 Found 2172 hits with Last Name = 'shi' and Initial = 'yj'
Found 2172 hits with Last Name = 'shi' and Initial = 'yj' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Prostacyclin receptor
(RAT) | BDBM23954

(5-[(2E,3aS,4R,5R,6aS)-5-hydroxy-4-[(1E,3S)-3-hydro...)Show SMILES [H][C@]12C[C@@H](O)[C@H](\C=C\[C@@H](O)C(C)CC#CC)[C@@]1([H])C\C(C2)=C\CCCC(O)=O Show InChI InChI=1S/C22H32O4/c1-3-4-7-15(2)20(23)11-10-18-19-13-16(8-5-6-9-22(25)26)12-17(19)14-21(18)24/h8,10-11,15,17-21,23-24H,5-7,9,12-14H2,1-2H3,(H,25,26)/b11-10+,16-8+/t15?,17-,18+,19-,20+,21+/m0/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Arena Pharmaceuticals
Curated by ChEMBL
| Assay Description
Displacement of [3H]-iloprost from recombinant rat IP receptor expressed in CHO-K1 cell membranes incubated for 1 hr by top count scintillation count... |
J Med Chem 60: 913-927 (2017)
Article DOI: 10.1021/acs.jmedchem.6b00871
BindingDB Entry DOI: 10.7270/Q2VX0JSM |
More data for this
Ligand-Target Pair | |
Prostacyclin receptor
(Homo sapiens (Human)) | BDBM50235385

(APD-811 | Ralinepag)Show SMILES OC(=O)COC[C@H]1CC[C@H](COC(=O)N(c2ccccc2)c2ccc(Cl)cc2)CC1 |r,wU:6.5,wD:9.9,(-11.29,-9.03,;-9.96,-9.8,;-9.96,-11.34,;-8.62,-9.03,;-7.29,-9.8,;-5.96,-9.03,;-4.62,-9.8,;-3.29,-9.03,;-1.96,-9.8,;-1.96,-11.34,;-.62,-12.11,;.71,-11.34,;2.04,-12.11,;2.04,-13.65,;3.38,-11.34,;3.38,-9.8,;2.04,-9.03,;2.04,-7.49,;3.38,-6.72,;4.71,-7.49,;4.71,-9.03,;4.71,-12.11,;6.05,-11.34,;7.38,-12.11,;7.38,-13.65,;8.71,-14.42,;6.05,-14.42,;4.71,-13.65,;-3.29,-12.11,;-4.62,-11.34,)| Show InChI InChI=1S/C23H26ClNO5/c24-19-10-12-21(13-11-19)25(20-4-2-1-3-5-20)23(28)30-15-18-8-6-17(7-9-18)14-29-16-22(26)27/h1-5,10-13,17-18H,6-9,14-16H2,(H,26,27)/t17-,18- | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Arena Pharmaceuticals
Curated by ChEMBL
| Assay Description
Displacement of [3H]-iloprost from recombinant human IP receptor expressed in CHO-K1 cell membranes incubated for 1 hr by top count scintillation cou... |
J Med Chem 60: 913-927 (2017)
Article DOI: 10.1021/acs.jmedchem.6b00871
BindingDB Entry DOI: 10.7270/Q2VX0JSM |
More data for this
Ligand-Target Pair | |
Prostacyclin receptor
(Homo sapiens (Human)) | BDBM23954

(5-[(2E,3aS,4R,5R,6aS)-5-hydroxy-4-[(1E,3S)-3-hydro...)Show SMILES [H][C@]12C[C@@H](O)[C@H](\C=C\[C@@H](O)C(C)CC#CC)[C@@]1([H])C\C(C2)=C\CCCC(O)=O Show InChI InChI=1S/C22H32O4/c1-3-4-7-15(2)20(23)11-10-18-19-13-16(8-5-6-9-22(25)26)12-17(19)14-21(18)24/h8,10-11,15,17-21,23-24H,5-7,9,12-14H2,1-2H3,(H,25,26)/b11-10+,16-8+/t15?,17-,18+,19-,20+,21+/m0/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Arena Pharmaceuticals
Curated by ChEMBL
| Assay Description
Displacement of [3H]-iloprost from recombinant human IP receptor expressed in CHO-K1 cell membranes incubated for 1 hr by top count scintillation cou... |
J Med Chem 60: 913-927 (2017)
Article DOI: 10.1021/acs.jmedchem.6b00871
BindingDB Entry DOI: 10.7270/Q2VX0JSM |
More data for this
Ligand-Target Pair | |
Prostacyclin receptor
(Homo sapiens (Human)) | BDBM50235370
(CHEMBL3933704)Show SMILES OC(=O)COC[C@H]1CC[C@H](COC(=O)N(c2ccccc2)c2cccc(F)c2)CC1 |r,wU:6.5,wD:9.9,(14.03,-33.02,;15.36,-32.25,;15.36,-30.71,;16.69,-33.02,;16.69,-34.56,;18.03,-35.33,;19.36,-34.56,;19.36,-33.02,;20.7,-32.25,;22.03,-33.02,;23.36,-32.25,;24.7,-33.02,;26.03,-32.25,;26.03,-30.71,;27.36,-33.02,;27.36,-34.56,;28.7,-35.33,;28.7,-36.87,;27.36,-37.64,;26.03,-36.87,;26.03,-35.33,;28.7,-32.25,;30.03,-33.02,;31.37,-32.25,;31.37,-30.71,;30.03,-29.94,;30.03,-28.4,;28.7,-30.71,;22.03,-34.56,;20.7,-35.33,)| Show InChI InChI=1S/C23H26FNO5/c24-19-5-4-8-21(13-19)25(20-6-2-1-3-7-20)23(28)30-15-18-11-9-17(10-12-18)14-29-16-22(26)27/h1-8,13,17-18H,9-12,14-16H2,(H,26,27)/t17-,18- | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 7 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Arena Pharmaceuticals
Curated by ChEMBL
| Assay Description
Displacement of [3H]-iloprost from recombinant human IP receptor expressed in CHO-K1 cell membranes incubated for 1 hr by top count scintillation cou... |
J Med Chem 60: 913-927 (2017)
Article DOI: 10.1021/acs.jmedchem.6b00871
BindingDB Entry DOI: 10.7270/Q2VX0JSM |
More data for this
Ligand-Target Pair | |
Cholesterol 24-hydroxylase
(Homo sapiens (Human)) | BDBM50590454
(CHEMBL5195220)Show SMILES OC[C@H]1O[C@@H](Oc2cc(\C=C\C(=O)NCCCNCCCCNC(=O)CCc3ccc(O)c(O)c3)ccc2O)[C@H](O)[C@@H](O)[C@@H]1O |r| | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 13 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2022.114600
BindingDB Entry DOI: 10.7270/Q2DB85V3 |
More data for this
Ligand-Target Pair | |
Hydroxycarboxylic acid receptor 2
(Homo sapiens (Human)) | BDBM23515

(CHEMBL573 | Niacin | Nicotinic Acid | [5, 6-3H]-ni...)Show InChI InChI=1S/C6H5NO2/c8-6(9)5-2-1-3-7-4-5/h1-4H,(H,8,9) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
PDB
Article
PubMed
| 50 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Arena Pharmaceuticals
Curated by ChEMBL
| Assay Description
Displacement of [3H]nicotinic acid from human GPR109a receptor expressed in CHO cells |
J Med Chem 51: 5101-8 (2008)
Article DOI: 10.1021/jm800258p
BindingDB Entry DOI: 10.7270/Q2CF9PWV |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Prostacyclin receptor
(RAT) | BDBM50235370

(CHEMBL3933704)Show SMILES OC(=O)COC[C@H]1CC[C@H](COC(=O)N(c2ccccc2)c2cccc(F)c2)CC1 |r,wU:6.5,wD:9.9,(14.03,-33.02,;15.36,-32.25,;15.36,-30.71,;16.69,-33.02,;16.69,-34.56,;18.03,-35.33,;19.36,-34.56,;19.36,-33.02,;20.7,-32.25,;22.03,-33.02,;23.36,-32.25,;24.7,-33.02,;26.03,-32.25,;26.03,-30.71,;27.36,-33.02,;27.36,-34.56,;28.7,-35.33,;28.7,-36.87,;27.36,-37.64,;26.03,-36.87,;26.03,-35.33,;28.7,-32.25,;30.03,-33.02,;31.37,-32.25,;31.37,-30.71,;30.03,-29.94,;30.03,-28.4,;28.7,-30.71,;22.03,-34.56,;20.7,-35.33,)| Show InChI InChI=1S/C23H26FNO5/c24-19-5-4-8-21(13-19)25(20-6-2-1-3-7-20)23(28)30-15-18-11-9-17(10-12-18)14-29-16-22(26)27/h1-8,13,17-18H,9-12,14-16H2,(H,26,27)/t17-,18- | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 51 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Arena Pharmaceuticals
Curated by ChEMBL
| Assay Description
Displacement of [3H]-iloprost from recombinant rat IP receptor expressed in CHO-K1 cell membranes incubated for 1 hr by top count scintillation count... |
J Med Chem 60: 913-927 (2017)
Article DOI: 10.1021/acs.jmedchem.6b00871
BindingDB Entry DOI: 10.7270/Q2VX0JSM |
More data for this
Ligand-Target Pair | |
Prostacyclin receptor
(RAT) | BDBM50235385

(APD-811 | Ralinepag)Show SMILES OC(=O)COC[C@H]1CC[C@H](COC(=O)N(c2ccccc2)c2ccc(Cl)cc2)CC1 |r,wU:6.5,wD:9.9,(-11.29,-9.03,;-9.96,-9.8,;-9.96,-11.34,;-8.62,-9.03,;-7.29,-9.8,;-5.96,-9.03,;-4.62,-9.8,;-3.29,-9.03,;-1.96,-9.8,;-1.96,-11.34,;-.62,-12.11,;.71,-11.34,;2.04,-12.11,;2.04,-13.65,;3.38,-11.34,;3.38,-9.8,;2.04,-9.03,;2.04,-7.49,;3.38,-6.72,;4.71,-7.49,;4.71,-9.03,;4.71,-12.11,;6.05,-11.34,;7.38,-12.11,;7.38,-13.65,;8.71,-14.42,;6.05,-14.42,;4.71,-13.65,;-3.29,-12.11,;-4.62,-11.34,)| Show InChI InChI=1S/C23H26ClNO5/c24-19-10-12-21(13-11-19)25(20-4-2-1-3-5-20)23(28)30-15-18-8-6-17(7-9-18)14-29-16-22(26)27/h1-5,10-13,17-18H,6-9,14-16H2,(H,26,27)/t17-,18- | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 76 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Arena Pharmaceuticals
Curated by ChEMBL
| Assay Description
Displacement of [3H]-iloprost from recombinant rat IP receptor expressed in CHO-K1 cell membranes incubated for 1 hr by top count scintillation count... |
J Med Chem 60: 913-927 (2017)
Article DOI: 10.1021/acs.jmedchem.6b00871
BindingDB Entry DOI: 10.7270/Q2VX0JSM |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C9
(Homo sapiens (Human)) | BDBM50343442

(CHEMBL1775179 | isopropyl 4-(1-(2-fluoro-4-(methyl...)Show SMILES CC(C)OC(=O)N1CCC(CC1)Oc1ncnc2n(ncc12)-c1ccc(cc1F)S(C)(=O)=O Show InChI InChI=1S/C21H24FN5O5S/c1-13(2)31-21(28)26-8-6-14(7-9-26)32-20-16-11-25-27(19(16)23-12-24-20)18-5-4-15(10-17(18)22)33(3,29)30/h4-5,10-14H,6-9H2,1-3H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Arena Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of CYP2C9 |
Bioorg Med Chem Lett 21: 3134-41 (2011)
Article DOI: 10.1016/j.bmcl.2011.03.007
BindingDB Entry DOI: 10.7270/Q24T6JQF |
More data for this
Ligand-Target Pair | |
Cholesterol 24-hydroxylase
(Homo sapiens (Human)) | BDBM50590453
(CHEMBL5186695)Show SMILES OC[C@H]1O[C@@H](Oc2ccc(\C=C\C(=O)NCCCNCCCCNC(=O)CCc3ccc(O)c(O)c3)cc2O)[C@H](O)[C@@H](O)[C@@H]1O |r| | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 106 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2022.114600
BindingDB Entry DOI: 10.7270/Q2DB85V3 |
More data for this
Ligand-Target Pair | |
Prostaglandin E2 receptor EP3 subtype
(Homo sapiens (Human)) | BDBM50235385

