELK catalytic domain (1 to 340 residues) (unknown origin) expressed in Escherichia coli BL21 (DE3) cells using Bcl-GL as s... |
Bioorg Med Chem 25: 2609-2616 (2017)
Article DOI: 10.1016/j.bmc.2017.03.018
BindingDB Entry DOI: 10.7270/Q2833VFF |
More data for this
Ligand-Target Pair | |
D(3) dopamine receptor
(Homo sapiens (Human)) | BDBM50042729

(CHEMBL3353914)Show SMILES COc1ccccc1N1CCN(CCCCCc2cn(nn2)-c2ccc(SC)cc2)CC1 Show InChI InChI=1S/C25H33N5OS/c1-31-25-10-6-5-9-24(25)29-18-16-28(17-19-29)15-7-3-4-8-21-20-30(27-26-21)22-11-13-23(32-2)14-12-22/h5-6,9-14,20H,3-4,7-8,15-19H2,1-2H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Washington University School of Medicine
Curated by ChEMBL
| Assay Description
Displacement of of [125I]-IABN from human D3 receptor expressed in HEK293 cell membranes after 60 mins by filtration binding assay |
Bioorg Med Chem Lett 25: 519-23 (2015)
Article DOI: 10.1016/j.bmcl.2014.12.023
BindingDB Entry DOI: 10.7270/Q27W6DS9 |
More data for this
Ligand-Target Pair | |
D(3) dopamine receptor
(Homo sapiens (Human)) | BDBM50339690
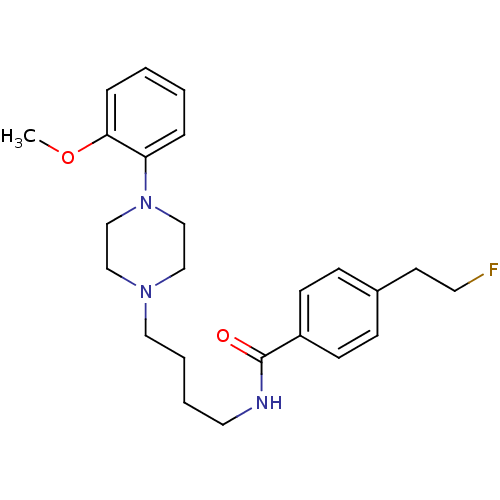
(4-(2-fluoroethyl)-N-(4-(4-(2-methoxyphenyl)piperaz...)Show SMILES COc1ccccc1N1CCN(CCCCNC(=O)c2ccc(CCF)cc2)CC1 Show InChI InChI=1S/C24H32FN3O2/c1-30-23-7-3-2-6-22(23)28-18-16-27(17-19-28)15-5-4-14-26-24(29)21-10-8-20(9-11-21)12-13-25/h2-3,6-11H,4-5,12-19H2,1H3,(H,26,29) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Washington University School of Medicine
Curated by ChEMBL
| Assay Description
Displacement of of [125I]-IABN from human D3 receptor expressed in HEK293 cell membranes after 60 mins by filtration binding assay |
Bioorg Med Chem Lett 25: 519-23 (2015)
Article DOI: 10.1016/j.bmcl.2014.12.023
BindingDB Entry DOI: 10.7270/Q27W6DS9 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 1A
(Homo sapiens (Human)) | BDBM50339690

