 Found 83 hits with Last Name = 'vogel' and Initial = 'rl'
Found 83 hits with Last Name = 'vogel' and Initial = 'rl' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
5-hydroxytryptamine receptor 1A
(Homo sapiens (Human)) | BDBM50099016

(CHEMBL275964 | Cyclohexanecarboxylic acid {(S)-1-b...)Show SMILES Clc1nsnc1N1CCN(C[C@H](Cc2ccccc2)NC(=O)C2CCCCC2)CC1 Show InChI InChI=1S/C22H30ClN5OS/c23-20-21(26-30-25-20)28-13-11-27(12-14-28)16-19(15-17-7-3-1-4-8-17)24-22(29)18-9-5-2-6-10-18/h1,3-4,7-8,18-19H,2,5-6,9-16H2,(H,24,29)/t19-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth-Ayerst Research
Curated by ChEMBL
| Assay Description
Ability to displace [3H]-8-OH-DPAT from CHO cells stably transfected with human 5-hydroxytryptamine 1A receptor |
Bioorg Med Chem Lett 11: 1069-71 (2001)
BindingDB Entry DOI: 10.7270/Q2SJ1JWX |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 1A
(Homo sapiens (Human)) | BDBM50099015
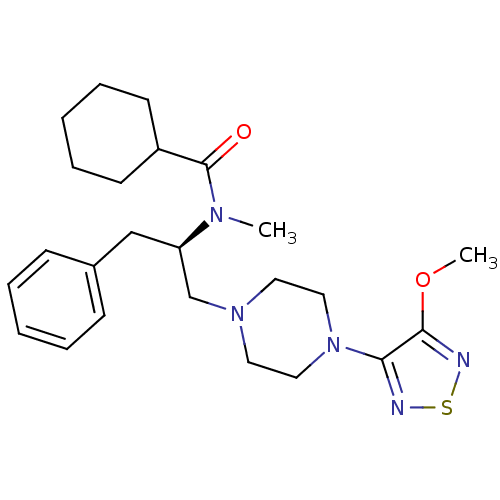
(CHEMBL10796 | Cyclohexanecarboxylic acid {(R)-1-be...)Show SMILES COc1nsnc1N1CCN(C[C@@H](Cc2ccccc2)N(C)C(=O)C2CCCCC2)CC1 Show InChI InChI=1S/C24H35N5O2S/c1-27(24(30)20-11-7-4-8-12-20)21(17-19-9-5-3-6-10-19)18-28-13-15-29(16-14-28)22-23(31-2)26-32-25-22/h3,5-6,9-10,20-21H,4,7-8,11-18H2,1-2H3/t21-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 1.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth-Ayerst Research
Curated by ChEMBL
| Assay Description
Ability to displace [3H]-8-OH-DPAT from CHO cells stably transfected with human 5-hydroxytryptamine 1A receptor |
Bioorg Med Chem Lett 11: 1069-71 (2001)
BindingDB Entry DOI: 10.7270/Q2SJ1JWX |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 1A
(Homo sapiens (Human)) | BDBM50099011
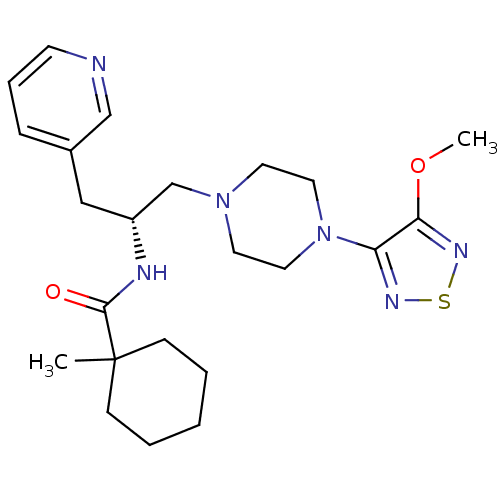
(1-Methyl-cyclohexanecarboxylic acid {(R)-2-[4-(4-m...)Show SMILES COc1nsnc1N1CCN(C[C@@H](Cc2cccnc2)NC(=O)C2(C)CCCCC2)CC1 Show InChI InChI=1S/C23H34N6O2S/c1-23(8-4-3-5-9-23)22(30)25-19(15-18-7-6-10-24-16-18)17-28-11-13-29(14-12-28)20-21(31-2)27-32-26-20/h6-7,10,16,19H,3-5,8-9,11-15,17H2,1-2H3,(H,25,30)/t19-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth-Ayerst Research
Curated by ChEMBL
| Assay Description
Ability to displace [3H]-8-OH-DPAT from CHO cells stably transfected with human 5-hydroxytryptamine 1A receptor |
Bioorg Med Chem Lett 11: 1069-71 (2001)
BindingDB Entry DOI: 10.7270/Q2SJ1JWX |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 1A
(Homo sapiens (Human)) | BDBM50099014
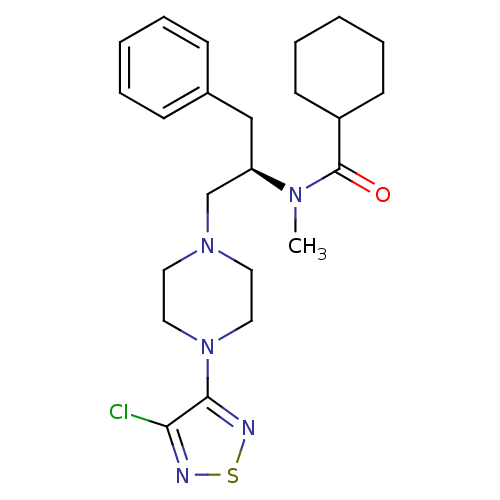
(CHEMBL10793 | Cyclohexanecarboxylic acid {(R)-1-be...)Show SMILES CN([C@@H](CN1CCN(CC1)c1nsnc1Cl)Cc1ccccc1)C(=O)C1CCCCC1 Show InChI InChI=1S/C23H32ClN5OS/c1-27(23(30)19-10-6-3-7-11-19)20(16-18-8-4-2-5-9-18)17-28-12-14-29(15-13-28)22-21(24)25-31-26-22/h2,4-5,8-9,19-20H,3,6-7,10-17H2,1H3/t20-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 4.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth-Ayerst Research
Curated by ChEMBL
| Assay Description
Ability to displace [3H]-8-OH-DPAT from CHO cells stably transfected with human 5-hydroxytryptamine 1A receptor |
Bioorg Med Chem Lett 11: 1069-71 (2001)
BindingDB Entry DOI: 10.7270/Q2SJ1JWX |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 1A
(Homo sapiens (Human)) | BDBM50099013
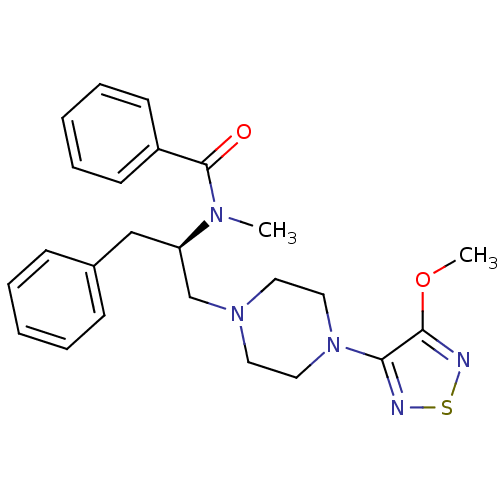
(CHEMBL10603 | N-{(R)-1-Benzyl-2-[4-(4-methoxy-[1,2...)Show SMILES COc1nsnc1N1CCN(C[C@@H](Cc2ccccc2)N(C)C(=O)c2ccccc2)CC1 Show InChI InChI=1S/C24H29N5O2S/c1-27(24(30)20-11-7-4-8-12-20)21(17-19-9-5-3-6-10-19)18-28-13-15-29(16-14-28)22-23(31-2)26-32-25-22/h3-12,21H,13-18H2,1-2H3/t21-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| PubMed
| 9.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth-Ayerst Research
Curated by ChEMBL
| Assay Description
Ability to displace [3H]-8-OH-DPAT from CHO cells stably transfected with human 5-hydroxytryptamine 1A receptor |
Bioorg Med Chem Lett 11: 1069-71 (2001)
BindingDB Entry DOI: 10.7270/Q2SJ1JWX |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2C
(Homo sapiens (Human)) | BDBM50145565
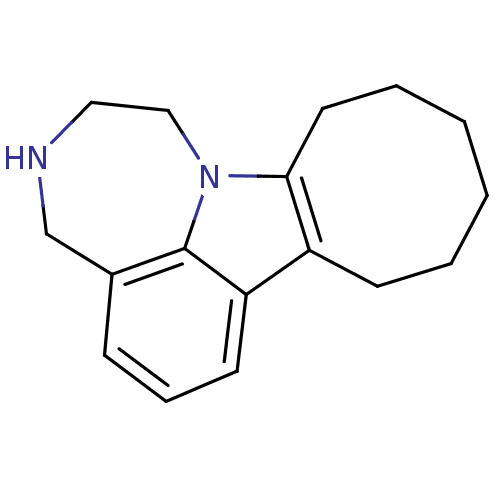
(1,2,3,4,8,9,10,11,12,13-decahydrocycloocta[b][1,4]...)Show InChI InChI=1S/C17H22N2/c1-2-4-9-16-14(7-3-1)15-8-5-6-13-12-18-10-11-19(16)17(13)15/h5-6,8,18H,1-4,7,9-12H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 13 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Agonistic binding affinity against human 5-hydroxytryptamine 2C receptor in CHO cells using [125I]- DOI |
Bioorg Med Chem Lett 14: 2603-7 (2004)
Article DOI: 10.1016/j.bmcl.2004.02.100
BindingDB Entry DOI: 10.7270/Q2QZ29F3 |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50296936
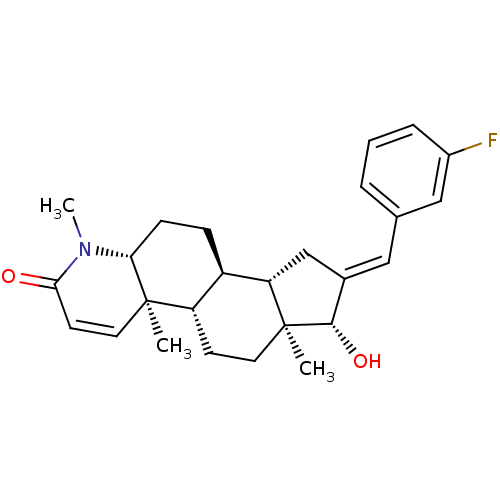
(16-[(3-Fluorophenyl)methylidene]-17beta-hydroxy-4-...)Show SMILES CN1[C@@H]2CC[C@H]3[C@@H]4C\C(=C/c5cccc(F)c5)[C@H](O)[C@@]4(C)CC[C@@H]3[C@@]2(C)C=CC1=O |r,c:30| Show InChI InChI=1S/C26H32FNO2/c1-25-12-10-23(29)28(3)22(25)8-7-19-20(25)9-11-26(2)21(19)15-17(24(26)30)13-16-5-4-6-18(27)14-16/h4-6,10,12-14,19-22,24,30H,7-9,11,15H2,1-3H3/b17-13+/t19-,20+,21+,22-,24+,25-,26+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 29 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Binding affinity to human ERG potassium channel |
J Med Chem 52: 4578-81 (2009)
Article DOI: 10.1021/jm900880r
BindingDB Entry DOI: 10.7270/Q2K35TPQ |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2A
(Homo sapiens (Human)) | BDBM50145565

