 Found 266 hits with Last Name = 'sheng' and Initial = 'z'
Found 266 hits with Last Name = 'sheng' and Initial = 'z' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Leucine-rich repeat serine/threonine-protein kinase 2
(Homo sapiens (Human)) | BDBM50448127
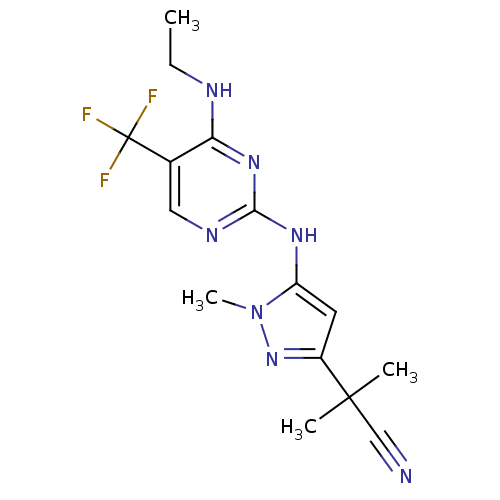
(CHEMBL3122119 | US9212173, 44)Show InChI InChI=1S/C15H18F3N7/c1-5-20-12-9(15(16,17)18)7-21-13(23-12)22-11-6-10(24-25(11)4)14(2,3)8-19/h6-7H,5H2,1-4H3,(H2,20,21,22,23) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 0.700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Inhibition of LRRK2 (unknown origin) |
J Med Chem 57: 921-36 (2014)
Article DOI: 10.1021/jm401654j
BindingDB Entry DOI: 10.7270/Q2N0181N |
More data for this
Ligand-Target Pair | |
Leucine-rich repeat serine/threonine-protein kinase 2
(Homo sapiens (Human)) | BDBM50448118
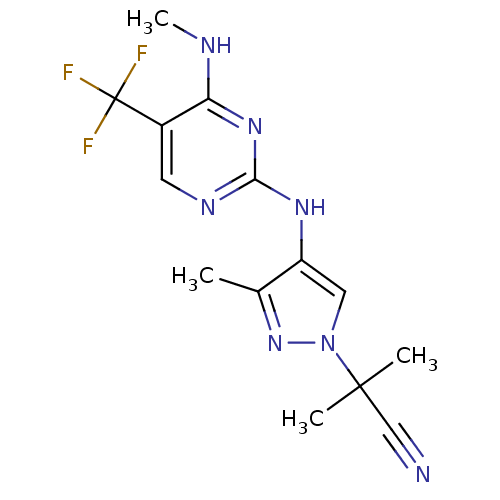
(CHEMBL3122113 | US10590114, No. 80 | US11111235, N...)Show InChI InChI=1S/C14H16F3N7/c1-8-10(6-24(23-8)13(2,3)7-18)21-12-20-5-9(14(15,16)17)11(19-4)22-12/h5-6H,1-4H3,(H2,19,20,21,22) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| 0.700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Inhibition of LRRK2 (unknown origin) |
J Med Chem 57: 921-36 (2014)
Article DOI: 10.1021/jm401654j
BindingDB Entry DOI: 10.7270/Q2N0181N |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Leucine-rich repeat serine/threonine-protein kinase 2
(Homo sapiens (Human)) | BDBM50448126
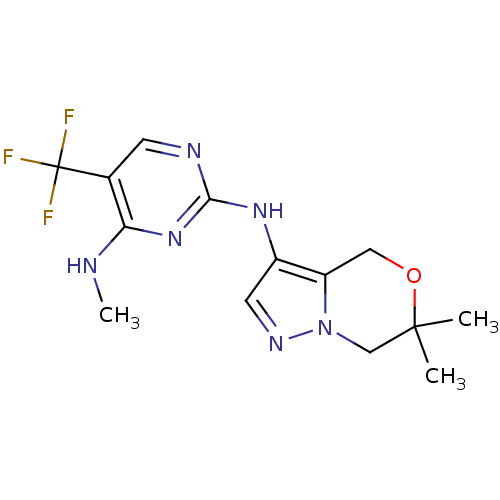
(CHEMBL3122105 | US9212186, 22)Show InChI InChI=1S/C14H17F3N6O/c1-13(2)7-23-10(6-24-13)9(5-20-23)21-12-19-4-8(14(15,16)17)11(18-3)22-12/h4-5H,6-7H2,1-3H3,(H2,18,19,21,22) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Inhibition of LRRK2 (unknown origin) |
J Med Chem 57: 921-36 (2014)
Article DOI: 10.1021/jm401654j
BindingDB Entry DOI: 10.7270/Q2N0181N |
More data for this
Ligand-Target Pair | |
Leucine-rich repeat serine/threonine-protein kinase 2
(Homo sapiens (Human)) | BDBM50398668
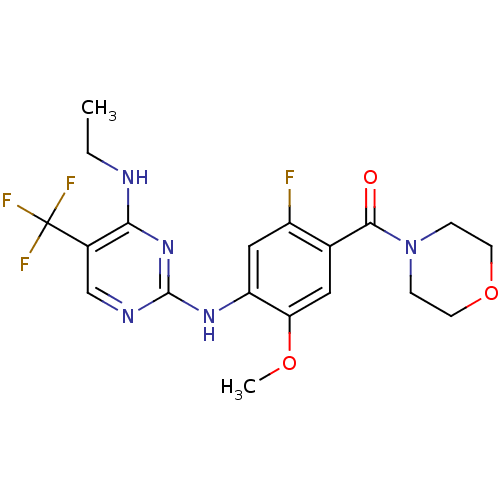
(CHEMBL2178134 | US8802674, 256)Show SMILES CCNc1nc(Nc2cc(F)c(cc2OC)C(=O)N2CCOCC2)ncc1C(F)(F)F Show InChI InChI=1S/C19H21F4N5O3/c1-3-24-16-12(19(21,22)23)10-25-18(27-16)26-14-9-13(20)11(8-15(14)30-2)17(29)28-4-6-31-7-5-28/h8-10H,3-7H2,1-2H3,(H2,24,25,26,27) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Inhibition of LRRK2 using FAM-LRRKtide as substrate after 120 mins by microfluidic capillary electrophoresis assay |
J Med Chem 55: 9416-33 (2012)
Article DOI: 10.1021/jm301020q
BindingDB Entry DOI: 10.7270/Q2P55PN7 |
More data for this
Ligand-Target Pair | |
Leucine-rich repeat serine/threonine-protein kinase 2
(Homo sapiens (Human)) | BDBM50398662
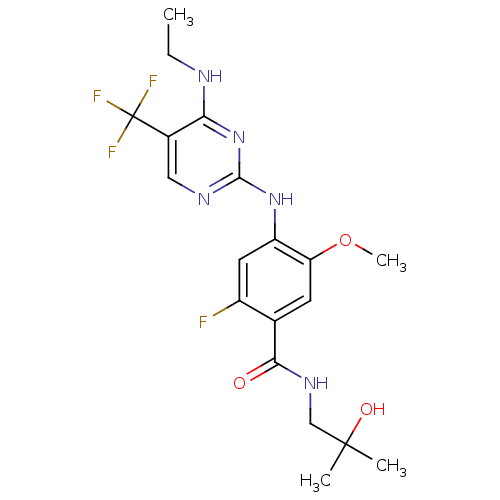
(CHEMBL2178140)Show SMILES CCNc1nc(Nc2cc(F)c(cc2OC)C(=O)NCC(C)(C)O)ncc1C(F)(F)F Show InChI InChI=1S/C19H23F4N5O3/c1-5-24-15-11(19(21,22)23)8-25-17(28-15)27-13-7-12(20)10(6-14(13)31-4)16(29)26-9-18(2,3)30/h6-8,30H,5,9H2,1-4H3,(H,26,29)(H2,24,25,27,28) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Inhibition of LRRK2 using FAM-LRRKtide as substrate after 120 mins by microfluidic capillary electrophoresis assay |
J Med Chem 55: 9416-33 (2012)
Article DOI: 10.1021/jm301020q
BindingDB Entry DOI: 10.7270/Q2P55PN7 |
More data for this
Ligand-Target Pair | |
Leucine-rich repeat serine/threonine-protein kinase 2
(Homo sapiens (Human)) | BDBM50448117
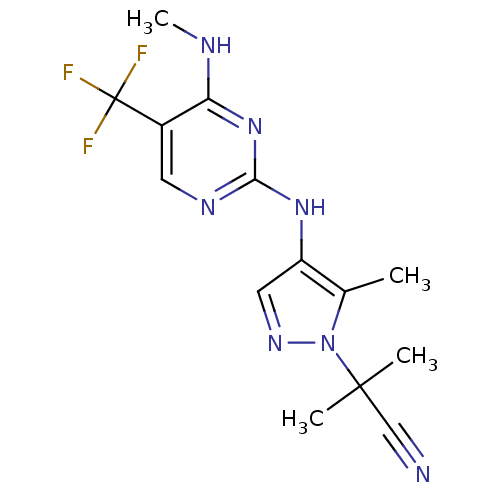
(CHEMBL3122114)Show InChI InChI=1S/C14H16F3N7/c1-8-10(6-21-24(8)13(2,3)7-18)22-12-20-5-9(14(15,16)17)11(19-4)23-12/h5-6H,1-4H3,(H2,19,20,22,23) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Inhibition of LRRK2 (unknown origin) |
J Med Chem 57: 921-36 (2014)
Article DOI: 10.1021/jm401654j
BindingDB Entry DOI: 10.7270/Q2N0181N |
More data for this
Ligand-Target Pair | |
Leucine-rich repeat serine/threonine-protein kinase 2
(Homo sapiens (Human)) | BDBM50398676
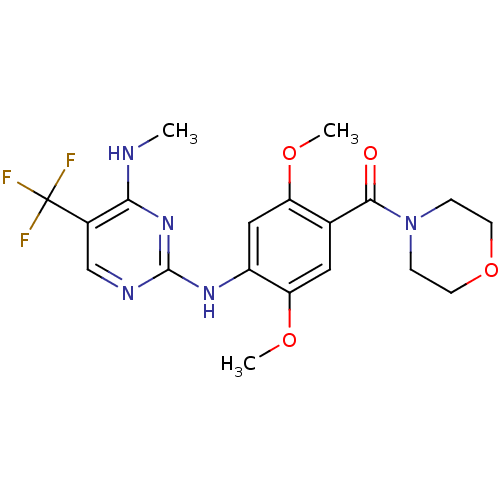
(CHEMBL2178125)Show SMILES CNc1nc(Nc2cc(OC)c(cc2OC)C(=O)N2CCOCC2)ncc1C(F)(F)F Show InChI InChI=1S/C19H22F3N5O4/c1-23-16-12(19(20,21)22)10-24-18(26-16)25-13-9-14(29-2)11(8-15(13)30-3)17(28)27-4-6-31-7-5-27/h8-10H,4-7H2,1-3H3,(H2,23,24,25,26) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Inhibition of LRRK2 using FAM-LRRKtide as substrate after 120 mins by microfluidic capillary electrophoresis assay |
