 Found 2073 hits with Last Name = 'hashimoto' and Initial = 'y'
Found 2073 hits with Last Name = 'hashimoto' and Initial = 'y' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Retinoic acid receptor gamma
(Homo sapiens (Human)) | BDBM31883

(9-cis-retinoic acid (9cRA) | ALL-TRANS-RETINOIC AC...)Show SMILES C\C(\C=C\C1=C(C)CCCC1(C)C)=C/C=C/C(/C)=C/C(O)=O |c:4| Show InChI InChI=1S/C20H28O2/c1-15(8-6-9-16(2)14-19(21)22)11-12-18-17(3)10-7-13-20(18,4)5/h6,8-9,11-12,14H,7,10,13H2,1-5H3,(H,21,22)/b9-6+,12-11+,15-8+,16-14+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
MMDB
PC cid
PC sid
UniChem
Patents
Similars
| MMDB
Article
PubMed
| 0.0400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Tokyo
Curated by ChEMBL
| Assay Description
Agonistic activity of the compound towards retinoic acid receptor-gamma |
J Med Chem 40: 4222-34 (1998)
Article DOI: 10.1021/jm9704309
BindingDB Entry DOI: 10.7270/Q21J98VC |
More data for this
Ligand-Target Pair | |
Nociceptin receptor
(MOUSE) | BDBM50333104
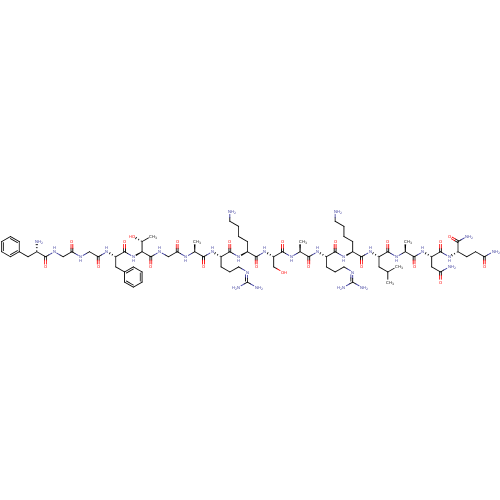
(CHEMBL389521 | H-FGGFTGARKSARKLANQ-NH2 | N/OFQ-NH2...)Show SMILES [#6]-[#6](-[#6])-[#6]-[#6@H](-[#7]-[#6](=O)-[#6@H](-[#6]-[#6]-[#6]-[#6]-[#7])-[#7]-[#6](=O)-[#6@H](-[#6]-[#6]-[#6]\[#7]=[#6](/[#7])-[#7])-[#7]-[#6](=O)-[#6@H](-[#6])-[#7]-[#6](=O)-[#6@H](-[#6]-[#8])-[#7]-[#6](=O)-[#6@H](-[#6]-[#6]-[#6]-[#6]-[#7])-[#7]-[#6](=O)-[#6@H](-[#6]-[#6]-[#6]\[#7]=[#6](/[#7])-[#7])-[#7]-[#6](=O)-[#6@H](-[#6])-[#7]-[#6](=O)-[#6]-[#7]-[#6](=O)-[#6@@H](-[#7]-[#6](=O)-[#6@H](-[#6]-c1ccccc1)-[#7]-[#6](=O)-[#6]-[#7]-[#6](=O)-[#6]-[#7]-[#6](=O)-[#6@@H](-[#7])-[#6]-c1ccccc1)-[#6@@H](-[#6])-[#8])-[#6](=O)-[#7]-[#6@@H](-[#6])-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6](-[#7])=O)-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6](-[#7])=O)-[#6](-[#7])=O Show InChI InChI=1S/C79H130N28O21/c1-41(2)33-54(73(124)96-44(5)67(118)104-56(36-59(84)111)74(125)99-49(64(85)115)27-28-58(83)110)105-71(122)50(23-13-15-29-80)102-70(121)53(26-18-32-91-79(88)89)101-66(117)43(4)97-76(127)57(40-108)106-72(123)51(24-14-16-30-81)103-69(120)52(25-17-31-90-78(86)87)100-65(116)42(3)95-61(113)39-94-77(128)63(45(6)109)107-75(126)55(35-47-21-11-8-12-22-47)98-62(114)38-92-60(112)37-93-68(119)48(82)34-46-19-9-7-10-20-46/h7-12,19-22,41-45,48-57,63,108-109H,13-18,23-40,80-82H2,1-6H3,(H2,83,110)(H2,84,111)(H2,85,115)(H,92,112)(H,93,119)(H,94,128)(H,95,113)(H,96,124)(H,97,127)(H,98,114)(H,99,125)(H,100,116)(H,101,117)(H,102,121)(H,103,120)(H,104,118)(H,105,122)(H,106,123)(H,107,126)(H4,86,87,90)(H4,88,89,91)/t42-,43-,44-,45+,48-,49-,50-,51-,52-,53-,54-,55-,56-,57-,63-/m0/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.794 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Ferrara
Curated by ChEMBL
| Assay Description
Affinity to nociceptin receptors on mouse forebrain membranes by [3H]NC-NH2 displacement. |
J Med Chem 43: 2805-13 (2000)
Article DOI: 10.1021/jm990075h
BindingDB Entry DOI: 10.7270/Q2028V88 |
More data for this
Ligand-Target Pair | |
Nociceptin receptor
(MOUSE) | BDBM50106479
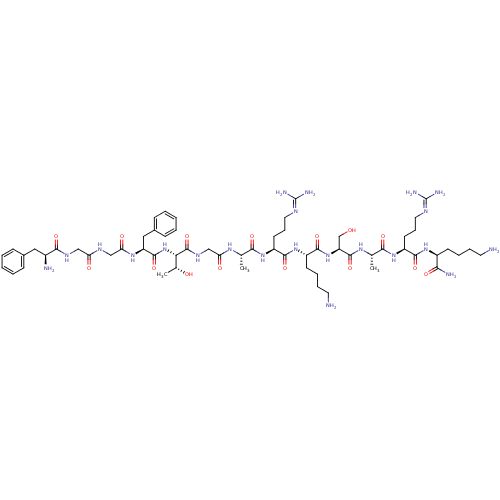
(CHEMBL384755 | FGGFTGARKSARK | H-FGGFTGARKSARK-NH2...)Show SMILES [#6]-[#6@@H](-[#8])-[#6@H](-[#7]-[#6](=O)-[#6@H](-[#6]-c1ccccc1)-[#7]-[#6](=O)-[#6]-[#7]-[#6](=O)-[#6]-[#7]-[#6](=O)-[#6@@H](-[#7])-[#6]-c1ccccc1)-[#6](=O)-[#7]-[#6]-[#6](=O)-[#7]-[#6@@H](-[#6])-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6]\[#7]=[#6](/[#7])-[#7])-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6]-[#6]-[#7])-[#6](=O)-[#7]-[#6@@H](-[#6]-[#8])-[#6](=O)-[#7]-[#6@@H](-[#6])-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6]\[#7]=[#6](/[#7])-[#7])-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6]-[#6]-[#7])-[#6](-[#7])=O |r| Show InChI InChI=1S/C61H100N22O15/c1-34(75-47(87)32-74-59(98)49(36(3)85)83-57(96)44(29-38-18-8-5-9-19-38)77-48(88)31-72-46(86)30-73-53(92)39(64)28-37-16-6-4-7-17-37)51(90)79-43(23-15-27-71-61(68)69)55(94)81-41(21-11-13-25-63)56(95)82-45(33-84)58(97)76-35(2)52(91)80-42(22-14-26-70-60(66)67)54(93)78-40(50(65)89)20-10-12-24-62/h4-9,16-19,34-36,39-45,49,84-85H,10-15,20-33,62-64H2,1-3H3,(H2,65,89)(H,72,86)(H,73,92)(H,74,98)(H,75,87)(H,76,97)(H,77,88)(H,78,93)(H,79,90)(H,80,91)(H,81,94)(H,82,95)(H,83,96)(H4,66,67,70)(H4,68,69,71)/t34-,35-,36+,39-,40-,41-,42-,43-,44-,45-,49-/m0/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.794 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Ferrara
Curated by ChEMBL
| Assay Description
Affinity to nociceptin receptors on mouse forebrain membranes by [3H]NC-NH2 displacement. |
J Med Chem 43: 2805-13 (2000)
Article DOI: 10.1021/jm990075h
BindingDB Entry DOI: 10.7270/Q2028V88 |
More data for this
Ligand-Target Pair | |
Retinoic acid receptor gamma
(Homo sapiens (Human)) | BDBM31892

