

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Carbonic anhydrase 4 (Homo sapiens (Human)) | BDBM50209283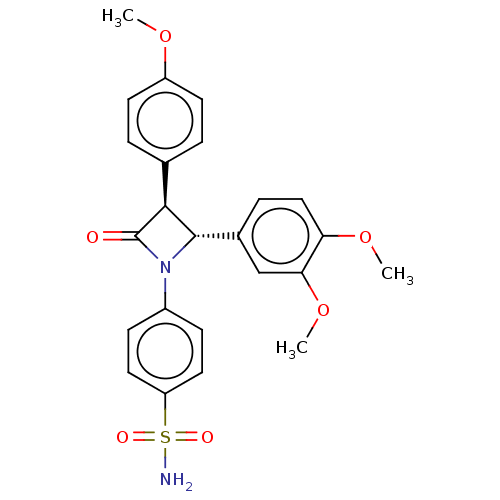 (CHEMBL3885230) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.530 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Pharmaceutical Education and Research (NIPER) Curated by ChEMBL | Assay Description Inhibition of human recombinant carbonic anhydrase-4 using CO2 as substrate preincubated for 10 mins by stopped-flow CO2 hydrase assay | Bioorg Med Chem 25: 539-544 (2017) Article DOI: 10.1016/j.bmc.2016.11.027 BindingDB Entry DOI: 10.7270/Q2G73GQQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1D (Homo sapiens (Human)) | BDBM50243699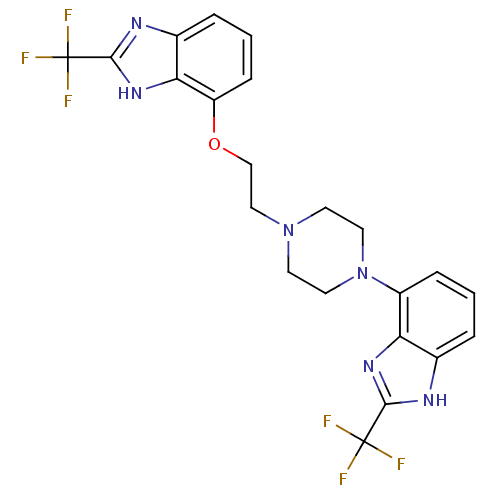 (2-(trifluoromethyl)-7-(2-(4-(2-(trifluoromethyl)-1...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.550 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research Curated by ChEMBL | Assay Description Binding affinity to human 5HT1D receptor | Bioorg Med Chem 16: 6617-40 (2008) Article DOI: 10.1016/j.bmc.2008.05.024 BindingDB Entry DOI: 10.7270/Q2FB52Q7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 4 (Homo sapiens (Human)) | BDBM50209288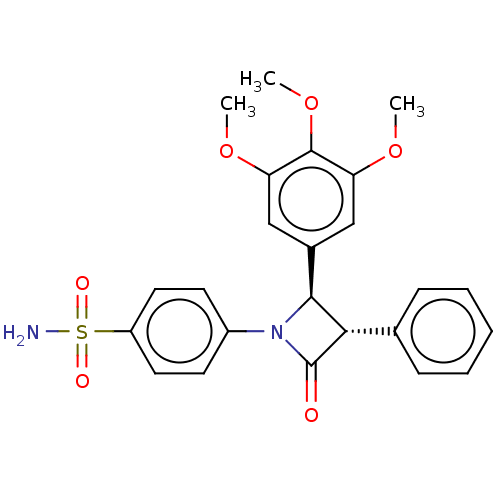 (CHEMBL3885451) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.640 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Pharmaceutical Education and Research (NIPER) Curated by ChEMBL | Assay Description Inhibition of human recombinant carbonic anhydrase-4 using CO2 as substrate preincubated for 10 mins by stopped-flow CO2 hydrase assay | Bioorg Med Chem 25: 539-544 (2017) Article DOI: 10.1016/j.bmc.2016.11.027 BindingDB Entry DOI: 10.7270/Q2G73GQQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 4 (Homo sapiens (Human)) | BDBM50209225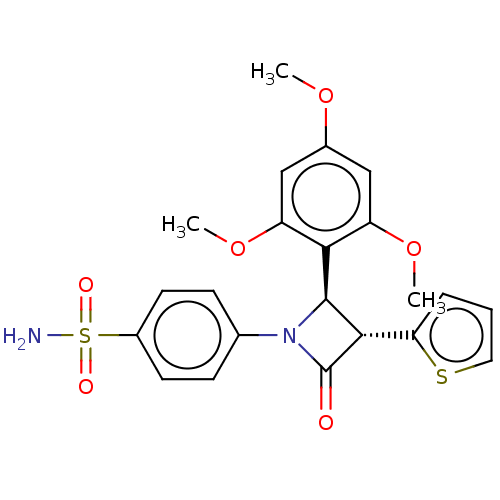 (CHEMBL3883807) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.650 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Pharmaceutical Education and Research (NIPER) Curated by ChEMBL | Assay Description Inhibition of human recombinant carbonic anhydrase-4 using CO2 as substrate preincubated for 10 mins by stopped-flow CO2 hydrase assay | Bioorg Med Chem 25: 539-544 (2017) Article DOI: 10.1016/j.bmc.2016.11.027 BindingDB Entry DOI: 10.7270/Q2G73GQQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 7 (Homo sapiens (Human)) | BDBM50209287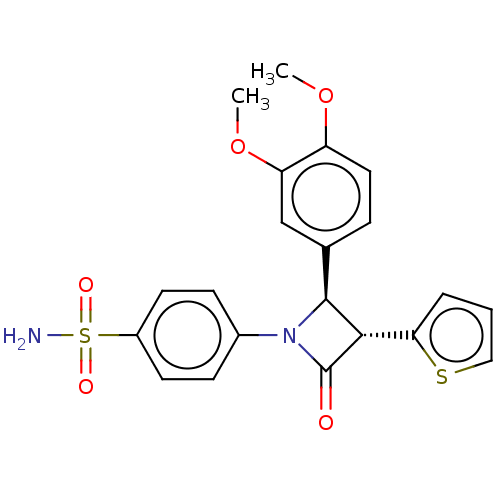 (CHEMBL3884217) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.680 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Pharmaceutical Education and Research (NIPER) Curated by ChEMBL | Assay Description Inhibition of human recombinant carbonic anhydrase-7 using CO2 as substrate preincubated for 10 mins by stopped-flow CO2 hydrase assay | Bioorg Med Chem 25: 539-544 (2017) Article DOI: 10.1016/j.bmc.2016.11.027 BindingDB Entry DOI: 10.7270/Q2G73GQQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 7 (Homo sapiens (Human)) | BDBM50209282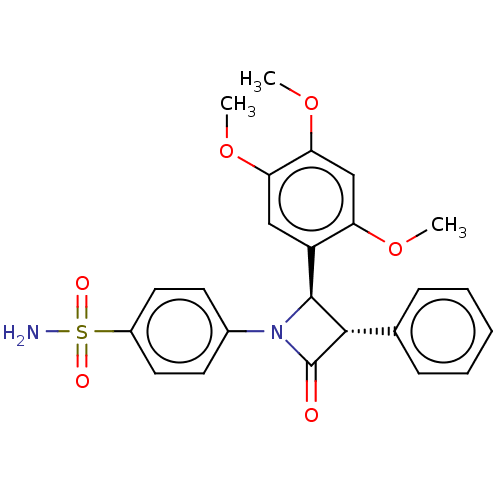 (CHEMBL3883491) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Pharmaceutical Education and Research (NIPER) Curated by ChEMBL | Assay Description Inhibition of human recombinant carbonic anhydrase-7 using CO2 as substrate preincubated for 10 mins by stopped-flow CO2 hydrase assay | Bioorg Med Chem 25: 539-544 (2017) Article DOI: 10.1016/j.bmc.2016.11.027 BindingDB Entry DOI: 