(APD-811 | Ralinepag)Show SMILES OC(=O)COC[C@H]1CC[C@H](COC(=O)N(c2ccccc2)c2ccc(Cl)cc2)CC1 |r,wU:6.5,wD:9.9,(-11.29,-9.03,;-9.96,-9.8,;-9.96,-11.34,;-8.62,-9.03,;-7.29,-9.8,;-5.96,-9.03,;-4.62,-9.8,;-3.29,-9.03,;-1.96,-9.8,;-1.96,-11.34,;-.62,-12.11,;.71,-11.34,;2.04,-12.11,;2.04,-13.65,;3.38,-11.34,;3.38,-9.8,;2.04,-9.03,;2.04,-7.49,;3.38,-6.72,;4.71,-7.49,;4.71,-9.03,;4.71,-12.11,;6.05,-11.34,;7.38,-12.11,;7.38,-13.65,;8.71,-14.42,;6.05,-14.42,;4.71,-13.65,;-3.29,-12.11,;-4.62,-11.34,)| Show InChI InChI=1S/C23H26ClNO5/c24-19-10-12-21(13-11-19)25(20-4-2-1-3-5-20)23(28)30-15-18-8-6-17(7-9-18)14-29-16-22(26)27/h1-5,10-13,17-18H,6-9,14-16H2,(H,26,27)/t17-,18- | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 143 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Arena Pharmaceuticals
Curated by ChEMBL
| Assay Description
Binding affinity to 5-hydroxytryptamine 3 receptor in rat entorhinal cortex using [3H]-BRL 43694 as radioligand |
J Med Chem 60: 913-927 (2017)
Article DOI: 10.1021/acs.jmedchem.6b00871
BindingDB Entry DOI: 10.7270/Q2VX0JSM |
More data for this
Ligand-Target Pair | |
Prostaglandin E2 receptor EP3 subtype
(Homo sapiens (Human)) | BDBM50235370

(CHEMBL3933704)Show SMILES OC(=O)COC[C@H]1CC[C@H](COC(=O)N(c2ccccc2)c2cccc(F)c2)CC1 |r,wU:6.5,wD:9.9,(14.03,-33.02,;15.36,-32.25,;15.36,-30.71,;16.69,-33.02,;16.69,-34.56,;18.03,-35.33,;19.36,-34.56,;19.36,-33.02,;20.7,-32.25,;22.03,-33.02,;23.36,-32.25,;24.7,-33.02,;26.03,-32.25,;26.03,-30.71,;27.36,-33.02,;27.36,-34.56,;28.7,-35.33,;28.7,-36.87,;27.36,-37.64,;26.03,-36.87,;26.03,-35.33,;28.7,-32.25,;30.03,-33.02,;31.37,-32.25,;31.37,-30.71,;30.03,-29.94,;30.03,-28.4,;28.7,-30.71,;22.03,-34.56,;20.7,-35.33,)| Show InChI InChI=1S/C23H26FNO5/c24-19-5-4-8-21(13-19)25(20-6-2-1-3-7-20)23(28)30-15-18-11-9-17(10-12-18)14-29-16-22(26)27/h1-8,13,17-18H,9-12,14-16H2,(H,26,27)/t17-,18- | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 210 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Arena Pharmaceuticals
Curated by ChEMBL
| Assay Description
Binding affinity to 5-hydroxytryptamine 3 receptor in rat entorhinal cortex using [3H]-BRL 43694 as radioligand |
J Med Chem 60: 913-927 (2017)
Article DOI: 10.1021/acs.jmedchem.6b00871
BindingDB Entry DOI: 10.7270/Q2VX0JSM |
More data for this
Ligand-Target Pair | |
Cholesterol 24-hydroxylase
(Homo sapiens (Human)) | BDBM50590452
(CHEMBL5206380)Show SMILES OC[C@H]1O[C@@H](Oc2ccc(CCC(=O)NCCCCNCCCNC(=O)\C=C\c3ccc(O)c(O)c3)cc2O)[C@H](O)[C@@H](O)[C@@H]1O |r| | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 258 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2022.114600
BindingDB Entry DOI: 10.7270/Q2DB85V3 |
More data for this
Ligand-Target Pair | |
Prostacyclin receptor
(Homo sapiens (Human)) | BDBM50235380

(CHEMBL3917503)Show SMILES OS(=O)(=O)CCNC(=O)COC[C@H]1CC[C@H](COC(=O)N(c2ccccc2)c2ccc(Cl)cc2)CC1 |r,wU:15.15,wD:12.11,(32.65,-26.35,;31.1,-26.35,;29.77,-25.58,;31.1,-24.81,;30.34,-27.69,;31.11,-29.02,;30.34,-30.36,;28.8,-30.36,;28.03,-29.02,;28.03,-31.69,;28.8,-33.02,;28.03,-34.36,;26.49,-34.36,;25.72,-33.02,;24.18,-33.02,;23.41,-34.36,;21.87,-34.36,;21.1,-35.69,;19.56,-35.69,;18.79,-34.36,;18.79,-37.02,;19.56,-38.36,;18.79,-39.69,;19.56,-41.02,;21.1,-41.02,;21.87,-39.69,;21.1,-38.36,;17.25,-37.02,;16.48,-35.69,;14.94,-35.69,;14.17,-37.02,;12.63,-37.02,;14.94,-38.36,;16.48,-38.36,;24.18,-35.69,;25.72,-35.69,)| Show InChI InChI=1S/C25H31ClN2O7S/c26-21-10-12-23(13-11-21)28(22-4-2-1-3-5-22)25(30)35-17-20-8-6-19(7-9-20)16-34-18-24(29)27-14-15-36(31,32)33/h1-5,10-13,19-20H,6-9,14-18H2,(H,27,29)(H,31,32,33)/t19-,20- | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Arena Pharmaceuticals
Curated by ChEMBL
| Assay Description
Displacement of [3H]-iloprost from recombinant human IP receptor expressed in CHO-K1 cell membranes incubated for 1 hr by top count scintillation cou... |
J Med Chem 60: 913-927 (2017)
Article DOI: 10.1021/acs.jmedchem.6b00871
BindingDB Entry DOI: 10.7270/Q2VX0JSM |
More data for this
Ligand-Target Pair | |
Hydroxycarboxylic acid receptor 2
(Homo sapiens (Human)) | BDBM50273099
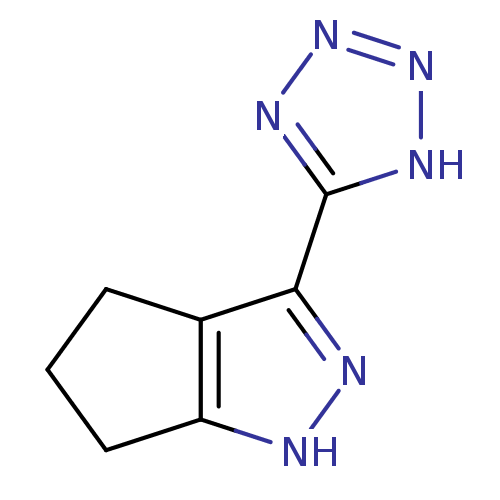
(3-(1H-tetrazol-5-yl)-1,4,5,6-tetrahydrocyclopenta[...)Show InChI InChI=1S/C7H8N6/c1-2-4-5(3-1)8-9-6(4)7-10-12-13-11-7/h1-3H2,(H,8,9)(H,10,11,12,13) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 505 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Arena Pharmaceuticals
Curated by ChEMBL
| Assay Description
Displacement of [3H]nicotinic acid from human GPR109a receptor expressed in CHO cells |
J Med Chem 51: 5101-8 (2008)
Article DOI: 10.1021/jm800258p
BindingDB Entry DOI: 10.7270/Q2CF9PWV |
More data for this
Ligand-Target Pair | |
Prostaglandin E2 receptor EP2 subtype
(Homo sapiens (Human)) | BDBM50235370

(CHEMBL3933704)Show SMILES OC(=O)COC[C@H]1CC[C@H](COC(=O)N(c2ccccc2)c2cccc(F)c2)CC1 |r,wU:6.5,wD:9.9,(14.03,-33.02,;15.36,-32.25,;15.36,-30.71,;16.69,-33.02,;16.69,-34.56,;18.03,-35.33,;19.36,-34.56,;19.36,-33.02,;20.7,-32.25,;22.03,-33.02,;23.36,-32.25,;24.7,-33.02,;26.03,-32.25,;26.03,-30.71,;27.36,-33.02,;27.36,-34.56,;28.7,-35.33,;28.7,-36.87,;27.36,-37.64,;26.03,-36.87,;26.03,-35.33,;28.7,-32.25,;30.03,-33.02,;31.37,-32.25,;31.37,-30.71,;30.03,-29.94,;30.03,-28.4,;28.7,-30.71,;22.03,-34.56,;20.7,-35.33,)| Show InChI InChI=1S/C23H26FNO5/c24-19-5-4-8-21(13-19)25(20-6-2-1-3-7-20)23(28)30-15-18-11-9-17(10-12-18)14-29-16-22(26)27/h1-8,13,17-18H,9-12,14-16H2,(H,26,27)/t17-,18- | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 570 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Arena Pharmaceuticals
Curated by ChEMBL
| Assay Description
Displacement of [3H]-PGE2 from recombinant human EP2 receptor expressed in HEK293 cell membranes incubated for 1 hr |
J Med Chem 60: 913-927 (2017)
Article DOI: 10.1021/acs.jmedchem.6b00871
BindingDB Entry DOI: 10.7270/Q2VX0JSM |
More data for this
Ligand-Target Pair | |
Prostaglandin E2 receptor EP2 subtype
(Homo sapiens (Human)) | BDBM50235385

(APD-811 | Ralinepag)Show SMILES OC(=O)COC[C@H]1CC[C@H](COC(=O)N(c2ccccc2)c2ccc(Cl)cc2)CC1 |r,wU:6.5,wD:9.9,(-11.29,-9.03,;-9.96,-9.8,;-9.96,-11.34,;-8.62,-9.03,;-7.29,-9.8,;-5.96,-9.03,;-4.62,-9.8,;-3.29,-9.03,;-1.96,-9.8,;-1.96,-11.34,;-.62,-12.11,;.71,-11.34,;2.04,-12.11,;2.04,-13.65,;3.38,-11.34,;3.38,-9.8,;2.04,-9.03,;2.04,-7.49,;3.38,-6.72,;4.71,-7.49,;4.71,-9.03,;4.71,-12.11,;6.05,-11.34,;7.38,-12.11,;7.38,-13.65,;8.71,-14.42,;6.05,-14.42,;4.71,-13.65,;-3.29,-12.11,;-4.62,-11.34,)| Show InChI InChI=1S/C23H26ClNO5/c24-19-10-12-21(13-11-19)25(20-4-2-1-3-5-20)23(28)30-15-18-8-6-17(7-9-18)14-29-16-22(26)27/h1-5,10-13,17-18H,6-9,14-16H2,(H,26,27)/t17-,18- | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 610 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Arena Pharmaceuticals
Curated by ChEMBL
| Assay Description
Binding affinity to 5-hydroxytryptamine 3 receptor in rat entorhinal cortex using [3H]-BRL 43694 as radioligand |
J Med Chem 60: 913-927 (2017)
Article DOI: 10.1021/acs.jmedchem.6b00871
BindingDB Entry DOI: 10.7270/Q2VX0JSM |
More data for this
Ligand-Target Pair | |
Prostaglandin E2 receptor EP4 subtype
(Homo sapiens (Human)) | BDBM50235385

(APD-811 | Ralinepag)Show SMILES OC(=O)COC[C@H]1CC[C@H](COC(=O)N(c2ccccc2)c2ccc(Cl)cc2)CC1 |r,wU:6.5,wD:9.9,(-11.29,-9.03,;-9.96,-9.8,;-9.96,-11.34,;-8.62,-9.03,;-7.29,-9.8,;-5.96,-9.03,;-4.62,-9.8,;-3.29,-9.03,;-1.96,-9.8,;-1.96,-11.34,;-.62,-12.11,;.71,-11.34,;2.04,-12.11,;2.04,-13.65,;3.38,-11.34,;3.38,-9.8,;2.04,-9.03,;2.04,-7.49,;3.38,-6.72,;4.71,-7.49,;4.71,-9.03,;4.71,-12.11,;6.05,-11.34,;7.38,-12.11,;7.38,-13.65,;8.71,-14.42,;6.05,-14.42,;4.71,-13.65,;-3.29,-12.11,;-4.62,-11.34,)| Show InChI InChI=1S/C23H26ClNO5/c24-19-10-12-21(13-11-19)25(20-4-2-1-3-5-20)23(28)30-15-18-8-6-17(7-9-18)14-29-16-22(26)27/h1-5,10-13,17-18H,6-9,14-16H2,(H,26,27)/t17-,18- | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 678 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Arena Pharmaceuticals
Curated by ChEMBL
| Assay Description
Binding affinity to 5-hydroxytryptamine 3 receptor in rat entorhinal cortex using [3H]-BRL 43694 as radioligand |
J Med Chem 60: 913-927 (2017)
Article DOI: 10.1021/acs.jmedchem.6b00871
BindingDB Entry DOI: 10.7270/Q2VX0JSM |
More data for this
Ligand-Target Pair | |
Prostaglandin E2 receptor EP4 subtype
(Homo sapiens (Human)) | BDBM50235370

