 Found 482 hits with Last Name = 'beer' and Initial = 'd'
Found 482 hits with Last Name = 'beer' and Initial = 'd' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM50260806
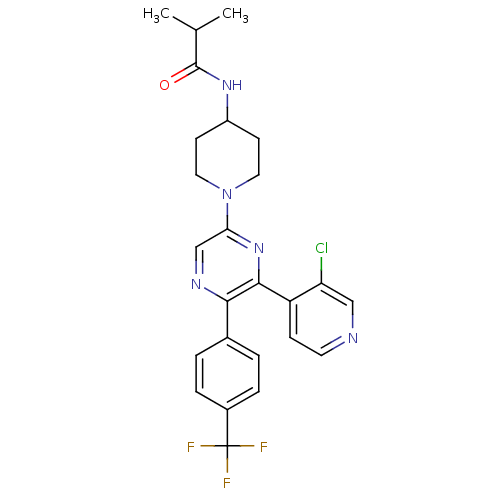
(CHEMBL497557 | N-(1-(6-(3-chloropyridin-4-yl)-5-(4...)Show SMILES CC(C)C(=O)NC1CCN(CC1)c1cnc(-c2ccc(cc2)C(F)(F)F)c(n1)-c1ccncc1Cl Show InChI InChI=1S/C25H25ClF3N5O/c1-15(2)24(35)32-18-8-11-34(12-9-18)21-14-31-22(16-3-5-17(6-4-16)25(27,28)29)23(33-21)19-7-10-30-13-20(19)26/h3-7,10,13-15,18H,8-9,11-12H2,1-2H3,(H,32,35) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.240 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Neurogen Corporation
Curated by ChEMBL
| Assay Description
Antagonist activity at human CB1 receptor expressed in SF9 cells assessed as inhibition of CP-55940-stimulated GTPgammaS binding |
Bioorg Med Chem Lett 18: 3376-81 (2008)
Article DOI: 10.1016/j.bmcl.2008.04.022
BindingDB Entry DOI: 10.7270/Q2QR4Z0T |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Rattus norvegicus (rat)) | BDBM21278

(5-(4-chlorophenyl)-1-(2,4-dichlorophenyl)-4-methyl...)Show SMILES Cc1c(nn(c1-c1ccc(Cl)cc1)-c1ccc(Cl)cc1Cl)C(=O)NN1CCCCC1 Show InChI InChI=1S/C22H21Cl3N4O/c1-14-20(22(30)27-28-11-3-2-4-12-28)26-29(19-10-9-17(24)13-18(19)25)21(14)15-5-7-16(23)8-6-15/h5-10,13H,2-4,11-12H2,1H3,(H,27,30) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 0.310 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Neurogen Corporation
Curated by ChEMBL
| Assay Description
Antagonist activity at rat CB1 receptor in SF9 cells assessed as inhibition of CP-55940-stimulated GTPgammaS binding |
Bioorg Med Chem Lett 18: 3376-81 (2008)
Article DOI: 10.1016/j.bmcl.2008.04.022
BindingDB Entry DOI: 10.7270/Q2QR4Z0T |
More data for this
Ligand-Target Pair | |
Beta-2 adrenergic receptor
(Homo sapiens (Human)) | BDBM25771
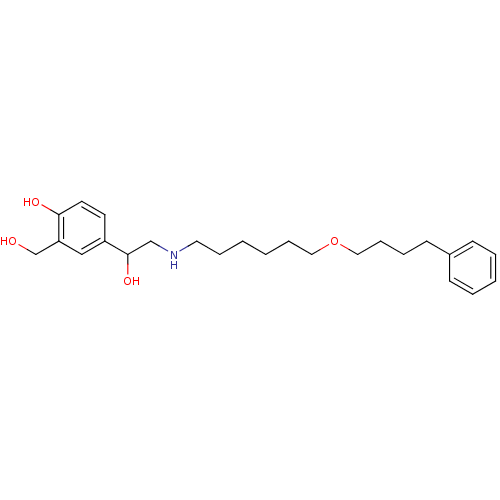
(1-hydroxy-2-naphthoic acid;4-[1-hydroxy-2-[6-(4-ph...)Show InChI InChI=1S/C25H37NO4/c27-20-23-18-22(13-14-24(23)28)25(29)19-26-15-7-1-2-8-16-30-17-9-6-12-21-10-4-3-5-11-21/h3-5,10-11,13-14,18,25-29H,1-2,6-9,12,15-17,19-20H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
PDB
Article
PubMed
| 0.390 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research
Curated by ChEMBL
| Assay Description
Displacement of [125I]cyanopindolol from human recombinant beta2 adrenergic receptor expressed in CHO cells by filtration assay |
J Med Chem 53: 3675-84 (2010)
Article DOI: 10.1021/jm100068m
BindingDB Entry DOI: 10.7270/Q27H1JRT |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Cannabinoid receptor 1
(Rattus norvegicus (rat)) | BDBM50260806

(CHEMBL497557 | N-(1-(6-(3-chloropyridin-4-yl)-5-(4...)Show SMILES CC(C)C(=O)NC1CCN(CC1)c1cnc(-c2ccc(cc2)C(F)(F)F)c(n1)-c1ccncc1Cl Show InChI InChI=1S/C25H25ClF3N5O/c1-15(2)24(35)32-18-8-11-34(12-9-18)21-14-31-22(16-3-5-17(6-4-16)25(27,28)29)23(33-21)19-7-10-30-13-20(19)26/h3-7,10,13-15,18H,8-9,11-12H2,1-2H3,(H,32,35) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.410 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Neurogen Corporation
Curated by ChEMBL
| Assay Description
Antagonist activity at rat CB1 receptor in SF9 cells assessed as inhibition of CP-55940-stimulated GTPgammaS binding |
Bioorg Med Chem Lett 18: 3376-81 (2008)
Article DOI: 10.1016/j.bmcl.2008.04.022
BindingDB Entry DOI: 10.7270/Q2QR4Z0T |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM21278

