

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Prothrombin (Homo sapiens (Human)) | BDBM50079482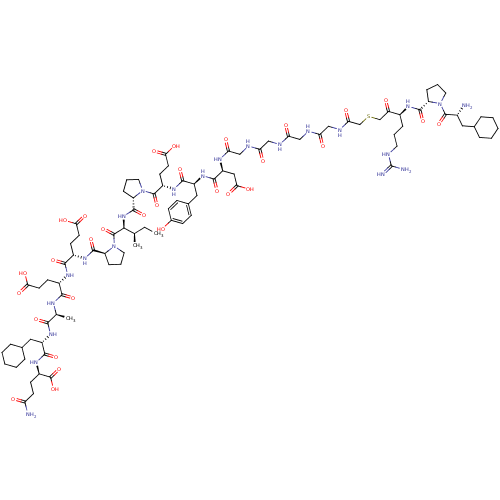 (Arginyl Ketomethylene analogue | CHEMBL410589) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.000350 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Research Council Canada Curated by ChEMBL | Assay Description Inhibitory constant against thrombin | J Med Chem 42: 3109-15 (1999) Article DOI: 10.1021/jm9807297 BindingDB Entry DOI: 10.7270/Q2VQ31WT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50079489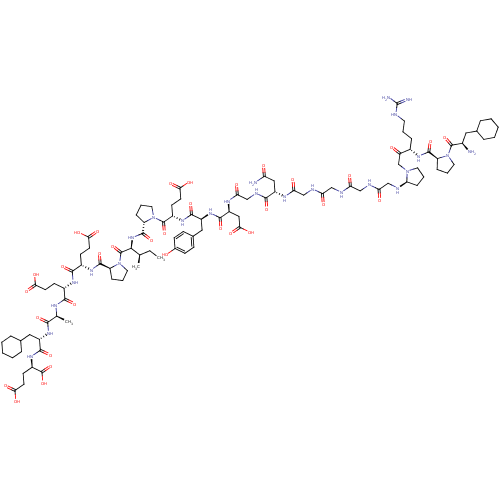 (Arginyl Ketomethylene analogue | CHEMBL428116) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | 0.000570 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Research Council Canada Curated by ChEMBL | Assay Description Inhibitory constant against thrombin | J Med Chem 42: 3109-15 (1999) Article DOI: 10.1021/jm9807297 BindingDB Entry DOI: 10.7270/Q2VQ31WT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50079476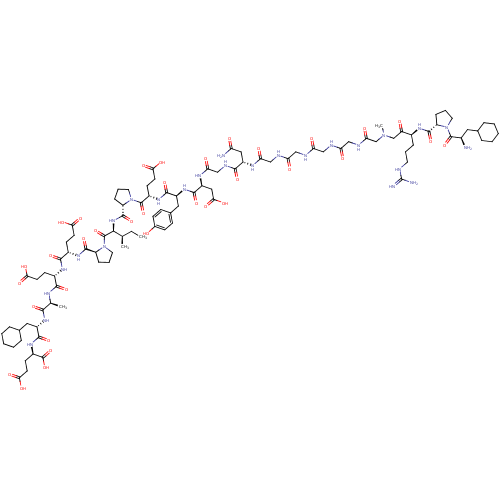 (Arginyl Ketomethylene analogue | CHEMBL437873) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | 0.000870 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Research Council Canada Curated by ChEMBL | Assay Description Inhibitory constant against thrombin | J Med Chem 42: 3109-15 (1999) Article DOI: 10.1021/jm9807297 BindingDB Entry DOI: 10.7270/Q2VQ31WT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50079479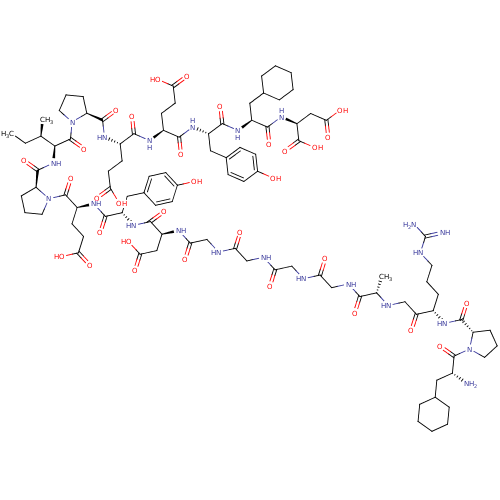 (Arginyl Ketomethylene analogue | CHEMBL407043) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | 0.00150 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Research Council Canada Curated by ChEMBL | Assay Description Inhibitory constant against thrombin | J Med Chem 42: 3109-15 (1999) Article DOI: 10.1021/jm9807297 BindingDB Entry DOI: 10.7270/Q2VQ31WT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50079485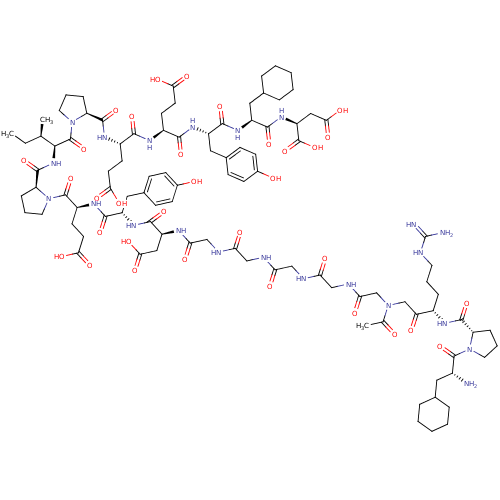 (Arginyl Ketomethylene analogue | CHEMBL414489) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | 0.00170 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Research Council Canada Curated by ChEMBL | Assay Description Inhibitory constant against thrombin | J Med Chem 42: 3109-15 (1999) Article DOI: 10.1021/jm9807297 BindingDB Entry DOI: 10.7270/Q2VQ31WT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50079478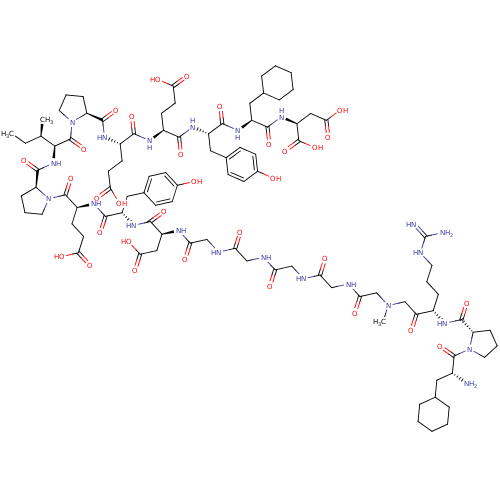 (Arginyl Ketomethylene analogue | CHEMBL414760) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | 0.00200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Research Council Canada Curated by ChEMBL | Assay Description Inhibitory constant against thrombin | J Med Chem 42: 3109-15 (1999) Article DOI: 10.1021/jm9807297 BindingDB Entry DOI: 10.7270/Q2VQ31WT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protease (Human immunodeficiency virus 1 (HIV-1)) | BDBM50480930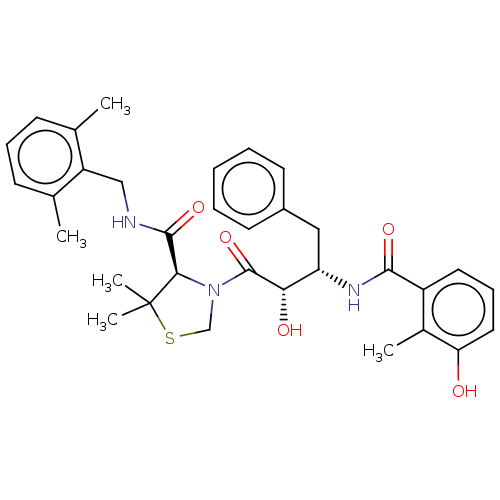 (CHEMBL584130 | KNI-814) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.00240 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kyoto Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of HIV1 recombinant protease after 15 mins by fluorescence assay | J Med Chem 52: 7604-17 (2009) Article DOI: 10.1021/jm9005115 BindingDB Entry DOI: 10.7270/Q2FR00F2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Rattus norvegicus (rat)) | BDBM50368723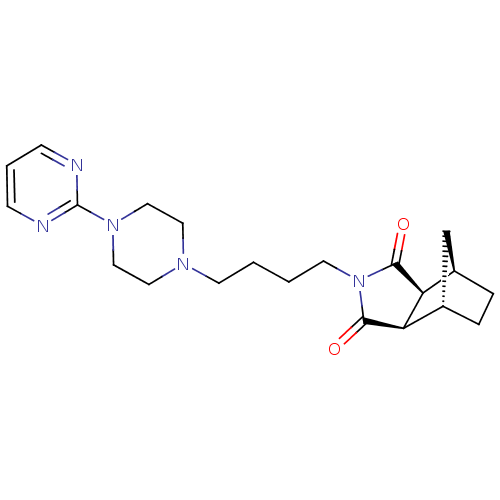 (Metanopirone | Sediel | TANDOSPIRONE HYDROCHLORIDE...