(1,2,3,4,8,9,10,11,12,13-decahydrocycloocta[b][1,4]...)Show InChI InChI=1S/C17H22N2/c1-2-4-9-16-14(7-3-1)15-8-5-6-13-12-18-10-11-19(16)17(13)15/h5-6,8,18H,1-4,7,9-12H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 36 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Agonistic binding affinity of the compound against human 5-hydroxytryptamine 2A receptor in CHO cells using 8-OH-DPA radioligand |
Bioorg Med Chem Lett 14: 2603-7 (2004)
Article DOI: 10.1016/j.bmcl.2004.02.100
BindingDB Entry DOI: 10.7270/Q2QZ29F3 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2C
(Homo sapiens (Human)) | BDBM50145564

(4,5,6,7,9,10,11,12-Octahydro-8H-5,7a-diaza-benzo[c...)Show InChI InChI=1S/C16H20N2/c1-2-6-13-14-7-4-5-12-11-17-9-10-18(16(12)14)15(13)8-3-1/h4-5,7,17H,1-3,6,8-11H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 38 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Agonistic binding affinity against human 5-hydroxytryptamine 2C receptor in CHO cells using [125I]- DOI |
Bioorg Med Chem Lett 14: 2603-7 (2004)
Article DOI: 10.1016/j.bmcl.2004.02.100
BindingDB Entry DOI: 10.7270/Q2QZ29F3 |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50296937
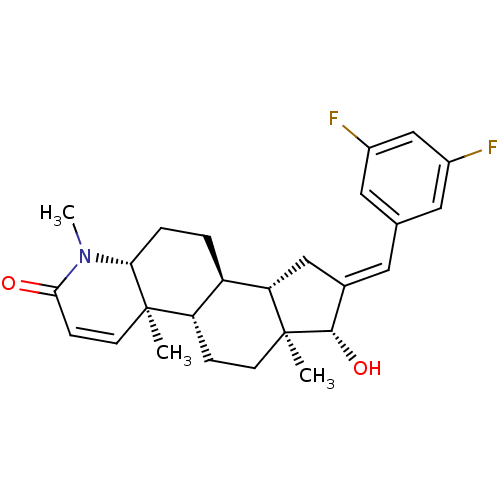
(16-[(3,5-Difluorophenyl)methylidene]-17beta-hydrox...)Show SMILES CN1[C@@H]2CC[C@H]3[C@@H]4C\C(=C/c5cc(F)cc(F)c5)[C@H](O)[C@@]4(C)CC[C@@H]3[C@@]2(C)C=CC1=O |r,c:31| Show InChI InChI=1S/C26H31F2NO2/c1-25-9-7-23(30)29(3)22(25)5-4-19-20(25)6-8-26(2)21(19)13-16(24(26)31)10-15-11-17(27)14-18(28)12-15/h7,9-12,14,19-22,24,31H,4-6,8,13H2,1-3H3/b16-10+/t19-,20+,21+,22-,24+,25-,26+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 46 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Binding affinity to human ERG potassium channel |
J Med Chem 52: 4578-81 (2009)
Article DOI: 10.1021/jm900880r
BindingDB Entry DOI: 10.7270/Q2K35TPQ |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2C
(Homo sapiens (Human)) | BDBM50145562
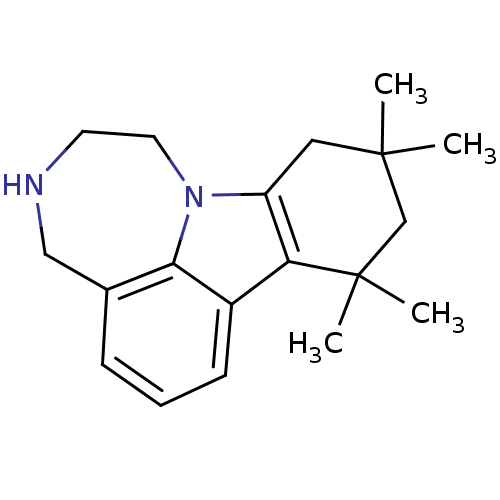
(8,8,10,10-Tetramethyl-1,2,3,4,8,9,10,11-octahydro-...)Show InChI InChI=1S/C19H26N2/c1-18(2)10-15-16(19(3,4)12-18)14-7-5-6-13-11-20-8-9-21(15)17(13)14/h5-7,20H,8-12H2,1-4H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 79 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Agonistic binding affinity against human 5-hydroxytryptamine 2C receptor in CHO cells using [125I]- DOI |
Bioorg Med Chem Lett 14: 2603-7 (2004)
Article DOI: 10.1016/j.bmcl.2004.02.100
BindingDB Entry DOI: 10.7270/Q2QZ29F3 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2C
(Homo sapiens (Human)) | BDBM50145568

