

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Serine/threonine-protein kinase pim-1 (Homo sapiens (Human)) | BDBM206677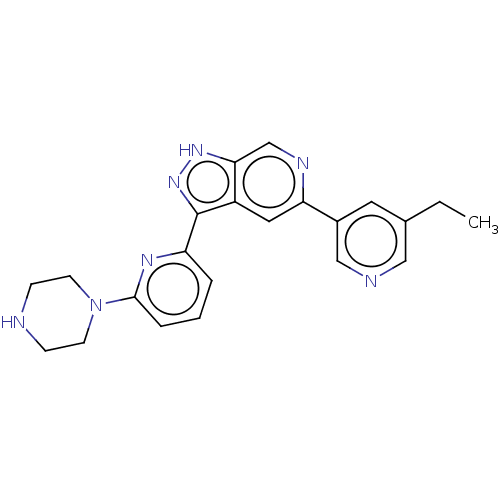 (US9260425, 438) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.00400 | n/a | n/a | n/a | n/a | n/a | n/a | 7.2 | n/a |
Genetech, Inc. US Patent | Assay Description PIM-1, PIM-2, and PIM-3 enzymes were generated as fusion proteins expressed in bacteria and purified by IMAC column chromatography (Sun, X., Chiu, J.... | US Patent US9260425 (2016) BindingDB Entry DOI: 10.7270/Q2RR1X3X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM17129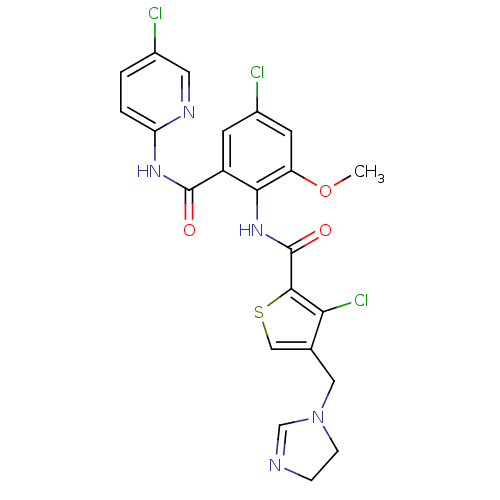 (3-chloro-N-{4-chloro-2-[(5-chloropyridin-2-yl)carb...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 0.00400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Berlex Biosciences | Assay Description The enzyme activities were determined kinetically as the initial rate of cleavage of a peptide p-nitroanilide. Km for enzyme and substrate was determ... | J Med Chem 50: 2967-80 (2007) Article DOI: 10.1021/jm070125f BindingDB Entry DOI: 10.7270/Q2J101F5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM17127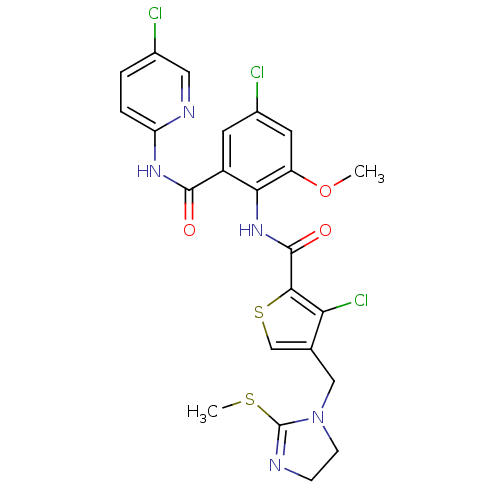 (3-chloro-N-{4-chloro-2-[(5-chloropyridin-2-yl)carb...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 0.00400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Berlex Biosciences | Assay Description The enzyme activities were determined kinetically as the initial rate of cleavage of a peptide p-nitroanilide. Km for enzyme and substrate was determ... | J Med Chem 50: 2967-80 (2007) Article DOI: 10.1021/jm070125f BindingDB Entry DOI: 10.7270/Q2J101F5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM17122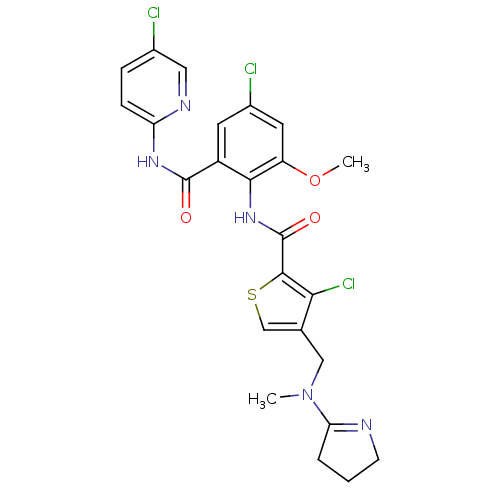 (3-chloro-N-{4-chloro-2-[(5-chloropyridin-2-yl)carb...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 0.00400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Berlex Biosciences | Assay Description The enzyme activities were determined kinetically as the initial rate of cleavage of a peptide p-nitroanilide. Km for enzyme and substrate was determ... | J Med Chem 50: 2967-80 (2007) Article DOI: 10.1021/jm070125f BindingDB Entry DOI: 10.7270/Q2J101F5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM17136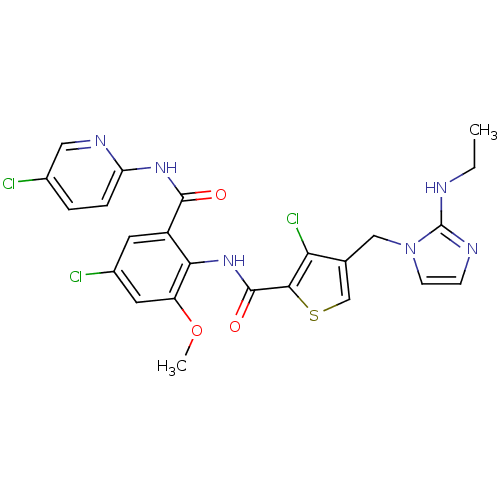 (3-chloro-N-{4-chloro-2-[(5-chloropyridin-2-yl)carb...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 0.00500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Berlex Biosciences | Assay Description The enzyme activities were determined kinetically as the initial rate of cleavage of a peptide p-nitroanilide. Km for enzyme and substrate was determ... | J Med Chem 50: 2967-80 (2007) Article DOI: 10.1021/jm070125f BindingDB Entry DOI: 10.7270/Q2J101F5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM17135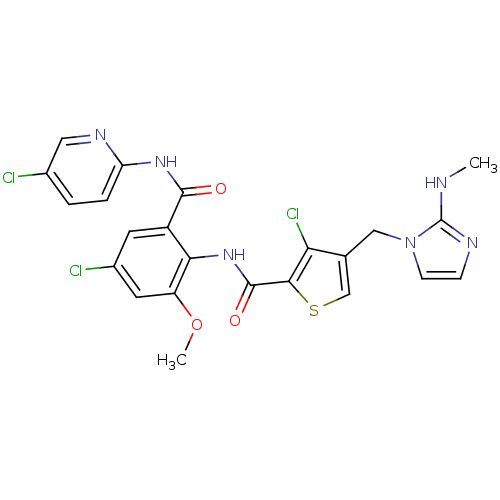 (3-chloro-N-{4-chloro-2-[(5-chloropyridin-2-yl)carb...