(5-(4-chlorophenyl)-1-(2,4-dichlorophenyl)-4-methyl...)Show SMILES Cc1c(nn(c1-c1ccc(Cl)cc1)-c1ccc(Cl)cc1Cl)C(=O)NN1CCCCC1 Show InChI InChI=1S/C22H21Cl3N4O/c1-14-20(22(30)27-28-11-3-2-4-12-28)26-29(19-10-9-17(24)13-18(19)25)21(14)15-5-7-16(23)8-6-15/h5-10,13H,2-4,11-12H2,1H3,(H,27,30) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
Article
PubMed
| 0.430 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Neurogen Corporation
Curated by ChEMBL
| Assay Description
Antagonist activity at human CB1 receptor expressed in SF9 cells assessed as inhibition of CP-55940-stimulated GTPgammaS binding |
Bioorg Med Chem Lett 18: 3376-81 (2008)
Article DOI: 10.1016/j.bmcl.2008.04.022
BindingDB Entry DOI: 10.7270/Q2QR4Z0T |
More data for this
Ligand-Target Pair | |
Beta-2 adrenergic receptor
(Homo sapiens (Human)) | BDBM50421329
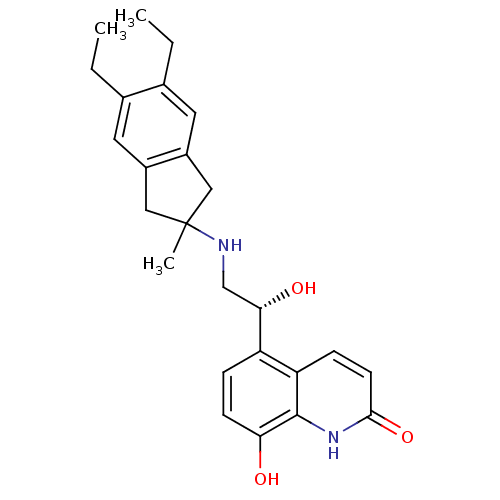
(CHEMBL2088201)Show SMILES CCc1cc2CC(C)(Cc2cc1CC)NC[C@H](O)c1ccc(O)c2[nH]c(=O)ccc12 |r| Show InChI InChI=1S/C25H30N2O3/c1-4-15-10-17-12-25(3,13-18(17)11-16(15)5-2)26-14-22(29)19-6-8-21(28)24-20(19)7-9-23(30)27-24/h6-11,22,26,28-29H,4-5,12-14H2,1-3H3,(H,27,30)/t22-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Binding affinity to human beta2-adrenoceptor by radioligand binding assay |
Bioorg Med Chem Lett 22: 6280-5 (2012)
Article DOI: 10.1016/j.bmcl.2012.07.096
BindingDB Entry DOI: 10.7270/Q2G73G09 |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM50260767
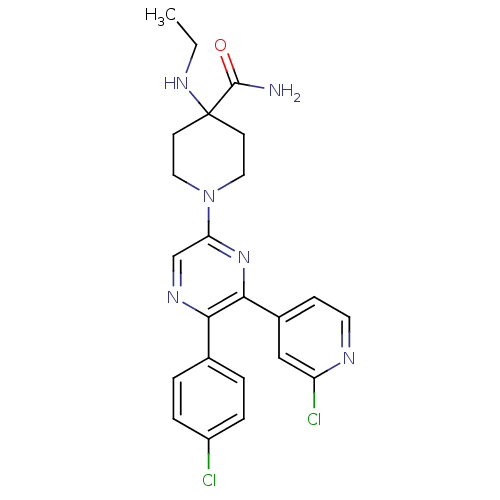
(1-(5-(4-chlorophenyl)-6-(2-chloropyridin-4-yl)pyra...)Show SMILES CCNC1(CCN(CC1)c1cnc(-c2ccc(Cl)cc2)c(n1)-c1ccnc(Cl)c1)C(N)=O Show InChI InChI=1S/C23H24Cl2N6O/c1-2-29-23(22(26)32)8-11-31(12-9-23)19-14-28-20(15-3-5-17(24)6-4-15)21(30-19)16-7-10-27-18(25)13-16/h3-7,10,13-14,29H,2,8-9,11-12H2,1H3,(H2,26,32) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.670 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Neurogen Corporation
Curated by ChEMBL
| Assay Description
Antagonist activity at human CB1 receptor expressed in SF9 cells assessed as inhibition of CP-55940-stimulated GTPgammaS binding |
Bioorg Med Chem Lett 18: 3376-81 (2008)
Article DOI: 10.1016/j.bmcl.2008.04.022
BindingDB Entry DOI: 10.7270/Q2QR4Z0T |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM50260805
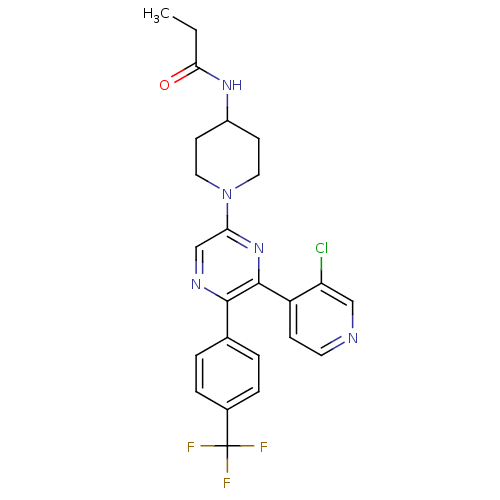
(CHEMBL524804 | N-(1-(6-(3-chloropyridin-4-yl)-5-(4...)Show SMILES CCC(=O)NC1CCN(CC1)c1cnc(-c2ccc(cc2)C(F)(F)F)c(n1)-c1ccncc1Cl Show InChI InChI=1S/C24H23ClF3N5O/c1-2-21(34)31-17-8-11-33(12-9-17)20-14-30-22(15-3-5-16(6-4-15)24(26,27)28)23(32-20)18-7-10-29-13-19(18)25/h3-7,10,13-14,17H,2,8-9,11-12H2,1H3,(H,31,34) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Neurogen Corporation
Curated by ChEMBL
| Assay Description
Antagonist activity at human CB1 receptor expressed in SF9 cells assessed as inhibition of CP-55940-stimulated GTPgammaS binding |
Bioorg Med Chem Lett 18: 3376-81 (2008)
Article DOI: 10.1016/j.bmcl.2008.04.022
BindingDB Entry DOI: 10.7270/Q2QR4Z0T |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Rattus norvegicus (rat)) | BDBM50260681
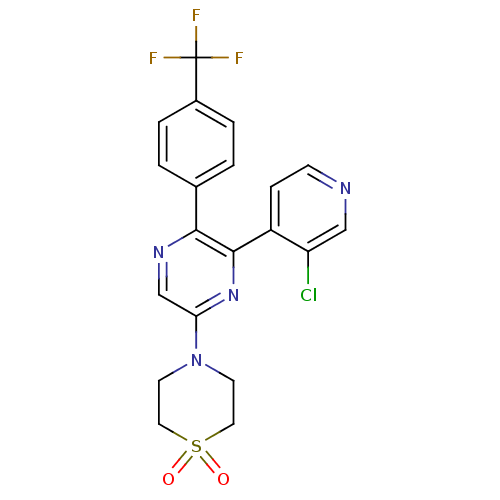
(4-[6-(3-Chloro-pyridin-4-yl)-5-(4-trifluoromethyl-...)Show SMILES FC(F)(F)c1ccc(cc1)-c1ncc(nc1-c1ccncc1Cl)N1CCS(=O)(=O)CC1 Show InChI InChI=1S/C20H16ClF3N4O2S/c21-16-11-25-6-5-15(16)19-18(13-1-3-14(4-2-13)20(22,23)24)26-12-17(27-19)28-7-9-31(29,30)10-8-28/h1-6,11-12H,7-10H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 0.830 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Neurogen Corporation
Curated by ChEMBL
| Assay Description
Antagonist activity at rat CB1 receptor in SF9 cells assessed as inhibition of CP-55940-stimulated GTPgammaS binding |
Bioorg Med Chem Lett 18: 3376-81 (2008)
Article DOI: 10.1016/j.bmcl.2008.04.022
BindingDB Entry DOI: 10.7270/Q2QR4Z0T |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM50260681

(4-[6-(3-Chloro-pyridin-4-yl)-5-(4-trifluoromethyl-...)Show SMILES FC(F)(F)c1ccc(cc1)-c1ncc(nc1-c1ccncc1Cl)N1CCS(=O)(=O)CC1 Show InChI InChI=1S/C20H16ClF3N4O2S/c21-16-11-25-6-5-15(16)19-18(13-1-3-14(4-2-13)20(22,23)24)26-12-17(27-19)28-7-9-31(29,30)10-8-28/h1-6,11-12H,7-10H2 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 0.830 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Neurogen Corporation
Curated by ChEMBL
| Assay Description
Antagonist activity at human CB1 receptor expressed in SF9 cells assessed as inhibition of CP-55940-stimulated GTPgammaS binding |
Bioorg Med Chem Lett 18: 3376-81 (2008)
Article DOI: 10.1016/j.bmcl.2008.04.022
BindingDB Entry DOI: 10.7270/Q2QR4Z0T |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM50260768
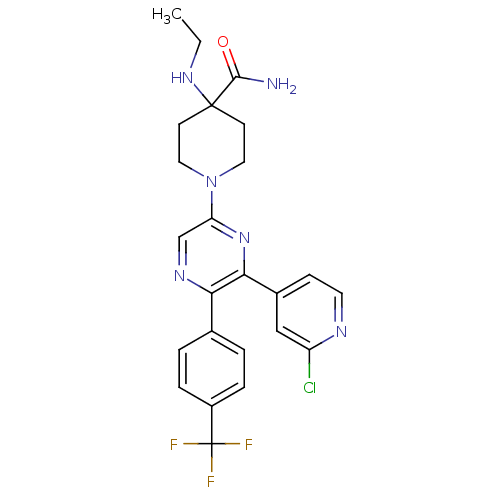
(1-(6-(2-chloropyridin-4-yl)-5-(4-(trifluoromethyl)...)Show SMILES CCNC1(CCN(CC1)c1cnc(-c2ccc(cc2)C(F)(F)F)c(n1)-c1ccnc(Cl)c1)C(N)=O Show InChI InChI=1S/C24H24ClF3N6O/c1-2-32-23(22(29)35)8-11-34(12-9-23)19-14-31-20(15-3-5-17(6-4-15)24(26,27)28)21(33-19)16-7-10-30-18(25)13-16/h3-7,10,13-14,32H,2,8-9,11-12H2,1H3,(H2,29,35) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.870 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Neurogen Corporation
Curated by ChEMBL
| Assay Description
Antagonist activity at human CB1 receptor expressed in SF9 cells assessed as inhibition of CP-55940-stimulated GTPgammaS binding |
Bioorg Med Chem Lett 18: 3376-81 (2008)
Article DOI: 10.1016/j.bmcl.2008.04.022
BindingDB Entry DOI: 10.7270/Q2QR4Z0T |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM50260682
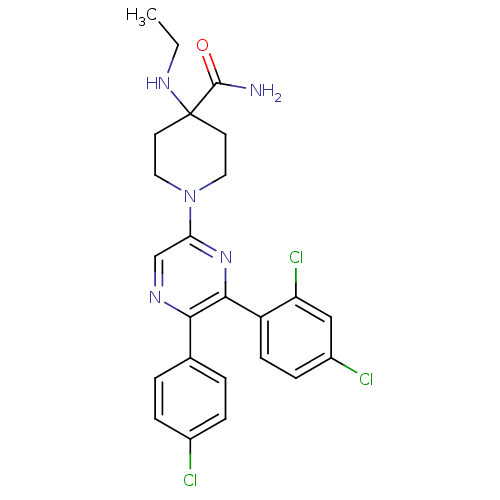
(1-(5-(4-chlorophenyl)-6-(2,4-dichlorophenyl)pyrazi...)Show SMILES CCNC1(CCN(CC1)c1cnc(-c2ccc(Cl)cc2)c(n1)-c1ccc(Cl)cc1Cl)C(N)=O Show InChI InChI=1S/C24H24Cl3N5O/c1-2-30-24(23(28)33)9-11-32(12-10-24)20-14-29-21(15-3-5-16(25)6-4-15)22(31-20)18-8-7-17(26)13-19(18)27/h3-8,13-14,30H,2,9-12H2,1H3,(H2,28,33) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.910 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Neurogen Corporation
Curated by ChEMBL
| Assay Description
Antagonist activity at human CB1 receptor expressed in SF9 cells assessed as inhibition of CP-55940-stimulated GTPgammaS binding |
Bioorg Med Chem Lett 18: 3376-81 (2008)
Article DOI: 10.1016/j.bmcl.2008.04.022
BindingDB Entry DOI: 10.7270/Q2QR4Z0T |
More data for this
Ligand-Target Pair | |
Beta-2 adrenergic receptor
(Homo sapiens (Human)) | BDBM25771

