

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Intestinal-type alkaline phosphatase (Bos taurus (Cattle)) | BDBM50437932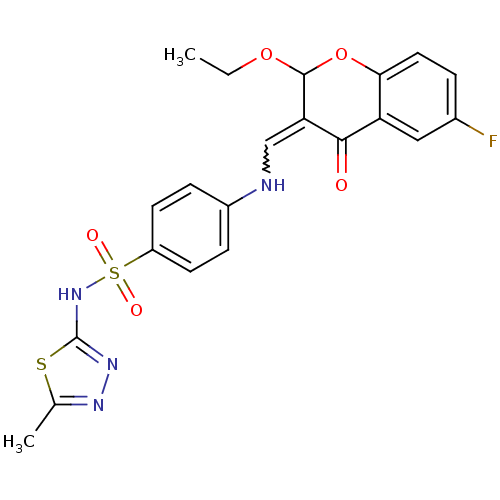 (CHEMBL2408704) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Forman Christian College (A Chartered University) Curated by ChEMBL | Assay Description Inhibition of bovine intestinal alkaline phosphatase using p-NPP as substrate treated 10 mins before substrate addition measured after 30 mins by spe... | Eur J Med Chem 66: 438-49 (2013) Article DOI: 10.1016/j.ejmech.2013.06.015 BindingDB Entry DOI: 10.7270/Q20K29ZH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Intestinal-type alkaline phosphatase (Bos taurus (Cattle)) | BDBM50437935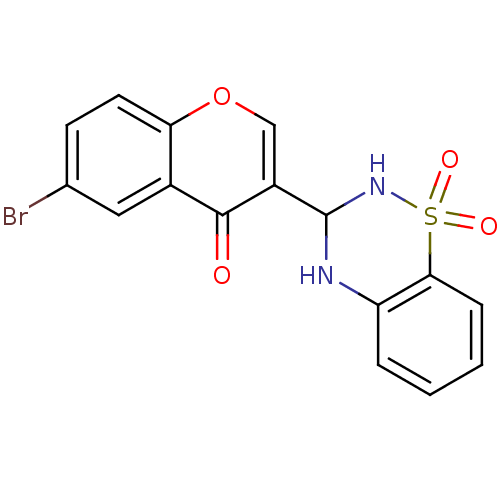 (CHEMBL2408701) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 21 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Forman Christian College (A Chartered University) Curated by ChEMBL | Assay Description Inhibition of bovine intestinal alkaline phosphatase using p-NPP as substrate treated 10 mins before substrate addition measured after 30 mins by spe... | Eur J Med Chem 66: 438-49 (2013) Article DOI: 10.1016/j.ejmech.2013.06.015 BindingDB Entry DOI: 10.7270/Q20K29ZH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Intestinal-type alkaline phosphatase (Bos taurus (Cattle)) | BDBM50437937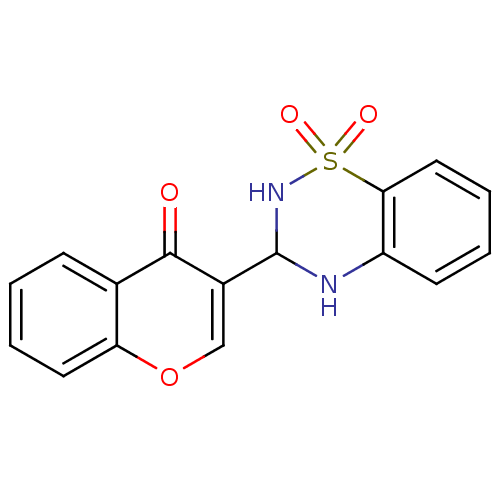 (CHEMBL2408699) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 49 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Forman Christian College (A Chartered University) Curated by ChEMBL | Assay Description Inhibition of bovine intestinal alkaline phosphatase using p-NPP as substrate treated 10 mins before substrate addition measured after 30 mins by spe... | Eur J Med Chem 66: 438-49 (2013) Article DOI: 10.1016/j.ejmech.2013.06.015 BindingDB Entry DOI: 10.7270/Q20K29ZH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Intestinal-type alkaline phosphatase (Bos taurus (Cattle)) | BDBM50437931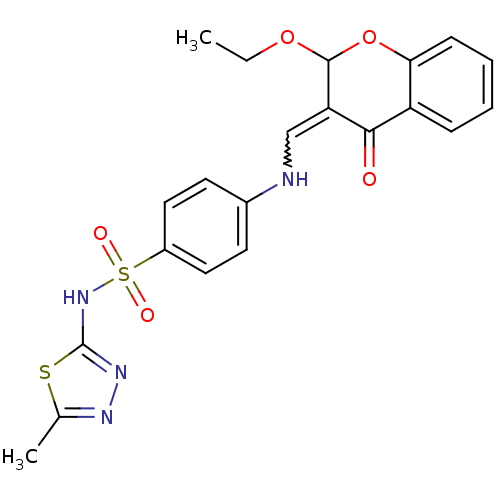 (CHEMBL2408705) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 85 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Forman Christian College (A Chartered University) Curated by ChEMBL | Assay Description Inhibition of bovine intestinal alkaline phosphatase using p-NPP as substrate treated 10 mins before substrate addition measured after 30 mins by spe... | Eur J Med Chem 66: 438-49 (2013) Article DOI: 10.1016/j.ejmech.2013.06.015 BindingDB Entry DOI: 10.7270/Q20K29ZH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alkaline phosphatase, tissue-nonspecific isozyme (Bos taurus (Cattle)) | BDBM50437935 (CHEMBL2408701) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 130 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Forman Christian College (A Chartered University) Curated by ChEMBL | Assay Description Inhibition of bovine kidney intestinal alkaline phosphatase using p-NPP as substrate treated 10 mins before substrate addition measured after 30 mins... | Eur J Med Chem 66: 438-49 (2013) Article DOI: 10.1016/j.ejmech.2013.06.015 BindingDB Entry DOI: 10.7270/Q20K29ZH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Intestinal-type alkaline phosphatase (Bos taurus (Cattle)) | BDBM50437934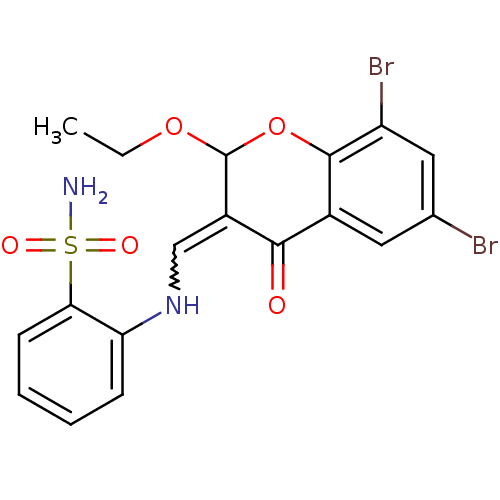 (CHEMBL2408702) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Forman Christian College (A Chartered University) Curated by ChEMBL | Assay Description Inhibition of bovine intestinal alkaline phosphatase using p-NPP as substrate treated 10 mins before substrate addition measured after 30 mins by spe... | Eur J Med Chem 66: 438-49 (2013) Article DOI: 10.1016/j.ejmech.2013.06.015 BindingDB Entry DOI: 10.7270/Q20K29ZH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 2 (Homo sapiens (Human)) | BDBM237231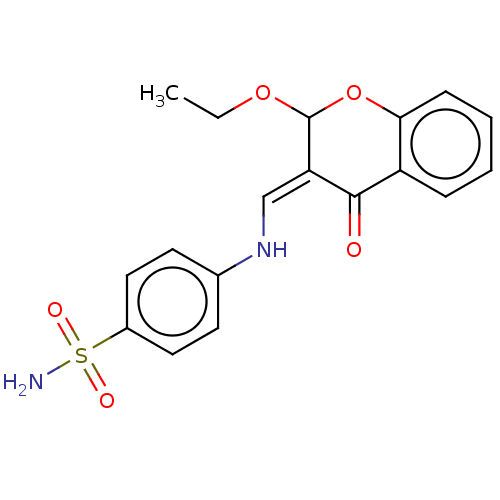 (hCA inhibitor, 1) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 228 | -37.9 | n/a | n/a | n/a | n/a | n/a | 7.4 | 25 |
