 Found 1751 hits with Last Name = 'li' and Initial = 'yl'
Found 1751 hits with Last Name = 'li' and Initial = 'yl' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Coagulation factor X
(Homo sapiens (Human)) | BDBM50377655
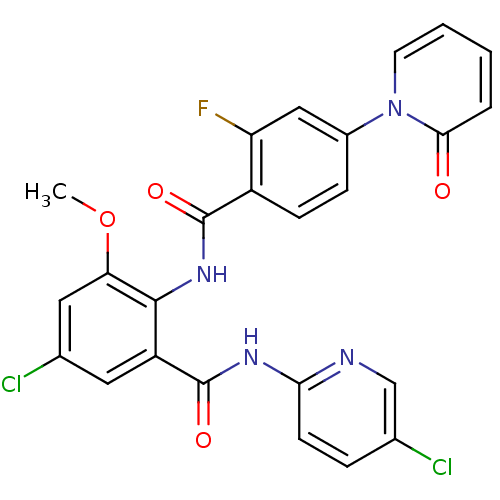
(CHEMBL260160)Show SMILES COc1cc(Cl)cc(C(=O)Nc2ccc(Cl)cn2)c1NC(=O)c1ccc(cc1F)-n1ccccc1=O Show InChI InChI=1S/C25H17Cl2FN4O4/c1-36-20-11-15(27)10-18(25(35)30-21-8-5-14(26)13-29-21)23(20)31-24(34)17-7-6-16(12-19(17)28)32-9-3-2-4-22(32)33/h2-13H,1H3,(H,31,34)(H,29,30,35) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.00500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of human factor 10a |
Bioorg Med Chem Lett 18: 2845-9 (2008)
Article DOI: 10.1016/j.bmcl.2008.03.092
BindingDB Entry DOI: 10.7270/Q2611169 |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50377635
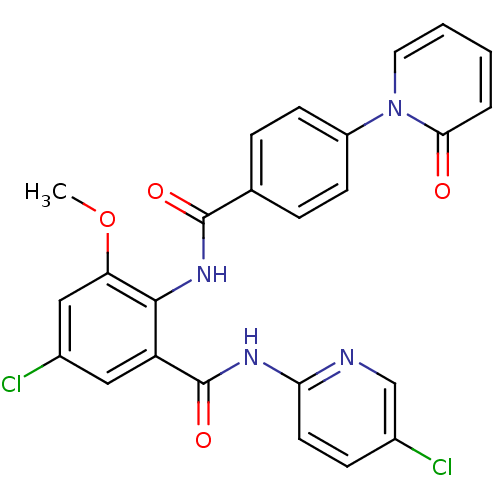
(CHEMBL402980)Show SMILES COc1cc(Cl)cc(C(=O)Nc2ccc(Cl)cn2)c1NC(=O)c1ccc(cc1)-n1ccccc1=O Show InChI InChI=1S/C25H18Cl2N4O4/c1-35-20-13-17(27)12-19(25(34)29-21-10-7-16(26)14-28-21)23(20)30-24(33)15-5-8-18(9-6-15)31-11-3-2-4-22(31)32/h2-14H,1H3,(H,30,33)(H,28,29,34) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0130 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of human factor 10a |
Bioorg Med Chem Lett 18: 2845-9 (2008)
Article DOI: 10.1016/j.bmcl.2008.03.092
BindingDB Entry DOI: 10.7270/Q2611169 |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50377637
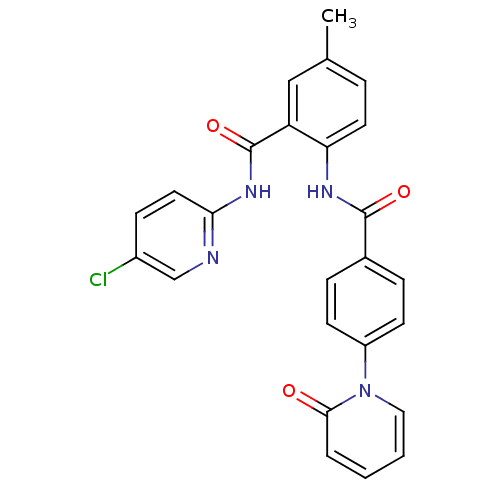
(CHEMBL257398)Show SMILES Cc1ccc(NC(=O)c2ccc(cc2)-n2ccccc2=O)c(c1)C(=O)Nc1ccc(Cl)cn1 Show InChI InChI=1S/C25H19ClN4O3/c1-16-5-11-21(20(14-16)25(33)29-22-12-8-18(26)15-27-22)28-24(32)17-6-9-19(10-7-17)30-13-3-2-4-23(30)31/h2-15H,1H3,(H,28,32)(H,27,29,33) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0280 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of human factor 10a |
Bioorg Med Chem Lett 18: 2845-9 (2008)
Article DOI: 10.1016/j.bmcl.2008.03.092
BindingDB Entry DOI: 10.7270/Q2611169 |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50377629
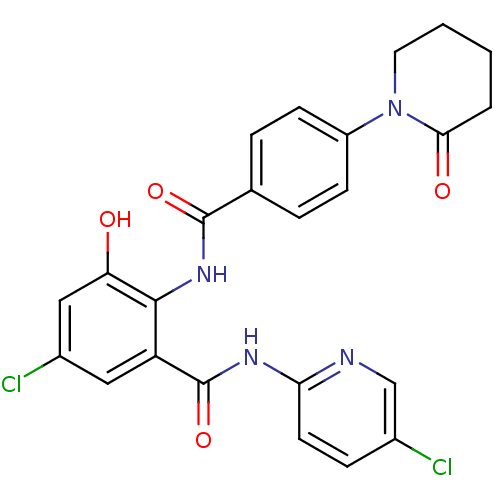
(CHEMBL260086)Show SMILES Oc1cc(Cl)cc(C(=O)Nc2ccc(Cl)cn2)c1NC(=O)c1ccc(cc1)N1CCCCC1=O Show InChI InChI=1S/C24H20Cl2N4O4/c25-15-6-9-20(27-13-15)28-24(34)18-11-16(26)12-19(31)22(18)29-23(33)14-4-7-17(8-5-14)30-10-2-1-3-21(30)32/h4-9,11-13,31H,1-3,10H2,(H,29,33)(H,27,28,34) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0470 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of human factor 10a |
Bioorg Med Chem Lett 18: 2845-9 (2008)
Article DOI: 10.1016/j.bmcl.2008.03.092
BindingDB Entry DOI: 10.7270/Q2611169 |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50328717

(5-Chloro-N-(5-chloro-pyridin-2-yl)-3-methoxy-2-[4-...)Show SMILES COc1cc(Cl)cc(C(=O)Nc2ccc(Cl)cn2)c1NC(=O)c1ccc(cc1)N1CCCCC1=O Show InChI InChI=1S/C25H22Cl2N4O4/c1-35-20-13-17(27)12-19(25(34)29-21-10-7-16(26)14-28-21)23(20)30-24(33)15-5-8-18(9-6-15)31-11-3-2-4-22(31)32/h5-10,12-14H,2-4,11H2,1H3,(H,30,33)(H,28,29,34) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0570 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of human factor 10a |
Bioorg Med Chem Lett 18: 2845-9 (2008)
Article DOI: 10.1016/j.bmcl.2008.03.092
BindingDB Entry DOI: 10.7270/Q2611169 |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50377638

(CHEMBL257400)Show SMILES Clc1ccc(NC(=O)c2cc(Cl)ccc2NC(=O)c2ccc(cc2)-n2ccccc2=O)nc1 Show InChI InChI=1S/C24H16Cl2N4O3/c25-16-6-10-20(19(13-16)24(33)29-21-11-7-17(26)14-27-21)28-23(32)15-4-8-18(9-5-15)30-12-2-1-3-22(30)31/h1-14H,(H,28,32)(H,27,29,33) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of human factor 10a |
Bioorg Med Chem Lett 18: 2845-9 (2008)
Article DOI: 10.1016/j.bmcl.2008.03.092
BindingDB Entry DOI: 10.7270/Q2611169 |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50377628
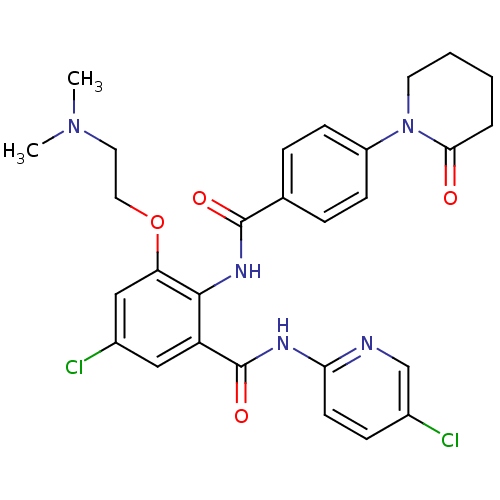
(CHEMBL261536)Show SMILES CN(C)CCOc1cc(Cl)cc(C(=O)Nc2ccc(Cl)cn2)c1NC(=O)c1ccc(cc1)N1CCCCC1=O Show InChI InChI=1S/C28H29Cl2N5O4/c1-34(2)13-14-39-23-16-20(30)15-22(28(38)32-24-11-8-19(29)17-31-24)26(23)33-27(37)18-6-9-21(10-7-18)35-12-4-3-5-25(35)36/h6-11,15-17H,3-5,12-14H2,1-2H3,(H,33,37)(H,31,32,38) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0650 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of human factor 10a |
Bioorg Med Chem Lett 18: 2845-9 (2008)
Article DOI: 10.1016/j.bmcl.2008.03.092
BindingDB Entry DOI: 10.7270/Q2611169 |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM19023