J Med Chem 55: 9416-33 (2012)
Article DOI: 10.1021/jm301020q
BindingDB Entry DOI: 10.7270/Q2P55PN7 |
More data for this
Ligand-Target Pair | |
Leucine-rich repeat serine/threonine-protein kinase 2
(Homo sapiens (Human)) | BDBM50398667

(CHEMBL2178135)Show SMILES COc1cc(C(=O)N2CCOCC2)c(F)cc1Nc1ncc(c(NC2CC2)n1)C(F)(F)F Show InChI InChI=1S/C20H21F4N5O3/c1-31-16-8-12(18(30)29-4-6-32-7-5-29)14(21)9-15(16)27-19-25-10-13(20(22,23)24)17(28-19)26-11-2-3-11/h8-11H,2-7H2,1H3,(H2,25,26,27,28) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Inhibition of LRRK2 using FAM-LRRKtide as substrate after 120 mins by microfluidic capillary electrophoresis assay |
J Med Chem 55: 9416-33 (2012)
Article DOI: 10.1021/jm301020q
BindingDB Entry DOI: 10.7270/Q2P55PN7 |
More data for this
Ligand-Target Pair | |
Leucine-rich repeat serine/threonine-protein kinase 2
(Homo sapiens (Human)) | BDBM50398665
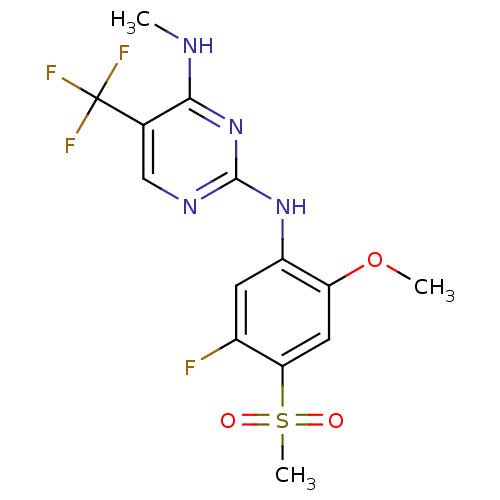
(CHEMBL2178137 | US9145402, 15)Show SMILES CNc1nc(Nc2cc(F)c(cc2OC)S(C)(=O)=O)ncc1C(F)(F)F Show InChI InChI=1S/C14H14F4N4O3S/c1-19-12-7(14(16,17)18)6-20-13(22-12)21-9-4-8(15)11(26(3,23)24)5-10(9)25-2/h4-6H,1-3H3,(H2,19,20,21,22) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Inhibition of LRRK2 using FAM-LRRKtide as substrate after 120 mins by microfluidic capillary electrophoresis assay |
J Med Chem 55: 9416-33 (2012)
Article DOI: 10.1021/jm301020q
BindingDB Entry DOI: 10.7270/Q2P55PN7 |
More data for this
Ligand-Target Pair | |
Leucine-rich repeat serine/threonine-protein kinase 2
(Homo sapiens (Human)) | BDBM50448115
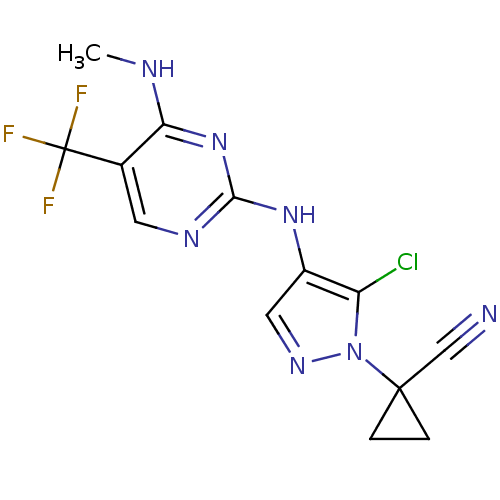
(CHEMBL3122118)Show InChI InChI=1S/C13H11ClF3N7/c1-19-10-7(13(15,16)17)4-20-11(23-10)22-8-5-21-24(9(8)14)12(6-18)2-3-12/h4-5H,2-3H2,1H3,(H2,19,20,22,23) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Inhibition of LRRK2 (unknown origin) |
J Med Chem 57: 921-36 (2014)
Article DOI: 10.1021/jm401654j
BindingDB Entry DOI: 10.7270/Q2N0181N |
More data for this
Ligand-Target Pair | |
Leucine-rich repeat serine/threonine-protein kinase 2
(Homo sapiens (Human)) | BDBM50396150
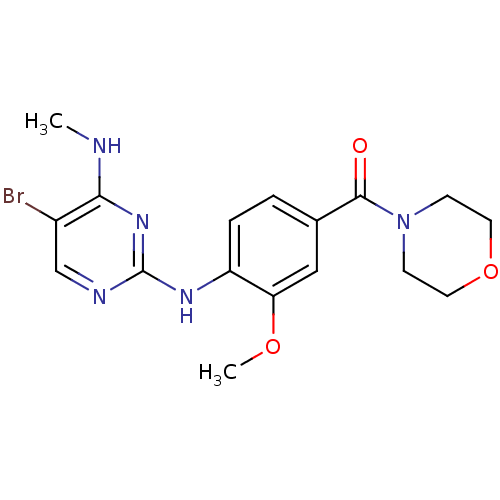
(CHEMBL2171743)Show InChI InChI=1S/C17H20BrN5O3/c1-19-15-12(18)10-20-17(22-15)21-13-4-3-11(9-14(13)25-2)16(24)23-5-7-26-8-6-23/h3-4,9-10H,5-8H2,1-2H3,(H2,19,20,21,22) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Inhibition of LRRK2 using FAM-LRRKtide as substrate after 120 mins by microfluidic capillary electrophoresis assay |
J Med Chem 55: 9416-33 (2012)
Article DOI: 10.1021/jm301020q
BindingDB Entry DOI: 10.7270/Q2P55PN7 |
More data for this
Ligand-Target Pair | |
Leucine-rich repeat serine/threonine-protein kinase 2
(Homo sapiens (Human)) | BDBM50448111
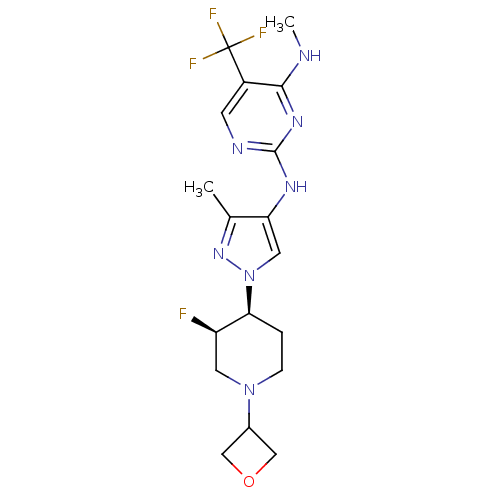
(CHEMBL3122116)Show SMILES CNc1nc(Nc2cn(nc2C)[C@H]2CCN(C[C@H]2F)C2COC2)ncc1C(F)(F)F |r| Show InChI InChI=1S/C18H23F4N7O/c1-10-14(25-17-24-5-12(18(20,21)22)16(23-2)26-17)7-29(27-10)15-3-4-28(6-13(15)19)11-8-30-9-11/h5,7,11,13,15H,3-4,6,8-9H2,1-2H3,(H2,23,24,25,26)/t13-,15+/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Inhibition of LRRK2 (unknown origin) |
J Med Chem 57: 921-36 (2014)
Article DOI: 10.1021/jm401654j
BindingDB Entry DOI: 10.7270/Q2N0181N |
More data for this
Ligand-Target Pair | |
Leucine-rich repeat serine/threonine-protein kinase 2
(Homo sapiens (Human)) | BDBM50448113
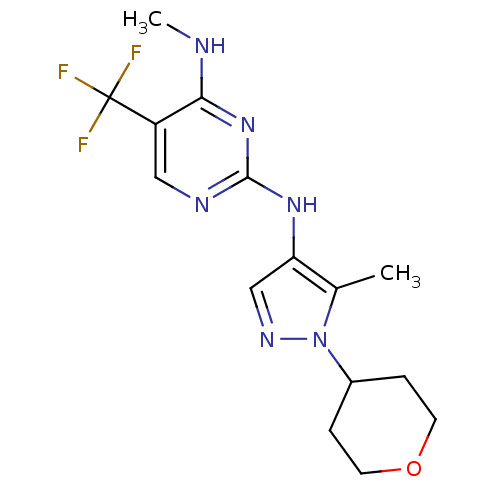
(CHEMBL3122121)Show InChI InChI=1S/C15H19F3N6O/c1-9-12(8-21-24(9)10-3-5-25-6-4-10)22-14-20-7-11(15(16,17)18)13(19-2)23-14/h7-8,10H,3-6H2,1-2H3,(H2,19,20,22,23) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Inhibition of LRRK2 (unknown origin) |
J Med Chem 57: 921-36 (2014)
Article DOI: 10.1021/jm401654j
BindingDB Entry DOI: 10.7270/Q2N0181N |
More data for this
Ligand-Target Pair | |
Leucine-rich repeat serine/threonine-protein kinase 2
(Homo sapiens (Human)) | BDBM50448114
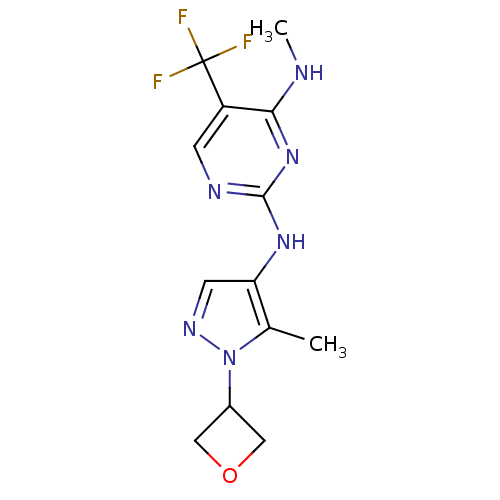
(CHEMBL3122120)Show InChI InChI=1S/C13H15F3N6O/c1-7-10(4-19-22(7)8-5-23-6-8)20-12-18-3-9(13(14,15)16)11(17-2)21-12/h3-4,8H,5-6H2,1-2H3,(H2,17,18,20,21) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Inhibition of LRRK2 (unknown origin) |
