 Found 143609 hits with Last Name = 'ma' and Initial = 't'
Found 143609 hits with Last Name = 'ma' and Initial = 't' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Prostaglandin E2 receptor EP3 subtype
(Mus musculus (Mouse)) | BDBM50101827
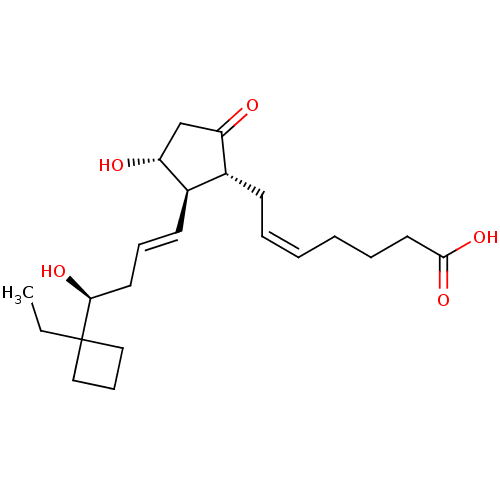
((Z)-7-{(1R,2R,3R)-2-[(E)-(S)-4-(1-Ethyl-cyclobutyl...)Show SMILES CCC1(CCC1)[C@@H](O)C\C=C\[C@H]1[C@H](O)CC(=O)[C@@H]1C\C=C/CCCC(O)=O Show InChI InChI=1S/C22H34O5/c1-2-22(13-8-14-22)20(25)11-7-10-17-16(18(23)15-19(17)24)9-5-3-4-6-12-21(26)27/h3,5,7,10,16-17,19-20,24-25H,2,4,6,8-9,11-15H2,1H3,(H,26,27)/b5-3-,10-7+/t16-,17-,19-,20+/m1/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| >0.000100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Minase Research Institute
Curated by ChEMBL
| Assay Description
Binding affinity towards mouse Prostanoid EP3 receptor in CHO cells. |
Bioorg Med Chem Lett 11: 2025-8 (2001)
BindingDB Entry DOI: 10.7270/Q2CV4H1T |
More data for this
Ligand-Target Pair | |
Prostacyclin receptor
(Homo sapiens (Human)) | BDBM50101832
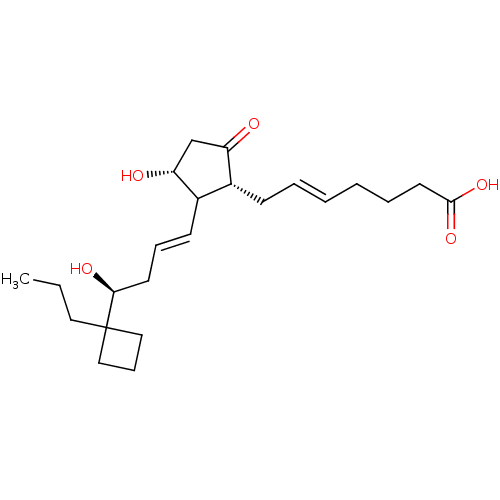
((E)-7-{(1R,3R)-3-Hydroxy-2-[(E)-(S)-4-hydroxy-4-(1...)Show SMILES CCCC1(CCC1)[C@@H](O)C\C=C\C1[C@H](O)CC(=O)[C@@H]1C\C=C\CCCC(O)=O Show InChI InChI=1S/C23H36O5/c1-2-13-23(14-8-15-23)21(26)11-7-10-18-17(19(24)16-20(18)25)9-5-3-4-6-12-22(27)28/h3,5,7,10,17-18,20-21,25-26H,2,4,6,8-9,11-16H2,1H3,(H,27,28)/b5-3+,10-7+/t17-,18?,20-,21+/m1/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| >0.000100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Minase Research Institute
Curated by ChEMBL
| Assay Description
Binding affinity towards human Prostanoid IP receptor in CHO cells. |
Bioorg Med Chem Lett 11: 2025-8 (2001)
BindingDB Entry DOI: 10.7270/Q2CV4H1T |
More data for this
Ligand-Target Pair | |
Prostacyclin receptor
(Homo sapiens (Human)) | BDBM50101825

((Z)-7-{(1R,2R,3R)-3-Hydroxy-2-[(E)-(S)-4-hydroxy-4...)Show SMILES CCCC1(CCCCC1)[C@@H](O)C\C=C\[C@H]1[C@H](O)CC(=O)[C@@H]1C\C=C/CCCC(O)=O Show InChI InChI=1S/C25H40O5/c1-2-15-25(16-8-5-9-17-25)23(28)13-10-12-20-19(21(26)18-22(20)27)11-6-3-4-7-14-24(29)30/h3,6,10,12,19-20,22-23,27-28H,2,4-5,7-9,11,13-18H2,1H3,(H,29,30)/b6-3-,12-10+/t19-,20-,22-,23+/m1/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| >0.000100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Minase Research Institute
Curated by ChEMBL
| Assay Description
Binding affinity towards human Prostanoid IP receptor in CHO cells. |
Bioorg Med Chem Lett 11: 2025-8 (2001)
BindingDB Entry DOI: 10.7270/Q2CV4H1T |
More data for this
Ligand-Target Pair | |
Prostaglandin E2 receptor EP3 subtype
(Mus musculus (Mouse)) | BDBM50101823
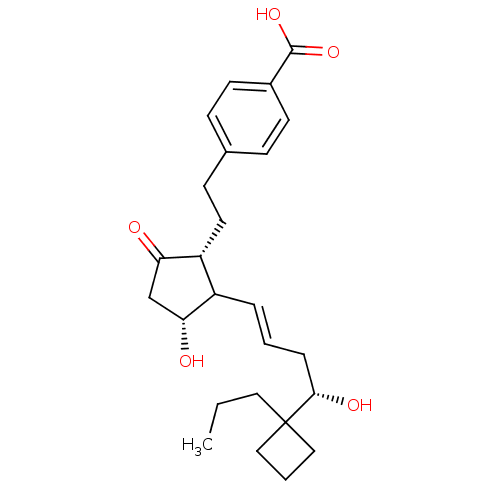
(4-(2-{(1R,3R)-3-Hydroxy-2-[(E)-(S)-4-hydroxy-4-(1-...)Show SMILES CCCC1(CCC1)[C@@H](O)C\C=C\C1[C@H](O)CC(=O)[C@@H]1CCc1ccc(cc1)C(O)=O Show InChI InChI=1S/C25H34O5/c1-2-13-25(14-4-15-25)23(28)6-3-5-19-20(22(27)16-21(19)26)12-9-17-7-10-18(11-8-17)24(29)30/h3,5,7-8,10-11,19-21,23,26,28H,2,4,6,9,12-16H2,1H3,(H,29,30)/b5-3+/t19?,20-,21-,23+/m1/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| PubMed
| >0.000100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Minase Research Institute
Curated by ChEMBL
| Assay Description
Binding affinity towards mouse Prostanoid EP3 receptor in CHO cells. |
Bioorg Med Chem Lett 11: 2025-8 (2001)
BindingDB Entry DOI: 10.7270/Q2CV4H1T |
More data for this
Ligand-Target Pair | |
Prostaglandin E2 receptor EP3 subtype
(Mus musculus (Mouse)) | BDBM50101825

((Z)-7-{(1R,2R,3R)-3-Hydroxy-2-[(E)-(S)-4-hydroxy-4...)Show SMILES CCCC1(CCCCC1)[C@@H](O)C\C=C\[C@H]1[C@H](O)CC(=O)[C@@H]1C\C=C/CCCC(O)=O Show InChI InChI=1S/C25H40O5/c1-2-15-25(16-8-5-9-17-25)23(28)13-10-12-20-19(21(26)18-22(20)27)11-6-3-4-7-14-24(29)30/h3,6,10,12,19-20,22-23,27-28H,2,4-5,7-9,11,13-18H2,1H3,(H,29,30)/b6-3-,12-10+/t19-,20-,22-,23+/m1/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| >0.000100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Minase Research Institute
Curated by ChEMBL
| Assay Description
Binding affinity towards mouse Prostanoid EP3 receptor in CHO cells. |
Bioorg Med Chem Lett 11: 2025-8 (2001)
BindingDB Entry DOI: 10.7270/Q2CV4H1T |
More data for this
Ligand-Target Pair | |
Prostaglandin E2 receptor EP3 subtype
(Mus musculus (Mouse)) | BDBM50101826
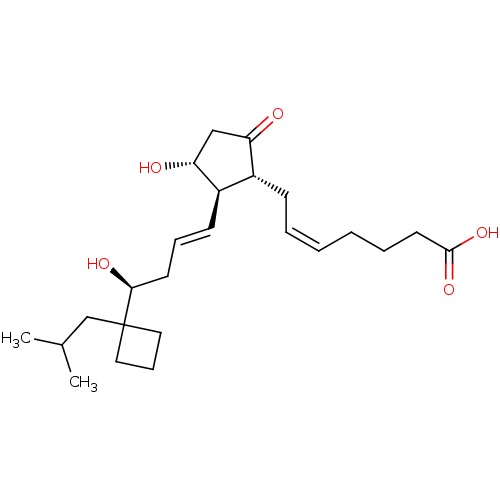
((Z)-7-{(1R,2R,3R)-3-Hydroxy-2-[(E)-(S)-4-hydroxy-4...)Show SMILES CC(C)CC1(CCC1)[C@@H](O)C\C=C\[C@H]1[C@H](O)CC(=O)[C@@H]1C\C=C/CCCC(O)=O Show InChI InChI=1S/C24H38O5/c1-17(2)16-24(13-8-14-24)22(27)11-7-10-19-18(20(25)15-21(19)26)9-5-3-4-6-12-23(28)29/h3,5,7,10,17-19,21-22,26-27H,4,6,8-9,11-16H2,1-2H3,(H,28,29)/b5-3-,10-7+/t18-,19-,21-,22+/m1/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| >0.000100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Minase Research Institute
Curated by ChEMBL
| Assay Description
Binding affinity towards mouse Prostanoid EP3 receptor in CHO cells. |
Bioorg Med Chem Lett 11: 2025-8 (2001)
BindingDB Entry DOI: 10.7270/Q2CV4H1T |
More data for this
Ligand-Target Pair | |
Prostacyclin receptor
(Homo sapiens (Human)) | BDBM50101823

(4-(2-{(1R,3R)-3-Hydroxy-2-[(E)-(S)-4-hydroxy-4-(1-...)Show SMILES CCCC1(CCC1)[C@@H](O)C\C=C\C1[C@H](O)CC(=O)[C@@H]1CCc1ccc(cc1)C(O)=O Show InChI InChI=1S/C25H34O5/c1-2-13-25(14-4-15-25)23(28)6-3-5-19-20(22(27)16-21(19)26)12-9-17-7-10-18(11-8-17)24(29)30/h3,5,7-8,10-11,19-21,23,26,28H,2,4,6,9,12-16H2,1H3,(H,29,30)/b5-3+/t19?,20-,21-,23+/m1/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| PubMed
| >0.000100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Minase Research Institute
Curated by ChEMBL
| Assay Description
Binding affinity towards human Prostanoid IP receptor in CHO cells. |
Bioorg Med Chem Lett 11: 2025-8 (2001)
BindingDB Entry DOI: 10.7270/Q2CV4H1T |
More data for this
Ligand-Target Pair | |
Prostaglandin E2 receptor EP3 subtype
(Mus musculus (Mouse)) | BDBM50101832