(4,5,6,7,9,10-Hexahydro-8H-5,7a-diaza-benzo[cd]cycl...)Show InChI InChI=1S/C14H16N2/c1-3-10-9-15-7-8-16-13-6-2-4-11(13)12(5-1)14(10)16/h1,3,5,15H,2,4,6-9H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 97 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Agonistic binding affinity against human 5-hydroxytryptamine 2C receptor in CHO cells using [125I]- DOI |
Bioorg Med Chem Lett 14: 2603-7 (2004)
Article DOI: 10.1016/j.bmcl.2004.02.100
BindingDB Entry DOI: 10.7270/Q2QZ29F3 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2C
(Homo sapiens (Human)) | BDBM50145569
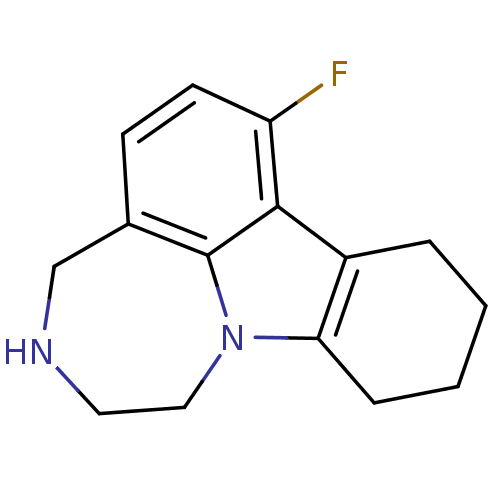
(7-Fluoro-1,2,3,4,8,9,10,11-octahydro-[1,4]diazepin...)Show InChI InChI=1S/C15H17FN2/c16-12-6-5-10-9-17-7-8-18-13-4-2-1-3-11(13)14(12)15(10)18/h5-6,17H,1-4,7-9H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 111 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Agonistic binding affinity against human 5-hydroxytryptamine 2C receptor in CHO cells using [125I]- DOI |
Bioorg Med Chem Lett 14: 2603-7 (2004)
Article DOI: 10.1016/j.bmcl.2004.02.100
BindingDB Entry DOI: 10.7270/Q2QZ29F3 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2A
(Homo sapiens (Human)) | BDBM50145562

(8,8,10,10-Tetramethyl-1,2,3,4,8,9,10,11-octahydro-...)Show InChI InChI=1S/C19H26N2/c1-18(2)10-15-16(19(3,4)12-18)14-7-5-6-13-11-20-8-9-21(15)17(13)14/h5-7,20H,8-12H2,1-4H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 152 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Agonistic binding affinity of the compound against human 5-hydroxytryptamine 2A receptor in CHO cells using 8-OH-DPA radioligand |
Bioorg Med Chem Lett 14: 2603-7 (2004)
Article DOI: 10.1016/j.bmcl.2004.02.100
BindingDB Entry DOI: 10.7270/Q2QZ29F3 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2C
(Homo sapiens (Human)) | BDBM50145559

((R)-2-Methyl-1,2,3,4,8,9,10,11-octahydro-[1,4]diaz...)Show InChI InChI=1S/C16H20N2/c1-11-10-18-15-8-3-2-6-13(15)14-7-4-5-12(9-17-11)16(14)18/h4-5,7,11,17H,2-3,6,8-10H2,1H3/t11-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 189 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Agonistic binding affinity against human 5-hydroxytryptamine 2C receptor in CHO cells using [125I]- DOI |
Bioorg Med Chem Lett 14: 2603-7 (2004)
Article DOI: 10.1016/j.bmcl.2004.02.100
BindingDB Entry DOI: 10.7270/Q2QZ29F3 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2A
(Homo sapiens (Human)) | BDBM50145564

