

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 5-hydroxytryptamine receptor 3A (Homo sapiens (Human)) | BDBM50307815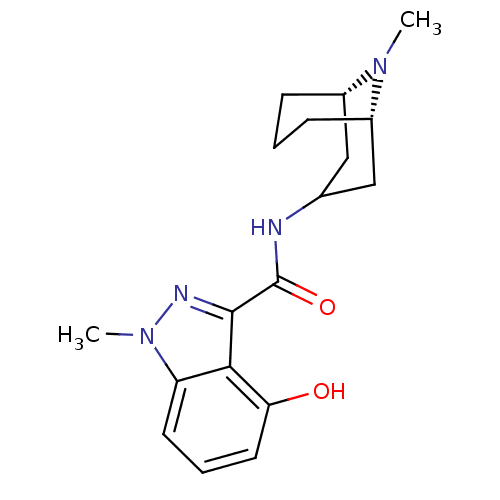 (4-Hydroxy-1-methyl-N-[(3-endo)-9-methyl-9-azabicyc...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.170 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Warwick Curated by ChEMBL | Assay Description Displacement of [3H]granisetron from human 5HT3A receptor expressed in HEK293 cells by scintillation counting | J Med Chem 53: 2324-8 (2010) Article DOI: 10.1021/jm901827x BindingDB Entry DOI: 10.7270/Q2JQ11Z5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 3A (Homo sapiens (Human)) | BDBM50307826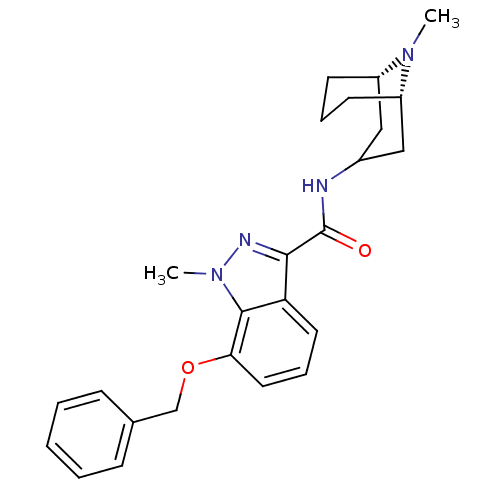 (1-Methyl-N-[(3-endo)-9-methyl-9-azabicyclo[3.3.1]n...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.230 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Warwick Curated by ChEMBL | Assay Description Displacement of [3H]granisetron from human 5HT3A receptor expressed in HEK293 cells by scintillation counting | J Med Chem 53: 2324-8 (2010) Article DOI: 10.1021/jm901827x BindingDB Entry DOI: 10.7270/Q2JQ11Z5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 3A (Homo sapiens (Human)) | BDBM50307824 (7-Hydroxy-1-methyl-N-[(3-endo)-9-methyl-9-azabicyc...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.670 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Warwick Curated by ChEMBL | Assay Description Displacement of [3H]granisetron from human 5HT3A receptor expressed in HEK293 cells by scintillation counting | J Med Chem 53: 2324-8 (2010) Article DOI: 10.1021/jm901827x BindingDB Entry DOI: 10.7270/Q2JQ11Z5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 3A (Homo sapiens (Human)) | BDBM50363283 (CHEMBL1945711) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bern Curated by ChEMBL | Assay Description Displacement of [3H]granisetron from human 5HT3A expressed in HEK293 cells after 1 hr by scintillation counting | Bioorg Med Chem Lett 22: 1151-5 (2012) Article DOI: 10.1016/j.bmcl.2011.11.097 BindingDB Entry DOI: 10.7270/Q237795K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 3A (Homo sapiens (Human)) | BDBM50363287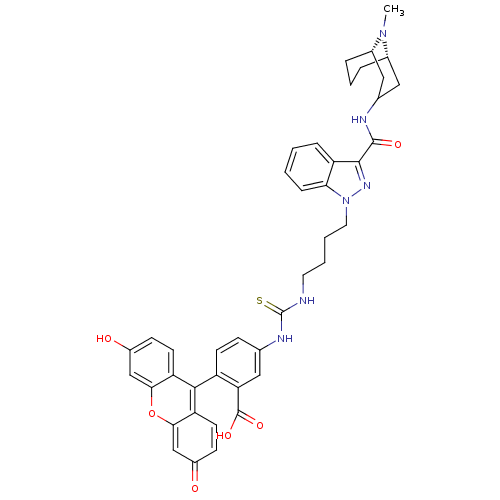 (CHEMBL1945830) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bern Curated by ChEMBL | Assay Description Displacement of [3H]granisetron from human 5HT3A expressed in HEK293 cells after 1 hr by scintillation counting | Bioorg Med Chem Lett 22: 1151-5 (2012) Article DOI: 10.1016/j.bmcl.2011.11.097 BindingDB Entry DOI: 10.7270/Q237795K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 3A (Homo sapiens (Human)) | BDBM50363291 (CHEMBL1945835) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bern Curated by ChEMBL | Assay Description Displacement of [3H]granisetron from human 5HT3A expressed in HEK293 cells after 1 hr by scintillation counting | Bioorg Med Chem Lett 22: 1151-5 (2012) Article DOI: 10.1016/j.bmcl.2011.11.097 BindingDB Entry DOI: 10.7270/Q237795K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 3A (Homo sapiens (Human)) | BDBM50000483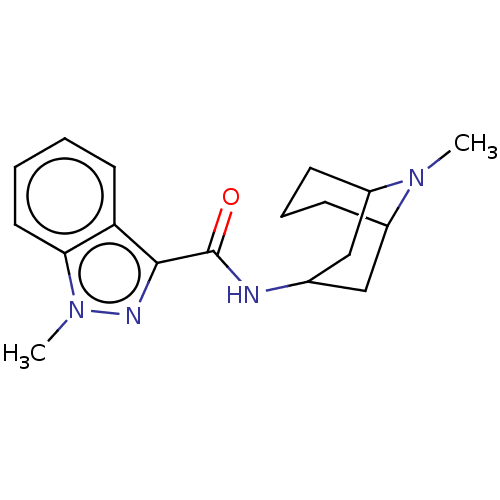 ((BRL 43694)1-Methyl-1H-indazole-3-carboxylic acid ...