 Found 587 hits with Last Name = 'abrams' and Initial = 't'
Found 587 hits with Last Name = 'abrams' and Initial = 't' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Estrogen receptor
(Homo sapiens (Human)) | BDBM50169743

((13S,17S)-13-Methyl-7-[9-(4,4,5,5,5-pentafluoro-pe...)Show SMILES C[C@]12CC[C@H]3[C@H]([C@@H]1CC[C@@H]2O)[C@H](CCCCCCCCCS(=O)CCCC(F)(F)C(F)(F)F)Cc1cc(O)ccc31 |r| Show InChI InChI=1S/C32H47F5O3S/c1-30-17-15-26-25-12-11-24(38)21-23(25)20-22(29(26)27(30)13-14-28(30)39)10-7-5-3-2-4-6-8-18-41(40)19-9-16-31(33,34)32(35,36)37/h11-12,21-22,26-29,38-39H,2-10,13-20H2,1H3/t22-,26-,27+,28+,29-,30+,41?/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.0600 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Induction of ERalpha degradation in human MCF7 cells assessed as inhibition of insulin-mediated cell proliferation after 6 days by Hoechst 33258 dye-... |
J Med Chem 60: 2790-2818 (2017)
Article DOI: 10.1021/acs.jmedchem.6b01468
BindingDB Entry DOI: 10.7270/Q2DB845Q |
More data for this
Ligand-Target Pair | |
Protein farnesyltransferase subunit beta/geranylgeranyltransferase type-1 subunit alpha
(Homo sapiens (Human)) | BDBM50101929
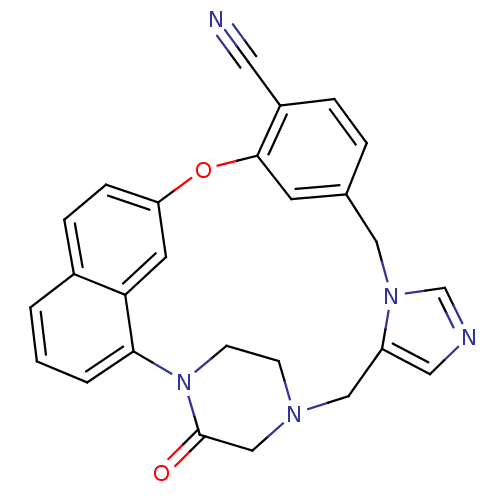
(3-oxo-18-oxa-2,5,9,11-tetraazahexacyclo[17.6.2.22,...)Show SMILES O=C1CN2CCN1c1cccc3ccc(Oc4cc(Cn5cncc5C2)ccc4C#N)cc13 Show InChI InChI=1S/C26H21N5O2/c27-12-20-5-4-18-10-25(20)33-22-7-6-19-2-1-3-24(23(19)11-22)31-9-8-29(16-26(31)32)15-21-13-28-17-30(21)14-18/h1-7,10-11,13,17H,8-9,14-16H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human Farnesyltransferase -catalyzed incorporation of [3H]-FPP into recombinant Ras-CVIM. |
Bioorg Med Chem Lett 14: 639-43 (2004)
BindingDB Entry DOI: 10.7270/Q2WM1CTK |
More data for this
Ligand-Target Pair | 
3D Structure (docked) |
Protein farnesyltransferase subunit beta/geranylgeranyltransferase type-1 subunit alpha
(Homo sapiens (Human)) | BDBM50139186
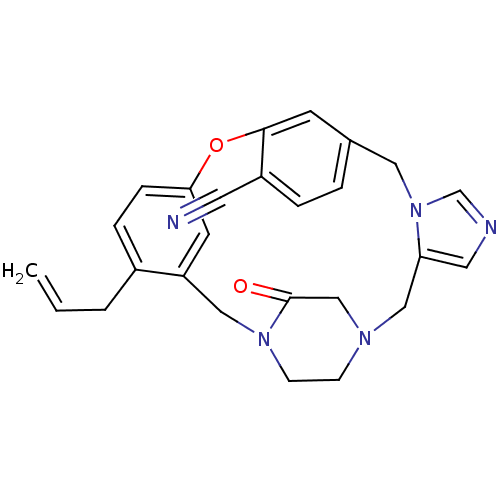
(4-allyl-23-oxo-8-oxa-1,15,17,21-tetraazapentacyclo...)Show SMILES C=CCc1ccc2Oc3cc(Cn4cncc4CN4CCN(Cc1c2)C(=O)C4)ccc3C#N Show InChI InChI=1S/C26H25N5O2/c1-2-3-20-6-7-24-11-22(20)15-30-9-8-29(17-26(30)32)16-23-13-28-18-31(23)14-19-4-5-21(12-27)25(10-19)33-24/h2,4-7,10-11,13,18H,1,3,8-9,14-17H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human Farnesyltransferase -catalyzed incorporation of [3H]-FPP into recombinant Ras-CVIM. |
Bioorg Med Chem Lett 14: 639-43 (2004)
BindingDB Entry DOI: 10.7270/Q2WM1CTK |
More data for this
Ligand-Target Pair | |
Protein farnesyltransferase subunit beta/geranylgeranyltransferase type-1 subunit alpha
(Homo sapiens (Human)) | BDBM50101929

(3-oxo-18-oxa-2,5,9,11-tetraazahexacyclo[17.6.2.22,...)Show SMILES O=C1CN2CCN1c1cccc3ccc(Oc4cc(Cn5cncc5C2)ccc4C#N)cc13 Show InChI InChI=1S/C26H21N5O2/c27-12-20-5-4-18-10-25(20)33-22-7-6-19-2-1-3-24(23(19)11-22)31-9-8-29(16-26(31)32)15-21-13-28-17-30(21)14-18/h1-7,10-11,13,17H,8-9,14-16H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity to reduce the human farnesyltransferase catalyzed incorporation of [3H]-FPP into recombinant Ras-CVIM |
Bioorg Med Chem Lett 11: 1817-21 (2001)
BindingDB Entry DOI: 10.7270/Q2R210PG |
More data for this
Ligand-Target Pair | 
3D Structure (docked) |
Geranylgeranyl transferase type-1 subunit beta/Protein farnesyltransferase/geranylgeranyltransferase type-1 subunit alpha
(Homo sapiens (Human)) | BDBM50115916