(1-(4-methoxyphenyl)-7-oxo-6-[4-(2-oxopiperidin-1-y...)Show SMILES COc1ccc(cc1)-n1nc(C(N)=O)c2CCN(C(=O)c12)c1ccc(cc1)N1CCCCC1=O Show InChI InChI=1S/C25H25N5O4/c1-34-19-11-9-18(10-12-19)30-23-20(22(27-30)24(26)32)13-15-29(25(23)33)17-7-5-16(6-8-17)28-14-3-2-4-21(28)31/h5-12H,2-4,13-15H2,1H3,(H2,26,32) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
MMDB
PDB
Article
PubMed
| 0.0800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of human factor 10a |
Bioorg Med Chem Lett 18: 2845-9 (2008)
Article DOI: 10.1016/j.bmcl.2008.03.092
BindingDB Entry DOI: 10.7270/Q2611169 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Coagulation factor X
(Homo sapiens (Human)) | BDBM12870
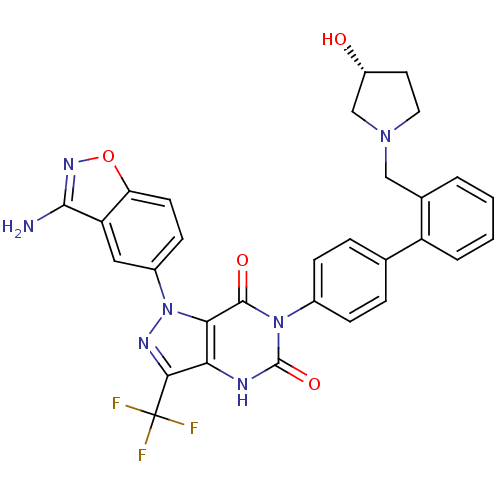
(1-(3-amino-1,2-benzoxazol-5-yl)-6-[4-(2-{[(3R)-3-h...)Show SMILES Nc1noc2ccc(cc12)-n1nc(c2[nH]c(=O)n(-c3ccc(cc3)-c3ccccc3CN3CC[C@@H](O)C3)c(=O)c12)C(F)(F)F |r| Show InChI InChI=1S/C30H24F3N7O4/c31-30(32,33)26-24-25(40(36-26)19-9-10-23-22(13-19)27(34)37-44-23)28(42)39(29(43)35-24)18-7-5-16(6-8-18)21-4-2-1-3-17(21)14-38-12-11-20(41)15-38/h1-10,13,20,41H,11-12,14-15H2,(H2,34,37)(H,35,43)/t20-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.110 | -56.3 | n/a | n/a | n/a | n/a | n/a | 7.0 | 22 |
Bristol-Myers Squibb Pharmaceutical Research Institute
| Assay Description
Ki values were obtained from human purified enzyme. All assays were run in microtiter plates. Plates were read for 30 min at 405 nm. Rates were deter... |
Bioorg Med Chem Lett 16: 5176-82 (2006)
Article DOI: 10.1016/j.bmcl.2006.07.002
BindingDB Entry DOI: 10.7270/Q29K48GC |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50377640
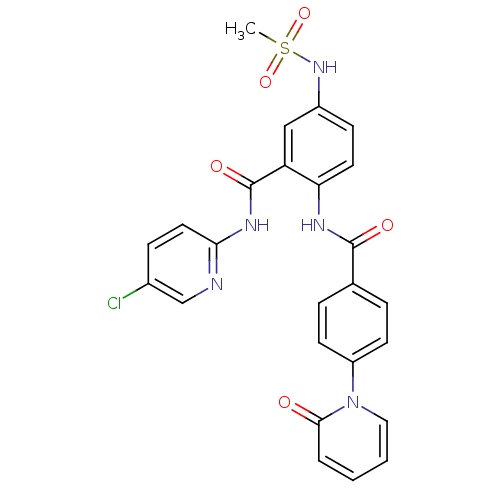
(CHEMBL258196)Show SMILES CS(=O)(=O)Nc1ccc(NC(=O)c2ccc(cc2)-n2ccccc2=O)c(c1)C(=O)Nc1ccc(Cl)cn1 Show InChI InChI=1S/C25H20ClN5O5S/c1-37(35,36)30-18-8-11-21(20(14-18)25(34)29-22-12-7-17(26)15-27-22)28-24(33)16-5-9-19(10-6-16)31-13-3-2-4-23(31)32/h2-15,30H,1H3,(H,28,33)(H,27,29,34) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 0.120 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of human factor 10a |
Bioorg Med Chem Lett 18: 2845-9 (2008)
Article DOI: 10.1016/j.bmcl.2008.03.092
BindingDB Entry DOI: 10.7270/Q2611169 |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50377631

(CHEMBL256152)Show SMILES Clc1ccc(NC(=O)c2cc(Br)ccc2NC(=O)c2ccc(cc2)N2CCCCC2=O)nc1 Show InChI InChI=1S/C24H20BrClN4O3/c25-16-6-10-20(19(13-16)24(33)29-21-11-7-17(26)14-27-21)28-23(32)15-4-8-18(9-5-15)30-12-2-1-3-22(30)31/h4-11,13-14H,1-3,12H2,(H,28,32)(H,27,29,33) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.140 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of human factor 10a |
Bioorg Med Chem Lett 18: 2845-9 (2008)
Article DOI: 10.1016/j.bmcl.2008.03.092
BindingDB Entry DOI: 10.7270/Q2611169 |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50377636
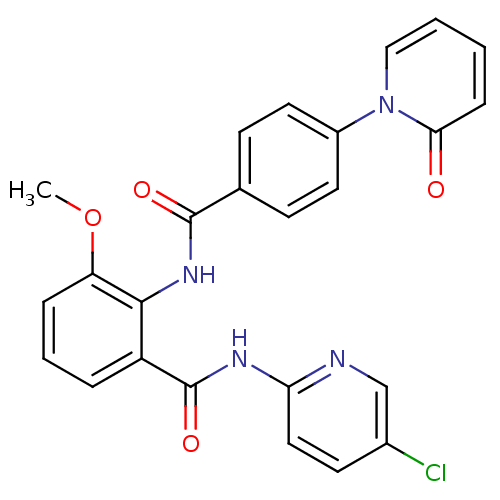
(CHEMBL257399)Show SMILES COc1cccc(C(=O)Nc2ccc(Cl)cn2)c1NC(=O)c1ccc(cc1)-n1ccccc1=O Show InChI InChI=1S/C25H19ClN4O4/c1-34-20-6-4-5-19(25(33)28-21-13-10-17(26)15-27-21)23(20)29-24(32)16-8-11-18(12-9-16)30-14-3-2-7-22(30)31/h2-15H,1H3,(H,29,32)(H,27,28,33) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.140 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of human factor 10a |
Bioorg Med Chem Lett 18: 2845-9 (2008)
Article DOI: 10.1016/j.bmcl.2008.03.092
BindingDB Entry DOI: 10.7270/Q2611169 |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50377656

(CHEMBL259534)Show SMILES Clc1ccc(NC(=O)c2ccccc2NC(=O)c2ccc(cc2)-n2ccccc2=O)nc1 Show InChI InChI=1S/C24H17ClN4O3/c25-17-10-13-21(26-15-17)28-24(32)19-5-1-2-6-20(19)27-23(31)16-8-11-18(12-9-16)29-14-4-3-7-22(29)30/h1-15H,(H,27,31)(H,26,28,32) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.140 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of human factor 10a |
Bioorg Med Chem Lett 18: 2845-9 (2008)
Article DOI: 10.1016/j.bmcl.2008.03.092
BindingDB Entry DOI: 10.7270/Q2611169 |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM12864
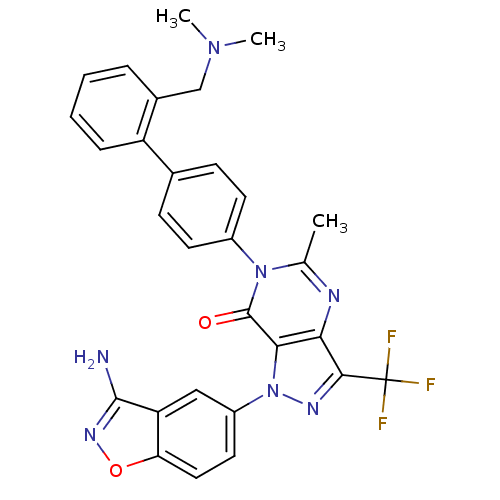
(1-(3-amino-1,2-benzoxazol-5-yl)-6-(4-{2-[(dimethyl...)Show SMILES CN(C)Cc1ccccc1-c1ccc(cc1)-n1c(C)nc2c(nn(-c3ccc4onc(N)c4c3)c2c1=O)C(F)(F)F Show InChI InChI=1S/C29H24F3N7O2/c1-16-34-24-25(39(35-26(24)29(30,31)32)20-12-13-23-22(14-20)27(33)36-41-23)28(40)38(16)19-10-8-17(9-11-19)21-7-5-4-6-18(21)15-37(2)3/h4-14H,15H2,1-3H3,(H2,33,36) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.170 | -55.2 | n/a | n/a | n/a | n/a | n/a | 7.0 | 22 |
Bristol-Myers Squibb Pharmaceutical Research Institute
| Assay Description
Ki values were obtained from human purified enzyme. All assays were run in microtiter plates. Plates were read for 30 min at 405 nm. Rates were deter... |