Ondokuz Mayis University | Assay Description Carbonic anhydrase activity was assayed by following the change in absorbance at 348 nm of 4-nitrophenylacetate (NPA) to 4-nitrophenylate ion over a ... | J Enzyme Inhib Med Chem 27: 744-7 (2012) Article DOI: 10.3109/14756366.2011.614607 BindingDB Entry DOI: 10.7270/Q2H70DQ2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alkaline phosphatase, tissue-nonspecific isozyme (Bos taurus (Cattle)) | BDBM50437931 (CHEMBL2408705) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 250 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Forman Christian College (A Chartered University) Curated by ChEMBL | Assay Description Inhibition of bovine kidney intestinal alkaline phosphatase using p-NPP as substrate treated 10 mins before substrate addition measured after 30 mins... | Eur J Med Chem 66: 438-49 (2013) Article DOI: 10.1016/j.ejmech.2013.06.015 BindingDB Entry DOI: 10.7270/Q20K29ZH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 2 (Homo sapiens (Human)) | BDBM237234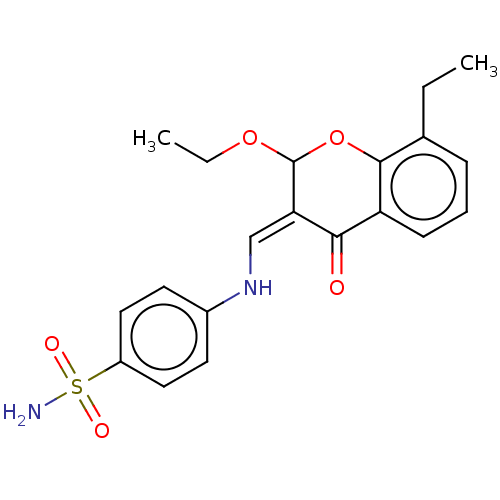 (hCA inhibitor, 5) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 286 | -37.3 | n/a | n/a | n/a | n/a | n/a | 7.4 | 25 |
Ondokuz Mayis University | Assay Description Carbonic anhydrase activity was assayed by following the change in absorbance at 348 nm of 4-nitrophenylacetate (NPA) to 4-nitrophenylate ion over a ... | J Enzyme Inhib Med Chem 27: 744-7 (2012) Article DOI: 10.3109/14756366.2011.614607 BindingDB Entry DOI: 10.7270/Q2H70DQ2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Intestinal-type alkaline phosphatase (Bos taurus (Cattle)) | BDBM50437933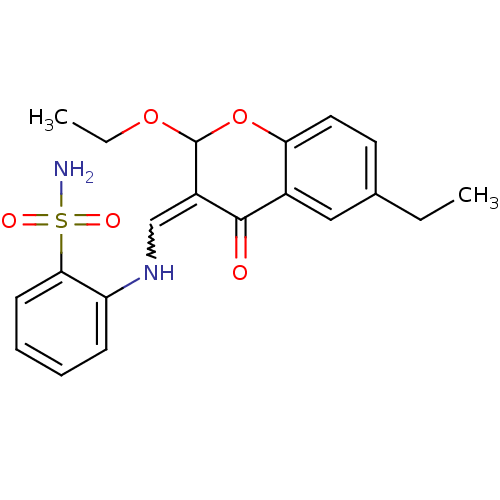 (CHEMBL2408703) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Forman Christian College (A Chartered University) Curated by ChEMBL | Assay Description Inhibition of bovine intestinal alkaline phosphatase using p-NPP as substrate treated 10 mins before substrate addition measured after 30 mins by spe... | Eur J Med Chem 66: 438-49 (2013) Article DOI: 10.1016/j.ejmech.2013.06.015 BindingDB Entry DOI: 10.7270/Q20K29ZH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Intestinal-type alkaline phosphatase (Bos taurus (Cattle)) | BDBM50437945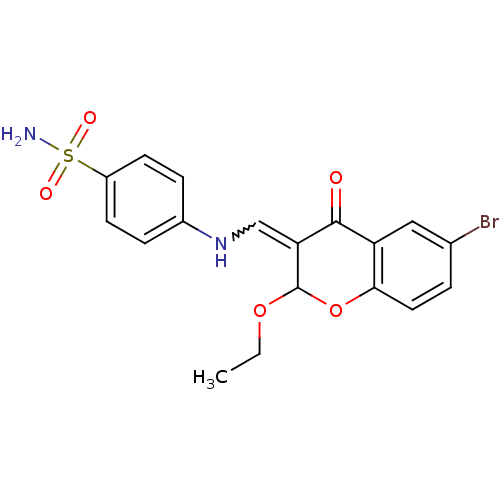 (CHEMBL1814393) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 410 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Forman Christian College (A Chartered University) Curated by ChEMBL | Assay Description Inhibition of bovine intestinal alkaline phosphatase using p-NPP as substrate treated 10 mins before substrate addition measured after 30 mins by spe... | Eur J Med Chem 66: 438-49 (2013) Article DOI: 10.1016/j.ejmech.2013.06.015 BindingDB Entry DOI: 10.7270/Q20K29ZH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Intestinal-type alkaline phosphatase (Bos taurus (Cattle)) | BDBM50437929 (CHEMBL2408560) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 410 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Forman Christian College (A Chartered University) Curated by ChEMBL | Assay Description Inhibition of bovine intestinal alkaline phosphatase using p-NPP as substrate treated 10 mins before substrate addition measured after 30 mins by spe... | Eur J Med Chem 66: 438-49 (2013) Article DOI: 10.1016/j.ejmech.2013.06.015 BindingDB Entry DOI: 10.7270/Q20K29ZH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 2 (Homo sapiens (Human)) | BDBM60962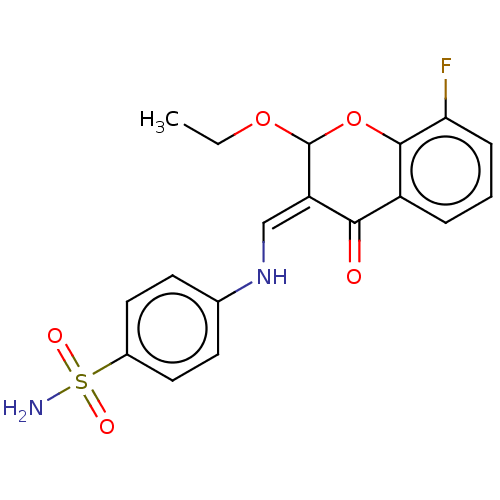 (hCA inhibitor, 2) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 437 | -36.3 | n/a | n/a | n/a | n/a | n/a | 7.4 | 25 |