(4-[3-(4-Cyano-benzyl)-3H-imidazol-4-ylmethyl]-pipe...)Show SMILES FC(F)(F)Oc1ccccc1COC(=O)N1CCN(Cc2cncn2Cc2ccc(cc2)C#N)CC1 Show InChI InChI=1S/C25H24F3N5O3/c26-25(27,28)36-23-4-2-1-3-21(23)17-35-24(34)32-11-9-31(10-12-32)16-22-14-30-18-33(22)15-20-7-5-19(13-29)6-8-20/h1-8,14,18H,9-12,15-17H2 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 0.160 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of [1-3H]-GGPP incorporation into biotinylated K4B-Ras peptide by geranylgeranyl transferase in the presence of 5 mM ATP |
Bioorg Med Chem Lett 12: 2027-30 (2002)
BindingDB Entry DOI: 10.7270/Q2TQ60VS |
More data for this
Ligand-Target Pair | |
Estrogen receptor
(Homo sapiens (Human)) | BDBM269467
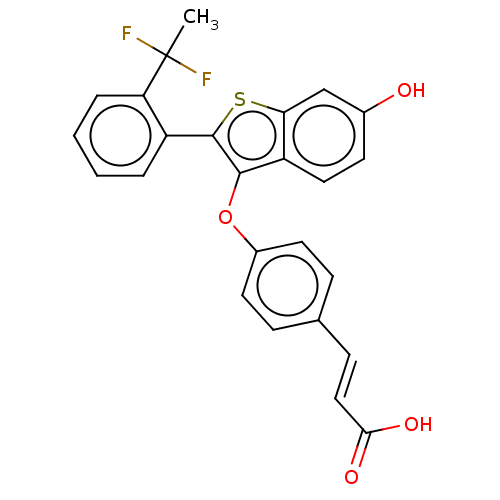
((E)-3-(4-((2-(2-(1,1- difluoroethyl)phenyl)-6- hyd...)Show SMILES CC(F)(F)c1ccccc1-c1sc2cc(O)ccc2c1Oc1ccc(\C=C\C(O)=O)cc1 Show InChI InChI=1S/C25H18F2O4S/c1-25(26,27)20-5-3-2-4-18(20)24-23(19-12-9-16(28)14-21(19)32-24)31-17-10-6-15(7-11-17)8-13-22(29)30/h2-14,28H,1H3,(H,29,30)/b13-8+ | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Induction of selective estrogen receptor alpha degradation in human MCF7 cells after 18 to 24 hrs by in-cell Western analysis |
J Med Chem 61: 2837-2864 (2018)
Article DOI: 10.1021/acs.jmedchem.7b01682
BindingDB Entry DOI: 10.7270/Q2TB1986 |
More data for this
Ligand-Target Pair | |
Estrogen receptor
(Homo sapiens (Human)) | BDBM269484
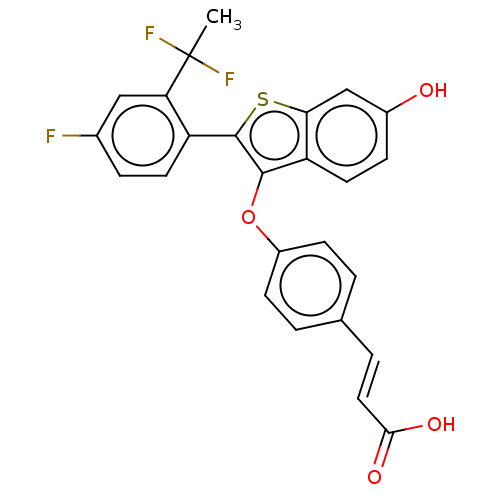
(US10058534, 139)Show SMILES CC(F)(F)c1cc(F)ccc1-c1sc2cc(O)ccc2c1Oc1ccc(\C=C\C(O)=O)cc1 Show InChI InChI=1S/C25H17F3O4S/c1-25(27,28)20-12-15(26)5-9-18(20)24-23(19-10-6-16(29)13-21(19)33-24)32-17-7-2-14(3-8-17)4-11-22(30)31/h2-13,29H,1H3,(H,30,31)/b11-4+ | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Induction of selective estrogen receptor alpha degradation in human MCF7 cells after 18 to 24 hrs by in-cell Western analysis |
J Med Chem 61: 2837-2864 (2018)
Article DOI: 10.1021/acs.jmedchem.7b01682
BindingDB Entry DOI: 10.7270/Q2TB1986 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Protein farnesyltransferase subunit beta/geranylgeranyltransferase type-1 subunit alpha
(Homo sapiens (Human)) | BDBM50139202
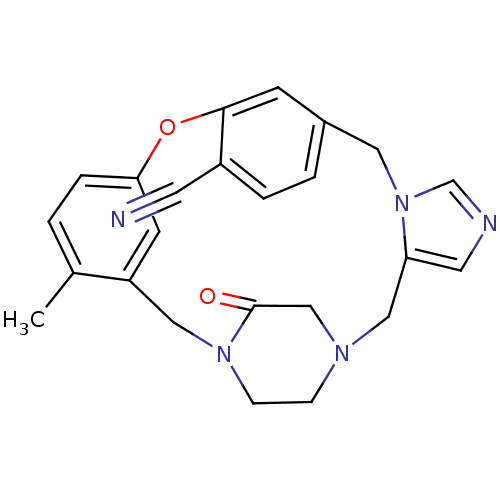
(4-methyl-23-oxo-8-oxa-1,15,17,21-tetraazapentacycl...)Show SMILES Cc1ccc2Oc3cc(Cn4cncc4CN4CCN(Cc1c2)C(=O)C4)ccc3C#N Show InChI InChI=1S/C24H23N5O2/c1-17-2-5-22-9-20(17)13-28-7-6-27(15-24(28)30)14-21-11-26-16-29(21)12-18-3-4-19(10-25)23(8-18)31-22/h2-5,8-9,11,16H,6-7,12-15H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 0.260 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human Farnesyltransferase -catalyzed incorporation of [3H]-FPP into recombinant Ras-CVIM. |
Bioorg Med Chem Lett 14: 639-43 (2004)
BindingDB Entry DOI: 10.7270/Q2WM1CTK |
More data for this
Ligand-Target Pair | |
Protein farnesyltransferase subunit beta/geranylgeranyltransferase type-1 subunit alpha
(Homo sapiens (Human)) | BDBM50101929

