 Found 716 hits with Last Name = 'kondo' and Initial = 't'
Found 716 hits with Last Name = 'kondo' and Initial = 't' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Tryptase beta-2/delta/gamma
(Homo sapiens (Human)) | BDBM50083552

(1,9-di{4-[5-amino(imino)methylbenzo[b]furan-2-ylca...)Show SMILES NC(=N)c1ccc2oc(cc2c1)C(=O)N1CCN(CC1)C(=O)CCCCCCCC(=O)N1CCN(CC1)C(=O)c1cc2cc(ccc2o1)C(N)=N Show InChI InChI=1S/C37H44N8O6/c38-34(39)24-8-10-28-26(20-24)22-30(50-28)36(48)44-16-12-42(13-17-44)32(46)6-4-2-1-3-5-7-33(47)43-14-18-45(19-15-43)37(49)31-23-27-21-25(35(40)41)9-11-29(27)51-31/h8-11,20-23H,1-7,12-19H2,(H3,38,39)(H3,40,41) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.0190 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Yoshitomi Pharmaceutical Industries, LTD.
Curated by ChEMBL
| Assay Description
Inhibition of tryptase activity |
Bioorg Med Chem Lett 9: 3285-90 (2000)
BindingDB Entry DOI: 10.7270/Q2TX3DK3 |
More data for this
Ligand-Target Pair | |
Tryptase beta-2/delta/gamma
(Homo sapiens (Human)) | BDBM50083561

(1-{4-[5-amino(imino)methylbenzo[b]thiophen-2-ylcar...)Show SMILES NC(=N)c1ccc2sc(cc2c1)C(=O)N1CCN(CC1)C(=O)COc1ccc(OCC(=O)N2CCN(CC2)C(=O)c2cc3cc(ccc3s2)C(N)=N)cc1 Show InChI InChI=1S/C38H38N8O6S2/c39-35(40)23-1-7-29-25(17-23)19-31(53-29)37(49)45-13-9-43(10-14-45)33(47)21-51-27-3-5-28(6-4-27)52-22-34(48)44-11-15-46(16-12-44)38(50)32-20-26-18-24(36(41)42)2-8-30(26)54-32/h1-8,17-20H,9-16,21-22H2,(H3,39,40)(H3,41,42) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| PubMed
| 0.0280 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Yoshitomi Pharmaceutical Industries, LTD.
Curated by ChEMBL
| Assay Description
Inhibition of tryptase activity |
Bioorg Med Chem Lett 9: 3285-90 (2000)
BindingDB Entry DOI: 10.7270/Q2TX3DK3 |
More data for this
Ligand-Target Pair | |
Tryptase beta-2/delta/gamma
(Homo sapiens (Human)) | BDBM50083556
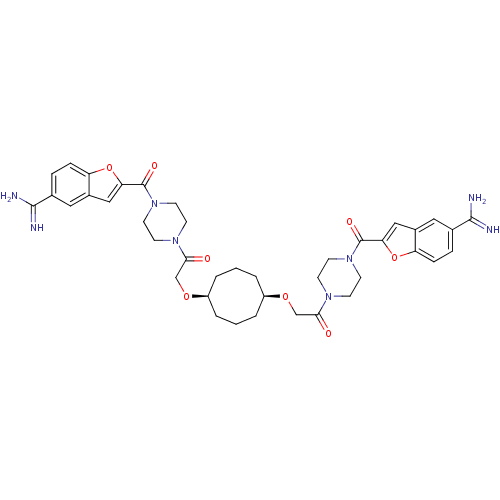
(1-{4-[5-amino(imino)methylbenzo[b]furan-2-ylcarbon...)Show SMILES NC(=N)c1ccc2oc(cc2c1)C(=O)N1CCN(CC1)C(=O)CO[C@H]1CCC[C@H](CCC1)OCC(=O)N1CCN(CC1)C(=O)c1cc2cc(ccc2o1)C(N)=N Show InChI InChI=1S/C40H48N8O8/c41-37(42)25-7-9-31-27(19-25)21-33(55-31)39(51)47-15-11-45(12-16-47)35(49)23-53-29-3-1-4-30(6-2-5-29)54-24-36(50)46-13-17-48(18-14-46)40(52)34-22-28-20-26(38(43)44)8-10-32(28)56-34/h7-10,19-22,29-30H,1-6,11-18,23-24H2,(H3,41,42)(H3,43,44)/t29-,30+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| PubMed
| 0.0290 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Yoshitomi Pharmaceutical Industries, LTD.
Curated by ChEMBL
| Assay Description
Inhibition of tryptase activity |
Bioorg Med Chem Lett 9: 3285-90 (2000)
BindingDB Entry DOI: 10.7270/Q2TX3DK3 |
More data for this
Ligand-Target Pair | |
Tryptase beta-2/delta/gamma
(Homo sapiens (Human)) | BDBM50083541
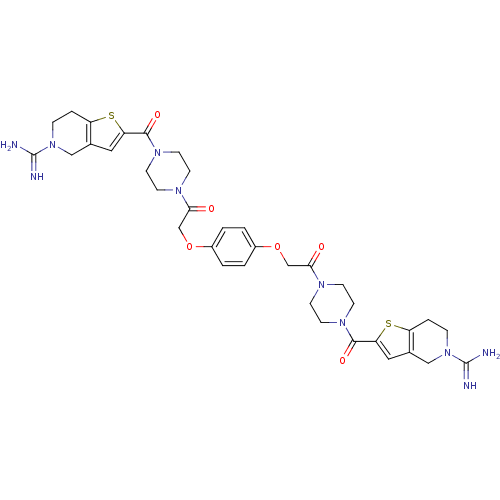
(1-{4-[5-amino(imino)methyl-4,5,6,7-tetrahydrothien...)Show SMILES NC(=N)N1CCc2sc(cc2C1)C(=O)N1CCN(CC1)C(=O)COc1ccc(OCC(=O)N2CCN(CC2)C(=O)c2cc3CN(CCc3s2)C(N)=N)cc1 Show InChI InChI=1S/C36H44N10O6S2/c37-35(38)45-7-5-27-23(19-45)17-29(53-27)33(49)43-13-9-41(10-14-43)31(47)21-51-25-1-2-26(4-3-25)52-22-32(48)42-11-15-44(16-12-42)34(50)30-18-24-20-46(36(39)40)8-6-28(24)54-30/h1-4,17-18H,5-16,19-22H2,(H3,37,38)(H3,39,40) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.0460 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Yoshitomi Pharmaceutical Industries, LTD.
Curated by ChEMBL
| Assay Description
Inhibition of tryptase activity |
Bioorg Med Chem Lett 9: 3285-90 (2000)
BindingDB Entry DOI: 10.7270/Q2TX3DK3 |
More data for this
Ligand-Target Pair | |
Tryptase beta-2/delta/gamma
(Homo sapiens (Human)) | BDBM50083548

(1-{4-[5-amino(imino)methylbenzo[b]furan-2-ylcarbon...)Show SMILES NC(=N)c1ccc2oc(cc2c1)C(=O)N1CCN(CC1)C(=O)COc1ccc(OCC(=O)N2CCN(CC2)C(=O)c2cc3cc(ccc3o2)C(N)=N)cc1 Show InChI InChI=1S/C38H38N8O8/c39-35(40)23-1-7-29-25(17-23)19-31(53-29)37(49)45-13-9-43(10-14-45)33(47)21-51-27-3-5-28(6-4-27)52-22-34(48)44-11-15-46(16-12-44)38(50)32-20-26-18-24(36(41)42)2-8-30(26)54-32/h1-8,17-20H,9-16,21-22H2,(H3,39,40)(H3,41,42) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.0570 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Yoshitomi Pharmaceutical Industries, LTD.
Curated by ChEMBL
| Assay Description
Inhibition of tryptase activity |
Bioorg Med Chem Lett 9: 3285-90 (2000)
BindingDB Entry DOI: 10.7270/Q2TX3DK3 |
More data for this
Ligand-Target Pair | |
Tryptase beta-2/delta/gamma
(Homo sapiens (Human)) | BDBM50217306

(CHEMBL112049)Show SMILES NC(=N)c1ccc2oc(cc2c1)C(=O)N1CCN(CC1)C(=O)CCCCCCC(=O)N1CCN(CC1)C(=O)c1cc2cc(ccc2o1)C(N)=N Show InChI InChI=1S/C36H42N8O6/c37-33(38)23-7-9-27-25(19-23)21-29(49-27)35(47)43-15-11-41(12-16-43)31(45)5-3-1-2-4-6-32(46)42-13-17-44(18-14-42)36(48)30-22-26-20-24(34(39)40)8-10-28(26)50-30/h7-10,19-22H,1-6,11-18H2,(H3,37,38)(H3,39,40) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.120 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Yoshitomi Pharmaceutical Industries, LTD.
Curated by ChEMBL
| Assay Description
Inhibition of tryptase activity |
Bioorg Med Chem Lett 9: 3285-90 (2000)
BindingDB Entry DOI: 10.7270/Q2TX3DK3 |
More data for this
Ligand-Target Pair | |
Sphingosine 1-phosphate receptor 5
(Homo sapiens (Human)) | BDBM50250631