(9-cis retinoic acid | 9C-RA | CHEMBL705 | Panretin...)Show SMILES C\C(\C=C\C1=C(C)CCCC1(C)C)=C\C=C\C(\C)=C\C(O)=O |c:4| Show InChI InChI=1S/C20H28O2/c1-15(8-6-9-16(2)14-19(21)22)11-12-18-17(3)10-7-13-20(18,4)5/h6,8-9,11-12,14H,7,10,13H2,1-5H3,(H,21,22)/b9-6+,12-11+,15-8-,16-14+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
PDB
Article
PubMed
| 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Tokyo
Curated by ChEMBL
| Assay Description
Agonistic activity of the compound towards retinoic acid receptor-gamma |
J Med Chem 40: 4222-34 (1998)
Article DOI: 10.1021/jm9704309
BindingDB Entry DOI: 10.7270/Q21J98VC |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Nociceptin receptor
(MOUSE) | BDBM50472736
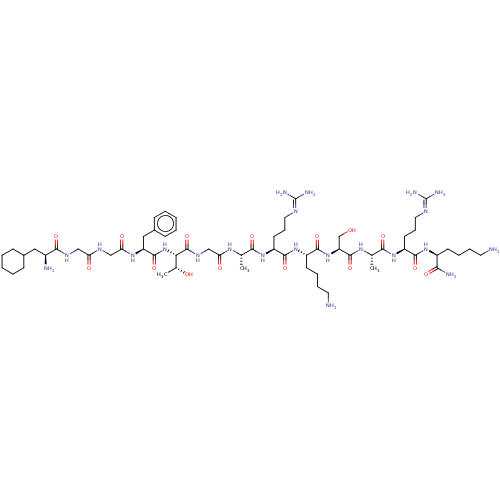
(CHEMBL406905)Show SMILES [#6]-[#6@@H](-[#8])-[#6@H](-[#7]-[#6](=O)-[#6@H](-[#6]-c1ccccc1)-[#7]-[#6](=O)-[#6]-[#7]-[#6](=O)-[#6]-[#7]-[#6](=O)-[#6@@H](-[#7])-[#6]-[#6]-1-[#6]-[#6]-[#6]-[#6]-[#6]-1)-[#6](=O)-[#7]-[#6]-[#6](=O)-[#7]-[#6@@H](-[#6])-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6]\[#7]=[#6](\[#7])-[#7])-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6]-[#6]-[#7])-[#6](=O)-[#7]-[#6@@H](-[#6]-[#8])-[#6](=O)-[#7]-[#6@@H](-[#6])-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6]\[#7]=[#6](\[#7])-[#7])-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6]-[#6]-[#7])-[#6](-[#7])=O Show InChI InChI=1S/C61H106N22O15/c1-34(75-47(87)32-74-59(98)49(36(3)85)83-57(96)44(29-38-18-8-5-9-19-38)77-48(88)31-72-46(86)30-73-53(92)39(64)28-37-16-6-4-7-17-37)51(90)79-43(23-15-27-71-61(68)69)55(94)81-41(21-11-13-25-63)56(95)82-45(33-84)58(97)76-35(2)52(91)80-42(22-14-26-70-60(66)67)54(93)78-40(50(65)89)20-10-12-24-62/h5,8-9,18-19,34-37,39-45,49,84-85H,4,6-7,10-17,20-33,62-64H2,1-3H3,(H2,65,89)(H,72,86)(H,73,92)(H,74,98)(H,75,87)(H,76,97)(H,77,88)(H,78,93)(H,79,90)(H,80,91)(H,81,94)(H,82,95)(H,83,96)(H4,66,67,70)(H4,68,69,71)/t34-,35-,36+,39-,40-,41-,42-,43-,44-,45-,49-/m0/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Ferrara
Curated by ChEMBL
| Assay Description
Affinity to nociceptin receptors on mouse forebrain membranes by [3H]NC-NH2 displacement. |
J Med Chem 43: 2805-13 (2000)
Article DOI: 10.1021/jm990075h
BindingDB Entry DOI: 10.7270/Q2028V88 |
More data for this
Ligand-Target Pair | |
Nociceptin receptor
(MOUSE) | BDBM50472738
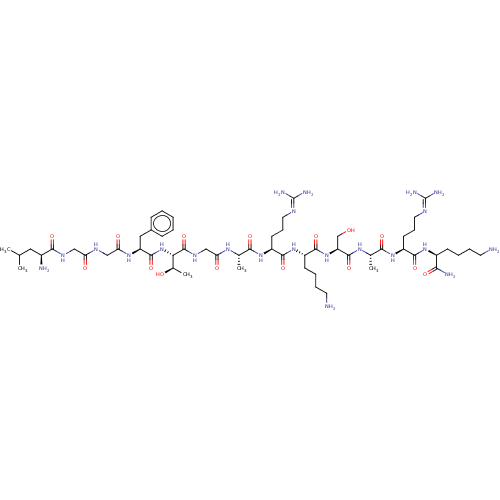
(CHEMBL412479)Show SMILES [#6]-[#6](-[#6])-[#6]-[#6@H](-[#7])-[#6](=O)-[#7]-[#6]-[#6](=O)-[#7]-[#6]-[#6](=O)-[#7]-[#6@@H](-[#6]-c1ccccc1)-[#6](=O)-[#7]-[#6@@H](-[#6@@H](-[#6])-[#8])-[#6](=O)-[#7]-[#6]-[#6](=O)-[#7]-[#6@@H](-[#6])-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6]\[#7]=[#6](\[#7])-[#7])-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6]-[#6]-[#7])-[#6](=O)-[#7]-[#6@@H](-[#6]-[#8])-[#6](=O)-[#7]-[#6@@H](-[#6])-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6]\[#7]=[#6](\[#7])-[#7])-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6]-[#6]-[#7])-[#6](-[#7])=O Show InChI InChI=1S/C58H102N22O15/c1-31(2)25-36(61)50(89)70-27-43(83)69-28-45(85)74-41(26-35-15-7-6-8-16-35)54(93)80-46(34(5)82)56(95)71-29-44(84)72-32(3)48(87)76-40(20-14-24-68-58(65)66)52(91)78-38(18-10-12-22-60)53(92)79-42(30-81)55(94)73-33(4)49(88)77-39(19-13-23-67-57(63)64)51(90)75-37(47(62)86)17-9-11-21-59/h6-8,15-16,31-34,36-42,46,81-82H,9-14,17-30,59-61H2,1-5H3,(H2,62,86)(H,69,83)(H,70,89)(H,71,95)(H,72,84)(H,73,94)(H,74,85)(H,75,90)(H,76,87)(H,77,88)(H,78,91)(H,79,92)(H,80,93)(H4,63,64,67)(H4,65,66,68)/t32-,33-,34+,36-,37-,38-,39-,40-,41-,42-,46-/m0/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 2.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Ferrara
Curated by ChEMBL
| Assay Description
Affinity to nociceptin receptors on mouse forebrain membranes by [3H]NC-NH2 displacement. |
J Med Chem 43: 2805-13 (2000)
Article DOI: 10.1021/jm990075h
BindingDB Entry DOI: 10.7270/Q2028V88 |
More data for this
Ligand-Target Pair | |
Retinoic acid receptor alpha
(Homo sapiens (Human)) | BDBM31883

(9-cis-retinoic acid (9cRA) | ALL-TRANS-RETINOIC AC...)Show SMILES C\C(\C=C\C1=C(C)CCCC1(C)C)=C/C=C/C(/C)=C/C(O)=O |c:4| Show InChI InChI=1S/C20H28O2/c1-15(8-6-9-16(2)14-19(21)22)11-12-18-17(3)10-7-13-20(18,4)5/h6,8-9,11-12,14H,7,10,13H2,1-5H3,(H,21,22)/b9-6+,12-11+,15-8+,16-14+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
MMDB
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 3.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Tokyo
Curated by ChEMBL
| Assay Description
Agonistic activity of the compound towards retinoic acid receptor-alpha |
J Med Chem 40: 4222-34 (1998)
Article DOI: 10.1021/jm9704309
BindingDB Entry DOI: 10.7270/Q21J98VC |
More data for this
Ligand-Target Pair | |
Retinoic acid receptor RXR-beta
(Homo sapiens (Human)) | BDBM31892

(9-cis retinoic acid | 9C-RA | CHEMBL705 | Panretin...)Show SMILES C\C(\C=C\C1=C(C)CCCC1(C)C)=C\C=C\C(\C)=C\C(O)=O |c:4| Show InChI InChI=1S/C20H28O2/c1-15(8-6-9-16(2)14-19(21)22)11-12-18-17(3)10-7-13-20(18,4)5/h6,8-9,11-12,14H,7,10,13H2,1-5H3,(H,21,22)/b9-6+,12-11+,15-8-,16-14+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
MMDB
PDB
Article
PubMed
| 3.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Tokyo
Curated by ChEMBL
| Assay Description
Agonistic activity of the compound towards retinoid X receptor-beta |
J Med Chem 40: 4222-34 (1998)
Article DOI: 10.1021/jm9704309
BindingDB Entry DOI: 10.7270/Q21J98VC |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Nociceptin receptor
(MOUSE) | BDBM50472742
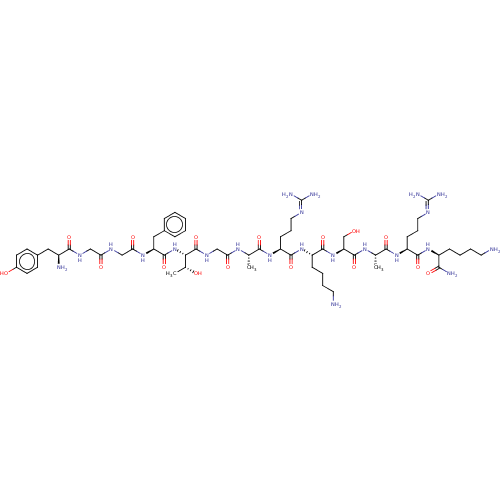
(CHEMBL429363)Show SMILES [#6]-[#6@@H](-[#8])-[#6@H](-[#7]-[#6](=O)-[#6@H](-[#6]-c1ccccc1)-[#7]-[#6](=O)-[#6]-[#7]-[#6](=O)-[#6]-[#7]-[#6](=O)-[#6@@H](-[#7])-[#6]-c1ccc(-[#8])cc1)-[#6](=O)-[#7]-[#6]-[#6](=O)-[#7]-[#6@@H](-[#6])-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6]\[#7]=[#6](\[#7])-[#7])-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6]-[#6]-[#7])-[#6](=O)-[#7]-[#6@@H](-[#6]-[#8])-[#6](=O)-[#7]-[#6@@H](-[#6])-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6]\[#7]=[#6](\[#7])-[#7])-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6]-[#6]-[#7])-[#6](-[#7])=O Show InChI InChI=1S/C61H100N22O16/c1-33(75-47(88)31-74-59(99)49(35(3)85)83-57(97)44(28-36-13-5-4-6-14-36)77-48(89)30-72-46(87)29-73-53(93)39(64)27-37-19-21-38(86)22-20-37)51(91)79-43(18-12-26-71-61(68)69)55(95)81-41(16-8-10-24-63)56(96)82-45(32-84)58(98)76-34(2)52(92)80-42(17-11-25-70-60(66)67)54(94)78-40(50(65)90)15-7-9-23-62/h4-6,13-14,19-22,33-35,39-45,49,84-86H,7-12,15-18,23-32,62-64H2,1-3H3,(H2,65,90)(H,72,87)(H,73,93)(H,74,99)(H,75,88)(H,76,98)(H,77,89)(H,78,94)(H,79,91)(H,80,92)(H,81,95)(H,82,96)(H,83,97)(H4,66,67,70)(H4,68,69,71)/t33-,34-,35+,39-,40-,41-,42-,43-,44-,45-,49-/m0/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Ferrara
Curated by ChEMBL
| Assay Description
Affinity to nociceptin receptors on mouse forebrain membranes by [3H]NC-NH2 displacement. |
J Med Chem 43: 2805-13 (2000)
Article DOI: 10.1021/jm990075h
BindingDB Entry DOI: 10.7270/Q2028V88 |
More data for this
Ligand-Target Pair | |
Retinoic acid receptor beta
(Homo sapiens (Human)) | BDBM31883

