 Found 866 hits with Last Name = 'blanz' and Initial = 'j'
Found 866 hits with Last Name = 'blanz' and Initial = 'j' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Metabotropic glutamate receptor 5
(Homo sapiens (Human)) | BDBM50336921
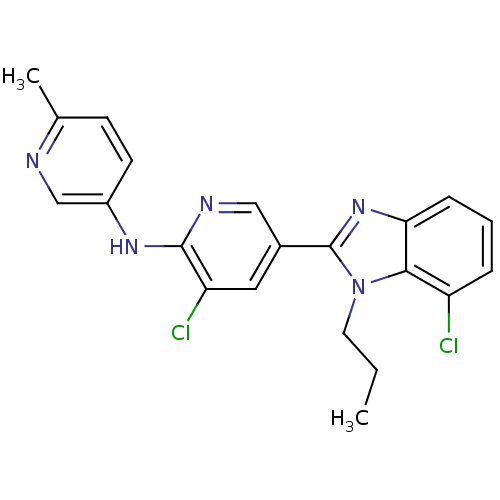
(3-chloro-5-(7-chloro-1-propyl-1H-benzo[d]imidazol-...)Show SMILES CCCn1c(nc2cccc(Cl)c12)-c1cnc(Nc2ccc(C)nc2)c(Cl)c1 Show InChI InChI=1S/C21H19Cl2N5/c1-3-9-28-19-16(22)5-4-6-18(19)27-21(28)14-10-17(23)20(25-11-14)26-15-8-7-13(2)24-12-15/h4-8,10-12H,3,9H2,1-2H3,(H,25,26) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Displacement of [3H]ABP688 from human recombinant mGlu5 receptor |
ACS Med Chem Lett 2: 58-62 (2011)
Article DOI: 10.1021/ml100215b
BindingDB Entry DOI: 10.7270/Q22R3RZM |
More data for this
Ligand-Target Pair | |
Metabotropic glutamate receptor 5
(Rattus norvegicus (Rat)) | BDBM50336921

