 Found 150 hits with Last Name = 'baroni' and Initial = 'm'
Found 150 hits with Last Name = 'baroni' and Initial = 'm' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Growth hormone secretagogue receptor type 1
(Homo sapiens (Human)) | BDBM50395751

(CHEMBL2164507)Show SMILES CCN1CCC(CC(=O)NC2(C(=O)Nc3cc(Cl)cc(Cl)c23)c2ccc(Cl)c(Cl)c2)CC1 Show InChI InChI=1S/C23H23Cl4N3O2/c1-2-30-7-5-13(6-8-30)9-20(31)29-23(14-3-4-16(25)17(26)10-14)21-18(27)11-15(24)12-19(21)28-22(23)32/h3-4,10-13H,2,5-9H2,1H3,(H,28,32)(H,29,31) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi Research
Curated by ChEMBL
| Assay Description
Displacement of [125I]-ghrelin from human GHSR1 expressed in CHO-CREluc cells after 1 hr by scintillation counting |
Bioorg Med Chem 20: 5623-36 (2012)
Article DOI: 10.1016/j.bmc.2012.07.018
BindingDB Entry DOI: 10.7270/Q2HD7WS4 |
More data for this
Ligand-Target Pair | |
Growth hormone secretagogue receptor type 1
(Homo sapiens (Human)) | BDBM50395753

(CHEMBL2164499)Show SMILES CCN1CCN(CC(=O)NC2(C(=O)Nc3cc(Cl)cc(Cl)c23)c2ccc(Cl)c(Cl)c2)CC1 Show InChI InChI=1S/C22H22Cl4N4O2/c1-2-29-5-7-30(8-6-29)12-19(31)28-22(13-3-4-15(24)16(25)9-13)20-17(26)10-14(23)11-18(20)27-21(22)32/h3-4,9-11H,2,5-8,12H2,1H3,(H,27,32)(H,28,31) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 8 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi Research
Curated by ChEMBL
| Assay Description
Displacement of [125I]-ghrelin from human GHSR1 expressed in CHO-CREluc cells after 1 hr by scintillation counting |
Bioorg Med Chem 20: 5623-36 (2012)
Article DOI: 10.1016/j.bmc.2012.07.018
BindingDB Entry DOI: 10.7270/Q2HD7WS4 |
More data for this
Ligand-Target Pair | |
Growth hormone secretagogue receptor type 1
(Homo sapiens (Human)) | BDBM50395744
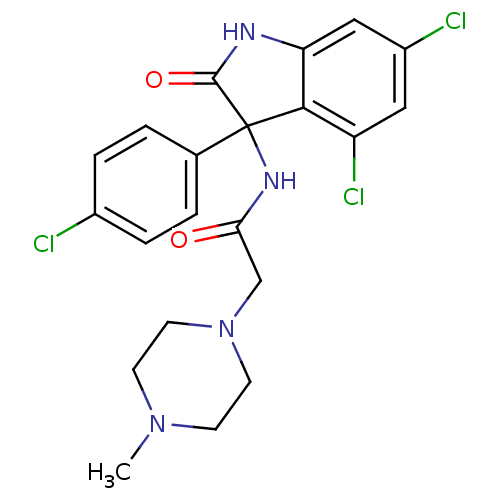
(CHEMBL2164831)Show SMILES CN1CCN(CC(=O)NC2(C(=O)Nc3cc(Cl)cc(Cl)c23)c2ccc(Cl)cc2)CC1 Show InChI InChI=1S/C21H21Cl3N4O2/c1-27-6-8-28(9-7-27)12-18(29)26-21(13-2-4-14(22)5-3-13)19-16(24)10-15(23)11-17(19)25-20(21)30/h2-5,10-11H,6-9,12H2,1H3,(H,25,30)(H,26,29) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 44 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi Research
Curated by ChEMBL
| Assay Description
Displacement of [125I]-ghrelin from human GHSR1 expressed in CHO-CREluc cells after 1 hr by scintillation counting |
Bioorg Med Chem 20: 5623-36 (2012)
Article DOI: 10.1016/j.bmc.2012.07.018
BindingDB Entry DOI: 10.7270/Q2HD7WS4 |
More data for this
Ligand-Target Pair | |
Growth hormone secretagogue receptor type 1
(Homo sapiens (Human)) | BDBM50395752
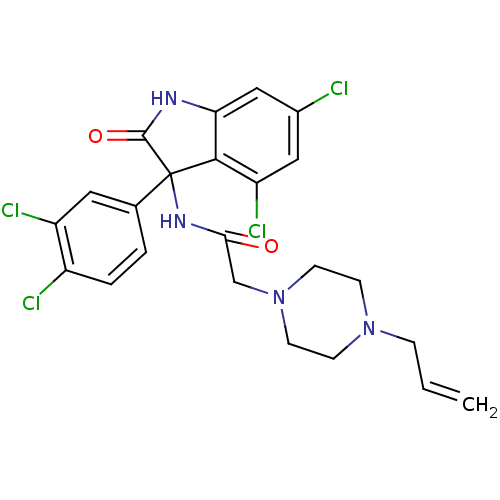
(CHEMBL2164500)Show SMILES Clc1cc2NC(=O)C(NC(=O)CN3CCN(CC=C)CC3)(c2c(Cl)c1)c1ccc(Cl)c(Cl)c1 Show InChI InChI=1S/C23H22Cl4N4O2/c1-2-5-30-6-8-31(9-7-30)13-20(32)29-23(14-3-4-16(25)17(26)10-14)21-18(27)11-15(24)12-19(21)28-22(23)33/h2-4,10-12H,1,5-9,13H2,(H,28,33)(H,29,32) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 121 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi Research
Curated by ChEMBL
| Assay Description
Displacement of [125I]-ghrelin from human GHSR1 expressed in CHO-CREluc cells after 1 hr by scintillation counting |
Bioorg Med Chem 20: 5623-36 (2012)
Article DOI: 10.1016/j.bmc.2012.07.018
BindingDB Entry DOI: 10.7270/Q2HD7WS4 |
More data for this
Ligand-Target Pair | |
Growth hormone secretagogue receptor type 1
(Homo sapiens (Human)) | BDBM50395758
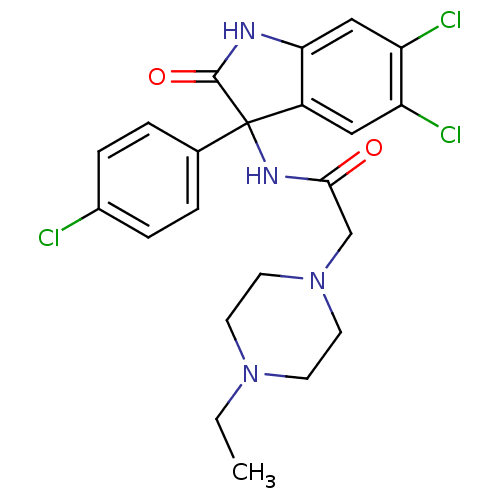
(CHEMBL2164823)Show SMILES CCN1CCN(CC(=O)NC2(C(=O)Nc3cc(Cl)c(Cl)cc23)c2ccc(Cl)cc2)CC1 Show InChI InChI=1S/C22H23Cl3N4O2/c1-2-28-7-9-29(10-8-28)13-20(30)27-22(14-3-5-15(23)6-4-14)16-11-17(24)18(25)12-19(16)26-21(22)31/h3-6,11-12H,2,7-10,13H2,1H3,(H,26,31)(H,27,30) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 202 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi Research
Curated by ChEMBL
| Assay Description
Displacement of [125I]-ghrelin from human GHSR1 expressed in CHO-CREluc cells after 1 hr by scintillation counting |
Bioorg Med Chem 20: 5623-36 (2012)
Article DOI: 10.1016/j.bmc.2012.07.018
BindingDB Entry DOI: 10.7270/Q2HD7WS4 |
More data for this
Ligand-Target Pair | |
Growth hormone secretagogue receptor type 1
(Homo sapiens (Human)) | BDBM50395749

