 Found 6722 hits with Last Name = 'zheng' and Initial = 'z'
Found 6722 hits with Last Name = 'zheng' and Initial = 'z' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Lactoylglutathione lyase
(Homo sapiens (Human)) | BDBM50126960
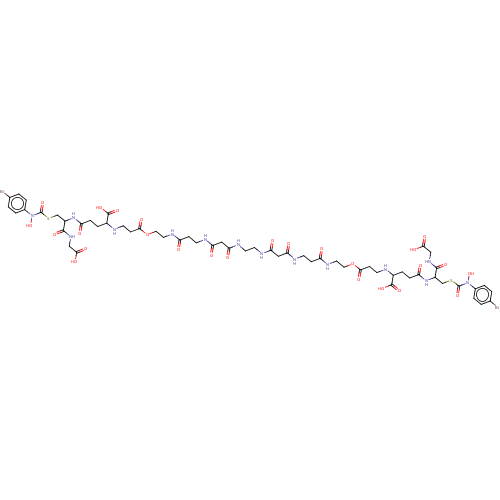
(CHEMBL3629116)Show SMILES ON(C(=O)SCC(NC(=O)CCC(NCCC(=O)OCCNC(=O)CCNC(=O)CC(=O)NCCNC(=O)CC(=O)NCCC(=O)NCCOC(=O)CCNC(CCC(=O)NC(CSC(=O)N(O)c1ccc(Br)cc1)C(=O)NCC(O)=O)C(O)=O)C(O)=O)C(=O)NCC(O)=O)c1ccc(Br)cc1 Show InChI InChI=1S/C58H78Br2N14O26S2/c59-33-1-5-35(6-2-33)73(97)57(95)101-31-39(53(89)69-29-49(83)84)71-43(77)11-9-37(55(91)92)61-19-15-51(87)99-25-23-67-41(75)13-17-63-45(79)27-47(81)65-21-22-66-48(82)28-46(80)64-18-14-42(76)68-24-26-100-52(88)16-20-62-38(56(93)94)10-12-44(78)72-40(54(90)70-30-50(85)86)32-102-58(96)74(98)36-7-3-34(60)4-8-36/h1-8,37-40,61-62,97-98H,9-32H2,(H,63,79)(H,64,80)(H,65,81)(H,66,82)(H,67,75)(H,68,76)(H,69,89)(H,70,90)(H,71,77)(H,72,78)(H,83,84)(H,85,86)(H,91,92)(H,93,94) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Chengdu University
Curated by ChEMBL
| Assay Description
Inhibition of 6-His tagged recombinant human glyoxalase 1 transfected in Escherichia coli BL21 (DE3) assessed as S-D-lactoylglutathione formation by ... |
Bioorg Med Chem Lett 25: 4724-7 (2015)
Article DOI: 10.1016/j.bmcl.2015.08.055
BindingDB Entry DOI: 10.7270/Q26H4K7B |
More data for this
Ligand-Target Pair | |
Lactoylglutathione lyase
(Homo sapiens (Human)) | BDBM50526945
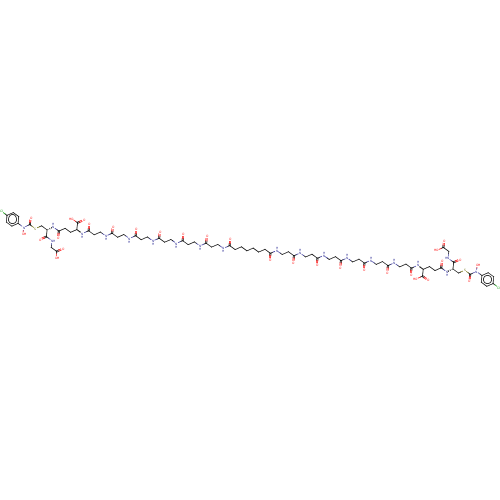
(CHEMBL4473806)Show SMILES ON(C(=O)SC[C@H](NC(=O)CC[C@H](NC(=O)CCNC(=O)CCNC(=O)CCNC(=O)CCNC(=O)CCNC(=O)CCNC(=O)CCCCCCC(=O)NCCC(=O)NCCC(=O)NCCC(=O)NCCC(=O)NCCC(=O)NCCC(=O)N[C@@H](CCC(=O)N[C@@H](CSC(=O)N(O)c1ccc(Cl)cc1)C(=O)NCC(O)=O)C(O)=O)C(O)=O)C(=O)NCC(O)=O)c1ccc(Cl)cc1 |r| Show InChI InChI=1S/C78H112Cl2N20O30S2/c79-47-7-11-49(12-8-47)99(129)77(127)131-45-53(73(121)93-43-71(117)118)97-67(113)17-15-51(75(123)124)95-69(115)29-41-91-65(111)27-39-89-63(109)25-37-87-61(107)23-35-85-59(105)21-33-83-57(103)19-31-81-55(101)5-3-1-2-4-6-56(102)82-32-20-58(104)84-34-22-60(106)86-36-24-62(108)88-38-26-64(110)90-40-28-66(112)92-42-30-70(116)96-52(76(125)126)16-18-68(114)98-54(74(122)94-44-72(119)120)46-132-78(128)100(130)50-13-9-48(80)10-14-50/h7-14,51-54,129-130H,1-6,15-46H2,(H,81,101)(H,82,102)(H,83,103)(H,84,104)(H,85,105)(H,86,106)(H,87,107)(H,88,108)(H,89,109)(H,90,110)(H,91,111)(H,92,112)(H,93,121)(H,94,122)(H,95,115)(H,96,116)(H,97,113)(H,98,114)(H,117,118)(H,119,120)(H,123,124)(H,125,126)/t51-,52-,53-,54-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.960 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Chengdu University
Curated by ChEMBL
| Assay Description
Competitive inhibition of human Glyoxalase-1 using GSH and MGO as substrate by Michaelis-Menten analysis |
Bioorg Med Chem 28: (2020)
Article DOI: 10.1016/j.bmc.2019.115243
BindingDB Entry DOI: 10.7270/Q2Z60SG5 |
More data for this
Ligand-Target Pair | |
Lactoylglutathione lyase
(Homo sapiens (Human)) | BDBM50126961
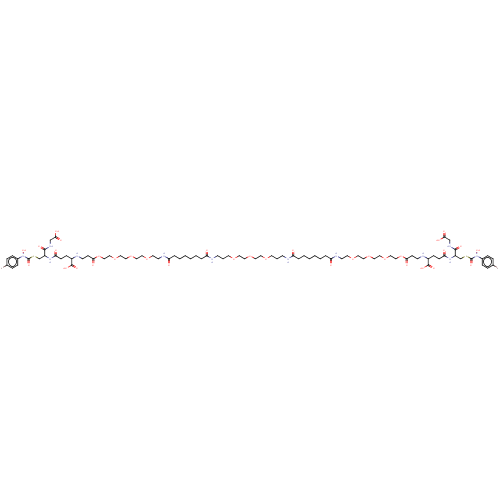
(CHEMBL3629115)Show SMILES ON(C(=O)SCC(NC(=O)CCC(NCCC(=O)OCCOCCOCCOCCNC(=O)CCCCCCC(=O)NCCCOCCOCCOCCCNC(=O)CCCCCCC(=O)NCCOCCOCCOCCOC(=O)CCNC(CCC(=O)NC(CSC(=O)N(O)c1ccc(Br)cc1)C(=O)NCC(O)=O)C(O)=O)C(O)=O)C(=O)NCC(O)=O)c1ccc(Br)cc1 Show InChI InChI=1S/C82H128Br2N12O33S2/c83-59-15-19-61(20-16-59)95(117)81(115)130-57-65(77(109)91-55-73(103)104)93-71(101)25-23-63(79(111)112)85-31-27-75(107)128-53-51-126-49-47-124-45-41-121-37-33-89-69(99)13-7-3-1-5-11-67(97)87-29-9-35-119-39-43-123-44-40-120-36-10-30-88-68(98)12-6-2-4-8-14-70(100)90-34-38-122-42-46-125-48-50-127-52-54-129-76(108)28-32-86-64(80(113)114)24-26-72(102)94-66(78(110)92-56-74(105)106)58-131-82(116)96(118)62-21-17-60(84)18-22-62/h15-22,63-66,85-86,117-118H,1-14,23-58H2,(H,87,97)(H,88,98)(H,89,99)(H,90,100)(H,91,109)(H,92,110)(H,93,101)(H,94,102)(H,103,104)(H,105,106)(H,111,112)(H,113,114) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Chengdu University
Curated by ChEMBL
| Assay Description
Inhibition of 6-His tagged recombinant human glyoxalase 1 transfected in Escherichia coli BL21 (DE3) assessed as S-D-lactoylglutathione formation by ... |
Bioorg Med Chem Lett 25: 4724-7 (2015)
Article DOI: 10.1016/j.bmcl.2015.08.055
BindingDB Entry DOI: 10.7270/Q26H4K7B |
More data for this
Ligand-Target Pair | |
Lactoylglutathione lyase
(Homo sapiens (Human)) | BDBM50526943
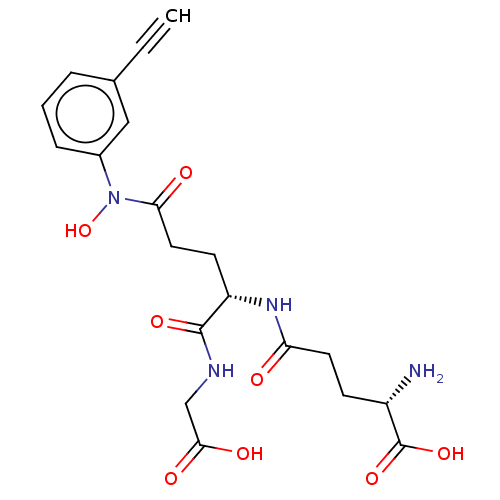
(CHEMBL4436073)Show SMILES N[C@@H](CCC(=O)N[C@@H](CCC(=O)N(O)c1cccc(c1)C#C)C(=O)NCC(O)=O)C(O)=O |r| Show InChI InChI=1S/C20H24N4O8/c1-2-12-4-3-5-13(10-12)24(32)17(26)9-7-15(19(29)22-11-18(27)28)23-16(25)8-6-14(21)20(30)31/h1,3-5,10,14-15,32H,6-9,11,21H2,(H,22,29)(H,23,25)(H,27,28)(H,30,31)/t14-,15-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| KEGG
PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Chengdu University
Curated by ChEMBL
| Assay Description
Competitive inhibition of human Glyoxalase-1 using GSH and MGO as substrate |
Bioorg Med Chem 28: (2020)
Article DOI: 10.1016/j.bmc.2019.115243
BindingDB Entry DOI: 10.7270/Q2Z60SG5 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Metabotropic glutamate receptor 1
(RAT) | BDBM50177063
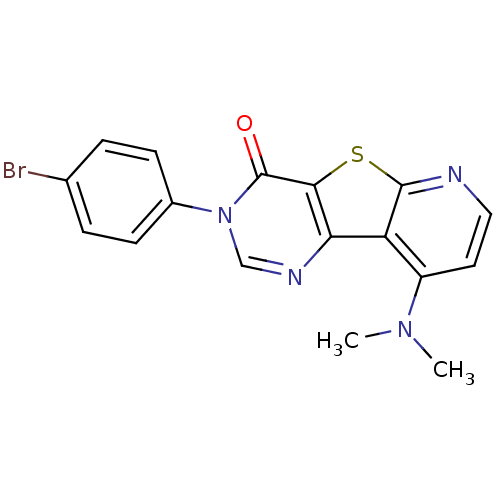
(3-(4-Bromo-phenyl)-9-dimethylamino-3H-pyrido[3',2'...)Show SMILES CN(C)c1ccnc2sc3c(ncn(-c4ccc(Br)cc4)c3=O)c12 Show InChI InChI=1S/C17H13BrN4OS/c1-21(2)12-7-8-19-16-13(12)14-15(24-16)17(23)22(9-20-14)11-5-3-10(18)4-6-11/h3-9H,1-2H3 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [3H]R214127 from mGluR1 in rat cerebellum membranes |
J Med Chem 48: 7374-88 (2005)
Article DOI: 10.1021/jm0504407
BindingDB Entry DOI: 10.7270/Q27P8XX0 |
More data for this
Ligand-Target Pair | |
Lactoylglutathione lyase
(Homo sapiens (Human)) | BDBM16452

((4-oxo-3-{[5-(trifluoromethyl)-1,3-benzothiazol-2-...)Show SMILES OC(=O)Cc1nn(Cc2nc3cc(ccc3s2)C(F)(F)F)c(=O)c2ccccc12 Show InChI InChI=1S/C19H12F3N3O3S/c20-19(21,22)10-5-6-15-14(7-10)23-16(29-15)9-25-18(28)12-4-2-1-3-11(12)13(24-25)8-17(26)27/h1-7H,8-9H2,(H,26,27) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| 1.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Chengdu University
Curated by ChEMBL
| Assay Description
Inhibition of human Glyoxalase-1 using GSH and MGO as substrate by Dixon plot analysis |
Bioorg Med Chem 28: (2020)
Article DOI: 10.1016/j.bmc.2019.115243
BindingDB Entry DOI: 10.7270/Q2Z60SG5 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Metabotropic glutamate receptor 1
(RAT) | BDBM50177097
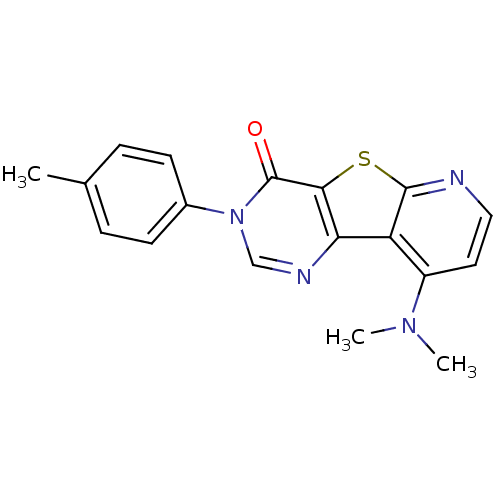
(9-Dimethylamino-3-p-tolyl-3H-pyrido[3',2':4,5]thie...)Show InChI InChI=1S/C18H16N4OS/c1-11-4-6-12(7-5-11)22-10-20-15-14-13(21(2)3)8-9-19-17(14)24-16(15)18(22)23/h4-10H,1-3H3 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [3H]R214127 from mGluR1 in rat cerebellum membranes |
J Med Chem 48: 7374-88 (2005)
Article DOI: 10.1021/jm0504407
BindingDB Entry DOI: 10.7270/Q27P8XX0 |
More data for this
Ligand-Target Pair | |
Metabotropic glutamate receptor 1
(RAT) | BDBM50177065
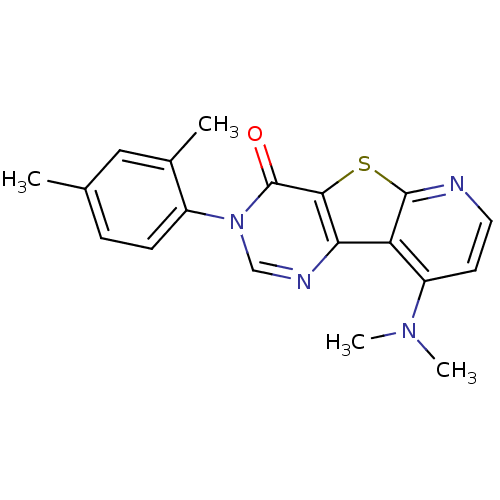
(9-Dimethylamino-3-(2,4-dimethyl-phenyl)-3H-pyrido[...)Show SMILES CN(C)c1ccnc2sc3c(ncn(-c4ccc(C)cc4C)c3=O)c12 Show InChI InChI=1S/C19H18N4OS/c1-11-5-6-13(12(2)9-11)23-10-21-16-15-14(22(3)4)7-8-20-18(15)25-17(16)19(23)24/h5-10H,1-4H3 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [3H]R214127 from mGluR1 in rat cerebellum membranes |
J Med Chem 48: 7374-88 (2005)
Article DOI: 10.1021/jm0504407
BindingDB Entry DOI: 10.7270/Q27P8XX0 |
More data for this
Ligand-Target Pair | |
Metabotropic glutamate receptor 1
(RAT) | BDBM50177076
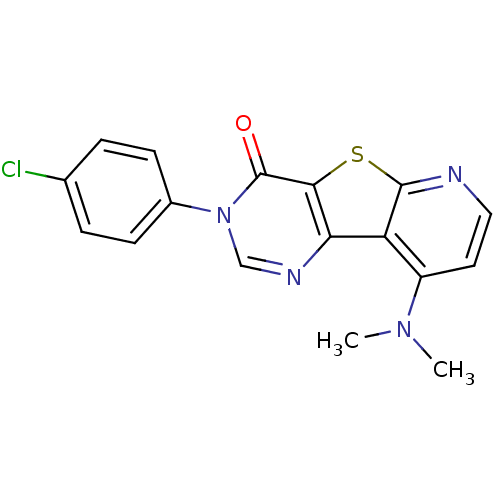
(3-(4-Chloro-phenyl)-9-dimethylamino-3H-pyrido[3',2...)Show SMILES CN(C)c1ccnc2sc3c(ncn(-c4ccc(Cl)cc4)c3=O)c12 Show InChI InChI=1S/C17H13ClN4OS/c1-21(2)12-7-8-19-16-13(12)14-15(24-16)17(23)22(9-20-14)11-5-3-10(18)4-6-11/h3-9H,1-2H3 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [3H]R214127 from mGluR1 in rat cerebellum membranes |
J Med Chem 48: 7374-88 (2005)
Article DOI: 10.1021/jm0504407
BindingDB Entry DOI: 10.7270/Q27P8XX0 |
More data for this
Ligand-Target Pair | |
Lactoylglutathione lyase
(Homo sapiens (Human)) | BDBM50140172

(CHEBI:3962 | CHEMBL140 | Curcumin | US9409845, Tab...)Show SMILES COc1cc(\C=C\C(=O)CC(=O)\C=C\c2ccc(O)c(OC)c2)ccc1O Show InChI InChI=1S/C21H20O6/c1-26-20-11-14(5-9-18(20)24)3-7-16(22)13-17(23)8-4-15-6-10-19(25)21(12-15)27-2/h3-12,24-25H,13H2,1-2H3/b7-3+,8-4+ | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Chengdu University
Curated by ChEMBL
| Assay Description
Inhibition of human erythrocyte Glyoxalase-1 using GSH and MGO as substrate by Dixon plot analysis |
Bioorg Med Chem 28: (2020)
Article DOI: 10.1016/j.bmc.2019.115243
BindingDB Entry DOI: 10.7270/Q2Z60SG5 |
More data for this
Ligand-Target Pair | |
Metabotropic glutamate receptor 1
(RAT) | BDBM50177053
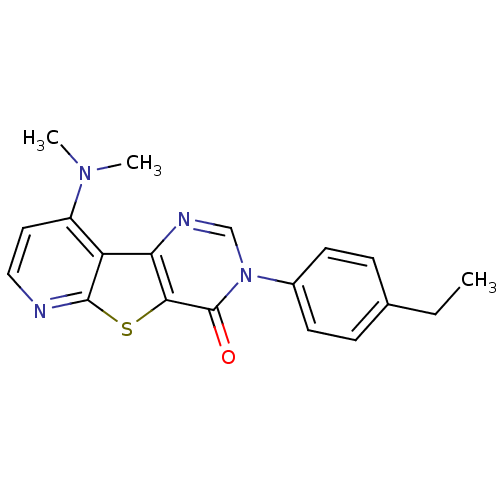
(9-Dimethylamino-3-(4-ethyl-phenyl)-3H-pyrido[3',2'...)Show SMILES CCc1ccc(cc1)-n1cnc2c(sc3nccc(N(C)C)c23)c1=O Show InChI InChI=1S/C19H18N4OS/c1-4-12-5-7-13(8-6-12)23-11-21-16-15-14(22(2)3)9-10-20-18(15)25-17(16)19(23)24/h5-11H,4H2,1-3H3 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [3H]R214127 from mGluR1 in rat cerebellum membranes |
J Med Chem 48: 7374-88 (2005)
Article DOI: 10.1021/jm0504407
BindingDB Entry DOI: 10.7270/Q27P8XX0 |
More data for this
Ligand-Target Pair | |
Metabotropic glutamate receptor 1
(RAT) | BDBM50177055
