

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Delta-type opioid receptor (Homo sapiens (Human)) | BDBM50144258 (4-[(8-Benzo[1,3]dioxol-5-ylmethyl-8-aza-bicyclo[3....) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson and Johnson Pharmaceutical Research and Development Curated by ChEMBL | Assay Description Binding affinity for delta opioid receptor | Bioorg Med Chem Lett 14: 2109-12 (2004) Article DOI: 10.1016/j.bmcl.2004.02.051 BindingDB Entry DOI: 10.7270/Q25B01X4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Homo sapiens (Human)) | BDBM50144236 (4-[(R)-(S)-8-Aza-bicyclo[3.2.1]oct-(3Z)-ylidene-ph...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0230 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson and Johnson Pharmaceutical Research and Development Curated by ChEMBL | Assay Description Binding affinity for delta opioid receptor | Bioorg Med Chem Lett 14: 2109-12 (2004) Article DOI: 10.1016/j.bmcl.2004.02.051 BindingDB Entry DOI: 10.7270/Q25B01X4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Homo sapiens (Human)) | BDBM50144229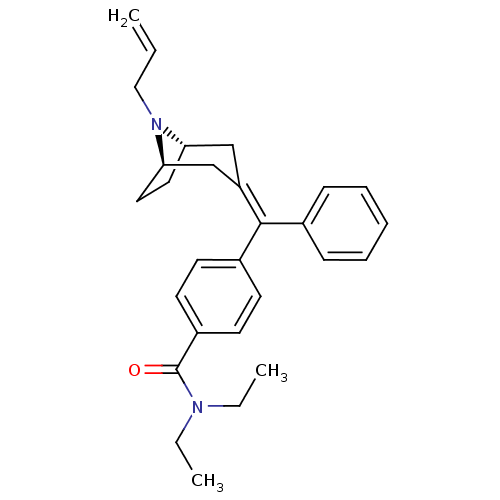 (4-{[(1S,5R)-8-Allyl-8-aza-bicyclo[3.2.1]oct-(3Z)-y...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0250 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson and Johnson Pharmaceutical Research and Development Curated by ChEMBL | Assay Description Effective concentration against stimulation of [35S]-GTP-gammaS, binding in CHO cells transfected with the human opioid receptor delta 1 | Bioorg Med Chem Lett 14: 2109-12 (2004) Article DOI: 10.1016/j.bmcl.2004.02.051 BindingDB Entry DOI: 10.7270/Q25B01X4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM50144281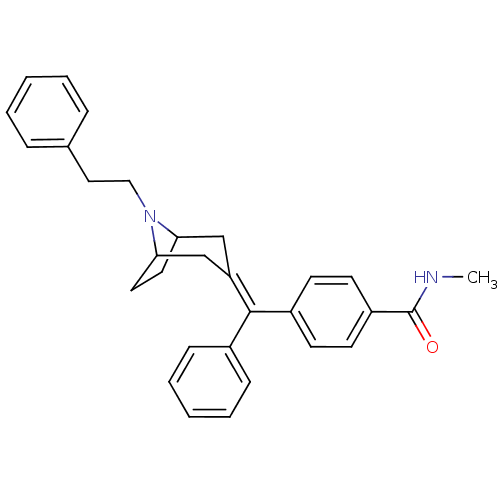 (CHEMBL63309 | N-Methyl-4-[(8-phenethyl-8-aza-bicyc...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.140 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson and Johnson Pharmaceutical Research and Development Curated by ChEMBL | Assay Description In vitro binding affinity towards opioid receptor mu 1 | Bioorg Med Chem Lett 14: 2113-6 (2004) Article DOI: 10.1016/j.bmcl.2004.02.052 BindingDB Entry DOI: 10.7270/Q21J997Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Homo sapiens (Human)) | BDBM50144284 (CHEMBL68412 | N-Ethyl-4-[(3-hydroxy-phenyl)-(8-thi...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.152 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson and Johnson Pharmaceutical Research and Development Curated by ChEMBL | Assay Description In vitro binding affinity towards opioid receptor delta 1 | Bioorg Med Chem Lett 14: 2113-6 (2004) Article DOI: 10.1016/j.bmcl.2004.02.052 BindingDB Entry DOI: 10.7270/Q21J997Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Rattus norvegicus (rat)) | BDBM50088712 (4-{[(1S,3S,5R)-8-(Benzo[1,3]dioxole-5-carbonyl)-8-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