(4,5,6,7,9,10,11,12-Octahydro-8H-5,7a-diaza-benzo[c...)Show InChI InChI=1S/C16H20N2/c1-2-6-13-14-7-4-5-12-11-17-9-10-18(16(12)14)15(13)8-3-1/h4-5,7,17H,1-3,6,8-11H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 199 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Agonistic binding affinity of the compound against human 5-hydroxytryptamine 2A receptor in CHO cells using 8-OH-DPA radioligand |
Bioorg Med Chem Lett 14: 2603-7 (2004)
Article DOI: 10.1016/j.bmcl.2004.02.100
BindingDB Entry DOI: 10.7270/Q2QZ29F3 |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50296938
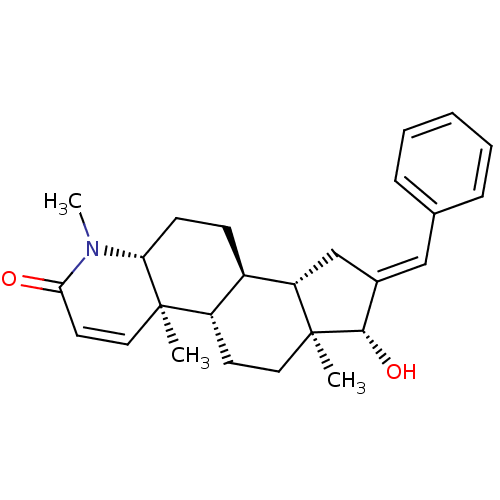
(16-(Phenylmethylidene)-17beta-hydroxy-4-methyl-4-a...)Show SMILES CN1[C@@H]2CC[C@H]3[C@@H]4C\C(=C/c5ccccc5)[C@H](O)[C@@]4(C)CC[C@@H]3[C@@]2(C)C=CC1=O |r,c:29| Show InChI InChI=1S/C26H33NO2/c1-25-14-12-23(28)27(3)22(25)10-9-19-20(25)11-13-26(2)21(19)16-18(24(26)29)15-17-7-5-4-6-8-17/h4-8,12,14-15,19-22,24,29H,9-11,13,16H2,1-3H3/b18-15+/t19-,20+,21+,22-,24+,25-,26+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 225 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Binding affinity to human ERG potassium channel |
J Med Chem 52: 4578-81 (2009)
Article DOI: 10.1021/jm900880r
BindingDB Entry DOI: 10.7270/Q2K35TPQ |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50296935
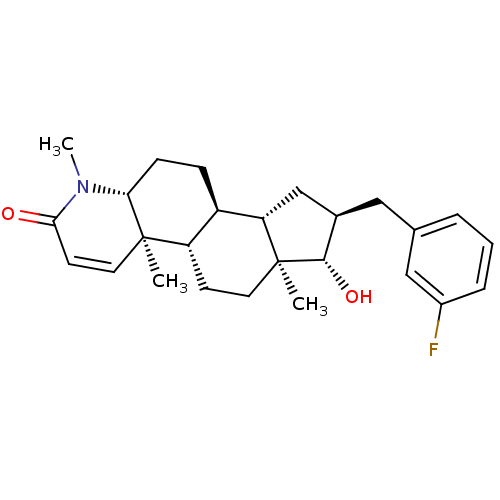
(16alpha-(3-Fluorobenzyl)-17beta-hydroxy-4-methyl-4...)Show SMILES CN1[C@@H]2CC[C@H]3[C@@H]4C[C@@H](Cc5cccc(F)c5)[C@H](O)[C@@]4(C)CC[C@@H]3[C@@]2(C)C=CC1=O |r,c:30| Show InChI InChI=1S/C26H34FNO2/c1-25-12-10-23(29)28(3)22(25)8-7-19-20(25)9-11-26(2)21(19)15-17(24(26)30)13-16-5-4-6-18(27)14-16/h4-6,10,12,14,17,19-22,24,30H,7-9,11,13,15H2,1-3H3/t17-,19-,20+,21+,22-,24+,25-,26+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 240 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Binding affinity to human ERG potassium channel |
J Med Chem 52: 4578-81 (2009)
Article DOI: 10.1021/jm900880r
BindingDB Entry DOI: 10.7270/Q2K35TPQ |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2C
(Homo sapiens (Human)) | BDBM50145563
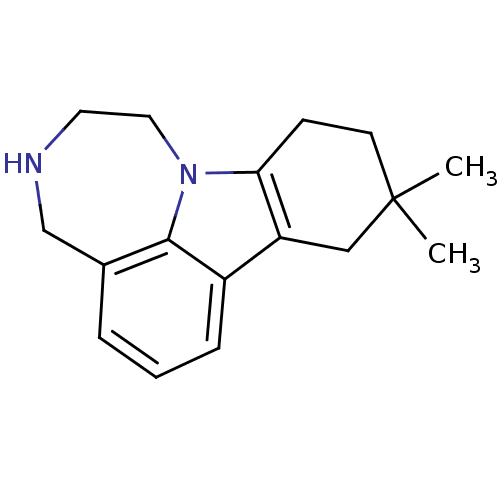
(9,9-Dimethyl-1,2,3,4,8,9,10,11-octahydro-[1,4]diaz...)Show InChI InChI=1S/C17H22N2/c1-17(2)7-6-15-14(10-17)13-5-3-4-12-11-18-8-9-19(15)16(12)13/h3-5,18H,6-11H2,1-2H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 243 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Agonistic binding affinity against human 5-hydroxytryptamine 2C receptor in CHO cells using [125I]- DOI |
Bioorg Med Chem Lett 14: 2603-7 (2004)
Article DOI: 10.1016/j.bmcl.2004.02.100
BindingDB Entry DOI: 10.7270/Q2QZ29F3 |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50296941
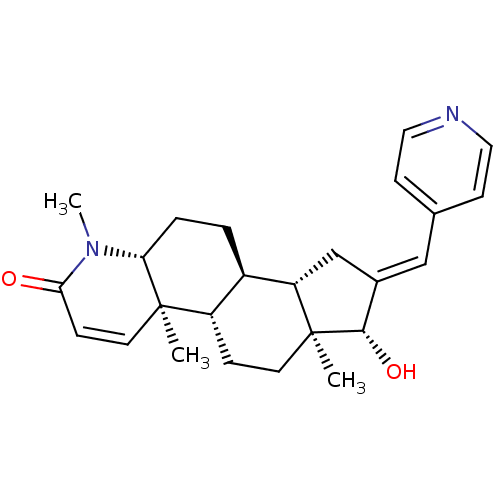
(16-(Pyridin-4-ylmethylidene)-17beta-hydroxy-4-meth...)Show SMILES CN1[C@@H]2CC[C@H]3[C@@H]4C\C(=C/c5ccncc5)[C@H](O)[C@@]4(C)CC[C@@H]3[C@@]2(C)C=CC1=O |r,c:29| Show InChI InChI=1S/C25H32N2O2/c1-24-11-7-22(28)27(3)21(24)5-4-18-19(24)6-10-25(2)20(18)15-17(23(25)29)14-16-8-12-26-13-9-16/h7-9,11-14,18-21,23,29H,4-6,10,15H2,1-3H3/b17-14+/t18-,19+,20+,21-,23+,24-,25+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 320 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Binding affinity to human ERG potassium channel |
J Med Chem 52: 4578-81 (2009)
Article DOI: 10.1021/jm900880r
BindingDB Entry DOI: 10.7270/Q2K35TPQ |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2C
(Homo sapiens (Human)) | BDBM50145561
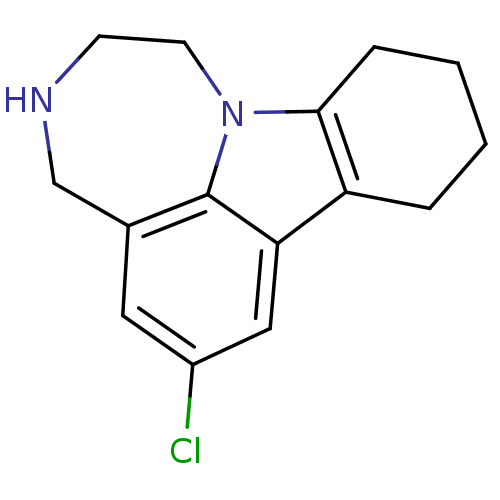
(6-Chloro-1,2,3,4,8,9,10,11-octahydro-[1,4]diazepin...)Show InChI InChI=1S/C15H17ClN2/c16-11-7-10-9-17-5-6-18-14-4-2-1-3-12(14)13(8-11)15(10)18/h7-8,17H,1-6,9H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 358 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Agonistic binding affinity against human 5-hydroxytryptamine 2C receptor in CHO cells using [125I]- DOI |
Bioorg Med Chem Lett 14: 2603-7 (2004)
Article DOI: 10.1016/j.bmcl.2004.02.100
BindingDB Entry DOI: 10.7270/Q2QZ29F3 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 1A
(Homo sapiens (Human)) | BDBM50099012

(CHEMBL10764 | N-{(S)-1-Benzyl-2-[4-(4-chloro-[1,2,...)Show SMILES Clc1nsnc1N1CCN(C[C@H](Cc2ccccc2)NC(=O)c2ccncc2)CC1 Show InChI InChI=1S/C21H23ClN6OS/c22-19-20(26-30-25-19)28-12-10-27(11-13-28)15-18(14-16-4-2-1-3-5-16)24-21(29)17-6-8-23-9-7-17/h1-9,18H,10-15H2,(H,24,29)/t18-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| PubMed
| 425 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth-Ayerst Research
Curated by ChEMBL
| Assay Description
Ability to displace [3H]-8-OH-DPAT from CHO cells stably transfected with human 5-hydroxytryptamine 1A receptor |
Bioorg Med Chem Lett 11: 1069-71 (2001)
BindingDB Entry DOI: 10.7270/Q2SJ1JWX |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2C
(Homo sapiens (Human)) | BDBM50145560