((E)-7-{(1R,3R)-3-Hydroxy-2-[(E)-(S)-4-hydroxy-4-(1...)Show SMILES CCCC1(CCC1)[C@@H](O)C\C=C\C1[C@H](O)CC(=O)[C@@H]1C\C=C\CCCC(O)=O Show InChI InChI=1S/C23H36O5/c1-2-13-23(14-8-15-23)21(26)11-7-10-18-17(19(24)16-20(18)25)9-5-3-4-6-12-22(27)28/h3,5,7,10,17-18,20-21,25-26H,2,4,6,8-9,11-16H2,1H3,(H,27,28)/b5-3+,10-7+/t17-,18?,20-,21+/m1/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| >0.000100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Minase Research Institute
Curated by ChEMBL
| Assay Description
Binding affinity towards mouse Prostanoid EP3 receptor in CHO cells. |
Bioorg Med Chem Lett 11: 2025-8 (2001)
BindingDB Entry DOI: 10.7270/Q2CV4H1T |
More data for this
Ligand-Target Pair | |
Prostaglandin E2 receptor EP3 subtype
(Mus musculus (Mouse)) | BDBM50101833
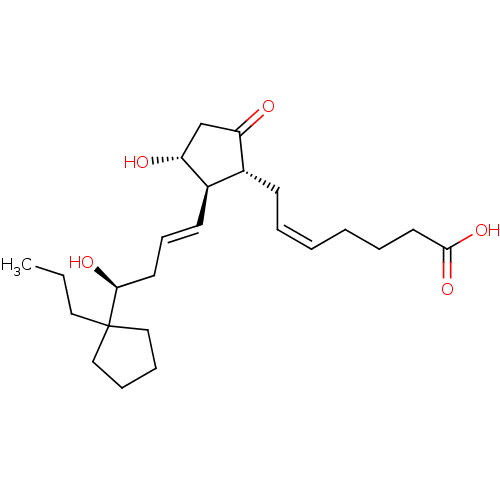
((Z)-7-{(1R,2R,3R)-3-Hydroxy-2-[(E)-(S)-4-hydroxy-4...)Show SMILES CCCC1(CCCC1)[C@@H](O)C\C=C\[C@H]1[C@H](O)CC(=O)[C@@H]1C\C=C/CCCC(O)=O Show InChI InChI=1S/C24H38O5/c1-2-14-24(15-7-8-16-24)22(27)12-9-11-19-18(20(25)17-21(19)26)10-5-3-4-6-13-23(28)29/h3,5,9,11,18-19,21-22,26-27H,2,4,6-8,10,12-17H2,1H3,(H,28,29)/b5-3-,11-9+/t18-,19-,21-,22+/m1/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| >0.000100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Minase Research Institute
Curated by ChEMBL
| Assay Description
Binding affinity towards mouse Prostanoid EP3 receptor in CHO cells. |
Bioorg Med Chem Lett 11: 2025-8 (2001)
BindingDB Entry DOI: 10.7270/Q2CV4H1T |
More data for this
Ligand-Target Pair | |
Substance-P receptor
(Homo sapiens (Human)) | BDBM50295070
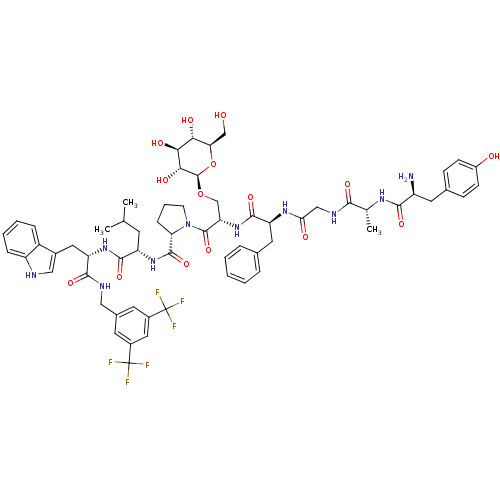
((S)-1-((2S,5S,11R,14S)-14-amino-5-benzyl-15-(4-hyd...)Show SMILES CC(C)C[C@H](NC(=O)[C@@H]1CCCN1C(=O)[C@H](CO[C@@H]1O[C@H](CO)[C@@H](O)[C@H](O)[C@H]1O)NC(=O)[C@H](Cc1ccccc1)NC(=O)CNC(=O)[C@@H](C)NC(=O)[C@@H](N)Cc1ccc(O)cc1)C(=O)N[C@@H](Cc1c[nH]c2ccccc12)C(=O)NCc1cc(cc(c1)C(F)(F)F)C(F)(F)F |r| Show InChI InChI=1S/C63H76F6N10O15/c1-32(2)20-44(57(89)76-46(25-37-28-71-43-13-8-7-12-41(37)43)56(88)72-27-36-21-38(62(64,65)66)26-39(22-36)63(67,68)69)77-59(91)48-14-9-19-79(48)60(92)47(31-93-61-53(85)52(84)51(83)49(30-80)94-61)78-58(90)45(24-34-10-5-4-6-11-34)75-50(82)29-73-54(86)33(3)74-55(87)42(70)23-35-15-17-40(81)18-16-35/h4-8,10-13,15-18,21-22,26,28,32-33,42,44-49,51-53,61,71,80-81,83-85H,9,14,19-20,23-25,27,29-31,70H2,1-3H3,(H,72,88)(H,73,86)(H,74,87)(H,75,82)(H,76,89)(H,77,91)(H,78,90)/t33-,42+,44+,45+,46+,47+,48+,49-,51-,52+,53-,61-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.00110 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Arizona
Curated by ChEMBL
| Assay Description
Displacement of [3H]substance P from human NK1 receptor expressed in CHO cell membrane |
J Med Chem 52: 5164-75 (2010)
Article DOI: 10.1021/jm900473p
BindingDB Entry DOI: 10.7270/Q2MC90ZZ |
More data for this
Ligand-Target Pair | |
Substance-P receptor
(Homo sapiens (Human)) | BDBM50295070

((S)-1-((2S,5S,11R,14S)-14-amino-5-benzyl-15-(4-hyd...)Show SMILES CC(C)C[C@H](NC(=O)[C@@H]1CCCN1C(=O)[C@H](CO[C@@H]1O[C@H](CO)[C@@H](O)[C@H](O)[C@H]1O)NC(=O)[C@H](Cc1ccccc1)NC(=O)CNC(=O)[C@@H](C)NC(=O)[C@@H](N)Cc1ccc(O)cc1)C(=O)N[C@@H](Cc1c[nH]c2ccccc12)C(=O)NCc1cc(cc(c1)C(F)(F)F)C(F)(F)F |r| Show InChI InChI=1S/C63H76F6N10O15/c1-32(2)20-44(57(89)76-46(25-37-28-71-43-13-8-7-12-41(37)43)56(88)72-27-36-21-38(62(64,65)66)26-39(22-36)63(67,68)69)77-59(91)48-14-9-19-79(48)60(92)47(31-93-61-53(85)52(84)51(83)49(30-80)94-61)78-58(90)45(24-34-10-5-4-6-11-34)75-50(82)29-73-54(86)33(3)74-55(87)42(70)23-35-15-17-40(81)18-16-35/h4-8,10-13,15-18,21-22,26,28,32-33,42,44-49,51-53,61,71,80-81,83-85H,9,14,19-20,23-25,27,29-31,70H2,1-3H3,(H,72,88)(H,73,86)(H,74,87)(H,75,82)(H,76,89)(H,77,91)(H,78,90)/t33-,42+,44+,45+,46+,47+,48+,49-,51-,52+,53-,61-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.00110 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Arizona
Curated by ChEMBL
| Assay Description
Displacement of [3H]substance P from human NK1 receptor expressed in CHO cell membrane |
J Med Chem 52: 5164-75 (2010)
Article DOI: 10.1021/jm900473p
BindingDB Entry DOI: 10.7270/Q2MC90ZZ |
More data for this
Ligand-Target Pair | |
Protease
(Human immunodeficiency virus 1 (HIV-1)) | BDBM50480930
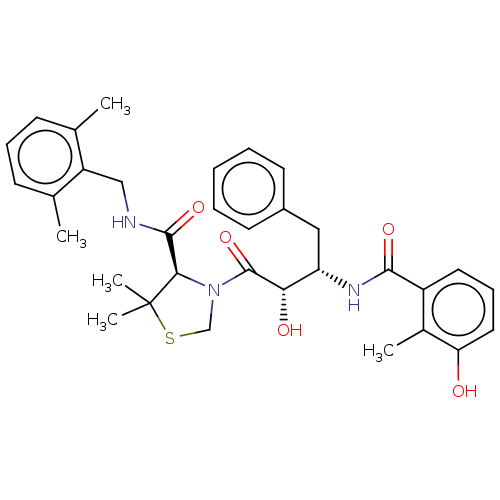
(CHEMBL584130 | KNI-814)Show SMILES Cc1cccc(C)c1CNC(=O)[C@H]1N(CSC1(C)C)C(=O)[C@@H](O)[C@H](Cc1ccccc1)NC(=O)c1cccc(O)c1C |r| Show InChI InChI=1S/C33H39N3O5S/c1-20-11-9-12-21(2)25(20)18-34-31(40)29-33(4,5)42-19-36(29)32(41)28(38)26(17-23-13-7-6-8-14-23)35-30(39)24-15-10-16-27(37)22(24)3/h6-16,26,28-29,37-38H,17-19H2,1-5H3,(H,34,40)(H,35,39)/t26-,28-,29+/m0/s1 | PDB
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.00240 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kyoto Pharmaceutical University
Curated by ChEMBL
| Assay Description
Inhibition of HIV1 recombinant protease after 15 mins by fluorescence assay |
J Med Chem 52: 7604-17 (2009)
Article DOI: 10.1021/jm9005115
BindingDB Entry DOI: 10.7270/Q2FR00F2 |
More data for this
Ligand-Target Pair | |
Adenosine deaminase
(Homo sapiens (Human)) | BDBM50407749

(CHEMBL2112110)Show SMILES OC[C@@H]1O[C@@H](C[C@H]1O)n1cnc2[C@H](O)CCC=Nc12 |c:17| Show InChI InChI=1S/C12H17N3O4/c16-5-9-8(18)4-10(19-9)15-6-14-11-7(17)2-1-3-13-12(11)15/h3,6-10,16-18H,1-2,4-5H2/t7-,8-,9+,10+/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.00250 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of California
Curated by ChEMBL
| Assay Description
Binding affinity towards Adenosine deaminase |
J Med Chem 39: 277-84 (1996)
Article DOI: 10.1021/jm9505674
BindingDB Entry DOI: 10.7270/Q2ZS2X6V |
More data for this
Ligand-Target Pair | |
Adenosine deaminase
(Homo sapiens (Human)) | BDBM50407749

