 Found 8387 hits with Last Name = 'singh' and Initial = 'r'
Found 8387 hits with Last Name = 'singh' and Initial = 'r' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Prothrombin
(Homo sapiens (Human)) | BDBM50123490
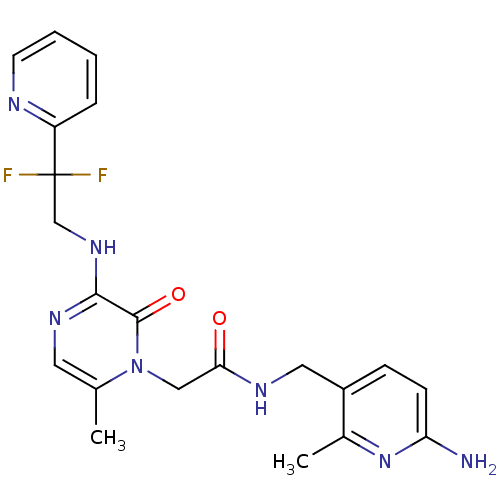
(CHEMBL143418 | N-(6-Amino-2-methyl-pyridin-3-ylmet...)Show SMILES Cc1cnc(NCC(F)(F)c2ccccn2)c(=O)n1CC(=O)NCc1ccc(N)nc1C Show InChI InChI=1S/C21H23F2N7O2/c1-13-9-27-19(28-12-21(22,23)16-5-3-4-8-25-16)20(32)30(13)11-18(31)26-10-15-6-7-17(24)29-14(15)2/h3-9H,10-12H2,1-2H3,(H2,24,29)(H,26,31)(H,27,28) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0420 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibitory constant against thrombin (IIa) |
J Med Chem 46: 461-73 (2003)
Article DOI: 10.1021/jm020311f
BindingDB Entry DOI: 10.7270/Q2W958J5 |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M3
(Homo sapiens (Human)) | BDBM50176723
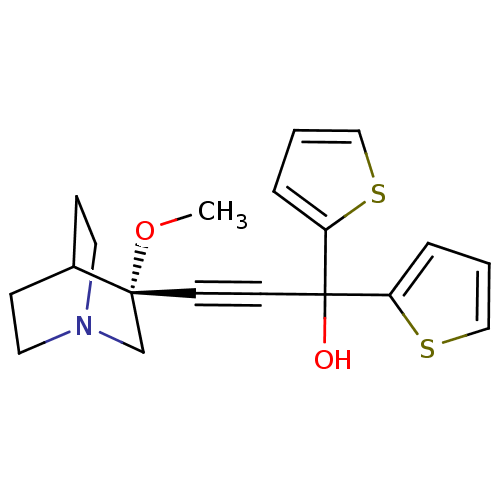
((R)-3-(3-methoxyquinuclidin-3-yl)-1,1-di(thiophen-...)Show SMILES CO[C@@]1(CN2CCC1CC2)C#CC(O)(c1cccs1)c1cccs1 |wU:2.1,wD:2.11,THB:1:2:5.6:9.8,(-4.55,6.62,;-3.06,6.24,;-2.65,4.75,;-2.46,3.37,;-.93,4.03,;.43,3.4,;.16,4.79,;-1.19,5.39,;-1.12,7.02,;-.67,5.92,;-4.15,4.35,;-5.64,3.96,;-7.13,3.56,;-7.52,5.04,;-8.62,3.16,;-9.16,1.72,;-10.68,1.79,;-11.09,3.3,;-9.81,4.14,;-6.73,2.08,;-5.29,1.53,;-5.36,.02,;-6.86,-.39,;-7.7,.89,)| Show InChI InChI=1S/C19H21NO2S2/c1-22-18(14-20-10-6-15(18)7-11-20)8-9-19(21,16-4-2-12-23-16)17-5-3-13-24-17/h2-5,12-13,15,21H,6-7,10-11,14H2,1H3/t18-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 0.0500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
UCB Pharma
Curated by ChEMBL
| Assay Description
Binding affinity to human muscarinic M3 receptor |
Bioorg Med Chem Lett 16: 373-7 (2005)
Article DOI: 10.1016/j.bmcl.2005.09.079
BindingDB Entry DOI: 10.7270/Q2T72J70 |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M3
(Homo sapiens (Human)) | BDBM50176735
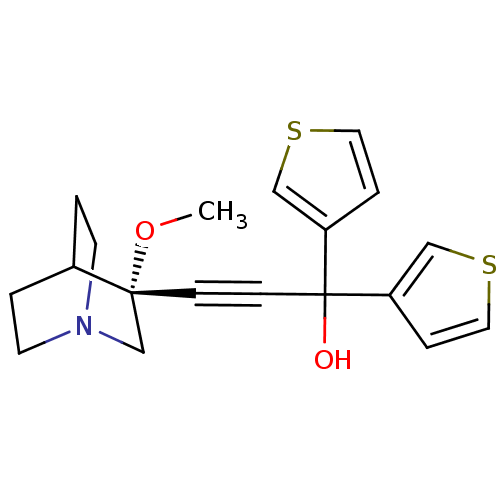
((R)-3-(3-methoxyquinuclidin-3-yl)-1,1-di(thiophen-...)Show SMILES CO[C@@]1(CN2CCC1CC2)C#CC(O)(c1ccsc1)c1ccsc1 |wU:2.1,wD:2.11,THB:1:2:5.6:9.8,(9.1,6.91,;10.59,6.52,;11,5.02,;11.19,3.64,;12.73,4.3,;14.09,3.67,;13.82,5.06,;12.47,5.67,;12.54,7.31,;12.98,6.2,;9.5,4.63,;8,4.23,;6.51,3.83,;6.11,5.32,;5.02,3.44,;4.48,2,;2.91,2.09,;2.52,3.58,;3.83,4.41,;6.91,2.35,;8.33,1.8,;8.24,.24,;6.76,-.15,;5.94,1.16,)| Show InChI InChI=1S/C19H21NO2S2/c1-22-18(14-20-8-2-15(18)3-9-20)6-7-19(21,16-4-10-23-12-16)17-5-11-24-13-17/h4-5,10-13,15,21H,2-3,8-9,14H2,1H3/t18-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 0.0800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
UCB Pharma
Curated by ChEMBL
| Assay Description
Binding affinity to human muscarinic M3 receptor |
Bioorg Med Chem Lett 16: 373-7 (2005)
Article DOI: 10.1016/j.bmcl.2005.09.079
BindingDB Entry DOI: 10.7270/Q2T72J70 |
More data for this
Ligand-Target Pair | |
Prothrombin
(Homo sapiens (Human)) | BDBM50126304
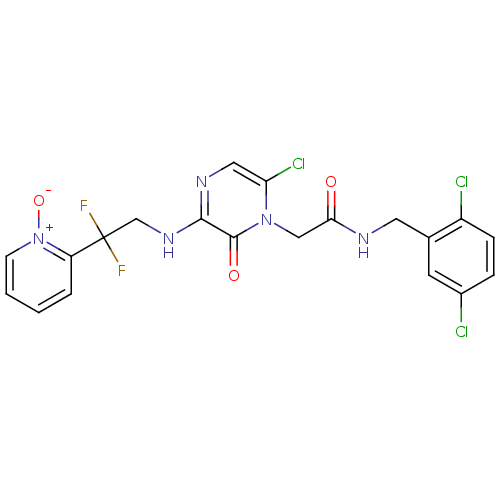
(2-{6-Chloro-3-[2,2-difluoro-2-(1-oxy-pyridin-2-yl)...)Show SMILES [O-][n+]1ccccc1C(F)(F)CNc1ncc(Cl)n(CC(=O)NCc2cc(Cl)ccc2Cl)c1=O Show InChI InChI=1S/C20H16Cl3F2N5O3/c21-13-4-5-14(22)12(7-13)8-26-17(31)10-29-16(23)9-27-18(19(29)32)28-11-20(24,25)15-3-1-2-6-30(15)33/h1-7,9H,8,10-11H2,(H,26,31)(H,27,28) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.0870 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibitory activity against human thrombin |
Bioorg Med Chem Lett 13: 1353-7 (2003)
BindingDB Entry DOI: 10.7270/Q2833RC9 |
More data for this
Ligand-Target Pair | |
Prothrombin
(Homo sapiens (Human)) | BDBM50123504
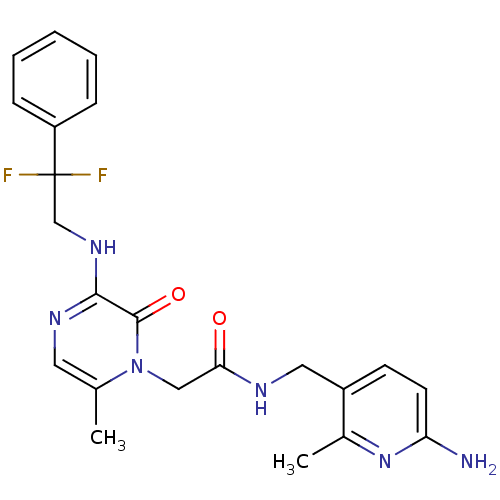
(CHEMBL142546 | N-((6-amino-2-methylpyridin-3-yl)me...)Show SMILES Cc1cnc(NCC(F)(F)c2ccccc2)c(=O)n1CC(=O)NCc1ccc(N)nc1C Show InChI InChI=1S/C22H24F2N6O2/c1-14-10-27-20(28-13-22(23,24)17-6-4-3-5-7-17)21(32)30(14)12-19(31)26-11-16-8-9-18(25)29-15(16)2/h3-10H,11-13H2,1-2H3,(H2,25,29)(H,26,31)(H,27,28) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibitory constant against thrombin (IIa) |
J Med Chem 46: 461-73 (2003)
Article DOI: 10.1021/jm020311f
BindingDB Entry DOI: 10.7270/Q2W958J5 |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M3
(Homo sapiens (Human)) | BDBM50176732
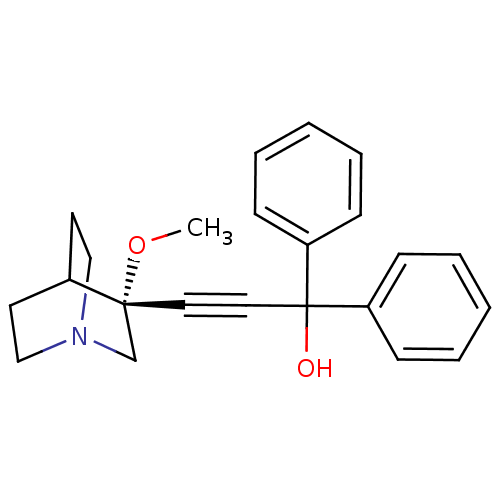
((R)-3-(3-methoxyquinuclidin-3-yl)-1,1-diphenylprop...)Show SMILES CO[C@@]1(CN2CCC1CC2)C#CC(O)(c1ccccc1)c1ccccc1 |wU:2.1,wD:2.11,THB:1:2:5.6:9.8,(-2.54,5.9,;-1.05,5.51,;-.64,4.02,;-.44,2.64,;1.09,3.3,;2.46,2.67,;2.18,4.06,;.83,4.66,;.9,6.3,;1.34,5.19,;-2.14,3.62,;-3.63,3.22,;-5.12,2.83,;-5.52,4.31,;-4.73,1.34,;-5.81,.25,;-5.42,-1.23,;-3.93,-1.63,;-2.84,-.54,;-3.24,.94,;-6.61,2.43,;-7.7,3.53,;-9.19,3.13,;-9.59,1.64,;-8.5,.54,;-7.01,.94,)| Show InChI InChI=1S/C23H25NO2/c1-26-22(18-24-16-12-19(22)13-17-24)14-15-23(25,20-8-4-2-5-9-20)21-10-6-3-7-11-21/h2-11,19,25H,12-13,16-18H2,1H3/t22-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.130 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
UCB Pharma
Curated by ChEMBL
| Assay Description
Binding affinity to human muscarinic M3 receptor |
Bioorg Med Chem Lett 16: 373-7 (2005)
