 Found 228 hits with Last Name = 'beevers' and Initial = 'r'
Found 228 hits with Last Name = 'beevers' and Initial = 'r' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM50265362
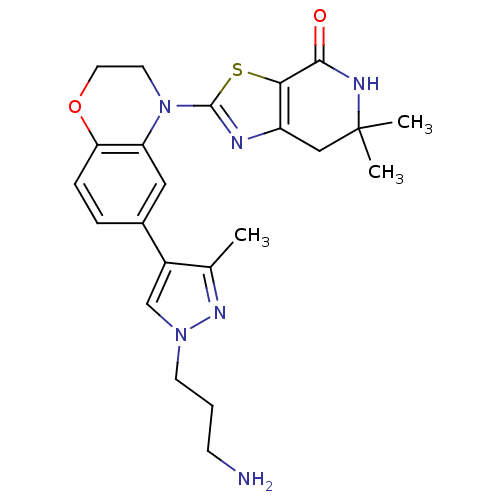
(2-(6-(1-(3-aminopropyl)-3-methyl-1H-pyrazol-4-yl)-...)Show SMILES Cc1nn(CCCN)cc1-c1ccc2OCCN(c3nc4CC(C)(C)NC(=O)c4s3)c2c1 Show InChI InChI=1S/C23H28N6O2S/c1-14-16(13-28(27-14)8-4-7-24)15-5-6-19-18(11-15)29(9-10-31-19)22-25-17-12-23(2,3)26-21(30)20(17)32-22/h5-6,11,13H,4,7-10,12,24H2,1-3H3,(H,26,30) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
UCB
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kdelta (unknown origin) |
Bioorg Med Chem Lett 18: 5299-302 (2008)
Article DOI: 10.1016/j.bmcl.2008.08.042
BindingDB Entry DOI: 10.7270/Q2WS8T3X |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM50265317
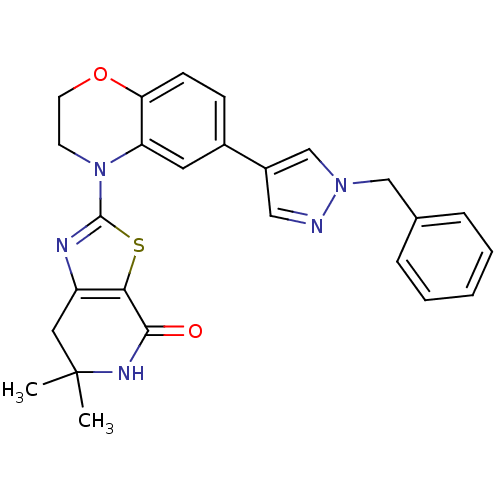
(2-(6-(1-benzyl-1H-pyrazol-4-yl)-2H-benzo[b][1,4]ox...)Show SMILES CC1(C)Cc2nc(sc2C(=O)N1)N1CCOc2ccc(cc12)-c1cnn(Cc2ccccc2)c1 Show InChI InChI=1S/C26H25N5O2S/c1-26(2)13-20-23(24(32)29-26)34-25(28-20)31-10-11-33-22-9-8-18(12-21(22)31)19-14-27-30(16-19)15-17-6-4-3-5-7-17/h3-9,12,14,16H,10-11,13,15H2,1-2H3,(H,29,32) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
UCB
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kdelta (unknown origin) |
Bioorg Med Chem Lett 18: 5299-302 (2008)
Article DOI: 10.1016/j.bmcl.2008.08.042
BindingDB Entry DOI: 10.7270/Q2WS8T3X |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM50265318
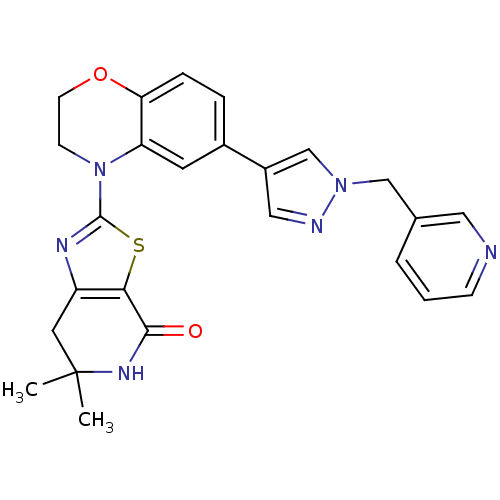
(6,6-dimethyl-2-(6-(1-(pyridin-3-ylmethyl)-1H-pyraz...)Show SMILES CC1(C)Cc2nc(sc2C(=O)N1)N1CCOc2ccc(cc12)-c1cnn(Cc2cccnc2)c1 Show InChI InChI=1S/C25H24N6O2S/c1-25(2)11-19-22(23(32)29-25)34-24(28-19)31-8-9-33-21-6-5-17(10-20(21)31)18-13-27-30(15-18)14-16-4-3-7-26-12-16/h3-7,10,12-13,15H,8-9,11,14H2,1-2H3,(H,29,32) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
UCB
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kdelta (unknown origin) |
Bioorg Med Chem Lett 18: 5299-302 (2008)
Article DOI: 10.1016/j.bmcl.2008.08.042
BindingDB Entry DOI: 10.7270/Q2WS8T3X |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM50264777
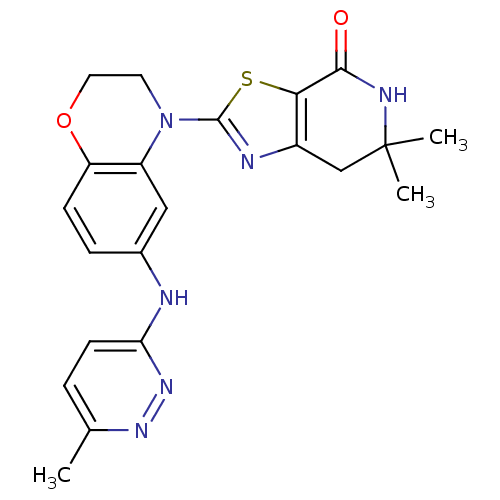
(6,6-dimethyl-2-(6-(6-methylpyridazin-3-ylamino)-2H...)Show SMILES Cc1ccc(Nc2ccc3OCCN(c4nc5CC(C)(C)NC(=O)c5s4)c3c2)nn1 Show InChI InChI=1S/C21H22N6O2S/c1-12-4-7-17(26-25-12)22-13-5-6-16-15(10-13)27(8-9-29-16)20-23-14-11-21(2,3)24-19(28)18(14)30-20/h4-7,10H,8-9,11H2,1-3H3,(H,22,26)(H,24,28) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
UCB
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kdelta (unknown origin) |
Bioorg Med Chem Lett 18: 5299-302 (2008)
Article DOI: 10.1016/j.bmcl.2008.08.042
BindingDB Entry DOI: 10.7270/Q2WS8T3X |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 10
(Homo sapiens (Human)) | BDBM50211428
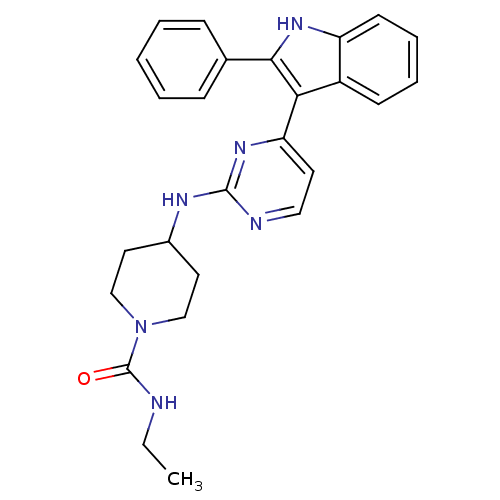
(CHEMBL245936 | N-ethyl-4-(4-(2-phenyl-1H-indol-3-y...)Show SMILES CCNC(=O)N1CCC(CC1)Nc1nccc(n1)-c1c([nH]c2ccccc12)-c1ccccc1 Show InChI InChI=1S/C26H28N6O/c1-2-27-26(33)32-16-13-19(14-17-32)29-25-28-15-12-22(31-25)23-20-10-6-7-11-21(20)30-24(23)18-8-4-3-5-9-18/h3-12,15,19,30H,2,13-14,16-17H2,1H3,(H,27,33)(H,28,29,31) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
UCB
Curated by ChEMBL
| Assay Description
Inhibition of JNK3 |
Bioorg Med Chem Lett 17: 3463-7 (2007)
Article DOI: 10.1016/j.bmcl.2007.03.078
BindingDB Entry DOI: 10.7270/Q2959H7W |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 9
(Homo sapiens (Human)) | BDBM50211428