(CHEMBL3933704)Show SMILES OC(=O)COC[C@H]1CC[C@H](COC(=O)N(c2ccccc2)c2cccc(F)c2)CC1 |r,wU:6.5,wD:9.9,(14.03,-33.02,;15.36,-32.25,;15.36,-30.71,;16.69,-33.02,;16.69,-34.56,;18.03,-35.33,;19.36,-34.56,;19.36,-33.02,;20.7,-32.25,;22.03,-33.02,;23.36,-32.25,;24.7,-33.02,;26.03,-32.25,;26.03,-30.71,;27.36,-33.02,;27.36,-34.56,;28.7,-35.33,;28.7,-36.87,;27.36,-37.64,;26.03,-36.87,;26.03,-35.33,;28.7,-32.25,;30.03,-33.02,;31.37,-32.25,;31.37,-30.71,;30.03,-29.94,;30.03,-28.4,;28.7,-30.71,;22.03,-34.56,;20.7,-35.33,)| Show InChI InChI=1S/C23H26FNO5/c24-19-5-4-8-21(13-19)25(20-6-2-1-3-7-20)23(28)30-15-18-11-9-17(10-12-18)14-29-16-22(26)27/h1-8,13,17-18H,9-12,14-16H2,(H,26,27)/t17-,18- | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 1.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Arena Pharmaceuticals
Curated by ChEMBL
| Assay Description
Binding affinity to 5-hydroxytryptamine 3 receptor in rat entorhinal cortex using [3H]-BRL 43694 as radioligand |
J Med Chem 60: 913-927 (2017)
Article DOI: 10.1021/acs.jmedchem.6b00871
BindingDB Entry DOI: 10.7270/Q2VX0JSM |
More data for this
Ligand-Target Pair | |
Prostaglandin D2 receptor
(Homo sapiens (Human)) | BDBM50235370

(CHEMBL3933704)Show SMILES OC(=O)COC[C@H]1CC[C@H](COC(=O)N(c2ccccc2)c2cccc(F)c2)CC1 |r,wU:6.5,wD:9.9,(14.03,-33.02,;15.36,-32.25,;15.36,-30.71,;16.69,-33.02,;16.69,-34.56,;18.03,-35.33,;19.36,-34.56,;19.36,-33.02,;20.7,-32.25,;22.03,-33.02,;23.36,-32.25,;24.7,-33.02,;26.03,-32.25,;26.03,-30.71,;27.36,-33.02,;27.36,-34.56,;28.7,-35.33,;28.7,-36.87,;27.36,-37.64,;26.03,-36.87,;26.03,-35.33,;28.7,-32.25,;30.03,-33.02,;31.37,-32.25,;31.37,-30.71,;30.03,-29.94,;30.03,-28.4,;28.7,-30.71,;22.03,-34.56,;20.7,-35.33,)| Show InChI InChI=1S/C23H26FNO5/c24-19-5-4-8-21(13-19)25(20-6-2-1-3-7-20)23(28)30-15-18-11-9-17(10-12-18)14-29-16-22(26)27/h1-8,13,17-18H,9-12,14-16H2,(H,26,27)/t17-,18- | UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 1.85E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Arena Pharmaceuticals
Curated by ChEMBL
| Assay Description
Displacement of [3H]-PGD2 from recombinant human DP1 receptor |
J Med Chem 60: 913-927 (2017)
Article DOI: 10.1021/acs.jmedchem.6b00871
BindingDB Entry DOI: 10.7270/Q2VX0JSM |
More data for this
Ligand-Target Pair | |
Prostaglandin D2 receptor
(Homo sapiens (Human)) | BDBM50235385

(APD-811 | Ralinepag)Show SMILES OC(=O)COC[C@H]1CC[C@H](COC(=O)N(c2ccccc2)c2ccc(Cl)cc2)CC1 |r,wU:6.5,wD:9.9,(-11.29,-9.03,;-9.96,-9.8,;-9.96,-11.34,;-8.62,-9.03,;-7.29,-9.8,;-5.96,-9.03,;-4.62,-9.8,;-3.29,-9.03,;-1.96,-9.8,;-1.96,-11.34,;-.62,-12.11,;.71,-11.34,;2.04,-12.11,;2.04,-13.65,;3.38,-11.34,;3.38,-9.8,;2.04,-9.03,;2.04,-7.49,;3.38,-6.72,;4.71,-7.49,;4.71,-9.03,;4.71,-12.11,;6.05,-11.34,;7.38,-12.11,;7.38,-13.65,;8.71,-14.42,;6.05,-14.42,;4.71,-13.65,;-3.29,-12.11,;-4.62,-11.34,)| Show InChI InChI=1S/C23H26ClNO5/c24-19-10-12-21(13-11-19)25(20-4-2-1-3-5-20)23(28)30-15-18-8-6-17(7-9-18)14-29-16-22(26)27/h1-5,10-13,17-18H,6-9,14-16H2,(H,26,27)/t17-,18- | UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Arena Pharmaceuticals
Curated by ChEMBL
| Assay Description
Displacement of [3H]-PGD2 from recombinant human DP1 receptor |
J Med Chem 60: 913-927 (2017)
Article DOI: 10.1021/acs.jmedchem.6b00871
BindingDB Entry DOI: 10.7270/Q2VX0JSM |
More data for this
Ligand-Target Pair | |
Prostaglandin E2 receptor EP3 subtype
(Homo sapiens (Human)) | BDBM23954

(5-[(2E,3aS,4R,5R,6aS)-5-hydroxy-4-[(1E,3S)-3-hydro...)Show SMILES [H][C@]12C[C@@H](O)[C@H](\C=C\[C@@H](O)C(C)CC#CC)[C@@]1([H])C\C(C2)=C\CCCC(O)=O Show InChI InChI=1S/C22H32O4/c1-3-4-7-15(2)20(23)11-10-18-19-13-16(8-5-6-9-22(25)26)12-17(19)14-21(18)24/h8,10-11,15,17-21,23-24H,5-7,9,12-14H2,1-2H3,(H,25,26)/b11-10+,16-8+/t15?,17-,18+,19-,20+,21+/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Arena Pharmaceuticals
Curated by ChEMBL
| Assay Description
Binding affinity to 5-hydroxytryptamine 3 receptor in rat entorhinal cortex using [3H]-BRL 43694 as radioligand |
J Med Chem 60: 913-927 (2017)
Article DOI: 10.1021/acs.jmedchem.6b00871
BindingDB Entry DOI: 10.7270/Q2VX0JSM |
More data for this
Ligand-Target Pair | |
Prostaglandin E2 receptor EP1 subtype
(Homo sapiens (Human)) | BDBM50235385