R. W. Johnson Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibition of [3H]-DPDPE radioligand binding to rat opioid receptor delta 1 site from rat brain membranes | Bioorg Med Chem Lett 10: 1109-11 (2000) BindingDB Entry DOI: 10.7270/Q2X0668B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(3) dopamine receptor (Homo sapiens (Human)) | BDBM50020217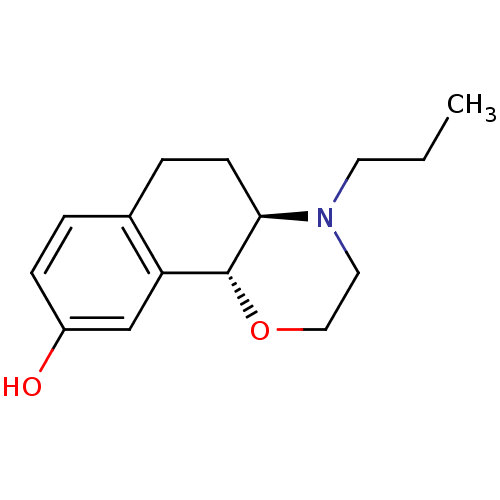 ((4aR,10bR)-4-propyl-3,4,4a,5,6,10b-hexahydro-2H-na...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Imperial College London Curated by ChEMBL | Assay Description Agonistic activity at DRD3 receptor | Bioorg Med Chem Lett 19: 5056-9 (2009) Article DOI: 10.1016/j.bmcl.2009.07.055 BindingDB Entry DOI: 10.7270/Q2PR7X7C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (MOUSE) | BDBM50155124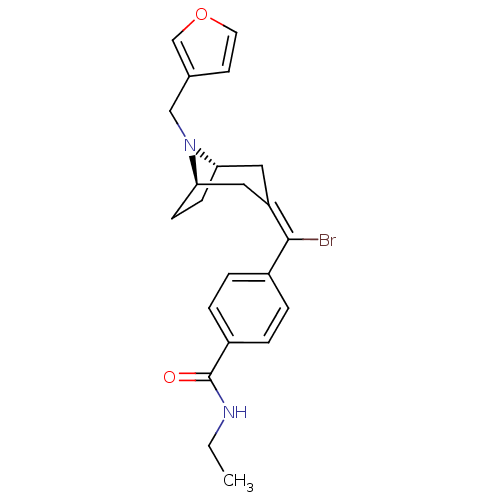 (4-{Bromo-[(1S,5R)-8-furan-3-ylmethyl-8-aza-bicyclo...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson and Johnson Pharmaceutical Research and Development Curated by ChEMBL | Assay Description Binding affinity against mu opioid receptor in mouse hot plate test | Bioorg Med Chem Lett 14: 5493-8 (2004) Article DOI: 10.1016/j.bmcl.2004.09.004 BindingDB Entry DOI: 10.7270/Q2X34WZV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM50144275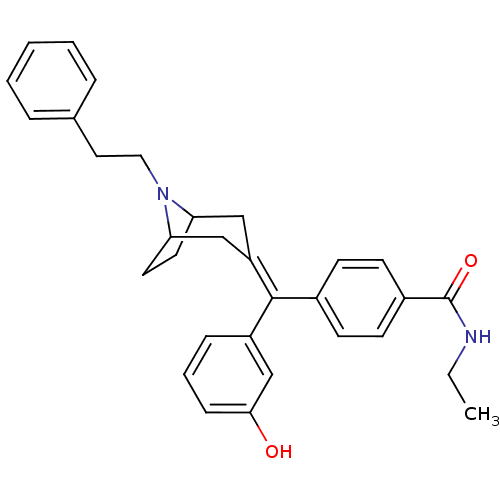 (CHEMBL419621 | N-Ethyl-4-[(3-hydroxy-phenyl)-(8-ph...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.222 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson and Johnson Pharmaceutical Research and Development Curated by ChEMBL | Assay Description In vitro binding affinity towards opioid receptor mu 1 | Bioorg Med Chem Lett 14: 2113-6 (2004) Article DOI: 10.1016/j.bmcl.2004.02.052 BindingDB Entry DOI: 10.7270/Q21J997Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Homo sapiens (Human)) | BDBM50144249 (CHEMBL302801 | N,N-Diethyl-4-{[(1S,5R)-8-phenethyl...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.240 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson and Johnson Pharmaceutical Research and Development Curated by ChEMBL | Assay Description In vitro binding affinity towards opioid receptor delta 1 | Bioorg Med Chem Lett 14: 2113-6 (2004) Article DOI: 10.1016/j.bmcl.2004.02.052 BindingDB Entry DOI: 10.7270/Q21J997Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Homo sapiens (Human)) | BDBM50144249 (CHEMBL302801 | N,N-Diethyl-4-{[(1S,5R)-8-phenethyl...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.240 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson and Johnson Pharmaceutical Research and Development Curated by ChEMBL | Assay Description Binding affinity for delta opioid receptor | Bioorg Med Chem Lett 14: 2109-12 (2004) Article DOI: 10.1016/j.bmcl.2004.02.051 BindingDB Entry DOI: 10.7270/Q25B01X4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Homo sapiens (Human)) | BDBM50076428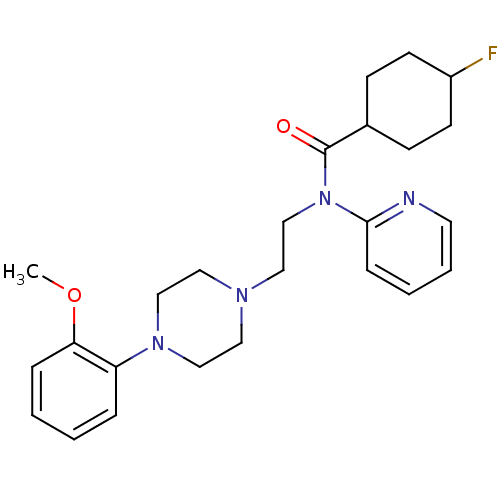 (4-Fluoro-cyclohexanecarboxylic acid {2-[4-(2-metho...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.247 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institutes of Health Curated by ChEMBL | Assay Description In vitro inhibition of [3H]- 8-OH-DPAT binding to cloned cell line containing human 5-hydroxytryptamine 1A receptor by Panlabs assay | J Med Chem 42: 1576-86 (1999) Article DOI: 10.1021/jm980456f BindingDB Entry DOI: 10.7270/Q23R0S2J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Homo sapiens (Human)) | BDBM50144291 (CHEMBL67790 | N-Ethyl-4-[(4-hydroxy-phenyl)-(8-thi...