

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50110291 ((7-Chloro-quinolin-4-yl)-[4-(3-piperidin-1-yl-prop...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 0.0860 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Freie Universität Berlin Curated by ChEMBL | Assay Description Affinity for displacement of [125I]-iodoproxyfan from human histamine H3 receptors stably expressed in CHO cells | J Med Chem 45: 1128-41 (2002) BindingDB Entry DOI: 10.7270/Q2H70F4V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50110288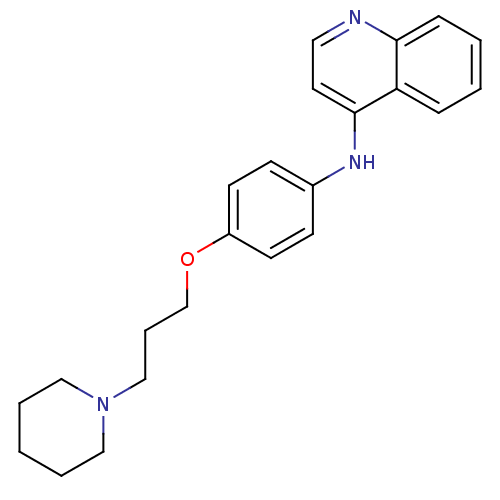 (CHEMBL15153 | N-(4-(3-(piperidin-1-yl)propoxy)phen...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.0910 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Freie Universität Berlin Curated by ChEMBL | Assay Description Affinity for displacement of [125I]-iodoproxyfan from human histamine H3 receptors stably expressed in CHO cells | J Med Chem 45: 1128-41 (2002) BindingDB Entry DOI: 10.7270/Q2H70F4V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hrh3 protein (RAT) | BDBM22911 (2-(3H-imidazol-4-yl)ethylsulfanylmethanimidamide |...) | KEGG UniProtKB/TrEMBL GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Patents Similars | PubMed | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
U. 109 Curated by PDSP Ki Database | J Pharmacol Exp Ther 271: 452-9 (1994) BindingDB Entry DOI: 10.7270/Q23X8556 | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Rattus norvegicus (rat)) | BDBM50098203 (1-{4-[2-(1H-Imidazol-4-yl)-ethylsulfanyl]-phenyl}-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.110 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Freie Universität Berlin Curated by ChEMBL | Assay Description Inhibition of [125I]-iodoproxyfan from rat histamine H3 receptor expressed in CHO-K1 cells | Bioorg Med Chem Lett 11: 951-4 (2001) BindingDB Entry DOI: 10.7270/Q28P5ZRB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Rattus norvegicus (rat)) | BDBM50074629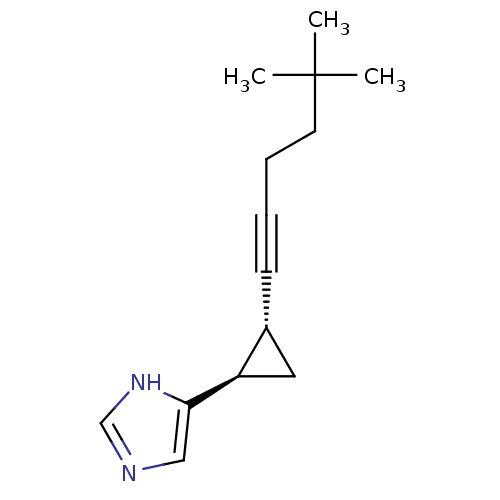 (4-[(1R,2R)-2-(5,5-Dimethyl-hex-1-ynyl)-cyclopropyl...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | PubMed | 0.120 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Freie Universität Berlin Curated by ChEMBL | Assay Description In vitro inhibitory activity against Histamine H3 receptor for K+ evoked depolarization-induced release of [3H]-histamine from synaptosomes of rat ce... | Bioorg Med Chem Lett 10: 2379-82 (2001) BindingDB Entry DOI: 10.7270/Q28G8JZ4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hrh3 protein (RAT) | BDBM22911 (2-(3H-imidazol-4-yl)ethylsulfanylmethanimidamide |...) | KEGG UniProtKB/TrEMBL GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Patents Similars | PubMed | 0.150 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