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Similars | PubMed | 0.00270 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Asahi Chemical Industry Company, Ltd. Curated by ChEMBL | Assay Description Binding affinity by measuring displacement of [3H]8-OH-DPAT from 5-hydroxytryptamine 1A receptor in rat hippocampus | J Med Chem 36: 3526-32 (1994) BindingDB Entry DOI: 10.7270/Q2D21Z74 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Melanocyte-stimulating hormone receptor (Homo sapiens (Human)) | BDBM250661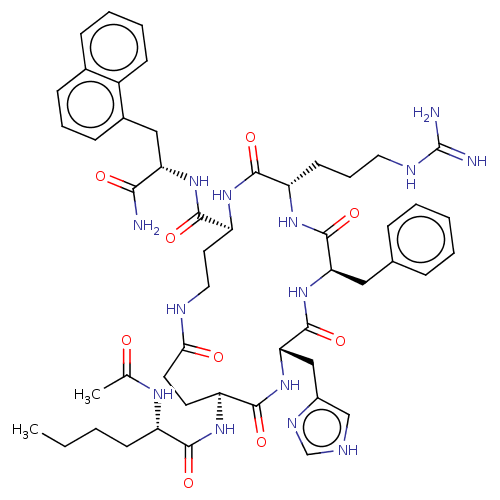 (US9447148, 9.30) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.00500 | -67.1 | n/a | n/a | n/a | n/a | n/a | 7.5 | 37 |
Palatin Technologies, Inc. US Patent | Assay Description A competitive inhibition binding assay was performed using membrane homogenates prepared from HEK-293 cells that express recombinant hMCR-1a or hMCR-... | US Patent US9447148 (2016) BindingDB Entry DOI: 10.7270/Q2RJ4HD2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase pim-1 (Homo sapiens (Human)) | BDBM602107 (US11643417, Ex. No. 120) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | 0.00600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Citation and Details BindingDB Entry DOI: 10.7270/Q2R49VQ0 | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Melatonin receptor type 1B (Homo sapiens (Human)) | BDBM50343590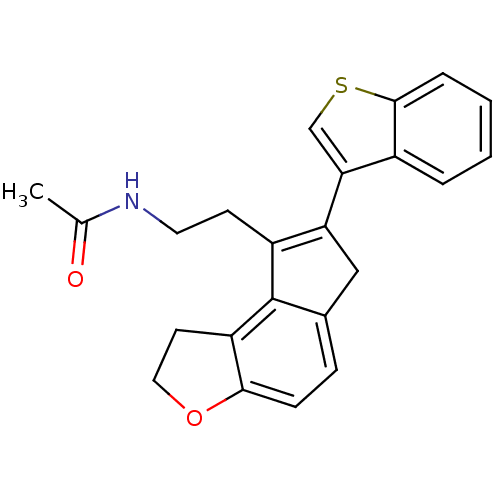 (CHEMBL1774531 | N-{2-[7-(1-Benzothien-3-yl)-1,6-di...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 0.00600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Limited Curated by ChEMBL | Assay Description Displacement of [125I]-2-iodomelatonin from human MT2 receptor expressed on CHO cells microscintillation counting | J Med Chem 54: 3436-44 (2011) Article DOI: 10.1021/jm200221q BindingDB Entry DOI: 10.7270/Q2028RVN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Melatonin receptor type 1B (Homo sapiens (Human)) | BDBM50343599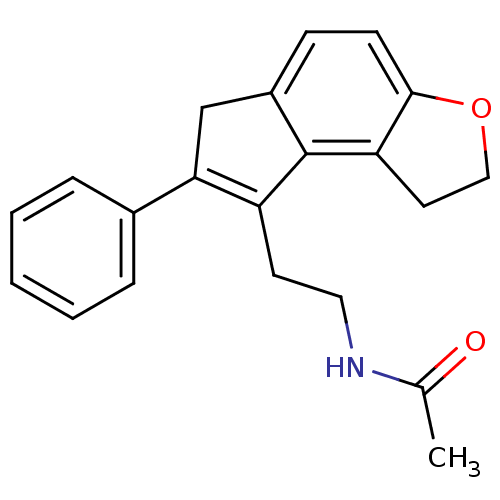 (CHEMBL1774522 | N-[2-(7-Phenyl-1,6-dihydro-2H-inde...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.00650 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Limited Curated by ChEMBL | Assay Description Displacement of 2-[125I]iodomelatonin from human MT2 receptor expressed in CHO cells | J Med Chem 54: 4207-18 (2011) Article DOI: 10.1021/jm200385u BindingDB Entry DOI: 10.7270/Q2HT2PN3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Melatonin receptor type 1A (Homo sapiens (Human)) | BDBM50343598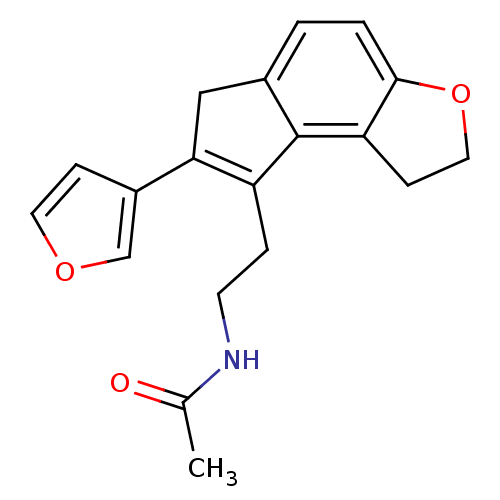 (CHEMBL1774523 | N-{2-[7-(3-Furyl)-1,6-dihydro-2H-i...