(CHEMBL245936 | N-ethyl-4-(4-(2-phenyl-1H-indol-3-y...)Show SMILES CCNC(=O)N1CCC(CC1)Nc1nccc(n1)-c1c([nH]c2ccccc12)-c1ccccc1 Show InChI InChI=1S/C26H28N6O/c1-2-27-26(33)32-16-13-19(14-17-32)29-25-28-15-12-22(31-25)23-20-10-6-7-11-21(20)30-24(23)18-8-4-3-5-9-18/h3-12,15,19,30H,2,13-14,16-17H2,1H3,(H,27,33)(H,28,29,31) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
UCB
Curated by ChEMBL
| Assay Description
Inhibition of JNK2 |
Bioorg Med Chem Lett 17: 3463-7 (2007)
Article DOI: 10.1016/j.bmcl.2007.03.078
BindingDB Entry DOI: 10.7270/Q2959H7W |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM50264873

(6,6-dimethyl-2-(6-(6-phenylpyridazin-3-ylamino)-2H...)Show SMILES CC1(C)Cc2nc(sc2C(=O)N1)N1CCOc2ccc(Nc3ccc(nn3)-c3ccccc3)cc12 Show InChI InChI=1S/C26H24N6O2S/c1-26(2)15-19-23(24(33)29-26)35-25(28-19)32-12-13-34-21-10-8-17(14-20(21)32)27-22-11-9-18(30-31-22)16-6-4-3-5-7-16/h3-11,14H,12-13,15H2,1-2H3,(H,27,31)(H,29,33) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
UCB
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kdelta (unknown origin) |
Bioorg Med Chem Lett 18: 5299-302 (2008)
Article DOI: 10.1016/j.bmcl.2008.08.042
BindingDB Entry DOI: 10.7270/Q2WS8T3X |
More data for this
Ligand-Target Pair | |
Peptidase 1
(Dermatophagoides pteronyssinus (European house dus...) | BDBM103023
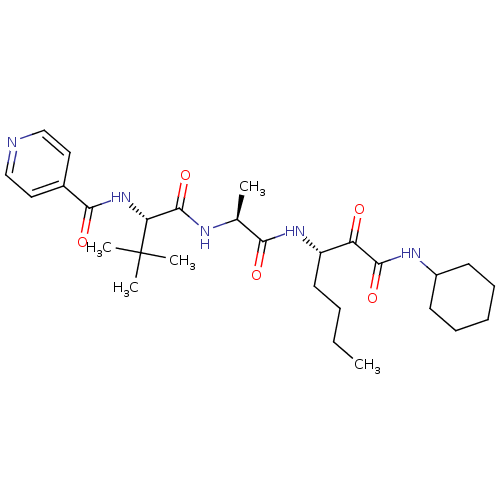
(US8541363, PVA-039)Show SMILES CCCC[C@H](NC(=O)[C@H](C)NC(=O)[C@@H](NC(=O)c1ccncc1)C(C)(C)C)C(=O)C(=O)NC1CCCCC1 |r| Show InChI InChI=1S/C28H43N5O5/c1-6-7-13-21(22(34)26(37)31-20-11-9-8-10-12-20)32-24(35)18(2)30-27(38)23(28(3,4)5)33-25(36)19-14-16-29-17-15-19/h14-18,20-21,23H,6-13H2,1-5H3,(H,30,38)(H,31,37)(H,32,35)(H,33,36)/t18-,21-,23+/m0/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 6.30 | n/a | n/a | n/a | n/a | n/a | n/a |
St George's Hosptial Medical School; The University of Manchester
US Patent
| Assay Description
The fluorogenic substrate used for measuring Der p 1 proteolytic activity was 2-aminobenzoylvalylalanylnorleucylseryl-(3-nitro)tyrosinyl aspartamide.... |
US Patent US8541363 (2013)
BindingDB Entry DOI: 10.7270/Q2PC3105 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM50264872

(2-(6-(6-methoxypyridazin-3-ylamino)-2H-benzo[b][1,...)Show SMILES COc1ccc(Nc2ccc3OCCN(c4nc5CC(C)(C)NC(=O)c5s4)c3c2)nn1 Show InChI InChI=1S/C21H22N6O3S/c1-21(2)11-13-18(19(28)24-21)31-20(23-13)27-8-9-30-15-5-4-12(10-14(15)27)22-16-6-7-17(29-3)26-25-16/h4-7,10H,8-9,11H2,1-3H3,(H,22,25)(H,24,28) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
UCB
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kdelta (unknown origin) |
Bioorg Med Chem Lett 18: 5299-302 (2008)
Article DOI: 10.1016/j.bmcl.2008.08.042
BindingDB Entry DOI: 10.7270/Q2WS8T3X |
More data for this
Ligand-Target Pair | |
Peptidase 1
(Dermatophagoides pteronyssinus (European house dus...) | BDBM103022
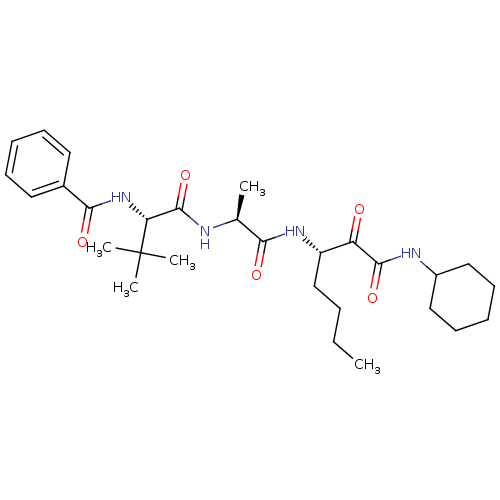
(US8541363, PVA-037)Show SMILES CCCC[C@H](NC(=O)[C@H](C)NC(=O)[C@@H](NC(=O)c1ccccc1)C(C)(C)C)C(=O)C(=O)NC1CCCCC1 |r| Show InChI InChI=1S/C29H44N4O5/c1-6-7-18-22(23(34)27(37)31-21-16-12-9-13-17-21)32-25(35)19(2)30-28(38)24(29(3,4)5)33-26(36)20-14-10-8-11-15-20/h8,10-11,14-15,19,21-22,24H,6-7,9,12-13,16-18H2,1-5H3,(H,30,38)(H,31,37)(H,32,35)(H,33,36)/t19-,22-,24+/m0/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 7.85 | n/a | n/a | n/a | n/a | n/a | n/a |
St George's Hosptial Medical School; The University of Manchester
US Patent
| Assay Description
The fluorogenic substrate used for measuring Der p 1 proteolytic activity was 2-aminobenzoylvalylalanylnorleucylseryl-(3-nitro)tyrosinyl aspartamide.... |
US Patent US8541363 (2013)
BindingDB Entry DOI: 10.7270/Q2PC3105 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM50265319
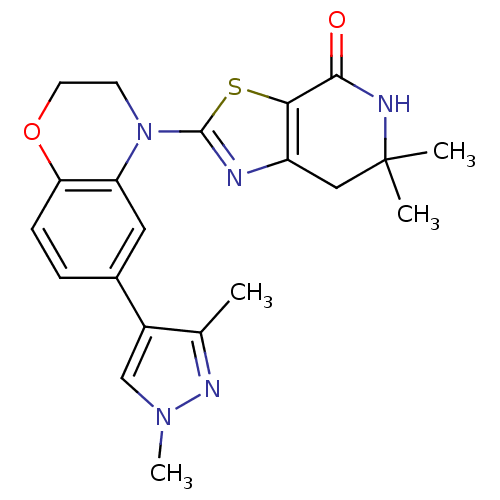
(2-(6-(1,3-dimethyl-1H-pyrazol-4-yl)-2H-benzo[b][1,...)Show SMILES Cc1nn(C)cc1-c1ccc2OCCN(c3nc4CC(C)(C)NC(=O)c4s3)c2c1 Show InChI InChI=1S/C21H23N5O2S/c1-12-14(11-25(4)24-12)13-5-6-17-16(9-13)26(7-8-28-17)20-22-15-10-21(2,3)23-19(27)18(15)29-20/h5-6,9,11H,7-8,10H2,1-4H3,(H,23,27) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
UCB
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kdelta (unknown origin) |
Bioorg Med Chem Lett 18: 5299-302 (2008)
Article DOI: 10.1016/j.bmcl.2008.08.042
BindingDB Entry DOI: 10.7270/Q2WS8T3X |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM50264748
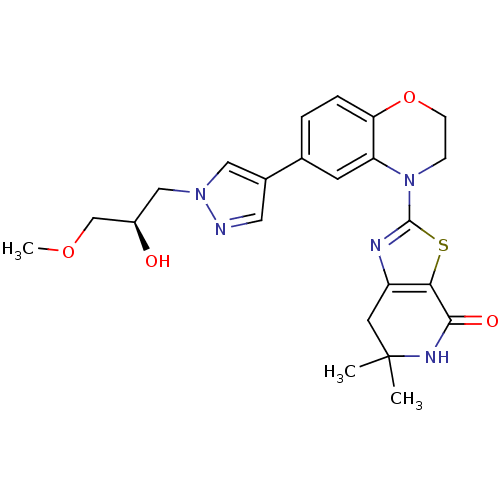
((R)-2-(6-(1-(2-hydroxy-3-methoxypropyl)-1H-pyrazol...)Show SMILES COC[C@H](O)Cn1cc(cn1)-c1ccc2OCCN(c3nc4CC(C)(C)NC(=O)c4s3)c2c1 |r| Show InChI InChI=1S/C23H27N5O4S/c1-23(2)9-17-20(21(30)26-23)33-22(25-17)28-6-7-32-19-5-4-14(8-18(19)28)15-10-24-27(11-15)12-16(29)13-31-3/h4-5,8,10-11,16,29H,6-7,9,12-13H2,1-3H3,(H,26,30)/t16-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
UCB
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kdelta (unknown origin) |
Bioorg Med Chem Lett 18: 5299-302 (2008)
Article DOI: 10.1016/j.bmcl.2008.08.042
BindingDB Entry DOI: 10.7270/Q2WS8T3X |
More data for this
Ligand-Target Pair | |
Genome polyprotein
(Hepatitis C virus) | BDBM50109999