(3-oxo-18-oxa-2,5,9,11-tetraazahexacyclo[17.6.2.22,...)Show SMILES O=C1CN2CCN1c1cccc3ccc(Oc4cc(Cn5cncc5C2)ccc4C#N)cc13 Show InChI InChI=1S/C26H21N5O2/c27-12-20-5-4-18-10-25(20)33-22-7-6-19-2-1-3-24(23(19)11-22)31-9-8-29(16-26(31)32)15-21-13-28-17-30(21)14-18/h1-7,10-11,13,17H,8-9,14-16H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 0.290 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of radiolabeled FTI from Farnesyltransferase in cultured Ha-ras transformed RAT1 cells. |
Bioorg Med Chem Lett 14: 639-43 (2004)
BindingDB Entry DOI: 10.7270/Q2WM1CTK |
More data for this
Ligand-Target Pair | 
3D Structure (docked) |
Protein farnesyltransferase subunit beta/geranylgeranyltransferase type-1 subunit alpha
(Homo sapiens (Human)) | BDBM50139196
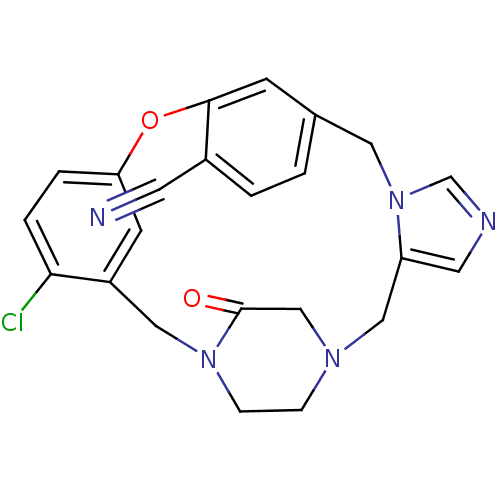
(4-chloro-23-oxo-8-oxa-1,15,17,21-tetraazapentacycl...)Show SMILES Clc1ccc2Oc3cc(Cn4cncc4CN4CCN(Cc1c2)C(=O)C4)ccc3C#N Show InChI InChI=1S/C23H20ClN5O2/c24-21-4-3-20-8-18(21)12-28-6-5-27(14-23(28)30)13-19-10-26-15-29(19)11-16-1-2-17(9-25)22(7-16)31-20/h1-4,7-8,10,15H,5-6,11-14H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of radiolabeled FTI from Farnesyltransferase in cultured Ha-ras transformed RAT1 cells. |
Bioorg Med Chem Lett 14: 639-43 (2004)
BindingDB Entry DOI: 10.7270/Q2WM1CTK |
More data for this
Ligand-Target Pair | |
Estrogen receptor
(Homo sapiens (Human)) | BDBM269456
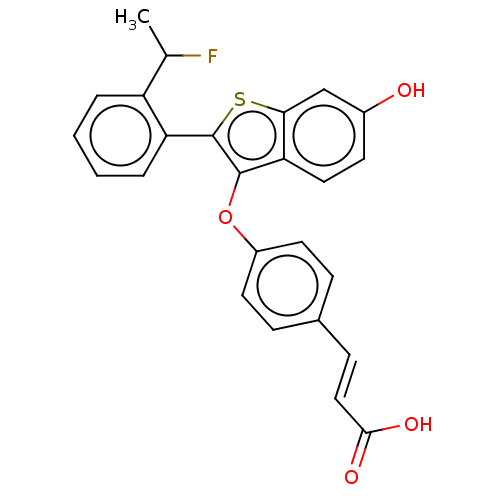
((E)-3-(4-((2-(2-(1- fluoroethyl)phenyl)-6- hydroxy...)Show SMILES CC(F)c1ccccc1-c1sc2cc(O)ccc2c1Oc1ccc(\C=C\C(O)=O)cc1 Show InChI InChI=1S/C25H19FO4S/c1-15(26)19-4-2-3-5-20(19)25-24(21-12-9-17(27)14-22(21)31-25)30-18-10-6-16(7-11-18)8-13-23(28)29/h2-15,27H,1H3,(H,28,29)/b13-8+ | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Induction of selective estrogen receptor alpha degradation in human MCF7 cells after 18 to 24 hrs by in-cell Western analysis |
J Med Chem 61: 2837-2864 (2018)
Article DOI: 10.1021/acs.jmedchem.7b01682
BindingDB Entry DOI: 10.7270/Q2TB1986 |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase Chk1
(Homo sapiens (Human)) | BDBM50223460
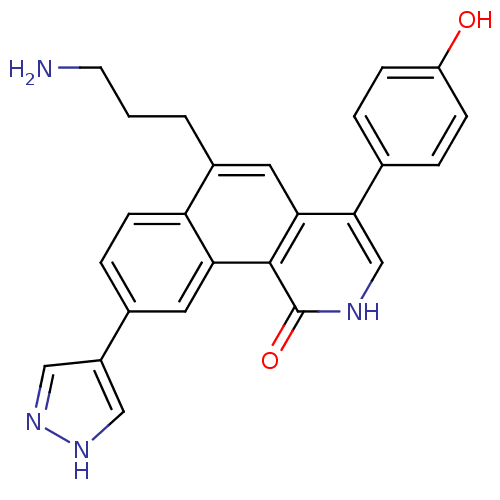
(6-(3-aminopropyl)-4-(4-hydroxyphenyl)-9-(1H-pyrazo...)Show SMILES NCCCc1cc2c(c[nH]c(=O)c2c2cc(ccc12)-c1cn[nH]c1)-c1ccc(O)cc1 Show InChI InChI=1S/C25H22N4O2/c26-9-1-2-17-11-22-23(15-3-6-19(30)7-4-15)14-27-25(31)24(22)21-10-16(5-8-20(17)21)18-12-28-29-13-18/h3-8,10-14,30H,1-2,9,26H2,(H,27,31)(H,28,29) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human Chk1 expressed in baculovirus by time-resolved fluorescence assay |
Bioorg Med Chem Lett 17: 6280-5 (2007)
Article DOI: 10.1016/j.bmcl.2007.09.007
BindingDB Entry DOI: 10.7270/Q2W958ZJ |
More data for this
Ligand-Target Pair | |
Protein farnesyltransferase subunit beta/geranylgeranyltransferase type-1 subunit alpha
(Homo sapiens (Human)) | BDBM50139190
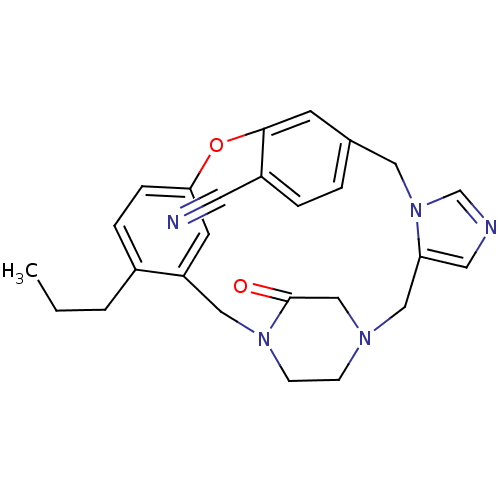
(4-propyl-23-oxo-8-oxa-1,15,17,21-tetraazapentacycl...)Show SMILES CCCc1ccc2Oc3cc(Cn4cncc4CN4CCN(Cc1c2)C(=O)C4)ccc3C#N Show InChI InChI=1S/C26H27N5O2/c1-2-3-20-6-7-24-11-22(20)15-30-9-8-29(17-26(30)32)16-23-13-28-18-31(23)14-19-4-5-21(12-27)25(10-19)33-24/h4-7,10-11,13,18H,2-3,8-9,14-17H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 0.310 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human Farnesyltransferase -catalyzed incorporation of [3H]-FPP into recombinant Ras-CVIM. |
Bioorg Med Chem Lett 14: 639-43 (2004)
BindingDB Entry DOI: 10.7270/Q2WM1CTK |
More data for this
Ligand-Target Pair | |
Geranylgeranyl transferase type-1 subunit beta/Protein farnesyltransferase/geranylgeranyltransferase type-1 subunit alpha
(Homo sapiens (Human)) | BDBM50115911
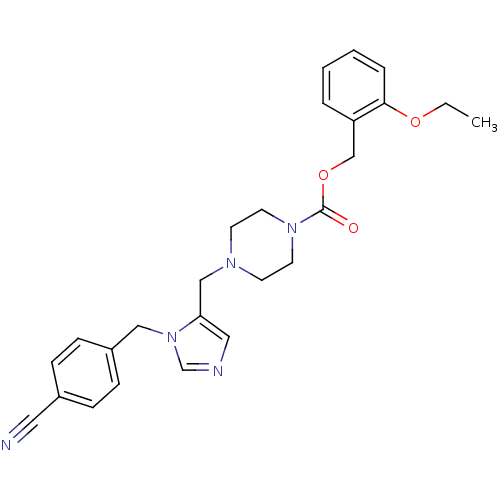
(4-[3-(4-Cyano-benzyl)-3H-imidazol-4-ylmethyl]-pipe...)Show SMILES CCOc1ccccc1COC(=O)N1CCN(Cc2cncn2Cc2ccc(cc2)C#N)CC1 Show InChI InChI=1S/C26H29N5O3/c1-2-33-25-6-4-3-5-23(25)19-34-26(32)30-13-11-29(12-14-30)18-24-16-28-20-31(24)17-22-9-7-21(15-27)8-10-22/h3-10,16,20H,2,11-14,17-19H2,1H3 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 0.320 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of [1-3H]-GGPP incorporation into biotinylated K4B-Ras peptide by geranylgeranyl transferase in the presence of 5 mM ATP |
Bioorg Med Chem Lett 12: 2027-30 (2002)
BindingDB Entry DOI: 10.7270/Q2TQ60VS |
More data for this
Ligand-Target Pair | |
Geranylgeranyl transferase type-1 subunit beta/Protein farnesyltransferase/geranylgeranyltransferase type-1 subunit alpha
(Homo sapiens (Human)) | BDBM50115919
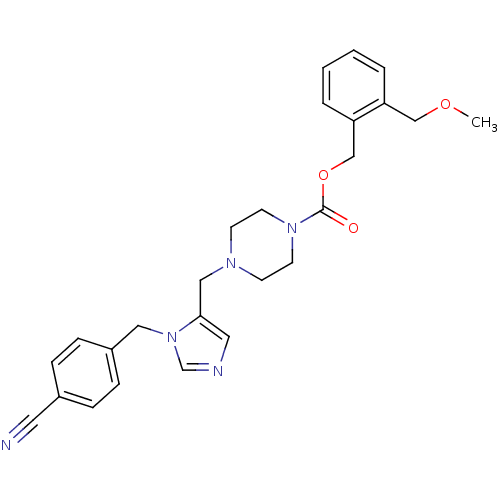
(4-[3-(4-Cyano-benzyl)-3H-imidazol-4-ylmethyl]-pipe...)Show SMILES COCc1ccccc1COC(=O)N1CCN(Cc2cncn2Cc2ccc(cc2)C#N)CC1 Show InChI InChI=1S/C26H29N5O3/c1-33-18-23-4-2-3-5-24(23)19-34-26(32)30-12-10-29(11-13-30)17-25-15-28-20-31(25)16-22-8-6-21(14-27)7-9-22/h2-9,15,20H,10-13,16-19H2,1H3 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 0.320 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of [1-3H]-GGPP incorporation into biotinylated K4B-Ras peptide by geranylgeranyl transferase in the presence of 5 mM ATP |
Bioorg Med Chem Lett 12: 2027-30 (2002)
BindingDB Entry DOI: 10.7270/Q2TQ60VS |
More data for this
Ligand-Target Pair | |
Protein farnesyltransferase subunit beta/geranylgeranyltransferase type-1 subunit alpha
(Homo sapiens (Human)) | BDBM50139202

(4-methyl-23-oxo-8-oxa-1,15,17,21-tetraazapentacycl...)Show SMILES Cc1ccc2Oc3cc(Cn4cncc4CN4CCN(Cc1c2)C(=O)C4)ccc3C#N Show InChI InChI=1S/C24H23N5O2/c1-17-2-5-22-9-20(17)13-28-7-6-27(15-24(28)30)14-21-11-26-16-29(21)12-18-3-4-19(10-25)23(8-18)31-22/h2-5,8-9,11,16H,6-7,12-15H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 0.330 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of radiolabeled FTI from Farnesyltransferase in cultured Ha-ras transformed RAT1 cells. |
Bioorg Med Chem Lett 14: 639-43 (2004)
BindingDB Entry DOI: 10.7270/Q2WM1CTK |
More data for this
Ligand-Target Pair | |
Protein farnesyltransferase subunit beta/geranylgeranyltransferase type-1 subunit alpha
(Homo sapiens (Human)) | BDBM50115927

(4-[3-(4-Cyano-benzyl)-2-methyl-3H-imidazol-4-ylmet...)Show SMILES Cc1ncc(CN2CCN(CC2)C(=O)OCc2ccccc2OC(F)(F)F)n1Cc1ccc(cc1)C#N Show InChI InChI=1S/C26H26F3N5O3/c1-19-31-15-23(34(19)16-21-8-6-20(14-30)7-9-21)17-32-10-12-33(13-11-32)25(35)36-18-22-4-2-3-5-24(22)37-26(27,28)29/h2-9,15H,10-13,16-18H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 0.330 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of Farnesyl protein transferase radiolabel [1-3H] incorporation |
Bioorg Med Chem Lett 12: 2027-30 (2002)
BindingDB Entry DOI: 10.7270/Q2TQ60VS |
More data for this
Ligand-Target Pair | |
Protein farnesyltransferase subunit beta/geranylgeranyltransferase type-1 subunit alpha
(Homo sapiens (Human)) | BDBM50115927

(4-[3-(4-Cyano-benzyl)-2-methyl-3H-imidazol-4-ylmet...)Show SMILES Cc1ncc(CN2CCN(CC2)C(=O)OCc2ccccc2OC(F)(F)F)n1Cc1ccc(cc1)C#N Show InChI InChI=1S/C26H26F3N5O3/c1-19-31-15-23(34(19)16-21-8-6-20(14-30)7-9-21)17-32-10-12-33(13-11-32)25(35)36-18-22-4-2-3-5-24(22)37-26(27,28)29/h2-9,15H,10-13,16-18H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 0.340 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of radiolabeled farnesyl transferase inhibitor |
Bioorg Med Chem Lett 12: 2027-30 (2002)
BindingDB Entry DOI: 10.7270/Q2TQ60VS |
More data for this
Ligand-Target Pair | |
Protein farnesyltransferase subunit beta/geranylgeranyltransferase type-1 subunit alpha
(Homo sapiens (Human)) | BDBM50115916

