

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Oxytocin receptor (Homo sapiens (Human)) | BDBM50326722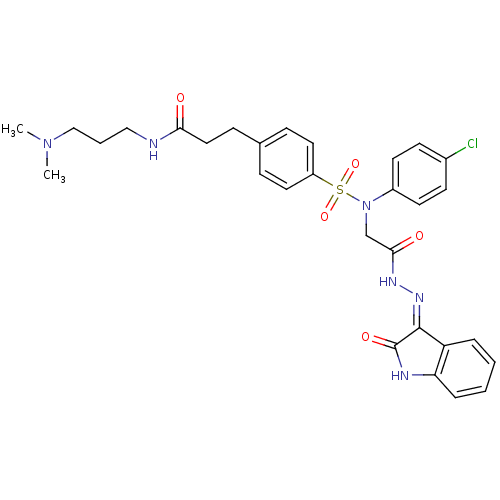 ((Z)-3-(4-(N-(4-chlorophenyl)-N-(2-oxo-2-(2-(2-oxoi...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.650 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Serono Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Displacement of [125I]OVTA antagonist from human oxytocin receptor expressed in HEK293-EBNA cells | J Med Chem 48: 7882-905 (2005) Article DOI: 10.1021/jm050645f BindingDB Entry DOI: 10.7270/Q2QF8TN3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxytocin receptor (RAT) | BDBM50326722 ((Z)-3-(4-(N-(4-chlorophenyl)-N-(2-oxo-2-(2-(2-oxoi...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.670 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Serono Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Displacement of [125I]OVTA antagonist from rat oxytocin receptor expressed in HEK293-EBNA cells | J Med Chem 48: 7882-905 (2005) Article DOI: 10.1021/jm050645f BindingDB Entry DOI: 10.7270/Q2QF8TN3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Homo sapiens (Human)) | BDBM50056445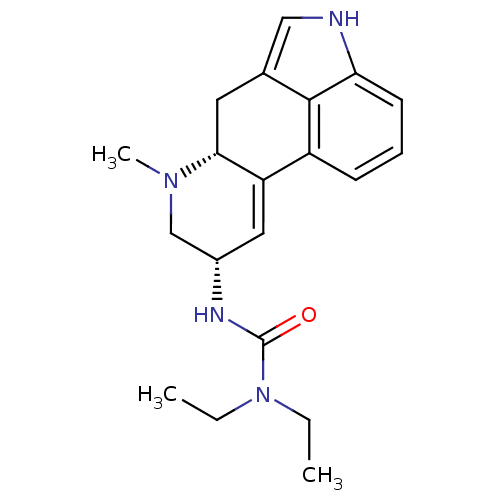 (1,1-diethyl-3-[(8alpha)-6-methyl-9,10-didehydroerg...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE PC cid PC sid PDB UniChem Patents Similars | DrugBank Article PubMed | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Texas at Austin Curated by ChEMBL | Assay Description Affinity towards Dopamine receptor D2 | J Med Chem 41: 4385-99 (1998) Article DOI: 10.1021/jm9800292 BindingDB Entry DOI: 10.7270/Q2R21227 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxytocin receptor (Homo sapiens (Human)) | BDBM50410616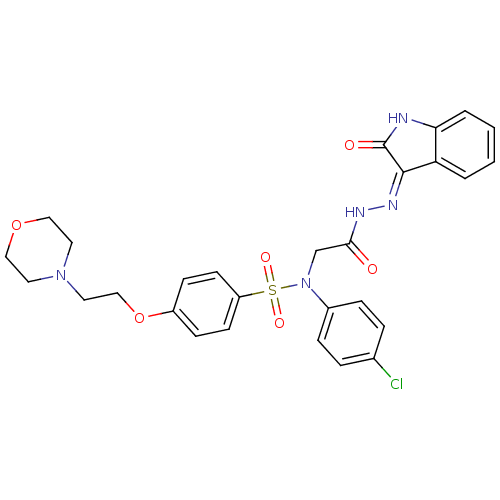 (CHEMBL2113185) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Serono Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Displacement of [125I]OVTA antagonist from human oxytocin receptor expressed in HEK293-EBNA cells | J Med Chem 48: 7882-905 (2005) Article DOI: 10.1021/jm050645f BindingDB Entry DOI: 10.7270/Q2QF8TN3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxytocin receptor (Homo sapiens (Human)) | BDBM50410618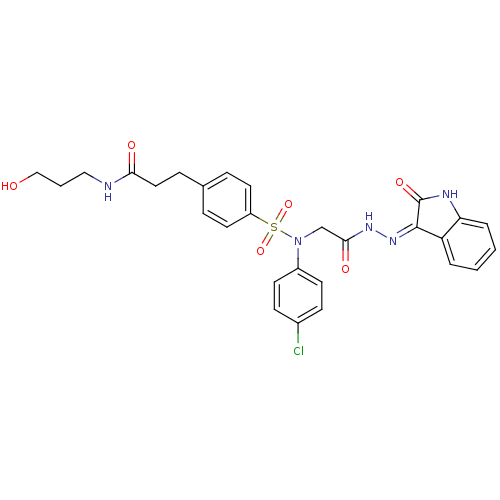 (CHEMBL2113181) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Serono Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Displacement of [125I]OVTA antagonist from human oxytocin receptor expressed in HEK293-EBNA cells | J Med Chem 48: 7882-905 (2005) Article DOI: 10.1021/jm050645f BindingDB Entry DOI: 10.7270/Q2QF8TN3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxytocin receptor (Homo sapiens (Human)) | BDBM50410625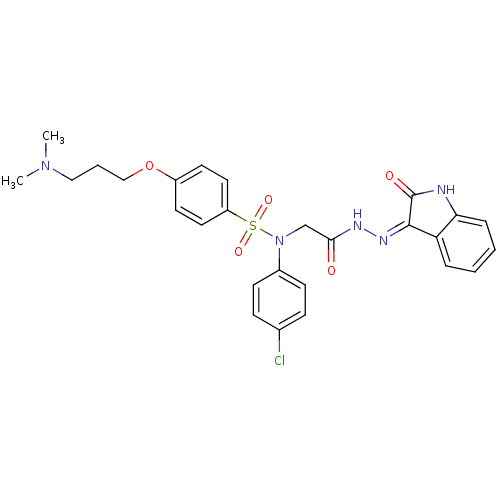 (CHEMBL2113201) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Serono Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Displacement of [125I]OVTA antagonist from human oxytocin receptor expressed in HEK293-EBNA cells | J Med Chem 48: 7882-905 (2005) Article DOI: 10.1021/jm050645f BindingDB Entry DOI: 10.7270/Q2QF8TN3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxytocin receptor (Homo sapiens (Human)) | BDBM50410634 (CHEMBL2113189) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Serono Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Displacement of [125I]OVTA antagonist from human oxytocin receptor expressed in HEK293-EBNA cells | J Med Chem 48: 7882-905 (2005) Article DOI: 10.1021/jm050645f BindingDB Entry DOI: 10.7270/Q2QF8TN3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Homo sapiens (Human)) | BDBM50067719 ((6aR,9R)-5-Bromo-9-carbamoyl-7-methyl-4,6,6a,7,8,9...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Texas at Austin Curated by ChEMBL | Assay Description Affinity towards Dopamine receptor D2 | J Med Chem 41: 4385-99 (1998) Article DOI: 10.1021/jm9800292 BindingDB Entry DOI: 10.7270/Q2R21227 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Translocator protein (Rattus norvegicus (rat)) | BDBM21363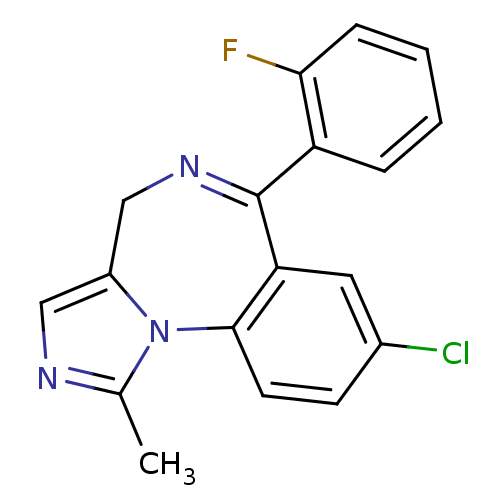 (12-chloro-9-(2-fluorophenyl)-3-methyl-2,4,8-triaza...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PubMed | 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Binding affinity of the compound was determined against rat benzodiazepine (BZD) receptor | Bioorg Med Chem Lett 12: 3219-22 (2002) BindingDB Entry DOI: 10.7270/Q2GQ6X4F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxytocin receptor (Homo sapiens (Human)) | BDBM50410622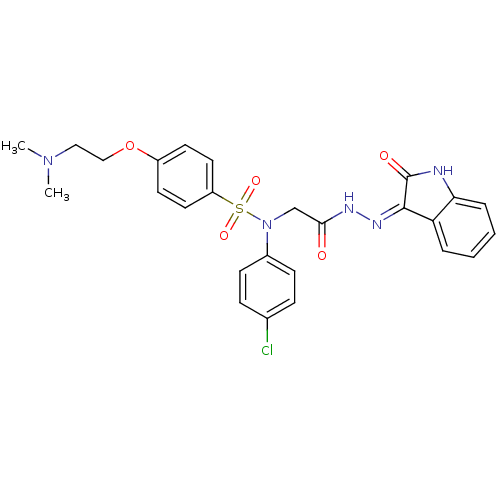 (CHEMBL2113203) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Serono Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Displacement of [125I]OVTA antagonist from human oxytocin receptor expressed in HEK293-EBNA cells | J Med Chem 48: 7882-905 (2005) Article DOI: 10.1021/jm050645f BindingDB Entry DOI: 10.7270/Q2QF8TN3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxytocin receptor (RAT) | BDBM50410634 (CHEMBL2113189) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 2.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Serono Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Displacement of [125I]OVTA antagonist from rat oxytocin receptor expressed in HEK293-EBNA cells | J Med Chem 48: 7882-905 (2005) Article DOI: 10.1021/jm050645f BindingDB Entry DOI: 10.7270/Q2QF8TN3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(1A) dopamine receptor (Homo sapiens (Human)) | BDBM50004921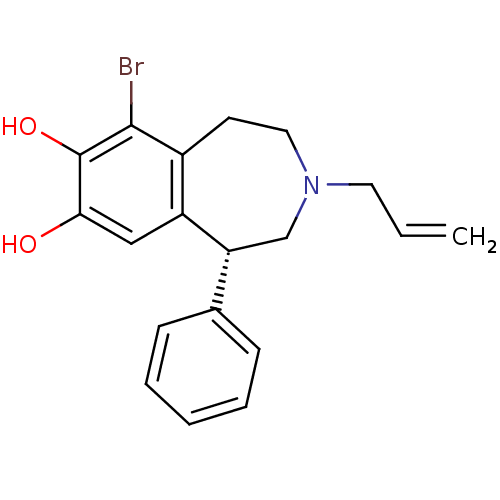 ((R)-3-Allyl-6-bromo-7,8-dihydroxy-1-phenyl-2,3,4,5...) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Texas at Austin Curated by ChEMBL | Assay Description Affinity towards Dopamine receptor D1 | J Med Chem 41: 4385-99 (1998) Article DOI: 10.1021/jm9800292 BindingDB Entry DOI: 10.7270/Q2R21227 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxytocin receptor (Homo sapiens (Human)) | BDBM50410619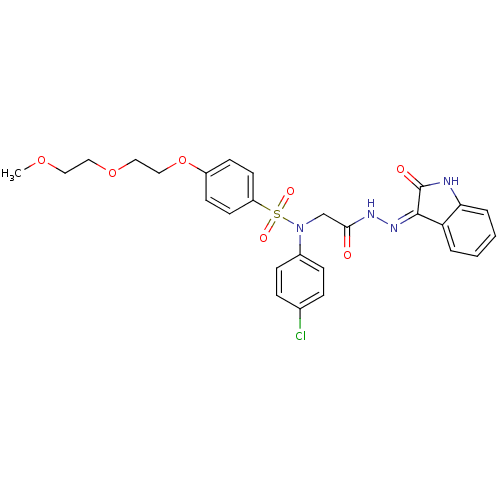 (CHEMBL2113208) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Serono Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Displacement of [125I]OVTA antagonist from human oxytocin receptor expressed in HEK293-EBNA cells | J Med Chem 48: 7882-905 (2005) Article DOI: 10.1021/jm050645f BindingDB Entry DOI: 10.7270/Q2QF8TN3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxytocin receptor (Homo sapiens (Human)) | BDBM50410640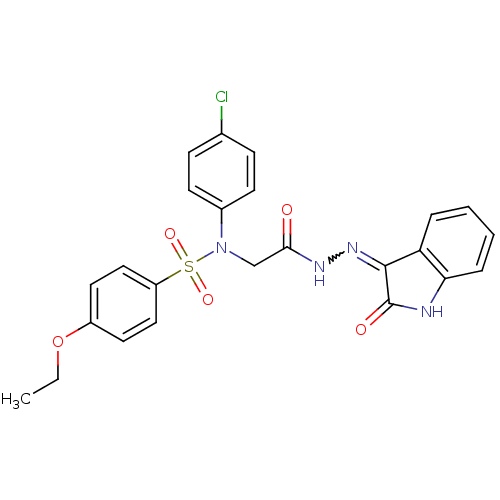 (CHEMBL2113198) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 3.