

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Aromatase (Homo sapiens (Human)) | BDBM19461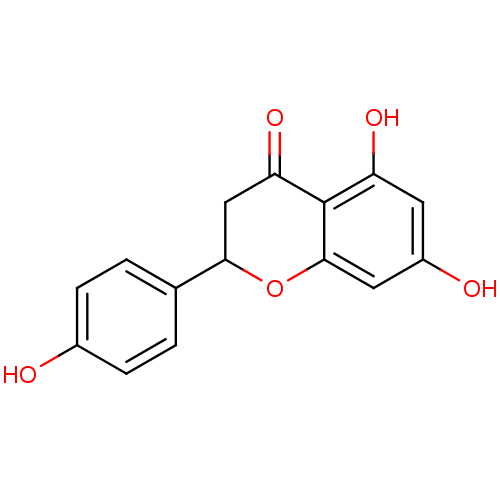 (α-CA inhibitor, 5 | 5,7-dihydroxy-2-(4-hydrox...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 0.00110 | n/a | 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Central Institute of Medicinal and Aromatic Plants | Assay Description Inhibition assay using aromatase enzyme. | Chem Biol Drug Des 80: 616-624 (2012) Article DOI: 10.1111/j.1747-0285.2012.01439.x BindingDB Entry DOI: 10.7270/Q24X56CG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Homo sapiens (Human)) | BDBM92556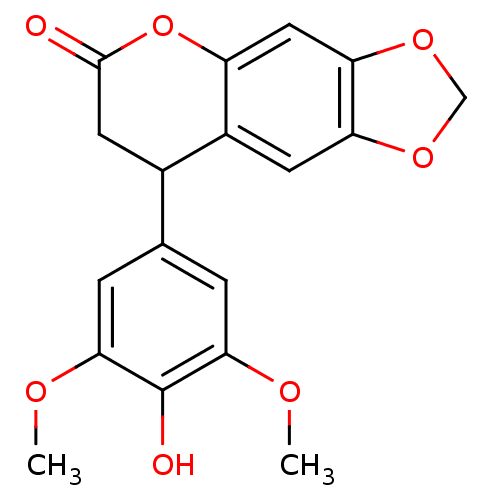 (Neoflavonoid, 8) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | Article PubMed | 0.00182 | n/a | 1.21E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Central Institute of Medicinal and Aromatic Plants | Assay Description Inhibition assay using aromatase enzyme. | Chem Biol Drug Des 80: 616-624 (2012) Article DOI: 10.1111/j.1747-0285.2012.01439.x BindingDB Entry DOI: 10.7270/Q24X56CG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Homo sapiens (Human)) | BDBM92555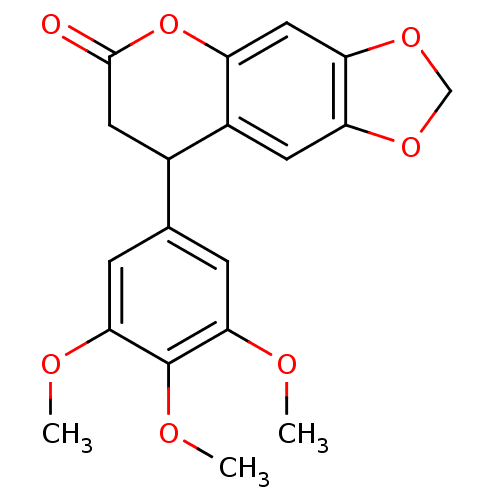 (Neoflavonoid, 7) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | Article PubMed | 0.00216 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Central Institute of Medicinal and Aromatic Plants | Assay Description Inhibition assay using aromatase enzyme. | Chem Biol Drug Des 80: 616-624 (2012) Article DOI: 10.1111/j.1747-0285.2012.01439.x BindingDB Entry DOI: 10.7270/Q24X56CG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Homo sapiens (Human)) | BDBM92557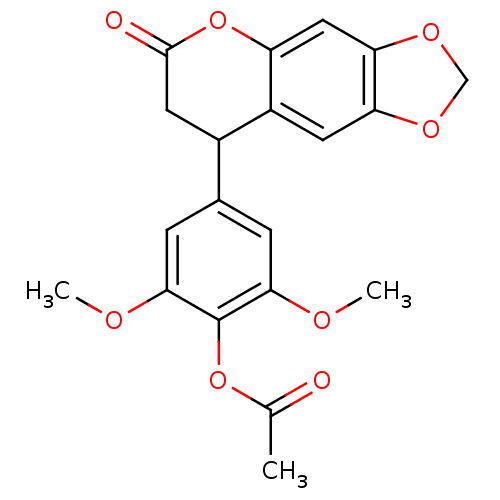 (Neoflavonoid, 9) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.00256 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Central Institute of Medicinal and Aromatic Plants | Assay Description Inhibition assay using aromatase enzyme. | Chem Biol Drug Des 80: 616-624 (2012) Article DOI: 10.1111/j.1747-0285.2012.01439.x BindingDB Entry DOI: 10.7270/Q24X56CG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Homo sapiens (Human)) | BDBM92559 (Neoflavonoid, 11) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.00425 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Central Institute of Medicinal and Aromatic Plants | Assay Description Inhibition assay using aromatase enzyme. | Chem Biol Drug Des 80: 616-624 (2012) Article DOI: 10.1111/j.1747-0285.2012.01439.x BindingDB Entry DOI: 10.7270/Q24X56CG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Homo sapiens (Human)) | BDBM92560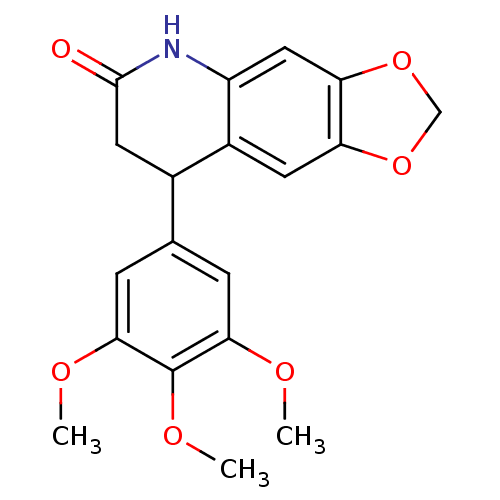 (Neoflavonoid, 19) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | Article PubMed | 0.00597 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Central Institute of Medicinal and Aromatic Plants | Assay Description Inhibition assay using aromatase enzyme. | Chem Biol Drug Des 80: 616-624 (2012) Article DOI: 10.1111/j.1747-0285.2012.01439.x BindingDB Entry DOI: 10.7270/Q24X56CG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Homo sapiens (Human)) | BDBM92558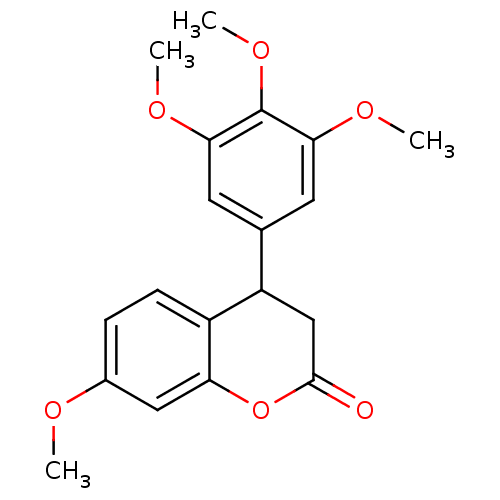 (Neoflavonoid, 10) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.00838 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Central Institute of Medicinal and Aromatic Plants | Assay Description Inhibition assay using aromatase enzyme. | Chem Biol Drug Des 80: 616-624 (2012) Article DOI: 10.1111/j.1747-0285.2012.01439.x BindingDB Entry DOI: 