(4-[3-(4-Cyano-benzyl)-3H-imidazol-4-ylmethyl]-pipe...)Show SMILES FC(F)(F)Oc1ccccc1COC(=O)N1CCN(Cc2cncn2Cc2ccc(cc2)C#N)CC1 Show InChI InChI=1S/C25H24F3N5O3/c26-25(27,28)36-23-4-2-1-3-21(23)17-35-24(34)32-11-9-31(10-12-32)16-22-14-30-18-33(22)15-20-7-5-19(13-29)6-8-20/h1-8,14,18H,9-12,15-17H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 0.360 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibitor of Farnesyl protein transferase(FTPase) required to reduce radiolabel [1-3H] incorporation by 50% |
Bioorg Med Chem Lett 12: 2027-30 (2002)
BindingDB Entry DOI: 10.7270/Q2TQ60VS |
More data for this
Ligand-Target Pair | |
Estrogen receptor
(Homo sapiens (Human)) | BDBM50237322
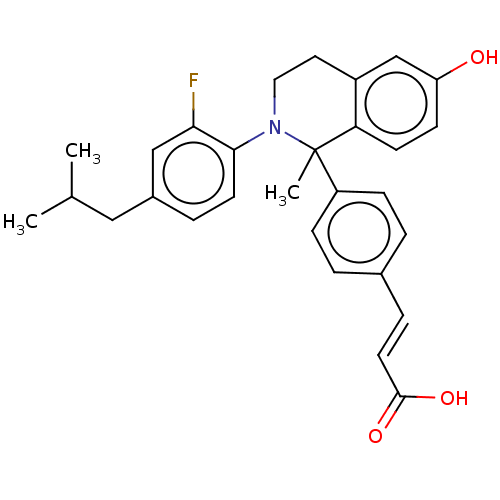
(CHEMBL4088736)Show SMILES CC(C)Cc1ccc(N2CCc3cc(O)ccc3C2(C)c2ccc(\C=C\C(O)=O)cc2)c(F)c1 Show InChI InChI=1S/C29H30FNO3/c1-19(2)16-21-6-12-27(26(30)17-21)31-15-14-22-18-24(32)10-11-25(22)29(31,3)23-8-4-20(5-9-23)7-13-28(33)34/h4-13,17-19,32H,14-16H2,1-3H3,(H,33,34)/b13-7+ | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Induction of ERalpha degradation in human MCF7 cells after 18 to 24 hrs by Western blot analysis |
J Med Chem 60: 2790-2818 (2017)
Article DOI: 10.1021/acs.jmedchem.6b01468
BindingDB Entry DOI: 10.7270/Q2DB845Q |
More data for this
Ligand-Target Pair | |
Estrogen receptor
(Homo sapiens (Human)) | BDBM50237304

(CHEMBL4093325)Show SMILES CC1(N(CCc2cc(O)ccc12)c1ccc(cc1)C1CC1)c1ccc(\C=C\C(O)=O)cc1 Show InChI InChI=1S/C28H27NO3/c1-28(23-9-2-19(3-10-23)4-15-27(31)32)26-14-13-25(30)18-22(26)16-17-29(28)24-11-7-21(8-12-24)20-5-6-20/h2-4,7-15,18,20,30H,5-6,16-17H2,1H3,(H,31,32)/b15-4+ | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
In vitro binding affinity at human cloned dopamine receptor D2 (short) stably expressed in CHO cells by [3H]spiperone displacement. |
J Med Chem 60: 2790-2818 (2017)
Article DOI: 10.1021/acs.jmedchem.6b01468
BindingDB Entry DOI: 10.7270/Q2DB845Q |
More data for this
Ligand-Target Pair | |
Estrogen receptor
(Homo sapiens (Human)) | BDBM269308
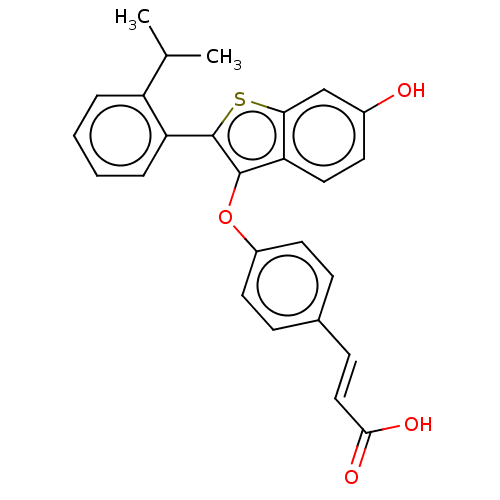
((E)-3-(4-((6-hydroxy-2- (2-isopropylphenyl)- benzo...)Show SMILES CC(C)c1ccccc1-c1sc2cc(O)ccc2c1Oc1ccc(\C=C\C(O)=O)cc1 Show InChI InChI=1S/C26H22O4S/c1-16(2)20-5-3-4-6-21(20)26-25(22-13-10-18(27)15-23(22)31-26)30-19-11-7-17(8-12-19)9-14-24(28)29/h3-16,27H,1-2H3,(H,28,29)/b14-9+ | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Induction of selective estrogen receptor alpha degradation in human MCF7 cells after 18 to 24 hrs by in-cell Western analysis |
J Med Chem 61: 2837-2864 (2018)
Article DOI: 10.1021/acs.jmedchem.7b01682
BindingDB Entry DOI: 10.7270/Q2TB1986 |
More data for this
Ligand-Target Pair | |
Protein farnesyltransferase subunit beta/geranylgeranyltransferase type-1 subunit alpha
(Homo sapiens (Human)) | BDBM50139190

(4-propyl-23-oxo-8-oxa-1,15,17,21-tetraazapentacycl...)Show SMILES CCCc1ccc2Oc3cc(Cn4cncc4CN4CCN(Cc1c2)C(=O)C4)ccc3C#N Show InChI InChI=1S/C26H27N5O2/c1-2-3-20-6-7-24-11-22(20)15-30-9-8-29(17-26(30)32)16-23-13-28-18-31(23)14-19-4-5-21(12-27)25(10-19)33-24/h4-7,10-11,13,18H,2-3,8-9,14-17H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 0.440 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of radiolabeled FTI from Farnesyltransferase in cultured Ha-ras transformed RAT1 cells. |
Bioorg Med Chem Lett 14: 639-43 (2004)
BindingDB Entry DOI: 10.7270/Q2WM1CTK |
More data for this
Ligand-Target Pair | |
Protein farnesyltransferase subunit beta/geranylgeranyltransferase type-1 subunit alpha
(Homo sapiens (Human)) | BDBM50139189
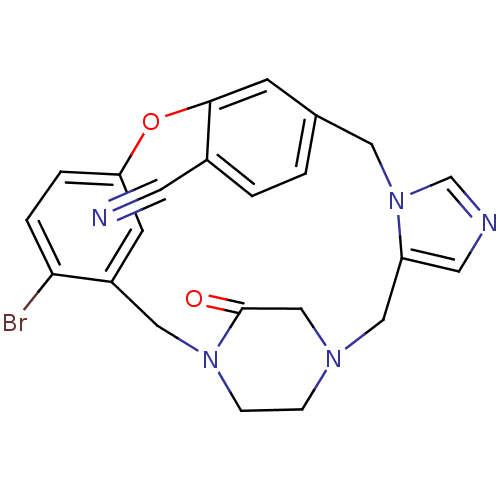
(4-bromo-23-oxo-8-oxa-1,15,17,21-tetraazapentacyclo...)Show SMILES Brc1ccc2Oc3cc(Cn4cncc4CN4CCN(Cc1c2)C(=O)C4)ccc3C#N Show InChI InChI=1S/C23H20BrN5O2/c24-21-4-3-20-8-18(21)12-28-6-5-27(14-23(28)30)13-19-10-26-15-29(19)11-16-1-2-17(9-25)22(7-16)31-20/h1-4,7-8,10,15H,5-6,11-14H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 0.450 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human Farnesyltransferase -catalyzed incorporation of [3H]-FPP into recombinant Ras-CVIM. |
Bioorg Med Chem Lett 14: 639-43 (2004)
BindingDB Entry DOI: 10.7270/Q2WM1CTK |
More data for this
Ligand-Target Pair | |
Protein farnesyltransferase subunit beta/geranylgeranyltransferase type-1 subunit alpha
(Homo sapiens (Human)) | BDBM50101925