10.7270/Q2G73GQQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 7 (Homo sapiens (Human)) | BDBM50209283 (CHEMBL3885230) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.730 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Pharmaceutical Education and Research (NIPER) Curated by ChEMBL | Assay Description Inhibition of human recombinant carbonic anhydrase-7 using CO2 as substrate preincubated for 10 mins by stopped-flow CO2 hydrase assay | Bioorg Med Chem 25: 539-544 (2017) Article DOI: 10.1016/j.bmc.2016.11.027 BindingDB Entry DOI: 10.7270/Q2G73GQQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 2 (Homo sapiens (Human)) | BDBM50209292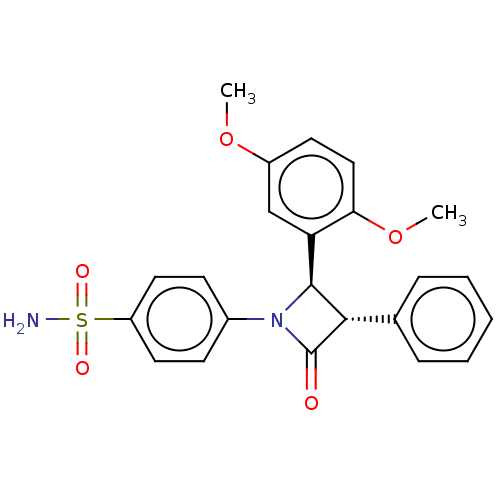 (CHEMBL3884793) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.760 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Pharmaceutical Education and Research (NIPER) Curated by ChEMBL | Assay Description Inhibition of human recombinant carbonic anhydrase-2 using CO2 as substrate preincubated for 10 mins by stopped-flow CO2 hydrase assay | Bioorg Med Chem 25: 539-544 (2017) Article DOI: 10.1016/j.bmc.2016.11.027 BindingDB Entry DOI: 10.7270/Q2G73GQQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 7 (Homo sapiens (Human)) | BDBM50209289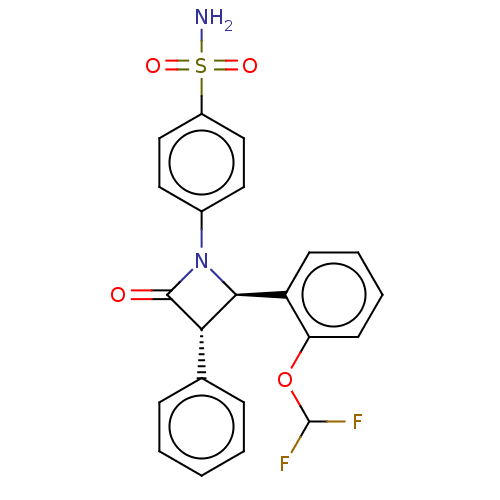 (CHEMBL3885091) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Pharmaceutical Education and Research (NIPER) Curated by ChEMBL | Assay Description Inhibition of human recombinant carbonic anhydrase-7 using CO2 as substrate preincubated for 10 mins by stopped-flow CO2 hydrase assay | Bioorg Med Chem 25: 539-544 (2017) Article DOI: 10.1016/j.bmc.2016.11.027 BindingDB Entry DOI: 10.7270/Q2G73GQQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 7 (Homo sapiens (Human)) | BDBM50209226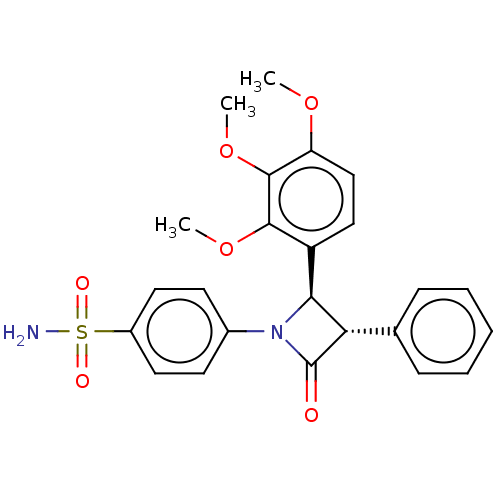 (CHEMBL3884755) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.810 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Pharmaceutical Education and Research (NIPER) Curated by ChEMBL | Assay Description Inhibition of human recombinant carbonic anhydrase-7 using CO2 as substrate preincubated for 10 mins by stopped-flow CO2 hydrase assay | Bioorg Med Chem 25: 539-544 (2017) Article DOI: 10.1016/j.bmc.2016.11.027 BindingDB Entry DOI: 10.7270/Q2G73GQQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 2 (Homo sapiens (Human)) | BDBM50209283 (CHEMBL3885230) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.870 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Pharmaceutical Education and Research (NIPER) Curated by ChEMBL | Assay Description Inhibition of human recombinant carbonic anhydrase-2 using CO2 as substrate preincubated for 10 mins by stopped-flow CO2 hydrase assay | Bioorg Med Chem 25: 539-544 (2017) Article DOI: 10.1016/j.bmc.2016.11.027 BindingDB Entry DOI: 10.7270/Q2G73GQQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 7 (Homo sapiens (Human)) | BDBM50209286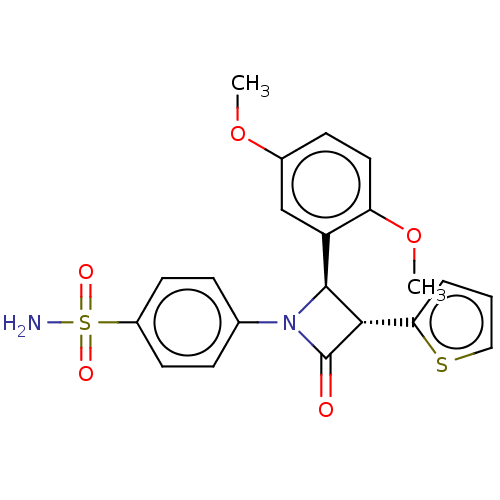 (CHEMBL3884261) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.910 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Pharmaceutical Education and Research (NIPER) Curated by ChEMBL | Assay Description Inhibition of human recombinant carbonic anhydrase-7 using CO2 as substrate preincubated for 10 mins by stopped-flow CO2 hydrase assay | Bioorg Med Chem 25: 539-544 (2017) Article DOI: 10.1016/j.bmc.2016.11.027 BindingDB Entry DOI: 10.7270/Q2G73GQQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 