J Med Chem 57: 921-36 (2014)
Article DOI: 10.1021/jm401654j
BindingDB Entry DOI: 10.7270/Q2N0181N |
More data for this
Ligand-Target Pair | |
Leucine-rich repeat serine/threonine-protein kinase 2
(Homo sapiens (Human)) | BDBM50396148

(CHEMBL2171745 | US8802674, 50)Show SMILES CNc1nc(Nc2ccc(cc2OC)C(=O)N2CCOCC2)ncc1C(F)(F)F Show InChI InChI=1S/C18H20F3N5O3/c1-22-15-12(18(19,20)21)10-23-17(25-15)24-13-4-3-11(9-14(13)28-2)16(27)26-5-7-29-8-6-26/h3-4,9-10H,5-8H2,1-2H3,(H2,22,23,24,25) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Inhibition of LRRK2 using FAM-LRRKtide as substrate after 120 mins by microfluidic capillary electrophoresis assay |
J Med Chem 55: 9416-33 (2012)
Article DOI: 10.1021/jm301020q
BindingDB Entry DOI: 10.7270/Q2P55PN7 |
More data for this
Ligand-Target Pair | |
Leucine-rich repeat serine/threonine-protein kinase 2
(Homo sapiens (Human)) | BDBM50448110
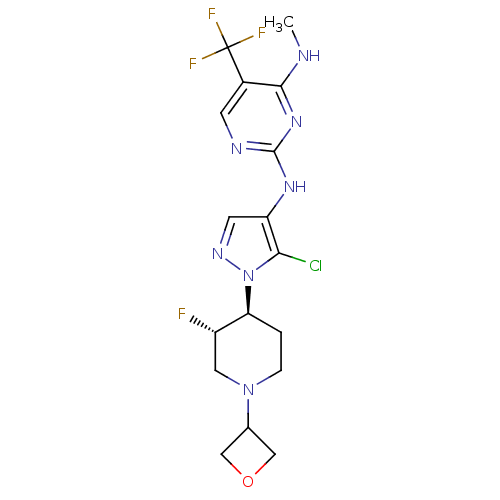
(CHEMBL3122117)Show SMILES CNc1nc(Nc2cnn([C@H]3CCN(C[C@@H]3F)C3COC3)c2Cl)ncc1C(F)(F)F |r| Show InChI InChI=1S/C17H20ClF4N7O/c1-23-15-10(17(20,21)22)4-24-16(27-15)26-12-5-25-29(14(12)18)13-2-3-28(6-11(13)19)9-7-30-8-9/h4-5,9,11,13H,2-3,6-8H2,1H3,(H2,23,24,26,27)/t11-,13-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Inhibition of LRRK2 (unknown origin) |
J Med Chem 57: 921-36 (2014)
Article DOI: 10.1021/jm401654j
BindingDB Entry DOI: 10.7270/Q2N0181N |
More data for this
Ligand-Target Pair | |
Leucine-rich repeat serine/threonine-protein kinase 2
(Homo sapiens (Human)) | BDBM50398672
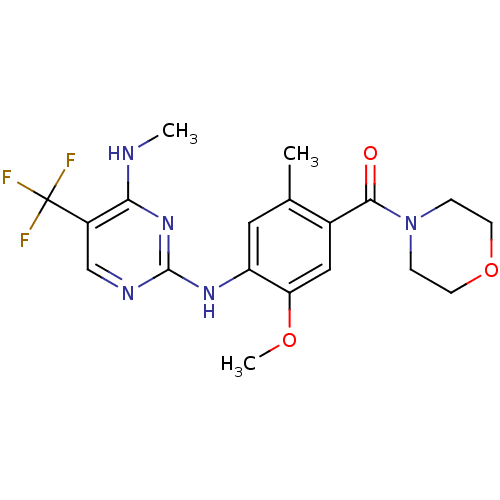
(CHEMBL2178130)Show SMILES CNc1nc(Nc2cc(C)c(cc2OC)C(=O)N2CCOCC2)ncc1C(F)(F)F Show InChI InChI=1S/C19H22F3N5O3/c1-11-8-14(25-18-24-10-13(19(20,21)22)16(23-2)26-18)15(29-3)9-12(11)17(28)27-4-6-30-7-5-27/h8-10H,4-7H2,1-3H3,(H2,23,24,25,26) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Inhibition of LRRK2 using FAM-LRRKtide as substrate after 120 mins by microfluidic capillary electrophoresis assay |
J Med Chem 55: 9416-33 (2012)
Article DOI: 10.1021/jm301020q
BindingDB Entry DOI: 10.7270/Q2P55PN7 |
More data for this
Ligand-Target Pair | |
Leucine-rich repeat serine/threonine-protein kinase 2
(Homo sapiens (Human)) | BDBM50398677
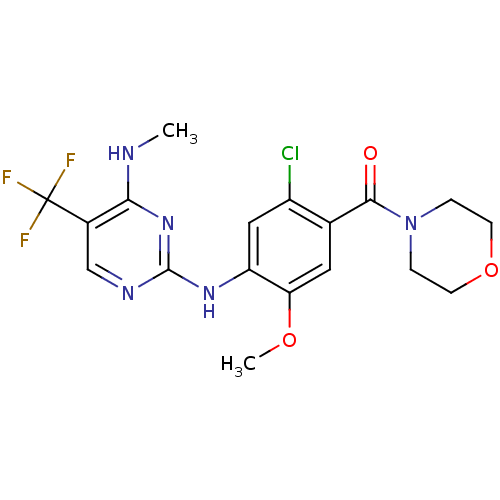
(CHEMBL2178124 | US8802674, 292)Show SMILES CNc1nc(Nc2cc(Cl)c(cc2OC)C(=O)N2CCOCC2)ncc1C(F)(F)F Show InChI InChI=1S/C18H19ClF3N5O3/c1-23-15-11(18(20,21)22)9-24-17(26-15)25-13-8-12(19)10(7-14(13)29-2)16(28)27-3-5-30-6-4-27/h7-9H,3-6H2,1-2H3,(H2,23,24,25,26) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Inhibition of LRRK2 using FAM-LRRKtide as substrate after 120 mins by microfluidic capillary electrophoresis assay |
J Med Chem 55: 9416-33 (2012)
Article DOI: 10.1021/jm301020q
BindingDB Entry DOI: 10.7270/Q2P55PN7 |
More data for this
Ligand-Target Pair | |
Leucine-rich repeat serine/threonine-protein kinase 2
(Homo sapiens (Human)) | BDBM50398674
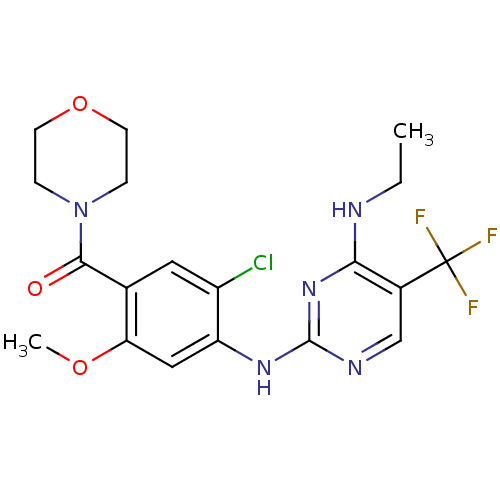
(CHEMBL2178127 | US8802674, 147)Show SMILES CCNc1nc(Nc2cc(OC)c(cc2Cl)C(=O)N2CCOCC2)ncc1C(F)(F)F Show InChI InChI=1S/C19H21ClF3N5O3/c1-3-24-16-12(19(21,22)23)10-25-18(27-16)26-14-9-15(30-2)11(8-13(14)20)17(29)28-4-6-31-7-5-28/h8-10H,3-7H2,1-2H3,(H2,24,25,26,27) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Inhibition of LRRK2 using FAM-LRRKtide as substrate after 120 mins by microfluidic capillary electrophoresis assay |
J Med Chem 55: 9416-33 (2012)
Article DOI: 10.1021/jm301020q
BindingDB Entry DOI: 10.7270/Q2P55PN7 |
More data for this
Ligand-Target Pair | |
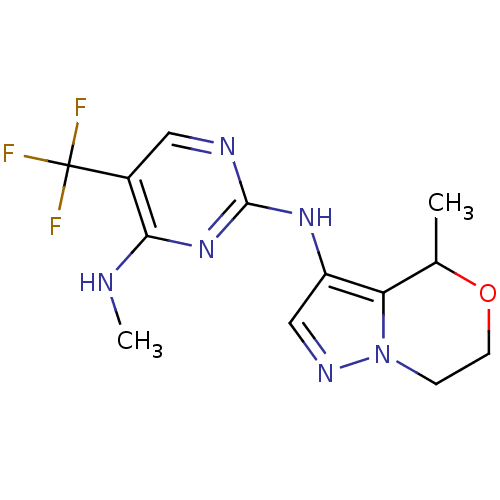
Leucine-rich repeat serine/threonine-protein kinase 2
(Homo sapiens (Human)) | BDBM50448123
(CHEMBL3122108 | US9212186, 16)Show InChI InChI=1S/C13H15F3N6O/c1-7-10-9(6-19-22(10)3-4-23-7)20-12-18-5-8(13(14,15)16)11(17-2)21-12/h5-7H,3-4H2,1-2H3,(H2,17,18,20,21) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Inhibition of LRRK2 (unknown origin) |
J Med Chem 57: 921-36 (2014)
Article DOI: 10.1021/jm401654j
BindingDB Entry DOI: 10.7270/Q2N0181N |
More data for this
Ligand-Target Pair | |
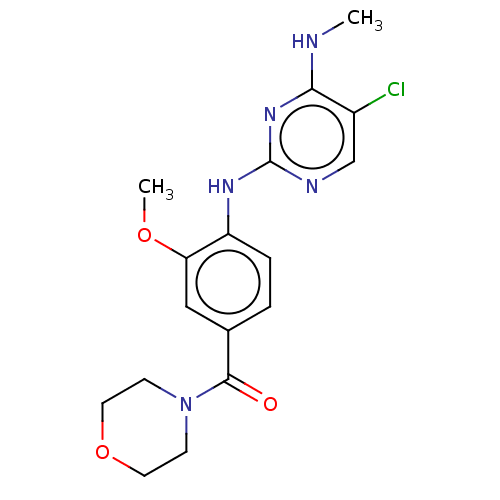
Leucine-rich repeat serine/threonine-protein kinase 2
(Homo sapiens (Human)) | BDBM482160