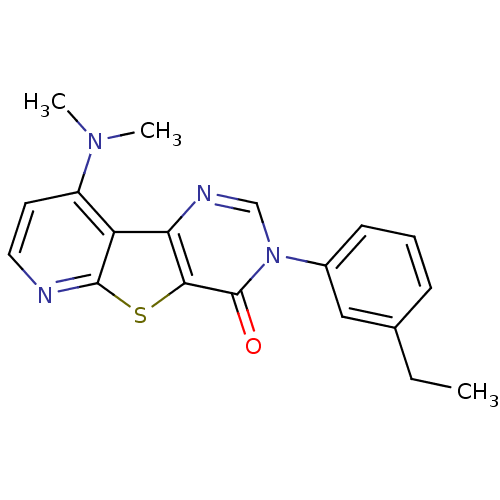
(9-Dimethylamino-3-(3-ethyl-phenyl)-3H-pyrido[3',2'...)Show SMILES CCc1cccc(c1)-n1cnc2c(sc3nccc(N(C)C)c23)c1=O Show InChI InChI=1S/C19H18N4OS/c1-4-12-6-5-7-13(10-12)23-11-21-16-15-14(22(2)3)8-9-20-18(15)25-17(16)19(23)24/h5-11H,4H2,1-3H3 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [3H]R214127 from mGluR1 in rat cerebellum membranes |
J Med Chem 48: 7374-88 (2005)
Article DOI: 10.1021/jm0504407
BindingDB Entry DOI: 10.7270/Q27P8XX0 |
More data for this
Ligand-Target Pair | |
Lactoylglutathione lyase
(Homo sapiens (Human)) | BDBM50526944
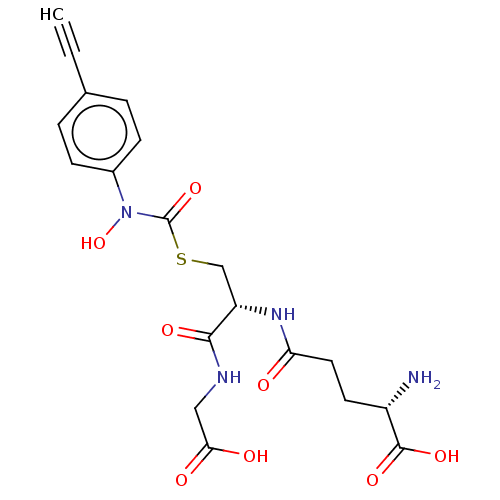
(CHEMBL4450158)Show SMILES N[C@@H](CCC(=O)N[C@@H](CSC(=O)N(O)c1ccc(cc1)C#C)C(=O)NCC(O)=O)C(O)=O |r| Show InChI InChI=1S/C19H22N4O8S/c1-2-11-3-5-12(6-4-11)23(31)19(30)32-10-14(17(27)21-9-16(25)26)22-15(24)8-7-13(20)18(28)29/h1,3-6,13-14,31H,7-10,20H2,(H,21,27)(H,22,24)(H,25,26)(H,28,29)/t13-,14-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| KEGG
PC cid
PC sid
UniChem
| Article
PubMed
| 7 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Chengdu University
Curated by ChEMBL
| Assay Description
Competitive inhibition of human Glyoxalase-1 using GSH and MGO as substrate |
Bioorg Med Chem 28: (2020)
Article DOI: 10.1016/j.bmc.2019.115243
BindingDB Entry DOI: 10.7270/Q2Z60SG5 |
More data for this
Ligand-Target Pair | |
Metabotropic glutamate receptor 1
(RAT) | BDBM50177078
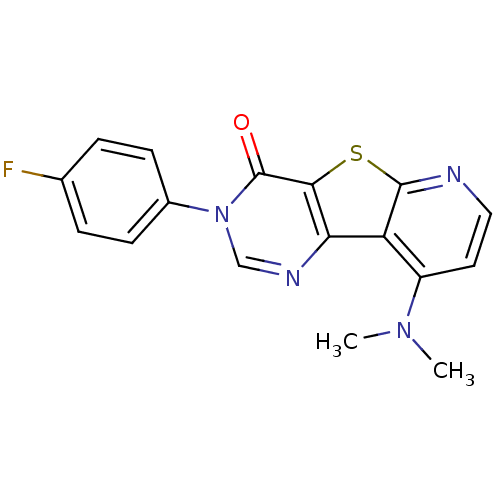
(9-Dimethylamino-3-(4-fluoro-phenyl)-3H-pyrido[3',2...)Show InChI InChI=1S/C17H13FN4OS/c1-21(2)12-7-8-19-16-13(12)14-15(24-16)17(23)22(9-20-14)11-5-3-10(18)4-6-11/h3-9H,1-2H3 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 8 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [3H]R214127 from mGluR1 in rat cerebellum membranes |
J Med Chem 48: 7374-88 (2005)
Article DOI: 10.1021/jm0504407
BindingDB Entry DOI: 10.7270/Q27P8XX0 |
More data for this
Ligand-Target Pair | |
Metabotropic glutamate receptor 1
(RAT) | BDBM50177057
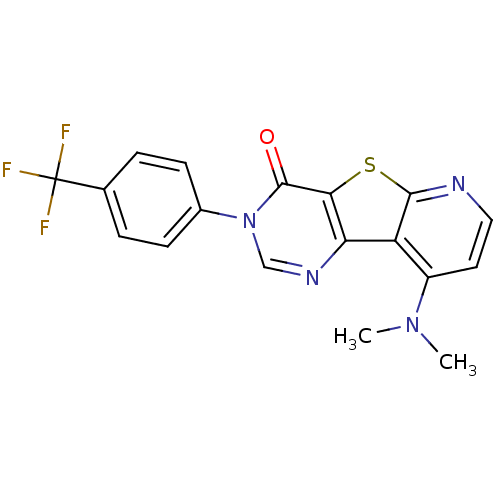
(9-Dimethylamino-3-(4-trifluoromethyl-phenyl)-3H-py...)Show SMILES CN(C)c1ccnc2sc3c(ncn(-c4ccc(cc4)C(F)(F)F)c3=O)c12 Show InChI InChI=1S/C18H13F3N4OS/c1-24(2)12-7-8-22-16-13(12)14-15(27-16)17(26)25(9-23-14)11-5-3-10(4-6-11)18(19,20)21/h3-9H,1-2H3 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 8 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [3H]R214127 from mGluR1 in rat cerebellum membranes |
J Med Chem 48: 7374-88 (2005)
Article DOI: 10.1021/jm0504407
BindingDB Entry DOI: 10.7270/Q27P8XX0 |
More data for this
Ligand-Target Pair | |
Lactoylglutathione lyase
(Homo sapiens (Human)) | BDBM50526942
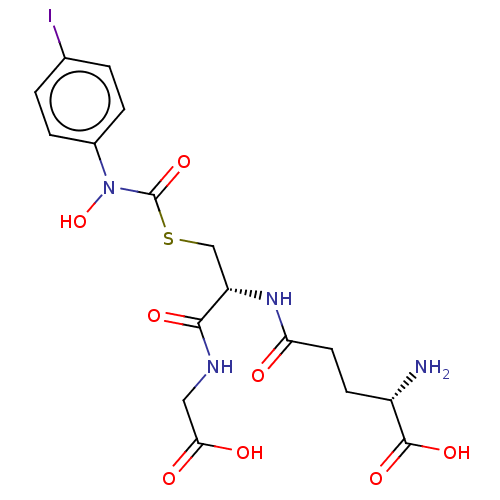
(CHEMBL4438930)Show SMILES N[C@@H](CCC(=O)N[C@@H](CSC(=O)N(O)c1ccc(I)cc1)C(=O)NCC(O)=O)C(O)=O |r| Show InChI InChI=1S/C17H21IN4O8S/c18-9-1-3-10(4-2-9)22(30)17(29)31-8-12(15(26)20-7-14(24)25)21-13(23)6-5-11(19)16(27)28/h1-4,11-12,30H,5-8,19H2,(H,20,26)(H,21,23)(H,24,25)(H,27,28)/t11-,12-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
KEGG
PC cid
PC sid
UniChem
| Article
PubMed
| 10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Chengdu University
Curated by ChEMBL
| Assay Description
Inhibition of human Glyoxalase-1 |
Bioorg Med Chem 28: (2020)
Article DOI: 10.1016/j.bmc.2019.115243
BindingDB Entry DOI: 10.7270/Q2Z60SG5 |
More data for this
Ligand-Target Pair | |
Metabotropic glutamate receptor 1
(RAT) | BDBM50177081
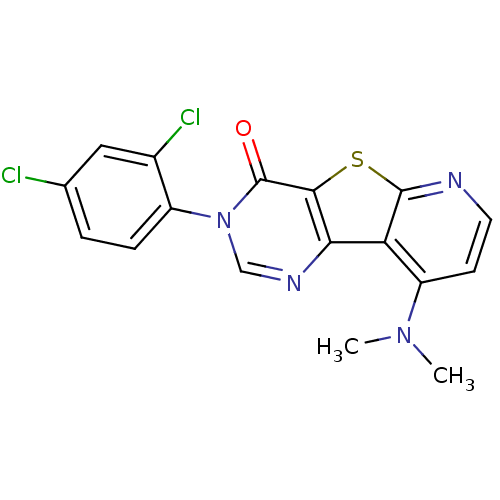
(3-(2,4-Dichloro-phenyl)-9-dimethylamino-3H-pyrido[...)Show SMILES CN(C)c1ccnc2sc3c(ncn(-c4ccc(Cl)cc4Cl)c3=O)c12 Show InChI InChI=1S/C17H12Cl2N4OS/c1-22(2)12-5-6-20-16-13(12)14-15(25-16)17(24)23(8-21-14)11-4-3-9(18)7-10(11)19/h3-8H,1-2H3 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 11 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [3H]R214127 from mGluR1 in rat cerebellum membranes |
J Med Chem 48: 7374-88 (2005)
Article DOI: 10.1021/jm0504407
BindingDB Entry DOI: 10.7270/Q27P8XX0 |
More data for this
Ligand-Target Pair | |
Lactoylglutathione lyase
(Homo sapiens (Human)) | BDBM50092826
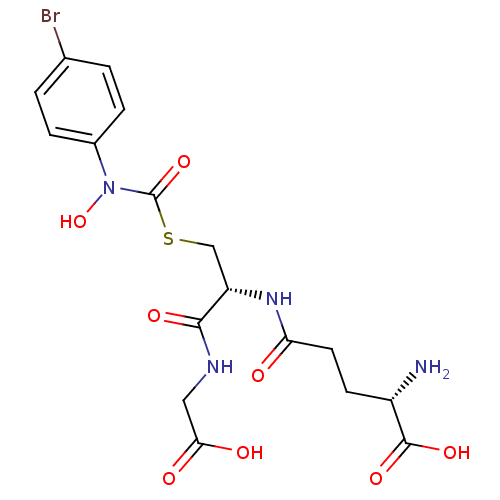
((2S)-2-amino-5-{[(1R)-1-[({[(4-bromophenyl)(hydrox...)Show SMILES N[C@@H](CCC(=O)N[C@@H](CSC(=O)N(O)c1ccc(Br)cc1)C(=O)NCC(O)=O)C(O)=O Show InChI InChI=1S/C17H21BrN4O8S/c18-9-1-3-10(4-2-9)22(30)17(29)31-8-12(15(26)20-7-14(24)25)21-13(23)6-5-11(19)16(27)28/h1-4,11-12,30H,5-8,19H2,(H,20,26)(H,21,23)(H,24,25)(H,27,28)/t11-,12-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
MMDB
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 14 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Chengdu University
Curated by ChEMBL
| Assay Description
Inhibition of human glyoxalase 1 |
Bioorg Med Chem Lett 25: 4724-7 (2015)