(9-cis-retinoic acid (9cRA) | ALL-TRANS-RETINOIC AC...)Show SMILES C\C(\C=C\C1=C(C)CCCC1(C)C)=C/C=C/C(/C)=C/C(O)=O |c:4| Show InChI InChI=1S/C20H28O2/c1-15(8-6-9-16(2)14-19(21)22)11-12-18-17(3)10-7-13-20(18,4)5/h6,8-9,11-12,14H,7,10,13H2,1-5H3,(H,21,22)/b9-6+,12-11+,15-8+,16-14+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
MMDB
PC cid
PC sid
UniChem
Patents
Similars
| MMDB
Article
PubMed
| 4.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Tokyo
Curated by ChEMBL
| Assay Description
Agonistic activity of the compound towards retinoic acid receptor-beta |
J Med Chem 40: 4222-34 (1998)
Article DOI: 10.1021/jm9704309
BindingDB Entry DOI: 10.7270/Q21J98VC |
More data for this
Ligand-Target Pair | |
Retinoic acid receptor RXR-beta
(Homo sapiens (Human)) | BDBM50032675

(4-[1-(3,5,5,8,8-pentamethyl-5,6,7,8-tetrahydronaph...)Show SMILES Cc1cc2c(cc1C(=C)c1ccc(cc1)C(O)=O)C(C)(C)CCC2(C)C Show InChI InChI=1S/C24H28O2/c1-15-13-20-21(24(5,6)12-11-23(20,3)4)14-19(15)16(2)17-7-9-18(10-8-17)22(25)26/h7-10,13-14H,2,11-12H2,1,3-6H3,(H,25,26) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
MMDB
PDB
Article
PubMed
| 5.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Tokyo
Curated by ChEMBL
| Assay Description
Selective activity of the compound towards retinoid X receptor-beta |
J Med Chem 40: 4222-34 (1998)
Article DOI: 10.1021/jm9704309
BindingDB Entry DOI: 10.7270/Q21J98VC |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Retinoic acid receptor alpha
(Homo sapiens (Human)) | BDBM50061625
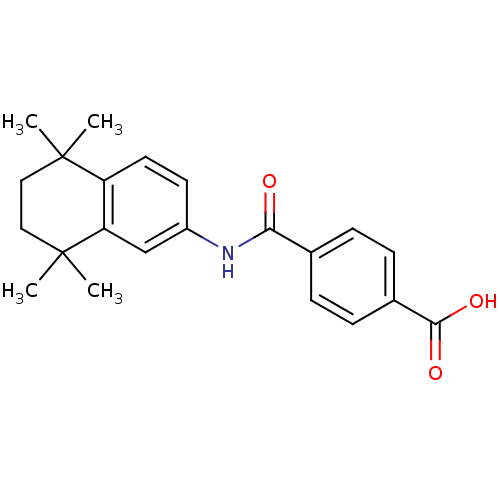
(4-[(5,5,8,8-tetramethyl-5,6,7,8-tetrahydronaphthal...)Show SMILES CC1(C)CCC(C)(C)c2cc(NC(=O)c3ccc(cc3)C(O)=O)ccc12 Show InChI InChI=1S/C22H25NO3/c1-21(2)11-12-22(3,4)18-13-16(9-10-17(18)21)23-19(24)14-5-7-15(8-6-14)20(25)26/h5-10,13H,11-12H2,1-4H3,(H,23,24)(H,25,26) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
Article
PubMed
| 6.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Tokyo
Curated by ChEMBL
| Assay Description
Agonistic activity of the compound towards retinoic acid receptor-alpha |
J Med Chem 40: 4222-34 (1998)
Article DOI: 10.1021/jm9704309
BindingDB Entry DOI: 10.7270/Q21J98VC |
More data for this
Ligand-Target Pair | |
Retinoic acid receptor RXR-gamma
(Homo sapiens (Human)) | BDBM50032675

(4-[1-(3,5,5,8,8-pentamethyl-5,6,7,8-tetrahydronaph...)Show SMILES Cc1cc2c(cc1C(=C)c1ccc(cc1)C(O)=O)C(C)(C)CCC2(C)C Show InChI InChI=1S/C24H28O2/c1-15-13-20-21(24(5,6)12-11-23(20,3)4)14-19(15)16(2)17-7-9-18(10-8-17)22(25)26/h7-10,13-14H,2,11-12H2,1,3-6H3,(H,25,26) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
MMDB
PDB
Article
PubMed
| 8.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Tokyo
Curated by ChEMBL
| Assay Description
Selective activity of the compound towards retinoid X receptor-gamma |
J Med Chem 40: 4222-34 (1998)
Article DOI: 10.1021/jm9704309
BindingDB Entry DOI: 10.7270/Q21J98VC |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Nociceptin receptor
(MOUSE) | BDBM50472729

(CHEMBL410396)Show SMILES [#6]-[#6@@H](-[#8])-[#6@H](-[#7]-[#6](=O)-[#6@H](-[#6]-c1ccccc1)-[#7]-[#6](=O)-[#6]-[#7]-[#6](=O)-[#6]-[#7]-[#6]-[#6@@H](-[#7])-[#6]-c1ccccc1)-[#6](=O)-[#7]-[#6]-[#6](=O)-[#7]-[#6@@H](-[#6])-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6]\[#7]=[#6](\[#7])-[#7])-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6]-[#6]-[#7])-[#6](=O)-[#7]-[#6@@H](-[#6]-[#8])-[#6](=O)-[#7]-[#6@@H](-[#6])-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6]\[#7]=[#6](\[#7])-[#7])-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6]-[#6]-[#7])-[#6](-[#7])=O Show InChI InChI=1S/C61H102N22O14/c1-35(75-48(87)33-74-59(97)50(37(3)85)83-57(95)45(29-39-18-8-5-9-19-39)77-49(88)32-73-47(86)31-70-30-40(64)28-38-16-6-4-7-17-38)52(90)79-44(23-15-27-72-61(68)69)55(93)81-42(21-11-13-25-63)56(94)82-46(34-84)58(96)76-36(2)53(91)80-43(22-14-26-71-60(66)67)54(92)78-41(51(65)89)20-10-12-24-62/h4-9,16-19,35-37,40-46,50,70,84-85H,10-15,20-34,62-64H2,1-3H3,(H2,65,89)(H,73,86)(H,74,97)(H,75,87)(H,76,96)(H,77,88)(H,78,92)(H,79,90)(H,80,91)(H,81,93)(H,82,94)(H,83,95)(H4,66,67,71)(H4,68,69,72)/t35-,36-,37+,40-,41-,42-,43-,44-,45-,46-,50-/m0/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
| Article
PubMed
| 10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Ferrara
Curated by ChEMBL
| Assay Description
Affinity to nociceptin receptors on mouse forebrain membranes by [3H]NC-NH2 displacement. |
J Med Chem 43: 2805-13 (2000)
Article DOI: 10.1021/jm990075h
BindingDB Entry DOI: 10.7270/Q2028V88 |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor gamma
(Homo sapiens (Human)) | BDBM50428854
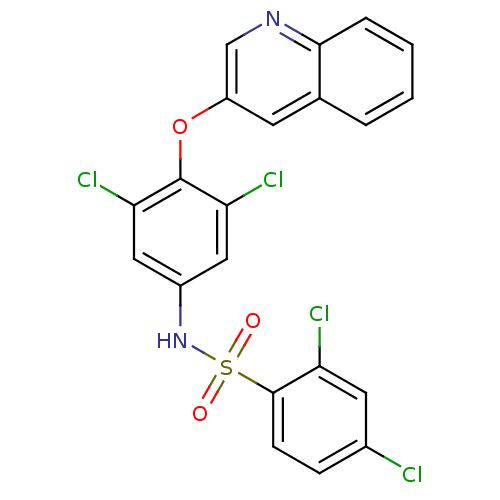
(CHEMBL1236924)Show SMILES Clc1ccc(c(Cl)c1)S(=O)(=O)Nc1cc(Cl)c(Oc2cnc3ccccc3c2)c(Cl)c1 Show InChI InChI=1S/C21H12Cl4N2O3S/c22-13-5-6-20(16(23)8-13)31(28,29)27-14-9-17(24)21(18(25)10-14)30-15-7-12-3-1-2-4-19(12)26-11-15/h1-11,27H | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| MMDB
PDB
Article
PubMed
| 10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Tokyo
Curated by ChEMBL
| Assay Description
Displacement of [3H]rosiglitazone from PPARgamma (unknown origin) |
Bioorg Med Chem 24: 5455-5461 (2016)
Article DOI: 10.1016/j.bmc.2016.08.067
BindingDB Entry DOI: 10.7270/Q21V5JGB |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Retinoic acid receptor RXR-gamma
(Homo sapiens (Human)) | BDBM31892

(9-cis retinoic acid | 9C-RA | CHEMBL705 | Panretin...)Show SMILES C\C(\C=C\C1=C(C)CCCC1(C)C)=C\C=C\C(\C)=C\C(O)=O |c:4| Show InChI InChI=1S/C20H28O2/c1-15(8-6-9-16(2)14-19(21)22)11-12-18-17(3)10-7-13-20(18,4)5/h6,8-9,11-12,14H,7,10,13H2,1-5H3,(H,21,22)/b9-6+,12-11+,15-8-,16-14+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
MMDB
PDB
Article
PubMed
| 11 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Tokyo
Curated by ChEMBL
| Assay Description
Agonistic activity of the compound towards retinoid X receptor-gamma |
J Med Chem 40: 4222-34 (1998)
Article DOI: 10.1021/jm9704309
BindingDB Entry DOI: 10.7270/Q21J98VC |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Vitamin D3 receptor
(Homo sapiens (Human)) | BDBM50329707
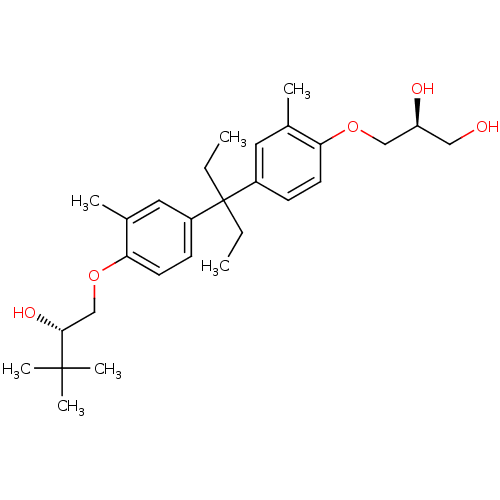
((S)-3-(4-(3-(4-((S)-2-hydroxy-3,3-dimethylbutoxy)-...)Show SMILES CCC(CC)(c1ccc(OC[C@@H](O)CO)c(C)c1)c1ccc(OC[C@@H](O)C(C)(C)C)c(C)c1 Show InChI InChI=1S/C28H42O5/c1-8-28(9-2,21-10-12-24(19(3)14-21)32-17-23(30)16-29)22-11-13-25(20(4)15-22)33-18-26(31)27(5,6)7/h10-15,23,26,29-31H,8-9,16-18H2,1-7H3/t23-,26+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 11 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Tokyo
Curated by ChEMBL
| Assay Description
Binding affinity to VDR receptor |
Bioorg Med Chem Lett 20: 6661-6 (2010)
Article DOI: 10.1016/j.bmcl.2010.09.011
BindingDB Entry DOI: 10.7270/Q20K29J4 |
More data for this
Ligand-Target Pair | |
Vitamin D3 receptor
(Homo sapiens (Human)) | BDBM50329706