(CHEMBL2112110)Show SMILES OC[C@@H]1O[C@@H](C[C@H]1O)n1cnc2[C@H](O)CCC=Nc12 |c:17| Show InChI InChI=1S/C12H17N3O4/c16-5-9-8(18)4-10(19-9)15-6-14-11-7(17)2-1-3-13-12(11)15/h3,6-10,16-18H,1-2,4-5H2/t7-,8-,9+,10+/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.00250 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of California
Curated by ChEMBL
| Assay Description
Binding affinity towards Adenosine deaminase |
J Med Chem 39: 277-84 (1996)
Article DOI: 10.1021/jm9505674
BindingDB Entry DOI: 10.7270/Q2ZS2X6V |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 1A
(Rattus norvegicus (rat)) | BDBM50368723
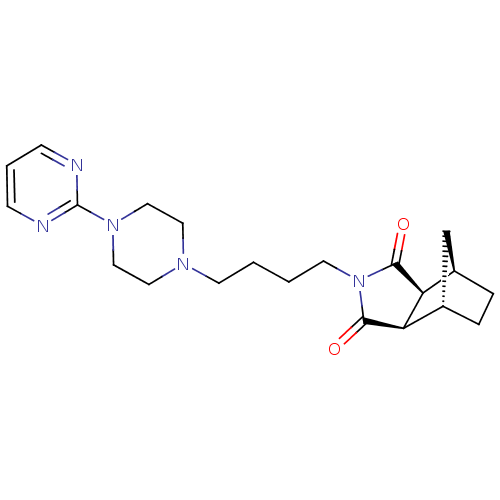
(Metanopirone | Sediel | TANDOSPIRONE HYDROCHLORIDE...)Show SMILES O=C1[C@H]2[C@@H]3CC[C@@H](C3)[C@H]2C(=O)N1CCCCN1CCN(CC1)c1ncccn1 |r| Show InChI InChI=1S/C21H29N5O2/c27-19-17-15-4-5-16(14-15)18(17)20(28)26(19)9-2-1-8-24-10-12-25(13-11-24)21-22-6-3-7-23-21/h3,6-7,15-18H,1-2,4-5,8-14H2/t15-,16+,17+,18- | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.00270 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Asahi Chemical Industry Company, Ltd.
Curated by ChEMBL
| Assay Description
Binding affinity by measuring displacement of [3H]8-OH-DPAT from 5-hydroxytryptamine 1A receptor in rat hippocampus |
J Med Chem 36: 3526-32 (1994)
BindingDB Entry DOI: 10.7270/Q2D21Z74 |
More data for this
Ligand-Target Pair | |
Substance-P receptor
(Homo sapiens (Human)) | BDBM50295069
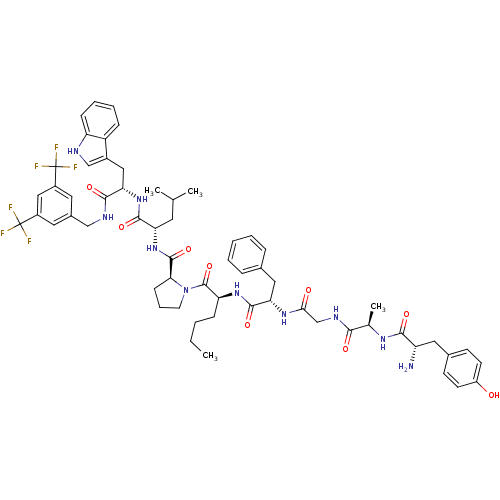
((S)-1-((2S,5S,11R,14S)-14-amino-5-benzyl-2-butyl-1...)Show SMILES CCCC[C@H](NC(=O)[C@H](Cc1ccccc1)NC(=O)CNC(=O)[C@@H](C)NC(=O)[C@@H](N)Cc1ccc(O)cc1)C(=O)N1CCC[C@H]1C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](Cc1c[nH]c2ccccc12)C(=O)NCc1cc(cc(c1)C(F)(F)F)C(F)(F)F |r| Show InChI InChI=1S/C60H72F6N10O9/c1-5-6-16-46(73-56(83)48(28-36-13-8-7-9-14-36)72-51(78)33-70-52(79)35(4)71-53(80)44(67)27-37-19-21-42(77)22-20-37)58(85)76-23-12-18-50(76)57(84)75-47(24-34(2)3)55(82)74-49(29-39-32-68-45-17-11-10-15-43(39)45)54(81)69-31-38-25-40(59(61,62)63)30-41(26-38)60(64,65)66/h7-11,13-15,17,19-22,25-26,30,32,34-35,44,46-50,68,77H,5-6,12,16,18,23-24,27-29,31,33,67H2,1-4H3,(H,69,81)(H,70,79)(H,71,80)(H,72,78)(H,73,83)(H,74,82)(H,75,84)/t35-,44+,46+,47+,48+,49+,50+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.00280 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Arizona
Curated by ChEMBL
| Assay Description
Displacement of [3H]substance P from human NK1 receptor expressed in CHO cell membrane |
J Med Chem 52: 5164-75 (2010)
Article DOI: 10.1021/jm900473p
BindingDB Entry DOI: 10.7270/Q2MC90ZZ |
More data for this
Ligand-Target Pair | |
Substance-P receptor
(Homo sapiens (Human)) | BDBM50295069

((S)-1-((2S,5S,11R,14S)-14-amino-5-benzyl-2-butyl-1...)Show SMILES CCCC[C@H](NC(=O)[C@H](Cc1ccccc1)NC(=O)CNC(=O)[C@@H](C)NC(=O)[C@@H](N)Cc1ccc(O)cc1)C(=O)N1CCC[C@H]1C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](Cc1c[nH]c2ccccc12)C(=O)NCc1cc(cc(c1)C(F)(F)F)C(F)(F)F |r| Show InChI InChI=1S/C60H72F6N10O9/c1-5-6-16-46(73-56(83)48(28-36-13-8-7-9-14-36)72-51(78)33-70-52(79)35(4)71-53(80)44(67)27-37-19-21-42(77)22-20-37)58(85)76-23-12-18-50(76)57(84)75-47(24-34(2)3)55(82)74-49(29-39-32-68-45-17-11-10-15-43(39)45)54(81)69-31-38-25-40(59(61,62)63)30-41(26-38)60(64,65)66/h7-11,13-15,17,19-22,25-26,30,32,34-35,44,46-50,68,77H,5-6,12,16,18,23-24,27-29,31,33,67H2,1-4H3,(H,69,81)(H,70,79)(H,71,80)(H,72,78)(H,73,83)(H,74,82)(H,75,84)/t35-,44+,46+,47+,48+,49+,50+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.00280 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Arizona
Curated by ChEMBL
| Assay Description
Displacement of [3H]substance P from human NK1 receptor expressed in CHO cell membrane |
J Med Chem 52: 5164-75 (2010)
Article DOI: 10.1021/jm900473p
BindingDB Entry DOI: 10.7270/Q2MC90ZZ |
More data for this
Ligand-Target Pair | |
Substance-P receptor
(Homo sapiens (Human)) | BDBM50498577
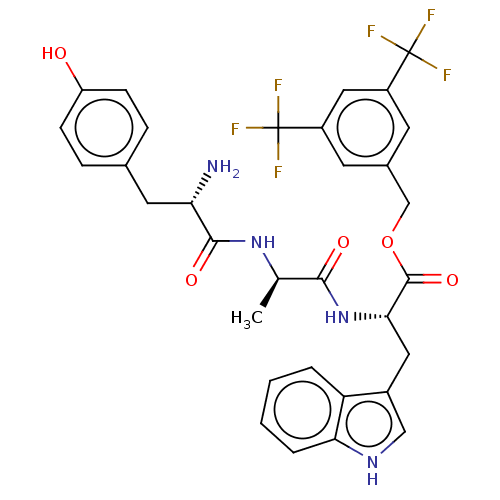
(CHEMBL3609619)Show SMILES C[C@@H](NC(=O)[C@@H](N)Cc1ccc(O)cc1)C(=O)N[C@@H](Cc1c[nH]c2ccccc12)C(=O)OCc1cc(cc(c1)C(F)(F)F)C(F)(F)F |r| Show InChI InChI=1S/C32H30F6N4O5/c1-17(41-29(45)25(39)12-18-6-8-23(43)9-7-18)28(44)42-27(13-20-15-40-26-5-3-2-4-24(20)26)30(46)47-16-19-10-21(31(33,34)35)14-22(11-19)32(36,37)38/h2-11,14-15,17,25,27,40,43H,12-13,16,39H2,1H3,(H,41,45)(H,42,44)/t17-,25+,27+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.00400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Arizona
Curated by ChEMBL
| Assay Description
Displacement of [3H]-substance P from human NK1 receptor transfected in CHO cells |
Bioorg Med Chem Lett 25: 3716-20 (2015)
Article DOI: 10.1016/j.bmcl.2015.06.030
BindingDB Entry DOI: 10.7270/Q2R78J7N |
More data for this
Ligand-Target Pair | |
Substance-P receptor
(Homo sapiens (Human)) | BDBM50498575
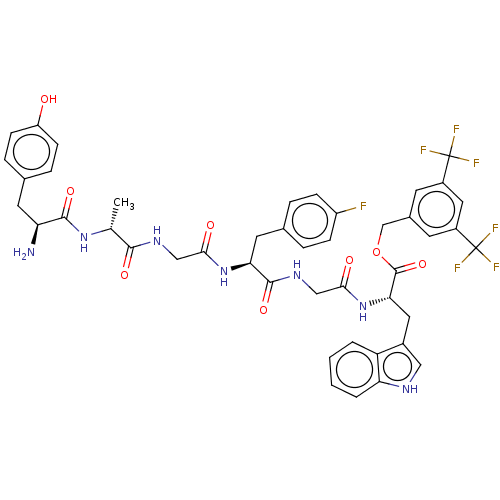
(CHEMBL3608939)Show SMILES C[C@@H](NC(=O)[C@@H](N)Cc1ccc(O)cc1)C(=O)NCC(=O)N[C@@H](Cc1ccc(F)cc1)C(=O)NCC(=O)N[C@@H](Cc1c[nH]c2ccccc12)C(=O)OCc1cc(cc(c1)C(F)(F)F)C(F)(F)F |r| Show InChI InChI=1S/C45H44F7N7O8/c1-24(57-41(64)34(53)16-25-8-12-32(60)13-9-25)40(63)55-21-38(61)58-36(17-26-6-10-31(46)11-7-26)42(65)56-22-39(62)59-37(18-28-20-54-35-5-3-2-4-33(28)35)43(66)67-23-27-14-29(44(47,48)49)19-30(15-27)45(50,51)52/h2-15,19-20,24,34,36-37,54,60H,16-18,21-23,53H2,1H3,(H,55,63)(H,56,65)(H,57,64)(H,58,61)(H,59,62)/t24-,34+,36+,37+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.00400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Arizona
Curated by ChEMBL
| Assay Description
Displacement of [3H]-substance P from human NK1 receptor transfected in CHO cells |
Bioorg Med Chem Lett 25: 3716-20 (2015)
Article DOI: 10.1016/j.bmcl.2015.06.030
BindingDB Entry DOI: 10.7270/Q2R78J7N |
More data for this
Ligand-Target Pair | |
Cathepsin K
(Homo sapiens (Human)) | BDBM50098576