U. 109 Curated by PDSP Ki Database | J Pharmacol Exp Ther 271: 452-9 (1994) BindingDB Entry DOI: 10.7270/Q23X8556 | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (GUINEA PIG) | BDBM50247053 (1-(3-(3-(4-chlorophenyl)propoxy)propyl)piperidine ...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | 0.160 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Jagiellonian University Medical College Curated by ChEMBL | Assay Description Antagonist activity at human histamine H3 receptor expressed in HEK293 cells by [35S]GTPgammaS binding assay | Bioorg Med Chem 17: 3037-42 (2009) Article DOI: 10.1016/j.bmc.2009.03.014 BindingDB Entry DOI: 10.7270/Q2SF2X3H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hrh3 protein (RAT) | BDBM22916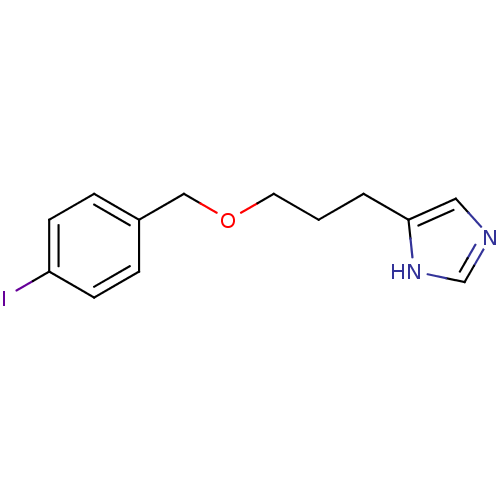 (5-{3-[(4-iodophenyl)methoxy]propyl}-1H-imidazole |...) | KEGG UniProtKB/TrEMBL GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
U. 109 Curated by PDSP Ki Database | J Pharmacol Exp Ther 271: 452-9 (1994) BindingDB Entry DOI: 10.7270/Q23X8556 | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Rattus norvegicus (rat)) | BDBM50091385 (1-{4-[3-(1H-Imidazol-4-yl)-propoxy]-phenyl}-ethano...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.260 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Freie Universität Berlin Curated by ChEMBL | Assay Description Histamine H3 receptor antagonist activity in an assay with K+ evoked depolarization-induced release of [3H]-histamine from rat synaptosomes. | J Med Chem 43: 3335-43 (2000) BindingDB Entry DOI: 10.7270/Q2RR1XGV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Rattus norvegicus (rat)) | BDBM50091373 (1-{2-Fluoro-4-[3-(1H-imidazol-4-yl)-propoxy]-pheny...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Freie Universität Berlin Curated by ChEMBL | Assay Description Histamine H3 receptor antagonist activity in an assay with K+ evoked depolarization-induced release of [3H]-histamine from rat synaptosomes. | J Med Chem 43: 3335-43 (2000) BindingDB Entry DOI: 10.7270/Q2RR1XGV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50110300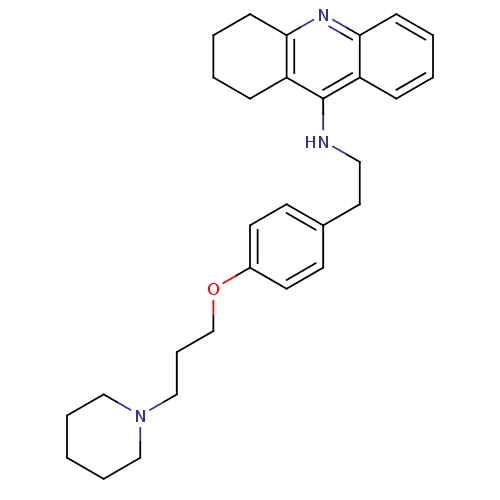 (CHEMBL15056 | N-(4-(3-(piperidin-1-yl)propoxy)phen...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.330 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Freie Universität Berlin Curated by ChEMBL | Assay Description Affinity for displacement of [125I]-iodoproxyfan from human histamine H3 receptors stably expressed in CHO cells | J Med Chem 45: 1128-41 (2002) BindingDB Entry DOI: 10.7270/Q2H70F4V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Rattus norvegicus (rat)) | BDBM50091396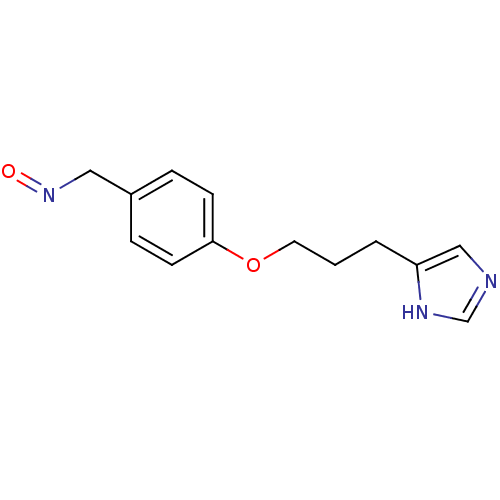 (4-[3-(1H-Imidazol-4-yl)-propoxy]-benzaldehyde oxim...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.440 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Freie Universität Berlin Curated by ChEMBL | Assay Description Histamine H3 receptor antagonist activity in an assay with K+ evoked depolarization-induced release of [3H]-histamine from rat synaptosomes. | J Med Chem 43: 3335-43 (2000) BindingDB Entry DOI: 10.7270/Q2RR1XGV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Rattus norvegicus (rat)) | BDBM50091382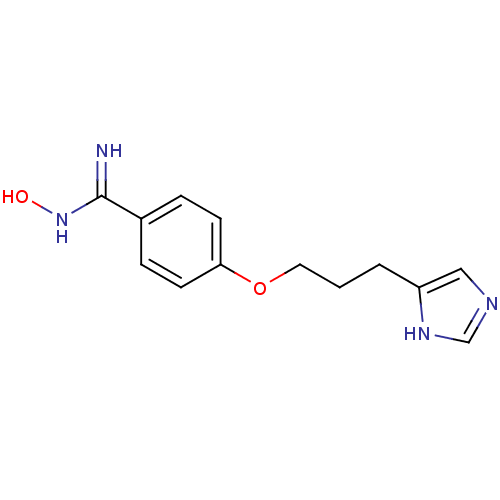 (CHEMBL324724 | N-Hydroxy-4-[3-(1H-imidazol-4-yl)-p...