((2S)-3-{4-[1-ethyl-1-(4-{[(2R)-2-hydroxy-3,3-dimet...)Show SMILES CCC(CC)(c1ccc(OC[C@@H](O)CO)c(C)c1)c1ccc(OC[C@H](O)C(C)(C)C)c(C)c1 |r| Show InChI InChI=1S/C28H42O5/c1-8-28(9-2,21-10-12-24(19(3)14-21)32-17-23(30)16-29)22-11-13-25(20(4)15-22)33-18-26(31)27(5,6)7/h10-15,23,26,29-31H,8-9,16-18H2,1-7H3/t23-,26-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| 12 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Tokyo
Curated by ChEMBL
| Assay Description
Binding affinity to VDR receptor |
Bioorg Med Chem Lett 20: 6661-6 (2010)
Article DOI: 10.1016/j.bmcl.2010.09.011
BindingDB Entry DOI: 10.7270/Q20K29J4 |
More data for this
Ligand-Target Pair | |
Retinoic acid receptor RXR-alpha
(Homo sapiens (Human)) | BDBM31892

(9-cis retinoic acid | 9C-RA | CHEMBL705 | Panretin...)Show SMILES C\C(\C=C\C1=C(C)CCCC1(C)C)=C\C=C\C(\C)=C\C(O)=O |c:4| Show InChI InChI=1S/C20H28O2/c1-15(8-6-9-16(2)14-19(21)22)11-12-18-17(3)10-7-13-20(18,4)5/h6,8-9,11-12,14H,7,10,13H2,1-5H3,(H,21,22)/b9-6+,12-11+,15-8-,16-14+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
MMDB
PDB
Article
PubMed
| 12 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Tokyo
Curated by ChEMBL
| Assay Description
Agonistic activity of the compound towards retinoid X receptor-alpha |
J Med Chem 40: 4222-34 (1998)
Article DOI: 10.1021/jm9704309
BindingDB Entry DOI: 10.7270/Q21J98VC |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Nociceptin receptor
(MOUSE) | BDBM50472741

(CHEMBL414792)Show SMILES [#6]-[#6](-[#6])-[#6]-[#6@H](-[#7])-[#6]-[#7]-[#6]-[#6](=O)-[#7]-[#6]-[#6](=O)-[#7]-[#6@@H](-[#6]-c1ccccc1)-[#6](=O)-[#7]-[#6@@H](-[#6@@H](-[#6])-[#8])-[#6](=O)-[#7]-[#6]-[#6](=O)-[#7]-[#6@@H](-[#6])-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6]\[#7]=[#6](\[#7])-[#7])-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6]-[#6]-[#7])-[#6](=O)-[#7]-[#6@@H](-[#6]-[#8])-[#6](=O)-[#7]-[#6@@H](-[#6])-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6]\[#7]=[#6](\[#7])-[#7])-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6]-[#6]-[#7])-[#6](-[#7])=O Show InChI InChI=1S/C58H104N22O14/c1-32(2)25-37(61)27-67-28-44(83)70-29-46(85)74-42(26-36-15-7-6-8-16-36)54(92)80-47(35(5)82)56(94)71-30-45(84)72-33(3)49(87)76-41(20-14-24-69-58(65)66)52(90)78-39(18-10-12-22-60)53(91)79-43(31-81)55(93)73-34(4)50(88)77-40(19-13-23-68-57(63)64)51(89)75-38(48(62)86)17-9-11-21-59/h6-8,15-16,32-35,37-43,47,67,81-82H,9-14,17-31,59-61H2,1-5H3,(H2,62,86)(H,70,83)(H,71,94)(H,72,84)(H,73,93)(H,74,85)(H,75,89)(H,76,87)(H,77,88)(H,78,90)(H,79,91)(H,80,92)(H4,63,64,68)(H4,65,66,69)/t33-,34-,35+,37-,38-,39-,40-,41-,42-,43-,47-/m0/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 16 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Ferrara
Curated by ChEMBL
| Assay Description
Affinity to nociceptin receptors on mouse forebrain membranes by [3H]NC-NH2 displacement. |
J Med Chem 43: 2805-13 (2000)
Article DOI: 10.1021/jm990075h
BindingDB Entry DOI: 10.7270/Q2028V88 |
More data for this
Ligand-Target Pair | |
Retinoic acid receptor RXR-alpha
(Homo sapiens (Human)) | BDBM50032675

(4-[1-(3,5,5,8,8-pentamethyl-5,6,7,8-tetrahydronaph...)Show SMILES Cc1cc2c(cc1C(=C)c1ccc(cc1)C(O)=O)C(C)(C)CCC2(C)C Show InChI InChI=1S/C24H28O2/c1-15-13-20-21(24(5,6)12-11-23(20,3)4)14-19(15)16(2)17-7-9-18(10-8-17)22(25)26/h7-10,13-14H,2,11-12H2,1,3-6H3,(H,25,26) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
MMDB
PDB
Article
PubMed
| 16 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Tokyo
Curated by ChEMBL
| Assay Description
Selective activity of the compound towards retinoid X receptor-alpha |
J Med Chem 40: 4222-34 (1998)
Article DOI: 10.1021/jm9704309
BindingDB Entry DOI: 10.7270/Q21J98VC |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Nociceptin receptor
(MOUSE) | BDBM50472747
(CHEMBL414767)Show SMILES [#6]-[#6](-[#6])-[#6]-[#6@H](-[#7]-[#6](=O)-[#6@H](-[#6]-[#6]-[#6]-[#6]-[#7])-[#7]-[#6](=O)-[#6@H](-[#6]-[#6]-[#6]\[#7]=[#6](\[#7])-[#7])-[#7]-[#6](=O)-[#6@H](-[#6])-[#7]-[#6](=O)-[#6@H](-[#6]-[#8])-[#7]-[#6](=O)-[#6@H](-[#6]-[#6]-[#6]-[#6]-[#7])-[#7]-[#6](=O)-[#6@H](-[#6]-[#6]-[#6]-[#7]=[#6](-[#7])-[#7])-[#7]-[#6](=O)-[#6@H](-[#6])-[#7]-[#6](=O)-[#6]-[#7]-[#6](=O)-[#6@@H](-[#7]-[#6](=O)-[#6@H](-[#6]-c1ccccc1)-[#7]-[#6](=O)-[#6]-[#7]-[#6](=O)-[#6]-[#7]-[#6]-[#6@@H](-[#7])-[#6]-c1ccccc1)-[#6@@H](-[#6])-[#8])-[#6](=O)-[#7]-[#6@@H](-[#6])-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6](-[#7])=O)-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6](-[#7])=O)-[#6](-[#7])=O Show InChI InChI=1S/C79H132N28O20/c1-42(2)33-55(73(123)96-45(5)68(118)104-57(36-60(84)111)74(124)99-50(65(85)115)27-28-59(83)110)105-71(121)51(23-13-15-29-80)102-70(120)54(26-18-32-92-79(88)89)101-67(117)44(4)97-76(126)58(41-108)106-72(122)52(24-14-16-30-81)103-69(119)53(25-17-31-91-78(86)87)100-66(116)43(3)95-62(113)40-94-77(127)64(46(6)109)107-75(125)56(35-48-21-11-8-12-22-48)98-63(114)39-93-61(112)38-90-37-49(82)34-47-19-9-7-10-20-47/h7-12,19-22,42-46,49-58,64,90,108-109H,13-18,23-41,80-82H2,1-6H3,(H2,83,110)(H2,84,111)(H2,85,115)(H,93,112)(H,94,127)(H,95,113)(H,96,123)(H,97,126)(H,98,114)(H,99,124)(H,100,116)(H,101,117)(H,102,120)(H,103,119)(H,104,118)(H,105,121)(H,106,122)(H,107,125)(H4,86,87,91)(H4,88,89,92)/t43-,44-,45-,46+,49-,50-,51-,52-,53-,54-,55-,56-,57-,58-,64-/m0/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Ferrara
Curated by ChEMBL
| Assay Description
Affinity to nociceptin receptors on mouse forebrain membranes by [3H]NC-NH2 displacement. |
J Med Chem 43: 2805-13 (2000)
Article DOI: 10.1021/jm990075h
BindingDB Entry DOI: 10.7270/Q2028V88 |
More data for this
Ligand-Target Pair | |
Nociceptin receptor
(MOUSE) | BDBM50472728

(CHEMBL413516)Show SMILES [#6]-[#6@@H](-[#8])-[#6@H](-[#7]-[#6](=O)-[#6@H](-[#6]-c1ccccc1)-[#7]-[#6](=O)-[#6]-[#7]-[#6](=O)-[#6]-[#7]-[#6](=O)-[#6@@H](-[#7])-[#6]-c1ccccc1)-[#6](=O)-[#7]-[#6]-[#6](=O)-[#7]-[#6@@H](-[#6])-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6]\[#7]=[#6](\[#7])-[#7])-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6]-[#6]-[#7])-[#6](=O)-[#7]-[#6@@H](-[#6]-[#8])-[#6](=O)-[#7]-[#6@@H](-[#6])-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6]\[#7]=[#6](\[#7])-[#7])-[#6](-[#7])=O Show InChI InChI=1S/C55H88N20O14/c1-30(68-42(79)28-67-53(89)44(32(3)77)75-51(87)39(25-34-16-8-5-9-17-34)70-43(80)27-65-41(78)26-66-48(84)35(57)24-33-14-6-4-7-15-33)46(82)72-38(20-13-23-64-55(61)62)49(85)73-37(18-10-11-21-56)50(86)74-40(29-76)52(88)69-31(2)47(83)71-36(45(58)81)19-12-22-63-54(59)60/h4-9,14-17,30-32,35-40,44,76-77H,10-13,18-29,56-57H2,1-3H3,(H2,58,81)(H,65,78)(H,66,84)(H,67,89)(H,68,79)(H,69,88)(H,70,80)(H,71,83)(H,72,82)(H,73,85)(H,74,86)(H,75,87)(H4,59,60,63)(H4,61,62,64)/t30-,31-,32+,35-,36-,37-,38-,39-,40-,44-/m0/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 25 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Ferrara
Curated by ChEMBL
| Assay Description
Affinity to nociceptin receptors on mouse forebrain membranes by [3H]NC-NH2 displacement. |
J Med Chem 43: 2805-13 (2000)
Article DOI: 10.1021/jm990075h
BindingDB Entry DOI: 10.7270/Q2028V88 |
More data for this
Ligand-Target Pair | |
Retinoic acid receptor beta
(Homo sapiens (Human)) | BDBM50061625