(1-hydroxy-2-naphthoic acid;4-[1-hydroxy-2-[6-(4-ph...)Show InChI InChI=1S/C25H37NO4/c27-20-23-18-22(13-14-24(23)28)25(29)19-26-15-7-1-2-8-16-30-17-9-6-12-21-10-4-3-5-11-21/h3-5,10-11,13-14,18,25-29H,1-2,6-9,12,15-17,19-20H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
PDB
Article
PubMed
| 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Horsham Research Centre
Curated by ChEMBL
| Assay Description
Binding affinity for human beta-2 adrenergic receptor |
Bioorg Med Chem Lett 14: 4705-10 (2004)
Article DOI: 10.1016/j.bmcl.2004.06.086
BindingDB Entry DOI: 10.7270/Q2416XSZ |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM50260803
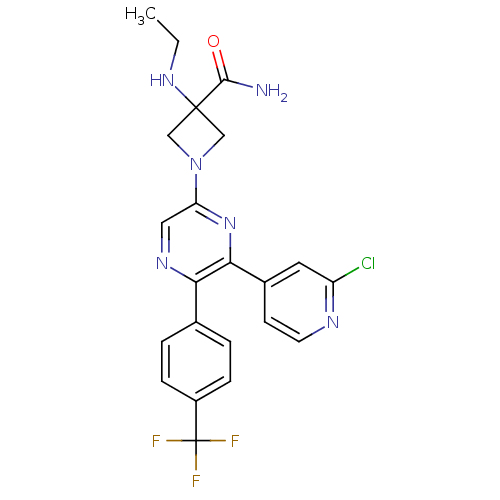
(1-(6-(2-chloropyridin-4-yl)-5-(4-(trifluoromethyl)...)Show SMILES CCNC1(CN(C1)c1cnc(-c2ccc(cc2)C(F)(F)F)c(n1)-c1ccnc(Cl)c1)C(N)=O Show InChI InChI=1S/C22H20ClF3N6O/c1-2-30-21(20(27)33)11-32(12-21)17-10-29-18(13-3-5-15(6-4-13)22(24,25)26)19(31-17)14-7-8-28-16(23)9-14/h3-10,30H,2,11-12H2,1H3,(H2,27,33) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Neurogen Corporation
Curated by ChEMBL
| Assay Description
Antagonist activity at human CB1 receptor expressed in SF9 cells assessed as inhibition of CP-55940-stimulated GTPgammaS binding |
Bioorg Med Chem Lett 18: 3376-81 (2008)
Article DOI: 10.1016/j.bmcl.2008.04.022
BindingDB Entry DOI: 10.7270/Q2QR4Z0T |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM50260718

(1-(6-(2-chlorophenyl)-5-(4-chlorophenyl)pyrazin-2-...)Show SMILES CCNC1(CCN(CC1)c1cnc(-c2ccc(Cl)cc2)c(n1)-c1ccccc1Cl)C(N)=O Show InChI InChI=1S/C24H25Cl2N5O/c1-2-29-24(23(27)32)11-13-31(14-12-24)20-15-28-21(16-7-9-17(25)10-8-16)22(30-20)18-5-3-4-6-19(18)26/h3-10,15,29H,2,11-14H2,1H3,(H2,27,32) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 1.66 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Neurogen Corporation
Curated by ChEMBL
| Assay Description
Antagonist activity at human CB1 receptor expressed in SF9 cells assessed as inhibition of CP-55940-stimulated GTPgammaS binding |
Bioorg Med Chem Lett 18: 3376-81 (2008)
Article DOI: 10.1016/j.bmcl.2008.04.022
BindingDB Entry DOI: 10.7270/Q2QR4Z0T |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM50260717
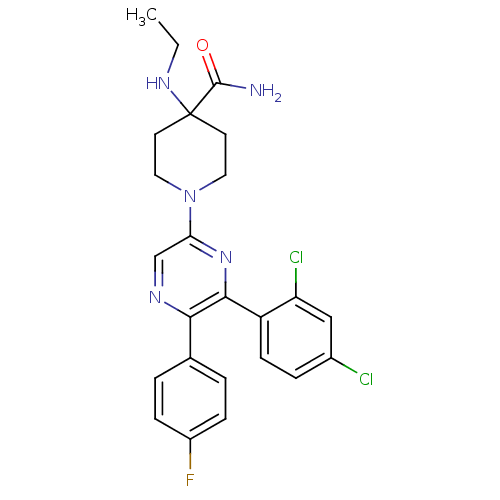
(1-(6-(2,4-dichlorophenyl)-5-(4-fluorophenyl)pyrazi...)Show SMILES CCNC1(CCN(CC1)c1cnc(-c2ccc(F)cc2)c(n1)-c1ccc(Cl)cc1Cl)C(N)=O Show InChI InChI=1S/C24H24Cl2FN5O/c1-2-30-24(23(28)33)9-11-32(12-10-24)20-14-29-21(15-3-6-17(27)7-4-15)22(31-20)18-8-5-16(25)13-19(18)26/h3-8,13-14,30H,2,9-12H2,1H3,(H2,28,33) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.75 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Neurogen Corporation
Curated by ChEMBL
| Assay Description
Antagonist activity at human CB1 receptor expressed in SF9 cells assessed as inhibition of CP-55940-stimulated GTPgammaS binding |
Bioorg Med Chem Lett 18: 3376-81 (2008)
Article DOI: 10.1016/j.bmcl.2008.04.022
BindingDB Entry DOI: 10.7270/Q2QR4Z0T |
More data for this
Ligand-Target Pair | |
Beta-1 adrenergic receptor
(Homo sapiens (Human)) | BDBM50421329

(CHEMBL2088201)Show SMILES CCc1cc2CC(C)(Cc2cc1CC)NC[C@H](O)c1ccc(O)c2[nH]c(=O)ccc12 |r| Show InChI InChI=1S/C25H30N2O3/c1-4-15-10-17-12-25(3,13-18(17)11-16(15)5-2)26-14-22(29)19-6-8-21(28)24-20(19)7-9-23(30)27-24/h6-11,22,26,28-29H,4-5,12-14H2,1-3H3,(H,27,30)/t22-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Binding affinity to human beta1-adrenoceptor by radioligand binding assay |
Bioorg Med Chem Lett 22: 6280-5 (2012)
Article DOI: 10.1016/j.bmcl.2012.07.096
BindingDB Entry DOI: 10.7270/Q2G73G09 |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM50260804
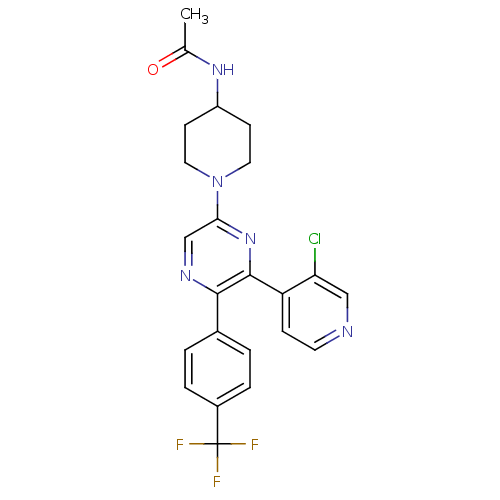
(CHEMBL497556 | N-(1-(6-(3-chloropyridin-4-yl)-5-(4...)Show SMILES CC(=O)NC1CCN(CC1)c1cnc(-c2ccc(cc2)C(F)(F)F)c(n1)-c1ccncc1Cl Show InChI InChI=1S/C23H21ClF3N5O/c1-14(33)30-17-7-10-32(11-8-17)20-13-29-21(15-2-4-16(5-3-15)23(25,26)27)22(31-20)18-6-9-28-12-19(18)24/h2-6,9,12-13,17H,7-8,10-11H2,1H3,(H,30,33) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.97 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Neurogen Corporation
Curated by ChEMBL
| Assay Description
Antagonist activity at human CB1 receptor expressed in SF9 cells assessed as inhibition of CP-55940-stimulated GTPgammaS binding |
Bioorg Med Chem Lett 18: 3376-81 (2008)
Article DOI: 10.1016/j.bmcl.2008.04.022
BindingDB Entry DOI: 10.7270/Q2QR4Z0T |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Rattus norvegicus (rat)) | BDBM50260803

(1-(6-(2-chloropyridin-4-yl)-5-(4-(trifluoromethyl)...)Show SMILES CCNC1(CN(C1)c1cnc(-c2ccc(cc2)C(F)(F)F)c(n1)-c1ccnc(Cl)c1)C(N)=O Show InChI InChI=1S/C22H20ClF3N6O/c1-2-30-21(20(27)33)11-32(12-21)17-10-29-18(13-3-5-15(6-4-13)22(24,25)26)19(31-17)14-7-8-28-16(23)9-14/h3-10,30H,2,11-12H2,1H3,(H2,27,33) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.06 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Neurogen Corporation
Curated by ChEMBL
| Assay Description
Antagonist activity at rat CB1 receptor in SF9 cells assessed as inhibition of CP-55940-stimulated GTPgammaS binding |
Bioorg Med Chem Lett 18: 3376-81 (2008)
Article DOI: 10.1016/j.bmcl.2008.04.022
BindingDB Entry DOI: 10.7270/Q2QR4Z0T |
More data for this
Ligand-Target Pair | |
Beta-2 adrenergic receptor
(Homo sapiens (Human)) | BDBM86453

