 Found 4021 hits with Last Name = 'kaku' and Initial = 't'
Found 4021 hits with Last Name = 'kaku' and Initial = 't' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Sodium channel subunit beta-2
(Homo sapiens) | BDBM145285
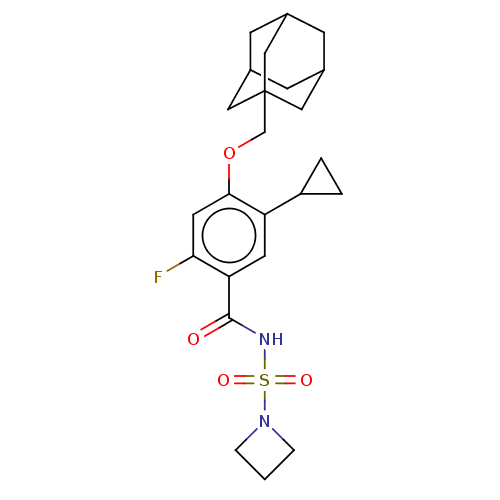
(US8952169, 64 | US9771376, Example 64)Show SMILES Fc1cc(OCC23CC4CC(CC(C4)C2)C3)c(cc1C(=O)NS(=O)(=O)N1CCC1)C1CC1 |TLB:5:6:9:13.12.11,15:6:13:9.10.11,THB:15:10:13:6.14.7,14:6:9:13.12.11,14:12:9:6.15.7| Show InChI InChI=1S/C24H31FN2O4S/c25-21-10-22(31-14-24-11-15-6-16(12-24)8-17(7-15)13-24)19(18-2-3-18)9-20(21)23(28)26-32(29,30)27-4-1-5-27/h9-10,15-18H,1-8,11-14H2,(H,26,28) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Similars
| | n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Androgen receptor
(Homo sapiens (Human)) | BDBM50366207
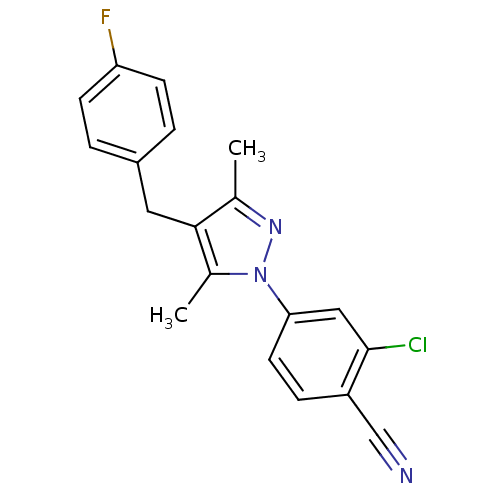
(CHEMBL1957612)Show SMILES Cc1nn(c(C)c1Cc1ccc(F)cc1)-c1ccc(C#N)c(Cl)c1 Show InChI InChI=1S/C19H15ClFN3/c1-12-18(9-14-3-6-16(21)7-4-14)13(2)24(23-12)17-8-5-15(11-22)19(20)10-17/h3-8,10H,9H2,1-2H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.470 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Ltd
Curated by ChEMBL
| Assay Description
Displacement of [17-alpha-methyl-3H]mibolerone from androgen receptor expressed in HEK293 cells after 3 hrs |
Bioorg Med Chem 20: 2338-52 (2012)
Article DOI: 10.1016/j.bmc.2012.02.005
BindingDB Entry DOI: 10.7270/Q2H995PT |
More data for this
Ligand-Target Pair | |
Sodium channel protein type 9 subunit alpha/subunit beta-1/subunit beta-2
(Mus musculus) | BDBM50257179
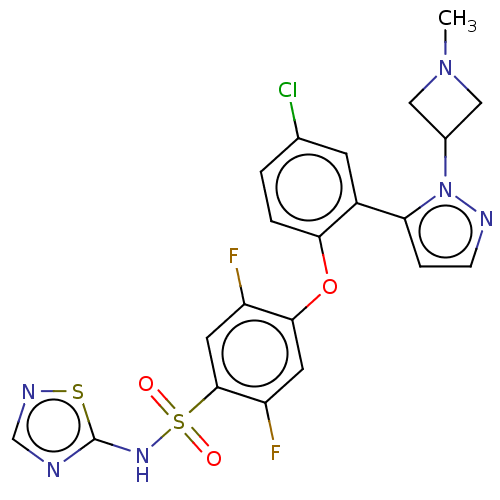
(CHEMBL2325622)Show SMILES CN1CC(C1)n1nccc1-c1cc(Cl)ccc1Oc1cc(F)c(cc1F)S(=O)(=O)Nc1ncns1 Show InChI InChI=1S/C21H17ClF2N6O3S2/c1-29-9-13(10-29)30-17(4-5-26-30)14-6-12(22)2-3-18(14)33-19-7-16(24)20(8-15(19)23)35(31,32)28-21-25-11-27-34-21/h2-8,11,13H,9-10H2,1H3,(H,25,27,28) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.550 | n/a | n/a | n/a | n/a | n/a | n/a |
Daiichi Sankyo Co., Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of mouse NaV1.7/beta1/beta2 expressed in HEK293A cells by Ionworks high-throughput electrophysiology method |
J Med Chem 63: 10204-10220 (2020)
Article DOI: 10.1021/acs.jmedchem.0c00259
BindingDB Entry DOI: 10.7270/Q2Q52T67 |
More data for this
Ligand-Target Pair | |
Sodium channel subunit beta-2
(Homo sapiens) | BDBM70937
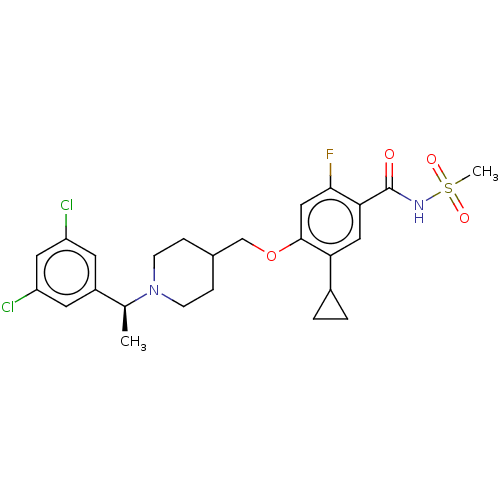
(US9546164, 100 | US9694002, 100)Show SMILES C[C@H](N1CCC(COc2cc(F)c(cc2C2CC2)C(=O)NS(C)(=O)=O)CC1)c1cc(Cl)cc(Cl)c1 |r| Show InChI InChI=1S/C25H29Cl2FN2O4S/c1-15(18-9-19(26)11-20(27)10-18)30-7-5-16(6-8-30)14-34-24-13-23(28)22(12-21(24)17-3-4-17)25(31)29-35(2,32)33/h9-13,15-17H,3-8,14H2,1-2H3,(H,29,31)/t15-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
Similars
| | n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Androgen receptor
(Homo sapiens (Human)) | BDBM50366208
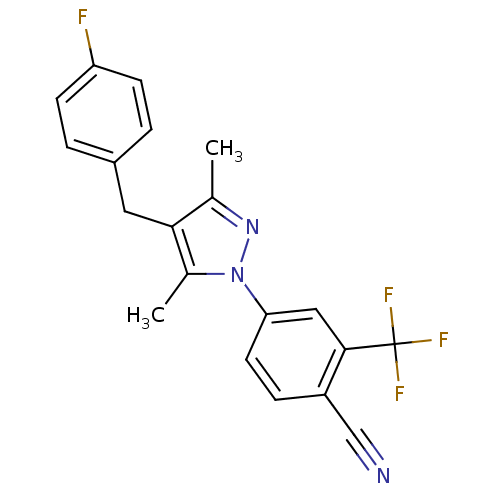
(CHEMBL1957613)Show SMILES Cc1nn(c(C)c1Cc1ccc(F)cc1)-c1ccc(C#N)c(c1)C(F)(F)F Show InChI InChI=1S/C20H15F4N3/c1-12-18(9-14-3-6-16(21)7-4-14)13(2)27(26-12)17-8-5-15(11-25)19(10-17)20(22,23)24/h3-8,10H,9H2,1-2H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| n/a | n/a | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Ltd
Curated by ChEMBL
| Assay Description
Displacement of [17-alpha-methyl-3H]mibolerone from androgen receptor expressed in HEK293 cells after 3 hrs |
Bioorg Med Chem 20: 2338-52 (2012)
Article DOI: 10.1016/j.bmc.2012.02.005
BindingDB Entry DOI: 10.7270/Q2H995PT |
More data for this
Ligand-Target Pair | |
Sodium channel subunit beta-2
(Homo sapiens) | BDBM50257179