(BDBM50396041 | HG-10-102-01)Show InChI InChI=1S/C17H20ClN5O3/c1-19-15-12(18)10-20-17(22-15)21-13-4-3-11(9-14(13)25-2)16(24)23-5-7-26-8-6-23/h3-4,9-10H,5-8H2,1-2H3,(H2,19,20,21,22) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Inhibition of LRRK2 using FAM-LRRKtide as substrate after 120 mins by microfluidic capillary electrophoresis assay |
J Med Chem 55: 9416-33 (2012)
Article DOI: 10.1021/jm301020q
BindingDB Entry DOI: 10.7270/Q2P55PN7 |
More data for this
Ligand-Target Pair | |
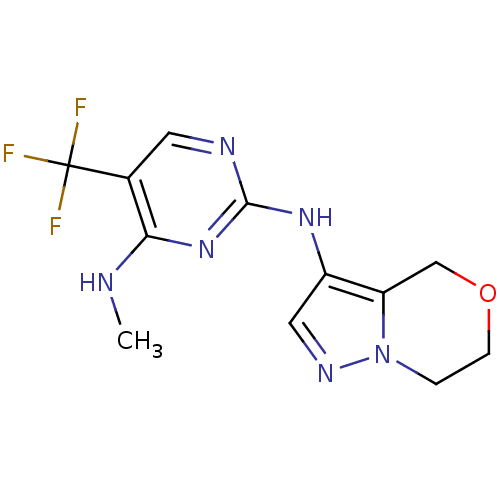
Leucine-rich repeat serine/threonine-protein kinase 2
(Homo sapiens (Human)) | BDBM50448125
(CHEMBL3122106 | US9212186, 17)Show InChI InChI=1S/C12H13F3N6O/c1-16-10-7(12(13,14)15)4-17-11(20-10)19-8-5-18-21-2-3-22-6-9(8)21/h4-5H,2-3,6H2,1H3,(H2,16,17,19,20) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Inhibition of LRRK2 (unknown origin) |
J Med Chem 57: 921-36 (2014)
Article DOI: 10.1021/jm401654j
BindingDB Entry DOI: 10.7270/Q2N0181N |
More data for this
Ligand-Target Pair | |
Leucine-rich repeat serine/threonine-protein kinase 2
(Homo sapiens (Human)) | BDBM50448116
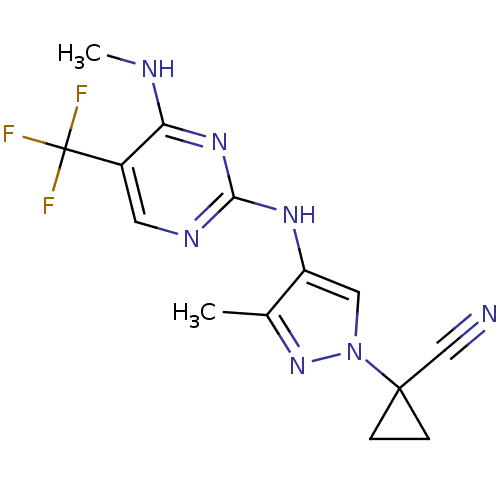
(CHEMBL3122115)Show InChI InChI=1S/C14H14F3N7/c1-8-10(6-24(23-8)13(7-18)3-4-13)21-12-20-5-9(14(15,16)17)11(19-2)22-12/h5-6H,3-4H2,1-2H3,(H2,19,20,21,22) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Inhibition of LRRK2 (unknown origin) |
J Med Chem 57: 921-36 (2014)
Article DOI: 10.1021/jm401654j
BindingDB Entry DOI: 10.7270/Q2N0181N |
More data for this
Ligand-Target Pair | |
Leucine-rich repeat serine/threonine-protein kinase 2
(Homo sapiens (Human)) | BDBM50398664
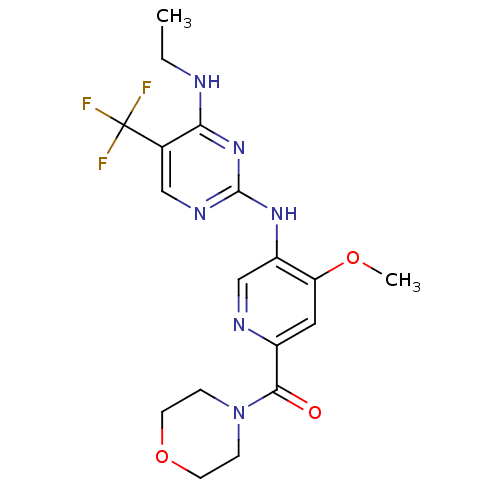
(CHEMBL2178138)Show SMILES CCNc1nc(Nc2cnc(cc2OC)C(=O)N2CCOCC2)ncc1C(F)(F)F Show InChI InChI=1S/C18H21F3N6O3/c1-3-22-15-11(18(19,20)21)9-24-17(26-15)25-13-10-23-12(8-14(13)29-2)16(28)27-4-6-30-7-5-27/h8-10H,3-7H2,1-2H3,(H2,22,24,25,26) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Inhibition of LRRK2 using FAM-LRRKtide as substrate after 120 mins by microfluidic capillary electrophoresis assay |
J Med Chem 55: 9416-33 (2012)
Article DOI: 10.1021/jm301020q
BindingDB Entry DOI: 10.7270/Q2P55PN7 |
More data for this
Ligand-Target Pair | |
Leucine-rich repeat serine/threonine-protein kinase 2
(Homo sapiens (Human)) | BDBM50398675
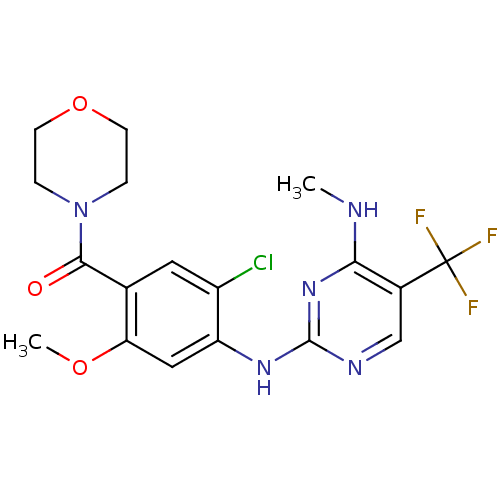
(CHEMBL2178126 | US8802674, 296)Show SMILES CNc1nc(Nc2cc(OC)c(cc2Cl)C(=O)N2CCOCC2)ncc1C(F)(F)F Show InChI InChI=1S/C18H19ClF3N5O3/c1-23-15-11(18(20,21)22)9-24-17(26-15)25-13-8-14(29-2)10(7-12(13)19)16(28)27-3-5-30-6-4-27/h7-9H,3-6H2,1-2H3,(H2,23,24,25,26) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Inhibition of LRRK2 using FAM-LRRKtide as substrate after 120 mins by microfluidic capillary electrophoresis assay |
J Med Chem 55: 9416-33 (2012)
Article DOI: 10.1021/jm301020q
BindingDB Entry DOI: 10.7270/Q2P55PN7 |
More data for this
Ligand-Target Pair | |
Leucine-rich repeat serine/threonine-protein kinase 2
(Homo sapiens (Human)) | BDBM50398661
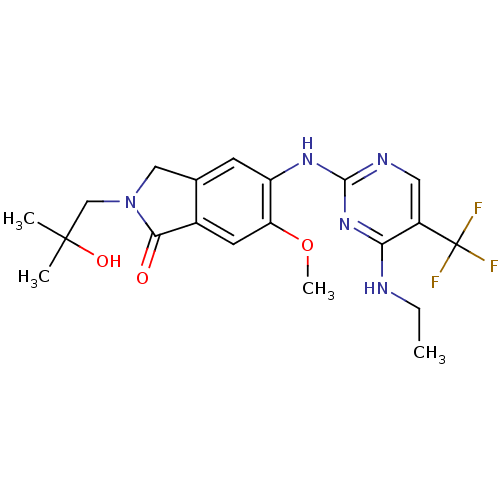
(CHEMBL2178141 | US8796296, 24)Show SMILES CCNc1nc(Nc2cc3CN(CC(C)(C)O)C(=O)c3cc2OC)ncc1C(F)(F)F Show InChI InChI=1S/C20H24F3N5O3/c1-5-24-16-13(20(21,22)23)8-25-18(27-16)26-14-6-11-9-28(10-19(2,3)30)17(29)12(11)7-15(14)31-4/h6-8,30H,5,9-10H2,1-4H3,(H2,24,25,26,27) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Inhibition of LRRK2 using FAM-LRRKtide as substrate after 120 mins by microfluidic capillary electrophoresis assay |
J Med Chem 55: 9416-33 (2012)
Article DOI: 10.1021/jm301020q
BindingDB Entry DOI: 10.7270/Q2P55PN7 |
More data for this
Ligand-Target Pair | |
Leucine-rich repeat serine/threonine-protein kinase 2
(Homo sapiens (Human)) | BDBM50448112
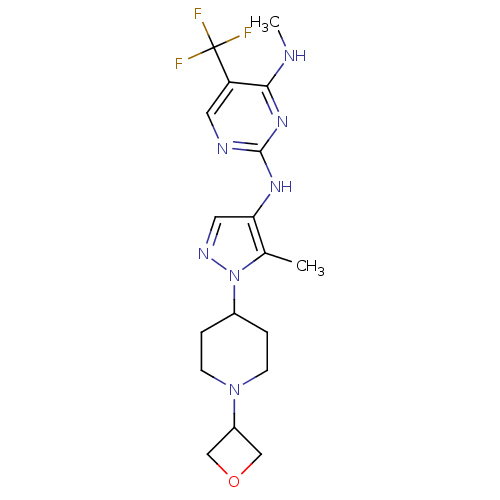
(CHEMBL3122122)Show SMILES CNc1nc(Nc2cnn(C3CCN(CC3)C3COC3)c2C)ncc1C(F)(F)F Show InChI InChI=1S/C18H24F3N7O/c1-11-15(25-17-23-7-14(18(19,20)21)16(22-2)26-17)8-24-28(11)12-3-5-27(6-4-12)13-9-29-10-13/h7-8,12-13H,3-6,9-10H2,1-2H3,(H2,22,23,25,26) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Inhibition of LRRK2 (unknown origin) |
J Med Chem 57: 921-36 (2014)
Article DOI: 10.1021/jm401654j
BindingDB Entry DOI: 10.7270/Q2N0181N |
More data for this
Ligand-Target Pair | |
Leucine-rich repeat serine/threonine-protein kinase 2