10.7270/Q24X56CG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Homo sapiens (Human)) | BDBM92561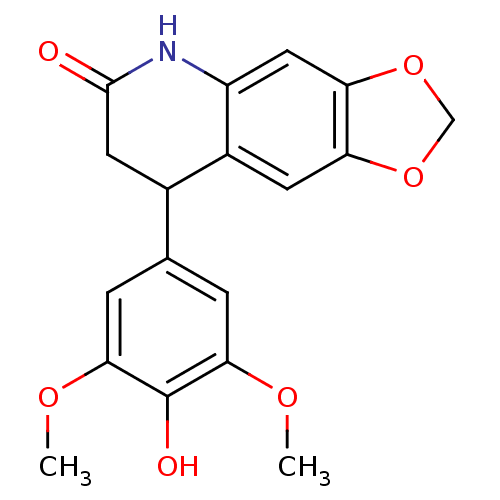 (Neoflavonoid, 20) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | Article PubMed | 0.0118 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Central Institute of Medicinal and Aromatic Plants | Assay Description Inhibition assay using aromatase enzyme. | Chem Biol Drug Des 80: 616-624 (2012) Article DOI: 10.1111/j.1747-0285.2012.01439.x BindingDB Entry DOI: 10.7270/Q24X56CG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Homo sapiens (Human)) | BDBM13061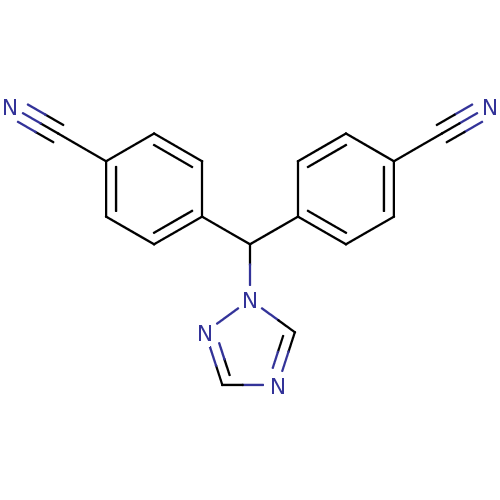 (4,4 -(1H-1,2,4-triazol-1-ylmethanediyl)dibenzonitr...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid UniChem Patents Similars | DrugBank Article PubMed | 0.0200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University Curated by ChEMBL | Assay Description Competitive inhibition of human aromatase using dibenzylfluorescein substrate after 10 mins preincubation measured every 10 sec for 5 mins by Michael... | Bioorg Med Chem 20: 2427-34 (2012) Article DOI: 10.1016/j.bmc.2012.01.047 BindingDB Entry DOI: 10.7270/Q2DJ5G3W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Homo sapiens (Human)) | BDBM8611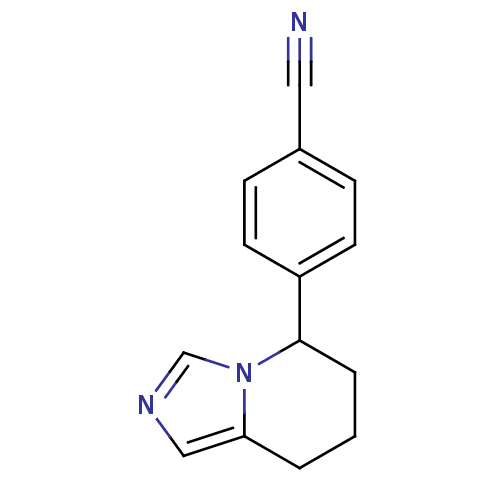 (4-{5H,6H,7H,8H-imidazo[1,5-a]pyridin-5-yl}benzonit...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 0.0500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University Curated by ChEMBL | Assay Description Competitive inhibition of human aromatase using dibenzylfluorescein substrate after 10 mins preincubation measured every 10 sec for 5 mins by Michael... | Bioorg Med Chem 20: 2427-34 (2012) Article DOI: 10.1016/j.bmc.2012.01.047 BindingDB Entry DOI: 10.7270/Q2DJ5G3W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Homo sapiens (Human)) | BDBM50366125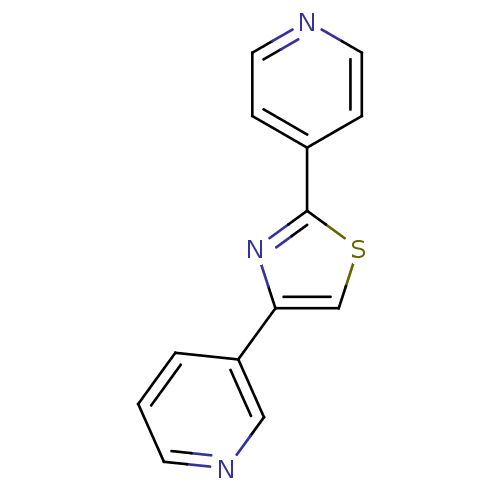 (CHEMBL1957214) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University Curated by ChEMBL | Assay Description Competitive inhibition of human aromatase using dibenzylfluorescein substrate after 10 mins preincubation measured every 10 sec for 5 mins by Michael... | Bioorg Med Chem 20: 2427-34 (2012) Article DOI: 10.1016/j.bmc.2012.01.047 BindingDB Entry DOI: 10.7270/Q2DJ5G3W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Homo sapiens (Human)) | BDBM10015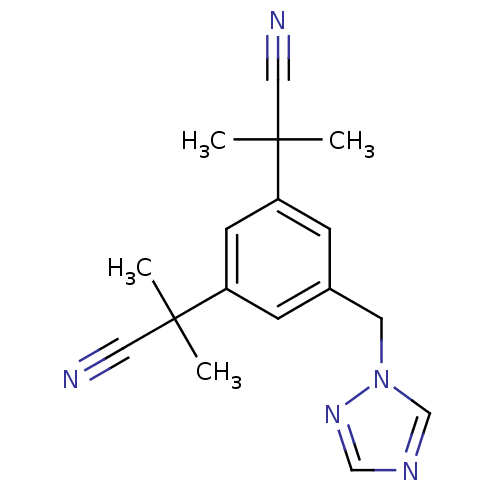 (2-[3-(1-cyano-1-methylethyl)-5-(1H-1,2,4-triazol-1...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid UniChem Patents Similars | DrugBank Article PubMed | 0.130 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University Curated by ChEMBL | Assay Description Competitive inhibition of human aromatase using dibenzylfluorescein substrate after 10 mins preincubation measured every 10 sec for 5 mins by Michael... | Bioorg Med Chem 20: 2427-34 (2012) Article DOI: 10.1016/j.bmc.2012.01.047 BindingDB Entry DOI: 10.7270/Q2DJ5G3W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelin-1 receptor (Homo sapiens (Human)) | BDBM50058126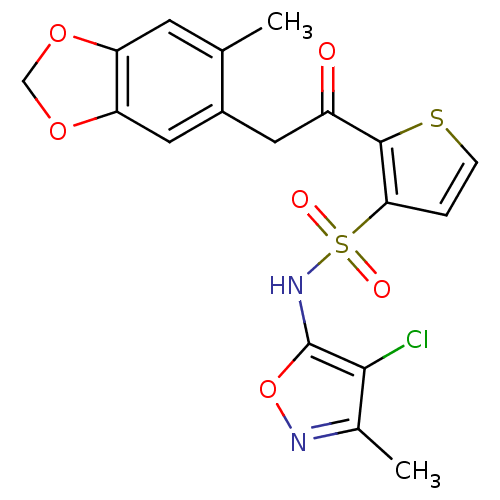 (2-[2-(6-Methyl-benzo[1,3]dioxol-5-yl)-acetyl]-thio...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE PC cid PC sid UniChem Patents Similars | Article PubMed | 0.430 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