((S)-4-[(S)-3-Carboxy-2-(3-carboxy-propionylamino)-...)Show SMILES CCCC[C@H](NC(=O)[C@H](CC(C)C)NC(=O)[C@@H](NC(=O)[C@H](Cc1ccccc1C)NC(=O)[C@H](CCC(O)=O)NC(=O)[C@H](CC(O)=O)NC(=O)CCC(O)=O)C(C)(C)C)C(=O)C(=O)N[C@@H](CC)c1ccccc1 Show InChI InChI=1S/C51H73N7O14/c1-9-11-21-34(43(66)49(71)53-33(10-2)31-18-13-12-14-19-31)54-46(68)36(26-29(3)4)57-50(72)44(51(6,7)8)58-48(70)37(27-32-20-16-15-17-30(32)5)56-45(67)35(22-24-40(60)61)55-47(69)38(28-42(64)65)52-39(59)23-25-41(62)63/h12-20,29,33-38,44H,9-11,21-28H2,1-8H3,(H,52,59)(H,53,71)(H,54,68)(H,55,69)(H,56,67)(H,57,72)(H,58,70)(H,60,61)(H,62,63)(H,64,65)/t33-,34-,35-,36-,37-,38-,44+/m0/s1 | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Discovery Welwyn
Curated by ChEMBL
| Assay Description
Inhibitory concentration of the compound against Hepatitis C virus NS3 proteinase |
Bioorg Med Chem Lett 12: 641-3 (2002)
BindingDB Entry DOI: 10.7270/Q2SN089N |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM50264871

(2-(6-(6-(dimethylamino)pyridazin-3-ylamino)-2H-ben...)Show SMILES CN(C)c1ccc(Nc2ccc3OCCN(c4nc5CC(C)(C)NC(=O)c5s4)c3c2)nn1 Show InChI InChI=1S/C22H25N7O2S/c1-22(2)12-14-19(20(30)25-22)32-21(24-14)29-9-10-31-16-6-5-13(11-15(16)29)23-17-7-8-18(27-26-17)28(3)4/h5-8,11H,9-10,12H2,1-4H3,(H,23,26)(H,25,30) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
UCB
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kdelta (unknown origin) |
Bioorg Med Chem Lett 18: 5299-302 (2008)
Article DOI: 10.1016/j.bmcl.2008.08.042
BindingDB Entry DOI: 10.7270/Q2WS8T3X |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 8
(Homo sapiens (Human)) | BDBM50211424

(4-(5-chloro-4-(1H-indol-3-yl)pyrimidin-2-ylamino)-...)Show SMILES CCNC(=O)N1CCC(CC1)Nc1ncc(Cl)c(n1)-c1c[nH]c2ccccc12 Show InChI InChI=1S/C20H23ClN6O/c1-2-22-20(28)27-9-7-13(8-10-27)25-19-24-12-16(21)18(26-19)15-11-23-17-6-4-3-5-14(15)17/h3-6,11-13,23H,2,7-10H2,1H3,(H,22,28)(H,24,25,26) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
DrugBank
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| MMDB
PDB
Article
PubMed
| n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
UCB
Curated by ChEMBL
| Assay Description
Inhibition of JNK1 |
Bioorg Med Chem Lett 17: 3463-7 (2007)
Article DOI: 10.1016/j.bmcl.2007.03.078
BindingDB Entry DOI: 10.7270/Q2959H7W |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Mitogen-activated protein kinase 8
(Homo sapiens (Human)) | BDBM50211436

(2-(4-(5-chloro-4-(1H-indol-3-yl)pyrimidin-2-ylamin...)Show SMILES CNC(=O)CN1CCC(CC1)Nc1ncc(Cl)c(n1)-c1c[nH]c2ccccc12 Show InChI InChI=1S/C20H23ClN6O/c1-22-18(28)12-27-8-6-13(7-9-27)25-20-24-11-16(21)19(26-20)15-10-23-17-5-3-2-4-14(15)17/h2-5,10-11,13,23H,6-9,12H2,1H3,(H,22,28)(H,24,25,26) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
UCB
Curated by ChEMBL
| Assay Description
Inhibition of JNK1 |
Bioorg Med Chem Lett 17: 3463-7 (2007)
Article DOI: 10.1016/j.bmcl.2007.03.078
BindingDB Entry DOI: 10.7270/Q2959H7W |
More data for this
Ligand-Target Pair | |
Peptidase 1
(Dermatophagoides pteronyssinus (European house dus...) | BDBM103021
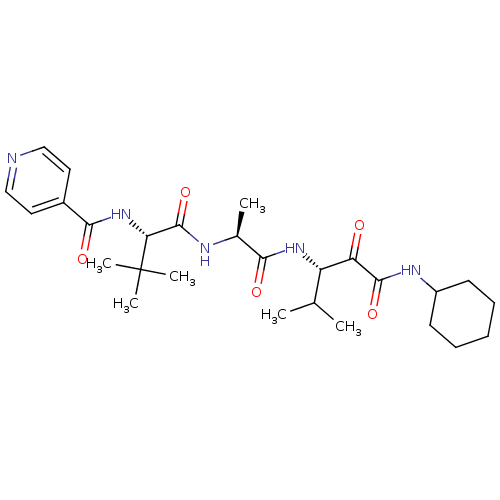
(US8541363, PVA-038)Show SMILES CC(C)[C@H](NC(=O)[C@H](C)NC(=O)[C@@H](NC(=O)c1ccncc1)C(C)(C)C)C(=O)C(=O)NC1CCCCC1 |r| Show InChI InChI=1S/C27H41N5O5/c1-16(2)20(21(33)25(36)30-19-10-8-7-9-11-19)31-23(34)17(3)29-26(37)22(27(4,5)6)32-24(35)18-12-14-28-15-13-18/h12-17,19-20,22H,7-11H2,1-6H3,(H,29,37)(H,30,36)(H,31,34)(H,32,35)/t17-,20-,22+/m0/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 13.3 | n/a | n/a | n/a | n/a | n/a | n/a |
St George's Hosptial Medical School; The University of Manchester
US Patent
| Assay Description
The fluorogenic substrate used for measuring Der p 1 proteolytic activity was 2-aminobenzoylvalylalanylnorleucylseryl-(3-nitro)tyrosinyl aspartamide.... |
US Patent US8541363 (2013)
BindingDB Entry DOI: 10.7270/Q2PC3105 |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 9
(Homo sapiens (Human)) | BDBM50211430
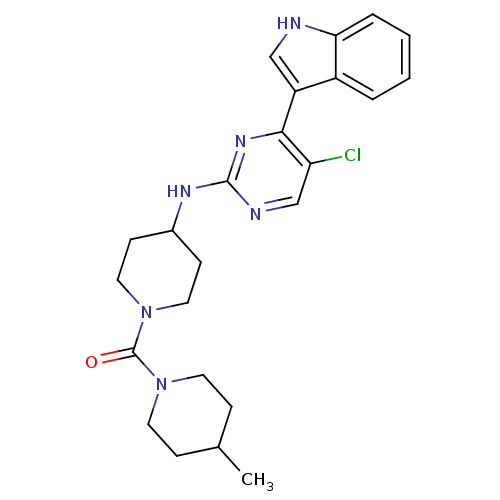
((4-(5-chloro-4-(1H-indol-3-yl)pyrimidin-2-ylamino)...)Show SMILES CC1CCN(CC1)C(=O)N1CCC(CC1)Nc1ncc(Cl)c(n1)-c1c[nH]c2ccccc12 Show InChI InChI=1S/C24H29ClN6O/c1-16-6-10-30(11-7-16)24(32)31-12-8-17(9-13-31)28-23-27-15-20(25)22(29-23)19-14-26-21-5-3-2-4-18(19)21/h2-5,14-17,26H,6-13H2,1H3,(H,27,28,29) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a |
UCB
Curated by ChEMBL
| Assay Description
Inhibition of JNK2 |
Bioorg Med Chem Lett 17: 3463-7 (2007)
Article DOI: 10.1016/j.bmcl.2007.03.078
BindingDB Entry DOI: 10.7270/Q2959H7W |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 10
(Homo sapiens (Human)) | BDBM50211436