(CHEMBL2325622)Show SMILES CN1CC(C1)n1nccc1-c1cc(Cl)ccc1Oc1cc(F)c(cc1F)S(=O)(=O)Nc1ncns1 Show InChI InChI=1S/C21H17ClF2N6O3S2/c1-29-9-13(10-29)30-17(4-5-26-30)14-6-12(22)2-3-18(14)33-19-7-16(24)20(8-15(19)23)35(31,32)28-21-25-11-27-34-21/h2-8,11,13H,9-10H2,1H3,(H,25,27,28) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Daiichi Sankyo Co., Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of human NaV1.7/beta1/beta2 expressed in HEK293A cells by Ionworks high-throughput electrophysiology method |
J Med Chem 63: 10204-10220 (2020)
Article DOI: 10.1021/acs.jmedchem.0c00259
BindingDB Entry DOI: 10.7270/Q2Q52T67 |
More data for this
Ligand-Target Pair | |
Androgen receptor
(Homo sapiens (Human)) | BDBM18161

((1S,2S,7S,10R,11S,14S,15S)-14-hydroxy-2,15-dimethy...)Show SMILES [H][C@@]12CC[C@H](O)[C@@]1(C)CC[C@@]1([H])[C@@]2([H])CC[C@@]2([H])CC(=O)CC[C@]12C |r| Show InChI InChI=1S/C19H30O2/c1-18-9-7-13(20)11-12(18)3-4-14-15-5-6-17(21)19(15,2)10-8-16(14)18/h12,14-17,21H,3-11H2,1-2H3/t12-,14-,15-,16-,17-,18-,19-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 2.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Ltd
Curated by ChEMBL
| Assay Description
Inhibition of wild type Androgen receptor (unknown origin) expressed in Freestyle293F cells |
Bioorg Med Chem 21: 70-83 (2012)
Article DOI: 10.1016/j.bmc.2012.11.001
BindingDB Entry DOI: 10.7270/Q2QC04VS |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Short transient receptor potential channel 6
(Homo sapiens (Human)) | BDBM50273013
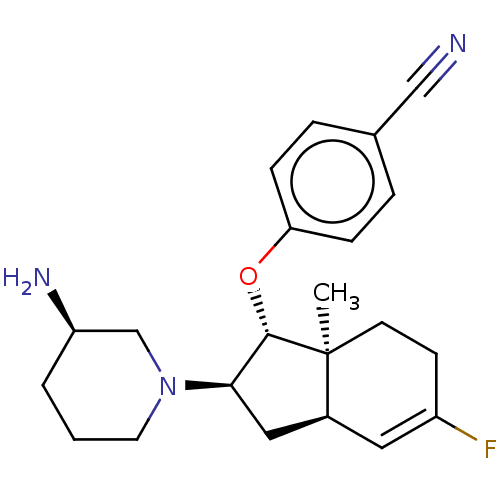
(CHEMBL4129456)Show SMILES [H][C@]12C[C@H]([C@H](Oc3ccc(cc3)C#N)[C@@]1(C)CCC(F)=C2)N1CCC[C@@H](N)C1 |r,c:21| Show InChI InChI=1S/C22H28FN3O/c1-22-9-8-17(23)11-16(22)12-20(26-10-2-3-18(25)14-26)21(22)27-19-6-4-15(13-24)5-7-19/h4-7,11,16,18,20-21H,2-3,8-10,12,14,25H2,1H3/t16-,18+,20+,21-,22-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Daiichi Sankyo Co., Ltd
Curated by ChEMBL
| Assay Description
Inhibition of human TRPC6 expressed in HEK293 cells assessed as decrease in intracellular calcium level after 24 hrs by Fluo-4 dye-based FLIPR assay |
Bioorg Med Chem Lett 28: 2222-2227 (2018)
Article DOI: 10.1016/j.bmcl.2018.03.056
BindingDB Entry DOI: 10.7270/Q2CF9SMH |
More data for this
Ligand-Target Pair | |
Sodium channel subunit beta-2
(Mus musculus) | BDBM50240267
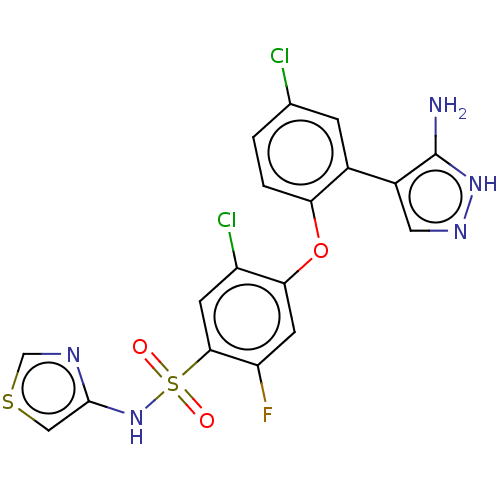
(CHEMBL2325014)Show SMILES Nc1[nH]ncc1-c1cc(Cl)ccc1Oc1cc(F)c(cc1Cl)S(=O)(=O)Nc1cscn1 Show InChI InChI=1S/C18H12Cl2FN5O3S2/c19-9-1-2-14(10(3-9)11-6-24-25-18(11)22)29-15-5-13(21)16(4-12(15)20)31(27,28)26-17-7-30-8-23-17/h1-8,26H,(H3,22,24,25) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
| PDB
Article
PubMed
| n/a | n/a | 4.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Daiichi Sankyo Co., Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of mouse NaV1.7/beta1/beta2 expressed in HEK293A cells by Ionworks high-throughput electrophysiology method |
J Med Chem 63: 10204-10220 (2020)
Article DOI: 10.1021/acs.jmedchem.0c00259
BindingDB Entry DOI: 10.7270/Q2Q52T67 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Retinol-binding protein 4
(Homo sapiens (Human)) | BDBM106687

(US8586571, 50)Show InChI InChI=1S/C21H26N2O2/c1-21(2,3)17-8-4-5-10-19(17)25-16-11-14-23(15-12-16)20(24)18-9-6-7-13-22-18/h4-10,13,16H,11-12,14-15H2,1-3H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Limited
US Patent
| Assay Description
The action of the compound of the present invention to inhibit binding of RBP4 and retinol and TTR was evaluated using the Retinol-RBP4-TTR ELISA (hu... |
US Patent US8586571 (2013)
BindingDB Entry DOI: 10.7270/Q26W98QK |
More data for this
Ligand-Target Pair | |
Steroid 17-alpha-hydroxylase/17,20 lyase
(Homo sapiens (Human)) | BDBM50358192
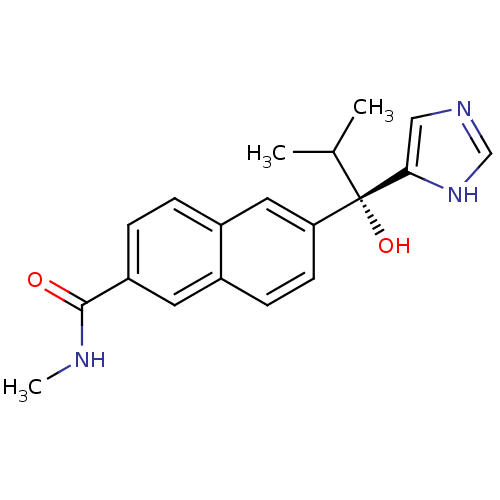
(CHEMBL1921975)Show SMILES CNC(=O)c1ccc2cc(ccc2c1)[C@@](O)(C(C)C)c1cnc[nH]1 |r| Show InChI InChI=1S/C19H21N3O2/c1-12(2)19(24,17-10-21-11-22-17)16-7-6-13-8-15(18(23)20-3)5-4-14(13)9-16/h4-12,24H,1-3H3,(H,20,23)(H,21,22)/t19-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company, Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of 17,20-lyase activity of human CYP17A1 |
Bioorg Med Chem 19: 6383-99 (2011)
Article DOI: 10.1016/j.bmc.2011.08.066
BindingDB Entry DOI: 10.7270/Q2X63NDF |
More data for this
Ligand-Target Pair | |
Steroid 17-alpha-hydroxylase/17,20 lyase
(Rattus norvegicus (Rat)) | BDBM50338363
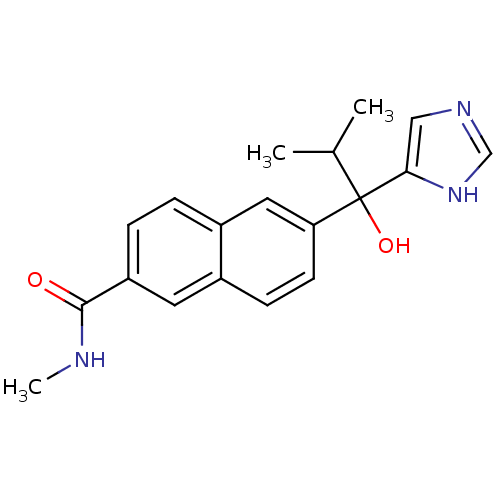
(CHEMBL1682893 | rac-6-[1-Hydroxy-1-(1H-imidazol-4-...)Show SMILES CNC(=O)c1ccc2cc(ccc2c1)C(O)(C(C)C)c1cnc[nH]1 Show InChI InChI=1S/C19H21N3O2/c1-12(2)19(24,17-10-21-11-22-17)16-7-6-13-8-15(18(23)20-3)5-4-14(13)9-16/h4-12,24H,1-3H3,(H,20,23)(H,21,22) | KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company, Ltd
Curated by ChEMBL
| Assay Description
Inhibition of 17,20 lyase activity in Sprague-Dawley rat testicular microsomes |
Bioorg Med Chem 19: 1751-70 (2011)
Article DOI: 10.1016/j.bmc.2011.01.017
BindingDB Entry DOI: 10.7270/Q2X92C9F |
More data for this
Ligand-Target Pair | |
Steroid 17-alpha-hydroxylase/17,20 lyase
(Rattus norvegicus (Rat)) | BDBM50338363

