

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cannabinoid receptor 2 (Homo sapiens (Human)) | BDBM50334011 (4-(3-(4-(3-(trifluoromethyl)phenyl)-1H-imidazol-2-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 42 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratory Curated by ChEMBL | Assay Description Displacement of [3H]-CP55940 from human cloned CB2 receptor expressed in CHO cells | Bioorg Med Chem Lett 21: 182-5 (2010) Article DOI: 10.1016/j.bmcl.2010.11.044 BindingDB Entry DOI: 10.7270/Q20P108G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM50334011 (4-(3-(4-(3-(trifluoromethyl)phenyl)-1H-imidazol-2-...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratory Curated by ChEMBL | Assay Description Displacement of [3H]-CP55940 from human cloned CB1 receptor expressed in CHO cells | Bioorg Med Chem Lett 21: 182-5 (2010) Article DOI: 10.1016/j.bmcl.2010.11.044 BindingDB Entry DOI: 10.7270/Q20P108G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| KiSS-1 receptor (Homo sapiens (Human)) | BDBM50082051 (CHEMBL3422514) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 2.90 | n/a | n/a | n/a | n/a |
UMR7247; Universit£ Fran£ois Rabelais Tours; IFCE) Curated by ChEMBL | Assay Description Agonist activity at human wild-type KISS1R expressed in HEK293 cells assessed as induction of intracellular Ca2+ mobilization after 30 mins by Fluo4 ... | J Med Chem 58: 3459-70 (2015) Article DOI: 10.1021/jm5019675 BindingDB Entry DOI: 10.7270/Q2JD4ZHJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| KiSS-1 receptor (Homo sapiens (Human)) | BDBM50082064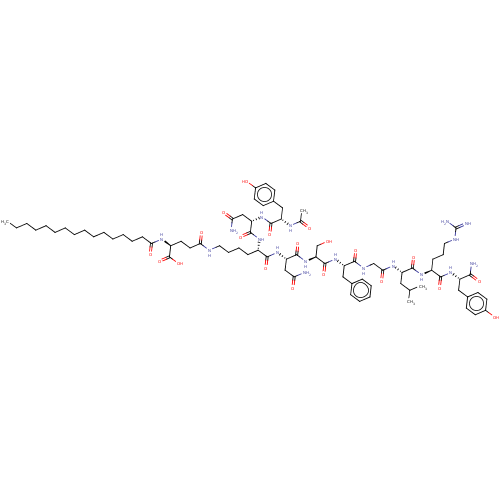 (CHEMBL3422515) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 24 | n/a | n/a | n/a | n/a |
UMR7247; Universit£ Fran£ois Rabelais Tours; IFCE) Curated by ChEMBL | Assay Description Agonist activity at human wild-type KISS1R expressed in HEK293 cells assessed as induction of intracellular Ca2+ mobilization after 30 mins by Fluo4 ... | J Med Chem 58: 3459-70 (2015) Article DOI: 10.1021/jm5019675 BindingDB Entry DOI: 10.7270/Q2JD4ZHJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| KiSS-1 receptor (Homo sapiens (Human)) | BDBM50082065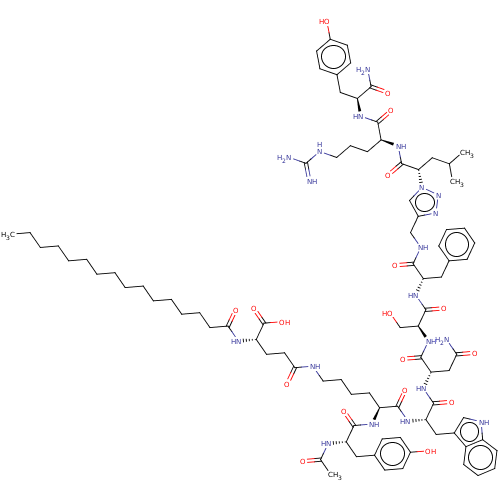 (CHEMBL3422516) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | 1.60 | n/a | n/a | n/a | n/a |
UMR7247; Universit£ Fran£ois Rabelais Tours; IFCE) Curated by ChEMBL | Assay Description Agonist activity at human wild-type KISS1R expressed in HEK293 cells assessed as induction of intracellular Ca2+ mobilization after 30 mins by Fluo4 ... | J Med Chem 58: 3459-70 (2015) Article DOI: 10.1021/jm5019675 BindingDB Entry DOI: 10.7270/Q2JD4ZHJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| KiSS-1 receptor (Homo sapiens (Human)) | BDBM50082066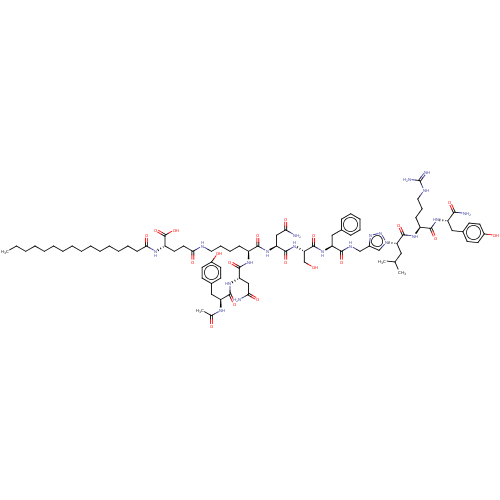 (CHEMBL3422517) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 216 | n/a | n/a | n/a | n/a |
UMR7247; Universit£ Fran£ois Rabelais Tours; IFCE) Curated by ChEMBL | Assay Description Agonist activity at human wild-type KISS1R expressed in HEK293 cells assessed as induction of intracellular Ca2+ mobilization after 30 mins by Fluo4 ... | J Med Chem 58: 3459-70 (2015) Article DOI: 10.1021/jm5019675 BindingDB Entry DOI: 10.7270/Q2JD4ZHJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| KiSS-1 receptor (Homo sapiens (Human)) | BDBM50082067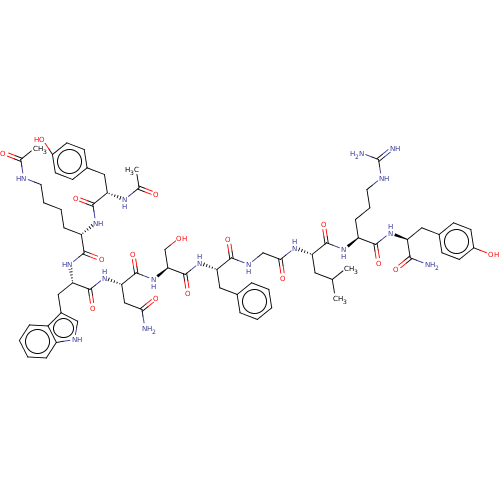 (CHEMBL3422413) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | 0.100 | n/a | n/a | n/a | n/a |