(3-chloro-5-(7-chloro-1-propyl-1H-benzo[d]imidazol-...)Show SMILES CCCn1c(nc2cccc(Cl)c12)-c1cnc(Nc2ccc(C)nc2)c(Cl)c1 Show InChI InChI=1S/C21H19Cl2N5/c1-3-9-28-19-16(22)5-4-6-18(19)27-21(28)14-10-17(23)20(25-11-14)26-15-8-7-13(2)24-12-15/h4-8,10-12H,3,9H2,1-2H3,(H,25,26) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Displacement of [3H]ABP688 from mGlu5 receptor in rat brain tissue |
ACS Med Chem Lett 2: 58-62 (2011)
Article DOI: 10.1021/ml100215b
BindingDB Entry DOI: 10.7270/Q22R3RZM |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase SYK
(Homo sapiens (Human)) | BDBM50076190
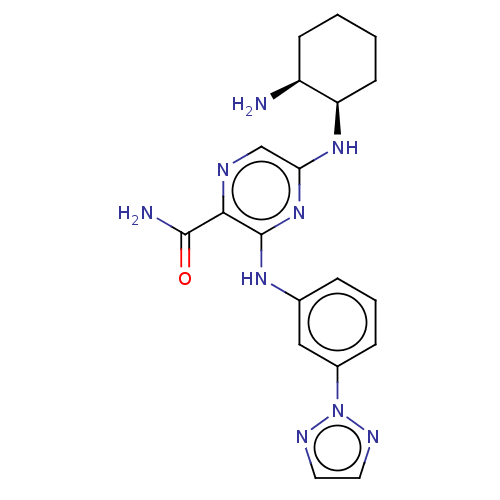
(CHEMBL3416023)Show SMILES N[C@H]1CCCC[C@H]1Nc1cnc(C(N)=O)c(Nc2cccc(c2)-n2nccn2)n1 |r| Show InChI InChI=1S/C19H23N9O/c20-14-6-1-2-7-15(14)26-16-11-22-17(18(21)29)19(27-16)25-12-4-3-5-13(10-12)28-23-8-9-24-28/h3-5,8-11,14-15H,1-2,6-7,20H2,(H2,21,29)(H2,25,26,27)/t14-,15+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research
Curated by ChEMBL
| Assay Description
Inhibition of Syk (unknown origin) using 5-Fluo-Ahx-GAPDYENLQELNKK-Amide as substrate after 60 mins by microfluidic mobility shift assay |
J Med Chem 58: 1950-63 (2015)
Article DOI: 10.1021/jm5018863
BindingDB Entry DOI: 10.7270/Q2G44S0C |
More data for this
Ligand-Target Pair | |
Gamma-aminobutyric acid receptor subunit alpha-1
(Homo sapiens (Human)) | BDBM84957
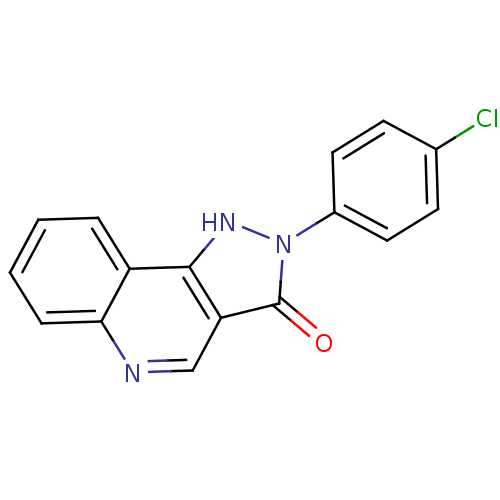
(CGS 9896 | CHEMBL20042)Show InChI InChI=1S/C16H10ClN3O/c17-10-5-7-11(8-6-10)20-16(21)13-9-18-14-4-2-1-3-12(14)15(13)19-20/h1-9,19H | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Displacement of [3H]flumazenil from GABAA receptor subunit alpha-1 expressed in CHO cells |
Bioorg Med Chem Lett 21: 1523-6 (2011)
Article DOI: 10.1016/j.bmcl.2010.12.107
BindingDB Entry DOI: 10.7270/Q289164V |
More data for this
Ligand-Target Pair | |
Gamma-aminobutyric acid receptor subunit alpha-1
(Homo sapiens (Human)) | BDBM50337263
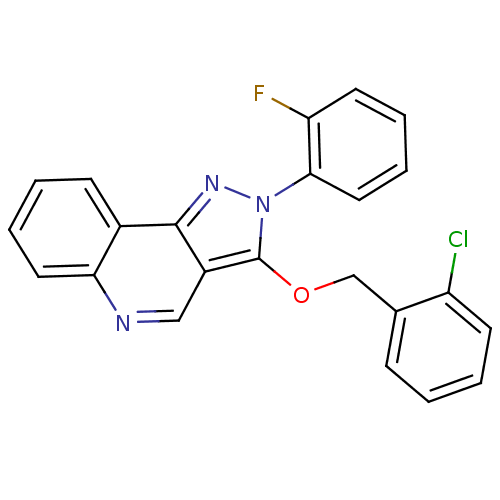
(3-(2-chlorobenzyloxy)-2-(2-fluorophenyl)-2H-pyrazo...)Show SMILES Fc1ccccc1-n1nc2c(cnc3ccccc23)c1OCc1ccccc1Cl Show InChI InChI=1S/C23H15ClFN3O/c24-18-9-3-1-7-15(18)14-29-23-17-13-26-20-11-5-2-8-16(20)22(17)27-28(23)21-12-6-4-10-19(21)25/h1-13H,14H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Displacement of [3H]flumazenil from GABAA receptor subunit alpha-1 expressed in CHO cells |
Bioorg Med Chem Lett 21: 1523-6 (2011)
Article DOI: 10.1016/j.bmcl.2010.12.107
BindingDB Entry DOI: 10.7270/Q289164V |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase SYK
(Homo sapiens (Human)) | BDBM50013170
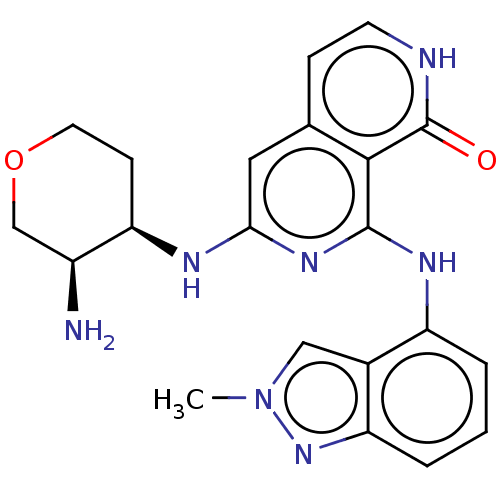
(CHEMBL3262622)Show SMILES Cn1cc2c(Nc3nc(N[C@@H]4CCOC[C@@H]4N)cc4cc[nH]c(=O)c34)cccc2n1 |r| Show InChI InChI=1S/C21H23N7O2/c1-28-10-13-15(3-2-4-16(13)27-28)25-20-19-12(5-7-23-21(19)29)9-18(26-20)24-17-6-8-30-11-14(17)22/h2-5,7,9-10,14,17H,6,8,11,22H2,1H3,(H,23,29)(H2,24,25,26)/t14-,17+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research
Curated by ChEMBL
| Assay Description
Inhibition of Syk (unknown origin) using 4 uM peptide assessed as product formation after 60 mins incubation by microfluidic mobility shift assay |
Bioorg Med Chem Lett 24: 2278-82 (2014)
Article DOI: 10.1016/j.bmcl.2014.03.075
BindingDB Entry DOI: 10.7270/Q2PN976C |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase SYK
(Homo sapiens (Human)) | BDBM50013169
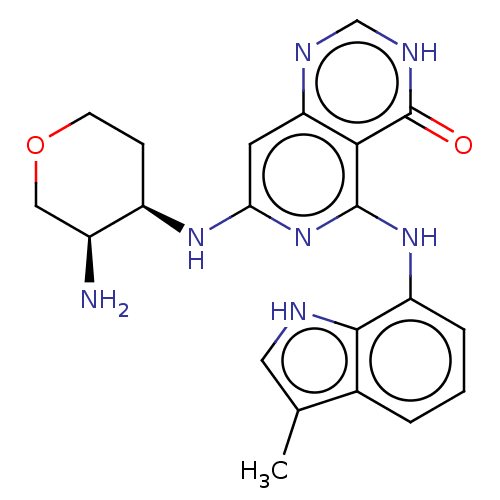
(CHEMBL3262620)Show SMILES Cc1c[nH]c2c(Nc3nc(N[C@@H]4CCOC[C@@H]4N)cc4nc[nH]c(=O)c34)cccc12 |r| Show InChI InChI=1S/C21H23N7O2/c1-11-8-23-19-12(11)3-2-4-15(19)27-20-18-16(24-10-25-21(18)29)7-17(28-20)26-14-5-6-30-9-13(14)22/h2-4,7-8,10,13-14,23H,5-6,9,22H2,1H3,(H,24,25,29)(H2,26,27,28)/t13-,14+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research
Curated by ChEMBL
| Assay Description
Inhibition of Syk (unknown origin) using 4 uM peptide assessed as product formation after 60 mins incubation by microfluidic mobility shift assay |
Bioorg Med Chem Lett 24: 2278-82 (2014)
Article DOI: 10.1016/j.bmcl.2014.03.075
BindingDB Entry DOI: 10.7270/Q2PN976C |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase SYK
(Homo sapiens (Human)) | BDBM50013165
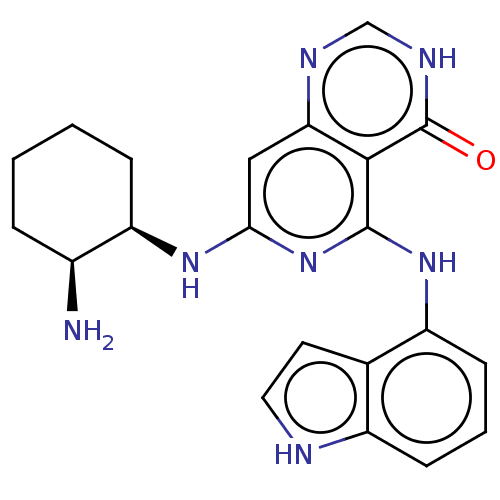
(CHEMBL3262616)Show SMILES N[C@H]1CCCC[C@H]1Nc1cc2nc[nH]c(=O)c2c(Nc2cccc3[nH]ccc23)n1 |r| Show InChI InChI=1S/C21H23N7O/c22-13-4-1-2-5-16(13)26-18-10-17-19(21(29)25-11-24-17)20(28-18)27-15-7-3-6-14-12(15)8-9-23-14/h3,6-11,13,16,23H,1-2,4-5,22H2,(H,24,25,29)(H2,26,27,28)/t13-,16+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research
Curated by ChEMBL
| Assay Description
Inhibition of Syk (unknown origin) using 4 uM peptide assessed as product formation after 60 mins incubation by microfluidic mobility shift assay |
Bioorg Med Chem Lett 24: 2278-82 (2014)
Article DOI: 10.1016/j.bmcl.2014.03.075
BindingDB Entry DOI: 10.7270/Q2PN976C |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase SYK
(Homo sapiens (Human)) | BDBM50076187
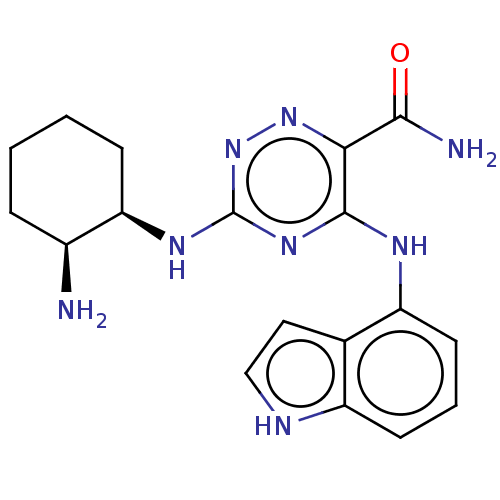
(CHEMBL3416027 | US9290481, 1.4)Show SMILES N[C@H]1CCCC[C@H]1Nc1nnc(C(N)=O)c(Nc2cccc3[nH]ccc23)n1 |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research
Curated by ChEMBL
| Assay Description
Inhibition of Syk (unknown origin) using 5-Fluo-Ahx-GAPDYENLQELNKK-Amide as substrate after 60 mins by microfluidic mobility shift assay |
J Med Chem 58: 1950-63 (2015)
Article DOI: 10.1021/jm5018863
BindingDB Entry DOI: 10.7270/Q2G44S0C |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase SYK
(Homo sapiens (Human)) | BDBM50013162
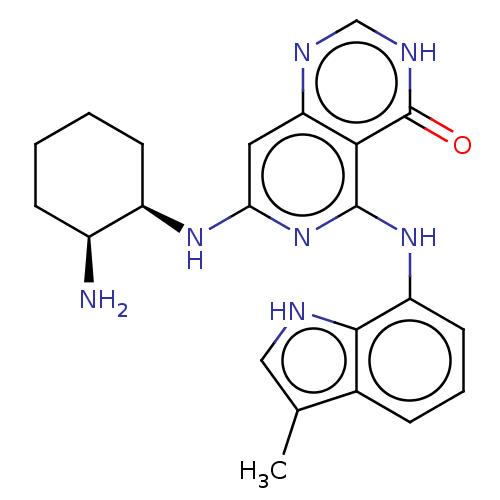
(CHEMBL3262357)Show SMILES Cc1c[nH]c2c(Nc3nc(N[C@@H]4CCCC[C@@H]4N)cc4nc[nH]c(=O)c34)cccc12 |r| Show InChI InChI=1S/C22H25N7O/c1-12-10-24-20-13(12)5-4-8-16(20)28-21-19-17(25-11-26-22(19)30)9-18(29-21)27-15-7-3-2-6-14(15)23/h4-5,8-11,14-15,24H,2-3,6-7,23H2,1H3,(H,25,26,30)(H2,27,28,29)/t14-,15+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research
Curated by ChEMBL
| Assay Description
Inhibition of Syk (unknown origin) using 4 uM peptide assessed as product formation after 60 mins incubation by microfluidic mobility shift assay |
Bioorg Med Chem Lett 24: 2278-82 (2014)
Article DOI: 10.1016/j.bmcl.2014.03.075
BindingDB Entry DOI: 10.7270/Q2PN976C |
More data for this
Ligand-Target Pair | |
Gamma-aminobutyric acid receptor subunit alpha-2
(Homo sapiens (Human)) | BDBM50337263

(3-(2-chlorobenzyloxy)-2-(2-fluorophenyl)-2H-pyrazo...)Show SMILES Fc1ccccc1-n1nc2c(cnc3ccccc23)c1OCc1ccccc1Cl Show InChI InChI=1S/C23H15ClFN3O/c24-18-9-3-1-7-15(18)14-29-23-17-13-26-20-11-5-2-8-16(20)22(17)27-28(23)21-12-6-4-10-19(21)25/h1-13H,14H2 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Displacement of [3H]flumazenil from GABAA receptor subunit alpha-2 expressed in CHO cells |
Bioorg Med Chem Lett 21: 1523-6 (2011)
Article DOI: 10.1016/j.bmcl.2010.12.107
BindingDB Entry DOI: 10.7270/Q289164V |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM50533774