(CHEMBL1682893 | rac-6-[1-Hydroxy-1-(1H-imidazol-4-...)Show SMILES CNC(=O)c1ccc2cc(ccc2c1)C(O)(C(C)C)c1cnc[nH]1 Show InChI InChI=1S/C19H21N3O2/c1-12(2)19(24,17-10-21-11-22-17)16-7-6-13-8-15(18(23)20-3)5-4-14(13)9-16/h4-12,24H,1-3H3,(H,20,23)(H,21,22) | KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 6.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company, Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of 17,20-lyase activity of Sprague-Dawley rat testicular microsomal CYP17A1 using [1,2-3H]-17a-hydroxyprogesterone as substrate after 15 m... |
Bioorg Med Chem 19: 6383-99 (2011)
Article DOI: 10.1016/j.bmc.2011.08.066
BindingDB Entry DOI: 10.7270/Q2X63NDF |
More data for this
Ligand-Target Pair | |
Short transient receptor potential channel 6
(Homo sapiens (Human)) | BDBM50273116
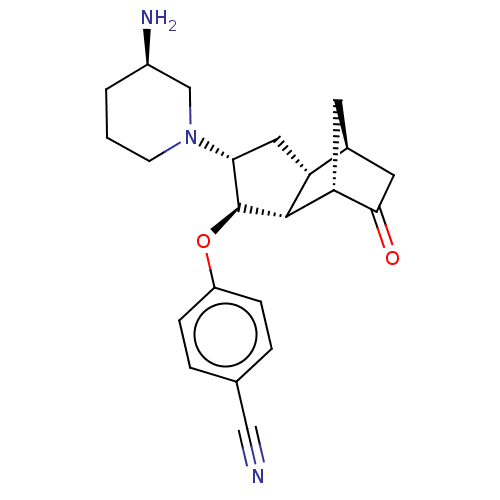
(CHEMBL4129805)Show SMILES [H][C@@]12CC(=O)[C@@]([H])(C1)[C@]1([H])[C@@H](Oc3ccc(cc3)C#N)[C@@H](C[C@]21[H])N1CCC[C@@H](N)C1 |r| Show InChI InChI=1S/C22H27N3O2/c23-11-13-3-5-16(6-4-13)27-22-19(25-7-1-2-15(24)12-25)10-17-14-8-18(21(17)22)20(26)9-14/h3-6,14-15,17-19,21-22H,1-2,7-10,12,24H2/t14-,15+,17+,18+,19+,21+,22-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
Daiichi Sankyo Co., Ltd
Curated by ChEMBL
| Assay Description
Inhibition of human TRPC6 expressed in HEK293 cells assessed as decrease in intracellular calcium level after 24 hrs by Fluo-4 dye-based FLIPR assay |
Bioorg Med Chem Lett 28: 2222-2227 (2018)
Article DOI: 10.1016/j.bmcl.2018.03.056
BindingDB Entry DOI: 10.7270/Q2CF9SMH |
More data for this
Ligand-Target Pair | |
Androgen receptor
(Homo sapiens (Human)) | BDBM50428555
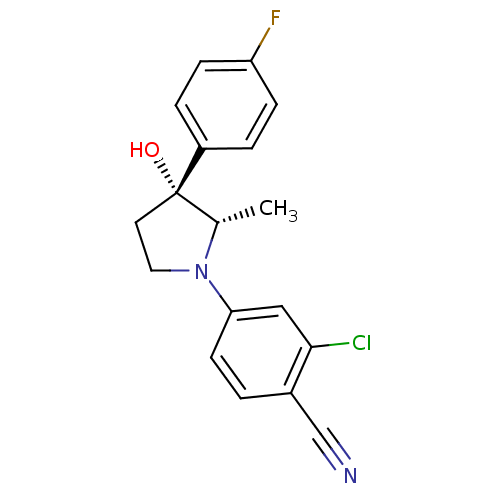
(CHEMBL2337509)Show SMILES C[C@@H]1N(CC[C@@]1(O)c1ccc(F)cc1)c1ccc(C#N)c(Cl)c1 |r| Show InChI InChI=1S/C18H16ClFN2O/c1-12-18(23,14-3-5-15(20)6-4-14)8-9-22(12)16-7-2-13(11-21)17(19)10-16/h2-7,10,12,23H,8-9H2,1H3/t12-,18-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Ltd
Curated by ChEMBL
| Assay Description
Inhibition of wild type Androgen receptor (unknown origin) expressed in Freestyle293F cells |
Bioorg Med Chem 21: 70-83 (2012)
Article DOI: 10.1016/j.bmc.2012.11.001
BindingDB Entry DOI: 10.7270/Q2QC04VS |
More data for this
Ligand-Target Pair | |
Steroid 17-alpha-hydroxylase/17,20 lyase
(Rattus norvegicus (Rat)) | BDBM50342169
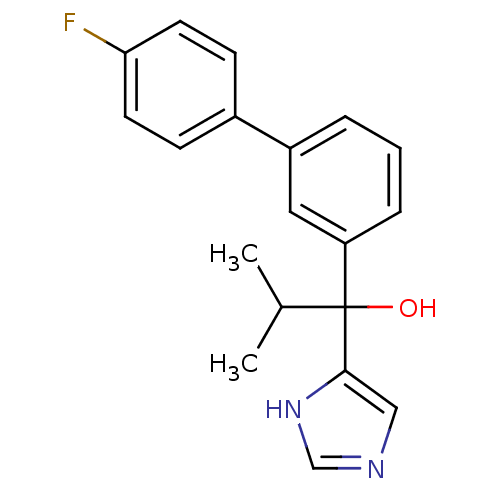
(CHEMBL1766170 | rac-1-(4'-Fluoro[1,1'-biphenyl]-3-...)Show SMILES CC(C)C(O)(c1cnc[nH]1)c1cccc(c1)-c1ccc(F)cc1 Show InChI InChI=1S/C19H19FN2O/c1-13(2)19(23,18-11-21-12-22-18)16-5-3-4-15(10-16)14-6-8-17(20)9-7-14/h3-13,23H,1-2H3,(H,21,22) | KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 8.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company, Ltd
Curated by ChEMBL
| Assay Description
Inhibition of 17,20 lyase activity in Sprague-Dawley rat testicular microsomes |
Bioorg Med Chem 19: 2428-42 (2011)
Article DOI: 10.1016/j.bmc.2011.02.009
BindingDB Entry DOI: 10.7270/Q2FJ2H3G |
More data for this
Ligand-Target Pair | |
Androgen receptor
(Homo sapiens (Human)) | BDBM50428553
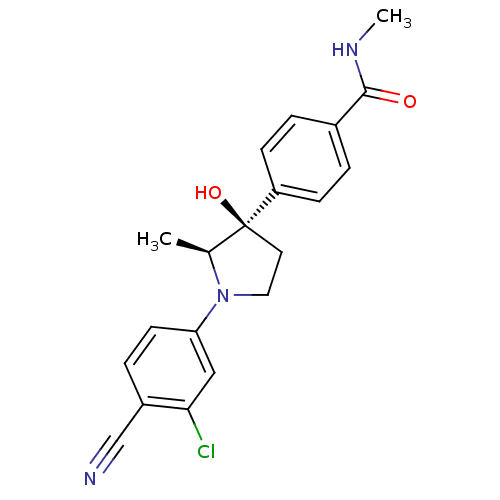
(CHEMBL2337511)Show SMILES CNC(=O)c1ccc(cc1)[C@]1(O)CCN([C@H]1C)c1ccc(C#N)c(Cl)c1 |r| Show InChI InChI=1S/C20H20ClN3O2/c1-13-20(26,16-6-3-14(4-7-16)19(25)23-2)9-10-24(13)17-8-5-15(12-22)18(21)11-17/h3-8,11,13,26H,9-10H2,1-2H3,(H,23,25)/t13-,20-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 8.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Ltd
Curated by ChEMBL
| Assay Description
Antagonist activity at wild type Androgen receptor (unknown origin) expressed in human Cos-7 cells co-expressing pGL3-MMTV-luc vector assessed as luc... |
Bioorg Med Chem 21: 70-83 (2012)
Article DOI: 10.1016/j.bmc.2012.11.001
BindingDB Entry DOI: 10.7270/Q2QC04VS |
More data for this
Ligand-Target Pair | |
Short transient receptor potential channel 6
(Homo sapiens (Human)) | BDBM50273009
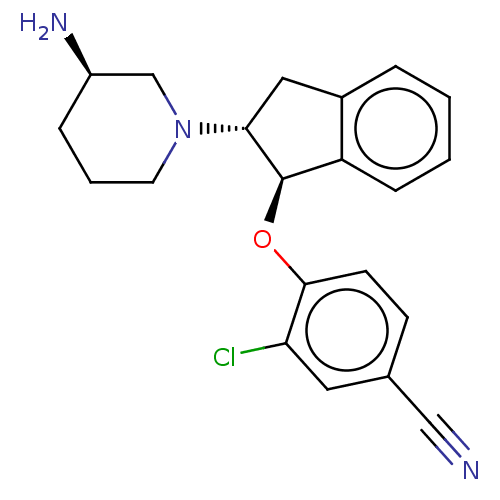
(CHEMBL4129809)Show SMILES N[C@@H]1CCCN(C1)[C@@H]1Cc2ccccc2[C@H]1Oc1ccc(cc1Cl)C#N |r| Show InChI InChI=1S/C21H22ClN3O/c22-18-10-14(12-23)7-8-20(18)26-21-17-6-2-1-4-15(17)11-19(21)25-9-3-5-16(24)13-25/h1-2,4,6-8,10,16,19,21H,3,5,9,11,13,24H2/t16-,19-,21-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | 9.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Daiichi Sankyo Co., Ltd
Curated by ChEMBL
| Assay Description
Inhibition of human TRPC6 expressed in HEK293 cells assessed as decrease in intracellular calcium level after 24 hrs by Fluo-4 dye-based FLIPR assay |
Bioorg Med Chem Lett 28: 2222-2227 (2018)
Article DOI: 10.1016/j.bmcl.2018.03.056
BindingDB Entry DOI: 10.7270/Q2CF9SMH |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Steroid 17-alpha-hydroxylase/17,20 lyase
(Rattus norvegicus (Rat)) | BDBM50342168
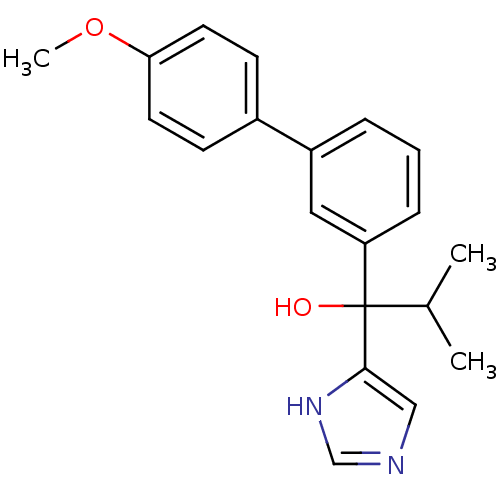
(CHEMBL1765101 | rac-1-(1H-Imidazol-4-yl)-1-(4'-met...)Show SMILES COc1ccc(cc1)-c1cccc(c1)C(O)(C(C)C)c1cnc[nH]1 Show InChI InChI=1S/C20H22N2O2/c1-14(2)20(23,19-12-21-13-22-19)17-6-4-5-16(11-17)15-7-9-18(24-3)10-8-15/h4-14,23H,1-3H3,(H,21,22) | KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company, Ltd
Curated by ChEMBL
| Assay Description
Inhibition of 17,20 lyase activity in Sprague-Dawley rat testicular microsomes |
Bioorg Med Chem 19: 2428-42 (2011)
Article DOI: 10.1016/j.bmc.2011.02.009
BindingDB Entry DOI: 10.7270/Q2FJ2H3G |
More data for this
Ligand-Target Pair | |
Retinol-binding protein 4
(Homo sapiens (Human)) | BDBM106685
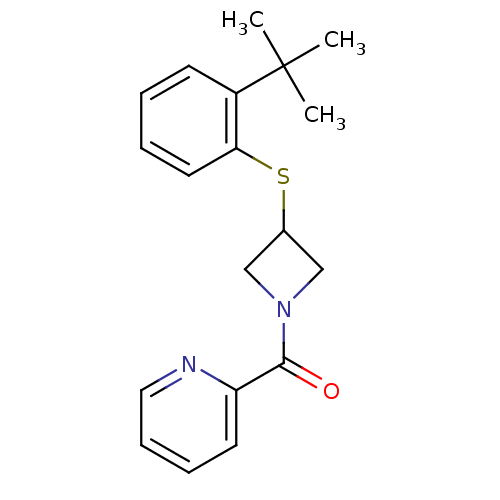
(US8586571, 17)Show InChI InChI=1S/C19H22N2OS/c1-19(2,3)15-8-4-5-10-17(15)23-14-12-21(13-14)18(22)16-9-6-7-11-20-16/h4-11,14H,12-13H2,1-3H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Limited
US Patent
| Assay Description
The action of the compound of the present invention to inhibit binding of RBP4 and retinol and TTR was evaluated using the Retinol-RBP4-TTR ELISA (hu... |
US Patent US8586571 (2013)
BindingDB Entry DOI: 10.7270/Q26W98QK |
More data for this
Ligand-Target Pair | |
Platelet-derived growth factor receptor alpha/beta
(Mus musculus (mouse)) | BDBM145285