((S)-2-Methyl-1,2,3,4,8,9,10,11-octahydro-[1,4]diaz...)Show InChI InChI=1S/C16H20N2/c1-11-10-18-15-8-3-2-6-13(15)14-7-4-5-12(9-17-11)16(14)18/h4-5,7,11,17H,2-3,6,8-10H2,1H3/t11-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 452 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Agonistic binding affinity against human 5-hydroxytryptamine 2C receptor in CHO cells using [125I]- DOI |
Bioorg Med Chem Lett 14: 2603-7 (2004)
Article DOI: 10.1016/j.bmcl.2004.02.100
BindingDB Entry DOI: 10.7270/Q2QZ29F3 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2C
(Homo sapiens (Human)) | BDBM50145566
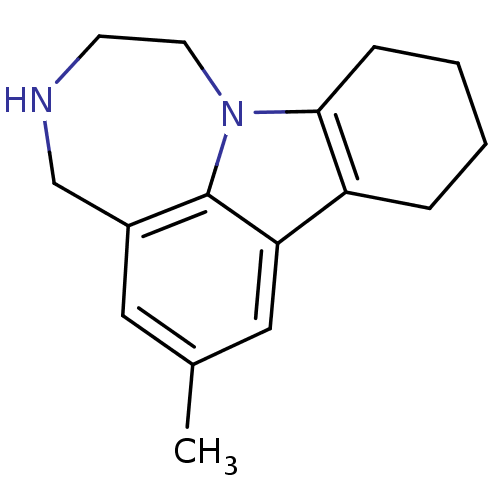
(6-Methyl-1,2,3,4,8,9,10,11-octahydro-[1,4]diazepin...)Show InChI InChI=1S/C16H20N2/c1-11-8-12-10-17-6-7-18-15-5-3-2-4-13(15)14(9-11)16(12)18/h8-9,17H,2-7,10H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 599 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Agonistic binding affinity against human 5-hydroxytryptamine 2C receptor in CHO cells using [125I]- DOI |
Bioorg Med Chem Lett 14: 2603-7 (2004)
Article DOI: 10.1016/j.bmcl.2004.02.100
BindingDB Entry DOI: 10.7270/Q2QZ29F3 |
More data for this
Ligand-Target Pair | |
D(1A) dopamine receptor
(RAT) | BDBM50145565

(1,2,3,4,8,9,10,11,12,13-decahydrocycloocta[b][1,4]...)Show InChI InChI=1S/C17H22N2/c1-2-4-9-16-14(7-3-1)15-8-5-6-13-12-18-10-11-19(16)17(13)15/h5-6,8,18H,1-4,7,9-12H2 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 665 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Binding affinity of the compound against dopamine receptor D1A |
Bioorg Med Chem Lett 14: 2603-7 (2004)
Article DOI: 10.1016/j.bmcl.2004.02.100
BindingDB Entry DOI: 10.7270/Q2QZ29F3 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2A
(Homo sapiens (Human)) | BDBM50145568

(4,5,6,7,9,10-Hexahydro-8H-5,7a-diaza-benzo[cd]cycl...)Show InChI InChI=1S/C14H16N2/c1-3-10-9-15-7-8-16-13-6-2-4-11(13)12(5-1)14(10)16/h1,3,5,15H,2,4,6-9H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 922 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Agonistic binding affinity of the compound against human 5-hydroxytryptamine 2A receptor in CHO cells using 8-OH-DPA radioligand |
Bioorg Med Chem Lett 14: 2603-7 (2004)
Article DOI: 10.1016/j.bmcl.2004.02.100
BindingDB Entry DOI: 10.7270/Q2QZ29F3 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2C
(Homo sapiens (Human)) | BDBM50145567

(2-Methyl-4,5,6,7,9,10-hexahydro-8H-5,7a-diaza-benz...)Show InChI InChI=1S/C15H18N2/c1-10-7-11-9-16-5-6-17-14-4-2-3-12(14)13(8-10)15(11)17/h7-8,16H,2-6,9H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 985 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Agonistic binding affinity against human 5-hydroxytryptamine 2C receptor in CHO cells using [125I]- DOI |
Bioorg Med Chem Lett 14: 2603-7 (2004)
Article DOI: 10.1016/j.bmcl.2004.02.100
BindingDB Entry DOI: 10.7270/Q2QZ29F3 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2A
(Homo sapiens (Human)) | BDBM50145566

(6-Methyl-1,2,3,4,8,9,10,11-octahydro-[1,4]diazepin...)Show InChI InChI=1S/C16H20N2/c1-11-8-12-10-17-6-7-18-15-5-3-2-4-13(15)14(9-11)16(12)18/h8-9,17H,2-7,10H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.23E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Agonistic binding affinity of the compound against human 5-hydroxytryptamine 2A receptor in CHO cells using 8-OH-DPA radioligand |
Bioorg Med Chem Lett 14: 2603-7 (2004)
Article DOI: 10.1016/j.bmcl.2004.02.100
BindingDB Entry DOI: 10.7270/Q2QZ29F3 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2A
(Homo sapiens (Human)) | BDBM50145559

((R)-2-Methyl-1,2,3,4,8,9,10,11-octahydro-[1,4]diaz...)Show InChI InChI=1S/C16H20N2/c1-11-10-18-15-8-3-2-6-13(15)14-7-4-5-12(9-17-11)16(14)18/h4-5,7,11,17H,2-3,6,8-10H2,1H3/t11-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.56E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Agonistic binding affinity of the compound against human 5-hydroxytryptamine 2A receptor in CHO cells using 8-OH-DPA radioligand |
Bioorg Med Chem Lett 14: 2603-7 (2004)
Article DOI: 10.1016/j.bmcl.2004.02.100
BindingDB Entry DOI: 10.7270/Q2QZ29F3 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2A
(Homo sapiens (Human)) | BDBM50145567

(2-Methyl-4,5,6,7,9,10-hexahydro-8H-5,7a-diaza-benz...)Show InChI InChI=1S/C15H18N2/c1-10-7-11-9-16-5-6-17-14-4-2-3-12(14)13(8-10)15(11)17/h7-8,16H,2-6,9H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.53E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Agonistic binding affinity of the compound against human 5-hydroxytryptamine 2A receptor in CHO cells using 8-OH-DPA radioligand |
Bioorg Med Chem Lett 14: 2603-7 (2004)
Article DOI: 10.1016/j.bmcl.2004.02.100
BindingDB Entry DOI: 10.7270/Q2QZ29F3 |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50296942

(16-(Pyridin-3-ylmethylidene)-17beta-hydroxy-4-meth...)Show SMILES CN1[C@@H]2CC[C@H]3[C@@H]4C\C(=C/c5cccnc5)[C@H](O)[C@@]4(C)CC[C@@H]3[C@@]2(C)C=CC1=O |r,c:29| Show InChI InChI=1S/C25H32N2O2/c1-24-11-9-22(28)27(3)21(24)7-6-18-19(24)8-10-25(2)20(18)14-17(23(25)29)13-16-5-4-12-26-15-16/h4-5,9,11-13,15,18-21,23,29H,6-8,10,14H2,1-3H3/b17-13+/t18-,19+,20+,21-,23+,24-,25+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Binding affinity to human ERG potassium channel |
J Med Chem 52: 4578-81 (2009)
Article DOI: 10.1021/jm900880r
BindingDB Entry DOI: 10.7270/Q2K35TPQ |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50296944

