 Found 5865 hits with Last Name = 'able' and Initial = 'j'
Found 5865 hits with Last Name = 'able' and Initial = 'j' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Dihydrofolate reductase
(Candida albicans) | BDBM50049912

(7-(1,1-Dimethyl-propyl)-8-methyl-7H-pyrrolo[3,2-f]...)Show InChI InChI=1S/C16H21N5/c1-5-16(3,4)21-9(2)8-10-12(21)7-6-11-13(10)14(17)20-15(18)19-11/h6-8H,5H2,1-4H3,(H4,17,18,19,20) | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.00710 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wellcome Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of Dihydrofolate reductase enzyme from Candida albicans |
J Med Chem 39: 892-903 (1996)
Article DOI: 10.1021/jm9505122
BindingDB Entry DOI: 10.7270/Q2XD10RC |
More data for this
Ligand-Target Pair | |
Dihydrofolate reductase
(Homo sapiens (Human)) | BDBM18224

(6-[(2,5-dimethoxyphenyl)methyl]-5-methylpyrido[2,3...)Show InChI InChI=1S/C17H19N5O2/c1-9-11(6-10-7-12(23-2)4-5-13(10)24-3)8-20-16-14(9)15(18)21-17(19)22-16/h4-5,7-8H,6H2,1-3H3,(H4,18,19,20,21,22) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| MMDB
PDB
Article
PubMed
| 0.0250 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wellcome Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant Dihydrofolate reductase enzyme |
J Med Chem 39: 892-903 (1996)
Article DOI: 10.1021/jm9505122
BindingDB Entry DOI: 10.7270/Q2XD10RC |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Dihydrofolate reductase
(Candida albicans) | BDBM50049905
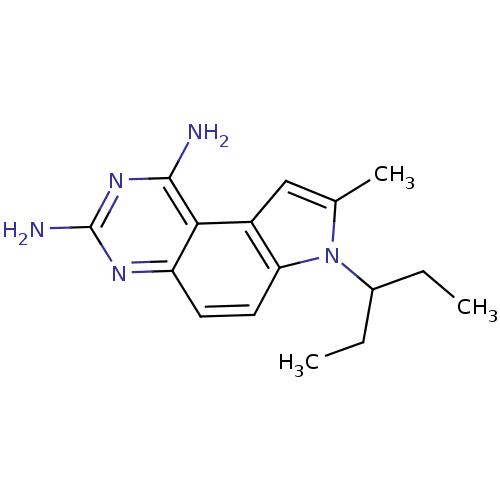
(7-(1-Ethyl-propyl)-8-methyl-7H-pyrrolo[3,2-f]quina...)Show InChI InChI=1S/C16H21N5/c1-4-10(5-2)21-9(3)8-11-13(21)7-6-12-14(11)15(17)20-16(18)19-12/h6-8,10H,4-5H2,1-3H3,(H4,17,18,19,20) | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.0300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wellcome Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of Dihydrofolate reductase enzyme from Candida albicans |
J Med Chem 39: 892-903 (1996)
Article DOI: 10.1021/jm9505122
BindingDB Entry DOI: 10.7270/Q2XD10RC |
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Homo sapiens (Human)) | BDBM50007344
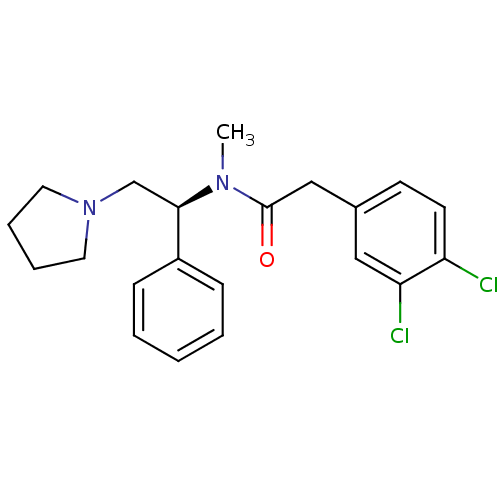
((S)-2-(3,4-Dichloro-phenyl)-N-methyl-N-(1-phenyl-2...)Show SMILES CN([C@H](CN1CCCC1)c1ccccc1)C(=O)Cc1ccc(Cl)c(Cl)c1 |r| Show InChI InChI=1S/C21H24Cl2N2O/c1-24(21(26)14-16-9-10-18(22)19(23)13-16)20(15-25-11-5-6-12-25)17-7-3-2-4-8-17/h2-4,7-10,13,20H,5-6,11-12,14-15H2,1H3/t20-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.0400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Adolor Corporation
Curated by ChEMBL
| Assay Description
Inhibitory constant against human Opioid receptor kappa using [3H]-diprenorphine as radio ligand |
Bioorg Med Chem Lett 15: 2647-52 (2005)
Article DOI: 10.1016/j.bmcl.2005.03.020
BindingDB Entry DOI: 10.7270/Q2PR7VH4 |
More data for this
Ligand-Target Pair | |
Dihydrofolate reductase
(Homo sapiens (Human)) | BDBM50049901
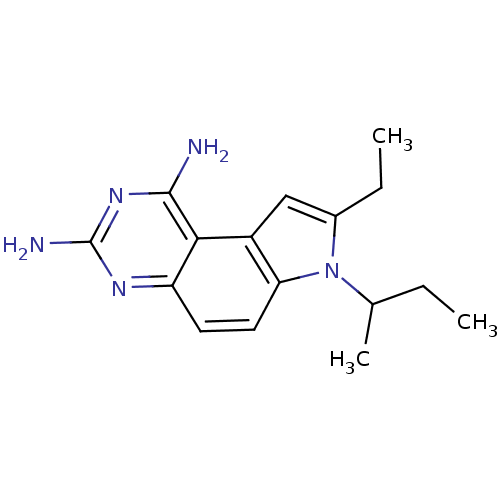
(7-sec-Butyl-8-ethyl-7H-pyrrolo[3,2-f]quinazoline-1...)Show InChI InChI=1S/C16H21N5/c1-4-9(3)21-10(5-2)8-11-13(21)7-6-12-14(11)15(17)20-16(18)19-12/h6-9H,4-5H2,1-3H3,(H4,17,18,19,20) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wellcome Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant Dihydrofolate reductase enzyme |
J Med Chem 39: 892-903 (1996)
Article DOI: 10.1021/jm9505122
BindingDB Entry DOI: 10.7270/Q2XD10RC |
More data for this
Ligand-Target Pair | |
Dihydrofolate reductase
(Homo sapiens (Human)) | BDBM50049906
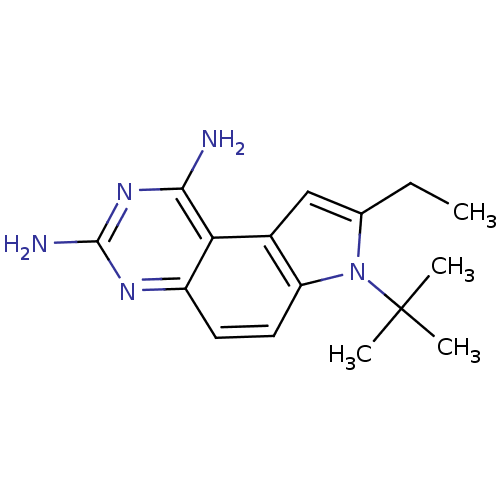
(7-tert-Butyl-8-ethyl-7H-pyrrolo[3,2-f]quinazoline-...)Show InChI InChI=1S/C16H21N5/c1-5-9-8-10-12(21(9)16(2,3)4)7-6-11-13(10)14(17)20-15(18)19-11/h6-8H,5H2,1-4H3,(H4,17,18,19,20) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wellcome Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant Dihydrofolate reductase enzyme |
J Med Chem 39: 892-903 (1996)
Article DOI: 10.1021/jm9505122
BindingDB Entry DOI: 10.7270/Q2XD10RC |
More data for this
Ligand-Target Pair | |
Dihydrofolate reductase
(Homo sapiens (Human)) | BDBM50049907