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.460 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Freie Universität Berlin Curated by ChEMBL | Assay Description Histamine H3 receptor antagonist activity in an assay with K+ evoked depolarization-induced release of [3H]-histamine from rat synaptosomes. | J Med Chem 43: 3335-43 (2000) BindingDB Entry DOI: 10.7270/Q2RR1XGV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hrh3 protein (RAT) | BDBM22541 (Clobenpropit | N''-[(4-chlorophenyl)methyl]{[3-(1H...) | KEGG UniProtKB/TrEMBL GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Patents Similars | PubMed | 0.480 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
U. 109 Curated by PDSP Ki Database | J Pharmacol Exp Ther 271: 452-9 (1994) BindingDB Entry DOI: 10.7270/Q23X8556 | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Rattus norvegicus (rat)) | BDBM27213 (4-[3-(4-cyclopropanecarbonylphenoxy)propyl]-1H-imi...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | PubMed | 0.490 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Freie Universität Berlin Curated by ChEMBL | Assay Description In vitro inhibitory activity against Histamine H3 receptor for K+ evoked depolarization-induced release of [3H]-histamine from synaptosomes of rat ce... | Bioorg Med Chem Lett 10: 2379-82 (2001) BindingDB Entry DOI: 10.7270/Q28G8JZ4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Rattus norvegicus (rat)) | BDBM27213 (4-[3-(4-cyclopropanecarbonylphenoxy)propyl]-1H-imi...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | PubMed | 0.490 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Freie Universit£t Berlin Curated by ChEMBL | Assay Description Effect on histamine H3 receptors in vitro, on synaptosomes of rat cerebral cortex for the release of [3H]histamine | J Med Chem 43: 3987-94 (2000) BindingDB Entry DOI: 10.7270/Q2833SRN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Rattus norvegicus (rat)) | BDBM27213 (4-[3-(4-cyclopropanecarbonylphenoxy)propyl]-1H-imi...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | PubMed | 0.490 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Freie Universität Berlin Curated by ChEMBL | Assay Description Histamine H3 receptor antagonist activity in an assay with K+ evoked depolarization-induced release of [3H]-histamine from rat synaptosomes. | J Med Chem 43: 3335-43 (2000) BindingDB Entry DOI: 10.7270/Q2RR1XGV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Rattus norvegicus (rat)) | BDBM27213 (4-[3-(4-cyclopropanecarbonylphenoxy)propyl]-1H-imi...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | 0.490 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Freie Universität Berlin Curated by ChEMBL | Assay Description In vitro binding affinity against rat histamine H3 receptor | J Med Chem 47: 2678-87 (2004) Article DOI: 10.1021/jm031065q BindingDB Entry DOI: 10.7270/Q2SQ8ZTC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hrh3 protein (RAT) | BDBM22904 ((2R)-1-(1H-imidazol-5-yl)propan-2-amine | (R)-alph...) | KEGG UniProtKB/TrEMBL GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | PubMed | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