Bioorg Med Chem Lett 16: 5176-82 (2006)
Article DOI: 10.1016/j.bmcl.2006.07.002
BindingDB Entry DOI: 10.7270/Q29K48GC |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM12866
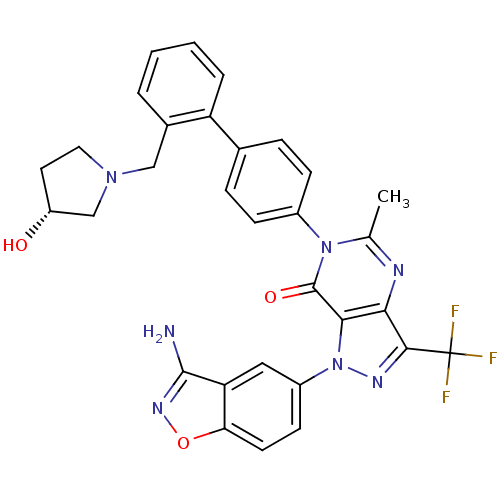
(1-(3-amino-1,2-benzoxazol-5-yl)-6-[4-(2-{[(3R)-3-h...)Show SMILES Cc1nc2c(nn(-c3ccc4onc(N)c4c3)c2c(=O)n1-c1ccc(cc1)-c1ccccc1CN1CC[C@@H](O)C1)C(F)(F)F |r| Show InChI InChI=1S/C31H26F3N7O3/c1-17-36-26-27(41(37-28(26)31(32,33)34)21-10-11-25-24(14-21)29(35)38-44-25)30(43)40(17)20-8-6-18(7-9-20)23-5-3-2-4-19(23)15-39-13-12-22(42)16-39/h2-11,14,22,42H,12-13,15-16H2,1H3,(H2,35,38)/t22-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.180 | -55.1 | n/a | n/a | n/a | n/a | n/a | 7.0 | 22 |
Bristol-Myers Squibb Pharmaceutical Research Institute
| Assay Description
Ki values were obtained from human purified enzyme. All assays were run in microtiter plates. Plates were read for 30 min at 405 nm. Rates were deter... |
Bioorg Med Chem Lett 16: 5176-82 (2006)
Article DOI: 10.1016/j.bmcl.2006.07.002
BindingDB Entry DOI: 10.7270/Q29K48GC |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50377630
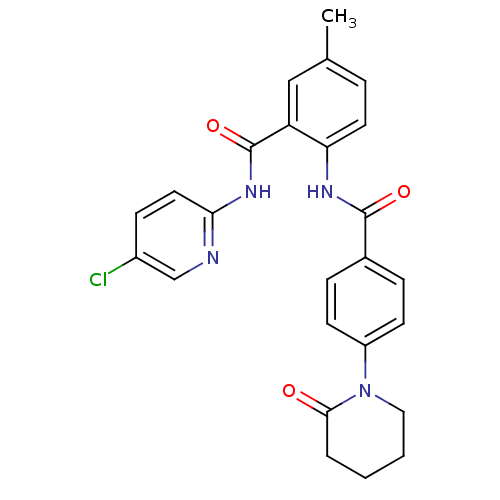
(CHEMBL404448)Show SMILES Cc1ccc(NC(=O)c2ccc(cc2)N2CCCCC2=O)c(c1)C(=O)Nc1ccc(Cl)cn1 Show InChI InChI=1S/C25H23ClN4O3/c1-16-5-11-21(20(14-16)25(33)29-22-12-8-18(26)15-27-22)28-24(32)17-6-9-19(10-7-17)30-13-3-2-4-23(30)31/h5-12,14-15H,2-4,13H2,1H3,(H,28,32)(H,27,29,33) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.190 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of human factor 10a |
Bioorg Med Chem Lett 18: 2845-9 (2008)
Article DOI: 10.1016/j.bmcl.2008.03.092
BindingDB Entry DOI: 10.7270/Q2611169 |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50377643
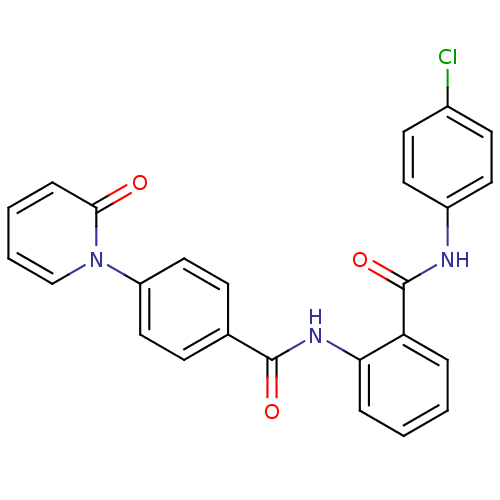
(CHEMBL257966)Show SMILES Clc1ccc(NC(=O)c2ccccc2NC(=O)c2ccc(cc2)-n2ccccc2=O)cc1 Show InChI InChI=1S/C25H18ClN3O3/c26-18-10-12-19(13-11-18)27-25(32)21-5-1-2-6-22(21)28-24(31)17-8-14-20(15-9-17)29-16-4-3-7-23(29)30/h1-16H,(H,27,32)(H,28,31) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.210 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of human factor 10a |
Bioorg Med Chem Lett 18: 2845-9 (2008)
Article DOI: 10.1016/j.bmcl.2008.03.092
BindingDB Entry DOI: 10.7270/Q2611169 |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM12868
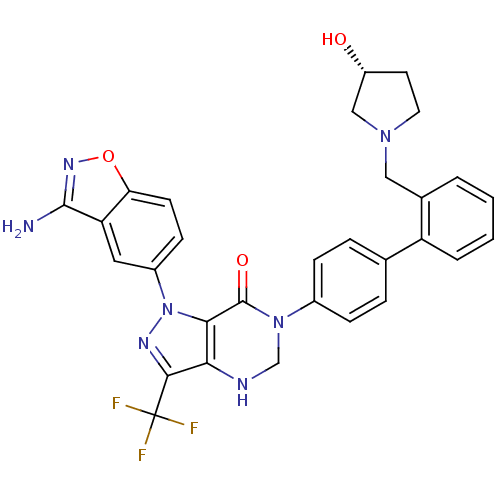
(1-(3-amino-1,2-benzoxazol-5-yl)-6-[4-(2-{[(3R)-3-h...)Show SMILES Nc1noc2ccc(cc12)-n1nc(c2NCN(C(=O)c12)c1ccc(cc1)-c1ccccc1CN1CC[C@@H](O)C1)C(F)(F)F |r| Show InChI InChI=1S/C30H26F3N7O3/c31-30(32,33)27-25-26(40(36-27)20-9-10-24-23(13-20)28(34)37-43-24)29(42)39(16-35-25)19-7-5-17(6-8-19)22-4-2-1-3-18(22)14-38-12-11-21(41)15-38/h1-10,13,21,35,41H,11-12,14-16H2,(H2,34,37)/t21-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.210 | -54.7 | n/a | n/a | n/a | n/a | n/a | 7.0 | 22 |
Bristol-Myers Squibb Pharmaceutical Research Institute
| Assay Description
Ki values were obtained from human purified enzyme. All assays were run in microtiter plates. Plates were read for 30 min at 405 nm. Rates were deter... |
Bioorg Med Chem Lett 16: 5176-82 (2006)
Article DOI: 10.1016/j.bmcl.2006.07.002
BindingDB Entry DOI: 10.7270/Q29K48GC |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM12871
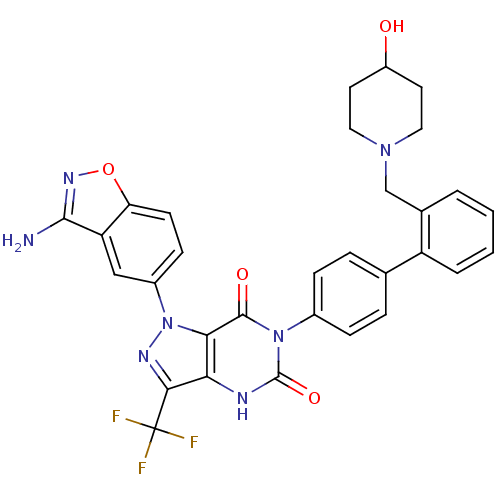
(1-(3-amino-1,2-benzoxazol-5-yl)-6-(4-{2-[(4-hydrox...)Show SMILES Nc1noc2ccc(cc12)-n1nc(c2[nH]c(=O)n(-c3ccc(cc3)-c3ccccc3CN3CCC(O)CC3)c(=O)c12)C(F)(F)F Show InChI InChI=1S/C31H26F3N7O4/c32-31(33,34)27-25-26(41(37-27)20-9-10-24-23(15-20)28(35)38-45-24)29(43)40(30(44)36-25)19-7-5-17(6-8-19)22-4-2-1-3-18(22)16-39-13-11-21(42)12-14-39/h1-10,15,21,42H,11-14,16H2,(H2,35,38)(H,36,44) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.220 | -54.6 | n/a | n/a | n/a | n/a | n/a | 7.0 | 22 |
Bristol-Myers Squibb Pharmaceutical Research Institute
| Assay Description
Ki values were obtained from human purified enzyme. All assays were run in microtiter plates. Plates were read for 30 min at 405 nm. Rates were deter... |
Bioorg Med Chem Lett 16: 5176-82 (2006)
Article DOI: 10.1016/j.bmcl.2006.07.002
BindingDB Entry DOI: 10.7270/Q29K48GC |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50377627

(CHEMBL260369)Show SMILES COc1cc(Cl)cc(C(=O)Nc2ccc(Cl)cn2)c1NC(=O)c1ccc(nc1)-n1ccccc1=O Show InChI InChI=1S/C24H17Cl2N5O4/c1-35-18-11-16(26)10-17(24(34)29-19-7-6-15(25)13-27-19)22(18)30-23(33)14-5-8-20(28-12-14)31-9-3-2-4-21(31)32/h2-13H,1H3,(H,30,33)(H,27,29,34) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.240 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of human factor 10a |