(CHEMBL4093489)Show SMILES CCCc1ccc(COc2ccc3C(C)=C(CN4CC(C4)C(O)=O)CCc3c2)c(OC)c1 |t:14| Show InChI InChI=1S/C27H33NO4/c1-4-5-19-6-7-22(26(12-19)31-3)17-32-24-10-11-25-18(2)21(9-8-20(25)13-24)14-28-15-23(16-28)27(29)30/h6-7,10-13,23H,4-5,8-9,14-17H2,1-3H3,(H,29,30) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.574 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ono Pharmaceutical Co., Ltd
Curated by ChEMBL
| Assay Description
Displacement of [33P]-S1P from human S1P5 receptor expressed in CHO-K1 cells after 60 mins by scintillation counting method |
J Med Chem 60: 9508-9530 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00785
BindingDB Entry DOI: 10.7270/Q2J968SK |
More data for this
Ligand-Target Pair | |
Sphingosine 1-phosphate receptor 1
(Homo sapiens (Human)) | BDBM50250631

(CHEMBL4093489)Show SMILES CCCc1ccc(COc2ccc3C(C)=C(CN4CC(C4)C(O)=O)CCc3c2)c(OC)c1 |t:14| Show InChI InChI=1S/C27H33NO4/c1-4-5-19-6-7-22(26(12-19)31-3)17-32-24-10-11-25-18(2)21(9-8-20(25)13-24)14-28-15-23(16-28)27(29)30/h6-7,10-13,23H,4-5,8-9,14-17H2,1-3H3,(H,29,30) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.626 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ono Pharmaceutical Co., Ltd
Curated by ChEMBL
| Assay Description
Displacement of [33P]-S1P from human S1P1 receptor expressed in CHO-K1 cells after 60 mins by scintillation counting method |
J Med Chem 60: 9508-9530 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00785
BindingDB Entry DOI: 10.7270/Q2J968SK |
More data for this
Ligand-Target Pair | |
Sphingosine 1-phosphate receptor 1
(Rattus norvegicus) | BDBM50250631

(CHEMBL4093489)Show SMILES CCCc1ccc(COc2ccc3C(C)=C(CN4CC(C4)C(O)=O)CCc3c2)c(OC)c1 |t:14| Show InChI InChI=1S/C27H33NO4/c1-4-5-19-6-7-22(26(12-19)31-3)17-32-24-10-11-25-18(2)21(9-8-20(25)13-24)14-28-15-23(16-28)27(29)30/h6-7,10-13,23H,4-5,8-9,14-17H2,1-3H3,(H,29,30) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.772 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ono Pharmaceutical Co., Ltd
Curated by ChEMBL
| Assay Description
Displacement of [33P]-S1P from rat S1P1 receptor after 60 mins by scintillation counting method |
J Med Chem 60: 9508-9530 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00785
BindingDB Entry DOI: 10.7270/Q2J968SK |
More data for this
Ligand-Target Pair | |
Corticotropin-releasing factor receptor 1
(Homo sapiens (Human)) | BDBM50087713
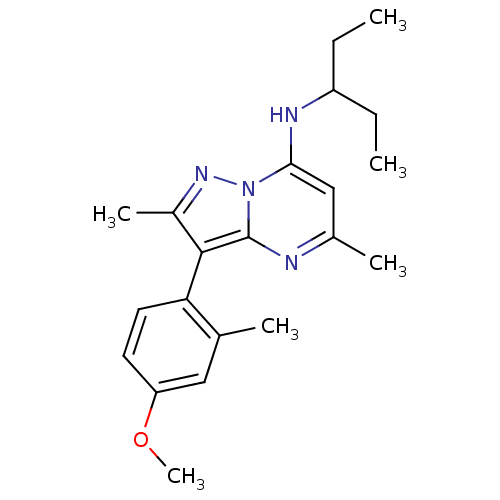
((1-Ethyl-propyl)-[3-(4-methoxy-2-methyl-phenyl)-2,...)Show SMILES CCC(CC)Nc1cc(C)nc2c(c(C)nn12)-c1ccc(OC)cc1C |(-4.03,1.25,;-2.68,.48,;-1.35,1.27,;-1.37,2.81,;-2.86,3.21,;-.02,.5,;-.02,-1.04,;-1.34,-1.81,;-1.34,-3.35,;-2.66,-4.12,;,-4.12,;1.33,-3.35,;2.8,-3.8,;3.7,-2.55,;5.24,-2.53,;2.77,-1.32,;1.33,-1.81,;3.3,-5.26,;2.28,-6.39,;2.77,-7.87,;4.28,-8.16,;4.78,-9.63,;3.76,-10.79,;5.3,-7,;4.79,-5.55,;5.82,-4.4,)| Show InChI InChI=1S/C21H28N4O/c1-7-16(8-2)23-19-12-14(4)22-21-20(15(5)24-25(19)21)18-10-9-17(26-6)11-13(18)3/h9-12,16,23H,7-8H2,1-6H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Minase Research Institute
Curated by ChEMBL
| Assay Description
Displacement of [125I]CRF from human corticotropin-releasing factor receptor 1 expressed in CHO-K1 cells after 2 hrs by gamma counting |
Bioorg Med Chem 19: 5432-45 (2011)
Article DOI: 10.1016/j.bmc.2011.07.055
BindingDB Entry DOI: 10.7270/Q2Z038JX |
More data for this
Ligand-Target Pair | |
Tryptase beta-2/delta/gamma
(Homo sapiens (Human)) | BDBM50083549
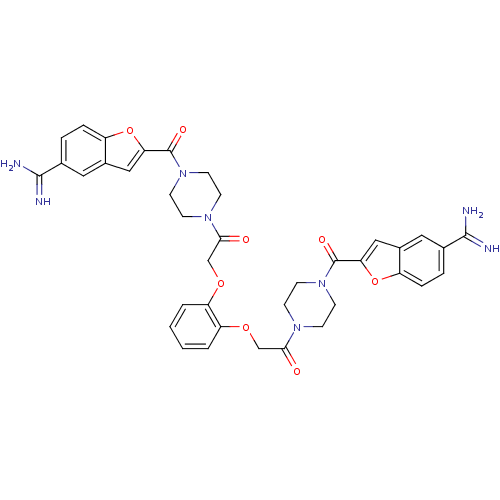
(1-{4-[5-amino(imino)methylbenzo[b]furan-2-ylcarbon...)Show SMILES NC(=N)c1ccc2oc(cc2c1)C(=O)N1CCN(CC1)C(=O)COc1ccccc1OCC(=O)N1CCN(CC1)C(=O)c1cc2cc(ccc2o1)C(N)=N Show InChI InChI=1S/C38H38N8O8/c39-35(40)23-5-7-27-25(17-23)19-31(53-27)37(49)45-13-9-43(10-14-45)33(47)21-51-29-3-1-2-4-30(29)52-22-34(48)44-11-15-46(16-12-44)38(50)32-20-26-18-24(36(41)42)6-8-28(26)54-32/h1-8,17-20H,9-16,21-22H2,(H3,39,40)(H3,41,42) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 1.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Yoshitomi Pharmaceutical Industries, LTD.
Curated by ChEMBL
| Assay Description
Inhibition of tryptase activity |
Bioorg Med Chem Lett 9: 3285-90 (2000)
BindingDB Entry DOI: 10.7270/Q2TX3DK3 |
More data for this
Ligand-Target Pair | |
Sphingosine 1-phosphate receptor 4
(Homo sapiens (Human)) | BDBM50250631

(CHEMBL4093489)Show SMILES CCCc1ccc(COc2ccc3C(C)=C(CN4CC(C4)C(O)=O)CCc3c2)c(OC)c1 |t:14| Show InChI InChI=1S/C27H33NO4/c1-4-5-19-6-7-22(26(12-19)31-3)17-32-24-10-11-25-18(2)21(9-8-20(25)13-24)14-28-15-23(16-28)27(29)30/h6-7,10-13,23H,4-5,8-9,14-17H2,1-3H3,(H,29,30) | NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 29 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ono Pharmaceutical Co., Ltd
Curated by ChEMBL
| Assay Description
Displacement of [33P]-S1P from human S1P4 receptor expressed in CHO-K1 cells after 60 mins by scintillation counting method |
J Med Chem 60: 9508-9530 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00785
BindingDB Entry DOI: 10.7270/Q2J968SK |
More data for this
Ligand-Target Pair | |
Tryptase beta-2/delta/gamma
(Homo sapiens (Human)) | BDBM50217313