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank PC cid PC sid PDB UniChem Patents Similars | DrugBank PDB Article PubMed | 1.45 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bern Curated by ChEMBL | Assay Description Displacement of [3H]granisetron from human 5HT3A expressed in HEK293 cells after 1 hr by scintillation counting | Bioorg Med Chem Lett 22: 1151-5 (2012) Article DOI: 10.1016/j.bmcl.2011.11.097 BindingDB Entry DOI: 10.7270/Q237795K | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| 5-hydroxytryptamine receptor 3A (Homo sapiens (Human)) | BDBM50000483 ((BRL 43694)1-Methyl-1H-indazole-3-carboxylic acid ...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank PC cid PC sid PDB UniChem Patents Similars | DrugBank PDB Article PubMed | 1.45 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Warwick Curated by ChEMBL | Assay Description Displacement of [3H]granisetron from human 5HT3A receptor expressed in HEK293 cells by scintillation counting | J Med Chem 53: 2324-8 (2010) Article DOI: 10.1021/jm901827x BindingDB Entry DOI: 10.7270/Q2JQ11Z5 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| 5-hydroxytryptamine receptor 3A (Homo sapiens (Human)) | BDBM50363292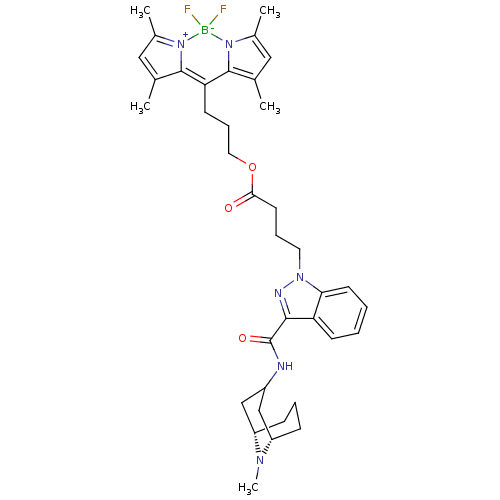 (CHEMBL1945836) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bern Curated by ChEMBL | Assay Description Displacement of [3H]granisetron from human 5HT3A expressed in HEK293 cells after 1 hr by scintillation counting | Bioorg Med Chem Lett 22: 1151-5 (2012) Article DOI: 10.1016/j.bmcl.2011.11.097 BindingDB Entry DOI: 10.7270/Q237795K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 3A (Homo sapiens (Human)) | BDBM50363285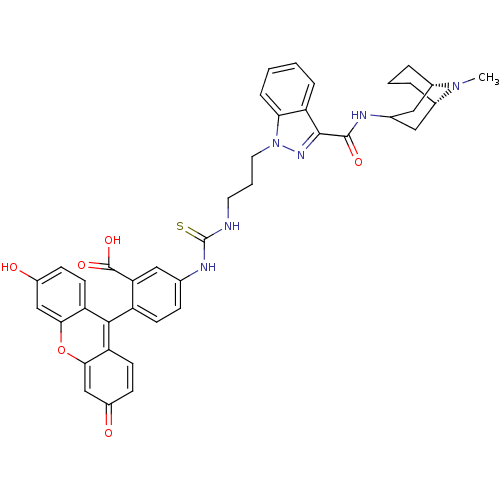 (CHEMBL1945713) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bern Curated by ChEMBL | Assay Description Displacement of [3H]granisetron from human 5HT3A expressed in HEK293 cells after 1 hr by scintillation counting | Bioorg Med Chem Lett 22: 1151-5 (2012) Article DOI: 10.1016/j.bmcl.2011.11.097 BindingDB Entry DOI: 10.7270/Q237795K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 3A (Homo sapiens (Human)) | BDBM50307828 (1-(3-Aminopropyl)-N-[(3-endo)-9-methyl-9-azabicycl...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.89 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Warwick Curated by ChEMBL | Assay Description Displacement of [3H]granisetron from human 5HT3A receptor expressed in HEK293 cells by scintillation counting | J Med Chem 53: 2324-8 (2010) Article DOI: 10.1021/jm901827x BindingDB Entry DOI: 10.7270/Q2JQ11Z5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 3A (Homo sapiens (Human)) | BDBM50363293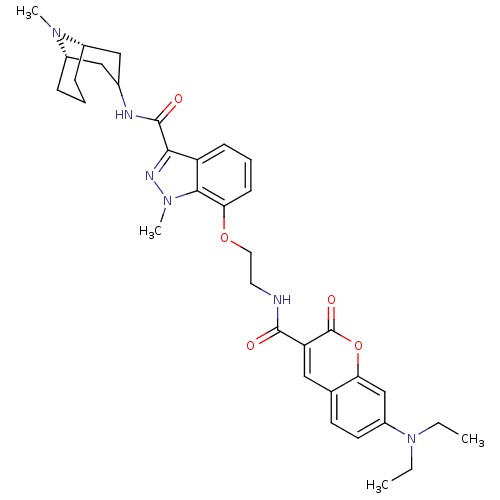 (CHEMBL1945837) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bern Curated by ChEMBL | Assay Description Displacement of [3H]granisetron from human 5HT3A expressed in HEK293 cells after 1 hr by scintillation counting | Bioorg Med Chem Lett 22: 1151-5 (2012) Article DOI: 10.1016/j.bmcl.2011.11.097 BindingDB Entry DOI: 10.7270/Q237795K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 3A (Homo sapiens (Human)) | BDBM50307829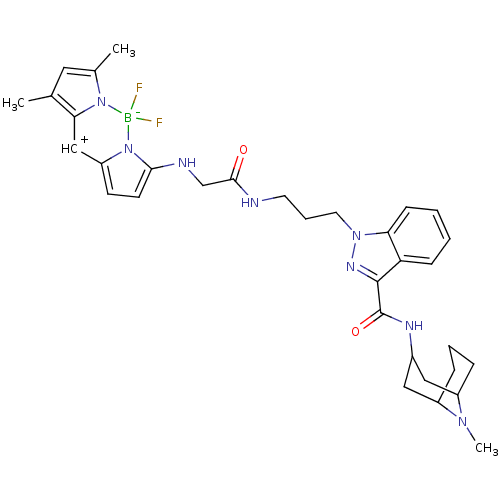 (5,5-difluoro-7,9-dimethyl-3-(2-(3-(3-((1S,5S)-9-me...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 2.