Bioorg Med Chem Lett 18: 2845-9 (2008)
Article DOI: 10.1016/j.bmcl.2008.03.092
BindingDB Entry DOI: 10.7270/Q2611169 |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM12687
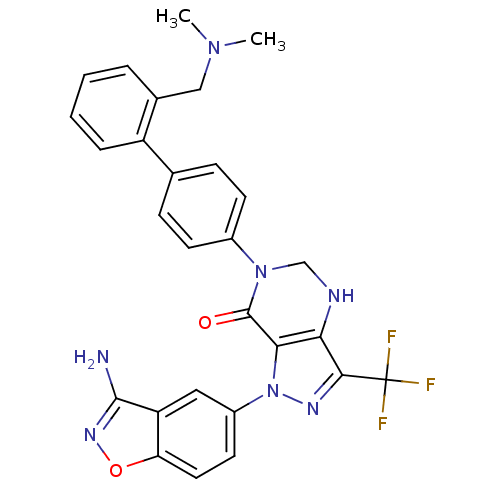
(1-(3-amino-1,2-benzoxazol-5-yl)-6-(4-{2-[(dimethyl...)Show SMILES CN(C)Cc1ccccc1-c1ccc(cc1)N1CNc2c(nn(c2C1=O)-c1ccc2onc(N)c2c1)C(F)(F)F Show InChI InChI=1S/C28H24F3N7O2/c1-36(2)14-17-5-3-4-6-20(17)16-7-9-18(10-8-16)37-15-33-23-24(27(37)39)38(34-25(23)28(29,30)31)19-11-12-22-21(13-19)26(32)35-40-22/h3-13,33H,14-15H2,1-2H3,(H2,32,35) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.25 | -54.3 | n/a | n/a | n/a | n/a | n/a | 7.0 | 22 |
Bristol-Myers Squibb Pharmaceutical Research Institute
| Assay Description
Ki values were obtained from human purified enzyme. All assays were run in microtiter plates. Plates were read for 30 min at 405 nm. Rates were deter... |
Bioorg Med Chem Lett 16: 5176-82 (2006)
Article DOI: 10.1016/j.bmcl.2006.07.002
BindingDB Entry DOI: 10.7270/Q29K48GC |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM12860
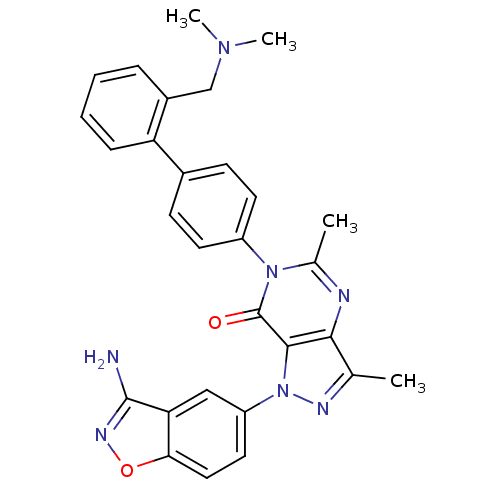
(1-(3-amino-1,2-benzoxazol-5-yl)-6-(4-{2-[(dimethyl...)Show SMILES CN(C)Cc1ccccc1-c1ccc(cc1)-n1c(C)nc2c(C)nn(-c3ccc4onc(N)c4c3)c2c1=O Show InChI InChI=1S/C29H27N7O2/c1-17-26-27(36(32-17)22-13-14-25-24(15-22)28(30)33-38-25)29(37)35(18(2)31-26)21-11-9-19(10-12-21)23-8-6-5-7-20(23)16-34(3)4/h5-15H,16H2,1-4H3,(H2,30,33) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.270 | -54.1 | n/a | n/a | n/a | n/a | n/a | 7.0 | 22 |
Bristol-Myers Squibb Pharmaceutical Research Institute
| Assay Description
Ki values were obtained from human purified enzyme. All assays were run in microtiter plates. Plates were read for 30 min at 405 nm. Rates were deter... |
Bioorg Med Chem Lett 16: 5176-82 (2006)
Article DOI: 10.1016/j.bmcl.2006.07.002
BindingDB Entry DOI: 10.7270/Q29K48GC |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50377632
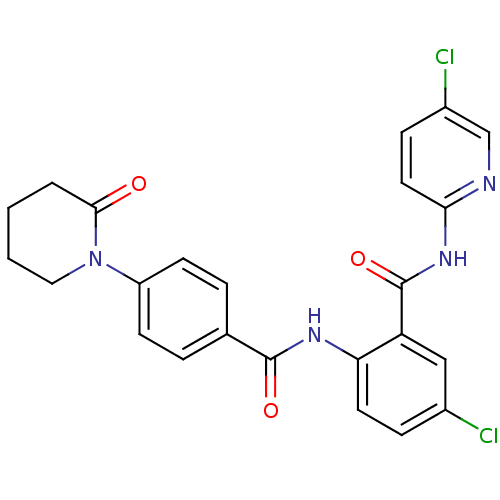
(CHEMBL404449)Show SMILES Clc1ccc(NC(=O)c2cc(Cl)ccc2NC(=O)c2ccc(cc2)N2CCCCC2=O)nc1 Show InChI InChI=1S/C24H20Cl2N4O3/c25-16-6-10-20(19(13-16)24(33)29-21-11-7-17(26)14-27-21)28-23(32)15-4-8-18(9-5-15)30-12-2-1-3-22(30)31/h4-11,13-14H,1-3,12H2,(H,28,32)(H,27,29,33) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.330 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of human factor 10a |
Bioorg Med Chem Lett 18: 2845-9 (2008)
Article DOI: 10.1016/j.bmcl.2008.03.092
BindingDB Entry DOI: 10.7270/Q2611169 |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM12862
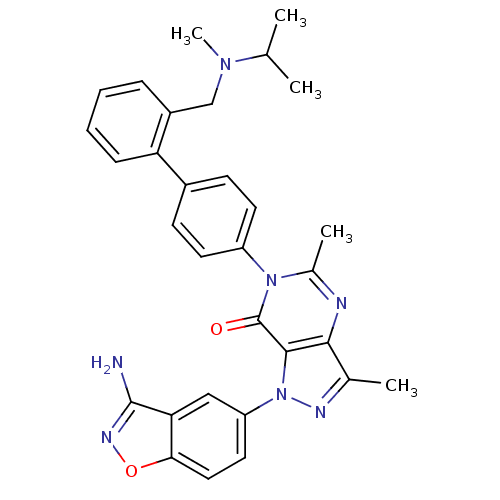
(1-(3-amino-1,2-benzoxazol-5-yl)-3,5-dimethyl-6-[4-...)Show SMILES CC(C)N(C)Cc1ccccc1-c1ccc(cc1)-n1c(C)nc2c(C)nn(-c3ccc4onc(N)c4c3)c2c1=O Show InChI InChI=1S/C31H31N7O2/c1-18(2)36(5)17-22-8-6-7-9-25(22)21-10-12-23(13-11-21)37-20(4)33-28-19(3)34-38(29(28)31(37)39)24-14-15-27-26(16-24)30(32)35-40-27/h6-16,18H,17H2,1-5H3,(H2,32,35) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.350 | -53.4 | n/a | n/a | n/a | n/a | n/a | 7.0 | 22 |
Bristol-Myers Squibb Pharmaceutical Research Institute
| Assay Description
Ki values were obtained from human purified enzyme. All assays were run in microtiter plates. Plates were read for 30 min at 405 nm. Rates were deter... |
Bioorg Med Chem Lett 16: 5176-82 (2006)
Article DOI: 10.1016/j.bmcl.2006.07.002
BindingDB Entry DOI: 10.7270/Q29K48GC |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM12869
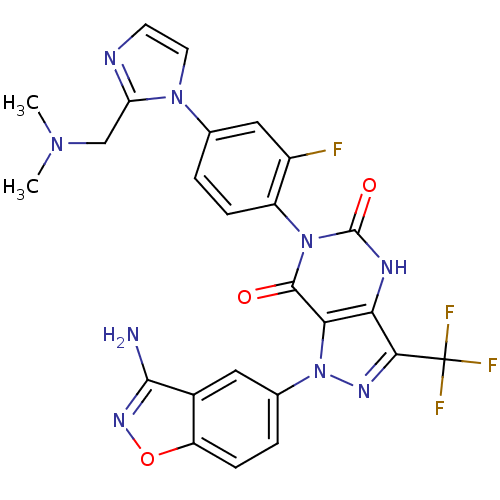
(1-(3-amino-1,2-benzoxazol-5-yl)-6-(4-{2-[(dimethyl...)Show SMILES CN(C)Cc1nccn1-c1ccc(c(F)c1)-n1c(=O)[nH]c2c(nn(-c3ccc4onc(N)c4c3)c2c1=O)C(F)(F)F |(5.96,5.32,;5.96,3.78,;7.29,3.01,;4.63,3.01,;4.63,1.47,;5.87,.57,;5.4,-.9,;3.86,-.9,;3.38,.57,;2.05,1.34,;.71,.57,;-.62,1.34,;-.62,2.88,;.71,3.65,;.71,5.19,;2.05,2.88,;-2.11,3.28,;-2.58,4.74,;-1.55,5.89,;-4.09,5.06,;-5.12,3.92,;-6.66,3.92,;-7.14,2.45,;-5.89,1.55,;-5.89,.01,;-7.23,-.76,;-7.23,-2.3,;-5.89,-3.07,;-5.57,-4.58,;-4.04,-4.74,;-3.41,-3.33,;-1.91,-3.01,;-4.56,-2.3,;-4.56,-.76,;-4.65,2.45,;-3.14,2.13,;-2.74,.65,;-7.57,5.16,;-8.34,6.5,;-6.28,6,;-8.86,4.33,)| Show InChI InChI=1S/C25H19F4N9O3/c1-35(2)11-18-31-7-8-36(18)12-3-5-16(15(26)10-12)37-23(39)20-19(32-24(37)40)21(25(27,28)29)33-38(20)13-4-6-17-14(9-13)22(30)34-41-17/h3-10H,11H2,1-2H3,(H2,30,34)(H,32,40) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.350 | -53.4 | n/a | n/a | n/a | n/a | n/a | 7.0 | 22 |
Bristol-Myers Squibb Pharmaceutical Research Institute
| Assay Description
Ki values were obtained from human purified enzyme. All assays were run in microtiter plates. Plates were read for 30 min at 405 nm. Rates were deter... |