Article DOI: 10.1016/j.bmcl.2015.08.055
BindingDB Entry DOI: 10.7270/Q26H4K7B |
More data for this
Ligand-Target Pair | |
Lactoylglutathione lyase
(Homo sapiens (Human)) | BDBM50092826

((2S)-2-amino-5-{[(1R)-1-[({[(4-bromophenyl)(hydrox...)Show SMILES N[C@@H](CCC(=O)N[C@@H](CSC(=O)N(O)c1ccc(Br)cc1)C(=O)NCC(O)=O)C(O)=O Show InChI InChI=1S/C17H21BrN4O8S/c18-9-1-3-10(4-2-9)22(30)17(29)31-8-12(15(26)20-7-14(24)25)21-13(23)6-5-11(19)16(27)28/h1-4,11-12,30H,5-8,19H2,(H,20,26)(H,21,23)(H,24,25)(H,27,28)/t11-,12-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
MMDB
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 14 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Chengdu University
Curated by ChEMBL
| Assay Description
Inhibition of human erythrocyte Glyoxalase-1 |
Bioorg Med Chem 28: (2020)
Article DOI: 10.1016/j.bmc.2019.115243
BindingDB Entry DOI: 10.7270/Q2Z60SG5 |
More data for this
Ligand-Target Pair | |
Lactoylglutathione lyase
(Homo sapiens (Human)) | BDBM50526948
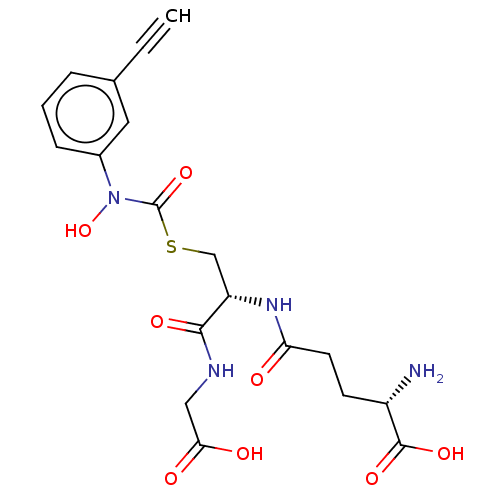
(CHEMBL4559486)Show SMILES N[C@@H](CCC(=O)N[C@@H](CSC(=O)N(O)c1cccc(c1)C#C)C(=O)NCC(O)=O)C(O)=O |r| Show InChI InChI=1S/C19H22N4O8S/c1-2-11-4-3-5-12(8-11)23(31)19(30)32-10-14(17(27)21-9-16(25)26)22-15(24)7-6-13(20)18(28)29/h1,3-5,8,13-14,31H,6-7,9-10,20H2,(H,21,27)(H,22,24)(H,25,26)(H,28,29)/t13-,14-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| KEGG
PC cid
PC sid
UniChem
| Article
PubMed
| 19 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Chengdu University
Curated by ChEMBL
| Assay Description
Competitive inhibition of human Glyoxalase-1 using GSH and MGO as substrate |
Bioorg Med Chem 28: (2020)
Article DOI: 10.1016/j.bmc.2019.115243
BindingDB Entry DOI: 10.7270/Q2Z60SG5 |
More data for this
Ligand-Target Pair | |
Lactoylglutathione lyase
(Homo sapiens (Human)) | BDBM50126957
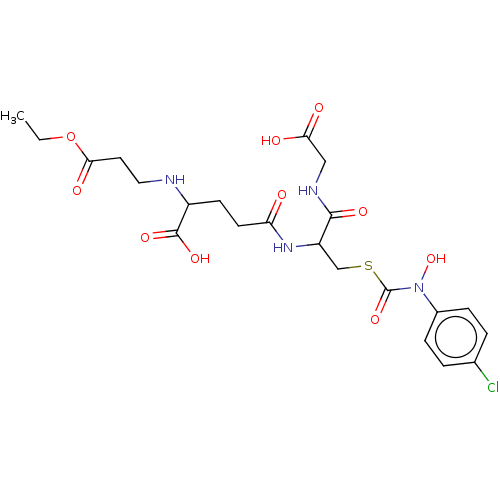
(CHEMBL3629119)Show SMILES CCOC(=O)CCNC(CCC(=O)NC(CSC(=O)N(O)c1ccc(Cl)cc1)C(=O)NCC(O)=O)C(O)=O Show InChI InChI=1S/C22H29ClN4O10S/c1-2-37-19(31)9-10-24-15(21(33)34)7-8-17(28)26-16(20(32)25-11-18(29)30)12-38-22(35)27(36)14-5-3-13(23)4-6-14/h3-6,15-16,24,36H,2,7-12H2,1H3,(H,25,32)(H,26,28)(H,29,30)(H,33,34) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 46 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Chengdu University
Curated by ChEMBL
| Assay Description
Inhibition of human glyoxalase 1 |
Bioorg Med Chem Lett 25: 4724-7 (2015)
Article DOI: 10.1016/j.bmcl.2015.08.055
BindingDB Entry DOI: 10.7270/Q26H4K7B |
More data for this
Ligand-Target Pair | |
Lactoylglutathione lyase
(Homo sapiens (Human)) | BDBM50092824
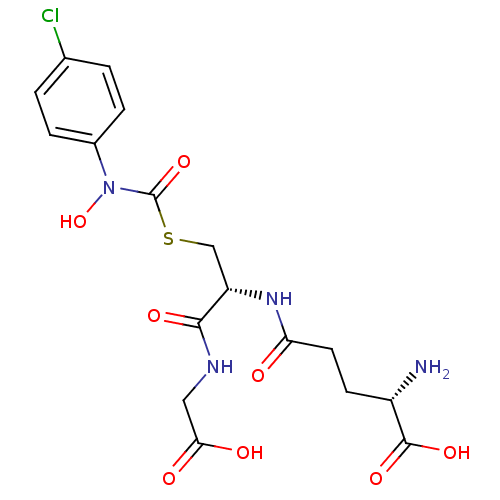
(CHEMBL131578 | S-(N-4chlorophenyl-N-hydroxycarbamo...)Show SMILES N[C@@H](CCC(=O)N[C@@H](CSC(=O)N(O)c1ccc(Cl)cc1)C(=O)NCC(O)=O)C(O)=O Show InChI InChI=1S/C17H21ClN4O8S/c18-9-1-3-10(4-2-9)22(30)17(29)31-8-12(15(26)20-7-14(24)25)21-13(23)6-5-11(19)16(27)28/h1-4,11-12,30H,5-8,19H2,(H,20,26)(H,21,23)(H,24,25)(H,27,28)/t11-,12-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 46 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Chengdu University
Curated by ChEMBL
| Assay Description
Inhibition of human glyoxalase 1 |
Bioorg Med Chem Lett 25: 4724-7 (2015)
Article DOI: 10.1016/j.bmcl.2015.08.055
BindingDB Entry DOI: 10.7270/Q26H4K7B |
More data for this
Ligand-Target Pair | |
Lactoylglutathione lyase
(Homo sapiens (Human)) | BDBM50092824

(CHEMBL131578 | S-(N-4chlorophenyl-N-hydroxycarbamo...)Show SMILES N[C@@H](CCC(=O)N[C@@H](CSC(=O)N(O)c1ccc(Cl)cc1)C(=O)NCC(O)=O)C(O)=O Show InChI InChI=1S/C17H21ClN4O8S/c18-9-1-3-10(4-2-9)22(30)17(29)31-8-12(15(26)20-7-14(24)25)21-13(23)6-5-11(19)16(27)28/h1-4,11-12,30H,5-8,19H2,(H,20,26)(H,21,23)(H,24,25)(H,27,28)/t11-,12-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 46 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Chengdu University
Curated by ChEMBL
| Assay Description
Inhibition of human erythrocyte Glyoxalase-1 |
Bioorg Med Chem 28: (2020)
Article DOI: 10.1016/j.bmc.2019.115243
BindingDB Entry DOI: 10.7270/Q2Z60SG5 |
More data for this
Ligand-Target Pair | |
Metabotropic glutamate receptor 1
(RAT) | BDBM50177082
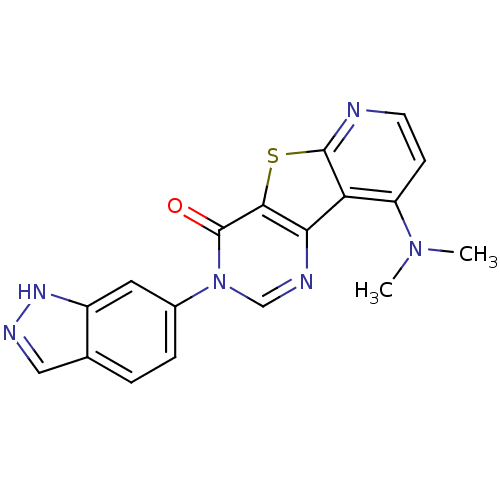
(9-dimethylamino-3-(1H-indazol-6-yl)-3H-5-thia-1,3,...)Show SMILES CN(C)c1ccnc2sc3c(ncn(-c4ccc5cn[nH]c5c4)c3=O)c12 Show InChI InChI=1S/C18H14N6OS/c1-23(2)13-5-6-19-17-14(13)15-16(26-17)18(25)24(9-20-15)11-4-3-10-8-21-22-12(10)7-11/h3-9H,1-2H3,(H,21,22) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 62 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [3H]R214127 from mGluR1 in rat cerebellum membranes |
J Med Chem 48: 7374-88 (2005)
Article DOI: 10.1021/jm0504407
BindingDB Entry DOI: 10.7270/Q27P8XX0 |
More data for this
Ligand-Target Pair | |