(APD-811 | Ralinepag)Show SMILES OC(=O)COC[C@H]1CC[C@H](COC(=O)N(c2ccccc2)c2ccc(Cl)cc2)CC1 |r,wU:6.5,wD:9.9,(-11.29,-9.03,;-9.96,-9.8,;-9.96,-11.34,;-8.62,-9.03,;-7.29,-9.8,;-5.96,-9.03,;-4.62,-9.8,;-3.29,-9.03,;-1.96,-9.8,;-1.96,-11.34,;-.62,-12.11,;.71,-11.34,;2.04,-12.11,;2.04,-13.65,;3.38,-11.34,;3.38,-9.8,;2.04,-9.03,;2.04,-7.49,;3.38,-6.72,;4.71,-7.49,;4.71,-9.03,;4.71,-12.11,;6.05,-11.34,;7.38,-12.11,;7.38,-13.65,;8.71,-14.42,;6.05,-14.42,;4.71,-13.65,;-3.29,-12.11,;-4.62,-11.34,)| Show InChI InChI=1S/C23H26ClNO5/c24-19-10-12-21(13-11-19)25(20-4-2-1-3-5-20)23(28)30-15-18-8-6-17(7-9-18)14-29-16-22(26)27/h1-5,10-13,17-18H,6-9,14-16H2,(H,26,27)/t17-,18- | KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 9.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Arena Pharmaceuticals
Curated by ChEMBL
| Assay Description
Binding affinity to 5-hydroxytryptamine 3 receptor in rat entorhinal cortex using [3H]-BRL 43694 as radioligand |
J Med Chem 60: 913-927 (2017)
Article DOI: 10.1021/acs.jmedchem.6b00871
BindingDB Entry DOI: 10.7270/Q2VX0JSM |
More data for this
Ligand-Target Pair | |
11-beta-hydroxysteroid dehydrogenase 1
(Homo sapiens (Human)) | BDBM250432
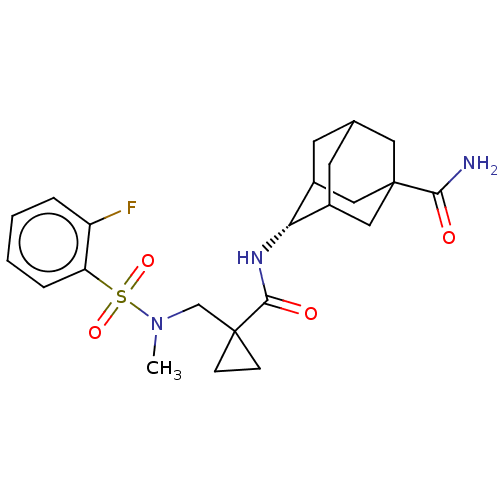
(US9464044, 22)Show SMILES CN(CC1(CC1)C(=O)N[C@H]1C2CC3CC1CC(C3)(C2)C(N)=O)S(=O)(=O)c1ccccc1F |r,wU:9.9,TLB:8:9:13:16.17.15,18:16:13:9.10.11,19:16:9:13.12.11,THB:18:10:13:16.17.15,17:16:9:13.12.11,17:12:9:16.18.15,(-3.29,2.5,;-3.29,.96,;-1.95,.19,;-.62,.96,;.15,2.3,;-1.39,2.3,;.72,.19,;.72,-1.35,;2.05,.96,;3.38,.19,;4.72,.96,;3.96,-.2,;3.94,-2.6,;2.55,-3.25,;3.38,-1.35,;4.72,-2.12,;6.04,-1.35,;5.15,-3.27,;6.04,.19,;7.53,-1.75,;8.62,-.66,;7.93,-3.23,;-4.62,.19,;-5.39,-1.14,;-3.85,-1.14,;-5.95,.96,;-5.95,2.5,;-7.29,3.27,;-8.62,2.5,;-8.62,.96,;-7.29,.19,;-7.29,-1.35,)| Show InChI InChI=1S/C23H30FN3O4S/c1-27(32(30,31)18-5-3-2-4-17(18)24)13-22(6-7-22)21(29)26-19-15-8-14-9-16(19)12-23(10-14,11-15)20(25)28/h2-5,14-16,19H,6-13H2,1H3,(H2,25,28)(H,26,29)/t14?,15?,16?,19-,23? | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 0.0200 | n/a | n/a | n/a | n/a | 6.0 | 25 |
AHN-GOOK PHARMACEUTICAL CO., LTD.; BAMICHEM CO., LTD; INCHEON UNIVERSITY INDUSTRY ACADEMIC COOPERATION FOUNDATION
US Patent
| Assay Description
The inhibiting activity of 11β-HSD1 derived from microsomal fractions was measured using the HTRF assay (62CO2PEB, Cisbio). Different concentrat... |
US Patent US9464044 (2016)
BindingDB Entry DOI: 10.7270/Q2SJ1JH0 |
More data for this
Ligand-Target Pair | |
11-beta-hydroxysteroid dehydrogenase 1
(Homo sapiens (Human)) | BDBM50112149
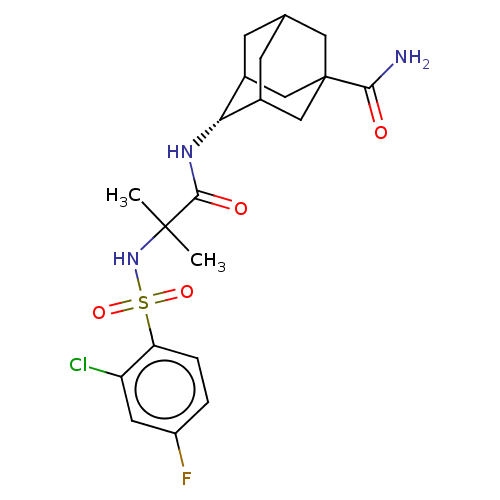
(CHEMBL3609877)Show SMILES CC(C)(NS(=O)(=O)c1ccc(F)cc1Cl)C(=O)N[C@H]1C2CC3CC1CC(C3)(C2)C(N)=O |r,wD:18.18,TLB:22:23:27:26.20.21,22:21:27:18.23.24,18:19:26:23.22.24,THB:17:18:27:26.20.21,18:23:26:27.20.19,(-7,-2.31,;-6.03,-1.55,;-5.85,-2.77,;-7.46,-.97,;-8.67,-1.93,;-7.53,-2.38,;-8.49,-3.15,;-10.1,-1.35,;-10.32,.17,;-11.75,.74,;-12.96,-.21,;-14.11,.25,;-12.74,-1.74,;-11.31,-2.31,;-11.14,-3.53,;-4.82,-.59,;-4.99,.63,;-3.39,-1.17,;-2.19,-.22,;-.95,.32,;-1,2.05,;.12,3.07,;-1.2,2.69,;-1.2,1.02,;.34,.44,;1.56,1.02,;1.56,2.59,;.56,-.22,;3.07,1.15,;3.78,.15,;3.59,2.27,)| Show InChI InChI=1S/C21H27ClFN3O4S/c1-20(2,26-31(29,30)16-4-3-14(23)7-15(16)22)19(28)25-17-12-5-11-6-13(17)10-21(8-11,9-12)18(24)27/h3-4,7,11-13,17,26H,5-6,8-10H2,1-2H3,(H2,24,27)(H,25,28)/t11?,12?,13?,17-,21? | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a |
Incheon National University
Curated by ChEMBL
| Assay Description
Inhibition of human 11beta-HSD1 |
Bioorg Med Chem Lett 25: 3501-6 (2015)
Article DOI: 10.1016/j.bmcl.2015.06.099
BindingDB Entry DOI: 10.7270/Q2BP04KW |
More data for this
Ligand-Target Pair | |
11-beta-hydroxysteroid dehydrogenase 1
(Homo sapiens (Human)) | BDBM250460
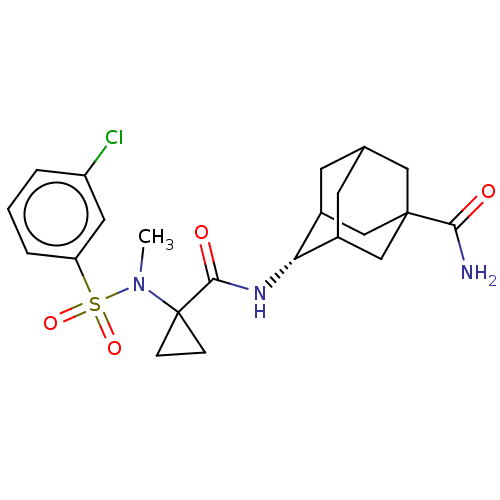
(US9464044, 62)Show SMILES CN(C1(CC1)C(=O)N[C@H]1C2CC3CC1CC(C3)(C2)C(N)=O)S(=O)(=O)c1cccc(Cl)c1 |r,wU:8.8,TLB:18:15:8:12.11.10,7:8:12:15.16.14,17:15:12:8.9.10,THB:16:15:8:12.11.10,16:11:8:15.17.14,17:9:12:15.16.14,(-1.95,-1.73,;-1.95,-.19,;-.62,.58,;.15,1.91,;-1.39,1.91,;.72,-.19,;.72,-1.73,;2.05,.58,;3.38,-.19,;4.72,.58,;3.96,-.59,;3.94,-2.99,;2.55,-3.63,;3.38,-1.73,;4.72,-2.5,;6.04,-1.73,;5.15,-3.66,;6.04,-.19,;7.53,-2.13,;8.62,-1.04,;7.93,-3.62,;-3.29,.58,;-2.52,1.91,;-4.06,-.76,;-4.62,1.35,;-4.62,2.89,;-5.95,3.66,;-7.29,2.89,;-7.29,1.35,;-8.62,.58,;-5.95,.58,)| Show InChI InChI=1S/C22H28ClN3O4S/c1-26(31(29,30)17-4-2-3-16(23)9-17)22(5-6-22)20(28)25-18-14-7-13-8-15(18)12-21(10-13,11-14)19(24)27/h2-4,9,13-15,18H,5-8,10-12H2,1H3,(H2,24,27)(H,25,28)/t13?,14?,15?,18-,21? | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 0.120 | n/a | n/a | n/a | n/a | 6.0 | 25 |
AHN-GOOK PHARMACEUTICAL CO., LTD.; BAMICHEM CO., LTD; INCHEON UNIVERSITY INDUSTRY ACADEMIC COOPERATION FOUNDATION
US Patent
| Assay Description
The inhibiting activity of 11β-HSD1 derived from microsomal fractions was measured using the HTRF assay (62CO2PEB, Cisbio). Different concentrat... |
US Patent US9464044 (2016)
BindingDB Entry DOI: 10.7270/Q2SJ1JH0 |
More data for this
Ligand-Target Pair | |
11-beta-hydroxysteroid dehydrogenase 1
(Homo sapiens (Human)) | BDBM50112154
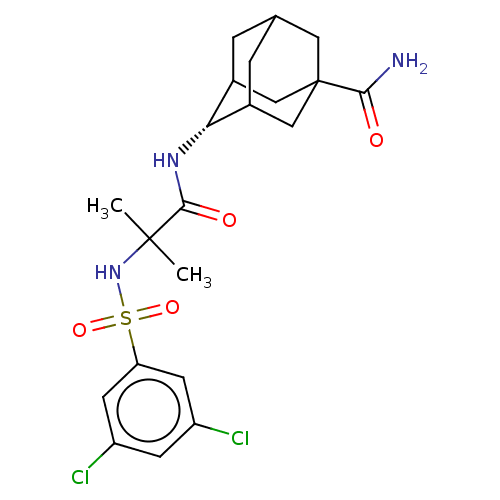
(CHEMBL3608403)Show SMILES CC(C)(NS(=O)(=O)c1cc(Cl)cc(Cl)c1)C(=O)N[C@H]1C2CC3CC1CC(C3)(C2)C(N)=O |r,wD:18.18,TLB:22:23:27:26.20.21,22:21:27:18.23.24,18:19:26:23.22.24,THB:17:18:27:26.20.21,18:23:26:27.20.19,(-7,-2.31,;-6.03,-1.55,;-5.85,-2.77,;-7.46,-.97,;-8.67,-1.93,;-7.53,-2.38,;-8.49,-3.15,;-10.1,-1.35,;-10.32,.17,;-11.75,.74,;-11.93,1.96,;-12.96,-.21,;-12.74,-1.74,;-13.71,-2.5,;-11.31,-2.31,;-4.82,-.59,;-4.99,.63,;-3.39,-1.17,;-2.19,-.22,;-.95,.32,;-1,2.05,;.12,3.07,;-1.2,2.69,;-1.2,1.02,;.34,.44,;1.56,1.02,;1.56,2.59,;.56,-.22,;3.07,1.15,;3.78,.15,;3.59,2.27,)| Show InChI InChI=1S/C21H27Cl2N3O4S/c1-20(2,26-31(29,30)16-6-14(22)5-15(23)7-16)19(28)25-17-12-3-11-4-13(17)10-21(8-11,9-12)18(24)27/h5-7,11-13,17,26H,3-4,8-10H2,1-2H3,(H2,24,27)(H,25,28)/t11?,12?,13?,17-,21? | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
Incheon National University
Curated by ChEMBL
| Assay Description
Inhibition of human 11beta-HSD1 |
Bioorg Med Chem Lett 25: 3501-6 (2015)
Article DOI: 10.1016/j.bmcl.2015.06.099
BindingDB Entry DOI: 10.7270/Q2BP04KW |
More data for this
Ligand-Target Pair | |
11-beta-hydroxysteroid dehydrogenase 1
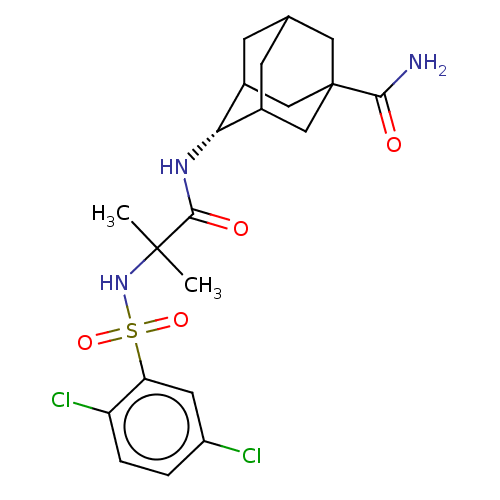
(Homo sapiens (Human)) | BDBM50112151
(CHEMBL3608400)Show SMILES CC(C)(NS(=O)(=O)c1cc(Cl)ccc1Cl)C(=O)N[C@H]1C2CC3CC1CC(C3)(C2)C(N)=O |r,wD:18.18,TLB:22:23:27:26.20.21,22:21:27:18.23.24,18:19:26:23.22.24,THB:17:18:27:26.20.21,18:23:26:27.20.19,(-7,-2.31,;-6.03,-1.55,;-5.85,-2.77,;-7.46,-.97,;-8.67,-1.93,;-7.53,-2.38,;-8.49,-3.15,;-10.1,-1.35,;-10.32,.17,;-11.75,.74,;-11.93,1.96,;-12.96,-.21,;-12.74,-1.74,;-11.31,-2.31,;-11.14,-3.53,;-4.82,-.59,;-4.99,.63,;-3.39,-1.17,;-2.19,-.22,;-.95,.32,;-1,2.05,;.12,3.07,;-1.2,2.69,;-1.2,1.02,;.34,.44,;1.56,1.02,;1.56,2.59,;.56,-.22,;3.07,1.15,;3.78,.15,;3.59,2.27,)| Show InChI InChI=1S/C21H27Cl2N3O4S/c1-20(2,26-31(29,30)16-7-14(22)3-4-15(16)23)19(28)25-17-12-5-11-6-13(17)10-21(8-11,9-12)18(24)27/h3-4,7,11-13,17,26H,5-6,8-10H2,1-2H3,(H2,24,27)(H,25,28)/t11?,12?,13?,17-,21? | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
Incheon National University
Curated by ChEMBL
| Assay Description
Inhibition of human 11beta-HSD1 |
Bioorg Med Chem Lett 25: 3501-6 (2015)
Article DOI: 10.1016/j.bmcl.2015.06.099
BindingDB Entry DOI: 10.7270/Q2BP04KW |
More data for this
Ligand-Target Pair | |
11-beta-hydroxysteroid dehydrogenase 1
(Homo sapiens (Human)) | BDBM250418
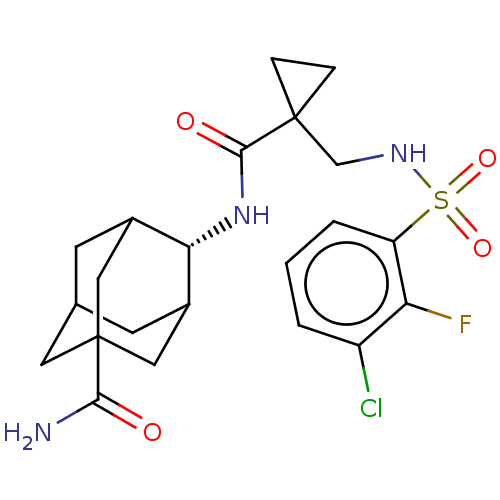
(US9464044, 8)Show SMILES NC(=O)C12CC3CC(C1)[C@H](NC(=O)C1(CNS(=O)(=O)c4cccc(Cl)c4F)CC1)C(C3)C2 |r,wU:9.10,TLB:10:9:30:3.4.31,8:3:30:9.7.6,1:3:9:30.5.6,THB:8:7:30:3.4.31,4:3:9:30.5.6,4:5:9:3.8.31,(9.29,-.66,;8.2,-1.75,;8.6,-3.23,;6.71,-1.35,;5.81,-3.27,;4.61,-2.6,;4.63,-.2,;5.38,.96,;6.71,.19,;4.05,.19,;2.72,.96,;1.38,.19,;1.38,-1.35,;.05,.96,;-1.29,.19,;-2.62,.96,;-3.95,.19,;-4.72,-1.14,;-3.18,-1.14,;-5.29,.96,;-5.29,2.5,;-6.62,3.27,;-7.95,2.5,;-7.95,.96,;-9.29,.19,;-6.62,.19,;-6.62,-1.35,;.82,2.3,;-.72,2.3,;4.05,-1.35,;3.22,-3.25,;5.38,-2.12,)| Show InChI InChI=1S/C22H27ClFN3O4S/c23-15-2-1-3-16(17(15)24)32(30,31)26-11-21(4-5-21)20(29)27-18-13-6-12-7-14(18)10-22(8-12,9-13)19(25)28/h1-3,12-14,18,26H,4-11H2,(H2,25,28)(H,27,29)/t12?,13?,14?,18-,22? | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | 6.0 | 25 |
AHN-GOOK PHARMACEUTICAL CO., LTD.; BAMICHEM CO., LTD; INCHEON UNIVERSITY INDUSTRY ACADEMIC COOPERATION FOUNDATION
US Patent
| Assay Description
The inhibiting activity of 11β-HSD1 derived from microsomal fractions was measured using the HTRF assay (62CO2PEB, Cisbio). Different concentrat... |
US Patent US9464044 (2016)
BindingDB Entry DOI: 10.7270/Q2SJ1JH0 |
More data for this
Ligand-Target Pair | |
11-beta-hydroxysteroid dehydrogenase 1
(Homo sapiens (Human)) | BDBM50112152
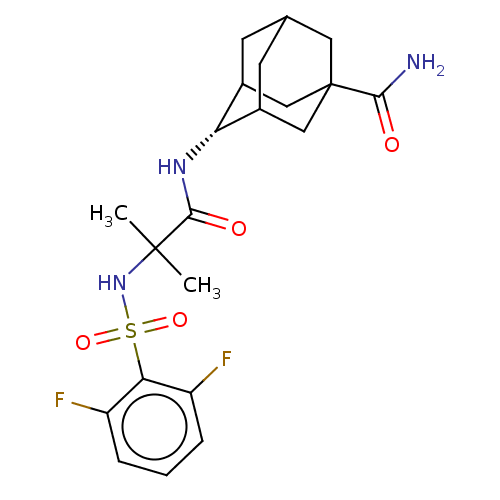
(CHEMBL3608401 | US9464044, 84)Show SMILES CC(C)(NS(=O)(=O)c1c(F)cccc1F)C(=O)N[C@H]1C2CC3CC1CC(C3)(C2)C(N)=O |r,wD:18.18,TLB:22:23:27:26.20.21,22:21:27:18.23.24,18:19:26:23.22.24,THB:17:18:27:26.20.21,18:23:26:27.20.19,(-7,-2.31,;-6.03,-1.55,;-5.85,-2.77,;-7.46,-.97,;-8.67,-1.93,;-7.53,-2.38,;-8.49,-3.15,;-10.1,-1.35,;-11.31,-2.31,;-11.14,-3.53,;-12.74,-1.74,;-12.96,-.21,;-11.75,.74,;-10.32,.17,;-9.35,.93,;-4.82,-.59,;-4.99,.63,;-3.39,-1.17,;-2.19,-.22,;-.95,.32,;-1,2.05,;.12,3.07,;-1.2,2.69,;-1.2,1.02,;.34,.44,;1.56,1.02,;1.56,2.59,;.56,-.22,;3.07,1.15,;3.78,.15,;3.59,2.27,)| Show InChI InChI=1S/C21H27F2N3O4S/c1-20(2,26-31(29,30)17-14(22)4-3-5-15(17)23)19(28)25-16-12-6-11-7-13(16)10-21(8-11,9-12)18(24)27/h3-5,11-13,16,26H,6-10H2,1-2H3,(H2,24,27)(H,25,28)/t11?,12?,13?,16-,21? | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 0.360 | n/a | n/a | n/a | n/a | 6.0 | 25 |
AHN-GOOK PHARMACEUTICAL CO., LTD.; BAMICHEM CO., LTD; INCHEON UNIVERSITY INDUSTRY ACADEMIC COOPERATION FOUNDATION
US Patent
| Assay Description
The inhibiting activity of 11β-HSD1 derived from microsomal fractions was measured using the HTRF assay (62CO2PEB, Cisbio). Different concentrat... |
US Patent US9464044 (2016)
BindingDB Entry DOI: 10.7270/Q2SJ1JH0 |
More data for this
Ligand-Target Pair | |
11-beta-hydroxysteroid dehydrogenase 1
(Homo sapiens (Human)) | BDBM250453
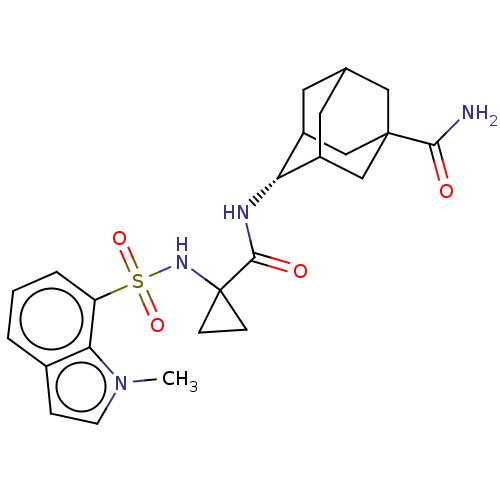
(US9464044, 50)Show SMILES Cn1ccc2cccc(c12)S(=O)(=O)NC1(CC1)C(=O)N[C@H]1C2CC3CC1CC(C3)(C2)C(N)=O |r,wU:20.22,TLB:29:27:24:20.21.22,30:27:20:24.23.22,19:20:24:27.28.26,THB:29:21:24:27.28.26,28:27:20:24.23.22,28:23:20:27.29.26,(-5.28,-2.02,;-6.37,-.93,;-7.9,-1.09,;-8.53,.32,;-7.38,1.35,;-7.38,2.89,;-6.05,3.66,;-4.71,2.89,;-4.71,1.35,;-6.05,.58,;-3.38,.58,;-2.61,1.91,;-4.15,-.76,;-2.05,-.19,;-.71,.58,;.06,1.91,;-1.48,1.91,;.62,-.19,;.62,-1.73,;1.95,.58,;3.29,-.19,;4.62,.58,;3.87,-.59,;3.85,-2.99,;2.45,-3.63,;3.29,-1.73,;4.62,-2.5,;5.95,-1.73,;5.05,-3.66,;5.95,-.19,;7.44,-2.13,;8.53,-1.04,;7.84,-3.62,)| Show InChI InChI=1S/C24H30N4O4S/c1-28-8-5-15-3-2-4-18(20(15)28)33(31,32)27-24(6-7-24)22(30)26-19-16-9-14-10-17(19)13-23(11-14,12-16)21(25)29/h2-5,8,14,16-17,19,27H,6-7,9-13H2,1H3,(H2,25,29)(H,26,30)/t14?,16?,17?,19-,23? | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | 6.0 | 25 |
AHN-GOOK PHARMACEUTICAL CO., LTD.; BAMICHEM CO., LTD; INCHEON UNIVERSITY INDUSTRY ACADEMIC COOPERATION FOUNDATION
US Patent
| Assay Description
The inhibiting activity of 11β-HSD1 derived from microsomal fractions was measured using the HTRF assay (62CO2PEB, Cisbio). Different concentrat... |
US Patent US9464044 (2016)
BindingDB Entry DOI: 10.7270/Q2SJ1JH0 |
More data for this
Ligand-Target Pair | |
11-beta-hydroxysteroid dehydrogenase 1
(Homo sapiens (Human)) | BDBM50112152