Bioorg Med Chem Lett 16: 5176-82 (2006)
Article DOI: 10.1016/j.bmcl.2006.07.002
BindingDB Entry DOI: 10.7270/Q29K48GC |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM12863
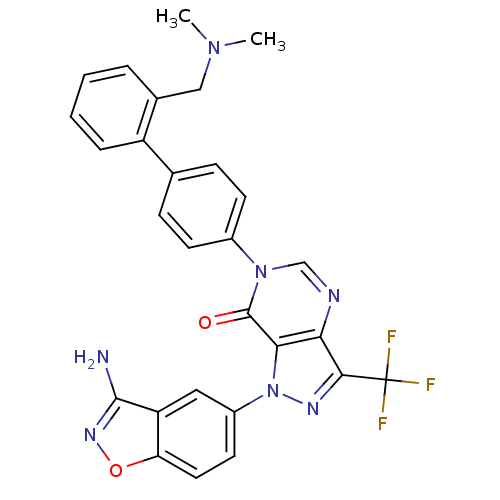
(1-(3-amino-1,2-benzoxazol-5-yl)-6-(4-{2-[(dimethyl...)Show SMILES CN(C)Cc1ccccc1-c1ccc(cc1)-n1cnc2c(nn(-c3ccc4onc(N)c4c3)c2c1=O)C(F)(F)F Show InChI InChI=1S/C28H22F3N7O2/c1-36(2)14-17-5-3-4-6-20(17)16-7-9-18(10-8-16)37-15-33-23-24(27(37)39)38(34-25(23)28(29,30)31)19-11-12-22-21(13-19)26(32)35-40-22/h3-13,15H,14H2,1-2H3,(H2,32,35) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.350 | -53.4 | n/a | n/a | n/a | n/a | n/a | 7.0 | 22 |
Bristol-Myers Squibb Pharmaceutical Research Institute
| Assay Description
Ki values were obtained from human purified enzyme. All assays were run in microtiter plates. Plates were read for 30 min at 405 nm. Rates were deter... |
Bioorg Med Chem Lett 16: 5176-82 (2006)
Article DOI: 10.1016/j.bmcl.2006.07.002
BindingDB Entry DOI: 10.7270/Q29K48GC |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM12861
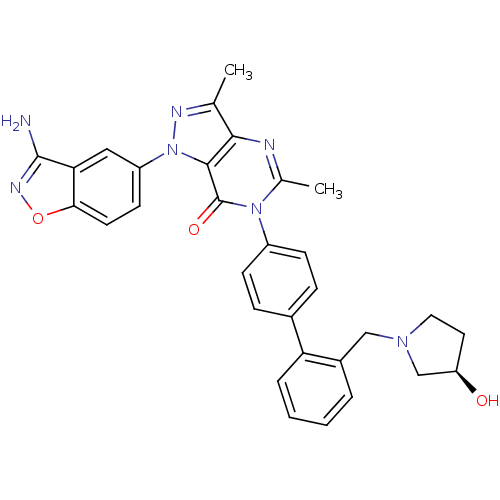
(1-(3-amino-1,2-benzoxazol-5-yl)-6-[4-(2-{[(3R)-3-h...)Show SMILES Cc1nn(-c2ccc3onc(N)c3c2)c2c1nc(C)n(-c1ccc(cc1)-c1ccccc1CN1CC[C@@H](O)C1)c2=O |r| Show InChI InChI=1S/C31H29N7O3/c1-18-28-29(38(34-18)23-11-12-27-26(15-23)30(32)35-41-27)31(40)37(19(2)33-28)22-9-7-20(8-10-22)25-6-4-3-5-21(25)16-36-14-13-24(39)17-36/h3-12,15,24,39H,13-14,16-17H2,1-2H3,(H2,32,35)/t24-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.370 | -53.3 | n/a | n/a | n/a | n/a | n/a | 7.0 | 22 |
Bristol-Myers Squibb Pharmaceutical Research Institute
| Assay Description
Ki values were obtained from human purified enzyme. All assays were run in microtiter plates. Plates were read for 30 min at 405 nm. Rates were deter... |
Bioorg Med Chem Lett 16: 5176-82 (2006)
Article DOI: 10.1016/j.bmcl.2006.07.002
BindingDB Entry DOI: 10.7270/Q29K48GC |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50377633

(CHEMBL403075)Show SMILES Clc1ccc(NC(=O)c2cc(ccc2NC(=O)c2ccc(cc2)N2CCCCC2=O)C#N)nc1 Show InChI InChI=1S/C25H20ClN5O3/c26-18-7-11-22(28-15-18)30-25(34)20-13-16(14-27)4-10-21(20)29-24(33)17-5-8-19(9-6-17)31-12-2-1-3-23(31)32/h4-11,13,15H,1-3,12H2,(H,29,33)(H,28,30,34) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.450 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of human factor 10a |
Bioorg Med Chem Lett 18: 2845-9 (2008)
Article DOI: 10.1016/j.bmcl.2008.03.092
BindingDB Entry DOI: 10.7270/Q2611169 |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM12865
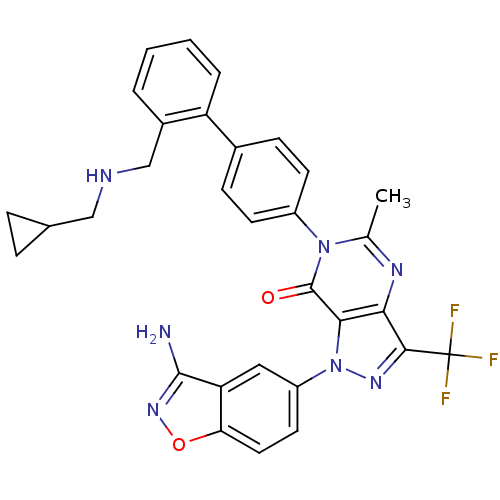
(1-(3-amino-1,2-benzoxazol-5-yl)-6-[4-(2-{[(cyclopr...)Show SMILES Cc1nc2c(nn(-c3ccc4onc(N)c4c3)c2c(=O)n1-c1ccc(cc1)-c1ccccc1CNCC1CC1)C(F)(F)F Show InChI InChI=1S/C31H26F3N7O2/c1-17-37-26-27(41(38-28(26)31(32,33)34)22-12-13-25-24(14-22)29(35)39-43-25)30(42)40(17)21-10-8-19(9-11-21)23-5-3-2-4-20(23)16-36-15-18-6-7-18/h2-5,8-14,18,36H,6-7,15-16H2,1H3,(H2,35,39) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.5 | -52.6 | n/a | n/a | n/a | n/a | n/a | 7.0 | 22 |
Bristol-Myers Squibb Pharmaceutical Research Institute
| Assay Description
Ki values were obtained from human purified enzyme. All assays were run in microtiter plates. Plates were read for 30 min at 405 nm. Rates were deter... |
Bioorg Med Chem Lett 16: 5176-82 (2006)
Article DOI: 10.1016/j.bmcl.2006.07.002
BindingDB Entry DOI: 10.7270/Q29K48GC |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM12857
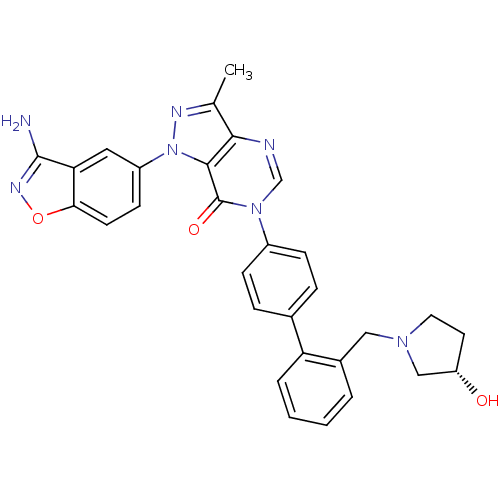
(1-(3-amino-1,2-benzoxazol-5-yl)-6-[4-(2-{[(3S)-3-h...)Show SMILES Cc1nn(-c2ccc3onc(N)c3c2)c2c1ncn(-c1ccc(cc1)-c1ccccc1CN1CC[C@H](O)C1)c2=O |r| Show InChI InChI=1S/C30H27N7O3/c1-18-27-28(37(33-18)22-10-11-26-25(14-22)29(31)34-40-26)30(39)36(17-32-27)21-8-6-19(7-9-21)24-5-3-2-4-20(24)15-35-13-12-23(38)16-35/h2-11,14,17,23,38H,12-13,15-16H2,1H3,(H2,31,34)/t23-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.5 | -52.6 | n/a | n/a | n/a | n/a | n/a | 7.0 | 22 |
Bristol-Myers Squibb Pharmaceutical Research Institute
| Assay Description
Ki values were obtained from human purified enzyme. All assays were run in microtiter plates. Plates were read for 30 min at 405 nm. Rates were deter... |
Bioorg Med Chem Lett 16: 5176-82 (2006)
Article DOI: 10.1016/j.bmcl.2006.07.002
BindingDB Entry DOI: 10.7270/Q29K48GC |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM12855
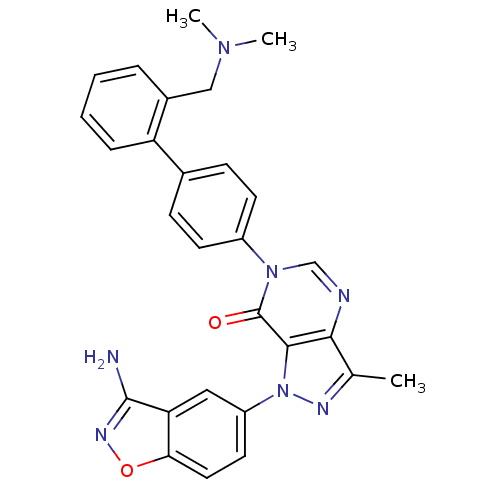