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.25 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson and Johnson Pharmaceutical Research and Development Curated by ChEMBL | Assay Description In vitro binding affinity towards opioid receptor delta 1 | Bioorg Med Chem Lett 14: 2113-6 (2004) Article DOI: 10.1016/j.bmcl.2004.02.052 BindingDB Entry DOI: 10.7270/Q21J997Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (MOUSE) | BDBM50155085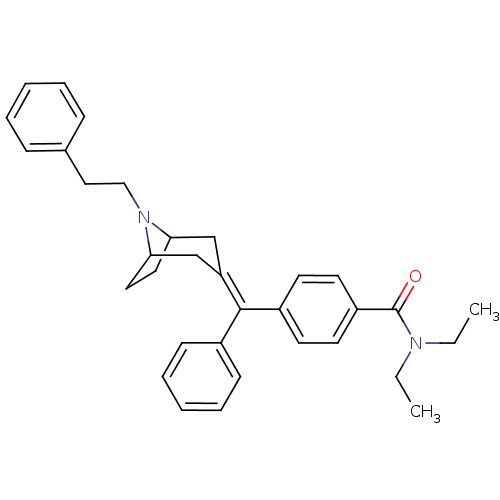 (CHEMBL362331 | N,N-Diethyl-4-[(8-phenethyl-8-aza-b...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.260 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson and Johnson Pharmaceutical Research and Development Curated by ChEMBL | Assay Description Binding affinity against mu opioid receptor in mouse hot plate test | Bioorg Med Chem Lett 14: 5493-8 (2004) Article DOI: 10.1016/j.bmcl.2004.09.004 BindingDB Entry DOI: 10.7270/Q2X34WZV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM50144276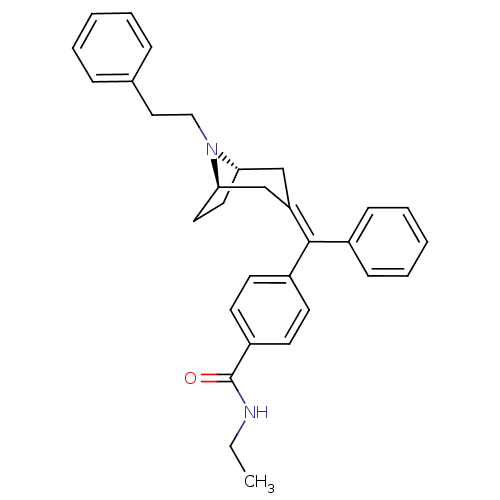 (CHEMBL63651 | N-Ethyl-4-{[(1S,5R)-8-phenethyl-8-az...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.260 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson and Johnson Pharmaceutical Research and Development Curated by ChEMBL | Assay Description In vitro binding affinity towards opioid receptor mu 1 | Bioorg Med Chem Lett 14: 2113-6 (2004) Article DOI: 10.1016/j.bmcl.2004.02.052 BindingDB Entry DOI: 10.7270/Q21J997Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Synaptic vesicle glycoprotein 2A (Rattus norvegicus) | BDBM582018 (US11518754, Compound (R)-3) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | US Patent | 0.270 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description For competition binding experiments, 0.5 mg of the rat brain homogenates in 800 μL of binding buffer (2 mM MgCl2 in 50 mM Tris*HCl, pH=7.4) were... | Citation and Details BindingDB Entry DOI: 10.7270/Q2FN1B15 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Homo sapiens (Human)) | BDBM50144234 (4-((R)-(S)-8-Aza-bicyclo[3.2.1]oct-3-yl-phenyl-ami...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson and Johnson Pharmaceutical Research and Development Curated by ChEMBL | Assay Description Effective concentration against stimulation of [35S]-GTP-gammaS, binding in CHO cells transfected with the human opioid receptor delta 1 | Bioorg Med Chem Lett 14: 2109-12 (2004) Article DOI: 10.1016/j.bmcl.2004.02.051 BindingDB Entry DOI: 10.7270/Q25B01X4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Homo sapiens (Human)) | BDBM50144246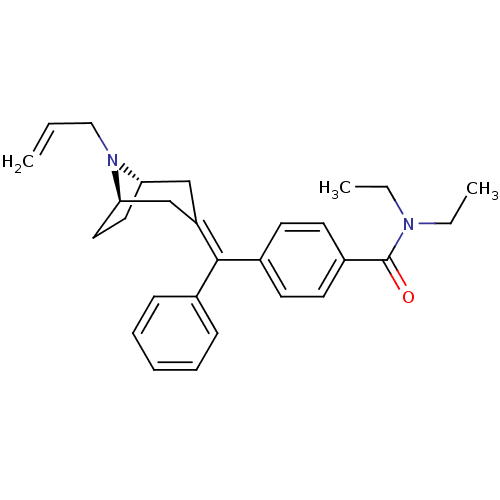 (4-{[(1R,5S)-8-Allyl-8-aza-bicyclo[3.2.1]oct-(3E)-y...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.320 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson and Johnson Pharmaceutical Research and Development Curated by ChEMBL | Assay Description Binding affinity for delta opioid receptor | Bioorg Med Chem Lett 14: 2109-12 (2004) Article DOI: 10.1016/j.bmcl.2004.02.051 BindingDB Entry DOI: 10.7270/Q25B01X4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1D (Mus musculus (Mouse)) | BDBM86708 (CAS_146714-97-8 | CHEMBL31354 | CHEMBL514874 | CHE...