(US8952169, 64 | US9771376, Example 64)Show SMILES Fc1cc(OCC23CC4CC(CC(C4)C2)C3)c(cc1C(=O)NS(=O)(=O)N1CCC1)C1CC1 |TLB:5:6:9:13.12.11,15:6:13:9.10.11,THB:15:10:13:6.14.7,14:6:9:13.12.11,14:12:9:6.15.7| Show InChI InChI=1S/C24H31FN2O4S/c25-21-10-22(31-14-24-11-15-6-16(12-24)8-17(7-15)13-24)19(18-2-3-18)9-20(21)23(28)26-32(29,30)27-4-1-5-27/h9-10,15-18H,1-8,11-14H2,(H,26,28) | MMDB
KEGG
UniProtKB/SwissProt
DrugBank
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Similars
| | n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Steroid 17-alpha-hydroxylase/17,20 lyase
(Rattus norvegicus (Rat)) | BDBM50338349
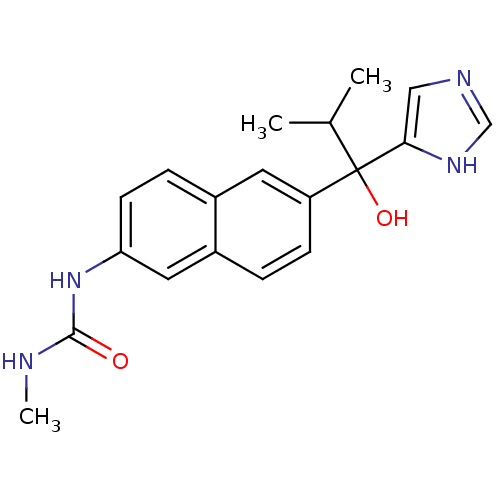
(CHEMBL1682890 | rac-N'-{6-[1-Hydroxy-1-(1H-imidazo...)Show SMILES CNC(=O)Nc1ccc2cc(ccc2c1)C(O)(C(C)C)c1cnc[nH]1 Show InChI InChI=1S/C19H22N4O2/c1-12(2)19(25,17-10-21-11-22-17)15-6-4-14-9-16(23-18(24)20-3)7-5-13(14)8-15/h4-12,25H,1-3H3,(H,21,22)(H2,20,23,24) | KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company, Ltd
Curated by ChEMBL
| Assay Description
Inhibition of 17,20 lyase activity in Sprague-Dawley rat testicular microsomes |
Bioorg Med Chem 19: 1751-70 (2011)
Article DOI: 10.1016/j.bmc.2011.01.017
BindingDB Entry DOI: 10.7270/Q2X92C9F |
More data for this
Ligand-Target Pair | |
Short transient receptor potential channel 6
(Homo sapiens (Human)) | BDBM50273012
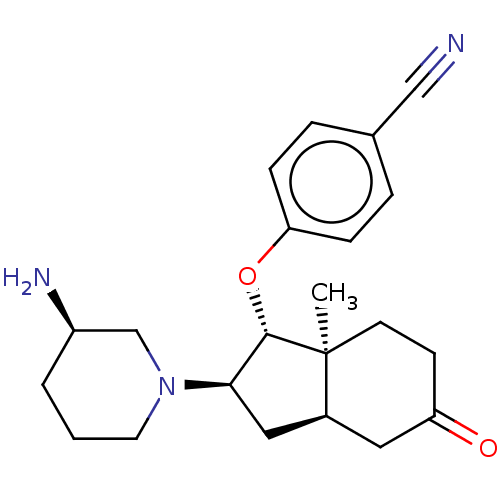
(CHEMBL4127600)Show SMILES [H][C@]12C[C@H]([C@H](Oc3ccc(cc3)C#N)[C@@]1(C)CCC(=O)C2)N1CCC[C@@H](N)C1 |r| Show InChI InChI=1S/C22H29N3O2/c1-22-9-8-18(26)11-16(22)12-20(25-10-2-3-17(24)14-25)21(22)27-19-6-4-15(13-23)5-7-19/h4-7,16-17,20-21H,2-3,8-12,14,24H2,1H3/t16-,17+,20+,21-,22-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
Daiichi Sankyo Co., Ltd
Curated by ChEMBL
| Assay Description
Inhibition of human TRPC6 expressed in HEK293 cells assessed as decrease in intracellular calcium level after 24 hrs by Fluo-4 dye-based FLIPR assay |
Bioorg Med Chem Lett 28: 2222-2227 (2018)
Article DOI: 10.1016/j.bmcl.2018.03.056
BindingDB Entry DOI: 10.7270/Q2CF9SMH |
More data for this
Ligand-Target Pair | |
Androgen receptor
(Homo sapiens (Human)) | BDBM50428548
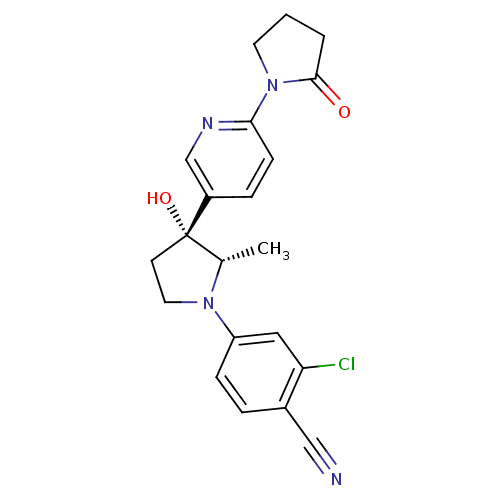
(CHEMBL2337516)Show SMILES C[C@@H]1N(CC[C@@]1(O)c1ccc(nc1)N1CCCC1=O)c1ccc(C#N)c(Cl)c1 |r| Show InChI InChI=1S/C21H21ClN4O2/c1-14-21(28,8-10-25(14)17-6-4-15(12-23)18(22)11-17)16-5-7-19(24-13-16)26-9-2-3-20(26)27/h4-7,11,13-14,28H,2-3,8-10H2,1H3/t14-,21-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Ltd
Curated by ChEMBL
| Assay Description
Antagonist activity at wild type Androgen receptor (unknown origin) expressed in human Cos-7 cells co-expressing pGL3-MMTV-luc vector assessed as luc... |
Bioorg Med Chem 21: 70-83 (2012)
Article DOI: 10.1016/j.bmc.2012.11.001
BindingDB Entry DOI: 10.7270/Q2QC04VS |
More data for this
Ligand-Target Pair | |
Steroid 17-alpha-hydroxylase/17,20 lyase
(Rattus norvegicus (Rat)) | BDBM50338355
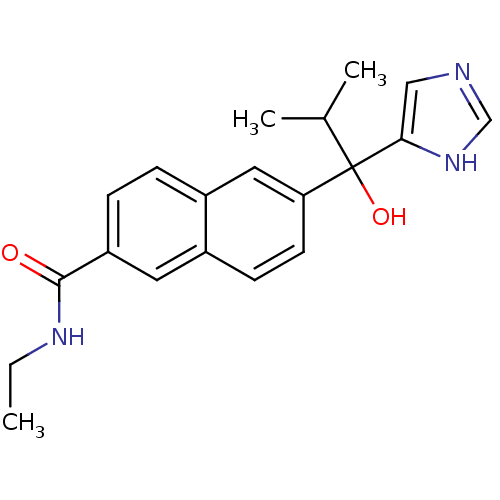
(CHEMBL1682894 | rac-N-Ethyl-6-[1-Hydroxy-1-(1H-imi...)Show SMILES CCNC(=O)c1ccc2cc(ccc2c1)C(O)(C(C)C)c1cnc[nH]1 Show InChI InChI=1S/C20H23N3O2/c1-4-22-19(24)16-6-5-15-10-17(8-7-14(15)9-16)20(25,13(2)3)18-11-21-12-23-18/h5-13,25H,4H2,1-3H3,(H,21,23)(H,22,24) | KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company, Ltd