Ondokuz Mayis University | Assay Description Carbonic anhydrase activity was assayed by following the change in absorbance at 348 nm of 4-nitrophenylacetate (NPA) to 4-nitrophenylate ion over a ... | J Enzyme Inhib Med Chem 27: 744-7 (2012) Article DOI: 10.3109/14756366.2011.614607 BindingDB Entry DOI: 10.7270/Q2H70DQ2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 2 (Homo sapiens (Human)) | BDBM10860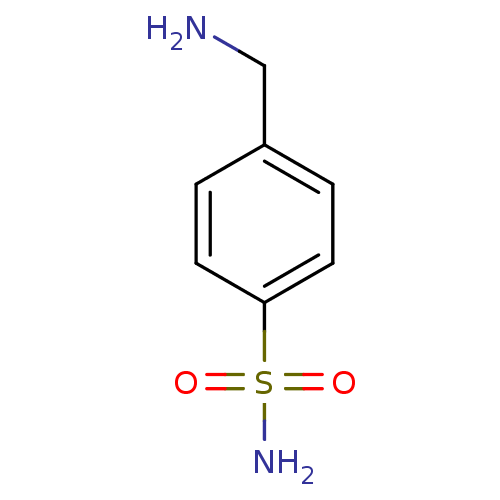 (4-(aminomethyl)benzene-1-sulfonamide | CHEMBL419 |...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 612 | -35.5 | n/a | n/a | n/a | n/a | n/a | 7.4 | 25 |
Ondokuz Mayis University | Assay Description Carbonic anhydrase activity was assayed by following the change in absorbance at 348 nm of 4-nitrophenylacetate (NPA) to 4-nitrophenylate ion over a ... | J Enzyme Inhib Med Chem 27: 744-7 (2012) Article DOI: 10.3109/14756366.2011.614607 BindingDB Entry DOI: 10.7270/Q2H70DQ2 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (docked) | ||||||||||||
| Carbonic anhydrase 2 (Homo sapiens (Human)) | BDBM10857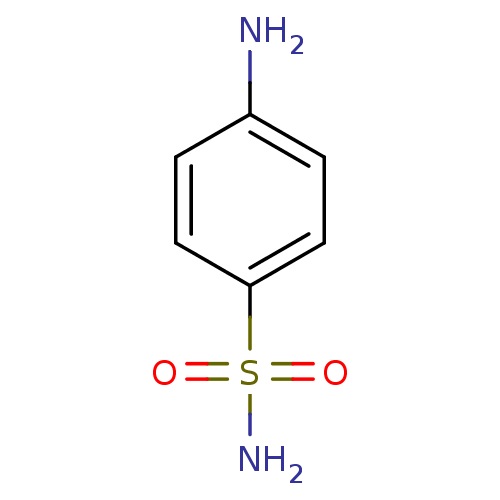 (4-aminobenzene-1-sulfonamide | CHEMBL21 | Sulfanil...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 628 | -35.4 | n/a | n/a | n/a | n/a | n/a | 7.4 | 25 |
Ondokuz Mayis University | Assay Description Carbonic anhydrase activity was assayed by following the change in absorbance at 348 nm of 4-nitrophenylacetate (NPA) to 4-nitrophenylate ion over a ... | J Enzyme Inhib Med Chem 27: 744-7 (2012) Article DOI: 10.3109/14756366.2011.614607 BindingDB Entry DOI: 10.7270/Q2H70DQ2 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (docked) | ||||||||||||
| Intestinal-type alkaline phosphatase (Bos taurus (Cattle)) | BDBM50437936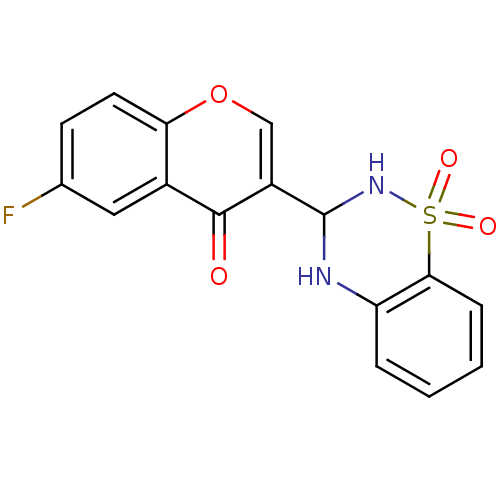 (CHEMBL2408700) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 780 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Forman Christian College (A Chartered University) Curated by ChEMBL | Assay Description Inhibition of bovine intestinal alkaline phosphatase using p-NPP as substrate treated 10 mins before substrate addition measured after 30 mins by spe... | Eur J Med Chem 66: 438-49 (2013) Article DOI: 10.1016/j.ejmech.2013.06.015 BindingDB Entry DOI: 10.7270/Q20K29ZH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Intestinal-type alkaline phosphatase (Bos taurus (Cattle)) | BDBM50437939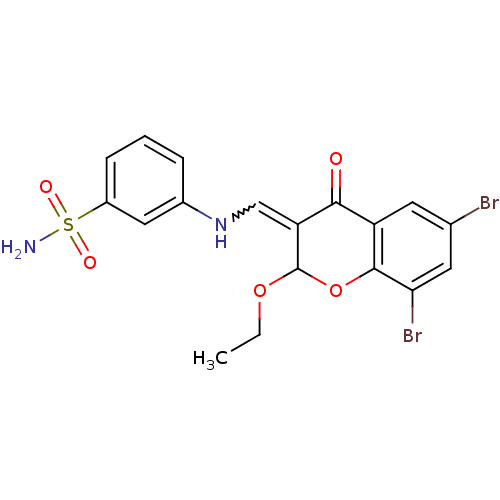 (CHEMBL1814399) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Forman Christian College (A Chartered University) Curated by ChEMBL | Assay Description Inhibition of bovine intestinal alkaline phosphatase using p-NPP as substrate treated 10 mins before substrate addition measured after 30 mins by spe... | Eur J Med Chem 66: 438-49 (2013) Article DOI: 10.1016/j.ejmech.2013.06.015 BindingDB Entry DOI: 10.7270/Q20K29ZH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 6 (Homo sapiens (Human)) | BDBM237231 (hCA inhibitor, 1) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 943 | -34.4 | n/a | n/a | n/a | n/a | n/a | 7.4 | 25 |
Ondokuz Mayis University | Assay Description Carbonic anhydrase activity was assayed by following the change in absorbance at 348 nm of 4-nitrophenylacetate (NPA) to 4-nitrophenylate ion over a ... | J Enzyme Inhib Med Chem 27: 744-7 (2012) Article DOI: 10.3109/14756366.2011.614607 BindingDB Entry DOI: 10.7270/Q2H70DQ2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 6 (Homo sapiens (Human)) | BDBM10860 (4-(aminomethyl)benzene-1-sulfonamide | CHEMBL419 |...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 1.01E+3 | -34.2 | n/a | n/a | n/a | n/a | n/a | 7.4 | 25 |