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.00650 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Limited Curated by ChEMBL | Assay Description Displacement of [125I]-2-iodomelatonin from human MT1 receptor expressed on CHO cells by microscintillation counting | J Med Chem 54: 3436-44 (2011) Article DOI: 10.1021/jm200221q BindingDB Entry DOI: 10.7270/Q2028RVN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Melatonin receptor type 1B (Homo sapiens (Human)) | BDBM50343599 (CHEMBL1774522 | N-[2-(7-Phenyl-1,6-dihydro-2H-inde...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.00650 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Limited Curated by ChEMBL | Assay Description Displacement of [125I]-2-iodomelatonin from human MT2 receptor expressed on CHO cells microscintillation counting | J Med Chem 54: 3436-44 (2011) Article DOI: 10.1021/jm200221q BindingDB Entry DOI: 10.7270/Q2028RVN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Melatonin receptor type 1B (Homo sapiens (Human)) | BDBM50343601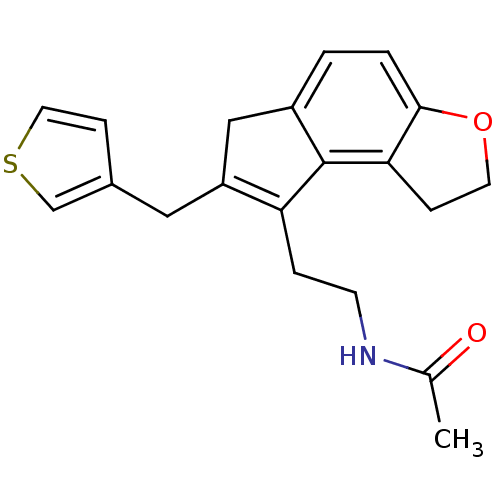 (CHEMBL1774520 | N-{2-[7-(3-Thienylmethyl)-1,6-dihy...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.00670 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Limited Curated by ChEMBL | Assay Description Displacement of [125I]-2-iodomelatonin from human MT2 receptor expressed on CHO cells microscintillation counting | J Med Chem 54: 3436-44 (2011) Article DOI: 10.1021/jm200221q BindingDB Entry DOI: 10.7270/Q2028RVN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Melatonin receptor type 1A (Homo sapiens (Human)) | BDBM50343599 (CHEMBL1774522 | N-[2-(7-Phenyl-1,6-dihydro-2H-inde...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.00820 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Limited Curated by ChEMBL | Assay Description Displacement of [125I]-2-iodomelatonin from human MT1 receptor expressed on CHO cells by microscintillation counting | J Med Chem 54: 3436-44 (2011) Article DOI: 10.1021/jm200221q BindingDB Entry DOI: 10.7270/Q2028RVN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Melatonin receptor type 1A (Homo sapiens (Human)) | BDBM50343599 (CHEMBL1774522 | N-[2-(7-Phenyl-1,6-dihydro-2H-inde...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.00820 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Limited Curated by ChEMBL | Assay Description Displacement of 2-[125I]iodomelatonin from human MT1 receptor expressed in CHO cells | J Med Chem 54: 4207-18 (2011) Article DOI: 10.1021/jm200385u BindingDB Entry DOI: 10.7270/Q2HT2PN3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Melatonin receptor type 1B (Homo sapiens (Human)) | BDBM50343603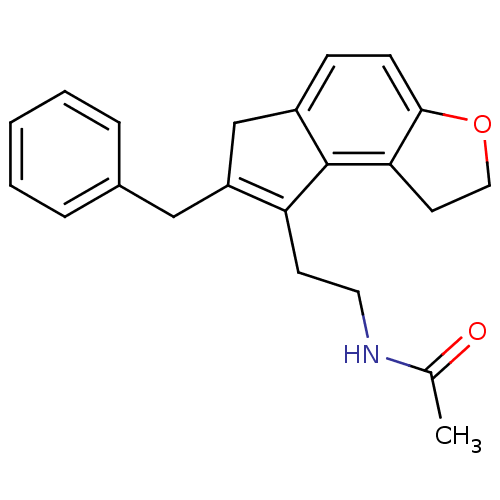 (CHEMBL1774518 | N-[2-(7-Benzyl-1,6-dihydro-2H-inde...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.00850 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Limited Curated by ChEMBL | Assay Description Displacement of [125I]-2-iodomelatonin from human MT2 receptor expressed on CHO cells microscintillation counting | J Med Chem 54: 3436-44 (2011) Article DOI: 10.1021/jm200221q BindingDB Entry DOI: 10.7270/Q2028RVN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Melatonin receptor type 1A (Homo sapiens (Human)) | BDBM50343600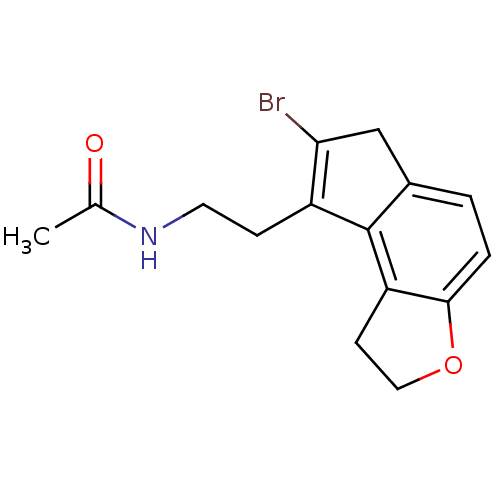 (CHEMBL1774521 | N-[2-(7-Bromo-1,6-dihydro-2H-inden...