(CHEMBL4469006)Show SMILES CC(=O)N1CCN(CC1)C(=O)c1cccc(c1)-c1ncnc2ccc(cc12)-c1cnc2ccccc2c1 Show InChI InChI=1S/C30H25N5O2/c1-20(36)34-11-13-35(14-12-34)30(37)24-7-4-6-23(16-24)29-26-17-21(9-10-28(26)32-19-33-29)25-15-22-5-2-3-8-27(22)31-18-25/h2-10,15-19H,11-14H2,1H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kdelta (unknown origin) assessed as reduction in ADP formation using phosphatidyl inositol as substrate after 30 to 60 mins by TR-FR... |
ACS Med Chem Lett 7: 762-7 (2016)
Article DOI: 10.1021/acsmedchemlett.6b00119
BindingDB Entry DOI: 10.7270/Q2V98CKP |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase SYK
(Homo sapiens (Human)) | BDBM50013173

(CHEMBL3262625)Show SMILES Cc1c[nH]c2c(Nc3nc(N[C@@H]4CCCC[C@@H]4N)cc4cc[nH]c(=O)c34)cccc12 |r| Show InChI InChI=1S/C23H26N6O/c1-13-12-26-21-15(13)5-4-8-18(21)28-22-20-14(9-10-25-23(20)30)11-19(29-22)27-17-7-3-2-6-16(17)24/h4-5,8-12,16-17,26H,2-3,6-7,24H2,1H3,(H,25,30)(H2,27,28,29)/t16-,17+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research
Curated by ChEMBL
| Assay Description
Inhibition of Syk (unknown origin) using 4 uM peptide assessed as product formation after 60 mins incubation by microfluidic mobility shift assay |
Bioorg Med Chem Lett 24: 2278-82 (2014)
Article DOI: 10.1016/j.bmcl.2014.03.075
BindingDB Entry DOI: 10.7270/Q2PN976C |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50112826
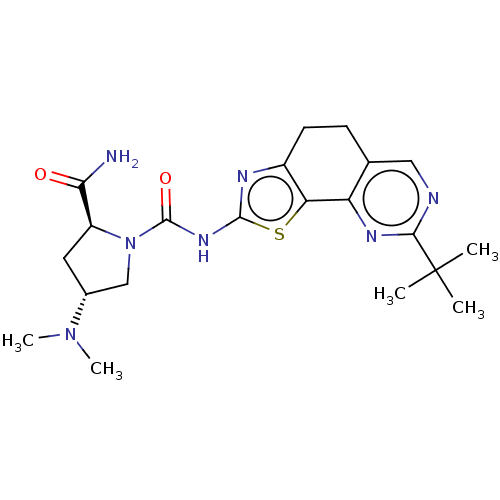
(CHEMBL3609529)Show SMILES CN(C)[C@@H]1C[C@H](N(C1)C(=O)Nc1nc2CCc3cnc(nc3-c2s1)C(C)(C)C)C(N)=O |r| Show InChI InChI=1S/C21H29N7O2S/c1-21(2,3)18-23-9-11-6-7-13-16(15(11)25-18)31-19(24-13)26-20(30)28-10-12(27(4)5)8-14(28)17(22)29/h9,12,14H,6-8,10H2,1-5H3,(H2,22,29)(H,24,26,30)/t12-,14+/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kalpha (unknown origin) using phosphatidylinositol as substrate preincubated for 15 mins followed by ATP addition measured after 1 h... |
Bioorg Med Chem Lett 25: 3575-81 (2015)
Article DOI: 10.1016/j.bmcl.2015.06.067
BindingDB Entry DOI: 10.7270/Q2DF6SZ5 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase SYK
(Homo sapiens (Human)) | BDBM50013171
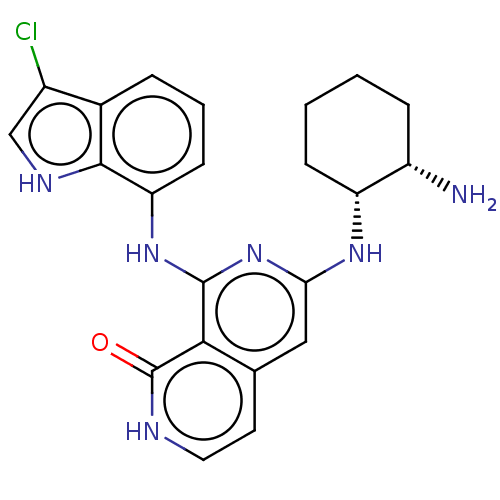
(CHEMBL3262623)Show SMILES N[C@H]1CCCC[C@H]1Nc1cc2cc[nH]c(=O)c2c(Nc2cccc3c(Cl)c[nH]c23)n1 |r| Show InChI InChI=1S/C22H23ClN6O/c23-14-11-26-20-13(14)4-3-7-17(20)28-21-19-12(8-9-25-22(19)30)10-18(29-21)27-16-6-2-1-5-15(16)24/h3-4,7-11,15-16,26H,1-2,5-6,24H2,(H,25,30)(H2,27,28,29)/t15-,16+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research
Curated by ChEMBL
| Assay Description
Inhibition of Syk (unknown origin) using 4 uM peptide assessed as product formation after 60 mins incubation by microfluidic mobility shift assay |
Bioorg Med Chem Lett 24: 2278-82 (2014)
Article DOI: 10.1016/j.bmcl.2014.03.075
BindingDB Entry DOI: 10.7270/Q2PN976C |
More data for this
Ligand-Target Pair | |
Gamma-aminobutyric acid receptor subunit alpha-2
(Homo sapiens (Human)) | BDBM84957

(CGS 9896 | CHEMBL20042)Show InChI InChI=1S/C16H10ClN3O/c17-10-5-7-11(8-6-10)20-16(21)13-9-18-14-4-2-1-3-12(14)15(13)19-20/h1-9,19H | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Displacement of [3H]flumazenil from GABAA receptor subunit alpha-2 expressed in CHO cells |
Bioorg Med Chem Lett 21: 1523-6 (2011)
Article DOI: 10.1016/j.bmcl.2010.12.107
BindingDB Entry DOI: 10.7270/Q289164V |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase SYK
(Homo sapiens (Human)) | BDBM50013161
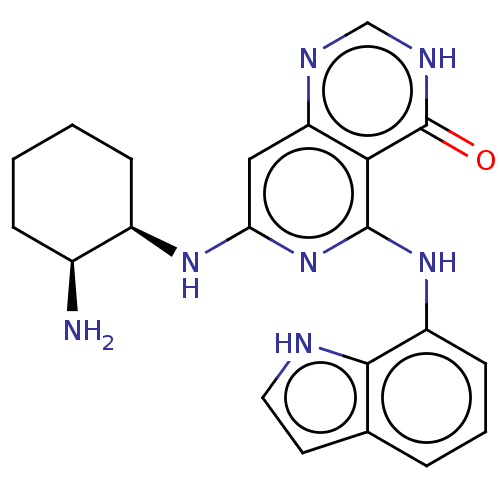
(CHEMBL3262356)Show SMILES N[C@H]1CCCC[C@H]1Nc1cc2nc[nH]c(=O)c2c(Nc2cccc3cc[nH]c23)n1 |r| Show InChI InChI=1S/C21H23N7O/c22-13-5-1-2-6-14(13)26-17-10-16-18(21(29)25-11-24-16)20(28-17)27-15-7-3-4-12-8-9-23-19(12)15/h3-4,7-11,13-14,23H,1-2,5-6,22H2,(H,24,25,29)(H2,26,27,28)/t13-,14+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research
Curated by ChEMBL
| Assay Description
Inhibition of Syk (unknown origin) using 4 uM peptide assessed as product formation after 60 mins incubation by microfluidic mobility shift assay |
Bioorg Med Chem Lett 24: 2278-82 (2014)
Article DOI: 10.1016/j.bmcl.2014.03.075
BindingDB Entry DOI: 10.7270/Q2PN976C |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50112820
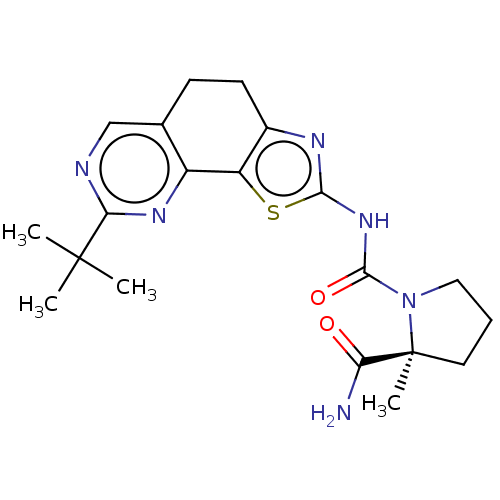
(CHEMBL3609523)Show SMILES CC(C)(C)c1ncc2CCc3nc(NC(=O)N4CCC[C@@]4(C)C(N)=O)sc3-c2n1 |r| Show InChI InChI=1S/C20H26N6O2S/c1-19(2,3)16-22-10-11-6-7-12-14(13(11)24-16)29-17(23-12)25-18(28)26-9-5-8-20(26,4)15(21)27/h10H,5-9H2,1-4H3,(H2,21,27)(H,23,25,28)/t20-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of N-terminal myristoylated human PI3Kalpha expressed in Rat1 cells assessed as inhibition of Akt phosphorylatuion at Ser473 by ELISA |
Bioorg Med Chem Lett 25: 3575-81 (2015)
Article DOI: 10.1016/j.bmcl.2015.06.067
BindingDB Entry DOI: 10.7270/Q2DF6SZ5 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM118300