Ondokuz Mayis University | Assay Description Carbonic anhydrase activity was assayed by following the change in absorbance at 348 nm of 4-nitrophenylacetate (NPA) to 4-nitrophenylate ion over a ... | J Enzyme Inhib Med Chem 27: 744-7 (2012) Article DOI: 10.3109/14756366.2011.614607 BindingDB Entry DOI: 10.7270/Q2H70DQ2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 2 (Homo sapiens (Human)) | BDBM12414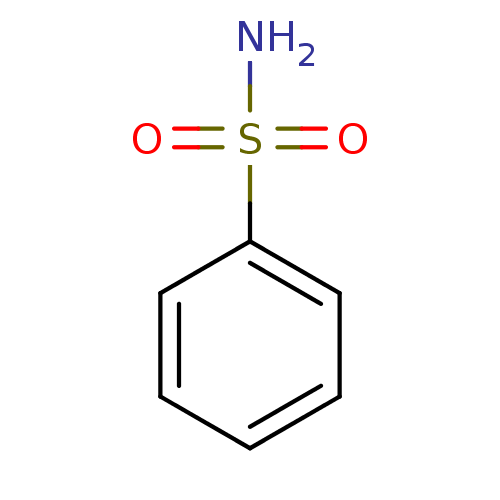 (CHEMBL27601 | benzenesulfonamide | hCA inhibitor, ...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE MMDB PC cid PC sid PDB UniChem Patents Similars | MMDB PDB Article PubMed | 1.10E+3 | -34.0 | n/a | n/a | n/a | n/a | n/a | 7.4 | 25 |
Ondokuz Mayis University | Assay Description Carbonic anhydrase activity was assayed by following the change in absorbance at 348 nm of 4-nitrophenylacetate (NPA) to 4-nitrophenylate ion over a ... | J Enzyme Inhib Med Chem 27: 744-7 (2012) Article DOI: 10.3109/14756366.2011.614607 BindingDB Entry DOI: 10.7270/Q2H70DQ2 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Carbonic anhydrase 2 (Homo sapiens (Human)) | BDBM237232 (hCA inhibitor, 3) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 1.13E+3 | -33.9 | n/a | n/a | n/a | n/a | n/a | 7.4 | 25 |
Ondokuz Mayis University | Assay Description Carbonic anhydrase activity was assayed by following the change in absorbance at 348 nm of 4-nitrophenylacetate (NPA) to 4-nitrophenylate ion over a ... | J Enzyme Inhib Med Chem 27: 744-7 (2012) Article DOI: 10.3109/14756366.2011.614607 BindingDB Entry DOI: 10.7270/Q2H70DQ2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 6 (Homo sapiens (Human)) | BDBM10857 (4-aminobenzene-1-sulfonamide | CHEMBL21 | Sulfanil...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 1.21E+3 | -33.8 | n/a | n/a | n/a | n/a | n/a | 7.4 | 25 |
Ondokuz Mayis University | Assay Description Carbonic anhydrase activity was assayed by following the change in absorbance at 348 nm of 4-nitrophenylacetate (NPA) to 4-nitrophenylate ion over a ... | J Enzyme Inhib Med Chem 27: 744-7 (2012) Article DOI: 10.3109/14756366.2011.614607 BindingDB Entry DOI: 10.7270/Q2H70DQ2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 6 (Homo sapiens (Human)) | BDBM237234 (hCA inhibitor, 5) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 1.25E+3 | -33.7 | n/a | n/a | n/a | n/a | n/a | 7.4 | 25 |
Ondokuz Mayis University | Assay Description Carbonic anhydrase activity was assayed by following the change in absorbance at 348 nm of 4-nitrophenylacetate (NPA) to 4-nitrophenylate ion over a ... | J Enzyme Inhib Med Chem 27: 744-7 (2012) Article DOI: 10.3109/14756366.2011.614607 BindingDB Entry DOI: 10.7270/Q2H70DQ2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Intestinal-type alkaline phosphatase (Bos taurus (Cattle)) | BDBM50437946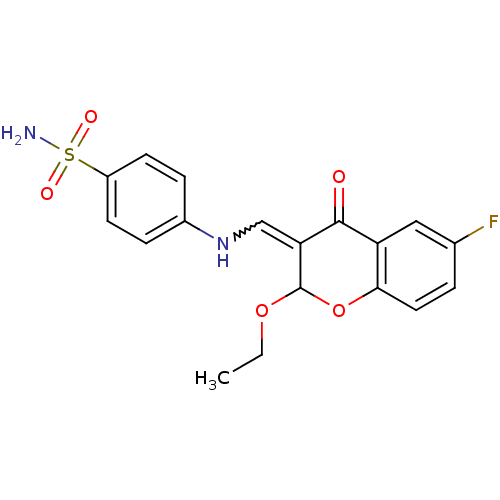 (CHEMBL1814392) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.43E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Forman Christian College (A Chartered University) Curated by ChEMBL | Assay Description Inhibition of bovine intestinal alkaline phosphatase using p-NPP as substrate treated 10 mins before substrate addition measured after 30 mins by spe... | Eur J Med Chem 66: 438-49 (2013) Article DOI: 10.1016/j.ejmech.2013.06.015 BindingDB Entry DOI: 10.7270/Q20K29ZH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 6 (Homo sapiens (Human)) | BDBM60962 (hCA inhibitor, 2) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 1.53E+3 | -33.2 | n/a | n/a | n/a | n/a | n/a | 7.4 | 25 |
Ondokuz Mayis University | Assay Description Carbonic anhydrase activity was assayed by following the change in absorbance at 348 nm of 4-nitrophenylacetate (NPA) to 4-nitrophenylate ion over a ... | J Enzyme Inhib Med Chem 27: 744-7 (2012) Article DOI: 10.3109/14756366.2011.614607 BindingDB Entry DOI: 10.7270/Q2H70DQ2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 2 (Homo sapiens (Human)) | BDBM237233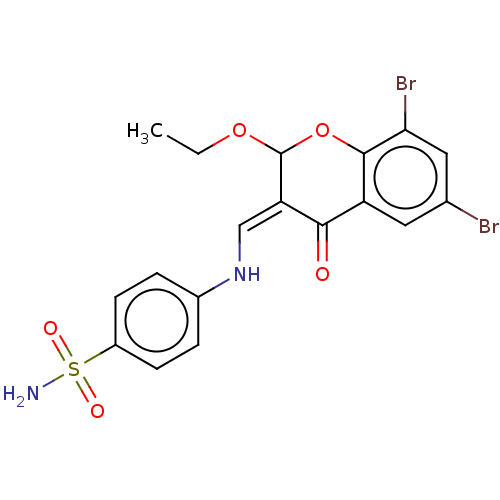 (hCA inhibitor, 4) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 1.86E+3 | -32.7 | n/a | n/a | n/a | n/a | n/a | 7.4 | 25 |