Hydroxyacylglutathione hydrolase, mitochondrial
(Bos taurus) | BDBM50126960

(CHEMBL3629116)Show SMILES ON(C(=O)SCC(NC(=O)CCC(NCCC(=O)OCCNC(=O)CCNC(=O)CC(=O)NCCNC(=O)CC(=O)NCCC(=O)NCCOC(=O)CCNC(CCC(=O)NC(CSC(=O)N(O)c1ccc(Br)cc1)C(=O)NCC(O)=O)C(O)=O)C(O)=O)C(=O)NCC(O)=O)c1ccc(Br)cc1 Show InChI InChI=1S/C58H78Br2N14O26S2/c59-33-1-5-35(6-2-33)73(97)57(95)101-31-39(53(89)69-29-49(83)84)71-43(77)11-9-37(55(91)92)61-19-15-51(87)99-25-23-67-41(75)13-17-63-45(79)27-47(81)65-21-22-66-48(82)28-46(80)64-18-14-42(76)68-24-26-100-52(88)16-20-62-38(56(93)94)10-12-44(78)72-40(54(90)70-30-50(85)86)32-102-58(96)74(98)36-7-3-34(60)4-8-36/h1-8,37-40,61-62,97-98H,9-32H2,(H,63,79)(H,64,80)(H,65,81)(H,66,82)(H,67,75)(H,68,76)(H,69,89)(H,70,90)(H,71,77)(H,72,78)(H,83,84)(H,85,86)(H,91,92)(H,93,94) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 81 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Chengdu University
Curated by ChEMBL
| Assay Description
Inhibition of bovine liver glyoxalase 2 |
Bioorg Med Chem Lett 25: 4724-7 (2015)
Article DOI: 10.1016/j.bmcl.2015.08.055
BindingDB Entry DOI: 10.7270/Q26H4K7B |
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Homo sapiens (Human)) | BDBM50265482
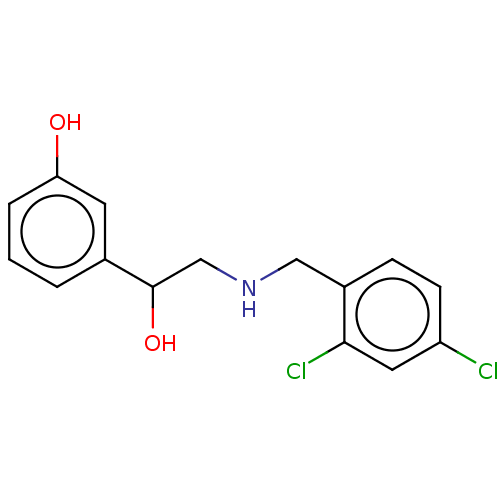
(CHEMBL4071661)Show InChI InChI=1S/C15H15Cl2NO2/c16-12-5-4-11(14(17)7-12)8-18-9-15(20)10-2-1-3-13(19)6-10/h1-7,15,18-20H,8-9H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 83 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Southern California
Curated by ChEMBL
| Assay Description
Displacement of 3H-U69593 from human KOR expressed in HEK293 cells after 90 mins by micro beta scintillation counting analysis |
J Med Chem 60: 3070-3081 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00109
BindingDB Entry DOI: 10.7270/Q2VQ354M |
More data for this
Ligand-Target Pair | |
Metabotropic glutamate receptor 1
(RAT) | BDBM50177064
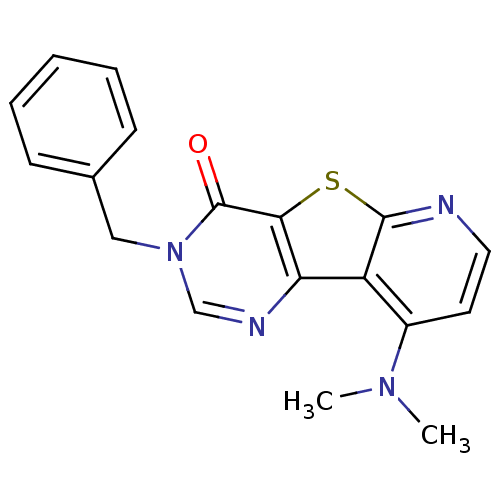
(3-Benzyl-9-dimethylamino-3H-pyrido[3',2':4,5]thien...)Show InChI InChI=1S/C18H16N4OS/c1-21(2)13-8-9-19-17-14(13)15-16(24-17)18(23)22(11-20-15)10-12-6-4-3-5-7-12/h3-9,11H,10H2,1-2H3 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 87 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [3H]R214127 from mGluR1 in rat cerebellum membranes |
J Med Chem 48: 7374-88 (2005)
Article DOI: 10.1021/jm0504407
BindingDB Entry DOI: 10.7270/Q27P8XX0 |
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Homo sapiens (Human)) | BDBM50265482

(CHEMBL4071661)Show InChI InChI=1S/C15H15Cl2NO2/c16-12-5-4-11(14(17)7-12)8-18-9-15(20)10-2-1-3-13(19)6-10/h1-7,15,18-20H,8-9H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Southern California
Curated by ChEMBL
| Assay Description
Displacement of 3H-U69593 from human KOR expressed in HEK293 cells after 90 mins by micro beta scintillation counting analysis |
J Med Chem 60: 3070-3081 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00109
BindingDB Entry DOI: 10.7270/Q2VQ354M |
More data for this
Ligand-Target Pair | |
Metabotropic glutamate receptor 1
(RAT) | BDBM50177067
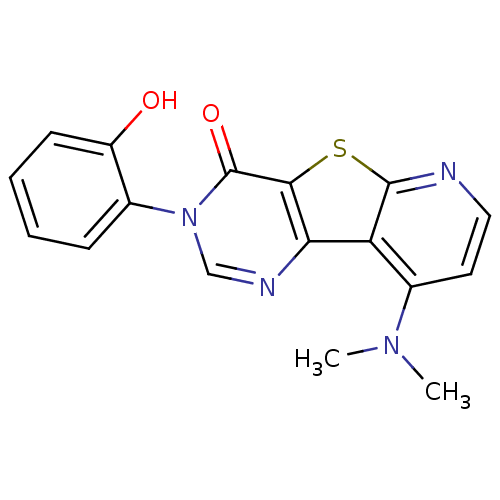
(9-Dimethylamino-3-(2-hydroxy-phenyl)-3H-pyrido[3',...)Show InChI InChI=1S/C17H14N4O2S/c1-20(2)11-7-8-18-16-13(11)14-15(24-16)17(23)21(9-19-14)10-5-3-4-6-12(10)22/h3-9,22H,1-2H3 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 99 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [3H]R214127 from mGluR1 in rat cerebellum membranes |
J Med Chem 48: 7374-88 (2005)
Article DOI: 10.1021/jm0504407
BindingDB Entry DOI: 10.7270/Q27P8XX0 |
More data for this
Ligand-Target Pair | |
Lactoylglutathione lyase
(Homo sapiens (Human)) | BDBM50126958
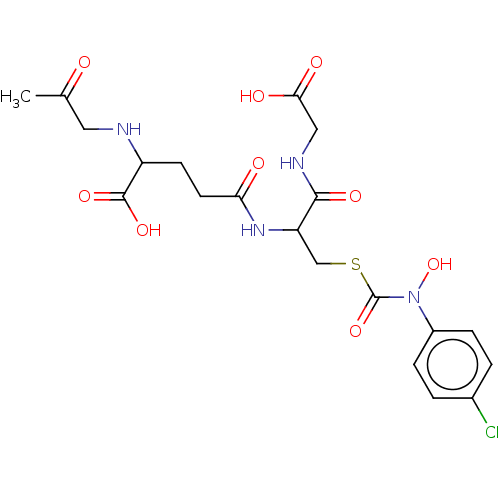
(CHEMBL3629118)Show SMILES CC(=O)CNC(CCC(=O)NC(CSC(=O)N(O)c1ccc(Cl)cc1)C(=O)NCC(O)=O)C(O)=O Show InChI InChI=1S/C20H25ClN4O9S/c1-11(26)8-22-14(19(31)32)6-7-16(27)24-15(18(30)23-9-17(28)29)10-35-20(33)25(34)13-4-2-12(21)3-5-13/h2-5,14-15,22,34H,6-10H2,1H3,(H,23,30)(H,24,27)(H,28,29)(H,31,32) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 130 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Chengdu University
Curated by ChEMBL
| Assay Description
Inhibition of human glyoxalase 1 |
Bioorg Med Chem Lett 25: 4724-7 (2015)
Article DOI: 10.1016/j.bmcl.2015.08.055
BindingDB Entry DOI: 10.7270/Q26H4K7B |
More data for this
Ligand-Target Pair | |
Hydroxyacylglutathione hydrolase, mitochondrial
(Bos taurus) | BDBM50126961
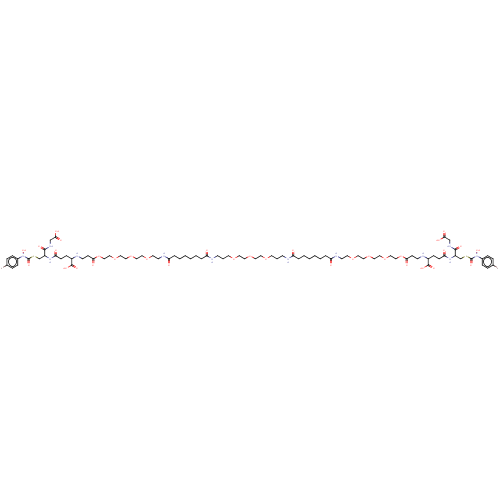