(5-(2-MORPHOLIN-4-YLETHOXY)BENZOFURAN-2-CARBOXYLIC ...)Show SMILES CC(C)C[C@H](NC(=O)c1cc2cc(OCCN3CCOCC3)ccc2o1)C(=O)N[C@H]1CCCN(CC1=O)C(=O)Cc1cccc(c1)-c1ccccn1 Show InChI InChI=1S/C40H47N5O7/c1-27(2)21-34(43-40(49)37-25-30-24-31(11-12-36(30)52-37)51-20-17-44-15-18-50-19-16-44)39(48)42-33-10-6-14-45(26-35(33)46)38(47)23-28-7-5-8-29(22-28)32-9-3-4-13-41-32/h3-5,7-9,11-13,22,24-25,27,33-34H,6,10,14-21,23,26H2,1-2H3,(H,42,48)(H,43,49)/t33-,34-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| PDB
PubMed
| 0.00480 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of Human cathepsin K |
J Med Chem 44: 1380-95 (2001)
BindingDB Entry DOI: 10.7270/Q2QR4WDC |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Substance-P receptor
(Homo sapiens (Human)) | BDBM50323869
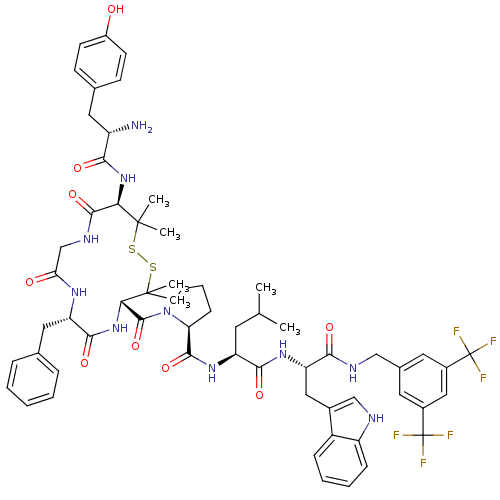
((S)-1-((4S,7S,13R)-13-((S)-2-amino-3-(4-hydroxyphe...)Show SMILES CC(C)C[C@H](NC(=O)[C@@H]1CCCN1C(=O)[C@@H]1NC(=O)[C@H](Cc2ccccc2)NC(=O)CNC(=O)[C@@H](NC(=O)[C@@H](N)Cc2ccc(O)cc2)C(C)(C)SSC1(C)C)C(=O)N[C@@H](Cc1c[nH]c2ccccc12)C(=O)NCc1cc(cc(c1)C(F)(F)F)C(F)(F)F |r| Show InChI InChI=1S/C61H72F6N10O9S2/c1-33(2)23-44(53(82)73-46(28-37-31-69-43-16-11-10-15-41(37)43)52(81)70-30-36-24-38(60(62,63)64)29-39(25-36)61(65,66)67)74-55(84)47-17-12-22-77(47)57(86)50-59(5,6)88-87-58(3,4)49(75-51(80)42(68)26-35-18-20-40(78)21-19-35)56(85)71-32-48(79)72-45(54(83)76-50)27-34-13-8-7-9-14-34/h7-11,13-16,18-21,24-25,29,31,33,42,44-47,49-50,69,78H,12,17,22-23,26-28,30,32,68H2,1-6H3,(H,70,81)(H,71,85)(H,72,79)(H,73,82)(H,74,84)(H,75,80)(H,76,83)/t42-,44-,45-,46-,47-,49+,50-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.00530 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Arizona
Curated by ChEMBL
| Assay Description
Displacement of [3H]substance P frome human NK1 receptor |
J Med Chem 53: 5491-501 (2010)
Article DOI: 10.1021/jm100157m
BindingDB Entry DOI: 10.7270/Q21N81BD |
More data for this
Ligand-Target Pair | |
Substance-P receptor
(Homo sapiens (Human)) | BDBM50323869

((S)-1-((4S,7S,13R)-13-((S)-2-amino-3-(4-hydroxyphe...)Show SMILES CC(C)C[C@H](NC(=O)[C@@H]1CCCN1C(=O)[C@@H]1NC(=O)[C@H](Cc2ccccc2)NC(=O)CNC(=O)[C@@H](NC(=O)[C@@H](N)Cc2ccc(O)cc2)C(C)(C)SSC1(C)C)C(=O)N[C@@H](Cc1c[nH]c2ccccc12)C(=O)NCc1cc(cc(c1)C(F)(F)F)C(F)(F)F |r| Show InChI InChI=1S/C61H72F6N10O9S2/c1-33(2)23-44(53(82)73-46(28-37-31-69-43-16-11-10-15-41(37)43)52(81)70-30-36-24-38(60(62,63)64)29-39(25-36)61(65,66)67)74-55(84)47-17-12-22-77(47)57(86)50-59(5,6)88-87-58(3,4)49(75-51(80)42(68)26-35-18-20-40(78)21-19-35)56(85)71-32-48(79)72-45(54(83)76-50)27-34-13-8-7-9-14-34/h7-11,13-16,18-21,24-25,29,31,33,42,44-47,49-50,69,78H,12,17,22-23,26-28,30,32,68H2,1-6H3,(H,70,81)(H,71,85)(H,72,79)(H,73,82)(H,74,84)(H,75,80)(H,76,83)/t42-,44-,45-,46-,47-,49+,50-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.00530 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Arizona
Curated by ChEMBL
| Assay Description
Displacement of [3H]substance P frome human NK1 receptor |
J Med Chem 53: 5491-501 (2010)
Article DOI: 10.1021/jm100157m
BindingDB Entry DOI: 10.7270/Q21N81BD |
More data for this
Ligand-Target Pair | |
Substance-P receptor
(Homo sapiens (Human)) | BDBM50439327
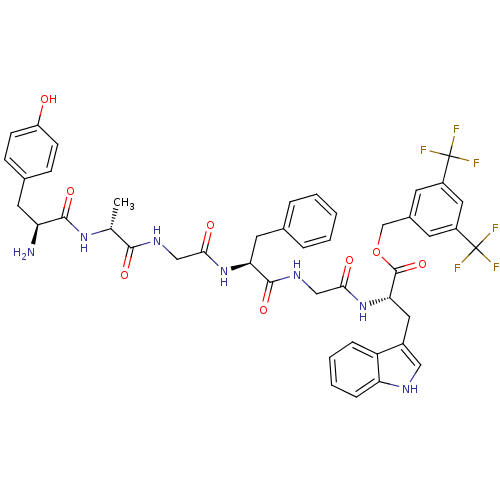
(CHEMBL2419544)Show SMILES C[C@@H](NC(=O)[C@@H](N)Cc1ccc(O)cc1)C(=O)NCC(=O)N[C@@H](Cc1ccccc1)C(=O)NCC(=O)N[C@@H](Cc1c[nH]c2ccccc12)C(=O)OCc1cc(cc(c1)C(F)(F)F)C(F)(F)F |r| Show InChI InChI=1S/C45H45F6N7O8/c1-25(56-41(63)34(52)17-27-11-13-32(59)14-12-27)40(62)54-22-38(60)57-36(18-26-7-3-2-4-8-26)42(64)55-23-39(61)58-37(19-29-21-53-35-10-6-5-9-33(29)35)43(65)66-24-28-15-30(44(46,47)48)20-31(16-28)45(49,50)51/h2-16,20-21,25,34,36-37,53,59H,17-19,22-24,52H2,1H3,(H,54,62)(H,55,64)(H,56,63)(H,57,60)(H,58,61)/t25-,34+,36+,37+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.00570 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Arizona
Curated by ChEMBL
| Assay Description
Displacement of [3H]substance P from human NK1 receptor expressed in CHO cells |
Bioorg Med Chem Lett 23: 4975-8 (2013)
Article DOI: 10.1016/j.bmcl.2013.06.065
BindingDB Entry DOI: 10.7270/Q2222W64 |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase pim-1
(Homo sapiens (Human)) | BDBM602107

(US11643417, Ex. No. 120)Show SMILES OCC1(O)CCC(CC1)C(F)(F)CN1CCNC(=O)c2oc3ccc(cc3c12)C(F)(F)F |(7.13,.13,;5.59,.13,;4.82,1.46,;6.31,1.86,;3.67,.45,;2.21,.95,;1.91,2.46,;3.07,3.47,;4.53,2.98,;.45,2.96,;-.56,4.12,;.95,4.42,;-.71,1.94,;-2.16,2.44,;-2.35,3.97,;-3.66,4.78,;-5.11,4.26,;-5.61,2.8,;-7.13,2.62,;-4.78,1.51,;-5.4,.1,;-4.26,-.93,;-4.26,-2.47,;-2.92,-3.24,;-1.59,-2.47,;-1.59,-.93,;-2.92,-.16,;-3.25,1.35,;-.26,-3.24,;1.08,-2.47,;-.26,-4.78,;1.08,-4.01,)| | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | 0.00600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2R49VQ0 |
More data for this
Ligand-Target Pair | |
Melatonin receptor type 1A/1B
(Homo sapiens (Human)) | BDBM50118462
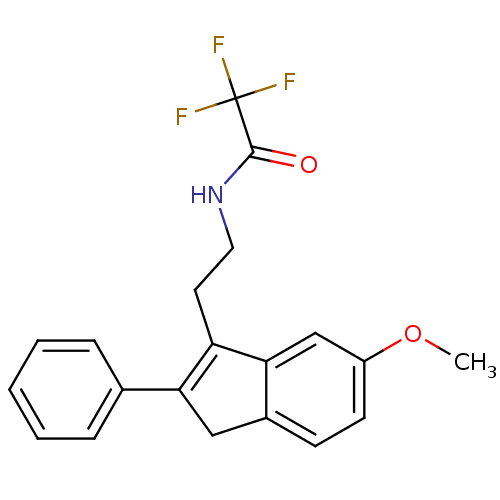
(2,2,2-Trifluoro-N-[2-(6-methoxy-2-phenyl-3H-inden-...)Show SMILES COc1ccc2CC(=C(CCNC(=O)C(F)(F)F)c2c1)c1ccccc1 |t:7| Show InChI InChI=1S/C20H18F3NO2/c1-26-15-8-7-14-11-17(13-5-3-2-4-6-13)16(18(14)12-15)9-10-24-19(25)20(21,22)23/h2-8,12H,9-11H2,1H3,(H,24,25) | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 0.00602 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Chemical Industries, Ltd.
Curated by ChEMBL
| Assay Description
Ability to inhibit 2-[125I]iodomelatonin specific binding to human melatonin receptor type 1A (MT1) expressed in CHO cells. |
J Med Chem 45: 4212-21 (2002)
BindingDB Entry DOI: 10.7270/Q2J102HG |
More data for this
Ligand-Target Pair | |
Substance-P receptor
(Homo sapiens (Human)) | BDBM50264406
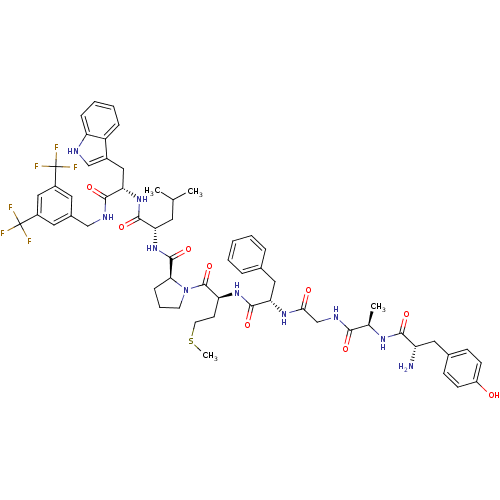
((S)-1-((2S,5S,11R,14S)-14-amino-5-benzyl-15-(4-hyd...)Show SMILES CSCC[C@H](NC(=O)[C@H](Cc1ccccc1)NC(=O)CNC(=O)[C@@H](C)NC(=O)[C@@H](N)Cc1ccc(O)cc1)C(=O)N1CCC[C@H]1C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](Cc1c[nH]c2ccccc12)C(=O)NCc1cc(cc(c1)C(F)(F)F)C(F)(F)F |r| Show InChI InChI=1S/C59H70F6N10O9S/c1-33(2)23-46(54(81)73-48(28-38-31-67-44-14-9-8-13-42(38)44)53(80)68-30-37-24-39(58(60,61)62)29-40(25-37)59(63,64)65)74-56(83)49-15-10-21-75(49)57(84)45(20-22-85-4)72-55(82)47(27-35-11-6-5-7-12-35)71-50(77)32-69-51(78)34(3)70-52(79)43(66)26-36-16-18-41(76)19-17-36/h5-9,11-14,16-19,24-25,29,31,33-34,43,45-49,67,76H,10,15,20-23,26-28,30,32,66H2,1-4H3,(H,68,80)(H,69,78)(H,70,79)(H,71,77)(H,72,82)(H,73,81)(H,74,83)/t34-,43+,45+,46+,47+,48+,49+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.00650 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Arizona
Curated by ChEMBL
| Assay Description
Displacement of [3H]substance P frome human NK1 receptor |
J Med Chem 53: 5491-501 (2010)
Article DOI: 10.1021/jm100157m
BindingDB Entry DOI: 10.7270/Q21N81BD |
More data for this
Ligand-Target Pair | |
Substance-P receptor
(Homo sapiens (Human)) | BDBM50264406