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | MMDB PC cid PC sid PDB UniChem Patents Similars | MMDB PDB Article PubMed | 0.00500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Berlex Biosciences | Assay Description The enzyme activities were determined kinetically as the initial rate of cleavage of a peptide p-nitroanilide. Km for enzyme and substrate was determ... | J Med Chem 50: 2967-80 (2007) Article DOI: 10.1021/jm070125f BindingDB Entry DOI: 10.7270/Q2J101F5 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Serine/threonine-protein kinase pim-3 (Homo sapiens (Human)) | BDBM50131278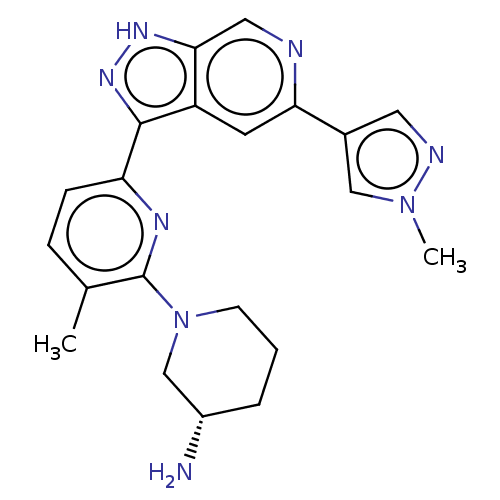 (CHEMBL3634760 | US9260425, 433) | KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.00600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech Curated by ChEMBL | Assay Description Inhibition of Pim3 (unknown origin) using 5FAM-ARKRRRHPSGPPTA as substrate after 90 mins | Bioorg Med Chem Lett 25: 5258-64 (2015) Article DOI: 10.1016/j.bmcl.2015.09.052 BindingDB Entry DOI: 10.7270/Q2TT4SR7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM17134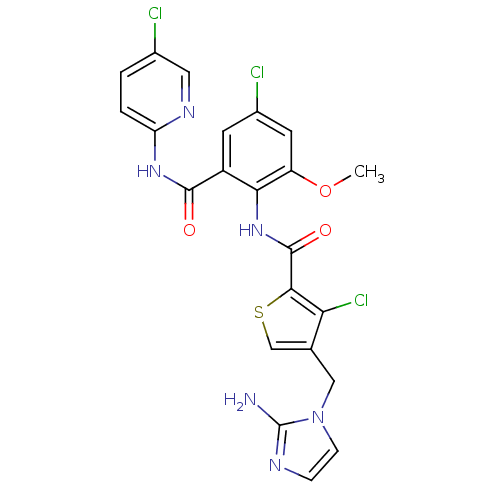 (4-[(2-amino-1H-imidazol-1-yl)methyl]-3-chloro-N-{4...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 0.00600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Berlex Biosciences | Assay Description The enzyme activities were determined kinetically as the initial rate of cleavage of a peptide p-nitroanilide. Km for enzyme and substrate was determ... | J Med Chem 50: 2967-80 (2007) Article DOI: 10.1021/jm070125f BindingDB Entry DOI: 10.7270/Q2J101F5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM17111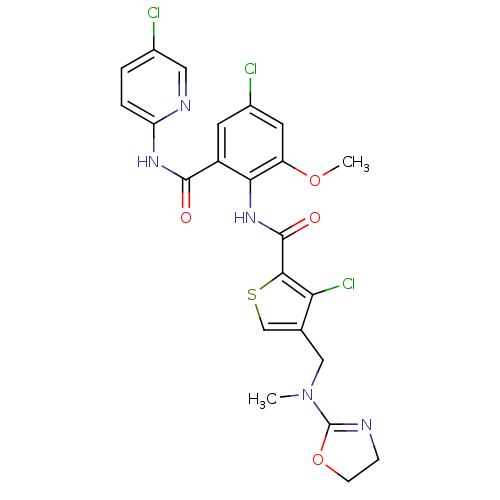 (3-chloro-N-{4-chloro-2-[(5-chloropyridin-2-yl)carb...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid PDB UniChem Similars | PDB Article PubMed | 0.00700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Berlex Biosciences | Assay Description The enzyme activities were determined kinetically as the initial rate of cleavage of a peptide p-nitroanilide. Km for enzyme and substrate was determ... | J Med Chem 50: 2967-80 (2007) Article DOI: 10.1021/jm070125f BindingDB Entry DOI: 10.7270/Q2J101F5 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Serine/threonine-protein kinase pim-1 (Homo sapiens (Human)) | BDBM206495 (US9260425, 245 | US9260425, 340) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.00790 | n/a | n/a | n/a | n/a | n/a | n/a | 7.2 | n/a |
Genetech, Inc. US Patent | Assay Description PIM-1, PIM-2, and PIM-3 enzymes were generated as fusion proteins expressed in bacteria and purified by IMAC column chromatography (Sun, X., Chiu, J.... | US Patent US9260425 (2016) BindingDB Entry DOI: 10.7270/Q2RR1X3X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM17137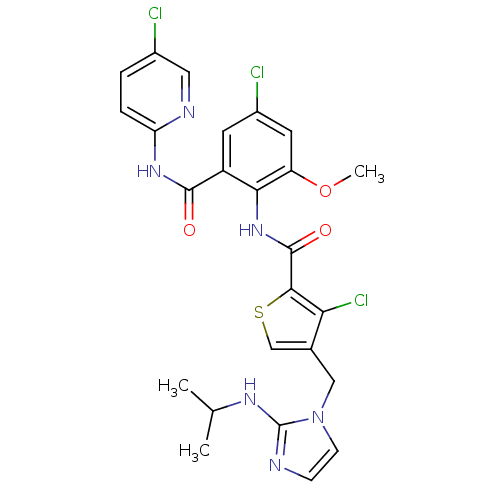 (3-chloro-N-{4-chloro-2-[(5-chloropyridin-2-yl)carb...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.00800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Berlex Biosciences | Assay Description The enzyme activities were determined kinetically as the initial rate of cleavage of a peptide p-nitroanilide. Km for enzyme and substrate was determ... | J Med Chem 50: 2967-80 (2007) Article DOI: 10.1021/jm070125f BindingDB Entry DOI: 10.7270/Q2J101F5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase pim-1 (Homo sapiens (Human)) | BDBM206562 (US9260425, 315) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.00806 | n/a | n/a | n/a | n/a | n/a | n/a | 7.2 | n/a |