Article DOI: 10.1016/j.bmcl.2005.09.079
BindingDB Entry DOI: 10.7270/Q2T72J70 |
More data for this
Ligand-Target Pair | |
Prothrombin
(Homo sapiens (Human)) | BDBM50126303

(2-{6-Chloro-3-[2,2-difluoro-2-(1-oxy-pyridin-2-yl)...)Show SMILES [O-][n+]1ccccc1C(F)(F)CNc1ncc(Cl)n(CC(=O)NCc2cc(Cl)ccc2F)c1=O Show InChI InChI=1S/C20H16Cl2F3N5O3/c21-13-4-5-14(23)12(7-13)8-26-17(31)10-29-16(22)9-27-18(19(29)32)28-11-20(24,25)15-3-1-2-6-30(15)33/h1-7,9H,8,10-11H2,(H,26,31)(H,27,28) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.160 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibitory activity against human thrombin |
Bioorg Med Chem Lett 13: 1353-7 (2003)
BindingDB Entry DOI: 10.7270/Q2833RC9 |
More data for this
Ligand-Target Pair | |
Prothrombin
(Homo sapiens (Human)) | BDBM50126309

(CHEMBL29744 | N-(3-Bromo-benzyl)-2-{6-chloro-3-[2,...)Show SMILES [O-][n+]1ccccc1C(F)(F)CNc1ncc(Cl)n(CC(=O)NCc2cccc(Br)c2)c1=O Show InChI InChI=1S/C20H17BrClF2N5O3/c21-14-5-3-4-13(8-14)9-25-17(30)11-28-16(22)10-26-18(19(28)31)27-12-20(23,24)15-6-1-2-7-29(15)32/h1-8,10H,9,11-12H2,(H,25,30)(H,26,27) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.190 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibitory activity against human thrombin |
Bioorg Med Chem Lett 13: 1353-7 (2003)
BindingDB Entry DOI: 10.7270/Q2833RC9 |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M3
(Homo sapiens (Human)) | BDBM50176718
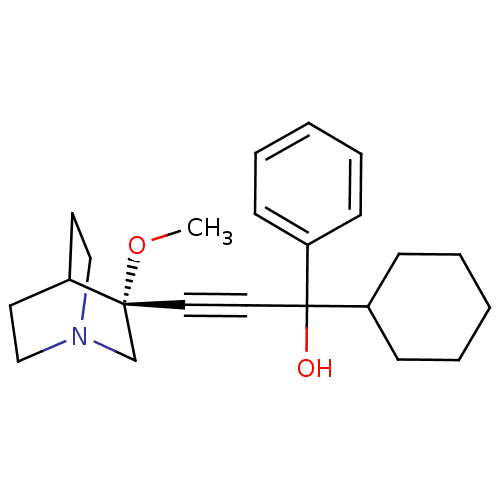
(1-cyclohexyl-3-((R)-3-methoxyquinuclidin-3-yl)-1-p...)Show SMILES CO[C@@]1(CN2CCC1CC2)C#CC(O)(C1CCCCC1)c1ccccc1 |wU:2.1,wD:2.11,THB:1:2:5.6:9.8,(11.35,5.74,;12.84,5.36,;13.25,3.86,;13.44,2.49,;14.97,3.14,;16.34,2.52,;16.06,3.9,;14.71,4.51,;14.78,6.14,;15.23,5.04,;11.75,3.47,;10.26,3.07,;8.77,2.68,;8.38,4.16,;9.16,1.19,;8.06,.11,;8.45,-1.37,;9.93,-1.78,;11.03,-.69,;10.64,.8,;7.28,2.28,;6.19,3.37,;4.71,2.98,;4.3,1.49,;5.39,.39,;6.88,.8,)| Show InChI InChI=1S/C23H31NO2/c1-26-22(18-24-16-12-19(22)13-17-24)14-15-23(25,20-8-4-2-5-9-20)21-10-6-3-7-11-21/h2,4-5,8-9,19,21,25H,3,6-7,10-13,16-18H2,1H3/t22-,23?/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
UCB Pharma
Curated by ChEMBL
| Assay Description
Binding affinity to human muscarinic M3 receptor |
Bioorg Med Chem Lett 16: 373-7 (2005)
Article DOI: 10.1016/j.bmcl.2005.09.079
BindingDB Entry DOI: 10.7270/Q2T72J70 |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M2
(Homo sapiens (Human)) | BDBM50176723

((R)-3-(3-methoxyquinuclidin-3-yl)-1,1-di(thiophen-...)Show SMILES CO[C@@]1(CN2CCC1CC2)C#CC(O)(c1cccs1)c1cccs1 |wU:2.1,wD:2.11,THB:1:2:5.6:9.8,(-4.55,6.62,;-3.06,6.24,;-2.65,4.75,;-2.46,3.37,;-.93,4.03,;.43,3.4,;.16,4.79,;-1.19,5.39,;-1.12,7.02,;-.67,5.92,;-4.15,4.35,;-5.64,3.96,;-7.13,3.56,;-7.52,5.04,;-8.62,3.16,;-9.16,1.72,;-10.68,1.79,;-11.09,3.3,;-9.81,4.14,;-6.73,2.08,;-5.29,1.53,;-5.36,.02,;-6.86,-.39,;-7.7,.89,)| Show InChI InChI=1S/C19H21NO2S2/c1-22-18(14-20-10-6-15(18)7-11-20)8-9-19(21,16-4-2-12-23-16)17-5-3-13-24-17/h2-5,12-13,15,21H,6-7,10-11,14H2,1H3/t18-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
UCB Pharma
Curated by ChEMBL
| Assay Description
Binding affinity to human muscarinic M2 receptor |
Bioorg Med Chem Lett 16: 373-7 (2005)
Article DOI: 10.1016/j.bmcl.2005.09.079
BindingDB Entry DOI: 10.7270/Q2T72J70 |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M3
(Homo sapiens (Human)) | BDBM50176712
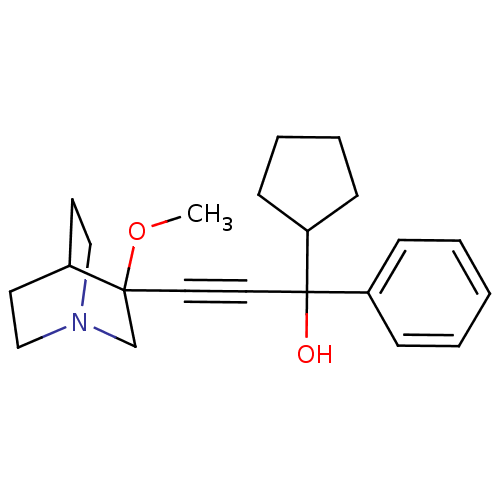
(1-cyclopentyl-3-(3-methoxyquinuclidin-3-yl)-1-phen...)Show SMILES COC1(CN2CCC1CC2)C#CC(O)(C1CCCC1)c1ccccc1 |THB:1:2:5.6:9.8,(26.6,5.4,;28.09,5.01,;28.5,3.51,;28.69,2.14,;30.23,2.79,;31.59,2.16,;31.32,3.55,;29.97,4.16,;30.04,5.8,;30.48,4.69,;27,3.12,;25.5,2.72,;24.01,2.33,;23.61,3.81,;24.41,.84,;23.43,-.35,;24.27,-1.64,;25.76,-1.25,;25.84,.29,;22.52,1.93,;21.42,3.02,;19.94,2.63,;19.53,1.13,;20.63,.04,;22.11,.44,)| Show InChI InChI=1S/C22H29NO2/c1-25-21(17-23-15-11-18(21)12-16-23)13-14-22(24,20-9-5-6-10-20)19-7-3-2-4-8-19/h2-4,7-8,18,20,24H,5-6,9-12,15-17H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.25 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
UCB Pharma
Curated by ChEMBL
| Assay Description
Binding affinity to human muscarinic M3 receptor |
Bioorg Med Chem Lett 16: 373-7 (2005)
Article DOI: 10.1016/j.bmcl.2005.09.079
BindingDB Entry DOI: 10.7270/Q2T72J70 |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M3
(Homo sapiens (Human)) | BDBM50176708
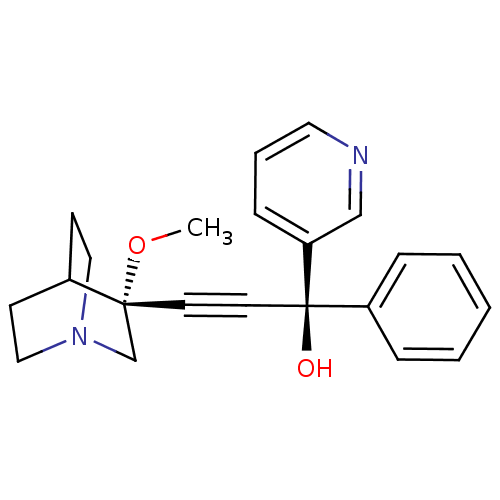
((R)-3-((R)-3-methoxyquinuclidin-3-yl)-1-phenyl-1-(...)Show SMILES CO[C@@]1(CN2CCC1CC2)C#C[C@@](O)(c1ccccc1)c1cccnc1 |wU:12.14,2.1,wD:12.13,2.11,THB:1:2:5.6:9.8,(16.23,-16.03,;17.77,-16,;18.57,-17.32,;19.13,-18.59,;20.42,-17.54,;21.91,-17.77,;21.26,-16.51,;19.8,-16.3,;19.42,-14.71,;20.15,-15.65,;17.23,-18.11,;15.91,-18.89,;14.58,-19.68,;13.8,-18.36,;13.26,-20.47,;11.91,-19.72,;10.59,-20.5,;10.61,-22.05,;11.96,-22.8,;13.28,-22.01,;15.37,-21,;16.9,-20.98,;17.68,-22.31,;16.93,-23.64,;15.4,-23.66,;14.6,-22.35,)| Show InChI InChI=1S/C22H24N2O2/c1-26-21(17-24-14-9-18(21)10-15-24)11-12-22(25,19-6-3-2-4-7-19)20-8-5-13-23-16-20/h2-8,13,16,18,25H,9-10,14-15,17H2,1H3/t21-,22-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.25 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