(CHEMBL3629115)Show SMILES ON(C(=O)SCC(NC(=O)CCC(NCCC(=O)OCCOCCOCCOCCNC(=O)CCCCCCC(=O)NCCCOCCOCCOCCCNC(=O)CCCCCCC(=O)NCCOCCOCCOCCOC(=O)CCNC(CCC(=O)NC(CSC(=O)N(O)c1ccc(Br)cc1)C(=O)NCC(O)=O)C(O)=O)C(O)=O)C(=O)NCC(O)=O)c1ccc(Br)cc1 Show InChI InChI=1S/C82H128Br2N12O33S2/c83-59-15-19-61(20-16-59)95(117)81(115)130-57-65(77(109)91-55-73(103)104)93-71(101)25-23-63(79(111)112)85-31-27-75(107)128-53-51-126-49-47-124-45-41-121-37-33-89-69(99)13-7-3-1-5-11-67(97)87-29-9-35-119-39-43-123-44-40-120-36-10-30-88-68(98)12-6-2-4-8-14-70(100)90-34-38-122-42-46-125-48-50-127-52-54-129-76(108)28-32-86-64(80(113)114)24-26-72(102)94-66(78(110)92-56-74(105)106)58-131-82(116)96(118)62-21-17-60(84)18-22-62/h15-22,63-66,85-86,117-118H,1-14,23-58H2,(H,87,97)(H,88,98)(H,89,99)(H,90,100)(H,91,109)(H,92,110)(H,93,101)(H,94,102)(H,103,104)(H,105,106)(H,111,112)(H,113,114) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 138 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Chengdu University
Curated by ChEMBL
| Assay Description
Inhibition of bovine liver glyoxalase 2 |
Bioorg Med Chem Lett 25: 4724-7 (2015)
Article DOI: 10.1016/j.bmcl.2015.08.055
BindingDB Entry DOI: 10.7270/Q26H4K7B |
More data for this
Ligand-Target Pair | |
Metabotropic glutamate receptor 1
(RAT) | BDBM50177056
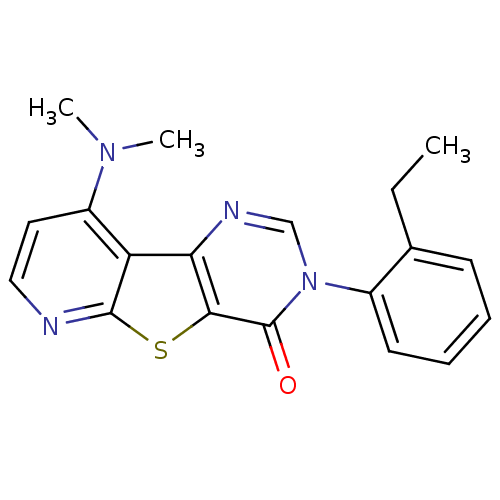
(9-Dimethylamino-3-(2-ethyl-phenyl)-3H-pyrido[3',2'...)Show InChI InChI=1S/C19H18N4OS/c1-4-12-7-5-6-8-13(12)23-11-21-16-15-14(22(2)3)9-10-20-18(15)25-17(16)19(23)24/h5-11H,4H2,1-3H3 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 142 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [3H]R214127 from mGluR1 in rat cerebellum membranes |
J Med Chem 48: 7374-88 (2005)
Article DOI: 10.1021/jm0504407
BindingDB Entry DOI: 10.7270/Q27P8XX0 |
More data for this
Ligand-Target Pair | |
Lactoylglutathione lyase
(Mus musculus) | BDBM50526947
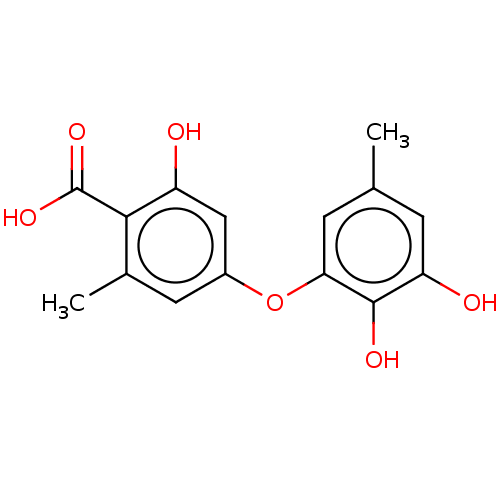
(CHEMBL4584432)Show InChI InChI=1S/C15H14O6/c1-7-3-11(17)14(18)12(4-7)21-9-5-8(2)13(15(19)20)10(16)6-9/h3-6,16-18H,1-2H3,(H,19,20) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
| Article
PubMed
| 150 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Chengdu University
Curated by ChEMBL
| Assay Description
Competitive inhibition of recombinant mouse His-tagged Glyoxalase-1 using GSH and MGO as substrate by spectrophotometric method |
Bioorg Med Chem 28: (2020)
Article DOI: 10.1016/j.bmc.2019.115243
BindingDB Entry DOI: 10.7270/Q2Z60SG5 |
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Homo sapiens (Human)) | BDBM50265524
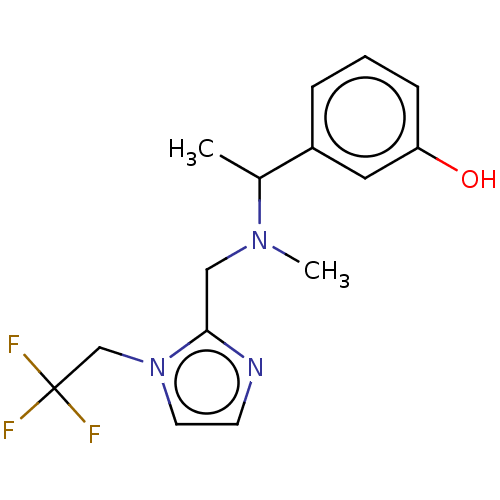
(CHEMBL4072651)Show InChI InChI=1S/C15H18F3N3O/c1-11(12-4-3-5-13(22)8-12)20(2)9-14-19-6-7-21(14)10-15(16,17)18/h3-8,11,22H,9-10H2,1-2H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 151 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Southern California
Curated by ChEMBL
| Assay Description
Displacement of 3H-U69593 from human KOR expressed in HEK293 cells after 90 mins by micro beta scintillation counting analysis |
J Med Chem 60: 3070-3081 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00109
BindingDB Entry DOI: 10.7270/Q2VQ354M |
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Homo sapiens (Human)) | BDBM50265524

(CHEMBL4072651)Show InChI InChI=1S/C15H18F3N3O/c1-11(12-4-3-5-13(22)8-12)20(2)9-14-19-6-7-21(14)10-15(16,17)18/h3-8,11,22H,9-10H2,1-2H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 151 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Southern California
Curated by ChEMBL
| Assay Description
Displacement of 3H-U69593 from human KOR expressed in HEK293 cells after 90 mins by micro beta scintillation counting analysis |
J Med Chem 60: 3070-3081 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00109
BindingDB Entry DOI: 10.7270/Q2VQ354M |
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Homo sapiens (Human)) | BDBM50265525
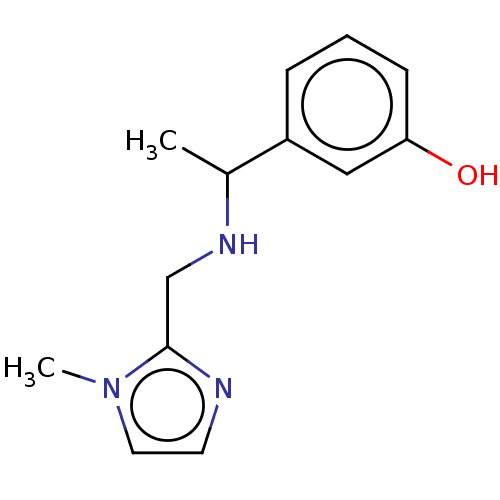
(CHEMBL4092454)Show InChI InChI=1S/C13H17N3O/c1-10(11-4-3-5-12(17)8-11)15-9-13-14-6-7-16(13)2/h3-8,10,15,17H,9H2,1-2H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 155 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Southern California
Curated by ChEMBL
| Assay Description
Displacement of 3H-U69593 from human KOR expressed in HEK293 cells after 90 mins by micro beta scintillation counting analysis |
J Med Chem 60: 3070-3081 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00109
BindingDB Entry DOI: 10.7270/Q2VQ354M |
More data for this
Ligand-Target Pair | |
Lactoylglutathione lyase
(Homo sapiens (Human)) | BDBM50092825
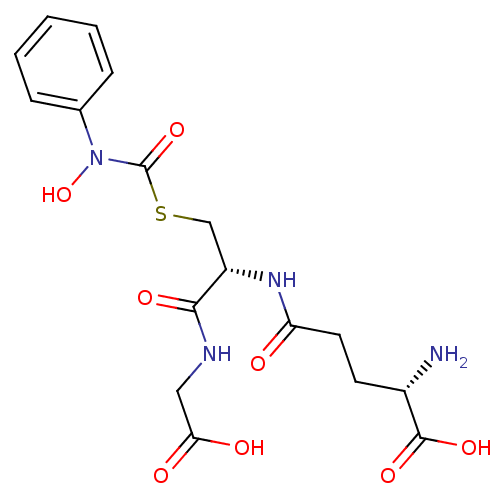
(CHEMBL128935 | S-(N-phenyl-N-hydroxycarbamoyl)glut...)Show SMILES N[C@@H](CCC(=O)N[C@@H](CSC(=O)N(O)c1ccccc1)C(=O)NCC(O)=O)C(O)=O Show InChI InChI=1S/C17H22N4O8S/c18-11(16(26)27)6-7-13(22)20-12(15(25)19-8-14(23)24)9-30-17(28)21(29)10-4-2-1-3-5-10/h1-5,11-12,29H,6-9,18H2,(H,19,25)(H,20,22)(H,23,24)(H,26,27)/t11-,12-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 160 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Chengdu University