Genetech, Inc. US Patent | Assay Description PIM-1, PIM-2, and PIM-3 enzymes were generated as fusion proteins expressed in bacteria and purified by IMAC column chromatography (Sun, X., Chiu, J.... | US Patent US9260425 (2016) BindingDB Entry DOI: 10.7270/Q2RR1X3X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase pim-1 (Homo sapiens (Human)) | BDBM206618 (US9260425, 376) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.00900 | n/a | n/a | n/a | n/a | n/a | n/a | 7.2 | n/a |
Genetech, Inc. US Patent | Assay Description PIM-1, PIM-2, and PIM-3 enzymes were generated as fusion proteins expressed in bacteria and purified by IMAC column chromatography (Sun, X., Chiu, J.... | US Patent US9260425 (2016) BindingDB Entry DOI: 10.7270/Q2RR1X3X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase pim-1 (Homo sapiens (Human)) | BDBM206724 (US9260425, 486) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.00900 | n/a | n/a | n/a | n/a | n/a | n/a | 7.2 | n/a |
Genetech, Inc. US Patent | Assay Description PIM-1, PIM-2, and PIM-3 enzymes were generated as fusion proteins expressed in bacteria and purified by IMAC column chromatography (Sun, X., Chiu, J.... | US Patent US9260425 (2016) BindingDB Entry DOI: 10.7270/Q2RR1X3X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM17123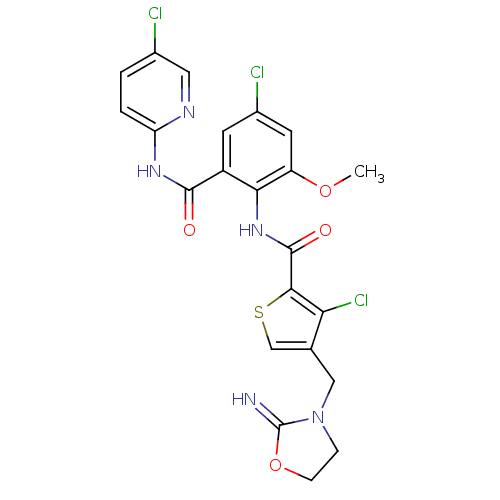 (3-chloro-N-{4-chloro-2-[(5-chloropyridin-2-yl)carb...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 0.0100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Berlex Biosciences | Assay Description The enzyme activities were determined kinetically as the initial rate of cleavage of a peptide p-nitroanilide. Km for enzyme and substrate was determ... | J Med Chem 50: 2967-80 (2007) Article DOI: 10.1021/jm070125f BindingDB Entry DOI: 10.7270/Q2J101F5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M3 (Homo sapiens (Human)) | BDBM50337878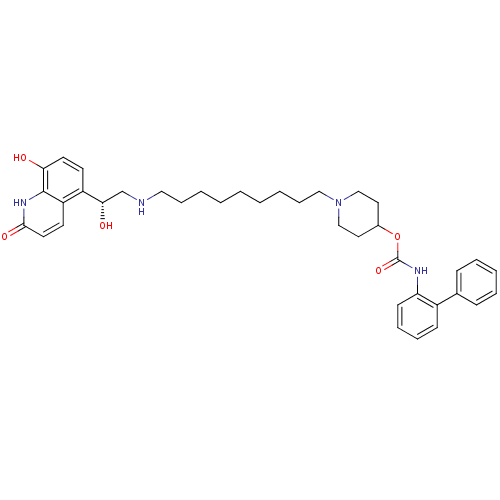 ((R)-1-(9-(2-hydroxy-2-(8-hydroxy-2-oxo-1,2-dihydro...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.0100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Theravance, Inc. Curated by ChEMBL | Assay Description Displacement of [3H]-N-methyl-scopolamine from human muscarinic M3 receptor after 6 hrs by cell based assay | Bioorg Med Chem Lett 21: 1354-8 (2011) Checked by Author Article DOI: 10.1016/j.bmcl.2011.01.043 BindingDB Entry DOI: 10.7270/Q2NC61HQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase pim-3 (Homo sapiens (Human)) | BDBM50131224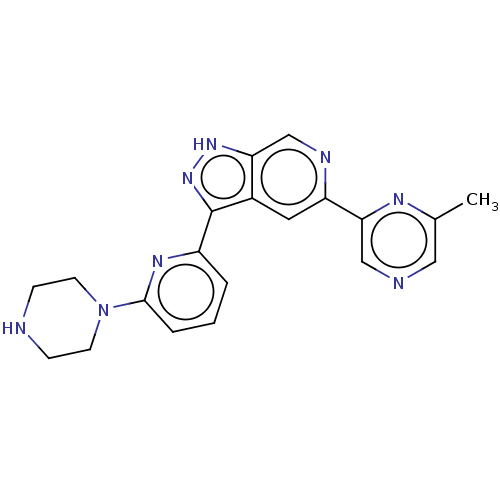 (CHEMBL3634783 | US9260425, 505) | KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech Curated by ChEMBL | Assay Description Inhibition of Pim3 (unknown origin) using 5FAM-ARKRRRHPSGPPTA as substrate after 90 mins | Bioorg Med Chem Lett 25: 5258-64 (2015) Article DOI: 10.1016/j.bmcl.2015.09.052 BindingDB Entry DOI: 10.7270/Q2TT4SR7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase pim-3 (Homo sapiens (Human)) | BDBM50131377 (CHEMBL3634781 | US9260425, 162) | KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech Curated by ChEMBL | Assay Description Inhibition of Pim3 (unknown origin) using 5FAM-ARKRRRHPSGPPTA as substrate after 90 mins | Bioorg Med Chem Lett 25: 5258-64 (2015) Article DOI: 10.1016/j.bmcl.2015.09.052 BindingDB Entry DOI: 10.7270/Q2TT4SR7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase pim-1 (Homo sapiens (Human)) | BDBM50131224 (CHEMBL3634783 | US9260425, 505) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech Curated by ChEMBL | Assay Description Inhibition of Pim1 (unknown origin) using 5FAM-ARKRRRHPSGPPTA as substrate after 90 mins | Bioorg Med Chem Lett 25: 5258-64 (2015) Article DOI: 10.1016/j.bmcl.2015.09.052 BindingDB Entry DOI: 10.7270/Q2TT4SR7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M2 (Homo sapiens (Human)) | BDBM50095105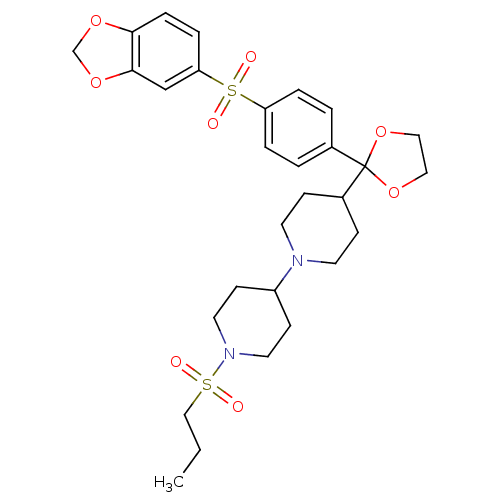 (4-{2-[4-(Benzo[1,3]dioxole-5-sulfonyl)-phenyl]-[1,...