UCB Pharma
Curated by ChEMBL
| Assay Description
Binding affinity to human muscarinic M3 receptor |
Bioorg Med Chem Lett 16: 373-7 (2005)
Article DOI: 10.1016/j.bmcl.2005.09.079
BindingDB Entry DOI: 10.7270/Q2T72J70 |
More data for this
Ligand-Target Pair | |
Prothrombin
(Homo sapiens (Human)) | BDBM50126300
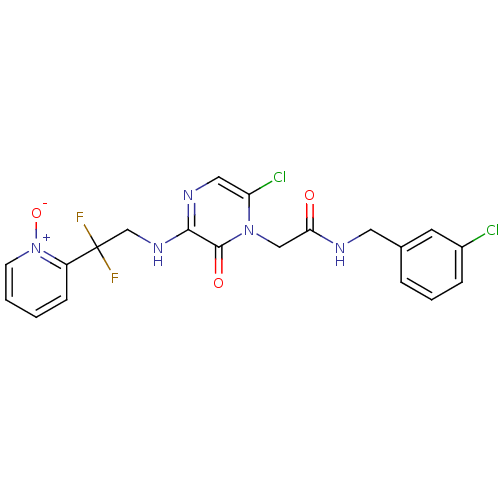
(CHEMBL25845 | N-(3-Chloro-benzyl)-2-{6-chloro-3-[2...)Show SMILES [O-][n+]1ccccc1C(F)(F)CNc1ncc(Cl)n(CC(=O)NCc2cccc(Cl)c2)c1=O Show InChI InChI=1S/C20H17Cl2F2N5O3/c21-14-5-3-4-13(8-14)9-25-17(30)11-28-16(22)10-26-18(19(28)31)27-12-20(23,24)15-6-1-2-7-29(15)32/h1-8,10H,9,11-12H2,(H,25,30)(H,26,27) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 0.260 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibitory activity against human thrombin |
Bioorg Med Chem Lett 13: 1353-7 (2003)
BindingDB Entry DOI: 10.7270/Q2833RC9 |
More data for this
Ligand-Target Pair | |
Replicase polyprotein 1ab
(2019-nCoV) | BDBM420298

(CVD-0006356 | PF-00835231 | PF-0835231 | US1152494...)Show SMILES COc1cccc2[nH]c(cc12)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](C[C@@H]1CCNC1=O)C(=O)CO Show InChI InChI=1S/C24H32N4O6/c1-13(2)9-18(23(32)27-17(20(30)12-29)10-14-7-8-25-22(14)31)28-24(33)19-11-15-16(26-19)5-4-6-21(15)34-3/h4-6,11,13-14,17-18,26,29H,7-10,12H2,1-3H3,(H,25,31)(H,27,32)(H,28,33)/t14-,17-,18-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
MCE
PDB
UniChem
| | 0.270 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer
| Assay Description
The respective human coronavirus Mpro in assay buffer (20 mM Tris-HCl, pH 7.3, 100 mM NaCl, 1 mM EDTA, 5 mM TCEP) and 0.1% BSA was added to assay-rea... |
Science 374: 1-13 (2021)
BindingDB Entry DOI: 10.7270/Q23T9MCM |
More data for this
Ligand-Target Pair | |
Prothrombin
(Homo sapiens (Human)) | BDBM50123496

(CHEMBL143138 | N-(6-Amino-2-methyl-pyridin-3-ylmet...)Show SMILES Cc1nc(N)ccc1CNC(=O)Cn1c(C)cnc(NCCc2ccccn2)c1=O Show InChI InChI=1S/C21H25N7O2/c1-14-11-26-20(24-10-8-17-5-3-4-9-23-17)21(30)28(14)13-19(29)25-12-16-6-7-18(22)27-15(16)2/h3-7,9,11H,8,10,12-13H2,1-2H3,(H2,22,27)(H,24,26)(H,25,29) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.270 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibitory constant against thrombin (IIa) |
J Med Chem 46: 461-73 (2003)
Article DOI: 10.1021/jm020311f
BindingDB Entry DOI: 10.7270/Q2W958J5 |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M3
(Homo sapiens (Human)) | BDBM50176706
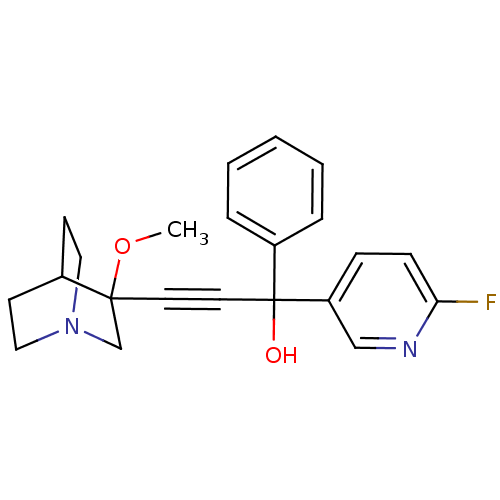
(1-(6-fluoropyridin-3-yl)-3-(3-methoxyquinuclidin-3...)Show SMILES COC1(CN2CCC1CC2)C#CC(O)(c1ccccc1)c1ccc(F)nc1 |THB:1:2:5.6:9.8,(7.82,-23.84,;9.35,-23.8,;10.15,-25.13,;10.72,-26.4,;12.01,-25.35,;13.49,-25.58,;12.85,-24.32,;11.39,-24.11,;11.01,-22.52,;11.74,-23.46,;8.82,-25.91,;7.5,-26.7,;6.17,-27.49,;5.39,-26.17,;4.85,-28.28,;3.5,-27.53,;2.18,-28.31,;2.2,-29.85,;3.55,-30.61,;4.87,-29.82,;6.96,-28.81,;8.48,-28.79,;9.27,-30.12,;8.52,-31.45,;9.31,-32.77,;6.98,-31.47,;6.19,-30.16,)| Show InChI InChI=1S/C22H23FN2O2/c1-27-21(16-25-13-9-17(21)10-14-25)11-12-22(26,18-5-3-2-4-6-18)19-7-8-20(23)24-15-19/h2-8,15,17,26H,9-10,13-14,16H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.320 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
UCB Pharma
Curated by ChEMBL
| Assay Description
Binding affinity to human muscarinic M3 receptor |
Bioorg Med Chem Lett 16: 373-7 (2005)
Article DOI: 10.1016/j.bmcl.2005.09.079
BindingDB Entry DOI: 10.7270/Q2T72J70 |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M2
(Homo sapiens (Human)) | BDBM50176735

((R)-3-(3-methoxyquinuclidin-3-yl)-1,1-di(thiophen-...)Show SMILES CO[C@@]1(CN2CCC1CC2)C#CC(O)(c1ccsc1)c1ccsc1 |wU:2.1,wD:2.11,THB:1:2:5.6:9.8,(9.1,6.91,;10.59,6.52,;11,5.02,;11.19,3.64,;12.73,4.3,;14.09,3.67,;13.82,5.06,;12.47,5.67,;12.54,7.31,;12.98,6.2,;9.5,4.63,;8,4.23,;6.51,3.83,;6.11,5.32,;5.02,3.44,;4.48,2,;2.91,2.09,;2.52,3.58,;3.83,4.41,;6.91,2.35,;8.33,1.8,;8.24,.24,;6.76,-.15,;5.94,1.16,)| Show InChI InChI=1S/C19H21NO2S2/c1-22-18(14-20-8-2-15(18)3-9-20)6-7-19(21,16-4-10-23-12-16)17-5-11-24-13-17/h4-5,10-13,15,21H,2-3,8-9,14H2,1H3/t18-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
UCB Pharma
Curated by ChEMBL
| Assay Description
Binding affinity to human muscarinic M2 receptor |
Bioorg Med Chem Lett 16: 373-7 (2005)
Article DOI: 10.1016/j.bmcl.2005.09.079
BindingDB Entry DOI: 10.7270/Q2T72J70 |
More data for this
Ligand-Target Pair | |
Prothrombin
(Homo sapiens (Human)) | BDBM50123486
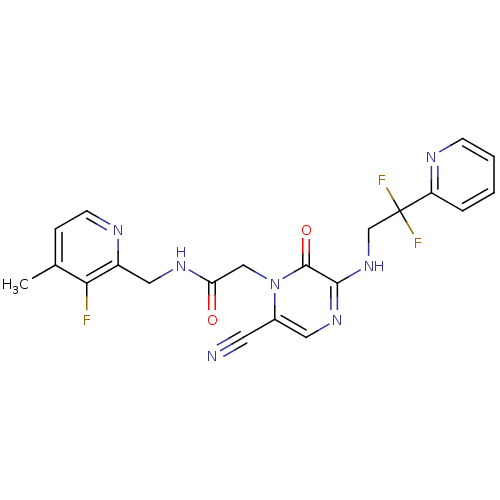
(2-[6-Cyano-3-(2,2-difluoro-2-pyridin-2-yl-ethylami...)Show SMILES Cc1ccnc(CNC(=O)Cn2c(cnc(NCC(F)(F)c3ccccn3)c2=O)C#N)c1F Show InChI InChI=1S/C21H18F3N7O2/c1-13-5-7-26-15(18(13)22)10-28-17(32)11-31-14(8-25)9-29-19(20(31)33)30-12-21(23,24)16-4-2-3-6-27-16/h2-7,9H,10-12H2,1H3,(H,28,32)(H,29,30) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibitory constant against thrombin (IIa) |
J Med Chem 46: 461-73 (2003)
Article DOI: 10.1021/jm020311f