Curated by ChEMBL
| Assay Description
Inhibition of human erythrocyte Glyoxalase-1 |
Bioorg Med Chem 28: (2020)
Article DOI: 10.1016/j.bmc.2019.115243
BindingDB Entry DOI: 10.7270/Q2Z60SG5 |
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Homo sapiens (Human)) | BDBM50265525

(CHEMBL4092454)Show InChI InChI=1S/C13H17N3O/c1-10(11-4-3-5-12(17)8-11)15-9-13-14-6-7-16(13)2/h3-8,10,15,17H,9H2,1-2H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 160 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Southern California
Curated by ChEMBL
| Assay Description
Displacement of 3H-U69593 from human KOR expressed in HEK293 cells after 90 mins by micro beta scintillation counting analysis |
J Med Chem 60: 3070-3081 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00109
BindingDB Entry DOI: 10.7270/Q2VQ354M |
More data for this
Ligand-Target Pair | |
Lactoylglutathione lyase
(Homo sapiens (Human)) | BDBM50241121
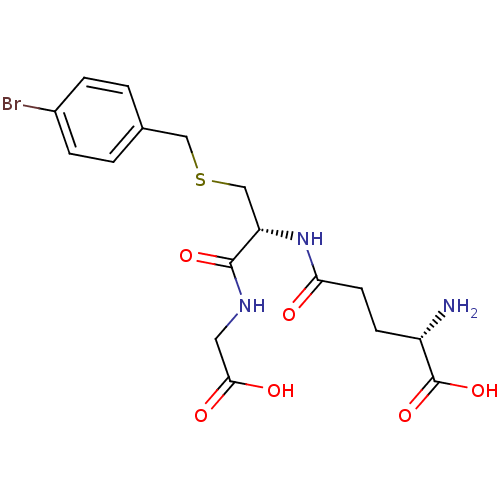
((S)-5-((R)-3-(4-bromobenzylthio)-1-(carboxymethyla...)Show SMILES N[C@@H](CCC(=O)N[C@@H](CSCc1ccc(Br)cc1)C(=O)NCC(O)=O)C(O)=O |r| Show InChI InChI=1S/C17H22BrN3O6S/c18-11-3-1-10(2-4-11)8-28-9-13(16(25)20-7-15(23)24)21-14(22)6-5-12(19)17(26)27/h1-4,12-13H,5-9,19H2,(H,20,25)(H,21,22)(H,23,24)(H,26,27)/t12-,13-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
KEGG
MMDB
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 170 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Chengdu University
Curated by ChEMBL
| Assay Description
Inhibition of Glyoxalase-1 (unknown origin) using GSH and MGO as substrates preincubated with substrates for 6 mins followed by enzyme addition by sp... |
Bioorg Med Chem 28: (2020)
Article DOI: 10.1016/j.bmc.2019.115243
BindingDB Entry DOI: 10.7270/Q2Z60SG5 |
More data for this
Ligand-Target Pair | |
Metabotropic glutamate receptor 5
(Rattus norvegicus (Rat)) | BDBM50177053

(9-Dimethylamino-3-(4-ethyl-phenyl)-3H-pyrido[3',2'...)Show SMILES CCc1ccc(cc1)-n1cnc2c(sc3nccc(N(C)C)c23)c1=O Show InChI InChI=1S/C19H18N4OS/c1-4-12-5-7-13(8-6-12)23-11-21-16-15-14(22(2)3)9-10-20-18(15)25-17(16)19(23)24/h5-11H,4H2,1-3H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 194 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [3H]MPEP from rat cortex mGluR5 |
J Med Chem 48: 7374-88 (2005)
Article DOI: 10.1021/jm0504407
BindingDB Entry DOI: 10.7270/Q27P8XX0 |
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Homo sapiens (Human)) | BDBM50265478
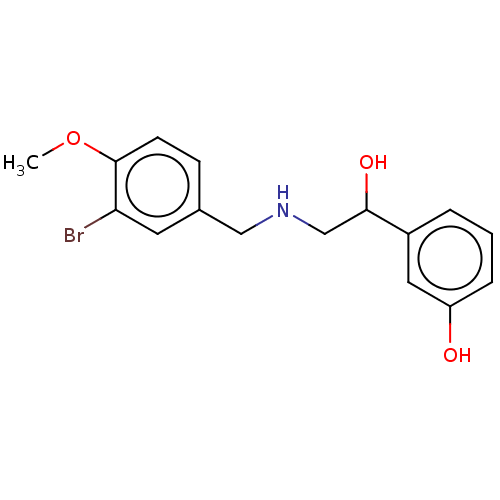
(CHEMBL3484893)Show InChI InChI=1S/C16H18BrNO3/c1-21-16-6-5-11(7-14(16)17)9-18-10-15(20)12-3-2-4-13(19)8-12/h2-8,15,18-20H,9-10H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 229 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Southern California
Curated by ChEMBL
| Assay Description
Displacement of 3H-U69593 from human KOR expressed in HEK293 cells after 90 mins by micro beta scintillation counting analysis |
J Med Chem 60: 3070-3081 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00109
BindingDB Entry DOI: 10.7270/Q2VQ354M |
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Homo sapiens (Human)) | BDBM50265478

(CHEMBL3484893)Show InChI InChI=1S/C16H18BrNO3/c1-21-16-6-5-11(7-14(16)17)9-18-10-15(20)12-3-2-4-13(19)8-12/h2-8,15,18-20H,9-10H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 230 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Southern California
Curated by ChEMBL
| Assay Description
Displacement of 3H-U69593 from human KOR expressed in HEK293 cells after 90 mins by micro beta scintillation counting analysis |
J Med Chem 60: 3070-3081 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00109
BindingDB Entry DOI: 10.7270/Q2VQ354M |
More data for this
Ligand-Target Pair | |
Lactoylglutathione lyase
(Mus musculus) | BDBM50517464
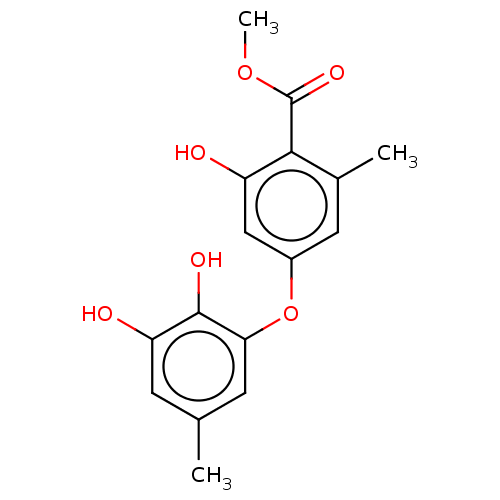
(CHEMBL1234300)Show InChI InChI=1S/C16H16O6/c1-8-4-12(18)15(19)13(5-8)22-10-6-9(2)14(11(17)7-10)16(20)21-3/h4-7,17-19H,1-3H3 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| 230 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Chengdu University
Curated by ChEMBL
| Assay Description
Competitive inhibition of recombinant mouse His-tagged Glyoxalase-1 using GSH and MGO as substrate by spectrophotometric method |
Bioorg Med Chem 28: (2020)
Article DOI: 10.1016/j.bmc.2019.115243
BindingDB Entry DOI: 10.7270/Q2Z60SG5 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Kappa-type opioid receptor
(Homo sapiens (Human)) | BDBM50265482

(CHEMBL4071661)Show InChI InChI=1S/C15H15Cl2NO2/c16-12-5-4-11(14(17)7-12)8-18-9-15(20)10-2-1-3-13(19)6-10/h1-7,15,18-20H,8-9H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 230 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Southern California