(16-[(2-cyclopropylpyrimidin-5-yl)methylidene]-17be...)Show SMILES CN1[C@@H]2CC[C@H]3[C@@H]4C\C(=C/c5cnc(nc5)C5CC5)[C@H](O)[C@@]4(C)CC[C@@H]3[C@@]2(C)C=CC1=O |r,c:33| Show InChI InChI=1S/C27H35N3O2/c1-26-11-9-23(31)30(3)22(26)7-6-19-20(26)8-10-27(2)21(19)13-18(24(27)32)12-16-14-28-25(29-15-16)17-4-5-17/h9,11-12,14-15,17,19-22,24,32H,4-8,10,13H2,1-3H3/b18-12+/t19-,20+,21+,22-,24+,26-,27+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 4.65E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Binding affinity to human ERG potassium channel |
J Med Chem 52: 4578-81 (2009)
Article DOI: 10.1021/jm900880r
BindingDB Entry DOI: 10.7270/Q2K35TPQ |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2B
(Homo sapiens (Human)) | BDBM50145565

(1,2,3,4,8,9,10,11,12,13-decahydrocycloocta[b][1,4]...)Show InChI InChI=1S/C17H22N2/c1-2-4-9-16-14(7-3-1)15-8-5-6-13-12-18-10-11-19(16)17(13)15/h5-6,8,18H,1-4,7,9-12H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| >5.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Binding affinity of the compound against 5-hydroxytryptamine 1A receptor |
Bioorg Med Chem Lett 14: 2603-7 (2004)
Article DOI: 10.1016/j.bmcl.2004.02.100
BindingDB Entry DOI: 10.7270/Q2QZ29F3 |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50296945
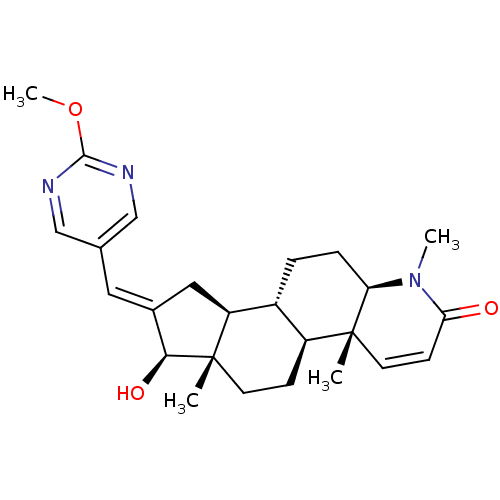
(16-[(2-Methoxypyrimidin-5-yl)methylidene]-17beta-h...)Show SMILES COc1ncc(\C=C2/C[C@H]3[C@@H]4CC[C@H]5N(C)C(=O)C=C[C@]5(C)[C@H]4CC[C@]3(C)[C@H]2O)cn1 |r,c:18| Show InChI InChI=1S/C25H33N3O3/c1-24-10-8-21(29)28(3)20(24)6-5-17-18(24)7-9-25(2)19(17)12-16(22(25)30)11-15-13-26-23(31-4)27-14-15/h8,10-11,13-14,17-20,22,30H,5-7,9,12H2,1-4H3/b16-11+/t17-,18+,19+,20-,22+,24-,25+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 7.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Binding affinity to human ERG potassium channel |
J Med Chem 52: 4578-81 (2009)
Article DOI: 10.1021/jm900880r
BindingDB Entry DOI: 10.7270/Q2K35TPQ |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50296939
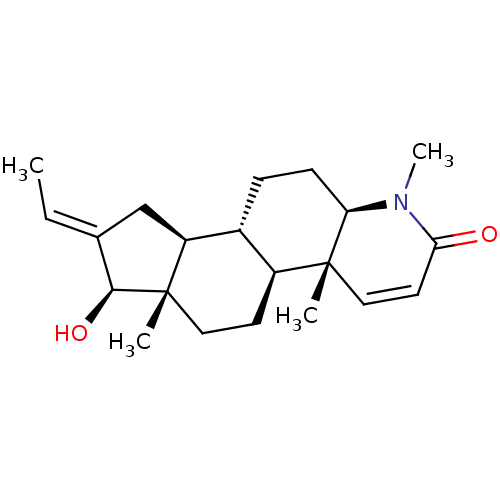
(16-(Methylmethylidene)-17beta-hydroxy-4-methyl-4-a...)Show SMILES C\C=C1/C[C@H]2[C@@H]3CC[C@H]4N(C)C(=O)C=C[C@]4(C)[C@H]3CC[C@]2(C)[C@H]1O |r,c:13| Show InChI InChI=1S/C21H31NO2/c1-5-13-12-16-14-6-7-17-20(2,11-9-18(23)22(17)4)15(14)8-10-21(16,3)19(13)24/h5,9,11,14-17,19,24H,6-8,10,12H2,1-4H3/b13-5+/t14-,15+,16+,17-,19+,20-,21+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 8.05E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Binding affinity to human ERG potassium channel |
J Med Chem 52: 4578-81 (2009)
Article DOI: 10.1021/jm900880r
BindingDB Entry DOI: 10.7270/Q2K35TPQ |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50296943
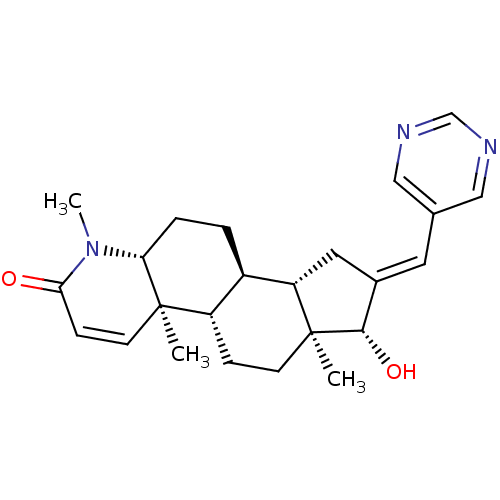
(16-(Pyrimidin-5-ylmethylidene)-17beta-hydroxy-4-me...)Show SMILES CN1[C@@H]2CC[C@H]3[C@@H]4C\C(=C/c5cncnc5)[C@H](O)[C@@]4(C)CC[C@@H]3[C@@]2(C)C=CC1=O |r,c:29| Show InChI InChI=1S/C24H31N3O2/c1-23-9-7-21(28)27(3)20(23)5-4-17-18(23)6-8-24(2)19(17)11-16(22(24)29)10-15-12-25-14-26-13-15/h7,9-10,12-14,17-20,22,29H,4-6,8,11H2,1-3H3/b16-10+/t17-,18+,19+,20-,22+,23-,24+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 9.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Binding affinity to human ERG potassium channel |
J Med Chem 52: 4578-81 (2009)
Article DOI: 10.1021/jm900880r
BindingDB Entry DOI: 10.7270/Q2K35TPQ |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50296946
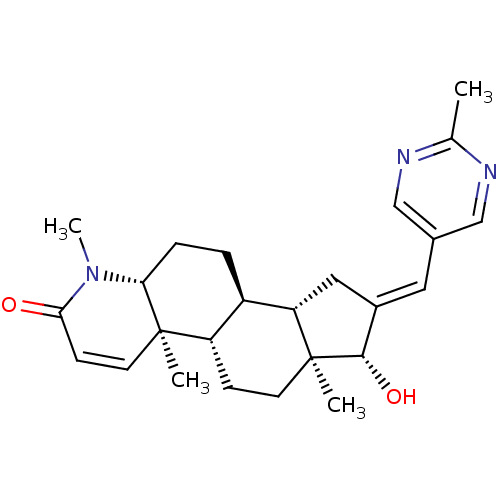
(16-[(2-Methylpyrimidin-5-yl)methylidene]-17beta-hy...)Show SMILES CN1[C@@H]2CC[C@H]3[C@@H]4C\C(=C/c5cnc(C)nc5)[C@H](O)[C@@]4(C)CC[C@@H]3[C@@]2(C)C=CC1=O |r,c:30| Show InChI InChI=1S/C25H33N3O2/c1-15-26-13-16(14-27-15)11-17-12-20-18-5-6-21-24(2,10-8-22(29)28(21)4)19(18)7-9-25(20,3)23(17)30/h8,10-11,13-14,18-21,23,30H,5-7,9,12H2,1-4H3/b17-11+/t18-,19+,20+,21-,23+,24-,25+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Binding affinity to human ERG potassium channel |
J Med Chem 52: 4578-81 (2009)
Article DOI: 10.1021/jm900880r
BindingDB Entry DOI: 10.7270/Q2K35TPQ |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50296933
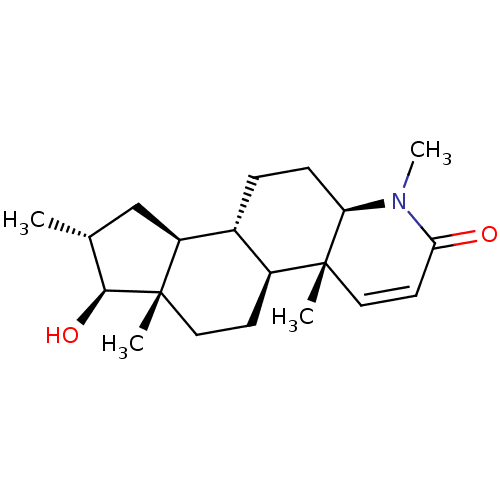
(16alpha-(Methyl)-17beta-hydroxy-4-methyl-4-aza-5al...)Show SMILES C[C@@H]1C[C@H]2[C@@H]3CC[C@H]4N(C)C(=O)C=C[C@]4(C)[C@H]3CC[C@]2(C)[C@H]1O |r,c:12| Show InChI InChI=1S/C20H31NO2/c1-12-11-15-13-5-6-16-19(2,10-8-17(22)21(16)4)14(13)7-9-20(15,3)18(12)23/h8,10,12-16,18,23H,5-7,9,11H2,1-4H3/t12-,13-,14+,15+,16-,18+,19-,20+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Binding affinity to human ERG potassium channel |
J Med Chem 52: 4578-81 (2009)
Article DOI: 10.1021/jm900880r
BindingDB Entry DOI: 10.7270/Q2K35TPQ |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50296947
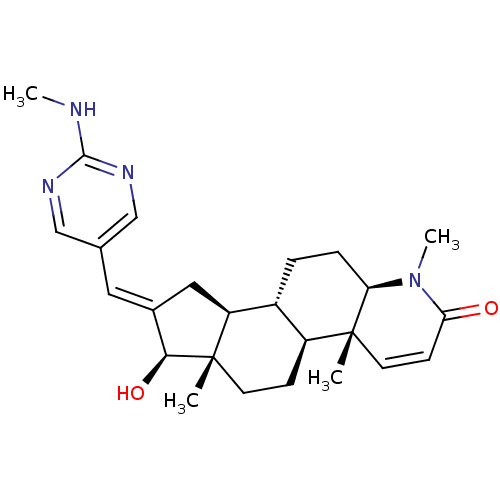
(16-{[(2-Methylamino)pyrimidin-5-yl]methylidene}-17...)Show SMILES CNc1ncc(\C=C2/C[C@H]3[C@@H]4CC[C@H]5N(C)C(=O)C=C[C@]5(C)[C@H]4CC[C@]3(C)[C@H]2O)cn1 |r,c:18| Show InChI InChI=1S/C25H34N4O2/c1-24-10-8-21(30)29(4)20(24)6-5-17-18(24)7-9-25(2)19(17)12-16(22(25)31)11-15-13-27-23(26-3)28-14-15/h8,10-11,13-14,17-20,22,31H,5-7,9,12H2,1-4H3,(H,26,27,28)/b16-11+/t17-,18+,19+,20-,22+,24-,25+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.25E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Binding affinity to human ERG potassium channel |
J Med Chem 52: 4578-81 (2009)
Article DOI: 10.1021/jm900880r
BindingDB Entry DOI: 10.7270/Q2K35TPQ |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50296934