(CAS_73573-87-2 | Formoterol | NSC_3083544)Show InChI InChI=1S/C19H24N2O4/c1-13(9-14-3-6-16(25-2)7-4-14)20-11-19(24)15-5-8-18(23)17(10-15)21-12-22/h3-8,10,12-13,19-20,23-24H,9,11H2,1-2H3,(H,21,22) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| DrugBank
Article
PubMed
| 2.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Horsham Research Centre
Curated by ChEMBL
| Assay Description
Binding affinity for human beta-2 adrenergic receptor |
Bioorg Med Chem Lett 14: 4705-10 (2004)
Article DOI: 10.1016/j.bmcl.2004.06.086
BindingDB Entry DOI: 10.7270/Q2416XSZ |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM50260720
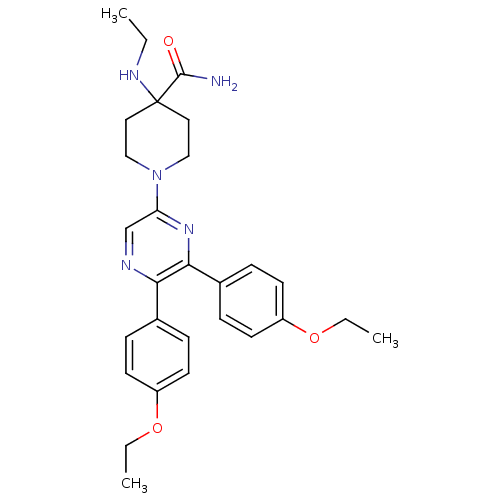
(1-(5,6-bis(4-ethoxyphenyl)pyrazin-2-yl)-4-(ethylam...)Show SMILES CCNC1(CCN(CC1)c1cnc(-c2ccc(OCC)cc2)c(n1)-c1ccc(OCC)cc1)C(N)=O Show InChI InChI=1S/C28H35N5O3/c1-4-31-28(27(29)34)15-17-33(18-16-28)24-19-30-25(20-7-11-22(12-8-20)35-5-2)26(32-24)21-9-13-23(14-10-21)36-6-3/h7-14,19,31H,4-6,15-18H2,1-3H3,(H2,29,34) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.84 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Neurogen Corporation
Curated by ChEMBL
| Assay Description
Antagonist activity at human CB1 receptor expressed in SF9 cells assessed as inhibition of CP-55940-stimulated GTPgammaS binding |
Bioorg Med Chem Lett 18: 3376-81 (2008)
Article DOI: 10.1016/j.bmcl.2008.04.022
BindingDB Entry DOI: 10.7270/Q2QR4Z0T |
More data for this
Ligand-Target Pair | |
Beta-2 adrenergic receptor
(Homo sapiens (Human)) | BDBM50318156
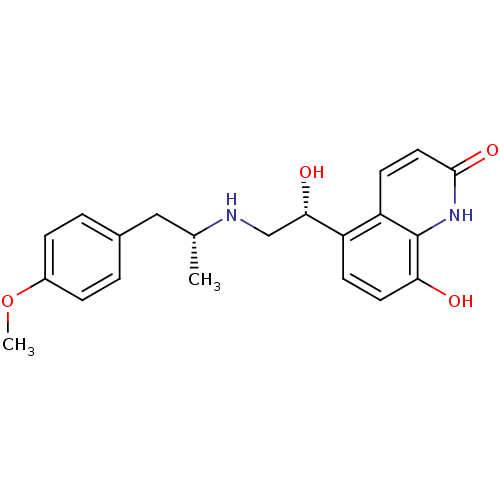
(CHEMBL1094785 | carmoterol)Show SMILES COc1ccc(C[C@@H](C)NC[C@H](O)c2ccc(O)c3[nH]c(=O)ccc23)cc1 |r| Show InChI InChI=1S/C21H24N2O4/c1-13(11-14-3-5-15(27-2)6-4-14)22-12-19(25)16-7-9-18(24)21-17(16)8-10-20(26)23-21/h3-10,13,19,22,24-25H,11-12H2,1-2H3,(H,23,26)/t13-,19+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 3.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research
Curated by ChEMBL
| Assay Description
Displacement of [125I]cyanopindolol from human recombinant beta2 adrenergic receptor expressed in CHO cells by filtration assay |
J Med Chem 53: 3675-84 (2010)
Article DOI: 10.1021/jm100068m
BindingDB Entry DOI: 10.7270/Q27H1JRT |
More data for this
Ligand-Target Pair | |
Beta-2 adrenergic receptor
(Homo sapiens (Human)) | BDBM50151724
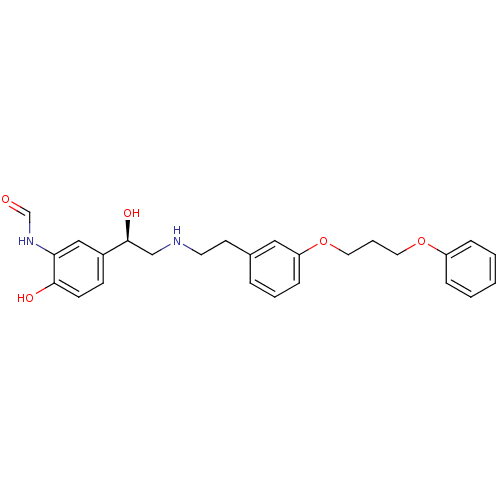
(CHEMBL183948 | N-[2-Hydroxy-5-(1-hydroxy-2-{2-[3-(...)Show SMILES O[C@@H](CNCCc1cccc(OCCCOc2ccccc2)c1)c1ccc(O)c(NC=O)c1 Show InChI InChI=1S/C26H30N2O5/c29-19-28-24-17-21(10-11-25(24)30)26(31)18-27-13-12-20-6-4-9-23(16-20)33-15-5-14-32-22-7-2-1-3-8-22/h1-4,6-11,16-17,19,26-27,30-31H,5,12-15,18H2,(H,28,29)/t26-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Horsham Research Centre
Curated by ChEMBL
| Assay Description
Binding affinity for human beta-2 adrenergic receptor |
Bioorg Med Chem Lett 14: 4705-10 (2004)
Article DOI: 10.1016/j.bmcl.2004.06.086
BindingDB Entry DOI: 10.7270/Q2416XSZ |
More data for this
Ligand-Target Pair | |
Beta-2 adrenergic receptor
(Homo sapiens (Human)) | BDBM50128690
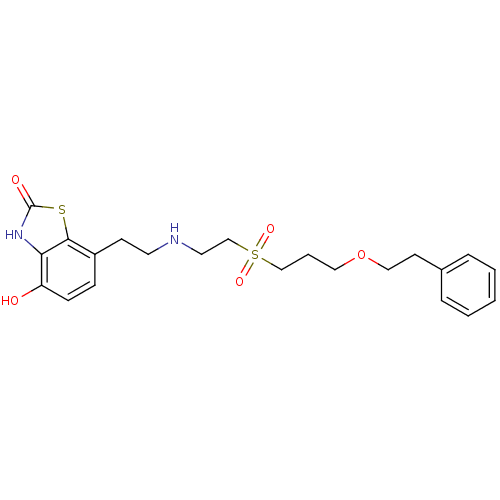
(4-Hydroxy-7-{2-[2-(3-phenethyloxy-propane-1-sulfon...)Show SMILES Oc1ccc(CCNCCS(=O)(=O)CCCOCCc2ccccc2)c2sc(=O)[nH]c12 Show InChI InChI=1S/C22H28N2O5S2/c25-19-8-7-18(21-20(19)24-22(26)30-21)9-11-23-12-16-31(27,28)15-4-13-29-14-10-17-5-2-1-3-6-17/h1-3,5-8,23,25H,4,9-16H2,(H,24,26) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Binding affinity to human beta2-adrenoceptor by radioligand binding assay |
Bioorg Med Chem Lett 22: 6280-5 (2012)
Article DOI: 10.1016/j.bmcl.2012.07.096
BindingDB Entry DOI: 10.7270/Q2G73G09 |
More data for this
Ligand-Target Pair | |
Beta-2 adrenergic receptor
(Homo sapiens (Human)) | BDBM50151719
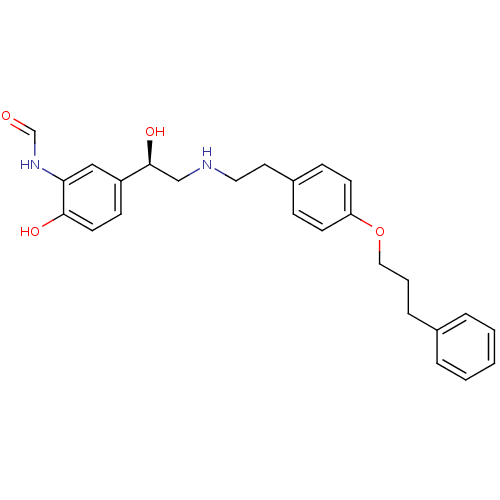
(CHEMBL363329 | N-[2-Hydroxy-5-(1-hydroxy-2-{2-[4-(...)Show SMILES O[C@@H](CNCCc1ccc(OCCCc2ccccc2)cc1)c1ccc(O)c(NC=O)c1 Show InChI InChI=1S/C26H30N2O4/c29-19-28-24-17-22(10-13-25(24)30)26(31)18-27-15-14-21-8-11-23(12-9-21)32-16-4-7-20-5-2-1-3-6-20/h1-3,5-6,8-13,17,19,26-27,30-31H,4,7,14-16,18H2,(H,28,29)/t26-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 5.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Horsham Research Centre
Curated by ChEMBL
| Assay Description
Binding affinity for human beta-2 adrenergic receptor |
Bioorg Med Chem Lett 14: 4705-10 (2004)
Article DOI: 10.1016/j.bmcl.2004.06.086
BindingDB Entry DOI: 10.7270/Q2416XSZ |
More data for this
Ligand-Target Pair | |
Beta-2 adrenergic receptor
(Homo sapiens (Human)) | BDBM50151721