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Warwick Curated by ChEMBL | Assay Description Binding affinity to human 5HT3A receptor in HEK293 cells assessed as cell labeling by confocal microscopy | J Med Chem 53: 2324-8 (2010) Article DOI: 10.1021/jm901827x BindingDB Entry DOI: 10.7270/Q2JQ11Z5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 3A (Homo sapiens (Human)) | BDBM50363286 (CHEMBL1945714) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 7.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bern Curated by ChEMBL | Assay Description Displacement of [3H]granisetron from human 5HT3A expressed in HEK293 cells after 1 hr by scintillation counting | Bioorg Med Chem Lett 22: 1151-5 (2012) Article DOI: 10.1016/j.bmcl.2011.11.097 BindingDB Entry DOI: 10.7270/Q237795K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 3A (Homo sapiens (Human)) | BDBM50307818 (5-Hydroxy-1-methyl-N-[(3-endo)-9-methyl-9-azabicyc...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 7.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Warwick Curated by ChEMBL | Assay Description Displacement of [3H]granisetron from human 5HT3A receptor expressed in HEK293 cells by scintillation counting | J Med Chem 53: 2324-8 (2010) Article DOI: 10.1021/jm901827x BindingDB Entry DOI: 10.7270/Q2JQ11Z5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 3A (Homo sapiens (Human)) | BDBM50363290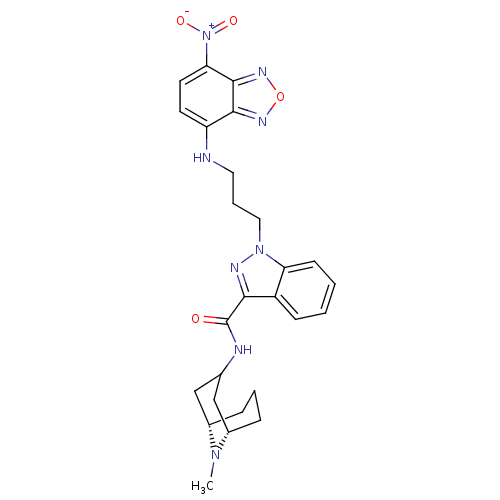 (CHEMBL1945834) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 8.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bern Curated by ChEMBL | Assay Description Displacement of [3H]granisetron from human 5HT3A expressed in HEK293 cells after 1 hr by scintillation counting | Bioorg Med Chem Lett 22: 1151-5 (2012) Article DOI: 10.1016/j.bmcl.2011.11.097 BindingDB Entry DOI: 10.7270/Q237795K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 3A (Homo sapiens (Human)) | BDBM50307816 (4-Methoxy-1-methyl-N-[(3-endo)-9-methyl-9-azabicyc...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 26.3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Warwick Curated by ChEMBL | Assay Description Displacement of [3H]granisetron from human 5HT3A receptor expressed in HEK293 cells by scintillation counting | J Med Chem 53: 2324-8 (2010) Article DOI: 10.1021/jm901827x BindingDB Entry DOI: 10.7270/Q2JQ11Z5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 3A (Homo sapiens (Human)) | BDBM50307827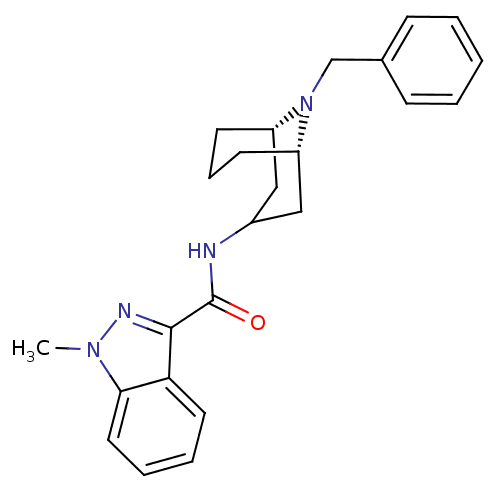 (1-Methyl-N-[(3-endo)-9-(phenylmethyl)-9-azabicyclo...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 59.3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Warwick Curated by ChEMBL | Assay Description Displacement of [3H]granisetron from human 5HT3A receptor expressed in HEK293 cells by scintillation counting | J Med Chem 53: 2324-8 (2010) Article DOI: 10.1021/jm901827x BindingDB Entry DOI: 10.7270/Q2JQ11Z5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 3A (Homo sapiens (Human)) | BDBM50307825 (7-Methoxy-1-methyl-N-[(3-endo)-9-methyl-9-azabicyc...