(7-(1-Ethyl-propyl)-8-isopropyl-7H-pyrrolo[3,2-f]qu...)Show InChI InChI=1S/C18H25N5/c1-5-11(6-2)23-14-8-7-13-16(17(19)22-18(20)21-13)12(14)9-15(23)10(3)4/h7-11H,5-6H2,1-4H3,(H4,19,20,21,22) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wellcome Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant Dihydrofolate reductase enzyme |
J Med Chem 39: 892-903 (1996)
Article DOI: 10.1021/jm9505122
BindingDB Entry DOI: 10.7270/Q2XD10RC |
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Homo sapiens (Human)) | BDBM50176375
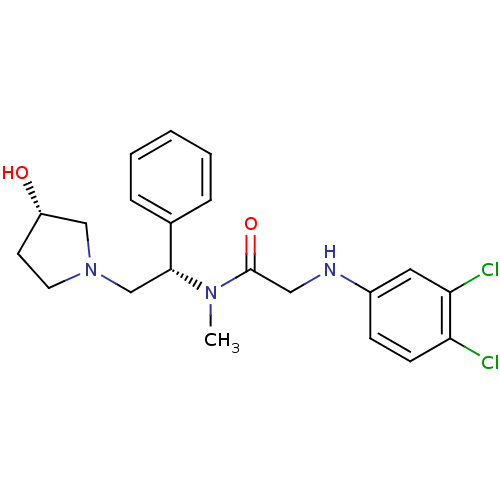
(2-(3,4-dichlorophenylamino)-N-((S)-2-((S)-3-hydrox...)Show SMILES CN([C@H](CN1CC[C@H](O)C1)c1ccccc1)C(=O)CNc1ccc(Cl)c(Cl)c1 Show InChI InChI=1S/C21H25Cl2N3O2/c1-25(21(28)12-24-16-7-8-18(22)19(23)11-16)20(15-5-3-2-4-6-15)14-26-10-9-17(27)13-26/h2-8,11,17,20,24,27H,9-10,12-14H2,1H3/t17-,20+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.110 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Adolor Corporation
Curated by ChEMBL
| Assay Description
Binding affinity to kappa opioid receptor |
Bioorg Med Chem Lett 16: 645-8 (2005)
Article DOI: 10.1016/j.bmcl.2005.10.034
BindingDB Entry DOI: 10.7270/Q26W99N3 |
More data for this
Ligand-Target Pair | |
Dihydrofolate reductase
(Candida albicans) | BDBM50049911

(8-tert-Butyl-7H-pyrrolo[3,2-f]quinazoline-1,3-diam...)Show InChI InChI=1S/C14H17N5/c1-14(2,3)10-6-7-8(17-10)4-5-9-11(7)12(15)19-13(16)18-9/h4-6,17H,1-3H3,(H4,15,16,18,19) | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.120 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wellcome Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of Dihydrofolate reductase enzyme from Candida albicans |
J Med Chem 39: 892-903 (1996)
Article DOI: 10.1021/jm9505122
BindingDB Entry DOI: 10.7270/Q2XD10RC |
More data for this
Ligand-Target Pair | |
Dihydrofolate reductase
(Candida albicans) | BDBM50049898

(8-Isopropyl-7H-pyrrolo[3,2-f]quinazoline-1,3-diami...)Show InChI InChI=1S/C13H15N5/c1-6(2)10-5-7-8(16-10)3-4-9-11(7)12(14)18-13(15)17-9/h3-6,16H,1-2H3,(H4,14,15,17,18) | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.160 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wellcome Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of Dihydrofolate reductase enzyme from Candida albicans |
J Med Chem 39: 892-903 (1996)
Article DOI: 10.1021/jm9505122
BindingDB Entry DOI: 10.7270/Q2XD10RC |
More data for this
Ligand-Target Pair | |
cAMP and cAMP-inhibited cGMP 3',5'-cyclic phosphodiesterase 10A
(Rattus norvegicus (rat)) | BDBM31592
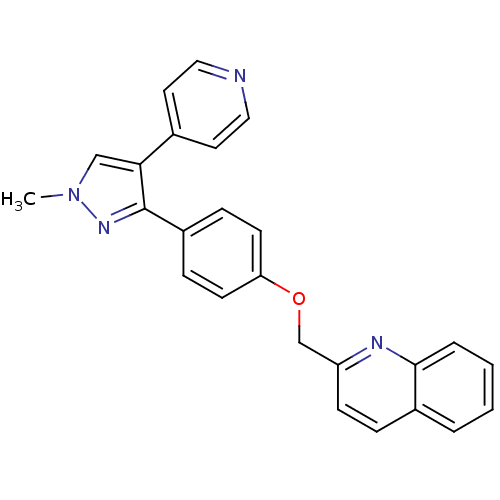
(PF-2545920 | US9138494, MP-10 | substituted pyraz...)Show SMILES Cn1cc(c(n1)-c1ccc(OCc2ccc3ccccc3n2)cc1)-c1ccncc1 Show InChI InChI=1S/C25H20N4O/c1-29-16-23(18-12-14-26-15-13-18)25(28-29)20-7-10-22(11-8-20)30-17-21-9-6-19-4-2-3-5-24(19)27-21/h2-16H,17H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| MMDB
PDB
Article
PubMed
| 0.170 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]5-(6,7-dimethoxycinnolin-4-yl)-N-isopropyl-3-methylpyridin-2-amine from PDE10A in Sprague-Dawley rat striatum |
J Med Chem 55: 4776-87 (2012)
Article DOI: 10.1021/jm3002372
BindingDB Entry DOI: 10.7270/Q2WM1FFG |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Kappa-type opioid receptor
(Homo sapiens (Human)) | BDBM50176369
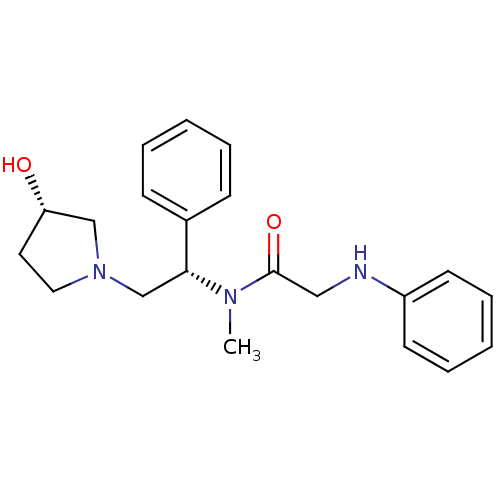
(CHEMBL201884 | N-((S)-2-((S)-3-hydroxypyrrolidin-1...)Show SMILES CN([C@H](CN1CC[C@H](O)C1)c1ccccc1)C(=O)CNc1ccccc1 Show InChI InChI=1S/C21H27N3O2/c1-23(21(26)14-22-18-10-6-3-7-11-18)20(17-8-4-2-5-9-17)16-24-13-12-19(25)15-24/h2-11,19-20,22,25H,12-16H2,1H3/t19-,20+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.170 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Adolor Corporation
Curated by ChEMBL
| Assay Description
Binding affinity to kappa opioid receptor |
Bioorg Med Chem Lett 16: 645-8 (2005)
Article DOI: 10.1016/j.bmcl.2005.10.034
BindingDB Entry DOI: 10.7270/Q26W99N3 |
More data for this
Ligand-Target Pair | |
Dihydrofolate reductase
(Homo sapiens (Human)) | BDBM50049909

(8-tert-Butyl-7-isopropyl-7H-pyrrolo[3,2-f]quinazol...)Show InChI InChI=1S/C17H23N5/c1-9(2)22-12-7-6-11-14(15(18)21-16(19)20-11)10(12)8-13(22)17(3,4)5/h6-9H,1-5H3,(H4,18,19,20,21) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wellcome Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant Dihydrofolate reductase enzyme |
J Med Chem 39: 892-903 (1996)
Article DOI: 10.1021/jm9505122
BindingDB Entry DOI: 10.7270/Q2XD10RC |
More data for this
Ligand-Target Pair | |
Dihydrofolate reductase
(Homo sapiens (Human)) | BDBM50049897