ImmunoPharmaceutics Inc. Curated by ChEMBL | Assay Description Binding affinity against human Endothelin A receptor | J Med Chem 40: 1690-7 (1997) Article DOI: 10.1021/jm9700068 BindingDB Entry DOI: 10.7270/Q2222SWV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Homo sapiens (Human)) | BDBM50366128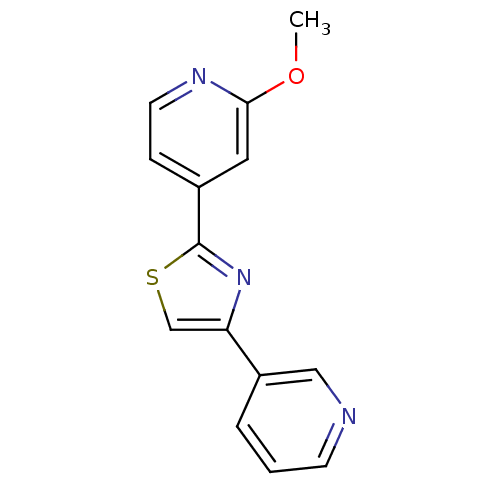 (CHEMBL1957217) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.650 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University Curated by ChEMBL | Assay Description Competitive inhibition of human aromatase using dibenzylfluorescein substrate after 10 mins preincubation measured every 10 sec for 5 mins by Michael... | Bioorg Med Chem 20: 2427-34 (2012) Article DOI: 10.1016/j.bmc.2012.01.047 BindingDB Entry DOI: 10.7270/Q2DJ5G3W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Homo sapiens (Human)) | BDBM50366129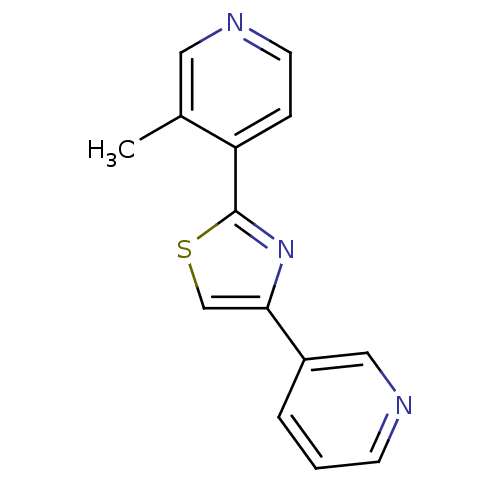 (CHEMBL1957218) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 3.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University Curated by ChEMBL | Assay Description Competitive inhibition of human aromatase using dibenzylfluorescein substrate after 10 mins preincubation measured every 10 sec for 5 mins by Michael... | Bioorg Med Chem 20: 2427-34 (2012) Article DOI: 10.1016/j.bmc.2012.01.047 BindingDB Entry DOI: 10.7270/Q2DJ5G3W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Homo sapiens (Human)) | BDBM50366127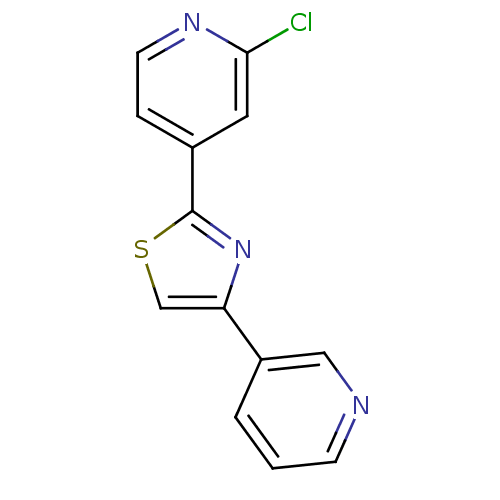 (CHEMBL1957216) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 8.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University Curated by ChEMBL | Assay Description Competitive inhibition of human aromatase using dibenzylfluorescein substrate after 10 mins preincubation measured every 10 sec for 5 mins by Michael... | Bioorg Med Chem 20: 2427-34 (2012) Article DOI: 10.1016/j.bmc.2012.01.047 BindingDB Entry DOI: 10.7270/Q2DJ5G3W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Geranylgeranyl pyrophosphate synthase BTS1 (Saccharomyces cerevisiae (Yeast)) | BDBM25297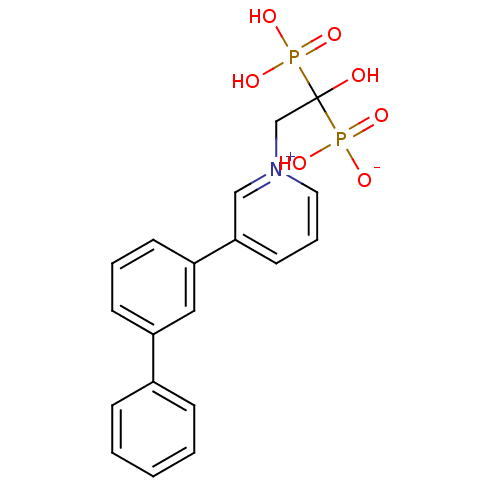 (1-(2-hydrogen phosphonato-2-hydroxy-2-phosphonoeth...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Biological Chemistry Curated by ChEMBL | Assay Description Binding affinity to Saccharomyces cerevisiae GGPPS | Proc Natl Acad Sci USA 104: 10022-7 (2007) Article DOI: 10.1073/pnas.0702254104 BindingDB Entry DOI: 10.7270/Q2MW2J2F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Geranylgeranyl pyrophosphate synthase (Homo sapiens (Human)) | BDBM25266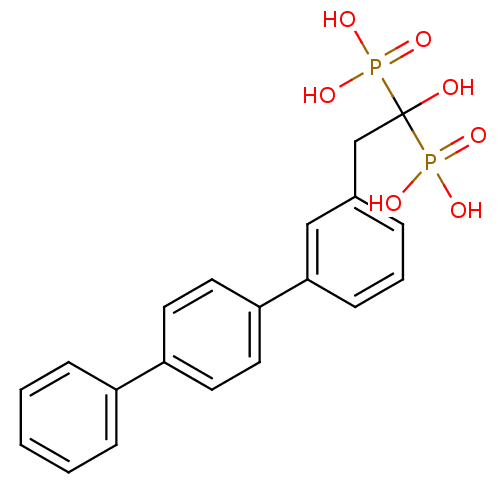 (BPH-628 | bisphosphonate, 21 | {1-hydroxy-2-[3-(4-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | DrugBank MMDB PC cid PC sid PDB UniChem Similars | Article PubMed | 20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Biological Chemistry Curated by ChEMBL | Assay Description Binding affinity to human GGPPS | Proc Natl Acad Sci USA 104: 10022-7 (2007) Article DOI: 10.1073/pnas.0702254104 BindingDB Entry DOI: 10.7270/Q2MW2J2F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Geranylgeranyl pyrophosphate synthase BTS1 (Saccharomyces cerevisiae (Yeast)) | BDBM25279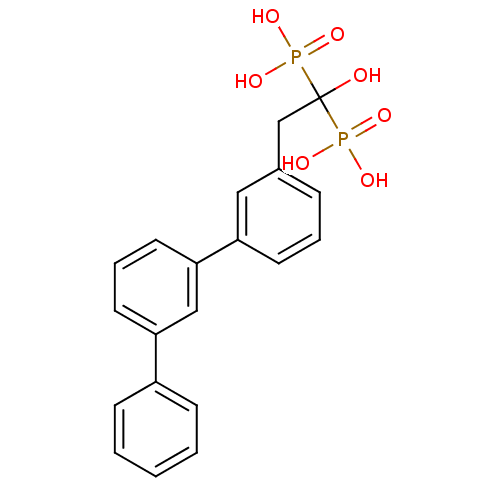 (BPH-608 | bisphosphonate, 31 | {1-hydroxy-2-[3-(3-...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | DrugBank MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Biological Chemistry Curated by ChEMBL | Assay Description Binding affinity to Saccharomyces cerevisiae GGPPS | Proc Natl Acad Sci USA 104: 10022-7 (2007) Article DOI: 10.1073/pnas.0702254104 BindingDB Entry DOI: 10.7270/Q2MW2J2F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Geranylgeranyl pyrophosphate synthase BTS1 (Saccharomyces cerevisiae (Yeast)) | BDBM25266 (BPH-628 | bisphosphonate, 21 | {1-hydroxy-2-[3-(4-...