(CHEMBL3608401 | US9464044, 84)Show SMILES CC(C)(NS(=O)(=O)c1c(F)cccc1F)C(=O)N[C@H]1C2CC3CC1CC(C3)(C2)C(N)=O |r,wD:18.18,TLB:22:23:27:26.20.21,22:21:27:18.23.24,18:19:26:23.22.24,THB:17:18:27:26.20.21,18:23:26:27.20.19,(-7,-2.31,;-6.03,-1.55,;-5.85,-2.77,;-7.46,-.97,;-8.67,-1.93,;-7.53,-2.38,;-8.49,-3.15,;-10.1,-1.35,;-11.31,-2.31,;-11.14,-3.53,;-12.74,-1.74,;-12.96,-.21,;-11.75,.74,;-10.32,.17,;-9.35,.93,;-4.82,-.59,;-4.99,.63,;-3.39,-1.17,;-2.19,-.22,;-.95,.32,;-1,2.05,;.12,3.07,;-1.2,2.69,;-1.2,1.02,;.34,.44,;1.56,1.02,;1.56,2.59,;.56,-.22,;3.07,1.15,;3.78,.15,;3.59,2.27,)| Show InChI InChI=1S/C21H27F2N3O4S/c1-20(2,26-31(29,30)17-14(22)4-3-5-15(17)23)19(28)25-16-12-6-11-7-13(16)10-21(8-11,9-12)18(24)27/h3-5,11-13,16,26H,6-10H2,1-2H3,(H2,24,27)(H,25,28)/t11?,12?,13?,16-,21? | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
Incheon National University
Curated by ChEMBL
| Assay Description
Inhibition of human 11beta-HSD1 |
Bioorg Med Chem Lett 25: 3501-6 (2015)
Article DOI: 10.1016/j.bmcl.2015.06.099
BindingDB Entry DOI: 10.7270/Q2BP04KW |
More data for this
Ligand-Target Pair | |
Lymphokine-activated killer T-cell-originated protein kinase
(Homo sapiens (Human)) | BDBM50520955

(CHEMBL4445123)Show SMILES C[C@@H](CN)c1ccc(cc1)-c1c(O)cc(C)c2[nH]c(=O)c3ccccc3c12 |r| Show InChI InChI=1S/C23H22N2O2/c1-13-11-19(26)20(16-9-7-15(8-10-16)14(2)12-24)21-17-5-3-4-6-18(17)23(27)25-22(13)21/h3-11,14,26H,12,24H2,1-2H3,(H,25,27)/t14-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Sichuan University
Curated by ChEMBL
| Assay Description
Inhibition of human TOPK using MBP as substrate after 2 hrs in presence of [gamma-33P]-ATP by filter-binding method |
Eur J Med Chem 162: 407-422 (2019)
Article DOI: 10.1016/j.ejmech.2018.11.007
BindingDB Entry DOI: 10.7270/Q28K7DGV |
More data for this
Ligand-Target Pair | |
11-beta-hydroxysteroid dehydrogenase 1
(Homo sapiens (Human)) | BDBM50112133
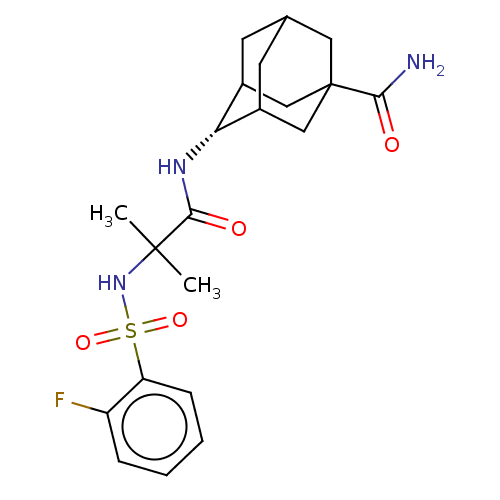
(CHEMBL3609861 | US9464044, 71)Show SMILES CC(C)(NS(=O)(=O)c1ccccc1F)C(=O)N[C@H]1C2CC3CC1CC(C3)(C2)C(N)=O |r,wD:17.17,TLB:16:17:21:23.25.24,26:24:21:19.18.17,THB:27:24:21:19.18.17,27:24:21.20.19:17,25:20:17:23.24.26,25:24:21.20.19:17,26:18:21:23.25.24,(-5.85,-2.77,;-6.03,-1.55,;-7,-2.31,;-7.46,-.97,;-8.67,-1.93,;-8.49,-3.15,;-7.53,-2.38,;-10.1,-1.35,;-11.31,-2.31,;-12.74,-1.74,;-12.96,-.21,;-11.75,.74,;-10.32,.17,;-9.35,.93,;-4.82,-.59,;-4.99,.63,;-3.39,-1.17,;-2.19,-.22,;-.95,.32,;-1,2.05,;.12,3.07,;-1.2,2.69,;-1.2,1.02,;.34,.44,;1.56,1.02,;1.56,2.59,;.56,-.22,;3.07,1.17,;3.58,2.29,;3.79,.17,)| Show InChI InChI=1S/C21H28FN3O4S/c1-20(2,25-30(28,29)16-6-4-3-5-15(16)22)19(27)24-17-13-7-12-8-14(17)11-21(9-12,10-13)18(23)26/h3-6,12-14,17,25H,7-11H2,1-2H3,(H2,23,26)(H,24,27)/t12?,13?,14?,17-,21? | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a |
Incheon National University
Curated by ChEMBL
| Assay Description
Inhibition of human 11beta-HSD1 |
Bioorg Med Chem Lett 25: 3501-6 (2015)
Article DOI: 10.1016/j.bmcl.2015.06.099
BindingDB Entry DOI: 10.7270/Q2BP04KW |
More data for this
Ligand-Target Pair | |
11-beta-hydroxysteroid dehydrogenase 1
(Homo sapiens (Human)) | BDBM50112133