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Serono Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Displacement of [125I]OVTA antagonist from human oxytocin receptor expressed in HEK293-EBNA cells | J Med Chem 48: 7882-905 (2005) Article DOI: 10.1021/jm050645f BindingDB Entry DOI: 10.7270/Q2QF8TN3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxytocin receptor (Homo sapiens (Human)) | BDBM50410623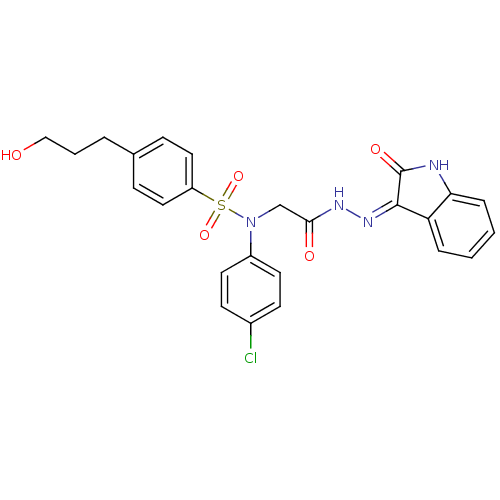 (CHEMBL2113179) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 4.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Serono Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Displacement of [125I]OVTA antagonist from human oxytocin receptor expressed in HEK293-EBNA cells | J Med Chem 48: 7882-905 (2005) Article DOI: 10.1021/jm050645f BindingDB Entry DOI: 10.7270/Q2QF8TN3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxytocin receptor (RAT) | BDBM50410625 (CHEMBL2113201) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 4.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Serono Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Displacement of [125I]OVTA antagonist from rat oxytocin receptor expressed in HEK293-EBNA cells | J Med Chem 48: 7882-905 (2005) Article DOI: 10.1021/jm050645f BindingDB Entry DOI: 10.7270/Q2QF8TN3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxytocin receptor (Homo sapiens (Human)) | BDBM50410621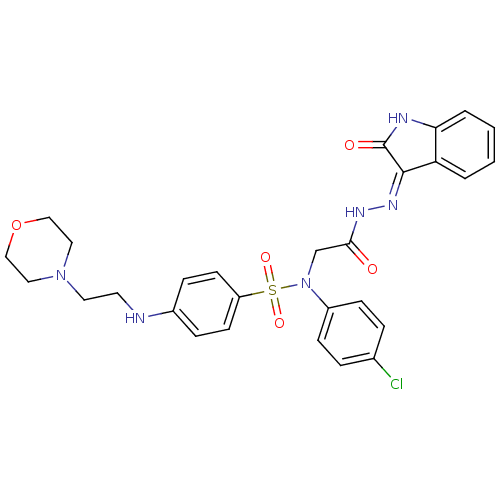 (CHEMBL2113177) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 7 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Serono Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Displacement of [125I]OVTA antagonist from human oxytocin receptor expressed in HEK293-EBNA cells | J Med Chem 48: 7882-905 (2005) Article DOI: 10.1021/jm050645f BindingDB Entry DOI: 10.7270/Q2QF8TN3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Translocator protein (Rattus norvegicus (rat)) | BDBM50120340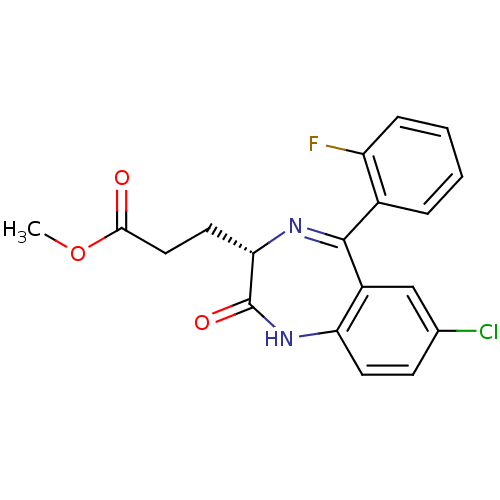 (3-[(S)-7-Chloro-5-(2-fluoro-phenyl)-2-oxo-2,3-dihy...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 7 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Binding affinity of the compound was determined against rat benzodiazepine (BZD) receptor | Bioorg Med Chem Lett 12: 3219-22 (2002) BindingDB Entry DOI: 10.7270/Q2GQ6X4F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Translocator protein (Rattus norvegicus (rat)) | BDBM50120343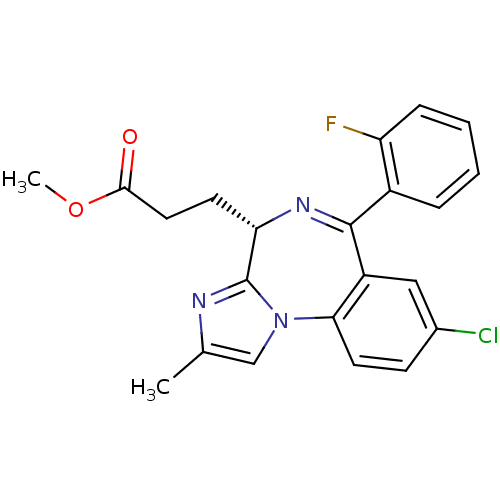 (3-[(S)-8-Chloro-6-(2-fluoro-phenyl)-2-methyl-4H-3,...