(8-Ethyl-7-(1-ethyl-propyl)-7H-pyrrolo[3,2-f]quinaz...)Show InChI InChI=1S/C17H23N5/c1-4-10(5-2)22-11(6-3)9-12-14(22)8-7-13-15(12)16(18)21-17(19)20-13/h7-10H,4-6H2,1-3H3,(H4,18,19,20,21) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wellcome Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant Dihydrofolate reductase enzyme |
J Med Chem 39: 892-903 (1996)
Article DOI: 10.1021/jm9505122
BindingDB Entry DOI: 10.7270/Q2XD10RC |
More data for this
Ligand-Target Pair | |
Histamine H4 receptor
(Homo sapiens (Human)) | BDBM50319300
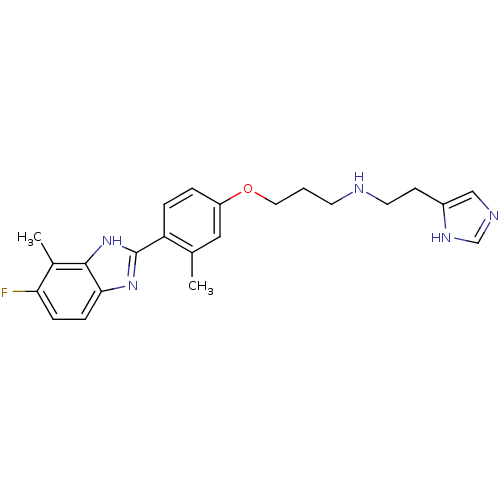
(CHEMBL1083162 | N-(2-(1H-imidazol-4-yl)ethyl)-3-(4...)Show SMILES Cc1cc(OCCCNCCc2cnc[nH]2)ccc1-c1nc2ccc(F)c(C)c2[nH]1 Show InChI InChI=1S/C23H26FN5O/c1-15-12-18(30-11-3-9-25-10-8-17-13-26-14-27-17)4-5-19(15)23-28-21-7-6-20(24)16(2)22(21)29-23/h4-7,12-14,25H,3,8-11H2,1-2H3,(H,26,27)(H,28,29) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.210 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development
Curated by ChEMBL
| Assay Description
Displacement of [3H]histamine from human recombinant histamine H4 receptor |
Bioorg Med Chem Lett 20: 3367-71 (2010)
Article DOI: 10.1016/j.bmcl.2010.04.017
BindingDB Entry DOI: 10.7270/Q23J3D4Z |
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Homo sapiens (Human)) | BDBM50176370
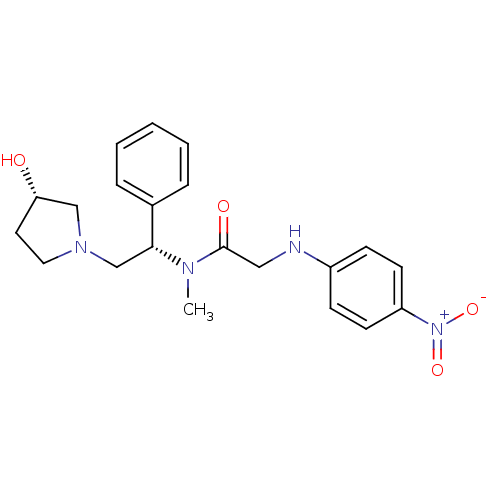
(CHEMBL201572 | N-((S)-2-((S)-3-hydroxypyrrolidin-1...)Show SMILES CN([C@H](CN1CC[C@H](O)C1)c1ccccc1)C(=O)CNc1ccc(cc1)[N+]([O-])=O Show InChI InChI=1S/C21H26N4O4/c1-23(21(27)13-22-17-7-9-18(10-8-17)25(28)29)20(16-5-3-2-4-6-16)15-24-12-11-19(26)14-24/h2-10,19-20,22,26H,11-15H2,1H3/t19-,20+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.210 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Adolor Corporation
Curated by ChEMBL
| Assay Description
Binding affinity to kappa opioid receptor |
Bioorg Med Chem Lett 16: 645-8 (2005)
Article DOI: 10.1016/j.bmcl.2005.10.034
BindingDB Entry DOI: 10.7270/Q26W99N3 |
More data for this
Ligand-Target Pair | |
Dihydrofolate reductase
(Candida albicans) | BDBM50049899
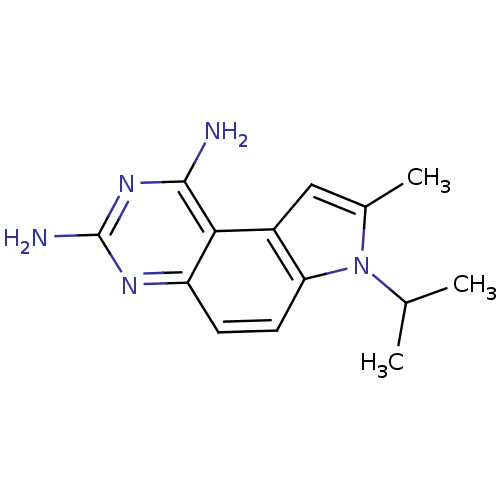
(7-Isopropyl-8-methyl-7H-pyrrolo[3,2-f]quinazoline-...)Show InChI InChI=1S/C14H17N5/c1-7(2)19-8(3)6-9-11(19)5-4-10-12(9)13(15)18-14(16)17-10/h4-7H,1-3H3,(H4,15,16,17,18) | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.220 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wellcome Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of Dihydrofolate reductase enzyme from Candida albicans |
J Med Chem 39: 892-903 (1996)
Article DOI: 10.1021/jm9505122
BindingDB Entry DOI: 10.7270/Q2XD10RC |
More data for this
Ligand-Target Pair | |
Dihydrofolate reductase
(Candida albicans) | BDBM18043
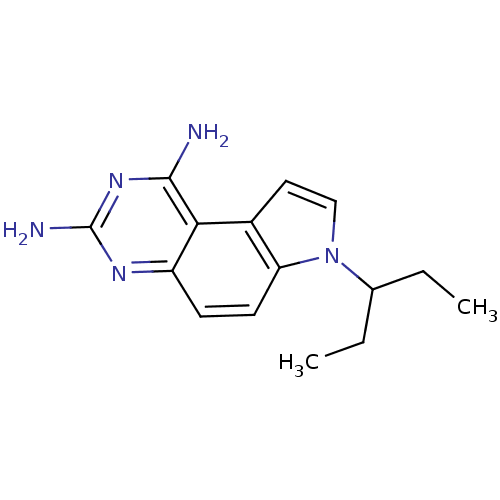
(1,3-DIAMINO-7-(1-ETHYEPROPYE)-7H-PYRRALO-[3,2-F]QU...)Show InChI InChI=1S/C15H19N5/c1-3-9(4-2)20-8-7-10-12(20)6-5-11-13(10)14(16)19-15(17)18-11/h5-9H,3-4H2,1-2H3,(H4,16,17,18,19) | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
DrugBank
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| MMDB
Article
PubMed
| 0.220 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wellcome Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of Dihydrofolate reductase enzyme from Candida albicans |
J Med Chem 39: 892-903 (1996)
Article DOI: 10.1021/jm9505122
BindingDB Entry DOI: 10.7270/Q2XD10RC |
More data for this
Ligand-Target Pair | |
Dihydrofolate reductase
(Candida albicans) | BDBM50049914

(7-(3,4,5-Trimethoxy-benzyl)-7H-pyrrolo[3,2-f]quina...)Show SMILES COc1cc(Cn2ccc3c2ccc2nc(N)nc(N)c32)cc(OC)c1OC Show InChI InChI=1S/C20H21N5O3/c1-26-15-8-11(9-16(27-2)18(15)28-3)10-25-7-6-12-14(25)5-4-13-17(12)19(21)24-20(22)23-13/h4-9H,10H2,1-3H3,(H4,21,22,23,24) | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.230 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wellcome Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of Dihydrofolate reductase enzyme from Candida albicans |
J Med Chem 39: 892-903 (1996)
Article DOI: 10.1021/jm9505122
BindingDB Entry DOI: 10.7270/Q2XD10RC |
More data for this
Ligand-Target Pair | |
Dihydrofolate reductase
(Homo sapiens (Human)) | BDBM50049905

(7-(1-Ethyl-propyl)-8-methyl-7H-pyrrolo[3,2-f]quina...)Show InChI InChI=1S/C16H21N5/c1-4-10(5-2)21-9(3)8-11-13(21)7-6-12-14(11)15(17)20-16(18)19-12/h6-8,10H,4-5H2,1-3H3,(H4,17,18,19,20) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wellcome Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant Dihydrofolate reductase enzyme |
J Med Chem 39: 892-903 (1996)
Article DOI: 10.1021/jm9505122
BindingDB Entry DOI: 10.7270/Q2XD10RC |
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Homo sapiens (Human)) | BDBM50176374