BindingDB Entry DOI: 10.7270/Q2W958J5 |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M3
(Homo sapiens (Human)) | BDBM50176729
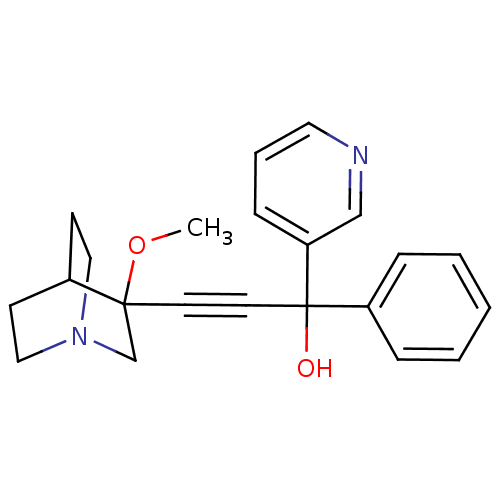
(3-(3-methoxyquinuclidin-3-yl)-1-phenyl-1-(pyridin-...)Show SMILES COC1(CN2CCC1CC2)C#CC(O)(c1ccccc1)c1cccnc1 |THB:1:2:5.6:9.8,(-5.03,-13.2,;-3.49,-13.16,;-2.69,-14.49,;-2.13,-15.76,;-.83,-14.71,;.65,-14.94,;0,-13.68,;-1.46,-13.46,;-1.84,-11.87,;-1.11,-12.82,;-4.02,-15.27,;-5.35,-16.06,;-6.67,-16.85,;-7.46,-15.53,;-8,-17.64,;-9.35,-16.88,;-10.67,-17.67,;-10.65,-19.21,;-9.3,-19.97,;-7.98,-19.18,;-5.89,-18.17,;-4.36,-18.15,;-3.58,-19.47,;-4.32,-20.81,;-5.86,-20.83,;-6.65,-19.52,)| Show InChI InChI=1S/C22H24N2O2/c1-26-21(17-24-14-9-18(21)10-15-24)11-12-22(25,19-6-3-2-4-7-19)20-8-5-13-23-16-20/h2-8,13,16,18,25H,9-10,14-15,17H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
UCB Pharma
Curated by ChEMBL
| Assay Description
Binding affinity to human muscarinic M3 receptor |
Bioorg Med Chem Lett 16: 373-7 (2005)
Article DOI: 10.1016/j.bmcl.2005.09.079
BindingDB Entry DOI: 10.7270/Q2T72J70 |
More data for this
Ligand-Target Pair | |
Prothrombin
(Homo sapiens (Human)) | BDBM50164264
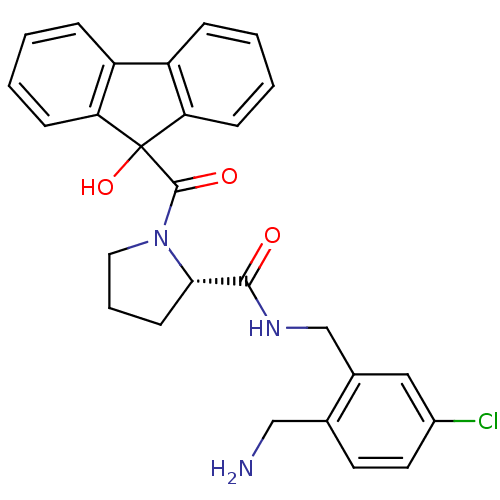
((S)-1-(9-Hydroxy-9H-fluorene-9-carbonyl)-pyrrolidi...)Show SMILES NCc1ccc(Cl)cc1CNC(=O)[C@@H]1CCCN1C(=O)C1(O)c2ccccc2-c2ccccc12 Show InChI InChI=1S/C27H26ClN3O3/c28-19-12-11-17(15-29)18(14-19)16-30-25(32)24-10-5-13-31(24)26(33)27(34)22-8-3-1-6-20(22)21-7-2-4-9-23(21)27/h1-4,6-9,11-12,14,24,34H,5,10,13,15-16,29H2,(H,30,32)/t24-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.490 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of coagulation factor II (thrombin) of human |
J Med Chem 48: 2282-93 (2005)
Article DOI: 10.1021/jm049423s
BindingDB Entry DOI: 10.7270/Q2BR8RPD |
More data for this
Ligand-Target Pair | |
Prothrombin
(Homo sapiens (Human)) | BDBM50123479
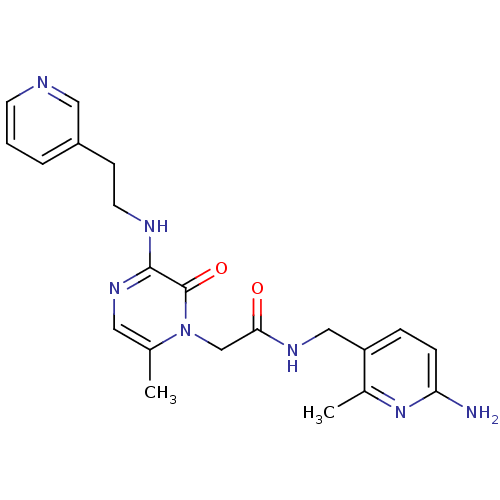
(CHEMBL143008 | N-(6-Amino-2-methyl-pyridin-3-ylmet...)Show SMILES Cc1nc(N)ccc1CNC(=O)Cn1c(C)cnc(NCCc2cccnc2)c1=O Show InChI InChI=1S/C21H25N7O2/c1-14-10-26-20(24-9-7-16-4-3-8-23-11-16)21(30)28(14)13-19(29)25-12-17-5-6-18(22)27-15(17)2/h3-6,8,10-11H,7,9,12-13H2,1-2H3,(H2,22,27)(H,24,26)(H,25,29) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.540 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibitory constant against thrombin (IIa) |
J Med Chem 46: 461-73 (2003)
Article DOI: 10.1021/jm020311f
BindingDB Entry DOI: 10.7270/Q2W958J5 |
More data for this
Ligand-Target Pair | |
Prothrombin
(Homo sapiens (Human)) | BDBM50164256
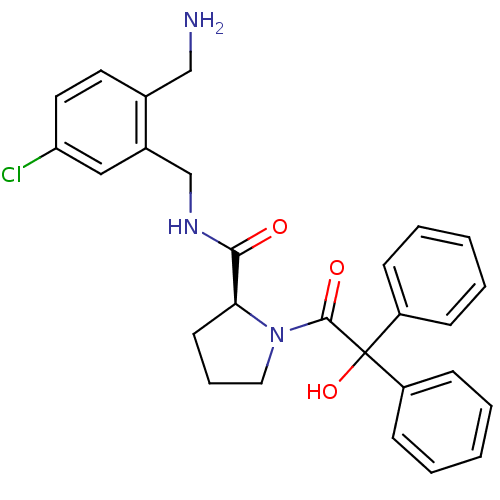
((S)-1-(2-Hydroxy-2,2-diphenyl-acetyl)-pyrrolidine-...)Show SMILES NCc1ccc(Cl)cc1CNC(=O)[C@@H]1CCCN1C(=O)C(O)(c1ccccc1)c1ccccc1 Show InChI InChI=1S/C27H28ClN3O3/c28-23-14-13-19(17-29)20(16-23)18-30-25(32)24-12-7-15-31(24)26(33)27(34,21-8-3-1-4-9-21)22-10-5-2-6-11-22/h1-6,8-11,13-14,16,24,34H,7,12,15,17-18,29H2,(H,30,32)/t24-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.550 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of coagulation factor II (thrombin) of human |
J Med Chem 48: 2282-93 (2005)
Article DOI: 10.1021/jm049423s
BindingDB Entry DOI: 10.7270/Q2BR8RPD |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M3
(Homo sapiens (Human)) | BDBM50176717
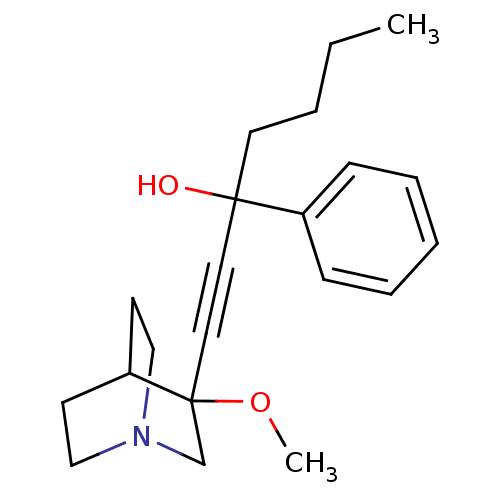
(1-(3-methoxyquinuclidin-3-yl)-3-phenylhept-1-yn-3-...)Show SMILES CCCCC(O)(C#CC1(CN2CCC1CC2)OC)c1ccccc1 |THB:16:8:11.12:15.14,(25.15,-12.89,;23.66,-12.49,;23.27,-11,;24.36,-9.92,;23.96,-8.43,;23.57,-6.94,;25.45,-8.03,;26.94,-7.64,;28.44,-7.24,;28.64,-8.62,;30.17,-7.96,;31.54,-8.59,;31.26,-7.2,;29.91,-6.6,;29.98,-4.96,;30.42,-6.07,;28.03,-5.75,;26.54,-5.36,;22.47,-8.83,;21.38,-7.73,;19.89,-8.13,;19.49,-9.62,;20.58,-10.72,;22.07,-10.31,)| Show InChI InChI=1S/C21H29NO2/c1-3-4-12-20(23,18-8-6-5-7-9-18)13-14-21(24-2)17-22-15-10-19(21)11-16-22/h5-9,19,23H,3-4,10-12,15-17H2,1-2H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.630 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
UCB Pharma
Curated by ChEMBL
| Assay Description
Binding affinity to human muscarinic M3 receptor |
Bioorg Med Chem Lett 16: 373-7 (2005)
Article DOI: 10.1016/j.bmcl.2005.09.079
BindingDB Entry DOI: 10.7270/Q2T72J70 |
More data for this
Ligand-Target Pair | |
Melanocyte-stimulating hormone receptor
(Homo sapiens (Human)) | BDBM50191554
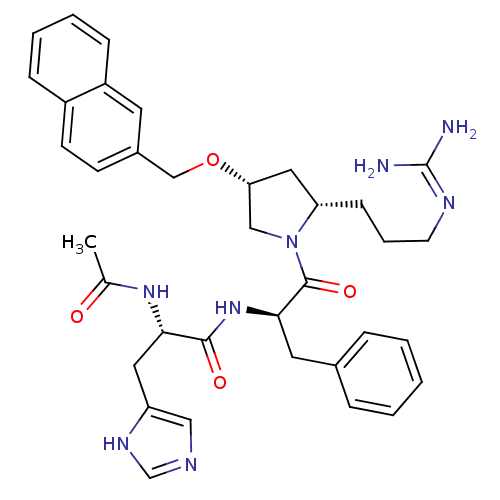
((S)-2-acetamido-N-((R)-1-((2S,4R)-2-(3-guanidinopr...)Show SMILES CC(=O)N[C@@H](Cc1cnc[nH]1)C(=O)N[C@H](Cc1ccccc1)C(=O)N1C[C@@H](C[C@@H]1CCCN=C(N)N)OCc1ccc2ccccc2c1 |wU:28.31,14.14,26.38,wD:4.3,(17.84,3.85,;16.5,3.08,;16.5,1.54,;15.17,3.85,;13.84,3.08,;12.5,3.85,;12.5,5.39,;11.25,6.28,;11.72,7.75,;13.26,7.75,;13.74,6.28,;13.84,1.54,;15.17,.77,;12.5,.77,;12.51,-.77,;11.17,-1.54,;9.84,-.77,;8.5,-1.55,;7.16,-.77,;7.17,.77,;8.49,1.54,;9.83,.78,;13.84,-1.54,;15.17,-.77,;13.84,-3.08,;12.59,-3.99,;13.07,-5.45,;14.61,-5.45,;15.09,-3.98,;16.43,-3.24,;17.75,-4.03,;19.1,-3.28,;20.42,-4.07,;21.77,-3.32,;23.09,-4.11,;21.79,-1.78,;12.17,-6.7,;12.8,-8.1,;12.03,-9.44,;12.8,-10.76,;12.04,-12.1,;10.49,-12.11,;9.73,-13.43,;8.2,-13.44,;7.43,-12.1,;8.2,-10.78,;9.73,-10.78,;10.49,-9.44,)| Show InChI InChI=1S/C36H44N8O4/c1-24(45)42-32(18-29-20-39-23-41-29)34(46)43-33(17-25-8-3-2-4-9-25)35(47)44-21-31(19-30(44)12-7-15-40-36(37)38)48-22-26-13-14-27-10-5-6-11-28(27)16-26/h2-6,8-11,13-14,16,20,23,30-33H,7,12,15,17-19,21-22H2,1H3,(H,39,41)(H,42,45)(H,43,46)(H4,37,38,40)/t30-,31+,32-,33+/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.650 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Procter & Gamble Pharmaceuticals