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 7 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Binding affinity of the compound was determined against rat benzodiazepine (BZD) receptor | Bioorg Med Chem Lett 12: 3219-22 (2002) BindingDB Entry DOI: 10.7270/Q2GQ6X4F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxytocin receptor (Homo sapiens (Human)) | BDBM50410635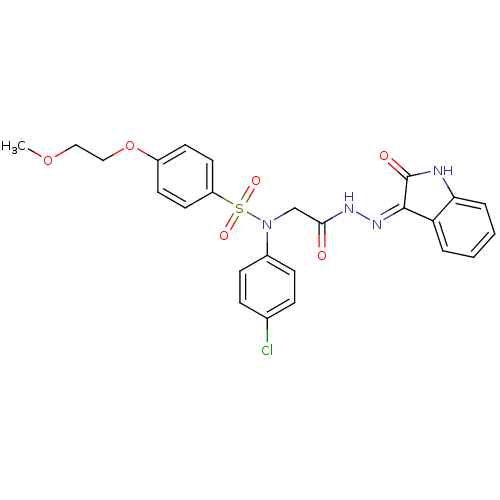 (CHEMBL2113211) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 7 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Serono Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Displacement of [125I]OVTA antagonist from human oxytocin receptor expressed in HEK293-EBNA cells | J Med Chem 48: 7882-905 (2005) Article DOI: 10.1021/jm050645f BindingDB Entry DOI: 10.7270/Q2QF8TN3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Translocator protein (Rattus norvegicus (rat)) | BDBM50120353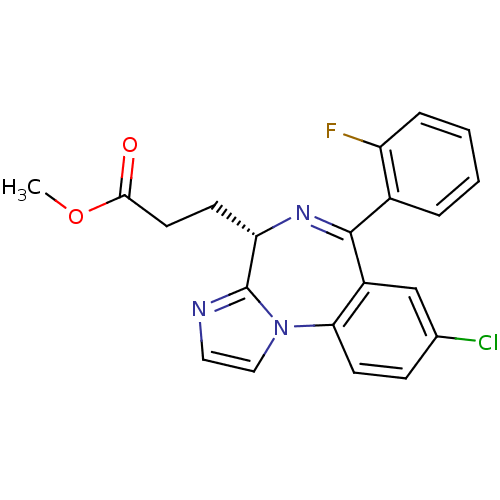 (3-[(S)-8-Chloro-6-(2-fluoro-phenyl)-4H-3,5,10b-tri...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 7 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Binding affinity of the compound was determined against rat benzodiazepine (BZD) receptor | Bioorg Med Chem Lett 12: 3219-22 (2002) BindingDB Entry DOI: 10.7270/Q2GQ6X4F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxytocin receptor (RAT) | BDBM50410616 (CHEMBL2113185) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 8 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Serono Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Displacement of [125I]OVTA antagonist from rat oxytocin receptor expressed in HEK293-EBNA cells | J Med Chem 48: 7882-905 (2005) Article DOI: 10.1021/jm050645f BindingDB Entry DOI: 10.7270/Q2QF8TN3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Translocator protein (Rattus norvegicus (rat)) | BDBM50120360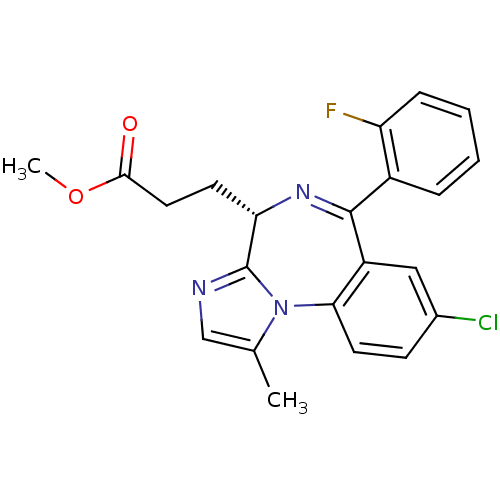 (3-[(S)-8-Chloro-6-(2-fluoro-phenyl)-1-methyl-4H-3,...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 8 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Binding affinity of the compound was determined against rat benzodiazepine (BZD) receptor | Bioorg Med Chem Lett 12: 3219-22 (2002) BindingDB Entry DOI: 10.7270/Q2GQ6X4F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxytocin receptor (Homo sapiens (Human)) | BDBM50410638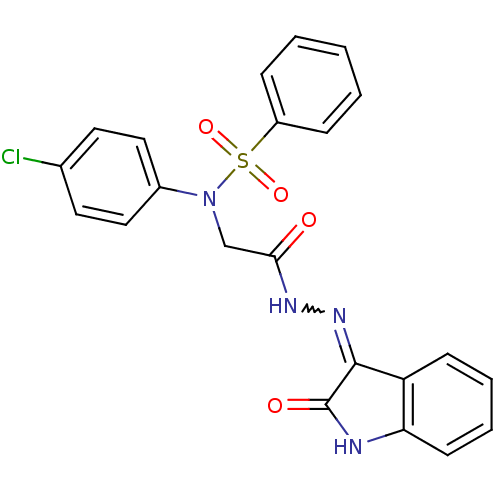 (CHEMBL2113207) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 8.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Serono Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Displacement of [125I]OVTA antagonist from human oxytocin receptor expressed in HEK293-EBNA cells | J Med Chem 48: 7882-905 (2005) Article DOI: 10.1021/jm050645f BindingDB Entry DOI: 10.7270/Q2QF8TN3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxytocin receptor (Homo sapiens (Human)) | BDBM50410630 (CHEMBL2113209) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Serono Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Displacement of [125I]OVTA antagonist from human oxytocin receptor expressed in HEK293-EBNA cells | J Med Chem 48: 7882-905 (2005) Article DOI: 10.1021/jm050645f BindingDB Entry DOI: 10.7270/Q2QF8TN3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Translocator protein (Rattus norvegicus (rat)) | BDBM50120344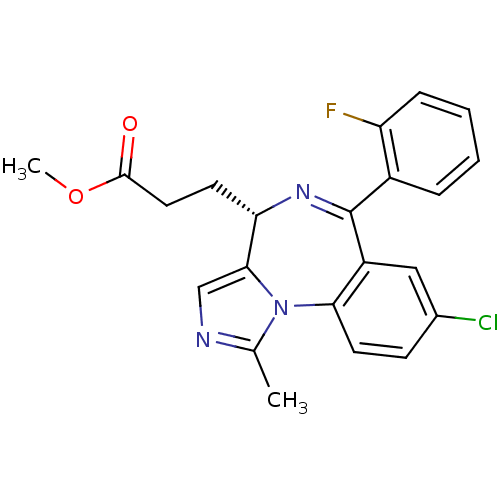 (3-[(S)-8-Chloro-6-(2-fluoro-phenyl)-1-methyl-4H-2,...