 Found 847 hits with Last Name = 'cowan' and Initial = 's'
Found 847 hits with Last Name = 'cowan' and Initial = 's' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Serine/threonine-protein kinase B-raf
(Homo sapiens (Human)) | BDBM50452149
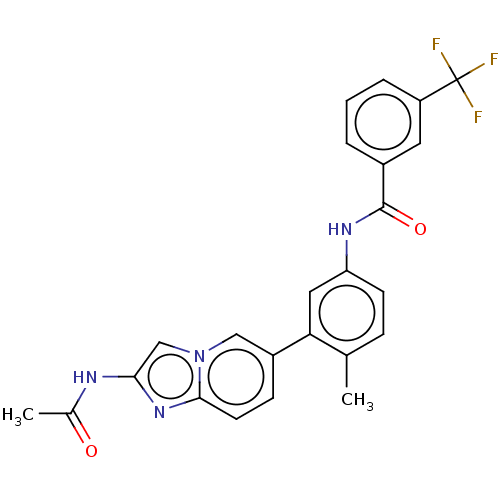
(CHEMBL4216073)Show SMILES CC(=O)Nc1cn2cc(ccc2n1)-c1cc(NC(=O)c2cccc(c2)C(F)(F)F)ccc1C Show InChI InChI=1S/C24H19F3N4O2/c1-14-6-8-19(29-23(33)16-4-3-5-18(10-16)24(25,26)27)11-20(14)17-7-9-22-30-21(28-15(2)32)13-31(22)12-17/h3-13H,1-2H3,(H,28,32)(H,29,33) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research
Curated by ChEMBL
| Assay Description
Inhibition of recombinant C-terminal his6-tagged B-Raf (437 to 765 residues) V600E mutant (unknown origin) catalytic domain expressed in baculovirus ... |
Bioorg Med Chem Lett 27: 5221-5224 (2017)
Article DOI: 10.1016/j.bmcl.2017.10.047
BindingDB Entry DOI: 10.7270/Q2SB4892 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Serine/threonine-protein kinase B-raf
(Homo sapiens (Human)) | BDBM50452150
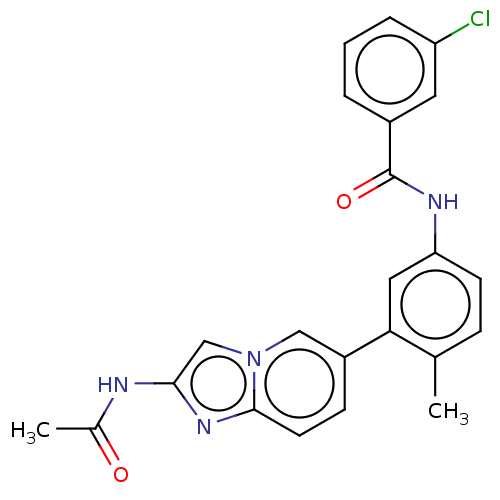
(CHEMBL4216386)Show SMILES CC(=O)Nc1cn2cc(ccc2n1)-c1cc(NC(=O)c2cccc(Cl)c2)ccc1C Show InChI InChI=1S/C23H19ClN4O2/c1-14-6-8-19(26-23(30)16-4-3-5-18(24)10-16)11-20(14)17-7-9-22-27-21(25-15(2)29)13-28(22)12-17/h3-13H,1-2H3,(H,25,29)(H,26,30) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research
Curated by ChEMBL
| Assay Description
Inhibition of recombinant C-terminal his6-tagged B-Raf (437 to 765 residues) V600E mutant (unknown origin) catalytic domain expressed in baculovirus ... |
Bioorg Med Chem Lett 27: 5221-5224 (2017)
Article DOI: 10.1016/j.bmcl.2017.10.047
BindingDB Entry DOI: 10.7270/Q2SB4892 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase ABL1
(Homo sapiens (Human)) | BDBM213578
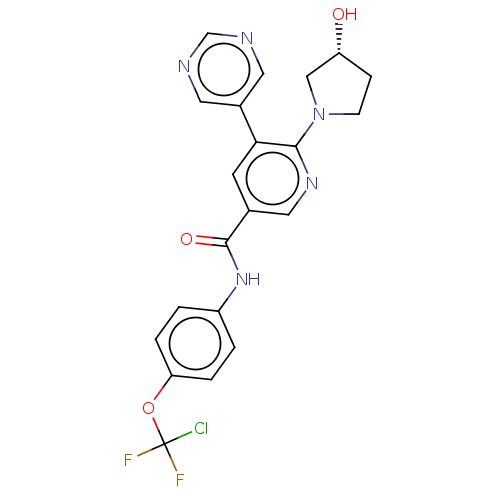
(US9278981, 170)Show SMILES O[C@@H]1CCN(C1)c1ncc(cc1-c1cncnc1)C(=O)Nc1ccc(OC(F)(F)Cl)cc1 |r| Show InChI InChI=1S/C21H18ClF2N5O3/c22-21(23,24)32-17-3-1-15(2-4-17)28-20(31)13-7-18(14-8-25-12-26-9-14)19(27-10-13)29-6-5-16(30)11-29/h1-4,7-10,12,16,30H,5-6,11H2,(H,28,31)/t16-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | <0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of ABL1 (64 to 515 residues)(unknown origin) expressed in Escherichia coli using FITC-Ahx-EAIYAAPFAKKK-NH2 peptide as substrate after 60 m... |
J Med Chem 61: 8120-8135 (2018)
Article DOI: 10.1021/acs.jmedchem.8b01040
BindingDB Entry DOI: 10.7270/Q2FX7D3X |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase B-raf
(Homo sapiens (Human)) | BDBM50452152
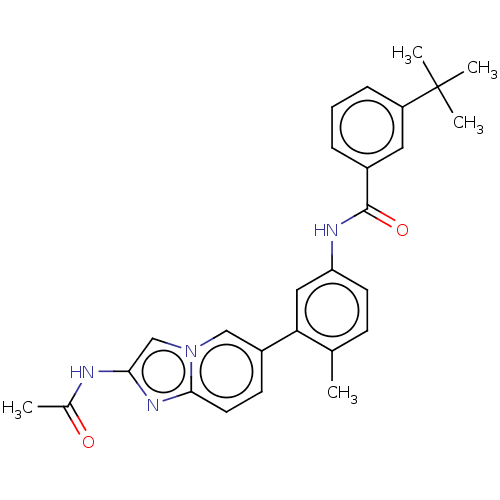
(CHEMBL4217462)Show SMILES CC(=O)Nc1cn2cc(ccc2n1)-c1cc(NC(=O)c2cccc(c2)C(C)(C)C)ccc1C Show InChI InChI=1S/C27H28N4O2/c1-17-9-11-22(29-26(33)19-7-6-8-21(13-19)27(3,4)5)14-23(17)20-10-12-25-30-24(28-18(2)32)16-31(25)15-20/h6-16H,1-5H3,(H,28,32)(H,29,33) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research
Curated by ChEMBL
| Assay Description
Inhibition of recombinant C-terminal his6-tagged B-Raf (437 to 765 residues) V600E mutant (unknown origin) catalytic domain expressed in baculovirus ... |
Bioorg Med Chem Lett 27: 5221-5224 (2017)
Article DOI: 10.1016/j.bmcl.2017.10.047
BindingDB Entry DOI: 10.7270/Q2SB4892 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase ABL1
(Homo sapiens (Human)) | BDBM213594

(US9278981, 186)Show SMILES O[C@@H]1CCN(C1)c1ncc(cc1-c1cncnc1)C(=O)Nc1ccc(SC(F)(F)F)cc1 |r| Show InChI InChI=1S/C21H18F3N5O2S/c22-21(23,24)32-17-3-1-15(2-4-17)28-20(31)13-7-18(14-8-25-12-26-9-14)19(27-10-13)29-6-5-16(30)11-29/h1-4,7-10,12,16,30H,5-6,11H2,(H,28,31)/t16-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of ABL1 (64 to 515 residues)(unknown origin) expressed in Escherichia coli using FITC-Ahx-EAIYAAPFAKKK-NH2 peptide as substrate after 60 m... |
J Med Chem 61: 8120-8135 (2018)
Article DOI: 10.1021/acs.jmedchem.8b01040
BindingDB Entry DOI: 10.7270/Q2FX7D3X |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase ABL1
(Homo sapiens (Human)) | BDBM50459091
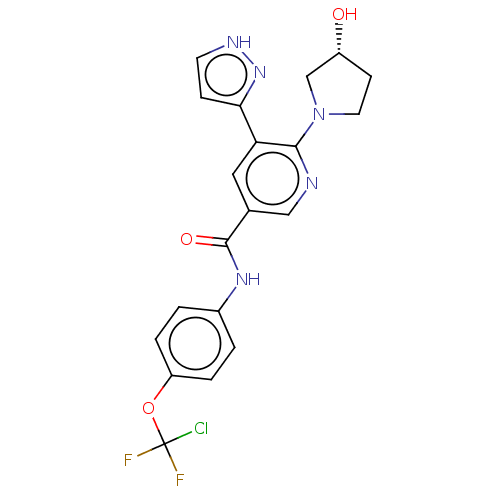
(ABL-001 | ABL001 | ABL001-NX | Asciminib | NVP-ABL...)Show SMILES O[C@@H]1CCN(C1)c1ncc(cc1-c1cc[nH]n1)C(=O)Nc1ccc(OC(F)(F)Cl)cc1 |r| Show InChI InChI=1S/C20H18ClF2N5O3/c21-20(22,23)31-15-3-1-13(2-4-15)26-19(30)12-9-16(17-5-7-25-27-17)18(24-10-12)28-8-6-14(29)11-28/h1-5,7,9-10,14,29H,6,8,11H2,(H,25,27)(H,26,30)/t14-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of ABL1 (64 to 515 residues)(unknown origin) expressed in Escherichia coli using FITC-Ahx-EAIYAAPFAKKK-NH2 peptide as substrate after 60 m... |
J Med Chem 61: 8120-8135 (2018)
Article DOI: 10.1021/acs.jmedchem.8b01040
BindingDB Entry DOI: 10.7270/Q2FX7D3X |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase ABL1
(Homo sapiens (Human)) | BDBM213656