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | 0.340 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institutes of Health Curated by ChEMBL | Assay Description Receptor-linked G protein activation at 5-hydroxytryptamine receptor was determined by measuring the stimulation of [35S]-GTP-gammaS, binding (Experi... | J Med Chem 42: 1576-86 (1999) Article DOI: 10.1021/jm980456f BindingDB Entry DOI: 10.7270/Q23R0S2J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Homo sapiens (Human)) | BDBM50144233 (4-[(S)-(R)-8-Aza-bicyclo[3.2.1]oct-(3E)-ylidene-ph...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.360 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson and Johnson Pharmaceutical Research and Development Curated by ChEMBL | Assay Description Binding affinity for delta opioid receptor | Bioorg Med Chem Lett 14: 2109-12 (2004) Article DOI: 10.1016/j.bmcl.2004.02.051 BindingDB Entry DOI: 10.7270/Q25B01X4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Homo sapiens (Human)) | BDBM50144240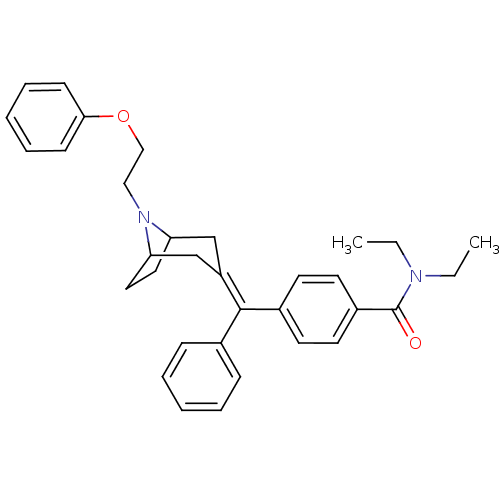 (CHEMBL65151 | N,N-Diethyl-4-{[8-(2-phenoxy-ethyl)-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.380 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson and Johnson Pharmaceutical Research and Development Curated by ChEMBL | Assay Description Binding affinity for delta opioid receptor | Bioorg Med Chem Lett 14: 2109-12 (2004) Article DOI: 10.1016/j.bmcl.2004.02.051 BindingDB Entry DOI: 10.7270/Q25B01X4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Homo sapiens (Human)) | BDBM50144282 (4-[(8-Allyl-8-aza-bicyclo[3.2.1]oct-3-ylidene)-(3-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.384 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson and Johnson Pharmaceutical Research and Development Curated by ChEMBL | Assay Description In vitro binding affinity towards opioid receptor delta 1 | Bioorg Med Chem Lett 14: 2113-6 (2004) Article DOI: 10.1016/j.bmcl.2004.02.052 BindingDB Entry DOI: 10.7270/Q21J997Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Homo sapiens (Human)) | BDBM50144244 (CHEMBL63571 | N,N-Diethyl-4-[phenyl-(8-propyl-8-az...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.390 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson and Johnson Pharmaceutical Research and Development Curated by ChEMBL | Assay Description Binding affinity for delta opioid receptor | Bioorg Med Chem Lett 14: 2109-12 (2004) Article DOI: 10.1016/j.bmcl.2004.02.051 BindingDB Entry DOI: 10.7270/Q25B01X4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Rattus norvegicus (rat)) | BDBM50088713 (4-((1S,3S,5R)-8-Aza-bicyclo[3.2.1]oct-3-yl-phenyl-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
R. W. Johnson Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibition of [3H]-DPDPE radioligand binding to rat opioid receptor delta 1 site from rat brain membranes | Bioorg Med Chem Lett 10: 1109-11 (2000) BindingDB Entry DOI: 10.7270/Q2X0668B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Rattus norvegicus (rat)) | BDBM50088715 (4-[((1R,3S,5S)-8-Allyl-8-aza-bicyclo[3.2.1]oct-3-y...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