UMR7247; Universit£ Fran£ois Rabelais Tours; IFCE) Curated by ChEMBL | Assay Description Agonist activity at human wild-type KISS1R expressed in HEK293 cells assessed as induction of intracellular Ca2+ mobilization after 30 mins by Fluo4 ... | J Med Chem 58: 3459-70 (2015) Article DOI: 10.1021/jm5019675 BindingDB Entry DOI: 10.7270/Q2JD4ZHJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| KiSS-1 receptor (Homo sapiens (Human)) | BDBM50082068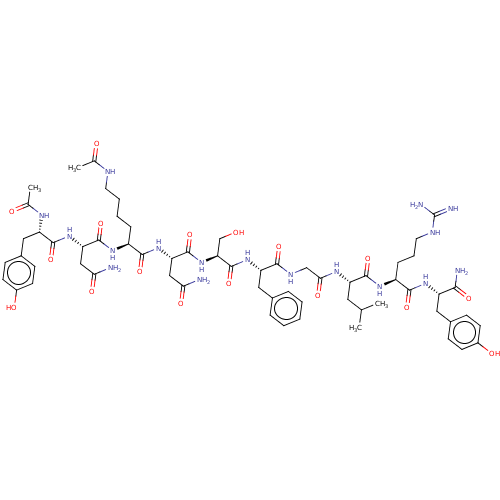 (CHEMBL3422414) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 0.0800 | n/a | n/a | n/a | n/a |
UMR7247; Universit£ Fran£ois Rabelais Tours; IFCE) Curated by ChEMBL | Assay Description Agonist activity at human wild-type KISS1R expressed in HEK293 cells assessed as induction of intracellular Ca2+ mobilization after 30 mins by Fluo4 ... | J Med Chem 58: 3459-70 (2015) Article DOI: 10.1021/jm5019675 BindingDB Entry DOI: 10.7270/Q2JD4ZHJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| KiSS-1 receptor (Homo sapiens (Human)) | BDBM50082069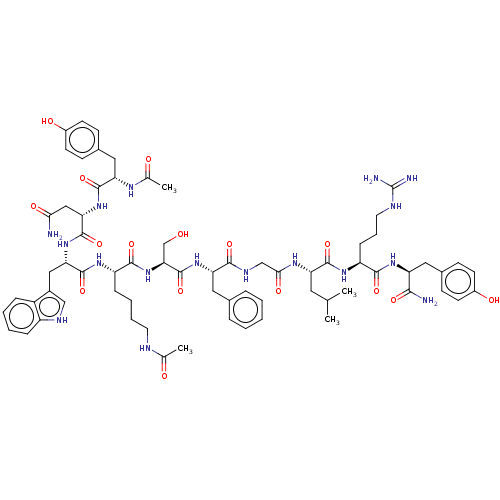 (CHEMBL3422510) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | 14 | n/a | n/a | n/a | n/a |
UMR7247; Universit£ Fran£ois Rabelais Tours; IFCE) Curated by ChEMBL | Assay Description Agonist activity at human wild-type KISS1R expressed in HEK293 cells assessed as induction of intracellular Ca2+ mobilization after 30 mins by Fluo4 ... | J Med Chem 58: 3459-70 (2015) Article DOI: 10.1021/jm5019675 BindingDB Entry DOI: 10.7270/Q2JD4ZHJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| KiSS-1 receptor (Homo sapiens (Human)) | BDBM50082070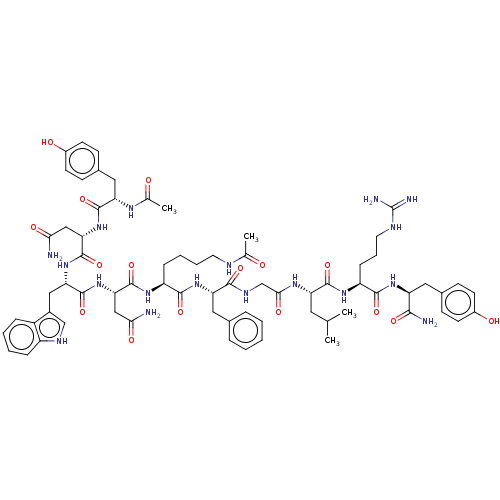 (CHEMBL3422511) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 7.20 | n/a | n/a | n/a | n/a |
UMR7247; Universit£ Fran£ois Rabelais Tours; IFCE) Curated by ChEMBL | Assay Description Agonist activity at human wild-type KISS1R expressed in HEK293 cells assessed as induction of intracellular Ca2+ mobilization after 30 mins by Fluo4 ... | J Med Chem 58: 3459-70 (2015) Article DOI: 10.1021/jm5019675 BindingDB Entry DOI: 10.7270/Q2JD4ZHJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| KiSS-1 receptor (Homo sapiens (Human)) | BDBM50082071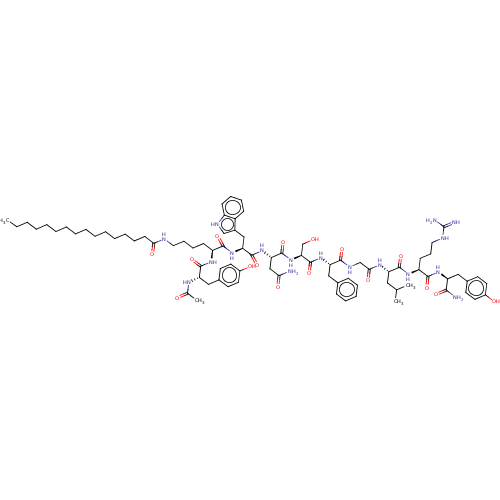 (CHEMBL3422512) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 182 | n/a | n/a | n/a | n/a |
UMR7247; Universit£ Fran£ois Rabelais Tours; IFCE) Curated by ChEMBL | Assay Description Agonist activity at human wild-type KISS1R expressed in HEK293 cells assessed as induction of intracellular Ca2+ mobilization after 30 mins by Fluo4 ... | J Med Chem 58: 3459-70 (2015) Article DOI: 10.1021/jm5019675 BindingDB Entry DOI: 10.7270/Q2JD4ZHJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| KiSS-1 receptor (Homo sapiens (Human)) | BDBM50082072 (CHEMBL3422513) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | >500 | n/a | n/a | n/a | n/a |
UMR7247; Universit£ Fran£ois Rabelais Tours; IFCE) Curated by ChEMBL | Assay Description Agonist activity at human wild-type KISS1R expressed in HEK293 cells assessed as induction of intracellular Ca2+ mobilization after 30 mins by Fluo4 ... | J Med Chem 58: 3459-70 (2015) Article DOI: 10.1021/jm5019675 BindingDB Entry DOI: 10.7270/Q2JD4ZHJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuropeptide FF receptor 1 (Homo sapiens (Human)) | BDBM50082073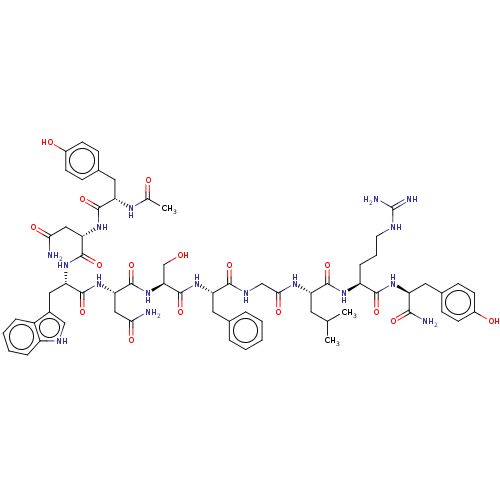 (CHEMBL3422407) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | >5.00E+3 | n/a | n/a | n/a | n/a |