(CHEMBL2164826)Show SMILES CN1CCN(CC(=O)N[C@@]2(C(=O)Nc3cc(Cl)c(Cl)cc23)c2ccc(Cl)cc2)CC1 |r| Show InChI InChI=1S/C21H21Cl3N4O2/c1-27-6-8-28(9-7-27)12-19(29)26-21(13-2-4-14(22)5-3-13)15-10-16(23)17(24)11-18(15)25-20(21)30/h2-5,10-11H,6-9,12H2,1H3,(H,25,30)(H,26,29)/t21-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 249 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi Research
Curated by ChEMBL
| Assay Description
Displacement of [125I]-ghrelin from human GHSR1 expressed in CHO-CREluc cells after 1 hr by scintillation counting |
Bioorg Med Chem 20: 5623-36 (2012)
Article DOI: 10.1016/j.bmc.2012.07.018
BindingDB Entry DOI: 10.7270/Q2HD7WS4 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C9
(Homo sapiens (Human)) | BDBM50367298
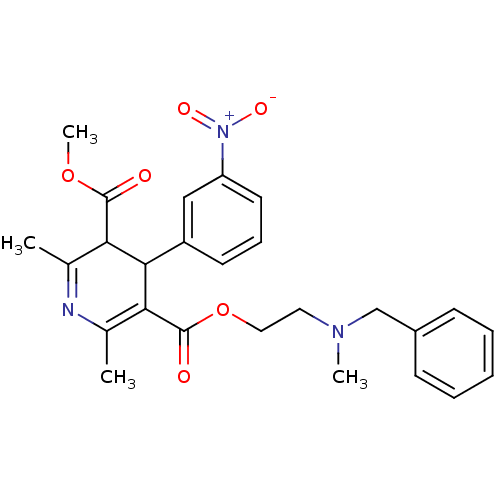
(Cardene | NICARDIPINE)Show SMILES COC(=O)C1C(C(C(=O)OCCN(C)Cc2ccccc2)=C(C)N=C1C)c1cccc(c1)[N+]([O-])=O |c:24,t:21| Show InChI InChI=1S/C26H29N3O6/c1-17-22(25(30)34-4)24(20-11-8-12-21(15-20)29(32)33)23(18(2)27-17)26(31)35-14-13-28(3)16-19-9-6-5-7-10-19/h5-12,15,22,24H,13-14,16H2,1-4H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 280 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca R&D
Curated by ChEMBL
| Assay Description
Inhibition of human cytochrome P450 2C9 |
J Med Chem 47: 907-14 (2004)
Article DOI: 10.1021/jm030972s
BindingDB Entry DOI: 10.7270/Q2ZK5HF3 |
More data for this
Ligand-Target Pair | |
Growth hormone secretagogue receptor type 1
(Homo sapiens (Human)) | BDBM50395750
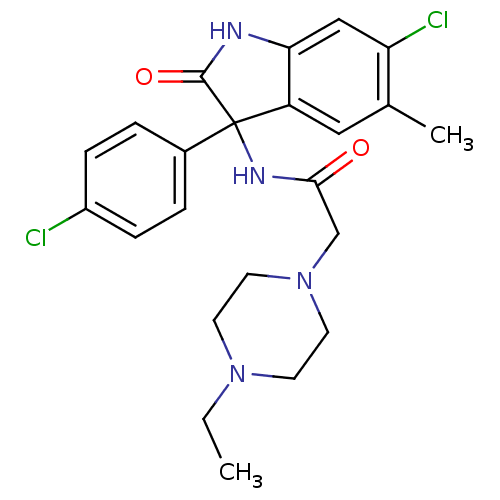
(CHEMBL2164508)Show SMILES CCN1CCN(CC(=O)NC2(C(=O)Nc3cc(Cl)c(C)cc23)c2ccc(Cl)cc2)CC1 Show InChI InChI=1S/C23H26Cl2N4O2/c1-3-28-8-10-29(11-9-28)14-21(30)27-23(16-4-6-17(24)7-5-16)18-12-15(2)19(25)13-20(18)26-22(23)31/h4-7,12-13H,3,8-11,14H2,1-2H3,(H,26,31)(H,27,30) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 286 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi Research
Curated by ChEMBL
| Assay Description
Displacement of [125I]-ghrelin from human GHSR1 expressed in CHO-CREluc cells after 1 hr by scintillation counting |
Bioorg Med Chem 20: 5623-36 (2012)
Article DOI: 10.1016/j.bmc.2012.07.018
BindingDB Entry DOI: 10.7270/Q2HD7WS4 |
More data for this
Ligand-Target Pair | |
Growth hormone secretagogue receptor type 1
(Homo sapiens (Human)) | BDBM50395755
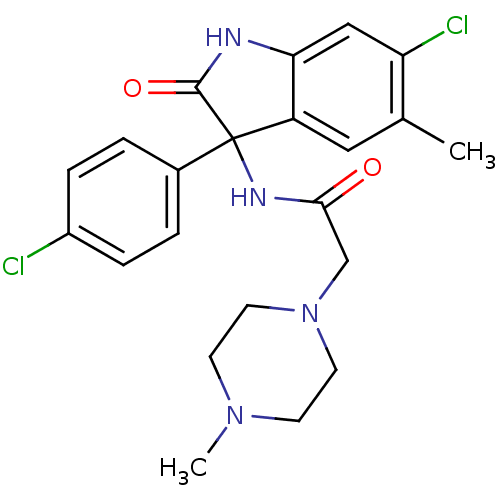
(CHEMBL2164494)Show SMILES CN1CCN(CC(=O)NC2(C(=O)Nc3cc(Cl)c(C)cc23)c2ccc(Cl)cc2)CC1 Show InChI InChI=1S/C22H24Cl2N4O2/c1-14-11-17-19(12-18(14)24)25-21(30)22(17,15-3-5-16(23)6-4-15)26-20(29)13-28-9-7-27(2)8-10-28/h3-6,11-12H,7-10,13H2,1-2H3,(H,25,30)(H,26,29) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 392 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi Research
Curated by ChEMBL
| Assay Description
Displacement of [125I]-ghrelin from human GHSR1 expressed in CHO-CREluc cells after 1 hr by scintillation counting |
Bioorg Med Chem 20: 5623-36 (2012)
Article DOI: 10.1016/j.bmc.2012.07.018
BindingDB Entry DOI: 10.7270/Q2HD7WS4 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C9
(Homo sapiens (Human)) | BDBM50090677

(4-Amino-N-(2-phenyl-2H-pyrazol-3-yl)-benzenesulfon...)Show InChI InChI=1S/C15H14N4O2S/c16-12-6-8-14(9-7-12)22(20,21)18-15-10-11-17-19(15)13-4-2-1-3-5-13/h1-11,18H,16H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
PC cid
PC sid
UniChem
Patents
Similars
| DrugBank
Article
PubMed
| 500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca R&D
Curated by ChEMBL
| Assay Description
Binding affinity towards cytochrome P450 2C9 |
J Med Chem 47: 907-14 (2004)
Article DOI: 10.1021/jm030972s
BindingDB Entry DOI: 10.7270/Q2ZK5HF3 |
More data for this
Ligand-Target Pair | |
Growth hormone secretagogue receptor type 1
(Homo sapiens (Human)) | BDBM50395754
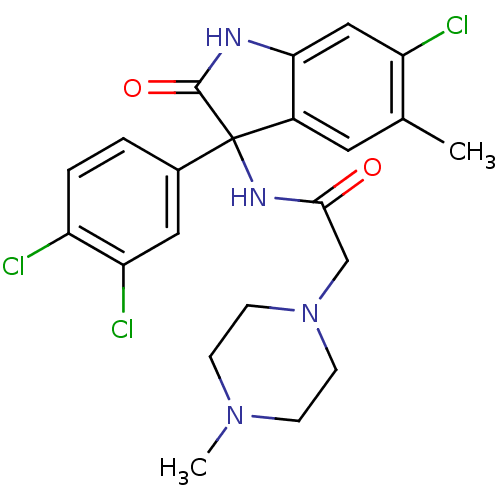
(CHEMBL2164497)Show SMILES CN1CCN(CC(=O)NC2(C(=O)Nc3cc(Cl)c(C)cc23)c2ccc(Cl)c(Cl)c2)CC1 Show InChI InChI=1S/C22H23Cl3N4O2/c1-13-9-15-19(11-17(13)24)26-21(31)22(15,14-3-4-16(23)18(25)10-14)27-20(30)12-29-7-5-28(2)6-8-29/h3-4,9-11H,5-8,12H2,1-2H3,(H,26,31)(H,27,30) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi Research
Curated by ChEMBL
| Assay Description
Displacement of [125I]-ghrelin from human GHSR1 expressed in CHO-CREluc cells after 1 hr by scintillation counting |
Bioorg Med Chem 20: 5623-36 (2012)
Article DOI: 10.1016/j.bmc.2012.07.018