Curated by ChEMBL
| Assay Description
Inhibition of 17,20 lyase activity in Sprague-Dawley rat testicular microsomes |
Bioorg Med Chem 19: 1751-70 (2011)
Article DOI: 10.1016/j.bmc.2011.01.017
BindingDB Entry DOI: 10.7270/Q2X92C9F |
More data for this
Ligand-Target Pair | |
Sodium channel subunit beta-2
(Homo sapiens) | BDBM258102
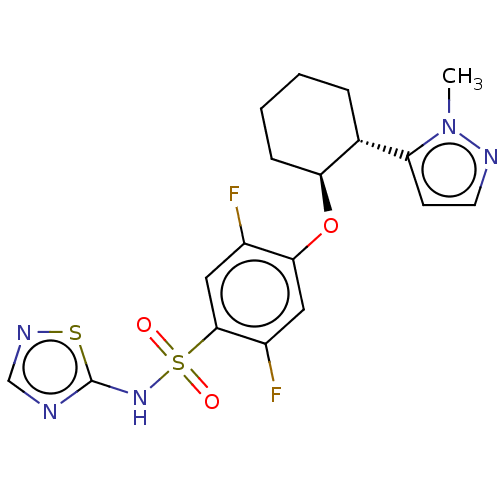
(US9493448, 4 | US9845313, Example 4)Show SMILES Cn1nccc1[C@H]1CCCC[C@@H]1Oc1cc(F)c(cc1F)S(=O)(=O)Nc1ncns1 |r| Show InChI InChI=1S/C18H19F2N5O3S2/c1-25-14(6-7-22-25)11-4-2-3-5-15(11)28-16-8-13(20)17(9-12(16)19)30(26,27)24-18-21-10-23-29-18/h6-11,15H,2-5H2,1H3,(H,21,23,24)/t11-,15+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
Daiichi Sankyo Co., Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of human NaV1.7/beta1/beta2 expressed in HEK293A cells by Ionworks high-throughput electrophysiology method |
J Med Chem 63: 10204-10220 (2020)
Article DOI: 10.1021/acs.jmedchem.0c00259
BindingDB Entry DOI: 10.7270/Q2Q52T67 |
More data for this
Ligand-Target Pair | |
Retinol-binding protein 4
(Homo sapiens (Human)) | BDBM106694
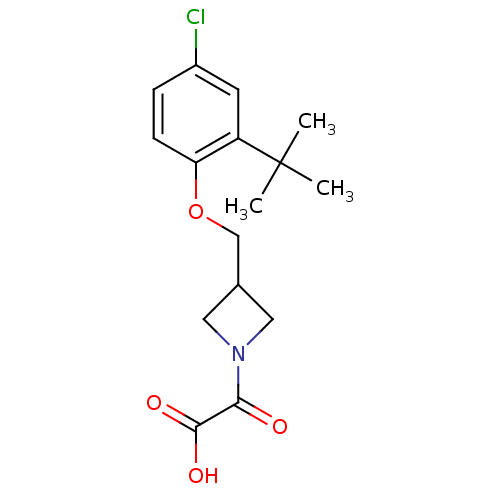
(US8586571, 207)Show InChI InChI=1S/C16H20ClNO4/c1-16(2,3)12-6-11(17)4-5-13(12)22-9-10-7-18(8-10)14(19)15(20)21/h4-6,10H,7-9H2,1-3H3,(H,20,21) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Limited
US Patent
| Assay Description
The action of the compound of the present invention to inhibit binding of RBP4 and retinol and TTR was evaluated using the Retinol-RBP4-TTR ELISA (hu... |
US Patent US8586571 (2013)
BindingDB Entry DOI: 10.7270/Q26W98QK |
More data for this
Ligand-Target Pair | |
Steroid 17-alpha-hydroxylase/17,20 lyase
(Rattus norvegicus (Rat)) | BDBM50338353
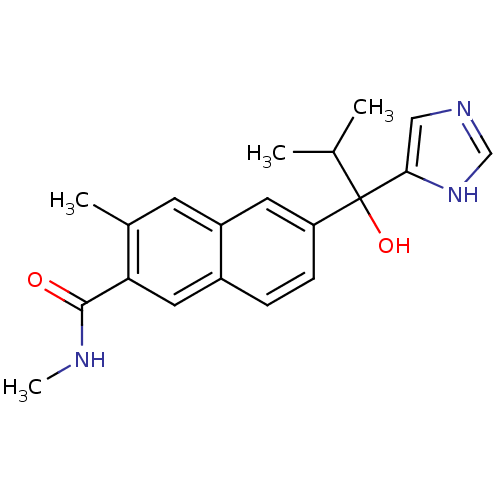
(CHEMBL1682899 | rac-6-[1-Hydroxy-1-(1H-imidazol-4-...)Show SMILES CNC(=O)c1cc2ccc(cc2cc1C)C(O)(C(C)C)c1cnc[nH]1 Show InChI InChI=1S/C20H23N3O2/c1-12(2)20(25,18-10-22-11-23-18)16-6-5-14-9-17(19(24)21-4)13(3)7-15(14)8-16/h5-12,25H,1-4H3,(H,21,24)(H,22,23) | KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company, Ltd
Curated by ChEMBL
| Assay Description
Inhibition of 17,20 lyase activity in Sprague-Dawley rat testicular microsomes |
Bioorg Med Chem 19: 1751-70 (2011)
Article DOI: 10.1016/j.bmc.2011.01.017
BindingDB Entry DOI: 10.7270/Q2X92C9F |
More data for this
Ligand-Target Pair | |
Steroid 17-alpha-hydroxylase/17,20 lyase
(Rattus norvegicus (Rat)) | BDBM50338358
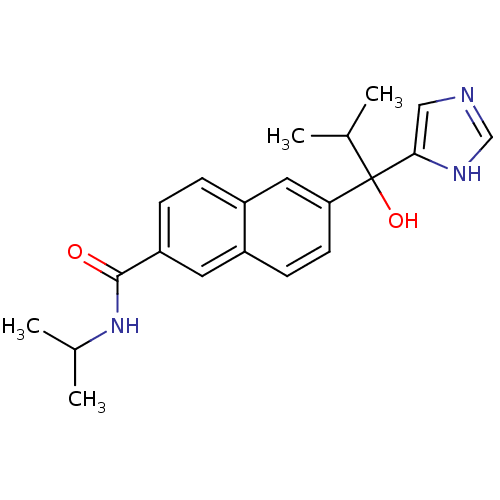
(CHEMBL1682896 | rac-6-[1-Hydroxy-1-(1H-imidazol-4-...)Show SMILES CC(C)NC(=O)c1ccc2cc(ccc2c1)C(O)(C(C)C)c1cnc[nH]1 Show InChI InChI=1S/C21H25N3O2/c1-13(2)21(26,19-11-22-12-23-19)18-8-7-15-9-17(6-5-16(15)10-18)20(25)24-14(3)4/h5-14,26H,1-4H3,(H,22,23)(H,24,25) | KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company, Ltd
Curated by ChEMBL
| Assay Description
Inhibition of 17,20 lyase activity in Sprague-Dawley rat testicular microsomes |
Bioorg Med Chem 19: 1751-70 (2011)
Article DOI: 10.1016/j.bmc.2011.01.017
BindingDB Entry DOI: 10.7270/Q2X92C9F |
More data for this
Ligand-Target Pair | |
Sodium channel subunit beta-2
(Homo sapiens) | BDBM50240267