(US9278981, 248)Show SMILES O[C@H]1CN(C[C@@H]1O)c1ncc(cc1-c1cncnc1)C(=O)Nc1ccc(OC(F)(F)Cl)cc1 |r| Show InChI InChI=1S/C21H18ClF2N5O4/c22-21(23,24)33-15-3-1-14(2-4-15)28-20(32)12-5-16(13-6-25-11-26-7-13)19(27-8-12)29-9-17(30)18(31)10-29/h1-8,11,17-18,30-31H,9-10H2,(H,28,32)/t17-,18-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of ABL1 (64 to 515 residues)(unknown origin) expressed in Escherichia coli using FITC-Ahx-EAIYAAPFAKKK-NH2 peptide as substrate after 60 m... |
J Med Chem 61: 8120-8135 (2018)
Article DOI: 10.1021/acs.jmedchem.8b01040
BindingDB Entry DOI: 10.7270/Q2FX7D3X |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase ABL1
(Homo sapiens (Human)) | BDBM50459090
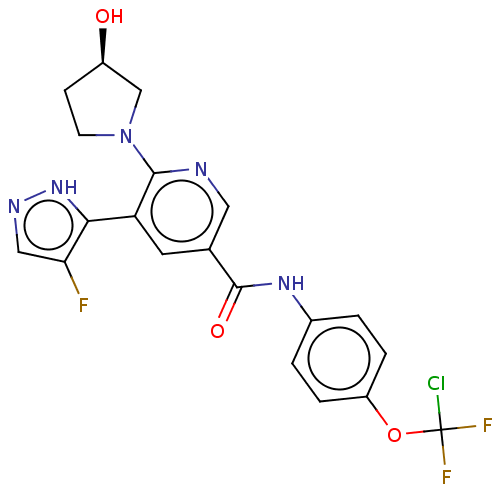
(CHEMBL4213152)Show SMILES O[C@@H]1CCN(C1)c1ncc(cc1-c1[nH]ncc1F)C(=O)Nc1ccc(OC(F)(F)Cl)cc1 |r| Show InChI InChI=1S/C20H17ClF3N5O3/c21-20(23,24)32-14-3-1-12(2-4-14)27-19(31)11-7-15(17-16(22)9-26-28-17)18(25-8-11)29-6-5-13(30)10-29/h1-4,7-9,13,30H,5-6,10H2,(H,26,28)(H,27,31)/t13-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of ABL1 (64 to 515 residues)(unknown origin) expressed in Escherichia coli using FITC-Ahx-EAIYAAPFAKKK-NH2 peptide as substrate after 60 m... |
J Med Chem 61: 8120-8135 (2018)
Article DOI: 10.1021/acs.jmedchem.8b01040
BindingDB Entry DOI: 10.7270/Q2FX7D3X |
More data for this
Ligand-Target Pair | |
Protein kinase C alpha type
(Homo sapiens (Human)) | BDBM33970
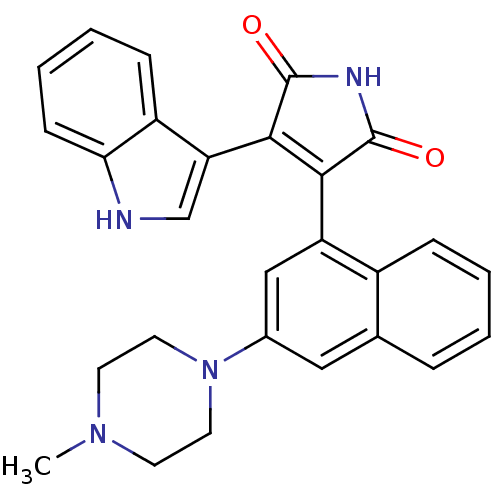
(maleimide derivative, 12)Show SMILES CN1CCN(CC1)c1cc(C2=C(C(=O)NC2=O)c2c[nH]c3ccccc23)c2ccccc2c1 |t:11| Show InChI InChI=1S/C27H24N4O2/c1-30-10-12-31(13-11-30)18-14-17-6-2-3-7-19(17)21(15-18)24-25(27(33)29-26(24)32)22-16-28-23-9-5-4-8-20(22)23/h2-9,14-16,28H,10-13H2,1H3,(H,29,32,33) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.900 | n/a | n/a | n/a | n/a | 7.4 | 23 |
Novartis
| Assay Description
Classical and novel PKC isotypes were assayed by scintillation proximity assay technology. In brief, the assay was performed in reaction buffer by in... |
J Med Chem 52: 6193-6 (2009)
Article DOI: 10.1021/jm901108b
BindingDB Entry DOI: 10.7270/Q25X278Q |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase B-raf
(Homo sapiens (Human)) | BDBM50452147
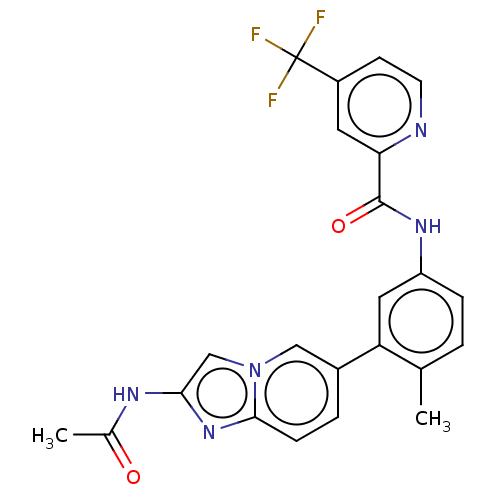
(CHEMBL4204192)Show SMILES CC(=O)Nc1cn2cc(ccc2n1)-c1cc(NC(=O)c2cc(ccn2)C(F)(F)F)ccc1C Show InChI InChI=1S/C23H18F3N5O2/c1-13-3-5-17(29-22(33)19-9-16(7-8-27-19)23(24,25)26)10-18(13)15-4-6-21-30-20(28-14(2)32)12-31(21)11-15/h3-12H,1-2H3,(H,28,32)(H,29,33) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research
Curated by ChEMBL
| Assay Description
Inhibition of recombinant C-terminal his6-tagged B-Raf (437 to 765 residues) V600E mutant (unknown origin) catalytic domain expressed in baculovirus ... |
Bioorg Med Chem Lett 27: 5221-5224 (2017)
Article DOI: 10.1016/j.bmcl.2017.10.047
BindingDB Entry DOI: 10.7270/Q2SB4892 |
More data for this
Ligand-Target Pair | |
Cathepsin K
(Homo sapiens (Human)) | BDBM50223939
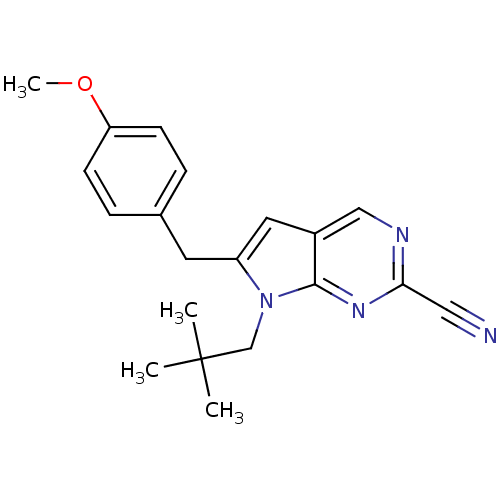
(6-(4-methoxybenzyl)-7-neopentyl-7H-pyrrolo[2,3-d]p...)Show InChI InChI=1S/C20H22N4O/c1-20(2,3)13-24-16(9-14-5-7-17(25-4)8-6-14)10-15-12-22-18(11-21)23-19(15)24/h5-8,10,12H,9,13H2,1-4H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of cathepsin K by fluorescence assay |
Bioorg Med Chem Lett 17: 6096-100 (2007)
Article DOI: 10.1016/j.bmcl.2007.09.047
BindingDB Entry DOI: 10.7270/Q2G160K6 |
More data for this
Ligand-Target Pair | |
Protein kinase C theta type
(Homo sapiens (Human)) | BDBM33971
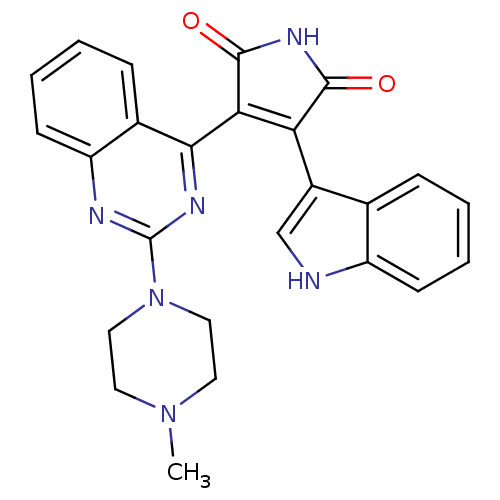