BindingDB Entry DOI: 10.7270/Q2HD7WS4 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C9
(Homo sapiens (Human)) | BDBM35525
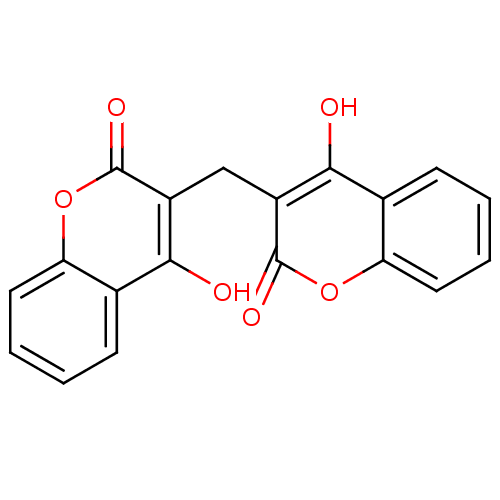
(3,3''''-methylenebis(4-hydroxy-coumarin | 3,3''''-...)Show InChI InChI=1S/C19H12O6/c20-16-10-5-1-3-7-14(10)24-18(22)12(16)9-13-17(21)11-6-2-4-8-15(11)25-19(13)23/h1-8,20-21H,9H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
UniChem
Similars
| DrugBank
Article
PubMed
| 1.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca R&D
Curated by ChEMBL
| Assay Description
Binding affinity towards cytochrome P450 2C9 |
J Med Chem 47: 907-14 (2004)
Article DOI: 10.1021/jm030972s
BindingDB Entry DOI: 10.7270/Q2ZK5HF3 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C9
(Homo sapiens (Human)) | BDBM50139812
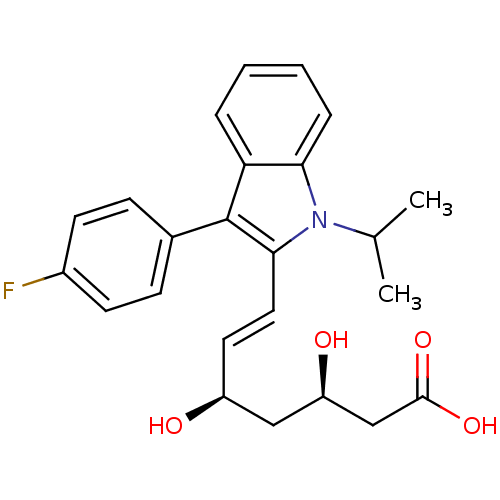
((3R,5R)-fluvastatin | (3R,5R,6E)-7-[3-(4-fluorophe...)Show SMILES CC(C)n1c(\C=C\[C@H](O)C[C@@H](O)CC(O)=O)c(-c2ccc(F)cc2)c2ccccc12 Show InChI InChI=1S/C24H26FNO4/c1-15(2)26-21-6-4-3-5-20(21)24(16-7-9-17(25)10-8-16)22(26)12-11-18(27)13-19(28)14-23(29)30/h3-12,15,18-19,27-28H,13-14H2,1-2H3,(H,29,30)/b12-11+/t18-,19+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca R&D
Curated by ChEMBL
| Assay Description
Binding affinity towards cytochrome P450 2C9 |
J Med Chem 47: 907-14 (2004)
Article DOI: 10.1021/jm030972s
BindingDB Entry DOI: 10.7270/Q2ZK5HF3 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C9
(Homo sapiens (Human)) | BDBM50009073
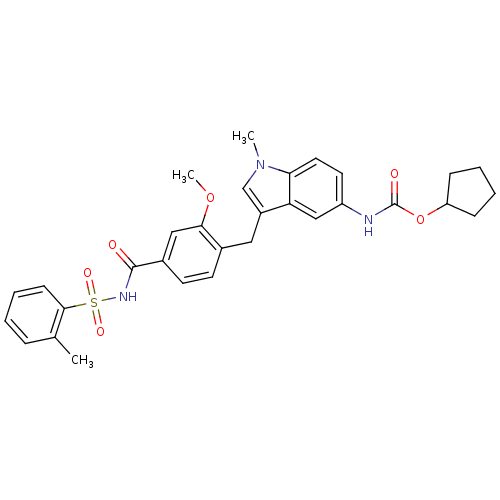
(4-(5-cyclopentyloxycarbonylamino-1-methyl-1H-indol...)Show SMILES COc1cc(ccc1Cc1cn(C)c2ccc(NC(=O)OC3CCCC3)cc12)C(=O)NS(=O)(=O)c1ccccc1C Show InChI InChI=1S/C31H33N3O6S/c1-20-8-4-7-11-29(20)41(37,38)33-30(35)22-13-12-21(28(17-22)39-3)16-23-19-34(2)27-15-14-24(18-26(23)27)32-31(36)40-25-9-5-6-10-25/h4,7-8,11-15,17-19,25H,5-6,9-10,16H2,1-3H3,(H,32,36)(H,33,35) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
Article
PubMed
| 2.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca R&D
Curated by ChEMBL
| Assay Description
Binding affinity towards cytochrome P450 2C9 |
J Med Chem 47: 907-14 (2004)
Article DOI: 10.1021/jm030972s
BindingDB Entry DOI: 10.7270/Q2ZK5HF3 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C9
(Homo sapiens (Human)) | BDBM50139803
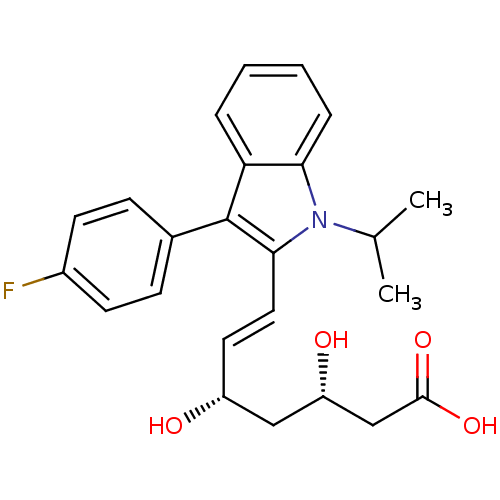
((3S,5S)-fluvastatin | (3S,5S,6E)-7-[3-(4-fluorophe...)Show SMILES CC(C)n1c(\C=C\[C@@H](O)C[C@H](O)CC(O)=O)c(-c2ccc(F)cc2)c2ccccc12 Show InChI InChI=1S/C24H26FNO4/c1-15(2)26-21-6-4-3-5-20(21)24(16-7-9-17(25)10-8-16)22(26)12-11-18(27)13-19(28)14-23(29)30/h3-12,15,18-19,27-28H,13-14H2,1-2H3,(H,29,30)/b12-11+/t18-,19+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
KEGG
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| 3.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca R&D
Curated by ChEMBL
| Assay Description
Binding affinity towards cytochrome P450 2C9 |
J Med Chem 47: 907-14 (2004)
Article DOI: 10.1021/jm030972s
BindingDB Entry DOI: 10.7270/Q2ZK5HF3 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C9
(Homo sapiens (Human)) | BDBM8903

((1S,2R,10S,11S,14S,15S)-14-acetyl-2,15-dimethyltet...)Show SMILES [H][C@@]12CC[C@H](C(C)=O)[C@@]1(C)CC[C@@]1([H])[C@@]2([H])CCC2=CC(=O)CC[C@]12C |r,t:20| Show InChI InChI=1S/C21H30O2/c1-13(22)17-6-7-18-16-5-4-14-12-15(23)8-10-20(14,2)19(16)9-11-21(17,18)3/h12,16-19H,4-11H2,1-3H3/t16-,17+,18-,19-,20-,21+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| DrugBank
Article
PubMed
| 5.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca R&D
Curated by ChEMBL
| Assay Description
Binding affinity towards cytochrome P450 2C9 |
J Med Chem 47: 907-14 (2004)
Article DOI: 10.1021/jm030972s
BindingDB Entry DOI: 10.7270/Q2ZK5HF3 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C9
(Homo sapiens (Human)) | BDBM50139808
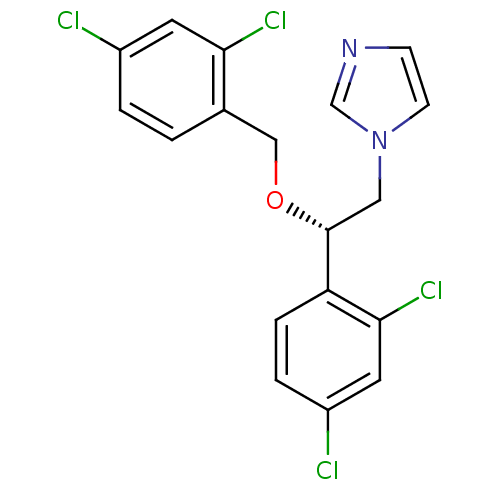
(1-[(S)-2-(2,4-Dichloro-benzyloxy)-2-(2,4-dichloro-...)Show SMILES Clc1ccc(CO[C@H](Cn2ccnc2)c2ccc(Cl)cc2Cl)c(Cl)c1 Show InChI InChI=1S/C18H14Cl4N2O/c19-13-2-1-12(16(21)7-13)10-25-18(9-24-6-5-23-11-24)15-4-3-14(20)8-17(15)22/h1-8,11,18H,9-10H2/t18-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 6.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca R&D
Curated by ChEMBL
| Assay Description
Binding affinity towards cytochrome P450 2C9 |