UMR7247; Universit£ Fran£ois Rabelais Tours; IFCE) Curated by ChEMBL | Assay Description Agonist activity at human NPFF1R expressed in CHO cells assessed as effect on forskolin-induced cAMP accumulation after 15 mins by luminescence based... | J Med Chem 58: 3459-70 (2015) Article DOI: 10.1021/jm5019675 BindingDB Entry DOI: 10.7270/Q2JD4ZHJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuropeptide FF receptor 1 (Homo sapiens (Human)) | BDBM50082074 (CHEMBL3422408) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | >5.00E+3 | n/a | n/a | n/a | n/a |
UMR7247; Universit£ Fran£ois Rabelais Tours; IFCE) Curated by ChEMBL | Assay Description Agonist activity at human NPFF1R expressed in CHO cells assessed as effect on forskolin-induced cAMP accumulation after 15 mins by luminescence based... | J Med Chem 58: 3459-70 (2015) Article DOI: 10.1021/jm5019675 BindingDB Entry DOI: 10.7270/Q2JD4ZHJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuropeptide FF receptor 1 (Homo sapiens (Human)) | BDBM50082065 (CHEMBL3422516) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | 222 | n/a | n/a | n/a | n/a |
UMR7247; Universit£ Fran£ois Rabelais Tours; IFCE) Curated by ChEMBL | Assay Description Agonist activity at human NPFF1R expressed in CHO cells assessed as effect on forskolin-induced cAMP accumulation after 15 mins by luminescence based... | J Med Chem 58: 3459-70 (2015) Article DOI: 10.1021/jm5019675 BindingDB Entry DOI: 10.7270/Q2JD4ZHJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| KiSS-1 receptor (Homo sapiens (Human)) | BDBM50082075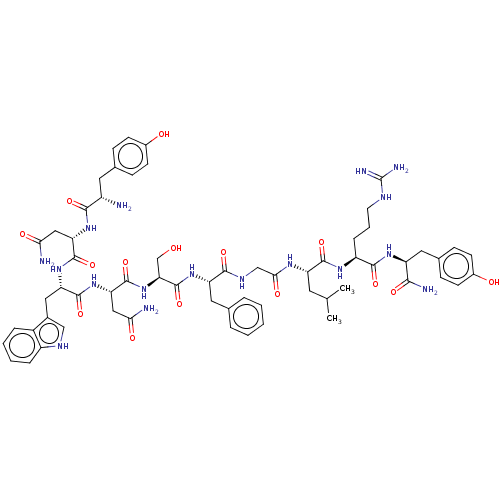 (CHEMBL3422406) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 2.5 | n/a | n/a | n/a | n/a |
UMR7247; Universit£ Fran£ois Rabelais Tours; IFCE) Curated by ChEMBL | Assay Description Agonist activity at human wild-type KISS1R expressed in HEK293 cells assessed as induction of intracellular Ca2+ mobilization after 30 mins by Fluo4 ... | J Med Chem 58: 3459-70 (2015) Article DOI: 10.1021/jm5019675 BindingDB Entry DOI: 10.7270/Q2JD4ZHJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| KiSS-1 receptor (Homo sapiens (Human)) | BDBM50082074 (CHEMBL3422408) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | 0.0700 | n/a | n/a | n/a | n/a |
UMR7247; Universit£ Fran£ois Rabelais Tours; IFCE) Curated by ChEMBL | Assay Description Agonist activity at human wild-type KISS1R expressed in HEK293 cells assessed as induction of intracellular Ca2+ mobilization after 30 mins by Fluo4 ... | J Med Chem 58: 3459-70 (2015) Article DOI: 10.1021/jm5019675 BindingDB Entry DOI: 10.7270/Q2JD4ZHJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| KiSS-1 receptor (Homo sapiens (Human)) | BDBM50082076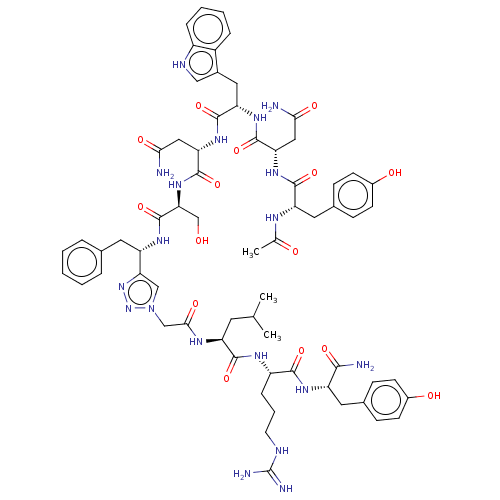 (CHEMBL3422409) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 0.600 | n/a | n/a | n/a | n/a |
UMR7247; Universit£ Fran£ois Rabelais Tours; IFCE) Curated by ChEMBL | Assay Description Agonist activity at human wild-type KISS1R expressed in HEK293 cells assessed as induction of intracellular Ca2+ mobilization after 30 mins by Fluo4 ... | J Med Chem 58: 3459-70 (2015) Article DOI: 10.1021/jm5019675 BindingDB Entry DOI: 10.7270/Q2JD4ZHJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| KiSS-1 receptor (Homo sapiens (Human)) | BDBM50082077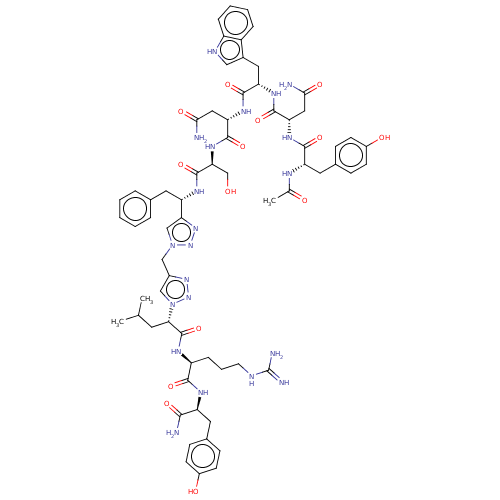 (CHEMBL3422410) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | 120 | n/a | n/a | n/a | n/a |