(2-(4-(5-chloro-4-(1H-indol-3-yl)pyrimidin-2-ylamin...)Show SMILES CNC(=O)CN1CCC(CC1)Nc1ncc(Cl)c(n1)-c1c[nH]c2ccccc12 Show InChI InChI=1S/C20H23ClN6O/c1-22-18(28)12-27-8-6-13(7-9-27)25-20-24-11-16(21)19(26-20)15-10-23-17-5-3-2-4-14(15)17/h2-5,10-11,13,23H,6-9,12H2,1H3,(H,22,28)(H,24,25,26) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a |
UCB
Curated by ChEMBL
| Assay Description
Inhibition of JNK3 |
Bioorg Med Chem Lett 17: 3463-7 (2007)
Article DOI: 10.1016/j.bmcl.2007.03.078
BindingDB Entry DOI: 10.7270/Q2959H7W |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM50265364
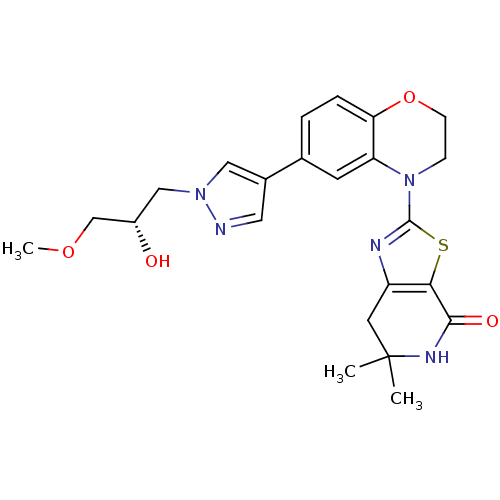
((S)-2-(6-(1-(2-hydroxy-3-methoxypropyl)-1H-pyrazol...)Show SMILES COC[C@@H](O)Cn1cc(cn1)-c1ccc2OCCN(c3nc4CC(C)(C)NC(=O)c4s3)c2c1 |r| Show InChI InChI=1S/C23H27N5O4S/c1-23(2)9-17-20(21(30)26-23)33-22(25-17)28-6-7-32-19-5-4-14(8-18(19)28)15-10-24-27(11-15)12-16(29)13-31-3/h4-5,8,10-11,16,29H,6-7,9,12-13H2,1-3H3,(H,26,30)/t16-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a |
UCB
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kdelta (unknown origin) |
Bioorg Med Chem Lett 18: 5299-302 (2008)
Article DOI: 10.1016/j.bmcl.2008.08.042
BindingDB Entry DOI: 10.7270/Q2WS8T3X |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM50265282
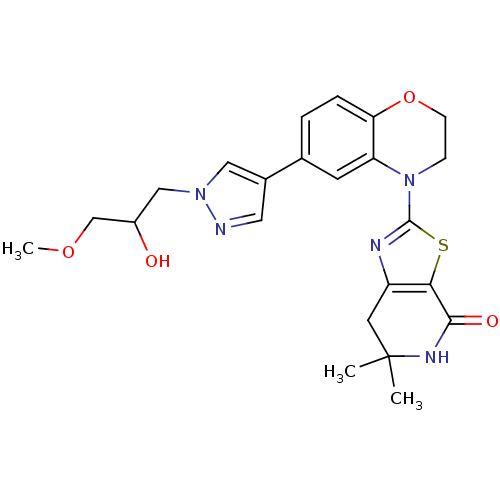
(2-(6-(1-(2-hydroxy-3-methoxypropyl)-1H-pyrazol-4-y...)Show SMILES COCC(O)Cn1cc(cn1)-c1ccc2OCCN(c3nc4CC(C)(C)NC(=O)c4s3)c2c1 Show InChI InChI=1S/C23H27N5O4S/c1-23(2)9-17-20(21(30)26-23)33-22(25-17)28-6-7-32-19-5-4-14(8-18(19)28)15-10-24-27(11-15)12-16(29)13-31-3/h4-5,8,10-11,16,29H,6-7,9,12-13H2,1-3H3,(H,26,30) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a |
UCB
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kdelta (unknown origin) |
Bioorg Med Chem Lett 18: 5299-302 (2008)
Article DOI: 10.1016/j.bmcl.2008.08.042
BindingDB Entry DOI: 10.7270/Q2WS8T3X |
More data for this
Ligand-Target Pair | |
Peptidase 1
(Dermatophagoides pteronyssinus (European house dus...) | BDBM103020
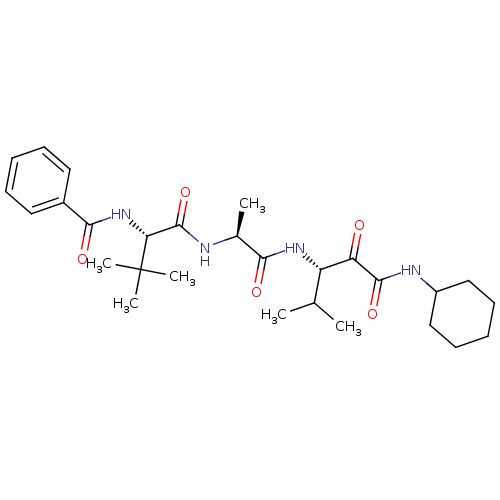
(US8541363, PVA-026)Show SMILES CC(C)[C@H](NC(=O)[C@H](C)NC(=O)[C@@H](NC(=O)c1ccccc1)C(C)(C)C)C(=O)C(=O)NC1CCCCC1 |r| Show InChI InChI=1S/C28H42N4O5/c1-17(2)21(22(33)26(36)30-20-15-11-8-12-16-20)31-24(34)18(3)29-27(37)23(28(4,5)6)32-25(35)19-13-9-7-10-14-19/h7,9-10,13-14,17-18,20-21,23H,8,11-12,15-16H2,1-6H3,(H,29,37)(H,30,36)(H,31,34)(H,32,35)/t18-,21-,23+/m0/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a |
St George's Hosptial Medical School; The University of Manchester
US Patent
| Assay Description
The fluorogenic substrate used for measuring Der p 1 proteolytic activity was 2-aminobenzoylvalylalanylnorleucylseryl-(3-nitro)tyrosinyl aspartamide.... |
US Patent US8541363 (2013)
BindingDB Entry DOI: 10.7270/Q2PC3105 |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 8
(Homo sapiens (Human)) | BDBM50211426