(CHEMBL363260 | N-(2-Hydroxy-5-{(R)-1-hydroxy-2-[2-...)Show SMILES O[C@@H](CNCCc1ccc(OCCc2ccccc2)cc1)c1ccc(O)c(NC=O)c1 Show InChI InChI=1S/C25H28N2O4/c28-18-27-23-16-21(8-11-24(23)29)25(30)17-26-14-12-20-6-9-22(10-7-20)31-15-13-19-4-2-1-3-5-19/h1-11,16,18,25-26,29-30H,12-15,17H2,(H,27,28)/t25-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 7.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Horsham Research Centre
Curated by ChEMBL
| Assay Description
Binding affinity for human beta-2 adrenergic receptor |
Bioorg Med Chem Lett 14: 4705-10 (2004)
Article DOI: 10.1016/j.bmcl.2004.06.086
BindingDB Entry DOI: 10.7270/Q2416XSZ |
More data for this
Ligand-Target Pair | |
Beta-2 adrenergic receptor
(Homo sapiens (Human)) | BDBM50151718
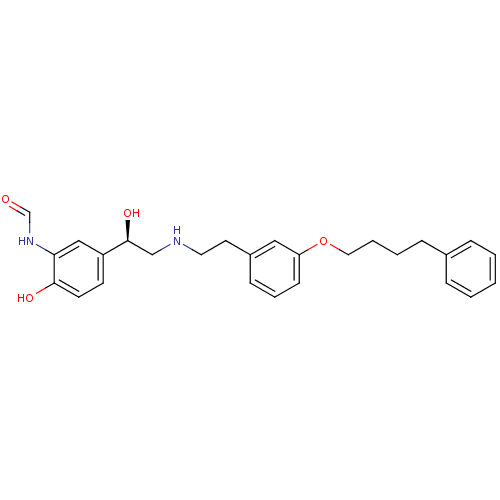
(CHEMBL440561 | N-[2-Hydroxy-5-((R)-1-hydroxy-2-{2-...)Show SMILES O[C@@H](CNCCc1cccc(OCCCCc2ccccc2)c1)c1ccc(O)c(NC=O)c1 Show InChI InChI=1S/C27H32N2O4/c30-20-29-25-18-23(12-13-26(25)31)27(32)19-28-15-14-22-10-6-11-24(17-22)33-16-5-4-9-21-7-2-1-3-8-21/h1-3,6-8,10-13,17-18,20,27-28,31-32H,4-5,9,14-16,19H2,(H,29,30)/t27-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 8.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Horsham Research Centre
Curated by ChEMBL
| Assay Description
Binding affinity for human beta-2 adrenergic receptor |
Bioorg Med Chem Lett 14: 4705-10 (2004)
Article DOI: 10.1016/j.bmcl.2004.06.086
BindingDB Entry DOI: 10.7270/Q2416XSZ |
More data for this
Ligand-Target Pair | |
Beta-2 adrenergic receptor
(Homo sapiens (Human)) | BDBM50151723
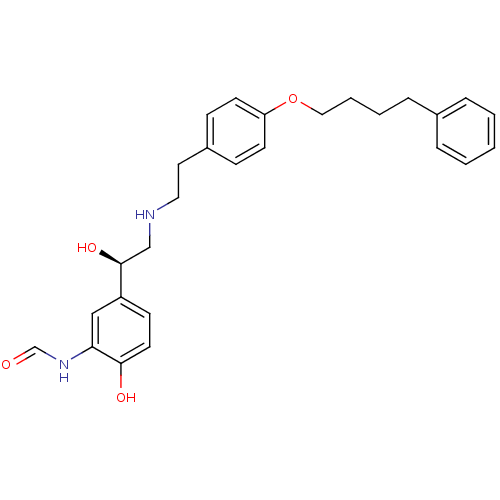
(CHEMBL185052 | N-[2-Hydroxy-5-((R)-1-hydroxy-2-{2-...)Show SMILES O[C@@H](CNCCc1ccc(OCCCCc2ccccc2)cc1)c1ccc(O)c(NC=O)c1 Show InChI InChI=1S/C27H32N2O4/c30-20-29-25-18-23(11-14-26(25)31)27(32)19-28-16-15-22-9-12-24(13-10-22)33-17-5-4-8-21-6-2-1-3-7-21/h1-3,6-7,9-14,18,20,27-28,31-32H,4-5,8,15-17,19H2,(H,29,30)/t27-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 9.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Horsham Research Centre
Curated by ChEMBL
| Assay Description
Binding affinity for human beta-2 adrenergic receptor |
Bioorg Med Chem Lett 14: 4705-10 (2004)
Article DOI: 10.1016/j.bmcl.2004.06.086
BindingDB Entry DOI: 10.7270/Q2416XSZ |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM50260766
(1-(5-(4-chlorophenyl)-6-(pyridin-4-yl)pyrazin-2-yl...)Show SMILES CCNC1(CCN(CC1)c1cnc(-c2ccc(Cl)cc2)c(n1)-c1ccncc1)C(N)=O Show InChI InChI=1S/C23H25ClN6O/c1-2-28-23(22(25)31)9-13-30(14-10-23)19-15-27-20(16-3-5-18(24)6-4-16)21(29-19)17-7-11-26-12-8-17/h3-8,11-12,15,28H,2,9-10,13-14H2,1H3,(H2,25,31) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 9.81 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Neurogen Corporation
Curated by ChEMBL
| Assay Description
Antagonist activity at human CB1 receptor expressed in SF9 cells assessed as inhibition of CP-55940-stimulated GTPgammaS binding |
Bioorg Med Chem Lett 18: 3376-81 (2008)
Article DOI: 10.1016/j.bmcl.2008.04.022
BindingDB Entry DOI: 10.7270/Q2QR4Z0T |
More data for this
Ligand-Target Pair | |
Beta-2 adrenergic receptor
(Homo sapiens (Human)) | BDBM50151717
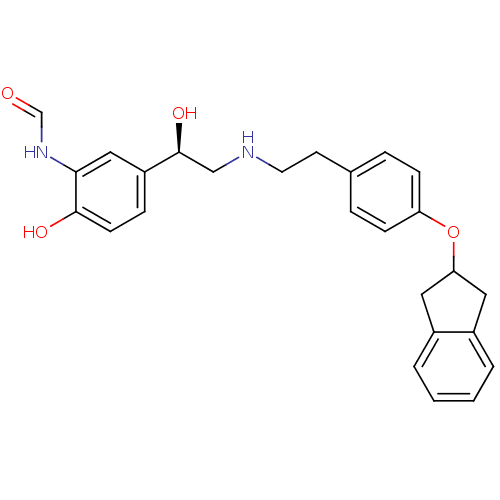
(CHEMBL184538 | N-[2-Hydroxy-5-(1-hydroxy-2-{2-[4-(...)Show SMILES O[C@@H](CNCCc1ccc(OC2Cc3ccccc3C2)cc1)c1ccc(O)c(NC=O)c1 Show InChI InChI=1S/C26H28N2O4/c29-17-28-24-15-21(7-10-25(24)30)26(31)16-27-12-11-18-5-8-22(9-6-18)32-23-13-19-3-1-2-4-20(19)14-23/h1-10,15,17,23,26-27,30-31H,11-14,16H2,(H,28,29)/t26-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 12 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Horsham Research Centre
Curated by ChEMBL
| Assay Description
Binding affinity for human beta-2 adrenergic receptor |
Bioorg Med Chem Lett 14: 4705-10 (2004)
Article DOI: 10.1016/j.bmcl.2004.06.086
BindingDB Entry DOI: 10.7270/Q2416XSZ |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM50260719
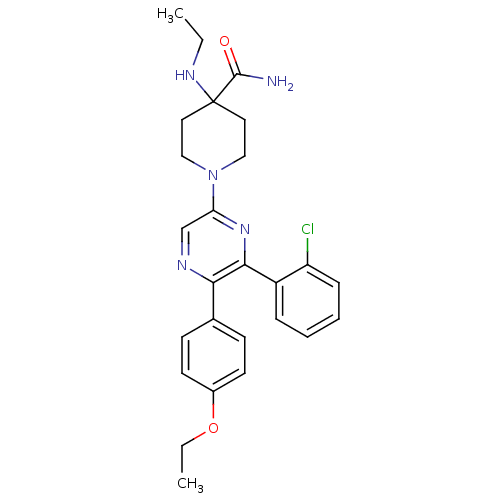
(1-(6-(2-chlorophenyl)-5-(4-ethoxyphenyl)pyrazin-2-...)Show SMILES CCNC1(CCN(CC1)c1cnc(-c2ccc(OCC)cc2)c(n1)-c1ccccc1Cl)C(N)=O Show InChI InChI=1S/C26H30ClN5O2/c1-3-30-26(25(28)33)13-15-32(16-14-26)22-17-29-23(18-9-11-19(12-10-18)34-4-2)24(31-22)20-7-5-6-8-21(20)27/h5-12,17,30H,3-4,13-16H2,1-2H3,(H2,28,33) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 15.0 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Neurogen Corporation