(Homo sapiens (Human)) | BDBM50398659
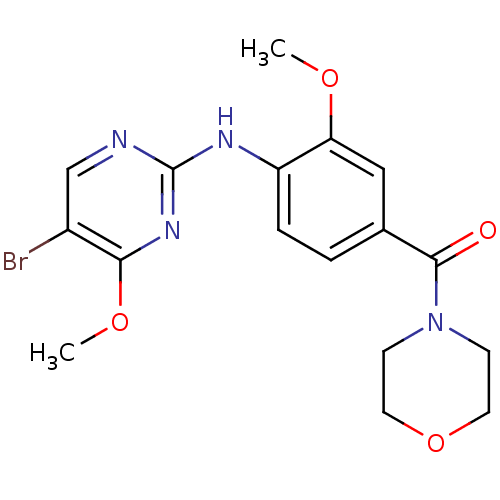
(CHEMBL2178123)Show InChI InChI=1S/C17H19BrN4O4/c1-24-14-9-11(16(23)22-5-7-26-8-6-22)3-4-13(14)20-17-19-10-12(18)15(21-17)25-2/h3-4,9-10H,5-8H2,1-2H3,(H,19,20,21) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Inhibition of LRRK2 using FAM-LRRKtide as substrate after 120 mins by microfluidic capillary electrophoresis assay |
J Med Chem 55: 9416-33 (2012)
Article DOI: 10.1021/jm301020q
BindingDB Entry DOI: 10.7270/Q2P55PN7 |
More data for this
Ligand-Target Pair | |
Leucine-rich repeat serine/threonine-protein kinase 2
(Homo sapiens (Human)) | BDBM50398669
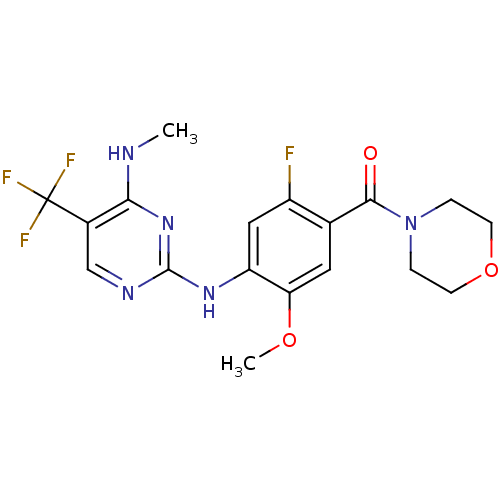
(CHEMBL2178133 | US8802674, 239)Show SMILES CNc1nc(Nc2cc(F)c(cc2OC)C(=O)N2CCOCC2)ncc1C(F)(F)F Show InChI InChI=1S/C18H19F4N5O3/c1-23-15-11(18(20,21)22)9-24-17(26-15)25-13-8-12(19)10(7-14(13)29-2)16(28)27-3-5-30-6-4-27/h7-9H,3-6H2,1-2H3,(H2,23,24,25,26) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Inhibition of LRRK2 using FAM-LRRKtide as substrate after 120 mins by microfluidic capillary electrophoresis assay |
J Med Chem 55: 9416-33 (2012)
Article DOI: 10.1021/jm301020q
BindingDB Entry DOI: 10.7270/Q2P55PN7 |
More data for this
Ligand-Target Pair | |
Leucine-rich repeat serine/threonine-protein kinase 2
(Homo sapiens (Human)) | BDBM50448124
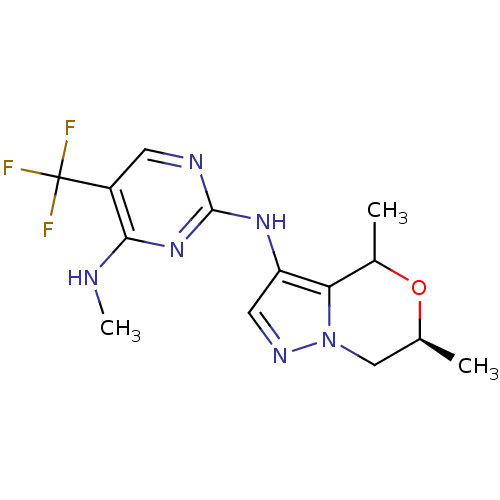
(CHEMBL3122107)Show SMILES CNc1nc(Nc2cnn3C[C@H](C)OC(C)c23)ncc1C(F)(F)F |r| Show InChI InChI=1S/C14H17F3N6O/c1-7-6-23-11(8(2)24-7)10(5-20-23)21-13-19-4-9(14(15,16)17)12(18-3)22-13/h4-5,7-8H,6H2,1-3H3,(H2,18,19,21,22)/t7-,8?/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Inhibition of LRRK2 (unknown origin) |
J Med Chem 57: 921-36 (2014)
Article DOI: 10.1021/jm401654j
BindingDB Entry DOI: 10.7270/Q2N0181N |
More data for this
Ligand-Target Pair | |
Leucine-rich repeat serine/threonine-protein kinase 2
(Homo sapiens (Human)) | BDBM50398673
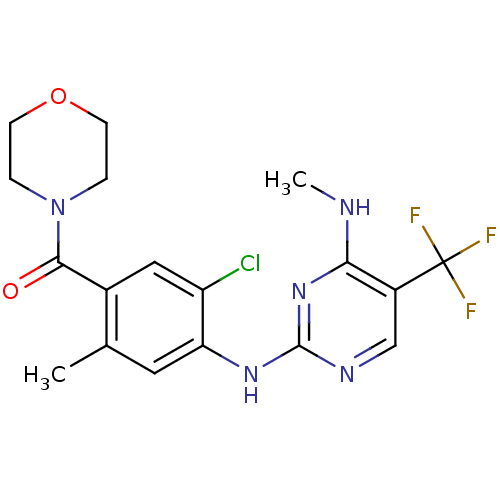
(CHEMBL2178129)Show SMILES CNc1nc(Nc2cc(C)c(cc2Cl)C(=O)N2CCOCC2)ncc1C(F)(F)F Show InChI InChI=1S/C18H19ClF3N5O2/c1-10-7-14(13(19)8-11(10)16(28)27-3-5-29-6-4-27)25-17-24-9-12(18(20,21)22)15(23-2)26-17/h7-9H,3-6H2,1-2H3,(H2,23,24,25,26) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 7 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Inhibition of LRRK2 using FAM-LRRKtide as substrate after 120 mins by microfluidic capillary electrophoresis assay |
J Med Chem 55: 9416-33 (2012)
Article DOI: 10.1021/jm301020q
BindingDB Entry DOI: 10.7270/Q2P55PN7 |
More data for this
Ligand-Target Pair | |
Leucine-rich repeat serine/threonine-protein kinase 2
(Homo sapiens (Human)) | BDBM50398671
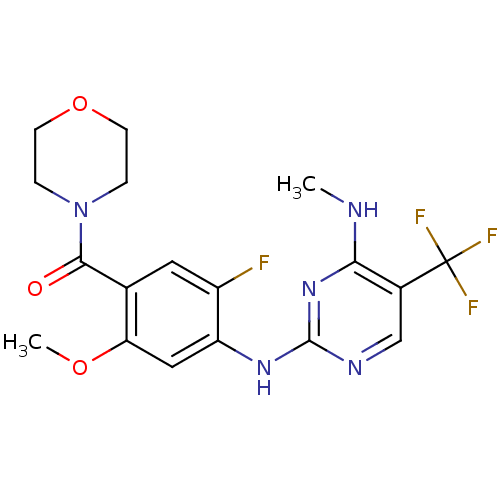
(CHEMBL2178131 | US8802674, 237)Show SMILES CNc1nc(Nc2cc(OC)c(cc2F)C(=O)N2CCOCC2)ncc1C(F)(F)F Show InChI InChI=1S/C18H19F4N5O3/c1-23-15-11(18(20,21)22)9-24-17(26-15)25-13-8-14(29-2)10(7-12(13)19)16(28)27-3-5-30-6-4-27/h7-9H,3-6H2,1-2H3,(H2,23,24,25,26) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 7 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Inhibition of LRRK2 using FAM-LRRKtide as substrate after 120 mins by microfluidic capillary electrophoresis assay |
J Med Chem 55: 9416-33 (2012)
Article DOI: 10.1021/jm301020q
BindingDB Entry DOI: 10.7270/Q2P55PN7 |
More data for this
Ligand-Target Pair | |
Leucine-rich repeat serine/threonine-protein kinase 2
(Homo sapiens (Human)) | BDBM50398666
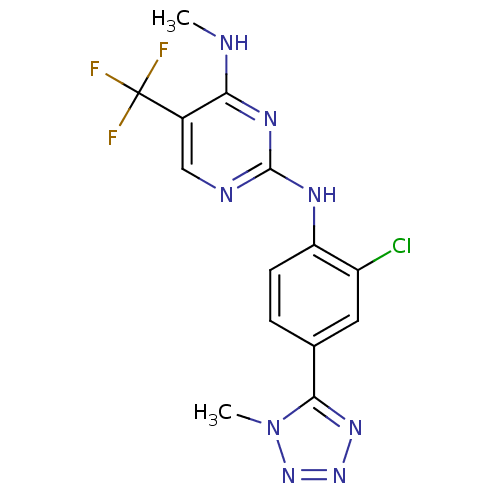
(CHEMBL2178136 | US8791130, 2)Show InChI InChI=1S/C14H12ClF3N8/c1-19-11-8(14(16,17)18)6-20-13(22-11)21-10-4-3-7(5-9(10)15)12-23-24-25-26(12)2/h3-6H,1-2H3,(H2,19,20,21,22) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 8 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Inhibition of LRRK2 using FAM-LRRKtide as substrate after 120 mins by microfluidic capillary electrophoresis assay |
J Med Chem 55: 9416-33 (2012)
Article DOI: 10.1021/jm301020q
BindingDB Entry DOI: 10.7270/Q2P55PN7 |
More data for this
Ligand-Target Pair | |
Leucine-rich repeat serine/threonine-protein kinase 2
(Homo sapiens (Human)) | BDBM50398660
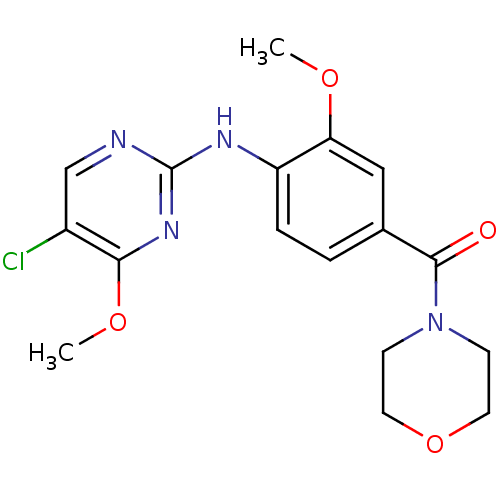