UMR7247; Universit£ Fran£ois Rabelais Tours; IFCE) Curated by ChEMBL | Assay Description Agonist activity at human wild-type KISS1R expressed in HEK293 cells assessed as induction of intracellular Ca2+ mobilization after 30 mins by Fluo4 ... | J Med Chem 58: 3459-70 (2015) Article DOI: 10.1021/jm5019675 BindingDB Entry DOI: 10.7270/Q2JD4ZHJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| KiSS-1 receptor (Homo sapiens (Human)) | BDBM50082078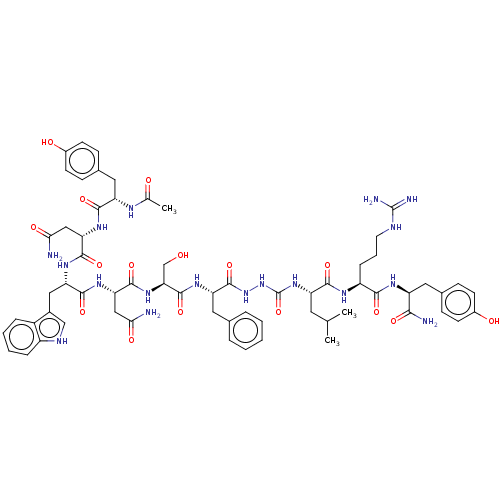 (CHEMBL3422411) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 0.00600 | n/a | n/a | n/a | n/a |
UMR7247; Universit£ Fran£ois Rabelais Tours; IFCE) Curated by ChEMBL | Assay Description Agonist activity at human wild-type KISS1R expressed in HEK293 cells assessed as induction of intracellular Ca2+ mobilization after 30 mins by Fluo4 ... | J Med Chem 58: 3459-70 (2015) Article DOI: 10.1021/jm5019675 BindingDB Entry DOI: 10.7270/Q2JD4ZHJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| KiSS-1 receptor (Homo sapiens (Human)) | BDBM50082079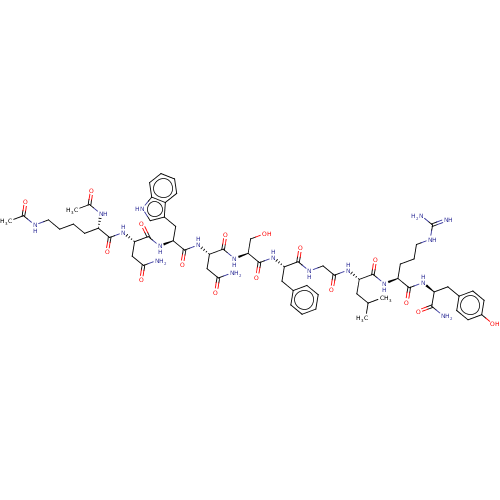 (CHEMBL3422412) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | 0.900 | n/a | n/a | n/a | n/a |
UMR7247; Universit£ Fran£ois Rabelais Tours; IFCE) Curated by ChEMBL | Assay Description Agonist activity at human wild-type KISS1R expressed in HEK293 cells assessed as induction of intracellular Ca2+ mobilization after 30 mins by Fluo4 ... | J Med Chem 58: 3459-70 (2015) Article DOI: 10.1021/jm5019675 BindingDB Entry DOI: 10.7270/Q2JD4ZHJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| KiSS-1 receptor (Homo sapiens (Human)) | BDBM50082073 (CHEMBL3422407) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 0.0700 | n/a | n/a | n/a | n/a |
UMR7247; Universit£ Fran£ois Rabelais Tours; IFCE) Curated by ChEMBL | Assay Description Agonist activity at human wild-type KISS1R expressed in HEK293 cells assessed as induction of intracellular Ca2+ mobilization after 30 mins by Fluo4 ... | J Med Chem 58: 3459-70 (2015) Article DOI: 10.1021/jm5019675 BindingDB Entry DOI: 10.7270/Q2JD4ZHJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM50334012 (4-(3-(1-isopropyl-4-(3-(trifluoromethyl)phenyl)-1H...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | >1.00E+3 | n/a | n/a | n/a | n/a |
Merck Research Laboratory Curated by ChEMBL | Assay Description Agonist activity at human cloned CB1 receptor expressed in CHO cells assessed as inhibition of forskolin-induced cAMP accumulation | Bioorg Med Chem Lett 21: 182-5 (2010) Article DOI: 10.1016/j.bmcl.2010.11.044 BindingDB Entry DOI: 10.7270/Q20P108G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM50334013 (4-(3-(1-(cyclopropylmethyl)-4-(3-(trifluoromethyl)...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | >1.00E+3 | n/a | n/a | n/a | n/a |
Merck Research Laboratory Curated by ChEMBL | Assay Description Agonist activity at human cloned CB1 receptor expressed in CHO cells assessed as inhibition of forskolin-induced cAMP accumulation | Bioorg Med Chem Lett 21: 182-5 (2010) Article DOI: 10.1016/j.bmcl.2010.11.044 BindingDB Entry DOI: 10.7270/Q20P108G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM50334014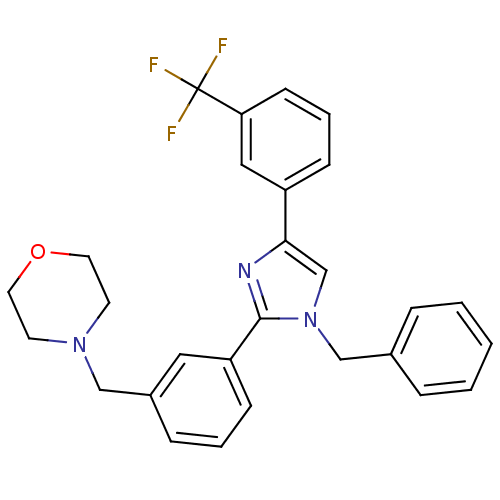 (4-(3-(1-benzyl-4-(3-(trifluoromethyl)phenyl)-1H-im...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | >1.00E+3 | n/a | n/a | n/a | n/a |