Ondokuz Mayis University | Assay Description Carbonic anhydrase activity was assayed by following the change in absorbance at 348 nm of 4-nitrophenylacetate (NPA) to 4-nitrophenylate ion over a ... | J Enzyme Inhib Med Chem 27: 744-7 (2012) Article DOI: 10.3109/14756366.2011.614607 BindingDB Entry DOI: 10.7270/Q2H70DQ2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Intestinal-type alkaline phosphatase (Bos taurus (Cattle)) | BDBM50437938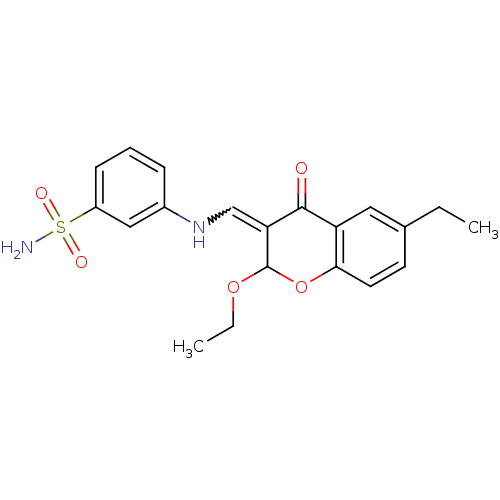 (CHEMBL1814400) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Forman Christian College (A Chartered University) Curated by ChEMBL | Assay Description Inhibition of bovine intestinal alkaline phosphatase using p-NPP as substrate treated 10 mins before substrate addition measured after 30 mins by spe... | Eur J Med Chem 66: 438-49 (2013) Article DOI: 10.1016/j.ejmech.2013.06.015 BindingDB Entry DOI: 10.7270/Q20K29ZH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 6 (Homo sapiens (Human)) | BDBM237232 (hCA inhibitor, 3) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 2.24E+3 | -32.3 | n/a | n/a | n/a | n/a | n/a | 7.4 | 25 |
Ondokuz Mayis University | Assay Description Carbonic anhydrase activity was assayed by following the change in absorbance at 348 nm of 4-nitrophenylacetate (NPA) to 4-nitrophenylate ion over a ... | J Enzyme Inhib Med Chem 27: 744-7 (2012) Article DOI: 10.3109/14756366.2011.614607 BindingDB Entry DOI: 10.7270/Q2H70DQ2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Intestinal-type alkaline phosphatase (Bos taurus (Cattle)) | BDBM50437943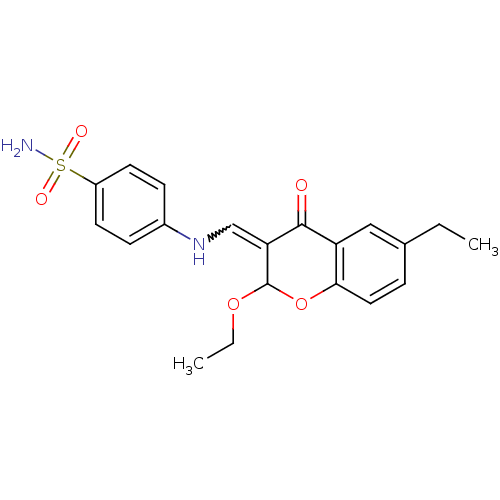 (CHEMBL1814395) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Forman Christian College (A Chartered University) Curated by ChEMBL | Assay Description Inhibition of bovine intestinal alkaline phosphatase using p-NPP as substrate treated 10 mins before substrate addition measured after 30 mins by spe... | Eur J Med Chem 66: 438-49 (2013) Article DOI: 10.1016/j.ejmech.2013.06.015 BindingDB Entry DOI: 10.7270/Q20K29ZH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 6 (Homo sapiens (Human)) | BDBM12414 (CHEMBL27601 | benzenesulfonamide | hCA inhibitor, ...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 4.11E+3 | -30.7 | n/a | n/a | n/a | n/a | n/a | 7.4 | 25 |
Ondokuz Mayis University | Assay Description Carbonic anhydrase activity was assayed by following the change in absorbance at 348 nm of 4-nitrophenylacetate (NPA) to 4-nitrophenylate ion over a ... | J Enzyme Inhib Med Chem 27: 744-7 (2012) Article DOI: 10.3109/14756366.2011.614607 BindingDB Entry DOI: 10.7270/Q2H70DQ2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 6 (Homo sapiens (Human)) | BDBM237233 (hCA inhibitor, 4) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 4.28E+3 | -30.6 | n/a | n/a | n/a | n/a | n/a | 7.4 | 25 |
Ondokuz Mayis University | Assay Description Carbonic anhydrase activity was assayed by following the change in absorbance at 348 nm of 4-nitrophenylacetate (NPA) to 4-nitrophenylate ion over a ... | J Enzyme Inhib Med Chem 27: 744-7 (2012) Article DOI: 10.3109/14756366.2011.614607 BindingDB Entry DOI: 10.7270/Q2H70DQ2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Intestinal-type alkaline phosphatase (Bos taurus (Cattle)) | BDBM50437944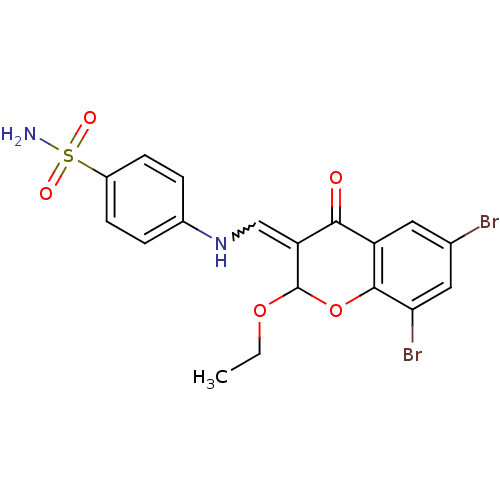 (CHEMBL1814394) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 5.