J Med Chem 47: 907-14 (2004)
Article DOI: 10.1021/jm030972s
BindingDB Entry DOI: 10.7270/Q2ZK5HF3 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C9
(Homo sapiens (Human)) | BDBM50003655
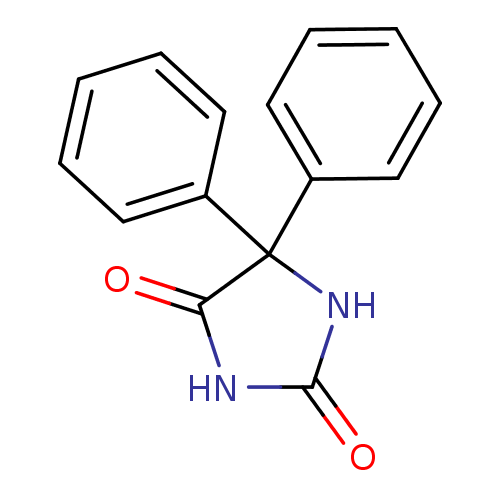
(Phenytoin)Show InChI InChI=1S/C15H12N2O2/c18-13-15(17-14(19)16-13,11-7-3-1-4-8-11)12-9-5-2-6-10-12/h1-10H,(H2,16,17,18,19) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
PC cid
PC sid
UniChem
Patents
Similars
| DrugBank
Article
PubMed
| 6.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca R&D
Curated by ChEMBL
| Assay Description
Binding affinity towards cytochrome P450 2C9 |
J Med Chem 47: 907-14 (2004)
Article DOI: 10.1021/jm030972s
BindingDB Entry DOI: 10.7270/Q2ZK5HF3 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C9
(Homo sapiens (Human)) | BDBM50139811
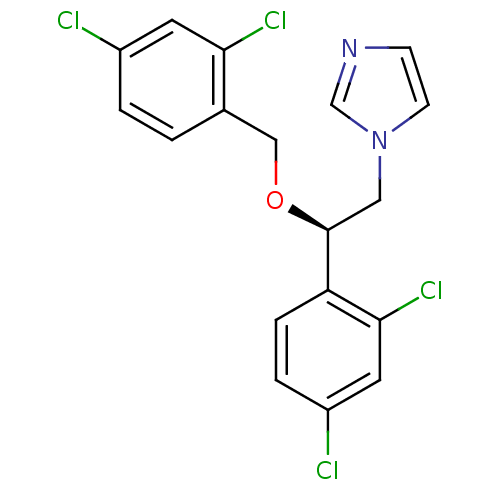
(1-[(R)-2-(2,4-Dichloro-benzyloxy)-2-(2,4-dichloro-...)Show SMILES Clc1ccc(CO[C@@H](Cn2ccnc2)c2ccc(Cl)cc2Cl)c(Cl)c1 Show InChI InChI=1S/C18H14Cl4N2O/c19-13-2-1-12(16(21)7-13)10-25-18(9-24-6-5-23-11-24)15-4-3-14(20)8-17(15)22/h1-8,11,18H,9-10H2/t18-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 6.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca R&D
Curated by ChEMBL
| Assay Description
Binding affinity towards cytochrome P450 2C9 |
J Med Chem 47: 907-14 (2004)
Article DOI: 10.1021/jm030972s
BindingDB Entry DOI: 10.7270/Q2ZK5HF3 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C9
(Homo sapiens (Human)) | BDBM7462

(3,5,7-trihydroxy-2-(4-hydroxyphenyl)-4H-chromen-4-...)Show InChI InChI=1S/C15H10O6/c16-8-3-1-7(2-4-8)15-14(20)13(19)12-10(18)5-9(17)6-11(12)21-15/h1-6,16-18,20H | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 6.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca R&D
Curated by ChEMBL
| Assay Description
Binding affinity towards cytochrome P450 2C9 |
J Med Chem 47: 907-14 (2004)
Article DOI: 10.1021/jm030972s
BindingDB Entry DOI: 10.7270/Q2ZK5HF3 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C9
(Homo sapiens (Human)) | BDBM50028091
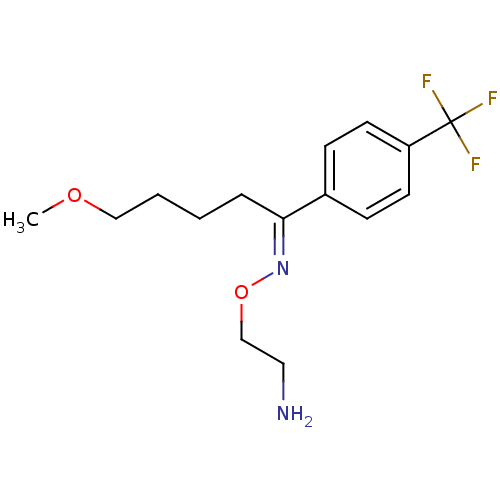
((1E)-5-methoxy-1-[4-(trifluoromethyl)phenyl]pentan...)Show InChI InChI=1S/C15H21F3N2O2/c1-21-10-3-2-4-14(20-22-11-9-19)12-5-7-13(8-6-12)15(16,17)18/h5-8H,2-4,9-11,19H2,1H3/b20-14+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 8.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca R&D
Curated by ChEMBL
| Assay Description
Binding affinity towards cytochrome P450 2C9 |
J Med Chem 47: 907-14 (2004)
Article DOI: 10.1021/jm030972s
BindingDB Entry DOI: 10.7270/Q2ZK5HF3 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C9
(Homo sapiens (Human)) | BDBM50139801
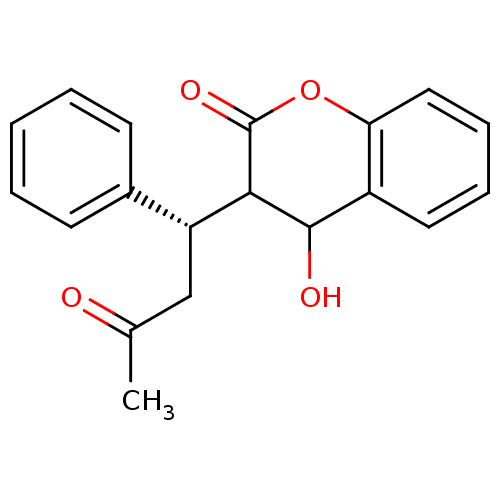
(4-Hydroxy-3-((S)-3-oxo-1-phenyl-butyl)-chroman-2-o...)Show InChI InChI=1S/C19H18O4/c1-12(20)11-15(13-7-3-2-4-8-13)17-18(21)14-9-5-6-10-16(14)23-19(17)22/h2-10,15,17-18,21H,11H2,1H3/t15-,17?,18?/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.36E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca R&D
Curated by ChEMBL
| Assay Description
Binding affinity towards cytochrome P450 2C9 |
J Med Chem 47: 907-14 (2004)
Article DOI: 10.1021/jm030972s
BindingDB Entry DOI: 10.7270/Q2ZK5HF3 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C9
(Homo sapiens (Human)) | BDBM50139802
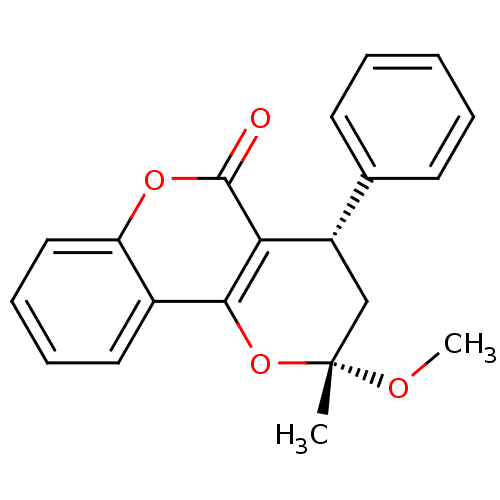
((2R,4R)-2-Methoxy-2-methyl-4-phenyl-3,4,4a,10b-tet...)Show SMILES CO[C@@]1(C)C[C@@H](c2ccccc2)c2c(O1)c1ccccc1oc2=O Show InChI InChI=1S/C20H18O4/c1-20(22-2)12-15(13-8-4-3-5-9-13)17-18(24-20)14-10-6-7-11-16(14)23-19(17)21/h3-11,15H,12H2,1-2H3/t15-,20+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.60E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca R&D
Curated by ChEMBL
| Assay Description
Binding affinity towards cytochrome P450 2C9 |
J Med Chem 47: 907-14 (2004)
Article DOI: 10.1021/jm030972s
BindingDB Entry DOI: 10.7270/Q2ZK5HF3 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C9
(Homo sapiens (Human)) | BDBM50139807
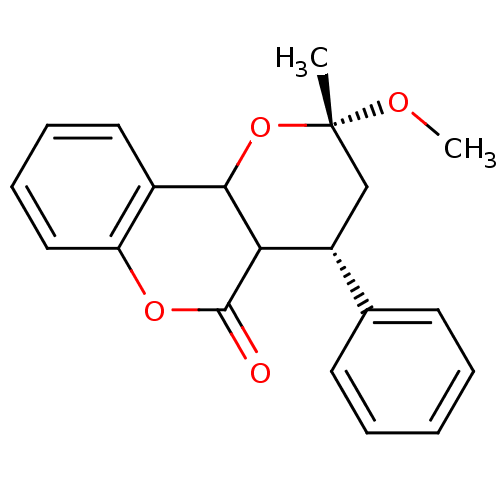
((2S,4S)-2-Methoxy-2-methyl-4-phenyl-3,4,4a,10b-tet...)Show SMILES CO[C@]1(C)C[C@@H](C2C(O1)c1ccccc1OC2=O)c1ccccc1 Show InChI InChI=1S/C20H20O4/c1-20(22-2)12-15(13-8-4-3-5-9-13)17-18(24-20)14-10-6-7-11-16(14)23-19(17)21/h3-11,15,17-18H,12H2,1-2H3/t15-,17?,18?,20+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 1.60E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca R&D