4 (Homo sapiens (Human)) | BDBM50209282 (CHEMBL3883491) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.950 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Pharmaceutical Education and Research (NIPER) Curated by ChEMBL | Assay Description Inhibition of human recombinant carbonic anhydrase-4 using CO2 as substrate preincubated for 10 mins by stopped-flow CO2 hydrase assay | Bioorg Med Chem 25: 539-544 (2017) Article DOI: 10.1016/j.bmc.2016.11.027 BindingDB Entry DOI: 10.7270/Q2G73GQQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 2 (Homo sapiens (Human)) | BDBM50209287 (CHEMBL3884217) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.970 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Pharmaceutical Education and Research (NIPER) Curated by ChEMBL | Assay Description Inhibition of human recombinant carbonic anhydrase-2 using CO2 as substrate preincubated for 10 mins by stopped-flow CO2 hydrase assay | Bioorg Med Chem 25: 539-544 (2017) Article DOI: 10.1016/j.bmc.2016.11.027 BindingDB Entry DOI: 10.7270/Q2G73GQQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 7 (Homo sapiens (Human)) | BDBM50209293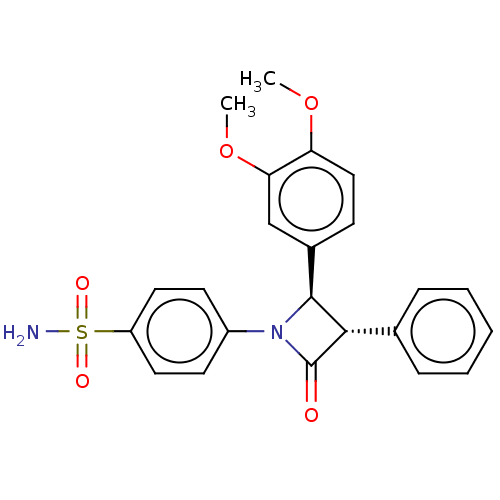 (CHEMBL3884597) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Pharmaceutical Education and Research (NIPER) Curated by ChEMBL | Assay Description Inhibition of human recombinant carbonic anhydrase-7 using CO2 as substrate preincubated for 10 mins by stopped-flow CO2 hydrase assay | Bioorg Med Chem 25: 539-544 (2017) Article DOI: 10.1016/j.bmc.2016.11.027 BindingDB Entry DOI: 10.7270/Q2G73GQQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 2 (Homo sapiens (Human)) | BDBM50209293 (CHEMBL3884597) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Pharmaceutical Education and Research (NIPER) Curated by ChEMBL | Assay Description Inhibition of human recombinant carbonic anhydrase-2 using CO2 as substrate preincubated for 10 mins by stopped-flow CO2 hydrase assay | Bioorg Med Chem 25: 539-544 (2017) Article DOI: 10.1016/j.bmc.2016.11.027 BindingDB Entry DOI: 10.7270/Q2G73GQQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 4 (Homo sapiens (Human)) | BDBM50209290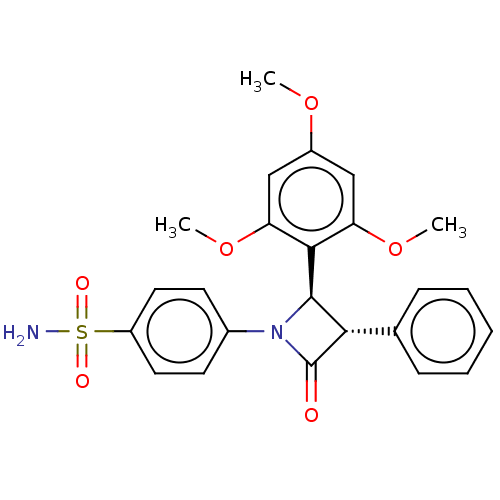 (CHEMBL3883755) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Pharmaceutical Education and Research (NIPER) Curated by ChEMBL | Assay Description Inhibition of human recombinant carbonic anhydrase-4 using CO2 as substrate preincubated for 10 mins by stopped-flow CO2 hydrase assay | Bioorg Med Chem 25: 539-544 (2017) Article DOI: 10.1016/j.bmc.2016.11.027 BindingDB Entry DOI: 10.7270/Q2G73GQQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 7 (Homo sapiens (Human)) | BDBM50209288 (CHEMBL3885451) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Pharmaceutical Education and Research (NIPER) Curated by ChEMBL | Assay Description Inhibition of human recombinant carbonic anhydrase-7 using CO2 as substrate preincubated for 10 mins by stopped-flow CO2 hydrase assay | Bioorg Med Chem 25: 539-544 (2017) Article DOI: 10.1016/j.bmc.2016.11.027 BindingDB Entry DOI: 10.7270/Q2G73GQQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 2 (Homo sapiens (Human)) | BDBM50209282 (CHEMBL3883491) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Pharmaceutical Education and Research (NIPER) Curated by ChEMBL | Assay Description Inhibition of human recombinant carbonic anhydrase-2 using CO2 as substrate preincubated for 10 mins by stopped-flow CO2 hydrase assay | Bioorg Med Chem 25: 539-544 (2017) Article DOI: 10.1016/j.bmc.2016.11.027 BindingDB Entry DOI: 10.7270/Q2G73GQQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 7 (Homo sapiens (Human)) | BDBM10880 (AZA | AZA2 | AZM acetazolamide | Acerazolamide, AA...