(CHEMBL3609861 | US9464044, 71)Show SMILES CC(C)(NS(=O)(=O)c1ccccc1F)C(=O)N[C@H]1C2CC3CC1CC(C3)(C2)C(N)=O |r,wD:17.17,TLB:16:17:21:23.25.24,26:24:21:19.18.17,THB:27:24:21:19.18.17,27:24:21.20.19:17,25:20:17:23.24.26,25:24:21.20.19:17,26:18:21:23.25.24,(-5.85,-2.77,;-6.03,-1.55,;-7,-2.31,;-7.46,-.97,;-8.67,-1.93,;-8.49,-3.15,;-7.53,-2.38,;-10.1,-1.35,;-11.31,-2.31,;-12.74,-1.74,;-12.96,-.21,;-11.75,.74,;-10.32,.17,;-9.35,.93,;-4.82,-.59,;-4.99,.63,;-3.39,-1.17,;-2.19,-.22,;-.95,.32,;-1,2.05,;.12,3.07,;-1.2,2.69,;-1.2,1.02,;.34,.44,;1.56,1.02,;1.56,2.59,;.56,-.22,;3.07,1.17,;3.58,2.29,;3.79,.17,)| Show InChI InChI=1S/C21H28FN3O4S/c1-20(2,25-30(28,29)16-6-4-3-5-15(16)22)19(27)24-17-13-7-12-8-14(17)11-21(9-12,10-13)18(23)26/h3-6,12-14,17,25H,7-11H2,1-2H3,(H2,23,26)(H,24,27)/t12?,13?,14?,17-,21? | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | 6.0 | 25 |
AHN-GOOK PHARMACEUTICAL CO., LTD.; BAMICHEM CO., LTD; INCHEON UNIVERSITY INDUSTRY ACADEMIC COOPERATION FOUNDATION
US Patent
| Assay Description
The inhibiting activity of 11β-HSD1 derived from microsomal fractions was measured using the HTRF assay (62CO2PEB, Cisbio). Different concentrat... |
US Patent US9464044 (2016)
BindingDB Entry DOI: 10.7270/Q2SJ1JH0 |
More data for this
Ligand-Target Pair | |
11-beta-hydroxysteroid dehydrogenase 1
(Homo sapiens (Human)) | BDBM50112143
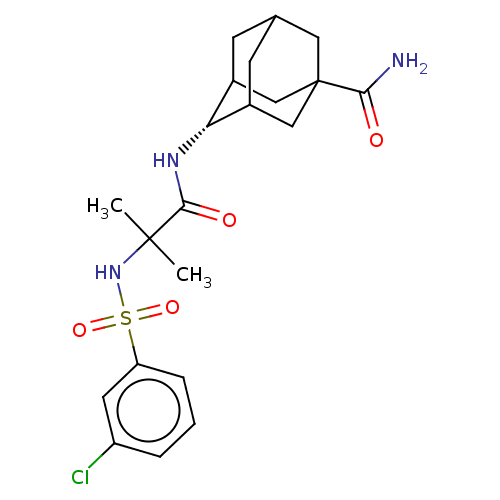
(CHEMBL3609871 | US9464044, 73)Show SMILES CC(C)(NS(=O)(=O)c1cccc(Cl)c1)C(=O)N[C@H]1C2CC3CC1CC(C3)(C2)C(N)=O |r,wD:17.17,TLB:21:22:26:25.19.20,21:20:26:17.22.23,17:18:25:22.21.23,THB:16:17:26:25.19.20,17:22:25:26.19.18,(-7,-2.31,;-6.03,-1.55,;-5.85,-2.77,;-7.46,-.97,;-8.67,-1.93,;-7.53,-2.38,;-8.49,-3.15,;-10.1,-1.35,;-10.32,.17,;-11.75,.74,;-12.96,-.21,;-12.74,-1.74,;-13.71,-2.5,;-11.31,-2.31,;-4.82,-.59,;-4.99,.63,;-3.39,-1.17,;-2.19,-.22,;-.95,.32,;-1,2.05,;.12,3.07,;-1.2,2.69,;-1.2,1.02,;.34,.44,;1.56,1.02,;1.56,2.59,;.56,-.22,;3.07,1.15,;3.78,.15,;3.59,2.27,)| Show InChI InChI=1S/C21H28ClN3O4S/c1-20(2,25-30(28,29)16-5-3-4-15(22)8-16)19(27)24-17-13-6-12-7-14(17)11-21(9-12,10-13)18(23)26/h3-5,8,12-14,17,25H,6-7,9-11H2,1-2H3,(H2,23,26)(H,24,27)/t12?,13?,14?,17-,21? | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 0.830 | n/a | n/a | n/a | n/a | 6.0 | 25 |
AHN-GOOK PHARMACEUTICAL CO., LTD.; BAMICHEM CO., LTD; INCHEON UNIVERSITY INDUSTRY ACADEMIC COOPERATION FOUNDATION
US Patent
| Assay Description
The inhibiting activity of 11β-HSD1 derived from microsomal fractions was measured using the HTRF assay (62CO2PEB, Cisbio). Different concentrat... |
US Patent US9464044 (2016)
BindingDB Entry DOI: 10.7270/Q2SJ1JH0 |
More data for this
Ligand-Target Pair | |
11-beta-hydroxysteroid dehydrogenase 1
(Homo sapiens (Human)) | BDBM250486

(US9464044, 119)Show SMILES NC(=O)C12CC3CC(C1)[C@H](NC(=O)c1cccc(NS(=O)(=O)c4cc(Cl)cc(Cl)c4)c1)C(C3)C2 |r,wU:9.10,TLB:10:9:32:3.4.33,8:3:32:9.7.6,1:3:9:32.5.6,THB:8:7:32:3.4.33,4:3:9:32.5.6,4:5:9:3.8.33,(9.95,-.46,;8.87,-1.55,;9.26,-3.04,;7.38,-1.15,;6.48,-3.08,;5.28,-2.41,;5.29,-.01,;6.05,1.15,;7.38,.38,;4.72,.38,;3.38,1.15,;2.05,.38,;2.05,-1.15,;.72,1.15,;.72,2.69,;-.62,3.47,;-1.95,2.69,;-1.95,1.15,;-3.29,.38,;-4.62,1.15,;-4.62,2.69,;-3.53,2.24,;-5.95,.38,;-7.29,1.15,;-8.62,.38,;-9.95,1.15,;-8.62,-1.15,;-7.29,-1.93,;-7.29,-3.47,;-5.95,-1.15,;-.62,.38,;4.72,-1.15,;3.88,-3.06,;6.05,-1.93,)| Show InChI InChI=1S/C24H25Cl2N3O4S/c25-17-7-18(26)9-20(8-17)34(32,33)29-19-3-1-2-14(6-19)22(30)28-21-15-4-13-5-16(21)12-24(10-13,11-15)23(27)31/h1-3,6-9,13,15-16,21,29H,4-5,10-12H2,(H2,27,31)(H,28,30)/t13?,15?,16?,21-,24? | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 0.900 | n/a | n/a | n/a | n/a | 6.0 | 25 |
AHN-GOOK PHARMACEUTICAL CO., LTD.; BAMICHEM CO., LTD; INCHEON UNIVERSITY INDUSTRY ACADEMIC COOPERATION FOUNDATION
US Patent
| Assay Description
The inhibiting activity of 11β-HSD1 derived from microsomal fractions was measured using the HTRF assay (62CO2PEB, Cisbio). Different concentrat... |
US Patent US9464044 (2016)
BindingDB Entry DOI: 10.7270/Q2SJ1JH0 |
More data for this
Ligand-Target Pair | |
11-beta-hydroxysteroid dehydrogenase 1
(Homo sapiens (Human)) | BDBM50112143

(CHEMBL3609871 | US9464044, 73)Show SMILES CC(C)(NS(=O)(=O)c1cccc(Cl)c1)C(=O)N[C@H]1C2CC3CC1CC(C3)(C2)C(N)=O |r,wD:17.17,TLB:21:22:26:25.19.20,21:20:26:17.22.23,17:18:25:22.21.23,THB:16:17:26:25.19.20,17:22:25:26.19.18,(-7,-2.31,;-6.03,-1.55,;-5.85,-2.77,;-7.46,-.97,;-8.67,-1.93,;-7.53,-2.38,;-8.49,-3.15,;-10.1,-1.35,;-10.32,.17,;-11.75,.74,;-12.96,-.21,;-12.74,-1.74,;-13.71,-2.5,;-11.31,-2.31,;-4.82,-.59,;-4.99,.63,;-3.39,-1.17,;-2.19,-.22,;-.95,.32,;-1,2.05,;.12,3.07,;-1.2,2.69,;-1.2,1.02,;.34,.44,;1.56,1.02,;1.56,2.59,;.56,-.22,;3.07,1.15,;3.78,.15,;3.59,2.27,)| Show InChI InChI=1S/C21H28ClN3O4S/c1-20(2,25-30(28,29)16-5-3-4-15(22)8-16)19(27)24-17-13-6-12-7-14(17)11-21(9-12,10-13)18(23)26/h3-5,8,12-14,17,25H,6-7,9-11H2,1-2H3,(H2,23,26)(H,24,27)/t12?,13?,14?,17-,21? | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Incheon National University
Curated by ChEMBL
| Assay Description
Inhibition of human 11beta-HSD1 |
Bioorg Med Chem Lett 25: 3501-6 (2015)
Article DOI: 10.1016/j.bmcl.2015.06.099
BindingDB Entry DOI: 10.7270/Q2BP04KW |
More data for this
Ligand-Target Pair | |
11-beta-hydroxysteroid dehydrogenase 1
(Homo sapiens (Human)) | BDBM50448235
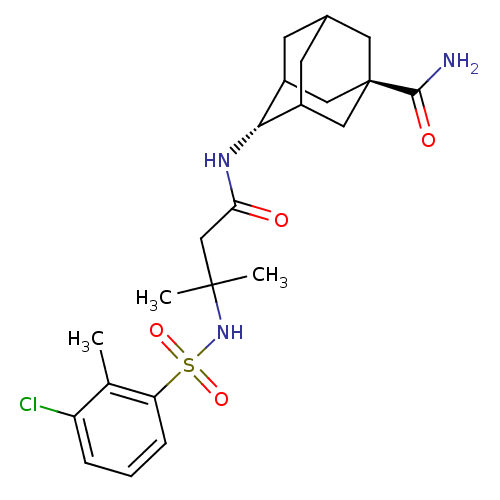
(CHEMBL3120758)Show SMILES Cc1c(Cl)cccc1S(=O)(=O)NC(C)(C)CC(=O)N[C@H]1C2CC3CC1C[C@](C3)(C2)C(N)=O |r,wU:19.19,wD:26.32,TLB:28:26:23:21.20.19,18:19:23:25.27.26,THB:27:22:19:25.26.28,27:26:23.22.21:19,28:20:23:25.27.26,29:26:23:21.20.19,(48.96,-12.34,;48.96,-10.8,;47.63,-10.03,;46.29,-10.8,;47.63,-8.49,;48.96,-7.71,;50.29,-8.48,;50.3,-10.03,;51.63,-10.8,;50.86,-12.13,;52.4,-12.13,;52.96,-10.02,;54.3,-10.79,;55.07,-12.13,;53.89,-12.27,;55.63,-10.02,;56.97,-10.79,;56.97,-12.33,;58.3,-10.02,;59.56,-10.89,;60.91,-10.41,;59.88,-11.65,;59.89,-13.23,;58.39,-13.66,;59.58,-12.38,;60.91,-12.86,;62.31,-12.51,;61.3,-13.79,;62.31,-10.98,;63.66,-13.24,;64.97,-12.44,;63.7,-14.78,)| Show InChI InChI=1S/C23H32ClN3O4S/c1-13-17(24)5-4-6-18(13)32(30,31)27-22(2,3)12-19(28)26-20-15-7-14-8-16(20)11-23(9-14,10-15)21(25)29/h4-6,14-16,20,27H,7-12H2,1-3H3,(H2,25,29)(H,26,28)/t14?,15?,16?,20-,23- | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Yonsei University
Curated by ChEMBL
| Assay Description
Inhibition of 11beta-HSD1 in human liver microsomes using cortisone as substrate after 2 hrs by HTRF assay |
Bioorg Med Chem Lett 24: 1421-5 (2014)
Article DOI: 10.1016/j.bmcl.2014.01.017
BindingDB Entry DOI: 10.7270/Q2BG2QG8 |
More data for this
Ligand-Target Pair | |
11-beta-hydroxysteroid dehydrogenase 1
(Homo sapiens (Human)) | BDBM50448232