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | DrugBank MMDB PC cid PC sid PDB UniChem Similars | Article PubMed | 30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Biological Chemistry Curated by ChEMBL | Assay Description Binding affinity to Saccharomyces cerevisiae GGPPS | Proc Natl Acad Sci USA 104: 10022-7 (2007) Article DOI: 10.1073/pnas.0702254104 BindingDB Entry DOI: 10.7270/Q2MW2J2F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Homo sapiens (Human)) | BDBM50366134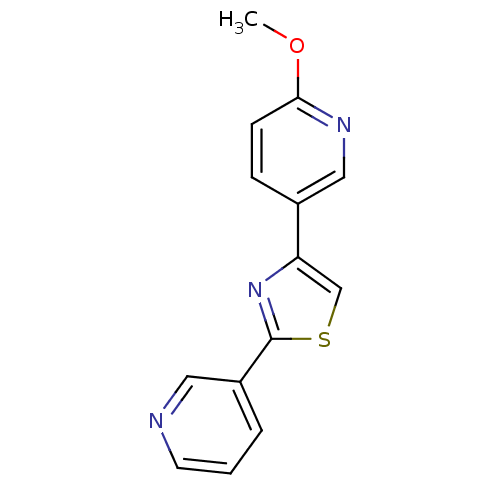 (CHEMBL1957223) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 35 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University Curated by ChEMBL | Assay Description Competitive inhibition of human aromatase using dibenzylfluorescein substrate after 10 mins preincubation measured every 10 sec for 5 mins by Michael... | Bioorg Med Chem 20: 2427-34 (2012) Article DOI: 10.1016/j.bmc.2012.01.047 BindingDB Entry DOI: 10.7270/Q2DJ5G3W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Replicase polyprotein 1ab (Human SARS coronavirus (SARS-CoV) (Severe acute re...) | BDBM92520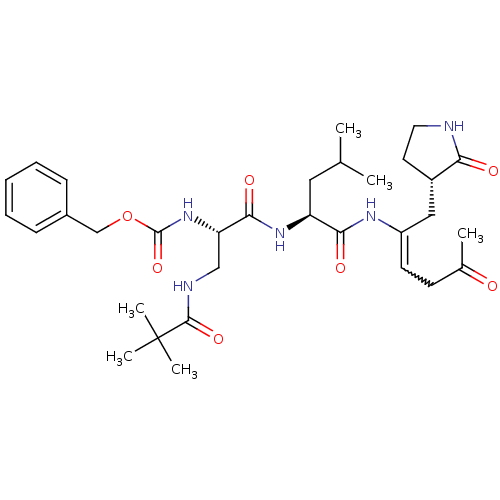 (TG-0204998) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 38 | -42.4 | n/a | n/a | n/a | n/a | n/a | 6.5 | 25 |
National Yang-Ming University | Assay Description Peptidomimetic inhibitors against CVB3 3Cpro and SARS-CoV 3CLpro. The inhibition constant of SARS 3CLpro was analyzed with reverse-phase HPLC using a... | J Biol Chem 284: 7646-55 (2009) Article DOI: 10.1074/jbc.M807947200 BindingDB Entry DOI: 10.7270/Q2SN07KT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Replicase polyprotein 1ab (Human SARS coronavirus (SARS-CoV) (Severe acute re...) | BDBM11233 (N-[(benzyloxy)carbonyl]-O-(tert-butyl)-L-threonyl-...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 54 | -41.5 | n/a | n/a | n/a | n/a | n/a | 6.5 | 25 |
National Yang-Ming University | Assay Description Peptidomimetic inhibitors against CVB3 3Cpro and SARS-CoV 3CLpro. The inhibition constant of SARS 3CLpro was analyzed with reverse-phase HPLC using a... | J Biol Chem 284: 7646-55 (2009) Article DOI: 10.1074/jbc.M807947200 BindingDB Entry DOI: 10.7270/Q2SN07KT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Replicase polyprotein 1ab (Human SARS coronavirus (SARS-CoV) (Severe acute re...) | BDBM11232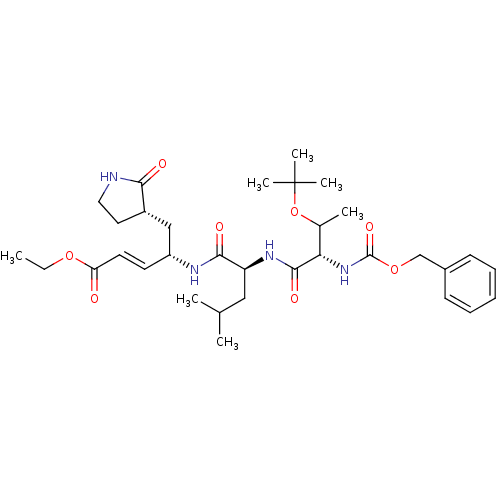 (N-[(benzyloxy)carbonyl]-O-(tert-butyl)-L-threonyl-...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 58 | -41.3 | n/a | n/a | n/a | n/a | n/a | 6.5 | 25 |
National Yang-Ming University | Assay Description Peptidomimetic inhibitors against CVB3 3Cpro and SARS-CoV 3CLpro. The inhibition constant of SARS 3CLpro was analyzed with reverse-phase HPLC using a... | J Biol Chem 284: 7646-55 (2009) Article DOI: 10.1074/jbc.M807947200 BindingDB Entry DOI: 10.7270/Q2SN07KT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Geranylgeranyl pyrophosphate synthase (Homo sapiens (Human)) | BDBM25279 (BPH-608 | bisphosphonate, 31 | {1-hydroxy-2-[3-(3-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | DrugBank MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Biological Chemistry Curated by ChEMBL | Assay Description Binding affinity to human GGPPS | Proc Natl Acad Sci USA 104: 10022-7 (2007) Article DOI: 10.1073/pnas.0702254104 BindingDB Entry DOI: 10.7270/Q2MW2J2F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Geranylgeranyl pyrophosphate synthase BTS1 (Saccharomyces cerevisiae (Yeast)) | BDBM25289 (bisphosphonate, 38 | {1-hydroxy-2-[3-(3-phenylphen...