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | DrugBank PDB Article PubMed | 2.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Pharmaceutical Education and Research (NIPER) Curated by ChEMBL | Assay Description Inhibition of human recombinant carbonic anhydrase-7 using CO2 as substrate preincubated for 10 mins by stopped-flow CO2 hydrase assay | Bioorg Med Chem 25: 539-544 (2017) Article DOI: 10.1016/j.bmc.2016.11.027 BindingDB Entry DOI: 10.7270/Q2G73GQQ | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| 5-hydroxytryptamine receptor 1A (Homo sapiens (Human)) | BDBM50243700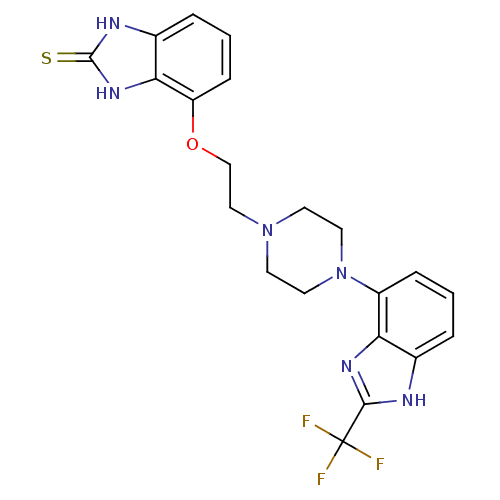 (4-(2-(4-(2-(trifluoromethyl)-1H-benzo[d]imidazol-4...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research Curated by ChEMBL | Assay Description Binding affinity to human 5HT1A receptor | Bioorg Med Chem 16: 6617-40 (2008) Article DOI: 10.1016/j.bmc.2008.05.024 BindingDB Entry DOI: 10.7270/Q2FB52Q7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 2 (Homo sapiens (Human)) | BDBM50209226 (CHEMBL3884755) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Pharmaceutical Education and Research (NIPER) Curated by ChEMBL | Assay Description Inhibition of human recombinant carbonic anhydrase-2 using CO2 as substrate preincubated for 10 mins by stopped-flow CO2 hydrase assay | Bioorg Med Chem 25: 539-544 (2017) Article DOI: 10.1016/j.bmc.2016.11.027 BindingDB Entry DOI: 10.7270/Q2G73GQQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 2 (Homo sapiens (Human)) | BDBM50209225 (CHEMBL3883807) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 3.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Pharmaceutical Education and Research (NIPER) Curated by ChEMBL | Assay Description Inhibition of human recombinant carbonic anhydrase-2 using CO2 as substrate preincubated for 10 mins by stopped-flow CO2 hydrase assay | Bioorg Med Chem 25: 539-544 (2017) Article DOI: 10.1016/j.bmc.2016.11.027 BindingDB Entry DOI: 10.7270/Q2G73GQQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 7 (Homo sapiens (Human)) | BDBM50209284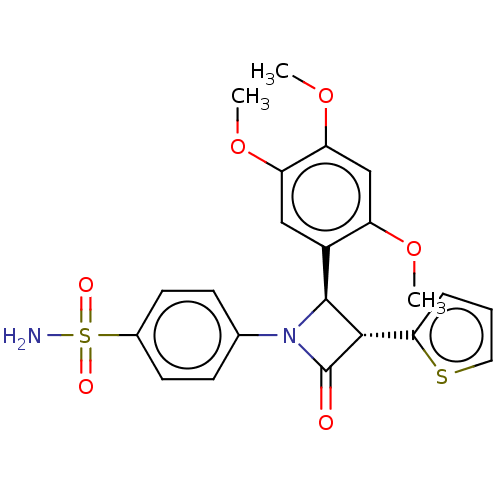 (CHEMBL3884126) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 3.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Pharmaceutical Education and Research (NIPER) Curated by ChEMBL | Assay Description Inhibition of human recombinant carbonic anhydrase-7 using CO2 as substrate preincubated for 10 mins by stopped-flow CO2 hydrase assay | Bioorg Med Chem 25: 539-544 (2017) Article DOI: 10.1016/j.bmc.2016.11.027 BindingDB Entry DOI: 10.7270/Q2G73GQQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 4 (Homo sapiens (Human)) | BDBM50209285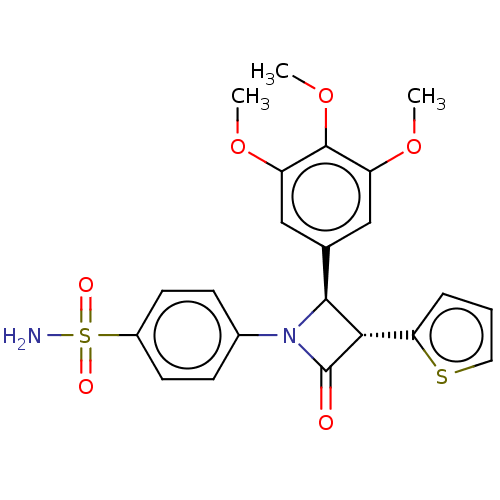 (CHEMBL3884696) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 3.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Pharmaceutical Education and Research (NIPER) Curated by ChEMBL | Assay Description Inhibition of human recombinant carbonic anhydrase-4 using CO2 as substrate preincubated for 10 mins by stopped-flow CO2 hydrase assay | Bioorg Med Chem 25: 539-544 (2017) Article DOI: 10.1016/j.bmc.2016.11.027 BindingDB Entry DOI: 10.7270/Q2G73GQQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 2 (Homo sapiens (Human)) | BDBM50209289 (CHEMBL3885091) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 3.