(4-(2-fluoroethyl)-N-(4-(4-(2-methoxyphenyl)piperaz...)Show SMILES COc1ccccc1N1CCN(CCCCNC(=O)c2ccc(CCF)cc2)CC1 Show InChI InChI=1S/C24H32FN3O2/c1-30-23-7-3-2-6-22(23)28-18-16-27(17-19-28)15-5-4-14-26-24(29)21-10-8-20(9-11-21)12-13-25/h2-3,6-11H,4-5,12-19H2,1H3,(H,26,29) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Washington University School of Medicine
Curated by ChEMBL
| Assay Description
Displacement of of [3H]8-OH-DPAT from human 5HT1A receptor after 60 mins by filtration binding assay |
Bioorg Med Chem Lett 25: 519-23 (2015)
Article DOI: 10.1016/j.bmcl.2014.12.023
BindingDB Entry DOI: 10.7270/Q27W6DS9 |
More data for this
Ligand-Target Pair | |
Protein kinase C alpha type
(Homo sapiens (Human)) | BDBM86435
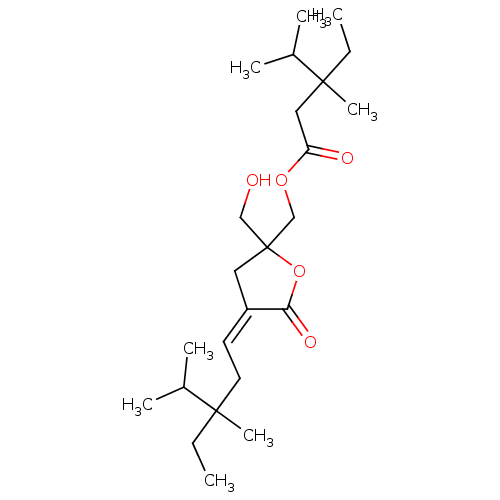
(B8-DL-B8 | B8-DL-B8 (low PS))Show SMILES CCC(C)(C\C=C1\CC(CO)(COC(=O)CC(C)(CC)C(C)C)OC1=O)C(C)C Show InChI InChI=1S/C24H42O5/c1-9-22(7,17(3)4)12-11-19-13-24(15-25,29-21(19)27)16-28-20(26)14-23(8,10-2)18(5)6/h11,17-18,25H,9-10,12-16H2,1-8H3/b19-11- | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Pittsburgh
Curated by PDSP Ki Database
| |
Mol Pharmacol 64: 1342-8 (2003)
Article DOI: 10.1124/mol.64.6.1342
BindingDB Entry DOI: 10.7270/Q270800T |
More data for this
Ligand-Target Pair | |
D(3) dopamine receptor
(Homo sapiens (Human)) | BDBM50042727
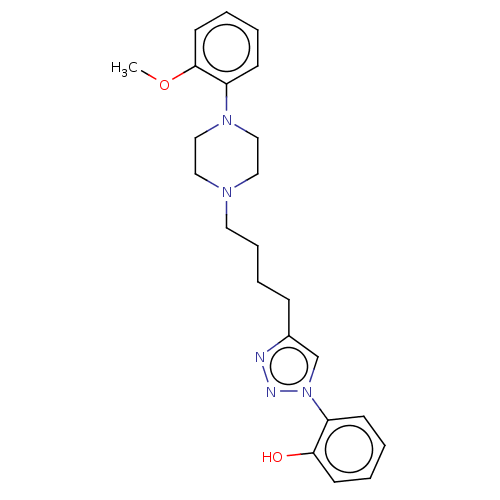
(CHEMBL3353916)Show SMILES COc1ccccc1N1CCN(CCCCc2cn(nn2)-c2ccccc2O)CC1 Show InChI InChI=1S/C23H29N5O2/c1-30-23-12-5-3-10-21(23)27-16-14-26(15-17-27)13-7-6-8-19-18-28(25-24-19)20-9-2-4-11-22(20)29/h2-5,9-12,18,29H,6-8,13-17H2,1H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Washington University School of Medicine
Curated by ChEMBL
| Assay Description
Displacement of of [125I]-IABN from human D3 receptor expressed in HEK293 cell membranes after 60 mins by filtration binding assay |
Bioorg Med Chem Lett 25: 519-23 (2015)
Article DOI: 10.1016/j.bmcl.2014.12.023
BindingDB Entry DOI: 10.7270/Q27W6DS9 |
More data for this
Ligand-Target Pair | |
Cyclin-dependent kinase 2
(Homo sapiens (Human)) | BDBM12621

(2,4-Diamino-5-ketopyrimidine 39 | 5-[(2,3-difluoro...)Show SMILES COc1ccc(F)c(F)c1C(=O)c1cnc(NC2CCN(CC2)S(C)(=O)=O)nc1N Show InChI InChI=1S/C18H21F2N5O4S/c1-29-13-4-3-12(19)15(20)14(13)16(26)11-9-22-18(24-17(11)21)23-10-5-7-25(8-6-10)30(2,27)28/h3-4,9-10H,5-8H2,1-2H3,(H3,21,22,23,24) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| MMDB
PDB
Article
PubMed
| 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c02190
BindingDB Entry DOI: 10.7270/Q2BV7MPD |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Maternal embryonic leucine zipper kinase
(Homo sapiens (Human)) | BDBM50248945
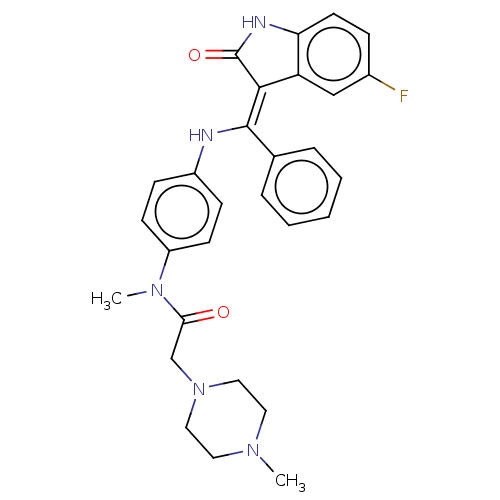
(CHEMBL4081410 | US10981896, Compound 19)Show SMILES CN(C(=O)CN1CCN(C)CC1)c1ccc(N\C(=C2/C(=O)Nc3ccc(F)cc23)c2ccccc2)cc1 Show InChI InChI=1S/C29H30FN5O2/c1-33-14-16-35(17-15-33)19-26(36)34(2)23-11-9-22(10-12-23)31-28(20-6-4-3-5-7-20)27-24-18-21(30)8-13-25(24)32-29(27)37/h3-13,18,31H,14-17,19H2,1-2H3,(H,32,37)/b28-27- | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 3.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Texas at Austin
Curated by ChEMBL
| Assay Description
Inhibition of His6-tagged MELK catalytic domain (1 to 340 residues) (unknown origin) expressed in Escherichia coli BL21 (DE3) cells using Bcl-GL as s... |
Bioorg Med Chem 25: 2609-2616 (2017)
Article DOI: 10.1016/j.bmc.2017.03.018
BindingDB Entry DOI: 10.7270/Q2833VFF |
More data for this
Ligand-Target Pair | |
D(3) dopamine receptor
(Homo sapiens (Human)) | BDBM50042721