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | DrugBank MMDB PC cid PC sid PDB UniChem | Article PubMed | 60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Biological Chemistry Curated by ChEMBL | Assay Description Binding affinity to Saccharomyces cerevisiae GGPPS | Proc Natl Acad Sci USA 104: 10022-7 (2007) Article DOI: 10.1073/pnas.0702254104 BindingDB Entry DOI: 10.7270/Q2MW2J2F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Homo sapiens (Human)) | BDBM50366120 (CHEMBL1601919) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 63 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University Curated by ChEMBL | Assay Description Competitive inhibition of human aromatase using dibenzylfluorescein substrate after 10 mins preincubation measured every 10 sec for 5 mins by Michael... | Bioorg Med Chem 20: 2427-34 (2012) Article DOI: 10.1016/j.bmc.2012.01.047 BindingDB Entry DOI: 10.7270/Q2DJ5G3W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Geranylgeranyl pyrophosphate synthase (Homo sapiens (Human)) | BDBM25284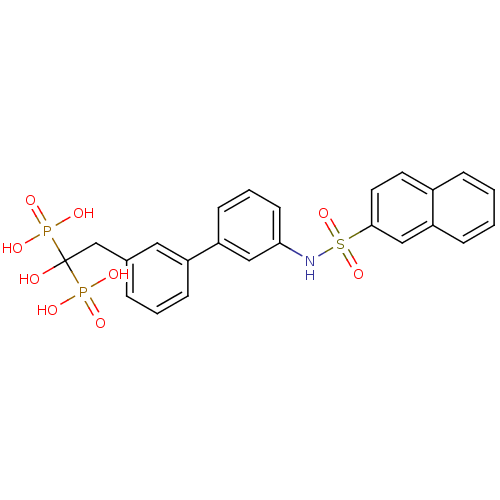 ((1-hydroxy-2-{3-[3-(naphthalene-2-sulfonamido)phen...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | MMDB PC cid PC sid PDB UniChem Similars | Article PubMed | 70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Biological Chemistry Curated by ChEMBL | Assay Description Binding affinity to human GGPPS | Proc Natl Acad Sci USA 104: 10022-7 (2007) Article DOI: 10.1073/pnas.0702254104 BindingDB Entry DOI: 10.7270/Q2MW2J2F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Geranylgeranyl pyrophosphate synthase BTS1 (Saccharomyces cerevisiae (Yeast)) | BDBM25284 ((1-hydroxy-2-{3-[3-(naphthalene-2-sulfonamido)phen...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | MMDB PC cid PC sid PDB UniChem Similars | PDB Article PubMed | 80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Biological Chemistry Curated by ChEMBL | Assay Description Binding affinity to Saccharomyces cerevisiae GGPPS | Proc Natl Acad Sci USA 104: 10022-7 (2007) Article DOI: 10.1073/pnas.0702254104 BindingDB Entry DOI: 10.7270/Q2MW2J2F | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Aromatase (Homo sapiens (Human)) | BDBM50366132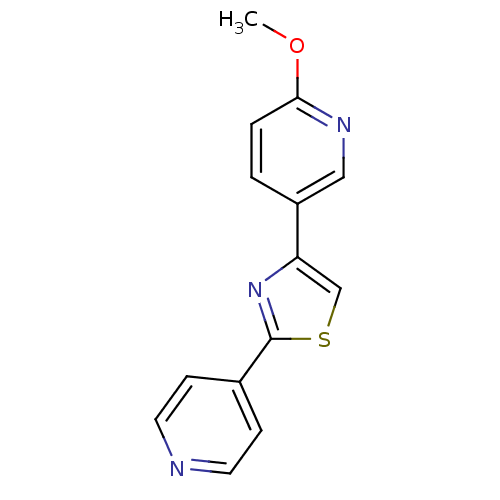 (CHEMBL1957221) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 83 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University Curated by ChEMBL | Assay Description Competitive inhibition of human aromatase using dibenzylfluorescein substrate after 10 mins preincubation measured every 10 sec for 5 mins by Michael... | Bioorg Med Chem 20: 2427-34 (2012) Article DOI: 10.1016/j.bmc.2012.01.047 BindingDB Entry DOI: 10.7270/Q2DJ5G3W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nitric oxide synthase, inducible (Homo sapiens (Human)) | BDBM50064015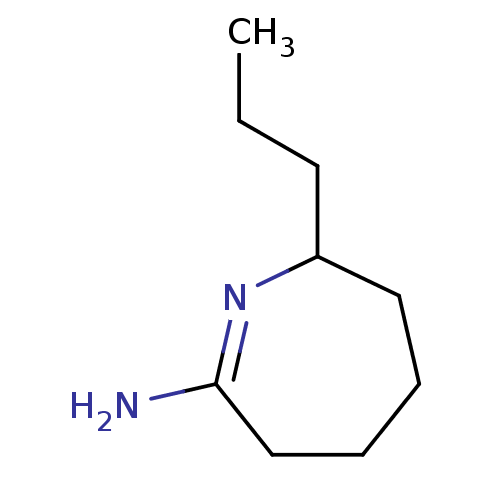 (7-Propyl-azepan-(2Z)-ylideneamine; hydrochloride |...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Inhibitory activity against L-arginine binding to Inducible nitric oxide synthase | J Med Chem 41: 1361-6 (1998) Article DOI: 10.1021/jm9704715 BindingDB Entry DOI: 10.7270/Q2348M29 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Replicase polyprotein 1ab (Human SARS coronavirus (SARS-CoV) (Severe acute re...) | BDBM92521 (TG-0205486) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 99 | -40.0 | n/a | n/a | n/a | n/a | n/a | 6.5 | 25 |