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 71.1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Warwick Curated by ChEMBL | Assay Description Displacement of [3H]granisetron from human 5HT3A receptor expressed in HEK293 cells by scintillation counting | J Med Chem 53: 2324-8 (2010) Article DOI: 10.1021/jm901827x BindingDB Entry DOI: 10.7270/Q2JQ11Z5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 3A (Homo sapiens (Human)) | BDBM50363288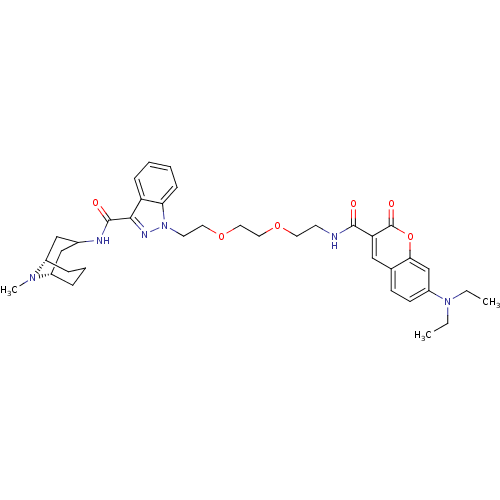 (CHEMBL1945832) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 142 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bern Curated by ChEMBL | Assay Description Displacement of [3H]granisetron from human 5HT3A expressed in HEK293 cells after 1 hr by scintillation counting | Bioorg Med Chem Lett 22: 1151-5 (2012) Article DOI: 10.1016/j.bmcl.2011.11.097 BindingDB Entry DOI: 10.7270/Q237795K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 3A (Homo sapiens (Human)) | BDBM50363294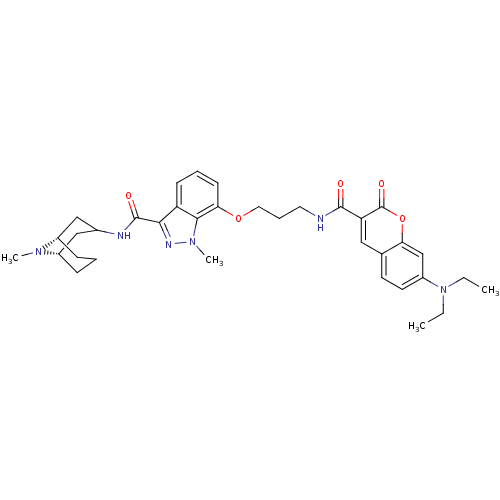 (CHEMBL1945838) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 157 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bern Curated by ChEMBL | Assay Description Displacement of [3H]granisetron from human 5HT3A expressed in HEK293 cells after 1 hr by scintillation counting | Bioorg Med Chem Lett 22: 1151-5 (2012) Article DOI: 10.1016/j.bmcl.2011.11.097 BindingDB Entry DOI: 10.7270/Q237795K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 3A (Homo sapiens (Human)) | BDBM50363284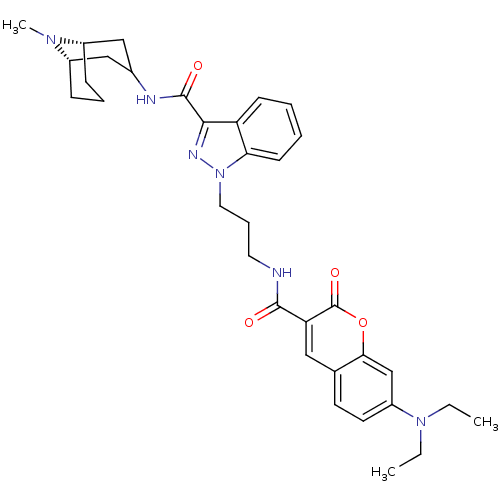 (CHEMBL1945712) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 199 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bern Curated by ChEMBL | Assay Description Displacement of [3H]granisetron from human 5HT3A expressed in HEK293 cells after 1 hr by scintillation counting | Bioorg Med Chem Lett 22: 1151-5 (2012) Article DOI: 10.1016/j.bmcl.2011.11.097 BindingDB Entry DOI: 10.7270/Q237795K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 3A (Homo sapiens (Human)) | BDBM50363296 (CHEMBL1946302) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 208 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bern Curated by ChEMBL | Assay Description Displacement of [3H]granisetron from human 5HT3A expressed in HEK293 cells after 1 hr by scintillation counting | Bioorg Med Chem Lett 22: 1151-5 (2012) Article DOI: 10.1016/j.bmcl.2011.11.097 BindingDB Entry DOI: 10.7270/Q237795K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 3A (Homo sapiens (Human)) | BDBM50307822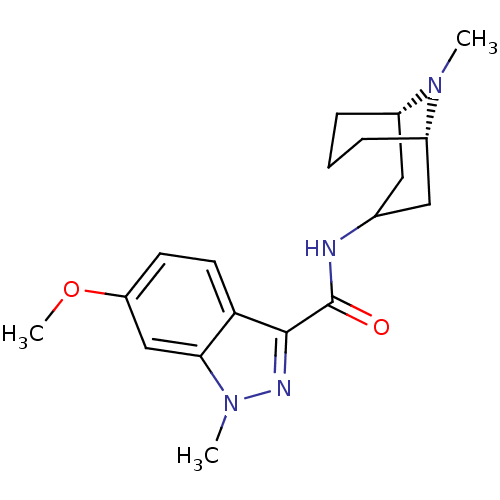 (6-Methoxy-1-methyl-N-[(3-endo)-9-methyl-9-azabicyc...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 237 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Warwick Curated by ChEMBL | Assay Description Displacement of [3H]granisetron from human 5HT3A receptor expressed in HEK293 cells by scintillation counting | J Med Chem 53: 2324-8 (2010) Article DOI: 10.1021/jm901827x BindingDB Entry DOI: 10.7270/Q2JQ11Z5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 3A (Homo sapiens (Human)) | BDBM50307821 (6-Hydroxy-1-methyl-N-[(3-endo)-9-methyl-9-azabicyc...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 279 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Warwick Curated by ChEMBL | Assay Description Displacement of [3H]granisetron from human 5HT3A receptor expressed in HEK293 cells by scintillation counting | J Med Chem 53: 2324-8 (2010) Article DOI: 10.1021/jm901827x BindingDB Entry DOI: 10.7270/Q2JQ11Z5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 3A (Homo sapiens (Human)) | BDBM50307817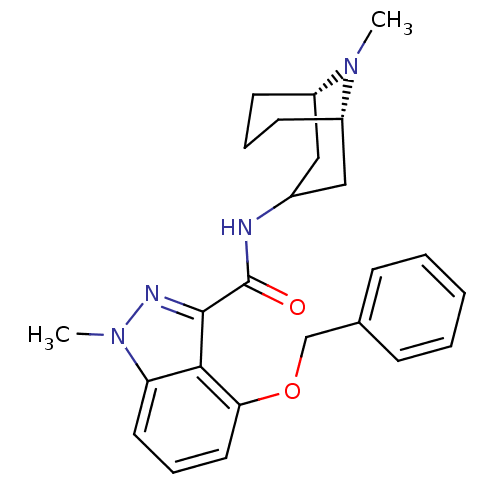 (1-Methyl-N-[(3-endo)-9-methyl-9-azabicyclo[3.3.1]n...