(2-((R)-3-(5-chloro-4-(1H-indol-3-yl)pyrimidin-2-yl...)Show SMILES CNC(=O)CN1CCC[C@H](C1)Nc1ncc(Cl)c(n1)-c1c[nH]c2ccccc12 Show InChI InChI=1S/C20H23ClN6O/c1-22-18(28)12-27-8-4-5-13(11-27)25-20-24-10-16(21)19(26-20)15-9-23-17-7-3-2-6-14(15)17/h2-3,6-7,9-10,13,23H,4-5,8,11-12H2,1H3,(H,22,28)(H,24,25,26)/t13-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
UCB
Curated by ChEMBL
| Assay Description
Inhibition of JNK1 |
Bioorg Med Chem Lett 17: 3463-7 (2007)
Article DOI: 10.1016/j.bmcl.2007.03.078
BindingDB Entry DOI: 10.7270/Q2959H7W |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 9
(Homo sapiens (Human)) | BDBM50211443
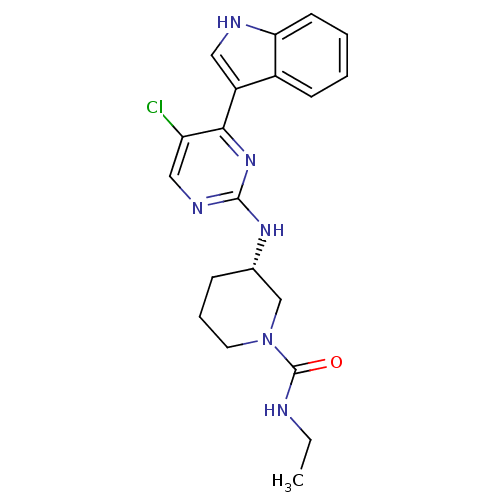
((3S)-3-(5-chloro-4-(1H-indol-3-yl)pyrimidin-2-ylam...)Show SMILES CCNC(=O)N1CCC[C@@H](C1)Nc1ncc(Cl)c(n1)-c1c[nH]c2ccccc12 Show InChI InChI=1S/C20H23ClN6O/c1-2-22-20(28)27-9-5-6-13(12-27)25-19-24-11-16(21)18(26-19)15-10-23-17-8-4-3-7-14(15)17/h3-4,7-8,10-11,13,23H,2,5-6,9,12H2,1H3,(H,22,28)(H,24,25,26)/t13-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
UCB
Curated by ChEMBL
| Assay Description
Inhibition of JNK2 |
Bioorg Med Chem Lett 17: 3463-7 (2007)
Article DOI: 10.1016/j.bmcl.2007.03.078
BindingDB Entry DOI: 10.7270/Q2959H7W |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 8
(Homo sapiens (Human)) | BDBM50211430

((4-(5-chloro-4-(1H-indol-3-yl)pyrimidin-2-ylamino)...)Show SMILES CC1CCN(CC1)C(=O)N1CCC(CC1)Nc1ncc(Cl)c(n1)-c1c[nH]c2ccccc12 Show InChI InChI=1S/C24H29ClN6O/c1-16-6-10-30(11-7-16)24(32)31-12-8-17(9-13-31)28-23-27-15-20(25)22(29-23)19-14-26-21-5-3-2-4-18(19)21/h2-5,14-17,26H,6-13H2,1H3,(H,27,28,29) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
UCB
Curated by ChEMBL
| Assay Description
Inhibition of JNK1 |
Bioorg Med Chem Lett 17: 3463-7 (2007)
Article DOI: 10.1016/j.bmcl.2007.03.078
BindingDB Entry DOI: 10.7270/Q2959H7W |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 8
(Homo sapiens (Human)) | BDBM50211438
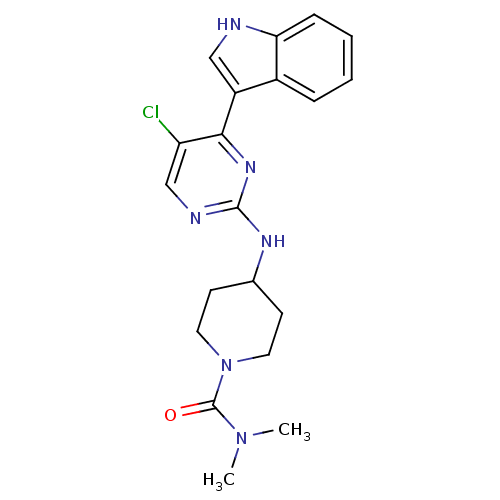
(4-(5-chloro-4-(1H-indol-3-yl)pyrimidin-2-ylamino)-...)Show SMILES CN(C)C(=O)N1CCC(CC1)Nc1ncc(Cl)c(n1)-c1c[nH]c2ccccc12 Show InChI InChI=1S/C20H23ClN6O/c1-26(2)20(28)27-9-7-13(8-10-27)24-19-23-12-16(21)18(25-19)15-11-22-17-6-4-3-5-14(15)17/h3-6,11-13,22H,7-10H2,1-2H3,(H,23,24,25) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
UCB
Curated by ChEMBL
| Assay Description
Inhibition of JNK1 |
Bioorg Med Chem Lett 17: 3463-7 (2007)
Article DOI: 10.1016/j.bmcl.2007.03.078
BindingDB Entry DOI: 10.7270/Q2959H7W |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM50262173
(2-(6-(1H-pyrazol-4-yl)-2,3-dihydrobenzo[b][1,4]oxa...)Show SMILES CC1(C)Cc2nc(sc2C(=O)N1)N1CCOc2ccc(cc12)-c1cn[nH]c1 Show InChI InChI=1S/C19H19N5O2S/c1-19(2)8-13-16(17(25)23-19)27-18(22-13)24-5-6-26-15-4-3-11(7-14(15)24)12-9-20-21-10-12/h3-4,7,9-10H,5-6,8H2,1-2H3,(H,20,21)(H,23,25) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 16 | n/a | n/a | n/a | n/a | n/a | n/a |
UCB
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kdelta (unknown origin) |
Bioorg Med Chem Lett 18: 5299-302 (2008)
Article DOI: 10.1016/j.bmcl.2008.08.042
BindingDB Entry DOI: 10.7270/Q2WS8T3X |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM50265280
(2-(6-(1-ethyl-1H-pyrazol-4-yl)-2H-benzo[b][1,4]oxa...)Show SMILES CCn1cc(cn1)-c1ccc2OCCN(c3nc4CC(C)(C)NC(=O)c4s3)c2c1 Show InChI InChI=1S/C21H23N5O2S/c1-4-25-12-14(11-22-25)13-5-6-17-16(9-13)26(7-8-28-17)20-23-15-10-21(2,3)24-19(27)18(15)29-20/h5-6,9,11-12H,4,7-8,10H2,1-3H3,(H,24,27) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 17 | n/a | n/a | n/a | n/a | n/a | n/a |
UCB
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kdelta (unknown origin) |
Bioorg Med Chem Lett 18: 5299-302 (2008)
Article DOI: 10.1016/j.bmcl.2008.08.042
BindingDB Entry DOI: 10.7270/Q2WS8T3X |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 8
(Homo sapiens (Human)) | BDBM50211429
((4-(5-chloro-4-(1H-indol-3-yl)pyrimidin-2-ylamino)...)Show SMILES CN1CCN(CC1)C(=O)N1CCC(CC1)Nc1ncc(Cl)c(n1)-c1c[nH]c2ccccc12 Show InChI InChI=1S/C23H28ClN7O/c1-29-10-12-31(13-11-29)23(32)30-8-6-16(7-9-30)27-22-26-15-19(24)21(28-22)18-14-25-20-5-3-2-4-17(18)20/h2-5,14-16,25H,6-13H2,1H3,(H,26,27,28) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 18 | n/a | n/a | n/a | n/a | n/a | n/a |
UCB
Curated by ChEMBL
| Assay Description
Inhibition of JNK1 |
Bioorg Med Chem Lett 17: 3463-7 (2007)
Article DOI: 10.1016/j.bmcl.2007.03.078
BindingDB Entry DOI: 10.7270/Q2959H7W |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform
(Homo sapiens (Human)) | BDBM50265319