(AEB071 | Sotrastaurin | med.21724, Compound 190)Show SMILES CN1CCN(CC1)c1nc(C2=C(C(=O)NC2=O)c2c[nH]c3ccccc23)c2ccccc2n1 |t:11| Show InChI InChI=1S/C25H22N6O2/c1-30-10-12-31(13-11-30)25-27-19-9-5-3-7-16(19)22(28-25)21-20(23(32)29-24(21)33)17-14-26-18-8-4-2-6-15(17)18/h2-9,14,26H,10-13H2,1H3,(H,29,32,33) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | 25 |
Novartis
| Assay Description
Classical and novel PKC isotypes were assayed by scintillation proximity assay technology. In brief, the assay was performed in reaction buffer by in... |
J Med Chem 52: 6193-6 (2009)
Article DOI: 10.1021/jm901108b
BindingDB Entry DOI: 10.7270/Q25X278Q |
More data for this
Ligand-Target Pair | |
Cathepsin K
(Homo sapiens (Human)) | BDBM50223914
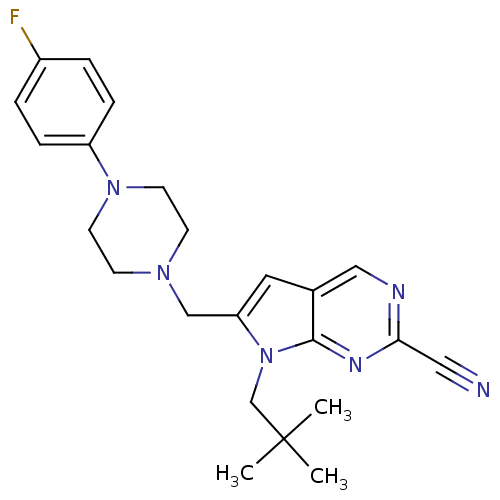
(6-((4-(4-fluorophenyl)piperazin-1-yl)methyl)-7-neo...)Show SMILES CC(C)(C)Cn1c(CN2CCN(CC2)c2ccc(F)cc2)cc2cnc(nc12)C#N Show InChI InChI=1S/C23H27FN6/c1-23(2,3)16-30-20(12-17-14-26-21(13-25)27-22(17)30)15-28-8-10-29(11-9-28)19-6-4-18(24)5-7-19/h4-7,12,14H,8-11,15-16H2,1-3H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of cathepsin K by fluorescence assay |
Bioorg Med Chem Lett 17: 6096-100 (2007)
Article DOI: 10.1016/j.bmcl.2007.09.047
BindingDB Entry DOI: 10.7270/Q2G160K6 |
More data for this
Ligand-Target Pair | |
Histamine H1 receptor
(Homo sapiens (Human)) | BDBM50112349
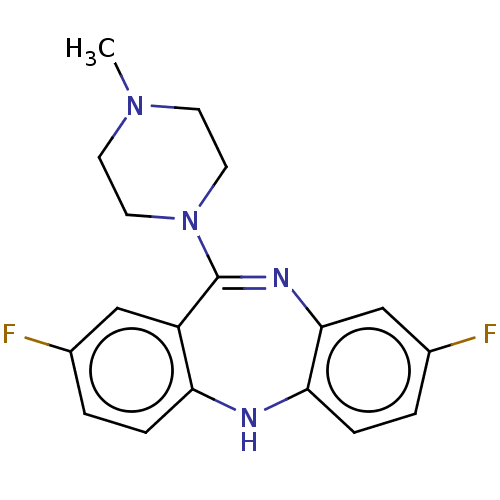
(CHEMBL3609328)Show SMILES CN1CCN(CC1)C1=Nc2cc(F)ccc2Nc2ccc(F)cc12 |t:8| Show InChI InChI=1S/C18H18F2N4/c1-23-6-8-24(9-7-23)18-14-10-12(19)2-4-15(14)21-16-5-3-13(20)11-17(16)22-18/h2-5,10-11,21H,6-9H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human histamine H1 receptor |
ACS Med Chem Lett 6: 776-81 (2015)
Article DOI: 10.1021/acsmedchemlett.5b00102
BindingDB Entry DOI: 10.7270/Q25X2BQS |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase ABL1
(Homo sapiens (Human)) | BDBM50459089
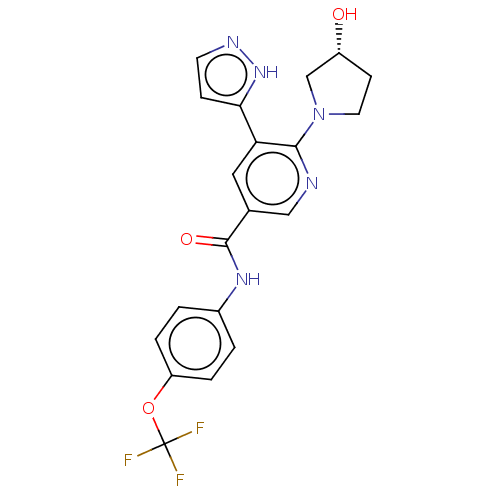
(CHEMBL4217559)Show SMILES O[C@@H]1CCN(C1)c1ncc(cc1-c1ccn[nH]1)C(=O)Nc1ccc(OC(F)(F)F)cc1 |r| Show InChI InChI=1S/C20H18F3N5O3/c21-20(22,23)31-15-3-1-13(2-4-15)26-19(30)12-9-16(17-5-7-25-27-17)18(24-10-12)28-8-6-14(29)11-28/h1-5,7,9-10,14,29H,6,8,11H2,(H,25,27)(H,26,30)/t14-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of ABL1 (64 to 515 residues)(unknown origin) expressed in Escherichia coli using FITC-Ahx-EAIYAAPFAKKK-NH2 peptide as substrate after 60 m... |
J Med Chem 61: 8120-8135 (2018)
Article DOI: 10.1021/acs.jmedchem.8b01040
BindingDB Entry DOI: 10.7270/Q2FX7D3X |
More data for this
Ligand-Target Pair | |
Cathepsin K
(Homo sapiens (Human)) | BDBM50223921
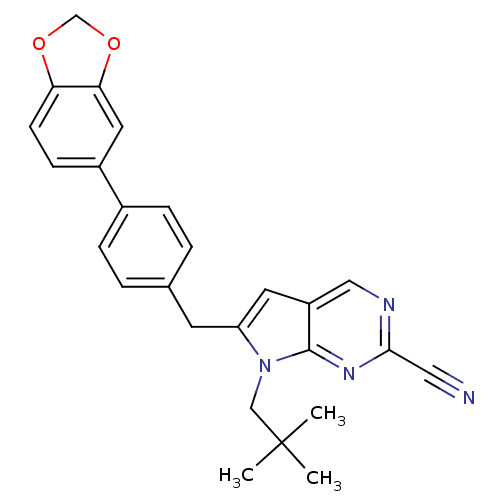
(6-(4-(benzo[d][1,3]dioxol-5-yl)benzyl)-7-neopentyl...)Show SMILES CC(C)(C)Cn1c(Cc2ccc(cc2)-c2ccc3OCOc3c2)cc2cnc(nc12)C#N Show InChI InChI=1S/C26H24N4O2/c1-26(2,3)15-30-21(11-20-14-28-24(13-27)29-25(20)30)10-17-4-6-18(7-5-17)19-8-9-22-23(12-19)32-16-31-22/h4-9,11-12,14H,10,15-16H2,1-3H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of cathepsin K by fluorescence assay |
Bioorg Med Chem Lett 17: 6096-100 (2007)
Article DOI: 10.1016/j.bmcl.2007.09.047
BindingDB Entry DOI: 10.7270/Q2G160K6 |
More data for this
Ligand-Target Pair | |
Cathepsin K
(Homo sapiens (Human)) | BDBM50223935
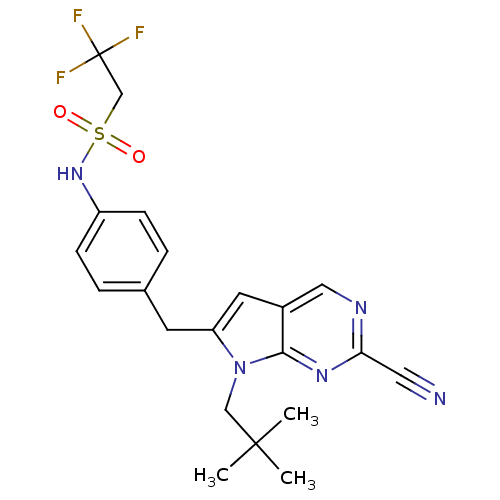
(CHEMBL399842 | N-(4-((2-cyano-7-neopentyl-7H-pyrro...)Show SMILES CC(C)(C)Cn1c(Cc2ccc(NS(=O)(=O)CC(F)(F)F)cc2)cc2cnc(nc12)C#N Show InChI InChI=1S/C21H22F3N5O2S/c1-20(2,3)12-29-17(9-15-11-26-18(10-25)27-19(15)29)8-14-4-6-16(7-5-14)28-32(30,31)13-21(22,23)24/h4-7,9,11,28H,8,12-13H2,1-3H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of cathepsin K by fluorescence assay |
Bioorg Med Chem Lett 17: 6096-100 (2007)
Article DOI: 10.1016/j.bmcl.2007.09.047
BindingDB Entry DOI: 10.7270/Q2G160K6 |
More data for this
Ligand-Target Pair | |
Cathepsin K
(Homo sapiens (Human)) | BDBM50223915