Curated by ChEMBL
| Assay Description
Binding affinity towards cytochrome P450 2C9 |
J Med Chem 47: 907-14 (2004)
Article DOI: 10.1021/jm030972s
BindingDB Entry DOI: 10.7270/Q2ZK5HF3 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C9
(Homo sapiens (Human)) | BDBM50139799

(2-(2,5-Dichloro-thiophene-3-sulfonylamino)-N-(1,3,...)Show SMILES Cc1nn(C)c(C)c1NC(=O)c1ccccc1NS(=O)(=O)c1cc(Cl)sc1Cl Show InChI InChI=1S/C17H16Cl2N4O3S2/c1-9-15(10(2)23(3)21-9)20-17(24)11-6-4-5-7-12(11)22-28(25,26)13-8-14(18)27-16(13)19/h4-8,22H,1-3H3,(H,20,24) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 1.70E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca R&D
Curated by ChEMBL
| Assay Description
Binding affinity towards cytochrome P450 2C9 |
J Med Chem 47: 907-14 (2004)
Article DOI: 10.1021/jm030972s
BindingDB Entry DOI: 10.7270/Q2ZK5HF3 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C9
(Homo sapiens (Human)) | BDBM50022309
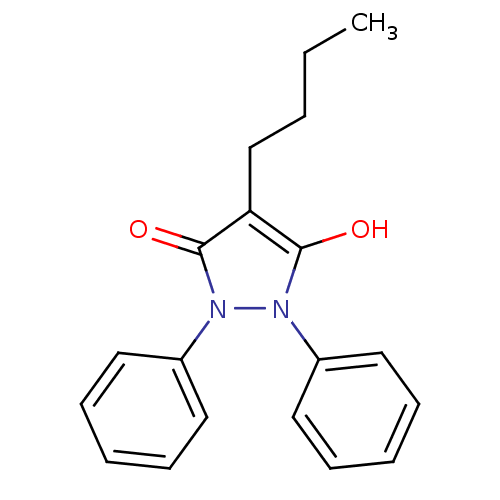
(3,5-Dioxo-1,2-diphenyl-4-n-butylpyrazolidine | 4-b...)Show InChI InChI=1S/C19H20N2O2/c1-2-3-14-17-18(22)20(15-10-6-4-7-11-15)21(19(17)23)16-12-8-5-9-13-16/h4-13,22H,2-3,14H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 1.90E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca R&D
Curated by ChEMBL
| Assay Description
Binding affinity towards cytochrome P450 2C9 |
J Med Chem 47: 907-14 (2004)
Article DOI: 10.1021/jm030972s
BindingDB Entry DOI: 10.7270/Q2ZK5HF3 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C9
(Homo sapiens (Human)) | BDBM50139809
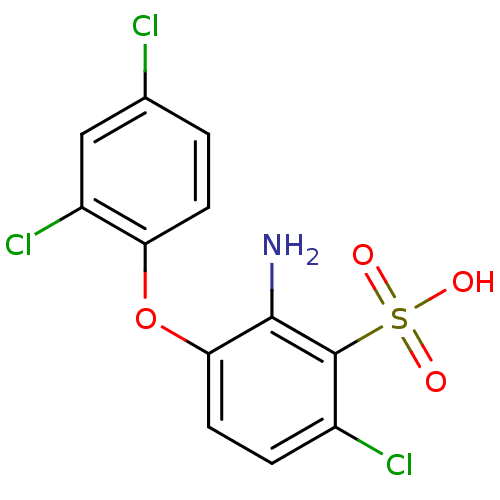
(2-Amino-6-chloro-3-(2,4-dichloro-phenoxy)-benzenes...)Show InChI InChI=1S/C12H8Cl3NO4S/c13-6-1-3-9(8(15)5-6)20-10-4-2-7(14)12(11(10)16)21(17,18)19/h1-5H,16H2,(H,17,18,19) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 2.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca R&D
Curated by ChEMBL
| Assay Description
Binding affinity towards cytochrome P450 2C9 |
J Med Chem 47: 907-14 (2004)
Article DOI: 10.1021/jm030972s
BindingDB Entry DOI: 10.7270/Q2ZK5HF3 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C9
(Homo sapiens (Human)) | BDBM50343352
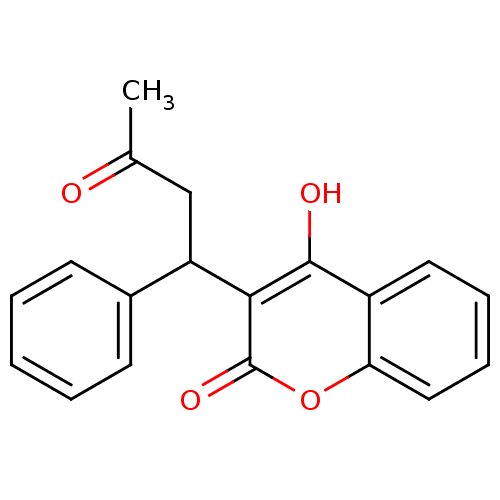
(2-hydroxy-3-(3-oxo-1-phenylbutyl)-4H-chromen-4-one...)Show InChI InChI=1S/C19H16O4/c1-12(20)11-15(13-7-3-2-4-8-13)17-18(21)14-9-5-6-10-16(14)23-19(17)22/h2-10,15,21H,11H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
KEGG
PC cid
PC sid
PDB
UniChem
Similars
| DrugBank
Article
PubMed
| 2.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca R&D
Curated by ChEMBL
| Assay Description
Binding affinity towards cytochrome P450 2C9 |
J Med Chem 47: 907-14 (2004)
Article DOI: 10.1021/jm030972s
BindingDB Entry DOI: 10.7270/Q2ZK5HF3 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C9
(Homo sapiens (Human)) | BDBM7460

(2-(3,4-dihydroxyphenyl)-3,5,7-trihydroxy-4H-chrome...)Show InChI InChI=1S/C15H10O7/c16-7-4-10(19)12-11(5-7)22-15(14(21)13(12)20)6-1-2-8(17)9(18)3-6/h1-5,16-19,21H | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 2.70E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca R&D
Curated by ChEMBL
| Assay Description
Binding affinity towards cytochrome P450 2C9 |
J Med Chem 47: 907-14 (2004)
Article DOI: 10.1021/jm030972s
BindingDB Entry DOI: 10.7270/Q2ZK5HF3 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C9
(Homo sapiens (Human)) | BDBM50017681
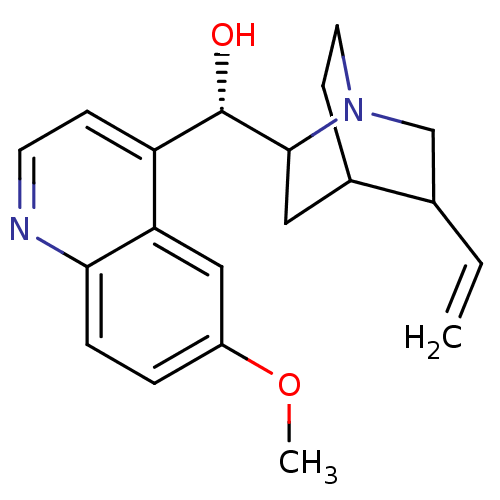
((1S,2R,4S,5R)-2-[(S)-Hydroxy-(6-methoxy-quinolin-4...)Show SMILES COc1ccc2nccc([C@H](O)C3CC4CCN3CC4C=C)c2c1 |TLB:10:12:18.19:16.15,20:19:12.13:16.15| Show InChI InChI=1S/C20H24N2O2/c1-3-13-12-22-9-7-14(13)10-19(22)20(23)16-6-8-21-18-5-4-15(24-2)11-17(16)18/h3-6,8,11,13-14,19-20,23H,1,7,9-10,12H2,2H3/t13?,14?,19?,20-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 3.20E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca R&D
Curated by ChEMBL
| Assay Description
Binding affinity towards cytochrome P450 2C9 |
J Med Chem 47: 907-14 (2004)
Article DOI: 10.1021/jm030972s
BindingDB Entry DOI: 10.7270/Q2ZK5HF3 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C9
(Homo sapiens (Human)) | BDBM50139805
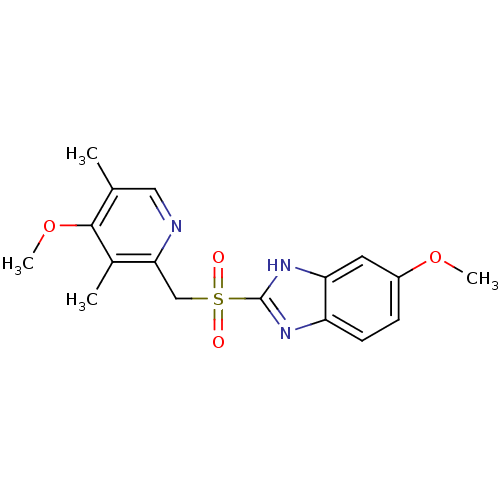