Merck Research Laboratory Curated by ChEMBL | Assay Description Agonist activity at human cloned CB1 receptor expressed in CHO cells assessed as inhibition of forskolin-induced cAMP accumulation | Bioorg Med Chem Lett 21: 182-5 (2010) Article DOI: 10.1016/j.bmcl.2010.11.044 BindingDB Entry DOI: 10.7270/Q20P108G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM50334015 (1-(2-(3-(morpholinomethyl)phenyl)-4-(3-(trifluorom...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | >1.00E+3 | n/a | n/a | n/a | n/a |
Merck Research Laboratory Curated by ChEMBL | Assay Description Agonist activity at human cloned CB1 receptor expressed in CHO cells assessed as inhibition of forskolin-induced cAMP accumulation | Bioorg Med Chem Lett 21: 182-5 (2010) Article DOI: 10.1016/j.bmcl.2010.11.044 BindingDB Entry DOI: 10.7270/Q20P108G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM50334011 (4-(3-(4-(3-(trifluoromethyl)phenyl)-1H-imidazol-2-...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | >1.00E+3 | n/a | n/a | n/a | n/a |
Merck Research Laboratory Curated by ChEMBL | Assay Description Agonist activity at human cloned CB1 receptor expressed in CHO cells assessed as inhibition of forskolin-induced cAMP accumulation | Bioorg Med Chem Lett 21: 182-5 (2010) Article DOI: 10.1016/j.bmcl.2010.11.044 BindingDB Entry DOI: 10.7270/Q20P108G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM50334016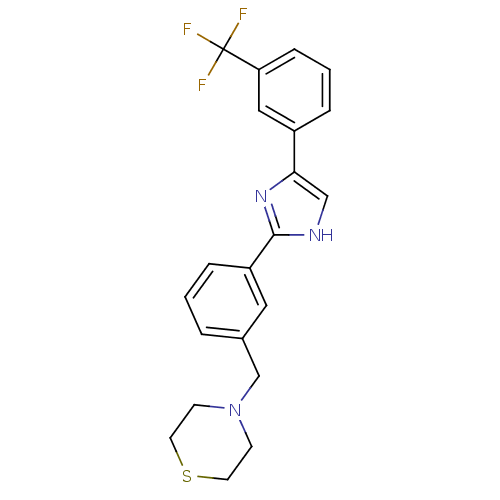 (4-(3-(4-(3-(trifluoromethyl)phenyl)-1H-imidazol-2-...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | n/a | >1.00E+3 | n/a | n/a | n/a | n/a |
Merck Research Laboratory Curated by ChEMBL | Assay Description Agonist activity at human cloned CB1 receptor expressed in CHO cells assessed as inhibition of forskolin-induced cAMP accumulation | Bioorg Med Chem Lett 21: 182-5 (2010) Article DOI: 10.1016/j.bmcl.2010.11.044 BindingDB Entry DOI: 10.7270/Q20P108G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM50334017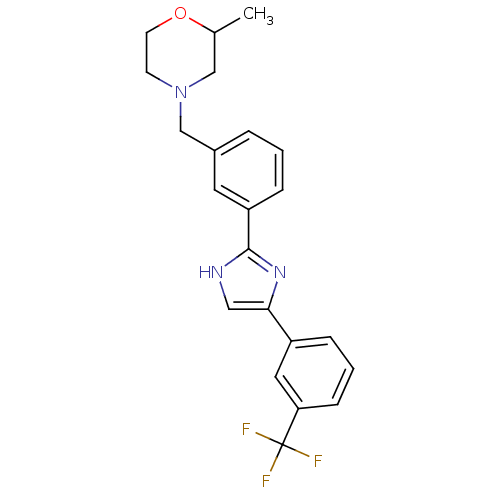 (2-methyl-4-(3-(4-(3-(trifluoromethyl)phenyl)-1H-im...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a |
Merck Research Laboratory Curated by ChEMBL | Assay Description Agonist activity at human cloned CB1 receptor expressed in CHO cells assessed as inhibition of forskolin-induced cAMP accumulation | Bioorg Med Chem Lett 21: 182-5 (2010) Article DOI: 10.1016/j.bmcl.2010.11.044 BindingDB Entry DOI: 10.7270/Q20P108G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM50334018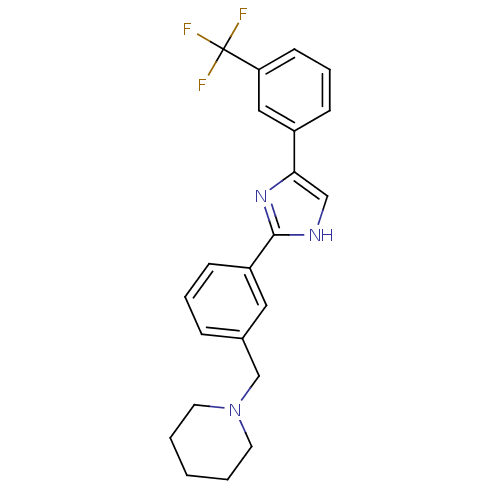 (1-(3-(4-(3-(trifluoromethyl)phenyl)-1H-imidazol-2-...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a |
Merck Research Laboratory Curated by ChEMBL | Assay Description Agonist activity at human cloned CB1 receptor expressed in CHO cells assessed as inhibition of forskolin-induced cAMP accumulation | Bioorg Med Chem Lett 21: 182-5 (2010) Article DOI: 10.1016/j.bmcl.2010.11.044 BindingDB Entry DOI: 10.7270/Q20P108G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM50334019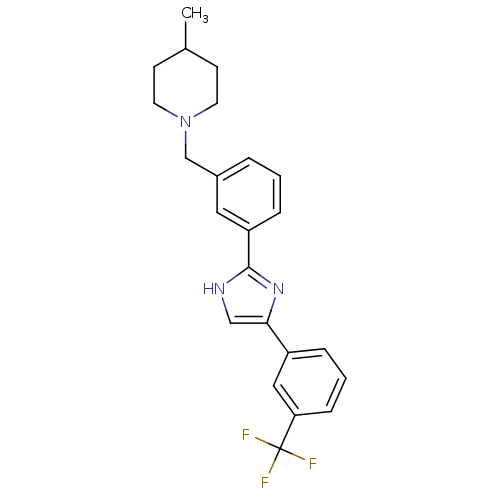 (4-methyl-1-(3-(4-(3-(trifluoromethyl)phenyl)-1H-im...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a |
Merck Research Laboratory Curated by ChEMBL | Assay Description Agonist activity at human cloned CB1 receptor expressed in CHO cells assessed as inhibition of forskolin-induced cAMP accumulation | Bioorg Med Chem Lett 21: 182-5 (2010) Article DOI: 10.1016/j.bmcl.2010.11.044 BindingDB Entry DOI: 10.7270/Q20P108G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM50334020 (4-fluoro-1-(3-(4-(3-(trifluoromethyl)phenyl)-1H-im...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 2.29E+3 | n/a | n/a | n/a | n/a |