(CHEMBL3120817)Show SMILES CC(C)(CC(=O)N[C@H]1C2CC3CC1C[C@](C3)(C2)C(N)=O)NS(=O)(=O)c1cccc(Cl)c1F |r,wU:7.6,wD:14.19,TLB:16:14:11:9.8.7,6:7:11:13.15.14,THB:15:10:7:13.14.16,15:14:11.10.9:7,16:8:11:13.15.14,17:14:11:9.8.7,(29.03,-26.58,;28.26,-25.25,;27.85,-26.73,;29.59,-24.48,;30.93,-25.25,;30.93,-26.79,;32.26,-24.47,;33.52,-25.35,;34.87,-24.87,;33.84,-26.11,;33.85,-27.69,;32.35,-28.12,;33.54,-26.84,;34.87,-27.32,;36.26,-26.97,;35.26,-28.25,;36.27,-25.44,;37.59,-27.75,;38.93,-26.98,;37.59,-29.29,;26.92,-24.48,;25.59,-25.25,;24.82,-26.59,;26.36,-26.59,;24.26,-24.49,;24.25,-22.94,;22.92,-22.17,;21.59,-22.94,;21.59,-24.49,;20.25,-25.26,;22.92,-25.26,;22.92,-26.8,)| Show InChI InChI=1S/C22H29ClFN3O4S/c1-21(2,27-32(30,31)16-5-3-4-15(23)18(16)24)11-17(28)26-19-13-6-12-7-14(19)10-22(8-12,9-13)20(25)29/h3-5,12-14,19,27H,6-11H2,1-2H3,(H2,25,29)(H,26,28)/t12?,13?,14?,19-,22- | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Yonsei University
Curated by ChEMBL
| Assay Description
Inhibition of 11beta-HSD1 in human liver microsomes using cortisone as substrate after 2 hrs by HTRF assay |
Bioorg Med Chem Lett 24: 1421-5 (2014)
Article DOI: 10.1016/j.bmcl.2014.01.017
BindingDB Entry DOI: 10.7270/Q2BG2QG8 |
More data for this
Ligand-Target Pair | |
11-beta-hydroxysteroid dehydrogenase 1
(Homo sapiens (Human)) | BDBM50448231
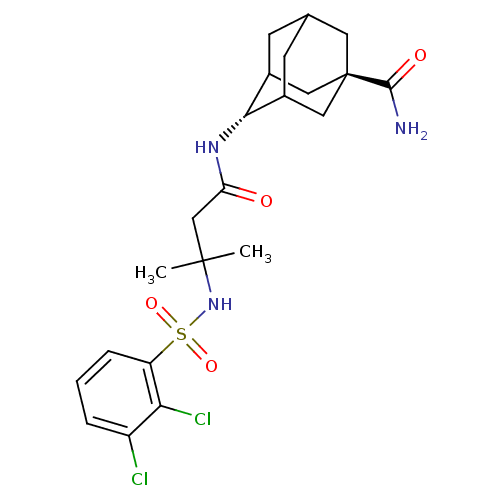
(CHEMBL3120818)Show SMILES CC(C)(CC(=O)N[C@H]1C2CC3CC1C[C@](C3)(C2)C(N)=O)NS(=O)(=O)c1cccc(Cl)c1Cl |r,wU:7.6,wD:14.19,TLB:16:14:11:9.8.7,6:7:11:13.15.14,THB:15:10:7:13.14.16,15:14:11.10.9:7,16:8:11:13.15.14,17:14:11:9.8.7,(10.27,-32,;9.5,-30.67,;9.09,-32.15,;10.83,-29.9,;12.17,-30.67,;12.17,-32.21,;13.5,-29.9,;14.76,-30.77,;16.11,-30.29,;15.08,-31.53,;15.09,-33.11,;13.59,-33.54,;14.78,-32.26,;16.11,-32.74,;17.51,-32.39,;16.5,-33.67,;17.51,-30.86,;18.86,-33.13,;20.17,-32.33,;18.89,-34.67,;8.17,-29.9,;6.83,-30.68,;6.06,-32.01,;7.6,-32.01,;5.5,-29.91,;5.49,-28.36,;4.16,-27.59,;2.83,-28.36,;2.83,-29.91,;1.49,-30.68,;4.16,-30.68,;4.16,-32.22,)| Show InChI InChI=1S/C22H29Cl2N3O4S/c1-21(2,27-32(30,31)16-5-3-4-15(23)18(16)24)11-17(28)26-19-13-6-12-7-14(19)10-22(8-12,9-13)20(25)29/h3-5,12-14,19,27H,6-11H2,1-2H3,(H2,25,29)(H,26,28)/t12?,13?,14?,19-,22- | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Yonsei University
Curated by ChEMBL
| Assay Description
Inhibition of 11beta-HSD1 in human liver microsomes using cortisone as substrate after 2 hrs by HTRF assay |
Bioorg Med Chem Lett 24: 1421-5 (2014)
Article DOI: 10.1016/j.bmcl.2014.01.017
BindingDB Entry DOI: 10.7270/Q2BG2QG8 |
More data for this
Ligand-Target Pair | |
11-beta-hydroxysteroid dehydrogenase 1
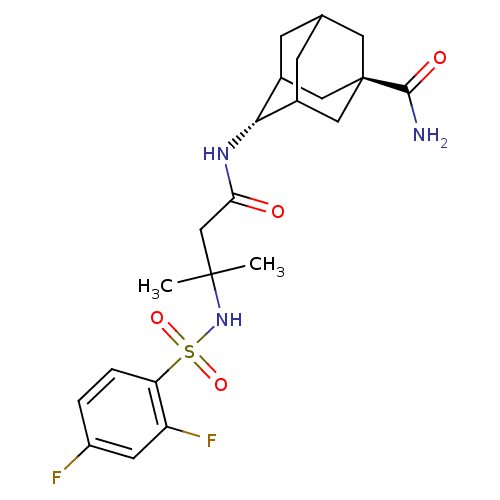
(Homo sapiens (Human)) | BDBM50448230
(CHEMBL3120819)Show SMILES CC(C)(CC(=O)N[C@H]1C2CC3CC1C[C@](C3)(C2)C(N)=O)NS(=O)(=O)c1ccc(F)cc1F |r,wU:7.6,wD:14.19,TLB:16:14:11:9.8.7,6:7:11:13.15.14,THB:15:10:7:13.14.16,15:14:11.10.9:7,16:8:11:13.15.14,17:14:11:9.8.7,(35.35,-34.25,;34.58,-32.91,;34.17,-34.39,;35.91,-32.14,;37.24,-32.91,;37.25,-34.45,;38.58,-32.14,;39.84,-33.01,;41.19,-32.53,;40.15,-33.77,;40.17,-35.35,;38.67,-35.78,;39.86,-34.5,;41.19,-34.98,;42.58,-34.63,;41.58,-35.91,;42.59,-33.1,;43.92,-35.4,;45.25,-34.63,;43.92,-36.94,;33.24,-32.15,;31.91,-32.92,;31.14,-34.25,;32.68,-34.25,;30.57,-32.15,;30.57,-30.6,;29.23,-29.84,;27.9,-30.61,;26.57,-29.84,;27.9,-32.15,;29.24,-32.92,;29.24,-34.46,)| Show InChI InChI=1S/C22H29F2N3O4S/c1-21(2,27-32(30,31)17-4-3-15(23)7-16(17)24)11-18(28)26-19-13-5-12-6-14(19)10-22(8-12,9-13)20(25)29/h3-4,7,12-14,19,27H,5-6,8-11H2,1-2H3,(H2,25,29)(H,26,28)/t12?,13?,14?,19-,22- | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Yonsei University
Curated by ChEMBL
| Assay Description
Inhibition of 11beta-HSD1 in human liver microsomes using cortisone as substrate after 2 hrs by HTRF assay |
Bioorg Med Chem Lett 24: 1421-5 (2014)
Article DOI: 10.1016/j.bmcl.2014.01.017
BindingDB Entry DOI: 10.7270/Q2BG2QG8 |
More data for this
Ligand-Target Pair | |
11-beta-hydroxysteroid dehydrogenase 1
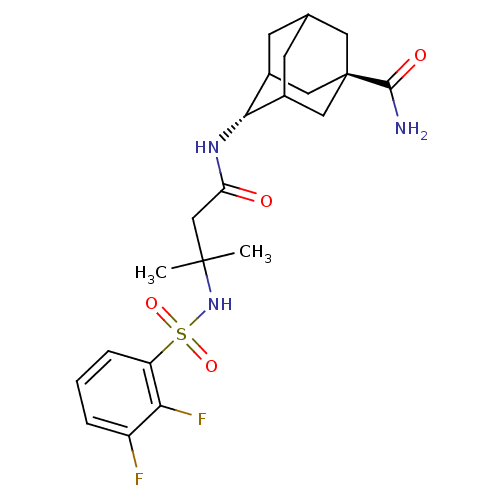
(Homo sapiens (Human)) | BDBM50448229
(CHEMBL3120820)Show SMILES CC(C)(CC(=O)N[C@H]1C2CC3CC1C[C@](C3)(C2)C(N)=O)NS(=O)(=O)c1cccc(F)c1F |r,wU:7.6,wD:14.19,TLB:16:14:11:9.8.7,6:7:11:13.15.14,THB:15:10:7:13.14.16,15:14:11.10.9:7,16:8:11:13.15.14,17:14:11:9.8.7,(11.78,-31.4,;11.01,-30.07,;10.6,-31.55,;12.34,-29.3,;13.68,-30.06,;13.68,-31.6,;15.01,-29.29,;16.27,-30.17,;17.62,-29.68,;16.59,-30.92,;16.6,-32.51,;15.1,-32.94,;16.29,-31.65,;17.62,-32.14,;19.01,-31.79,;18.01,-33.06,;19.02,-30.25,;20.35,-32.56,;21.68,-31.79,;20.34,-34.1,;9.67,-29.3,;8.34,-30.07,;7.57,-31.41,;9.11,-31.4,;7.01,-29.3,;7,-27.75,;5.67,-26.99,;4.34,-27.76,;4.34,-29.3,;3,-30.07,;5.67,-30.07,;5.67,-31.61,)| Show InChI InChI=1S/C22H29F2N3O4S/c1-21(2,27-32(30,31)16-5-3-4-15(23)18(16)24)11-17(28)26-19-13-6-12-7-14(19)10-22(8-12,9-13)20(25)29/h3-5,12-14,19,27H,6-11H2,1-2H3,(H2,25,29)(H,26,28)/t12?,13?,14?,19-,22- | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Yonsei University
Curated by ChEMBL
| Assay Description
Inhibition of 11beta-HSD1 in human liver microsomes using cortisone as substrate after 2 hrs by HTRF assay |
Bioorg Med Chem Lett 24: 1421-5 (2014)
Article DOI: 10.1016/j.bmcl.2014.01.017
BindingDB Entry DOI: 10.7270/Q2BG2QG8 |
More data for this
Ligand-Target Pair | |
11-beta-hydroxysteroid dehydrogenase 1
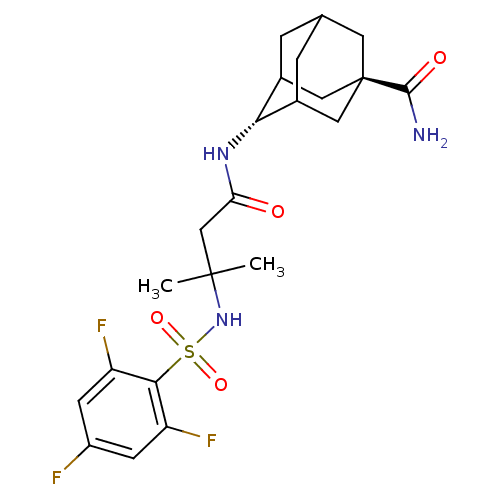
(Homo sapiens (Human)) | BDBM50448225
(CHEMBL3120824)Show SMILES CC(C)(CC(=O)N[C@H]1C2CC3CC1C[C@](C3)(C2)C(N)=O)NS(=O)(=O)c1c(F)cc(F)cc1F |r,wU:7.6,wD:14.19,TLB:16:14:11:9.8.7,6:7:11:13.15.14,THB:15:10:7:13.14.16,15:14:11.10.9:7,16:8:11:13.15.14,17:14:11:9.8.7,(35.27,-35.23,;34.5,-33.9,;34.09,-35.38,;35.83,-33.13,;37.16,-33.9,;37.17,-35.44,;38.5,-33.12,;39.76,-34,;41.11,-33.51,;40.07,-34.76,;40.09,-36.34,;38.59,-36.77,;39.78,-35.49,;41.11,-35.97,;42.5,-35.62,;41.5,-36.9,;42.51,-34.09,;43.83,-36.4,;45.17,-35.64,;43.81,-37.94,;33.16,-33.13,;31.83,-33.9,;31.06,-35.24,;32.6,-35.24,;30.49,-33.14,;29.16,-33.91,;29.16,-35.45,;27.82,-33.14,;27.83,-31.59,;26.49,-30.82,;29.15,-30.82,;30.49,-31.59,;31.82,-30.81,)| Show InChI InChI=1S/C22H28F3N3O4S/c1-21(2,28-33(31,32)19-15(24)5-14(23)6-16(19)25)10-17(29)27-18-12-3-11-4-13(18)9-22(7-11,8-12)20(26)30/h5-6,11-13,18,28H,3-4,7-10H2,1-2H3,(H2,26,30)(H,27,29)/t11?,12?,13?,18-,22- | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Yonsei University
Curated by ChEMBL
| Assay Description
Inhibition of 11beta-HSD1 in human liver microsomes using cortisone as substrate after 2 hrs by HTRF assay |
Bioorg Med Chem Lett 24: 1421-5 (2014)
Article DOI: 10.1016/j.bmcl.2014.01.017
BindingDB Entry DOI: 10.7270/Q2BG2QG8 |
More data for this
Ligand-Target Pair | |
11-beta-hydroxysteroid dehydrogenase 1
(Homo sapiens (Human)) | BDBM50448224
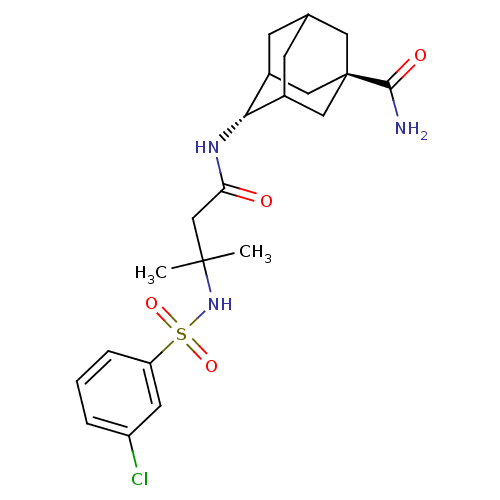
(CHEMBL3120825)Show SMILES CC(C)(CC(=O)N[C@H]1C2CC3CC1C[C@](C3)(C2)C(N)=O)NS(=O)(=O)c1cccc(Cl)c1 |r,wU:7.6,wD:14.19,TLB:16:14:11:9.8.7,6:7:11:13.15.14,THB:15:10:7:13.14.16,15:14:11.10.9:7,16:8:11:13.15.14,17:14:11:9.8.7,(11.44,-47.23,;10.67,-45.9,;10.26,-47.38,;12,-45.12,;13.33,-45.89,;13.34,-47.43,;14.67,-45.12,;15.93,-46,;17.28,-45.51,;16.25,-46.75,;16.26,-48.34,;14.76,-48.76,;15.95,-47.48,;17.28,-47.96,;18.67,-47.61,;17.67,-48.89,;18.68,-46.08,;20.01,-48.37,;21.34,-47.59,;20.03,-49.91,;9.33,-45.13,;8,-45.9,;7.23,-47.23,;8.77,-47.23,;6.66,-45.13,;6.66,-43.58,;5.32,-42.82,;4,-43.59,;3.99,-45.13,;2.66,-45.9,;5.33,-45.9,)| Show InChI InChI=1S/C22H30ClN3O4S/c1-21(2,26-31(29,30)17-5-3-4-16(23)8-17)12-18(27)25-19-14-6-13-7-15(19)11-22(9-13,10-14)20(24)28/h3-5,8,13-15,19,26H,6-7,9-12H2,1-2H3,(H2,24,28)(H,25,27)/t13?,14?,15?,19-,22- | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Yonsei University
Curated by ChEMBL
| Assay Description
Inhibition of 11beta-HSD1 in human liver microsomes using cortisone as substrate after 2 hrs by HTRF assay |
Bioorg Med Chem Lett 24: 1421-5 (2014)
Article DOI: 10.1016/j.bmcl.2014.01.017
BindingDB Entry DOI: 10.7270/Q2BG2QG8 |
More data for this
Ligand-Target Pair | |
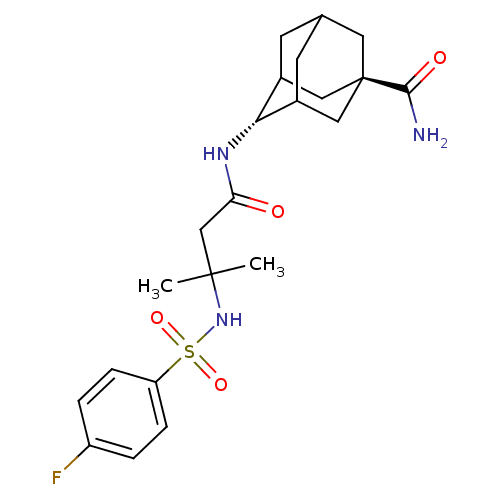
11-beta-hydroxysteroid dehydrogenase 1
(Homo sapiens (Human)) | BDBM50448221
(CHEMBL3120828)Show SMILES CC(C)(CC(=O)N[C@H]1C2CC3CC1C[C@](C3)(C2)C(N)=O)NS(=O)(=O)c1ccc(F)cc1 |r,wU:7.6,wD:14.19,TLB:16:14:11:9.8.7,6:7:11:13.15.14,THB:15:10:7:13.14.16,15:14:11.10.9:7,16:8:11:13.15.14,17:14:11:9.8.7,(16.74,-56.49,;15.96,-55.16,;15.56,-56.64,;17.3,-54.38,;18.63,-55.15,;18.63,-56.69,;19.96,-54.38,;21.23,-55.26,;22.57,-54.77,;21.54,-56.01,;21.55,-57.6,;20.05,-58.02,;21.24,-56.74,;22.58,-57.22,;23.97,-56.87,;22.96,-58.15,;23.97,-55.34,;25.29,-57.66,;26.63,-56.9,;25.28,-59.2,;14.63,-54.39,;13.3,-55.16,;12.53,-56.49,;14.07,-56.49,;11.96,-54.39,;10.62,-55.16,;9.29,-54.39,;9.29,-52.85,;7.96,-52.08,;10.62,-52.08,;11.96,-52.84,)| Show InChI InChI=1S/C22H30FN3O4S/c1-21(2,26-31(29,30)17-5-3-16(23)4-6-17)12-18(27)25-19-14-7-13-8-15(19)11-22(9-13,10-14)20(24)28/h3-6,13-15,19,26H,7-12H2,1-2H3,(H2,24,28)(H,25,27)/t13?,14?,15?,19-,22- | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Yonsei University
Curated by ChEMBL
| Assay Description
Inhibition of 11beta-HSD1 in human liver microsomes using cortisone as substrate after 2 hrs by HTRF assay |
Bioorg Med Chem Lett 24: 1421-5 (2014)
Article DOI: 10.1016/j.bmcl.2014.01.017
BindingDB Entry DOI: 10.7270/Q2BG2QG8 |
More data for this
Ligand-Target Pair | |
Metabotropic glutamate receptor 5
(Homo sapiens (Human)) | BDBM50431696
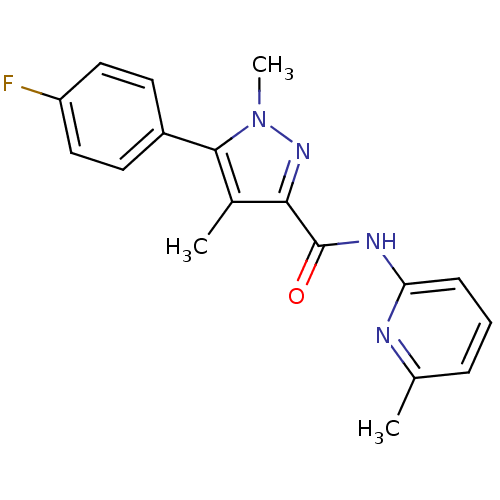
(CHEMBL2349534)Show InChI InChI=1S/C18H17FN4O/c1-11-5-4-6-15(20-11)21-18(24)16-12(2)17(23(3)22-16)13-7-9-14(19)10-8-13/h4-10H,1-3H3,(H,20,21,24) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a |
SK Biopharmaceuticals
Curated by ChEMBL
| Assay Description
Negative allosteric modulation of human mGluR5 expressed in HEK293 cells assessed as calcium mobilization by FLIPR assay |
Bioorg Med Chem Lett 23: 2134-9 (2013)
Article DOI: 10.1016/j.bmcl.2013.01.116
BindingDB Entry DOI: 10.7270/Q20R9QR5 |
More data for this
Ligand-Target Pair | |
11-beta-hydroxysteroid dehydrogenase 1
(Homo sapiens (Human)) | BDBM250474