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 375 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Warwick Curated by ChEMBL | Assay Description Displacement of [3H]granisetron from human 5HT3A receptor expressed in HEK293 cells by scintillation counting | J Med Chem 53: 2324-8 (2010) Article DOI: 10.1021/jm901827x BindingDB Entry DOI: 10.7270/Q2JQ11Z5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 3A (Homo sapiens (Human)) | BDBM50307823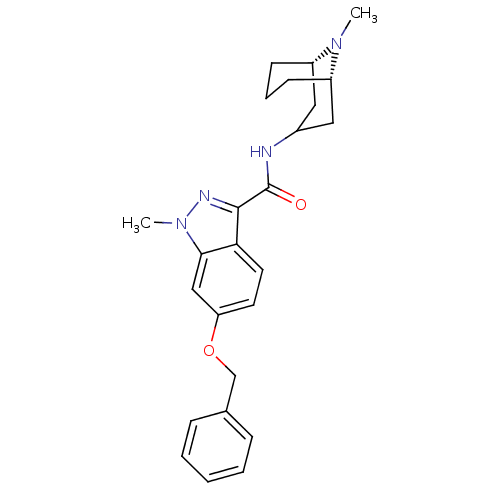 (1-Methyl-N-[(3-endo)-9-methyl-9-azabicyclo[3.3.1]n...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 749 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Warwick Curated by ChEMBL | Assay Description Displacement of [3H]granisetron from human 5HT3A receptor expressed in HEK293 cells by scintillation counting | J Med Chem 53: 2324-8 (2010) Article DOI: 10.1021/jm901827x BindingDB Entry DOI: 10.7270/Q2JQ11Z5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 3A (Homo sapiens (Human)) | BDBM50363295 (CHEMBL1946154) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bern Curated by ChEMBL | Assay Description Displacement of [3H]granisetron from human 5HT3A expressed in HEK293 cells after 1 hr by scintillation counting | Bioorg Med Chem Lett 22: 1151-5 (2012) Article DOI: 10.1016/j.bmcl.2011.11.097 BindingDB Entry DOI: 10.7270/Q237795K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 3A (Homo sapiens (Human)) | BDBM50307820 (1-Methyl-N-[(3-endo)-9-methyl-9-azabicyclo[3.3.1]n...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 3.03E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Warwick Curated by ChEMBL | Assay Description Displacement of [3H]granisetron from human 5HT3A receptor expressed in HEK293 cells by scintillation counting | J Med Chem 53: 2324-8 (2010) Article DOI: 10.1021/jm901827x BindingDB Entry DOI: 10.7270/Q2JQ11Z5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 3A (Homo sapiens (Human)) | BDBM50307819 (5-Methoxy-1-methyl-N-[(3-endo)-9-methyl-9-azabicyc...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 5.31E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Warwick Curated by ChEMBL | Assay Description Displacement of [3H]granisetron from human 5HT3A receptor expressed in HEK293 cells by scintillation counting | J Med Chem 53: 2324-8 (2010) Article DOI: 10.1021/jm901827x BindingDB Entry DOI: 10.7270/Q2JQ11Z5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 3A (Homo sapiens (Human)) | BDBM50363289 (CHEMBL1945833) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 6.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bern Curated by ChEMBL | Assay Description Displacement of [3H]granisetron from human 5HT3A expressed in HEK293 cells after 1 hr by scintillation counting | Bioorg Med Chem Lett 22: 1151-5 (2012) Article DOI: 10.1016/j.bmcl.2011.11.097 BindingDB Entry DOI: 10.7270/Q237795K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase (Human immunodeficiency virus 1) | BDBM50075207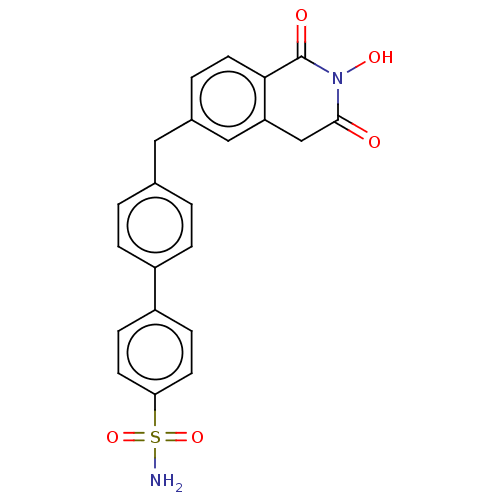 (CHEMBL3414871) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 200 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Minnesota Curated by ChEMBL | Assay Description Inhibition of HIV1 reverse transcriptase L100I/K103N mutant