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.00870 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Limited Curated by ChEMBL | Assay Description Displacement of [125I]-2-iodomelatonin from human MT1 receptor expressed on CHO cells by microscintillation counting | J Med Chem 54: 3436-44 (2011) Article DOI: 10.1021/jm200221q BindingDB Entry DOI: 10.7270/Q2028RVN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Melatonin receptor type 1B (Homo sapiens (Human)) | BDBM50343595 (CHEMBL1774526 | N-{2-[7-(3-Methoxyphenyl)-1,6-dihy...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.00900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Limited Curated by ChEMBL | Assay Description Displacement of [125I]-2-iodomelatonin from human MT2 receptor expressed on CHO cells microscintillation counting | J Med Chem 54: 3436-44 (2011) Article DOI: 10.1021/jm200221q BindingDB Entry DOI: 10.7270/Q2028RVN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase pim-1 (Homo sapiens (Human)) | BDBM602075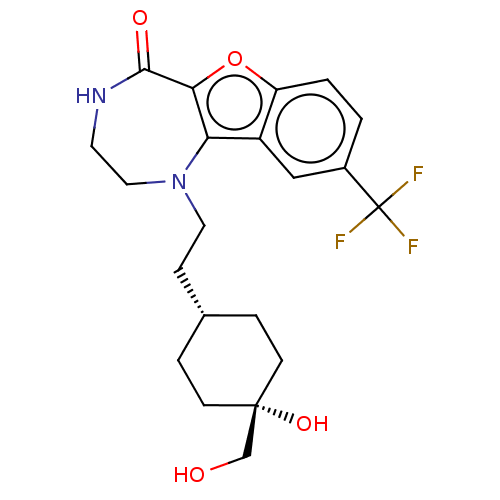 (US11643417, Ex. No. 90) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | 0.00900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Citation and Details BindingDB Entry DOI: 10.7270/Q2R49VQ0 | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase pim-1 (Homo sapiens (Human)) | BDBM602103 (US11643417, Ex. No. 116) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | 0.00900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Citation and Details BindingDB Entry DOI: 10.7270/Q2R49VQ0 | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50079491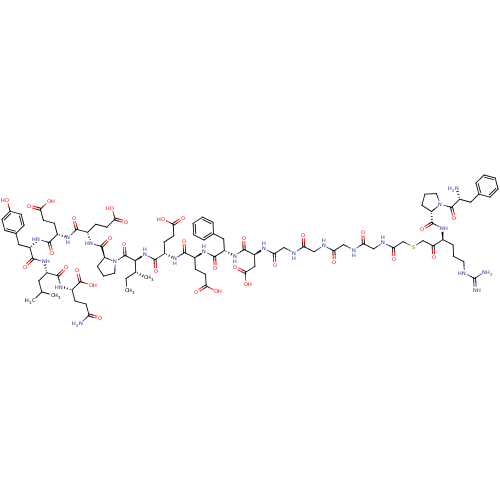 (Arginyl Ketomethylene analogue | CHEMBL412457) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | 0.00940 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Research Council Canada Curated by ChEMBL | Assay Description Inhibitory constant against thrombin | J Med Chem 42: 3109-15 (1999) Article DOI: 10.1021/jm9807297 BindingDB Entry DOI: 10.7270/Q2VQ31WT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Melatonin receptor type 1B (Homo sapiens (Human)) | BDBM50343598 (CHEMBL1774523 | N-{2-[7-(3-Furyl)-1,6-dihydro-2H-i...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.00960 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Limited Curated by ChEMBL | Assay Description Displacement of [125I]-2-iodomelatonin from human MT2 receptor expressed on CHO cells microscintillation counting | J Med Chem 54: 3436-44 (2011) Article DOI: 10.1021/jm200221q BindingDB Entry DOI: 10.7270/Q2028RVN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase pim-1 (Homo sapiens (Human)) | BDBM602160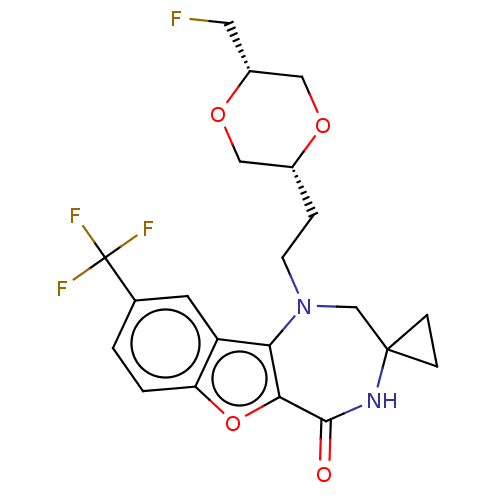 (US11643417, Ex. No. 156) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | 0.0100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Citation and Details BindingDB Entry DOI: 10.7270/Q2R49VQ0 | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Melanocyte-stimulating hormone receptor (Homo sapiens (Human)) | BDBM250652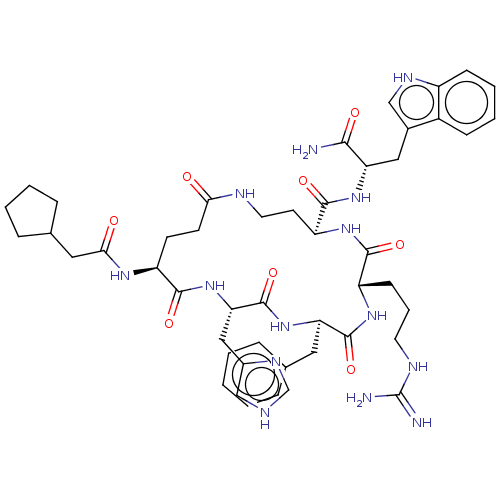 (US9447148, 9.21) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.0100 | -65.3 | n/a | n/a | n/a | n/a | n/a | 7.5 | 37 |