(US8653092, 68)Show SMILES COc1ncc(cc1C(F)(F)F)N1CCc2ncnc(N[C@H]3CCN(C3)C(=O)C3CCOCC3)c2C1 |r| Show InChI InChI=1S/C24H29F3N6O3/c1-35-22-19(24(25,26)27)10-17(11-28-22)32-7-3-20-18(13-32)21(30-14-29-20)31-16-2-6-33(12-16)23(34)15-4-8-36-9-5-15/h10-11,14-16H,2-9,12-13H2,1H3,(H,29,30,31)/t16-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kdelta (unknown origin) using phosphatidyl inositol as substrate measured after 60 mins by Alexa Fluor647-labelled ADP tracer based ... |
ACS Med Chem Lett 8: 975-980 (2017)
Article DOI: 10.1021/acsmedchemlett.7b00293
BindingDB Entry DOI: 10.7270/Q2SX6GR0 |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase kinase kinase 8
(Homo sapiens (Human)) | BDBM50191770
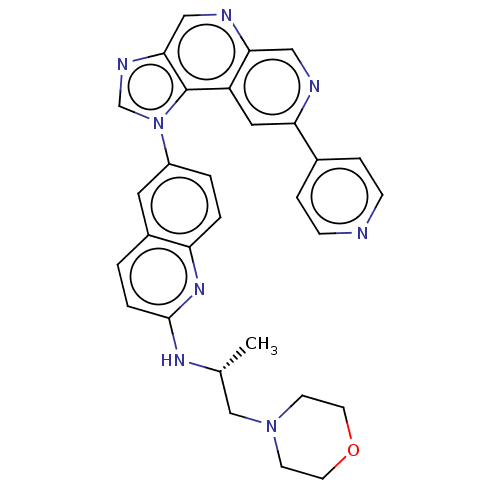
(CHEMBL3912476)Show SMILES C[C@H](CN1CCOCC1)Nc1ccc2cc(ccc2n1)-n1cnc2cnc3cnc(cc3c12)-c1ccncc1 |r| Show InChI InChI=1S/C30H28N8O/c1-20(18-37-10-12-39-13-11-37)35-29-5-2-22-14-23(3-4-25(22)36-29)38-19-34-28-17-33-27-16-32-26(15-24(27)30(28)38)21-6-8-31-9-7-21/h2-9,14-17,19-20H,10-13,18H2,1H3,(H,35,36)/t20-/m1/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of human COT (66 to 395 residues) expressed in Sf21 cells using 5-Fluo-Ahx-AGAGSGQLIDSNleANSFVGTR-NH2 as substrate after 60 mins by calipe... |
J Med Chem 59: 7544-60 (2016)
Article DOI: 10.1021/acs.jmedchem.6b00598
BindingDB Entry DOI: 10.7270/Q29P33MR |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase SYK
(Homo sapiens (Human)) | BDBM50013161

(CHEMBL3262356)Show SMILES N[C@H]1CCCC[C@H]1Nc1cc2nc[nH]c(=O)c2c(Nc2cccc3cc[nH]c23)n1 |r| Show InChI InChI=1S/C21H23N7O/c22-13-5-1-2-6-14(13)26-17-10-16-18(21(29)25-11-24-16)20(28-17)27-15-7-3-4-12-8-9-23-19(12)15/h3-4,7-11,13-14,23H,1-2,5-6,22H2,(H,24,25,29)(H2,26,27,28)/t13-,14+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research
Curated by ChEMBL
| Assay Description
Inhibition of Syk (unknown origin) using 5-Fluo-Ahx-GAPDYENLQELNKK-Amide as substrate after 60 mins by microfluidic mobility shift assay |
J Med Chem 58: 1950-63 (2015)
Article DOI: 10.1021/jm5018863
BindingDB Entry DOI: 10.7270/Q2G44S0C |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50112823

(CHEMBL3609526)Show SMILES C[C@@H]1CCN([C@@H]1C(N)=O)C(=O)Nc1nc2CCc3cnc(nc3-c2s1)C(C)(C)C(F)(F)F |r| Show InChI InChI=1S/C20H23F3N6O2S/c1-9-6-7-29(13(9)15(24)30)18(31)28-17-26-11-5-4-10-8-25-16(19(2,3)20(21,22)23)27-12(10)14(11)32-17/h8-9,13H,4-7H2,1-3H3,(H2,24,30)(H,26,28,31)/t9-,13+/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of N-terminal myristoylated human PI3Kalpha expressed in Rat1 cells assessed as inhibition of Akt phosphorylatuion at Ser473 by ELISA |
Bioorg Med Chem Lett 25: 3575-81 (2015)
Article DOI: 10.1016/j.bmcl.2015.06.067
BindingDB Entry DOI: 10.7270/Q2DF6SZ5 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50112819