Curated by ChEMBL
| Assay Description
Antagonist activity at human CB1 receptor expressed in SF9 cells assessed as inhibition of CP-55940-stimulated GTPgammaS binding |
Bioorg Med Chem Lett 18: 3376-81 (2008)
Article DOI: 10.1016/j.bmcl.2008.04.022
BindingDB Entry DOI: 10.7270/Q2QR4Z0T |
More data for this
Ligand-Target Pair | |
Beta-2 adrenergic receptor
(Homo sapiens (Human)) | BDBM50318159
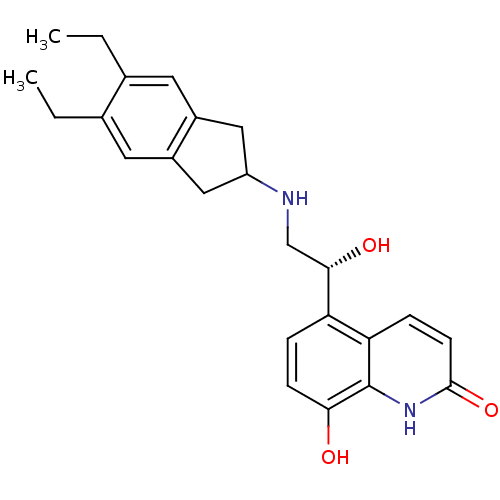
(8-Hydroxy-5-[(R)-1-hydroxy-2-(5,6-diethylindan-2-y...)Show SMILES CCc1cc2CC(Cc2cc1CC)NC[C@H](O)c1ccc(O)c2[nH]c(=O)ccc12 |r| Show InChI InChI=1S/C24H28N2O3/c1-3-14-9-16-11-18(12-17(16)10-15(14)4-2)25-13-22(28)19-5-7-21(27)24-20(19)6-8-23(29)26-24/h5-10,18,22,25,27-28H,3-4,11-13H2,1-2H3,(H,26,29)/t22-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
PC cid
PC sid
UniChem
Patents
Similars
| DrugBank
Article
PubMed
| 16 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Binding affinity to human beta2-adrenoceptor by radioligand binding assay |
Bioorg Med Chem Lett 22: 6280-5 (2012)
Article DOI: 10.1016/j.bmcl.2012.07.096
BindingDB Entry DOI: 10.7270/Q2G73G09 |
More data for this
Ligand-Target Pair | |
Beta-2 adrenergic receptor
(Homo sapiens (Human)) | BDBM50421327
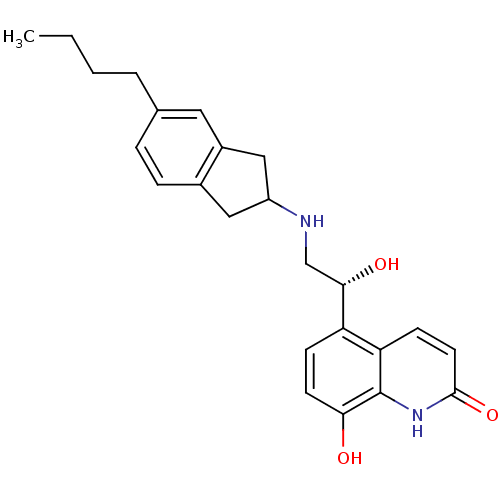
(CHEMBL2088199)Show SMILES CCCCc1ccc2CC(Cc2c1)NC[C@H](O)c1ccc(O)c2[nH]c(=O)ccc12 |r| Show InChI InChI=1S/C24H28N2O3/c1-2-3-4-15-5-6-16-12-18(13-17(16)11-15)25-14-22(28)19-7-9-21(27)24-20(19)8-10-23(29)26-24/h5-11,18,22,25,27-28H,2-4,12-14H2,1H3,(H,26,29)/t18?,22-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 19 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Binding affinity to human beta2-adrenoceptor by radioligand binding assay |
Bioorg Med Chem Lett 22: 6280-5 (2012)
Article DOI: 10.1016/j.bmcl.2012.07.096
BindingDB Entry DOI: 10.7270/Q2G73G09 |
More data for this
Ligand-Target Pair | |
Prostaglandin D2 receptor 2
(Homo sapiens (Human)) | BDBM50213911
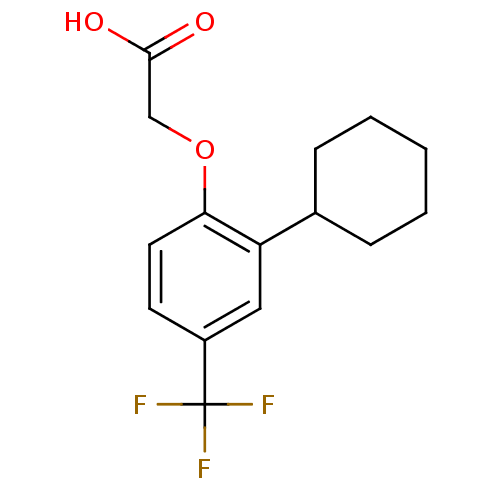
(2-(2-cyclohexyl-4-(trifluoromethyl)phenoxy)acetic ...)Show InChI InChI=1S/C15H17F3O3/c16-15(17,18)11-6-7-13(21-9-14(19)20)12(8-11)10-4-2-1-3-5-10/h6-8,10H,1-5,9H2,(H,19,20) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes of Biomedical Research
Curated by ChEMBL
| Assay Description
Displacement of [3H]PG2 from human CRTh2 receptor expressed in CHO cells |
Bioorg Med Chem Lett 17: 4347-50 (2007)
Article DOI: 10.1016/j.bmcl.2007.05.019
BindingDB Entry DOI: 10.7270/Q2N58M2M |
More data for this
Ligand-Target Pair | |
Beta-2 adrenergic receptor
(Homo sapiens (Human)) | BDBM50151720
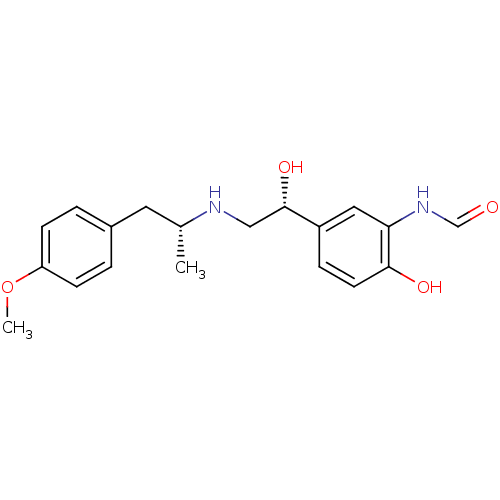
(ARFORMOTEROL TARTRATE | CHEMBL1363 | CHEMBL605993 ...)Show SMILES COc1ccc(C[C@@H](C)NC[C@H](O)c2ccc(O)c(NC=O)c2)cc1 |r| Show InChI InChI=1S/C19H24N2O4/c1-13(9-14-3-6-16(25-2)7-4-14)20-11-19(24)15-5-8-18(23)17(10-15)21-12-22/h3-8,10,12-13,19-20,23-24H,9,11H2,1-2H3,(H,21,22)/t13-,19+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
PDB
Article
PubMed
| 23 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research
Curated by ChEMBL
| Assay Description
Displacement of [125I]cyanopindolol from human recombinant beta2 adrenergic receptor expressed in CHO cells by filtration assay |
J Med Chem 53: 3675-84 (2010)
Article DOI: 10.1021/jm100068m
BindingDB Entry DOI: 10.7270/Q27H1JRT |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM50260765
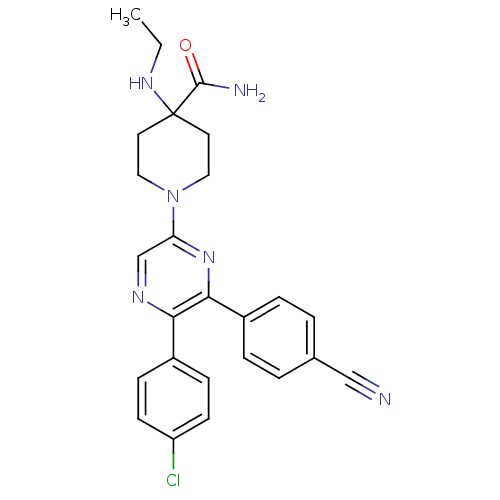
(1-(5-(4-chlorophenyl)-6-(4-cyanophenyl)pyrazin-2-y...)Show SMILES CCNC1(CCN(CC1)c1cnc(-c2ccc(Cl)cc2)c(n1)-c1ccc(cc1)C#N)C(N)=O Show InChI InChI=1S/C25H25ClN6O/c1-2-30-25(24(28)33)11-13-32(14-12-25)21-16-29-22(18-7-9-20(26)10-8-18)23(31-21)19-5-3-17(15-27)4-6-19/h3-10,16,30H,2,11-14H2,1H3,(H2,28,33) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 32.3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Neurogen Corporation
Curated by ChEMBL
| Assay Description
Antagonist activity at human CB1 receptor expressed in SF9 cells assessed as inhibition of CP-55940-stimulated GTPgammaS binding |
Bioorg Med Chem Lett 18: 3376-81 (2008)
Article DOI: 10.1016/j.bmcl.2008.04.022
BindingDB Entry DOI: 10.7270/Q2QR4Z0T |
More data for this
Ligand-Target Pair | |
Beta-2 adrenergic receptor
(Homo sapiens (Human)) | BDBM50151722