National Yang-Ming University | Assay Description Peptidomimetic inhibitors against CVB3 3Cpro and SARS-CoV 3CLpro. The inhibition constant of SARS 3CLpro was analyzed with reverse-phase HPLC using a... | J Biol Chem 284: 7646-55 (2009) Article DOI: 10.1074/jbc.M807947200 BindingDB Entry DOI: 10.7270/Q2SN07KT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Homo sapiens (Human)) | BDBM50366133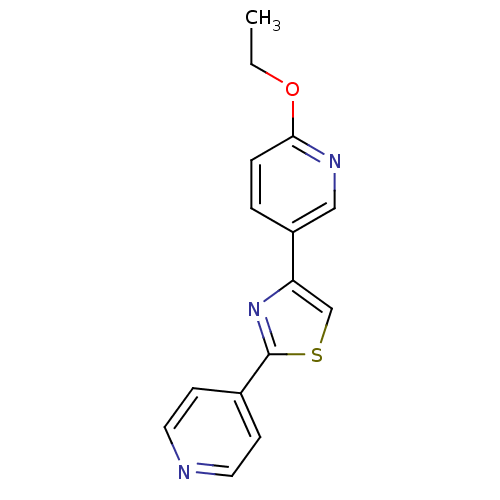 (CHEMBL1957222) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University Curated by ChEMBL | Assay Description Competitive inhibition of human aromatase using dibenzylfluorescein substrate after 10 mins preincubation measured every 10 sec for 5 mins by Michael... | Bioorg Med Chem 20: 2427-34 (2012) Article DOI: 10.1016/j.bmc.2012.01.047 BindingDB Entry DOI: 10.7270/Q2DJ5G3W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Geranylgeranyl pyrophosphate synthase (Homo sapiens (Human)) | BDBM25289 (bisphosphonate, 38 | {1-hydroxy-2-[3-(3-phenylphen...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | DrugBank MMDB PC cid PC sid PDB UniChem | Article PubMed | 110 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Biological Chemistry Curated by ChEMBL | Assay Description Binding affinity to human GGPPS | Proc Natl Acad Sci USA 104: 10022-7 (2007) Article DOI: 10.1073/pnas.0702254104 BindingDB Entry DOI: 10.7270/Q2MW2J2F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Geranylgeranyl pyrophosphate synthase BTS1 (Saccharomyces cerevisiae (Yeast)) | BDBM25288 (BPH-629 | [1-hydroxy-2-(3-{8-oxatricyclo[7.4.0.0^{...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | DrugBank MMDB PC cid PC sid PDB UniChem | PDB Article PubMed | 110 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Biological Chemistry Curated by ChEMBL | Assay Description Binding affinity to Saccharomyces cerevisiae GGPPS | Proc Natl Acad Sci USA 104: 10022-7 (2007) Article DOI: 10.1073/pnas.0702254104 BindingDB Entry DOI: 10.7270/Q2MW2J2F | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Geranylgeranyl pyrophosphate synthase (Homo sapiens (Human)) | BDBM25288 (BPH-629 | [1-hydroxy-2-(3-{8-oxatricyclo[7.4.0.0^{...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | DrugBank MMDB PC cid PC sid PDB UniChem | Article PubMed | 110 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Biological Chemistry Curated by ChEMBL | Assay Description Binding affinity to human GGPPS | Proc Natl Acad Sci USA 104: 10022-7 (2007) Article DOI: 10.1073/pnas.0702254104 BindingDB Entry DOI: 10.7270/Q2MW2J2F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Geranylgeranyl pyrophosphate synthase BTS1 (Saccharomyces cerevisiae (Yeast)) | BDBM25308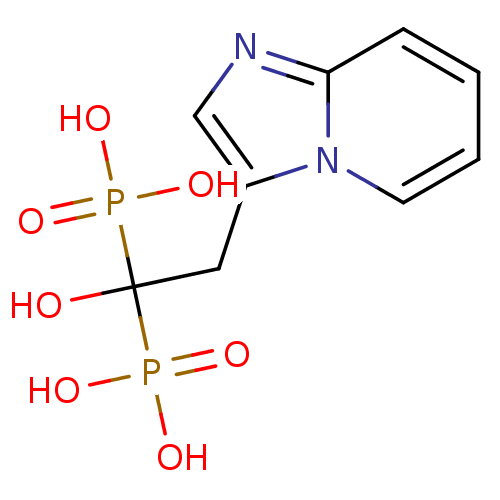 ((1-hydroxy-2-{imidazo[1,2-a]pyridin-3-yl}-1-phosph...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase MCE MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | 130 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Biological Chemistry Curated by ChEMBL | Assay Description Binding affinity to Saccharomyces cerevisiae GGPPS | Proc Natl Acad Sci USA 104: 10022-7 (2007) Article DOI: 10.1073/pnas.0702254104 BindingDB Entry DOI: 10.7270/Q2MW2J2F | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Geranylgeranyl pyrophosphate synthase (Homo sapiens (Human)) | BDBM25297 (1-(2-hydrogen phosphonato-2-hydroxy-2-phosphonoeth...