(16-benzyl-17-oxo-(16R)-2-oxa-9,11,15,18-tetraazape...)Show SMILES O=C1Nc2cccc3ccc(Oc4cc(Cn5cncc5CN[C@@H]1Cc1ccccc1)ccc4C#N)cc23 Show InChI InChI=1S/C31H25N5O2/c32-16-24-10-9-22-14-30(24)38-26-12-11-23-7-4-8-28(27(23)15-26)35-31(37)29(13-21-5-2-1-3-6-21)34-18-25-17-33-20-36(25)19-22/h1-12,14-15,17,20,29,34H,13,18-19H2,(H,35,37)/t29-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 0.470 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity to reduce the human farnesyltransferase catalyzed incorporation of [3H]-FPP into recombinant Ras-CVIM |
Bioorg Med Chem Lett 11: 1817-21 (2001)
BindingDB Entry DOI: 10.7270/Q2R210PG |
More data for this
Ligand-Target Pair | |
Protein farnesyltransferase subunit beta/geranylgeranyltransferase type-1 subunit alpha
(Homo sapiens (Human)) | BDBM50139195
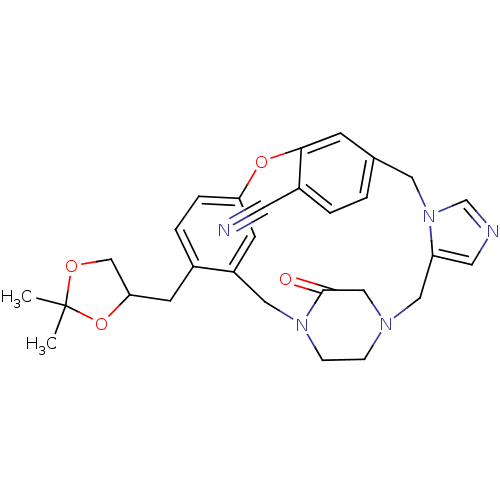
(4-(2,2-dimethyl-1,3-dioxolan-4-ylmethyl)-23-oxo-8-...)Show SMILES CC1(C)OCC(Cc2ccc3Oc4cc(Cn5cncc5CN5CCN(Cc2c3)C(=O)C5)ccc4C#N)O1 Show InChI InChI=1S/C29H31N5O4/c1-29(2)36-18-26(38-29)10-21-5-6-25-11-23(21)15-33-8-7-32(17-28(33)35)16-24-13-31-19-34(24)14-20-3-4-22(12-30)27(9-20)37-25/h3-6,9,11,13,19,26H,7-8,10,14-18H2,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human Farnesyltransferase -catalyzed incorporation of [3H]-FPP into recombinant Ras-CVIM. |
Bioorg Med Chem Lett 14: 639-43 (2004)
BindingDB Entry DOI: 10.7270/Q2WM1CTK |
More data for this
Ligand-Target Pair | |
Protein farnesyltransferase subunit beta/geranylgeranyltransferase type-1 subunit alpha
(Homo sapiens (Human)) | BDBM50139198
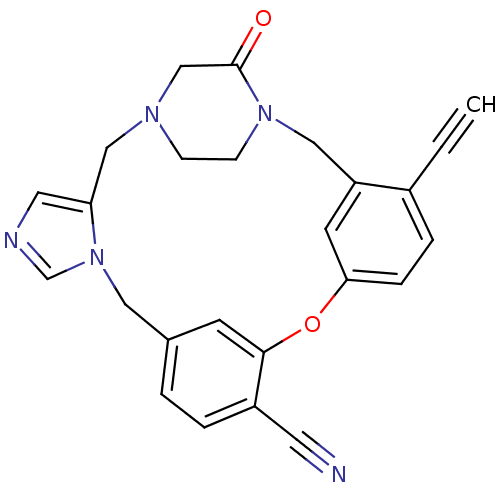
(4-(1-ethynyl)-23-oxo-8-oxa-1,15,17,21-tetraazapent...)Show SMILES O=C1CN2CCN1Cc1cc(Oc3cc(Cn4cncc4C2)ccc3C#N)ccc1C#C Show InChI InChI=1S/C25H21N5O2/c1-2-19-5-6-23-10-21(19)14-29-8-7-28(16-25(29)31)15-22-12-27-17-30(22)13-18-3-4-20(11-26)24(9-18)32-23/h1,3-6,9-10,12,17H,7-8,13-16H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human Farnesyltransferase -catalyzed incorporation of [3H]-FPP into recombinant Ras-CVIM. |
Bioorg Med Chem Lett 14: 639-43 (2004)
BindingDB Entry DOI: 10.7270/Q2WM1CTK |
More data for this
Ligand-Target Pair | |
Estrogen receptor
(Homo sapiens (Human)) | BDBM50237317

(CHEMBL4098286)Show SMILES CC1(N(CCc2cc(O)ccc12)c1ccc(CC(F)(F)F)cc1)c1ccc(\C=C\C(O)=O)cc1 Show InChI InChI=1S/C27H24F3NO3/c1-26(21-7-2-18(3-8-21)6-13-25(33)34)24-12-11-23(32)16-20(24)14-15-31(26)22-9-4-19(5-10-22)17-27(28,29)30/h2-13,16,32H,14-15,17H2,1H3,(H,33,34)/b13-6+ | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Induction of ERalpha degradation in human MCF7 cells after 18 to 24 hrs by Western blot analysis |
J Med Chem 60: 2790-2818 (2017)
Article DOI: 10.1021/acs.jmedchem.6b01468
BindingDB Entry DOI: 10.7270/Q2DB845Q |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase Chk1
(Homo sapiens (Human)) | BDBM50223478
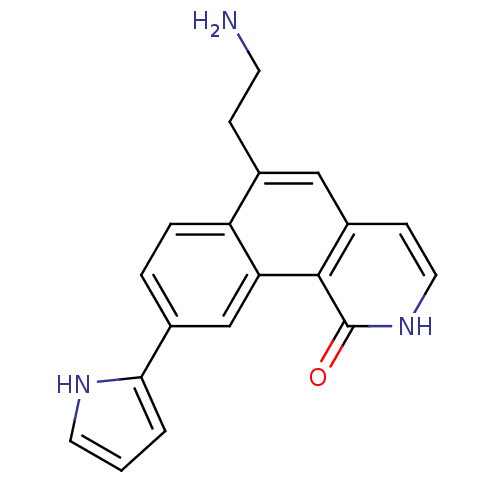
(6-(2-aminoethyl)-9-(1H-pyrrol-2-yl)benzo[h]isoquin...)Show SMILES NCCc1cc2cc[nH]c(=O)c2c2cc(ccc12)-c1ccc[nH]1 Show InChI InChI=1S/C19H17N3O/c20-7-5-12-10-14-6-9-22-19(23)18(14)16-11-13(3-4-15(12)16)17-2-1-8-21-17/h1-4,6,8-11,21H,5,7,20H2,(H,22,23) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human Chk1 expressed in baculovirus by time-resolved fluorescence assay |
Bioorg Med Chem Lett 17: 6280-5 (2007)
Article DOI: 10.1016/j.bmcl.2007.09.007
BindingDB Entry DOI: 10.7270/Q2W958ZJ |
More data for this
Ligand-Target Pair | |
Estrogen receptor
(Homo sapiens (Human)) | BDBM50090462
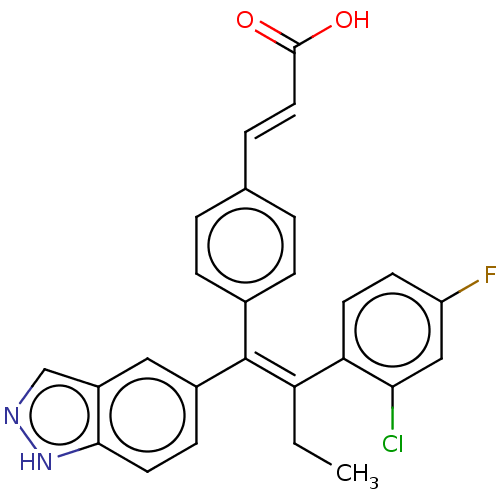
(CHEMBL3581693 | US20240043442, Example GDC-0810)Show SMILES CC\C(=C(\c1ccc(\C=C\C(O)=O)cc1)c1ccc2[nH]ncc2c1)c1ccc(F)cc1Cl Show InChI InChI=1S/C26H20ClFN2O2/c1-2-21(22-10-9-20(28)14-23(22)27)26(18-8-11-24-19(13-18)15-29-30-24)17-6-3-16(4-7-17)5-12-25(31)32/h3-15H,2H2,1H3,(H,29,30)(H,31,32)/b12-5+,26-21+ | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Induction of selective estrogen receptor alpha degradation in human MCF7 cells after 18 to 24 hrs by in-cell Western analysis |
J Med Chem 61: 2837-2864 (2018)
Article DOI: 10.1021/acs.jmedchem.7b01682
BindingDB Entry DOI: 10.7270/Q2TB1986 |
More data for this
Ligand-Target Pair | |
Protein farnesyltransferase subunit beta/geranylgeranyltransferase type-1 subunit alpha
(Homo sapiens (Human)) | BDBM50139186