U. 109 Curated by PDSP Ki Database | J Pharmacol Exp Ther 271: 452-9 (1994) BindingDB Entry DOI: 10.7270/Q23X8556 | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50110311 (CHEMBL276938 | {2-[4-(3-Piperidin-1-yl-propoxy)-ph...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.530 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Freie Universität Berlin Curated by ChEMBL | Assay Description Affinity for displacement of [125I]-iodoproxyfan from human histamine H3 receptors stably expressed in CHO cells | J Med Chem 45: 1128-41 (2002) BindingDB Entry DOI: 10.7270/Q2H70F4V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Rattus norvegicus (rat)) | BDBM50093224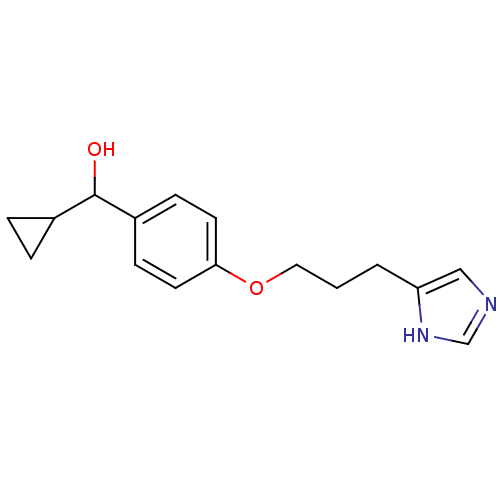 (CHEMBL309302 | Cyclopropyl-{4-[3-(1H-imidazol-4-yl...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.580 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Freie Universität Berlin Curated by ChEMBL | Assay Description In vitro inhibitory activity against Histamine H3 receptor for K+ evoked depolarization-induced release of [3H]-histamine from synaptosomes of rat ce... | Bioorg Med Chem Lett 10: 2379-82 (2001) BindingDB Entry DOI: 10.7270/Q28G8JZ4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Rattus norvegicus (rat)) | BDBM22541 (Clobenpropit | N''-[(4-chlorophenyl)methyl]{[3-(1H...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Patents Similars | Article PubMed | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Freie Universität Berlin Curated by ChEMBL | Assay Description Effect at histamine H3 receptors (in vitro) on synaptosomes of rat cerebral cortex assayed by functional H3-receptor assay. | J Med Chem 42: 593-600 (1999) Article DOI: 10.1021/jm9804376 BindingDB Entry DOI: 10.7270/Q2GH9H4J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Rattus norvegicus (rat)) | BDBM22541 (Clobenpropit | N''-[(4-chlorophenyl)methyl]{[3-(1H...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Patents Similars | PubMed | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Freie Universität Berlin Curated by ChEMBL | Assay Description In vitro inhibitory activity against Histamine H3 receptor for K+ evoked depolarization-induced release of [3H]-histamine from synaptosomes of rat ce... | Bioorg Med Chem Lett 10: 2379-82 (2001) BindingDB Entry DOI: 10.7270/Q28G8JZ4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Rattus norvegicus (rat)) | BDBM22541 (Clobenpropit | N''-[(4-chlorophenyl)methyl]{[3-(1H...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Patents Similars | PubMed | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Freie Universität Berlin Curated by ChEMBL | Assay Description In vitro Histamine H3 receptor antagonist activity in an assay with K+-evoked depolarization-induced release of [3H]-histamine from synaptosomes of r... | Bioorg Med Chem Lett 8: 2011-6 (1999) BindingDB Entry DOI: 10.7270/Q28K788H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hrh3 protein (RAT) | BDBM22541 (Clobenpropit | N''-[(4-chlorophenyl)methyl]{[3-(1H...) | KEGG UniProtKB/TrEMBL GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Patents Similars | PubMed | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