(4-[(5,5,8,8-tetramethyl-5,6,7,8-tetrahydronaphthal...)Show SMILES CC1(C)CCC(C)(C)c2cc(NC(=O)c3ccc(cc3)C(O)=O)ccc12 Show InChI InChI=1S/C22H25NO3/c1-21(2)11-12-22(3,4)18-13-16(9-10-17(18)21)23-19(24)14-5-7-15(8-6-14)20(25)26/h5-10,13H,11-12H2,1-4H3,(H,23,24)(H,25,26) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
Article
PubMed
| 30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Tokyo
Curated by ChEMBL
| Assay Description
Agonistic activity of the compound towards retinoic acid receptor-beta |
J Med Chem 40: 4222-34 (1998)
Article DOI: 10.1021/jm9704309
BindingDB Entry DOI: 10.7270/Q21J98VC |
More data for this
Ligand-Target Pair | |
Retinoic acid receptor beta
(Homo sapiens (Human)) | BDBM50032675

(4-[1-(3,5,5,8,8-pentamethyl-5,6,7,8-tetrahydronaph...)Show SMILES Cc1cc2c(cc1C(=C)c1ccc(cc1)C(O)=O)C(C)(C)CCC2(C)C Show InChI InChI=1S/C24H28O2/c1-15-13-20-21(24(5,6)12-11-23(20,3)4)14-19(15)16(2)17-7-9-18(10-8-17)22(25)26/h7-10,13-14H,2,11-12H2,1,3-6H3,(H,25,26) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 50 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Tokyo
Curated by ChEMBL
| Assay Description
Selective activity of the compound towards retinoic acid receptor-beta |
J Med Chem 40: 4222-34 (1998)
Article DOI: 10.1021/jm9704309
BindingDB Entry DOI: 10.7270/Q21J98VC |
More data for this
Ligand-Target Pair | |
Retinoic acid receptor RXR-beta
(Homo sapiens (Human)) | BDBM31883

(9-cis-retinoic acid (9cRA) | ALL-TRANS-RETINOIC AC...)Show SMILES C\C(\C=C\C1=C(C)CCCC1(C)C)=C/C=C/C(/C)=C/C(O)=O |c:4| Show InChI InChI=1S/C20H28O2/c1-15(8-6-9-16(2)14-19(21)22)11-12-18-17(3)10-7-13-20(18,4)5/h6,8-9,11-12,14H,7,10,13H2,1-5H3,(H,21,22)/b9-6+,12-11+,15-8+,16-14+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
MMDB
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 54 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Tokyo
Curated by ChEMBL
| Assay Description
Agonistic activity of the compound towards retinoid X receptor-beta |
J Med Chem 40: 4222-34 (1998)
Article DOI: 10.1021/jm9704309
BindingDB Entry DOI: 10.7270/Q21J98VC |
More data for this
Ligand-Target Pair | |
Androgen receptor
(Homo sapiens (Human)) | BDBM50252332

(4-[(Benzyl)(4-nitrophenyl)amino]-1-methylpyrrole-2...)Show SMILES Cn1cc(cc1C(O)=O)N(Cc1ccccc1)c1ccc(cc1)[N+]([O-])=O Show InChI InChI=1S/C19H17N3O4/c1-20-13-17(11-18(20)19(23)24)21(12-14-5-3-2-4-6-14)15-7-9-16(10-8-15)22(25)26/h2-11,13H,12H2,1H3,(H,23,24) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 75 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Tokyo
Curated by ChEMBL
| Assay Description
Displacement of [3H]testosterone from wild type human androgen receptor |
Bioorg Med Chem 16: 6799-812 (2008)
Article DOI: 10.1016/j.bmc.2008.05.063
BindingDB Entry DOI: 10.7270/Q2MK6CPV |
More data for this
Ligand-Target Pair | |
Nociceptin receptor
(MOUSE) | BDBM50472731
(CHEMBL406946)Show SMILES [#6]-[#6@@H](-[#8])-[#6@H](-[#7]-[#6](=O)-[#6@H](-[#6]-c1ccccc1)-[#7]-[#6](=O)-[#6]-[#7]-[#6](=O)-[#6]-[#7]-[#6](=O)-[#6]-[#7]-[#6]-c1ccccc1)-[#6](=O)-[#7]-[#6]-[#6](=O)-[#7]-[#6@@H](-[#6])-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6]\[#7]=[#6](\[#7])-[#7])-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6]-[#6]-[#7])-[#6](=O)-[#7]-[#6@@H](-[#6]-[#8])-[#6](=O)-[#7]-[#6@@H](-[#6])-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6]\[#7]=[#6](\[#7])-[#7])-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6]-[#6]-[#7])-[#6](-[#7])=O Show InChI InChI=1S/C61H100N22O15/c1-35(75-48(88)33-74-59(98)50(37(3)85)83-57(96)44(28-38-16-6-4-7-17-38)77-49(89)32-73-47(87)31-72-46(86)30-69-29-39-18-8-5-9-19-39)52(91)79-43(23-15-27-71-61(67)68)55(94)81-41(21-11-13-25-63)56(95)82-45(34-84)58(97)76-36(2)53(92)80-42(22-14-26-70-60(65)66)54(93)78-40(51(64)90)20-10-12-24-62/h4-9,16-19,35-37,40-45,50,69,84-85H,10-15,20-34,62-63H2,1-3H3,(H2,64,90)(H,72,86)(H,73,87)(H,74,98)(H,75,88)(H,76,97)(H,77,89)(H,78,93)(H,79,91)(H,80,92)(H,81,94)(H,82,95)(H,83,96)(H4,65,66,70)(H4,67,68,71)/t35-,36-,37+,40-,41-,42-,43-,44-,45-,50-/m0/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
| Article
PubMed
| 100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Ferrara
Curated by ChEMBL
| Assay Description
Affinity to nociceptin receptors on mouse forebrain membranes by [3H]NC-NH2 displacement. |
J Med Chem 43: 2805-13 (2000)
Article DOI: 10.1021/jm990075h
BindingDB Entry DOI: 10.7270/Q2028V88 |
More data for this
Ligand-Target Pair | |
Androgen receptor
(Homo sapiens (Human)) | BDBM50252385
(CHEMBL481139 | N,N-Diethyl 4-[(4-nitrophenyl)(benz...)Show SMILES CCN(CC)C(=O)c1cc(cn1C)N(Cc1ccccc1)c1ccc(cc1)[N+]([O-])=O Show InChI InChI=1S/C23H26N4O3/c1-4-25(5-2)23(28)22-15-21(17-24(22)3)26(16-18-9-7-6-8-10-18)19-11-13-20(14-12-19)27(29)30/h6-15,17H,4-5,16H2,1-3H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Tokyo
Curated by ChEMBL
| Assay Description
Displacement of [3H]testosterone from wild type human androgen receptor |
Bioorg Med Chem 16: 6799-812 (2008)
Article DOI: 10.1016/j.bmc.2008.05.063
BindingDB Entry DOI: 10.7270/Q2MK6CPV |
More data for this
Ligand-Target Pair | |
Nociceptin receptor
(MOUSE) | BDBM50472734

(CHEMBL407461)Show SMILES [#6]-[#6@@H](-[#8])-[#6@H](-[#7]-[#6](=O)-[#6@H](-[#6]-c1ccccc1)-[#7]-[#6](=O)-[#6]-[#7]-[#6](=O)-[#6]-[#7]-[#6]-[#6@@H](-[#7])-[#6]-c1ccc(-[#8])cc1)-[#6](=O)-[#7]-[#6]-[#6](=O)-[#7]-[#6@@H](-[#6])-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6]\[#7]=[#6](\[#7])-[#7])-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6]-[#6]-[#7])-[#6](=O)-[#7]-[#6@@H](-[#6]-[#8])-[#6](=O)-[#7]-[#6@@H](-[#6])-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6]\[#7]=[#6](\[#7])-[#7])-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6]-[#6]-[#7])-[#6](-[#7])=O Show InChI InChI=1S/C61H102N22O15/c1-34(75-48(88)32-74-59(98)50(36(3)85)83-57(96)45(28-37-13-5-4-6-14-37)77-49(89)31-73-47(87)30-70-29-39(64)27-38-19-21-40(86)22-20-38)52(91)79-44(18-12-26-72-61(68)69)55(94)81-42(16-8-10-24-63)56(95)82-46(33-84)58(97)76-35(2)53(92)80-43(17-11-25-71-60(66)67)54(93)78-41(51(65)90)15-7-9-23-62/h4-6,13-14,19-22,34-36,39,41-46,50,70,84-86H,7-12,15-18,23-33,62-64H2,1-3H3,(H2,65,90)(H,73,87)(H,74,98)(H,75,88)(H,76,97)(H,77,89)(H,78,93)(H,79,91)(H,80,92)(H,81,94)(H,82,95)(H,83,96)(H4,66,67,71)(H4,68,69,72)/t34-,35-,36+,39-,41-,42-,43-,44-,45-,46-,50-/m0/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Ferrara
Curated by ChEMBL
| Assay Description
Affinity to nociceptin receptors on mouse forebrain membranes by [3H]NC-NH2 displacement. |
J Med Chem 43: 2805-13 (2000)
Article DOI: 10.1021/jm990075h
BindingDB Entry DOI: 10.7270/Q2028V88 |
More data for this
Ligand-Target Pair | |
Androgen receptor
(Homo sapiens (Human)) | BDBM50252176
(CHEMBL518945 | N-[4-[(Benzyl)(4-nitrophenyl)amino]...)Show SMILES Cn1cc(cc1C(=O)N1CCCC1)N(Cc1ccccc1)c1ccc(cc1)[N+]([O-])=O Show InChI InChI=1S/C23H24N4O3/c1-24-17-21(15-22(24)23(28)25-13-5-6-14-25)26(16-18-7-3-2-4-8-18)19-9-11-20(12-10-19)27(29)30/h2-4,7-12,15,17H,5-6,13-14,16H2,1H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 110 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Tokyo
Curated by ChEMBL
| Assay Description
Displacement of [3H]testosterone from wild type human androgen receptor |
Bioorg Med Chem 16: 6799-812 (2008)
Article DOI: 10.1016/j.bmc.2008.05.063
BindingDB Entry DOI: 10.7270/Q2MK6CPV |
More data for this
Ligand-Target Pair | |
Androgen receptor
(Homo sapiens (Human)) | BDBM50252387
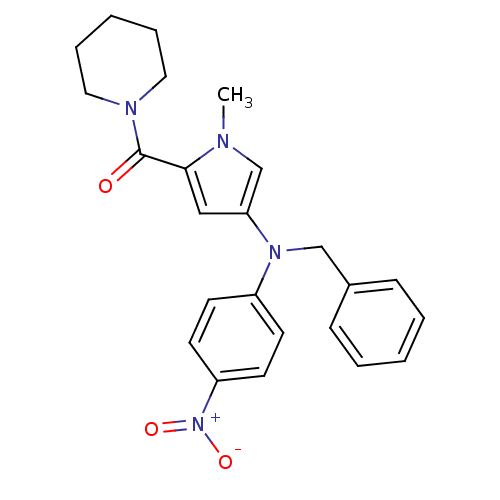
(CHEMBL520428 | N-[4-[(Benzyl)(4-nitrophenyl)amino]...)Show SMILES Cn1cc(cc1C(=O)N1CCCCC1)N(Cc1ccccc1)c1ccc(cc1)[N+]([O-])=O Show InChI InChI=1S/C24H26N4O3/c1-25-18-22(16-23(25)24(29)26-14-6-3-7-15-26)27(17-19-8-4-2-5-9-19)20-10-12-21(13-11-20)28(30)31/h2,4-5,8-13,16,18H,3,6-7,14-15,17H2,1H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 110 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Tokyo
Curated by ChEMBL
| Assay Description
Displacement of [3H]testosterone from wild type human androgen receptor |
Bioorg Med Chem 16: 6799-812 (2008)
Article DOI: 10.1016/j.bmc.2008.05.063
BindingDB Entry DOI: 10.7270/Q2MK6CPV |
More data for this
Ligand-Target Pair | |
Retinoic acid receptor RXR-gamma
(Homo sapiens (Human)) | BDBM31883