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Pharmaceutical Education and Research (NIPER) Curated by ChEMBL | Assay Description Inhibition of human recombinant carbonic anhydrase-2 using CO2 as substrate preincubated for 10 mins by stopped-flow CO2 hydrase assay | Bioorg Med Chem 25: 539-544 (2017) Article DOI: 10.1016/j.bmc.2016.11.027 BindingDB Entry DOI: 10.7270/Q2G73GQQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 7 (Homo sapiens (Human)) | BDBM50209225 (CHEMBL3883807) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Pharmaceutical Education and Research (NIPER) Curated by ChEMBL | Assay Description Inhibition of human recombinant carbonic anhydrase-7 using CO2 as substrate preincubated for 10 mins by stopped-flow CO2 hydrase assay | Bioorg Med Chem 25: 539-544 (2017) Article DOI: 10.1016/j.bmc.2016.11.027 BindingDB Entry DOI: 10.7270/Q2G73GQQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 4 (Homo sapiens (Human)) | BDBM50209284 (CHEMBL3884126) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Pharmaceutical Education and Research (NIPER) Curated by ChEMBL | Assay Description Inhibition of human recombinant carbonic anhydrase-4 using CO2 as substrate preincubated for 10 mins by stopped-flow CO2 hydrase assay | Bioorg Med Chem 25: 539-544 (2017) Article DOI: 10.1016/j.bmc.2016.11.027 BindingDB Entry DOI: 10.7270/Q2G73GQQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 2 (Homo sapiens (Human)) | BDBM50209224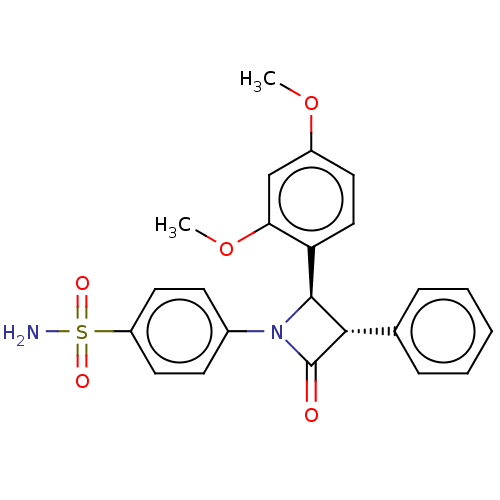 (CHEMBL3885126) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 5.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Pharmaceutical Education and Research (NIPER) Curated by ChEMBL | Assay Description Inhibition of human recombinant carbonic anhydrase-2 using CO2 as substrate preincubated for 10 mins by stopped-flow CO2 hydrase assay | Bioorg Med Chem 25: 539-544 (2017) Article DOI: 10.1016/j.bmc.2016.11.027 BindingDB Entry DOI: 10.7270/Q2G73GQQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 12 (Homo sapiens (Human)) | BDBM10880 (AZA | AZA2 | AZM acetazolamide | Acerazolamide, AA...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | 5.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of recombinant human carbonic anhydrase 12 preincubated for 10 mins by stopped flow CO2 hydrase assay | Citation and Details Article DOI: 10.1016/j.bmcl.2021.127877 BindingDB Entry DOI: 10.7270/Q29Z98MV | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Carbonic anhydrase 12 (Homo sapiens (Human)) | BDBM10880 (AZA | AZA2 | AZM acetazolamide | Acerazolamide, AA...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | 6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Pharmaceutical Education and Research (NIPER) Curated by ChEMBL | Assay Description Inhibition of recombinant human carbonic anhydrase12 preincubated for 10 mins followed by CO2 solution addition by phenol red dye based stopped flow ... | Bioorg Med Chem 27: (2019) Article DOI: 10.1016/j.bmc.2019.115090 BindingDB Entry DOI: 10.7270/Q2RV0S2G | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Carbonic anhydrase 2 (Homo sapiens (Human)) | BDBM50209288 (CHEMBL3885451) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 6.