(16alpha-(Methoxymethyl)-17beta-hydroxy-4-methyl-4-...)Show SMILES COC[C@@H]1C[C@H]2[C@@H]3CC[C@H]4N(C)C(=O)C=C[C@]4(C)[C@H]3CC[C@]2(C)[C@H]1O |r,c:14| Show InChI InChI=1S/C21H33NO3/c1-20-10-8-18(23)22(3)17(20)6-5-14-15(20)7-9-21(2)16(14)11-13(12-25-4)19(21)24/h8,10,13-17,19,24H,5-7,9,11-12H2,1-4H3/t13-,14+,15-,16-,17+,19-,20+,21-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3.90E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Binding affinity to human ERG potassium channel |
J Med Chem 52: 4578-81 (2009)
Article DOI: 10.1021/jm900880r
BindingDB Entry DOI: 10.7270/Q2K35TPQ |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50296932

((4aR,4bS,6aS,7S,9aS,9bR,11aR)-7-hydroxy-1,4a,6a-tr...)Show SMILES CN1[C@@H]2CC[C@H]3[C@@H]4CC[C@H](O)[C@@]4(C)CC[C@@H]3[C@@]2(C)C=CC1=O |r,c:21| Show InChI InChI=1S/C19H29NO2/c1-18-11-9-17(22)20(3)15(18)6-4-12-13-5-7-16(21)19(13,2)10-8-14(12)18/h9,11-16,21H,4-8,10H2,1-3H3/t12-,13-,14-,15+,16-,18+,19-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.30E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Binding affinity to human ERG potassium channel |
J Med Chem 52: 4578-81 (2009)
Article DOI: 10.1021/jm900880r
BindingDB Entry DOI: 10.7270/Q2K35TPQ |
More data for this
Ligand-Target Pair | |
Androgen receptor
(Homo sapiens (Human)) | BDBM18161

((1S,2S,7S,10R,11S,14S,15S)-14-hydroxy-2,15-dimethy...)Show SMILES [H][C@@]12CC[C@H](O)[C@@]1(C)CC[C@@]1([H])[C@@]2([H])CC[C@@]2([H])CC(=O)CC[C@]12C |r| Show InChI InChI=1S/C19H30O2/c1-18-9-7-13(20)11-12(18)3-4-14-15-5-6-17(21)19(15,2)10-8-16(14)18/h12,14-17,21H,3-11H2,1-2H3/t12-,14-,15-,16-,17-,18-,19-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [3H]methyltrienolone from androgen receptor in human MDA-MB-453 cells |
J Med Chem 52: 4578-81 (2009)
Article DOI: 10.1021/jm900880r
BindingDB Entry DOI: 10.7270/Q2K35TPQ |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Androgen receptor
(Homo sapiens (Human)) | BDBM50296932

((4aR,4bS,6aS,7S,9aS,9bR,11aR)-7-hydroxy-1,4a,6a-tr...)Show SMILES CN1[C@@H]2CC[C@H]3[C@@H]4CC[C@H](O)[C@@]4(C)CC[C@@H]3[C@@]2(C)C=CC1=O |r,c:21| Show InChI InChI=1S/C19H29NO2/c1-18-11-9-17(22)20(3)15(18)6-4-12-13-5-7-16(21)19(13,2)10-8-14(12)18/h9,11-16,21H,4-8,10H2,1-3H3/t12-,13-,14-,15+,16-,18+,19-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [3H]methyltrienolone from androgen receptor in human MDA-MB-453 cells |
J Med Chem 52: 4578-81 (2009)
Article DOI: 10.1021/jm900880r
BindingDB Entry DOI: 10.7270/Q2K35TPQ |
More data for this
Ligand-Target Pair | |
Androgen receptor
(Homo sapiens (Human)) | BDBM50296945