(CHEMBL3353896)Show SMILES COc1ccccc1N1CCN(CCCCCc2cn(nn2)-c2ccccc2)CC1 Show InChI InChI=1S/C24H31N5O/c1-30-24-14-8-7-13-23(24)28-18-16-27(17-19-28)15-9-3-4-10-21-20-29(26-25-21)22-11-5-2-6-12-22/h2,5-8,11-14,20H,3-4,9-10,15-19H2,1H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Washington University School of Medicine
Curated by ChEMBL
| Assay Description
Displacement of of [125I]-IABN from human D3 receptor expressed in HEK293 cell membranes after 60 mins by filtration binding assay |
Bioorg Med Chem Lett 25: 519-23 (2015)
Article DOI: 10.1016/j.bmcl.2014.12.023
BindingDB Entry DOI: 10.7270/Q27W6DS9 |
More data for this
Ligand-Target Pair | |
D(3) dopamine receptor
(Homo sapiens (Human)) | BDBM50042840
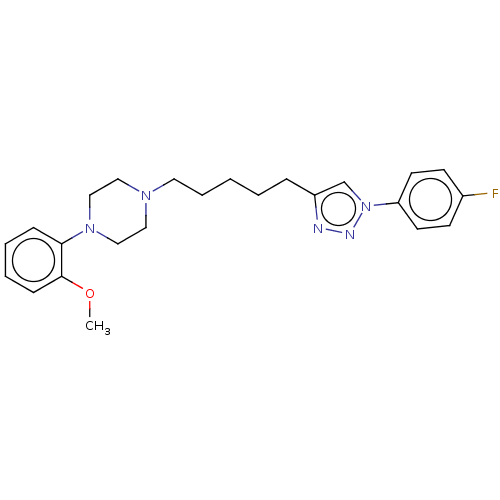
(CHEMBL3353905)Show SMILES COc1ccccc1N1CCN(CCCCCc2cn(nn2)-c2ccc(F)cc2)CC1 Show InChI InChI=1S/C24H30FN5O/c1-31-24-9-5-4-8-23(24)29-17-15-28(16-18-29)14-6-2-3-7-21-19-30(27-26-21)22-12-10-20(25)11-13-22/h4-5,8-13,19H,2-3,6-7,14-18H2,1H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Washington University School of Medicine
Curated by ChEMBL
| Assay Description
Displacement of of [125I]-IABN from human D3 receptor expressed in HEK293 cell membranes after 60 mins by filtration binding assay |
Bioorg Med Chem Lett 25: 519-23 (2015)
Article DOI: 10.1016/j.bmcl.2014.12.023
BindingDB Entry DOI: 10.7270/Q27W6DS9 |
More data for this
Ligand-Target Pair | |
Polycystin-1
(Homo sapiens (Human)) | BDBM86429