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 0.0100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute Curated by ChEMBL | Assay Description Antagonistic activity of the compound against Muscarinic acetylcholine receptor M2 | Bioorg Med Chem Lett 11: 2311-4 (2001) BindingDB Entry DOI: 10.7270/Q28S4P7Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase pim-1 (Homo sapiens (Human)) | BDBM50131224 (CHEMBL3634783 | US9260425, 505) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | US Patent | 0.0100 | n/a | n/a | n/a | n/a | n/a | n/a | 7.2 | n/a |
Genetech, Inc. US Patent | Assay Description PIM-1, PIM-2, and PIM-3 enzymes were generated as fusion proteins expressed in bacteria and purified by IMAC column chromatography (Sun, X., Chiu, J.... | US Patent US9260425 (2016) BindingDB Entry DOI: 10.7270/Q2RR1X3X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase pim-1 (Homo sapiens (Human)) | BDBM206587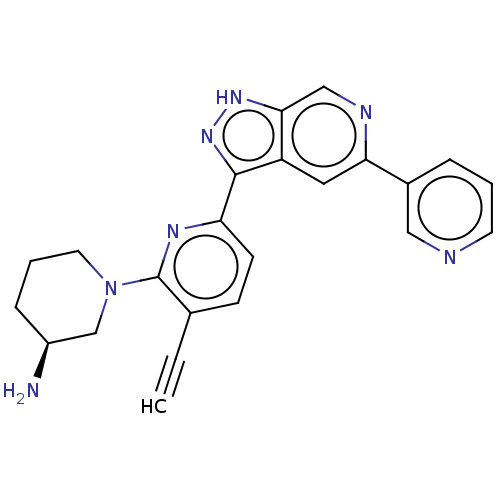 (US9260425, 342) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.0100 | n/a | n/a | n/a | n/a | n/a | n/a | 7.2 | n/a |
Genetech, Inc. US Patent | Assay Description PIM-1, PIM-2, and PIM-3 enzymes were generated as fusion proteins expressed in bacteria and purified by IMAC column chromatography (Sun, X., Chiu, J.... | US Patent US9260425 (2016) BindingDB Entry DOI: 10.7270/Q2RR1X3X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase pim-1 (Homo sapiens (Human)) | BDBM206555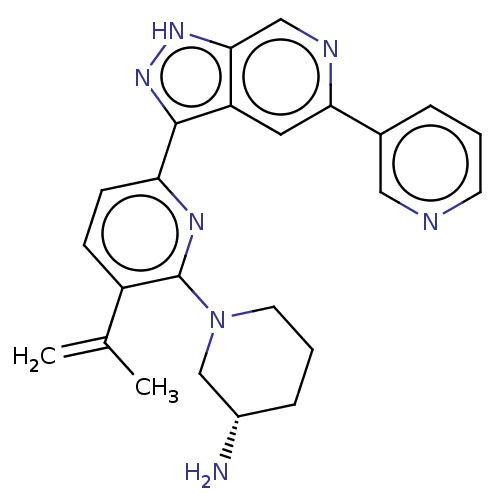 (US9260425, 308) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.0100 | n/a | n/a | n/a | n/a | n/a | n/a | 7.2 | n/a |
Genetech, Inc. US Patent | Assay Description PIM-1, PIM-2, and PIM-3 enzymes were generated as fusion proteins expressed in bacteria and purified by IMAC column chromatography (Sun, X., Chiu, J.... | US Patent US9260425 (2016) BindingDB Entry DOI: 10.7270/Q2RR1X3X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM17133 (3-chloro-N-{4-chloro-2-[(5-chloropyridin-2-yl)carb...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 0.0100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Berlex Biosciences | Assay Description The enzyme activities were determined kinetically as the initial rate of cleavage of a peptide p-nitroanilide. Km for enzyme and substrate was determ... | J Med Chem 50: 2967-80 (2007) Article DOI: 10.1021/jm070125f BindingDB Entry DOI: 10.7270/Q2J101F5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM17131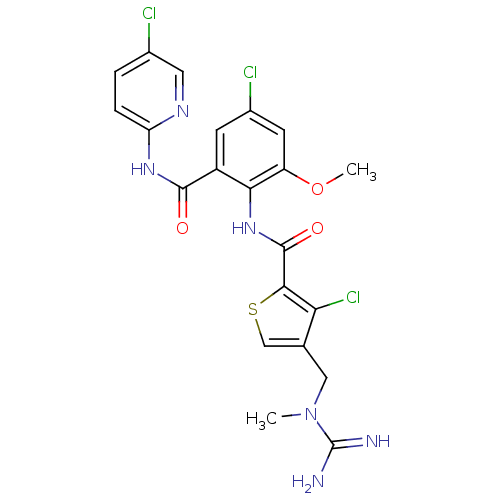 (3-chloro-N-{4-chloro-2-[(5-chloropyridin-2-yl)carb...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 0.0100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Berlex Biosciences | Assay Description The enzyme activities were determined kinetically as the initial rate of cleavage of a peptide p-nitroanilide. Km for enzyme and substrate was determ... | J Med Chem 50: 2967-80 (2007) Article DOI: 10.1021/jm070125f BindingDB Entry DOI: 10.7270/Q2J101F5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM17130 (3-chloro-N-{4-chloro-2-[(5-chloropyridin-2-yl)carb...