(CHEMBL2325014)Show SMILES Nc1[nH]ncc1-c1cc(Cl)ccc1Oc1cc(F)c(cc1Cl)S(=O)(=O)Nc1cscn1 Show InChI InChI=1S/C18H12Cl2FN5O3S2/c19-9-1-2-14(10(3-9)11-6-24-25-18(11)22)29-15-5-13(21)16(4-12(15)20)31(27,28)26-17-7-30-8-23-17/h1-8,26H,(H3,22,24,25) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
| PDB
Article
PubMed
| n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
Daiichi Sankyo Co., Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of human NaV1.7/beta1/beta2 expressed in HEK293A cells by Ionworks high-throughput electrophysiology method |
J Med Chem 63: 10204-10220 (2020)
Article DOI: 10.1021/acs.jmedchem.0c00259
BindingDB Entry DOI: 10.7270/Q2Q52T67 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Steroid 11-beta-monooxygenase
(Rattus norvegicus) | BDBM50338356

((+)-7-[1-Hydroxy-1-(1H-imidazol-4-yl)-2-methylprop...)Show SMILES CC(C)C(O)(c1cnc[nH]1)c1ccc2c3CNC(=O)c3ccc2c1 Show InChI InChI=1S/C19H19N3O2/c1-11(2)19(24,17-9-20-10-22-17)13-4-6-14-12(7-13)3-5-15-16(14)8-21-18(15)23/h3-7,9-11,24H,8H2,1-2H3,(H,20,22)(H,21,23) | UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company, Ltd
Curated by ChEMBL
| Assay Description
Inhibition of 11-hydroxylase activity in Sprague-Dawley rat adrenal gland |
Bioorg Med Chem 19: 1751-70 (2011)
Article DOI: 10.1016/j.bmc.2011.01.017
BindingDB Entry DOI: 10.7270/Q2X92C9F |
More data for this
Ligand-Target Pair | |
Steroid 17-alpha-hydroxylase/17,20 lyase
(Homo sapiens (Human)) | BDBM50358195
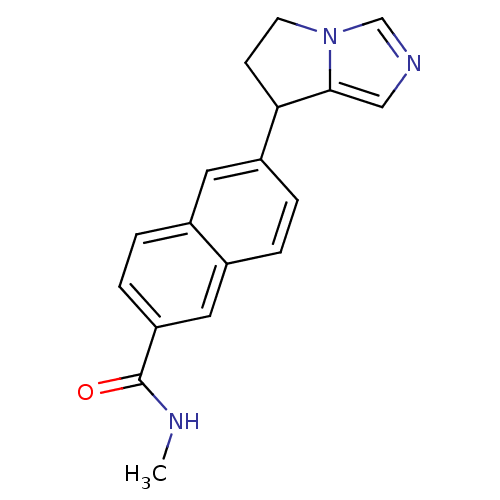
(CHEMBL1921979)Show InChI InChI=1S/C18H17N3O/c1-19-18(22)15-5-3-12-8-14(4-2-13(12)9-15)16-6-7-21-11-20-10-17(16)21/h2-5,8-11,16H,6-7H2,1H3,(H,19,22) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company, Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of 17,20-lyase activity of human CYP17A1 |
Bioorg Med Chem 19: 6383-99 (2011)
Article DOI: 10.1016/j.bmc.2011.08.066
BindingDB Entry DOI: 10.7270/Q2X63NDF |
More data for this
Ligand-Target Pair | |
Androgen receptor
(Homo sapiens (Human)) | BDBM50428548

(CHEMBL2337516)Show SMILES C[C@@H]1N(CC[C@@]1(O)c1ccc(nc1)N1CCCC1=O)c1ccc(C#N)c(Cl)c1 |r| Show InChI InChI=1S/C21H21ClN4O2/c1-14-21(28,8-10-25(14)17-6-4-15(12-23)18(22)11-17)16-5-7-19(24-13-16)26-9-2-3-20(26)27/h4-7,11,13-14,28H,2-3,8-10H2,1H3/t14-,21-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Ltd
Curated by ChEMBL
| Assay Description
Inhibition of wild type Androgen receptor (unknown origin) expressed in Freestyle293F cells |
Bioorg Med Chem 21: 70-83 (2012)
Article DOI: 10.1016/j.bmc.2012.11.001
BindingDB Entry DOI: 10.7270/Q2QC04VS |
More data for this
Ligand-Target Pair | |
Steroid 17-alpha-hydroxylase/17,20 lyase
(Rattus norvegicus (Rat)) | BDBM50338351
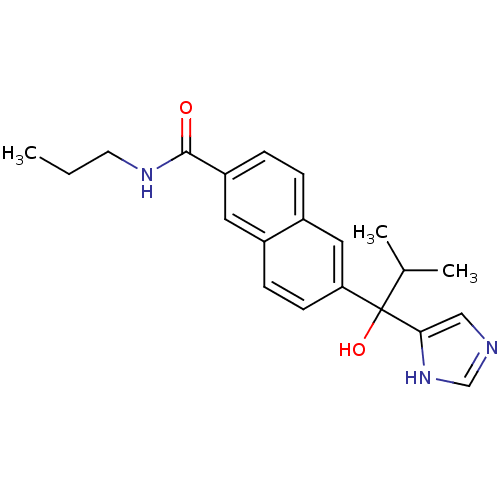
(CHEMBL1682895 | rac-6-[1-Hydroxy-1-(1H-imidazol-4-...)Show SMILES CCCNC(=O)c1ccc2cc(ccc2c1)C(O)(C(C)C)c1cnc[nH]1 Show InChI InChI=1S/C21H25N3O2/c1-4-9-23-20(25)17-6-5-16-11-18(8-7-15(16)10-17)21(26,14(2)3)19-12-22-13-24-19/h5-8,10-14,26H,4,9H2,1-3H3,(H,22,24)(H,23,25) | KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company, Ltd
Curated by ChEMBL
| Assay Description
Inhibition of 17,20 lyase activity in Sprague-Dawley rat testicular microsomes |
Bioorg Med Chem 19: 1751-70 (2011)
Article DOI: 10.1016/j.bmc.2011.01.017
BindingDB Entry DOI: 10.7270/Q2X92C9F |
More data for this
Ligand-Target Pair | |
Steroid 11-beta-monooxygenase
(Rattus norvegicus) | BDBM50338356