polymerase activity using [3H]TTP and poly(rA)-oligo(dT)16 substrate incubated for 20 min... | J Med Chem 58: 651-64 (2015) Article DOI: 10.1021/jm501132s BindingDB Entry DOI: 10.7270/Q2W95BWG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase (Human immunodeficiency virus 1) | BDBM50075207 (CHEMBL3414871) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 200 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Minnesota Curated by ChEMBL | Assay Description Inhibition of HIV1 reverse transcriptase Y181C mutant associated RNase H domain activity assessed as reduction in internal cleavage using HTS-1 RNA/D... | J Med Chem 58: 651-64 (2015) Article DOI: 10.1021/jm501132s BindingDB Entry DOI: 10.7270/Q2W95BWG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase (Human immunodeficiency virus 1) | BDBM50075204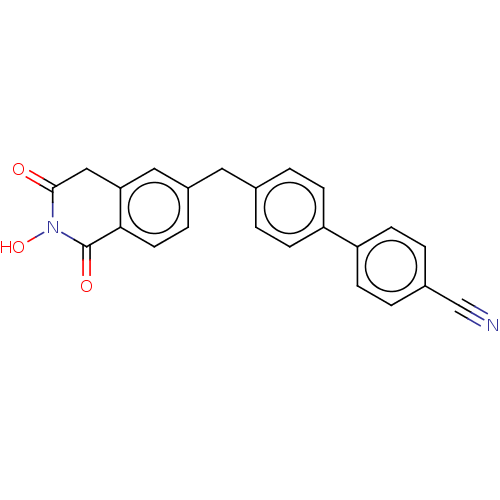 (CHEMBL3414868) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 300 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Minnesota Curated by ChEMBL | Assay Description Inhibition of HIV1 reverse transcriptase L100I/K103N mutant polymerase activity using [3H]TTP and poly(rA)-oligo(dT)16 substrate incubated for 20 min... | J Med Chem 58: 651-64 (2015) Article DOI: 10.1021/jm501132s BindingDB Entry DOI: 10.7270/Q2W95BWG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase (Human immunodeficiency virus 1) | BDBM50075207 (CHEMBL3414871) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 300 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Minnesota Curated by ChEMBL | Assay Description Inhibition of HIV1 reverse transcriptase L100I/K103N mutant associated RNase H domain activity assessed as reduction in reduction in DNA 3' end direc... | J Med Chem 58: 651-64 (2015) Article DOI: 10.1021/jm501132s BindingDB Entry DOI: 10.7270/Q2W95BWG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase (Human immunodeficiency virus 1) | BDBM50075207 (CHEMBL3414871) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 300 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Minnesota Curated by ChEMBL | Assay Description Inhibition of HIV1 reverse transcriptase Y181C mutant polymerase activity using [3H]TTP and poly(rA)-oligo(dT)16 substrate incubated for 20 mins by l... | J Med Chem 58: 651-64 (2015) Article DOI: 10.1021/jm501132s BindingDB Entry DOI: 10.7270/Q2W95BWG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase (Human immunodeficiency virus 1) | BDBM50075207 (CHEMBL3414871) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 300 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Minnesota Curated by ChEMBL | Assay Description Inhibition of HIV1 reverse transcriptase Y181C mutant associated RNase H domain activity assessed as reduction in reduction in DNA 3' end directed cl... | J Med Chem 58: 651-64 (2015) Article DOI: 10.1021/jm501132s BindingDB Entry DOI: 10.7270/Q2W95BWG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase/RNaseH (Human immunodeficiency virus 1) | BDBM50075207 (CHEMBL3414871) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 400 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Minnesota Curated by ChEMBL | Assay Description Inhibition of reconstituted HIV1 RNase H using RNA/DNA duplex substrate by fluorescence assay | J Med Chem 58: 651-64 (2015) Article DOI: 