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 11 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Binding affinity of the compound was determined against rat benzodiazepine (BZD) receptor | Bioorg Med Chem Lett 12: 3219-22 (2002) BindingDB Entry DOI: 10.7270/Q2GQ6X4F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxytocin receptor (RAT) | BDBM50410619 (CHEMBL2113208) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 12 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Serono Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Displacement of [125I]OVTA antagonist from rat oxytocin receptor expressed in HEK293-EBNA cells | J Med Chem 48: 7882-905 (2005) Article DOI: 10.1021/jm050645f BindingDB Entry DOI: 10.7270/Q2QF8TN3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxytocin receptor (Homo sapiens (Human)) | BDBM50178153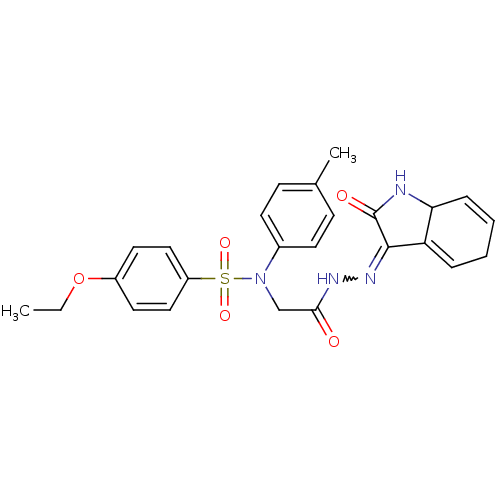 (4-ethoxy-N-(4-methylphenyl)-N-{2-oxo-2-[2-(2-oxo-1...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 14 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Serono Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Displacement of [125I]OVTA antagonist from human oxytocin receptor expressed in HEK293-EBNA cells | J Med Chem 48: 7882-905 (2005) Article DOI: 10.1021/jm050645f BindingDB Entry DOI: 10.7270/Q2QF8TN3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxytocin receptor (RAT) | BDBM50410622 (CHEMBL2113203) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 15 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Serono Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Displacement of [125I]OVTA antagonist from rat oxytocin receptor expressed in HEK293-EBNA cells | J Med Chem 48: 7882-905 (2005) Article DOI: 10.1021/jm050645f BindingDB Entry DOI: 10.7270/Q2QF8TN3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxytocin receptor (Homo sapiens (Human)) | BDBM50178186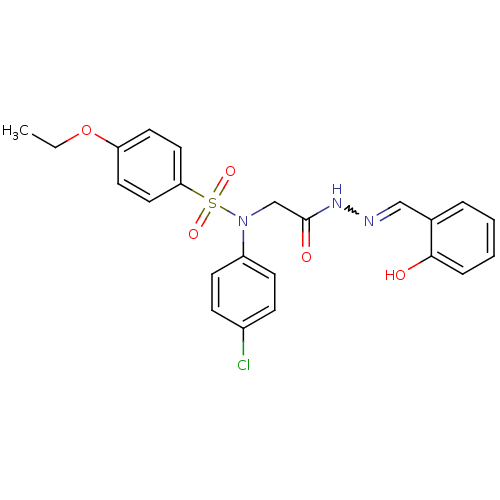 (CHEMBL372122 | N-(4-chlorophenyl)-4-ethoxy-N-{2-[(...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 15 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Serono Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Displacement of [125I]OVTA antagonist from human oxytocin receptor expressed in HEK293-EBNA cells | J Med Chem 48: 7882-905 (2005) Article DOI: 10.1021/jm050645f BindingDB Entry DOI: 10.7270/Q2QF8TN3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxytocin receptor (Homo sapiens (Human)) | BDBM50410637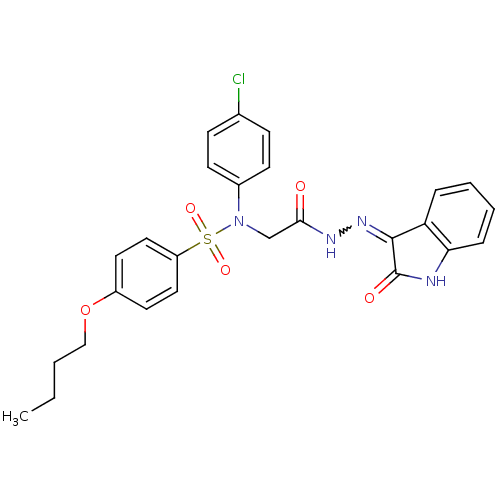 (CHEMBL2113210) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 18 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Serono Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Displacement of [125I]OVTA antagonist from human oxytocin receptor expressed in HEK293-EBNA cells | J Med Chem 48: 7882-905 (2005) Article DOI: 10.1021/jm050645f BindingDB Entry DOI: 10.7270/Q2QF8TN3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxytocin receptor (Homo sapiens (Human)) | BDBM50178209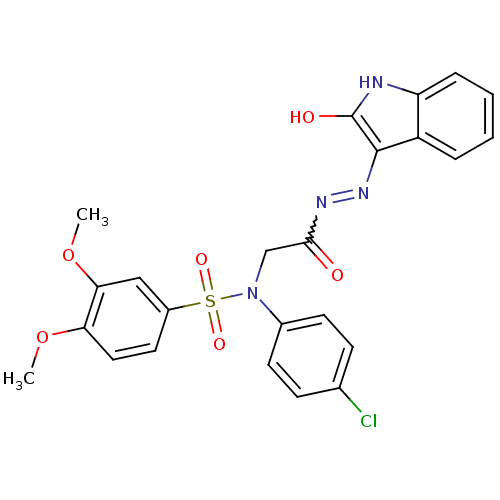 (CHEMBL371223 | N-(4-chlorophenyl)-3,4-dimethoxy-N-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 18 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Serono Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Displacement of [125I]OVTA antagonist from human oxytocin receptor