(CHEMBL2178142 | US8802674, 61)Show InChI InChI=1S/C17H19ClN4O4/c1-24-14-9-11(16(23)22-5-7-26-8-6-22)3-4-13(14)20-17-19-10-12(18)15(21-17)25-2/h3-4,9-10H,5-8H2,1-2H3,(H,19,20,21) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 9 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Inhibition of LRRK2 using FAM-LRRKtide as substrate after 120 mins by microfluidic capillary electrophoresis assay |
J Med Chem 55: 9416-33 (2012)
Article DOI: 10.1021/jm301020q
BindingDB Entry DOI: 10.7270/Q2P55PN7 |
More data for this
Ligand-Target Pair | |
Leucine-rich repeat serine/threonine-protein kinase 2
(Homo sapiens (Human)) | BDBM50398663
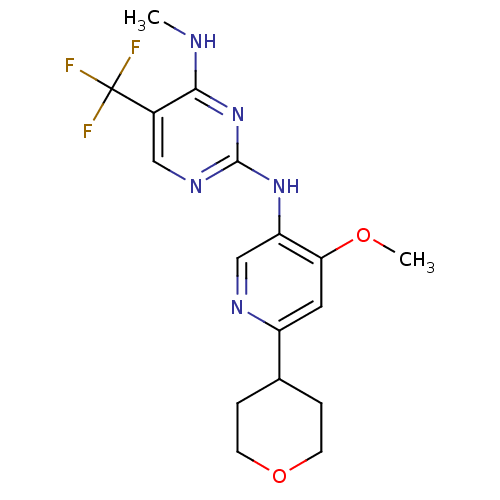
(CHEMBL2178139)Show InChI InChI=1S/C17H20F3N5O2/c1-21-15-11(17(18,19)20)8-23-16(25-15)24-13-9-22-12(7-14(13)26-2)10-3-5-27-6-4-10/h7-10H,3-6H2,1-2H3,(H2,21,23,24,25) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 9 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Inhibition of LRRK2 using FAM-LRRKtide as substrate after 120 mins by microfluidic capillary electrophoresis assay |
J Med Chem 55: 9416-33 (2012)
Article DOI: 10.1021/jm301020q
BindingDB Entry DOI: 10.7270/Q2P55PN7 |
More data for this
Ligand-Target Pair | |
Leucine-rich repeat serine/threonine-protein kinase 2
(Homo sapiens (Human)) | BDBM50396147
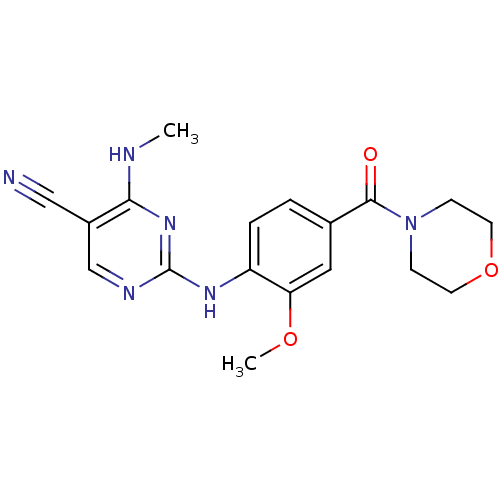
(CHEMBL2171746 | US8802674, 314)Show InChI InChI=1S/C18H20N6O3/c1-20-16-13(10-19)11-21-18(23-16)22-14-4-3-12(9-15(14)26-2)17(25)24-5-7-27-8-6-24/h3-4,9,11H,5-8H2,1-2H3,(H2,20,21,22,23) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 11 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Inhibition of LRRK2 using FAM-LRRKtide as substrate after 120 mins by microfluidic capillary electrophoresis assay |
J Med Chem 55: 9416-33 (2012)
Article DOI: 10.1021/jm301020q
BindingDB Entry DOI: 10.7270/Q2P55PN7 |
More data for this
Ligand-Target Pair | |
Leucine-rich repeat serine/threonine-protein kinase 2
(Homo sapiens (Human)) | BDBM50398670
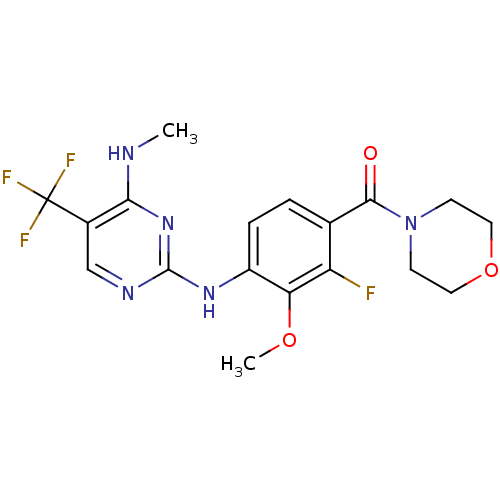
(CHEMBL2178132 | US8802674, 297)Show SMILES CNc1nc(Nc2ccc(C(=O)N3CCOCC3)c(F)c2OC)ncc1C(F)(F)F Show InChI InChI=1S/C18H19F4N5O3/c1-23-15-11(18(20,21)22)9-24-17(26-15)25-12-4-3-10(13(19)14(12)29-2)16(28)27-5-7-30-8-6-27/h3-4,9H,5-8H2,1-2H3,(H2,23,24,25,26) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 12 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Inhibition of LRRK2 using FAM-LRRKtide as substrate after 120 mins by microfluidic capillary electrophoresis assay |
J Med Chem 55: 9416-33 (2012)
Article DOI: 10.1021/jm301020q
BindingDB Entry DOI: 10.7270/Q2P55PN7 |
More data for this
Ligand-Target Pair | |
Dual specificity protein kinase TTK
(Homo sapiens (Human)) | BDBM50396148

(CHEMBL2171745 | US8802674, 50)Show SMILES CNc1nc(Nc2ccc(cc2OC)C(=O)N2CCOCC2)ncc1C(F)(F)F Show InChI InChI=1S/C18H20F3N5O3/c1-22-15-12(18(19,20)21)10-23-17(25-15)24-13-4-3-11(9-14(13)28-2)16(27)26-5-7-29-8-6-26/h3-4,9-10H,5-8H2,1-2H3,(H2,22,23,24,25) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 25 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Inhibition of TTK |
J Med Chem 55: 9416-33 (2012)
Article DOI: 10.1021/jm301020q
BindingDB Entry DOI: 10.7270/Q2P55PN7 |
More data for this
Ligand-Target Pair | |
Leucine-rich repeat serine/threonine-protein kinase 2
(Homo sapiens (Human)) | BDBM50396149
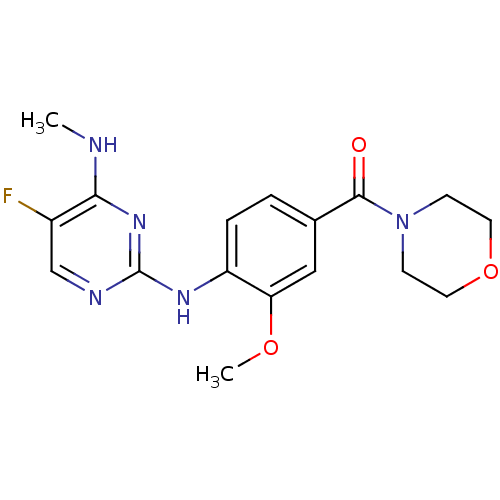
(CHEMBL2171744)Show InChI InChI=1S/C17H20FN5O3/c1-19-15-12(18)10-20-17(22-15)21-13-4-3-11(9-14(13)25-2)16(24)23-5-7-26-8-6-23/h3-4,9-10H,5-8H2,1-2H3,(H2,19,20,21,22) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 58 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Inhibition of LRRK2 using FAM-LRRKtide as substrate after 120 mins by microfluidic capillary electrophoresis assay |
J Med Chem 55: 9416-33 (2012)
Article DOI: 10.1021/jm301020q
BindingDB Entry DOI: 10.7270/Q2P55PN7 |
More data for this
Ligand-Target Pair | |
Dual specificity protein kinase TTK
(Homo sapiens (Human)) | BDBM50448118

(CHEMBL3122113 | US10590114, No. 80 | US11111235, N...)Show InChI InChI=1S/C14H16F3N7/c1-8-10(6-24(23-8)13(2,3)7-18)21-12-20-5-9(14(15,16)17)11(19-4)22-12/h5-6H,1-4H3,(H2,19,20,21,22) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| 150 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Inhibition of TTK (unknown origin) |
J Med Chem 57: 921-36 (2014)