(CHEMBL382932 | N-((S)-2-((S)-3-hydroxypyrrolidin-1...)Show SMILES CN(CC(=O)N(C)[C@H](CN1CC[C@H](O)C1)c1ccccc1)c1ccccc1 Show InChI InChI=1S/C22H29N3O2/c1-23(19-11-7-4-8-12-19)17-22(27)24(2)21(18-9-5-3-6-10-18)16-25-14-13-20(26)15-25/h3-12,20-21,26H,13-17H2,1-2H3/t20-,21+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.320 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Adolor Corporation
Curated by ChEMBL
| Assay Description
Binding affinity to kappa opioid receptor |
Bioorg Med Chem Lett 16: 645-8 (2005)
Article DOI: 10.1016/j.bmcl.2005.10.034
BindingDB Entry DOI: 10.7270/Q26W99N3 |
More data for this
Ligand-Target Pair | |
Dihydrofolate reductase
(Candida albicans) | BDBM50049902

(7,8-Diethyl-7H-pyrrolo[3,2-f]quinazoline-1,3-diami...)Show InChI InChI=1S/C14H17N5/c1-3-8-7-9-11(19(8)4-2)6-5-10-12(9)13(15)18-14(16)17-10/h5-7H,3-4H2,1-2H3,(H4,15,16,17,18) | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.330 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wellcome Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of Dihydrofolate reductase enzyme from Candida albicans |
J Med Chem 39: 892-903 (1996)
Article DOI: 10.1021/jm9505122
BindingDB Entry DOI: 10.7270/Q2XD10RC |
More data for this
Ligand-Target Pair | |
Dihydrofolate reductase
(Candida albicans) | BDBM50049913
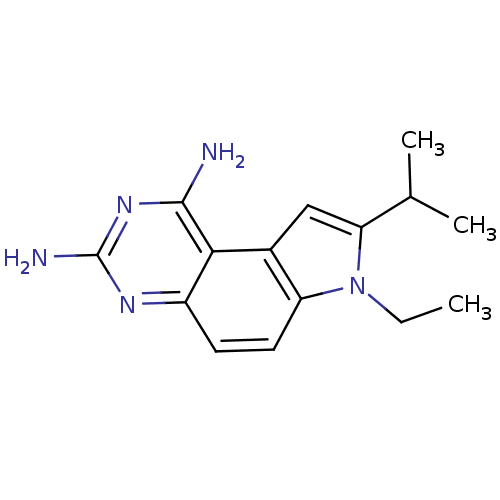
(7-Ethyl-8-isopropyl-7H-pyrrolo[3,2-f]quinazoline-1...)Show InChI InChI=1S/C15H19N5/c1-4-20-11-6-5-10-13(14(16)19-15(17)18-10)9(11)7-12(20)8(2)3/h5-8H,4H2,1-3H3,(H4,16,17,18,19) | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.330 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wellcome Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of Dihydrofolate reductase enzyme from Candida albicans |
J Med Chem 39: 892-903 (1996)
Article DOI: 10.1021/jm9505122
BindingDB Entry DOI: 10.7270/Q2XD10RC |
More data for this
Ligand-Target Pair | |
Dihydrofolate reductase
(Candida albicans) | BDBM50049896
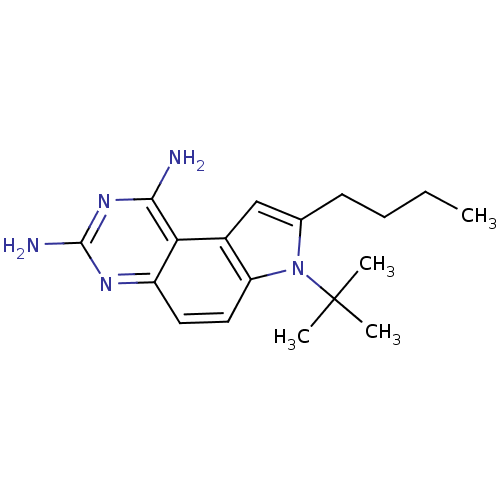
(8-Butyl-7-tert-butyl-7H-pyrrolo[3,2-f]quinazoline-...)Show InChI InChI=1S/C18H25N5/c1-5-6-7-11-10-12-14(23(11)18(2,3)4)9-8-13-15(12)16(19)22-17(20)21-13/h8-10H,5-7H2,1-4H3,(H4,19,20,21,22) | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.380 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wellcome Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of Dihydrofolate reductase enzyme from Candida albicans |
J Med Chem 39: 892-903 (1996)
Article DOI: 10.1021/jm9505122
BindingDB Entry DOI: 10.7270/Q2XD10RC |
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Homo sapiens (Human)) | BDBM50176373
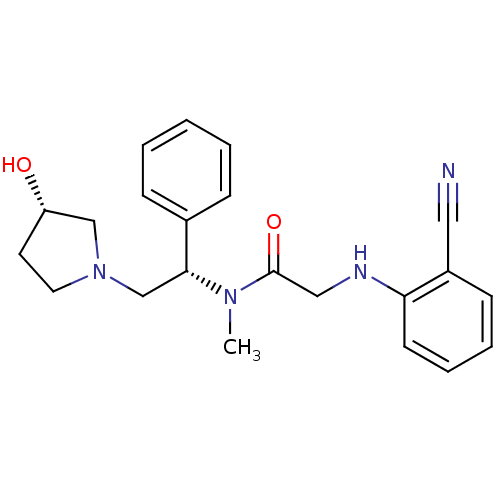
(2-(2-cyanophenylamino)-N-((S)-2-((S)-3-hydroxypyrr...)Show SMILES CN([C@H](CN1CC[C@H](O)C1)c1ccccc1)C(=O)CNc1ccccc1C#N Show InChI InChI=1S/C22H26N4O2/c1-25(22(28)14-24-20-10-6-5-9-18(20)13-23)21(17-7-3-2-4-8-17)16-26-12-11-19(27)15-26/h2-10,19,21,24,27H,11-12,14-16H2,1H3/t19-,21+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.390 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Adolor Corporation
Curated by ChEMBL
| Assay Description
Binding affinity to kappa opioid receptor |
Bioorg Med Chem Lett 16: 645-8 (2005)
Article DOI: 10.1016/j.bmcl.2005.10.034
BindingDB Entry DOI: 10.7270/Q26W99N3 |
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Homo sapiens (Human)) | BDBM50176365
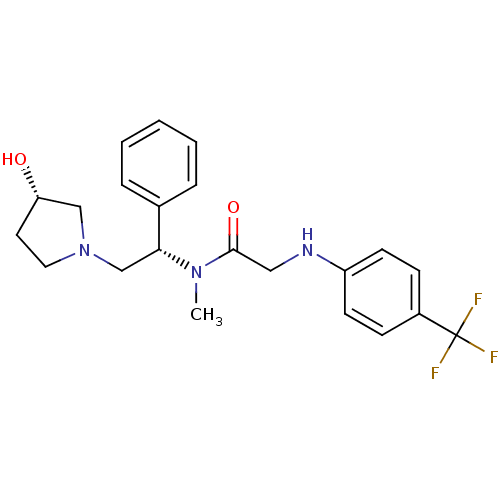
(CHEMBL201905 | N-((S)-2-((S)-3-hydroxypyrrolidin-1...)Show SMILES CN([C@H](CN1CC[C@H](O)C1)c1ccccc1)C(=O)CNc1ccc(cc1)C(F)(F)F Show InChI InChI=1S/C22H26F3N3O2/c1-27(21(30)13-26-18-9-7-17(8-10-18)22(23,24)25)20(16-5-3-2-4-6-16)15-28-12-11-19(29)14-28/h2-10,19-20,26,29H,11-15H2,1H3/t19-,20+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.390 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Adolor Corporation
Curated by ChEMBL
| Assay Description
Binding affinity to kappa opioid receptor |
Bioorg Med Chem Lett 16: 645-8 (2005)
Article DOI: 10.1016/j.bmcl.2005.10.034
BindingDB Entry DOI: 10.7270/Q26W99N3 |
More data for this
Ligand-Target Pair | |
Dihydrofolate reductase
(Homo sapiens (Human)) | BDBM50049903
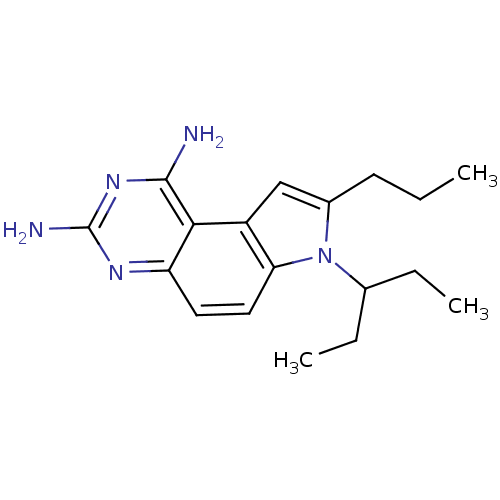
(7-(1-Ethyl-propyl)-8-propyl-7H-pyrrolo[3,2-f]quina...)Show InChI InChI=1S/C18H25N5/c1-4-7-12-10-13-15(23(12)11(5-2)6-3)9-8-14-16(13)17(19)22-18(20)21-14/h8-11H,4-7H2,1-3H3,(H4,19,20,21,22) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wellcome Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant Dihydrofolate reductase enzyme |
J Med Chem 39: 892-903 (1996)
Article DOI: 10.1021/jm9505122
BindingDB Entry DOI: 10.7270/Q2XD10RC |
More data for this
Ligand-Target Pair | |
Dihydrofolate reductase
(Homo sapiens (Human)) | BDBM50049912