R. W. Johnson Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibition of [3H]-DPDPE radioligand binding to rat opioid receptor delta 1 site from rat brain membranes | Bioorg Med Chem Lett 10: 1109-11 (2000) BindingDB Entry DOI: 10.7270/Q2X0668B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Homo sapiens (Human)) | BDBM50144247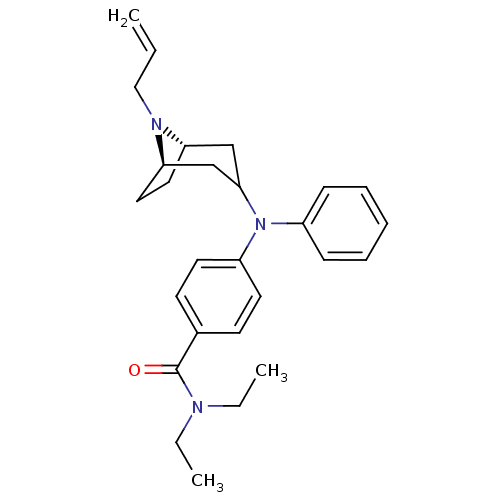 (4-[((1S,5R)-8-Allyl-8-aza-bicyclo[3.2.1]oct-3-yl)-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson and Johnson Pharmaceutical Research and Development Curated by ChEMBL | Assay Description Effective concentration against stimulation of [35S]-GTP-gammaS, binding in CHO cells transfected with the human opioid receptor delta 1 | Bioorg Med Chem Lett 14: 2109-12 (2004) Article DOI: 10.1016/j.bmcl.2004.02.051 BindingDB Entry DOI: 10.7270/Q25B01X4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Homo sapiens (Human)) | BDBM50144273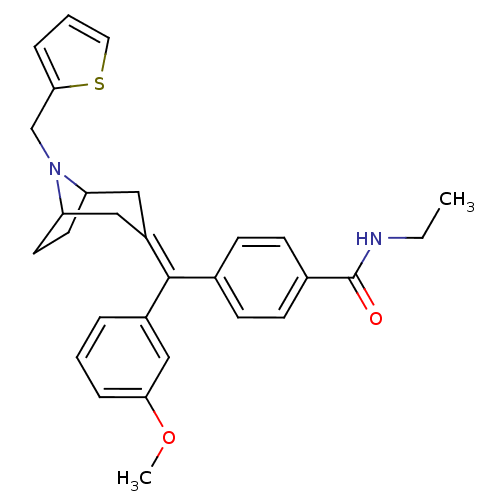 (CHEMBL66741 | N-Ethyl-4-[(3-methoxy-phenyl)-(8-thi...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.510 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson and Johnson Pharmaceutical Research and Development Curated by ChEMBL | Assay Description In vitro binding affinity towards opioid receptor delta 1 | Bioorg Med Chem Lett 14: 2113-6 (2004) Article DOI: 10.1016/j.bmcl.2004.02.052 BindingDB Entry DOI: 10.7270/Q21J997Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Homo sapiens (Human)) | BDBM50076428 (4-Fluoro-cyclohexanecarboxylic acid {2-[4-(2-metho...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.515 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institutes of Health Curated by ChEMBL | Assay Description Compound was tested in vitro for the inhibition of [3H]- 8-OH-DPAT binding to cloned cell line containing human 5-hydroxytryptamine 1A receptor | J Med Chem 42: 1576-86 (1999) Article DOI: 10.1021/jm980456f BindingDB Entry DOI: 10.7270/Q23R0S2J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Homo sapiens (Human)) | BDBM50144235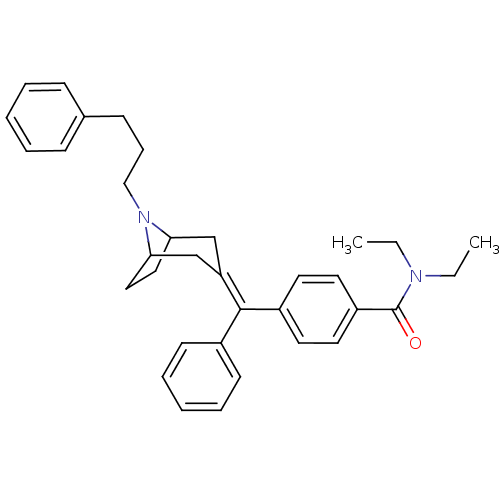 (CHEMBL63750 | N,N-Diethyl-4-{phenyl-[8-(3-phenyl-p...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.560 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson and Johnson Pharmaceutical Research and Development Curated by ChEMBL | Assay Description Binding affinity for delta opioid receptor | Bioorg Med Chem Lett 14: 2109-12 (2004) Article DOI: 10.1016/j.bmcl.2004.02.051 BindingDB Entry DOI: 10.7270/Q25B01X4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Synaptic vesicle glycoprotein 2A (Rattus norvegicus) | BDBM582020 (US11518754, Compound (R)-5) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.580 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description For competition binding experiments, 0.5 mg of the rat brain homogenates in 800 μL of binding buffer (2 mM MgCl2 in 50 mM Tris*HCl, pH=7.4) were... | Citation and Details BindingDB Entry DOI: 10.7270/Q2FN1B15 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Homo sapiens (Human)) | BDBM50144245 (4-[(8-Aza-bicyclo[3.2.1]oct-3-ylidene)-(4-hydroxy-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.580 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson and Johnson Pharmaceutical Research and Development Curated by ChEMBL | Assay Description Binding affinity for delta opioid receptor | Bioorg Med Chem Lett 14: 2109-12 (2004) Article DOI: 10.1016/j.bmcl.2004.02.051 BindingDB Entry DOI: 10.7270/Q25B01X4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (MOUSE) | BDBM50155068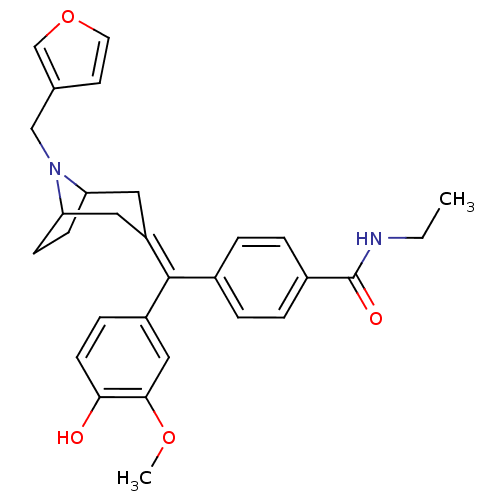 (CHEMBL361720 | N-Ethyl-4-[(8-furan-3-ylmethyl-8-az...