(2-(6-(1,3-dimethyl-1H-pyrazol-4-yl)-2H-benzo[b][1,...)Show SMILES Cc1nn(C)cc1-c1ccc2OCCN(c3nc4CC(C)(C)NC(=O)c4s3)c2c1 Show InChI InChI=1S/C21H23N5O2S/c1-12-14(11-25(4)24-12)13-5-6-17-16(9-13)26(7-8-28-17)20-22-15-10-21(2,3)23-19(27)18(15)29-20/h5-6,9,11H,7-8,10H2,1-4H3,(H,23,27) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 18 | n/a | n/a | n/a | n/a | n/a | n/a |
UCB
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kgamma (unknown origin) |
Bioorg Med Chem Lett 18: 5299-302 (2008)
Article DOI: 10.1016/j.bmcl.2008.08.042
BindingDB Entry DOI: 10.7270/Q2WS8T3X |
More data for this
Ligand-Target Pair | |
Genome polyprotein
(Hepatitis C virus) | BDBM50110000
((S)-N-[(S)-1-((S)-1-{(S)-1-[(S)-1-((S)-1-Aminooxal...)Show SMILES CCCC[C@H](NC(=O)[C@H](CC(C)C)NC(=O)[C@@H](NC(=O)[C@H](Cc1ccccc1C)NC(=O)[C@@H](NC(=O)[C@H](CC(O)=O)NC(=O)CCC(O)=O)C(C)C)C(C)(C)C)C(=O)C(N)=O Show InChI InChI=1S/C42H65N7O12/c1-10-11-16-26(34(55)36(43)56)45-37(57)27(19-22(2)3)47-41(61)35(42(7,8)9)49-39(59)28(20-25-15-13-12-14-24(25)6)46-40(60)33(23(4)5)48-38(58)29(21-32(53)54)44-30(50)17-18-31(51)52/h12-15,22-23,26-29,33,35H,10-11,16-21H2,1-9H3,(H2,43,56)(H,44,50)(H,45,57)(H,46,60)(H,47,61)(H,48,58)(H,49,59)(H,51,52)(H,53,54)/t26-,27-,28-,29-,33-,35+/m0/s1 | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Discovery Welwyn
Curated by ChEMBL
| Assay Description
Inhibitory concentration of the compound against Hepatitis C virus NS3 proteinase |
Bioorg Med Chem Lett 12: 641-3 (2002)
BindingDB Entry DOI: 10.7270/Q2SN089N |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform
(Homo sapiens (Human)) | BDBM50264777

(6,6-dimethyl-2-(6-(6-methylpyridazin-3-ylamino)-2H...)Show SMILES Cc1ccc(Nc2ccc3OCCN(c4nc5CC(C)(C)NC(=O)c5s4)c3c2)nn1 Show InChI InChI=1S/C21H22N6O2S/c1-12-4-7-17(26-25-12)22-13-5-6-16-15(10-13)27(8-9-29-16)20-23-14-11-21(2,3)24-19(28)18(14)30-20/h4-7,10H,8-9,11H2,1-3H3,(H,22,26)(H,24,28) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
UCB
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kgamma (unknown origin) |
Bioorg Med Chem Lett 18: 5299-302 (2008)
Article DOI: 10.1016/j.bmcl.2008.08.042
BindingDB Entry DOI: 10.7270/Q2WS8T3X |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform
(Homo sapiens (Human)) | BDBM50264872

(2-(6-(6-methoxypyridazin-3-ylamino)-2H-benzo[b][1,...)Show SMILES COc1ccc(Nc2ccc3OCCN(c4nc5CC(C)(C)NC(=O)c5s4)c3c2)nn1 Show InChI InChI=1S/C21H22N6O3S/c1-21(2)11-13-18(19(28)24-21)31-20(23-13)27-8-9-30-15-5-4-12(10-14(15)27)22-16-6-7-17(29-3)26-25-16/h4-7,10H,8-9,11H2,1-3H3,(H,22,25)(H,24,28) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
UCB
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kgamma (unknown origin) |
Bioorg Med Chem Lett 18: 5299-302 (2008)
Article DOI: 10.1016/j.bmcl.2008.08.042
BindingDB Entry DOI: 10.7270/Q2WS8T3X |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM50264749
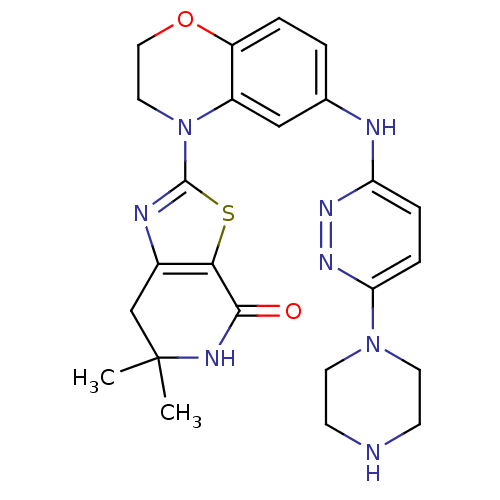
(6,6-dimethyl-2-(6-(6-(piperazin-1-yl)pyridazin-3-y...)Show SMILES CC1(C)Cc2nc(sc2C(=O)N1)N1CCOc2ccc(Nc3ccc(nn3)N3CCNCC3)cc12 Show InChI InChI=1S/C24H28N8O2S/c1-24(2)14-16-21(22(33)28-24)35-23(27-16)32-11-12-34-18-4-3-15(13-17(18)32)26-19-5-6-20(30-29-19)31-9-7-25-8-10-31/h3-6,13,25H,7-12,14H2,1-2H3,(H,26,29)(H,28,33) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 21 | n/a | n/a | n/a | n/a | n/a | n/a |
UCB
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kdelta (unknown origin) |
Bioorg Med Chem Lett 18: 5299-302 (2008)
Article DOI: 10.1016/j.bmcl.2008.08.042
BindingDB Entry DOI: 10.7270/Q2WS8T3X |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform
(Homo sapiens (Human)) | BDBM50262173

(2-(6-(1H-pyrazol-4-yl)-2,3-dihydrobenzo[b][1,4]oxa...)Show SMILES CC1(C)Cc2nc(sc2C(=O)N1)N1CCOc2ccc(cc12)-c1cn[nH]c1 Show InChI InChI=1S/C19H19N5O2S/c1-19(2)8-13-16(17(25)23-19)27-18(22-13)24-5-6-26-15-4-3-11(7-14(15)24)12-9-20-21-10-12/h3-4,7,9-10H,5-6,8H2,1-2H3,(H,20,21)(H,23,25) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 22 | n/a | n/a | n/a | n/a | n/a | n/a |
UCB
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kgamma (unknown origin) |
Bioorg Med Chem Lett 18: 5299-302 (2008)
Article DOI: 10.1016/j.bmcl.2008.08.042
BindingDB Entry DOI: 10.7270/Q2WS8T3X |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 8
(Homo sapiens (Human)) | BDBM50211428

(CHEMBL245936 | N-ethyl-4-(4-(2-phenyl-1H-indol-3-y...)Show SMILES CCNC(=O)N1CCC(CC1)Nc1nccc(n1)-c1c([nH]c2ccccc12)-c1ccccc1 Show InChI InChI=1S/C26H28N6O/c1-2-27-26(33)32-16-13-19(14-17-32)29-25-28-15-12-22(31-25)23-20-10-6-7-11-21(20)30-24(23)18-8-4-3-5-9-18/h3-12,15,19,30H,2,13-14,16-17H2,1H3,(H,27,33)(H,28,29,31) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 22 | n/a | n/a | n/a | n/a | n/a | n/a |
UCB
Curated by ChEMBL
| Assay Description
Inhibition of JNK1 |
Bioorg Med Chem Lett 17: 3463-7 (2007)
Article DOI: 10.1016/j.bmcl.2007.03.078
BindingDB Entry DOI: 10.7270/Q2959H7W |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 9
(Homo sapiens (Human)) | BDBM50211436