Curated by ChEMBL
| Assay Description
Displacement of europium labeled NDP-alpha-MSH from human MC1R expressed in HEK293 cells |
J Med Chem 49: 4745-61 (2006)
Article DOI: 10.1021/jm060384p
BindingDB Entry DOI: 10.7270/Q29W0F4J |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M3
(Homo sapiens (Human)) | BDBM50176726
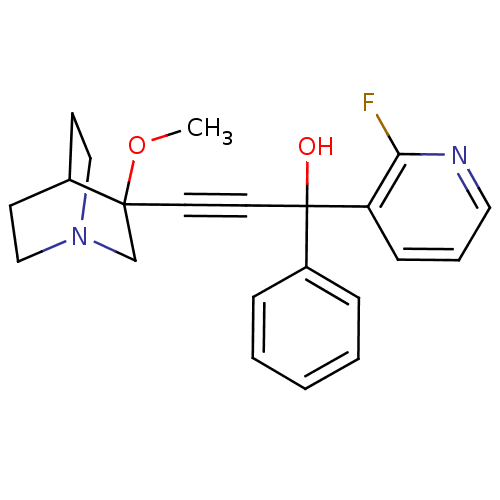
(1-(2-fluoropyridin-3-yl)-3-(3-methoxyquinuclidin-3...)Show SMILES COC1(CN2CCC1CC2)C#CC(O)(c1ccccc1)c1cccnc1F |THB:1:2:5.6:9.8,(22.68,-23.08,;24.21,-23.04,;25.02,-24.36,;25.58,-25.63,;26.87,-24.58,;28.36,-24.82,;27.71,-23.56,;26.25,-23.34,;25.87,-21.75,;26.6,-22.69,;23.68,-25.15,;22.36,-25.94,;21.03,-26.73,;20.25,-25.41,;19.71,-27.52,;18.36,-26.76,;17.04,-27.55,;17.06,-29.09,;18.41,-29.85,;19.73,-29.06,;21.82,-28.05,;21.07,-29.39,;21.85,-30.71,;23.39,-30.69,;24.14,-29.35,;23.35,-28.03,;24.1,-26.68,)| Show InChI InChI=1S/C22H23FN2O2/c1-27-21(16-25-14-9-17(21)10-15-25)11-12-22(26,18-6-3-2-4-7-18)19-8-5-13-24-20(19)23/h2-8,13,17,26H,9-10,14-16H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.790 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
UCB Pharma
Curated by ChEMBL
| Assay Description
Binding affinity to human muscarinic M3 receptor |
Bioorg Med Chem Lett 16: 373-7 (2005)
Article DOI: 10.1016/j.bmcl.2005.09.079
BindingDB Entry DOI: 10.7270/Q2T72J70 |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M2
(Homo sapiens (Human)) | BDBM50176732

((R)-3-(3-methoxyquinuclidin-3-yl)-1,1-diphenylprop...)Show SMILES CO[C@@]1(CN2CCC1CC2)C#CC(O)(c1ccccc1)c1ccccc1 |wU:2.1,wD:2.11,THB:1:2:5.6:9.8,(-2.54,5.9,;-1.05,5.51,;-.64,4.02,;-.44,2.64,;1.09,3.3,;2.46,2.67,;2.18,4.06,;.83,4.66,;.9,6.3,;1.34,5.19,;-2.14,3.62,;-3.63,3.22,;-5.12,2.83,;-5.52,4.31,;-4.73,1.34,;-5.81,.25,;-5.42,-1.23,;-3.93,-1.63,;-2.84,-.54,;-3.24,.94,;-6.61,2.43,;-7.7,3.53,;-9.19,3.13,;-9.59,1.64,;-8.5,.54,;-7.01,.94,)| Show InChI InChI=1S/C23H25NO2/c1-26-22(18-24-16-12-19(22)13-17-24)14-15-23(25,20-8-4-2-5-9-20)21-10-6-3-7-11-21/h2-11,19,25H,12-13,16-18H2,1H3/t22-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.790 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
UCB Pharma
Curated by ChEMBL
| Assay Description
Binding affinity to human muscarinic M2 receptor |
Bioorg Med Chem Lett 16: 373-7 (2005)
Article DOI: 10.1016/j.bmcl.2005.09.079
BindingDB Entry DOI: 10.7270/Q2T72J70 |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M3
(Homo sapiens (Human)) | BDBM50176711
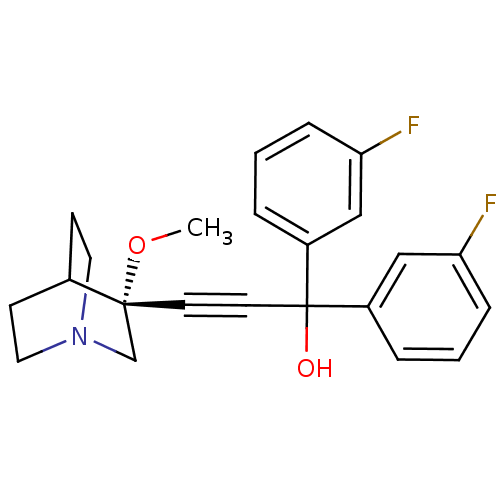
((R)-1,1-bis(3-fluorophenyl)-3-(3-methoxyquinuclidi...)Show SMILES CO[C@@]1(CN2CCC1CC2)C#CC(O)(c1cccc(F)c1)c1cccc(F)c1 |wU:2.1,wD:2.11,THB:1:2:5.6:9.8,(26.22,-26.56,;27.7,-26.94,;28.12,-28.44,;28.31,-29.81,;29.84,-29.16,;31.21,-29.79,;30.93,-28.4,;29.58,-27.79,;29.65,-26.16,;30.1,-27.26,;26.62,-28.83,;25.13,-29.23,;23.63,-29.62,;23.24,-28.14,;22.14,-30.02,;21.74,-31.51,;20.25,-31.91,;19.16,-30.81,;19.57,-29.32,;18.48,-28.23,;21.05,-28.93,;24.03,-31.11,;22.94,-32.2,;23.34,-33.68,;24.82,-34.09,;25.91,-32.99,;27.4,-33.39,;25.51,-31.51,)| Show InChI InChI=1S/C23H23F2NO2/c1-28-22(16-26-12-8-17(22)9-13-26)10-11-23(27,18-4-2-6-20(24)14-18)19-5-3-7-21(25)15-19/h2-7,14-15,17,27H,8-9,12-13,16H2,1H3/t22-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.790 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
UCB Pharma
Curated by ChEMBL
| Assay Description
Binding affinity to human muscarinic M3 receptor |
Bioorg Med Chem Lett 16: 373-7 (2005)
Article DOI: 10.1016/j.bmcl.2005.09.079
BindingDB Entry DOI: 10.7270/Q2T72J70 |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M2
(Homo sapiens (Human)) | BDBM50176708

((R)-3-((R)-3-methoxyquinuclidin-3-yl)-1-phenyl-1-(...)Show SMILES CO[C@@]1(CN2CCC1CC2)C#C[C@@](O)(c1ccccc1)c1cccnc1 |wU:12.14,2.1,wD:12.13,2.11,THB:1:2:5.6:9.8,(16.23,-16.03,;17.77,-16,;18.57,-17.32,;19.13,-18.59,;20.42,-17.54,;21.91,-17.77,;21.26,-16.51,;19.8,-16.3,;19.42,-14.71,;20.15,-15.65,;17.23,-18.11,;15.91,-18.89,;14.58,-19.68,;13.8,-18.36,;13.26,-20.47,;11.91,-19.72,;10.59,-20.5,;10.61,-22.05,;11.96,-22.8,;13.28,-22.01,;15.37,-21,;16.9,-20.98,;17.68,-22.31,;16.93,-23.64,;15.4,-23.66,;14.6,-22.35,)| Show InChI InChI=1S/C22H24N2O2/c1-26-21(17-24-14-9-18(21)10-15-24)11-12-22(25,19-6-3-2-4-7-19)20-8-5-13-23-16-20/h2-8,13,16,18,25H,9-10,14-15,17H2,1H3/t21-,22-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.790 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
UCB Pharma
Curated by ChEMBL
| Assay Description
Binding affinity to human muscarinic M2 receptor |
Bioorg Med Chem Lett 16: 373-7 (2005)
Article DOI: 10.1016/j.bmcl.2005.09.079
BindingDB Entry DOI: 10.7270/Q2T72J70 |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M3