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.590 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson and Johnson Pharmaceutical Research and Development Curated by ChEMBL | Assay Description Binding affinity against mu opioid receptor in mouse hot plate test | Bioorg Med Chem Lett 14: 5493-8 (2004) Article DOI: 10.1016/j.bmcl.2004.09.004 BindingDB Entry DOI: 10.7270/Q2X34WZV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Homo sapiens (Human)) | BDBM86708 (CAS_146714-97-8 | CHEMBL31354 | CHEMBL514874 | CHE...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | 0.590 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institutes of Health Curated by ChEMBL | Assay Description Compound was tested in vitro for the inhibition of [3H]- 8-OH-DPAT binding to cloned cell line containing human 5-hydroxytryptamine 1A receptor | J Med Chem 42: 1576-86 (1999) Article DOI: 10.1021/jm980456f BindingDB Entry DOI: 10.7270/Q23R0S2J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Homo sapiens (Human)) | BDBM50144243 (CHEMBL67996 | N,N-Diethyl-4-[(3-hydroxy-phenyl)-(8...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson and Johnson Pharmaceutical Research and Development Curated by ChEMBL | Assay Description Binding affinity for delta opioid receptor | Bioorg Med Chem Lett 14: 2109-12 (2004) Article DOI: 10.1016/j.bmcl.2004.02.051 BindingDB Entry DOI: 10.7270/Q25B01X4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(3) dopamine receptor (Homo sapiens (Human)) | BDBM50414563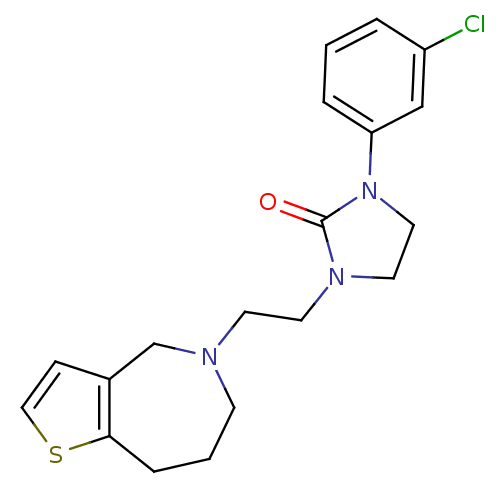 (CHEMBL559873) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 0.631 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Imperial College London Curated by ChEMBL | Assay Description Binding affinity to human DRD3 receptor by GTPgammaS binding assay | Bioorg Med Chem Lett 19: 5056-9 (2009) Article DOI: 10.1016/j.bmcl.2009.07.055 BindingDB Entry DOI: 10.7270/Q2PR7X7C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM50144271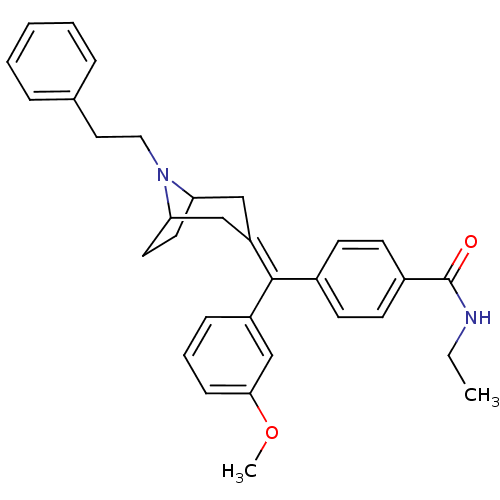 (CHEMBL67289 | N-Ethyl-4-[(3-methoxy-phenyl)-(8-phe...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.654 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson and Johnson Pharmaceutical Research and Development Curated by ChEMBL | Assay Description In vitro binding affinity towards opioid receptor mu 1 | Bioorg Med Chem Lett 14: 2113-6 (2004) Article DOI: 10.1016/j.bmcl.2004.02.052 BindingDB Entry DOI: 10.7270/Q21J997Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM50144284 (CHEMBL68412 | N-Ethyl-4-[(3-hydroxy-phenyl)-(8-thi...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.664 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson and Johnson Pharmaceutical Research and Development Curated by ChEMBL | Assay Description In vitro binding affinity towards opioid receptor mu 1 | Bioorg Med Chem Lett 14: 2113-6 (2004) Article DOI: 10.1016/j.bmcl.2004.02.052 BindingDB Entry DOI: 10.7270/Q21J997Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Homo sapiens (Human)) | BDBM50144293 (CHEMBL67240 | N-Ethyl-4-{(3-methoxy-phenyl)-[8-(3-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.720 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson and Johnson Pharmaceutical Research and Development Curated by ChEMBL | Assay Description In vitro binding affinity towards opioid receptor delta 1 | Bioorg Med Chem Lett 14: 2113-6 (2004) Article DOI: 10.1016/j.bmcl.2004.02.052 BindingDB Entry DOI: 10.7270/Q21J997Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Homo sapiens (Human)) | BDBM50076429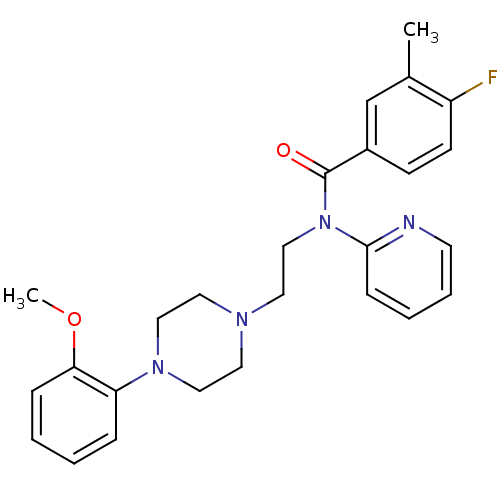 (4-Fluoro-N-{2-[4-(2-methoxy-phenyl)-piperazin-1-yl...