(CHEMBL110856)Show SMILES NC(=N)c1ccc2oc(cc2c1)C(=O)N1CCN(CC1)C(=O)COc1ccc(OCC(=O)N2CCCCC2)cc1 Show InChI InChI=1S/C29H33N5O6/c30-28(31)20-4-9-24-21(16-20)17-25(40-24)29(37)34-14-12-33(13-15-34)27(36)19-39-23-7-5-22(6-8-23)38-18-26(35)32-10-2-1-3-11-32/h4-9,16-17H,1-3,10-15,18-19H2,(H3,30,31) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Yoshitomi Pharmaceutical Industries, LTD.
Curated by ChEMBL
| Assay Description
Inhibition of tryptase activity |
Bioorg Med Chem Lett 9: 3285-90 (2000)
BindingDB Entry DOI: 10.7270/Q2TX3DK3 |
More data for this
Ligand-Target Pair | |
Tryptase beta-2/delta/gamma
(Homo sapiens (Human)) | BDBM50217310
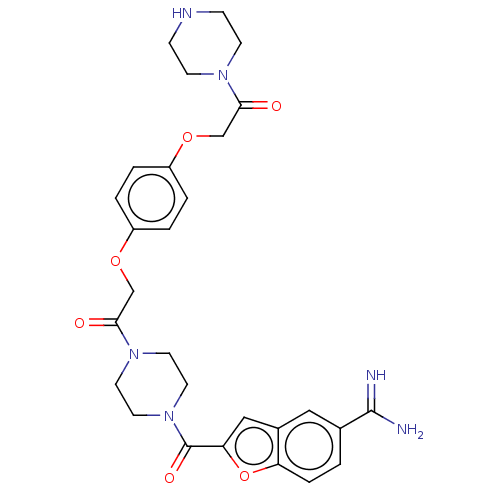
(CHEMBL109688)Show SMILES NC(=N)c1ccc2oc(cc2c1)C(=O)N1CCN(CC1)C(=O)COc1ccc(OCC(=O)N2CCNCC2)cc1 Show InChI InChI=1S/C28H32N6O6/c29-27(30)19-1-6-23-20(15-19)16-24(40-23)28(37)34-13-11-33(12-14-34)26(36)18-39-22-4-2-21(3-5-22)38-17-25(35)32-9-7-31-8-10-32/h1-6,15-16,31H,7-14,17-18H2,(H3,29,30) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 67 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Yoshitomi Pharmaceutical Industries, LTD.
Curated by ChEMBL
| Assay Description
Inhibition of tryptase activity |
Bioorg Med Chem Lett 9: 3285-90 (2000)
BindingDB Entry DOI: 10.7270/Q2TX3DK3 |
More data for this
Ligand-Target Pair | |
Tryptase beta-2/delta/gamma
(Homo sapiens (Human)) | BDBM50217308

(CHEMBL424436)Show SMILES CCOC(=O)COc1ccc(OCC(=O)N2CCN(CC2)C(=O)c2cc3cc(ccc3o2)C(N)=N)cc1 Show InChI InChI=1S/C26H28N4O7/c1-2-34-24(32)16-36-20-6-4-19(5-7-20)35-15-23(31)29-9-11-30(12-10-29)26(33)22-14-18-13-17(25(27)28)3-8-21(18)37-22/h3-8,13-14H,2,9-12,15-16H2,1H3,(H3,27,28) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 210 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Yoshitomi Pharmaceutical Industries, LTD.
Curated by ChEMBL
| Assay Description
Inhibition of tryptase activity |
Bioorg Med Chem Lett 9: 3285-90 (2000)
BindingDB Entry DOI: 10.7270/Q2TX3DK3 |
More data for this
Ligand-Target Pair | |
Tryptase beta-2/delta/gamma
(Homo sapiens (Human)) | BDBM50217305

(CHEMBL323935)Show SMILES NC(=N)c1ccc2oc(cc2c1)C(=O)N1CCN(CC1)C(=O)N1CCN(CC1)C(=O)c1cc2cc(ccc2o1)C(N)=N Show InChI InChI=1S/C29H30N8O5/c30-25(31)17-1-3-21-19(13-17)15-23(41-21)27(38)34-5-9-36(10-6-34)29(40)37-11-7-35(8-12-37)28(39)24-16-20-14-18(26(32)33)2-4-22(20)42-24/h1-4,13-16H,5-12H2,(H3,30,31)(H3,32,33) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 460 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Yoshitomi Pharmaceutical Industries, LTD.
Curated by ChEMBL
| Assay Description
Inhibition of tryptase activity |
Bioorg Med Chem Lett 9: 3285-90 (2000)
BindingDB Entry DOI: 10.7270/Q2TX3DK3 |
More data for this
Ligand-Target Pair | |
Tryptase beta-2/delta/gamma
(Homo sapiens (Human)) | BDBM50217315
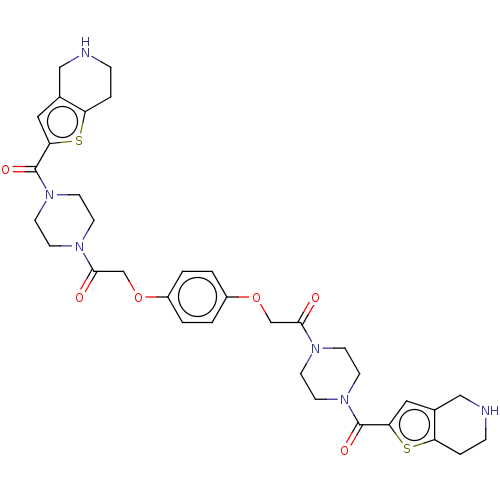
(CHEMBL30862)Show SMILES O=C(COc1ccc(OCC(=O)N2CCN(CC2)C(=O)c2cc3CNCCc3s2)cc1)N1CCN(CC1)C(=O)c1cc2CNCCc2s1 Show InChI InChI=1S/C34H40N6O6S2/c41-31(37-9-13-39(14-10-37)33(43)29-17-23-19-35-7-5-27(23)47-29)21-45-25-1-2-26(4-3-25)46-22-32(42)38-11-15-40(16-12-38)34(44)30-18-24-20-36-8-6-28(24)48-30/h1-4,17-18,35-36H,5-16,19-22H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 970 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Yoshitomi Pharmaceutical Industries, LTD.
Curated by ChEMBL
| Assay Description
Inhibition of tryptase activity |
Bioorg Med Chem Lett 9: 3285-90 (2000)
BindingDB Entry DOI: 10.7270/Q2TX3DK3 |
More data for this