10.1021/jm501132s BindingDB Entry DOI: 10.7270/Q2W95BWG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase (Human immunodeficiency virus 1) | BDBM50075207 (CHEMBL3414871) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 400 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Minnesota Curated by ChEMBL | Assay Description Inhibition of HIV1 reverse transcriptase L100I/K103N mutant associated RNase H domain activity assessed as reduction in internal cleavage using HTS-1... | J Med Chem 58: 651-64 (2015) Article DOI: 10.1021/jm501132s BindingDB Entry DOI: 10.7270/Q2W95BWG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase/RNaseH (Human immunodeficiency virus 1) | BDBM50075207 (CHEMBL3414871) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 400 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Minnesota Curated by ChEMBL | Assay Description Inhibition of HIV1 recombinant reverse transcriptase associated catalytically active RNase H domain assessed as reduction in internal cleavage using ... | J Med Chem 58: 651-64 (2015) Article DOI: 10.1021/jm501132s BindingDB Entry DOI: 10.7270/Q2W95BWG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase (Human immunodeficiency virus 1) | BDBM50075207 (CHEMBL3414871) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 500 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Minnesota Curated by ChEMBL | Assay Description Inhibition of HIV1 recombinant reverse transcriptase polymerase activity using [3H]TTP and poly(rA)-oligo(dT)16 substrate incubated for 20 mins by li... | J Med Chem 58: 651-64 (2015) Article DOI: 10.1021/jm501132s BindingDB Entry DOI: 10.7270/Q2W95BWG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase/RNaseH (Human immunodeficiency virus 1) | BDBM33410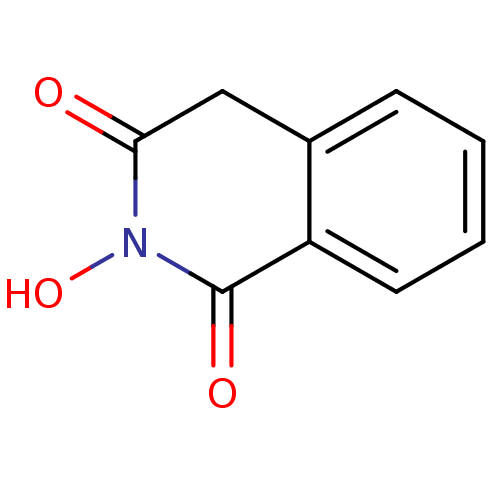 (CHEMBL16755 | N-hydroxyisoquinolinedione, 2) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL MCE MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 500 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Minnesota Curated by ChEMBL | Assay Description Inhibition of reconstituted HIV1 RNase H using RNA/DNA duplex substrate by fluorescence assay | J Med Chem 58: 651-64 (2015) Article DOI: 10.1021/jm501132s BindingDB Entry DOI: 10.7270/Q2W95BWG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase/RNaseH (Human immunodeficiency virus 1) | BDBM50075204 (CHEMBL3414868) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 500 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Minnesota Curated by ChEMBL | Assay Description Inhibition of reconstituted HIV1 RNase H using RNA/DNA duplex substrate by fluorescence assay | J Med Chem 58: 651-64 (2015) Article DOI: 10.1021/jm501132s BindingDB Entry DOI: 10.7270/Q2W95BWG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase/RNaseH (Human immunodeficiency virus 1) | BDBM50075209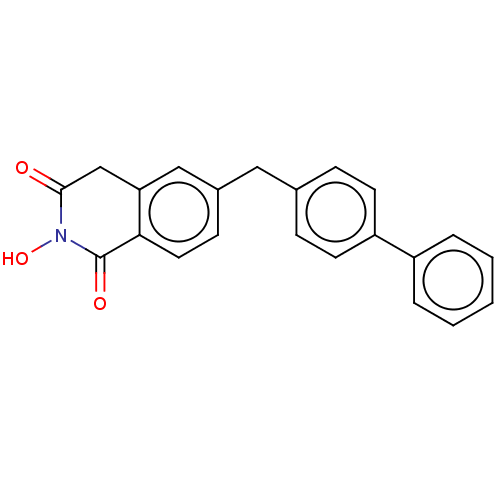 (CHEMBL3414865) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 500 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Minnesota Curated by ChEMBL | Assay Description Inhibition of reconstituted HIV1 RNase H using RNA/DNA duplex substrate by fluorescence assay | J Med Chem 58: 651-64 (2015) Article DOI: 10.1021/jm501132s BindingDB Entry DOI: 10.7270/Q2W95BWG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase/RNaseH (Human immunodeficiency virus 1) | BDBM50075216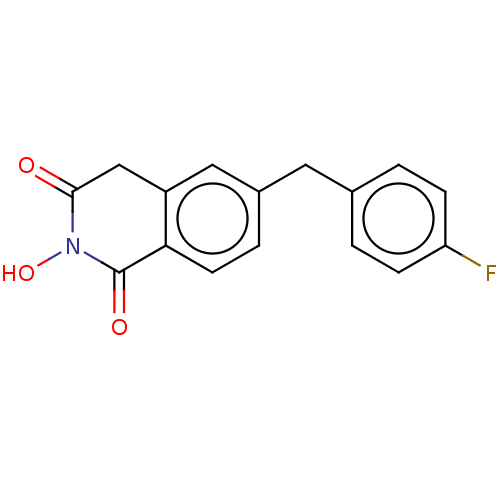 (CHEMBL3414859) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 500 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Minnesota Curated by ChEMBL | Assay Description Inhibition of reconstituted HIV1 RNase H using RNA/DNA duplex substrate by fluorescence assay | J Med Chem 58: 651-64 (2015) Article DOI: 10.1021/jm501132s BindingDB Entry DOI: 10.7270/Q2W95BWG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase/RNaseH (Human immunodeficiency virus 1) | BDBM50075207 (CHEMBL3414871) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 500 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Minnesota Curated by ChEMBL | Assay Description Inhibition of HIV1 recombinant reverse transcriptase associated catalytically active RNase H domain assessed as reduction in RNA 5' end directed clea... | J Med Chem 58: 651-64 (2015) Article DOI: 10.1021/jm501132s BindingDB Entry DOI: 10.7270/Q2W95BWG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase/RNaseH (Human immunodeficiency virus 1) | BDBM50075214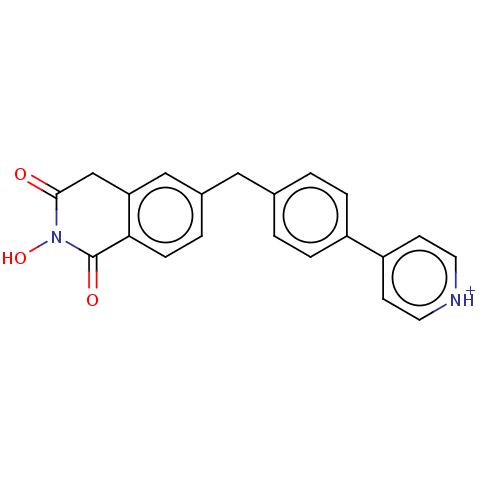 (CHEMBL3414876) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 500 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Minnesota Curated by ChEMBL | Assay Description Inhibition of HIV1 recombinant reverse transcriptase associated catalytically active RNase H domain assessed as reduction in internal cleavage using ... | J Med Chem 58: 651-64 (2015) Article DOI: 10.1021/jm501132s BindingDB Entry DOI: 10.7270/Q2W95BWG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase/RNaseH (Human immunodeficiency virus 1) | BDBM50075207 (CHEMBL3414871) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 500 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Minnesota Curated by ChEMBL | Assay Description Inhibition of HIV1 recombinant reverse transcriptase associated catalytically active RNase H domain assessed as reduction in DNA 3' end directed clea... | J Med Chem 58: 651-64 (2015) Article DOI: 10.1021/jm501132s BindingDB Entry DOI: 10.7270/Q2W95BWG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase (Human immunodeficiency virus 1) | BDBM50075206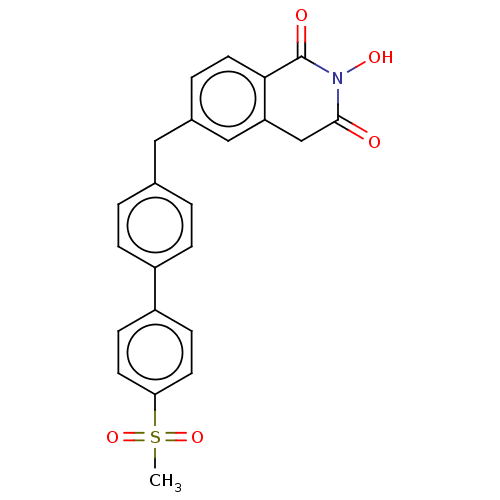 (CHEMBL3414870) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 500 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Minnesota Curated by ChEMBL | Assay Description Inhibition of HIV1 reverse transcriptase L100I/K103N mutant polymerase activity using [3H]TTP and poly(rA)-oligo(dT)16 substrate incubated for 20 min... | J Med Chem 58: 651-64 (2015) Article DOI: 10.1021/jm501132s BindingDB Entry DOI: 10.7270/Q2W95BWG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase (Human immunodeficiency virus 1) | BDBM50075209 (CHEMBL3414865) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 500 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Minnesota Curated by ChEMBL | Assay Description Inhibition of HIV1 reverse transcriptase L100I/K103N mutant polymerase activity using [3H]TTP and poly(rA)-oligo(dT)16 substrate incubated for 20 min... | J Med Chem 58: 651-64 (2015) Article DOI: 10.1021/jm501132s BindingDB Entry DOI: 10.7270/Q2W95BWG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 284 total ) | Next | Last >> |