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 0.0100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Berlex Biosciences | Assay Description The enzyme activities were determined kinetically as the initial rate of cleavage of a peptide p-nitroanilide. Km for enzyme and substrate was determ... | J Med Chem 50: 2967-80 (2007) Article DOI: 10.1021/jm070125f BindingDB Entry DOI: 10.7270/Q2J101F5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM17128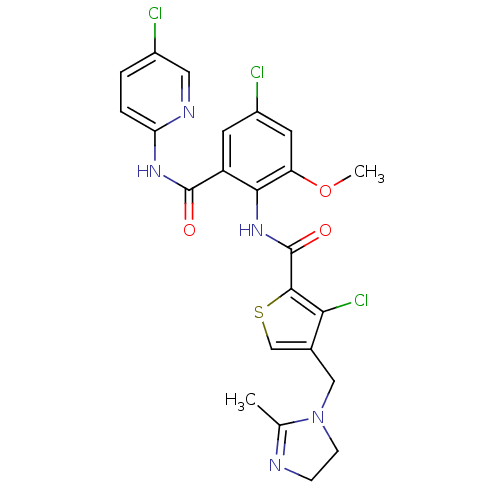 (3-chloro-N-{4-chloro-2-[(5-chloropyridin-2-yl)carb...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 0.0100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Berlex Biosciences | Assay Description The enzyme activities were determined kinetically as the initial rate of cleavage of a peptide p-nitroanilide. Km for enzyme and substrate was determ... | J Med Chem 50: 2967-80 (2007) Article DOI: 10.1021/jm070125f BindingDB Entry DOI: 10.7270/Q2J101F5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM17125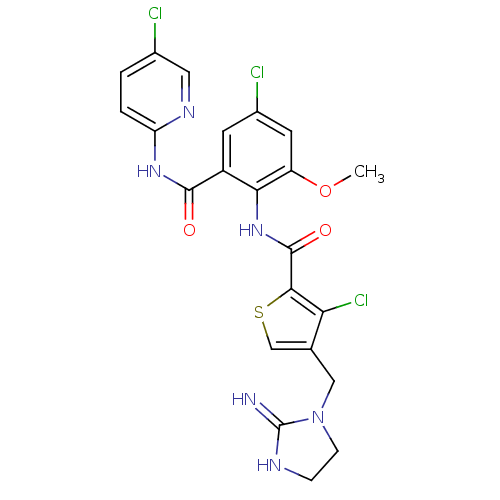 (3-chloro-N-{4-chloro-2-[(5-chloropyridin-2-yl)carb...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 0.0100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Berlex Biosciences | Assay Description The enzyme activities were determined kinetically as the initial rate of cleavage of a peptide p-nitroanilide. Km for enzyme and substrate was determ... | J Med Chem 50: 2967-80 (2007) Article DOI: 10.1021/jm070125f BindingDB Entry DOI: 10.7270/Q2J101F5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM17124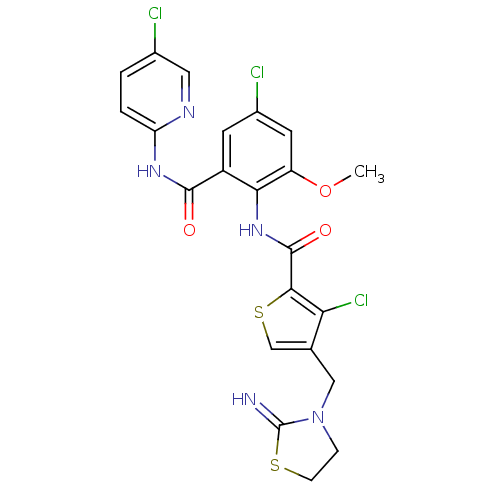 (3-chloro-N-{4-chloro-2-[(5-chloropyridin-2-yl)carb...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 0.0100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Berlex Biosciences | Assay Description The enzyme activities were determined kinetically as the initial rate of cleavage of a peptide p-nitroanilide. Km for enzyme and substrate was determ... | J Med Chem 50: 2967-80 (2007) Article DOI: 10.1021/jm070125f BindingDB Entry DOI: 10.7270/Q2J101F5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM17118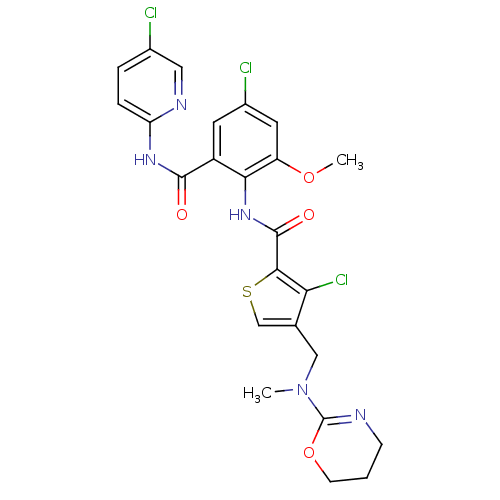 (3-chloro-N-{4-chloro-2-[(5-chloropyridin-2-yl)carb...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.0120 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Berlex Biosciences | Assay Description The enzyme activities were determined kinetically as the initial rate of cleavage of a peptide p-nitroanilide. Km for enzyme and substrate was determ... | J Med Chem 50: 2967-80 (2007) Article DOI: 10.1021/jm070125f BindingDB Entry DOI: 10.7270/Q2J101F5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM17112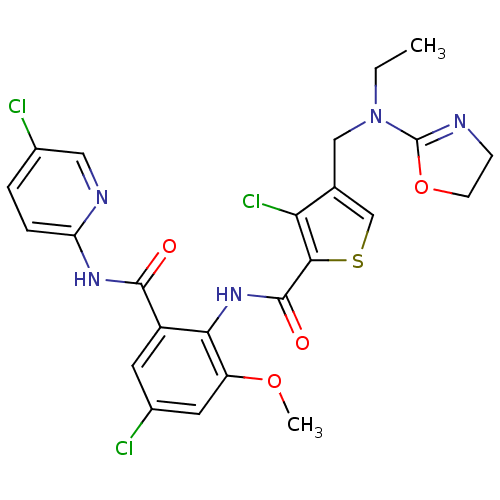 (3-chloro-N-{4-chloro-2-[(5-chloropyridin-2-yl)carb...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 0.0120 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Berlex Biosciences | Assay Description The enzyme activities were determined kinetically as the initial rate of cleavage of a peptide p-nitroanilide. Km for enzyme and substrate was determ... | J Med Chem 50: 2967-80 (2007) Article DOI: 10.1021/jm070125f BindingDB Entry DOI: 10.7270/Q2J101F5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase pim-1 (Homo sapiens (Human)) | BDBM50131278 (CHEMBL3634760 | US9260425, 433) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | US Patent | 0.0130 | n/a | n/a | n/a | n/a | n/a | n/a | 7.2 | n/a |