(4-allyl-23-oxo-8-oxa-1,15,17,21-tetraazapentacyclo...)Show SMILES C=CCc1ccc2Oc3cc(Cn4cncc4CN4CCN(Cc1c2)C(=O)C4)ccc3C#N Show InChI InChI=1S/C26H25N5O2/c1-2-3-20-6-7-24-11-22(20)15-30-9-8-29(17-26(30)32)16-23-13-28-18-31(23)14-19-4-5-21(12-27)25(10-19)33-24/h2,4-7,10-11,13,18H,1,3,8-9,14-17H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 0.590 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of radiolabeled FTI from Farnesyltransferase in cultured Ha-ras transformed RAT1 cells. |
Bioorg Med Chem Lett 14: 639-43 (2004)
BindingDB Entry DOI: 10.7270/Q2WM1CTK |
More data for this
Ligand-Target Pair | |
Estrogen receptor
(Homo sapiens (Human)) | BDBM50237332
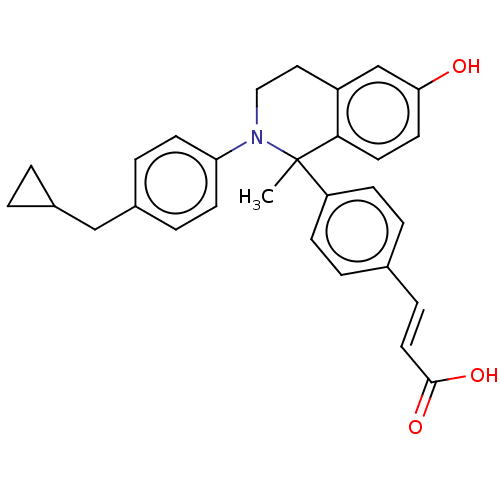
(CHEMBL4078937)Show SMILES CC1(N(CCc2cc(O)ccc12)c1ccc(CC2CC2)cc1)c1ccc(\C=C\C(O)=O)cc1 Show InChI InChI=1S/C29H29NO3/c1-29(24-9-4-20(5-10-24)8-15-28(32)33)27-14-13-26(31)19-23(27)16-17-30(29)25-11-6-22(7-12-25)18-21-2-3-21/h4-15,19,21,31H,2-3,16-18H2,1H3,(H,32,33)/b15-8+ | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Induction of ERalpha degradation in human MCF7 cells after 18 to 24 hrs by Western blot analysis |
J Med Chem 60: 2790-2818 (2017)
Article DOI: 10.1021/acs.jmedchem.6b01468
BindingDB Entry DOI: 10.7270/Q2DB845Q |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase Chk1
(Homo sapiens (Human)) | BDBM50223480
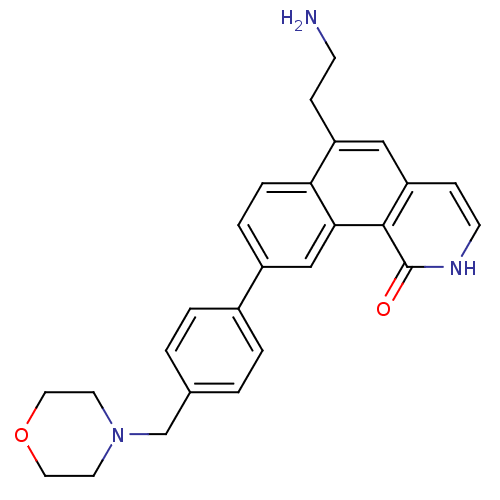
(6-(2-aminoethyl)-9-(4-(morpholinomethyl)phenyl)ben...)Show SMILES NCCc1cc2cc[nH]c(=O)c2c2cc(ccc12)-c1ccc(CN2CCOCC2)cc1 Show InChI InChI=1S/C26H27N3O2/c27-9-7-21-15-22-8-10-28-26(30)25(22)24-16-20(5-6-23(21)24)19-3-1-18(2-4-19)17-29-11-13-31-14-12-29/h1-6,8,10,15-16H,7,9,11-14,17,27H2,(H,28,30) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human Chk1 expressed in baculovirus by time-resolved fluorescence assay |
Bioorg Med Chem Lett 17: 6280-5 (2007)
Article DOI: 10.1016/j.bmcl.2007.09.007
BindingDB Entry DOI: 10.7270/Q2W958ZJ |
More data for this
Ligand-Target Pair | |
Estrogen receptor
(Homo sapiens (Human)) | BDBM50237305
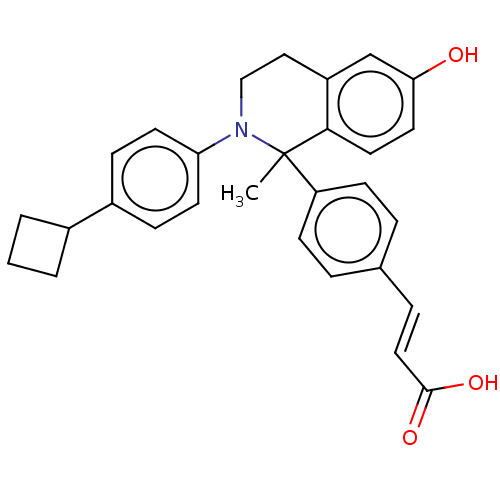
(CHEMBL4072611)Show SMILES CC1(N(CCc2cc(O)ccc12)c1ccc(cc1)C1CCC1)c1ccc(\C=C\C(O)=O)cc1 Show InChI InChI=1S/C29H29NO3/c1-29(24-10-5-20(6-11-24)7-16-28(32)33)27-15-14-26(31)19-23(27)17-18-30(29)25-12-8-22(9-13-25)21-3-2-4-21/h5-16,19,21,31H,2-4,17-18H2,1H3,(H,32,33)/b16-7+ | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Affinity of compound for Dopamine receptor D2 in rat striatal membrane determined for antagonist state (low affinity state, D2 Low) with [3H]spiperon... |
J Med Chem 60: 2790-2818 (2017)
Article DOI: 10.1021/acs.jmedchem.6b01468
BindingDB Entry DOI: 10.7270/Q2DB845Q |
More data for this
Ligand-Target Pair | |
Kinesin-like protein KIF11
(Homo sapiens (Human)) | BDBM103307