(2-(4-(5-chloro-4-(1H-indol-3-yl)pyrimidin-2-ylamin...)Show SMILES CNC(=O)CN1CCC(CC1)Nc1ncc(Cl)c(n1)-c1c[nH]c2ccccc12 Show InChI InChI=1S/C20H23ClN6O/c1-22-18(28)12-27-8-6-13(7-9-27)25-20-24-11-16(21)19(26-20)15-10-23-17-5-3-2-4-14(15)17/h2-5,10-11,13,23H,6-9,12H2,1H3,(H,22,28)(H,24,25,26) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 22 | n/a | n/a | n/a | n/a | n/a | n/a |
UCB
Curated by ChEMBL
| Assay Description
Inhibition of JNK2 |
Bioorg Med Chem Lett 17: 3463-7 (2007)
Article DOI: 10.1016/j.bmcl.2007.03.078
BindingDB Entry DOI: 10.7270/Q2959H7W |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit beta isoform
(Homo sapiens (Human)) | BDBM50264777

(6,6-dimethyl-2-(6-(6-methylpyridazin-3-ylamino)-2H...)Show SMILES Cc1ccc(Nc2ccc3OCCN(c4nc5CC(C)(C)NC(=O)c5s4)c3c2)nn1 Show InChI InChI=1S/C21H22N6O2S/c1-12-4-7-17(26-25-12)22-13-5-6-16-15(10-13)27(8-9-29-16)20-23-14-11-21(2,3)24-19(28)18(14)30-20/h4-7,10H,8-9,11H2,1-3H3,(H,22,26)(H,24,28) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 23 | n/a | n/a | n/a | n/a | n/a | n/a |
UCB
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kbeta (unknown origin) |
Bioorg Med Chem Lett 18: 5299-302 (2008)
Article DOI: 10.1016/j.bmcl.2008.08.042
BindingDB Entry DOI: 10.7270/Q2WS8T3X |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50264777

(6,6-dimethyl-2-(6-(6-methylpyridazin-3-ylamino)-2H...)Show SMILES Cc1ccc(Nc2ccc3OCCN(c4nc5CC(C)(C)NC(=O)c5s4)c3c2)nn1 Show InChI InChI=1S/C21H22N6O2S/c1-12-4-7-17(26-25-12)22-13-5-6-16-15(10-13)27(8-9-29-16)20-23-14-11-21(2,3)24-19(28)18(14)30-20/h4-7,10H,8-9,11H2,1-3H3,(H,22,26)(H,24,28) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 24 | n/a | n/a | n/a | n/a | n/a | n/a |
UCB
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kalpha (unknown origin) |
Bioorg Med Chem Lett 18: 5299-302 (2008)
Article DOI: 10.1016/j.bmcl.2008.08.042
BindingDB Entry DOI: 10.7270/Q2WS8T3X |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform
(Homo sapiens (Human)) | BDBM50264871

(2-(6-(6-(dimethylamino)pyridazin-3-ylamino)-2H-ben...)Show SMILES CN(C)c1ccc(Nc2ccc3OCCN(c4nc5CC(C)(C)NC(=O)c5s4)c3c2)nn1 Show InChI InChI=1S/C22H25N7O2S/c1-22(2)12-14-19(20(30)25-22)32-21(24-14)29-9-10-31-16-6-5-13(11-15(16)29)23-17-7-8-18(27-26-17)28(3)4/h5-8,11H,9-10,12H2,1-4H3,(H,23,26)(H,25,30) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 24 | n/a | n/a | n/a | n/a | n/a | n/a |
UCB
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kgamma (unknown origin) |
Bioorg Med Chem Lett 18: 5299-302 (2008)
Article DOI: 10.1016/j.bmcl.2008.08.042
BindingDB Entry DOI: 10.7270/Q2WS8T3X |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 9
(Homo sapiens (Human)) | BDBM50211424

(4-(5-chloro-4-(1H-indol-3-yl)pyrimidin-2-ylamino)-...)Show SMILES CCNC(=O)N1CCC(CC1)Nc1ncc(Cl)c(n1)-c1c[nH]c2ccccc12 Show InChI InChI=1S/C20H23ClN6O/c1-2-22-20(28)27-9-7-13(8-10-27)25-19-24-12-16(21)18(26-19)15-11-23-17-6-4-3-5-14(15)17/h3-6,11-13,23H,2,7-10H2,1H3,(H,22,28)(H,24,25,26) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
DrugBank
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| MMDB
PDB
Article
PubMed
| n/a | n/a | 25 | n/a | n/a | n/a | n/a | n/a | n/a |
UCB
Curated by ChEMBL
| Assay Description
Inhibition of JNK2 |
Bioorg Med Chem Lett 17: 3463-7 (2007)
Article DOI: 10.1016/j.bmcl.2007.03.078
BindingDB Entry DOI: 10.7270/Q2959H7W |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Mitogen-activated protein kinase 9
(Homo sapiens (Human)) | BDBM50211429

((4-(5-chloro-4-(1H-indol-3-yl)pyrimidin-2-ylamino)...)Show SMILES CN1CCN(CC1)C(=O)N1CCC(CC1)Nc1ncc(Cl)c(n1)-c1c[nH]c2ccccc12 Show InChI InChI=1S/C23H28ClN7O/c1-29-10-12-31(13-11-29)23(32)30-8-6-16(7-9-30)27-22-26-15-19(24)21(28-22)18-14-25-20-5-3-2-4-17(18)20/h2-5,14-16,25H,6-13H2,1H3,(H,26,27,28) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 26 | n/a | n/a | n/a | n/a | n/a | n/a |
UCB
Curated by ChEMBL
| Assay Description
Inhibition of JNK2 |
Bioorg Med Chem Lett 17: 3463-7 (2007)
Article DOI: 10.1016/j.bmcl.2007.03.078
BindingDB Entry DOI: 10.7270/Q2959H7W |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 8
(Homo sapiens (Human)) | BDBM50211444