((+)-7-[1-Hydroxy-1-(1H-imidazol-4-yl)-2-methylprop...)Show SMILES CC(C)C(O)(c1cnc[nH]1)c1ccc2c3CNC(=O)c3ccc2c1 Show InChI InChI=1S/C19H19N3O2/c1-11(2)19(24,17-9-20-10-22-17)13-4-6-14-12(7-13)3-5-15-16(14)8-21-18(15)23/h3-7,9-11,24H,8H2,1-2H3,(H,20,22)(H,21,23) | UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company, Ltd
Curated by ChEMBL
| Assay Description
Inhibition of 11-hydroxylase activity in Sprague-Dawley rat adrenal gland |
Bioorg Med Chem 19: 1751-70 (2011)
Article DOI: 10.1016/j.bmc.2011.01.017
BindingDB Entry DOI: 10.7270/Q2X92C9F |
More data for this
Ligand-Target Pair | |
Sodium channel subunit beta-2
(Homo sapiens) | BDBM50613335
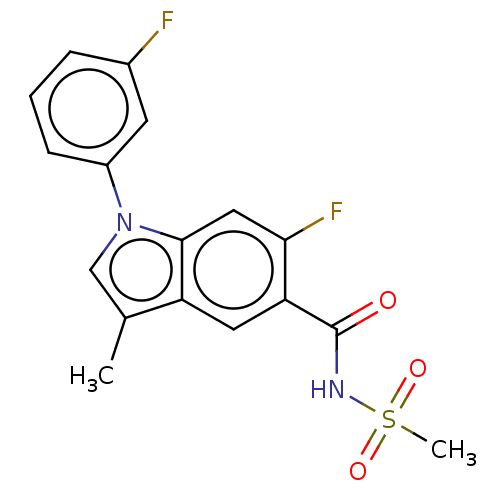
(CHEMBL5288162)Show SMILES Cc1cn(-c2cccc(F)c2)c2cc(F)c(cc12)C(=O)NS(C)(=O)=O | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Steroid 17-alpha-hydroxylase/17,20 lyase
(Rattus norvegicus (Rat)) | BDBM50342186
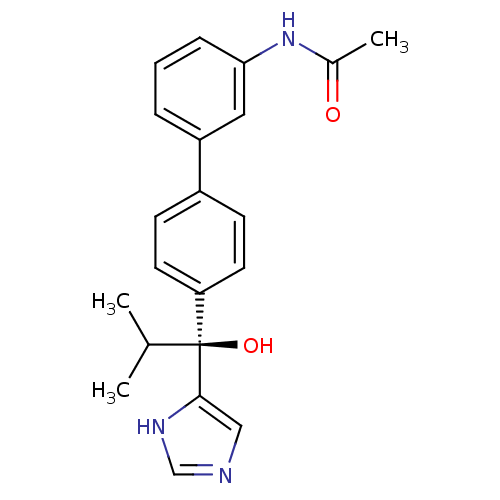
((S)-N-(4'-(1-hydroxy-1-(1H-imidazol-4-yl)-2-methyl...)Show SMILES CC(C)[C@@](O)(c1cnc[nH]1)c1ccc(cc1)-c1cccc(NC(C)=O)c1 |r| Show InChI InChI=1S/C21H23N3O2/c1-14(2)21(26,20-12-22-13-23-20)18-9-7-16(8-10-18)17-5-4-6-19(11-17)24-15(3)25/h4-14,26H,1-3H3,(H,22,23)(H,24,25)/t21-/m0/s1 | KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company, Ltd
Curated by ChEMBL
| Assay Description
Inhibition of 17,20 lyase activity in Sprague-Dawley rat testicular microsomes |
Bioorg Med Chem 19: 2428-42 (2011)
Article DOI: 10.1016/j.bmc.2011.02.009
BindingDB Entry DOI: 10.7270/Q2FJ2H3G |
More data for this
Ligand-Target Pair | |
Steroid 17-alpha-hydroxylase/17,20 lyase
(Rattus norvegicus (Rat)) | BDBM50338362
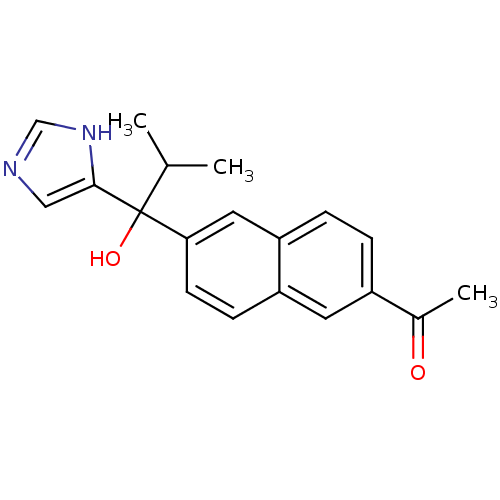
(CHEMBL1682888 | rac-1-{6-[1-Hydroxy-1-(1H-imidazol...)Show SMILES CC(C)C(O)(c1cnc[nH]1)c1ccc2cc(ccc2c1)C(C)=O Show InChI InChI=1S/C19H20N2O2/c1-12(2)19(23,18-10-20-11-21-18)17-7-6-15-8-14(13(3)22)4-5-16(15)9-17/h4-12,23H,1-3H3,(H,20,21) | KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company, Ltd
Curated by ChEMBL
| Assay Description
Inhibition of 17,20 lyase activity in Sprague-Dawley rat testicular microsomes |
Bioorg Med Chem 19: 1751-70 (2011)
Article DOI: 10.1016/j.bmc.2011.01.017
BindingDB Entry DOI: 10.7270/Q2X92C9F |
More data for this
Ligand-Target Pair | |
Sodium channel subunit beta-2
(Mus musculus) | BDBM258162

(US9493448, 64 | US9597330, Example 21 | US9845313,...)Show SMILES Cc1cc(c(F)cc1O[C@H]1CCCC[C@@H]1c1ccnn1C)S(=O)(=O)Nc1ccncn1 |r| Show InChI InChI=1S/C21H24FN5O3S/c1-14-11-20(31(28,29)26-21-8-9-23-13-24-21)16(22)12-19(14)30-18-6-4-3-5-15(18)17-7-10-25-27(17)2/h7-13,15,18H,3-6H2,1-2H3,(H,23,24,26)/t15-,18+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
Daiichi Sankyo Co., Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of mouse NaV1.7/beta1/beta2 expressed in HEK293A cells by Ionworks high-throughput electrophysiology method |
J Med Chem 63: 10204-10220 (2020)
Article DOI: 10.1021/acs.jmedchem.0c00259
BindingDB Entry DOI: 10.7270/Q2Q52T67 |
More data for this
Ligand-Target Pair | |
Sodium channel subunit beta-2
(Homo sapiens) | BDBM50613342
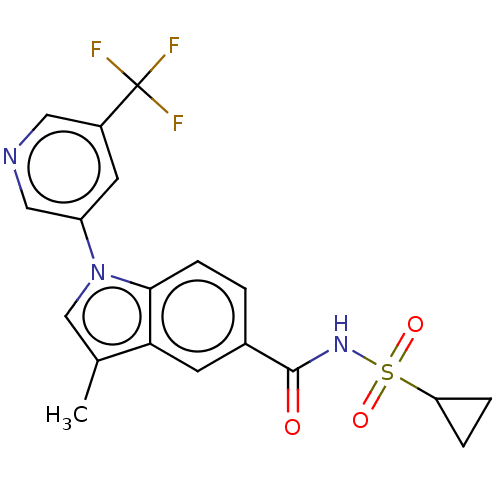
(CHEMBL5286433)Show SMILES Cc1cn(-c2cncc(c2)C(F)(F)F)c2ccc(cc12)C(=O)NS(=O)(=O)C1CC1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Sodium channel subunit beta-2
(Mus musculus) | BDBM50613344