Palatin Technologies, Inc. US Patent | Assay Description A competitive inhibition binding assay was performed using membrane homogenates prepared from HEK-293 cells that express recombinant hMCR-1a or hMCR-... | US Patent US9447148 (2016) BindingDB Entry DOI: 10.7270/Q2RJ4HD2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Melanocyte-stimulating hormone receptor (Homo sapiens (Human)) | BDBM250653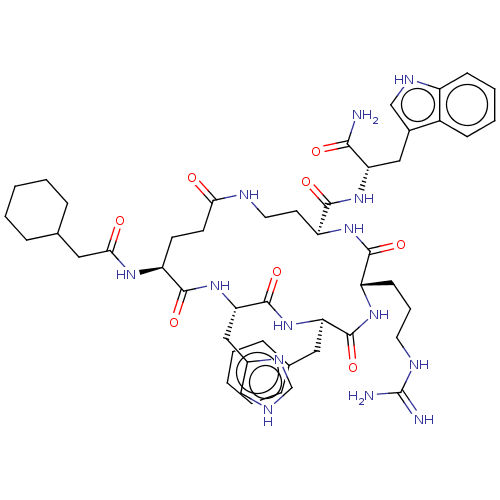 (US9447148, 9.22) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.0100 | -65.3 | n/a | n/a | n/a | n/a | n/a | 7.5 | 37 |
Palatin Technologies, Inc. US Patent | Assay Description A competitive inhibition binding assay was performed using membrane homogenates prepared from HEK-293 cells that express recombinant hMCR-1a or hMCR-... | US Patent US9447148 (2016) BindingDB Entry DOI: 10.7270/Q2RJ4HD2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Melatonin receptor type 1B (Homo sapiens (Human)) | BDBM50343605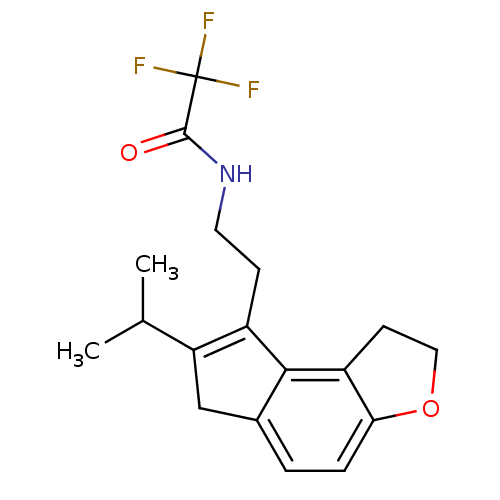 (2,2,2-Trifluoro-N-[2-(7-isopropyl-1,6-dihydro-2H-i...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Limited Curated by ChEMBL | Assay Description Displacement of [125I]-2-iodomelatonin from human MT2 receptor expressed on CHO cells microscintillation counting | J Med Chem 54: 3436-44 (2011) Article DOI: 10.1021/jm200221q BindingDB Entry DOI: 10.7270/Q2028RVN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Melanocyte-stimulating hormone receptor (Homo sapiens (Human)) | BDBM250642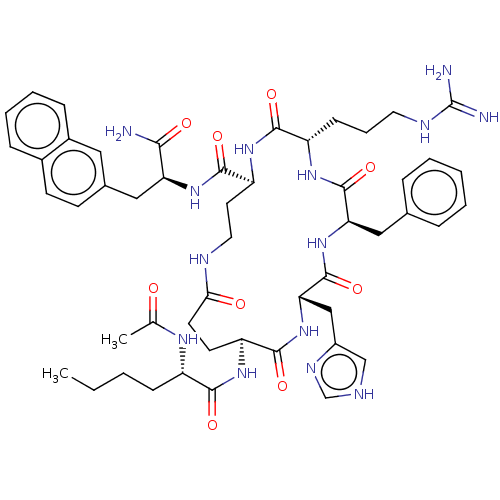 (US9447148, 9.10) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.0100 | -65.3 | n/a | n/a | n/a | n/a | n/a | 7.5 | 37 |
Palatin Technologies, Inc. US Patent | Assay Description A competitive inhibition binding assay was performed using membrane homogenates prepared from HEK-293 cells that express recombinant hMCR-1a or hMCR-... | US Patent US9447148 (2016) BindingDB Entry DOI: 10.7270/Q2RJ4HD2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Melanocyte-stimulating hormone receptor (Homo sapiens (Human)) | BDBM250633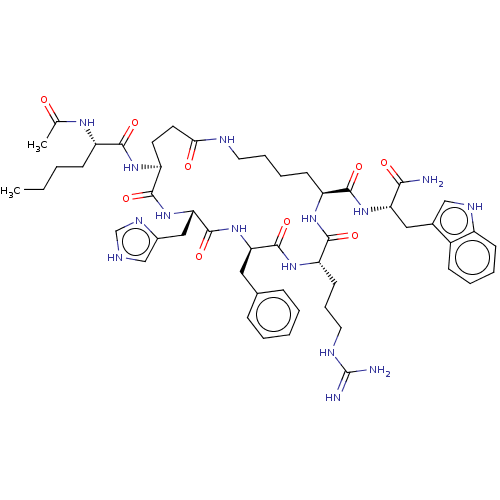 (US9447148, 9.1) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.0100 | -65.3 | n/a | n/a | n/a | n/a | n/a | 7.5 | 37 |
Palatin Technologies, Inc. US Patent | Assay Description A competitive inhibition binding assay was performed using membrane homogenates prepared from HEK-293 cells that express recombinant hMCR-1a or hMCR-... | US Patent US9447148 (2016) BindingDB Entry DOI: 10.7270/Q2RJ4HD2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Melatonin receptor type 1B (Homo sapiens (Human)) | BDBM50343607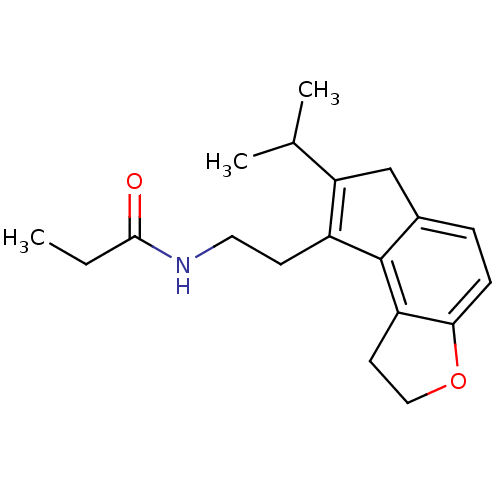 (CHEMBL1774514 | N-[2-(7-Isopropyl-1,6-dihydro-2H-i...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0110 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Limited Curated by ChEMBL | Assay Description Displacement of [125I]-2-iodomelatonin from human MT2 receptor expressed on CHO cells microscintillation counting | J Med Chem 54: 3436-44 (2011) Article DOI: 10.1021/jm200221q BindingDB Entry DOI: 10.7270/Q2028RVN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Melatonin receptor type 1B (Homo sapiens (Human)) | BDBM50343592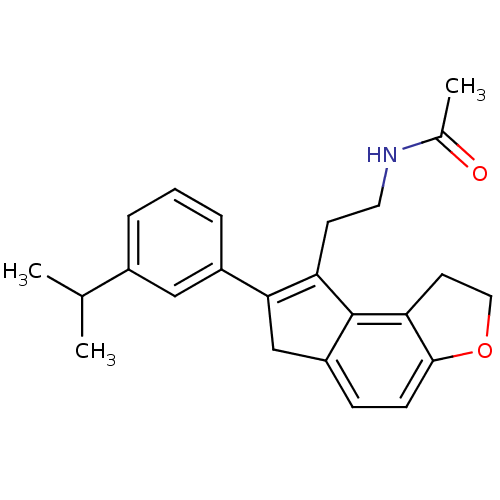 (CHEMBL1774529 | N-{2-[7-(3-Isopropylphenyl)-1,6-di...