Genetech, Inc. US Patent | Assay Description PIM-1, PIM-2, and PIM-3 enzymes were generated as fusion proteins expressed in bacteria and purified by IMAC column chromatography (Sun, X., Chiu, J.... | US Patent US9260425 (2016) BindingDB Entry DOI: 10.7270/Q2RR1X3X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase pim-1 (Homo sapiens (Human)) | BDBM50131278 (CHEMBL3634760 | US9260425, 433) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0130 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech Curated by ChEMBL | Assay Description Inhibition of Pim1 (unknown origin) using 5FAM-ARKRRRHPSGPPTA as substrate after 90 mins | Bioorg Med Chem Lett 25: 5258-64 (2015) Article DOI: 10.1016/j.bmcl.2015.09.052 BindingDB Entry DOI: 10.7270/Q2TT4SR7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase pim-3 (Homo sapiens (Human)) | BDBM50131261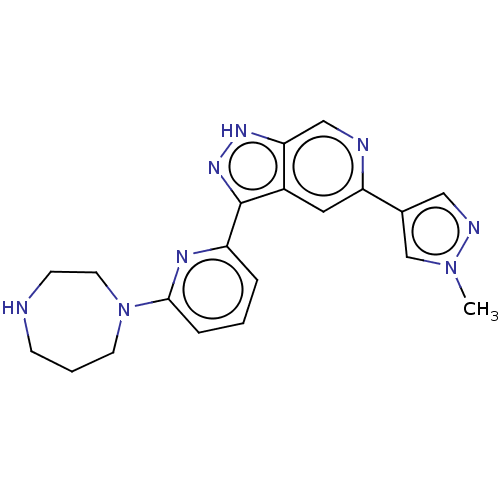 (CHEMBL3634767) | KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0130 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech Curated by ChEMBL | Assay Description Transcriptional activation of Retinoid X receptor RXR alpha | Bioorg Med Chem Lett 25: 5258-64 (2015) Article DOI: 10.1016/j.bmcl.2015.09.052 BindingDB Entry DOI: 10.7270/Q2TT4SR7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase pim-1 (Homo sapiens (Human)) | BDBM206715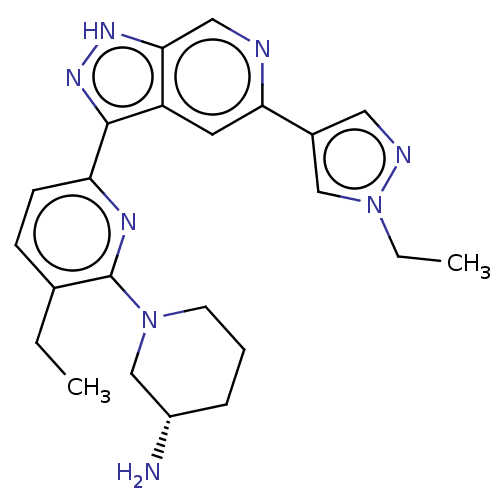 (US9260425, 477) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.0130 | n/a | n/a | n/a | n/a | n/a | n/a | 7.2 | n/a |
Genetech, Inc. US Patent | Assay Description PIM-1, PIM-2, and PIM-3 enzymes were generated as fusion proteins expressed in bacteria and purified by IMAC column chromatography (Sun, X., Chiu, J.... | US Patent US9260425 (2016) BindingDB Entry DOI: 10.7270/Q2RR1X3X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase pim-3 (Homo sapiens (Human)) | BDBM50131227 (CHEMBL3634771 | US9260425, 473) | KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0130 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech Curated by ChEMBL | Assay Description Inhibition of Pim3 (unknown origin) using 5FAM-ARKRRRHPSGPPTA as substrate after 90 mins | Bioorg Med Chem Lett 25: 5258-64 (2015) Article DOI: 10.1016/j.bmcl.2015.09.052 BindingDB Entry DOI: 10.7270/Q2TT4SR7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase pim-1 (Homo sapiens (Human)) | BDBM206554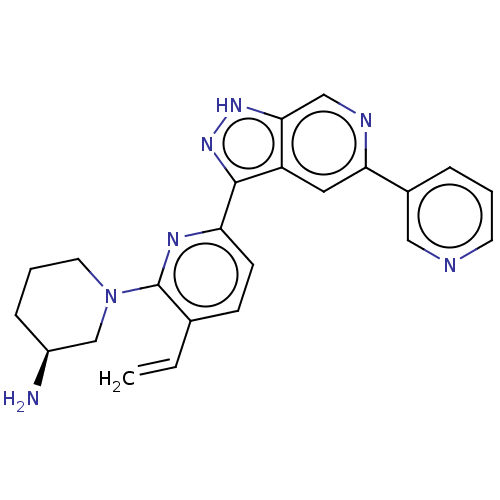 (US9260425, 307) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.0140 | n/a | n/a | n/a | n/a | n/a | n/a | 7.2 | n/a |
Genetech, Inc. US Patent | Assay Description PIM-1, PIM-2, and PIM-3 enzymes were generated as fusion proteins expressed in bacteria and purified by IMAC column chromatography (Sun, X., Chiu, J.... | US Patent US9260425 (2016) BindingDB Entry DOI: 10.7270/Q2RR1X3X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase pim-1 (Homo sapiens (Human)) | BDBM206651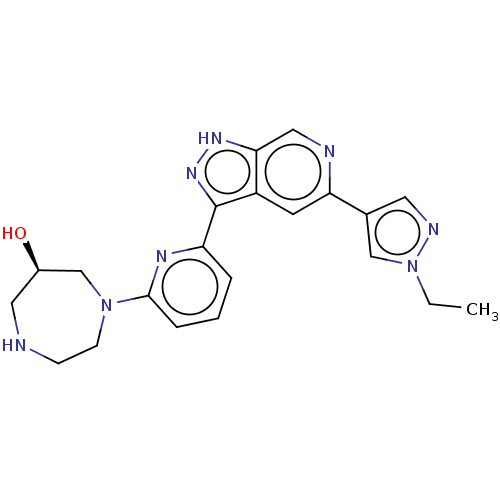 (US9260425, 411) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.0140 | n/a | n/a | n/a | n/a | n/a | n/a | 7.2 | n/a |