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.791 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institutes of Health Curated by ChEMBL | Assay Description In vitro inhibition of [3H]- 8-OH-DPAT binding to cloned cell line containing human 5-hydroxytryptamine 1A receptor (Experiment 2) | J Med Chem 42: 1576-86 (1999) Article DOI: 10.1021/jm980456f BindingDB Entry DOI: 10.7270/Q23R0S2J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(3) dopamine receptor (Rattus norvegicus (Rat)) | BDBM50331549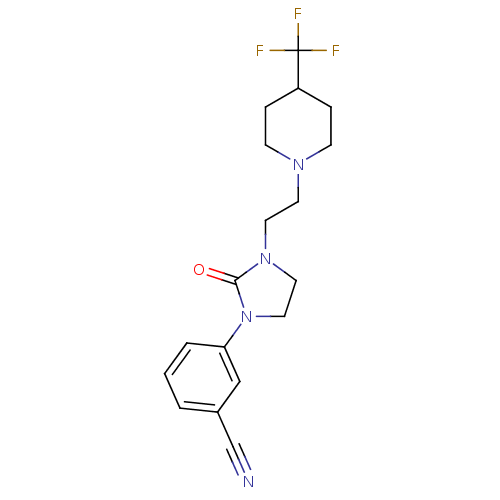 (CHEMBL567286 | [11C]-3-(2-oxo-3-(2-(4-(trifluorome...) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.794 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Imperial College London Curated by ChEMBL | Assay Description Antagonistic activity at rat DRD3 receptor | Bioorg Med Chem Lett 19: 5056-9 (2009) Article DOI: 10.1016/j.bmcl.2009.07.055 BindingDB Entry DOI: 10.7270/Q2PR7X7C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(3) dopamine receptor (Homo sapiens (Human)) | BDBM50331549 (CHEMBL567286 | [11C]-3-(2-oxo-3-(2-(4-(trifluorome...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.794 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Imperial College London Curated by ChEMBL | Assay Description Antagonistic activity at human DRD3 receptor by filtration binding assay | Bioorg Med Chem Lett 19: 5056-9 (2009) Article DOI: 10.1016/j.bmcl.2009.07.055 BindingDB Entry DOI: 10.7270/Q2PR7X7C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (MOUSE) | BDBM50155120 (4-[(4-Acetylamino-phenyl)-(8-furan-3-ylmethyl-8-az...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson and Johnson Pharmaceutical Research and Development Curated by ChEMBL | Assay Description Binding affinity against mu opioid receptor in mouse hot plate test | Bioorg Med Chem Lett 14: 5493-8 (2004) Article DOI: 10.1016/j.bmcl.2004.09.004 BindingDB Entry DOI: 10.7270/Q2X34WZV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (MOUSE) | BDBM50155082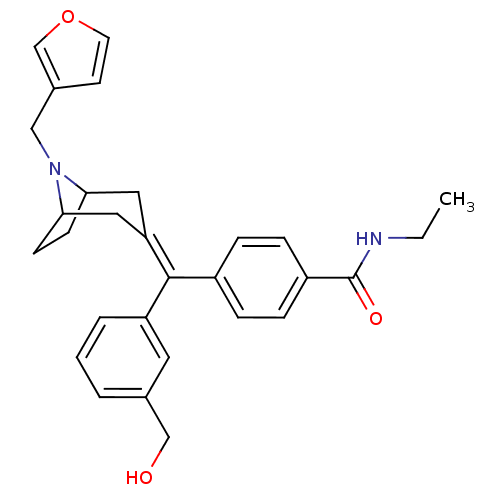 (CHEMBL363287 | N-Ethyl-4-[(8-furan-3-ylmethyl-8-az...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.810 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson and Johnson Pharmaceutical Research and Development Curated by ChEMBL | Assay Description Binding affinity against mu opioid receptor in mouse hot plate test | Bioorg Med Chem Lett 14: 5493-8 (2004) Article DOI: 10.1016/j.bmcl.2004.09.004 BindingDB Entry DOI: 10.7270/Q2X34WZV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Homo sapiens (Human)) | BDBM50144266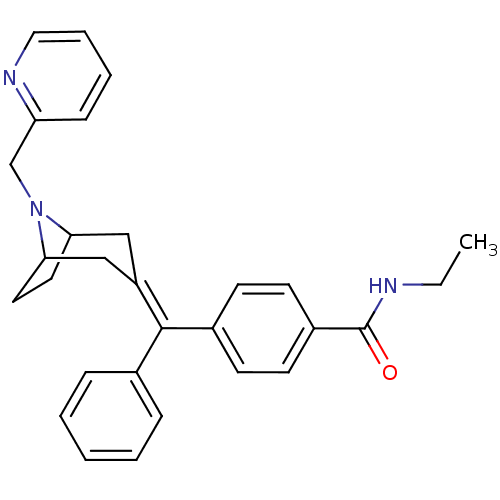 (CHEMBL291357 | N-Ethyl-4-[phenyl-(8-pyridin-2-ylme...