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Pharmaceutical Education and Research (NIPER) Curated by ChEMBL | Assay Description Inhibition of human recombinant carbonic anhydrase-2 using CO2 as substrate preincubated for 10 mins by stopped-flow CO2 hydrase assay | Bioorg Med Chem 25: 539-544 (2017) Article DOI: 10.1016/j.bmc.2016.11.027 BindingDB Entry DOI: 10.7270/Q2G73GQQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 7 (Homo sapiens (Human)) | BDBM50209224 (CHEMBL3885126) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 6.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Pharmaceutical Education and Research (NIPER) Curated by ChEMBL | Assay Description Inhibition of human recombinant carbonic anhydrase-7 using CO2 as substrate preincubated for 10 mins by stopped-flow CO2 hydrase assay | Bioorg Med Chem 25: 539-544 (2017) Article DOI: 10.1016/j.bmc.2016.11.027 BindingDB Entry DOI: 10.7270/Q2G73GQQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 1 (Homo sapiens (Human)) | BDBM50209293 (CHEMBL3884597) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 7.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Pharmaceutical Education and Research (NIPER) Curated by ChEMBL | Assay Description Inhibition of human recombinant carbonic anhydrase-1 using CO2 as substrate preincubated for 10 mins by stopped-flow CO2 hydrase assay | Bioorg Med Chem 25: 539-544 (2017) Article DOI: 10.1016/j.bmc.2016.11.027 BindingDB Entry DOI: 10.7270/Q2G73GQQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 4 (Homo sapiens (Human)) | BDBM50209287 (CHEMBL3884217) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 7.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Pharmaceutical Education and Research (NIPER) Curated by ChEMBL | Assay Description Inhibition of human recombinant carbonic anhydrase-4 using CO2 as substrate preincubated for 10 mins by stopped-flow CO2 hydrase assay | Bioorg Med Chem 25: 539-544 (2017) Article DOI: 10.1016/j.bmc.2016.11.027 BindingDB Entry DOI: 10.7270/Q2G73GQQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 1 (Homo sapiens (Human)) | BDBM50209283 (CHEMBL3885230) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 7.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Pharmaceutical Education and Research (NIPER) Curated by ChEMBL | Assay Description Inhibition of human recombinant carbonic anhydrase-1 using CO2 as substrate preincubated for 10 mins by stopped-flow CO2 hydrase assay | Bioorg Med Chem 25: 539-544 (2017) Article DOI: 10.1016/j.bmc.2016.11.027 BindingDB Entry DOI: 10.7270/Q2G73GQQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 7 (Homo sapiens (Human)) | BDBM50209285 (CHEMBL3884696) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 7.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Pharmaceutical Education and Research (NIPER) Curated by ChEMBL | Assay Description Inhibition of human recombinant carbonic anhydrase-7 using CO2 as substrate preincubated for 10 mins by stopped-flow CO2 hydrase assay | Bioorg Med Chem 25: 539-544 (2017) Article DOI: 10.1016/j.bmc.2016.11.027 BindingDB Entry DOI: 10.7270/Q2G73GQQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 2 (Homo sapiens (Human)) | BDBM50209284 (CHEMBL3884126) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 7.