U. 109 Curated by PDSP Ki Database | J Pharmacol Exp Ther 271: 452-9 (1994) BindingDB Entry DOI: 10.7270/Q23X8556 | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Rattus norvegicus (rat)) | BDBM22541 (Clobenpropit | N''-[(4-chlorophenyl)methyl]{[3-(1H...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Patents Similars | Article PubMed | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Freie Universität Berlin Curated by ChEMBL | Assay Description In vitro antagonist potency at Histamine H3 receptor measured as K+-evoked [3H]-histamine release from synaptosomes of rat cerebral cortex. | J Med Chem 41: 4171-6 (1998) Article DOI: 10.1021/jm9802970 BindingDB Entry DOI: 10.7270/Q2MK6C0W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Rattus norvegicus (rat)) | BDBM22541 (Clobenpropit | N''-[(4-chlorophenyl)methyl]{[3-(1H...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Patents Similars | Article PubMed | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Freie Universität Berlin Curated by ChEMBL | Assay Description In vitro antagonistic activity of the compound against Histamine H3 receptor on Synaptosomes of rat cerebral cortex. | J Med Chem 39: 1157-63 (1996) Article DOI: 10.1021/jm9507688 BindingDB Entry DOI: 10.7270/Q2SF2V7G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Rattus norvegicus (rat)) | BDBM22541 (Clobenpropit | N''-[(4-chlorophenyl)methyl]{[3-(1H...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Patents Similars | PubMed | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Freie Universit£t Berlin Curated by ChEMBL | Assay Description Effect on histamine H3 receptors in vitro, on synaptosomes of rat cerebral cortex for the release of [3H]histamine | J Med Chem 43: 3987-94 (2000) BindingDB Entry DOI: 10.7270/Q2833SRN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Rattus norvegicus (rat)) | BDBM22541 (Clobenpropit | N''-[(4-chlorophenyl)methyl]{[3-(1H...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Patents Similars | Article | 0.631 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description In vitro antagonistic activity against histamine H3-receptor in an assay with K+-evoked depolarisation-induced release of [3H]histamine of synaptosom... | Bioorg Med Chem Lett 6: 2013-2018 (1996) Article DOI: 10.1016/0960-894X(96)00361-7 BindingDB Entry DOI: 10.7270/Q2C82BGZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM22911 (2-(3H-imidazol-4-yl)ethylsulfanylmethanimidamide |...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Jagiellonian University Medical College Curated by ChEMBL | Assay Description Displacement of [125I]iodoproxyfan from human full-length histamine H3 receptor expressed in HEK293 cells after 60 mins | Bioorg Med Chem 19: 2850-8 (2011) Article DOI: 10.1016/j.bmc.2011.03.046 BindingDB Entry DOI: 10.7270/Q2X63N8N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hrh3 protein (RAT) | BDBM22530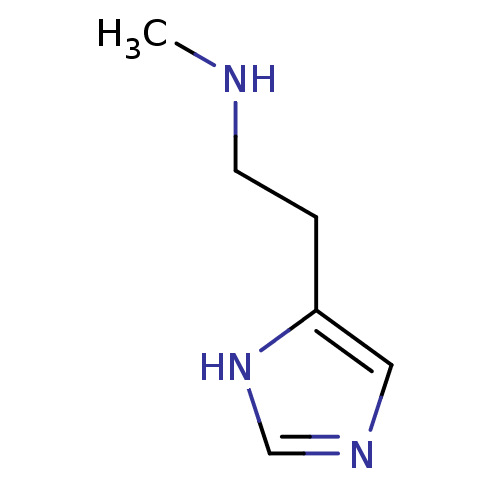 (N(alpha)-Methylhistamine | N-alpha-methylhistamine...) | KEGG UniProtKB/TrEMBL GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Patents Similars | PubMed | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