(5-Methoxy-2-(4-methoxy-3,5-dimethyl-pyridin-2-ylme...)Show SMILES COc1ccc2nc([nH]c2c1)S(=O)(=O)Cc1ncc(C)c(OC)c1C Show InChI InChI=1S/C17H19N3O4S/c1-10-8-18-15(11(2)16(10)24-4)9-25(21,22)17-19-13-6-5-12(23-3)7-14(13)20-17/h5-8H,9H2,1-4H3,(H,19,20) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 3.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca R&D
Curated by ChEMBL
| Assay Description
Binding affinity towards cytochrome P450 2C9 |
J Med Chem 47: 907-14 (2004)
Article DOI: 10.1021/jm030972s
BindingDB Entry DOI: 10.7270/Q2ZK5HF3 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C9
(Homo sapiens (Human)) | BDBM50409892
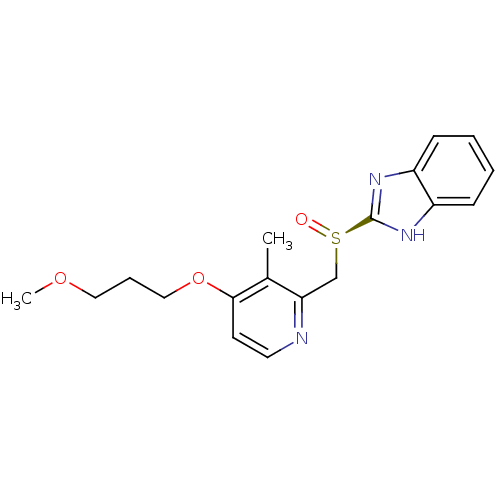
(CHEMBL1615209)Show SMILES COCCCOc1ccnc(C[S@@](=O)c2nc3ccccc3[nH]2)c1C |r| Show InChI InChI=1S/C18H21N3O3S/c1-13-16(19-9-8-17(13)24-11-5-10-23-2)12-25(22)18-20-14-6-3-4-7-15(14)21-18/h3-4,6-9H,5,10-12H2,1-2H3,(H,20,21)/t25-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| 3.60E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca R&D
Curated by ChEMBL
| Assay Description
Binding affinity towards cytochrome P450 2C9 |
J Med Chem 47: 907-14 (2004)
Article DOI: 10.1021/jm030972s
BindingDB Entry DOI: 10.7270/Q2ZK5HF3 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C9
(Homo sapiens (Human)) | BDBM50409894
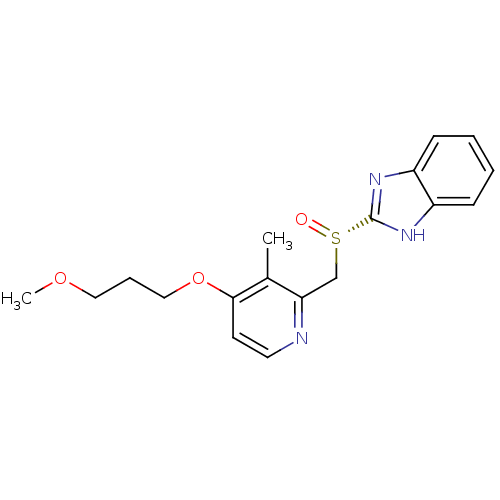
(CHEMBL1627299)Show SMILES COCCCOc1ccnc(C[S@](=O)c2nc3ccccc3[nH]2)c1C |r| Show InChI InChI=1S/C18H21N3O3S/c1-13-16(19-9-8-17(13)24-11-5-10-23-2)12-25(22)18-20-14-6-3-4-7-15(14)21-18/h3-4,6-9H,5,10-12H2,1-2H3,(H,20,21)/t25-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3.70E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca R&D
Curated by ChEMBL
| Assay Description
Binding affinity towards cytochrome P450 2C9 |
J Med Chem 47: 907-14 (2004)
Article DOI: 10.1021/jm030972s
BindingDB Entry DOI: 10.7270/Q2ZK5HF3 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C9
(Homo sapiens (Human)) | BDBM50241343
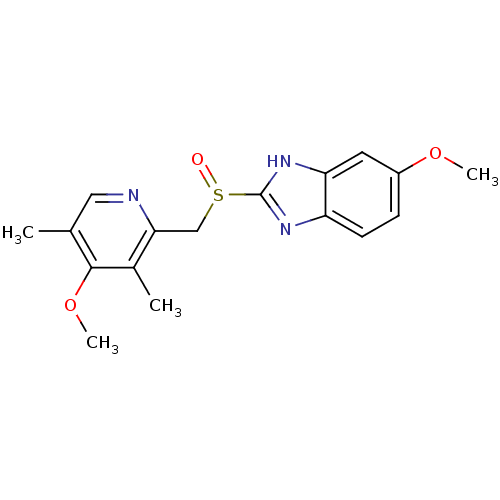
((RS)-6-methoxy-2-((4-methoxy-3,5-dimethylpyridin-2...)Show InChI InChI=1S/C17H19N3O3S/c1-10-8-18-15(11(2)16(10)23-4)9-24(21)17-19-13-6-5-12(22-3)7-14(13)20-17/h5-8H,9H2,1-4H3,(H,19,20) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
PC cid
PC sid
UniChem
Patents
Similars
| DrugBank
Article
PubMed
| 4.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca R&D
Curated by ChEMBL
| Assay Description
Binding affinity towards cytochrome P450 2C9 |
J Med Chem 47: 907-14 (2004)
Article DOI: 10.1021/jm030972s
BindingDB Entry DOI: 10.7270/Q2ZK5HF3 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C9
(Homo sapiens (Human)) | BDBM18512
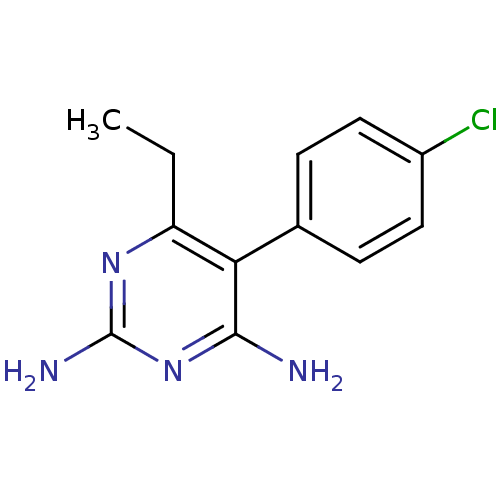
(5-(4-chlorophenyl)-6-ethylpyrimidine-2,4-diamine |...)Show InChI InChI=1S/C12H13ClN4/c1-2-9-10(11(14)17-12(15)16-9)7-3-5-8(13)6-4-7/h3-6H,2H2,1H3,(H4,14,15,16,17) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
Article
PubMed
| 5.15E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca R&D
Curated by ChEMBL
| Assay Description
Binding affinity towards cytochrome P450 2C9 |
J Med Chem 47: 907-14 (2004)
Article DOI: 10.1021/jm030972s
BindingDB Entry DOI: 10.7270/Q2ZK5HF3 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C9
(Homo sapiens (Human)) | BDBM50409895
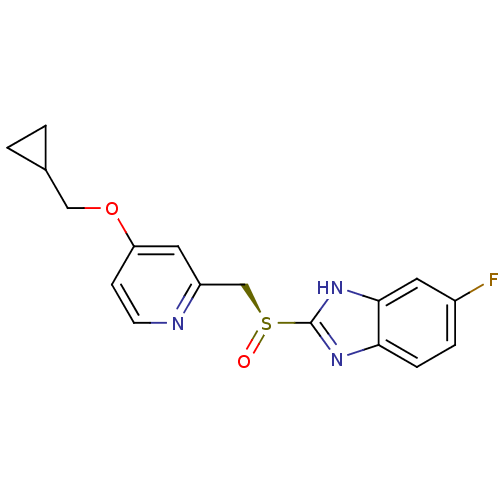
(CHEMBL2110248)Show SMILES Fc1ccc2nc([nH]c2c1)[S@@](=O)Cc1cc(OCC2CC2)ccn1 |r| Show InChI InChI=1S/C17H16FN3O2S/c18-12-3-4-15-16(7-12)21-17(20-15)24(22)10-13-8-14(5-6-19-13)23-9-11-1-2-11/h3-8,11H,1-2,9-10H2,(H,20,21)/t24-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 6.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca R&D
Curated by ChEMBL
| Assay Description
Binding affinity towards cytochrome P450 2C9 |
J Med Chem 47: 907-14 (2004)
Article DOI: 10.1021/jm030972s
BindingDB Entry DOI: 10.7270/Q2ZK5HF3 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C9
(Homo sapiens (Human)) | BDBM50409890

(CHEMBL2110339)Show SMILES COC(=O)c1ccc2nc([nH]c2c1)[S@@](=O)Cc1nccc(OC)c1OC |r| Show InChI InChI=1S/C17H17N3O5S/c1-23-14-6-7-18-13(15(14)24-2)9-26(22)17-19-11-5-4-10(16(21)25-3)8-12(11)20-17/h4-8H,9H2,1-3H3,(H,19,20)/t26-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 6.40E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca R&D
Curated by ChEMBL
| Assay Description
Binding affinity towards cytochrome P450 2C9 |