(6-benzyl-7-neopentyl-7H-pyrrolo[2,3-d]pyrimidine-2...)Show InChI InChI=1S/C19H20N4/c1-19(2,3)13-23-16(9-14-7-5-4-6-8-14)10-15-12-21-17(11-20)22-18(15)23/h4-8,10,12H,9,13H2,1-3H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of cathepsin K by fluorescence assay |
Bioorg Med Chem Lett 17: 6096-100 (2007)
Article DOI: 10.1016/j.bmcl.2007.09.047
BindingDB Entry DOI: 10.7270/Q2G160K6 |
More data for this
Ligand-Target Pair | |
Protein kinase C delta type
(Homo sapiens (Human)) | BDBM33971

(AEB071 | Sotrastaurin | med.21724, Compound 190)Show SMILES CN1CCN(CC1)c1nc(C2=C(C(=O)NC2=O)c2c[nH]c3ccccc23)c2ccccc2n1 |t:11| Show InChI InChI=1S/C25H22N6O2/c1-30-10-12-31(13-11-30)25-27-19-9-5-3-7-16(19)22(28-25)21-20(23(32)29-24(21)33)17-14-26-18-8-4-2-6-15(17)18/h2-9,14,26H,10-13H2,1H3,(H,29,32,33) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.30 | n/a | n/a | n/a | n/a | 7.4 | 23 |
Novartis
| Assay Description
Classical and novel PKC isotypes were assayed by scintillation proximity assay technology. In brief, the assay was performed in reaction buffer by in... |
J Med Chem 52: 6193-6 (2009)
Article DOI: 10.1021/jm901108b
BindingDB Entry DOI: 10.7270/Q25X278Q |
More data for this
Ligand-Target Pair | |
Cathepsin K
(Homo sapiens (Human)) | BDBM50223925
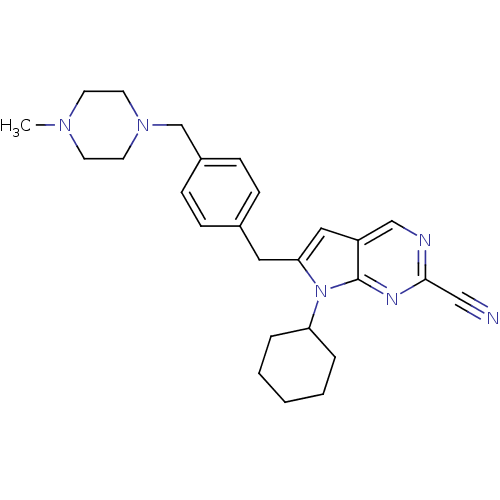
(6-(4-((4-methylpiperazin-1-yl)methyl)benzyl)-7-cyc...)Show SMILES CN1CCN(Cc2ccc(Cc3cc4cnc(nc4n3C3CCCCC3)C#N)cc2)CC1 Show InChI InChI=1S/C26H32N6/c1-30-11-13-31(14-12-30)19-21-9-7-20(8-10-21)15-24-16-22-18-28-25(17-27)29-26(22)32(24)23-5-3-2-4-6-23/h7-10,16,18,23H,2-6,11-15,19H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of cathepsin K by fluorescence assay |
Bioorg Med Chem Lett 17: 6096-100 (2007)
Article DOI: 10.1016/j.bmcl.2007.09.047
BindingDB Entry DOI: 10.7270/Q2G160K6 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase ABL1
(Homo sapiens (Human)) | BDBM230760
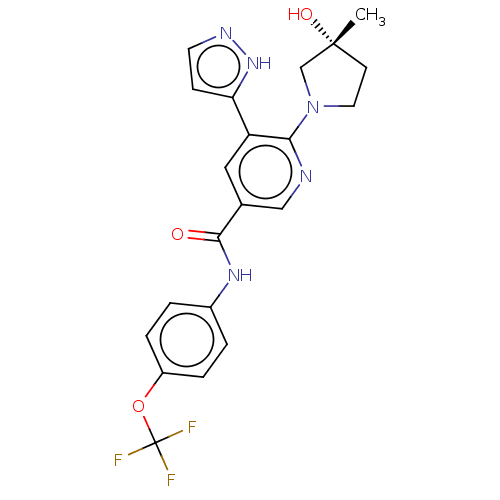
(US9340537, 14 | US9896444, Example 14)Show SMILES C[C@@]1(O)CCN(C1)c1ncc(cc1-c1ccn[nH]1)C(=O)Nc1ccc(OC(F)(F)F)cc1 |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of ABL1 (64 to 515 residues)(unknown origin) expressed in Escherichia coli using FITC-Ahx-EAIYAAPFAKKK-NH2 peptide as substrate after 60 m... |
J Med Chem 61: 8120-8135 (2018)
Article DOI: 10.1021/acs.jmedchem.8b01040
BindingDB Entry DOI: 10.7270/Q2FX7D3X |
More data for this
Ligand-Target Pair | |
Protein kinase C beta type
(Homo sapiens (Human)) | BDBM2683
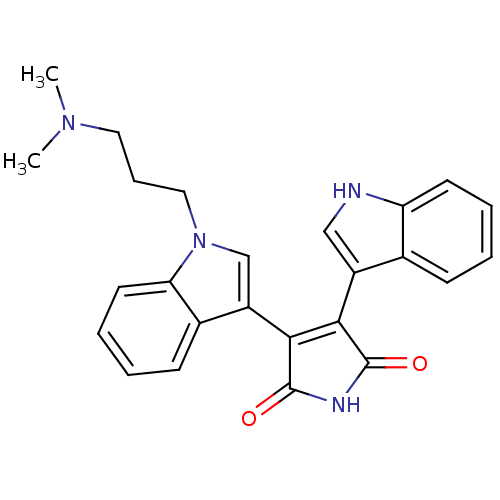
(2-[1-(3-dimethylaminopropyl)-indol-3-yl]-3-(indol-...)Show SMILES CN(C)CCCn1cc(C2=C(C(=O)NC2=O)c2c[nH]c3ccccc23)c2ccccc12 |t:9| Show InChI InChI=1S/C25H24N4O2/c1-28(2)12-7-13-29-15-19(17-9-4-6-11-21(17)29)23-22(24(30)27-25(23)31)18-14-26-20-10-5-3-8-16(18)20/h3-6,8-11,14-15,26H,7,12-13H2,1-2H3,(H,27,30,31) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.60 | n/a | n/a | n/a | n/a | 7.4 | 23 |
Novartis
| Assay Description
Classical and novel PKC isotypes were assayed by scintillation proximity assay technology. In brief, the assay was performed in reaction buffer by in... |
J Med Chem 52: 6193-6 (2009)
Article DOI: 10.1021/jm901108b
BindingDB Entry DOI: 10.7270/Q25X278Q |
More data for this
Ligand-Target Pair | |
Cathepsin K
(Homo sapiens (Human)) | BDBM50223919
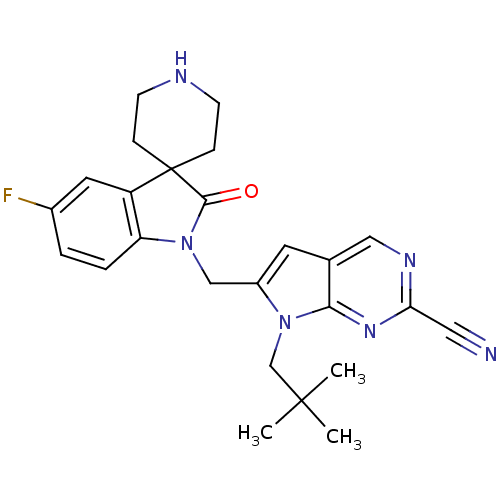
(7-(2,2-dimethylpropyl)-6-[(5-fluoro-2-oxospiro[ind...)Show SMILES CC(C)(C)Cn1c(CN2C(=O)C3(CCNCC3)c3cc(F)ccc23)cc2cnc(nc12)C#N Show InChI InChI=1S/C25H27FN6O/c1-24(2,3)15-32-18(10-16-13-29-21(12-27)30-22(16)32)14-31-20-5-4-17(26)11-19(20)25(23(31)33)6-8-28-9-7-25/h4-5,10-11,13,28H,6-9,14-15H2,1-3H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of cathepsin K by fluorescence assay |
Bioorg Med Chem Lett 17: 6096-100 (2007)
Article DOI: 10.1016/j.bmcl.2007.09.047
BindingDB Entry DOI: 10.7270/Q2G160K6 |
More data for this
Ligand-Target Pair | |
Protein kinase C beta type
(Homo sapiens (Human)) | BDBM33970