Merck Research Laboratory Curated by ChEMBL | Assay Description Agonist activity at human cloned CB1 receptor expressed in CHO cells assessed as inhibition of forskolin-induced cAMP accumulation | Bioorg Med Chem Lett 21: 182-5 (2010) Article DOI: 10.1016/j.bmcl.2010.11.044 BindingDB Entry DOI: 10.7270/Q20P108G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM50334021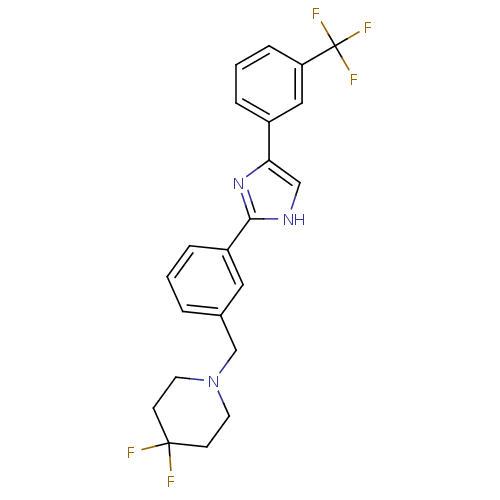 (4,4-difluoro-1-(3-(4-(3-(trifluoromethyl)phenyl)-1...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 2.55E+3 | n/a | n/a | n/a | n/a |
Merck Research Laboratory Curated by ChEMBL | Assay Description Agonist activity at human cloned CB1 receptor expressed in CHO cells assessed as inhibition of forskolin-induced cAMP accumulation | Bioorg Med Chem Lett 21: 182-5 (2010) Article DOI: 10.1016/j.bmcl.2010.11.044 BindingDB Entry DOI: 10.7270/Q20P108G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM50334022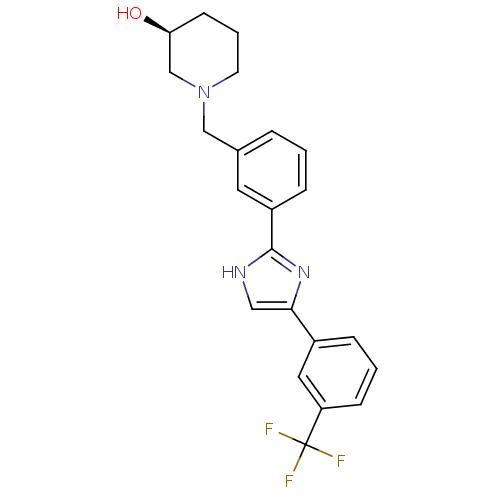 ((S)-1-(3-(4-(3-(trifluoromethyl)phenyl)-1H-imidazo...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a |
Merck Research Laboratory Curated by ChEMBL | Assay Description Agonist activity at human cloned CB1 receptor expressed in CHO cells assessed as inhibition of forskolin-induced cAMP accumulation | Bioorg Med Chem Lett 21: 182-5 (2010) Article DOI: 10.1016/j.bmcl.2010.11.044 BindingDB Entry DOI: 10.7270/Q20P108G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM50334023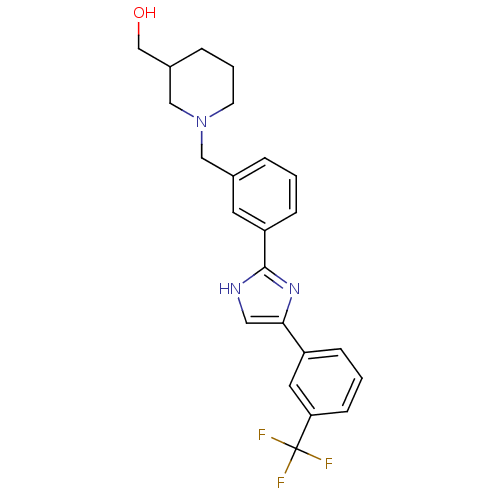 ((1-(3-(4-(3-(trifluoromethyl)phenyl)-1H-imidazol-2...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | >1.00E+3 | n/a | n/a | n/a | n/a |
Merck Research Laboratory Curated by ChEMBL | Assay Description Agonist activity at human cloned CB1 receptor expressed in CHO cells assessed as inhibition of forskolin-induced cAMP accumulation | Bioorg Med Chem Lett 21: 182-5 (2010) Article DOI: 10.1016/j.bmcl.2010.11.044 BindingDB Entry DOI: 10.7270/Q20P108G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM50334024 (4-(3-(4-(3-bromophenyl)-1H-imidazol-2-yl)benzyl)mo...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | n/a | 665 | n/a | n/a | n/a | n/a |
Merck Research Laboratory Curated by ChEMBL | Assay Description Agonist activity at human cloned CB1 receptor expressed in CHO cells assessed as inhibition of forskolin-induced cAMP accumulation | Bioorg Med Chem Lett 21: 182-5 (2010) Article DOI: 10.1016/j.bmcl.2010.11.044 BindingDB Entry DOI: 10.7270/Q20P108G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM50334025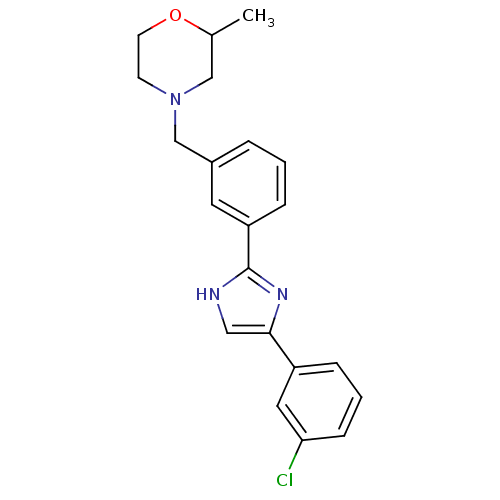 (4-(3-(4-(3-chlorophenyl)-1H-imidazol-2-yl)benzyl)-...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 3.25E+3 | n/a | n/a | n/a | n/a |
Merck Research Laboratory Curated by ChEMBL | Assay Description Agonist activity at human cloned CB1 receptor expressed in CHO cells assessed as inhibition of forskolin-induced cAMP accumulation | Bioorg Med Chem Lett 21: 182-5 (2010) Article DOI: 10.1016/j.bmcl.2010.11.044 BindingDB Entry DOI: 10.7270/Q20P108G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM50334026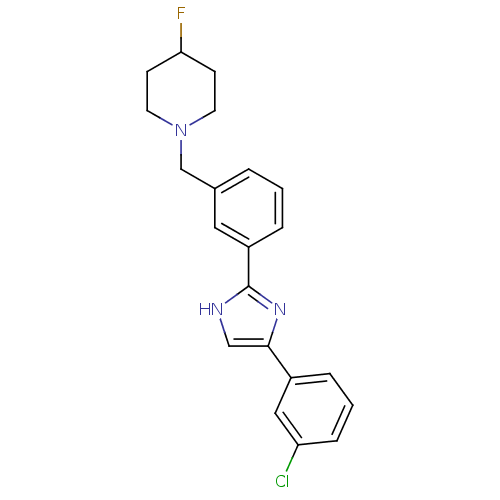 (1-(3-(4-(3-chlorophenyl)-1H-imidazol-2-yl)benzyl)-...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 130 | n/a | n/a | n/a | n/a |