(9-cis-retinoic acid (9cRA) | ALL-TRANS-RETINOIC AC...)Show SMILES C\C(\C=C\C1=C(C)CCCC1(C)C)=C/C=C/C(/C)=C/C(O)=O |c:4| Show InChI InChI=1S/C20H28O2/c1-15(8-6-9-16(2)14-19(21)22)11-12-18-17(3)10-7-13-20(18,4)5/h6,8-9,11-12,14H,7,10,13H2,1-5H3,(H,21,22)/b9-6+,12-11+,15-8+,16-14+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
MMDB
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 120 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Tokyo
Curated by ChEMBL
| Assay Description
Agonistic activity of the compound towards retinoid X receptor-gamma |
J Med Chem 40: 4222-34 (1998)
Article DOI: 10.1021/jm9704309
BindingDB Entry DOI: 10.7270/Q21J98VC |
More data for this
Ligand-Target Pair | |
Protein kinase C alpha type
(Homo sapiens (Human)) | BDBM50146559

(But-3-enoic acid (R)-1-acetoxymethyl-1-hydroxymeth...)Show SMILES CC(=O)OC[C@@]1(CO)OC(=O)c2c1cccc2OC(=O)CC=C Show InChI InChI=1S/C16H16O7/c1-3-5-13(19)22-12-7-4-6-11-14(12)15(20)23-16(11,8-17)9-21-10(2)18/h3-4,6-7,17H,1,5,8-9H2,2H3/t16-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 122 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Tohoku University
Curated by ChEMBL
| Assay Description
Binding affinity for protein kinase C alpha |
Bioorg Med Chem Lett 14: 2963-7 (2004)
Article DOI: 10.1016/j.bmcl.2004.02.097
BindingDB Entry DOI: 10.7270/Q2GB23GP |
More data for this
Ligand-Target Pair | |
Protein kinase C alpha type
(Homo sapiens (Human)) | BDBM50146555

(Acetic acid (S)-4-decyloxy-1-hydroxymethyl-3-oxo-1...)Show SMILES CCCCCCCCCCOc1cccc2c1C(=O)O[C@@]2(CO)COC(C)=O Show InChI InChI=1S/C22H32O6/c1-3-4-5-6-7-8-9-10-14-26-19-13-11-12-18-20(19)21(25)28-22(18,15-23)16-27-17(2)24/h11-13,23H,3-10,14-16H2,1-2H3/t22-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 122 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Tohoku University
Curated by ChEMBL
| Assay Description
Binding affinity for protein kinase C alpha |
Bioorg Med Chem Lett 14: 2963-7 (2004)
Article DOI: 10.1016/j.bmcl.2004.02.097
BindingDB Entry DOI: 10.7270/Q2GB23GP |
More data for this
Ligand-Target Pair | |
Protein kinase C alpha type
(Homo sapiens (Human)) | BDBM50146556

(Acetic acid (R)-4-decyloxy-1-hydroxymethyl-3-oxo-1...)Show SMILES CCCCCCCCCCOc1cccc2c1C(=O)O[C@]2(CO)COC(C)=O Show InChI InChI=1S/C22H32O6/c1-3-4-5-6-7-8-9-10-14-26-19-13-11-12-18-20(19)21(25)28-22(18,15-23)16-27-17(2)24/h11-13,23H,3-10,14-16H2,1-2H3/t22-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 122 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Tohoku University
Curated by ChEMBL
| Assay Description
Binding affinity for protein kinase C alpha |
Bioorg Med Chem Lett 14: 2963-7 (2004)
Article DOI: 10.1016/j.bmcl.2004.02.097
BindingDB Entry DOI: 10.7270/Q2GB23GP |
More data for this
Ligand-Target Pair | |
Protein kinase C alpha type
(Homo sapiens (Human)) | BDBM50146558
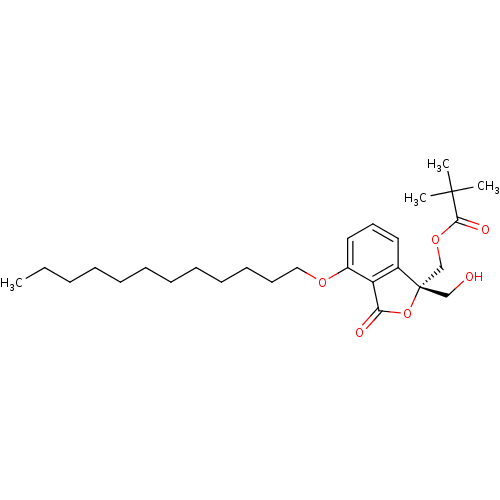
(2,2-Dimethyl-propionic acid (S)-4-dodecyloxy-1-hyd...)Show SMILES CCCCCCCCCCCCOc1cccc2c1C(=O)O[C@@]2(CO)COC(=O)C(C)(C)C Show InChI InChI=1S/C27H42O6/c1-5-6-7-8-9-10-11-12-13-14-18-31-22-17-15-16-21-23(22)24(29)33-27(21,19-28)20-32-25(30)26(2,3)4/h15-17,28H,5-14,18-20H2,1-4H3/t27-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 122 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Tohoku University
Curated by ChEMBL
| Assay Description
Binding affinity for protein kinase C alpha |
Bioorg Med Chem Lett 14: 2963-7 (2004)
Article DOI: 10.1016/j.bmcl.2004.02.097
BindingDB Entry DOI: 10.7270/Q2GB23GP |
More data for this
Ligand-Target Pair | |
Nociceptin receptor
(MOUSE) | BDBM50472746
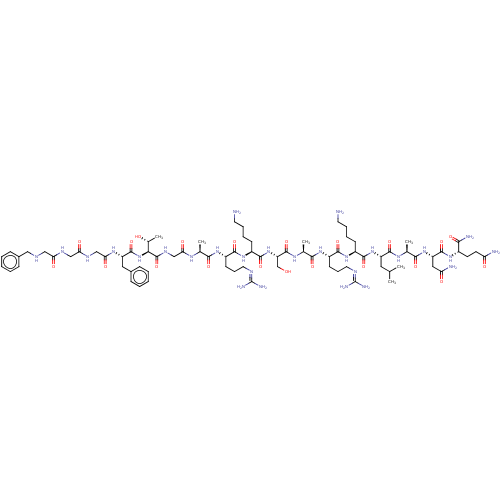
(CHEMBL411621)Show SMILES [#6]-[#6](-[#6])-[#6]-[#6@H](-[#7]-[#6](=O)-[#6@H](-[#6]-[#6]-[#6]-[#6]-[#7])-[#7]-[#6](=O)-[#6@H](-[#6]-[#6]-[#6]\[#7]=[#6](\[#7])-[#7])-[#7]-[#6](=O)-[#6@H](-[#6])-[#7]-[#6](=O)-[#6@H](-[#6]-[#8])-[#7]-[#6](=O)-[#6@H](-[#6]-[#6]-[#6]-[#6]-[#7])-[#7]-[#6](=O)-[#6@H](-[#6]-[#6]-[#6]-[#7]=[#6](-[#7])-[#7])-[#7]-[#6](=O)-[#6@H](-[#6])-[#7]-[#6](=O)-[#6]-[#7]-[#6](=O)-[#6@@H](-[#7]-[#6](=O)-[#6@H](-[#6]-c1ccccc1)-[#7]-[#6](=O)-[#6]-[#7]-[#6](=O)-[#6]-[#7]-[#6](=O)-[#6]-[#7]-[#6]-c1ccccc1)-[#6@@H](-[#6])-[#8])-[#6](=O)-[#7]-[#6@@H](-[#6])-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6](-[#7])=O)-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6](-[#7])=O)-[#6](-[#7])=O Show InChI InChI=1S/C79H130N28O21/c1-42(2)33-54(73(124)96-45(5)68(119)104-56(35-59(83)111)74(125)99-49(65(84)116)27-28-58(82)110)105-71(122)50(23-13-15-29-80)102-70(121)53(26-18-32-91-79(87)88)101-67(118)44(4)97-76(127)57(41-108)106-72(123)51(24-14-16-30-81)103-69(120)52(25-17-31-90-78(85)86)100-66(117)43(3)95-62(114)40-94-77(128)64(46(6)109)107-75(126)55(34-47-19-9-7-10-20-47)98-63(115)39-93-61(113)38-92-60(112)37-89-36-48-21-11-8-12-22-48/h7-12,19-22,42-46,49-57,64,89,108-109H,13-18,23-41,80-81H2,1-6H3,(H2,82,110)(H2,83,111)(H2,84,116)(H,92,112)(H,93,113)(H,94,128)(H,95,114)(H,96,124)(H,97,127)(H,98,115)(H,99,125)(H,100,117)(H,101,118)(H,102,121)(H,103,120)(H,104,119)(H,105,122)(H,106,123)(H,107,126)(H4,85,86,90)(H4,87,88,91)/t43-,44-,45-,46+,49-,50-,51-,52-,53-,54-,55-,56-,57-,64-/m0/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 126 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Ferrara
Curated by ChEMBL
| Assay Description
Affinity to nociceptin receptors on mouse forebrain membranes by [3H]NC-NH2 displacement. |
J Med Chem 43: 2805-13 (2000)
Article DOI: 10.1021/jm990075h
BindingDB Entry DOI: 10.7270/Q2028V88 |
More data for this
Ligand-Target Pair | |
Retinoic acid receptor gamma
(Homo sapiens (Human)) | BDBM50032675