(maleimide derivative, 12)Show SMILES CN1CCN(CC1)c1cc(C2=C(C(=O)NC2=O)c2c[nH]c3ccccc23)c2ccccc2c1 |t:11| Show InChI InChI=1S/C27H24N4O2/c1-30-10-12-31(13-11-30)18-14-17-6-2-3-7-19(17)21(15-18)24-25(27(33)29-26(24)32)22-16-28-23-9-5-4-8-20(22)23/h2-9,14-16,28H,10-13H2,1H3,(H,29,32,33) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.80 | n/a | n/a | n/a | n/a | 7.4 | 23 |
Novartis
| Assay Description
Classical and novel PKC isotypes were assayed by scintillation proximity assay technology. In brief, the assay was performed in reaction buffer by in... |
J Med Chem 52: 6193-6 (2009)
Article DOI: 10.1021/jm901108b
BindingDB Entry DOI: 10.7270/Q25X278Q |
More data for this
Ligand-Target Pair | |
Proto-oncogene tyrosine-protein kinase Src
(Homo sapiens (Human)) | BDBM50320214
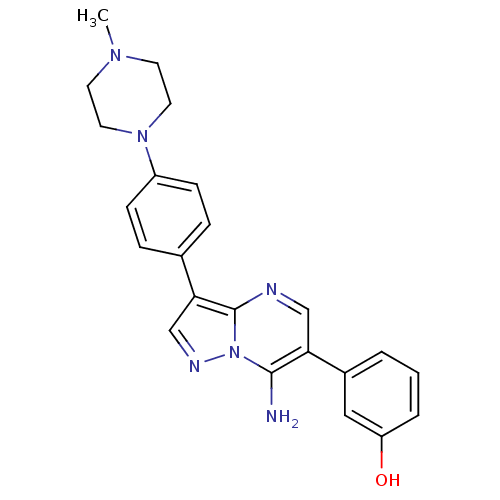
(3-(7-amino-3-(4-(4-methylpiperazin-1-yl)phenyl)pyr...)Show SMILES CN1CCN(CC1)c1ccc(cc1)-c1cnn2c(N)c(cnc12)-c1cccc(O)c1 Show InChI InChI=1S/C23H24N6O/c1-27-9-11-28(12-10-27)18-7-5-16(6-8-18)21-15-26-29-22(24)20(14-25-23(21)29)17-3-2-4-19(30)13-17/h2-8,13-15,30H,9-12,24H2,1H3 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institute of Biomedical Research
Curated by ChEMBL
| Assay Description
Inhibition of SRC |
Bioorg Med Chem Lett 20: 3628-31 (2010)
Article DOI: 10.1016/j.bmcl.2010.04.112
BindingDB Entry DOI: 10.7270/Q2XP754D |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 11/12/13/14
(Homo sapiens (Human)) | BDBM50452151
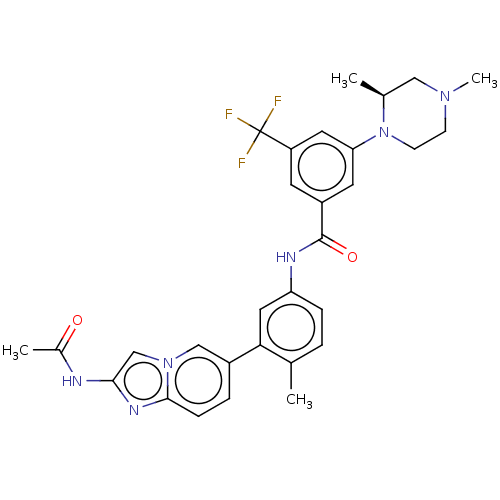
(CHEMBL4208527)Show SMILES C[C@H]1CN(C)CCN1c1cc(cc(c1)C(F)(F)F)C(=O)Nc1ccc(C)c(c1)-c1ccc2nc(NC(C)=O)cn2c1 |r| Show InChI InChI=1S/C30H31F3N6O2/c1-18-5-7-24(14-26(18)21-6-8-28-36-27(34-20(3)40)17-38(28)16-21)35-29(41)22-11-23(30(31,32)33)13-25(12-22)39-10-9-37(4)15-19(39)2/h5-8,11-14,16-17,19H,9-10,15H2,1-4H3,(H,34,40)(H,35,41)/t19-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research
Curated by ChEMBL
| Assay Description
Inhibition of P38 (unknown origin) |
Bioorg Med Chem Lett 27: 5221-5224 (2017)
Article DOI: 10.1016/j.bmcl.2017.10.047
BindingDB Entry DOI: 10.7270/Q2SB4892 |
More data for this
Ligand-Target Pair | |
Protein kinase C eta type
(Homo sapiens (Human)) | BDBM50391897
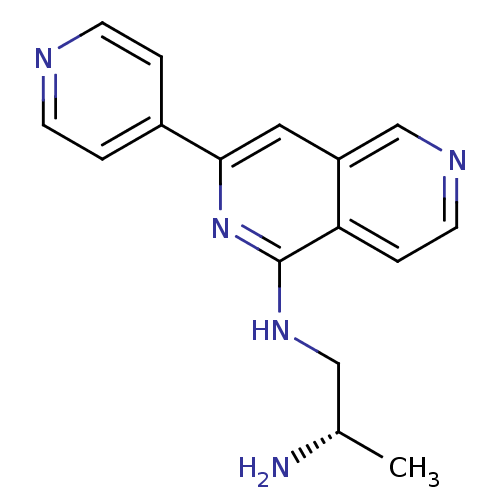
(CHEMBL2147537)Show InChI InChI=1S/C16H17N5/c1-11(17)9-20-16-14-4-7-19-10-13(14)8-15(21-16)12-2-5-18-6-3-12/h2-8,10-11H,9,17H2,1H3,(H,20,21)/t11-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human PKCeta assessed as [33P]-ATP incorporation into tridecapeptide substrate after 60 mins by scintillation proximity ass... |
Bioorg Med Chem Lett 21: 7367-72 (2011)
Article DOI: 10.1016/j.bmcl.2011.10.025
BindingDB Entry DOI: 10.7270/Q22J6CZ4 |
More data for this
Ligand-Target Pair | |
Protein kinase C beta type
(Homo sapiens (Human)) | BDBM33971

(AEB071 | Sotrastaurin | med.21724, Compound 190)Show SMILES CN1CCN(CC1)c1nc(C2=C(C(=O)NC2=O)c2c[nH]c3ccccc23)c2ccccc2n1 |t:11| Show InChI InChI=1S/C25H22N6O2/c1-30-10-12-31(13-11-30)25-27-19-9-5-3-7-16(19)22(28-25)21-20(23(32)29-24(21)33)17-14-26-18-8-4-2-6-15(17)18/h2-9,14,26H,10-13H2,1H3,(H,29,32,33) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | 7.4 | 23 |
Novartis
| Assay Description
Classical and novel PKC isotypes were assayed by scintillation proximity assay technology. In brief, the assay was performed in reaction buffer by in... |
J Med Chem 52: 6193-6 (2009)
Article DOI: 10.1021/jm901108b
BindingDB Entry DOI: 10.7270/Q25X278Q |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 11/12/13/14
(Homo sapiens (Human)) | BDBM50452152

(CHEMBL4217462)Show SMILES CC(=O)Nc1cn2cc(ccc2n1)-c1cc(NC(=O)c2cccc(c2)C(C)(C)C)ccc1C Show InChI InChI=1S/C27H28N4O2/c1-17-9-11-22(29-26(33)19-7-6-8-21(13-19)27(3,4)5)14-23(17)20-10-12-25-30-24(28-18(2)32)16-31(25)15-20/h6-16H,1-5H3,(H,28,32)(H,29,33) | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research
Curated by ChEMBL
| Assay Description
Inhibition of P38 (unknown origin) |
Bioorg Med Chem Lett 27: 5221-5224 (2017)
Article DOI: 10.1016/j.bmcl.2017.10.047
BindingDB Entry DOI: 10.7270/Q2SB4892 |
More data for this
Ligand-Target Pair | |
Protein kinase C beta type
(Homo sapiens (Human)) | BDBM33968
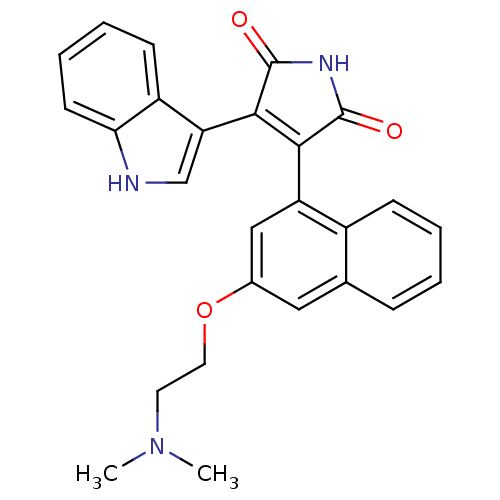
(maleimide derivative, 10)Show SMILES CN(C)CCOc1cc(C2=C(C(=O)NC2=O)c2c[nH]c3ccccc23)c2ccccc2c1 |t:9| Show InChI InChI=1S/C26H23N3O3/c1-29(2)11-12-32-17-13-16-7-3-4-8-18(16)20(14-17)23-24(26(31)28-25(23)30)21-15-27-22-10-6-5-9-19(21)22/h3-10,13-15,27H,11-12H2,1-2H3,(H,28,30,31) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2.10 | n/a | n/a | n/a | n/a | 7.4 | 23 |
Novartis
| Assay Description
Classical and novel PKC isotypes were assayed by scintillation proximity assay technology. In brief, the assay was performed in reaction buffer by in... |
J Med Chem 52: 6193-6 (2009)
Article DOI: 10.1021/jm901108b
BindingDB Entry DOI: 10.7270/Q25X278Q |
More data for this
Ligand-Target Pair | |
Protein kinase C alpha type
(Homo sapiens (Human)) | BDBM33971