((S)-1-((2S,5S,11R,14S)-14-amino-5-benzyl-15-(4-hyd...)Show SMILES CSCC[C@H](NC(=O)[C@H](Cc1ccccc1)NC(=O)CNC(=O)[C@@H](C)NC(=O)[C@@H](N)Cc1ccc(O)cc1)C(=O)N1CCC[C@H]1C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](Cc1c[nH]c2ccccc12)C(=O)NCc1cc(cc(c1)C(F)(F)F)C(F)(F)F |r| Show InChI InChI=1S/C59H70F6N10O9S/c1-33(2)23-46(54(81)73-48(28-38-31-67-44-14-9-8-13-42(38)44)53(80)68-30-37-24-39(58(60,61)62)29-40(25-37)59(63,64)65)74-56(83)49-15-10-21-75(49)57(84)45(20-22-85-4)72-55(82)47(27-35-11-6-5-7-12-35)71-50(77)32-69-51(78)34(3)70-52(79)43(66)26-36-16-18-41(76)19-17-36/h5-9,11-14,16-19,24-25,29,31,33-34,43,45-49,67,76H,10,15,20-23,26-28,30,32,66H2,1-4H3,(H,68,80)(H,69,78)(H,70,79)(H,71,77)(H,72,82)(H,73,81)(H,74,83)/t34-,43+,45+,46+,47+,48+,49+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.00650 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Arizona
Curated by ChEMBL
| Assay Description
Displacement of [3H]substance P from human NK1 receptor expressed in CHO cell membrane by liquid scintillation counting |
J Med Chem 51: 6334-47 (2008)
Article DOI: 10.1021/jm800389v
BindingDB Entry DOI: 10.7270/Q26T0MF1 |
More data for this
Ligand-Target Pair | |
Substance-P receptor
(Homo sapiens (Human)) | BDBM50264406

((S)-1-((2S,5S,11R,14S)-14-amino-5-benzyl-15-(4-hyd...)Show SMILES CSCC[C@H](NC(=O)[C@H](Cc1ccccc1)NC(=O)CNC(=O)[C@@H](C)NC(=O)[C@@H](N)Cc1ccc(O)cc1)C(=O)N1CCC[C@H]1C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](Cc1c[nH]c2ccccc12)C(=O)NCc1cc(cc(c1)C(F)(F)F)C(F)(F)F |r| Show InChI InChI=1S/C59H70F6N10O9S/c1-33(2)23-46(54(81)73-48(28-38-31-67-44-14-9-8-13-42(38)44)53(80)68-30-37-24-39(58(60,61)62)29-40(25-37)59(63,64)65)74-56(83)49-15-10-21-75(49)57(84)45(20-22-85-4)72-55(82)47(27-35-11-6-5-7-12-35)71-50(77)32-69-51(78)34(3)70-52(79)43(66)26-36-16-18-41(76)19-17-36/h5-9,11-14,16-19,24-25,29,31,33-34,43,45-49,67,76H,10,15,20-23,26-28,30,32,66H2,1-4H3,(H,68,80)(H,69,78)(H,70,79)(H,71,77)(H,72,82)(H,73,81)(H,74,83)/t34-,43+,45+,46+,47+,48+,49+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.00700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Arizona
Curated by ChEMBL
| Assay Description
Displacement of [3H]substance P from human NK1 receptor expressed in CHO cell membranes |
Bioorg Med Chem 17: 7337-43 (2009)
Article DOI: 10.1016/j.bmc.2009.08.035
BindingDB Entry DOI: 10.7270/Q2CJ8DK5 |
More data for this
Ligand-Target Pair | |
Substance-P receptor
(Homo sapiens (Human)) | BDBM50264406

((S)-1-((2S,5S,11R,14S)-14-amino-5-benzyl-15-(4-hyd...)Show SMILES CSCC[C@H](NC(=O)[C@H](Cc1ccccc1)NC(=O)CNC(=O)[C@@H](C)NC(=O)[C@@H](N)Cc1ccc(O)cc1)C(=O)N1CCC[C@H]1C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](Cc1c[nH]c2ccccc12)C(=O)NCc1cc(cc(c1)C(F)(F)F)C(F)(F)F |r| Show InChI InChI=1S/C59H70F6N10O9S/c1-33(2)23-46(54(81)73-48(28-38-31-67-44-14-9-8-13-42(38)44)53(80)68-30-37-24-39(58(60,61)62)29-40(25-37)59(63,64)65)74-56(83)49-15-10-21-75(49)57(84)45(20-22-85-4)72-55(82)47(27-35-11-6-5-7-12-35)71-50(77)32-69-51(78)34(3)70-52(79)43(66)26-36-16-18-41(76)19-17-36/h5-9,11-14,16-19,24-25,29,31,33-34,43,45-49,67,76H,10,15,20-23,26-28,30,32,66H2,1-4H3,(H,68,80)(H,69,78)(H,70,79)(H,71,77)(H,72,82)(H,73,81)(H,74,83)/t34-,43+,45+,46+,47+,48+,49+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.00700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Arizona
Curated by ChEMBL
| Assay Description
Displacement of [3H]substance P from human NK1 receptor expressed in CHO cell membranes |
Bioorg Med Chem 17: 7337-43 (2009)
Article DOI: 10.1016/j.bmc.2009.08.035
BindingDB Entry DOI: 10.7270/Q2CJ8DK5 |
More data for this
Ligand-Target Pair | |
Substance-P receptor
(Homo sapiens (Human)) | BDBM50341318
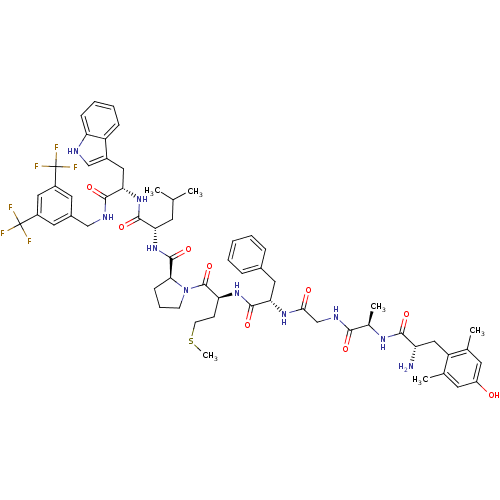
((S)-1-((2S,5S,11R,14S)-14-amino-5-benzyl-15-(4-hyd...)Show SMILES CSCC[C@H](NC(=O)[C@H](Cc1ccccc1)NC(=O)CNC(=O)[C@@H](C)NC(=O)[C@@H](N)Cc1c(C)cc(O)cc1C)C(=O)N1CCC[C@H]1C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](Cc1c[nH]c2ccccc12)C(=O)NCc1cc(cc(c1)C(F)(F)F)C(F)(F)F |r| Show InChI InChI=1S/C61H74F6N10O9S/c1-33(2)21-48(56(83)75-50(27-39-31-69-46-16-11-10-15-43(39)46)55(82)70-30-38-24-40(60(62,63)64)28-41(25-38)61(65,66)67)76-58(85)51-17-12-19-77(51)59(86)47(18-20-87-6)74-57(84)49(26-37-13-8-7-9-14-37)73-52(79)32-71-53(80)36(5)72-54(81)45(68)29-44-34(3)22-42(78)23-35(44)4/h7-11,13-16,22-25,28,31,33,36,45,47-51,69,78H,12,17-21,26-27,29-30,32,68H2,1-6H3,(H,70,82)(H,71,80)(H,72,81)(H,73,79)(H,74,84)(H,75,83)(H,76,85)/t36-,45+,47+,48+,49+,50+,51+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.00790 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Arizona
Curated by ChEMBL
| Assay Description
Displacement of [3H]substance P from human NK1 receptor expressed in CHO cells |
J Med Chem 54: 2029-38 (2011)
Article DOI: 10.1021/jm101023r
BindingDB Entry DOI: 10.7270/Q2862GRK |
More data for this
Ligand-Target Pair | |
Substance-P receptor
(Homo sapiens (Human)) | BDBM50341318