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Pharmaceutical Education and Research (NIPER) Curated by ChEMBL | Assay Description Inhibition of human recombinant carbonic anhydrase-2 using CO2 as substrate preincubated for 10 mins by stopped-flow CO2 hydrase assay | Bioorg Med Chem 25: 539-544 (2017) Article DOI: 10.1016/j.bmc.2016.11.027 BindingDB Entry DOI: 10.7270/Q2G73GQQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 1 (Homo sapiens (Human)) | BDBM50209286 (CHEMBL3884261) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 8.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Pharmaceutical Education and Research (NIPER) Curated by ChEMBL | Assay Description Inhibition of human recombinant carbonic anhydrase-1 using CO2 as substrate preincubated for 10 mins by stopped-flow CO2 hydrase assay | Bioorg Med Chem 25: 539-544 (2017) Article DOI: 10.1016/j.bmc.2016.11.027 BindingDB Entry DOI: 10.7270/Q2G73GQQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 2 (Homo sapiens (Human)) | BDBM50209286 (CHEMBL3884261) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 8.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Pharmaceutical Education and Research (NIPER) Curated by ChEMBL | Assay Description Inhibition of human recombinant carbonic anhydrase-2 using CO2 as substrate preincubated for 10 mins by stopped-flow CO2 hydrase assay | Bioorg Med Chem 25: 539-544 (2017) Article DOI: 10.1016/j.bmc.2016.11.027 BindingDB Entry DOI: 10.7270/Q2G73GQQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 7 (Homo sapiens (Human)) | BDBM50209290 (CHEMBL3883755) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 9.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Pharmaceutical Education and Research (NIPER) Curated by ChEMBL | Assay Description Inhibition of human recombinant carbonic anhydrase-7 using CO2 as substrate preincubated for 10 mins by stopped-flow CO2 hydrase assay | Bioorg Med Chem 25: 539-544 (2017) Article DOI: 10.1016/j.bmc.2016.11.027 BindingDB Entry DOI: 10.7270/Q2G73GQQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 7 (Homo sapiens (Human)) | BDBM50209292 (CHEMBL3884793) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 9.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Pharmaceutical Education and Research (NIPER) Curated by ChEMBL | Assay Description Inhibition of human recombinant carbonic anhydrase-7 using CO2 as substrate preincubated for 10 mins by stopped-flow CO2 hydrase assay | Bioorg Med Chem 25: 539-544 (2017) Article DOI: 10.1016/j.bmc.2016.11.027 BindingDB Entry DOI: 10.7270/Q2G73GQQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 2 (Homo sapiens (Human)) | BDBM10880 (AZA | AZA2 | AZM acetazolamide | Acerazolamide, AA...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | DrugBank MMDB PDB Article PubMed | 12 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Pharmaceutical Education and Research (NIPER) Curated by ChEMBL | Assay Description Inhibition of recombinant human carbonic anhydrase2 preincubated for 10 mins followed by CO2 solution addition by phenol red dye based stopped flow C... | Bioorg Med Chem 27: (2019) Article DOI: 10.1016/j.bmc.2019.115090 BindingDB Entry DOI: 10.7270/Q2RV0S2G | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Carbonic anhydrase 2 (Homo sapiens (Human)) | BDBM10880 (AZA | AZA2 | AZM acetazolamide | Acerazolamide, AA...