(US8546434, 4)Show SMILES C[C@H](O)C(=O)N(C[C@@H]1CNC[C@@H]1F)[C@@H](c1nc(nn1Cc1ccccc1)-c1cc(F)ccc1F)C(C)(C)C |r| Show InChI InChI=1S/C28H34F3N5O2/c1-17(37)27(38)35(16-19-13-32-14-23(19)31)24(28(2,3)4)26-33-25(21-12-20(29)10-11-22(21)30)34-36(26)15-18-8-6-5-7-9-18/h5-12,17,19,23-24,32,37H,13-16H2,1-4H3/t17-,19-,23-,24-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | 7.5 | 37 |
Novartis AG
US Patent
| Assay Description
In vitro activity assay using KSP. |
US Patent US8546434 (2013)
BindingDB Entry DOI: 10.7270/Q25D8QG9 |
More data for this
Ligand-Target Pair | |
Protein farnesyltransferase subunit beta/geranylgeranyltransferase type-1 subunit alpha
(Homo sapiens (Human)) | BDBM50139191
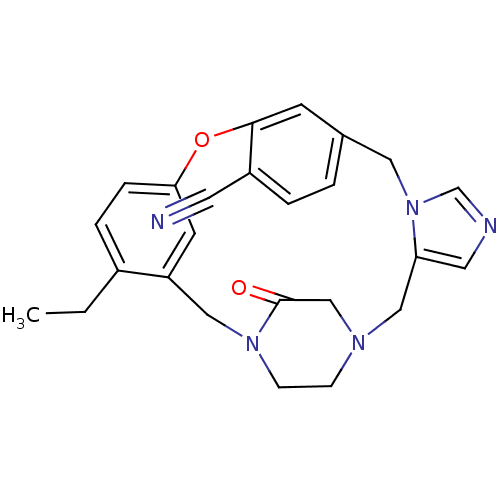
(4-ethyl-23-oxo-8-oxa-1,15,17,21-tetraazapentacyclo...)Show SMILES CCc1ccc2Oc3cc(Cn4cncc4CN4CCN(Cc1c2)C(=O)C4)ccc3C#N Show InChI InChI=1S/C25H25N5O2/c1-2-19-5-6-23-10-21(19)14-29-8-7-28(16-25(29)31)15-22-12-27-17-30(22)13-18-3-4-20(11-26)24(9-18)32-23/h3-6,9-10,12,17H,2,7-8,13-16H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 0.660 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human Farnesyltransferase -catalyzed incorporation of [3H]-FPP into recombinant Ras-CVIM. |
Bioorg Med Chem Lett 14: 639-43 (2004)
BindingDB Entry DOI: 10.7270/Q2WM1CTK |
More data for this
Ligand-Target Pair | |
Protein farnesyltransferase subunit beta/geranylgeranyltransferase type-1 subunit alpha
(Homo sapiens (Human)) | BDBM50139200
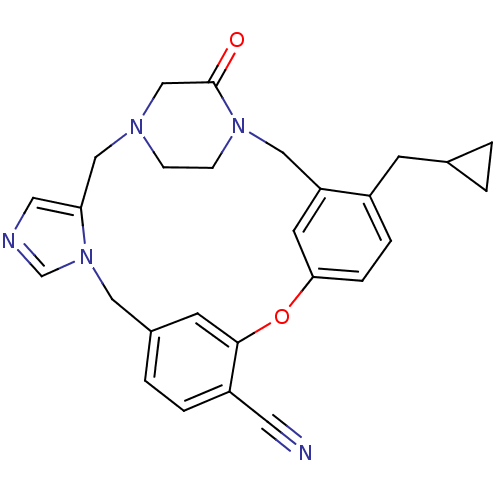
(4-cyclopropylmethyl-23-oxo-8-oxa-1,15,17,21-tetraa...)Show SMILES O=C1CN2CCN1Cc1cc(Oc3cc(Cn4cncc4C2)ccc3C#N)ccc1CC1CC1 Show InChI InChI=1S/C27H27N5O2/c28-12-22-4-3-20-10-26(22)34-25-6-5-21(9-19-1-2-19)23(11-25)15-31-8-7-30(17-27(31)33)16-24-13-29-18-32(24)14-20/h3-6,10-11,13,18-19H,1-2,7-9,14-17H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 0.660 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human Farnesyltransferase -catalyzed incorporation of [3H]-FPP into recombinant Ras-CVIM. |
Bioorg Med Chem Lett 14: 639-43 (2004)
BindingDB Entry DOI: 10.7270/Q2WM1CTK |
More data for this
Ligand-Target Pair | |
Geranylgeranyl transferase type-1 subunit beta/Protein farnesyltransferase/geranylgeranyltransferase type-1 subunit alpha
(Homo sapiens (Human)) | BDBM50115914
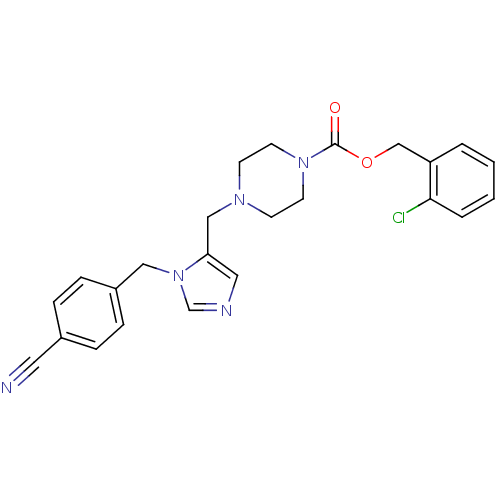
(4-[3-(4-Cyano-benzyl)-3H-imidazol-4-ylmethyl]-pipe...)Show SMILES Clc1ccccc1COC(=O)N1CCN(Cc2cncn2Cc2ccc(cc2)C#N)CC1 Show InChI InChI=1S/C24H24ClN5O2/c25-23-4-2-1-3-21(23)17-32-24(31)29-11-9-28(10-12-29)16-22-14-27-18-30(22)15-20-7-5-19(13-26)6-8-20/h1-8,14,18H,9-12,15-17H2 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 0.680 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of geranyl-geranylation of C-terminal CAAX sequence of Rap 1a in PSN-1 cells |
Bioorg Med Chem Lett 12: 2027-30 (2002)
BindingDB Entry DOI: 10.7270/Q2TQ60VS |
More data for this
Ligand-Target Pair | |
Kinesin-like protein KIF11
(Homo sapiens (Human)) | BDBM34539
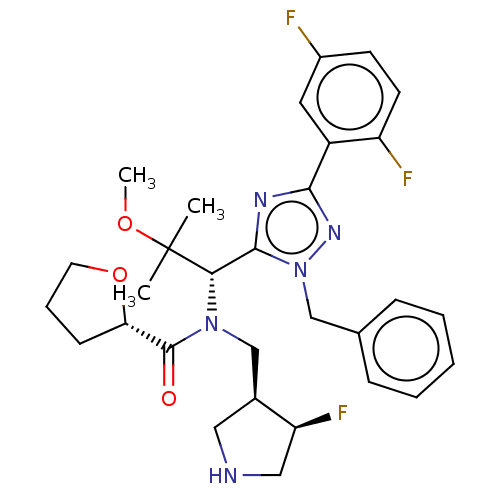
(US8546434, 13)Show SMILES COC(C)(C)[C@@H](N(C[C@@H]1CNC[C@@H]1F)C(=O)[C@@H]1CCCO1)c1nc(nn1Cc1ccccc1)-c1cc(F)ccc1F |r| Show InChI InChI=1S/C13H17N3O3S2/c1-13(2,3)11-14-15-12(20-11)16-21(17,18)10-7-5-9(19-4)6-8-10/h5-8H,1-4H3,(H,15,16) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 0.690 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis AG
US Patent
| Assay Description
In vitro activity assay using KSP. |
US Patent US8546434 (2013)
BindingDB Entry DOI: 10.7270/Q25D8QG9 |
More data for this
Ligand-Target Pair | |
Kinesin-like protein KIF11
(Homo sapiens (Human)) | BDBM103306

(US8546434, 3)Show SMILES C[C@H]1CN(C[C@@H](C)O1)C(=O)N(C[C@@H]1CNC[C@@H]1F)[C@@H](c1nc(nn1Cc1cccc(F)c1)-c1cc(F)ccc1F)C(C)(C)C |r| Show InChI InChI=1S/C32H40F4N6O2/c1-19-15-40(16-20(2)44-19)31(43)41(18-22-13-37-14-27(22)36)28(32(3,4)5)30-38-29(25-12-24(34)9-10-26(25)35)39-42(30)17-21-7-6-8-23(33)11-21/h6-12,19-20,22,27-28,37H,13-18H2,1-5H3/t19-,20+,22-,27-,28-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 0.740 | n/a | n/a | n/a | n/a | 7.5 | 37 |
Novartis AG
US Patent
| Assay Description
In vitro activity assay using KSP. |
US Patent US8546434 (2013)
BindingDB Entry DOI: 10.7270/Q25D8QG9 |
More data for this
Ligand-Target Pair | |
Estrogen receptor
(Homo sapiens (Human)) | BDBM50237306
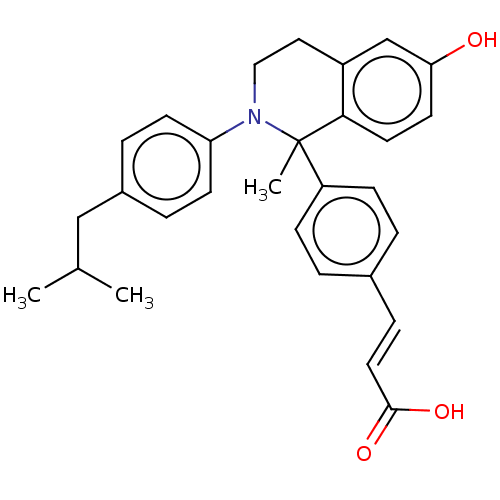
(CHEMBL4076352)Show SMILES CC(C)Cc1ccc(cc1)N1CCc2cc(O)ccc2C1(C)c1ccc(\C=C\C(O)=O)cc1 Show InChI InChI=1S/C29H31NO3/c1-20(2)18-22-6-11-25(12-7-22)30-17-16-23-19-26(31)13-14-27(23)29(30,3)24-9-4-21(5-10-24)8-15-28(32)33/h4-15,19-20,31H,16-18H2,1-3H3,(H,32,33)/b15-8+ | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Induction of ERalpha degradation in human MCF7 cells after 18 to 24 hrs by Western blot analysis |
J Med Chem 60: 2790-2818 (2017)
Article DOI: 10.1021/acs.jmedchem.6b01468
BindingDB Entry DOI: 10.7270/Q2DB845Q |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase Chk1
(Homo sapiens (Human)) | BDBM50223454

(6-(3-aminopropyl)-4-(2-chlorophenyl)-9-(1H-pyrazol...)Show SMILES NCCCc1cc2c(c[nH]c(=O)c2c2cc(ccc12)-c1cn[nH]c1)-c1ccccc1Cl Show InChI InChI=1S/C25H21ClN4O/c26-23-6-2-1-5-19(23)22-14-28-25(31)24-20-10-15(17-12-29-30-13-17)7-8-18(20)16(4-3-9-27)11-21(22)24/h1-2,5-8,10-14H,3-4,9,27H2,(H,28,31)(H,29,30) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human Chk1 expressed in baculovirus by time-resolved fluorescence assay |
Bioorg Med Chem Lett 17: 6280-5 (2007)
Article DOI: 10.1016/j.bmcl.2007.09.007
BindingDB Entry DOI: 10.7270/Q2W958ZJ |
More data for this
Ligand-Target Pair | |
Kinesin-like protein KIF11
(Homo sapiens (Human)) | BDBM103309