J Med Chem 47: 907-14 (2004)
Article DOI: 10.1021/jm030972s
BindingDB Entry DOI: 10.7270/Q2ZK5HF3 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C9
(Homo sapiens (Human)) | BDBM50409891
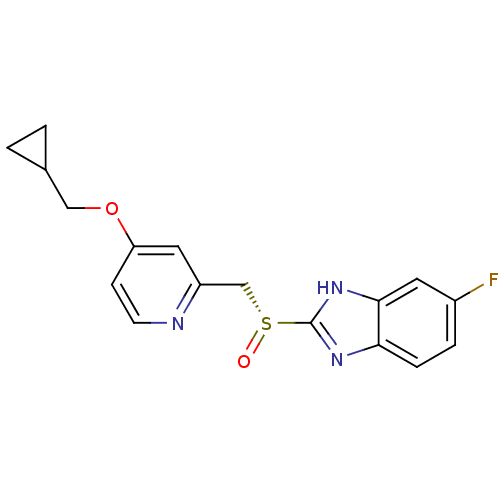
(CHEMBL2110340)Show SMILES Fc1ccc2nc([nH]c2c1)[S@](=O)Cc1cc(OCC2CC2)ccn1 |r| Show InChI InChI=1S/C17H16FN3O2S/c18-12-3-4-15-16(7-12)21-17(20-15)24(22)10-13-8-14(5-6-19-13)23-9-11-1-2-11/h3-8,11H,1-2,9-10H2,(H,20,21)/t24-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 9.30E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca R&D
Curated by ChEMBL
| Assay Description
Binding affinity towards cytochrome P450 2C9 |
J Med Chem 47: 907-14 (2004)
Article DOI: 10.1021/jm030972s
BindingDB Entry DOI: 10.7270/Q2ZK5HF3 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C9
(Homo sapiens (Human)) | BDBM50040780
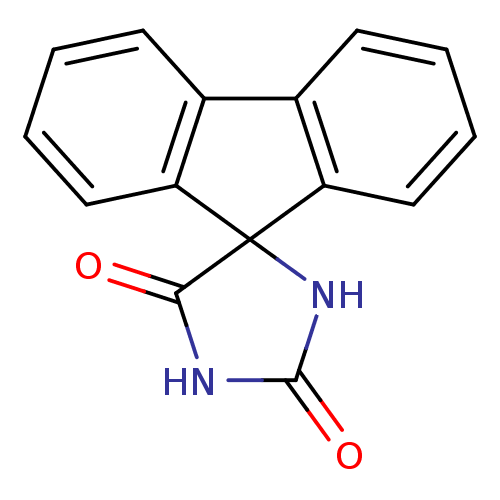
(CHEMBL163958 | cid_234512 | spiro[9H-fluorene-9,4'...)Show InChI InChI=1S/C15H10N2O2/c18-13-15(17-14(19)16-13)11-7-3-1-5-9(11)10-6-2-4-8-12(10)15/h1-8H,(H2,16,17,18,19) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 1.25E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca R&D
Curated by ChEMBL
| Assay Description
Binding affinity towards cytochrome P450 2C9 |
J Med Chem 47: 907-14 (2004)
Article DOI: 10.1021/jm030972s
BindingDB Entry DOI: 10.7270/Q2ZK5HF3 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C9
(Homo sapiens (Human)) | BDBM50409896

(CHEMBL2110247)Show SMILES COC(=O)c1ccc2nc([nH]c2c1)[S@](=O)Cc1nccc(OC)c1OC |r| Show InChI InChI=1S/C17H17N3O5S/c1-23-14-6-7-18-13(15(14)24-2)9-26(22)17-19-11-5-4-10(16(21)25-3)8-12(11)20-17/h4-8H,9H2,1-3H3,(H,19,20)/t26-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.40E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca R&D
Curated by ChEMBL
| Assay Description
Binding affinity towards cytochrome P450 2C9 |
J Med Chem 47: 907-14 (2004)
Article DOI: 10.1021/jm030972s
BindingDB Entry DOI: 10.7270/Q2ZK5HF3 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C9
(Homo sapiens (Human)) | BDBM50409893
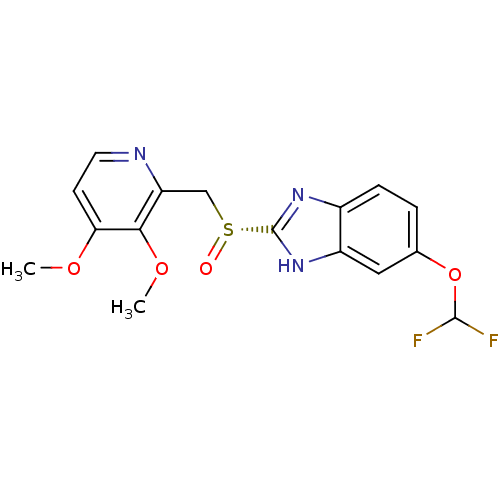
(CHEMBL2096747)Show SMILES COc1ccnc(C[S@@](=O)c2nc3ccc(OC(F)F)cc3[nH]2)c1OC |r| Show InChI InChI=1S/C16H15F2N3O4S/c1-23-13-5-6-19-12(14(13)24-2)8-26(22)16-20-10-4-3-9(25-15(17)18)7-11(10)21-16/h3-7,15H,8H2,1-2H3,(H,20,21)/t26-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.45E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca R&D
Curated by ChEMBL
| Assay Description
Binding affinity towards cytochrome P450 2C9 |
J Med Chem 47: 907-14 (2004)
Article DOI: 10.1021/jm030972s
BindingDB Entry DOI: 10.7270/Q2ZK5HF3 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C9
(Homo sapiens (Human)) | BDBM50409893

(CHEMBL2096747)Show SMILES COc1ccnc(C[S@@](=O)c2nc3ccc(OC(F)F)cc3[nH]2)c1OC |r| Show InChI InChI=1S/C16H15F2N3O4S/c1-23-13-5-6-19-12(14(13)24-2)8-26(22)16-20-10-4-3-9(25-15(17)18)7-11(10)21-16/h3-7,15H,8H2,1-2H3,(H,20,21)/t26-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.45E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca R&D
Curated by ChEMBL
| Assay Description
Binding affinity towards cytochrome P450 2C9 |
J Med Chem 47: 907-14 (2004)
Article DOI: 10.1021/jm030972s
BindingDB Entry DOI: 10.7270/Q2ZK5HF3 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C9
(Homo sapiens (Human)) | BDBM50121347
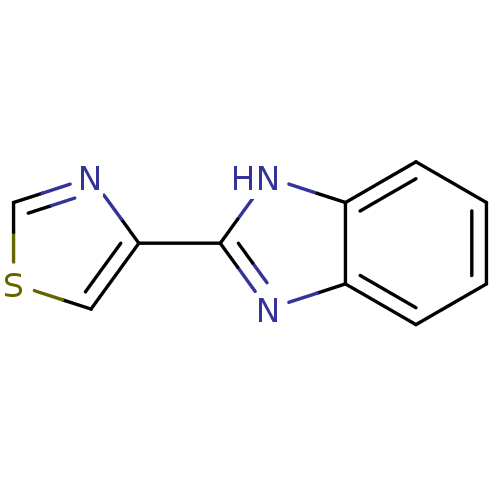
(CHEMBL625 | THIABENDAZOLE)Show InChI InChI=1S/C10H7N3S/c1-2-4-8-7(3-1)12-10(13-8)9-5-14-6-11-9/h1-6H,(H,12,13) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 2.45E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca R&D
Curated by ChEMBL
| Assay Description
Binding affinity towards cytochrome P450 2C9 |
J Med Chem 47: 907-14 (2004)
Article DOI: 10.1021/jm030972s
BindingDB Entry DOI: 10.7270/Q2ZK5HF3 |
More data for this
Ligand-Target Pair | |
Growth hormone secretagogue receptor type 1
(Homo sapiens (Human)) | BDBM50395751

(CHEMBL2164507)Show SMILES CCN1CCC(CC(=O)NC2(C(=O)Nc3cc(Cl)cc(Cl)c23)c2ccc(Cl)c(Cl)c2)CC1 Show InChI InChI=1S/C23H23Cl4N3O2/c1-2-30-7-5-13(6-8-30)9-20(31)29-23(14-3-4-16(25)17(26)10-14)21-18(27)11-15(24)12-19(21)28-22(23)32/h3-4,10-13H,2,5-9H2,1H3,(H,28,32)(H,29,31) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi Research
Curated by ChEMBL