(Homo sapiens (Human)) | BDBM50403947
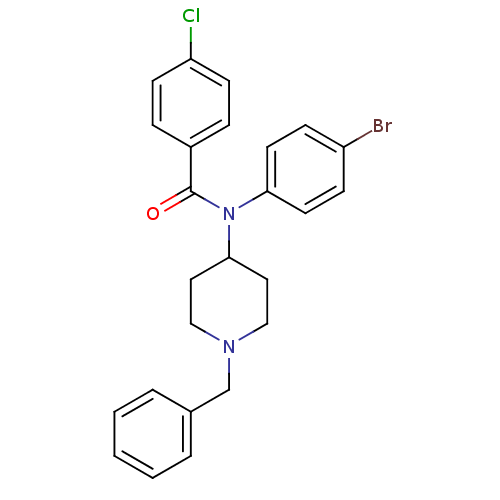
(CHEMBL85996)Show SMILES Clc1ccc(cc1)C(=O)N(C1CCN(Cc2ccccc2)CC1)c1ccc(Br)cc1 Show InChI InChI=1S/C25H24BrClN2O/c26-21-8-12-23(13-9-21)29(25(30)20-6-10-22(27)11-7-20)24-14-16-28(17-15-24)18-19-4-2-1-3-5-19/h1-13,24H,14-18H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.794 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ Libre de Bruxelles
Curated by ChEMBL
| Assay Description
In vitro affinity for muscarinic M3 receptor. |
Bioorg Med Chem Lett 12: 2535-9 (2002)
BindingDB Entry DOI: 10.7270/Q2GT5PBW |
More data for this
Ligand-Target Pair | |
Prothrombin
(Homo sapiens (Human)) | BDBM50067797
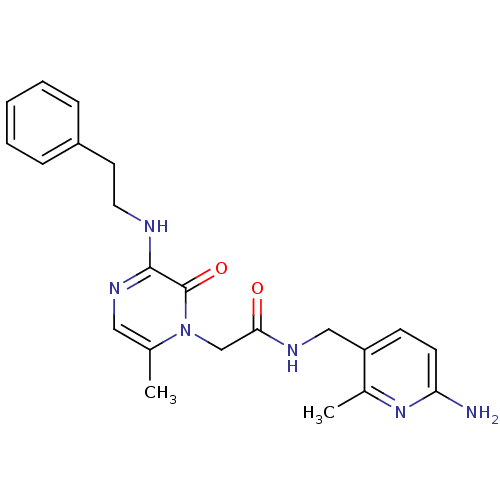
(CHEMBL19080 | L-37378 | N-(6-Amino-2-methyl-pyridi...)Show SMILES Cc1nc(N)ccc1CNC(=O)Cn1c(C)cnc(NCCc2ccccc2)c1=O Show InChI InChI=1S/C22H26N6O2/c1-15-12-26-21(24-11-10-17-6-4-3-5-7-17)22(30)28(15)14-20(29)25-13-18-8-9-19(23)27-16(18)2/h3-9,12H,10-11,13-14H2,1-2H3,(H2,23,27)(H,24,26)(H,25,29) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibitory constant against thrombin (IIa) |
J Med Chem 46: 461-73 (2003)
Article DOI: 10.1021/jm020311f
BindingDB Entry DOI: 10.7270/Q2W958J5 |
More data for this
Ligand-Target Pair | |
72 kDa type IV collagenase
(Homo sapiens (Human)) | BDBM50090364

(3-[2-Hydroxycarbamoyl-1-(4-methoxy-benzenesulfonyl...)Show SMILES CCOC(=O)C1CCCC(C1)C(CC(=O)NO)S(=O)(=O)c1ccc(OC)cc1 Show InChI InChI=1S/C19H27NO7S/c1-3-27-19(22)14-6-4-5-13(11-14)17(12-18(21)20-23)28(24,25)16-9-7-15(26-2)8-10-16/h7-10,13-14,17,23H,3-6,11-12H2,1-2H3,(H,20,21) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Rhone Poulenc Rorer
Curated by ChEMBL
| Assay Description
In vitro inhibition of human recombinant Matrix metalloproteinase-2. |
Bioorg Med Chem Lett 10: 1637-40 (2000)
BindingDB Entry DOI: 10.7270/Q20G3JDK |
More data for this
Ligand-Target Pair | |
TGF-beta receptor type-1
(Homo sapiens (Human)) | BDBM282825
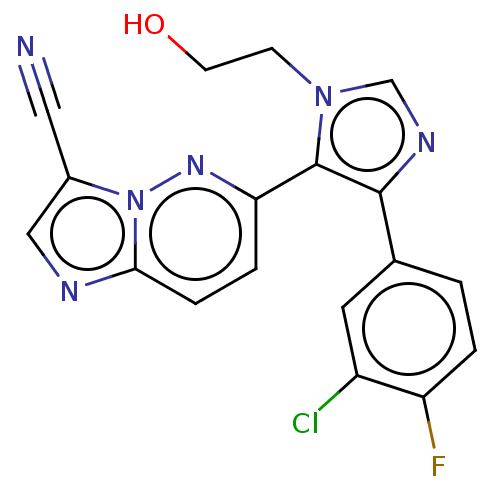
(6-(4-(3-chloro-4-fluorophenyl)-1-(2-hydroxyethyl)-...)Show SMILES OCCn1cnc(c1-c1ccc2ncc(C#N)n2n1)-c1ccc(F)c(Cl)c1 Show InChI InChI=1S/C18H12ClFN6O/c19-13-7-11(1-2-14(13)20)17-18(25(5-6-27)10-23-17)15-3-4-16-22-9-12(8-21)26(16)24-15/h1-4,7,9-10,27H,5-6H2 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| 0.800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research & Development
Curated by ChEMBL
| Assay Description
Inhibition of TGFBR1 in human whole blood assessed as apparent inhibition constant by measuring reduction in TGFbeta-induced SMAD phosphorylation |
ACS Med Chem Lett 11: 172-178 (2020)
Article DOI: 10.1021/acsmedchemlett.9b00552
BindingDB Entry DOI: 10.7270/Q2MP56K3 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Prothrombin
(Homo sapiens (Human)) | BDBM50126295

(2-{6-Chloro-3-[2,2-difluoro-2-(1-oxy-pyridin-2-yl)...)Show SMILES Cc1cccc(CNC(=O)Cn2c(Cl)cnc(NCC(F)(F)c3cccc[n+]3[O-])c2=O)c1 Show InChI InChI=1S/C21H20ClF2N5O3/c1-14-5-4-6-15(9-14)10-25-18(30)12-28-17(22)11-26-19(20(28)31)27-13-21(23,24)16-7-2-3-8-29(16)32/h2-9,11H,10,12-13H2,1H3,(H,25,30)(H,26,27) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 0.800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibitory activity against human thrombin |
Bioorg Med Chem Lett 13: 1353-7 (2003)
BindingDB Entry DOI: 10.7270/Q2833RC9 |
More data for this
Ligand-Target Pair | |
Prothrombin
(Homo sapiens (Human)) | BDBM50126294
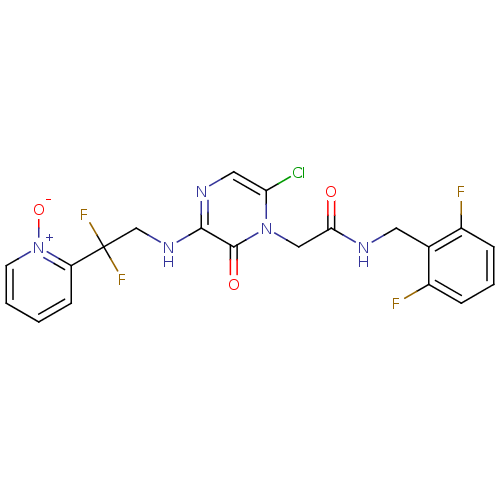
(2-{6-Chloro-3-[2,2-difluoro-2-(1-oxy-pyridin-2-yl)...)Show SMILES [O-][n+]1ccccc1C(F)(F)CNc1ncc(Cl)n(CC(=O)NCc2c(F)cccc2F)c1=O Show InChI InChI=1S/C20H16ClF4N5O3/c21-16-9-27-18(28-11-20(24,25)15-6-1-2-7-30(15)33)19(32)29(16)10-17(31)26-8-12-13(22)4-3-5-14(12)23/h1-7,9H,8,10-11H2,(H,26,31)(H,27,28) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.850 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibitory activity against human thrombin |
Bioorg Med Chem Lett 13: 1353-7 (2003)
BindingDB Entry DOI: 10.7270/Q2833RC9 |
More data for this
Ligand-Target Pair | |
Prothrombin
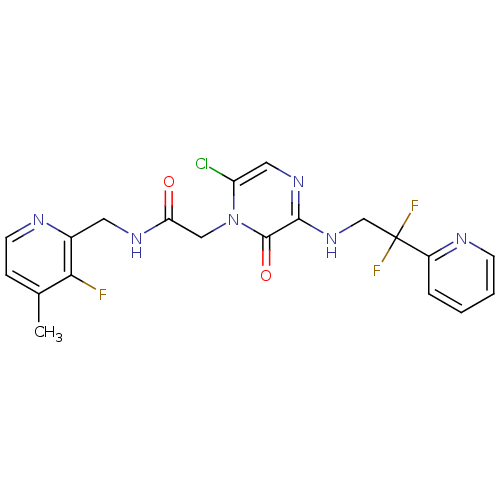
(Homo sapiens (Human)) | BDBM50123497
(2-[6-Chloro-3-(2,2-difluoro-2-pyridin-2-yl-ethylam...)Show SMILES Cc1ccnc(CNC(=O)Cn2c(Cl)cnc(NCC(F)(F)c3ccccn3)c2=O)c1F Show InChI InChI=1S/C20H18ClF3N6O2/c1-12-5-7-25-13(17(12)22)8-27-16(31)10-30-15(21)9-28-18(19(30)32)29-11-20(23,24)14-4-2-3-6-26-14/h2-7,9H,8,10-11H2,1H3,(H,27,31)(H,28,29) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibitory constant against thrombin (IIa) |
J Med Chem 46: 461-73 (2003)
Article DOI: 10.1021/jm020311f
BindingDB Entry DOI: 10.7270/Q2W958J5 |
More data for this
Ligand-Target Pair | |
Histamine H1 receptor
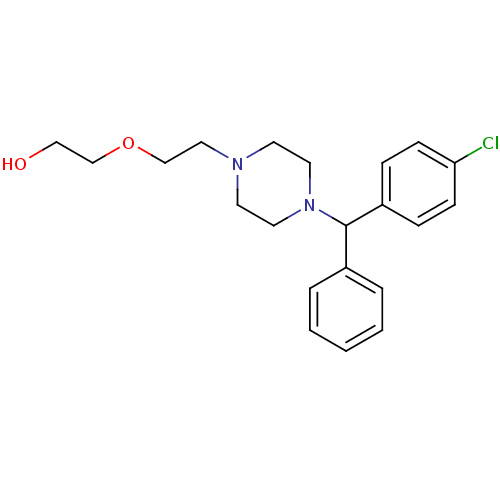
(Homo sapiens (Human)) | BDBM22875
(2-(2-{4-[(4-chlorophenyl)(phenyl)methyl]piperazin-...)Show InChI InChI=1S/C21H27ClN2O2/c22-20-8-6-19(7-9-20)21(18-4-2-1-3-5-18)24-12-10-23(11-13-24)14-16-26-17-15-25/h1-9,21,25H,10-17H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| DrugBank
Article
PubMed
| 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
UCB S.A.