((S)-1-((2S,5S,11R,14S)-14-amino-5-benzyl-15-(4-hyd...)Show SMILES CSCC[C@H](NC(=O)[C@H](Cc1ccccc1)NC(=O)CNC(=O)[C@@H](C)NC(=O)[C@@H](N)Cc1c(C)cc(O)cc1C)C(=O)N1CCC[C@H]1C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](Cc1c[nH]c2ccccc12)C(=O)NCc1cc(cc(c1)C(F)(F)F)C(F)(F)F |r| Show InChI InChI=1S/C61H74F6N10O9S/c1-33(2)21-48(56(83)75-50(27-39-31-69-46-16-11-10-15-43(39)46)55(82)70-30-38-24-40(60(62,63)64)28-41(25-38)61(65,66)67)76-58(85)51-17-12-19-77(51)59(86)47(18-20-87-6)74-57(84)49(26-37-13-8-7-9-14-37)73-52(79)32-71-53(80)36(5)72-54(81)45(68)29-44-34(3)22-42(78)23-35(44)4/h7-11,13-16,22-25,28,31,33,36,45,47-51,69,78H,12,17-21,26-27,29-30,32,68H2,1-6H3,(H,70,82)(H,71,80)(H,72,81)(H,73,79)(H,74,84)(H,75,83)(H,76,85)/t36-,45+,47+,48+,49+,50+,51+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.00790 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Arizona
Curated by ChEMBL
| Assay Description
Displacement of [3H]substance P from human NK1 receptor expressed in CHO cells |
J Med Chem 54: 2029-38 (2011)
Article DOI: 10.1021/jm101023r
BindingDB Entry DOI: 10.7270/Q2862GRK |
More data for this
Ligand-Target Pair | |
Endothelin receptor type B
(Sus scrofa) | BDBM50000558
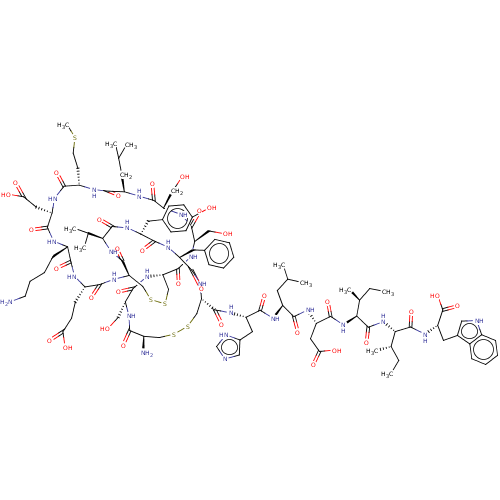
(CHEMBL437472 | ET-1 | Endothelin -1 | Endothelin 1...)Show SMILES CC[C@H](C)[C@H](NC(=O)[C@H](CC(O)=O)NC(=O)[C@H](CC(C)C)NC(=O)[C@H](Cc1cnc[nH]1)NC(=O)[C@@H]1CSSC[C@@H](N)C(=O)N[C@H](CO)C(=O)N[C@H]2CSSC[C@@H](NC(=O)[C@H](CCC(O)=O)NC(=O)[C@H](CCCCN)NC(=O)[C@@H](CC(O)=O)NC(=O)[C@H](CCSC)NC(=O)[C@@H](CC(C)C)NC(=O)[C@H](CO)NC(=O)[C@@H](CO)NC2=O)C(=O)N[C@@H](C(C)C)C(=O)N[C@H](Cc2ccc(O)cc2)C(=O)N[C@@H](Cc2ccccc2)C(=O)N1)C(=O)N[C@@H]([C@@H](C)CC)C(=O)N[C@@H](Cc1c[nH]c2ccccc12)C(O)=O Show InChI InChI=1S/C109H159N25O32S5/c1-12-56(9)87(107(163)125-76(109(165)166)39-60-43-113-65-24-18-17-23-63(60)65)134-108(164)88(57(10)13-2)133-99(155)75(42-85(143)144)123-94(150)70(36-54(5)6)118-97(153)73(40-61-44-112-52-114-61)121-103(159)80-49-169-168-48-64(111)89(145)126-77(45-135)102(158)131-81-50-170-171-51-82(105(161)132-86(55(7)8)106(162)124-72(38-59-26-28-62(138)29-27-59)95(151)120-71(96(152)130-80)37-58-21-15-14-16-22-58)129-91(147)67(30-31-83(139)140)116-90(146)66(25-19-20-33-110)115-98(154)74(41-84(141)142)122-92(148)68(32-34-167-11)117-93(149)69(35-53(3)4)119-100(156)78(46-136)127-101(157)79(47-137)128-104(81)160/h14-18,21-24,26-29,43-44,52-57,64,66-82,86-88,113,135-138H,12-13,19-20,25,30-42,45-51,110-111H2,1-11H3,(H,112,114)(H,115,154)(H,116,146)(H,117,149)(H,118,153)(H,119,156)(H,120,151)(H,121,159)(H,122,148)(H,123,150)(H,124,162)(H,125,163)(H,126,145)(H,127,157)(H,128,160)(H,129,147)(H,130,152)(H,131,158)(H,132,161)(H,133,155)(H,134,164)(H,139,140)(H,141,142)(H,143,144)(H,165,166)/t56-,57-,64+,66-,67-,68-,69+,70-,71-,72+,73-,74+,75-,76-,77+,78-,79+,80-,81-,82+,86-,87-,88-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| 0.00800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Ability to inhibit [125I]ET-3 binding to the Endothelin B receptor from porcine lung membranes was evaluated |
Bioorg Med Chem Lett 6: 2323-2328 (1996)
Article DOI: 10.1016/0960-894X(96)00421-0
BindingDB Entry DOI: 10.7270/Q21J9B8K |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase pim-1
(Homo sapiens (Human)) | BDBM602075
(US11643417, Ex. No. 90)Show SMILES OC[C@@]1(O)CC[C@H](CCN2CCNC(=O)c3oc4ccc(cc4c23)C(F)(F)F)CC1 |r,wU:6.6,2.2,wD:2.1,(7.13,.13,;5.59,.13,;4.82,1.46,;6.16,2.23,;4.53,2.98,;3.07,3.47,;1.91,2.46,;.45,2.96,;-.71,1.94,;-2.16,2.44,;-2.35,3.97,;-3.66,4.78,;-5.11,4.26,;-5.61,2.8,;-7.13,2.62,;-4.78,1.51,;-5.4,.1,;-4.26,-.93,;-4.26,-2.47,;-2.92,-3.24,;-1.59,-2.47,;-1.59,-.93,;-2.92,-.16,;-3.25,1.35,;-.26,-3.24,;1.08,-2.47,;-.26,-4.78,;1.08,-4.01,;2.21,.95,;3.67,.45,)| | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | 0.00900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2R49VQ0 |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase pim-1
(Homo sapiens (Human)) | BDBM602103

(US11643417, Ex. No. 116)Show SMILES OCC1(O)CCC(CCN2CC3(CC3)NC(=O)c3oc4ccc(cc4c23)C(F)(F)F)CC1 |(7.29,1.28,;5.83,1.77,;4.67,.76,;6.13,.26,;3.51,-.25,;2.05,.25,;1.76,1.76,;.3,2.25,;-.86,1.24,;-2.32,1.74,;-2.5,3.27,;-3.81,4.08,;-3.19,5.48,;-4.72,5.32,;-5.26,3.56,;-5.76,2.1,;-7.29,1.92,;-4.93,.8,;-5.56,-.6,;-4.41,-1.63,;-4.41,-3.17,;-3.08,-3.94,;-1.74,-3.17,;-1.74,-1.63,;-3.08,-.86,;-3.4,.64,;-.41,-3.94,;.92,-3.17,;-.41,-5.48,;-.41,-2.4,;2.92,2.77,;4.37,2.27,)| | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | 0.00900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2R49VQ0 |
More data for this
Ligand-Target Pair | |
Substance-P receptor
(Homo sapiens (Human)) | BDBM50439329
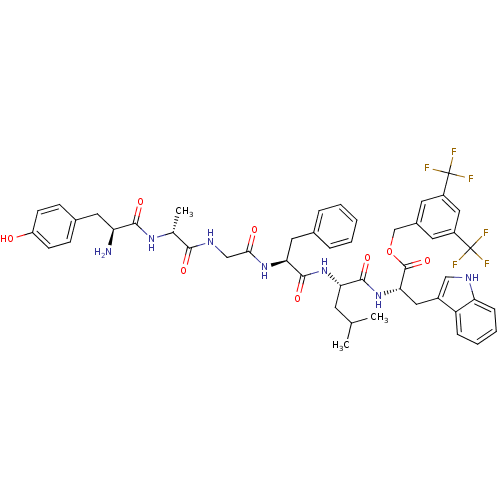
(CHEMBL2419542)Show SMILES CC(C)C[C@H](NC(=O)[C@H](Cc1ccccc1)NC(=O)CNC(=O)[C@@H](C)NC(=O)[C@@H](N)Cc1ccc(O)cc1)C(=O)N[C@@H](Cc1c[nH]c2ccccc12)C(=O)OCc1cc(cc(c1)C(F)(F)F)C(F)(F)F |r| Show InChI InChI=1S/C49H53F6N7O8/c1-27(2)17-39(45(67)62-41(22-32-24-57-38-12-8-7-11-36(32)38)47(69)70-26-31-18-33(48(50,51)52)23-34(19-31)49(53,54)55)61-46(68)40(21-29-9-5-4-6-10-29)60-42(64)25-58-43(65)28(3)59-44(66)37(56)20-30-13-15-35(63)16-14-30/h4-16,18-19,23-24,27-28,37,39-41,57,63H,17,20-22,25-26,56H2,1-3H3,(H,58,65)(H,59,66)(H,60,64)(H,61,68)(H,62,67)/t28-,37+,39+,40+,41+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.00900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Arizona
Curated by ChEMBL
| Assay Description
Displacement of [3H]substance P from human NK1 receptor expressed in CHO cells |
Bioorg Med Chem Lett 23: 4975-8 (2013)
Article DOI: 10.1016/j.bmcl.2013.06.065
BindingDB Entry DOI: 10.7270/Q2222W64 |
More data for this
Ligand-Target Pair | |
Cathepsin K
(Homo sapiens (Human)) | BDBM19770
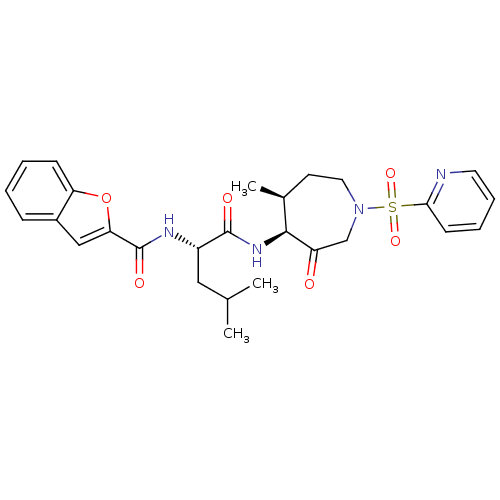
((2S)-2-(1-benzofuran-2-ylformamido)-4-methyl-N-[(4...)Show SMILES CC(C)C[C@H](NC(=O)c1cc2ccccc2o1)C(=O)N[C@H]1[C@@H](C)CCN(CC1=O)S(=O)(=O)c1ccccn1 |r| Show InChI InChI=1S/C27H32N4O6S/c1-17(2)14-20(29-27(34)23-15-19-8-4-5-9-22(19)37-23)26(33)30-25-18(3)11-13-31(16-21(25)32)38(35,36)24-10-6-7-12-28-24/h4-10,12,15,17-18,20,25H,11,13-14,16H2,1-3H3,(H,29,34)(H,30,33)/t18-,20-,25-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.00990 | -62.2 | n/a | n/a | n/a | n/a | n/a | 5.5 | 22 |
GSK
| Assay Description
Potential inhibitors were evaluated using the progress curve method. Assays were carried out in the presence of variable concentrations of test compo... |
J Med Chem 49: 1597-612 (2006)
Article DOI: 10.1021/jm050915u
BindingDB Entry DOI: 10.7270/Q2SQ8XP5 |
More data for this
Ligand-Target Pair | 
3D Structure (docked) |
Genome polyprotein
(Hepatitis C Virus (Virus)) | BDBM403592
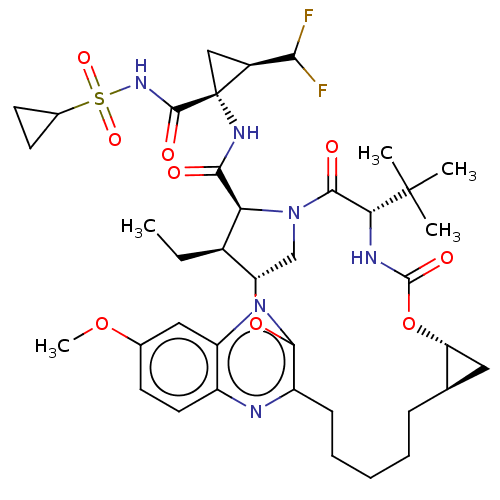
(Preparation of (1aR,5S,8S,9S,10R,22aR)-5-tert-buty...)Show SMILES CC[C@@H]1[C@@H]2CN([C@@H]1C(=O)N[C@@]1(C[C@H]1C(F)F)C(=O)NS(=O)(=O)C1CC1)C(=O)[C@@H](NC(=O)O[C@@H]1C[C@H]1CCCCCc1nc3ccc(OC)cc3nc1O2)C(C)(C)C |r| Show InChI InChI=1S/C39H52F2N6O9S/c1-6-23-29-19-47(30(23)33(48)45-39(18-24(39)32(40)41)36(50)46-57(52,53)22-13-14-22)35(49)31(38(2,3)4)44-37(51)56-28-16-20(28)10-8-7-9-11-26-34(55-29)43-27-17-21(54-5)12-15-25(27)42-26/h12,15,17,20,22-24,28-32H,6-11,13-14,16,18-19H2,1-5H3,(H,44,51)(H,45,48)(H,46,50)/t20-,23-,24+,28-,29+,30+,31-,39-/m1/s1 | PDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| 0.0100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
SRMLSC
Curated by PubChem BioAssay
| Assay Description
Purified NS3 protease domain (amino acids 1-181) of the genotype 1b and 3a virus were generated as above. The internally quenched fluorogenic depsipe... |
PubChem Bioassay (2006)
BindingDB Entry DOI: 10.7270/Q2930WJC |
More data for this
Ligand-Target Pair | |
Adenosine deaminase
(Homo sapiens (Human)) | BDBM50369126
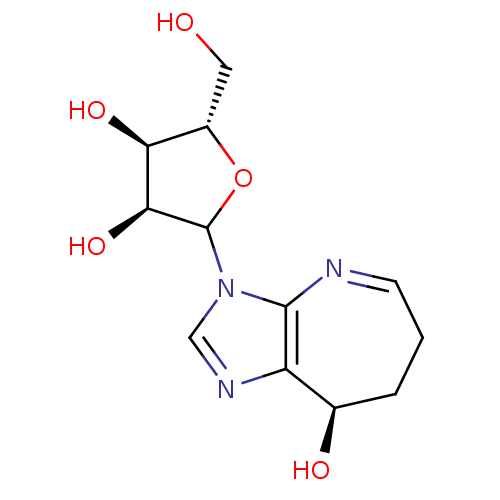
(CONFORMYCIN)Show SMILES OC[C@@H]1OC([C@@H](O)[C@H]1O)n1cnc2[C@H](O)CCC=Nc12 |r,c:18| Show InChI InChI=1S/C12H17N3O5/c16-4-7-9(18)10(19)12(20-7)15-5-14-8-6(17)2-1-3-13-11(8)15/h3,5-7,9-10,12,16-19H,1-2,4H2/t6-,7+,9+,10+,12?/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of California
Curated by ChEMBL
| Assay Description
Binding affinity towards Adenosine deaminase |
J Med Chem 39: 277-84 (1996)
Article DOI: 10.1021/jm9505674
BindingDB Entry DOI: 10.7270/Q2ZS2X6V |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase pim-1
(Homo sapiens (Human)) | BDBM602160
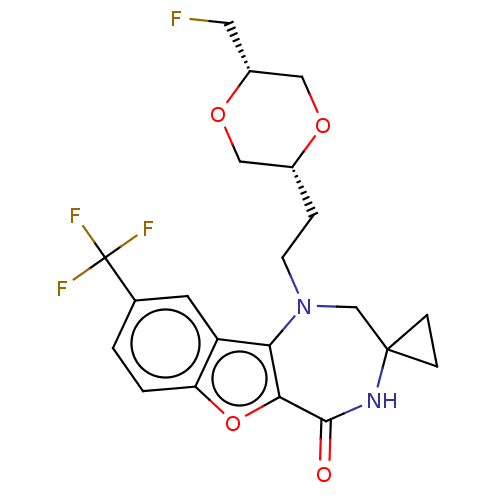
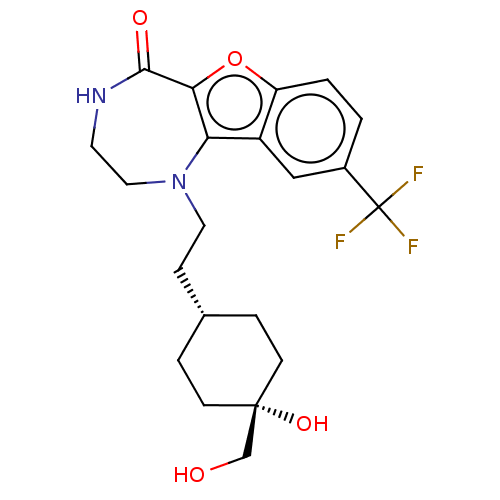
(US11643417, Ex. No. 156)Show SMILES FC[C@@H]1CO[C@H](CCN2CC3(CC3)NC(=O)c3oc4ccc(cc4c23)C(F)(F)F)CO1 |r| | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | 0.0100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2R49VQ0 |
More data for this
Ligand-Target Pair | |
Adenosine deaminase
(Homo sapiens (Human)) | BDBM50369126