Article DOI: 10.1021/jm401654j
BindingDB Entry DOI: 10.7270/Q2N0181N |
More data for this
Ligand-Target Pair | |
Sodium/glucose cotransporter 2
(Homo sapiens (Human)) | BDBM50321299
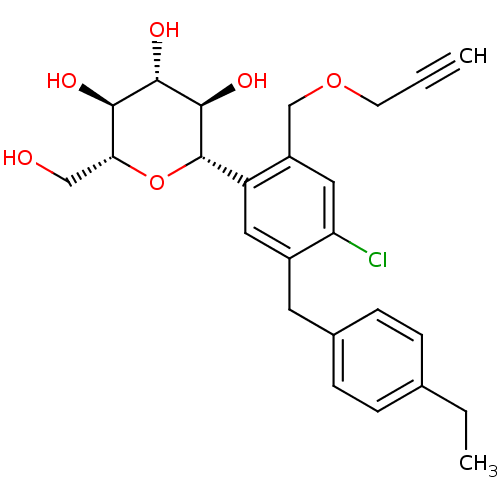
((2S,3R,4R,5S,6R)-2-(4-chloro-5-(4-ethylbenzyl)-2-(...)Show SMILES CCc1ccc(Cc2cc([C@@H]3O[C@H](CO)[C@@H](O)[C@H](O)[C@H]3O)c(COCC#C)cc2Cl)cc1 |r| Show InChI InChI=1S/C25H29ClO6/c1-3-9-31-14-18-12-20(26)17(10-16-7-5-15(4-2)6-8-16)11-19(18)25-24(30)23(29)22(28)21(13-27)32-25/h1,5-8,11-12,21-25,27-30H,4,9-10,13-14H2,2H3/t21-,22-,23+,24-,25+/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
Chinese Academy of Sciences
Curated by ChEMBL
| Assay Description
Inhibition of human SGLT2 expressed in HEK293 cells assessed as inhibition of [14C]-alpha-methyl-D-glucopyranoside uptake after 1.5 hrs by liquid sci... |
Bioorg Med Chem 18: 4422-32 (2010)
Article DOI: 10.1016/j.bmc.2010.04.088
BindingDB Entry DOI: 10.7270/Q2TM7B8N |
More data for this
Ligand-Target Pair | |
Sodium/glucose cotransporter 2
(Homo sapiens (Human)) | BDBM50303246

((1S,3'R,4'S,5'S,6'R)-5-chloro-6'-(hydroxymethyl)-6...)Show SMILES OC[C@H]1O[C@]2(OCc3cc(Cl)c(Cc4ccc(OC(F)(F)F)cc4)cc23)[C@H](O)[C@@H](O)[C@@H]1O |r| Show InChI InChI=1S/C21H20ClF3O7/c22-15-7-12-9-30-20(19(29)18(28)17(27)16(8-26)32-20)14(12)6-11(15)5-10-1-3-13(4-2-10)31-21(23,24)25/h1-4,6-7,16-19,26-29H,5,8-9H2/t16-,17-,18+,19-,20+/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
Chinese Academy of Sciences
Curated by ChEMBL
| Assay Description
Inhibition of human SGLT2 transfected in HEK293.ETN cells assessed as AMG uptake after 1.5 hrs by scintillation counting |
Bioorg Med Chem Lett 19: 6877-81 (2009)
Article DOI: 10.1016/j.bmcl.2009.10.088
BindingDB Entry DOI: 10.7270/Q2MC903R |
More data for this
Ligand-Target Pair | |
Sodium/glucose cotransporter 2
(Homo sapiens (Human)) | BDBM50308469
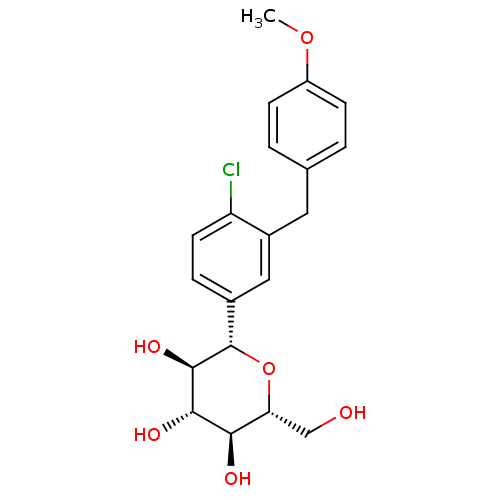
((2S,3R,4R,5S,6R)-2-(4-chloro-3-(4-methoxybenzyl)ph...)Show SMILES COc1ccc(Cc2cc(ccc2Cl)[C@@H]2O[C@H](CO)[C@@H](O)[C@H](O)[C@H]2O)cc1 |r| Show InChI InChI=1S/C20H23ClO6/c1-26-14-5-2-11(3-6-14)8-13-9-12(4-7-15(13)21)20-19(25)18(24)17(23)16(10-22)27-20/h2-7,9,16-20,22-25H,8,10H2,1H3/t16-,17-,18+,19-,20+/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a |
Fudan University
Curated by ChEMBL
| Assay Description
Inhibition of full length human SGLT2 assessed as methyl-alpha-D-[U-14C]glucopyranoside uptake after 1.5 hrs by cell-based topcount scintillation cou... |
J Med Chem 57: 1236-51 (2014)
Article DOI: 10.1021/jm401780b
BindingDB Entry DOI: 10.7270/Q2D50PF5 |
More data for this
Ligand-Target Pair | |
Sodium/glucose cotransporter 2
(Homo sapiens (Human)) | BDBM50303254
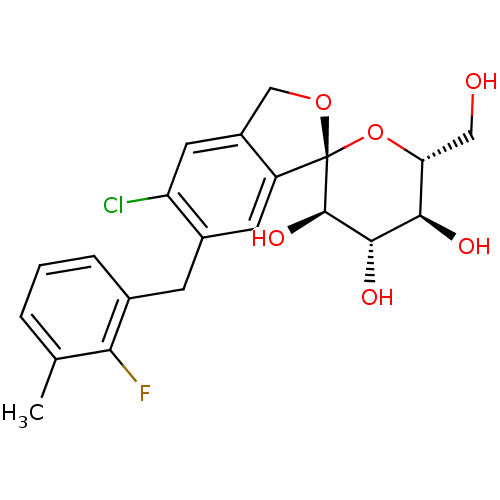
((1S,3'R,4'S,5'S,6'R)-5-chloro-6-(2-fluoro-3-methyl...)Show SMILES Cc1cccc(Cc2cc3c(CO[C@]33O[C@H](CO)[C@@H](O)[C@H](O)[C@H]3O)cc2Cl)c1F |r| Show InChI InChI=1S/C21H22ClFO6/c1-10-3-2-4-11(17(10)23)5-12-6-14-13(7-15(12)22)9-28-21(14)20(27)19(26)18(25)16(8-24)29-21/h2-4,6-7,16,18-20,24-27H,5,8-9H2,1H3/t16-,18-,19+,20-,21+/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a |
Chinese Academy of Sciences
Curated by ChEMBL
| Assay Description
Inhibition of human SGLT2 transfected in HEK293.ETN cells assessed as AMG uptake after 1.5 hrs by scintillation counting |
Bioorg Med Chem Lett 19: 6877-81 (2009)
Article DOI: 10.1016/j.bmcl.2009.10.088
BindingDB Entry DOI: 10.7270/Q2MC903R |
More data for this
Ligand-Target Pair | |
Sodium/glucose cotransporter 2
(Homo sapiens (Human)) | BDBM50321300

((2S,3R,4R,5S,6R)-2-(4-chloro-5-(4-ethoxybenzyl)-2-...)Show SMILES CCOc1ccc(Cc2cc([C@@H]3O[C@H](CO)[C@@H](O)[C@H](O)[C@H]3O)c(CCOC)cc2Cl)cc1 |r| Show InChI InChI=1S/C24H31ClO7/c1-3-31-17-6-4-14(5-7-17)10-16-11-18(15(8-9-30-2)12-19(16)25)24-23(29)22(28)21(27)20(13-26)32-24/h4-7,11-12,20-24,26-29H,3,8-10,13H2,1-2H3/t20-,21-,22+,23-,24+/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a |
Chinese Academy of Sciences
Curated by ChEMBL
| Assay Description
Inhibition of human SGLT2 expressed in HEK293 cells assessed as inhibition of [14C]-alpha-methyl-D-glucopyranoside uptake after 1.5 hrs by liquid sci... |
Bioorg Med Chem 18: 4422-32 (2010)
Article DOI: 10.1016/j.bmc.2010.04.088
BindingDB Entry DOI: 10.7270/Q2TM7B8N |