(CHEMBL245535 | N-(2-(4-(5-chloro-4-(1H-indol-3-yl)...)Show SMILES CC(=O)NCC(=O)N1CCC(CC1)Nc1ncc(Cl)c(n1)-c1c[nH]c2ccccc12 Show InChI InChI=1S/C21H23ClN6O2/c1-13(29)23-12-19(30)28-8-6-14(7-9-28)26-21-25-11-17(22)20(27-21)16-10-24-18-5-3-2-4-15(16)18/h2-5,10-11,14,24H,6-9,12H2,1H3,(H,23,29)(H,25,26,27) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 28 | n/a | n/a | n/a | n/a | n/a | n/a |
UCB
Curated by ChEMBL
| Assay Description
Inhibition of JNK1 |
Bioorg Med Chem Lett 17: 3463-7 (2007)
Article DOI: 10.1016/j.bmcl.2007.03.078
BindingDB Entry DOI: 10.7270/Q2959H7W |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM50265283
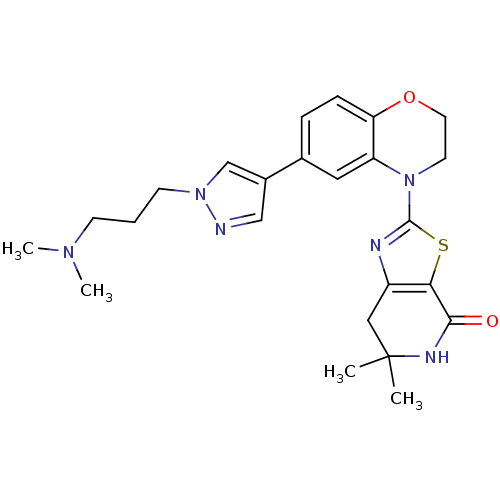
(2-(6-(1-(3-(dimethylamino)propyl)-1H-pyrazol-4-yl)...)Show SMILES CN(C)CCCn1cc(cn1)-c1ccc2OCCN(c3nc4CC(C)(C)NC(=O)c4s3)c2c1 Show InChI InChI=1S/C24H30N6O2S/c1-24(2)13-18-21(22(31)27-24)33-23(26-18)30-10-11-32-20-7-6-16(12-19(20)30)17-14-25-29(15-17)9-5-8-28(3)4/h6-7,12,14-15H,5,8-11,13H2,1-4H3,(H,27,31) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 29 | n/a | n/a | n/a | n/a | n/a | n/a |
UCB
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kdelta (unknown origin) |
Bioorg Med Chem Lett 18: 5299-302 (2008)
Article DOI: 10.1016/j.bmcl.2008.08.042
BindingDB Entry DOI: 10.7270/Q2WS8T3X |
More data for this
Ligand-Target Pair | |
Genome polyprotein
(Hepatitis C virus) | BDBM50109998
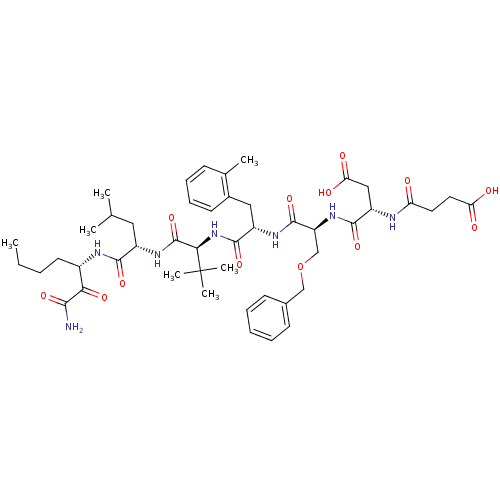
((S)-N-[(S)-1-((S)-1-{(S)-1-[(S)-1-((S)-1-Aminooxal...)Show SMILES CCCC[C@H](NC(=O)[C@H](CC(C)C)NC(=O)[C@@H](NC(=O)[C@H](Cc1ccccc1C)NC(=O)[C@H](COCc1ccccc1)NC(=O)[C@H](CC(O)=O)NC(=O)CCC(O)=O)C(C)(C)C)C(=O)C(N)=O Show InChI InChI=1S/C47H67N7O13/c1-8-9-19-31(39(60)41(48)61)50-42(62)32(22-27(2)3)52-46(66)40(47(5,6)7)54-44(64)33(23-30-18-14-13-15-28(30)4)51-45(65)35(26-67-25-29-16-11-10-12-17-29)53-43(63)34(24-38(58)59)49-36(55)20-21-37(56)57/h10-18,27,31-35,40H,8-9,19-26H2,1-7H3,(H2,48,61)(H,49,55)(H,50,62)(H,51,65)(H,52,66)(H,53,63)(H,54,64)(H,56,57)(H,58,59)/t31-,32-,33-,34-,35-,40+/m0/s1 | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| PubMed
| n/a | n/a | 29 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Discovery Welwyn
Curated by ChEMBL
| Assay Description
Inhibitory concentration of the compound against Hepatitis C virus NS3 proteinase |
Bioorg Med Chem Lett 12: 641-3 (2002)
BindingDB Entry DOI: 10.7270/Q2SN089N |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 8
(Homo sapiens (Human)) | BDBM50211443

((3S)-3-(5-chloro-4-(1H-indol-3-yl)pyrimidin-2-ylam...)Show SMILES CCNC(=O)N1CCC[C@@H](C1)Nc1ncc(Cl)c(n1)-c1c[nH]c2ccccc12 Show InChI InChI=1S/C20H23ClN6O/c1-2-22-20(28)27-9-5-6-13(12-27)25-19-24-11-16(21)18(26-19)15-10-23-17-8-4-3-7-14(15)17/h3-4,7-8,10-11,13,23H,2,5-6,9,12H2,1H3,(H,22,28)(H,24,25,26)/t13-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 29 | n/a | n/a | n/a | n/a | n/a | n/a |
UCB
Curated by ChEMBL
| Assay Description
Inhibition of JNK1 |
Bioorg Med Chem Lett 17: 3463-7 (2007)
Article DOI: 10.1016/j.bmcl.2007.03.078
BindingDB Entry DOI: 10.7270/Q2959H7W |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM50264750
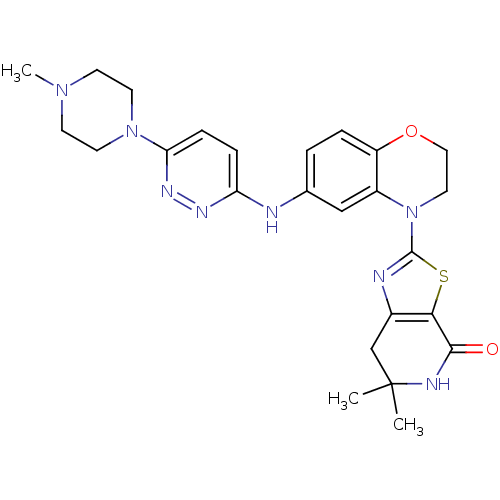
(6,6-dimethyl-2-(6-(6-(4-methylpiperazin-1-yl)pyrid...)Show SMILES CN1CCN(CC1)c1ccc(Nc2ccc3OCCN(c4nc5CC(C)(C)NC(=O)c5s4)c3c2)nn1 Show InChI InChI=1S/C25H30N8O2S/c1-25(2)15-17-22(23(34)28-25)36-24(27-17)33-12-13-35-19-5-4-16(14-18(19)33)26-20-6-7-21(30-29-20)32-10-8-31(3)9-11-32/h4-7,14H,8-13,15H2,1-3H3,(H,26,29)(H,28,34) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a |
UCB
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kdelta (unknown origin) |
Bioorg Med Chem Lett 18: 5299-302 (2008)
Article DOI: 10.1016/j.bmcl.2008.08.042
BindingDB Entry DOI: 10.7270/Q2WS8T3X |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 10
(Homo sapiens (Human)) | BDBM50211426

(2-((R)-3-(5-chloro-4-(1H-indol-3-yl)pyrimidin-2-yl...)Show SMILES CNC(=O)CN1CCC[C@H](C1)Nc1ncc(Cl)c(n1)-c1c[nH]c2ccccc12 Show InChI InChI=1S/C20H23ClN6O/c1-22-18(28)12-27-8-4-5-13(11-27)25-20-24-10-16(21)19(26-20)15-9-23-17-7-3-2-6-14(15)17/h2-3,6-7,9-10,13,23H,4-5,8,11-12H2,1H3,(H,22,28)(H,24,25,26)/t13-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 31 | n/a | n/a | n/a | n/a | n/a | n/a |
UCB
Curated by ChEMBL
| Assay Description
Inhibition of JNK3 |
Bioorg Med Chem Lett 17: 3463-7 (2007)
Article DOI: 10.1016/j.bmcl.2007.03.078
BindingDB Entry DOI: 10.7270/Q2959H7W |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 9
(Homo sapiens (Human)) | BDBM50211426

(2-((R)-3-(5-chloro-4-(1H-indol-3-yl)pyrimidin-2-yl...)Show SMILES CNC(=O)CN1CCC[C@H](C1)Nc1ncc(Cl)c(n1)-c1c[nH]c2ccccc12 Show InChI InChI=1S/C20H23ClN6O/c1-22-18(28)12-27-8-4-5-13(11-27)25-20-24-10-16(21)19(26-20)15-9-23-17-7-3-2-6-14(15)17/h2-3,6-7,9-10,13,23H,4-5,8,11-12H2,1H3,(H,22,28)(H,24,25,26)/t13-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 31 | n/a | n/a | n/a | n/a | n/a | n/a |
UCB
Curated by ChEMBL
| Assay Description
Inhibition of JNK2 |
Bioorg Med Chem Lett 17: 3463-7 (2007)
Article DOI: 10.1016/j.bmcl.2007.03.078
BindingDB Entry DOI: 10.7270/Q2959H7W |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM50265243

(2-(6-(3,5-dimethyl-1H-pyrazol-4-yl)-2H-benzo[b][1,...)Show SMILES Cc1n[nH]c(C)c1-c1ccc2OCCN(c3nc4CC(C)(C)NC(=O)c4s3)c2c1 Show InChI InChI=1S/C21H23N5O2S/c1-11-17(12(2)25-24-11)13-5-6-16-15(9-13)26(7-8-28-16)20-22-14-10-21(3,4)23-19(27)18(14)29-20/h5-6,9H,7-8,10H2,1-4H3,(H,23,27)(H,24,25) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 32 | n/a | n/a | n/a | n/a | n/a | n/a |
UCB
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kdelta (unknown origin) |
Bioorg Med Chem Lett 18: 5299-302 (2008)
Article DOI: 10.1016/j.bmcl.2008.08.042
BindingDB Entry DOI: 10.7270/Q2WS8T3X |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data