(CHEMBL3609522)Show SMILES CC(C)(C)c1ncc2CCc3nc(NC(=O)N4CCC[C@H]4C(N)=O)sc3-c2n1 |r| Show InChI InChI=1S/C19H24N6O2S/c1-19(2,3)16-21-9-10-6-7-11-14(13(10)23-16)28-17(22-11)24-18(27)25-8-4-5-12(25)15(20)26/h9,12H,4-8H2,1-3H3,(H2,20,26)(H,22,24,27)/t12-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kalpha (unknown origin) using phosphatidylinositol as substrate preincubated for 15 mins followed by ATP addition measured after 1 h... |
Bioorg Med Chem Lett 25: 3575-81 (2015)
Article DOI: 10.1016/j.bmcl.2015.06.067
BindingDB Entry DOI: 10.7270/Q2DF6SZ5 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50112822
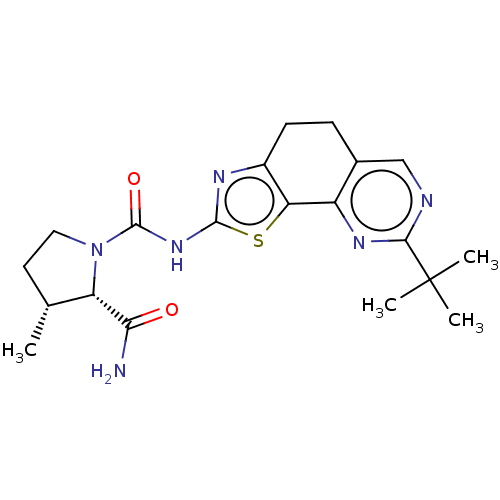
(CHEMBL3609525)Show SMILES C[C@@H]1CCN([C@@H]1C(N)=O)C(=O)Nc1nc2CCc3cnc(nc3-c2s1)C(C)(C)C |r| Show InChI InChI=1S/C20H26N6O2S/c1-10-7-8-26(14(10)16(21)27)19(28)25-18-23-12-6-5-11-9-22-17(20(2,3)4)24-13(11)15(12)29-18/h9-10,14H,5-8H2,1-4H3,(H2,21,27)(H,23,25,28)/t10-,14+/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of N-terminal myristoylated human PI3Kalpha expressed in Rat1 cells assessed as inhibition of Akt phosphorylatuion at Ser473 by ELISA |
Bioorg Med Chem Lett 25: 3575-81 (2015)
Article DOI: 10.1016/j.bmcl.2015.06.067
BindingDB Entry DOI: 10.7270/Q2DF6SZ5 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Tyrosine-protein kinase SYK
(Homo sapiens (Human)) | BDBM50076189
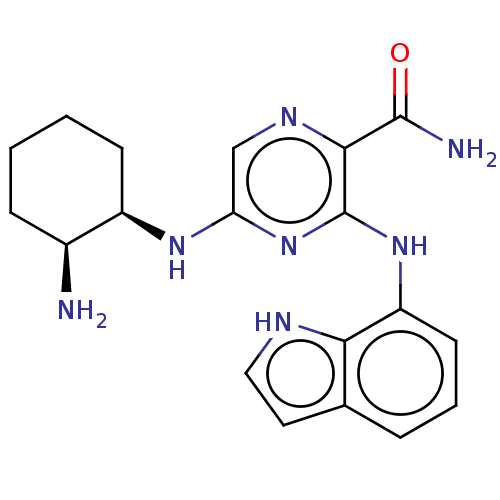
(CHEMBL3416024 | US9290481, 4.1)Show SMILES N[C@H]1CCCC[C@H]1Nc1cnc(C(N)=O)c(Nc2cccc3cc[nH]c23)n1 |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research
Curated by ChEMBL
| Assay Description
Inhibition of Syk (unknown origin) using 5-Fluo-Ahx-GAPDYENLQELNKK-Amide as substrate after 60 mins by microfluidic mobility shift assay |
J Med Chem 58: 1950-63 (2015)
Article DOI: 10.1021/jm5018863
BindingDB Entry DOI: 10.7270/Q2G44S0C |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase SYK
(Homo sapiens (Human)) | BDBM50076188
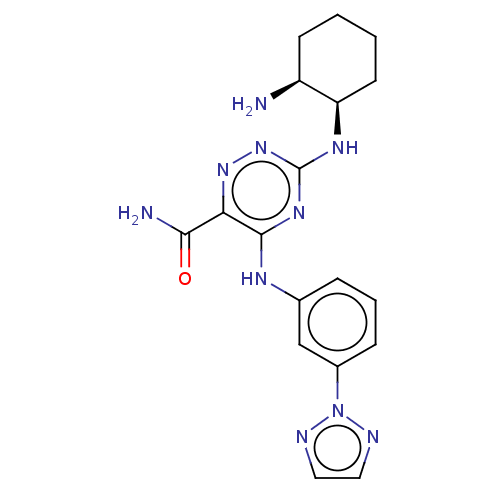
(CHEMBL3416025)Show SMILES N[C@H]1CCCC[C@H]1Nc1nnc(C(N)=O)c(Nc2cccc(c2)-n2nccn2)n1 |r| Show InChI InChI=1S/C18H22N10O/c19-13-6-1-2-7-14(13)24-18-25-17(15(16(20)29)26-27-18)23-11-4-3-5-12(10-11)28-21-8-9-22-28/h3-5,8-10,13-14H,1-2,6-7,19H2,(H2,20,29)(H2,23,24,25,27)/t13-,14+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research
Curated by ChEMBL
| Assay Description
Inhibition of Syk (unknown origin) using 5-Fluo-Ahx-GAPDYENLQELNKK-Amide as substrate after 60 mins by microfluidic mobility shift assay |
J Med Chem 58: 1950-63 (2015)
Article DOI: 10.1021/jm5018863
BindingDB Entry DOI: 10.7270/Q2G44S0C |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Tyrosine-protein kinase SYK
(Homo sapiens (Human)) | BDBM50013160
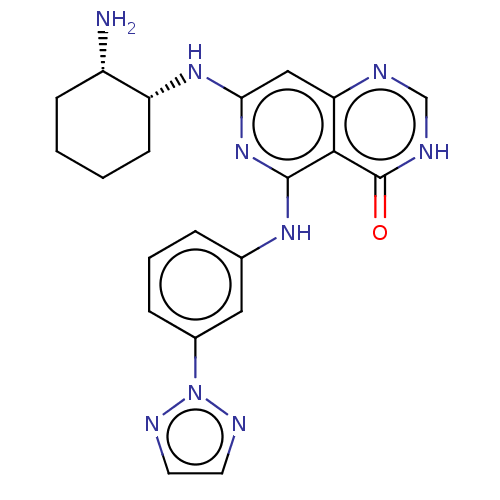
(CHEMBL3262355)Show SMILES N[C@H]1CCCC[C@H]1Nc1cc2nc[nH]c(=O)c2c(Nc2cccc(c2)-n2nccn2)n1 |r| Show InChI InChI=1S/C21H23N9O/c22-15-6-1-2-7-16(15)28-18-11-17-19(21(31)24-12-23-17)20(29-18)27-13-4-3-5-14(10-13)30-25-8-9-26-30/h3-5,8-12,15-16H,1-2,6-7,22H2,(H,23,24,31)(H2,27,28,29)/t15-,16+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research
Curated by ChEMBL
| Assay Description
Inhibition of Syk (unknown origin) using 5-Fluo-Ahx-GAPDYENLQELNKK-Amide as substrate after 60 mins by microfluidic mobility shift assay |
J Med Chem 58: 1950-63 (2015)
Article DOI: 10.1021/jm5018863
BindingDB Entry DOI: 10.7270/Q2G44S0C |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase SYK
(Homo sapiens (Human)) | BDBM50123580
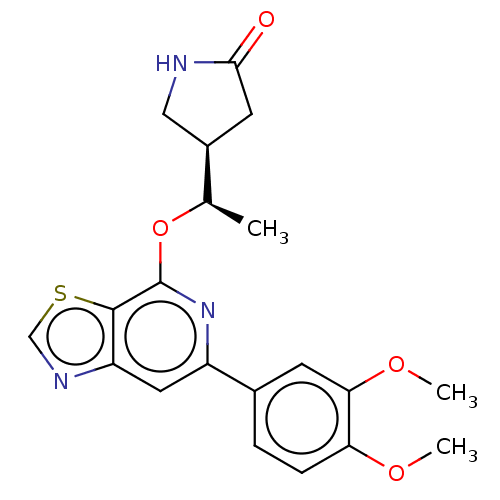
(CHEMBL3622208)Show SMILES [H][C@@]1(CNC(=O)C1)[C@@H](C)Oc1nc(cc2ncsc12)-c1ccc(OC)c(OC)c1 |r| Show InChI InChI=1S/C20H21N3O4S/c1-11(13-7-18(24)21-9-13)27-20-19-15(22-10-28-19)8-14(23-20)12-4-5-16(25-2)17(6-12)26-3/h4-6,8,10-11,13H,7,9H2,1-3H3,(H,21,24)/t11-,13-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research
Curated by ChEMBL
| Assay Description
Inhibition of Syk (unknown origin) using 5-Fluo-Ahx-GAPDYENLQELNKK-Amide as substrate after 60 mins by microfluidic mobility shift assay |
Bioorg Med Chem Lett 25: 4642-7 (2015)
Article DOI: 10.1016/j.bmcl.2015.08.037
BindingDB Entry DOI: 10.7270/Q2XD13HG |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50112817
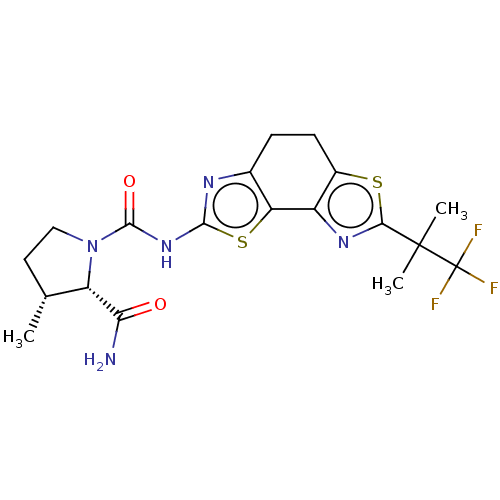
(CHEMBL3609520)Show SMILES C[C@@H]1CCN([C@@H]1C(N)=O)C(=O)Nc1nc2CCc3sc(nc3-c2s1)C(C)(C)C(F)(F)F |r| Show InChI InChI=1S/C19H22F3N5O2S2/c1-8-6-7-27(12(8)14(23)28)17(29)26-16-24-9-4-5-10-11(13(9)31-16)25-15(30-10)18(2,3)19(20,21)22/h8,12H,4-7H2,1-3H3,(H2,23,28)(H,24,26,29)/t8-,12+/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of N-terminal myristoylated human PI3Kalpha expressed in Rat1 cells assessed as inhibition of Akt phosphorylatuion at Ser473 by ELISA |
Bioorg Med Chem Lett 25: 3575-81 (2015)
Article DOI: 10.1016/j.bmcl.2015.06.067
BindingDB Entry DOI: 10.7270/Q2DF6SZ5 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase SYK
(Homo sapiens (Human)) | BDBM50013160