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Forman Christian College (A Chartered University) Curated by ChEMBL | Assay Description Inhibition of bovine intestinal alkaline phosphatase using p-NPP as substrate treated 10 mins before substrate addition measured after 30 mins by spe... | Eur J Med Chem 66: 438-49 (2013) Article DOI: 10.1016/j.ejmech.2013.06.015 BindingDB Entry DOI: 10.7270/Q20K29ZH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alkaline phosphatase, tissue-nonspecific isozyme (Bos taurus (Cattle)) | BDBM50437947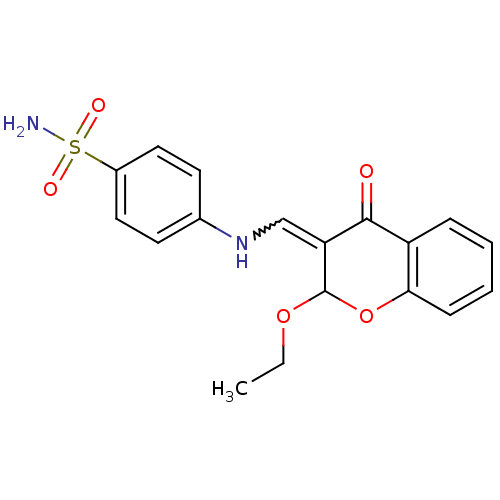 (CHEMBL1814391) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 6.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Forman Christian College (A Chartered University) Curated by ChEMBL | Assay Description Inhibition of bovine kidney intestinal alkaline phosphatase using p-NPP as substrate treated 10 mins before substrate addition measured after 30 mins... | Eur J Med Chem 66: 438-49 (2013) Article DOI: 10.1016/j.ejmech.2013.06.015 BindingDB Entry DOI: 10.7270/Q20K29ZH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Intestinal-type alkaline phosphatase (Bos taurus (Cattle)) | BDBM50437928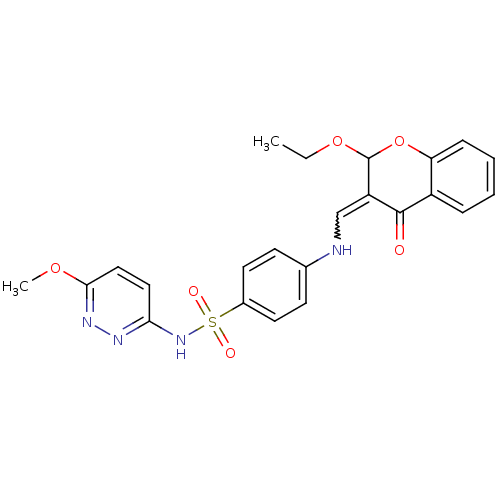 (CHEMBL2408697) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 7.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Forman Christian College (A Chartered University) Curated by ChEMBL | Assay Description Inhibition of bovine intestinal alkaline phosphatase using p-NPP as substrate treated 10 mins before substrate addition measured after 30 mins by spe... | Eur J Med Chem 66: 438-49 (2013) Article DOI: 10.1016/j.ejmech.2013.06.015 BindingDB Entry DOI: 10.7270/Q20K29ZH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Intestinal-type alkaline phosphatase (Bos taurus (Cattle)) | BDBM50437927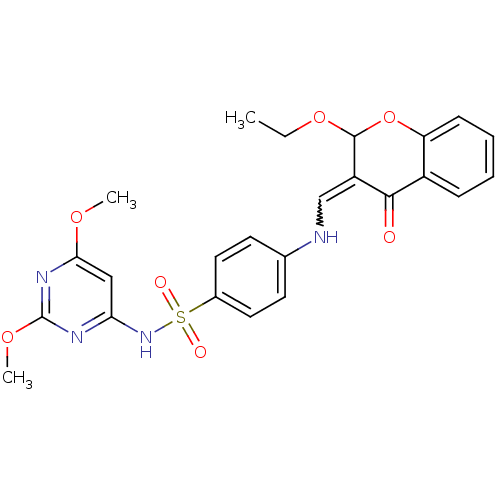 (CHEMBL2408698) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 7.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Forman Christian College (A Chartered University) Curated by ChEMBL | Assay Description Inhibition of bovine intestinal alkaline phosphatase using p-NPP as substrate treated 10 mins before substrate addition measured after 30 mins by spe... | Eur J Med Chem 66: 438-49 (2013) Article DOI: 10.1016/j.ejmech.2013.06.015 BindingDB Entry DOI: 10.7270/Q20K29ZH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 2 (Homo sapiens (Human)) | BDBM10859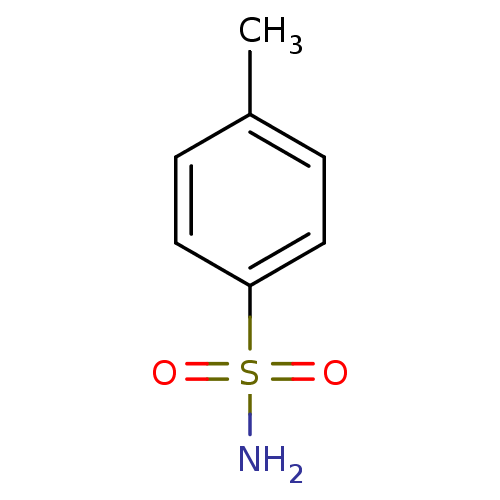 (4-methylbenzene-1-sulfonamide | CHEMBL574 | aromat...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 8.14E+3 | -29.0 | n/a | n/a | n/a | n/a | n/a | 7.4 | 25 |
Ondokuz Mayis University | Assay Description Carbonic anhydrase activity was assayed by following the change in absorbance at 348 nm of 4-nitrophenylacetate (NPA) to 4-nitrophenylate ion over a ... | J Enzyme Inhib Med Chem 27: 744-7 (2012) Article DOI: 10.3109/14756366.2011.614607 BindingDB Entry DOI: 10.7270/Q2H70DQ2 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (docked) | ||||||||||||
| Intestinal-type alkaline phosphatase (Bos taurus (Cattle)) | BDBM50437940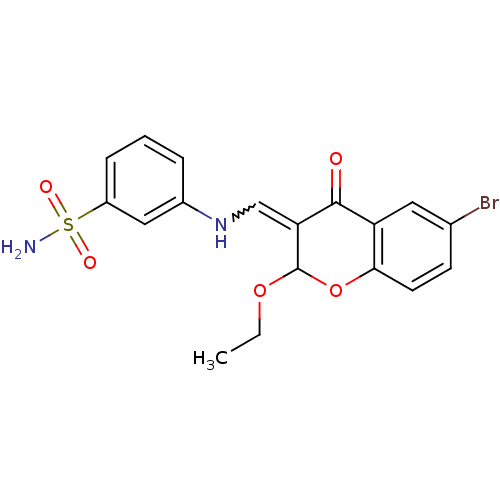 (CHEMBL1814398) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 8.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Forman Christian College (A Chartered University) Curated by ChEMBL | Assay Description Inhibition of bovine intestinal alkaline phosphatase using p-NPP as substrate treated 10 mins before substrate addition measured after 30 mins by spe... | Eur J Med Chem 66: 438-49 (2013) Article DOI: 10.1016/j.ejmech.2013.06.015 BindingDB Entry DOI: 10.7270/Q20K29ZH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Intestinal-type alkaline phosphatase (Bos taurus (Cattle)) | BDBM50437930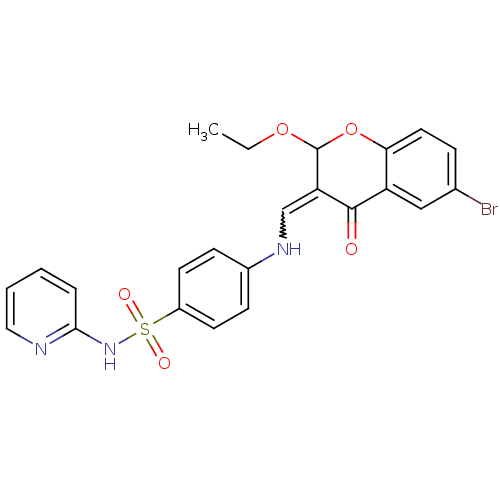 (CHEMBL2408706) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 9.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Forman Christian College (A Chartered University) Curated by ChEMBL | Assay Description Inhibition of bovine intestinal alkaline phosphatase using p-NPP as substrate treated 10 mins before substrate addition measured after 30 mins by spe... | Eur J Med Chem 66: 438-49 (2013) Article DOI: 10.1016/j.ejmech.2013.06.015 BindingDB Entry DOI: 10.7270/Q20K29ZH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 1 (Homo sapiens (Human)) | BDBM237231 (hCA inhibitor, 1) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 1.37E+4 | -27.8 | n/a | n/a | n/a | n/a | n/a | 7.4 | 25 |