Ligand-Target Pair | |
Tryptase beta-2/delta/gamma
(Homo sapiens (Human)) | BDBM50217312
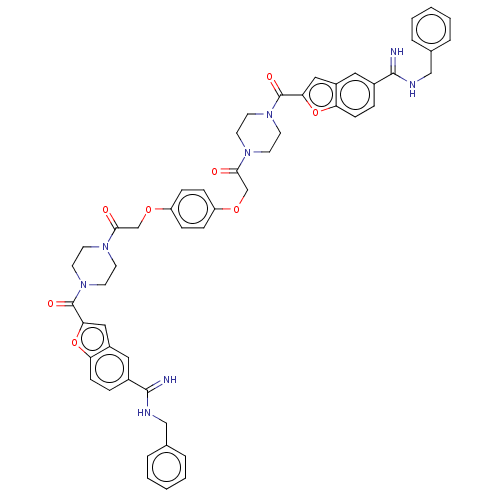
(CHEMBL324918)Show SMILES N=C(NCc1ccccc1)c1ccc2oc(cc2c1)C(=O)N1CCN(CC1)C(=O)COc1ccc(OCC(=O)N2CCN(CC2)C(=O)c2cc3cc(ccc3o2)C(=N)NCc2ccccc2)cc1 Show InChI InChI=1S/C52H50N8O8/c53-49(55-31-35-7-3-1-4-8-35)37-11-17-43-39(27-37)29-45(67-43)51(63)59-23-19-57(20-24-59)47(61)33-65-41-13-15-42(16-14-41)66-34-48(62)58-21-25-60(26-22-58)52(64)46-30-40-28-38(12-18-44(40)68-46)50(54)56-32-36-9-5-2-6-10-36/h1-18,27-30H,19-26,31-34H2,(H2,53,55)(H2,54,56) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 1.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Yoshitomi Pharmaceutical Industries, LTD.
Curated by ChEMBL
| Assay Description
Inhibition of tryptase activity |
Bioorg Med Chem Lett 9: 3285-90 (2000)
BindingDB Entry DOI: 10.7270/Q2TX3DK3 |
More data for this
Ligand-Target Pair | |
Tryptase beta-2/delta/gamma
(Homo sapiens (Human)) | BDBM50083563
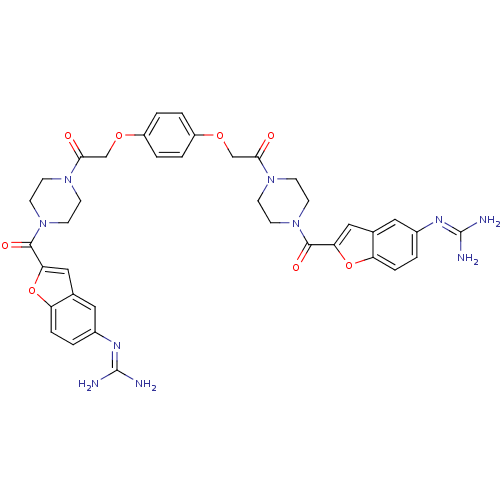
(1-{4-[5-amino(imino)methylaminobenzo[b]furan-2-ylc...)Show SMILES [#7]\[#6](-[#7])=[#7]/c1ccc2oc(cc2c1)-[#6](=O)-[#7]-1-[#6]-[#6]-[#7](-[#6]-[#6]-1)-[#6](=O)-[#6]-[#8]-c1ccc(-[#8]-[#6]-[#6](=O)-[#7]-2-[#6]-[#6]-[#7](-[#6]-[#6]-2)-[#6](=O)-c2cc3cc(ccc3o2)\[#7]=[#6](/[#7])-[#7])cc1 Show InChI InChI=1S/C38H40N10O8/c39-37(40)43-25-1-7-29-23(17-25)19-31(55-29)35(51)47-13-9-45(10-14-47)33(49)21-53-27-3-5-28(6-4-27)54-22-34(50)46-11-15-48(16-12-46)36(52)32-20-24-18-26(44-38(41)42)2-8-30(24)56-32/h1-8,17-20H,9-16,21-22H2,(H4,39,40,43)(H4,41,42,44) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| PubMed
| 1.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Yoshitomi Pharmaceutical Industries, LTD.
Curated by ChEMBL
| Assay Description
Inhibition of tryptase activity |
Bioorg Med Chem Lett 9: 3285-90 (2000)
BindingDB Entry DOI: 10.7270/Q2TX3DK3 |
More data for this
Ligand-Target Pair | |
Tryptase beta-2/delta/gamma
(Homo sapiens (Human)) | BDBM50217317
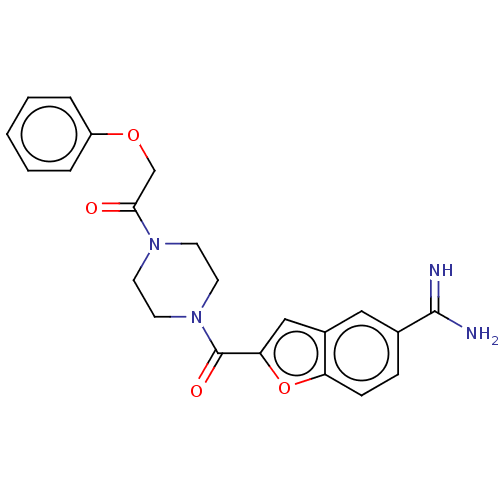
(CHEMBL109903)Show SMILES NC(=N)c1ccc2oc(cc2c1)C(=O)N1CCN(CC1)C(=O)COc1ccccc1 Show InChI InChI=1S/C22H22N4O4/c23-21(24)15-6-7-18-16(12-15)13-19(30-18)22(28)26-10-8-25(9-11-26)20(27)14-29-17-4-2-1-3-5-17/h1-7,12-13H,8-11,14H2,(H3,23,24) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 3.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Yoshitomi Pharmaceutical Industries, LTD.
Curated by ChEMBL
| Assay Description
Inhibition of tryptase activity |
Bioorg Med Chem Lett 9: 3285-90 (2000)
BindingDB Entry DOI: 10.7270/Q2TX3DK3 |
More data for this
Ligand-Target Pair | |
Prothrombin
(Homo sapiens (Human)) | BDBM50083562
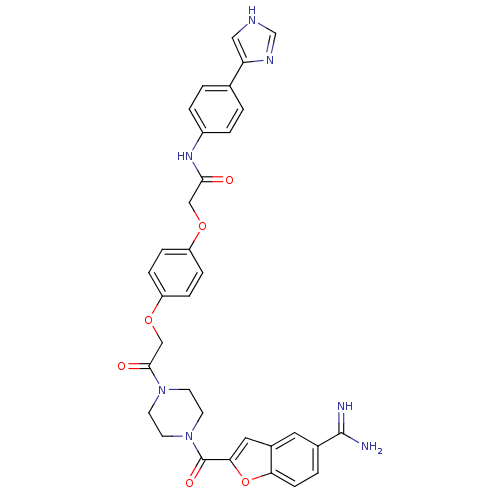
(2-(4-{2-[4-(5-Carbamimidoyl-benzofuran-2-carbonyl)...)Show SMILES NC(=N)c1ccc2oc(cc2c1)C(=O)N1CCN(CC1)C(=O)COc1ccc(OCC(=O)Nc2ccc(cc2)-c2c[nH]cn2)cc1 Show InChI InChI=1S/C33H31N7O6/c34-32(35)22-3-10-28-23(15-22)16-29(46-28)33(43)40-13-11-39(12-14-40)31(42)19-45-26-8-6-25(7-9-26)44-18-30(41)38-24-4-1-21(2-5-24)27-17-36-20-37-27/h1-10,15-17,20H,11-14,18-19H2,(H3,34,35)(H,36,37)(H,38,41) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| PubMed
| 5.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Yoshitomi Pharmaceutical Industries, LTD.
Curated by ChEMBL
| Assay Description
Inhibition of thrombin |
Bioorg Med Chem Lett 9: 3285-90 (2000)
BindingDB Entry DOI: 10.7270/Q2TX3DK3 |
More data for this
Ligand-Target Pair | |
Sphingosine 1-phosphate receptor 2
(Homo sapiens (Human)) | BDBM50250631