U. 109 Curated by PDSP Ki Database | J Pharmacol Exp Ther 271: 452-9 (1994) BindingDB Entry DOI: 10.7270/Q23X8556 | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H2 receptor (Cavia porcellus (domestic guinea pig)) | BDBM50406642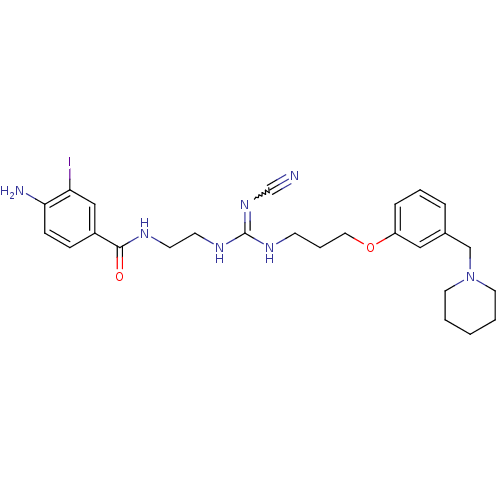 (CHEMBL72193) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.708 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Freie Universit£t Berlin Curated by ChEMBL | Assay Description Inhibition of [125I]APT binding to histamine H2 receptor in guinea pig cerebral membranes. | J Med Chem 35: 2231-8 (1992) BindingDB Entry DOI: 10.7270/Q23T9JFP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Rattus norvegicus (rat)) | BDBM50092853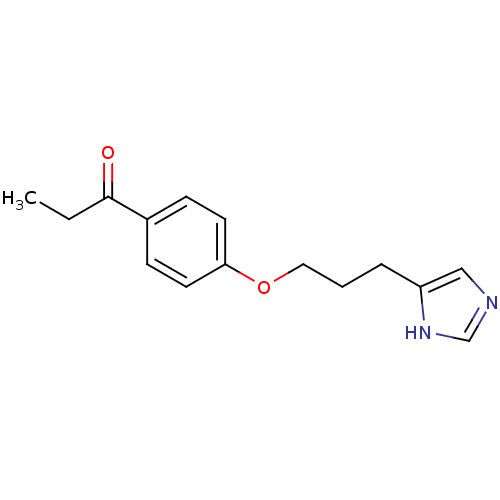 (1-{4-[3-(1H-Imidazol-4-yl)-propoxy]-phenyl}-propan...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.740 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Freie Universit£t Berlin Curated by ChEMBL | Assay Description Effect on histamine H3 receptors in vitro, on synaptosomes of rat cerebral cortex for the release of [3H]histamine | J Med Chem 43: 3987-94 (2000) BindingDB Entry DOI: 10.7270/Q2833SRN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (GUINEA PIG) | BDBM50283867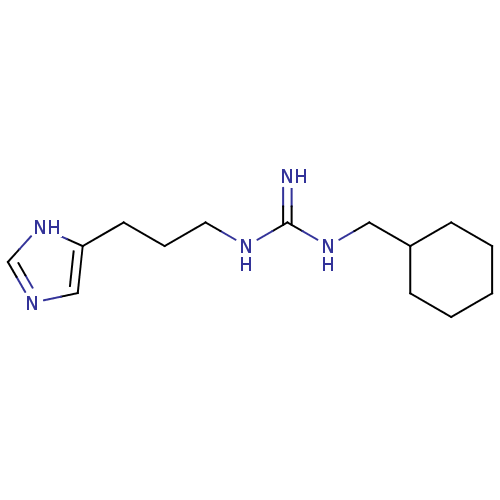 (CHEMBL98165 | N-Cyclohexylmethyl-N'-[3-(1H-imidazo...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article | 0.740 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Antagonistic activity for Histamine H3 receptor binding in guinea pig | Bioorg Med Chem Lett 4: 2907-2912 (1994) Article DOI: 10.1016/S0960-894X(01)80838-6 BindingDB Entry DOI: 10.7270/Q20K28H8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50110285 (CHEMBL274041 | {3-[4-(3-Piperidin-1-yl-propoxy)-ph...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.75 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Freie Universität Berlin Curated by ChEMBL | Assay Description Affinity for displacement of [125I]-iodoproxyfan from human histamine H3 receptors stably expressed in CHO cells | J Med Chem 45: 1128-41 (2002) BindingDB Entry DOI: 10.7270/Q2H70F4V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (GUINEA PIG) | BDBM50283867 (CHEMBL98165 | N-Cyclohexylmethyl-N'-[3-(1H-imidazo...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article | 0.794 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Antagonistic activity for Histamine H3 receptor on electrically evoked guinea-pig ileum contraction | Bioorg Med Chem Lett 4: 2907-2912 (1994) Article DOI: 10.1016/S0960-894X(01)80838-6 BindingDB Entry DOI: 10.7270/Q20K28H8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hrh3 protein (RAT) | BDBM22530 (N(alpha)-Methylhistamine | N-alpha-methylhistamine...) | KEGG UniProtKB/TrEMBL GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Patents Similars | PubMed | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