(4-[1-(3,5,5,8,8-pentamethyl-5,6,7,8-tetrahydronaph...)Show SMILES Cc1cc2c(cc1C(=C)c1ccc(cc1)C(O)=O)C(C)(C)CCC2(C)C Show InChI InChI=1S/C24H28O2/c1-15-13-20-21(24(5,6)12-11-23(20,3)4)14-19(15)16(2)17-7-9-18(10-8-17)22(25)26/h7-10,13-14H,2,11-12H2,1,3-6H3,(H,25,26) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 130 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Tokyo
Curated by ChEMBL
| Assay Description
Selective activity of the compound towards retinoic acid receptor-gamma |
J Med Chem 40: 4222-34 (1998)
Article DOI: 10.1021/jm9704309
BindingDB Entry DOI: 10.7270/Q21J98VC |
More data for this
Ligand-Target Pair | |
Androgen receptor
(Homo sapiens (Human)) | BDBM50252276

(CHEMBL480955 | N-{4-[(Benzyl)(4-cyanophenyl)amino]...)Show SMILES Cn1cc(cc1C(=O)N1CCCC1)N(Cc1ccccc1)c1ccc(cc1)C#N Show InChI InChI=1S/C24H24N4O/c1-26-18-22(15-23(26)24(29)27-13-5-6-14-27)28(17-20-7-3-2-4-8-20)21-11-9-19(16-25)10-12-21/h2-4,7-12,15,18H,5-6,13-14,17H2,1H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 130 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Tokyo
Curated by ChEMBL
| Assay Description
Displacement of [3H]testosterone from wild type human androgen receptor |
Bioorg Med Chem 16: 6799-812 (2008)
Article DOI: 10.1016/j.bmc.2008.05.063
BindingDB Entry DOI: 10.7270/Q2MK6CPV |
More data for this
Ligand-Target Pair | |
Androgen receptor
(Homo sapiens (Human)) | BDBM50252328
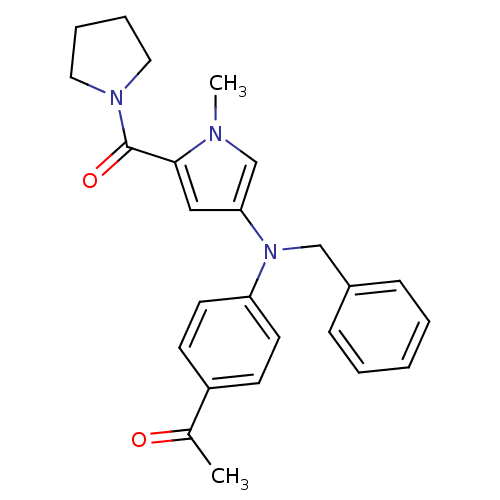
(CHEMBL479988 | N-{4-[(4-Acetylphenyl)(benzyl)amino...)Show SMILES CC(=O)c1ccc(cc1)N(Cc1ccccc1)c1cc(C(=O)N2CCCC2)n(C)c1 Show InChI InChI=1S/C25H27N3O2/c1-19(29)21-10-12-22(13-11-21)28(17-20-8-4-3-5-9-20)23-16-24(26(2)18-23)25(30)27-14-6-7-15-27/h3-5,8-13,16,18H,6-7,14-15,17H2,1-2H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 140 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Tokyo
Curated by ChEMBL
| Assay Description
Displacement of [3H]testosterone from wild type human androgen receptor |
Bioorg Med Chem 16: 6799-812 (2008)
Article DOI: 10.1016/j.bmc.2008.05.063
BindingDB Entry DOI: 10.7270/Q2MK6CPV |
More data for this
Ligand-Target Pair | |
Androgen receptor
(Homo sapiens (Human)) | BDBM50252331

(4-[(Benzyl)(4-nitrophenyl)amino]-2-hydroxymethyl-1...)Show SMILES Cn1cc(cc1CO)N(Cc1ccccc1)c1ccc(cc1)[N+]([O-])=O Show InChI InChI=1S/C19H19N3O3/c1-20-13-18(11-19(20)14-23)21(12-15-5-3-2-4-6-15)16-7-9-17(10-8-16)22(24)25/h2-11,13,23H,12,14H2,1H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 170 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Tokyo
Curated by ChEMBL
| Assay Description
Displacement of [3H]testosterone from wild type human androgen receptor |
Bioorg Med Chem 16: 6799-812 (2008)
Article DOI: 10.1016/j.bmc.2008.05.063
BindingDB Entry DOI: 10.7270/Q2MK6CPV |
More data for this
Ligand-Target Pair | |
Androgen receptor
(Homo sapiens (Human)) | BDBM50252330
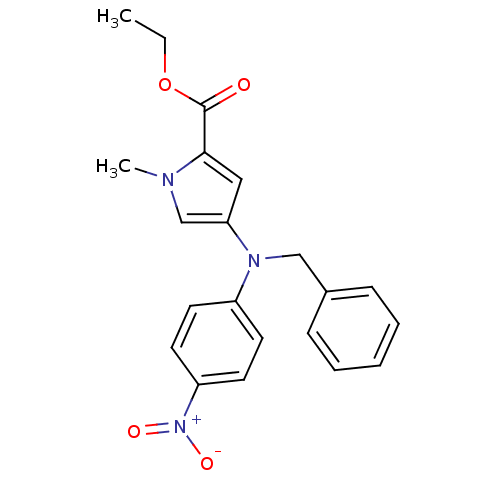
(CHEMBL521251 | Ethyl 4-[(benzyl)(4-nitrophenyl)ami...)Show SMILES CCOC(=O)c1cc(cn1C)N(Cc1ccccc1)c1ccc(cc1)[N+]([O-])=O Show InChI InChI=1S/C21H21N3O4/c1-3-28-21(25)20-13-19(15-22(20)2)23(14-16-7-5-4-6-8-16)17-9-11-18(12-10-17)24(26)27/h4-13,15H,3,14H2,1-2H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 180 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Tokyo
Curated by ChEMBL
| Assay Description
Displacement of [3H]testosterone from wild type human androgen receptor |
Bioorg Med Chem 16: 6799-812 (2008)
Article DOI: 10.1016/j.bmc.2008.05.063
BindingDB Entry DOI: 10.7270/Q2MK6CPV |
More data for this
Ligand-Target Pair | |
Retinoic acid receptor alpha
(Homo sapiens (Human)) | BDBM50032675