Curated by ChEMBL
| Assay Description
Antagonist activity at KOR (unknown origin) expressed in HTLA cells assessed as inhibition of Sal A-induced beta-arrestin recruitment preincubated fo... |
J Med Chem 60: 3070-3081 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00109
BindingDB Entry DOI: 10.7270/Q2VQ354M |
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Homo sapiens (Human)) | BDBM50265482

(CHEMBL4071661)Show InChI InChI=1S/C15H15Cl2NO2/c16-12-5-4-11(14(17)7-12)8-18-9-15(20)10-2-1-3-13(19)6-10/h1-7,15,18-20H,8-9H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 234 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Southern California
Curated by ChEMBL
| Assay Description
Antagonist activity at KOR (unknown origin) expressed in HTLA cells assessed as inhibition of Sal A-induced beta-arrestin recruitment preincubated fo... |
J Med Chem 60: 3070-3081 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00109
BindingDB Entry DOI: 10.7270/Q2VQ354M |
More data for this
Ligand-Target Pair | |
Metabotropic glutamate receptor 1
(RAT) | BDBM50177084
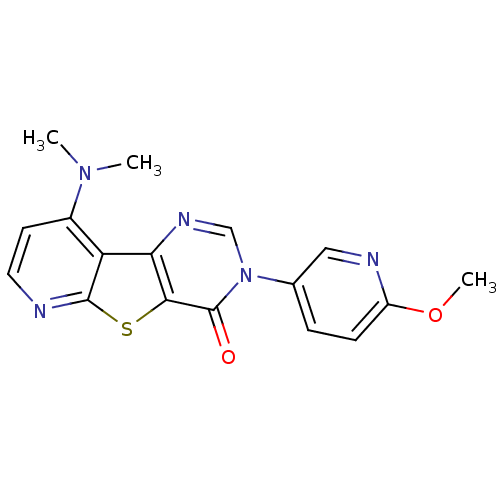
(9-Dimethylamino-3-(6-methoxy-pyridin-3-yl)-3H-pyri...)Show SMILES COc1ccc(cn1)-n1cnc2c(sc3nccc(N(C)C)c23)c1=O Show InChI InChI=1S/C17H15N5O2S/c1-21(2)11-6-7-18-16-13(11)14-15(25-16)17(23)22(9-20-14)10-4-5-12(24-3)19-8-10/h4-9H,1-3H3 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 261 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [3H]R214127 from mGluR1 in rat cerebellum membranes |
J Med Chem 48: 7374-88 (2005)
Article DOI: 10.1021/jm0504407
BindingDB Entry DOI: 10.7270/Q27P8XX0 |
More data for this
Ligand-Target Pair | |
Lactoylglutathione lyase
(Mus musculus) | BDBM50233538

(18beta-glycyrrhetic acid | 3beta-hydroxy-11-oxoole...)Show SMILES CC1(C)[C@@H](O)CC[C@@]2(C)[C@H]1CC[C@]1(C)[C@@H]2C(=O)C=C2[C@@H]3C[C@](C)(CC[C@]3(C)CC[C@@]12C)C(O)=O |r,t:19| Show InChI InChI=1S/C30H46O4/c1-25(2)21-8-11-30(7)23(28(21,5)10-9-22(25)32)20(31)16-18-19-17-27(4,24(33)34)13-12-26(19,3)14-15-29(18,30)6/h16,19,21-23,32H,8-15,17H2,1-7H3,(H,33,34)/t19-,21-,22-,23+,26+,27-,28-,29+,30+/m0/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| 290 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Chengdu University
Curated by ChEMBL
| Assay Description
Inhibition of recombinant mouse Glyoxalase-1 expressed in Escherichia coli BL21 (DE3) pLysS cells using GSH and MGO as substrate by Dixon plot |
Bioorg Med Chem 28: (2020)
Article DOI: 10.1016/j.bmc.2019.115243
BindingDB Entry DOI: 10.7270/Q2Z60SG5 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Lactoylglutathione lyase
(Homo sapiens (Human)) | BDBM50126959
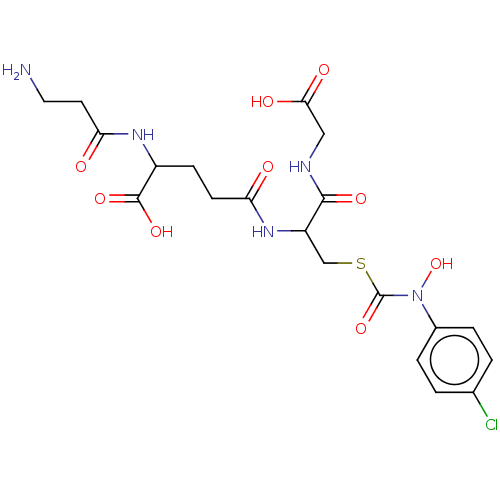
(CHEMBL3629117)Show SMILES NCCC(=O)NC(CCC(=O)NC(CSC(=O)N(O)c1ccc(Cl)cc1)C(=O)NCC(O)=O)C(O)=O Show InChI InChI=1S/C20H26ClN5O9S/c21-11-1-3-12(4-2-11)26(35)20(34)36-10-14(18(31)23-9-17(29)30)25-15(27)6-5-13(19(32)33)24-16(28)7-8-22/h1-4,13-14,35H,5-10,22H2,(H,23,31)(H,24,28)(H,25,27)(H,29,30)(H,32,33) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 330 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Chengdu University
Curated by ChEMBL
| Assay Description
Inhibition of human glyoxalase 1 |
Bioorg Med Chem Lett 25: 4724-7 (2015)
Article DOI: 10.1016/j.bmcl.2015.08.055
BindingDB Entry DOI: 10.7270/Q26H4K7B |
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Homo sapiens (Human)) | BDBM50265477
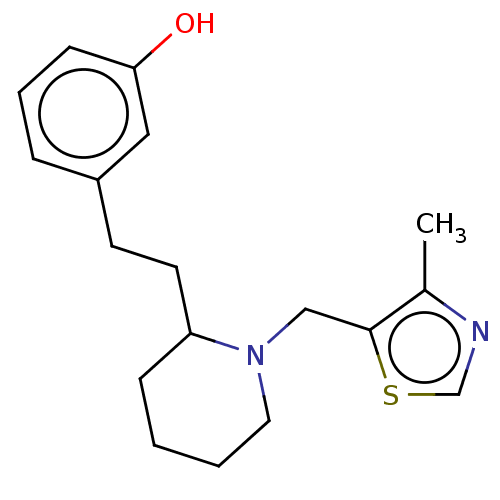
(CHEMBL4084706)Show InChI InChI=1S/C18H24N2OS/c1-14-18(22-13-19-14)12-20-10-3-2-6-16(20)9-8-15-5-4-7-17(21)11-15/h4-5,7,11,13,16,21H,2-3,6,8-10,12H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 347 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Southern California
Curated by ChEMBL
| Assay Description
Displacement of 3H-U69593 from human KOR expressed in HEK293 cells after 90 mins by micro beta scintillation counting analysis |
J Med Chem 60: 3070-3081 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00109
BindingDB Entry DOI: 10.7270/Q2VQ354M |
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Homo sapiens (Human)) | BDBM50265477

(CHEMBL4084706)Show InChI InChI=1S/C18H24N2OS/c1-14-18(22-13-19-14)12-20-10-3-2-6-16(20)9-8-15-5-4-7-17(21)11-15/h4-5,7,11,13,16,21H,2-3,6,8-10,12H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 350 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Southern California
Curated by ChEMBL
| Assay Description
Displacement of 3H-U69593 from human KOR expressed in HEK293 cells after 90 mins by micro beta scintillation counting analysis |
J Med Chem 60: 3070-3081 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00109
BindingDB Entry DOI: 10.7270/Q2VQ354M |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data