(7-(1,1-Dimethyl-propyl)-8-methyl-7H-pyrrolo[3,2-f]...)Show InChI InChI=1S/C16H21N5/c1-5-16(3,4)21-9(2)8-10-12(21)7-6-11-13(10)14(17)20-15(18)19-11/h6-8H,5H2,1-4H3,(H4,17,18,19,20) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wellcome Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant Dihydrofolate reductase enzyme |
J Med Chem 39: 892-903 (1996)
Article DOI: 10.1021/jm9505122
BindingDB Entry DOI: 10.7270/Q2XD10RC |
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Homo sapiens (Human)) | BDBM50166621
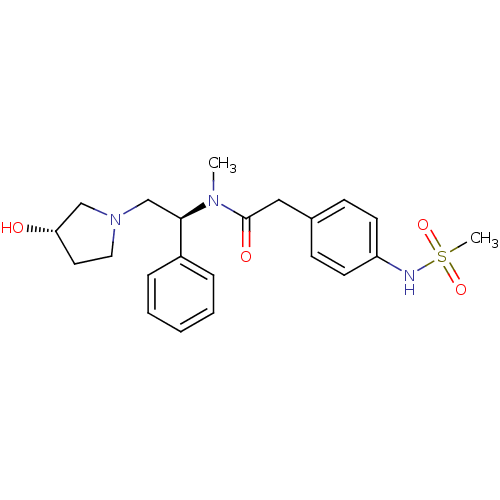
(CHEMBL191987 | N-[(S)-2-((S)-3-Hydroxy-pyrrolidin-...)Show SMILES CN([C@H](CN1CC[C@H](O)C1)c1ccccc1)C(=O)Cc1ccc(NS(C)(=O)=O)cc1 Show InChI InChI=1S/C22H29N3O4S/c1-24(22(27)14-17-8-10-19(11-9-17)23-30(2,28)29)21(18-6-4-3-5-7-18)16-25-13-12-20(26)15-25/h3-11,20-21,23,26H,12-16H2,1-2H3/t20-,21+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Adolor Corporation
Curated by ChEMBL
| Assay Description
Inhibitory constant against human Opioid receptor kappa using [3H]-diprenorphine as radio ligand |
Bioorg Med Chem Lett 15: 2647-52 (2005)
Article DOI: 10.1016/j.bmcl.2005.03.020
BindingDB Entry DOI: 10.7270/Q2PR7VH4 |
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Homo sapiens (Human)) | BDBM50176367
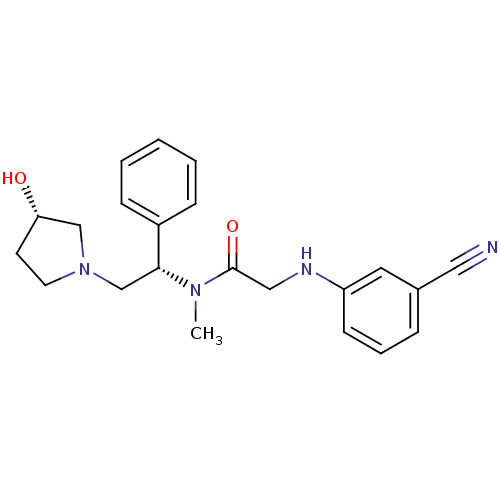
(2-(3-cyanophenylamino)-N-((S)-2-((S)-3-hydroxypyrr...)Show SMILES CN([C@H](CN1CC[C@H](O)C1)c1ccccc1)C(=O)CNc1cccc(c1)C#N Show InChI InChI=1S/C22H26N4O2/c1-25(22(28)14-24-19-9-5-6-17(12-19)13-23)21(18-7-3-2-4-8-18)16-26-11-10-20(27)15-26/h2-9,12,20-21,24,27H,10-11,14-16H2,1H3/t20-,21+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Adolor Corporation
Curated by ChEMBL
| Assay Description
Binding affinity to kappa opioid receptor |
Bioorg Med Chem Lett 16: 645-8 (2005)
Article DOI: 10.1016/j.bmcl.2005.10.034
BindingDB Entry DOI: 10.7270/Q26W99N3 |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Homo sapiens (Human)) | BDBM50136591
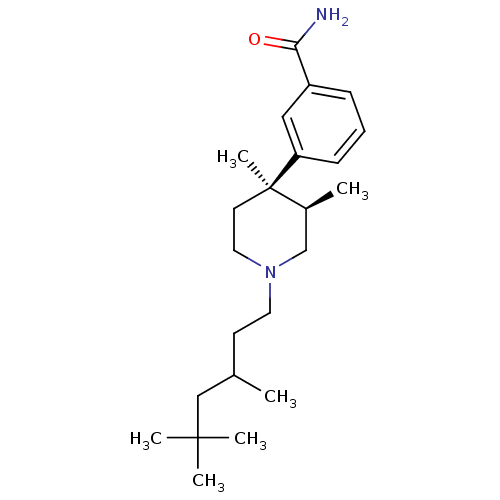
(3-[(3R,4R)-3,4-Dimethyl-1-(3,5,5-trimethyl-hexyl)-...)Show SMILES CC(CCN1CC[C@](C)([C@@H](C)C1)c1cccc(c1)C(N)=O)CC(C)(C)C Show InChI InChI=1S/C23H38N2O/c1-17(15-22(3,4)5)10-12-25-13-11-23(6,18(2)16-25)20-9-7-8-19(14-20)21(24)26/h7-9,14,17-18H,10-13,15-16H2,1-6H3,(H2,24,26)/t17?,18-,23+/m0/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Adolor Corporation
Curated by ChEMBL
| Assay Description
Inhibition of binding of the non-selective opioid antagonist, [3H]diprenorphine, to cloned human mu opioid receptor |
Bioorg Med Chem Lett 13: 4459-62 (2003)
BindingDB Entry DOI: 10.7270/Q2RV0P7G |
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Homo sapiens (Human)) | BDBM50166619
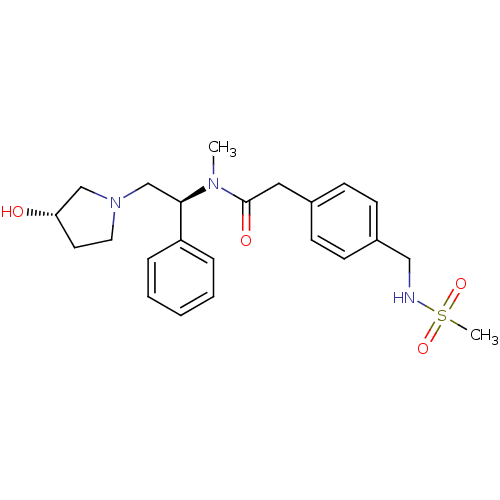
(CHEMBL425897 | N-[(S)-2-((S)-3-Hydroxy-pyrrolidin-...)Show SMILES CN([C@H](CN1CC[C@H](O)C1)c1ccccc1)C(=O)Cc1ccc(CNS(C)(=O)=O)cc1 Show InChI InChI=1S/C23H31N3O4S/c1-25(22(20-6-4-3-5-7-20)17-26-13-12-21(27)16-26)23(28)14-18-8-10-19(11-9-18)15-24-31(2,29)30/h3-11,21-22,24,27H,12-17H2,1-2H3/t21-,22+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Adolor Corporation
Curated by ChEMBL
| Assay Description
Inhibitory constant against human Opioid receptor kappa using [3H]-diprenorphine as radio ligand |
Bioorg Med Chem Lett 15: 2647-52 (2005)
Article DOI: 10.1016/j.bmcl.2005.03.020
BindingDB Entry DOI: 10.7270/Q2PR7VH4 |
More data for this
Ligand-Target Pair | |
Sigma non-opioid intracellular receptor 1
(Homo sapiens (Human)) | BDBM50063800
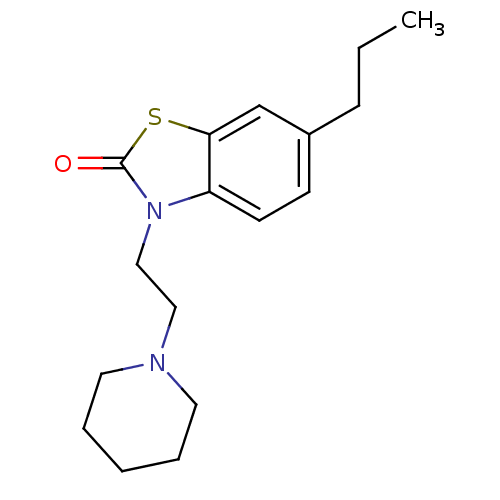
(3-(2-Piperidin-1-yl-ethyl)-6-propyl-3H-benzothiazo...)Show InChI InChI=1S/C17H24N2OS/c1-2-6-14-7-8-15-16(13-14)21-17(20)19(15)12-11-18-9-4-3-5-10-18/h7-8,13H,2-6,9-12H2,1H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Louvain
Curated by ChEMBL
| Assay Description
The compound was tested for the affinity on sigma 1 receptor using [3H]- pentazocine as radioligand |
J Med Chem 41: 1138-45 (1998)
Article DOI: 10.1021/jm970682+
BindingDB Entry DOI: 10.7270/Q29S1Q5N |
More data for this
Ligand-Target Pair | |
Dihydrofolate reductase
(Candida albicans) | BDBM50049910