(CHEMBL3262355)Show SMILES N[C@H]1CCCC[C@H]1Nc1cc2nc[nH]c(=O)c2c(Nc2cccc(c2)-n2nccn2)n1 |r| Show InChI InChI=1S/C21H23N9O/c22-15-6-1-2-7-16(15)28-18-11-17-19(21(31)24-12-23-17)20(29-18)27-13-4-3-5-14(10-13)30-25-8-9-26-30/h3-5,8-12,15-16H,1-2,6-7,22H2,(H,23,24,31)(H2,27,28,29)/t15-,16+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research
Curated by ChEMBL
| Assay Description
Inhibition of Syk (unknown origin) using 4 uM peptide assessed as product formation after 60 mins incubation by microfluidic mobility shift assay |
Bioorg Med Chem Lett 24: 2278-82 (2014)
Article DOI: 10.1016/j.bmcl.2014.03.075
BindingDB Entry DOI: 10.7270/Q2PN976C |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50112823

(CHEMBL3609526)Show SMILES C[C@@H]1CCN([C@@H]1C(N)=O)C(=O)Nc1nc2CCc3cnc(nc3-c2s1)C(C)(C)C(F)(F)F |r| Show InChI InChI=1S/C20H23F3N6O2S/c1-9-6-7-29(13(9)15(24)30)18(31)28-17-26-11-5-4-10-8-25-16(19(2,3)20(21,22)23)27-12(10)14(11)32-17/h8-9,13H,4-7H2,1-3H3,(H2,24,30)(H,26,28,31)/t9-,13+/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kalpha (unknown origin) using phosphatidylinositol as substrate preincubated for 15 mins followed by ATP addition measured after 1 h... |
Bioorg Med Chem Lett 25: 3575-81 (2015)
Article DOI: 10.1016/j.bmcl.2015.06.067
BindingDB Entry DOI: 10.7270/Q2DF6SZ5 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform
(Homo sapiens (Human)) | BDBM50112822

(CHEMBL3609525)Show SMILES C[C@@H]1CCN([C@@H]1C(N)=O)C(=O)Nc1nc2CCc3cnc(nc3-c2s1)C(C)(C)C |r| Show InChI InChI=1S/C20H26N6O2S/c1-10-7-8-26(14(10)16(21)27)19(28)25-18-23-12-6-5-11-9-22-17(20(2,3)4)24-13(11)15(12)29-18/h9-10,14H,5-8H2,1-4H3,(H2,21,27)(H,23,25,28)/t10-,14+/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kgamma (unknown origin) using PI or PIP2:PS as substrate by TR-FRET assay |
Bioorg Med Chem Lett 25: 3575-81 (2015)
Article DOI: 10.1016/j.bmcl.2015.06.067
BindingDB Entry DOI: 10.7270/Q2DF6SZ5 |
More data for this
Ligand-Target Pair | |
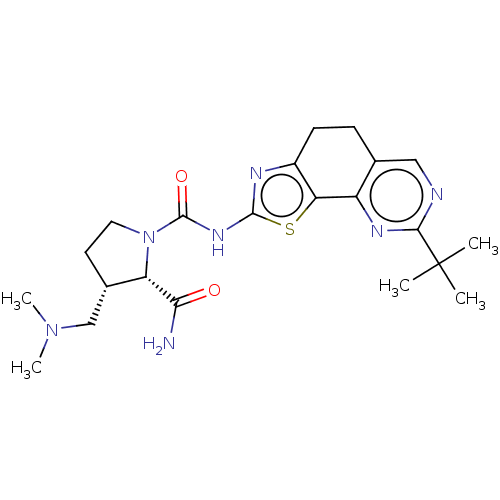
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50112824
(CHEMBL3609527)Show SMILES CN(C)C[C@@H]1CCN([C@@H]1C(N)=O)C(=O)Nc1nc2CCc3cnc(nc3-c2s1)C(C)(C)C |r| Show InChI InChI=1S/C22H31N7O2S/c1-22(2,3)19-24-10-12-6-7-14-17(15(12)26-19)32-20(25-14)27-21(31)29-9-8-13(11-28(4)5)16(29)18(23)30/h10,13,16H,6-9,11H2,1-5H3,(H2,23,30)(H,25,27,31)/t13-,16-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kalpha (unknown origin) using phosphatidylinositol as substrate preincubated for 15 mins followed by ATP addition measured after 1 h... |
Bioorg Med Chem Lett 25: 3575-81 (2015)
Article DOI: 10.1016/j.bmcl.2015.06.067
BindingDB Entry DOI: 10.7270/Q2DF6SZ5 |
More data for this
Ligand-Target Pair | |
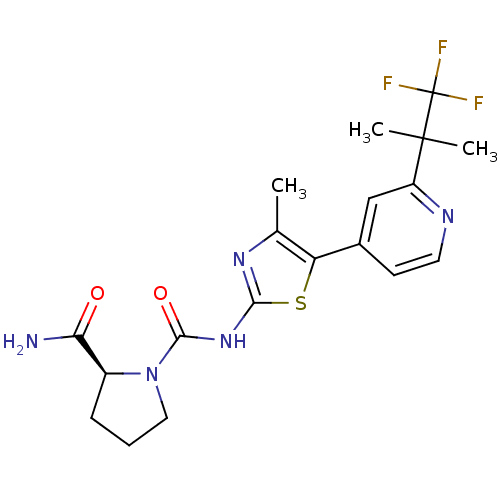
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50436459
(CHEMBL2396661)Show SMILES Cc1nc(NC(=O)N2CCC[C@H]2C(N)=O)sc1-c1ccnc(c1)C(C)(C)C(F)(F)F |r| Show InChI InChI=1S/C19H22F3N5O2S/c1-10-14(11-6-7-24-13(9-11)18(2,3)19(20,21)22)30-16(25-10)26-17(29)27-8-4-5-12(27)15(23)28/h6-7,9,12H,4-5,8H2,1-3H3,(H2,23,28)(H,25,26,29)/t12-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of P110alpha (unknown origin) using L-a-phosphatidylinositol as substrate by luminescence assay |
Bioorg Med Chem Lett 23: 3741-8 (2013)
Article DOI: 10.1016/j.bmcl.2013.05.007
BindingDB Entry DOI: 10.7270/Q2M046VD |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM50533772

(CHEMBL4521888)Show SMILES COc1ncc(cc1C(F)(F)F)-c1ccc2ncnc(-c3cccc(c3)C(=O)N3CCN(CC3)C(C)=O)c2c1 Show InChI InChI=1S/C28H24F3N5O3/c1-17(37)35-8-10-36(11-9-35)27(38)20-5-3-4-19(12-20)25-22-13-18(6-7-24(22)33-16-34-25)21-14-23(28(29,30)31)26(39-2)32-15-21/h3-7,12-16H,8-11H2,1-2H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kdelta (unknown origin) assessed as reduction in ADP formation using phosphatidyl inositol as substrate after 30 to 60 mins by TR-FR... |
ACS Med Chem Lett 7: 762-7 (2016)
Article DOI: 10.1021/acsmedchemlett.6b00119
BindingDB Entry DOI: 10.7270/Q2V98CKP |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50112820

(CHEMBL3609523)Show SMILES CC(C)(C)c1ncc2CCc3nc(NC(=O)N4CCC[C@@]4(C)C(N)=O)sc3-c2n1 |r| Show InChI InChI=1S/C20H26N6O2S/c1-19(2,3)16-22-10-11-6-7-12-14(13(11)24-16)29-17(23-12)25-18(28)26-9-5-8-20(26,4)15(21)27/h10H,5-9H2,1-4H3,(H2,21,27)(H,23,25,28)/t20-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kalpha (unknown origin) using phosphatidylinositol as substrate preincubated for 15 mins followed by ATP addition measured after 1 h... |
Bioorg Med Chem Lett 25: 3575-81 (2015)
Article DOI: 10.1016/j.bmcl.2015.06.067
BindingDB Entry DOI: 10.7270/Q2DF6SZ5 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50112821

(CHEMBL3609524)Show SMILES [H][C@@]12C[C@@]1(N(CC2)C(=O)Nc1nc2CCc3cnc(nc3-c2s1)C(C)(C)C)C(N)=O |r| Show InChI InChI=1S/C20H24N6O2S/c1-19(2,3)16-22-9-10-4-5-12-14(13(10)24-16)29-17(23-12)25-18(28)26-7-6-11-8-20(11,26)15(21)27/h9,11H,4-8H2,1-3H3,(H2,21,27)(H,23,25,28)/t11-,20+/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kalpha (unknown origin) using phosphatidylinositol as substrate preincubated for 15 mins followed by ATP addition measured after 1 h... |
Bioorg Med Chem Lett 25: 3575-81 (2015)
Article DOI: 10.1016/j.bmcl.2015.06.067
BindingDB Entry DOI: 10.7270/Q2DF6SZ5 |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase mTOR
(Homo sapiens (Human)) | BDBM92862