(CONFORMYCIN)Show SMILES OC[C@@H]1OC([C@@H](O)[C@H]1O)n1cnc2[C@H](O)CCC=Nc12 |r,c:18| Show InChI InChI=1S/C12H17N3O5/c16-4-7-9(18)10(19)12(20-7)15-5-14-8-6(17)2-1-3-13-11(8)15/h3,5-7,9-10,12,16-19H,1-2,4H2/t6-,7+,9+,10+,12?/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of California
Curated by ChEMBL
| Assay Description
Binding affinity towards Adenosine deaminase |
J Med Chem 39: 277-84 (1996)
Article DOI: 10.1021/jm9505674
BindingDB Entry DOI: 10.7270/Q2ZS2X6V |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M1
(RAT) | BDBM50055978
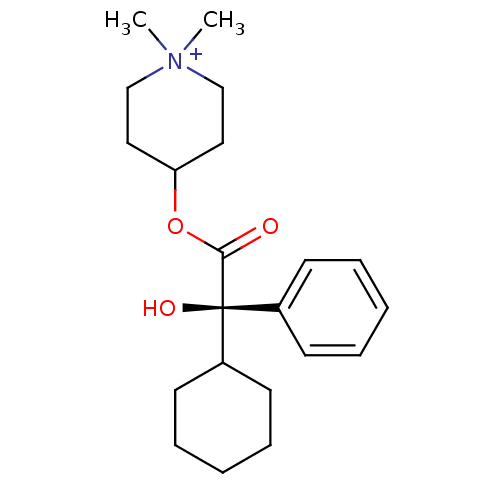
(4-((R)-2-Cyclohexyl-2-hydroxy-2-phenyl-acetoxy)-1,...)Show SMILES C[N+]1(C)CCC(CC1)OC(=O)[C@@](O)(C1CCCCC1)c1ccccc1 Show InChI InChI=1S/C21H32NO3/c1-22(2)15-13-19(14-16-22)25-20(23)21(24,17-9-5-3-6-10-17)18-11-7-4-8-12-18/h3,5-6,9-10,18-19,24H,4,7-8,11-16H2,1-2H3/q+1/t21-/m0/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Groningen University Hospital
Curated by ChEMBL
| Assay Description
Binding affinity towards rat Muscarinic acetylcholine receptor M1 was determined |
J Med Chem 40: 117-24 (1997)
Article DOI: 10.1021/jm960374w
BindingDB Entry DOI: 10.7270/Q2VH5PG0 |
More data for this
Ligand-Target Pair | |
Muscarinic receptor M1
(Bos taurus) | BDBM50055976
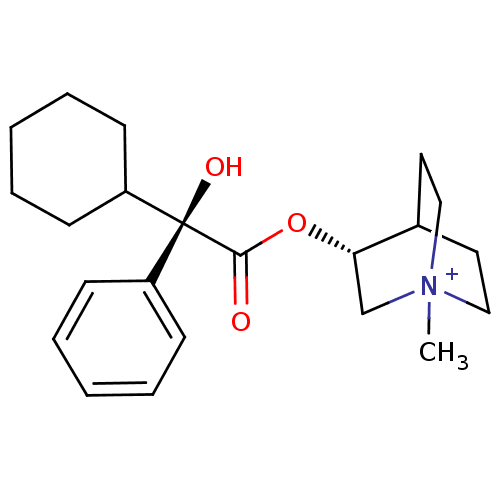
((R)-3-((R)-2-Cyclohexyl-2-hydroxy-2-phenyl-acetoxy...)Show SMILES C[N+]12CCC(CC1)[C@H](C2)OC(=O)[C@@](O)(C1CCCCC1)c1ccccc1 |wU:12.14,wD:12.13,7.10,TLB:9:7:2.3:6.5,(13.94,-4.53,;13.94,-2.99,;14.71,-1.66,;13.24,-1.26,;14.01,.06,;15.34,-.72,;15.34,-2.26,;12.68,-.71,;12.68,-2.25,;11.35,.06,;10.02,-.71,;10.02,-2.26,;8.66,.09,;9.46,1.42,;7.33,.86,;6,.09,;4.67,.86,;4.67,2.4,;6,3.17,;7.33,2.4,;7.89,-1.27,;6.35,-1.27,;5.58,-2.6,;6.35,-3.94,;7.89,-3.95,;8.66,-2.62,)| Show InChI InChI=1S/C22H32NO3/c1-23-14-12-17(13-15-23)20(16-23)26-21(24)22(25,18-8-4-2-5-9-18)19-10-6-3-7-11-19/h2,4-5,8-9,17,19-20,25H,3,6-7,10-16H2,1H3/q+1/t17?,20-,22-,23?/m0/s1 | KEGG
UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Groningen University Hospital
Curated by ChEMBL
| Assay Description
Binding affinity towards Muscarinic acetylcholine receptor M1 was determined in calf brain membrane |
J Med Chem 40: 117-24 (1997)
Article DOI: 10.1021/jm960374w
BindingDB Entry DOI: 10.7270/Q2VH5PG0 |
More data for this
Ligand-Target Pair | |
Genome polyprotein
(Hepatitis C virus genotype 3a (isolate k3a) (HCV)) | BDBM403653
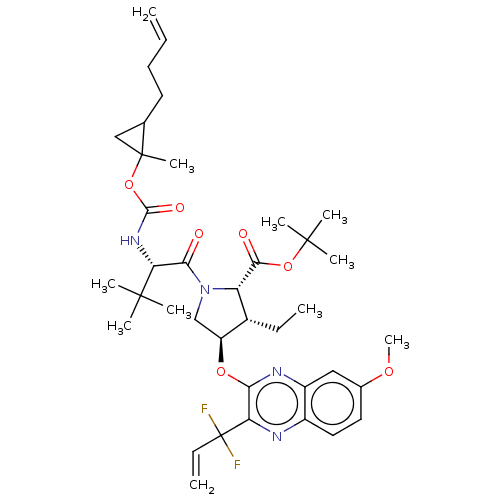
((1aR,5S,8S,9S,10R,22aR)-5-tert-butyl-N-[(1R,2R)-2-...)Show SMILES CC[C@@H]1[C@H](CN([C@@H]1C(=O)OC(C)(C)C)C(=O)[C@@H](NC(=O)OC1(C)CC1CCC=C)C(C)(C)C)Oc1nc2cc(OC)ccc2nc1C(F)(F)C=C |r| Show InChI InChI=1S/C38H52F2N4O7/c1-12-15-16-22-20-37(22,10)51-34(47)43-30(35(4,5)6)32(45)44-21-27(24(13-2)28(44)33(46)50-36(7,8)9)49-31-29(38(39,40)14-3)41-25-18-17-23(48-11)19-26(25)42-31/h12,14,17-19,22,24,27-28,30H,1,3,13,15-16,20-21H2,2,4-11H3,(H,43,47)/t22?,24-,27+,28+,30-,37?/m1/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| 0.0100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
SRMLSC
Curated by PubChem BioAssay
| Assay Description
Purified NS3 protease domain (amino acids 1-181) of the genotype 1b and 3a virus were generated as above. The internally quenched fluorogenic depsipe... |
PubChem Bioassay (2006)
BindingDB Entry DOI: 10.7270/Q2930WJC |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase pim-1
(Homo sapiens (Human)) | BDBM602097
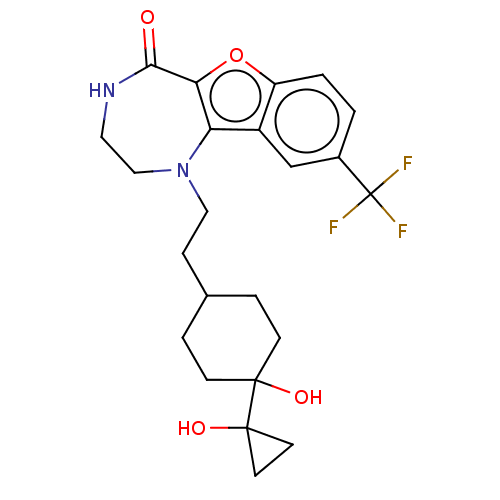
(US11643417, Ex. No. 111)Show SMILES OC1(CC1)C1(O)CCC(CCN2CCNC(=O)c3oc4ccc(cc4c23)C(F)(F)F)CC1 |(7.29,1.98,;6.13,.97,;5.63,-.49,;7.14,-.19,;4.67,1.46,;5.44,2.8,;3.51,.45,;2.05,.95,;1.76,2.46,;.3,2.96,;-.86,1.94,;-2.32,2.44,;-2.5,3.97,;-3.81,4.78,;-5.26,4.26,;-5.76,2.8,;-7.29,2.62,;-4.93,1.51,;-5.56,.1,;-4.41,-.93,;-4.41,-2.47,;-3.08,-3.24,;-1.74,-2.47,;-1.74,-.93,;-3.08,-.16,;-3.4,1.35,;-.41,-3.24,;.92,-2.47,;-.41,-4.78,;.92,-4.01,;2.92,3.47,;4.37,2.98,)| | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | 0.0110 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2R49VQ0 |
More data for this
Ligand-Target Pair | |
Prostaglandin E2 receptor EP4 subtype
(Mus musculus (Mouse)) | BDBM50366138
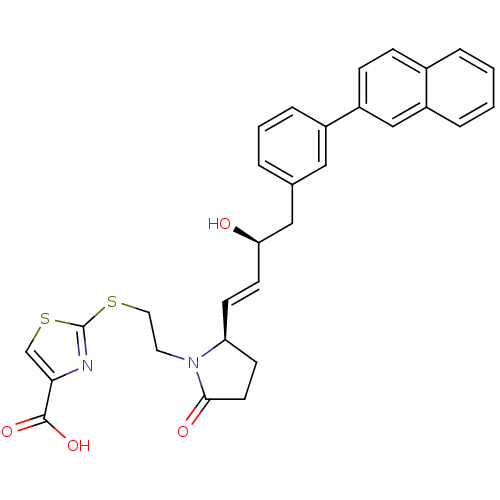
(CHEMBL1957437)Show SMILES O[C@@H](Cc1cccc(c1)-c1ccc2ccccc2c1)\C=C\[C@H]1CCC(=O)N1CCSc1nc(cs1)C(O)=O |r| Show InChI InChI=1S/C30H28N2O4S2/c33-26(17-20-4-3-7-22(16-20)24-9-8-21-5-1-2-6-23(21)18-24)12-10-25-11-13-28(34)32(25)14-15-37-30-31-27(19-38-30)29(35)36/h1-10,12,16,18-19,25-26,33H,11,13-15,17H2,(H,35,36)/b12-10+/t25-,26+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.0120 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Minase Research Institute
Curated by ChEMBL
| Assay Description
Displacement of [3H]PGE2 from mouse EP4 receptor expressed in CHO cells after 60 mins by scintillation counting |
Bioorg Med Chem 20: 3502-22 (2012)
Article DOI: 10.1016/j.bmc.2012.04.008
BindingDB Entry DOI: 10.7270/Q2D50P0B |
More data for this
Ligand-Target Pair | |
Prostaglandin E2 receptor EP4 subtype
(Mus musculus (Mouse)) | BDBM50366138