Curated by PDSP Ki Database
| |
Mol Pharmacol 61: 391-9 (2002)
Article DOI: 10.1124/mol.61.2.391
BindingDB Entry DOI: 10.7270/Q2D50KJ8 |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M3
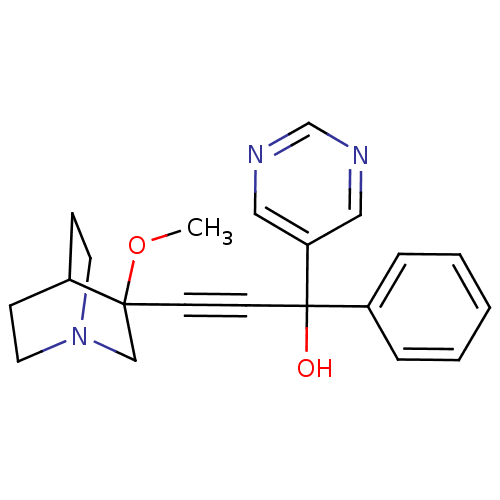
(Homo sapiens (Human)) | BDBM50176704
(3-(3-methoxyquinuclidin-3-yl)-1-phenyl-1-(pyrimidi...)Show SMILES COC1(CN2CCC1CC2)C#CC(O)(c1ccccc1)c1cncnc1 |THB:1:2:5.6:9.8,(8.21,-34.77,;9.74,-34.74,;10.54,-36.06,;11.11,-37.33,;12.4,-36.28,;13.88,-36.51,;13.24,-35.25,;11.78,-35.04,;11.4,-33.45,;12.13,-34.39,;9.21,-36.85,;7.89,-37.63,;6.56,-38.42,;5.78,-37.1,;5.24,-39.21,;3.89,-38.46,;2.57,-39.24,;2.59,-40.78,;3.94,-41.54,;5.26,-40.75,;7.35,-39.74,;8.88,-39.72,;9.66,-41.04,;8.91,-42.38,;7.37,-42.4,;6.58,-41.09,)| Show InChI InChI=1S/C21H23N3O2/c1-26-20(15-24-11-7-17(20)8-12-24)9-10-21(25,18-5-3-2-4-6-18)19-13-22-16-23-14-19/h2-6,13-14,16-17,25H,7-8,11-12,15H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
UCB Pharma
Curated by ChEMBL
| Assay Description
Binding affinity to human muscarinic M3 receptor |
Bioorg Med Chem Lett 16: 373-7 (2005)
Article DOI: 10.1016/j.bmcl.2005.09.079
BindingDB Entry DOI: 10.7270/Q2T72J70 |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M3
(Homo sapiens (Human)) | BDBM50403940

(CHEMBL85892)Show SMILES Brc1ccc(cc1)N(C1CCN(Cc2ccccc2)CC1)C(=O)c1ccccc1 Show InChI InChI=1S/C25H25BrN2O/c26-22-11-13-23(14-12-22)28(25(29)21-9-5-2-6-10-21)24-15-17-27(18-16-24)19-20-7-3-1-4-8-20/h1-14,24H,15-19H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ Libre de Bruxelles
Curated by ChEMBL
| Assay Description
In vitro affinity for muscarinic M3 receptor. |
Bioorg Med Chem Lett 12: 2535-9 (2002)
BindingDB Entry DOI: 10.7270/Q2GT5PBW |
More data for this
Ligand-Target Pair | |
Prothrombin
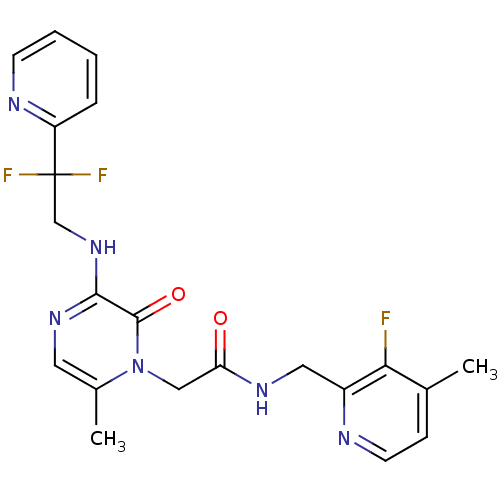
(Homo sapiens (Human)) | BDBM50123503
(2-[3-(2,2-Difluoro-2-pyridin-2-yl-ethylamino)-6-me...)Show SMILES Cc1ccnc(CNC(=O)Cn2c(C)cnc(NCC(F)(F)c3ccccn3)c2=O)c1F Show InChI InChI=1S/C21H21F3N6O2/c1-13-6-8-25-15(18(13)22)10-27-17(31)11-30-14(2)9-28-19(20(30)32)29-12-21(23,24)16-5-3-4-7-26-16/h3-9H,10-12H2,1-2H3,(H,27,31)(H,28,29) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibitory constant against thrombin (IIa) |
J Med Chem 46: 461-73 (2003)
Article DOI: 10.1021/jm020311f
BindingDB Entry DOI: 10.7270/Q2W958J5 |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M3
(Homo sapiens (Human)) | BDBM50176722
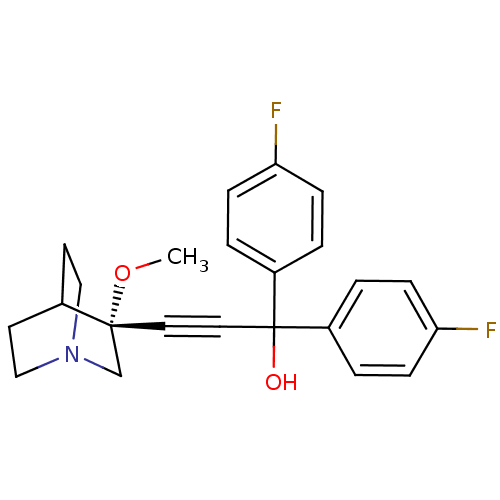
((R)-1,1-bis(4-fluorophenyl)-3-(3-methoxyquinuclidi...)Show SMILES CO[C@@]1(CN2CCC1CC2)C#CC(O)(c1ccc(F)cc1)c1ccc(F)cc1 |wU:2.1,wD:2.11,THB:1:2:5.6:9.8,(25.27,-13.99,;26.76,-14.38,;27.17,-15.87,;27.37,-17.25,;28.9,-16.59,;30.27,-17.22,;29.99,-15.83,;28.64,-15.23,;28.71,-13.59,;29.15,-14.7,;25.67,-16.27,;24.18,-16.66,;22.69,-17.06,;22.3,-15.57,;21.2,-17.46,;20.11,-16.36,;18.62,-16.76,;18.22,-18.25,;16.73,-18.64,;19.31,-19.34,;20.8,-18.94,;23.09,-18.55,;22,-19.63,;22.39,-21.12,;23.88,-21.52,;24.28,-23.01,;24.97,-20.43,;24.57,-18.95,)| Show InChI InChI=1S/C23H23F2NO2/c1-28-22(16-26-14-10-17(22)11-15-26)12-13-23(27,18-2-6-20(24)7-3-18)19-4-8-21(25)9-5-19/h2-9,17,27H,10-11,14-16H2,1H3/t22-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
UCB Pharma
Curated by ChEMBL
| Assay Description
Binding affinity to human muscarinic M3 receptor |
Bioorg Med Chem Lett 16: 373-7 (2005)
Article DOI: 10.1016/j.bmcl.2005.09.079
BindingDB Entry DOI: 10.7270/Q2T72J70 |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M3
(Homo sapiens (Human)) | BDBM50176714
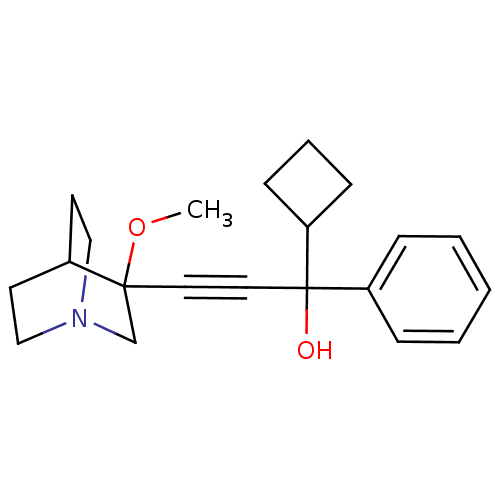
(1-cyclobutyl-3-(3-methoxyquinuclidin-3-yl)-1-pheny...)Show SMILES COC1(CN2CCC1CC2)C#CC(O)(C1CCC1)c1ccccc1 |THB:1:2:5.6:9.8,(-3.4,-5.54,;-1.91,-5.93,;-1.5,-7.42,;-1.31,-8.8,;.23,-8.14,;1.59,-8.77,;1.32,-7.38,;-.03,-6.78,;.04,-5.14,;.48,-6.25,;-3,-7.82,;-4.49,-8.21,;-5.98,-8.61,;-6.38,-7.12,;-5.59,-10.1,;-6.36,-11.43,;-5.03,-12.2,;-4.26,-10.87,;-7.48,-9.01,;-8.57,-7.91,;-10.05,-8.31,;-10.46,-9.8,;-9.36,-10.9,;-7.88,-10.5,)| Show InChI InChI=1S/C21H27NO2/c1-24-20(16-22-14-10-17(20)11-15-22)12-13-21(23,19-8-5-9-19)18-6-3-2-4-7-18/h2-4,6-7,17,19,23H,5,8-11,14-16H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
UCB Pharma
Curated by ChEMBL
| Assay Description
Binding affinity to human muscarinic M3 receptor |
Bioorg Med Chem Lett 16: 373-7 (2005)
Article DOI: 10.1016/j.bmcl.2005.09.079
BindingDB Entry DOI: 10.7270/Q2T72J70 |
More data for this
Ligand-Target Pair | |
Prothrombin
(Homo sapiens (Human)) | BDBM50126306

(2-{6-Chloro-3-[2,2-difluoro-2-(1-oxy-pyridin-2-yl)...)Show SMILES [O-][n+]1ccccc1C(F)(F)CNc1ncc(Cl)n(CC(=O)NCc2c(F)ccc(F)c2F)c1=O Show InChI InChI=1S/C20H15ClF5N5O3/c21-15-8-28-18(29-10-20(25,26)14-3-1-2-6-31(14)34)19(33)30(15)9-16(32)27-7-11-12(22)4-5-13(23)17(11)24/h1-6,8H,7,9-10H2,(H,27,32)(H,28,29) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 1.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibitory activity against human thrombin |
Bioorg Med Chem Lett 13: 1353-7 (2003)
BindingDB Entry DOI: 10.7270/Q2833RC9 |
More data for this
Ligand-Target Pair | |
Prothrombin
(Homo sapiens (Human)) | BDBM50164260
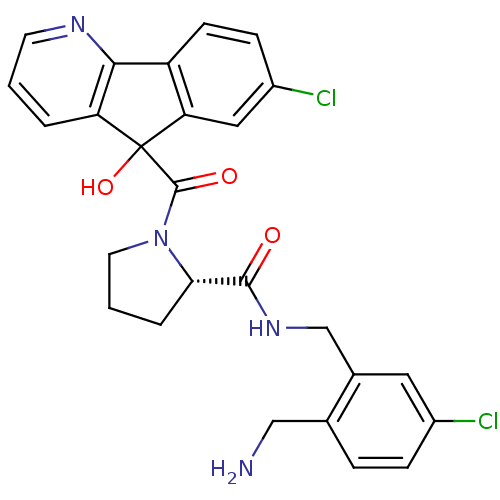