(AEB071 | Sotrastaurin | med.21724, Compound 190)Show SMILES CN1CCN(CC1)c1nc(C2=C(C(=O)NC2=O)c2c[nH]c3ccccc23)c2ccccc2n1 |t:11| Show InChI InChI=1S/C25H22N6O2/c1-30-10-12-31(13-11-30)25-27-19-9-5-3-7-16(19)22(28-25)21-20(23(32)29-24(21)33)17-14-26-18-8-4-2-6-15(17)18/h2-9,14,26H,10-13H2,1H3,(H,29,32,33) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| MMDB
PDB
Article
PubMed
| n/a | n/a | 2.10 | n/a | n/a | n/a | n/a | 7.4 | 23 |
Novartis
| Assay Description
Classical and novel PKC isotypes were assayed by scintillation proximity assay technology. In brief, the assay was performed in reaction buffer by in... |
J Med Chem 52: 6193-6 (2009)
Article DOI: 10.1021/jm901108b
BindingDB Entry DOI: 10.7270/Q25X278Q |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Protein kinase C alpha type
(Homo sapiens (Human)) | BDBM33968

(maleimide derivative, 10)Show SMILES CN(C)CCOc1cc(C2=C(C(=O)NC2=O)c2c[nH]c3ccccc23)c2ccccc2c1 |t:9| Show InChI InChI=1S/C26H23N3O3/c1-29(2)11-12-32-17-13-16-7-3-4-8-18(16)20(14-17)23-24(26(31)28-25(23)30)21-15-27-22-10-6-5-9-19(21)22/h3-10,13-15,27H,11-12H2,1-2H3,(H,28,30,31) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2.20 | n/a | n/a | n/a | n/a | 7.4 | 23 |
Novartis
| Assay Description
Classical and novel PKC isotypes were assayed by scintillation proximity assay technology. In brief, the assay was performed in reaction buffer by in... |
J Med Chem 52: 6193-6 (2009)
Article DOI: 10.1021/jm901108b
BindingDB Entry DOI: 10.7270/Q25X278Q |
More data for this
Ligand-Target Pair | |
Protein kinase C alpha type
(Homo sapiens (Human)) | BDBM2683

(2-[1-(3-dimethylaminopropyl)-indol-3-yl]-3-(indol-...)Show SMILES CN(C)CCCn1cc(C2=C(C(=O)NC2=O)c2c[nH]c3ccccc23)c2ccccc12 |t:9| Show InChI InChI=1S/C25H24N4O2/c1-28(2)12-7-13-29-15-19(17-9-4-6-11-21(17)29)23-22(24(30)27-25(23)31)18-14-26-20-10-5-3-8-16(18)20/h3-6,8-11,14-15,26H,7,12-13H2,1-2H3,(H,27,30,31) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2.30 | n/a | n/a | n/a | n/a | 7.4 | 23 |
Novartis
| Assay Description
Classical and novel PKC isotypes were assayed by scintillation proximity assay technology. In brief, the assay was performed in reaction buffer by in... |
J Med Chem 52: 6193-6 (2009)
Article DOI: 10.1021/jm901108b
BindingDB Entry DOI: 10.7270/Q25X278Q |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase ABL1
(Homo sapiens (Human)) | BDBM213441
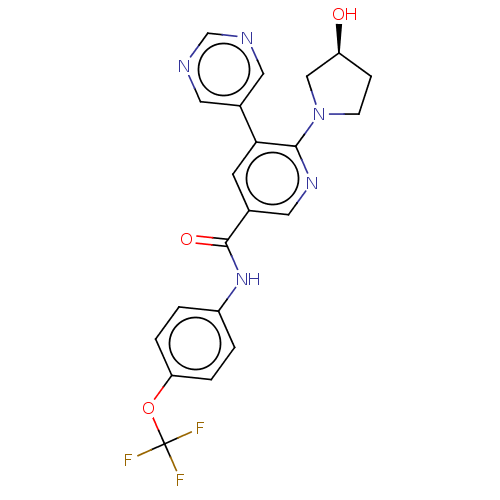
(US9278981, 33)Show SMILES O[C@H]1CCN(C1)c1ncc(cc1-c1cncnc1)C(=O)Nc1ccc(OC(F)(F)F)cc1 |r| Show InChI InChI=1S/C21H18F3N5O3/c22-21(23,24)32-17-3-1-15(2-4-17)28-20(31)13-7-18(14-8-25-12-26-9-14)19(27-10-13)29-6-5-16(30)11-29/h1-4,7-10,12,16,30H,5-6,11H2,(H,28,31)/t16-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of ABL1 (64 to 515 residues)(unknown origin) expressed in Escherichia coli using FITC-Ahx-EAIYAAPFAKKK-NH2 peptide as substrate after 60 m... |
J Med Chem 61: 8120-8135 (2018)
Article DOI: 10.1021/acs.jmedchem.8b01040
BindingDB Entry DOI: 10.7270/Q2FX7D3X |
More data for this
Ligand-Target Pair | |
Cathepsin K
(Homo sapiens (Human)) | BDBM50223910

(6-(4-chlorobenzyl)-7-cyclohexyl-7H-pyrrolo[2,3-d]p...)Show InChI InChI=1S/C20H19ClN4/c21-16-8-6-14(7-9-16)10-18-11-15-13-23-19(12-22)24-20(15)25(18)17-4-2-1-3-5-17/h6-9,11,13,17H,1-5,10H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of cathepsin K by fluorescence assay |
Bioorg Med Chem Lett 17: 6096-100 (2007)
Article DOI: 10.1016/j.bmcl.2007.09.047
BindingDB Entry DOI: 10.7270/Q2G160K6 |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase B-raf
(Homo sapiens (Human)) | BDBM50452151

(CHEMBL4208527)Show SMILES C[C@H]1CN(C)CCN1c1cc(cc(c1)C(F)(F)F)C(=O)Nc1ccc(C)c(c1)-c1ccc2nc(NC(C)=O)cn2c1 |r| Show InChI InChI=1S/C30H31F3N6O2/c1-18-5-7-24(14-26(18)21-6-8-28-36-27(34-20(3)40)17-38(28)16-21)35-29(41)22-11-23(30(31,32)33)13-25(12-22)39-10-9-37(4)15-19(39)2/h5-8,11-14,16-17,19H,9-10,15H2,1-4H3,(H,34,40)(H,35,41)/t19-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research
Curated by ChEMBL
| Assay Description
Inhibition of recombinant C-terminal his6-tagged B-Raf (437 to 765 residues) V600E mutant (unknown origin) catalytic domain expressed in baculovirus ... |
Bioorg Med Chem Lett 27: 5221-5224 (2017)
Article DOI: 10.1016/j.bmcl.2017.10.047
BindingDB Entry DOI: 10.7270/Q2SB4892 |
More data for this
Ligand-Target Pair | |
Cathepsin K
(Homo sapiens (Human)) | BDBM50223911

(7-neopentyl-6-((pyridin-4-yloxy)methyl)-7H-pyrrolo...)Show InChI InChI=1S/C18H19N5O/c1-18(2,3)12-23-14(11-24-15-4-6-20-7-5-15)8-13-10-21-16(9-19)22-17(13)23/h4-8,10H,11-12H2,1-3H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of cathepsin K by fluorescence assay |
Bioorg Med Chem Lett 17: 6096-100 (2007)
Article DOI: 10.1016/j.bmcl.2007.09.047
BindingDB Entry DOI: 10.7270/Q2G160K6 |
More data for this
Ligand-Target Pair | |
Protein kinase C theta type
(Homo sapiens (Human)) | BDBM33968