U. 109 Curated by PDSP Ki Database | J Pharmacol Exp Ther 271: 452-9 (1994) BindingDB Entry DOI: 10.7270/Q23X8556 | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Rattus norvegicus (rat)) | BDBM50091386 (1-{4-[3-(1H-Imidazol-4-yl)-propoxy]-phenyl}-ethano...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Freie Universität Berlin Curated by ChEMBL | Assay Description Histamine H3 receptor antagonist activity in an assay with K+ evoked depolarization-induced release of [3H]-histamine from rat synaptosomes. | J Med Chem 43: 3335-43 (2000) BindingDB Entry DOI: 10.7270/Q2RR1XGV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Rattus norvegicus (rat)) | BDBM50091386 (1-{4-[3-(1H-Imidazol-4-yl)-propoxy]-phenyl}-ethano...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Freie Universit£t Berlin Curated by ChEMBL | Assay Description Effect on histamine H3 receptors in vitro, on synaptosomes of rat cerebral cortex for the release of [3H]histamine | J Med Chem 43: 3987-94 (2000) BindingDB Entry DOI: 10.7270/Q2833SRN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Rattus norvegicus (rat)) | BDBM50092841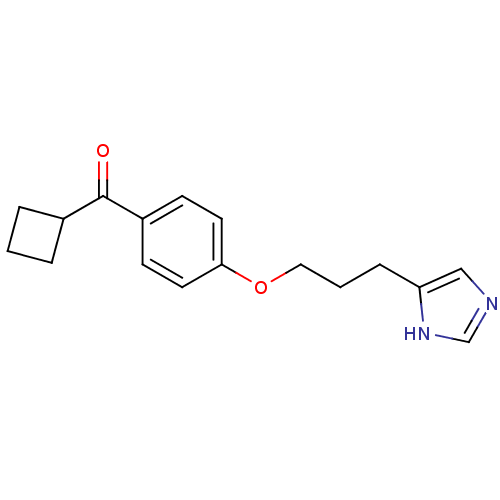 (CHEMBL128690 | Cyclobutyl-{4-[3-(1H-imidazol-4-yl)...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.890 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Freie Universit£t Berlin Curated by ChEMBL | Assay Description Effect on histamine H3 receptors in vitro, on synaptosomes of rat cerebral cortex for the release of [3H]histamine | J Med Chem 43: 3987-94 (2000) BindingDB Entry DOI: 10.7270/Q2833SRN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hrh3 protein (RAT) | BDBM22916 (5-{3-[(4-iodophenyl)methoxy]propyl}-1H-imidazole |...) | KEGG UniProtKB/TrEMBL GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
U. 109 Curated by PDSP Ki Database | J Pharmacol Exp Ther 271: 452-9 (1994) BindingDB Entry DOI: 10.7270/Q23X8556 | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Rattus norvegicus (rat)) | BDBM50091372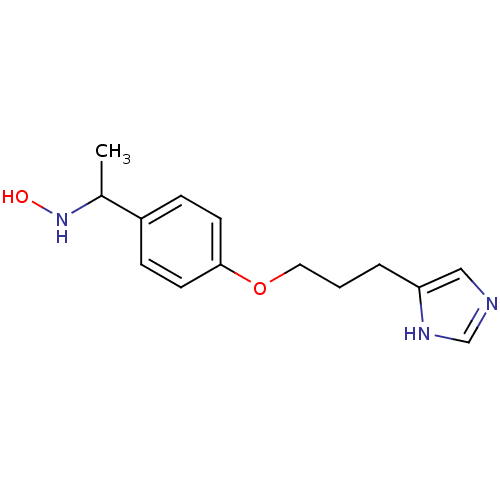 (CHEMBL322481 | N-(1-{4-[3-(1H-Imidazol-4-yl)-propo...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.930 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Freie Universität Berlin Curated by ChEMBL | Assay Description Histamine H3 receptor antagonist activity in an assay with K+ evoked depolarization-induced release of [3H]-histamine from rat synaptosomes. | J Med Chem 43: 3335-43 (2000) BindingDB Entry DOI: 10.7270/Q2RR1XGV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hrh3 protein (RAT) | BDBM22911 (2-(3H-imidazol-4-yl)ethylsulfanylmethanimidamide |...) | KEGG UniProtKB/TrEMBL GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Patents Similars | PubMed | 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