Ondokuz Mayis University | Assay Description Carbonic anhydrase activity was assayed by following the change in absorbance at 348 nm of 4-nitrophenylacetate (NPA) to 4-nitrophenylate ion over a ... | J Enzyme Inhib Med Chem 27: 744-7 (2012) Article DOI: 10.3109/14756366.2011.614607 BindingDB Entry DOI: 10.7270/Q2H70DQ2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alkaline phosphatase, tissue-nonspecific isozyme (Bos taurus (Cattle)) | BDBM50437938 (CHEMBL1814400) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.68E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Forman Christian College (A Chartered University) Curated by ChEMBL | Assay Description Inhibition of bovine kidney intestinal alkaline phosphatase using p-NPP as substrate treated 10 mins before substrate addition measured after 30 mins... | Eur J Med Chem 66: 438-49 (2013) Article DOI: 10.1016/j.ejmech.2013.06.015 BindingDB Entry DOI: 10.7270/Q20K29ZH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Intestinal-type alkaline phosphatase (Bos taurus (Cattle)) | BDBM50437941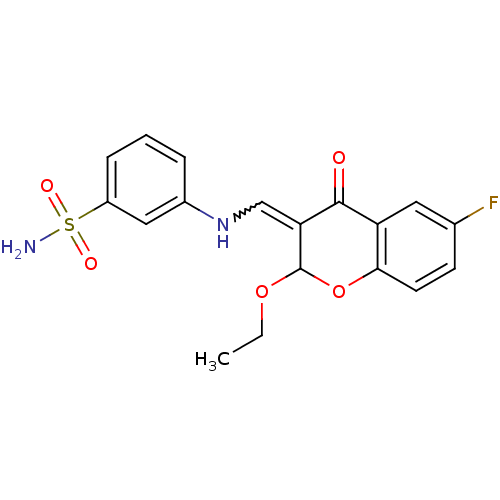 (CHEMBL1814397) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.71E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Forman Christian College (A Chartered University) Curated by ChEMBL | Assay Description Inhibition of bovine intestinal alkaline phosphatase using p-NPP as substrate treated 10 mins before substrate addition measured after 30 mins by spe... | Eur J Med Chem 66: 438-49 (2013) Article DOI: 10.1016/j.ejmech.2013.06.015 BindingDB Entry DOI: 10.7270/Q20K29ZH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alkaline phosphatase, tissue-nonspecific isozyme (Bos taurus (Cattle)) | BDBM50437941 (CHEMBL1814397) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.80E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Forman Christian College (A Chartered University) Curated by ChEMBL | Assay Description Inhibition of bovine kidney intestinal alkaline phosphatase using p-NPP as substrate treated 10 mins before substrate addition measured after 30 mins... | Eur J Med Chem 66: 438-49 (2013) Article DOI: 10.1016/j.ejmech.2013.06.015 BindingDB Entry DOI: 10.7270/Q20K29ZH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alkaline phosphatase, tissue-nonspecific isozyme (Bos taurus (Cattle)) | BDBM50437932 (CHEMBL2408704) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.10E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Forman Christian College (A Chartered University) Curated by ChEMBL | Assay Description Inhibition of bovine kidney intestinal alkaline phosphatase using p-NPP as substrate treated 10 mins before substrate addition measured after 30 mins... | Eur J Med Chem 66: 438-49 (2013) Article DOI: 10.1016/j.ejmech.2013.06.015 BindingDB Entry DOI: 10.7270/Q20K29ZH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 6 (Homo sapiens (Human)) | BDBM10859 (4-methylbenzene-1-sulfonamide | CHEMBL574 | aromat...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 2.12E+4 | -26.7 | n/a | n/a | n/a | n/a | n/a | 7.4 | 25 |