(CHEMBL1957437)Show SMILES O[C@@H](Cc1cccc(c1)-c1ccc2ccccc2c1)\C=C\[C@H]1CCC(=O)N1CCSc1nc(cs1)C(O)=O |r| Show InChI InChI=1S/C30H28N2O4S2/c33-26(17-20-4-3-7-22(16-20)24-9-8-21-5-1-2-6-23(21)18-24)12-10-25-11-13-28(34)32(25)14-15-37-30-31-27(19-38-30)29(35)36/h1-10,12,16,18-19,25-26,33H,11,13-15,17H2,(H,35,36)/b12-10+/t25-,26+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.0120 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Minase Research Institute
Curated by ChEMBL
| Assay Description
Displacement of [3H]PGE2 from mouse EP4 receptor expressed in CHO cells after 60 mins by scintillation counting |
Bioorg Med Chem 20: 3502-22 (2012)
Article DOI: 10.1016/j.bmc.2012.04.008
BindingDB Entry DOI: 10.7270/Q2D50P0B |
More data for this
Ligand-Target Pair | |
Prostaglandin E2 receptor EP4 subtype
(Mus musculus (Mouse)) | BDBM50366138

(CHEMBL1957437)Show SMILES O[C@@H](Cc1cccc(c1)-c1ccc2ccccc2c1)\C=C\[C@H]1CCC(=O)N1CCSc1nc(cs1)C(O)=O |r| Show InChI InChI=1S/C30H28N2O4S2/c33-26(17-20-4-3-7-22(16-20)24-9-8-21-5-1-2-6-23(21)18-24)12-10-25-11-13-28(34)32(25)14-15-37-30-31-27(19-38-30)29(35)36/h1-10,12,16,18-19,25-26,33H,11,13-15,17H2,(H,35,36)/b12-10+/t25-,26+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.0120 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Minase Research Institute
Curated by ChEMBL
| Assay Description
Displacement of [3H]-PGE2 from mouse EP4 receptor expressed in CHO cells after 60 mins by scintillation counting |
Bioorg Med Chem 20: 2235-51 (2012)
Article DOI: 10.1016/j.bmc.2012.02.018
BindingDB Entry DOI: 10.7270/Q2542P2G |
More data for this
Ligand-Target Pair | |
Prostaglandin E2 receptor EP4 subtype
(Mus musculus (Mouse)) | BDBM50366138

(CHEMBL1957437)Show SMILES O[C@@H](Cc1cccc(c1)-c1ccc2ccccc2c1)\C=C\[C@H]1CCC(=O)N1CCSc1nc(cs1)C(O)=O |r| Show InChI InChI=1S/C30H28N2O4S2/c33-26(17-20-4-3-7-22(16-20)24-9-8-21-5-1-2-6-23(21)18-24)12-10-25-11-13-28(34)32(25)14-15-37-30-31-27(19-38-30)29(35)36/h1-10,12,16,18-19,25-26,33H,11,13-15,17H2,(H,35,36)/b12-10+/t25-,26+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.0120 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Minase Research Institute
Curated by ChEMBL
| Assay Description
Displacement of [3H]-PGE2 from mouse EP4 receptor expressed in CHO cells after 60 mins by scintillation counting |
Bioorg Med Chem 20: 2235-51 (2012)
Article DOI: 10.1016/j.bmc.2012.02.018
BindingDB Entry DOI: 10.7270/Q2542P2G |
More data for this
Ligand-Target Pair | |
Melatonin receptor type 1A/1B
(Homo sapiens (Human)) | BDBM50118435
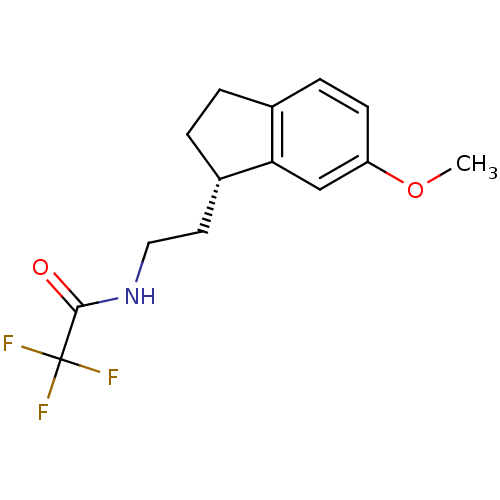
(2,2,2-Trifluoro-N-[2-(6-methoxy-indan-1-yl)-ethyl]...)Show InChI InChI=1S/C14H16F3NO2/c1-20-11-5-4-9-2-3-10(12(9)8-11)6-7-18-13(19)14(15,16)17/h4-5,8,10H,2-3,6-7H2,1H3,(H,18,19)/t10-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 0.0123 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Chemical Industries, Ltd.
Curated by ChEMBL
| Assay Description
Ability to inhibit 2-[125I]iodomelatonin specific binding to human melatonin receptor type 1A (MT1) expressed in CHO cells. |
J Med Chem 45: 4212-21 (2002)
BindingDB Entry DOI: 10.7270/Q2J102HG |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Rattus norvegicus (rat)) | BDBM50166065
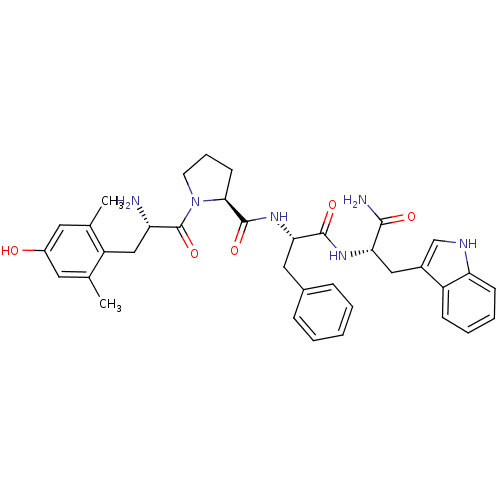
((S)-1-[(S)-2-Amino-3-(4-hydroxy-2,6-dimethyl-pheny...)Show SMILES Cc1cc(O)cc(C)c1C[C@H](N)C(=O)N1CCC[C@H]1C(=O)N[C@@H](Cc1ccccc1)C(=O)N[C@@H](Cc1c[nH]c2ccccc12)C(N)=O Show InChI InChI=1S/C36H42N6O5/c1-21-15-25(43)16-22(2)27(21)19-28(37)36(47)42-14-8-13-32(42)35(46)41-31(17-23-9-4-3-5-10-23)34(45)40-30(33(38)44)18-24-20-39-29-12-7-6-11-26(24)29/h3-7,9-12,15-16,20,28,30-32,39,43H,8,13-14,17-19,37H2,1-2H3,(H2,38,44)(H,40,45)(H,41,46)/t28-,30-,31-,32-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0140 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Szeged
Curated by ChEMBL
| Assay Description
Inhibition of DAMGO (Tyr-[D-Ala]-Gly-[NMe-Phe]-Gly-ol) binding to rat brain mu opioid receptor |
J Med Chem 48: 3239-50 (2005)
Article DOI: 10.1021/jm049157i
BindingDB Entry DOI: 10.7270/Q28P601Q |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data