(US8546434, 6)Show SMILES C[C@H](O)C(=O)N(C[C@@H]1CNC[C@@H]1F)[C@@H](c1nc(nn1Cc1cccc(F)c1)-c1cc(F)ccc1F)C(C)(C)C |r| Show InChI InChI=1S/C28H33F4N5O2/c1-16(38)27(39)36(15-18-12-33-13-23(18)32)24(28(2,3)4)26-34-25(21-11-20(30)8-9-22(21)31)35-37(26)14-17-6-5-7-19(29)10-17/h5-11,16,18,23-24,33,38H,12-15H2,1-4H3/t16-,18-,23-,24-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 0.830 | n/a | n/a | n/a | n/a | 7.5 | 37 |
Novartis AG
US Patent
| Assay Description
In vitro activity assay using KSP. |
US Patent US8546434 (2013)
BindingDB Entry DOI: 10.7270/Q25D8QG9 |
More data for this
Ligand-Target Pair | |
Kinesin-like protein KIF11
(Homo sapiens (Human)) | BDBM103305
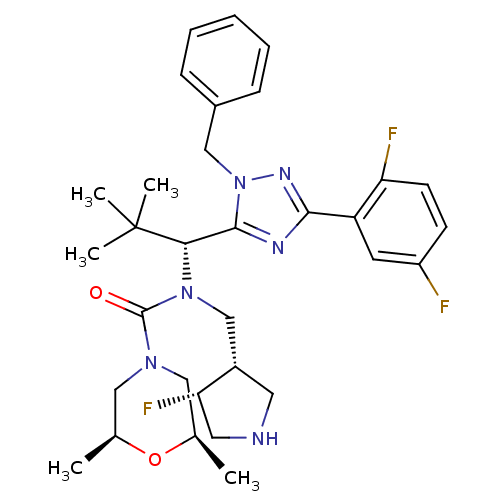
(US8546434, 2)Show SMILES C[C@H]1CN(C[C@@H](C)O1)C(=O)N(C[C@@H]1CNC[C@@H]1F)[C@@H](c1nc(nn1Cc1ccccc1)-c1cc(F)ccc1F)C(C)(C)C |r| Show InChI InChI=1S/C32H41F3N6O2/c1-20-16-39(17-21(2)43-20)31(42)40(19-23-14-36-15-27(23)35)28(32(3,4)5)30-37-29(25-13-24(33)11-12-26(25)34)38-41(30)18-22-9-7-6-8-10-22/h6-13,20-21,23,27-28,36H,14-19H2,1-5H3/t20-,21+,23-,27-,28-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 0.830 | n/a | n/a | n/a | n/a | 7.5 | 37 |
Novartis AG
US Patent
| Assay Description
In vitro activity assay using KSP. |
US Patent US8546434 (2013)
BindingDB Entry DOI: 10.7270/Q25D8QG9 |
More data for this
Ligand-Target Pair | |
Geranylgeranyl transferase type-1 subunit beta/Protein farnesyltransferase/geranylgeranyltransferase type-1 subunit alpha
(Homo sapiens (Human)) | BDBM50115925

(4-[3-(4-Cyano-benzyl)-3H-imidazol-4-ylmethyl]-pipe...)Show SMILES COc1ccccc1COC(=O)N1CCN(Cc2cncn2Cc2ccc(cc2)C#N)CC1 Show InChI InChI=1S/C25H27N5O3/c1-32-24-5-3-2-4-22(24)18-33-25(31)29-12-10-28(11-13-29)17-23-15-27-19-30(23)16-21-8-6-20(14-26)7-9-21/h2-9,15,19H,10-13,16-18H2,1H3 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 0.840 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of [1-3H]-GGPP incorporation into biotinylated K4B-Ras peptide by geranylgeranyl transferase in the presence of 5 mM ATP |
Bioorg Med Chem Lett 12: 2027-30 (2002)
BindingDB Entry DOI: 10.7270/Q2TQ60VS |
More data for this
Ligand-Target Pair | |
Protein farnesyltransferase subunit beta/geranylgeranyltransferase type-1 subunit alpha
(Homo sapiens (Human)) | BDBM50115928

(4-[3-(4-Cyano-benzyl)-3H-imidazol-4-ylmethyl]-pipe...)Show SMILES FC(F)(F)Cc1ccccc1COC(=O)N1CCN(Cc2cncn2Cc2ccc(cc2)C#N)CC1 Show InChI InChI=1S/C26H26F3N5O2/c27-26(28,29)13-22-3-1-2-4-23(22)18-36-25(35)33-11-9-32(10-12-33)17-24-15-31-19-34(24)16-21-7-5-20(14-30)6-8-21/h1-8,15,19H,9-13,16-18H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 0.850 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
The concentration required to displace 50% of a highly potent radiolabeled FPTase inhibitor |
Bioorg Med Chem Lett 12: 2027-30 (2002)
BindingDB Entry DOI: 10.7270/Q2TQ60VS |
More data for this
Ligand-Target Pair | |
Protein farnesyltransferase subunit beta/geranylgeranyltransferase type-1 subunit alpha
(Homo sapiens (Human)) | BDBM50139195

(4-(2,2-dimethyl-1,3-dioxolan-4-ylmethyl)-23-oxo-8-...)Show SMILES CC1(C)OCC(Cc2ccc3Oc4cc(Cn5cncc5CN5CCN(Cc2c3)C(=O)C5)ccc4C#N)O1 Show InChI InChI=1S/C29H31N5O4/c1-29(2)36-18-26(38-29)10-21-5-6-25-11-23(21)15-33-8-7-32(17-28(33)35)16-24-13-31-19-34(24)14-20-3-4-22(12-30)27(9-20)37-25/h3-6,9,11,13,19,26H,7-8,10,14-18H2,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 0.860 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of radiolabeled FTI from Farnesyltransferase in cultured Ha-ras transformed RAT1 cells. |
Bioorg Med Chem Lett 14: 639-43 (2004)
BindingDB Entry DOI: 10.7270/Q2WM1CTK |
More data for this
Ligand-Target Pair | |
Kinesin-like protein KIF11
(Homo sapiens (Human)) | BDBM34538
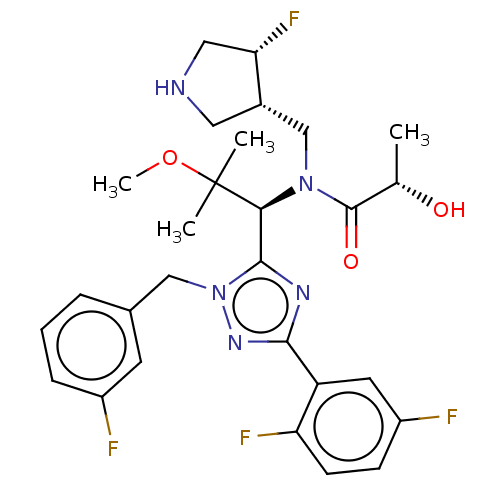
(US8546434, 12)Show SMILES COC(C)(C)[C@@H](N(C[C@@H]1CNC[C@@H]1F)C(=O)[C@H](C)O)c1nc(nn1Cc1cccc(F)c1)-c1cc(F)ccc1F |r| Show InChI InChI=1S/C22H19ClN4O3S/c23-14-4-3-5-15(12-14)27-21(29)20-19(16-6-1-2-7-17(16)24-20)25-22(27)31-13-18(28)26-8-10-30-11-9-26/h1-7,12,24H,8-11,13H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 0.870 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis AG
US Patent
| Assay Description
In vitro activity assay using KSP. |
US Patent US8546434 (2013)
BindingDB Entry DOI: 10.7270/Q25D8QG9 |
More data for this
Ligand-Target Pair | |
Estrogen receptor
(Homo sapiens (Human)) | BDBM50237302

(CHEMBL4101697)Show SMILES CC(C)c1ccc(cc1)N1CCc2cc(O)ccc2C1(C)c1ccc(\C=C\C(O)=O)cc1 Show InChI InChI=1S/C28H29NO3/c1-19(2)21-7-11-24(12-8-21)29-17-16-22-18-25(30)13-14-26(22)28(29,3)23-9-4-20(5-10-23)6-15-27(31)32/h4-15,18-19,30H,16-17H2,1-3H3,(H,31,32)/b15-6+ | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Induction of ERalpha degradation in human MCF7 cells after 18 to 24 hrs by Western blot analysis |
J Med Chem 60: 2790-2818 (2017)
Article DOI: 10.1021/acs.jmedchem.6b01468
BindingDB Entry DOI: 10.7270/Q2DB845Q |
More data for this
Ligand-Target Pair | |
Kinesin-like protein KIF11
(Homo sapiens (Human)) | BDBM34536
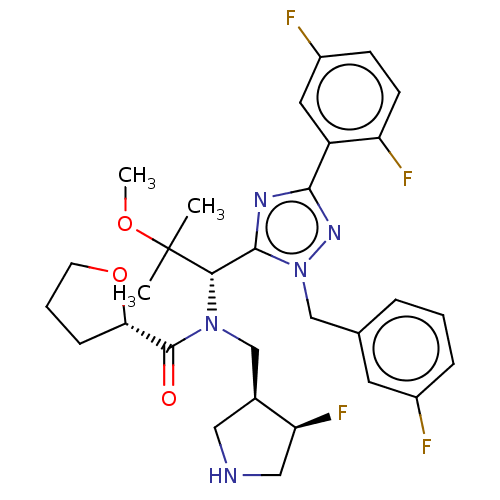
(US8546434, 10)Show SMILES COC(C)(C)[C@@H](N(C[C@@H]1CNC[C@@H]1F)C(=O)[C@@H]1CCCO1)c1nc(nn1Cc1cccc(F)c1)-c1cc(F)ccc1F |r| Show InChI InChI=1S/C15H23N5O4S/c1-18-12-11(13(21)17-14(18)22)20(5-7-23-2)15(16-12)25-10-6-19-3-8-24-9-4-19/h3-10H2,1-2H3,(H,17,21,22) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis AG
US Patent
| Assay Description
In vitro activity assay using KSP. |
US Patent US8546434 (2013)
BindingDB Entry DOI: 10.7270/Q25D8QG9 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data