| Assay Description
Antagonist activity at human GHSR1 expressed in CHO-CREluc cells after 4 hrs by Luciferase reporter assay |
Bioorg Med Chem 20: 5623-36 (2012)
Article DOI: 10.1016/j.bmc.2012.07.018
BindingDB Entry DOI: 10.7270/Q2HD7WS4 |
More data for this
Ligand-Target Pair | |
Reverse transcriptase/RNaseH
(Human immunodeficiency virus 1) | BDBM1803
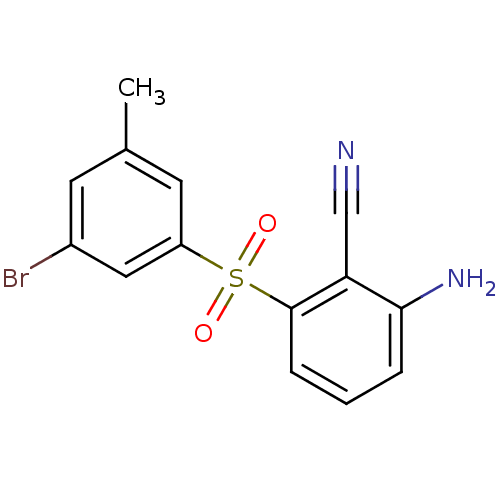
(2-Amino-6-arylthiobenzonitrile deriv. 3w | 2-amino...)Show InChI InChI=1S/C14H11BrN2O2S/c1-9-5-10(15)7-11(6-9)20(18,19)14-4-2-3-13(17)12(14)8-16/h2-7H,17H2,1H3 | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Perugia
Curated by ChEMBL
| Assay Description
Inhibitory concentration against Reverse transcriptase |
J Med Chem 48: 3756-67 (2005)
Article DOI: 10.1021/jm049162m
BindingDB Entry DOI: 10.7270/Q2XS5WQ0 |
More data for this
Ligand-Target Pair | |
Reverse transcriptase/RNaseH
(Human immunodeficiency virus 1) | BDBM1804
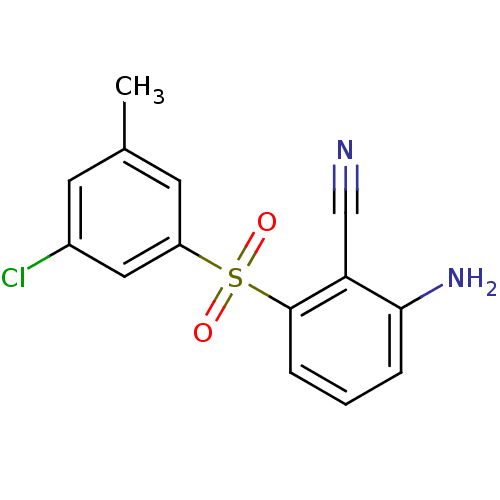
(2-Amino-6-arylthiobenzonitrile deriv. 3x | 2-amino...)Show InChI InChI=1S/C14H11ClN2O2S/c1-9-5-10(15)7-11(6-9)20(18,19)14-4-2-3-13(17)12(14)8-16/h2-7H,17H2,1H3 | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Perugia
Curated by ChEMBL
| Assay Description
Inhibitory concentration against Reverse transcriptase |
J Med Chem 48: 3756-67 (2005)
Article DOI: 10.1021/jm049162m
BindingDB Entry DOI: 10.7270/Q2XS5WQ0 |
More data for this
Ligand-Target Pair | |
Growth hormone secretagogue receptor type 1
(Homo sapiens (Human)) | BDBM50395753

(CHEMBL2164499)Show SMILES CCN1CCN(CC(=O)NC2(C(=O)Nc3cc(Cl)cc(Cl)c23)c2ccc(Cl)c(Cl)c2)CC1 Show InChI InChI=1S/C22H22Cl4N4O2/c1-2-29-5-7-30(8-6-29)12-19(31)28-22(13-3-4-15(24)16(25)9-13)20-17(26)10-14(23)11-18(20)27-21(22)32/h3-4,9-11H,2,5-8,12H2,1H3,(H,27,32)(H,28,31) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi Research
Curated by ChEMBL
| Assay Description
Antagonist activity at human GHSR1 expressed in CHO-CREluc cells after 4 hrs by Luciferase reporter assay |
Bioorg Med Chem 20: 5623-36 (2012)
Article DOI: 10.1016/j.bmc.2012.07.018
BindingDB Entry DOI: 10.7270/Q2HD7WS4 |
More data for this
Ligand-Target Pair | |
Reverse transcriptase/RNaseH
(Human immunodeficiency virus 1) | BDBM1802
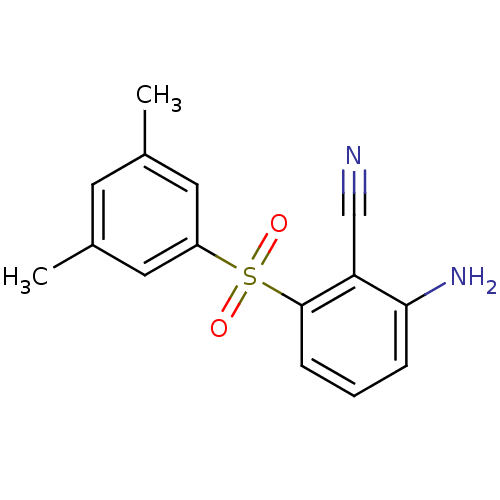
(2-Amino-6-arylthiobenzonitrile deriv. 3v, 739W94 |...)Show InChI InChI=1S/C15H14N2O2S/c1-10-6-11(2)8-12(7-10)20(18,19)15-5-3-4-14(17)13(15)9-16/h3-8H,17H2,1-2H3 | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| DrugBank
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| MMDB
PDB
Article
PubMed
| n/a | n/a | 7.10 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Perugia
Curated by ChEMBL
| Assay Description
Inhibitory concentration against Reverse transcriptase |
J Med Chem 48: 3756-67 (2005)
Article DOI: 10.1021/jm049162m
BindingDB Entry DOI: 10.7270/Q2XS5WQ0 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Reverse transcriptase/RNaseH
(Human immunodeficiency virus 1) | BDBM1805
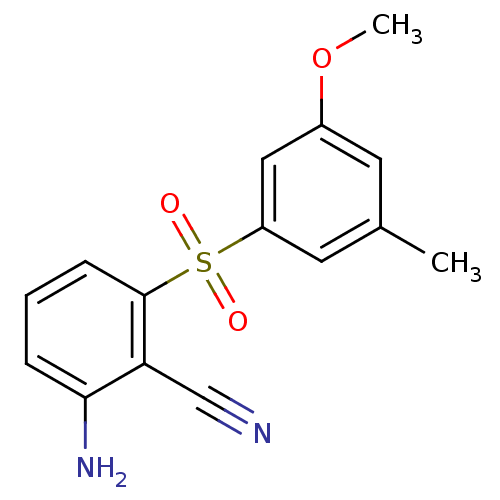
(2-Amino-6-arylthiobenzonitrile deriv. 3y | 2-amino...)Show InChI InChI=1S/C15H14N2O3S/c1-10-6-11(20-2)8-12(7-10)21(18,19)15-5-3-4-14(17)13(15)9-16/h3-8H,17H2,1-2H3 | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Perugia
Curated by ChEMBL
| Assay Description
Inhibitory concentration against Reverse transcriptase |
J Med Chem 48: 3756-67 (2005)
Article DOI: 10.1021/jm049162m
BindingDB Entry DOI: 10.7270/Q2XS5WQ0 |
More data for this
Ligand-Target Pair | |
Growth hormone secretagogue receptor type 1
(Homo sapiens (Human)) | BDBM50395743
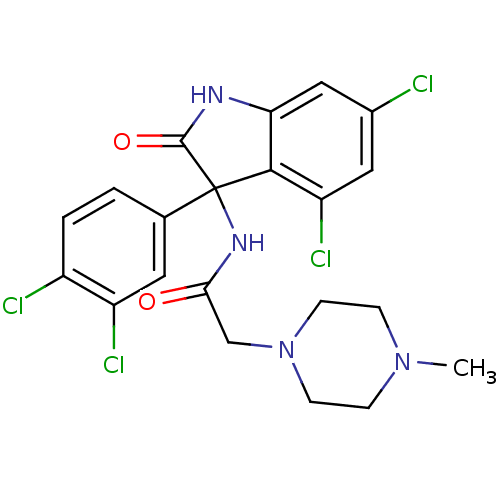
(CHEMBL2164832)Show SMILES CN1CCN(CC(=O)NC2(C(=O)Nc3cc(Cl)cc(Cl)c23)c2ccc(Cl)c(Cl)c2)CC1 Show InChI InChI=1S/C21H20Cl4N4O2/c1-28-4-6-29(7-5-28)11-18(30)27-21(12-2-3-14(23)15(24)8-12)19-16(25)9-13(22)10-17(19)26-20(21)31/h2-3,8-10H,4-7,11H2,1H3,(H,26,31)(H,27,30) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 24 | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi Research
Curated by ChEMBL
| Assay Description
Antagonist activity at human GHSR1 expressed in CHO-CREluc cells after 4 hrs by Luciferase reporter assay |
Bioorg Med Chem 20: 5623-36 (2012)
Article DOI: 10.1016/j.bmc.2012.07.018
BindingDB Entry DOI: 10.7270/Q2HD7WS4 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data