(1-(3-amino-1,2-benzoxazol-5-yl)-6-(4-{2-[(dimethyl...)Show SMILES CN(C)Cc1ccccc1-c1ccc(cc1)-n1cnc2c(C)nn(-c3ccc4onc(N)c4c3)c2c1=O Show InChI InChI=1S/C28H25N7O2/c1-17-25-26(35(31-17)21-12-13-24-23(14-21)27(29)32-37-24)28(36)34(16-30-25)20-10-8-18(9-11-20)22-7-5-4-6-19(22)15-33(2)3/h4-14,16H,15H2,1-3H3,(H2,29,32) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.530 | -52.4 | n/a | n/a | n/a | n/a | n/a | 7.0 | 22 |
Bristol-Myers Squibb Pharmaceutical Research Institute
| Assay Description
Ki values were obtained from human purified enzyme. All assays were run in microtiter plates. Plates were read for 30 min at 405 nm. Rates were deter... |
Bioorg Med Chem Lett 16: 5176-82 (2006)
Article DOI: 10.1016/j.bmcl.2006.07.002
BindingDB Entry DOI: 10.7270/Q29K48GC |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50377646
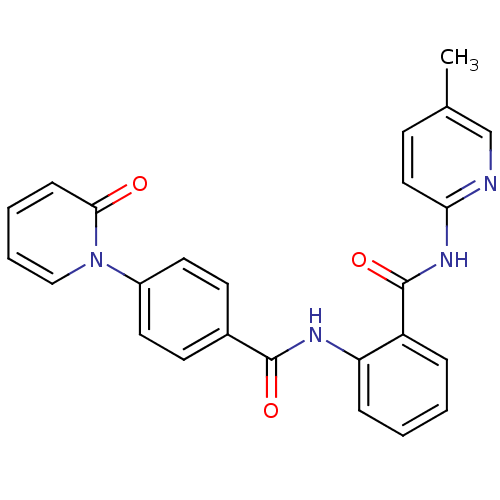
(CHEMBL259535)Show SMILES Cc1ccc(NC(=O)c2ccccc2NC(=O)c2ccc(cc2)-n2ccccc2=O)nc1 Show InChI InChI=1S/C25H20N4O3/c1-17-9-14-22(26-16-17)28-25(32)20-6-2-3-7-21(20)27-24(31)18-10-12-19(13-11-18)29-15-5-4-8-23(29)30/h2-16H,1H3,(H,27,31)(H,26,28,32) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.580 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of human factor 10a |
Bioorg Med Chem Lett 18: 2845-9 (2008)
Article DOI: 10.1016/j.bmcl.2008.03.092
BindingDB Entry DOI: 10.7270/Q2611169 |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50377641

(CHEMBL402836)Show SMILES Clc1ccc(NCc2ccccc2NC(=O)c2ccc(cc2)-n2ccccc2=O)nc1 Show InChI InChI=1S/C24H19ClN4O2/c25-19-10-13-22(27-16-19)26-15-18-5-1-2-6-21(18)28-24(31)17-8-11-20(12-9-17)29-14-4-3-7-23(29)30/h1-14,16H,15H2,(H,26,27)(H,28,31) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.620 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of human factor 10a |
Bioorg Med Chem Lett 18: 2845-9 (2008)
Article DOI: 10.1016/j.bmcl.2008.03.092
BindingDB Entry DOI: 10.7270/Q2611169 |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM12873

(5-amino-1-(3-amino-1,2-benzoxazol-5-yl)-6-(4-{2-[(...)Show SMILES Nc1noc2ccc(cc12)-n1nc(c2nc(N)c(-c3ccc(cc3)-c3ccccc3CN3CCC(O)CC3)c(O)c12)C(F)(F)F Show InChI InChI=1S/C32H28F3N7O3/c33-32(34,35)29-26-27(42(39-29)20-9-10-24-23(15-20)30(36)40-45-24)28(44)25(31(37)38-26)18-7-5-17(6-8-18)22-4-2-1-3-19(22)16-41-13-11-21(43)12-14-41/h1-10,15,21,43H,11-14,16H2,(H2,36,40)(H3,37,38,44) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.770 | -51.5 | n/a | n/a | n/a | n/a | n/a | 7.0 | 22 |
Bristol-Myers Squibb Pharmaceutical Research Institute
| Assay Description
Ki values were obtained from human purified enzyme. All assays were run in microtiter plates. Plates were read for 30 min at 405 nm. Rates were deter... |
Bioorg Med Chem Lett 16: 5176-82 (2006)
Article DOI: 10.1016/j.bmcl.2006.07.002
BindingDB Entry DOI: 10.7270/Q29K48GC |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 1A
(Rattus norvegicus (rat)) | BDBM21393

(7-(dipropylamino)-5,6,7,8-tetrahydronaphthalen-1-o...)Show InChI InChI=1S/C16H25NO/c1-3-10-17(11-4-2)14-9-8-13-6-5-7-16(18)15(13)12-14/h5-7,14,18H,3-4,8-12H2,1-2H3 | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Binding affinity of the compound towards 5-hydroxytryptamine 1A receptor sites in cortical membranes using [3H]-8-OH-DPAT as radioligand |
J Med Chem 33: 1541-4 (1990)
BindingDB Entry DOI: 10.7270/Q2QJ7G8P |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50377647

(CHEMBL411044)Show SMILES Fc1ccc(NC(=O)c2ccccc2NC(=O)c2ccc(cc2)-n2ccccc2=O)nc1 Show InChI InChI=1S/C24H17FN4O3/c25-17-10-13-21(26-15-17)28-24(32)19-5-1-2-6-20(19)27-23(31)16-8-11-18(12-9-16)29-14-4-3-7-22(29)30/h1-15H,(H,27,31)(H,26,28,32) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of human factor 10a |
Bioorg Med Chem Lett 18: 2845-9 (2008)
Article DOI: 10.1016/j.bmcl.2008.03.092
BindingDB Entry DOI: 10.7270/Q2611169 |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50377652
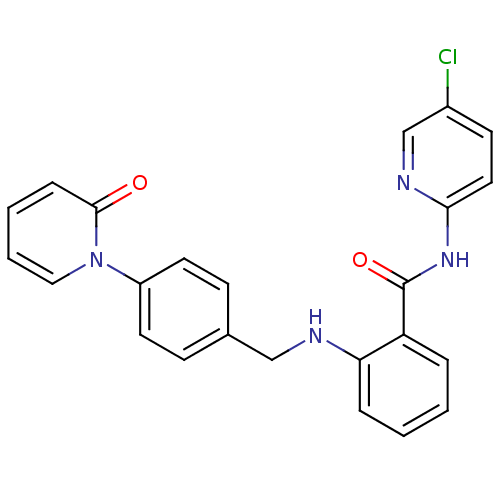
(CHEMBL256573)Show SMILES Clc1ccc(NC(=O)c2ccccc2NCc2ccc(cc2)-n2ccccc2=O)nc1 Show InChI InChI=1S/C24H19ClN4O2/c25-18-10-13-22(27-16-18)28-24(31)20-5-1-2-6-21(20)26-15-17-8-11-19(12-9-17)29-14-4-3-7-23(29)30/h1-14,16,26H,15H2,(H,27,28,31) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of human factor 10a |
Bioorg Med Chem Lett 18: 2845-9 (2008)
Article DOI: 10.1016/j.bmcl.2008.03.092
BindingDB Entry DOI: 10.7270/Q2611169 |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50377650

(CHEMBL260354)Show SMILES Clc1ccc(NC(=O)c2ccccc2NC(=O)c2ccc(cc2)N2CCCCC2=O)nc1 Show InChI InChI=1S/C24H21ClN4O3/c25-17-10-13-21(26-15-17)28-24(32)19-5-1-2-6-20(19)27-23(31)16-8-11-18(12-9-16)29-14-4-3-7-22(29)30/h1-2,5-6,8-13,15H,3-4,7,14H2,(H,27,31)(H,26,28,32) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of human factor 10a |