(7-Ethyl-8-propyl-7H-pyrrolo[3,2-f]quinazoline-1,3-...)Show InChI InChI=1S/C15H19N5/c1-3-5-9-8-10-12(20(9)4-2)7-6-11-13(10)14(16)19-15(17)18-11/h6-8H,3-5H2,1-2H3,(H4,16,17,18,19) | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.650 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wellcome Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of Dihydrofolate reductase enzyme from Candida albicans |
J Med Chem 39: 892-903 (1996)
Article DOI: 10.1021/jm9505122
BindingDB Entry DOI: 10.7270/Q2XD10RC |
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Homo sapiens (Human)) | BDBM50176376
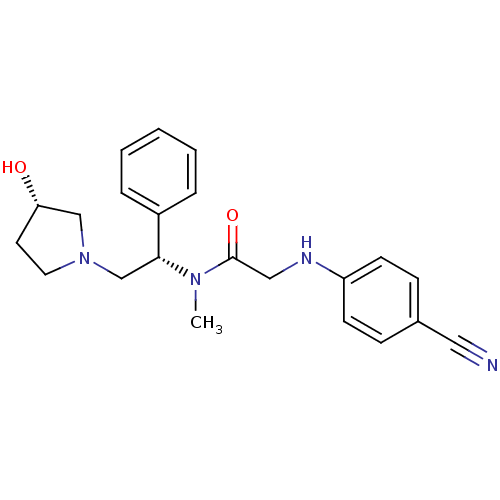
(2-(4-cyanophenylamino)-N-((S)-2-((S)-3-hydroxypyrr...)Show SMILES CN([C@H](CN1CC[C@H](O)C1)c1ccccc1)C(=O)CNc1ccc(cc1)C#N Show InChI InChI=1S/C22H26N4O2/c1-25(22(28)14-24-19-9-7-17(13-23)8-10-19)21(18-5-3-2-4-6-18)16-26-12-11-20(27)15-26/h2-10,20-21,24,27H,11-12,14-16H2,1H3/t20-,21+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.660 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Adolor Corporation
Curated by ChEMBL
| Assay Description
Binding affinity to kappa opioid receptor |
Bioorg Med Chem Lett 16: 645-8 (2005)
Article DOI: 10.1016/j.bmcl.2005.10.034
BindingDB Entry DOI: 10.7270/Q26W99N3 |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM22904

((2R)-1-(1H-imidazol-5-yl)propan-2-amine | (R)-alph...)Show InChI InChI=1S/C6H11N3/c1-5(7)2-6-3-8-4-9-6/h3-5H,2,7H2,1H3,(H,8,9)/t5-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research and Development
Curated by ChEMBL
| Assay Description
Binding affinity to the human histamine H3 receptor |
J Med Chem 46: 3957-60 (2003)
Article DOI: 10.1021/jm0341047
BindingDB Entry DOI: 10.7270/Q2QJ7J1C |
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Homo sapiens (Human)) | BDBM50176372
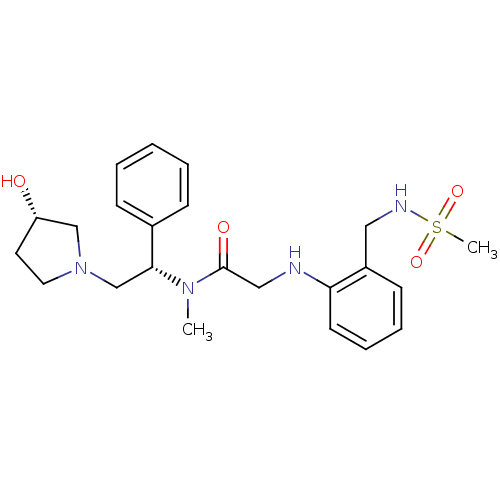
(CHEMBL201283 | N-((S)-2-((S)-3-hydroxypyrrolidin-1...)Show SMILES CN([C@H](CN1CC[C@H](O)C1)c1ccccc1)C(=O)CNc1ccccc1CNS(C)(=O)=O Show InChI InChI=1S/C23H32N4O4S/c1-26(22(18-8-4-3-5-9-18)17-27-13-12-20(28)16-27)23(29)15-24-21-11-7-6-10-19(21)14-25-32(2,30)31/h3-11,20,22,24-25,28H,12-17H2,1-2H3/t20-,22+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.780 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Adolor Corporation
Curated by ChEMBL
| Assay Description
Binding affinity to kappa opioid receptor |
Bioorg Med Chem Lett 16: 645-8 (2005)
Article DOI: 10.1016/j.bmcl.2005.10.034
BindingDB Entry DOI: 10.7270/Q26W99N3 |
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Homo sapiens (Human)) | BDBM50166613
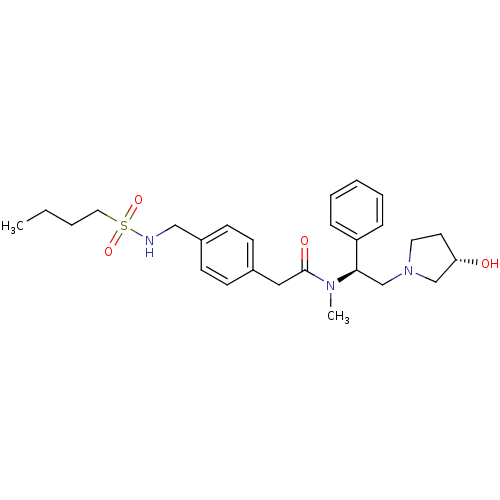
(2-{4-[(Butane-1-sulfonylamino)-methyl]-phenyl}-N-[...)Show SMILES CCCCS(=O)(=O)NCc1ccc(CC(=O)N(C)[C@H](CN2CC[C@H](O)C2)c2ccccc2)cc1 Show InChI InChI=1S/C26H37N3O4S/c1-3-4-16-34(32,33)27-18-22-12-10-21(11-13-22)17-26(31)28(2)25(23-8-6-5-7-9-23)20-29-15-14-24(30)19-29/h5-13,24-25,27,30H,3-4,14-20H2,1-2H3/t24-,25+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Adolor Corporation
Curated by ChEMBL
| Assay Description
Inhibitory constant against human Opioid receptor kappa using [3H]-diprenorphine as radio ligand |
Bioorg Med Chem Lett 15: 2647-52 (2005)
Article DOI: 10.1016/j.bmcl.2005.03.020
BindingDB Entry DOI: 10.7270/Q2PR7VH4 |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 2
(Homo sapiens (Human)) | BDBM50213144
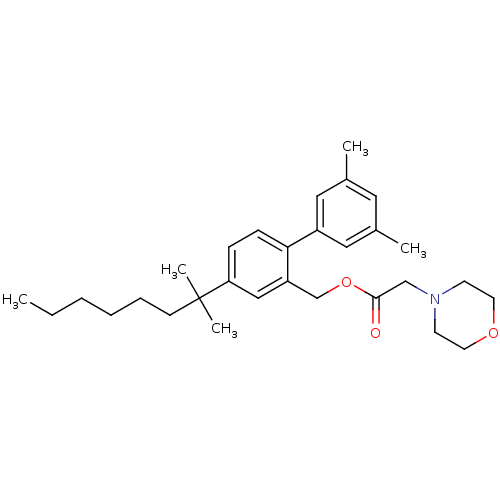
(CHEMBL233184 | morpholin-4-yl-acetic acid 4-(1,1-d...)Show SMILES CCCCCCC(C)(C)c1ccc(c(COC(=O)CN2CCOCC2)c1)-c1cc(C)cc(C)c1 Show InChI InChI=1S/C30H43NO3/c1-6-7-8-9-12-30(4,5)27-10-11-28(25-18-23(2)17-24(3)19-25)26(20-27)22-34-29(32)21-31-13-15-33-16-14-31/h10-11,17-20H,6-9,12-16,21-22H2,1-5H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 0.810 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Adolor Corporation
Curated by ChEMBL
| Assay Description
Displacement of [3H]CP55940 from cloned human CB2 receptor |
Bioorg Med Chem Lett 17: 3652-6 (2007)
Article DOI: 10.1016/j.bmcl.2007.04.059
BindingDB Entry DOI: 10.7270/Q2GM8703 |
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Homo sapiens (Human)) | BDBM50176362