expressed in HEK293-EBNA cells | J Med Chem 48: 7882-905 (2005) Article DOI: 10.1021/jm050645f BindingDB Entry DOI: 10.7270/Q2QF8TN3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxytocin receptor (Homo sapiens (Human)) | BDBM50410624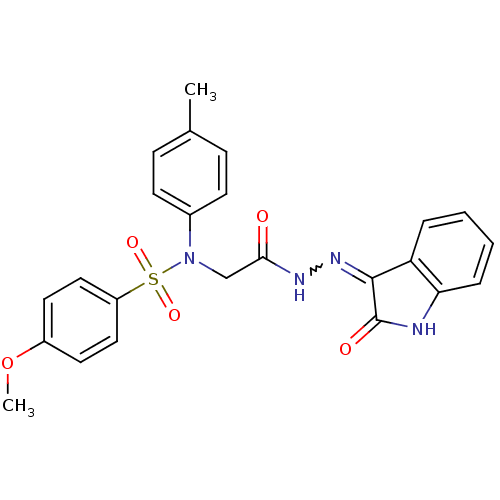 (CHEMBL2113215) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Serono Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Displacement of [125I]OVTA antagonist from human oxytocin receptor expressed in HEK293-EBNA cells | J Med Chem 48: 7882-905 (2005) Article DOI: 10.1021/jm050645f BindingDB Entry DOI: 10.7270/Q2QF8TN3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Homo sapiens (Human)) | BDBM50010289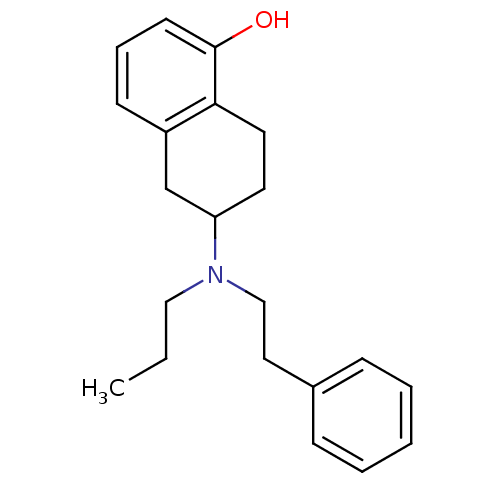 ((R)6-(Phenethyl-propyl-amino)-5,6,7,8-tetrahydro-n...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Texas at Austin Curated by ChEMBL | Assay Description Affinity towards Dopamine receptor D2 | J Med Chem 41: 4385-99 (1998) Article DOI: 10.1021/jm9800292 BindingDB Entry DOI: 10.7270/Q2R21227 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(1A) dopamine receptor (Homo sapiens (Human)) | BDBM50049048 ((R)-3-Allyl-6-chloro-1-phenyl-2,3,4,5-tetrahydro-1...) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | 20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Texas at Austin Curated by ChEMBL | Assay Description Affinity towards Dopamine receptor D1 | J Med Chem 41: 4385-99 (1998) Article DOI: 10.1021/jm9800292 BindingDB Entry DOI: 10.7270/Q2R21227 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxytocin receptor (Homo sapiens (Human)) | BDBM50410628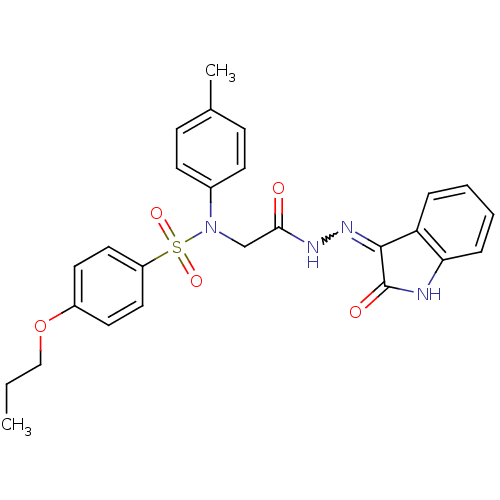 (CHEMBL2113216) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 21 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Serono Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Displacement of [125I]OVTA antagonist from human oxytocin receptor expressed in HEK293-EBNA cells | J Med Chem 48: 7882-905 (2005) Article DOI: 10.1021/jm050645f BindingDB Entry DOI: 10.7270/Q2QF8TN3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dihydroxyacetone phosphate acyltransferase (Homo sapiens (Human)) | BDBM50068943 ((S)-2-(3-Carboxy-propionylamino)-6-hydrazino-hepta...) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | 22 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bristol Curated by ChEMBL | Assay Description Compound was tested for 50% inhibition of N-Succinyl Diaminopimelic Acid Aminotransferase (DAP-AT) from E. coli. | Bioorg Med Chem Lett 8: 945-50 (1999) BindingDB Entry DOI: 10.7270/Q23F4NR8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxytocin receptor (RAT) | BDBM50178153 (4-ethoxy-N-(4-methylphenyl)-N-{2-oxo-2-[2-(2-oxo-1...