(maleimide derivative, 10)Show SMILES CN(C)CCOc1cc(C2=C(C(=O)NC2=O)c2c[nH]c3ccccc23)c2ccccc2c1 |t:9| Show InChI InChI=1S/C26H23N3O3/c1-29(2)11-12-32-17-13-16-7-3-4-8-18(16)20(14-17)23-24(26(31)28-25(23)30)21-15-27-22-10-6-5-9-19(21)22/h3-10,13-15,27H,11-12H2,1-2H3,(H,28,30,31) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 3.30 | n/a | n/a | n/a | n/a | n/a | 25 |
Novartis
| Assay Description
Classical and novel PKC isotypes were assayed by scintillation proximity assay technology. In brief, the assay was performed in reaction buffer by in... |
J Med Chem 52: 6193-6 (2009)
Article DOI: 10.1021/jm901108b
BindingDB Entry DOI: 10.7270/Q25X278Q |
More data for this
Ligand-Target Pair | |
Cathepsin K
(Homo sapiens (Human)) | BDBM50223920
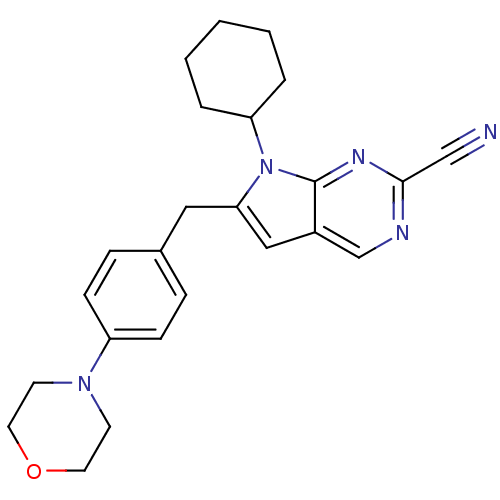
(6-(4-morpholinobenzyl)-7-cyclohexyl-7H-pyrrolo[2,3...)Show SMILES N#Cc1ncc2cc(Cc3ccc(cc3)N3CCOCC3)n(C3CCCCC3)c2n1 Show InChI InChI=1S/C24H27N5O/c25-16-23-26-17-19-15-22(29(24(19)27-23)21-4-2-1-3-5-21)14-18-6-8-20(9-7-18)28-10-12-30-13-11-28/h6-9,15,17,21H,1-5,10-14H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of cathepsin K by fluorescence assay |
Bioorg Med Chem Lett 17: 6096-100 (2007)
Article DOI: 10.1016/j.bmcl.2007.09.047
BindingDB Entry DOI: 10.7270/Q2G160K6 |
More data for this
Ligand-Target Pair | |
Cathepsin K
(Homo sapiens (Human)) | BDBM50156086

(9-CYCLOPENTYL-6-[2-(3-IMIDAZOL-1-YL-PROPOXY)-PHENY...)Show SMILES N#Cc1nc(Nc2ccccc2OCCCn2ccnc2)c2ncn(C3CCCC3)c2n1 Show InChI InChI=1S/C23H24N8O/c24-14-20-28-22(21-23(29-20)31(16-26-21)17-6-1-2-7-17)27-18-8-3-4-9-19(18)32-13-5-11-30-12-10-25-15-30/h3-4,8-10,12,15-17H,1-2,5-7,11,13H2,(H,27,28,29) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
DrugBank
PC cid
PC sid
PDB
UniChem
Similars
| DrugBank
PDB
Article
PubMed
| n/a | n/a | 3.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibitory concentration against recombinant human cathepsin K by using Z-Phe-Arg-AMC as synthetic substrate |
J Med Chem 47: 5833-6 (2004)
Article DOI: 10.1021/jm0493111
BindingDB Entry DOI: 10.7270/Q2862FWZ |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Epithelial discoidin domain-containing receptor 1
(Homo sapiens (Human)) | BDBM50237710
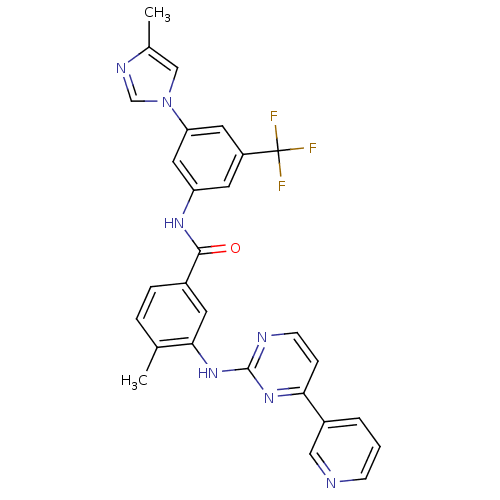
(4-methyl-N-[3-(4-methyl-1H-imidazol-1-yl)-5-(trifl...)Show SMILES Cc1cn(cn1)-c1cc(NC(=O)c2ccc(C)c(Nc3nccc(n3)-c3cccnc3)c2)cc(c1)C(F)(F)F Show InChI InChI=1S/C28H22F3N7O/c1-17-5-6-19(10-25(17)37-27-33-9-7-24(36-27)20-4-3-8-32-14-20)26(39)35-22-11-21(28(29,30)31)12-23(13-22)38-15-18(2)34-16-38/h3-16H,1-2H3,(H,35,39)(H,33,36,37) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 3.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of autophosphorylation of DDR1 expressed in HEK293 cells by ELISA |
Bioorg Med Chem 18: 6977-86 (2010)
Article DOI: 10.1016/j.bmc.2010.08.026
BindingDB Entry DOI: 10.7270/Q2930TC9 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase ABL1
(Homo sapiens (Human)) | BDBM213443
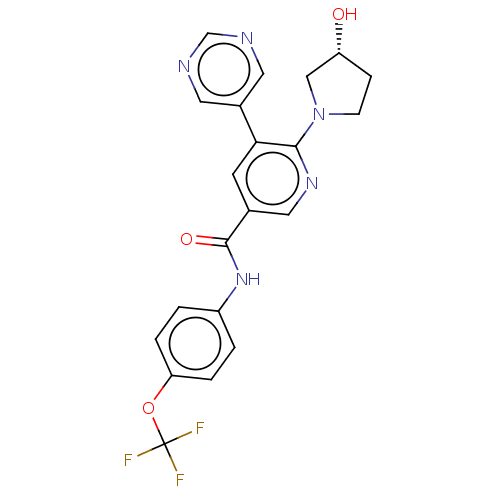
(US9278981, 35)Show SMILES O[C@@H]1CCN(C1)c1ncc(cc1-c1cncnc1)C(=O)Nc1ccc(OC(F)(F)F)cc1 |r| Show InChI InChI=1S/C21H18F3N5O3/c22-21(23,24)32-17-3-1-15(2-4-17)28-20(31)13-7-18(14-8-25-12-26-9-14)19(27-10-13)29-6-5-16(30)11-29/h1-4,7-10,12,16,30H,5-6,11H2,(H,28,31)/t16-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of ABL1 (64 to 515 residues)(unknown origin) expressed in Escherichia coli using FITC-Ahx-EAIYAAPFAKKK-NH2 peptide as substrate after 60 m... |
J Med Chem 61: 8120-8135 (2018)
Article DOI: 10.1021/acs.jmedchem.8b01040
BindingDB Entry DOI: 10.7270/Q2FX7D3X |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Cathepsin K
(Homo sapiens (Human)) | BDBM50223940
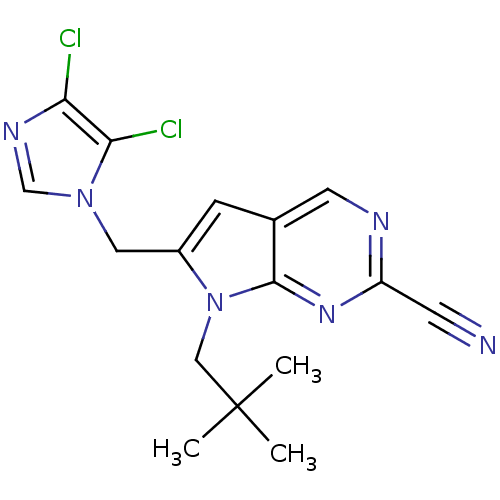
(6-((4,5-dichloro-1H-imidazol-1-yl)methyl)-7-neopen...)Show SMILES CC(C)(C)Cn1c(Cn2cnc(Cl)c2Cl)cc2cnc(nc12)C#N Show InChI InChI=1S/C16H16Cl2N6/c1-16(2,3)8-24-11(7-23-9-21-13(17)14(23)18)4-10-6-20-12(5-19)22-15(10)24/h4,6,9H,7-8H2,1-3H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of cathepsin K by fluorescence assay |
Bioorg Med Chem Lett 17: 6096-100 (2007)
Article DOI: 10.1016/j.bmcl.2007.09.047
BindingDB Entry DOI: 10.7270/Q2G160K6 |
More data for this
Ligand-Target Pair | |
Cathepsin K
(Homo sapiens (Human)) | BDBM50223936