(4-[1-(3,5,5,8,8-pentamethyl-5,6,7,8-tetrahydronaph...)Show SMILES Cc1cc2c(cc1C(=C)c1ccc(cc1)C(O)=O)C(C)(C)CCC2(C)C Show InChI InChI=1S/C24H28O2/c1-15-13-20-21(24(5,6)12-11-23(20,3)4)14-19(15)16(2)17-7-9-18(10-8-17)22(25)26/h7-10,13-14H,2,11-12H2,1,3-6H3,(H,25,26) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 180 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Tokyo
Curated by ChEMBL
| Assay Description
Selective activity of the compound towards retinoic acid receptor-alpha |
J Med Chem 40: 4222-34 (1998)
Article DOI: 10.1021/jm9704309
BindingDB Entry DOI: 10.7270/Q21J98VC |
More data for this
Ligand-Target Pair | |
Androgen receptor
(Homo sapiens (Human)) | BDBM50252329
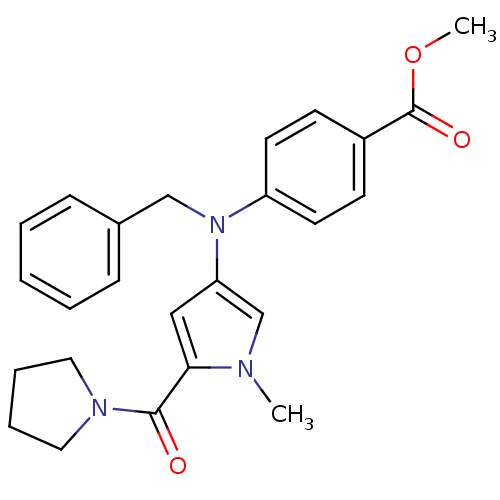
(CHEMBL480959 | N-{4-[(Benzyl)(4-methoxycarbonylphe...)Show SMILES COC(=O)c1ccc(cc1)N(Cc1ccccc1)c1cc(C(=O)N2CCCC2)n(C)c1 Show InChI InChI=1S/C25H27N3O3/c1-26-18-22(16-23(26)24(29)27-14-6-7-15-27)28(17-19-8-4-3-5-9-19)21-12-10-20(11-13-21)25(30)31-2/h3-5,8-13,16,18H,6-7,14-15,17H2,1-2H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 180 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Tokyo
Curated by ChEMBL
| Assay Description
Displacement of [3H]testosterone from wild type human androgen receptor |
Bioorg Med Chem 16: 6799-812 (2008)
Article DOI: 10.1016/j.bmc.2008.05.063
BindingDB Entry DOI: 10.7270/Q2MK6CPV |
More data for this
Ligand-Target Pair | |
Nociceptin receptor
(MOUSE) | BDBM50472733
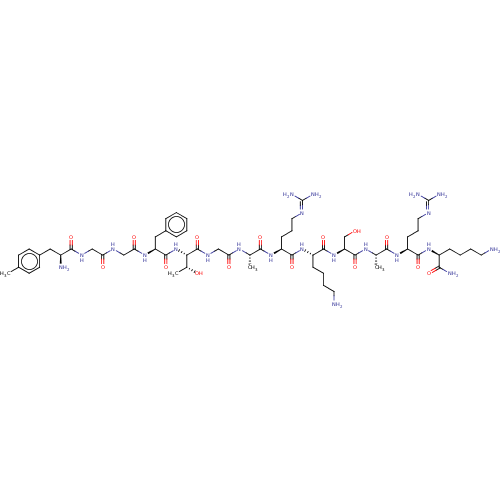
(CHEMBL413178)Show SMILES [#6]-[#6@@H](-[#8])-[#6@H](-[#7]-[#6](=O)-[#6@H](-[#6]-c1ccccc1)-[#7]-[#6](=O)-[#6]-[#7]-[#6](=O)-[#6]-[#7]-[#6](=O)-[#6@@H](-[#7])-[#6]-c1ccc(-[#6])cc1)-[#6](=O)-[#7]-[#6]-[#6](=O)-[#7]-[#6@@H](-[#6])-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6]\[#7]=[#6](\[#7])-[#7])-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6]-[#6]-[#7])-[#6](=O)-[#7]-[#6@@H](-[#6]-[#8])-[#6](=O)-[#7]-[#6@@H](-[#6])-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6]\[#7]=[#6](\[#7])-[#7])-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6]-[#6]-[#7])-[#6](-[#7])=O Show InChI InChI=1S/C62H102N22O15/c1-34-20-22-39(23-21-34)28-40(65)54(93)74-30-47(87)73-31-49(89)78-45(29-38-14-6-5-7-15-38)58(97)84-50(37(4)86)60(99)75-32-48(88)76-35(2)52(91)80-44(19-13-27-72-62(69)70)56(95)82-42(17-9-11-25-64)57(96)83-46(33-85)59(98)77-36(3)53(92)81-43(18-12-26-71-61(67)68)55(94)79-41(51(66)90)16-8-10-24-63/h5-7,14-15,20-23,35-37,40-46,50,85-86H,8-13,16-19,24-33,63-65H2,1-4H3,(H2,66,90)(H,73,87)(H,74,93)(H,75,99)(H,76,88)(H,77,98)(H,78,89)(H,79,94)(H,80,91)(H,81,92)(H,82,95)(H,83,96)(H,84,97)(H4,67,68,71)(H4,69,70,72)/t35-,36-,37+,40-,41-,42-,43-,44-,45-,46-,50-/m0/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Ferrara
Curated by ChEMBL
| Assay Description
Affinity to nociceptin receptors on mouse forebrain membranes by [3H]NC-NH2 displacement. |
J Med Chem 43: 2805-13 (2000)
Article DOI: 10.1021/jm990075h
BindingDB Entry DOI: 10.7270/Q2028V88 |
More data for this
Ligand-Target Pair | |
Retinoic acid receptor beta
(Homo sapiens (Human)) | BDBM50061616
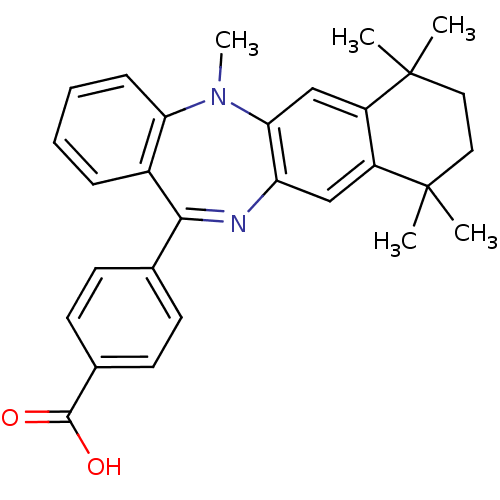
(4-(5,7,7,10,10-Pentamethyl-7,8,9,10-tetrahydro-5H-...)Show SMILES CN1c2cc3c(cc2N=C(c2ccc(cc2)C(O)=O)c2ccccc12)C(C)(C)CCC3(C)C |t:9| Show InChI InChI=1S/C29H30N2O2/c1-28(2)14-15-29(3,4)22-17-25-23(16-21(22)28)30-26(18-10-12-19(13-11-18)27(32)33)20-8-6-7-9-24(20)31(25)5/h6-13,16-17H,14-15H2,1-5H3,(H,32,33) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 220 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Tokyo
Curated by ChEMBL
| Assay Description
Antagonistic activity of the compound towards retinoic acid receptor-beta |
J Med Chem 40: 4222-34 (1998)
Article DOI: 10.1021/jm9704309
BindingDB Entry DOI: 10.7270/Q21J98VC |
More data for this
Ligand-Target Pair | |
Androgen receptor
(Homo sapiens (Human)) | BDBM50252384
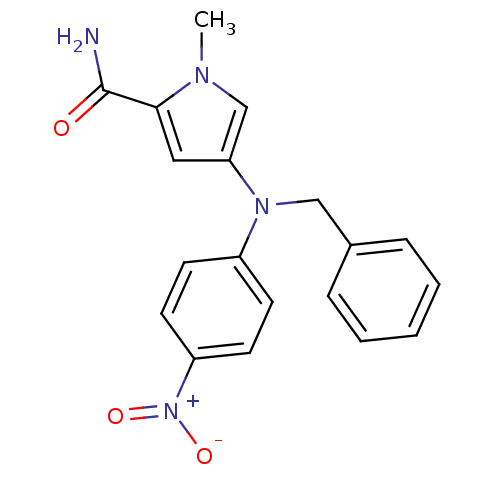
(4-[(Benzyl)(4-nitrophenyl)amino]-1-methylpyrrole-2...)Show SMILES Cn1cc(cc1C(N)=O)N(Cc1ccccc1)c1ccc(cc1)[N+]([O-])=O Show InChI InChI=1S/C19H18N4O3/c1-21-13-17(11-18(21)19(20)24)22(12-14-5-3-2-4-6-14)15-7-9-16(10-8-15)23(25)26/h2-11,13H,12H2,1H3,(H2,20,24) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 220 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Tokyo
Curated by ChEMBL
| Assay Description
Displacement of [3H]testosterone from wild type human androgen receptor |
Bioorg Med Chem 16: 6799-812 (2008)
Article DOI: 10.1016/j.bmc.2008.05.063
BindingDB Entry DOI: 10.7270/Q2MK6CPV |
More data for this
Ligand-Target Pair | |
Androgen receptor
(Homo sapiens (Human)) | BDBM50252277
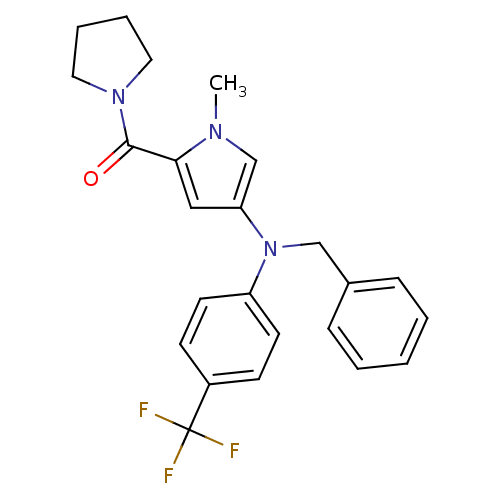
(CHEMBL521249 | N-{4-[(Benzyl)(4-trifluoromethylphe...)Show SMILES Cn1cc(cc1C(=O)N1CCCC1)N(Cc1ccccc1)c1ccc(cc1)C(F)(F)F Show InChI InChI=1S/C24H24F3N3O/c1-28-17-21(15-22(28)23(31)29-13-5-6-14-29)30(16-18-7-3-2-4-8-18)20-11-9-19(10-12-20)24(25,26)27/h2-4,7-12,15,17H,5-6,13-14,16H2,1H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 270 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Tokyo
Curated by ChEMBL
| Assay Description
Displacement of [3H]testosterone from wild type human androgen receptor |
Bioorg Med Chem 16: 6799-812 (2008)
Article DOI: 10.1016/j.bmc.2008.05.063
BindingDB Entry DOI: 10.7270/Q2MK6CPV |
More data for this
Ligand-Target Pair | |
Nociceptin receptor
(MOUSE) | BDBM50472745
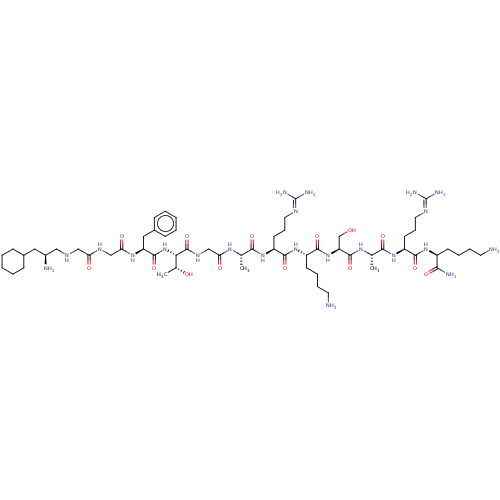
(CHEMBL407671)Show SMILES [#6]-[#6@@H](-[#8])-[#6@H](-[#7]-[#6](=O)-[#6@H](-[#6]-c1ccccc1)-[#7]-[#6](=O)-[#6]-[#7]-[#6](=O)-[#6]-[#7]-[#6]-[#6@@H](-[#7])-[#6]-[#6]-1-[#6]-[#6]-[#6]-[#6]-[#6]-1)-[#6](=O)-[#7]-[#6]-[#6](=O)-[#7]-[#6@@H](-[#6])-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6]\[#7]=[#6](\[#7])-[#7])-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6]-[#6]-[#7])-[#6](=O)-[#7]-[#6@@H](-[#6]-[#8])-[#6](=O)-[#7]-[#6@@H](-[#6])-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6]\[#7]=[#6](\[#7])-[#7])-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6]-[#6]-[#7])-[#6](-[#7])=O Show InChI InChI=1S/C61H108N22O14/c1-35(75-48(87)33-74-59(97)50(37(3)85)83-57(95)45(29-39-18-8-5-9-19-39)77-49(88)32-73-47(86)31-70-30-40(64)28-38-16-6-4-7-17-38)52(90)79-44(23-15-27-72-61(68)69)55(93)81-42(21-11-13-25-63)56(94)82-46(34-84)58(96)76-36(2)53(91)80-43(22-14-26-71-60(66)67)54(92)78-41(51(65)89)20-10-12-24-62/h5,8-9,18-19,35-38,40-46,50,70,84-85H,4,6-7,10-17,20-34,62-64H2,1-3H3,(H2,65,89)(H,73,86)(H,74,97)(H,75,87)(H,76,96)(H,77,88)(H,78,92)(H,79,90)(H,80,91)(H,81,93)(H,82,94)(H,83,95)(H4,66,67,71)(H4,68,69,72)/t35-,36-,37+,40-,41-,42-,43-,44-,45-,46-,50-/m0/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 316 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Ferrara
Curated by ChEMBL
| Assay Description
Affinity to nociceptin receptors on mouse forebrain membranes by [3H]NC-NH2 displacement. |
J Med Chem 43: 2805-13 (2000)
Article DOI: 10.1021/jm990075h
BindingDB Entry DOI: 10.7270/Q2028V88 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data