(CAS_6437389 | NSC_6437389 | Thymeleatoxin | Thymel...)Show SMILES CC1C(OC(=O)C=Cc2ccccc2)C2(OC3(OC2C2C4OC4(CO)C(O)C4(O)C(C=C(C)C4=O)C12O3)c1ccccc1)C(C)=C |w:6.5,t:33,TLB:1:35:17:14.15,THB:2:14:19.35.36:17,20:19:17:14.15,20:19:1.2.14:36.16.17| Show InChI InChI=1S/C36H36O10/c1-19(2)34-28(42-25(38)16-15-22-11-7-5-8-12-22)21(4)35-24-17-20(3)27(39)33(24,41)31(40)32(18-37)29(43-32)26(35)30(34)44-36(45-34,46-35)23-13-9-6-10-14-23/h5-17,21,24,26,28-31,37,40-41H,1,18H2,2-4H3 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Pittsburgh
Curated by PDSP Ki Database
| |
Mol Pharmacol 64: 1342-8 (2003)
Article DOI: 10.1124/mol.64.6.1342
BindingDB Entry DOI: 10.7270/Q270800T |
More data for this
Ligand-Target Pair | |
Protein kinase C alpha type
(Homo sapiens (Human)) | BDBM86435

(B8-DL-B8 | B8-DL-B8 (low PS))Show SMILES CCC(C)(C\C=C1\CC(CO)(COC(=O)CC(C)(CC)C(C)C)OC1=O)C(C)C Show InChI InChI=1S/C24H42O5/c1-9-22(7,17(3)4)12-11-19-13-24(15-25,29-21(19)27)16-28-20(26)14-23(8,10-2)18(5)6/h11,17-18,25H,9-10,12-16H2,1-8H3/b19-11- | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3.57 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Pittsburgh
Curated by PDSP Ki Database
| |
Mol Pharmacol 64: 1342-8 (2003)
Article DOI: 10.1124/mol.64.6.1342
BindingDB Entry DOI: 10.7270/Q270800T |
More data for this
Ligand-Target Pair | |
D(3) dopamine receptor
(Homo sapiens (Human)) | BDBM50042709
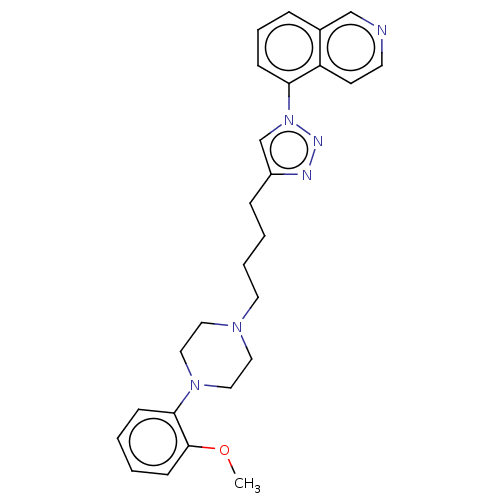
(CHEMBL3353898)Show SMILES COc1ccccc1N1CCN(CCCCc2cn(nn2)-c2cccc3cnccc23)CC1 Show InChI InChI=1S/C26H30N6O/c1-33-26-11-3-2-9-25(26)31-17-15-30(16-18-31)14-5-4-8-22-20-32(29-28-22)24-10-6-7-21-19-27-13-12-23(21)24/h2-3,6-7,9-13,19-20H,4-5,8,14-18H2,1H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Washington University School of Medicine
Curated by ChEMBL
| Assay Description
Displacement of of [125I]-IABN from human D3 receptor expressed in HEK293 cell membranes after 60 mins by filtration binding assay |
Bioorg Med Chem Lett 25: 519-23 (2015)
Article DOI: 10.1016/j.bmcl.2014.12.023
BindingDB Entry DOI: 10.7270/Q27W6DS9 |
More data for this
Ligand-Target Pair | |
Cathepsin B
(Homo sapiens (Human)) | BDBM50137736

((1S,5S,6R)-2-((S)-3,3-Dimethyl-2-{(S)-3-methyl-2-[...)Show SMILES CCC[C@H](NC(=O)[C@@H]1[C@H]2CCC[C@H]2CN1C(=O)[C@@H](NC(=O)[C@@H](NC(=O)c1cnccn1)C(C)C)C(C)(C)C)C(=O)C(=O)NC(C)CC Show InChI InChI=1S/C34H53N7O6/c1-9-12-23(27(42)32(46)37-20(5)10-2)38-31(45)26-22-14-11-13-21(22)18-41(26)33(47)28(34(6,7)8)40-30(44)25(19(3)4)39-29(43)24-17-35-15-16-36-24/h15-17,19-23,25-26,28H,9-14,18H2,1-8H3,(H,37,46)(H,38,45)(H,39,43)(H,40,44)/t20?,21-,22-,23-,25-,26-,28+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 3.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratory
Curated by ChEMBL
| Assay Description
Inhibitory activity of the compound against cathepsin B |
Bioorg Med Chem Lett 14: 257-61 (2003)
BindingDB Entry DOI: 10.7270/Q20P0ZDG |
More data for this
Ligand-Target Pair | |
Polycystin-1
(Homo sapiens (Human)) | BDBM86429