(US9464044, 102)Show SMILES NC(=O)C12CC3CC(C1)[C@H](NC(=O)c1cccc(NS(=O)(=O)c4cccs4)c1)C(C3)C2 |r,wU:9.10,TLB:10:9:29:3.4.30,8:3:29:9.7.6,1:3:9:29.5.6,THB:8:7:29:3.4.30,4:3:9:29.5.6,4:5:9:3.8.30,(9.14,-.66,;8.05,-1.75,;8.45,-3.23,;6.56,-1.35,;5.67,-3.27,;4.46,-2.6,;4.48,-.2,;5.23,.96,;6.56,.19,;3.9,.19,;2.57,.96,;1.23,.19,;1.23,-1.35,;-.1,.96,;-.1,2.5,;-1.43,3.27,;-2.77,2.5,;-2.77,.96,;-4.1,.19,;-5.44,.96,;-5.44,2.5,;-4.35,2.05,;-6.77,.19,;-6.77,-1.35,;-8.23,-1.82,;-9.14,-.58,;-8.23,.67,;-1.43,.19,;3.9,-1.35,;3.07,-3.25,;5.23,-2.12,)| Show InChI InChI=1S/C22H25N3O4S2/c23-21(27)22-10-13-7-15(11-22)19(16(8-13)12-22)24-20(26)14-3-1-4-17(9-14)25-31(28,29)18-5-2-6-30-18/h1-6,9,13,15-16,19,25H,7-8,10-12H2,(H2,23,27)(H,24,26)/t13?,15?,16?,19-,22? | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | 6.0 | 25 |
AHN-GOOK PHARMACEUTICAL CO., LTD.; BAMICHEM CO., LTD; INCHEON UNIVERSITY INDUSTRY ACADEMIC COOPERATION FOUNDATION
US Patent
| Assay Description
The inhibiting activity of 11β-HSD1 derived from microsomal fractions was measured using the HTRF assay (62CO2PEB, Cisbio). Different concentrat... |
US Patent US9464044 (2016)
BindingDB Entry DOI: 10.7270/Q2SJ1JH0 |
More data for this
Ligand-Target Pair | |
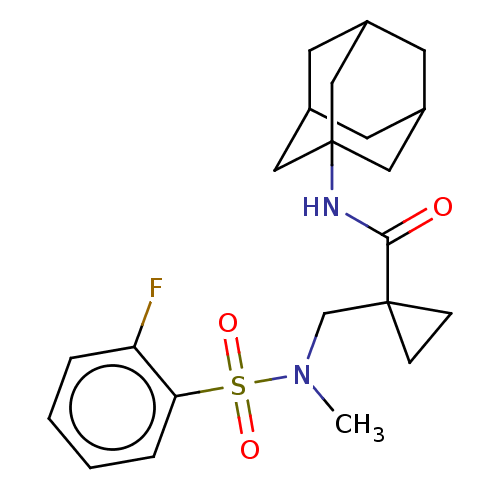
11-beta-hydroxysteroid dehydrogenase 1
(Homo sapiens (Human)) | BDBM250437
(US9464044, 31)Show SMILES CN(CC1(CC1)C(=O)NC12CC3CC(CC(C3)C1)C2)S(=O)(=O)c1ccccc1F |TLB:12:13:11.10.16:17,16:11:18:15.14.17,16:15:18:12.11.10,THB:12:11:18.13.14:17| Show InChI InChI=1S/C22H29FN2O3S/c1-25(29(27,28)19-5-3-2-4-18(19)23)14-21(6-7-21)20(26)24-22-11-15-8-16(12-22)10-17(9-15)13-22/h2-5,15-17H,6-14H2,1H3,(H,24,26) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | 6.0 | 25 |
AHN-GOOK PHARMACEUTICAL CO., LTD.; BAMICHEM CO., LTD; INCHEON UNIVERSITY INDUSTRY ACADEMIC COOPERATION FOUNDATION
US Patent
| Assay Description
The inhibiting activity of 11β-HSD1 derived from microsomal fractions was measured using the HTRF assay (62CO2PEB, Cisbio). Different concentrat... |
US Patent US9464044 (2016)
BindingDB Entry DOI: 10.7270/Q2SJ1JH0 |
More data for this
Ligand-Target Pair | |
11-beta-hydroxysteroid dehydrogenase 1
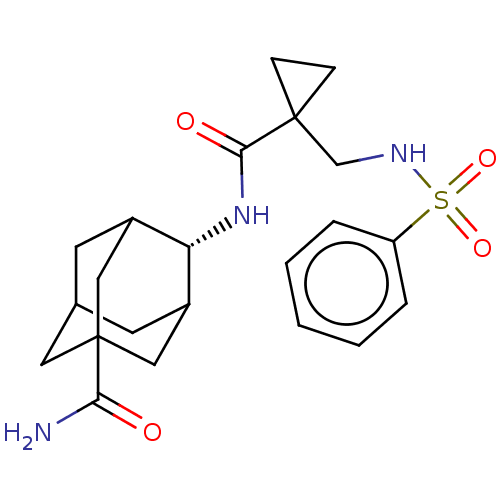
(Homo sapiens (Human)) | BDBM250436
(US9464044, 28)Show SMILES NC(=O)C12CC3CC(C1)[C@H](NC(=O)C1(CNS(=O)(=O)c4ccccc4)CC1)C(C3)C2 |r,wU:9.10,TLB:1:3:9:28.5.6,10:9:28:3.4.29,8:3:28:9.7.6,THB:4:3:9:28.5.6,4:5:9:3.8.29,8:7:28:3.4.29,(8.62,-.66,;7.53,-1.75,;7.93,-3.23,;6.04,-1.35,;5.15,-3.27,;3.94,-2.6,;3.96,-.2,;4.72,.96,;6.04,.19,;3.38,.19,;2.05,.96,;.72,.19,;.72,-1.35,;-.62,.96,;-1.95,.19,;-3.29,.96,;-4.62,.19,;-5.39,-1.14,;-3.85,-1.14,;-5.95,.96,;-5.95,2.5,;-7.29,3.27,;-8.62,2.5,;-8.62,.96,;-7.29,.19,;.15,2.3,;-1.39,2.3,;3.38,-1.35,;2.55,-3.25,;4.72,-2.12,)| Show InChI InChI=1S/C22H29N3O4S/c23-19(26)22-10-14-8-15(11-22)18(16(9-14)12-22)25-20(27)21(6-7-21)13-24-30(28,29)17-4-2-1-3-5-17/h1-5,14-16,18,24H,6-13H2,(H2,23,26)(H,25,27)/t14?,15?,16?,18-,22? | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | 6.0 | 25 |
AHN-GOOK PHARMACEUTICAL CO., LTD.; BAMICHEM CO., LTD; INCHEON UNIVERSITY INDUSTRY ACADEMIC COOPERATION FOUNDATION
US Patent
| Assay Description
The inhibiting activity of 11β-HSD1 derived from microsomal fractions was measured using the HTRF assay (62CO2PEB, Cisbio). Different concentrat... |
US Patent US9464044 (2016)
BindingDB Entry DOI: 10.7270/Q2SJ1JH0 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data