More data for this
Ligand-Target Pair | |
Sodium/glucose cotransporter 2
(Homo sapiens (Human)) | BDBM50321288

((2S,3R,4R,5S,6R)-2-(4-chloro-5-(4-ethylbenzyl)-2-(...)Show SMILES CCc1ccc(Cc2cc([C@@H]3O[C@H](CO)[C@@H](O)[C@H](O)[C@H]3O)c(COC)cc2Cl)cc1 |r| Show InChI InChI=1S/C23H29ClO6/c1-3-13-4-6-14(7-5-13)8-15-9-17(16(12-29-2)10-18(15)24)23-22(28)21(27)20(26)19(11-25)30-23/h4-7,9-10,19-23,25-28H,3,8,11-12H2,1-2H3/t19-,20-,21+,22-,23+/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a |
Chinese Academy of Sciences
Curated by ChEMBL
| Assay Description
Inhibition of human SGLT2 expressed in HEK293 cells assessed as inhibition of [14C]-alpha-methyl-D-glucopyranoside uptake after 1.5 hrs by liquid sci... |
Bioorg Med Chem 18: 4422-32 (2010)
Article DOI: 10.1016/j.bmc.2010.04.088
BindingDB Entry DOI: 10.7270/Q2TM7B8N |
More data for this
Ligand-Target Pair | |
Sodium/glucose cotransporter 2
(Homo sapiens (Human)) | BDBM50321290
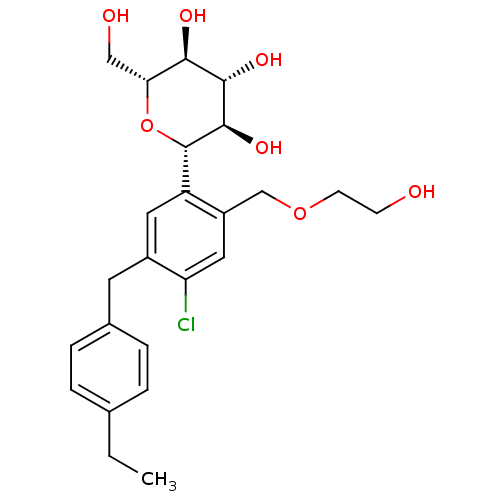
((2S,3R,4R,5S,6R)-2-(4-chloro-5-(4-ethylbenzyl)-2-(...)Show SMILES CCc1ccc(Cc2cc([C@@H]3O[C@H](CO)[C@@H](O)[C@H](O)[C@H]3O)c(COCCO)cc2Cl)cc1 |r| Show InChI InChI=1S/C24H31ClO7/c1-2-14-3-5-15(6-4-14)9-16-10-18(17(11-19(16)25)13-31-8-7-26)24-23(30)22(29)21(28)20(12-27)32-24/h3-6,10-11,20-24,26-30H,2,7-9,12-13H2,1H3/t20-,21-,22+,23-,24+/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Chinese Academy of Sciences
Curated by ChEMBL
| Assay Description
Inhibition of human SGLT2 expressed in HEK293 cells assessed as inhibition of [14C]-alpha-methyl-D-glucopyranoside uptake after 1.5 hrs by liquid sci... |
Bioorg Med Chem 18: 4422-32 (2010)
Article DOI: 10.1016/j.bmc.2010.04.088
BindingDB Entry DOI: 10.7270/Q2TM7B8N |
More data for this
Ligand-Target Pair | |
Sodium/glucose cotransporter 2
(Homo sapiens (Human)) | BDBM50303239

((1S,3'R,4'S,5'S,6'R)-5-chloro-4-(1-(4-ethylphenyl)...)Show SMILES CCc1ccc(cc1)C1(CC1)c1c2CO[C@]3(O[C@H](CO)[C@@H](O)[C@H](O)[C@H]3O)c2ccc1Cl |r| Show InChI InChI=1S/C24H27ClO6/c1-2-13-3-5-14(6-4-13)23(9-10-23)19-15-12-30-24(16(15)7-8-17(19)25)22(29)21(28)20(27)18(11-26)31-24/h3-8,18,20-22,26-29H,2,9-12H2,1H3/t18-,20-,21+,22-,24+/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Chinese Academy of Sciences
Curated by ChEMBL
| Assay Description
Inhibition of human SGLT2 transfected in HEK293.ETN cells assessed as AMG uptake after 1.5 hrs by scintillation counting |
Bioorg Med Chem Lett 19: 6877-81 (2009)
Article DOI: 10.1016/j.bmcl.2009.10.088
BindingDB Entry DOI: 10.7270/Q2MC903R |
More data for this
Ligand-Target Pair | |
Sodium/glucose cotransporter 2
(Homo sapiens (Human)) | BDBM50321292

((2S,3R,4R,5S,6R)-2-(4-chloro-5-(4-ethylbenzyl)-2-(...)Show SMILES CCc1ccc(Cc2cc([C@@H]3O[C@H](CO)[C@@H](O)[C@H](O)[C@H]3O)c(COCCF)cc2Cl)cc1 |r| Show InChI InChI=1S/C24H30ClFO6/c1-2-14-3-5-15(6-4-14)9-16-10-18(17(11-19(16)25)13-31-8-7-26)24-23(30)22(29)21(28)20(12-27)32-24/h3-6,10-11,20-24,27-30H,2,7-9,12-13H2,1H3/t20-,21-,22+,23-,24+/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Chinese Academy of Sciences
Curated by ChEMBL
| Assay Description
Inhibition of human SGLT2 expressed in HEK293 cells assessed as inhibition of [14C]-alpha-methyl-D-glucopyranoside uptake after 1.5 hrs by liquid sci... |
Bioorg Med Chem 18: 4422-32 (2010)
Article DOI: 10.1016/j.bmc.2010.04.088
BindingDB Entry DOI: 10.7270/Q2TM7B8N |
More data for this
Ligand-Target Pair | |
Sodium/glucose cotransporter 1
(Homo sapiens (Human)) | BDBM50349240
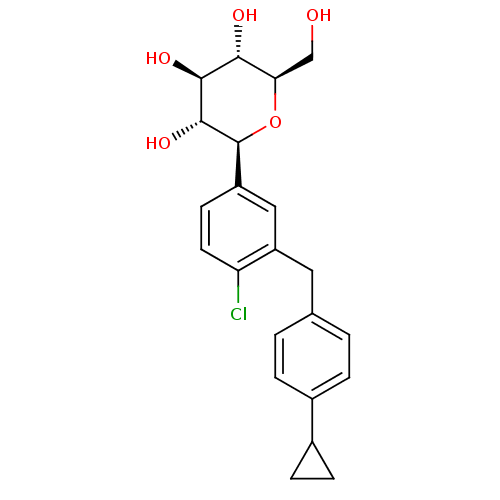
(CHEMBL1807551)Show SMILES OC[C@H]1O[C@H]([C@H](O)[C@@H](O)[C@@H]1O)c1ccc(Cl)c(Cc2ccc(cc2)C2CC2)c1 |r| Show InChI InChI=1S/C22H25ClO5/c23-17-8-7-15(22-21(27)20(26)19(25)18(11-24)28-22)10-16(17)9-12-1-3-13(4-2-12)14-5-6-14/h1-4,7-8,10,14,18-22,24-27H,5-6,9,11H2/t18-,19-,20+,21-,22+/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Chinese Academy of Sciences
Curated by ChEMBL
| Assay Description
Inhibition of human SGLT2 expressed in african green monkey COS7 cells assessed as inhibition of [14C]-methyl-alpha-D-glucopyranoside uptake after 2 ... |
Bioorg Med Chem Lett 21: 4465-70 (2011)
Article DOI: 10.1016/j.bmcl.2011.06.032
BindingDB Entry DOI: 10.7270/Q29W0FVZ |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data