(CAS_6437389 | NSC_6437389 | Thymeleatoxin | Thymel...)Show SMILES CC1C(OC(=O)C=Cc2ccccc2)C2(OC3(OC2C2C4OC4(CO)C(O)C4(O)C(C=C(C)C4=O)C12O3)c1ccccc1)C(C)=C |w:6.5,t:33,TLB:1:35:17:14.15,THB:2:14:19.35.36:17,20:19:17:14.15,20:19:1.2.14:36.16.17| Show InChI InChI=1S/C36H36O10/c1-19(2)34-28(42-25(38)16-15-22-11-7-5-8-12-22)21(4)35-24-17-20(3)27(39)33(24,41)31(40)32(18-37)29(43-32)26(35)30(34)44-36(45-34,46-35)23-13-9-6-10-14-23/h5-17,21,24,26,28-31,37,40-41H,1,18H2,2-4H3 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3.85 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Pittsburgh
Curated by PDSP Ki Database
| |
Mol Pharmacol 64: 1342-8 (2003)
Article DOI: 10.1124/mol.64.6.1342
BindingDB Entry DOI: 10.7270/Q270800T |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 1A
(Homo sapiens (Human)) | BDBM50042729

(CHEMBL3353914)Show SMILES COc1ccccc1N1CCN(CCCCCc2cn(nn2)-c2ccc(SC)cc2)CC1 Show InChI InChI=1S/C25H33N5OS/c1-31-25-10-6-5-9-24(25)29-18-16-28(17-19-29)15-7-3-4-8-21-20-30(27-26-21)22-11-13-23(32-2)14-12-22/h5-6,9-14,20H,3-4,7-8,15-19H2,1-2H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Washington University School of Medicine
Curated by ChEMBL
| Assay Description
Displacement of of [3H]8-OH-DPAT from human 5HT1A receptor after 60 mins by filtration binding assay |
Bioorg Med Chem Lett 25: 519-23 (2015)
Article DOI: 10.1016/j.bmcl.2014.12.023
BindingDB Entry DOI: 10.7270/Q27W6DS9 |
More data for this
Ligand-Target Pair | |
Cathepsin B
(Homo sapiens (Human)) | BDBM50137720

((1S,3aR,6aS)-2-((S)-3-Methyl-2-{(S)-3-methyl-2-[(p...)Show SMILES CCCC(NC(=O)[C@@H]1[C@H]2CCC[C@H]2CN1C(=O)[C@@H](NC(=O)[C@@H](NC(=O)c1cnccn1)C(C)C)C(C)C)C(=O)C(=O)NC1CC1 Show InChI InChI=1S/C32H47N7O6/c1-6-8-22(27(40)31(44)35-20-11-12-20)36-30(43)26-21-10-7-9-19(21)16-39(26)32(45)25(18(4)5)38-29(42)24(17(2)3)37-28(41)23-15-33-13-14-34-23/h13-15,17-22,24-26H,6-12,16H2,1-5H3,(H,35,44)(H,36,43)(H,37,41)(H,38,42)/t19-,21-,22?,24-,25-,26-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratory
Curated by ChEMBL
| Assay Description
Inhibitory activity of the compound against cathepsin B |
Bioorg Med Chem Lett 14: 257-61 (2003)
BindingDB Entry DOI: 10.7270/Q20P0ZDG |
More data for this
Ligand-Target Pair | |
Metabotropic glutamate receptor 3
(Homo sapiens (Human)) | BDBM50151435

((1R,2R,3R,5R,6R)-2-Amino-3-(3,4-dichloro-benzyloxy...)Show SMILES N[C@@]1([C@H]2[C@@H](C[C@H]1OCc1ccc(Cl)c(Cl)c1)[C@]2(F)C(O)=O)C(O)=O Show InChI InChI=1S/C15H14Cl2FNO5/c16-8-2-1-6(3-9(8)17)5-24-10-4-7-11(14(7,18)12(20)21)15(10,19)13(22)23/h1-3,7,10-11H,4-5,19H2,(H,20,21)(H,22,23)/t7-,10-,11+,14-,15+/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 4.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Massachusetts General Hospital
Curated by ChEMBL
| Assay Description
Binding affinity to mGLUR3 |
Bioorg Med Chem Lett 22: 1958-62 (2012)
Article DOI: 10.1016/j.bmcl.2012.01.039
BindingDB Entry DOI: 10.7270/Q2T72JJ1 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data