(CHEMBL185262 | N-(2-Hydroxy-5-{1-hydroxy-2-[2-(4-p...)Show SMILES O[C@@H](CNCCc1ccc(Oc2ccccc2)cc1)c1ccc(O)c(NC=O)c1 Show InChI InChI=1S/C23H24N2O4/c26-16-25-21-14-18(8-11-22(21)27)23(28)15-24-13-12-17-6-9-20(10-7-17)29-19-4-2-1-3-5-19/h1-11,14,16,23-24,27-28H,12-13,15H2,(H,25,26)/t23-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 35 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Horsham Research Centre
Curated by ChEMBL
| Assay Description
Binding affinity for human beta-2 adrenergic receptor |
Bioorg Med Chem Lett 14: 4705-10 (2004)
Article DOI: 10.1016/j.bmcl.2004.06.086
BindingDB Entry DOI: 10.7270/Q2416XSZ |
More data for this
Ligand-Target Pair | |
Genome polyprotein
(Dengue virus 2) | BDBM50175864

(Bz-Nle-Lys-Arg-Arg-B(OH)2 | CHEMBL199845)Show SMILES [#6]-[#6]-[#6]-[#6]-[#6@H](-[#7]-[#6](=O)-c1ccccc1)-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6]-[#6]-[#7])-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6]\[#7]=[#6](\[#7])-[#7])-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6]\[#7]=[#6](\[#7])-[#7])-[#6](=O)-[#5](-[#8])-[#8] Show InChI InChI=1S/C31H54BN11O7/c1-2-3-13-22(41-26(45)20-11-5-4-6-12-20)28(47)42-23(14-7-8-17-33)29(48)43-24(16-10-19-39-31(36)37)27(46)40-21(25(44)32(49)50)15-9-18-38-30(34)35/h4-6,11-12,21-24,49-50H,2-3,7-10,13-19,33H2,1H3,(H,40,46)(H,41,45)(H,42,47)(H,43,48)(H4,34,35,38)(H4,36,37,39)/t21-,22-,23-,24-/m0/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 43 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institute for Tropical Diseases
Curated by ChEMBL
| Assay Description
Inhibitory activity against CF40.NS3pro from Dengue virus type 2 |
Bioorg Med Chem Lett 16: 36-9 (2005)
Article DOI: 10.1016/j.bmcl.2005.09.062
BindingDB Entry DOI: 10.7270/Q2639P97 |
More data for this
Ligand-Target Pair | |
Beta-2 adrenergic receptor
(Homo sapiens (Human)) | BDBM50421325

(CHEMBL2088197)Show SMILES CCc1cc2CC(Cc2cc1CC)NC[C@H](O)c1ccc(O)c2NC(=O)CCc12 |r| Show InChI InChI=1S/C24H30N2O3/c1-3-14-9-16-11-18(12-17(16)10-15(14)4-2)25-13-22(28)19-5-7-21(27)24-20(19)6-8-23(29)26-24/h5,7,9-10,18,22,25,27-28H,3-4,6,8,11-13H2,1-2H3,(H,26,29)/t22-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 45 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Binding affinity to human beta2-adrenoceptor by radioligand binding assay |
Bioorg Med Chem Lett 22: 6280-5 (2012)
Article DOI: 10.1016/j.bmcl.2012.07.096
BindingDB Entry DOI: 10.7270/Q2G73G09 |
More data for this
Ligand-Target Pair | |
Prostaglandin D2 receptor 2
(Homo sapiens (Human)) | BDBM50213919
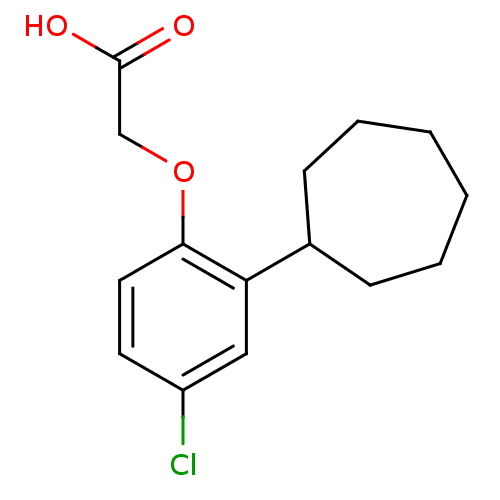
(2-(4-chloro-2-cycloheptylphenoxy)acetic acid | CHE...)Show InChI InChI=1S/C15H19ClO3/c16-12-7-8-14(19-10-15(17)18)13(9-12)11-5-3-1-2-4-6-11/h7-9,11H,1-6,10H2,(H,17,18) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 59 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes of Biomedical Research
Curated by ChEMBL
| Assay Description
Displacement of [3H]PG2 from human CRTh2 receptor expressed in CHO cells |
Bioorg Med Chem Lett 17: 4347-50 (2007)
Article DOI: 10.1016/j.bmcl.2007.05.019
BindingDB Entry DOI: 10.7270/Q2N58M2M |
More data for this
Ligand-Target Pair | |
Prostaglandin D2 receptor 2
(Homo sapiens (Human)) | BDBM50213912
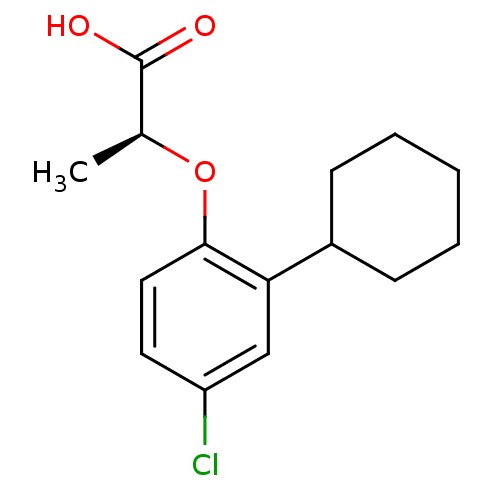
((S)-2-(4-chloro-2-cyclohexylphenoxy)propanoic acid...)Show InChI InChI=1S/C15H19ClO3/c1-10(15(17)18)19-14-8-7-12(16)9-13(14)11-5-3-2-4-6-11/h7-11H,2-6H2,1H3,(H,17,18)/t10-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 75 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes of Biomedical Research
Curated by ChEMBL
| Assay Description
Displacement of [3H]PG2 from human CRTh2 receptor expressed in CHO cells |
Bioorg Med Chem Lett 17: 4347-50 (2007)
Article DOI: 10.1016/j.bmcl.2007.05.019
BindingDB Entry DOI: 10.7270/Q2N58M2M |
More data for this
Ligand-Target Pair | |
Beta-2 adrenergic receptor
(Homo sapiens (Human)) | BDBM50318159

(8-Hydroxy-5-[(R)-1-hydroxy-2-(5,6-diethylindan-2-y...)Show SMILES CCc1cc2CC(Cc2cc1CC)NC[C@H](O)c1ccc(O)c2[nH]c(=O)ccc12 |r| Show InChI InChI=1S/C24H28N2O3/c1-3-14-9-16-11-18(12-17(16)10-15(14)4-2)25-13-22(28)19-5-7-21(27)24-20(19)6-8-23(29)26-24/h5-10,18,22,25,27-28H,3-4,11-13H2,1-2H3,(H,26,29)/t22-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
PC cid
PC sid
UniChem
Patents
Similars
| DrugBank
Article
PubMed
| 76 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research
Curated by ChEMBL
| Assay Description
Displacement of [125I]cyanopindolol from human recombinant beta2 adrenergic receptor expressed in CHO cells by filtration assay |
J Med Chem 53: 3675-84 (2010)
Article DOI: 10.1021/jm100068m
BindingDB Entry DOI: 10.7270/Q27H1JRT |
More data for this
Ligand-Target Pair | |
Beta-2 adrenergic receptor
(Homo sapiens (Human)) | BDBM50421330
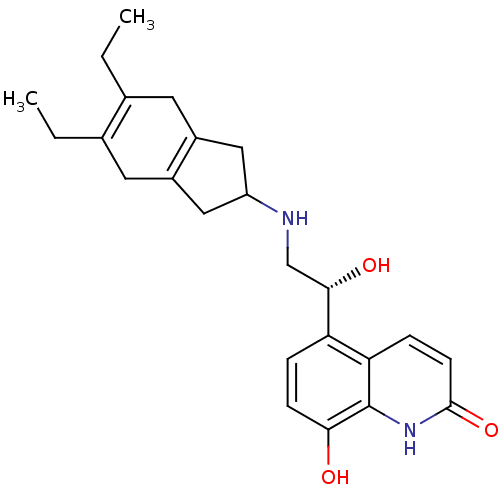
(CHEMBL2088202)Show SMILES CCC1=C(CC)CC2=C(CC(C2)NC[C@H](O)c2ccc(O)c3[nH]c(=O)ccc23)C1 |r,c:2,t:7| Show InChI InChI=1S/C24H30N2O3/c1-3-14-9-16-11-18(12-17(16)10-15(14)4-2)25-13-22(28)19-5-7-21(27)24-20(19)6-8-23(29)26-24/h5-8,18,22,25,27-28H,3-4,9-13H2,1-2H3,(H,26,29)/t22-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 77 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Binding affinity to human beta2-adrenoceptor by radioligand binding assay |
Bioorg Med Chem Lett 22: 6280-5 (2012)
Article DOI: 10.1016/j.bmcl.2012.07.096
BindingDB Entry DOI: 10.7270/Q2G73G09 |
More data for this
Ligand-Target Pair | |
Prostaglandin D2 receptor 2
(Homo sapiens (Human)) | BDBM50213922