Merck Research Laboratory Curated by ChEMBL | Assay Description Agonist activity at human cloned CB1 receptor expressed in CHO cells assessed as inhibition of forskolin-induced cAMP accumulation | Bioorg Med Chem Lett 21: 182-5 (2010) Article DOI: 10.1016/j.bmcl.2010.11.044 BindingDB Entry DOI: 10.7270/Q20P108G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM50334027 (1-(3-(4-(2,3-dichlorophenyl)-1H-imidazol-2-yl)benz...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a |
Merck Research Laboratory Curated by ChEMBL | Assay Description Agonist activity at human cloned CB1 receptor expressed in CHO cells assessed as inhibition of forskolin-induced cAMP accumulation | Bioorg Med Chem Lett 21: 182-5 (2010) Article DOI: 10.1016/j.bmcl.2010.11.044 BindingDB Entry DOI: 10.7270/Q20P108G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM50334028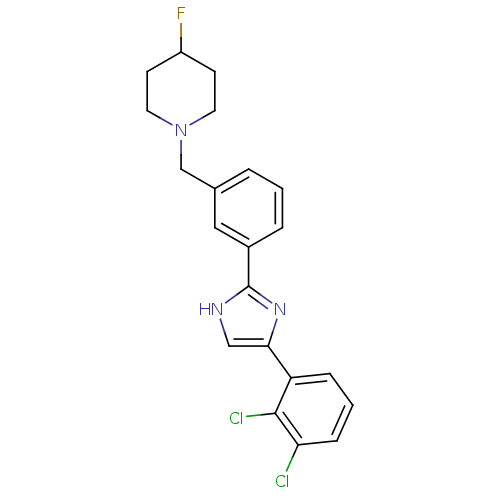 (1-(3-(4-(2,3-dichlorophenyl)-1H-imidazol-2-yl)benz...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 1.29E+3 | n/a | n/a | n/a | n/a |
Merck Research Laboratory Curated by ChEMBL | Assay Description Agonist activity at human cloned CB1 receptor expressed in CHO cells assessed as inhibition of forskolin-induced cAMP accumulation | Bioorg Med Chem Lett 21: 182-5 (2010) Article DOI: 10.1016/j.bmcl.2010.11.044 BindingDB Entry DOI: 10.7270/Q20P108G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM50334029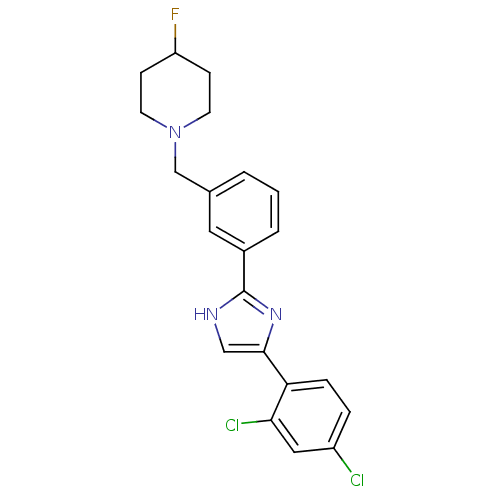 (1-(3-(4-(2,4-dichlorophenyl)-1H-imidazol-2-yl)benz...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 2.45E+3 | n/a | n/a | n/a | n/a |
Merck Research Laboratory Curated by ChEMBL | Assay Description Agonist activity at human cloned CB1 receptor expressed in CHO cells assessed as inhibition of forskolin-induced cAMP accumulation | Bioorg Med Chem Lett 21: 182-5 (2010) Article DOI: 10.1016/j.bmcl.2010.11.044 BindingDB Entry DOI: 10.7270/Q20P108G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM50334030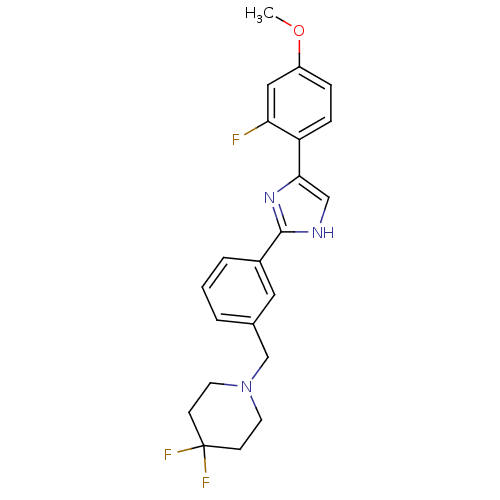 (4,4-difluoro-1-(3-(4-(2-fluoro-4-methoxyphenyl)-1H...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 39 | n/a | n/a | n/a | n/a |
Merck Research Laboratory Curated by ChEMBL | Assay Description Agonist activity at human cloned CB1 receptor expressed in CHO cells assessed as inhibition of forskolin-induced cAMP accumulation | Bioorg Med Chem Lett 21: 182-5 (2010) Article DOI: 10.1016/j.bmcl.2010.11.044 BindingDB Entry DOI: 10.7270/Q20P108G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM50334031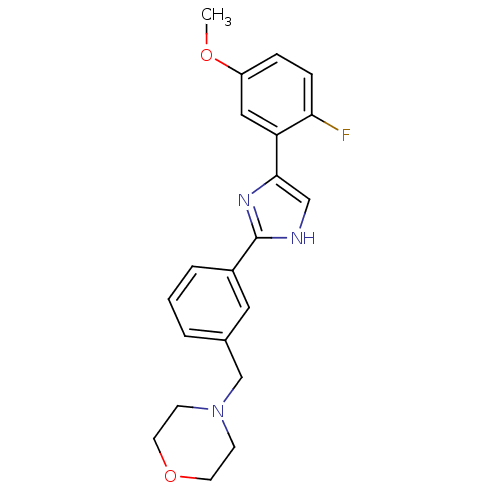 (4-(3-(4-(2-fluoro-5-methoxyphenyl)-1H-imidazol-2-y...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 140 | n/a | n/a | n/a | n/a |
Merck Research Laboratory Curated by ChEMBL | Assay Description Agonist activity at human cloned CB1 receptor expressed in CHO cells assessed as inhibition of forskolin-induced cAMP accumulation | Bioorg Med Chem Lett 21: 182-5 (2010) Article DOI: 10.1016/j.bmcl.2010.11.044 BindingDB Entry DOI: 10.7270/Q20P108G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM50334032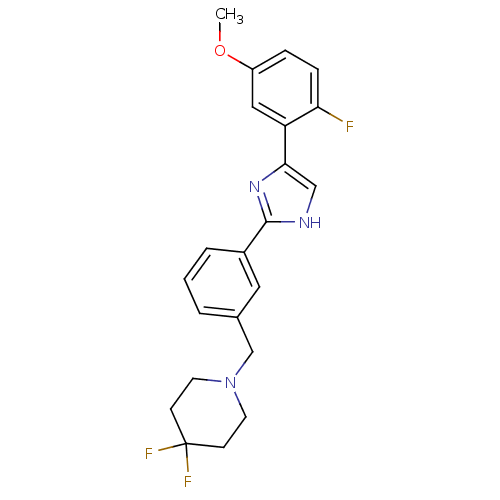 (4,4-difluoro-1-(3-(4-(2-fluoro-5-methoxyphenyl)-1H...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 25 | n/a | n/a | n/a | n/a |