(6-((5,5-dimethyl-2,4-dioxooxazolidin-3-yl)methyl)-...)Show SMILES CC(C)(C)Cn1c(CN2C(=O)OC(C)(C)C2=O)cc2cnc(nc12)C#N Show InChI InChI=1S/C18H21N5O3/c1-17(2,3)10-23-12(6-11-8-20-13(7-19)21-14(11)23)9-22-15(24)18(4,5)26-16(22)25/h6,8H,9-10H2,1-5H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of cathepsin K by fluorescence assay |
Bioorg Med Chem Lett 17: 6096-100 (2007)
Article DOI: 10.1016/j.bmcl.2007.09.047
BindingDB Entry DOI: 10.7270/Q2G160K6 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase Lck
(Homo sapiens (Human)) | BDBM50320205
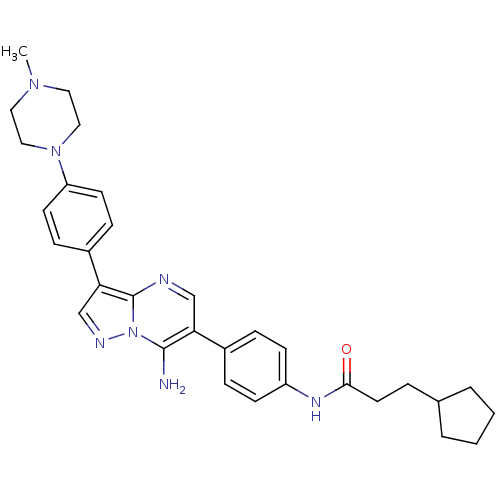
(CHEMBL1083001 | N-(4-(7-amino-3-(4-(4-methylpipera...)Show SMILES CN1CCN(CC1)c1ccc(cc1)-c1cnn2c(N)c(cnc12)-c1ccc(NC(=O)CCC2CCCC2)cc1 Show InChI InChI=1S/C31H37N7O/c1-36-16-18-37(19-17-36)26-13-9-24(10-14-26)28-21-34-38-30(32)27(20-33-31(28)38)23-7-11-25(12-8-23)35-29(39)15-6-22-4-2-3-5-22/h7-14,20-22H,2-6,15-19,32H2,1H3,(H,35,39) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institute of Biomedical Research
Curated by ChEMBL
| Assay Description
Inhibition of LCK |
Bioorg Med Chem Lett 20: 3628-31 (2010)
Article DOI: 10.1016/j.bmcl.2010.04.112
BindingDB Entry DOI: 10.7270/Q2XP754D |
More data for this
Ligand-Target Pair | |
Protein kinase C eta type
(Homo sapiens (Human)) | BDBM50391902
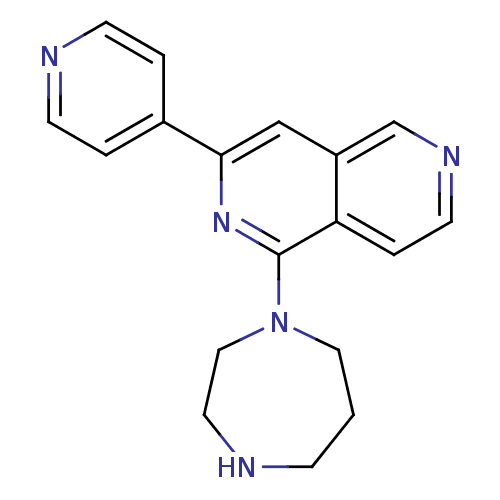
(CHEMBL2147542)Show InChI InChI=1S/C18H19N5/c1-5-19-9-11-23(10-1)18-16-4-8-21-13-15(16)12-17(22-18)14-2-6-20-7-3-14/h2-4,6-8,12-13,19H,1,5,9-11H2 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human PKCeta assessed as [33P]-ATP incorporation into tridecapeptide substrate after 60 mins by scintillation proximity ass... |
Bioorg Med Chem Lett 21: 7367-72 (2011)
Article DOI: 10.1016/j.bmcl.2011.10.025
BindingDB Entry DOI: 10.7270/Q22J6CZ4 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase Lck
(Homo sapiens (Human)) | BDBM50320225
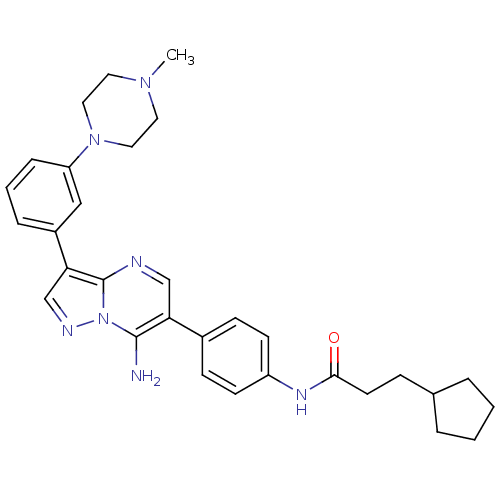
(CHEMBL1085316 | N-(4-(7-amino-3-(3-(4-methylpipera...)Show SMILES CN1CCN(CC1)c1cccc(c1)-c1cnn2c(N)c(cnc12)-c1ccc(NC(=O)CCC2CCCC2)cc1 Show InChI InChI=1S/C31H37N7O/c1-36-15-17-37(18-16-36)26-8-4-7-24(19-26)28-21-34-38-30(32)27(20-33-31(28)38)23-10-12-25(13-11-23)35-29(39)14-9-22-5-2-3-6-22/h4,7-8,10-13,19-22H,2-3,5-6,9,14-18,32H2,1H3,(H,35,39) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institute of Biomedical Research
Curated by ChEMBL
| Assay Description
Inhibition of LCK |
Bioorg Med Chem Lett 20: 3628-31 (2010)
Article DOI: 10.1016/j.bmcl.2010.04.112
BindingDB Entry DOI: 10.7270/Q2XP754D |
More data for this
Ligand-Target Pair | |
Protein kinase C delta type
(Homo sapiens (Human)) | BDBM33968

(maleimide derivative, 10)Show SMILES CN(C)CCOc1cc(C2=C(C(=O)NC2=O)c2c[nH]c3ccccc23)c2ccccc2c1 |t:9| Show InChI InChI=1S/C26H23N3O3/c1-29(2)11-12-32-17-13-16-7-3-4-8-18(16)20(14-17)23-24(26(31)28-25(23)30)21-15-27-22-10-6-5-9-19(21)22/h3-10,13-15,27H,11-12H2,1-2H3,(H,28,30,31) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 4.40 | n/a | n/a | n/a | n/a | 7.4 | 23 |
Novartis
| Assay Description
Classical and novel PKC isotypes were assayed by scintillation proximity assay technology. In brief, the assay was performed in reaction buffer by in... |
J Med Chem 52: 6193-6 (2009)
Article DOI: 10.1021/jm901108b
BindingDB Entry DOI: 10.7270/Q25X278Q |
More data for this
Ligand-Target Pair | |
Cathepsin K
(Homo sapiens (Human)) | BDBM50223941
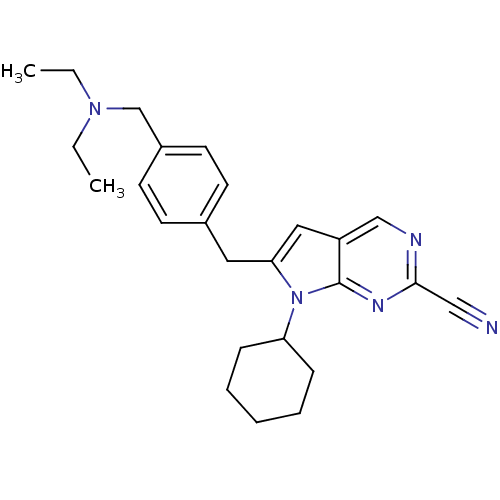
(6-(4-((diethylamino)methyl)benzyl)-7-cyclohexyl-7H...)Show SMILES CCN(CC)Cc1ccc(Cc2cc3cnc(nc3n2C2CCCCC2)C#N)cc1 Show InChI InChI=1S/C25H31N5/c1-3-29(4-2)18-20-12-10-19(11-13-20)14-23-15-21-17-27-24(16-26)28-25(21)30(23)22-8-6-5-7-9-22/h10-13,15,17,22H,3-9,14,18H2,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 4.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of cathepsin K by fluorescence assay |
Bioorg Med Chem Lett 17: 6096-100 (2007)
Article DOI: 10.1016/j.bmcl.2007.09.047
BindingDB Entry DOI: 10.7270/Q2G160K6 |
More data for this
Ligand-Target Pair | |
Protein kinase C theta type
(Homo sapiens (Human)) | BDBM33970

(maleimide derivative, 12)Show SMILES CN1CCN(CC1)c1cc(C2=C(C(=O)NC2=O)c2c[nH]c3ccccc23)c2ccccc2c1 |t:11| Show InChI InChI=1S/C27H24N4O2/c1-30-10-12-31(13-11-30)18-14-17-6-2-3-7-19(17)21(15-18)24-25(27(33)29-26(24)32)22-16-28-23-9-5-4-8-20(22)23/h2-9,14-16,28H,10-13H2,1H3,(H,29,32,33) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4.80 | n/a | n/a | n/a | n/a | n/a | 25 |
Novartis
| Assay Description
Classical and novel PKC isotypes were assayed by scintillation proximity assay technology. In brief, the assay was performed in reaction buffer by in... |
J Med Chem 52: 6193-6 (2009)
Article DOI: 10.1021/jm901108b
BindingDB Entry DOI: 10.7270/Q25X278Q |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data