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | DrugBank MMDB PDB Article PubMed | 12 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Pharmaceutical Education and Research (NIPER) Curated by ChEMBL | Assay Description Inhibition of human recombinant carbonic anhydrase-2 using CO2 as substrate preincubated for 10 mins by stopped-flow CO2 hydrase assay | Bioorg Med Chem 25: 539-544 (2017) Article DOI: 10.1016/j.bmc.2016.11.027 BindingDB Entry DOI: 10.7270/Q2G73GQQ | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Carbonic anhydrase 2 (Homo sapiens (Human)) | BDBM10880 (AZA | AZA2 | AZM acetazolamide | Acerazolamide, AA...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | DrugBank MMDB PDB Article PubMed | 12 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of recombinant human carbonic anhydrase 2 pre-incubated for 10 mins by stopped flow CO2 hydrase assay | Citation and Details Article DOI: 10.1016/j.bmcl.2021.127877 BindingDB Entry DOI: 10.7270/Q29Z98MV | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| 5-hydroxytryptamine receptor 1A (Homo sapiens (Human)) | BDBM50243699 (2-(trifluoromethyl)-7-(2-(4-(2-(trifluoromethyl)-1...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 19 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research Curated by ChEMBL | Assay Description Binding affinity to human 5HT1A receptor | Bioorg Med Chem 16: 6617-40 (2008) Article DOI: 10.1016/j.bmc.2008.05.024 BindingDB Entry DOI: 10.7270/Q2FB52Q7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 2 (Homo sapiens (Human)) | BDBM50209290 (CHEMBL3883755) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Pharmaceutical Education and Research (NIPER) Curated by ChEMBL | Assay Description Inhibition of human recombinant carbonic anhydrase-2 using CO2 as substrate preincubated for 10 mins by stopped-flow CO2 hydrase assay | Bioorg Med Chem 25: 539-544 (2017) Article DOI: 10.1016/j.bmc.2016.11.027 BindingDB Entry DOI: 10.7270/Q2G73GQQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 1 (Homo sapiens (Human)) | BDBM50209289 (CHEMBL3885091) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 22 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Pharmaceutical Education and Research (NIPER) Curated by ChEMBL | Assay Description Inhibition of human recombinant carbonic anhydrase-1 using CO2 as substrate preincubated for 10 mins by stopped-flow CO2 hydrase assay | Bioorg Med Chem 25: 539-544 (2017) Article DOI: 10.1016/j.bmc.2016.11.027 BindingDB Entry DOI: 10.7270/Q2G73GQQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 1 (Homo sapiens (Human)) | BDBM50209287 (CHEMBL3884217) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 24 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Pharmaceutical Education and Research (NIPER) Curated by ChEMBL | Assay Description Inhibition of human recombinant carbonic anhydrase-1 using CO2 as substrate preincubated for 10 mins by stopped-flow CO2 hydrase assay | Bioorg Med Chem 25: 539-544 (2017) Article DOI: 10.1016/j.bmc.2016.11.027 BindingDB Entry DOI: 10.7270/Q2G73GQQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 9 (Homo sapiens (Human)) | BDBM10880 (AZA | AZA2 | AZM acetazolamide | Acerazolamide, AA...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 25 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of recombinant human carbonic anhydrase 9 preincubated for 10 mins by stopped flow CO2 hydrase assay | Citation and Details Article DOI: 10.1016/j.bmcl.2021.127877 BindingDB Entry DOI: 10.7270/Q29Z98MV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 529 total ) | Next | Last >> |