(CHEMBL5284082)Show SMILES Cc1cn(-c2cncc(c2)C(F)(F)F)c2cc(F)c(cc12)C(=O)NS(=O)(=O)C1CC1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Steroid 17-alpha-hydroxylase/17,20 lyase
(Rattus norvegicus (Rat)) | BDBM50338360

((+)-(R)-7-(1-hydroxy-1-(1H-imidazol-4-yl)-2-methyl...)Show SMILES CC(C)[C@](O)(c1cnc[nH]1)c1ccc2c3CN(C)C(=O)c3ccc2c1 |r| Show InChI InChI=1S/C20H21N3O2/c1-12(2)20(25,18-9-21-11-22-18)14-5-7-15-13(8-14)4-6-16-17(15)10-23(3)19(16)24/h4-9,11-12,25H,10H2,1-3H3,(H,21,22)/t20-/m1/s1 | KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company, Ltd
Curated by ChEMBL
| Assay Description
Inhibition of 17,20 lyase activity in Sprague-Dawley rat testicular microsomes |
Bioorg Med Chem 19: 1751-70 (2011)
Article DOI: 10.1016/j.bmc.2011.01.017
BindingDB Entry DOI: 10.7270/Q2X92C9F |
More data for this
Ligand-Target Pair | |
Steroid 17-alpha-hydroxylase/17,20 lyase
(Rattus norvegicus (Rat)) | BDBM50358195

(CHEMBL1921979)Show InChI InChI=1S/C18H17N3O/c1-19-18(22)15-5-3-12-8-14(4-2-13(12)9-15)16-6-7-21-11-20-10-17(16)21/h2-5,8-11,16H,6-7H2,1H3,(H,19,22) | KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company, Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of 17,20-lyase activity of Sprague-Dawley rat testicular microsomal CYP17A1 using [1,2-3H]-17a-hydroxyprogesterone as substrate after 15 m... |
Bioorg Med Chem 19: 6383-99 (2011)
Article DOI: 10.1016/j.bmc.2011.08.066
BindingDB Entry DOI: 10.7270/Q2X63NDF |
More data for this
Ligand-Target Pair | |
Steroid 17-alpha-hydroxylase/17,20 lyase
(Homo sapiens (Human)) | BDBM50338356

((+)-7-[1-Hydroxy-1-(1H-imidazol-4-yl)-2-methylprop...)Show SMILES CC(C)C(O)(c1cnc[nH]1)c1ccc2c3CNC(=O)c3ccc2c1 Show InChI InChI=1S/C19H19N3O2/c1-11(2)19(24,17-9-20-10-22-17)13-4-6-14-12(7-13)3-5-15-16(14)8-21-18(15)23/h3-7,9-11,24H,8H2,1-2H3,(H,20,22)(H,21,23) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company, Ltd
Curated by ChEMBL
| Assay Description
Inhibition of 17,20 lyase activity in human |
Bioorg Med Chem 19: 1751-70 (2011)
Article DOI: 10.1016/j.bmc.2011.01.017
BindingDB Entry DOI: 10.7270/Q2X92C9F |
More data for this
Ligand-Target Pair | |
Retinol-binding protein 4
(Homo sapiens (Human)) | BDBM106691

(US8586571, 164)Show InChI InChI=1S/C19H21ClN2O2/c1-19(2,3)15-10-13(20)7-8-17(15)24-14-11-22(12-14)18(23)16-6-4-5-9-21-16/h4-10,14H,11-12H2,1-3H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Limited
US Patent
| Assay Description
The action of the compound of the present invention to inhibit binding of RBP4 and retinol and TTR was evaluated using the Retinol-RBP4-TTR ELISA (hu... |
US Patent US8586571 (2013)
BindingDB Entry DOI: 10.7270/Q26W98QK |
More data for this
Ligand-Target Pair | |
Steroid 17-alpha-hydroxylase/17,20 lyase
(Rattus norvegicus (Rat)) | BDBM50342167

(CHEMBL1766169 | rac-1-[1,1'-biphenyl]-3-yl-1-(1H-i...)Show InChI InChI=1S/C19H20N2O/c1-14(2)19(22,18-12-20-13-21-18)17-10-6-9-16(11-17)15-7-4-3-5-8-15/h3-14,22H,1-2H3,(H,20,21) | KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company, Ltd
Curated by ChEMBL
| Assay Description
Inhibition of 17,20 lyase activity in Sprague-Dawley rat testicular microsomes |
Bioorg Med Chem 19: 2428-42 (2011)
Article DOI: 10.1016/j.bmc.2011.02.009
BindingDB Entry DOI: 10.7270/Q2FJ2H3G |
More data for this
Ligand-Target Pair | |
Steroid 17-alpha-hydroxylase/17,20 lyase
(Homo sapiens (Human)) | BDBM50338356

((+)-7-[1-Hydroxy-1-(1H-imidazol-4-yl)-2-methylprop...)Show SMILES CC(C)C(O)(c1cnc[nH]1)c1ccc2c3CNC(=O)c3ccc2c1 Show InChI InChI=1S/C19H19N3O2/c1-11(2)19(24,17-9-20-10-22-17)13-4-6-14-12(7-13)3-5-15-16(14)8-21-18(15)23/h3-7,9-11,24H,8H2,1-2H3,(H,20,22)(H,21,23) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company, Ltd
Curated by ChEMBL
| Assay Description
Inhibition of 17,20 lyase activity in human |
Bioorg Med Chem 19: 1751-70 (2011)
Article DOI: 10.1016/j.bmc.2011.01.017
BindingDB Entry DOI: 10.7270/Q2X92C9F |
More data for this
Ligand-Target Pair | |
Sodium channel subunit beta-2
(Mus musculus) | BDBM50613336
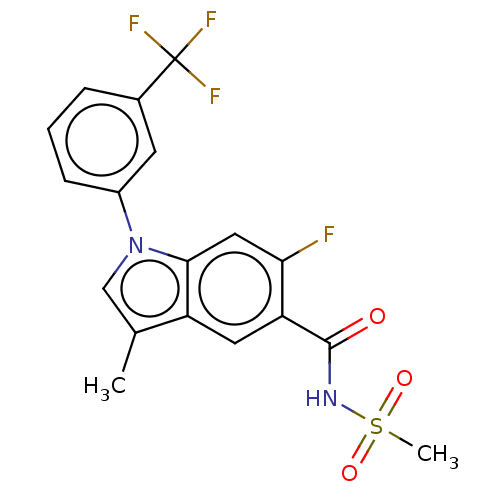
(CHEMBL5276801)Show SMILES Cc1cn(-c2cccc(c2)C(F)(F)F)c2cc(F)c(cc12)C(=O)NS(C)(=O)=O | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 16 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Steroid 17-alpha-hydroxylase/17,20 lyase
(Homo sapiens (Human)) | BDBM50338363

(CHEMBL1682893 | rac-6-[1-Hydroxy-1-(1H-imidazol-4-...)Show SMILES CNC(=O)c1ccc2cc(ccc2c1)C(O)(C(C)C)c1cnc[nH]1 Show InChI InChI=1S/C19H21N3O2/c1-12(2)19(24,17-10-21-11-22-17)16-7-6-13-8-15(18(23)20-3)5-4-14(13)9-16/h4-12,24H,1-3H3,(H,20,23)(H,21,22) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 16 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company, Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of 17,20-lyase activity of human CYP17A1 |
Bioorg Med Chem 19: 6383-99 (2011)
Article DOI: 10.1016/j.bmc.2011.08.066
BindingDB Entry DOI: 10.7270/Q2X63NDF |
More data for this
Ligand-Target Pair | |
Steroid 17-alpha-hydroxylase/17,20 lyase
(Homo sapiens (Human)) | BDBM50338363

(CHEMBL1682893 | rac-6-[1-Hydroxy-1-(1H-imidazol-4-...)Show SMILES CNC(=O)c1ccc2cc(ccc2c1)C(O)(C(C)C)c1cnc[nH]1 Show InChI InChI=1S/C19H21N3O2/c1-12(2)19(24,17-10-21-11-22-17)16-7-6-13-8-15(18(23)20-3)5-4-14(13)9-16/h4-12,24H,1-3H3,(H,20,23)(H,21,22) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 16 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company, Ltd
Curated by ChEMBL
| Assay Description
Inhibition of 17,20 lyase activity in human |
Bioorg Med Chem 19: 1751-70 (2011)
Article DOI: 10.1016/j.bmc.2011.01.017
BindingDB Entry DOI: 10.7270/Q2X92C9F |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data