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0110 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Limited Curated by ChEMBL | Assay Description Displacement of [125I]-2-iodomelatonin from human MT2 receptor expressed on CHO cells microscintillation counting | J Med Chem 54: 3436-44 (2011) Article DOI: 10.1021/jm200221q BindingDB Entry DOI: 10.7270/Q2028RVN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase pim-1 (Homo sapiens (Human)) | BDBM602097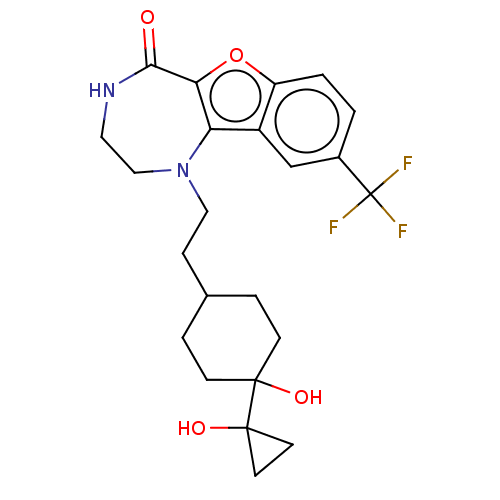 (US11643417, Ex. No. 111) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | 0.0110 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Citation and Details BindingDB Entry DOI: 10.7270/Q2R49VQ0 | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Melanocyte-stimulating hormone receptor (Homo sapiens (Human)) | BDBM250634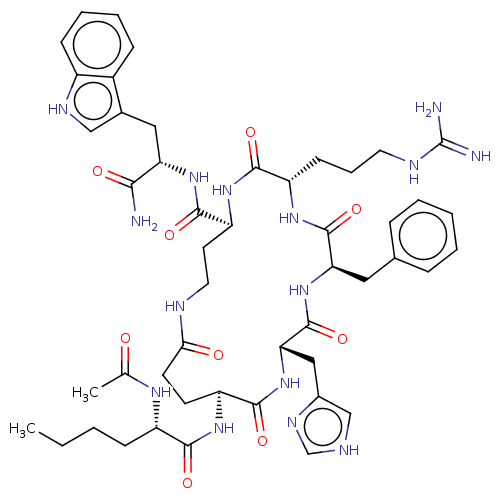 (US9447148, 9.2 | US9447148, 9.31) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.0120 | -64.8 | n/a | n/a | n/a | n/a | n/a | 7.5 | 37 |
Palatin Technologies, Inc. US Patent | Assay Description A competitive inhibition binding assay was performed using membrane homogenates prepared from HEK-293 cells that express recombinant hMCR-1a or hMCR-... | US Patent US9447148 (2016) BindingDB Entry DOI: 10.7270/Q2RJ4HD2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Melatonin receptor type 1B (Homo sapiens (Human)) | BDBM50343602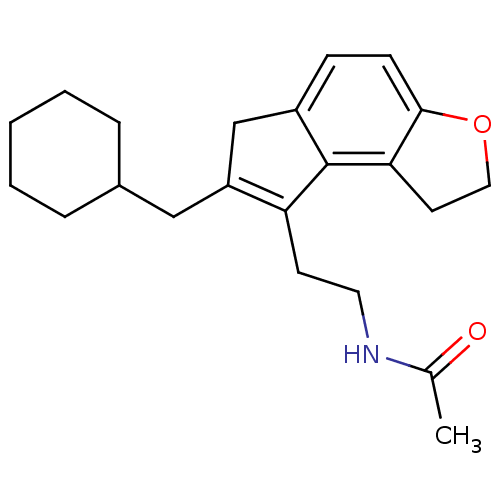 (CHEMBL1774519 | N-{2-[7-(Cyclohexylmethyl)-1,6-dih...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0120 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Limited Curated by ChEMBL | Assay Description Displacement of [125I]-2-iodomelatonin from human MT2 receptor expressed on CHO cells microscintillation counting | J Med Chem 54: 3436-44 (2011) Article DOI: 10.1021/jm200221q BindingDB Entry DOI: 10.7270/Q2028RVN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Melatonin receptor type 1A (Homo sapiens (Human)) | BDBM50343607 (CHEMBL1774514 | N-[2-(7-Isopropyl-1,6-dihydro-2H-i...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0130 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Limited Curated by ChEMBL | Assay Description Displacement of [125I]-2-iodomelatonin from human MT1 receptor expressed on CHO cells by microscintillation counting | J Med Chem 54: 3436-44 (2011) Article DOI: 10.1021/jm200221q BindingDB Entry DOI: 10.7270/Q2028RVN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Melanocyte-stimulating hormone receptor (Homo sapiens (Human)) | BDBM250668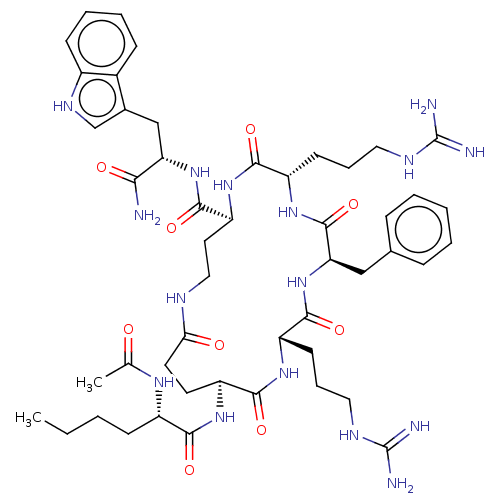 (US9447148, 9.37 | US9447148, 9.50) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.0140 | -64.4 | n/a | n/a | n/a | n/a | n/a | 7.5 | 37 |
Palatin Technologies, Inc. US Patent | Assay Description A competitive inhibition binding assay was performed using membrane homogenates prepared from HEK-293 cells that express recombinant hMCR-1a or hMCR-... | US Patent US9447148 (2016) BindingDB Entry DOI: 10.7270/Q2RJ4HD2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Melanocyte-stimulating hormone receptor (Homo sapiens (Human)) | BDBM250668 (US9447148, 9.37 | US9447148, 9.50) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.0140 | -64.4 | n/a | n/a | n/a | n/a | n/a | 7.5 | 37 |