Ondokuz Mayis University | Assay Description Carbonic anhydrase activity was assayed by following the change in absorbance at 348 nm of 4-nitrophenylacetate (NPA) to 4-nitrophenylate ion over a ... | J Enzyme Inhib Med Chem 27: 744-7 (2012) Article DOI: 10.3109/14756366.2011.614607 BindingDB Entry DOI: 10.7270/Q2H70DQ2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alkaline phosphatase, tissue-nonspecific isozyme (Bos taurus (Cattle)) | BDBM50437929 (CHEMBL2408560) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.20E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Forman Christian College (A Chartered University) Curated by ChEMBL | Assay Description Inhibition of bovine kidney intestinal alkaline phosphatase using p-NPP as substrate treated 10 mins before substrate addition measured after 30 mins... | Eur J Med Chem 66: 438-49 (2013) Article DOI: 10.1016/j.ejmech.2013.06.015 BindingDB Entry DOI: 10.7270/Q20K29ZH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alkaline phosphatase, tissue-nonspecific isozyme (Bos taurus (Cattle)) | BDBM50437927 (CHEMBL2408698) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 2.30E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Forman Christian College (A Chartered University) Curated by ChEMBL | Assay Description Inhibition of bovine kidney intestinal alkaline phosphatase using p-NPP as substrate treated 10 mins before substrate addition measured after 30 mins... | Eur J Med Chem 66: 438-49 (2013) Article DOI: 10.1016/j.ejmech.2013.06.015 BindingDB Entry DOI: 10.7270/Q20K29ZH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 1 (Homo sapiens (Human)) | BDBM237234 (hCA inhibitor, 5) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 2.43E+4 | -26.3 | n/a | n/a | n/a | n/a | n/a | 7.4 | 25 |
Ondokuz Mayis University | Assay Description Carbonic anhydrase activity was assayed by following the change in absorbance at 348 nm of 4-nitrophenylacetate (NPA) to 4-nitrophenylate ion over a ... | J Enzyme Inhib Med Chem 27: 744-7 (2012) Article DOI: 10.3109/14756366.2011.614607 BindingDB Entry DOI: 10.7270/Q2H70DQ2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alkaline phosphatase, tissue-nonspecific isozyme (Bos taurus (Cattle)) | BDBM50437939 (CHEMBL1814399) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.60E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Forman Christian College (A Chartered University) Curated by ChEMBL | Assay Description Inhibition of bovine kidney intestinal alkaline phosphatase using p-NPP as substrate treated 10 mins before substrate addition measured after 30 mins... | Eur J Med Chem 66: 438-49 (2013) Article DOI: 10.1016/j.ejmech.2013.06.015 BindingDB Entry DOI: 10.7270/Q20K29ZH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alkaline phosphatase, tissue-nonspecific isozyme (Bos taurus (Cattle)) | BDBM50437937 (CHEMBL2408699) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.70E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Forman Christian College (A Chartered University) Curated by ChEMBL | Assay Description Inhibition of bovine kidney intestinal alkaline phosphatase using p-NPP as substrate treated 10 mins before substrate addition measured after 30 mins... | Eur J Med Chem 66: 438-49 (2013) Article DOI: 10.1016/j.ejmech.2013.06.015 BindingDB Entry DOI: 10.7270/Q20K29ZH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alkaline phosphatase, tissue-nonspecific isozyme (Bos taurus (Cattle)) | BDBM50437933 (CHEMBL2408703) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.90E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Forman Christian College (A Chartered University) Curated by ChEMBL | Assay Description Inhibition of bovine kidney intestinal alkaline phosphatase using p-NPP as substrate treated 10 mins before substrate addition measured after 30 mins... | Eur J Med Chem 66: 438-49 (2013) Article DOI: 10.1016/j.ejmech.2013.06.015 BindingDB Entry DOI: 10.7270/Q20K29ZH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 72 total ) | Next | Last >> |