((S)-1-(7-Chloro-5-hydroxy-5H-indeno[1,2-b]pyridine...)Show SMILES NCc1ccc(Cl)cc1CNC(=O)[C@@H]1CCCN1C(=O)C1(O)c2cc(Cl)ccc2-c2ncccc12 Show InChI InChI=1S/C26H24Cl2N4O3/c27-17-6-5-15(13-29)16(11-17)14-31-24(33)22-4-2-10-32(22)25(34)26(35)20-3-1-9-30-23(20)19-8-7-18(28)12-21(19)26/h1,3,5-9,11-12,22,35H,2,4,10,13-14,29H2,(H,31,33)/t22-,26?/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 1.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of coagulation factor II (thrombin) of human |
J Med Chem 48: 2282-93 (2005)
Article DOI: 10.1021/jm049423s
BindingDB Entry DOI: 10.7270/Q2BR8RPD |
More data for this
Ligand-Target Pair | |
Prothrombin
(Homo sapiens (Human)) | BDBM50123493
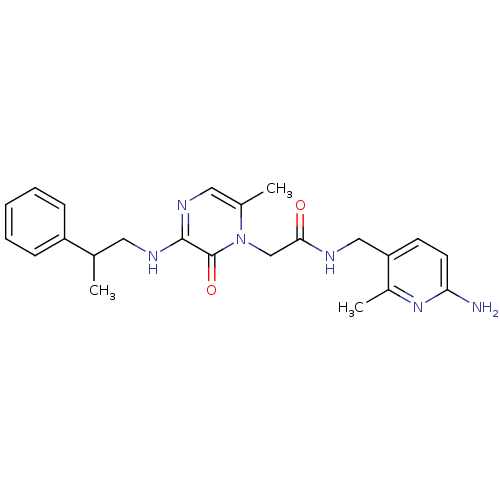
(CHEMBL142566 | N-(6-Amino-2-methyl-pyridin-3-ylmet...)Show SMILES CC(CNc1ncc(C)n(CC(=O)NCc2ccc(N)nc2C)c1=O)c1ccccc1 Show InChI InChI=1S/C23H28N6O2/c1-15(18-7-5-4-6-8-18)11-26-22-23(31)29(16(2)12-27-22)14-21(30)25-13-19-9-10-20(24)28-17(19)3/h4-10,12,15H,11,13-14H2,1-3H3,(H2,24,28)(H,25,30)(H,26,27) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibitory constant against thrombin (IIa) |
J Med Chem 46: 461-73 (2003)
Article DOI: 10.1021/jm020311f
BindingDB Entry DOI: 10.7270/Q2W958J5 |
More data for this
Ligand-Target Pair | |
Prothrombin
(Homo sapiens (Human)) | BDBM50123500

(CHEMBL143139 | N-(6-Amino-2-methyl-pyridin-3-ylmet...)Show SMILES Cc1nc(N)ccc1CNC(=O)Cn1c(C)cnc(NCC(C)(C)c2ccccc2)c1=O Show InChI InChI=1S/C24H30N6O2/c1-16-12-27-22(28-15-24(3,4)19-8-6-5-7-9-19)23(32)30(16)14-21(31)26-13-18-10-11-20(25)29-17(18)2/h5-12H,13-15H2,1-4H3,(H2,25,29)(H,26,31)(H,27,28) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibitory constant against thrombin (IIa) |
J Med Chem 46: 461-73 (2003)
Article DOI: 10.1021/jm020311f
BindingDB Entry DOI: 10.7270/Q2W958J5 |
More data for this
Ligand-Target Pair | |
Prothrombin
(Homo sapiens (Human)) | BDBM50164260

((S)-1-(7-Chloro-5-hydroxy-5H-indeno[1,2-b]pyridine...)Show SMILES NCc1ccc(Cl)cc1CNC(=O)[C@@H]1CCCN1C(=O)C1(O)c2cc(Cl)ccc2-c2ncccc12 Show InChI InChI=1S/C26H24Cl2N4O3/c27-17-6-5-15(13-29)16(11-17)14-31-24(33)22-4-2-10-32(22)25(34)26(35)20-3-1-9-30-23(20)19-8-7-18(28)12-21(19)26/h1,3,5-9,11-12,22,35H,2,4,10,13-14,29H2,(H,31,33)/t22-,26?/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 1.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of coagulation factor II (thrombin) of human |
J Med Chem 48: 2282-93 (2005)
Article DOI: 10.1021/jm049423s
BindingDB Entry DOI: 10.7270/Q2BR8RPD |
More data for this
Ligand-Target Pair | |
Prothrombin
(Homo sapiens (Human)) | BDBM50123495
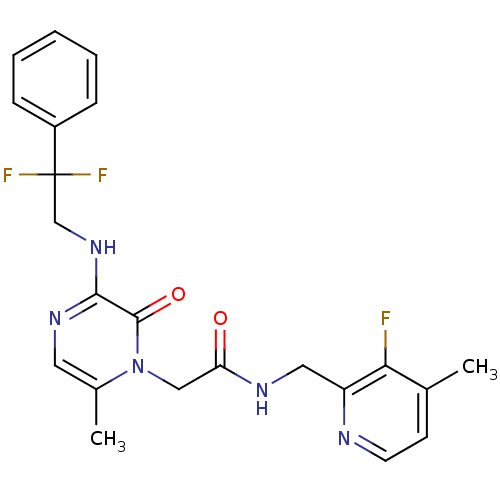
(2-[3-(2,2-Difluoro-2-phenyl-ethylamino)-6-methyl-2...)Show SMILES Cc1ccnc(CNC(=O)Cn2c(C)cnc(NCC(F)(F)c3ccccc3)c2=O)c1F Show InChI InChI=1S/C22H22F3N5O2/c1-14-8-9-26-17(19(14)23)11-27-18(31)12-30-15(2)10-28-20(21(30)32)29-13-22(24,25)16-6-4-3-5-7-16/h3-10H,11-13H2,1-2H3,(H,27,31)(H,28,29) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibitory constant against thrombin (IIa) |
J Med Chem 46: 461-73 (2003)
Article DOI: 10.1021/jm020311f
BindingDB Entry DOI: 10.7270/Q2W958J5 |
More data for this
Ligand-Target Pair | |
Prothrombin
(Homo sapiens (Human)) | BDBM50126297
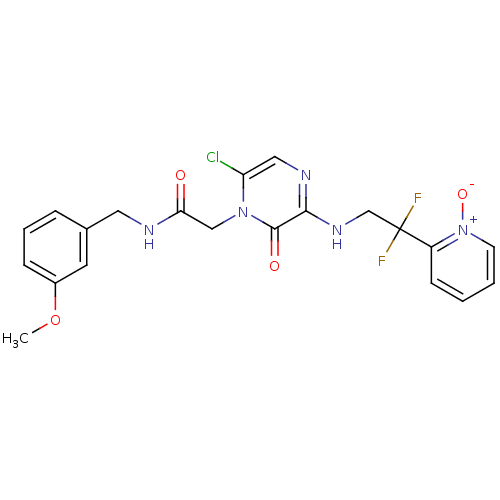
(2-{6-Chloro-3-[2,2-difluoro-2-(1-oxy-pyridin-2-yl)...)Show SMILES COc1cccc(CNC(=O)Cn2c(Cl)cnc(NCC(F)(F)c3cccc[n+]3[O-])c2=O)c1 Show InChI InChI=1S/C21H20ClF2N5O4/c1-33-15-6-4-5-14(9-15)10-25-18(30)12-28-17(22)11-26-19(20(28)31)27-13-21(23,24)16-7-2-3-8-29(16)32/h2-9,11H,10,12-13H2,1H3,(H,25,30)(H,26,27) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 1.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibitory activity against human thrombin |
Bioorg Med Chem Lett 13: 1353-7 (2003)
BindingDB Entry DOI: 10.7270/Q2833RC9 |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M3
(Homo sapiens (Human)) | BDBM50109647
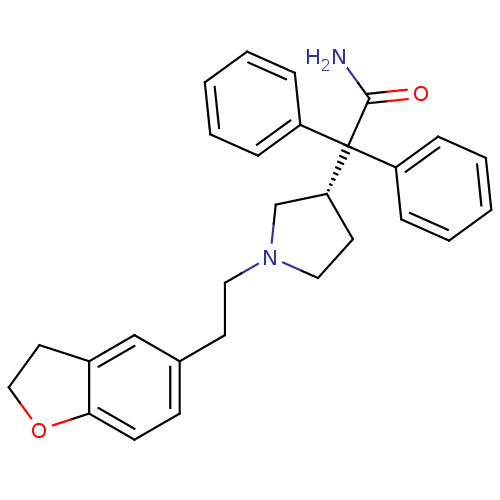
(2-{1-[2-(2,3-Dihydro-benzofuran-5-yl)-ethyl]-pyrro...)Show SMILES NC(=O)C([C@@H]1CCN(CCc2ccc3OCCc3c2)C1)(c1ccccc1)c1ccccc1 Show InChI InChI=1S/C28H30N2O2/c29-27(31)28(23-7-3-1-4-8-23,24-9-5-2-6-10-24)25-14-17-30(20-25)16-13-21-11-12-26-22(19-21)15-18-32-26/h1-12,19,25H,13-18,20H2,(H2,29,31)/t25-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
PC cid
PC sid
UniChem
Patents
Similars
| DrugBank
Article
PubMed
| 1.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
UCB Pharma
Curated by ChEMBL
| Assay Description
Binding affinity to human muscarinic M3 receptor |
Bioorg Med Chem Lett 16: 373-7 (2005)
Article DOI: 10.1016/j.bmcl.2005.09.079
BindingDB Entry DOI: 10.7270/Q2T72J70 |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M3
(Homo sapiens (Human)) | BDBM50165019
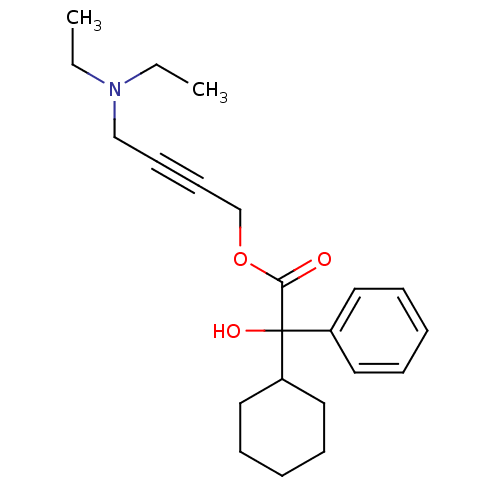
(4-(Diethylamino)-2-butynyl alpha-phenylcyclohexane...)Show InChI InChI=1S/C22H31NO3/c1-3-23(4-2)17-11-12-18-26-21(24)22(25,19-13-7-5-8-14-19)20-15-9-6-10-16-20/h5,7-8,13-14,20,25H,3-4,6,9-10,15-18H2,1-2H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| DrugBank
Article
PubMed
| 1.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
UCB Pharma
Curated by ChEMBL
| Assay Description
Binding affinity to human muscarinic M3 receptor |
Bioorg Med Chem Lett 16: 373-7 (2005)
Article DOI: 10.1016/j.bmcl.2005.09.079
BindingDB Entry DOI: 10.7270/Q2T72J70 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data