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.860 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson and Johnson Pharmaceutical Research and Development Curated by ChEMBL | Assay Description In vitro binding affinity towards opioid receptor delta 1 | Bioorg Med Chem Lett 14: 2113-6 (2004) Article DOI: 10.1016/j.bmcl.2004.02.052 BindingDB Entry DOI: 10.7270/Q21J997Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Homo sapiens (Human)) | BDBM50144289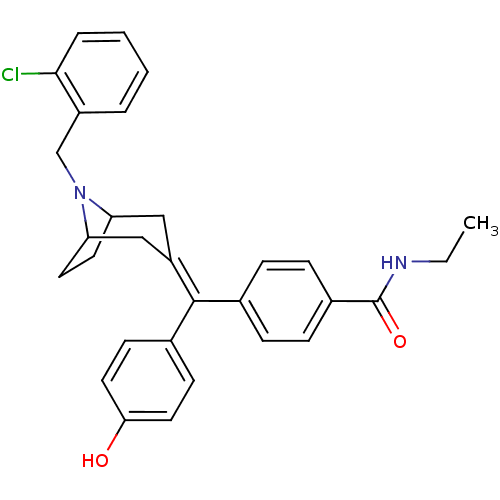 (4-[[8-(2-Chloro-benzyl)-8-aza-bicyclo[3.2.1]oct-3-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.930 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson and Johnson Pharmaceutical Research and Development Curated by ChEMBL | Assay Description In vitro binding affinity towards opioid receptor delta 1 | Bioorg Med Chem Lett 14: 2113-6 (2004) Article DOI: 10.1016/j.bmcl.2004.02.052 BindingDB Entry DOI: 10.7270/Q21J997Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (MOUSE) | BDBM50155069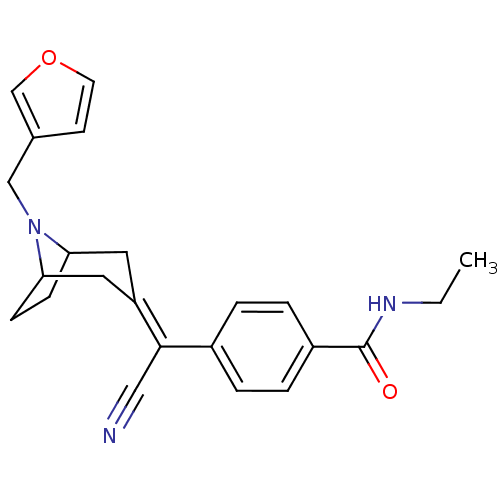 (4-[Cyano-(8-furan-3-ylmethyl-8-aza-bicyclo[3.2.1]o...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson and Johnson Pharmaceutical Research and Development Curated by ChEMBL | Assay Description Binding affinity against mu opioid receptor in mouse hot plate test | Bioorg Med Chem Lett 14: 5493-8 (2004) Article DOI: 10.1016/j.bmcl.2004.09.004 BindingDB Entry DOI: 10.7270/Q2X34WZV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1D (Mus musculus (Mouse)) | BDBM50076428 (4-Fluoro-cyclohexanecarboxylic acid {2-[4-(2-metho...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institutes of Health Curated by ChEMBL | Assay Description Receptor-linked G protein activation at 5-hydroxytryptamine receptor was determined by measuring the stimulation of [35S]-GTP-gammaS, binding | J Med Chem 42: 1576-86 (1999) Article DOI: 10.1021/jm980456f BindingDB Entry DOI: 10.7270/Q23R0S2J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM50144268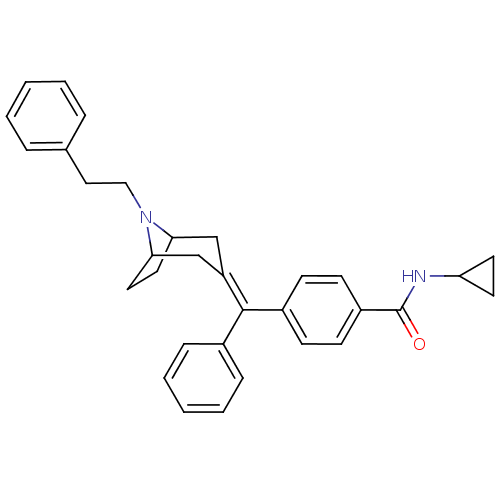 (CHEMBL307973 | N-Cyclopropyl-4-[(8-phenethyl-8-aza...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson and Johnson Pharmaceutical Research and Development Curated by ChEMBL | Assay Description In vitro binding affinity towards opioid receptor mu 1 | Bioorg Med Chem Lett 14: 2113-6 (2004) Article DOI: 10.1016/j.bmcl.2004.02.052 BindingDB Entry DOI: 10.7270/Q21J997Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Homo sapiens (Human)) | BDBM50076429 (4-Fluoro-N-{2-[4-(2-methoxy-phenyl)-piperazin-1-yl...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institutes of Health Curated by ChEMBL | Assay Description Compound was tested in vitro for the inhibition of [3H]- 8-OH-DPAT binding to cloned cell line containing human 5-hydroxytryptamine 1A receptor (Expe... | J Med Chem 42: 1576-86 (1999) Article DOI: 10.1021/jm980456f BindingDB Entry DOI: 10.7270/Q23R0S2J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (MOUSE) | BDBM50155096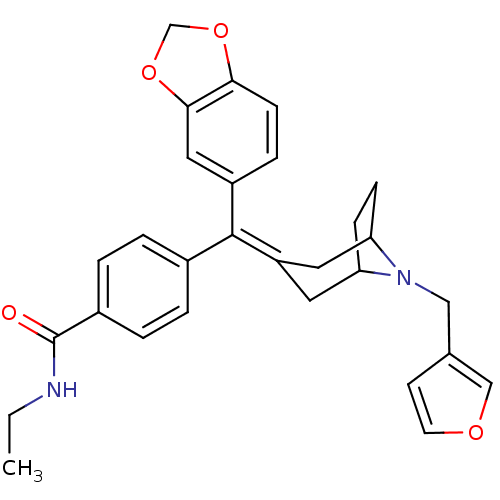 (4-[Benzo[1,3]dioxol-5-yl-(8-furan-3-ylmethyl-8-aza...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson and Johnson Pharmaceutical Research and Development Curated by ChEMBL | Assay Description Binding affinity against mu opioid receptor in mouse hot plate test | Bioorg Med Chem Lett 14: 5493-8 (2004) Article DOI: 10.1016/j.bmcl.2004.09.004 BindingDB Entry DOI: 10.7270/Q2X34WZV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 628 total ) | Next | Last >> |