Genetech, Inc. US Patent | Assay Description PIM-1, PIM-2, and PIM-3 enzymes were generated as fusion proteins expressed in bacteria and purified by IMAC column chromatography (Sun, X., Chiu, J.... | US Patent US9260425 (2016) BindingDB Entry DOI: 10.7270/Q2RR1X3X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase pim-1 (Homo sapiens (Human)) | BDBM206727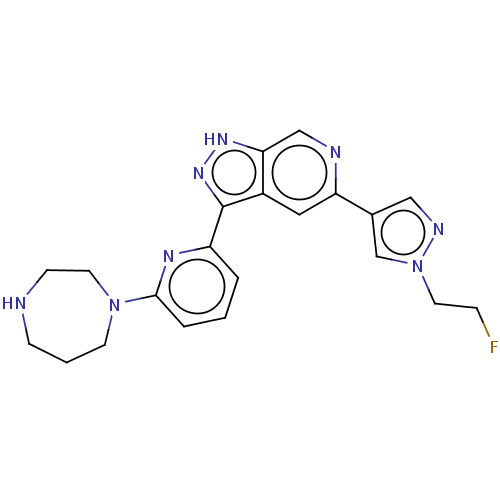 (US9260425, 489) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.0140 | n/a | n/a | n/a | n/a | n/a | n/a | 7.2 | n/a |
Genetech, Inc. US Patent | Assay Description PIM-1, PIM-2, and PIM-3 enzymes were generated as fusion proteins expressed in bacteria and purified by IMAC column chromatography (Sun, X., Chiu, J.... | US Patent US9260425 (2016) BindingDB Entry DOI: 10.7270/Q2RR1X3X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase pim-1 (Homo sapiens (Human)) | BDBM206442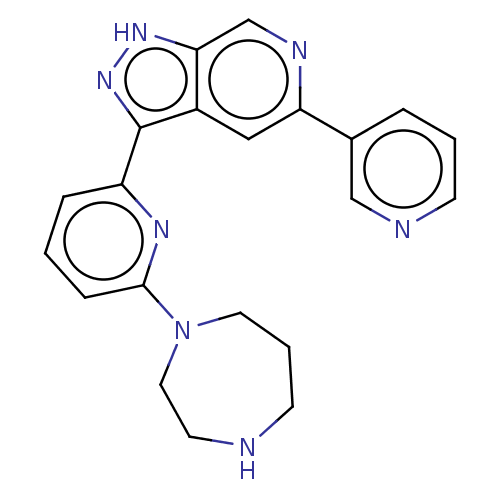 (US9260425, 187) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.0150 | n/a | n/a | n/a | n/a | n/a | n/a | 7.2 | n/a |
Genetech, Inc. US Patent | Assay Description PIM-1, PIM-2, and PIM-3 enzymes were generated as fusion proteins expressed in bacteria and purified by IMAC column chromatography (Sun, X., Chiu, J.... | US Patent US9260425 (2016) BindingDB Entry DOI: 10.7270/Q2RR1X3X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase pim-1 (Homo sapiens (Human)) | BDBM50131225 (CHEMBL3634769 | US9260425, 515) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | US Patent | 0.0150 | n/a | n/a | n/a | n/a | n/a | n/a | 7.2 | n/a |
Genetech, Inc. US Patent | Assay Description PIM-1, PIM-2, and PIM-3 enzymes were generated as fusion proteins expressed in bacteria and purified by IMAC column chromatography (Sun, X., Chiu, J.... | US Patent US9260425 (2016) BindingDB Entry DOI: 10.7270/Q2RR1X3X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase pim-3 (Homo sapiens (Human)) | BDBM50131265 (CHEMBL3634757 | US9260425, 189) | KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 0.0150 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech Curated by ChEMBL | Assay Description Inhibition of Pim3 (unknown origin) using 5FAM-ARKRRRHPSGPPTA as substrate after 90 mins | Bioorg Med Chem Lett 25: 5258-64 (2015) Article DOI: 10.1016/j.bmcl.2015.09.052 BindingDB Entry DOI: 10.7270/Q2TT4SR7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase pim-3 (Homo sapiens (Human)) | BDBM50131266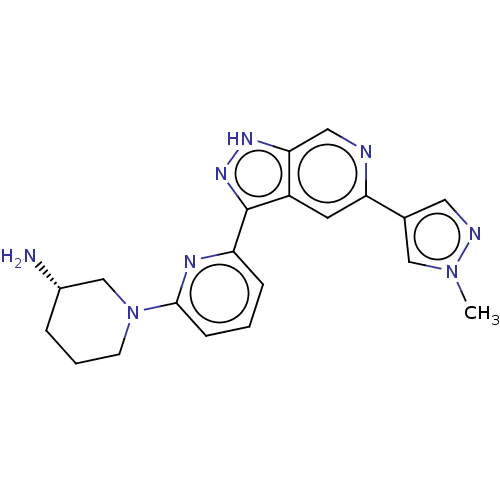 (CHEMBL3634758 | US9260425, 173) | KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0150 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech Curated by ChEMBL | Assay Description Inhibition of Pim3 (unknown origin) using 5FAM-ARKRRRHPSGPPTA as substrate after 90 mins | Bioorg Med Chem Lett 25: 5258-64 (2015) Article DOI: 10.1016/j.bmcl.2015.09.052 BindingDB Entry DOI: 10.7270/Q2TT4SR7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase pim-1 (Homo sapiens (Human)) | BDBM50131225 (CHEMBL3634769 | US9260425, 515) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0150 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech Curated by ChEMBL | Assay Description Inhibition of Pim1 (unknown origin) using 5FAM-ARKRRRHPSGPPTA as substrate after 90 mins | Bioorg Med Chem Lett 25: 5258-64 (2015) Article DOI: 10.1016/j.bmcl.2015.09.052 BindingDB Entry DOI: 10.7270/Q2TT4SR7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase pim-3 (Homo sapiens (Human)) | BDBM50131228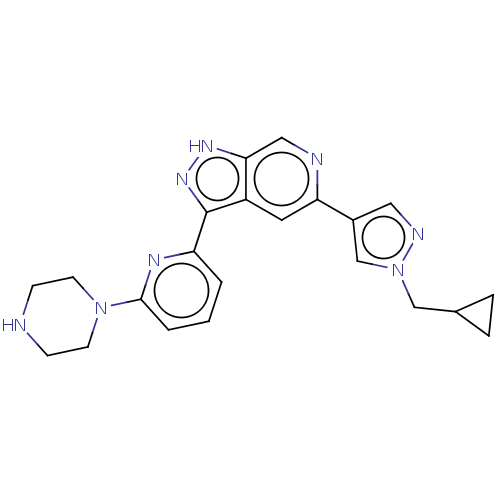 (CHEMBL3634772 | US9260425, 497) | KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 0.0160 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech Curated by ChEMBL | Assay Description Inhibition of Pim3 (unknown origin) using 5FAM-ARKRRRHPSGPPTA as substrate after 90 mins | Bioorg Med Chem Lett 25: 5258-64 (2015) Article DOI: 10.1016/j.bmcl.2015.09.052 BindingDB Entry DOI: 10.7270/Q2TT4SR7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase pim-1 (Homo sapiens (Human)) | BDBM206747 (US9260425, 511 | US9260425, 512) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.0160 | n/a | n/a | n/a | n/a | n/a | n/a | 7.2 | n/a |
Genetech, Inc. US Patent | Assay Description PIM-1, PIM-2, and PIM-3 enzymes were generated as fusion proteins expressed in bacteria and purified by IMAC column chromatography (Sun, X., Chiu, J.... | US Patent US9260425 (2016) BindingDB Entry DOI: 10.7270/Q2RR1X3X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase pim-1 (Homo sapiens (Human)) | BDBM206667 (US9260425, 427) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.0160 | n/a | n/a | n/a | n/a | n/a | n/a | 7.2 | n/a |
Genetech, Inc. US Patent | Assay Description PIM-1, PIM-2, and PIM-3 enzymes were generated as fusion proteins expressed in bacteria and purified by IMAC column chromatography (Sun, X., Chiu, J.... | US Patent US9260425 (2016) BindingDB Entry DOI: 10.7270/Q2RR1X3X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase pim-1 (Homo sapiens (Human)) | BDBM206536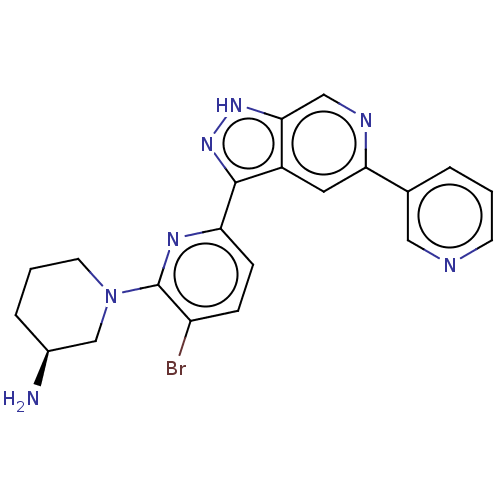 (US9260425, 289) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.0170 | n/a | n/a | n/a | n/a | n/a | n/a | 7.2 | n/a |
Genetech, Inc. US Patent | Assay Description PIM-1, PIM-2, and PIM-3 enzymes were generated as fusion proteins expressed in bacteria and purified by IMAC column chromatography (Sun, X., Chiu, J.... | US Patent US9260425 (2016) BindingDB Entry DOI: 10.7270/Q2RR1X3X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase pim-3 (Homo sapiens (Human)) | BDBM50131226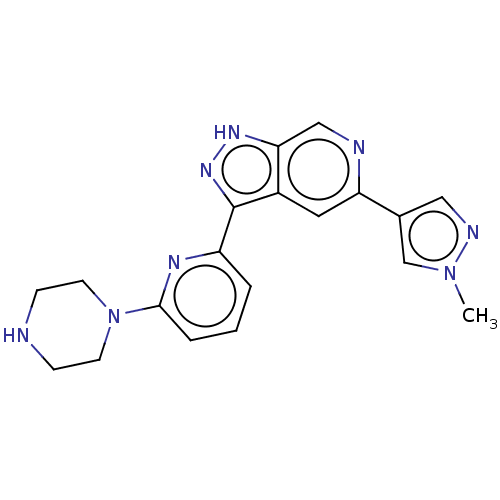 (CHEMBL3634770 | US9260425, 234) | KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0170 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech Curated by ChEMBL | Assay Description Inhibition of Pim3 (unknown origin) using 5FAM-ARKRRRHPSGPPTA as substrate after 90 mins | Bioorg Med Chem Lett 25: 5258-64 (2015) Article DOI: 10.1016/j.bmcl.2015.09.052 BindingDB Entry DOI: 10.7270/Q2TT4SR7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase pim-1 (Homo sapiens (Human)) | BDBM206512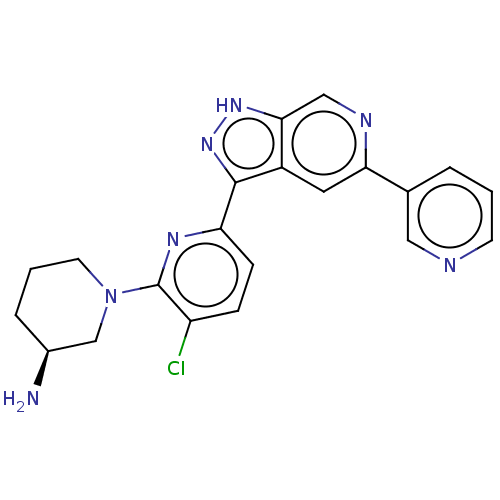 (US9260425, 265) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.0190 | n/a | n/a | n/a | n/a | n/a | n/a | 7.2 | n/a |
Genetech, Inc. US Patent | Assay Description PIM-1, PIM-2, and PIM-3 enzymes were generated as fusion proteins expressed in bacteria and purified by IMAC column chromatography (Sun, X., Chiu, J.... | US Patent US9260425 (2016) BindingDB Entry DOI: 10.7270/Q2RR1X3X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 21329 total ) | Next | Last >> |