U. 109 Curated by PDSP Ki Database | J Pharmacol Exp Ther 271: 452-9 (1994) BindingDB Entry DOI: 10.7270/Q23X8556 | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Rattus norvegicus (rat)) | BDBM22916 (5-{3-[(4-iodophenyl)methoxy]propyl}-1H-imidazole |...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Freie Universität Berlin Curated by ChEMBL | Assay Description Binding affinity towards Histamine H3 receptor on synaptosomes from rat cerebral cortex | J Med Chem 39: 1220-6 (1996) Article DOI: 10.1021/jm9504767 BindingDB Entry DOI: 10.7270/Q28K7855 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Rattus norvegicus (rat)) | BDBM50092846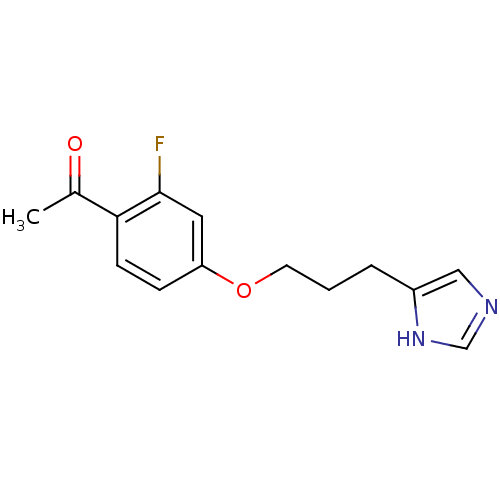 (1-{2-Fluoro-4-[3-(1H-imidazol-4-yl)-propoxy]-pheny...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Freie Universit£t Berlin Curated by ChEMBL | Assay Description Effect on histamine H3 receptors in vitro, on synaptosomes of rat cerebral cortex for the release of [3H]histamine | J Med Chem 43: 3987-94 (2000) BindingDB Entry DOI: 10.7270/Q2833SRN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50098203 (1-{4-[2-(1H-Imidazol-4-yl)-ethylsulfanyl]-phenyl}-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Freie Universität Berlin Curated by ChEMBL | Assay Description Inhibition of [125I]-iodoproxyfan binding to human histamine H3 receptor of CHO-K1 cells | Bioorg Med Chem Lett 11: 951-4 (2001) BindingDB Entry DOI: 10.7270/Q28P5ZRB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H1 receptor (Cavia porcellus (domestic guinea pig)) | BDBM86174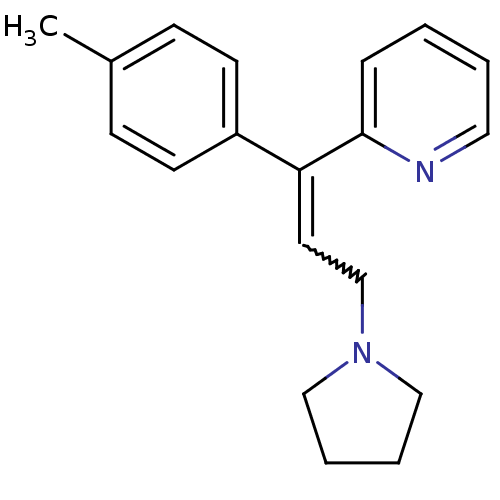 (CAS_486-12-4 | NSC_5282443 | Triprolidine) | UniProtKB/SwissProt GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | Article PubMed | 1.15 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Kansas Curated by PDSP Ki Database | J Pharmacol Exp Ther 305: 1104-15 (2003) Article DOI: 10.1124/jpet.103.049619 BindingDB Entry DOI: 10.7270/Q2HM5708 | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Rattus norvegicus (rat)) | BDBM50092848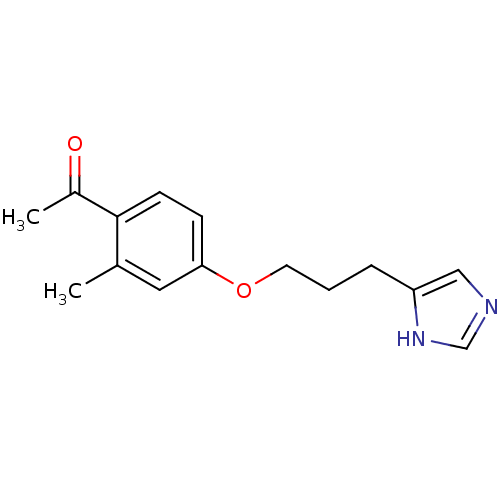 (1-{4-[3-(1H-Imidazol-4-yl)-propoxy]-2-methyl-pheny...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Freie Universit£t Berlin Curated by ChEMBL | Assay Description Effect on histamine H3 receptors in vitro, on synaptosomes of rat cerebral cortex for the release of [3H]histamine | J Med Chem 43: 3987-94 (2000) BindingDB Entry DOI: 10.7270/Q2833SRN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Rattus norvegicus (rat)) | BDBM50050180 (4-[3-(4-Bromo-benzyloxy)-propyl]-1H-imidazole | CH...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Freie Universität Berlin Curated by ChEMBL | Assay Description Binding affinity towards Histamine H3 receptor on synaptosomes from rat cerebral cortex | J Med Chem 39: 1220-6 (1996) Article DOI: 10.1021/jm9504767 BindingDB Entry DOI: 10.7270/Q28K7855 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Rattus norvegicus (rat)) | BDBM50050173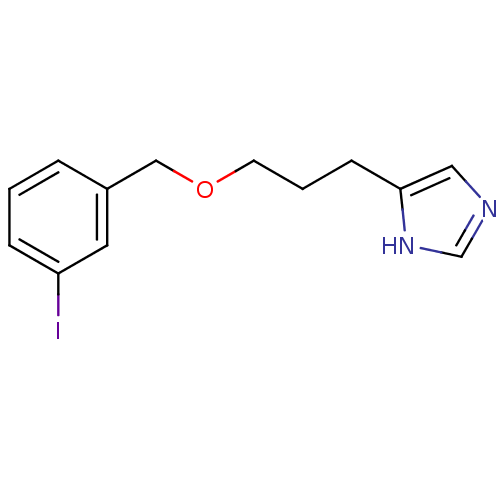 (4-[3-(3-Iodo-benzyloxy)-propyl]-1H-imidazole | CHE...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Freie Universität Berlin Curated by ChEMBL | Assay Description Binding affinity towards Histamine H3 receptor on synaptosomes from rat cerebral cortex | J Med Chem 39: 1220-6 (1996) Article DOI: 10.1021/jm9504767 BindingDB Entry DOI: 10.7270/Q28K7855 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 874 total ) | Next | Last >> |