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 25 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Serono Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Displacement of [125I]OVTA antagonist from rat oxytocin receptor expressed in HEK293-EBNA cells | J Med Chem 48: 7882-905 (2005) Article DOI: 10.1021/jm050645f BindingDB Entry DOI: 10.7270/Q2QF8TN3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxytocin receptor (RAT) | BDBM50410621 (CHEMBL2113177) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 25 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Serono Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Displacement of [125I]OVTA antagonist from rat oxytocin receptor expressed in HEK293-EBNA cells | J Med Chem 48: 7882-905 (2005) Article DOI: 10.1021/jm050645f BindingDB Entry DOI: 10.7270/Q2QF8TN3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Inositol 1,4,5-trisphosphate receptor type 3 (Bos taurus) | BDBM50132166 (1,2,4,5-InsP4 | CHEMBL100498 | Phosphoric acid mon...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Similars | Article | 26.4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Compound was evaluated for its ability to displace [3H]Ins(1,4,5)P3 from membranes prepared from bovine adrenal corticles | Bioorg Med Chem Lett 3: 1505-1510 (1993) Article DOI: 10.1016/S0960-894X(00)80007-4 BindingDB Entry DOI: 10.7270/Q27M08F4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxytocin receptor (Homo sapiens (Human)) | BDBM50177595 ((2S)-5-amino-2-{[(2S)-1-{[(4R,7S,10S,13S,16R)-13-[...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | 27 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Serono Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Displacement of [125I]OVTA antagonist from human oxytocin receptor expressed in HEK293-EBNA cells | J Med Chem 48: 7882-905 (2005) Article DOI: 10.1021/jm050645f BindingDB Entry DOI: 10.7270/Q2QF8TN3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(1A) dopamine receptor (Homo sapiens (Human)) | BDBM50067726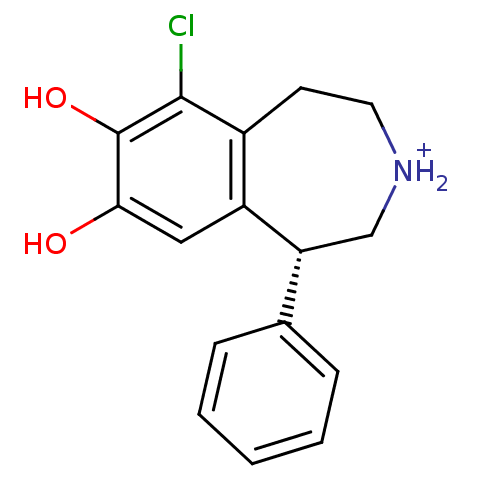 ((R)-6-Chloro-7,8-dihydroxy-1-phenyl-2,3,4,5-tetrah...) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Similars | Article PubMed | 30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Texas at Austin Curated by ChEMBL | Assay Description Affinity towards Dopamine receptor D1 | J Med Chem 41: 4385-99 (1998) Article DOI: 10.1021/jm9800292 BindingDB Entry DOI: 10.7270/Q2R21227 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxytocin receptor (RAT) | BDBM50410635 (CHEMBL2113211) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 33 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Serono Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Displacement of [125I]OVTA antagonist from rat oxytocin receptor expressed in HEK293-EBNA cells | J Med Chem 48: 7882-905 (2005) Article DOI: 10.1021/jm050645f BindingDB Entry DOI: 10.7270/Q2QF8TN3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxytocin receptor (Homo sapiens (Human)) | BDBM50410636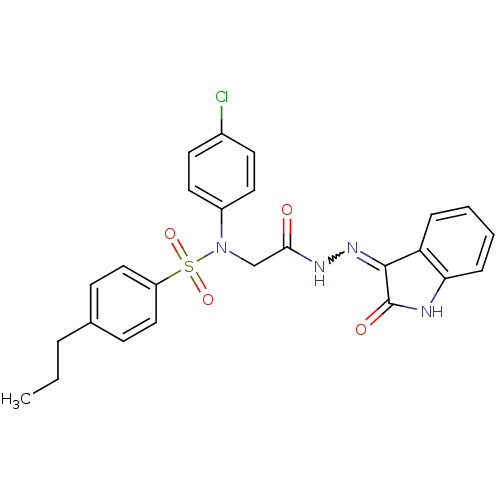 (CHEMBL2113212) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 36 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Serono Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Displacement of [125I]OVTA antagonist from human oxytocin receptor expressed in HEK293-EBNA cells | J Med Chem 48: 7882-905 (2005) Article DOI: 10.1021/jm050645f BindingDB Entry DOI: 10.7270/Q2QF8TN3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxytocin receptor (Homo sapiens (Human)) | BDBM50178197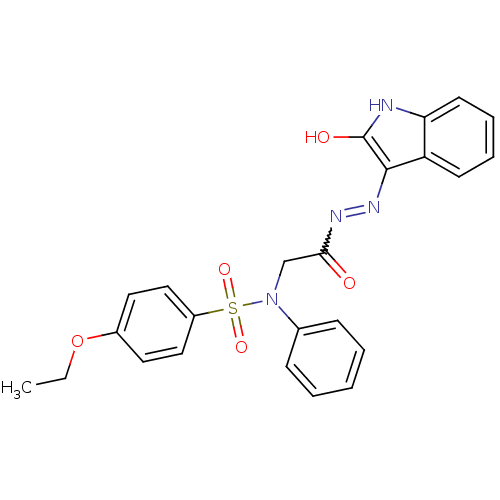 (4-ethoxy-N-{2-oxo-2-[2-(2-oxo-1,2-dihydro-3H-indol...