Bioorg Med Chem Lett 18: 2845-9 (2008)
Article DOI: 10.1016/j.bmcl.2008.03.092
BindingDB Entry DOI: 10.7270/Q2611169 |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM12856
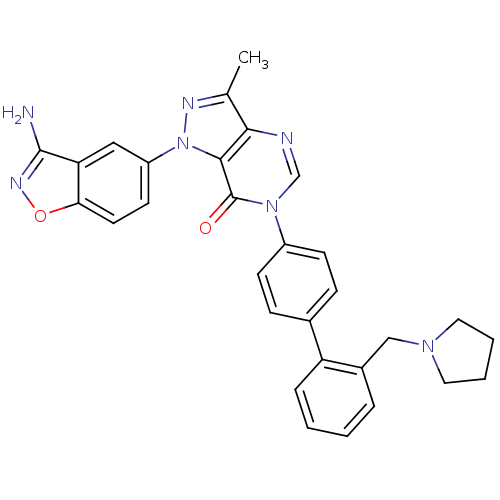
(1-(3-amino-1,2-benzoxazol-5-yl)-3-methyl-6-{4-[2-(...)Show SMILES Cc1nn(-c2ccc3onc(N)c3c2)c2c1ncn(-c1ccc(cc1)-c1ccccc1CN1CCCC1)c2=O Show InChI InChI=1S/C30H27N7O2/c1-19-27-28(37(33-19)23-12-13-26-25(16-23)29(31)34-39-26)30(38)36(18-32-27)22-10-8-20(9-11-22)24-7-3-2-6-21(24)17-35-14-4-5-15-35/h2-3,6-13,16,18H,4-5,14-15,17H2,1H3,(H2,31,34) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 1.30 | -50.2 | n/a | n/a | n/a | n/a | n/a | 7.0 | 22 |
Bristol-Myers Squibb Pharmaceutical Research Institute
| Assay Description
Ki values were obtained from human purified enzyme. All assays were run in microtiter plates. Plates were read for 30 min at 405 nm. Rates were deter... |
Bioorg Med Chem Lett 16: 5176-82 (2006)
Article DOI: 10.1016/j.bmcl.2006.07.002
BindingDB Entry DOI: 10.7270/Q29K48GC |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50377644

(CHEMBL1162978)Show SMILES Clc1cnc(NC(=O)c2ccccc2NC(=O)c2ccc(cc2)-n2ccccc2=O)nc1 Show InChI InChI=1S/C23H16ClN5O3/c24-16-13-25-23(26-14-16)28-22(32)18-5-1-2-6-19(18)27-21(31)15-8-10-17(11-9-15)29-12-4-3-7-20(29)30/h1-14H,(H,27,31)(H,25,26,28,32) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of human factor 10a |
Bioorg Med Chem Lett 18: 2845-9 (2008)
Article DOI: 10.1016/j.bmcl.2008.03.092
BindingDB Entry DOI: 10.7270/Q2611169 |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM12859
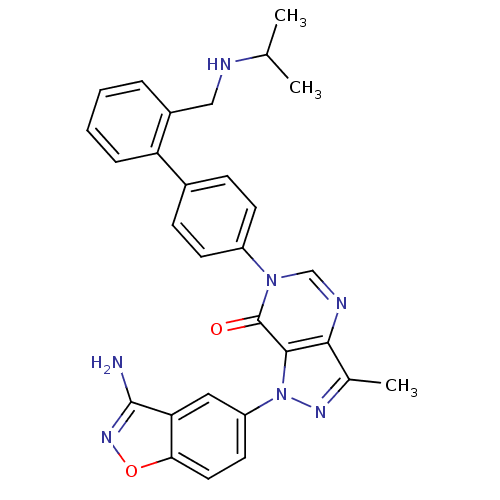
(1-(3-amino-1,2-benzoxazol-5-yl)-3-methyl-6-(4-{2-[...)Show SMILES CC(C)NCc1ccccc1-c1ccc(cc1)-n1cnc2c(C)nn(-c3ccc4onc(N)c4c3)c2c1=O Show InChI InChI=1S/C29H27N7O2/c1-17(2)31-15-20-6-4-5-7-23(20)19-8-10-21(11-9-19)35-16-32-26-18(3)33-36(27(26)29(35)37)22-12-13-25-24(14-22)28(30)34-38-25/h4-14,16-17,31H,15H2,1-3H3,(H2,30,34) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 1.5 | -49.9 | n/a | n/a | n/a | n/a | n/a | 7.0 | 22 |
Bristol-Myers Squibb Pharmaceutical Research Institute
| Assay Description
Ki values were obtained from human purified enzyme. All assays were run in microtiter plates. Plates were read for 30 min at 405 nm. Rates were deter... |
Bioorg Med Chem Lett 16: 5176-82 (2006)
Article DOI: 10.1016/j.bmcl.2006.07.002
BindingDB Entry DOI: 10.7270/Q29K48GC |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM12854
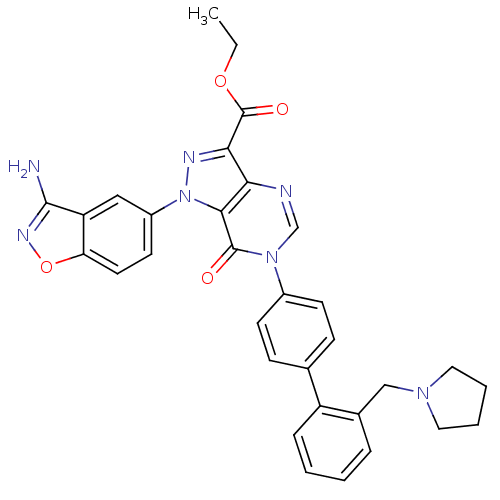
(Pyrazolo[4,3-d]pyrimidinone 12 | ethyl 1-(3-amino-...)Show SMILES CCOC(=O)c1nn(-c2ccc3onc(N)c3c2)c2c1ncn(-c1ccc(cc1)-c1ccccc1CN1CCCC1)c2=O Show InChI InChI=1S/C32H29N7O4/c1-2-42-32(41)28-27-29(39(35-28)23-13-14-26-25(17-23)30(33)36-43-26)31(40)38(19-34-27)22-11-9-20(10-12-22)24-8-4-3-7-21(24)18-37-15-5-6-16-37/h3-4,7-14,17,19H,2,5-6,15-16,18H2,1H3,(H2,33,36) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 1.60 | -49.7 | n/a | n/a | n/a | n/a | n/a | 7.0 | 22 |
Bristol-Myers Squibb Pharmaceutical Research Institute
| Assay Description
Ki values were obtained from human purified enzyme. All assays were run in microtiter plates. Plates were read for 30 min at 405 nm. Rates were deter... |
Bioorg Med Chem Lett 16: 5176-82 (2006)
Article DOI: 10.1016/j.bmcl.2006.07.002
BindingDB Entry DOI: 10.7270/Q29K48GC |
More data for this
Ligand-Target Pair | |
Coagulation factor X
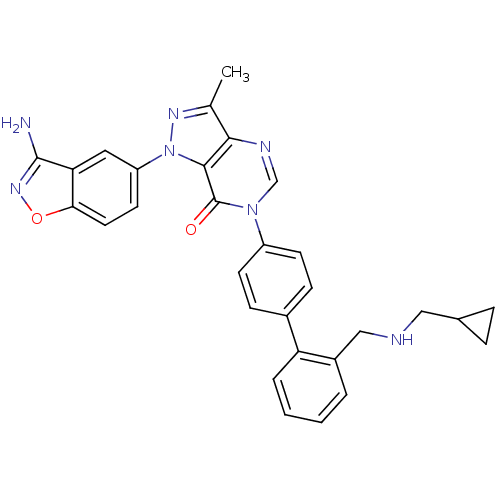
(Homo sapiens (Human)) | BDBM12858
(1-(3-amino-1,2-benzoxazol-5-yl)-6-[4-(2-{[(cyclopr...)Show SMILES Cc1nn(-c2ccc3onc(N)c3c2)c2c1ncn(-c1ccc(cc1)-c1ccccc1CNCC1CC1)c2=O Show InChI InChI=1S/C30H27N7O2/c1-18-27-28(37(34-18)23-12-13-26-25(14-23)29(31)35-39-26)30(38)36(17-33-27)22-10-8-20(9-11-22)24-5-3-2-4-21(24)16-32-15-19-6-7-19/h2-5,8-14,17,19,32H,6-7,15-16H2,1H3,(H2,31,35) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 1.70 | -49.6 | n/a | n/a | n/a | n/a | n/a | 7.0 | 22 |
Bristol-Myers Squibb Pharmaceutical Research Institute
| Assay Description
Ki values were obtained from human purified enzyme. All assays were run in microtiter plates. Plates were read for 30 min at 405 nm. Rates were deter... |
Bioorg Med Chem Lett 16: 5176-82 (2006)
Article DOI: 10.1016/j.bmcl.2006.07.002
BindingDB Entry DOI: 10.7270/Q29K48GC |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50377634

(CHEMBL428432)Show SMILES CS(=O)(=O)Nc1ccc(NC(=O)c2ccc(cc2)N2CCCCC2=O)c(c1)C(=O)Nc1ccc(Cl)cn1 Show InChI InChI=1S/C25H24ClN5O5S/c1-37(35,36)30-18-8-11-21(20(14-18)25(34)29-22-12-7-17(26)15-27-22)28-24(33)16-5-9-19(10-6-16)31-13-3-2-4-23(31)32/h5-12,14-15,30H,2-4,13H2,1H3,(H,28,33)(H,27,29,34) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of human factor 10a |