(16-[(2-Methoxypyrimidin-5-yl)methylidene]-17beta-h...)Show SMILES COc1ncc(\C=C2/C[C@H]3[C@@H]4CC[C@H]5N(C)C(=O)C=C[C@]5(C)[C@H]4CC[C@]3(C)[C@H]2O)cn1 |r,c:18| Show InChI InChI=1S/C25H33N3O3/c1-24-10-8-21(29)28(3)20(24)6-5-17-18(24)7-9-25(2)19(17)12-16(22(25)30)11-15-13-26-23(31-4)27-14-15/h8,10-11,13-14,17-20,22,30H,5-7,9,12H2,1-4H3/b16-11+/t17-,18+,19+,20-,22+,24-,25+/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [3H]methyltrienolone from androgen receptor in human MDA-MB-453 cells |
J Med Chem 52: 4578-81 (2009)
Article DOI: 10.1021/jm900880r
BindingDB Entry DOI: 10.7270/Q2K35TPQ |
More data for this
Ligand-Target Pair | |
Androgen receptor
(Homo sapiens (Human)) | BDBM50296935

(16alpha-(3-Fluorobenzyl)-17beta-hydroxy-4-methyl-4...)Show SMILES CN1[C@@H]2CC[C@H]3[C@@H]4C[C@@H](Cc5cccc(F)c5)[C@H](O)[C@@]4(C)CC[C@@H]3[C@@]2(C)C=CC1=O |r,c:30| Show InChI InChI=1S/C26H34FNO2/c1-25-12-10-23(29)28(3)22(25)8-7-19-20(25)9-11-26(2)21(19)15-17(24(26)30)13-16-5-4-6-18(27)14-16/h4-6,10,12,14,17,19-22,24,30H,7-9,11,13,15H2,1-3H3/t17-,19-,20+,21+,22-,24+,25-,26+/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 23 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [3H]methyltrienolone from androgen receptor in human MDA-MB-453 cells |
J Med Chem 52: 4578-81 (2009)
Article DOI: 10.1021/jm900880r
BindingDB Entry DOI: 10.7270/Q2K35TPQ |
More data for this
Ligand-Target Pair | |
Androgen receptor
(Homo sapiens (Human)) | BDBM50296934

(16alpha-(Methoxymethyl)-17beta-hydroxy-4-methyl-4-...)Show SMILES COC[C@@H]1C[C@H]2[C@@H]3CC[C@H]4N(C)C(=O)C=C[C@]4(C)[C@H]3CC[C@]2(C)[C@H]1O |r,c:14| Show InChI InChI=1S/C21H33NO3/c1-20-10-8-18(23)22(3)17(20)6-5-14-15(20)7-9-21(2)16(14)11-13(12-25-4)19(21)24/h8,10,13-17,19,24H,5-7,9,11-12H2,1-4H3/t13-,14+,15-,16-,17+,19-,20+,21-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 24 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [3H]methyltrienolone from androgen receptor in human MDA-MB-453 cells |
J Med Chem 52: 4578-81 (2009)
Article DOI: 10.1021/jm900880r
BindingDB Entry DOI: 10.7270/Q2K35TPQ |
More data for this
Ligand-Target Pair | |
Androgen receptor
(Homo sapiens (Human)) | BDBM50296936

(16-[(3-Fluorophenyl)methylidene]-17beta-hydroxy-4-...)Show SMILES CN1[C@@H]2CC[C@H]3[C@@H]4C\C(=C/c5cccc(F)c5)[C@H](O)[C@@]4(C)CC[C@@H]3[C@@]2(C)C=CC1=O |r,c:30| Show InChI InChI=1S/C26H32FNO2/c1-25-12-10-23(29)28(3)22(25)8-7-19-20(25)9-11-26(2)21(19)15-17(24(26)30)13-16-5-4-6-18(27)14-16/h4-6,10,12-14,19-22,24,30H,7-9,11,15H2,1-3H3/b17-13+/t19-,20+,21+,22-,24+,25-,26+/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 27 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [3H]methyltrienolone from androgen receptor in human MDA-MB-453 cells |
J Med Chem 52: 4578-81 (2009)
Article DOI: 10.1021/jm900880r
BindingDB Entry DOI: 10.7270/Q2K35TPQ |
More data for this
Ligand-Target Pair | |
Androgen receptor
(Homo sapiens (Human)) | BDBM50296933

(16alpha-(Methyl)-17beta-hydroxy-4-methyl-4-aza-5al...)Show SMILES C[C@@H]1C[C@H]2[C@@H]3CC[C@H]4N(C)C(=O)C=C[C@]4(C)[C@H]3CC[C@]2(C)[C@H]1O |r,c:12| Show InChI InChI=1S/C20H31NO2/c1-12-11-15-13-5-6-16-19(2,10-8-17(22)21(16)4)14(13)7-9-20(15,3)18(12)23/h8,10,12-16,18,23H,5-7,9,11H2,1-4H3/t12-,13-,14+,15+,16-,18+,19-,20+/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 31 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [3H]methyltrienolone from androgen receptor in human MDA-MB-453 cells |
J Med Chem 52: 4578-81 (2009)
Article DOI: 10.1021/jm900880r
BindingDB Entry DOI: 10.7270/Q2K35TPQ |
More data for this
Ligand-Target Pair | |
Androgen receptor
(Homo sapiens (Human)) | BDBM50296947

(16-{[(2-Methylamino)pyrimidin-5-yl]methylidene}-17...)Show SMILES CNc1ncc(\C=C2/C[C@H]3[C@@H]4CC[C@H]5N(C)C(=O)C=C[C@]5(C)[C@H]4CC[C@]3(C)[C@H]2O)cn1 |r,c:18| Show InChI InChI=1S/C25H34N4O2/c1-24-10-8-21(30)29(4)20(24)6-5-17-18(24)7-9-25(2)19(17)12-16(22(25)31)11-15-13-27-23(26-3)28-14-15/h8,10-11,13-14,17-20,22,31H,5-7,9,12H2,1-4H3,(H,26,27,28)/b16-11+/t17-,18+,19+,20-,22+,24-,25+/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 31 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [3H]methyltrienolone from androgen receptor in human MDA-MB-453 cells |
J Med Chem 52: 4578-81 (2009)
Article DOI: 10.1021/jm900880r
BindingDB Entry DOI: 10.7270/Q2K35TPQ |
More data for this
Ligand-Target Pair | |
Androgen receptor
(Homo sapiens (Human)) | BDBM50296939

(16-(Methylmethylidene)-17beta-hydroxy-4-methyl-4-a...)Show SMILES C\C=C1/C[C@H]2[C@@H]3CC[C@H]4N(C)C(=O)C=C[C@]4(C)[C@H]3CC[C@]2(C)[C@H]1O |r,c:13| Show InChI InChI=1S/C21H31NO2/c1-5-13-12-16-14-6-7-17-20(2,11-9-18(23)22(17)4)15(14)8-10-21(16,3)19(13)24/h5,9,11,14-17,19,24H,6-8,10,12H2,1-4H3/b13-5+/t14-,15+,16+,17-,19+,20-,21+/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 35 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [3H]methyltrienolone from androgen receptor in human MDA-MB-453 cells |
J Med Chem 52: 4578-81 (2009)
Article DOI: 10.1021/jm900880r
BindingDB Entry DOI: 10.7270/Q2K35TPQ |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data