Merck Research Laboratory Curated by ChEMBL | Assay Description Agonist activity at human cloned CB1 receptor expressed in CHO cells assessed as inhibition of forskolin-induced cAMP accumulation | Bioorg Med Chem Lett 21: 182-5 (2010) Article DOI: 10.1016/j.bmcl.2010.11.044 BindingDB Entry DOI: 10.7270/Q20P108G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM50334033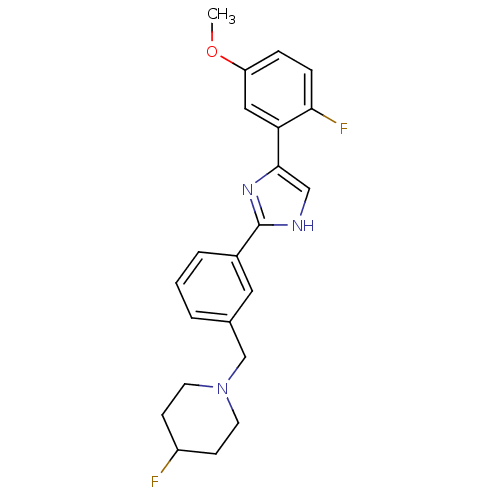 (4-fluoro-1-(3-(4-(2-fluoro-5-methoxyphenyl)-1H-imi...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 87 | n/a | n/a | n/a | n/a |
Merck Research Laboratory Curated by ChEMBL | Assay Description Agonist activity at human cloned CB1 receptor expressed in CHO cells assessed as inhibition of forskolin-induced cAMP accumulation | Bioorg Med Chem Lett 21: 182-5 (2010) Article DOI: 10.1016/j.bmcl.2010.11.044 BindingDB Entry DOI: 10.7270/Q20P108G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM50334034 (4-fluoro-1-(2-(4-(3-(trifluoromethyl)phenyl)-1H-im...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a |
Merck Research Laboratory Curated by ChEMBL | Assay Description Agonist activity at human cloned CB1 receptor expressed in CHO cells assessed as inhibition of forskolin-induced cAMP accumulation | Bioorg Med Chem Lett 21: 182-5 (2010) Article DOI: 10.1016/j.bmcl.2010.11.044 BindingDB Entry DOI: 10.7270/Q20P108G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM50334035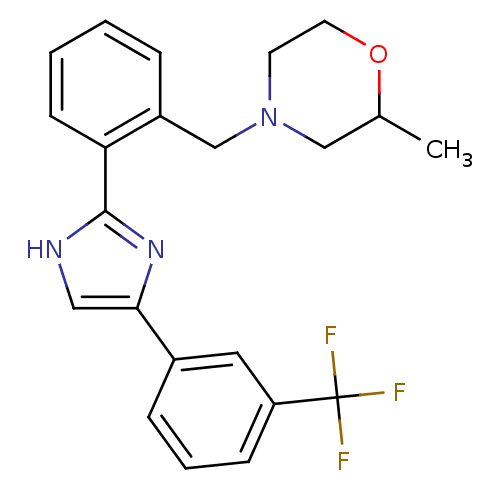 (2-methyl-4-(2-(4-(3-(trifluoromethyl)phenyl)-1H-im...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a |
Merck Research Laboratory Curated by ChEMBL | Assay Description Agonist activity at human cloned CB1 receptor expressed in CHO cells assessed as inhibition of forskolin-induced cAMP accumulation | Bioorg Med Chem Lett 21: 182-5 (2010) Article DOI: 10.1016/j.bmcl.2010.11.044 BindingDB Entry DOI: 10.7270/Q20P108G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 2 (Homo sapiens (Human)) | BDBM50334012 (4-(3-(1-isopropyl-4-(3-(trifluoromethyl)phenyl)-1H...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 218 | n/a | n/a | n/a | n/a |
Merck Research Laboratory Curated by ChEMBL | Assay Description Agonist activity at human cloned CB2 receptor expressed in CHO cells assessed as inhibition of forskolin-induced cAMP accumulation | Bioorg Med Chem Lett 21: 182-5 (2010) Article DOI: 10.1016/j.bmcl.2010.11.044 BindingDB Entry DOI: 10.7270/Q20P108G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 2 (Homo sapiens (Human)) | BDBM50334013 (4-(3-(1-(cyclopropylmethyl)-4-(3-(trifluoromethyl)...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 412 | n/a | n/a | n/a | n/a |
Merck Research Laboratory Curated by ChEMBL | Assay Description Agonist activity at human cloned CB2 receptor expressed in CHO cells assessed as inhibition of forskolin-induced cAMP accumulation | Bioorg Med Chem Lett 21: 182-5 (2010) Article DOI: 10.1016/j.bmcl.2010.11.044 BindingDB Entry DOI: 10.7270/Q20P108G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 2 (Homo sapiens (Human)) | BDBM50334014 (4-(3-(1-benzyl-4-(3-(trifluoromethyl)phenyl)-1H-im...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | >1.00E+3 | n/a | n/a | n/a | n/a |
Merck Research Laboratory Curated by ChEMBL | Assay Description Agonist activity at human cloned CB2 receptor expressed in CHO cells assessed as inhibition of forskolin-induced cAMP accumulation | Bioorg Med Chem Lett 21: 182-5 (2010) Article DOI: 10.1016/j.bmcl.2010.11.044 BindingDB Entry DOI: 10.7270/Q20P108G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 89 total ) | Next | Last >> |