Bioorg Med Chem Lett 18: 2845-9 (2008)
Article DOI: 10.1016/j.bmcl.2008.03.092
BindingDB Entry DOI: 10.7270/Q2611169 |
More data for this
Ligand-Target Pair | |
Coagulation factor X
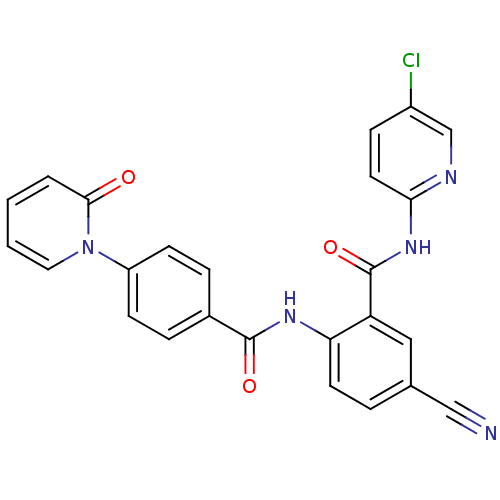
(Homo sapiens (Human)) | BDBM50377639
(CHEMBL258188)Show SMILES Clc1ccc(NC(=O)c2cc(ccc2NC(=O)c2ccc(cc2)-n2ccccc2=O)C#N)nc1 Show InChI InChI=1S/C25H16ClN5O3/c26-18-7-11-22(28-15-18)30-25(34)20-13-16(14-27)4-10-21(20)29-24(33)17-5-8-19(9-6-17)31-12-2-1-3-23(31)32/h1-13,15H,(H,29,33)(H,28,30,34) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of human factor 10a |
Bioorg Med Chem Lett 18: 2845-9 (2008)
Article DOI: 10.1016/j.bmcl.2008.03.092
BindingDB Entry DOI: 10.7270/Q2611169 |
More data for this
Ligand-Target Pair | |
Coagulation factor X
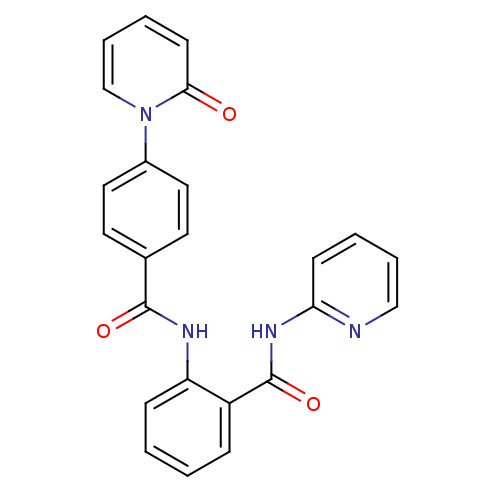
(Homo sapiens (Human)) | BDBM50377649
(CHEMBL260912)Show SMILES O=C(Nc1ccccc1C(=O)Nc1ccccn1)c1ccc(cc1)-n1ccccc1=O Show InChI InChI=1S/C24H18N4O3/c29-22-10-4-6-16-28(22)18-13-11-17(12-14-18)23(30)26-20-8-2-1-7-19(20)24(31)27-21-9-3-5-15-25-21/h1-16H,(H,26,30)(H,25,27,31) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of human factor 10a |
Bioorg Med Chem Lett 18: 2845-9 (2008)
Article DOI: 10.1016/j.bmcl.2008.03.092
BindingDB Entry DOI: 10.7270/Q2611169 |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM12872
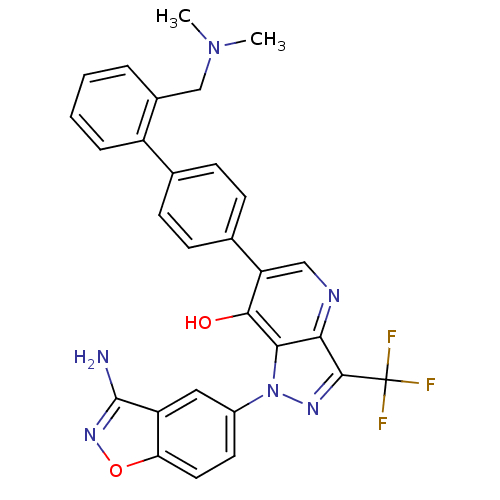
(1-(3-amino-1,2-benzoxazol-5-yl)-6-(4-{2-[(dimethyl...)Show SMILES CN(C)Cc1ccccc1-c1ccc(cc1)-c1cnc2c(nn(-c3ccc4onc(N)c4c3)c2c1O)C(F)(F)F Show InChI InChI=1S/C29H23F3N6O2/c1-37(2)15-18-5-3-4-6-20(18)16-7-9-17(10-8-16)22-14-34-24-25(26(22)39)38(35-27(24)29(30,31)32)19-11-12-23-21(13-19)28(33)36-40-23/h3-14H,15H2,1-2H3,(H2,33,36)(H,34,39) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3.20 | -48.0 | n/a | n/a | n/a | n/a | n/a | 7.0 | 22 |
Bristol-Myers Squibb Pharmaceutical Research Institute
| Assay Description
Ki values were obtained from human purified enzyme. All assays were run in microtiter plates. Plates were read for 30 min at 405 nm. Rates were deter... |
Bioorg Med Chem Lett 16: 5176-82 (2006)
Article DOI: 10.1016/j.bmcl.2006.07.002
BindingDB Entry DOI: 10.7270/Q29K48GC |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50377642
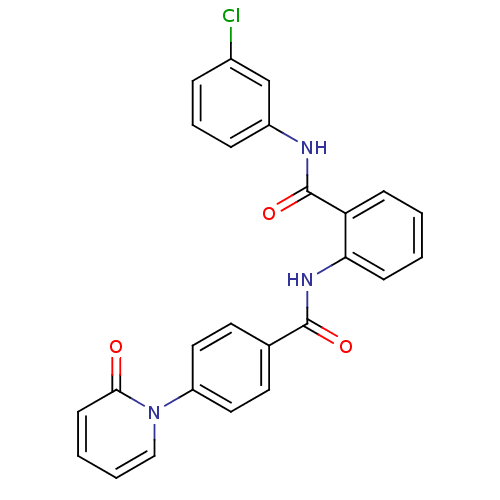
(CHEMBL257967)Show SMILES Clc1cccc(NC(=O)c2ccccc2NC(=O)c2ccc(cc2)-n2ccccc2=O)c1 Show InChI InChI=1S/C25H18ClN3O3/c26-18-6-5-7-19(16-18)27-25(32)21-8-1-2-9-22(21)28-24(31)17-11-13-20(14-12-17)29-15-4-3-10-23(29)30/h1-16H,(H,27,32)(H,28,31) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 4.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of human factor 10a |
Bioorg Med Chem Lett 18: 2845-9 (2008)
Article DOI: 10.1016/j.bmcl.2008.03.092
BindingDB Entry DOI: 10.7270/Q2611169 |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50377653
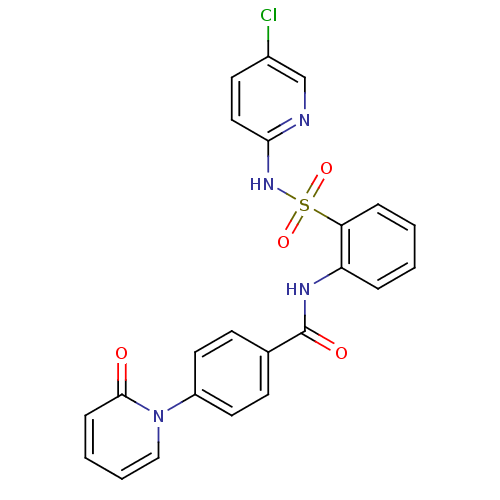
(CHEMBL402761)Show SMILES Clc1ccc(NS(=O)(=O)c2ccccc2NC(=O)c2ccc(cc2)-n2ccccc2=O)nc1 Show InChI InChI=1S/C23H17ClN4O4S/c24-17-10-13-21(25-15-17)27-33(31,32)20-6-2-1-5-19(20)26-23(30)16-8-11-18(12-9-16)28-14-4-3-7-22(28)29/h1-15H,(H,25,27)(H,26,30) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
DrugBank
MMDB
PC cid
PC sid
PDB
UniChem
| MMDB
PDB
Article
PubMed
| 5.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of human factor 10a |
Bioorg Med Chem Lett 18: 2845-9 (2008)
Article DOI: 10.1016/j.bmcl.2008.03.092
BindingDB Entry DOI: 10.7270/Q2611169 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Coagulation factor X
(Homo sapiens (Human)) | BDBM12874
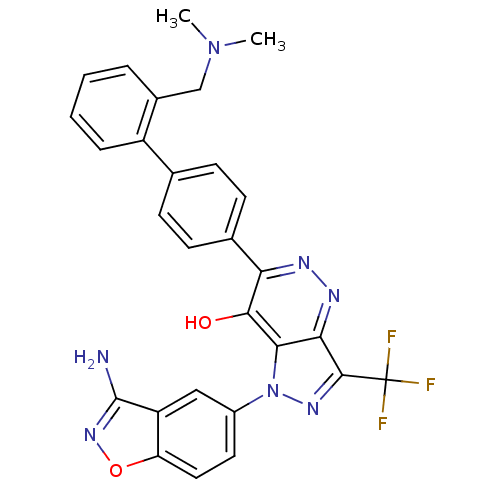
(1-(3-amino-1,2-benzoxazol-5-yl)-6-(4-{2-[(dimethyl...)Show SMILES CN(C)Cc1ccccc1-c1ccc(cc1)-c1nnc2c(nn(-c3ccc4onc(N)c4c3)c2c1O)C(F)(F)F Show InChI InChI=1S/C28H22F3N7O2/c1-37(2)14-17-5-3-4-6-19(17)15-7-9-16(10-8-15)22-25(39)24-23(34-33-22)26(28(29,30)31)35-38(24)18-11-12-21-20(13-18)27(32)36-40-21/h3-13H,14H2,1-2H3,(H2,32,36)(H,34,39) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 5.5 | -46.7 | n/a | n/a | n/a | n/a | n/a | 7.0 | 22 |
Bristol-Myers Squibb Pharmaceutical Research Institute
| Assay Description
Ki values were obtained from human purified enzyme. All assays were run in microtiter plates. Plates were read for 30 min at 405 nm. Rates were deter... |
Bioorg Med Chem Lett 16: 5176-82 (2006)
Article DOI: 10.1016/j.bmcl.2006.07.002
BindingDB Entry DOI: 10.7270/Q29K48GC |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data