(2-((4-cyanophenyl)(methyl)amino)-N-((S)-2-((S)-3-h...)Show SMILES CN(CC(=O)N(C)[C@H](CN1CC[C@H](O)C1)c1ccccc1)c1ccc(cc1)C#N Show InChI InChI=1S/C23H28N4O2/c1-25(20-10-8-18(14-24)9-11-20)17-23(29)26(2)22(19-6-4-3-5-7-19)16-27-13-12-21(28)15-27/h3-11,21-22,28H,12-13,15-17H2,1-2H3/t21-,22+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Adolor Corporation
Curated by ChEMBL
| Assay Description
Binding affinity to kappa opioid receptor |
Bioorg Med Chem Lett 16: 645-8 (2005)
Article DOI: 10.1016/j.bmcl.2005.10.034
BindingDB Entry DOI: 10.7270/Q26W99N3 |
More data for this
Ligand-Target Pair | |
Histamine H4 receptor
(Homo sapiens (Human)) | BDBM50006742
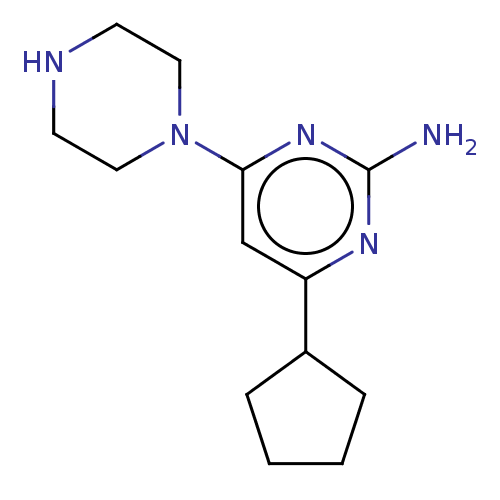
(CHEMBL3236553)Show InChI InChI=1S/C13H21N5/c14-13-16-11(10-3-1-2-4-10)9-12(17-13)18-7-5-15-6-8-18/h9-10,15H,1-8H2,(H2,14,16,17) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Janssen Pharmaceutical Research& Development, LLC
Curated by ChEMBL
| Assay Description
Displacement of [3H]histamine from human recombinant histamine H4 receptor |
Bioorg Med Chem Lett 24: 5489-92 (2015)
Article DOI: 10.1016/j.bmcl.2014.10.013
BindingDB Entry DOI: 10.7270/Q25M67B1 |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 2
(Homo sapiens (Human)) | BDBM50213141
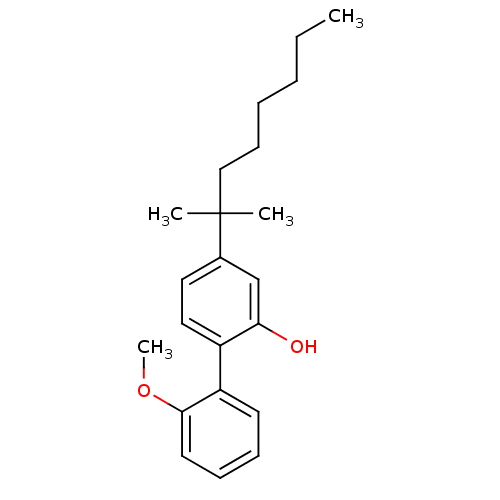
(4-(1,1-dimethyl-heptyl)-2'-methoxy-biphenyl-2-ol |...)Show InChI InChI=1S/C22H30O2/c1-5-6-7-10-15-22(2,3)17-13-14-18(20(23)16-17)19-11-8-9-12-21(19)24-4/h8-9,11-14,16,23H,5-7,10,15H2,1-4H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Adolor Corporation
Curated by ChEMBL
| Assay Description
Displacement of [3H]CP55940 from cloned human CB2 receptor |
Bioorg Med Chem Lett 17: 3652-6 (2007)
Article DOI: 10.1016/j.bmcl.2007.04.059
BindingDB Entry DOI: 10.7270/Q2GM8703 |
More data for this
Ligand-Target Pair | |
cAMP and cAMP-inhibited cGMP 3',5'-cyclic phosphodiesterase 10A
(Rattus norvegicus (rat)) | BDBM50365964
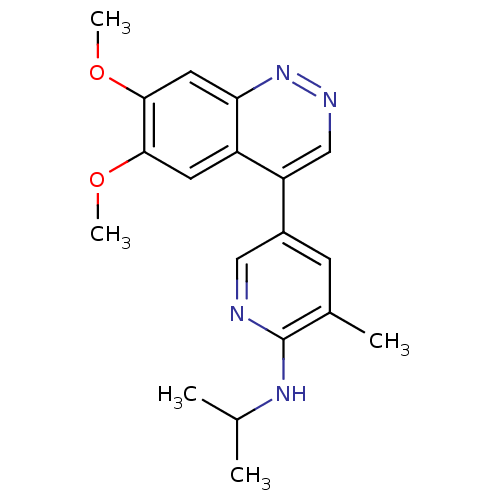
(CHEMBL1956235 | CHEMBL2070530)Show InChI InChI=1S/C19H22N4O2/c1-11(2)22-19-12(3)6-13(9-20-19)15-10-21-23-16-8-18(25-5)17(24-4)7-14(15)16/h6-11H,1-5H3,(H,20,22) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.18 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]5-(6,7-dimethoxycinnolin-4-yl)-N-isopropyl-3-methylpyridin-2-amine from PDE10A in Sprague-Dawley rat striatum |
J Med Chem 55: 4776-87 (2012)
Article DOI: 10.1021/jm3002372
BindingDB Entry DOI: 10.7270/Q2WM1FFG |
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Homo sapiens (Human)) | BDBM50166615