(CHEMBL4093489)Show SMILES CCCc1ccc(COc2ccc3C(C)=C(CN4CC(C4)C(O)=O)CCc3c2)c(OC)c1 |t:14| Show InChI InChI=1S/C27H33NO4/c1-4-5-19-6-7-22(26(12-19)31-3)17-32-24-10-11-25-18(2)21(9-8-20(25)13-24)14-28-15-23(16-28)27(29)30/h6-7,10-13,23H,4-5,8-9,14-17H2,1-3H3,(H,29,30) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >5.45E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ono Pharmaceutical Co., Ltd
Curated by ChEMBL
| Assay Description
Displacement of [33P]-S1P from human S1P2 receptor expressed in CHO-K1 cells after 60 mins by scintillation counting method |
J Med Chem 60: 9508-9530 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00785
BindingDB Entry DOI: 10.7270/Q2J968SK |
More data for this
Ligand-Target Pair | |
Sphingosine 1-phosphate receptor 3
(Homo sapiens (Human)) | BDBM50250631

(CHEMBL4093489)Show SMILES CCCc1ccc(COc2ccc3C(C)=C(CN4CC(C4)C(O)=O)CCc3c2)c(OC)c1 |t:14| Show InChI InChI=1S/C27H33NO4/c1-4-5-19-6-7-22(26(12-19)31-3)17-32-24-10-11-25-18(2)21(9-8-20(25)13-24)14-28-15-23(16-28)27(29)30/h6-7,10-13,23H,4-5,8-9,14-17H2,1-3H3,(H,29,30) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >5.63E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ono Pharmaceutical Co., Ltd
Curated by ChEMBL
| Assay Description
Displacement of [33P]-S1P from human S1P3 receptor expressed in CHO-K1 cells after 60 mins by scintillation counting method |
J Med Chem 60: 9508-9530 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00785
BindingDB Entry DOI: 10.7270/Q2J968SK |
More data for this
Ligand-Target Pair | |
Tryptase beta-2/delta/gamma
(Homo sapiens (Human)) | BDBM50217309
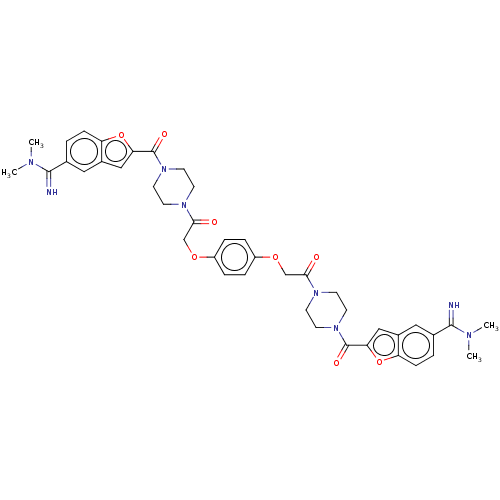
(CHEMBL111451)Show SMILES CN(C)C(=N)c1ccc2oc(cc2c1)C(=O)N1CCN(CC1)C(=O)COc1ccc(OCC(=O)N2CCN(CC2)C(=O)c2cc3cc(ccc3o2)C(=N)N(C)C)cc1 Show InChI InChI=1S/C42H46N8O8/c1-45(2)39(43)27-5-11-33-29(21-27)23-35(57-33)41(53)49-17-13-47(14-18-49)37(51)25-55-31-7-9-32(10-8-31)56-26-38(52)48-15-19-50(20-16-48)42(54)36-24-30-22-28(40(44)46(3)4)6-12-34(30)58-36/h5-12,21-24,43-44H,13-20,25-26H2,1-4H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 6.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Yoshitomi Pharmaceutical Industries, LTD.
Curated by ChEMBL
| Assay Description
Inhibition of tryptase activity |
Bioorg Med Chem Lett 9: 3285-90 (2000)
BindingDB Entry DOI: 10.7270/Q2TX3DK3 |
More data for this
Ligand-Target Pair | |
Tryptase beta-2/delta/gamma
(Homo sapiens (Human)) | BDBM50217314
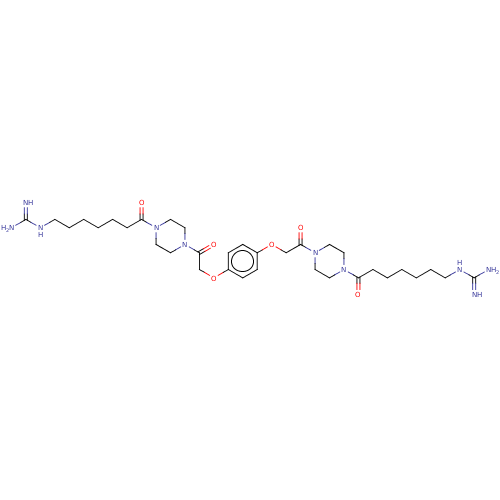
(CHEMBL112894)Show SMILES NC(=N)NCCCCCCC(=O)N1CCN(CC1)C(=O)COc1ccc(OCC(=O)N2CCN(CC2)C(=O)CCCCCCNC(N)=N)cc1 Show InChI InChI=1S/C34H56N10O6/c35-33(36)39-15-7-3-1-5-9-29(45)41-17-21-43(22-18-41)31(47)25-49-27-11-13-28(14-12-27)50-26-32(48)44-23-19-42(20-24-44)30(46)10-6-2-4-8-16-40-34(37)38/h11-14H,1-10,15-26H2,(H4,35,36,39)(H4,37,38,40) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 1.30E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Yoshitomi Pharmaceutical Industries, LTD.
Curated by ChEMBL
| Assay Description
Inhibition of tryptase activity |
Bioorg Med Chem Lett 9: 3285-90 (2000)
BindingDB Entry DOI: 10.7270/Q2TX3DK3 |
More data for this
Ligand-Target Pair | |
Tryptase beta-2/delta/gamma
(Homo sapiens (Human)) | BDBM50217307
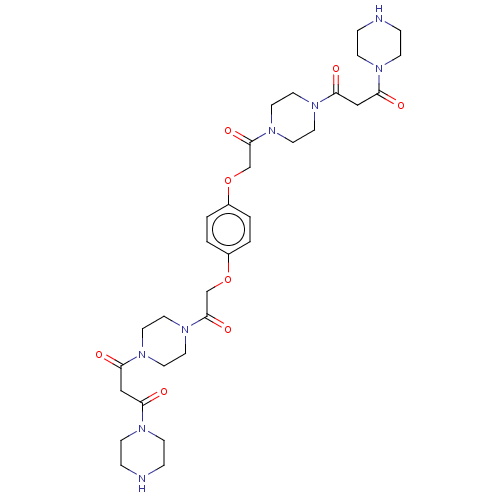
(CHEMBL112900)Show SMILES O=C(COc1ccc(OCC(=O)N2CCN(CC2)C(=O)CC(=O)N2CCNCC2)cc1)N1CCN(CC1)C(=O)CC(=O)N1CCNCC1 Show InChI InChI=1S/C32H46N8O8/c41-27(35-9-5-33-6-10-35)21-29(43)37-13-17-39(18-14-37)31(45)23-47-25-1-2-26(4-3-25)48-24-32(46)40-19-15-38(16-20-40)30(44)22-28(42)36-11-7-34-8-12-36/h1-4,33-34H,5-24H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 1.30E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Yoshitomi Pharmaceutical Industries, LTD.
Curated by ChEMBL
| Assay Description
Inhibition of tryptase activity |
Bioorg Med Chem Lett 9: 3285-90 (2000)
BindingDB Entry DOI: 10.7270/Q2TX3DK3 |
More data for this
Ligand-Target Pair | |
Prothrombin
(Homo sapiens (Human)) | BDBM50083556

(1-{4-[5-amino(imino)methylbenzo[b]furan-2-ylcarbon...)Show SMILES NC(=N)c1ccc2oc(cc2c1)C(=O)N1CCN(CC1)C(=O)CO[C@H]1CCC[C@H](CCC1)OCC(=O)N1CCN(CC1)C(=O)c1cc2cc(ccc2o1)C(N)=N Show InChI InChI=1S/C40H48N8O8/c41-37(42)25-7-9-31-27(19-25)21-33(55-31)39(51)47-15-11-45(12-16-47)35(49)23-53-29-3-1-4-30(6-2-5-29)54-24-36(50)46-13-17-48(18-14-46)40(52)34-22-28-20-26(38(43)44)8-10-32(28)56-34/h7-10,19-22,29-30H,1-6,11-18,23-24H2,(H3,41,42)(H3,43,44)/t29-,30+ | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| PubMed
| 1.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Yoshitomi Pharmaceutical Industries, LTD.
Curated by ChEMBL
| Assay Description
Inhibition of thrombin |
Bioorg Med Chem Lett 9: 3285-90 (2000)
BindingDB Entry DOI: 10.7270/Q2TX3DK3 |
More data for this
Ligand-Target Pair | |
Prothrombin
(Homo sapiens (Human)) | BDBM50083561

(1-{4-[5-amino(imino)methylbenzo[b]thiophen-2-ylcar...)Show SMILES NC(=N)c1ccc2sc(cc2c1)C(=O)N1CCN(CC1)C(=O)COc1ccc(OCC(=O)N2CCN(CC2)C(=O)c2cc3cc(ccc3s2)C(N)=N)cc1 Show InChI InChI=1S/C38H38N8O6S2/c39-35(40)23-1-7-29-25(17-23)19-31(53-29)37(49)45-13-9-43(10-14-45)33(47)21-51-27-3-5-28(6-4-27)52-22-34(48)44-11-15-46(16-12-44)38(50)32-20-26-18-24(36(41)42)2-8-30(26)54-32/h1-8,17-20H,9-16,21-22H2,(H3,39,40)(H3,41,42) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| PubMed
| 2.20E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Yoshitomi Pharmaceutical Industries, LTD.
Curated by ChEMBL
| Assay Description
Inhibition of thrombin |
Bioorg Med Chem Lett 9: 3285-90 (2000)
BindingDB Entry DOI: 10.7270/Q2TX3DK3 |
More data for this
Ligand-Target Pair | |
Tryptase beta-2/delta/gamma
(Homo sapiens (Human)) | BDBM50217316
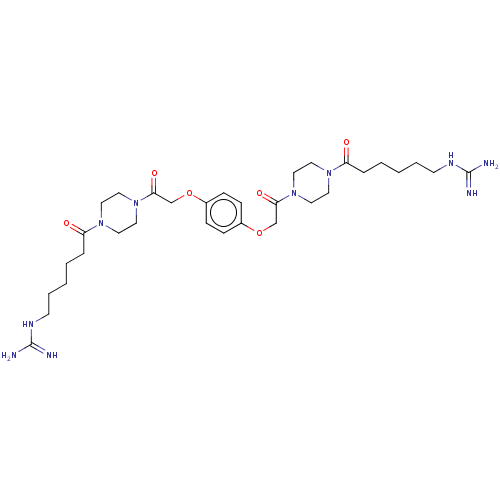
(CHEMBL113084)Show SMILES NC(=N)NCCCCCC(=O)N1CCN(CC1)C(=O)COc1ccc(OCC(=O)N2CCN(CC2)C(=O)CCCCCNC(N)=N)cc1 Show InChI InChI=1S/C32H52N10O6/c33-31(34)37-13-5-1-3-7-27(43)39-15-19-41(20-16-39)29(45)23-47-25-9-11-26(12-10-25)48-24-30(46)42-21-17-40(18-22-42)28(44)8-4-2-6-14-38-32(35)36/h9-12H,1-8,13-24H2,(H4,33,34,37)(H4,35,36,38) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 2.30E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Yoshitomi Pharmaceutical Industries, LTD.
Curated by ChEMBL
| Assay Description
Inhibition of tryptase activity |
Bioorg Med Chem Lett 9: 3285-90 (2000)
BindingDB Entry DOI: 10.7270/Q2TX3DK3 |
More data for this
Ligand-Target Pair | |
Prothrombin
(Homo sapiens (Human)) | BDBM50083547
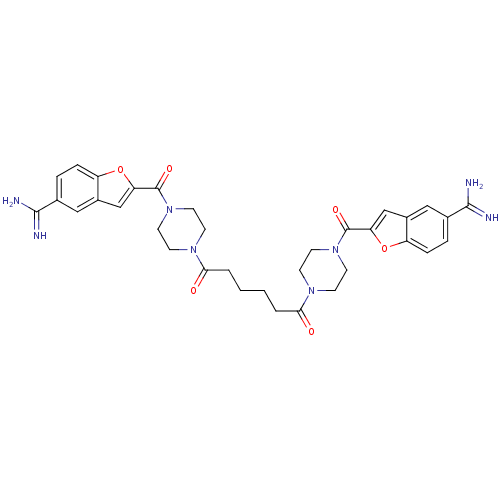
(1,5-di(4-(5-amino(imino)methylbenzo[b]furan-2-ylca...)Show SMILES NC(=N)c1ccc2oc(cc2c1)C(=O)N1CCN(CC1)C(=O)CCCCC(=O)N1CCN(CC1)C(=O)c1cc2cc(ccc2o1)C(N)=N Show InChI InChI=1S/C34H38N8O6/c35-31(36)21-5-7-25-23(17-21)19-27(47-25)33(45)41-13-9-39(10-14-41)29(43)3-1-2-4-30(44)40-11-15-42(16-12-40)34(46)28-20-24-18-22(32(37)38)6-8-26(24)48-28/h5-8,17-20H,1-4,9-16H2,(H3,35,36)(H3,37,38) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 2.30E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Yoshitomi Pharmaceutical Industries, LTD.
Curated by ChEMBL
| Assay Description
Inhibition of thrombin |
Bioorg Med Chem Lett 9: 3285-90 (2000)
BindingDB Entry DOI: 10.7270/Q2TX3DK3 |
More data for this
Ligand-Target Pair | |
Prothrombin
(Homo sapiens (Human)) | BDBM50083563