Palatin Technologies, Inc. US Patent | Assay Description A competitive inhibition binding assay was performed using membrane homogenates prepared from HEK-293 cells that express recombinant hMCR-1a or hMCR-... | US Patent US9447148 (2016) BindingDB Entry DOI: 10.7270/Q2RJ4HD2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Melatonin receptor type 1A (Homo sapiens (Human)) | BDBM50118470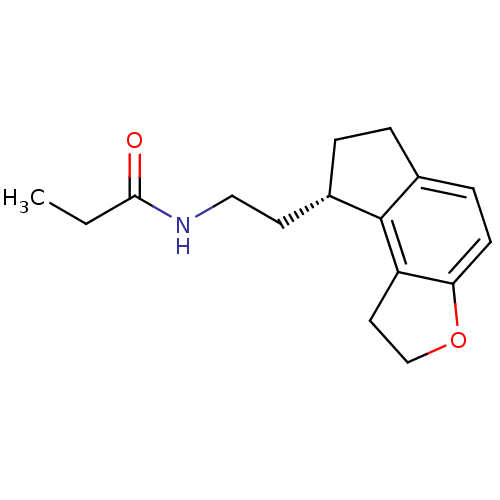 (CHEMBL1218 | N-[2-(1,6,7,8-Tetrahydro-2H-3-oxa-as-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE PC cid PC sid PDB UniChem Patents Similars | DrugBank PDB Article PubMed | 0.0140 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Binding affinity to human MT1 expressed in CHO cells | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c01836 BindingDB Entry DOI: 10.7270/Q25H7M2B | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Melatonin receptor type 1B (Homo sapiens (Human)) | BDBM50343600 (CHEMBL1774521 | N-[2-(7-Bromo-1,6-dihydro-2H-inden...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0140 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Limited Curated by ChEMBL | Assay Description Displacement of [125I]-2-iodomelatonin from human MT2 receptor expressed on CHO cells microscintillation counting | J Med Chem 54: 3436-44 (2011) Article DOI: 10.1021/jm200221q BindingDB Entry DOI: 10.7270/Q2028RVN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Melanocyte-stimulating hormone receptor (Homo sapiens (Human)) | BDBM250673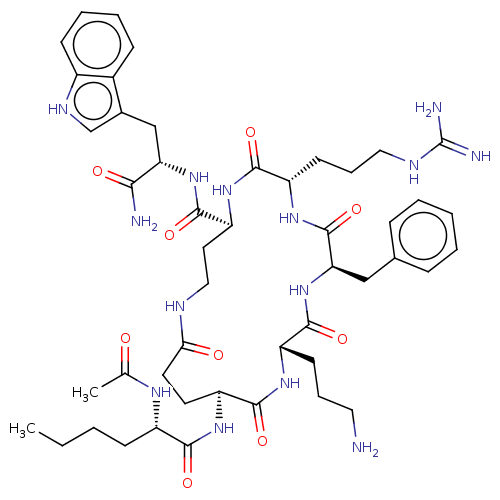 (US9447148, 9.42 | US9447148, 9.55) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.0150 | -64.3 | n/a | n/a | n/a | n/a | n/a | 7.5 | 37 |
Palatin Technologies, Inc. US Patent | Assay Description A competitive inhibition binding assay was performed using membrane homogenates prepared from HEK-293 cells that express recombinant hMCR-1a or hMCR-... | US Patent US9447148 (2016) BindingDB Entry DOI: 10.7270/Q2RJ4HD2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Melanocyte-stimulating hormone receptor (Homo sapiens (Human)) | BDBM250658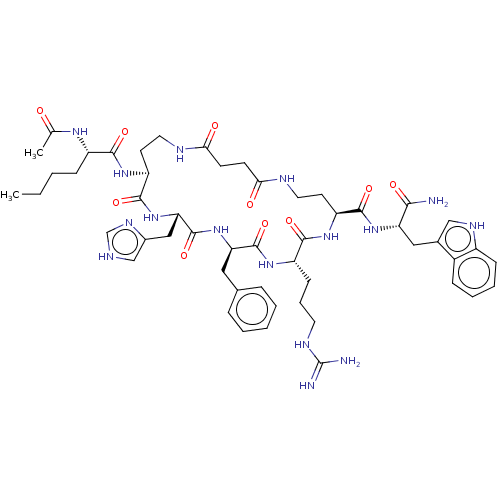 (US9447148, 9.27) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.0150 | -64.3 | n/a | n/a | n/a | n/a | n/a | 7.5 | 37 |
Palatin Technologies, Inc. US Patent | Assay Description A competitive inhibition binding assay was performed using membrane homogenates prepared from HEK-293 cells that express recombinant hMCR-1a or hMCR-... | US Patent US9447148 (2016) BindingDB Entry DOI: 10.7270/Q2RJ4HD2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Melanocyte-stimulating hormone receptor (Homo sapiens (Human)) | BDBM250673 (US9447148, 9.42 | US9447148, 9.55) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.0150 | -64.3 | n/a | n/a | n/a | n/a | n/a | 7.5 | 37 |