(US9284315, BEZ-235 | mTOR Inhibitor, BEZ235)Show SMILES Cn1c2cnc3ccc(cc3c2n(-c2ccc(cc2)C(C)(C)C#N)c1=O)-c1cnc2ccccc2c1 Show InChI InChI=1S/C30H23N5O/c1-30(2,18-31)22-9-11-23(12-10-22)35-28-24-15-19(21-14-20-6-4-5-7-25(20)32-16-21)8-13-26(24)33-17-27(28)34(3)29(35)36/h4-17H,1-3H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of human mTOR after 60 mins in presence of [gamma-33P]-ATP by microplate scintillation counting |
ACS Med Chem Lett 7: 762-7 (2016)
Article DOI: 10.1021/acsmedchemlett.6b00119
BindingDB Entry DOI: 10.7270/Q2V98CKP |
More data for this
Ligand-Target Pair | |
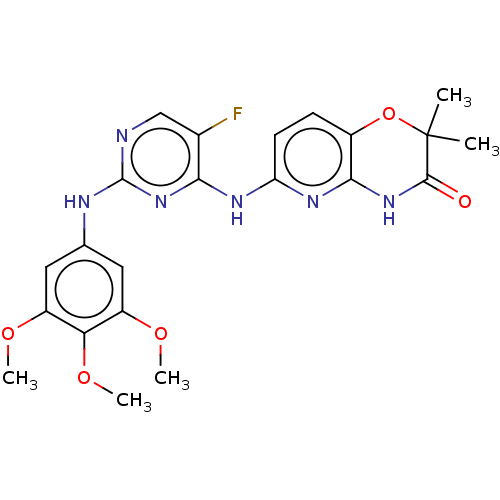
Tyrosine-protein kinase JAK2
(Homo sapiens (Human)) | BDBM60665
(BDBM50249542 | US9145414, R406 | US9212178, R406)Show SMILES COc1cc(Nc2ncc(F)c(Nc3ccc4OC(C)(C)C(=O)Nc4n3)n2)cc(OC)c1OC Show InChI InChI=1S/C22H23FN6O5/c1-22(2)20(30)28-19-13(34-22)6-7-16(27-19)26-18-12(23)10-24-21(29-18)25-11-8-14(31-3)17(33-5)15(9-11)32-4/h6-10H,1-5H3,(H3,24,25,26,27,28,29,30) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research
Curated by ChEMBL
| Assay Description
Inhibition of JAK2 (unknown origin) |
Bioorg Med Chem Lett 24: 2278-82 (2014)
Article DOI: 10.1016/j.bmcl.2014.03.075
BindingDB Entry DOI: 10.7270/Q2PN976C |
More data for this
Ligand-Target Pair | |
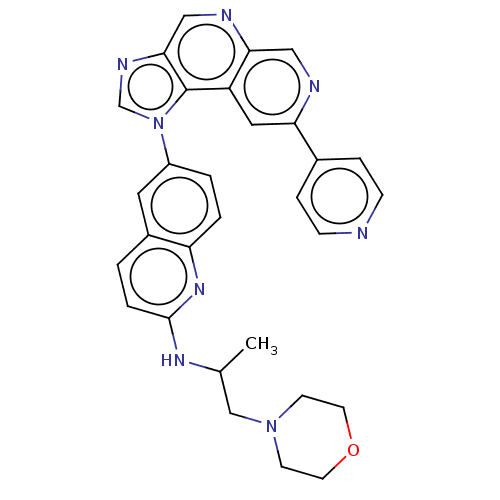
Mitogen-activated protein kinase kinase kinase 8
(Homo sapiens (Human)) | BDBM50191681
(CHEMBL3939592)Show SMILES CC(CN1CCOCC1)Nc1ccc2cc(ccc2n1)-n1cnc2cnc3cnc(cc3c12)-c1ccncc1 Show InChI InChI=1S/C30H28N8O/c1-20(18-37-10-12-39-13-11-37)35-29-5-2-22-14-23(3-4-25(22)36-29)38-19-34-28-17-33-27-16-32-26(15-24(27)30(28)38)21-6-8-31-9-7-21/h2-9,14-17,19-20H,10-13,18H2,1H3,(H,35,36) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of human COT (66 to 395 residues) expressed in Sf21 cells using 5-Fluo-Ahx-AGAGSGQLIDSNleANSFVGTR-NH2 as substrate after 60 mins by calipe... |
J Med Chem 59: 7544-60 (2016)
Article DOI: 10.1021/acs.jmedchem.6b00598
BindingDB Entry DOI: 10.7270/Q29P33MR |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50112822

(CHEMBL3609525)Show SMILES C[C@@H]1CCN([C@@H]1C(N)=O)C(=O)Nc1nc2CCc3cnc(nc3-c2s1)C(C)(C)C |r| Show InChI InChI=1S/C20H26N6O2S/c1-10-7-8-26(14(10)16(21)27)19(28)25-18-23-12-6-5-11-9-22-17(20(2,3)4)24-13(11)15(12)29-18/h9-10,14H,5-8H2,1-4H3,(H2,21,27)(H,23,25,28)/t10-,14+/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kalpha (unknown origin) using phosphatidylinositol as substrate preincubated for 15 mins followed by ATP addition measured after 1 h... |
Bioorg Med Chem Lett 25: 3575-81 (2015)
Article DOI: 10.1016/j.bmcl.2015.06.067
BindingDB Entry DOI: 10.7270/Q2DF6SZ5 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50112819

(CHEMBL3609522)Show SMILES CC(C)(C)c1ncc2CCc3nc(NC(=O)N4CCC[C@H]4C(N)=O)sc3-c2n1 |r| Show InChI InChI=1S/C19H24N6O2S/c1-19(2,3)16-21-9-10-6-7-11-14(13(10)23-16)28-17(22-11)24-18(27)25-8-4-5-12(25)15(20)26/h9,12H,4-8H2,1-3H3,(H2,20,26)(H,22,24,27)/t12-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of N-terminal myristoylated human PI3Kalpha expressed in Rat1 cells assessed as inhibition of Akt phosphorylatuion at Ser473 by ELISA |
Bioorg Med Chem Lett 25: 3575-81 (2015)
Article DOI: 10.1016/j.bmcl.2015.06.067
BindingDB Entry DOI: 10.7270/Q2DF6SZ5 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50436448
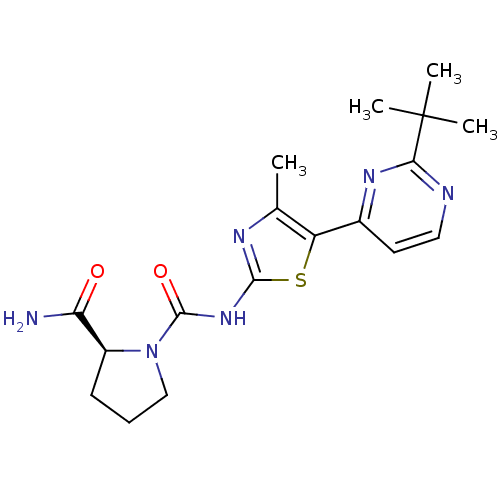
(CHEMBL2397186)Show SMILES Cc1nc(NC(=O)N2CCC[C@H]2C(N)=O)sc1-c1ccnc(n1)C(C)(C)C |r| Show InChI InChI=1S/C18H24N6O2S/c1-10-13(11-7-8-20-15(22-11)18(2,3)4)27-16(21-10)23-17(26)24-9-5-6-12(24)14(19)25/h7-8,12H,5-6,9H2,1-4H3,(H2,19,25)(H,21,23,26)/t12-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kalpha (unknown origin) using phosphatidylinositol as substrate preincubated for 15 mins followed by ATP addition measured after 1 h... |
Bioorg Med Chem Lett 25: 3575-81 (2015)
Article DOI: 10.1016/j.bmcl.2015.06.067
BindingDB Entry DOI: 10.7270/Q2DF6SZ5 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50436448