(1-{4-[5-amino(imino)methylaminobenzo[b]furan-2-ylc...)Show SMILES [#7]\[#6](-[#7])=[#7]/c1ccc2oc(cc2c1)-[#6](=O)-[#7]-1-[#6]-[#6]-[#7](-[#6]-[#6]-1)-[#6](=O)-[#6]-[#8]-c1ccc(-[#8]-[#6]-[#6](=O)-[#7]-2-[#6]-[#6]-[#7](-[#6]-[#6]-2)-[#6](=O)-c2cc3cc(ccc3o2)\[#7]=[#6](/[#7])-[#7])cc1 Show InChI InChI=1S/C38H40N10O8/c39-37(40)43-25-1-7-29-23(17-25)19-31(55-29)35(51)47-13-9-45(10-14-47)33(49)21-53-27-3-5-28(6-4-27)54-22-34(50)46-11-15-48(16-12-46)36(52)32-20-24-18-26(44-38(41)42)2-8-30(24)56-32/h1-8,17-20H,9-16,21-22H2,(H4,39,40,43)(H4,41,42,44) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| PubMed
| 2.40E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Yoshitomi Pharmaceutical Industries, LTD.
Curated by ChEMBL
| Assay Description
Inhibition of thrombin |
Bioorg Med Chem Lett 9: 3285-90 (2000)
BindingDB Entry DOI: 10.7270/Q2TX3DK3 |
More data for this
Ligand-Target Pair | |
Prothrombin
(Homo sapiens (Human)) | BDBM50083557
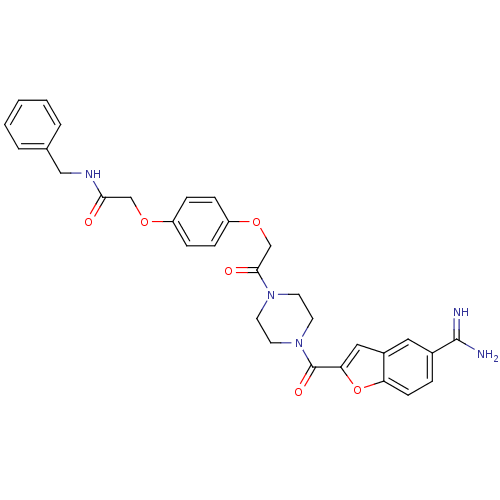
(CHEMBL113531 | N-Benzyl-2-(4-{2-[4-(5-carbamimidoy...)Show SMILES NC(=N)c1ccc2oc(cc2c1)C(=O)N1CCN(CC1)C(=O)COc1ccc(OCC(=O)NCc2ccccc2)cc1 Show InChI InChI=1S/C31H31N5O6/c32-30(33)22-6-11-26-23(16-22)17-27(42-26)31(39)36-14-12-35(13-15-36)29(38)20-41-25-9-7-24(8-10-25)40-19-28(37)34-18-21-4-2-1-3-5-21/h1-11,16-17H,12-15,18-20H2,(H3,32,33)(H,34,37) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 2.80E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Yoshitomi Pharmaceutical Industries, LTD.
Curated by ChEMBL
| Assay Description
Inhibition of thrombin |
Bioorg Med Chem Lett 9: 3285-90 (2000)
BindingDB Entry DOI: 10.7270/Q2TX3DK3 |
More data for this
Ligand-Target Pair | |
Prothrombin
(Homo sapiens (Human)) | BDBM50083541

(1-{4-[5-amino(imino)methyl-4,5,6,7-tetrahydrothien...)Show SMILES NC(=N)N1CCc2sc(cc2C1)C(=O)N1CCN(CC1)C(=O)COc1ccc(OCC(=O)N2CCN(CC2)C(=O)c2cc3CN(CCc3s2)C(N)=N)cc1 Show InChI InChI=1S/C36H44N10O6S2/c37-35(38)45-7-5-27-23(19-45)17-29(53-27)33(49)43-13-9-41(10-14-43)31(47)21-51-25-1-2-26(4-3-25)52-22-32(48)42-11-15-44(16-12-42)34(50)30-18-24-20-46(36(39)40)8-6-28(24)54-30/h1-4,17-18H,5-16,19-22H2,(H3,37,38)(H3,39,40) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 2.90E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Yoshitomi Pharmaceutical Industries, LTD.
Curated by ChEMBL
| Assay Description
Inhibition of thrombin |
Bioorg Med Chem Lett 9: 3285-90 (2000)
BindingDB Entry DOI: 10.7270/Q2TX3DK3 |
More data for this
Ligand-Target Pair | |
Prothrombin
(Homo sapiens (Human)) | BDBM50083542

(1,5-di{4-[5-amino(imino)methylbenzo[b]furan-2-ylca...)Show SMILES NC(=N)c1ccc2oc(cc2c1)C(=O)N1CCN(CC1)C(=O)CCCC(=O)N1CCN(CC1)C(=O)c1cc2cc(ccc2o1)C(N)=N Show InChI InChI=1S/C33H36N8O6/c34-30(35)20-4-6-24-22(16-20)18-26(46-24)32(44)40-12-8-38(9-13-40)28(42)2-1-3-29(43)39-10-14-41(15-11-39)33(45)27-19-23-17-21(31(36)37)5-7-25(23)47-27/h4-7,16-19H,1-3,8-15H2,(H3,34,35)(H3,36,37) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 3.20E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Yoshitomi Pharmaceutical Industries, LTD.
Curated by ChEMBL
| Assay Description
Inhibition of thrombin |
Bioorg Med Chem Lett 9: 3285-90 (2000)
BindingDB Entry DOI: 10.7270/Q2TX3DK3 |
More data for this
Ligand-Target Pair | |
Prothrombin
(Homo sapiens (Human)) | BDBM50083548