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 230 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Biological Chemistry Curated by ChEMBL | Assay Description Binding affinity to human GGPPS | Proc Natl Acad Sci USA 104: 10022-7 (2007) Article DOI: 10.1073/pnas.0702254104 BindingDB Entry DOI: 10.7270/Q2MW2J2F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Homo sapiens (Human)) | BDBM50366122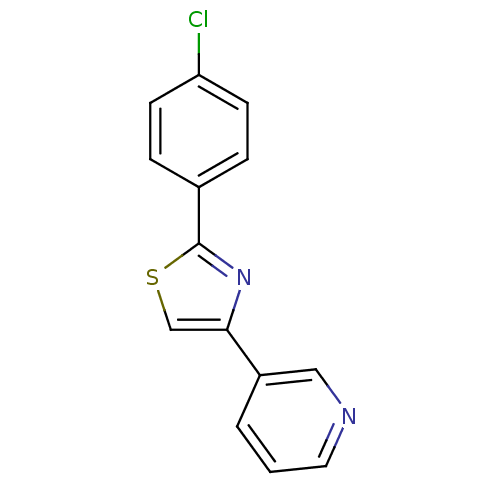 (CHEMBL1957211) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 234 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University Curated by ChEMBL | Assay Description Competitive inhibition of human aromatase using dibenzylfluorescein substrate after 10 mins preincubation measured every 10 sec for 5 mins by Michael... | Bioorg Med Chem 20: 2427-34 (2012) Article DOI: 10.1016/j.bmc.2012.01.047 BindingDB Entry DOI: 10.7270/Q2DJ5G3W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Geranylgeranyl pyrophosphate synthase BTS1 (Saccharomyces cerevisiae (Yeast)) | BDBM12578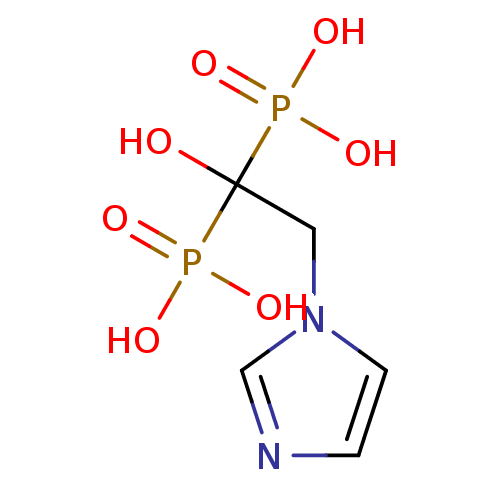 (2-(imidazol-1-yl)-1-hydroxyethylidene-1,1-bisphosp...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | 260 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Biological Chemistry Curated by ChEMBL | Assay Description Binding affinity to Saccharomyces cerevisiae GGPPS | Proc Natl Acad Sci USA 104: 10022-7 (2007) Article DOI: 10.1073/pnas.0702254104 BindingDB Entry DOI: 10.7270/Q2MW2J2F | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Aromatase (Homo sapiens (Human)) | BDBM50366130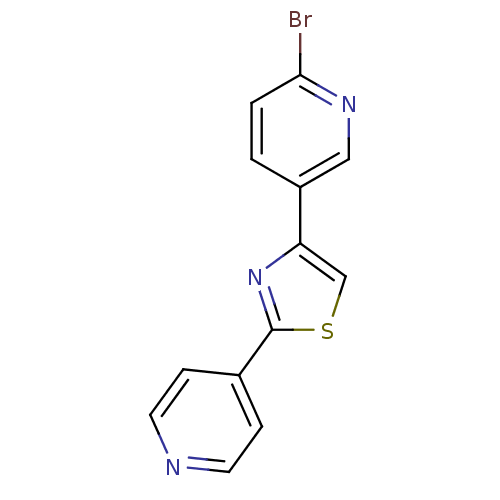 (CHEMBL1957219) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 285 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University Curated by ChEMBL | Assay Description Competitive inhibition of human aromatase using dibenzylfluorescein substrate after 10 mins preincubation measured every 10 sec for 5 mins by Michael... | Bioorg Med Chem 20: 2427-34 (2012) Article DOI: 10.1016/j.bmc.2012.01.047 BindingDB Entry DOI: 10.7270/Q2DJ5G3W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Coxsackievirus B3 (strain Nancy)) | BDBM92521 (TG-0205486) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 400 | -36.5 | n/a | n/a | n/a | n/a | n/a | 6.5 | 25 |
National Yang-Ming University | Assay Description Peptidomimetic inhibitors against CVB3 3Cpro and SARS-CoV 3CLpro. The inhibition constant of SARS 3CLpro was analyzed with reverse-phase HPLC using a... | J Biol Chem 284: 7646-55 (2009) Article DOI: 10.1074/jbc.M807947200 BindingDB Entry DOI: 10.7270/Q2SN07KT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Coxsackievirus B3 (strain Nancy)) | BDBM92520 (TG-0204998) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 800 | -34.8 | n/a | n/a | n/a | n/a | n/a | 6.5 | 25 |
National Yang-Ming University | Assay Description Peptidomimetic inhibitors against CVB3 3Cpro and SARS-CoV 3CLpro. The inhibition constant of SARS 3CLpro was analyzed with reverse-phase HPLC using a... | J Biol Chem 284: 7646-55 (2009) Article DOI: 10.1074/jbc.M807947200 BindingDB Entry DOI: 10.7270/Q2SN07KT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Homo sapiens (Human)) | BDBM50366118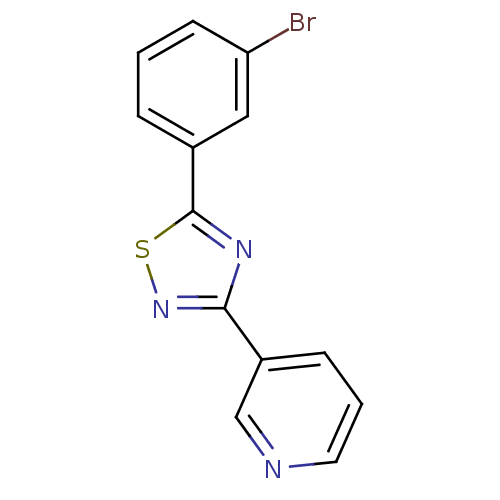 (CHEMBL1957208) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.16E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University Curated by ChEMBL | Assay Description Competitive inhibition of human aromatase using dibenzylfluorescein substrate after 10 mins preincubation measured every 10 sec for 5 mins by Michael... | Bioorg Med Chem 20: 2427-34 (2012) Article DOI: 10.1016/j.bmc.2012.01.047 BindingDB Entry DOI: 10.7270/Q2DJ5G3W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Homo sapiens (Human)) | BDBM50366131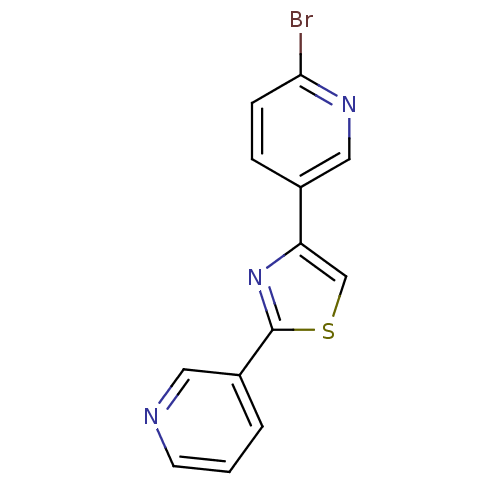 (CHEMBL1957220) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.48E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University Curated by ChEMBL | Assay Description Competitive inhibition of human aromatase using dibenzylfluorescein substrate after 10 mins preincubation measured every 10 sec for 5 mins by Michael... | Bioorg Med Chem 20: 2427-34 (2012) Article DOI: 10.1016/j.bmc.2012.01.047 BindingDB Entry DOI: 10.7270/Q2DJ5G3W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Coxsackievirus B3 (strain Nancy)) | BDBM11232 (N-[(benzyloxy)carbonyl]-O-(tert-butyl)-L-threonyl-...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 1.50E+3 | -33.2 | n/a | n/a | n/a | n/a | n/a | 6.5 | 25 |
National Yang-Ming University | Assay Description Peptidomimetic inhibitors against CVB3 3Cpro and SARS-CoV 3CLpro. The inhibition constant of SARS 3CLpro was analyzed with reverse-phase HPLC using a... | J Biol Chem 284: 7646-55 (2009) Article DOI: 10.1074/jbc.M807947200 BindingDB Entry DOI: 10.7270/Q2SN07KT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Homo sapiens (Human)) | BDBM50366123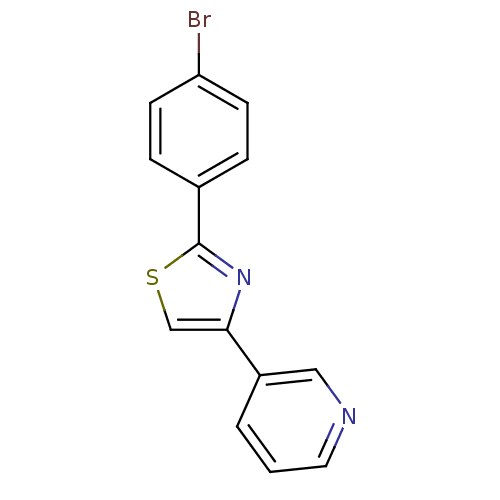 (CHEMBL1957212) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.72E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University Curated by ChEMBL | Assay Description Competitive inhibition of human aromatase using dibenzylfluorescein substrate after 10 mins preincubation measured every 10 sec for 5 mins by Michael... | Bioorg Med Chem 20: 2427-34 (2012) Article DOI: 10.1016/j.bmc.2012.01.047 BindingDB Entry DOI: 10.7270/Q2DJ5G3W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Geranylgeranyl pyrophosphate synthase (Homo sapiens (Human)) | BDBM25308 ((1-hydroxy-2-{imidazo[1,2-a]pyridin-3-yl}-1-phosph...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 1.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Biological Chemistry Curated by ChEMBL | Assay Description Binding affinity to human GGPPS | Proc Natl Acad Sci USA 104: 10022-7 (2007) Article DOI: 10.1073/pnas.0702254104 BindingDB Entry DOI: 10.7270/Q2MW2J2F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Coxsackievirus B3 (strain Nancy)) | BDBM11233 (N-[(benzyloxy)carbonyl]-O-(tert-butyl)-L-threonyl-...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 2.50E+3 | -32.0 | n/a | n/a | n/a | n/a | n/a | 6.5 | 25 |
National Yang-Ming University | Assay Description Peptidomimetic inhibitors against CVB3 3Cpro and SARS-CoV 3CLpro. The inhibition constant of SARS 3CLpro was analyzed with reverse-phase HPLC using a... | J Biol Chem 284: 7646-55 (2009) Article DOI: 10.1074/jbc.M807947200 BindingDB Entry DOI: 10.7270/Q2SN07KT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Homo sapiens (Human)) | BDBM50366126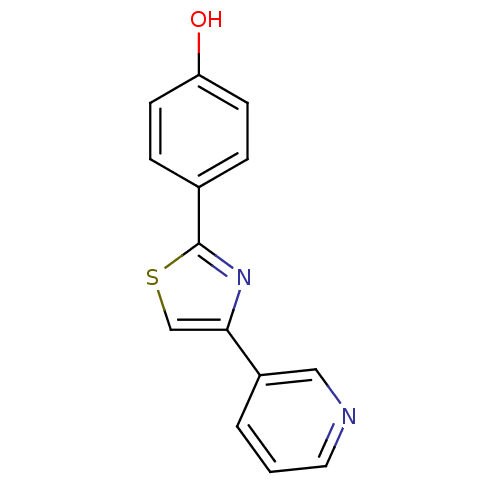 (CHEMBL1957215) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.55E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University Curated by ChEMBL | Assay Description Competitive inhibition of human aromatase using dibenzylfluorescein substrate after 10 mins preincubation measured every 10 sec for 5 mins by Michael... | Bioorg Med Chem 20: 2427-34 (2012) Article DOI: 10.1016/j.bmc.2012.01.047 BindingDB Entry DOI: 10.7270/Q2DJ5G3W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 928 total ) | Next | Last >> |