(CHEMBL2397186)Show SMILES Cc1nc(NC(=O)N2CCC[C@H]2C(N)=O)sc1-c1ccnc(n1)C(C)(C)C |r| Show InChI InChI=1S/C18H24N6O2S/c1-10-13(11-7-8-20-15(22-11)18(2,3)4)27-16(21-10)23-17(26)24-9-5-6-12(24)14(19)25/h7-8,12H,5-6,9H2,1-4H3,(H2,19,25)(H,21,23,26)/t12-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 7.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of P110alpha (unknown origin) using L-a-phosphatidylinositol as substrate by luminescence assay |
Bioorg Med Chem Lett 23: 3741-8 (2013)
Article DOI: 10.1016/j.bmcl.2013.05.007
BindingDB Entry DOI: 10.7270/Q2M046VD |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50112829
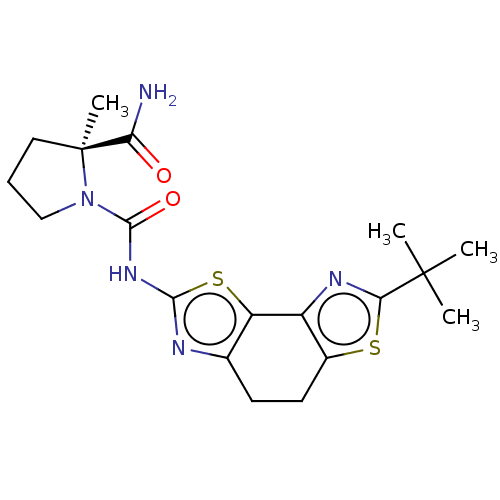
(CHEMBL3609516)Show SMILES CC(C)(C)c1nc-2c(CCc3nc(NC(=O)N4CCC[C@@]4(C)C(N)=O)sc-23)s1 |r| Show InChI InChI=1S/C19H25N5O2S2/c1-18(2,3)15-22-12-11(27-15)7-6-10-13(12)28-16(21-10)23-17(26)24-9-5-8-19(24,4)14(20)25/h5-9H2,1-4H3,(H2,20,25)(H,21,23,26)/t19-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kalpha (unknown origin) using phosphatidylinositol as substrate preincubated for 15 mins followed by ATP addition measured after 1 h... |
Bioorg Med Chem Lett 25: 3575-81 (2015)
Article DOI: 10.1016/j.bmcl.2015.06.067
BindingDB Entry DOI: 10.7270/Q2DF6SZ5 |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase kinase kinase 8
(Homo sapiens (Human)) | BDBM50191771
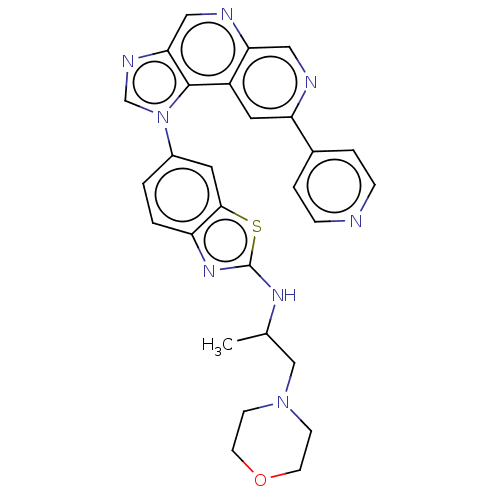
(CHEMBL3890505)Show SMILES CC(CN1CCOCC1)Nc1nc2ccc(cc2s1)-n1cnc2cnc3cnc(cc3c12)-c1ccncc1 Show InChI InChI=1S/C28H26N8OS/c1-18(16-35-8-10-37-11-9-35)33-28-34-22-3-2-20(12-26(22)38-28)36-17-32-25-15-31-24-14-30-23(13-21(24)27(25)36)19-4-6-29-7-5-19/h2-7,12-15,17-18H,8-11,16H2,1H3,(H,33,34) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of human COT (66 to 395 residues) expressed in Sf21 cells using 5-Fluo-Ahx-AGAGSGQLIDSNleANSFVGTR-NH2 as substrate after 60 mins by calipe... |
J Med Chem 59: 7544-60 (2016)
Article DOI: 10.1021/acs.jmedchem.6b00598
BindingDB Entry DOI: 10.7270/Q29P33MR |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase kinase kinase 8
(Homo sapiens (Human)) | BDBM50191769

(CHEMBL3927501)Show SMILES CC(CN1CCOCC1)Nc1nc2ccc(cc2s1)-n1cnc2cnc3cnc(cc3c12)-c1ccccc1 Show InChI InChI=1S/C29H27N7OS/c1-19(17-35-9-11-37-12-10-35)33-29-34-23-8-7-21(13-27(23)38-29)36-18-32-26-16-31-25-15-30-24(14-22(25)28(26)36)20-5-3-2-4-6-20/h2-8,13-16,18-19H,9-12,17H2,1H3,(H,33,34) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of human COT (66 to 395 residues) expressed in Sf21 cells using 5-Fluo-Ahx-AGAGSGQLIDSNleANSFVGTR-NH2 as substrate after 60 mins by calipe... |
J Med Chem 59: 7544-60 (2016)
Article DOI: 10.1021/acs.jmedchem.6b00598
BindingDB Entry DOI: 10.7270/Q29P33MR |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50112830
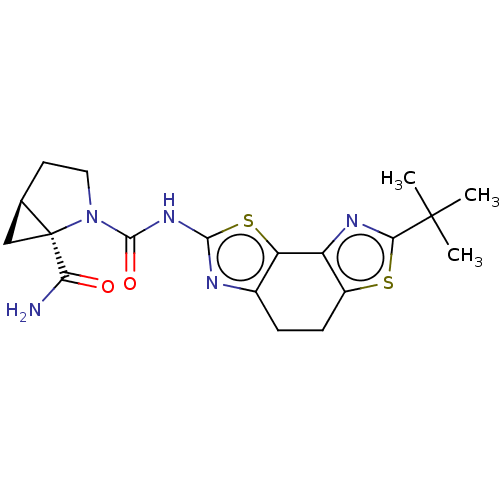
(CHEMBL3609517)Show SMILES [H][C@@]12C[C@@]1(N(CC2)C(=O)Nc1nc2CCc3sc(nc3-c2s1)C(C)(C)C)C(N)=O |r| Show InChI InChI=1S/C19H23N5O2S2/c1-18(2,3)15-22-12-11(27-15)5-4-10-13(12)28-16(21-10)23-17(26)24-7-6-9-8-19(9,24)14(20)25/h9H,4-8H2,1-3H3,(H2,20,25)(H,21,23,26)/t9-,19+/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kalpha (unknown origin) using phosphatidylinositol as substrate preincubated for 15 mins followed by ATP addition measured after 1 h... |
Bioorg Med Chem Lett 25: 3575-81 (2015)
Article DOI: 10.1016/j.bmcl.2015.06.067
BindingDB Entry DOI: 10.7270/Q2DF6SZ5 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM50203699
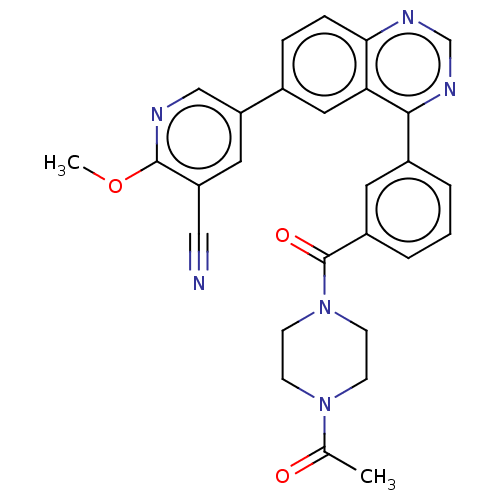
(CHEMBL3977066)Show SMILES COc1ncc(cc1C#N)-c1ccc2ncnc(-c3cccc(c3)C(=O)N3CCN(CC3)C(C)=O)c2c1 Show InChI InChI=1S/C28H24N6O3/c1-18(35)33-8-10-34(11-9-33)28(36)21-5-3-4-20(12-21)26-24-14-19(6-7-25(24)31-17-32-26)23-13-22(15-29)27(37-2)30-16-23/h3-7,12-14,16-17H,8-11H2,1-2H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kdelta (unknown origin) using phosphatidyl inositol as substrate measured after 60 mins by Alexa Fluor647-labelled ADP tracer based ... |
ACS Med Chem Lett 8: 975-980 (2017)
Article DOI: 10.1021/acsmedchemlett.7b00293
BindingDB Entry DOI: 10.7270/Q2SX6GR0 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50112817

(CHEMBL3609520)Show SMILES C[C@@H]1CCN([C@@H]1C(N)=O)C(=O)Nc1nc2CCc3sc(nc3-c2s1)C(C)(C)C(F)(F)F |r| Show InChI InChI=1S/C19H22F3N5O2S2/c1-8-6-7-27(12(8)14(23)28)17(29)26-16-24-9-4-5-10-11(13(9)31-16)25-15(30-10)18(2,3)19(20,21)22/h8,12H,4-7H2,1-3H3,(H2,23,28)(H,24,26,29)/t8-,12+/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kalpha (unknown origin) using phosphatidylinositol as substrate preincubated for 15 mins followed by ATP addition measured after 1 h... |
Bioorg Med Chem Lett 25: 3575-81 (2015)
Article DOI: 10.1016/j.bmcl.2015.06.067
BindingDB Entry DOI: 10.7270/Q2DF6SZ5 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data