(1-{4-[5-amino(imino)methylbenzo[b]furan-2-ylcarbon...)Show SMILES NC(=N)c1ccc2oc(cc2c1)C(=O)N1CCN(CC1)C(=O)COc1ccc(OCC(=O)N2CCN(CC2)C(=O)c2cc3cc(ccc3o2)C(N)=N)cc1 Show InChI InChI=1S/C38H38N8O8/c39-35(40)23-1-7-29-25(17-23)19-31(53-29)37(49)45-13-9-43(10-14-45)33(47)21-51-27-3-5-28(6-4-27)52-22-34(48)44-11-15-46(16-12-44)38(50)32-20-26-18-24(36(41)42)2-8-30(26)54-32/h1-8,17-20H,9-16,21-22H2,(H3,39,40)(H3,41,42) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 3.20E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Yoshitomi Pharmaceutical Industries, LTD.
Curated by ChEMBL
| Assay Description
Inhibition of thrombin |
Bioorg Med Chem Lett 9: 3285-90 (2000)
BindingDB Entry DOI: 10.7270/Q2TX3DK3 |
More data for this
Ligand-Target Pair | |
Prothrombin
(Homo sapiens (Human)) | BDBM50083543
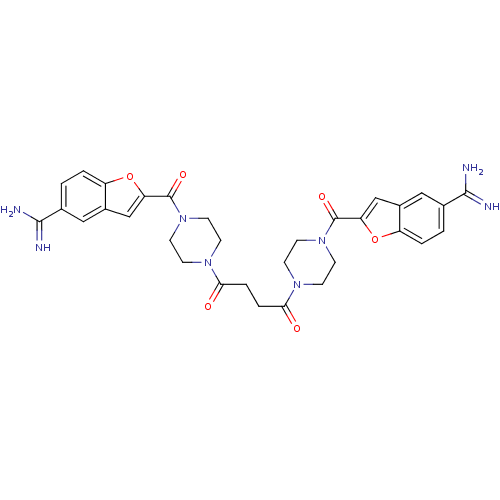
(1,4-di{4-[5-amino(imino)methylbenzo[b]furan-2-ylca...)Show SMILES NC(=N)c1ccc2oc(cc2c1)C(=O)N1CCN(CC1)C(=O)CCC(=O)N1CCN(CC1)C(=O)c1cc2cc(ccc2o1)C(N)=N Show InChI InChI=1S/C32H34N8O6/c33-29(34)19-1-3-23-21(15-19)17-25(45-23)31(43)39-11-7-37(8-12-39)27(41)5-6-28(42)38-9-13-40(14-10-38)32(44)26-18-22-16-20(30(35)36)2-4-24(22)46-26/h1-4,15-18H,5-14H2,(H3,33,34)(H3,35,36) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 5.10E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Yoshitomi Pharmaceutical Industries, LTD.
Curated by ChEMBL
| Assay Description
Inhibition of thrombin |
Bioorg Med Chem Lett 9: 3285-90 (2000)
BindingDB Entry DOI: 10.7270/Q2TX3DK3 |
More data for this
Ligand-Target Pair | |
Tryptase beta-2/delta/gamma
(Homo sapiens (Human)) | BDBM50217311
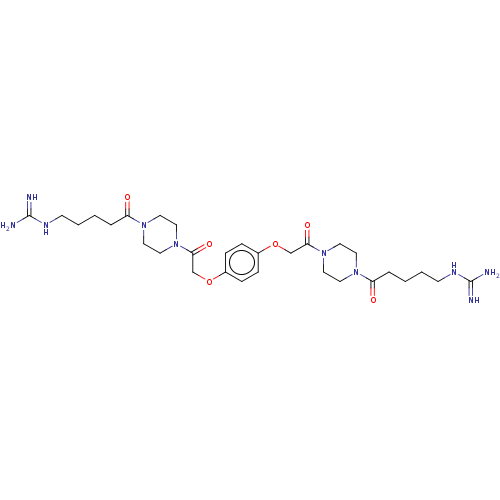
(CHEMBL109660)Show SMILES NC(=N)NCCCCC(=O)N1CCN(CC1)C(=O)COc1ccc(OCC(=O)N2CCN(CC2)C(=O)CCCCNC(N)=N)cc1 Show InChI InChI=1S/C30H48N10O6/c31-29(32)35-11-3-1-5-25(41)37-13-17-39(18-14-37)27(43)21-45-23-7-9-24(10-8-23)46-22-28(44)40-19-15-38(16-20-40)26(42)6-2-4-12-36-30(33)34/h7-10H,1-6,11-22H2,(H4,31,32,35)(H4,33,34,36) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 8.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Yoshitomi Pharmaceutical Industries, LTD.
Curated by ChEMBL
| Assay Description
Inhibition of tryptase activity |
Bioorg Med Chem Lett 9: 3285-90 (2000)
BindingDB Entry DOI: 10.7270/Q2TX3DK3 |
More data for this
Ligand-Target Pair | |
Prothrombin
(Homo sapiens (Human)) | BDBM50083553
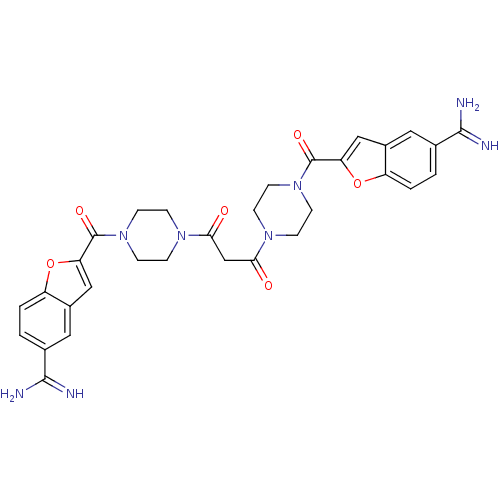
(1,3-di{4-[5-amino(imino)methylbenzo[b]furan-2-ylca...)Show SMILES NC(=N)c1ccc2oc(cc2c1)C(=O)N1CCN(CC1)C(=O)CC(=O)N1CCN(CC1)C(=O)c1cc2cc(ccc2o1)C(N)=N Show InChI InChI=1S/C31H32N8O6/c32-28(33)18-1-3-22-20(13-18)15-24(44-22)30(42)38-9-5-36(6-10-38)26(40)17-27(41)37-7-11-39(12-8-37)31(43)25-16-21-14-19(29(34)35)2-4-23(21)45-25/h1-4,13-16H,5-12,17H2,(H3,32,33)(H3,34,35) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 8.20E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Yoshitomi Pharmaceutical Industries, LTD.
Curated by ChEMBL
| Assay Description
Inhibition of thrombin |
Bioorg Med Chem Lett 9: 3285-90 (2000)
BindingDB Entry DOI: 10.7270/Q2TX3DK3 |
More data for this
Ligand-Target Pair | |
Prothrombin
(Homo sapiens (Human)) | BDBM50083545
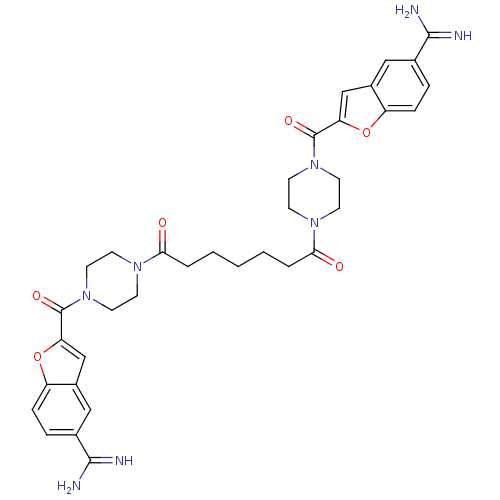
(1,7-di{4-[5-amino(imino)methylbenzo[b]furan-2-ylca...)Show SMILES NC(=N)c1ccc2oc(cc2c1)C(=O)N1CCN(CC1)C(=O)CCCCCC(=O)N1CCN(CC1)C(=O)c1cc2cc(ccc2o1)C(N)=N Show InChI InChI=1S/C35H40N8O6/c36-32(37)22-6-8-26-24(18-22)20-28(48-26)34(46)42-14-10-40(11-15-42)30(44)4-2-1-3-5-31(45)41-12-16-43(17-13-41)35(47)29-21-25-19-23(33(38)39)7-9-27(25)49-29/h6-9,18-21H,1-5,10-17H2,(H3,36,37)(H3,38,39) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 8.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Yoshitomi Pharmaceutical Industries, LTD.
Curated by ChEMBL
| Assay Description
Inhibition of thrombin |
Bioorg Med Chem Lett 9: 3285-90 (2000)
BindingDB Entry DOI: 10.7270/Q2TX3DK3 |
More data for this
Ligand-Target Pair | |
Prothrombin
(Homo sapiens (Human)) | BDBM50083549

(1-{4-[5-amino(imino)methylbenzo[b]furan-2-ylcarbon...)Show SMILES NC(=N)c1ccc2oc(cc2c1)C(=O)N1CCN(CC1)C(=O)COc1ccccc1OCC(=O)N1CCN(CC1)C(=O)c1cc2cc(ccc2o1)C(N)=N Show InChI InChI=1S/C38H38N8O8/c39-35(40)23-5-7-27-25(17-23)19-31(53-27)37(49)45-13-9-43(10-14-45)33(47)21-51-29-3-1-2-4-30(29)52-22-34(48)44-11-15-46(16-12-44)38(50)32-20-26-18-24(36(41)42)6-8-28(26)54-32/h1-8,17-20H,9-16,21-22H2,(H3,39,40)(H3,41,42) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 8.80E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Yoshitomi Pharmaceutical Industries, LTD.
Curated by ChEMBL
| Assay Description
Inhibition of thrombin |
Bioorg Med Chem Lett 9: 3285-90 (2000)
BindingDB Entry DOI: 10.7270/Q2TX3DK3 |
More data for this
Ligand-Target Pair | |
Prothrombin
(Homo sapiens (Human)) | BDBM50083552