(2-(4-bromo-2-cyclohexylphenoxy)acetic acid | CHEMB...)Show InChI InChI=1S/C14H17BrO3/c15-11-6-7-13(18-9-14(16)17)12(8-11)10-4-2-1-3-5-10/h6-8,10H,1-5,9H2,(H,16,17) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 83 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes of Biomedical Research
Curated by ChEMBL
| Assay Description
Displacement of [3H]PG2 from human CRTh2 receptor expressed in CHO cells |
Bioorg Med Chem Lett 17: 4347-50 (2007)
Article DOI: 10.1016/j.bmcl.2007.05.019
BindingDB Entry DOI: 10.7270/Q2N58M2M |
More data for this
Ligand-Target Pair | |
Prostaglandin D2 receptor 2
(Homo sapiens (Human)) | BDBM50213908

(2-(4-chloro-2-(1-methylcyclohexyl)phenoxy)acetic a...)Show InChI InChI=1S/C15H19ClO3/c1-15(7-3-2-4-8-15)12-9-11(16)5-6-13(12)19-10-14(17)18/h5-6,9H,2-4,7-8,10H2,1H3,(H,17,18) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes of Biomedical Research
Curated by ChEMBL
| Assay Description
Displacement of [3H]PG2 from human CRTh2 receptor expressed in CHO cells |
Bioorg Med Chem Lett 17: 4347-50 (2007)
Article DOI: 10.1016/j.bmcl.2007.05.019
BindingDB Entry DOI: 10.7270/Q2N58M2M |
More data for this
Ligand-Target Pair | |
Prostaglandin D2 receptor 2
(Homo sapiens (Human)) | BDBM50213907

((3-cyclohexyl-4'-fluoro-biphenyl-4-yloxy)-acetic a...)Show InChI InChI=1S/C20H21FO3/c21-17-9-6-14(7-10-17)16-8-11-19(24-13-20(22)23)18(12-16)15-4-2-1-3-5-15/h6-12,15H,1-5,13H2,(H,22,23) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 99 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes of Biomedical Research
Curated by ChEMBL
| Assay Description
Displacement of [3H]PG2 from human CRTh2 receptor expressed in CHO cells |
Bioorg Med Chem Lett 17: 4347-50 (2007)
Article DOI: 10.1016/j.bmcl.2007.05.019
BindingDB Entry DOI: 10.7270/Q2N58M2M |
More data for this
Ligand-Target Pair | |
Beta-2 adrenergic receptor
(Homo sapiens (Human)) | BDBM50318157
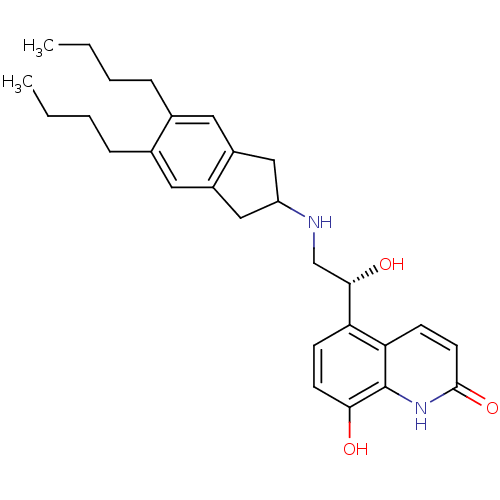
(8-Hydroxy-5-[(R)-1-hydroxy-2-(5,6-di-n-butylindan-...)Show SMILES CCCCc1cc2CC(Cc2cc1CCCC)NC[C@H](O)c1ccc(O)c2[nH]c(=O)ccc12 |r| Show InChI InChI=1S/C28H36N2O3/c1-3-5-7-18-13-20-15-22(16-21(20)14-19(18)8-6-4-2)29-17-26(32)23-9-11-25(31)28-24(23)10-12-27(33)30-28/h9-14,22,26,29,31-32H,3-8,15-17H2,1-2H3,(H,30,33)/t26-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 112 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research
Curated by ChEMBL
| Assay Description
Displacement of [125I]cyanopindolol from human recombinant beta2 adrenergic receptor expressed in CHO cells by filtration assay |
J Med Chem 53: 3675-84 (2010)
Article DOI: 10.1021/jm100068m
BindingDB Entry DOI: 10.7270/Q27H1JRT |
More data for this
Ligand-Target Pair | |
Beta-2 adrenergic receptor
(Homo sapiens (Human)) | BDBM50318158
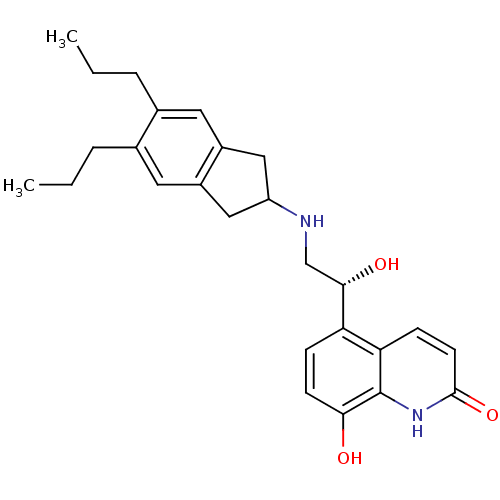
(8-Hydroxy-5-[(R)-1-hydroxy-2-(5,6-di-n-propylindan...)Show SMILES CCCc1cc2CC(Cc2cc1CCC)NC[C@H](O)c1ccc(O)c2[nH]c(=O)ccc12 |r| Show InChI InChI=1S/C26H32N2O3/c1-3-5-16-11-18-13-20(14-19(18)12-17(16)6-4-2)27-15-24(30)21-7-9-23(29)26-22(21)8-10-25(31)28-26/h7-12,20,24,27,29-30H,3-6,13-15H2,1-2H3,(H,28,31)/t24-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 119 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research
Curated by ChEMBL
| Assay Description
Displacement of [125I]cyanopindolol from human recombinant beta2 adrenergic receptor expressed in CHO cells by filtration assay |
J Med Chem 53: 3675-84 (2010)
Article DOI: 10.1021/jm100068m
BindingDB Entry DOI: 10.7270/Q27H1JRT |
More data for this
Ligand-Target Pair | |
Beta-1 adrenergic receptor
(Homo sapiens (Human)) | BDBM50421330

(CHEMBL2088202)Show SMILES CCC1=C(CC)CC2=C(CC(C2)NC[C@H](O)c2ccc(O)c3[nH]c(=O)ccc23)C1 |r,c:2,t:7| Show InChI InChI=1S/C24H30N2O3/c1-3-14-9-16-11-18(12-17(16)10-15(14)4-2)25-13-22(28)19-5-7-21(27)24-20(19)6-8-23(29)26-24/h5-8,18,22,25,27-28H,3-4,9-13H2,1-2H3,(H,26,29)/t22-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 133 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Binding affinity to human beta1-adrenoceptor by radioligand binding assay |
Bioorg Med Chem Lett 22: 6280-5 (2012)
Article DOI: 10.1016/j.bmcl.2012.07.096
BindingDB Entry DOI: 10.7270/Q2G73G09 |
More data for this
Ligand-Target Pair | |
Prostaglandin D2 receptor 2
(Homo sapiens (Human)) | BDBM50213917
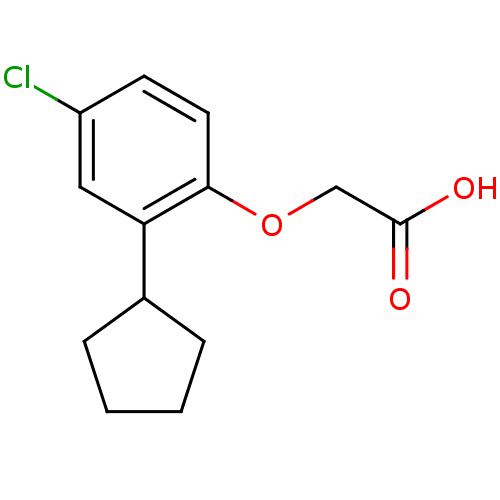
(2-(4-chloro-2-cyclopentylphenoxy)acetic acid | CHE...)Show InChI InChI=1S/C13H15ClO3/c14-10-5-6-12(17-8-13(15)16)11(7-10)9-3-1-2-4-9/h5-7,9H,1-4,8H2,(H,15,16) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 150 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes of Biomedical Research
Curated by ChEMBL
| Assay Description
Displacement of [3H]PG2 from human CRTh2 receptor expressed in CHO cells |
Bioorg Med Chem Lett 17: 4347-50 (2007)
Article DOI: 10.1016/j.bmcl.2007.05.019
BindingDB Entry DOI: 10.7270/Q2N58M2M |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data