(2-[4-(Ethanesulfonylamino-methyl)-phenyl]-N-[(S)-2...)Show SMILES CCS(=O)(=O)NCc1ccc(CC(=O)N(C)[C@H](CN2CC[C@H](O)C2)c2ccccc2)cc1 Show InChI InChI=1S/C24H33N3O4S/c1-3-32(30,31)25-16-20-11-9-19(10-12-20)15-24(29)26(2)23(21-7-5-4-6-8-21)18-27-14-13-22(28)17-27/h4-12,22-23,25,28H,3,13-18H2,1-2H3/t22-,23+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Adolor Corporation
Curated by ChEMBL
| Assay Description
Inhibitory constant against human Opioid receptor kappa using [3H]-diprenorphine as radio ligand |
Bioorg Med Chem Lett 15: 2647-52 (2005)
Article DOI: 10.1016/j.bmcl.2005.03.020
BindingDB Entry DOI: 10.7270/Q2PR7VH4 |
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
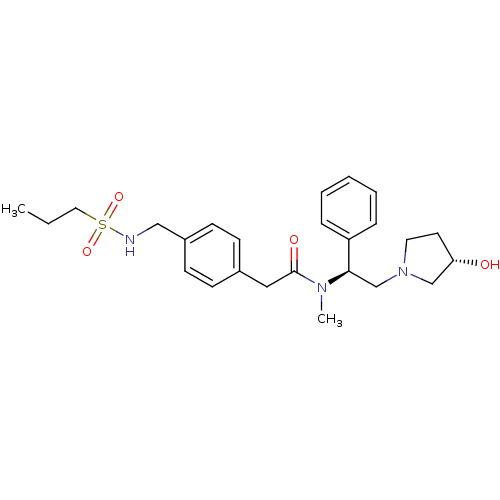
(Homo sapiens (Human)) | BDBM50166626
(CHEMBL363822 | N-[(S)-2-((S)-3-Hydroxy-pyrrolidin-...)Show SMILES CCCS(=O)(=O)NCc1ccc(CC(=O)N(C)[C@H](CN2CC[C@H](O)C2)c2ccccc2)cc1 Show InChI InChI=1S/C25H35N3O4S/c1-3-15-33(31,32)26-17-21-11-9-20(10-12-21)16-25(30)27(2)24(22-7-5-4-6-8-22)19-28-14-13-23(29)18-28/h4-12,23-24,26,29H,3,13-19H2,1-2H3/t23-,24+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Adolor Corporation
Curated by ChEMBL
| Assay Description
Inhibitory constant against human Opioid receptor kappa using [3H]-diprenorphine as radio ligand |
Bioorg Med Chem Lett 15: 2647-52 (2005)
Article DOI: 10.1016/j.bmcl.2005.03.020
BindingDB Entry DOI: 10.7270/Q2PR7VH4 |
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
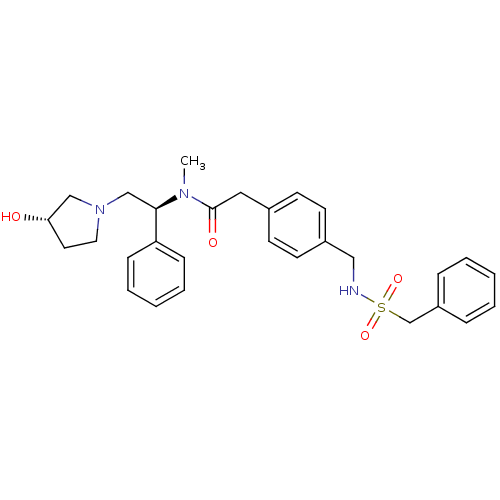
(Homo sapiens (Human)) | BDBM50166614
(CHEMBL189643 | N-[(S)-2-((S)-3-Hydroxy-pyrrolidin-...)Show SMILES CN([C@H](CN1CC[C@H](O)C1)c1ccccc1)C(=O)Cc1ccc(CNS(=O)(=O)Cc2ccccc2)cc1 Show InChI InChI=1S/C29H35N3O4S/c1-31(28(26-10-6-3-7-11-26)21-32-17-16-27(33)20-32)29(34)18-23-12-14-24(15-13-23)19-30-37(35,36)22-25-8-4-2-5-9-25/h2-15,27-28,30,33H,16-22H2,1H3/t27-,28+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Adolor Corporation
Curated by ChEMBL
| Assay Description
Inhibitory constant against human Opioid receptor kappa using [3H]-diprenorphine as radio ligand |
Bioorg Med Chem Lett 15: 2647-52 (2005)
Article DOI: 10.1016/j.bmcl.2005.03.020
BindingDB Entry DOI: 10.7270/Q2PR7VH4 |
More data for this
Ligand-Target Pair | |
Dihydrofolate reductase
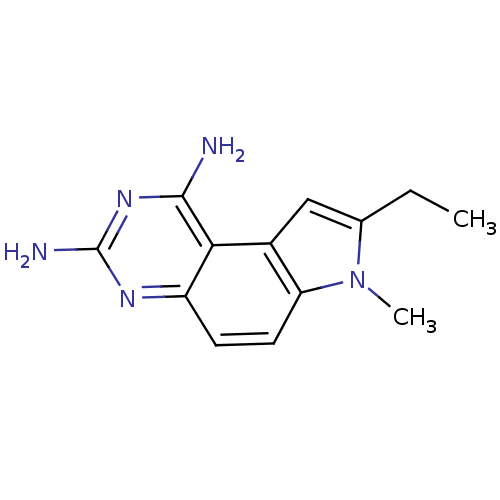
(Candida albicans) | BDBM50049915
(8-Ethyl-7-methyl-7H-pyrrolo[3,2-f]quinazoline-1,3-...)Show InChI InChI=1S/C13H15N5/c1-3-7-6-8-10(18(7)2)5-4-9-11(8)12(14)17-13(15)16-9/h4-6H,3H2,1-2H3,(H4,14,15,16,17) | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wellcome Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of Dihydrofolate reductase enzyme from Candida albicans |
J Med Chem 39: 892-903 (1996)
Article DOI: 10.1021/jm9505122
BindingDB Entry DOI: 10.7270/Q2XD10RC |
More data for this
Ligand-Target Pair | |
Dihydrofolate reductase
(Homo sapiens (Human)) | BDBM18268

(5-methyl-6-{[(3,4,5-trimethoxyphenyl)amino]methyl}...)Show InChI InChI=1S/C19H23N5O3/c1-10-11(5-6-13-16(10)18(20)24-19(21)23-13)9-22-12-7-14(25-2)17(27-4)15(8-12)26-3/h5-8,22H,9H2,1-4H3,(H4,20,21,23,24) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
Article
PubMed
| 1.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wellcome Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant Dihydrofolate reductase enzyme |
J Med Chem 39: 892-903 (1996)
Article DOI: 10.1021/jm9505122
BindingDB Entry DOI: 10.7270/Q2XD10RC |
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Homo sapiens (Human)) | BDBM50176371

(CHEMBL202267 | N-((S)-2-((S)-3-hydroxypyrrolidin-1...)Show SMILES CCCS(=O)(=O)Nc1ccc(NCC(=O)N(C)[C@H](CN2CC[C@H](O)C2)c2ccccc2)cc1 Show InChI InChI=1S/C24H34N4O4S/c1-3-15-33(31,32)26-21-11-9-20(10-12-21)25-16-24(30)27(2)23(19-7-5-4-6-8-19)18-28-14-13-22(29)17-28/h4-12,22-23,25-26,29H,3,13-18H2,1-2H3/t22-,23+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 1.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Adolor Corporation
Curated by ChEMBL
| Assay Description
Binding affinity to kappa opioid receptor |
Bioorg Med Chem Lett 16: 645-8 (2005)
Article DOI: 10.1016/j.bmcl.2005.10.034
BindingDB Entry DOI: 10.7270/Q26W99N3 |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM50213141

(4-(1,1-dimethyl-heptyl)-2'-methoxy-biphenyl-2-ol |...)Show InChI InChI=1S/C22H30O2/c1-5-6-7-10-15-22(2,3)17-13-14-18(20(23)16-17)19-11-8-9-12-21(19)24-4/h8-9,11-14,16,23H,5-7,10,15H2,1-4H3 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Adolor Corporation
Curated by ChEMBL
| Assay Description
Displacement of [3H]CP55940 from cloned human CB1 receptor |
Bioorg Med Chem Lett 17: 3652-6 (2007)
Article DOI: 10.1016/j.bmcl.2007.04.059
BindingDB Entry DOI: 10.7270/Q2GM8703 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data