(1,9-di{4-[5-amino(imino)methylbenzo[b]furan-2-ylca...)Show SMILES NC(=N)c1ccc2oc(cc2c1)C(=O)N1CCN(CC1)C(=O)CCCCCCCC(=O)N1CCN(CC1)C(=O)c1cc2cc(ccc2o1)C(N)=N Show InChI InChI=1S/C37H44N8O6/c38-34(39)24-8-10-28-26(20-24)22-30(50-28)36(48)44-16-12-42(13-17-44)32(46)6-4-2-1-3-5-7-33(47)43-14-18-45(19-15-43)37(49)31-23-27-21-25(35(40)41)9-11-29(27)51-31/h8-11,20-23H,1-7,12-19H2,(H3,38,39)(H3,40,41) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 2.30E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Yoshitomi Pharmaceutical Industries, LTD.
Curated by ChEMBL
| Assay Description
Inhibition of thrombin |
Bioorg Med Chem Lett 9: 3285-90 (2000)
BindingDB Entry DOI: 10.7270/Q2TX3DK3 |
More data for this
Ligand-Target Pair | |
Coagulation factor XI
(Homo sapiens (Human)) | BDBM335414
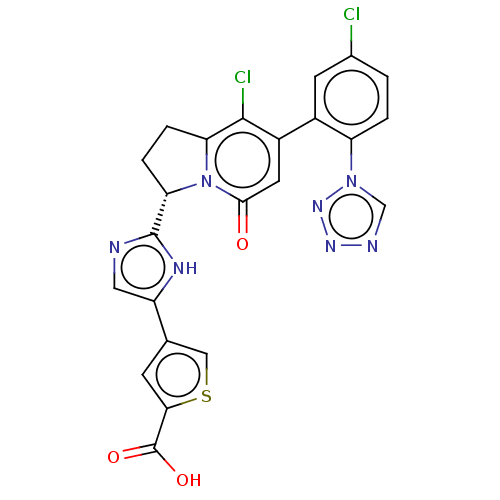
(4-(2-{(3S)-8-chloro-7-[5-chloro-2-(1H-tetrazol-1-y...)Show SMILES OC(=O)c1cc(cs1)-c1cnc([nH]1)[C@@H]1CCc2c(Cl)c(cc(=O)n12)-c1cc(Cl)ccc1-n1cnnn1 |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a |
ONO PHARMACEUTICAL CO., LTD.
US Patent
| Assay Description
Human Factor XIa activity was measured at an enzyme concentration of 0.1 U/mL in 150 mM NaCl, 5 mM KCl, 1 mg/mL PEG6000, 50 mM HEPES-NaOH (pH7.4) wit... |
US Patent US10717738 (2020)
BindingDB Entry DOI: 10.7270/Q2X92FBQ |
More data for this
Ligand-Target Pair | |
Coagulation factor XI
(Homo sapiens (Human)) | BDBM335414

(4-(2-{(3S)-8-chloro-7-[5-chloro-2-(1H-tetrazol-1-y...)Show SMILES OC(=O)c1cc(cs1)-c1cnc([nH]1)[C@@H]1CCc2c(Cl)c(cc(=O)n12)-c1cc(Cl)ccc1-n1cnnn1 |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a |
ONO PHARMACEUTICAL CO., LTD.
US Patent
| Assay Description
Human Factor XIa (Haematologic Technologies Inc.) activity was measured at an enzyme concentration of 0.1 U/mL in 150 mM NaCl, 5 mM KCl, 1 mg/mL PEG6... |
US Patent US9732085 (2017)
BindingDB Entry DOI: 10.7270/Q25X2C2P |
More data for this
Ligand-Target Pair | |
Coagulation factor XI
(Homo sapiens (Human)) | BDBM335416

(5-(2-{(3S)-7-[5-chloro-2-(1H-tetrazol-1-yl)phenyl]...)Show SMILES OC(=O)c1ccc(s1)-c1[nH]c(nc1F)[C@@H]1CCc2cc(cc(=O)n12)-c1cc(Cl)ccc1-n1cnnn1 |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a |
ONO PHARMACEUTICAL CO., LTD.
US Patent
| Assay Description
Human Factor XIa (Haematologic Technologies Inc.) activity was measured at an enzyme concentration of 0.1 U/mL in 150 mM NaCl, 5 mM KCl, 1 mg/mL PEG6... |
US Patent US9732085 (2017)
BindingDB Entry DOI: 10.7270/Q25X2C2P |
More data for this
Ligand-Target Pair | |
Coagulation factor XI
(Homo sapiens (Human)) | BDBM335416

(5-(2-{(3S)-7-[5-chloro-2-(1H-tetrazol-1-yl)phenyl]...)Show SMILES OC(=O)c1ccc(s1)-c1[nH]c(nc1F)[C@@H]1CCc2cc(cc(=O)n12)-c1cc(Cl)ccc1-n1cnnn1 |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a |
ONO PHARMACEUTICAL CO., LTD.
US Patent
| Assay Description
Human Factor XIa activity was measured at an enzyme concentration of 0.1 U/mL in 150 mM NaCl, 5 mM KCl, 1 mg/mL PEG6000, 50 mM HEPES-NaOH (pH7.4) wit... |
US Patent US10717738 (2020)
BindingDB Entry DOI: 10.7270/Q2X92FBQ |
More data for this
Ligand-Target Pair | |
Coagulation factor XI
(Homo sapiens (Human)) | BDBM294112

(US10336741, Example 99 | US10882855, Example 99 | ...)Show SMILES COC(=O)Nc1ccc(cc1)-c1[nH]c(nc1Cl)[C@@H]1C[C@@H](CN1C(=O)C1CCN(CC1)C(N)=N)N1CCN(CC1)S(C)(=O)=O |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a |
ONO PHARMACEUTICAL CO., LTD.
US Patent
| Assay Description
Human Factor XIa (Haematologic Technologies Inc.) activity was measured at an enzyme concentration of 0.1 U/mL in 150 mM NaCl, 5 mM KCl, 1 mg/mL PEG6... |
US Patent US9585881 (2017)
BindingDB Entry DOI: 10.7270/Q2D50Q0R |
More data for this
Ligand-Target Pair | |
Coagulation factor XI
(Homo sapiens (Human)) | BDBM294112

(US10336741, Example 99 | US10882855, Example 99 | ...)Show SMILES COC(=O)Nc1ccc(cc1)-c1[nH]c(nc1Cl)[C@@H]1C[C@@H](CN1C(=O)C1CCN(CC1)C(N)=N)N1CCN(CC1)S(C)(=O)=O |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a |
ONO PHARMACEUTICAL CO., LTD.
US Patent
| Assay Description
Human Factor XIa (Haematologic Technologies Inc.) activity was measured at an enzyme concentration of 0.1 U/mL in 150 mM NaCl, 5 mM KCl, 1 mg/mL PEG6... |
US Patent US10882855 (2021)
BindingDB Entry DOI: 10.7270/Q2XG9V7Q |
More data for this
Ligand-Target Pair | |
Coagulation factor XI
(Homo sapiens (Human)) | BDBM294112

(US10336741, Example 99 | US10882855, Example 99 | ...)Show SMILES COC(=O)Nc1ccc(cc1)-c1[nH]c(nc1Cl)[C@@H]1C[C@@H](CN1C(=O)C1CCN(CC1)C(N)=N)N1CCN(CC1)S(C)(=O)=O |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Ono Pharmaceutical Co., Ltd
US Patent
| Assay Description
Inhibitory activities of compounds of the present invention against factor XIa, Xa, XIIa, IXa, VIIa, plasma kallikrein or thrombin were evaluated usi... |
US Patent US10336741 (2019)
BindingDB Entry DOI: 10.7270/Q2CR5WQP |
More data for this
Ligand-Target Pair | |
Coagulation factor XI
(Homo sapiens (Human)) | BDBM238267

(US9394250, 99)Show SMILES COC(=O)Nc1ccc(cc1)-c1[nH]c(nc1Cl)[C@@H]1C[C@@H](CN1C(=O)C1CCC(CC1)C(N)=N)N1CCN(CC1)S(C)(=O)=O |r,wU:19.36,17.18,(10.48,2.25,;9.14,3.02,;7.81,2.25,;7.81,.71,;6.48,3.02,;5.14,2.25,;5.14,.71,;3.81,-.06,;2.47,.71,;2.47,2.25,;3.81,3.02,;1.14,-.06,;-.32,.41,;-1.23,-.83,;-.32,-2.08,;1.14,-1.6,;2.47,-2.37,;-2.56,-.53,;-3.04,.93,;-4.58,.93,;-5.05,-.53,;-3.81,-1.44,;-3.81,-2.98,;-2.47,-3.75,;-5.14,-3.75,;-6.48,-2.98,;-7.81,-3.75,;-7.81,-5.29,;-6.48,-6.06,;-5.14,-5.29,;-9.14,-6.06,;-9.14,-7.6,;-10.48,-5.29,;-5.35,2.26,;-4.58,3.6,;-5.35,4.93,;-6.89,4.93,;-7.66,3.6,;-6.89,2.26,;-7.66,6.27,;-8.43,7.6,;-6.32,7.04,;-8.99,5.5,)| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a |
ONO PHARMACEUTICAL CO., LTD.
US Patent
| Assay Description
Human Factor XIa (Haematologic Technologies Inc.) activity was measured at an enzyme concentration of 0.1 U/mL in 150 mM NaCl, 5 mM KCl, 1 mg/mL PEG6... |
US Patent US9394250 (2016)
BindingDB Entry DOI: 10.7270/Q2222SP5 |
More data for this
Ligand-Target Pair | |
Coagulation factor XI
(Homo sapiens (Human)) | BDBM335412

(4-(2-{(3S)-7-[5-chloro-2-(1H-tetrazol-1-yl)phenyl]...)Show SMILES OC(=O)c1cc(cs1)-c1cnc([nH]1)[C@@H]1CCc2cc(cc(=O)n12)-c1cc(Cl)ccc1-n1cnnn1 |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a |
ONO PHARMACEUTICAL CO., LTD.
US Patent
| Assay Description
Human Factor XIa activity was measured at an enzyme concentration of 0.1 U/mL in 150 mM NaCl, 5 mM KCl, 1 mg/mL PEG6000, 50 mM HEPES-NaOH (pH7.4) wit... |
US Patent US10717738 (2020)
BindingDB Entry DOI: 10.7270/Q2X92FBQ |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data