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 36 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Serono Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Displacement of [125I]OVTA antagonist from human oxytocin receptor expressed in HEK293-EBNA cells | J Med Chem 48: 7882-905 (2005) Article DOI: 10.1021/jm050645f BindingDB Entry DOI: 10.7270/Q2QF8TN3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxytocin receptor (Homo sapiens (Human)) | BDBM50410631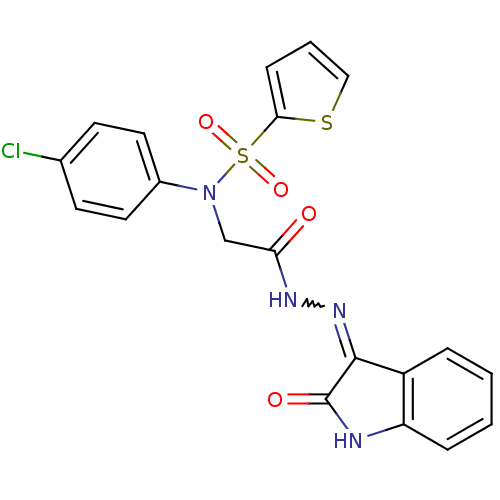 (CHEMBL2113204) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 37 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Serono Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Displacement of [125I]OVTA antagonist from human oxytocin receptor expressed in HEK293-EBNA cells | J Med Chem 48: 7882-905 (2005) Article DOI: 10.1021/jm050645f BindingDB Entry DOI: 10.7270/Q2QF8TN3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(1A) dopamine receptor (Homo sapiens (Human)) | BDBM60917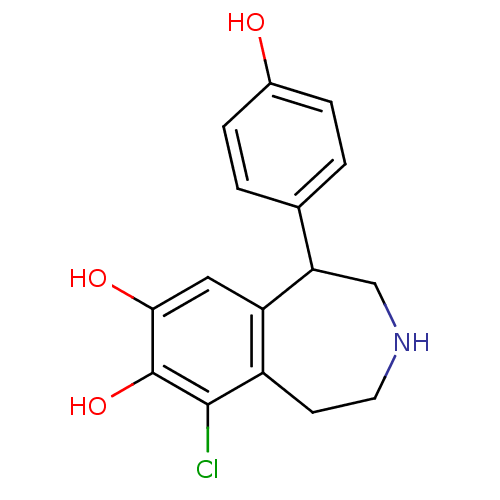 (9-chloranyl-5-(4-hydroxyphenyl)-2,3,4,5-tetrahydro...) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase DrugBank MCE KEGG PC cid PC sid PDB UniChem Similars | DrugBank PDB Article PubMed | 40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Texas at Austin Curated by ChEMBL | Assay Description Affinity towards Dopamine receptor D1 | J Med Chem 41: 4385-99 (1998) Article DOI: 10.1021/jm9800292 BindingDB Entry DOI: 10.7270/Q2R21227 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| D(2) dopamine receptor (Homo sapiens (Human)) | BDBM50017543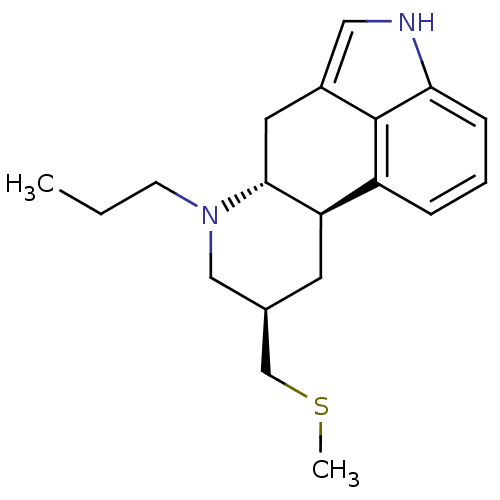 ((6aR,9R,10aR)-9-Methylsulfanylmethyl-7-propyl-4,6,...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid UniChem Patents Similars | DrugBank Article PubMed | 50 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Texas at Austin Curated by ChEMBL | Assay Description Affinity towards Dopamine receptor D2 | J Med Chem 41: 4385-99 (1998) Article DOI: 10.1021/jm9800292 BindingDB Entry DOI: 10.7270/Q2R21227 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Homo sapiens (Human)) | BDBM50007422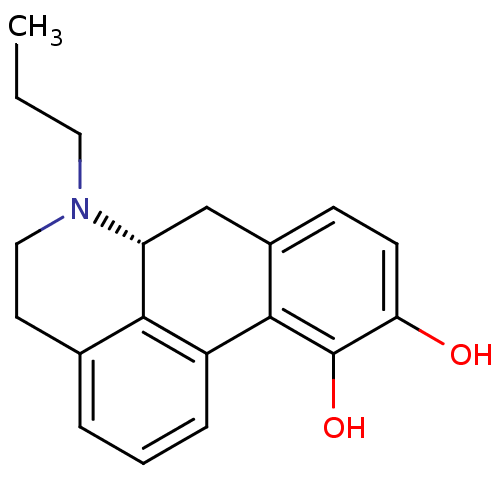 ((+)-6-Propyl-5,6,6a,7-tetrahydro-4H-dibenzo[de,g]q...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 50 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Texas at Austin Curated by ChEMBL | Assay Description Affinity towards Dopamine receptor D2 | J Med Chem 41: 4385-99 (1998) Article DOI: 10.1021/jm9800292 BindingDB Entry DOI: 10.7270/Q2R21227 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Translocator protein (Rattus norvegicus (rat)) | BDBM50120359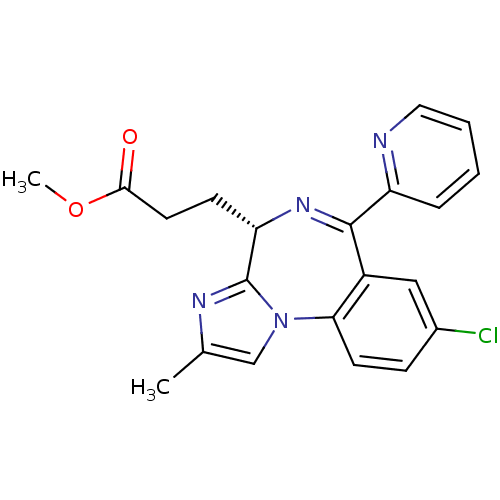 (3-((S)-8-Chloro-2-methyl-6-pyridin-2-yl-4H-3,5,10b...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Binding affinity of the compound was determined against rat benzodiazepine (BZD) receptor | Bioorg Med Chem Lett 12: 3219-22 (2002) BindingDB Entry DOI: 10.7270/Q2GQ6X4F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 958 total ) | Next | Last >> |