Palatin Technologies, Inc. US Patent | Assay Description A competitive inhibition binding assay was performed using membrane homogenates prepared from HEK-293 cells that express recombinant hMCR-1a or hMCR-... | US Patent US9447148 (2016) BindingDB Entry DOI: 10.7270/Q2RJ4HD2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase pim-1 (Homo sapiens (Human)) | BDBM602126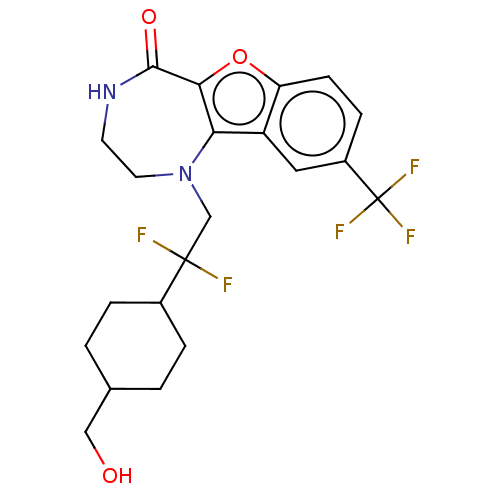 (US11643417, Ex. No. 138) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | 0.0150 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Citation and Details BindingDB Entry DOI: 10.7270/Q2R49VQ0 | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Melanocyte-stimulating hormone receptor (Homo sapiens (Human)) | BDBM250649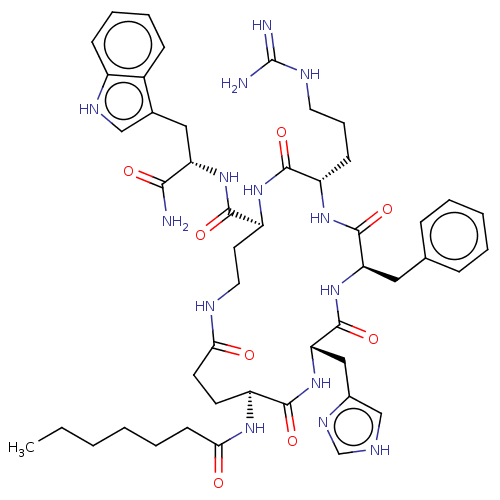 (US9447148, 9.18) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.0150 | -64.3 | n/a | n/a | n/a | n/a | n/a | 7.5 | 37 |
Palatin Technologies, Inc. US Patent | Assay Description A competitive inhibition binding assay was performed using membrane homogenates prepared from HEK-293 cells that express recombinant hMCR-1a or hMCR-... | US Patent US9447148 (2016) BindingDB Entry DOI: 10.7270/Q2RJ4HD2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase pim-1 (Homo sapiens (Human)) | BDBM601978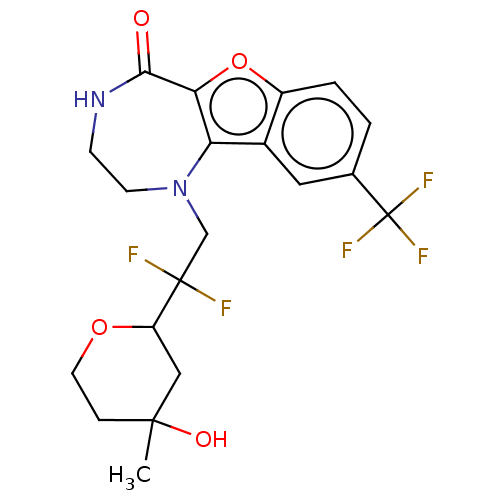 (US11643417, Ex. No. 11 | US11643417, Ex. No. 12 | ...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | 0.0170 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Citation and Details BindingDB Entry DOI: 10.7270/Q2R49VQ0 | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Melatonin receptor type 1B (Homo sapiens (Human)) | BDBM50343608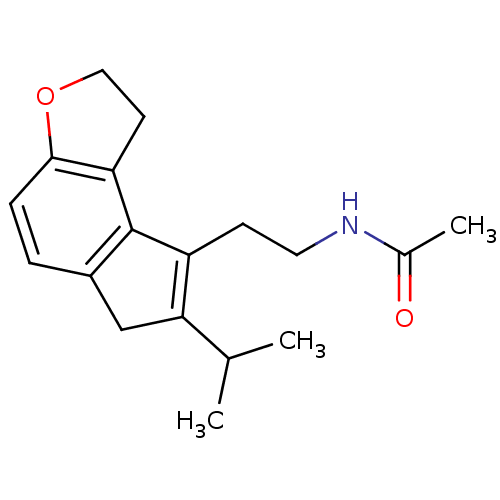 (CHEMBL1774513 | N-[2-(7-Isopropyl-1,6-dihydro-2H-i...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0180 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Limited Curated by ChEMBL | Assay Description Displacement of [125I]-2-iodomelatonin from human MT2 receptor expressed on CHO cells microscintillation counting | J Med Chem 54: 3436-44 (2011) Article DOI: 10.1021/jm200221q BindingDB Entry DOI: 10.7270/Q2028RVN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase pim-1 (Homo sapiens (Human)) | BDBM602169 (US11643417, Ex. No. 164) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | 0.0190 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Citation and Details BindingDB Entry DOI: 10.7270/Q2R49VQ0 | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM50325534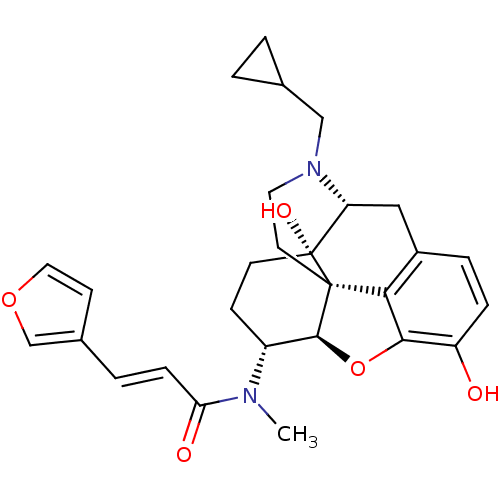 (CHEMBL267495 | nalfurafine) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | 0.0190 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Citation and Details Article DOI: 10.1016/j.bmcl.2022.128527 BindingDB Entry DOI: 10.7270/Q2HT2TDM | ||||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Substance-P receptor (Homo sapiens (Human)) | BDBM50186527 ((R)-3-((1-(3,5-bis(trifluoromethyl)phenyl)ethoxy)m...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.0200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute Curated by ChEMBL | Assay Description Displacement of [3H]Sar-Met substance P from human recombinant NK1 receptor expressed in CHO cells | Bioorg Med Chem Lett 16: 3859-63 (2006) Article DOI: 10.1016/j.bmcl.2006.04.031 BindingDB Entry DOI: 10.7270/Q2QV3M44 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 42418 total ) | Next | Last >> |