 Found 91 hits with Last Name = 'bowen' and Initial = 'jp'
Found 91 hits with Last Name = 'bowen' and Initial = 'jp' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
D(1A) dopamine receptor
(Homo sapiens (Human)) | BDBM82247

(8-Chloro-3-methyl-5-phenyl-2,3,4,5-tetrahydro-1H-b...)Show InChI InChI=1S/C17H18ClNO/c1-19-8-7-13-9-16(18)17(20)10-14(13)15(11-19)12-5-3-2-4-6-12/h2-6,9-10,15,20H,7-8,11H2,1H3/t15-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.427 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of North Carolina
Curated by ChEMBL
| Assay Description
In vitro inhibition of rat liver dihydrofolate reductase. |
J Med Chem 32: 2050-8 (1989)
BindingDB Entry DOI: 10.7270/Q2RV0QXV |
More data for this
Ligand-Target Pair | |
D(1A) dopamine receptor
(RAT) | BDBM82247

(8-Chloro-3-methyl-5-phenyl-2,3,4,5-tetrahydro-1H-b...)Show InChI InChI=1S/C17H18ClNO/c1-19-8-7-13-9-16(18)17(20)10-14(13)15(11-19)12-5-3-2-4-6-12/h2-6,9-10,15,20H,7-8,11H2,1H3/t15-/m1/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.430 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of North Carolina
Curated by ChEMBL
| Assay Description
Inhibition of [3H]SCH-23,390 binding to Dopamine receptor D1 at 0.25 nM |
J Med Chem 31: 1941-6 (1988)
BindingDB Entry DOI: 10.7270/Q20002PD |
More data for this
Ligand-Target Pair | |
D(1A) dopamine receptor
(RAT) | BDBM50004796
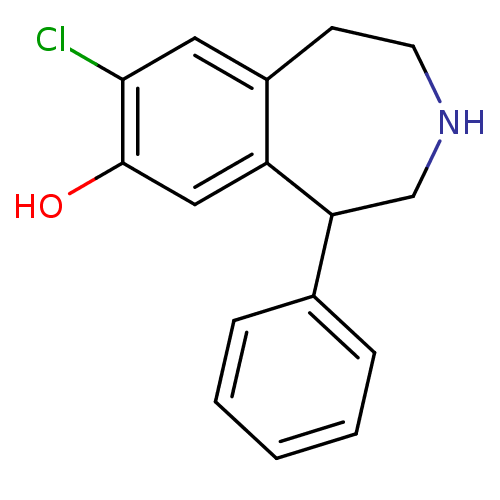
(8-Chloro-5-phenyl-2,3,4,5-tetrahydro-1H-benzo[d]az...)Show InChI InChI=1S/C16H16ClNO/c17-15-8-12-6-7-18-10-14(13(12)9-16(15)19)11-4-2-1-3-5-11/h1-5,8-9,14,18-19H,6-7,10H2 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 2.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of North Carolina
Curated by ChEMBL
| Assay Description
Inhibition of [3H]SCH-23,390 binding to Dopamine receptor D1 at 0.25 nM |
J Med Chem 31: 1941-6 (1988)
BindingDB Entry DOI: 10.7270/Q20002PD |
More data for this
Ligand-Target Pair | |
D(2) dopamine receptor
(Rattus norvegicus (rat)) | BDBM50001888

((chloropromazine) [3-(2-Chloro-phenothiazin-10-yl)...)Show InChI InChI=1S/C17H19ClN2S/c1-19(2)10-5-11-20-14-6-3-4-7-16(14)21-17-9-8-13(18)12-15(17)20/h3-4,6-9,12H,5,10-11H2,1-2H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PubMed
| 5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of North Carolina
Curated by ChEMBL
| Assay Description
Inhibition of [3H]spiperone binding to Dopamine receptor D2 at 0.02 nM |
J Med Chem 31: 1941-6 (1988)
BindingDB Entry DOI: 10.7270/Q20002PD |
More data for this
Ligand-Target Pair | |
D(1A) dopamine receptor
(RAT) | BDBM50038349
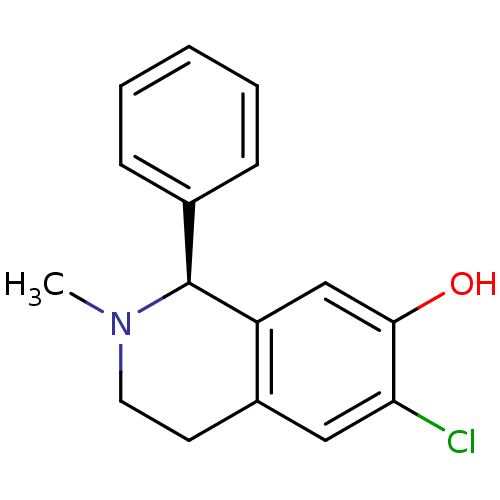
((S)-6-Chloro-2-methyl-1-phenyl-1,2,3,4-tetrahydro-...)Show InChI InChI=1S/C16H16ClNO/c1-18-8-7-12-9-14(17)15(19)10-13(12)16(18)11-5-3-2-4-6-11/h2-6,9-10,16,19H,7-8H2,1H3/t16-/m0/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 6.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of North Carolina
Curated by ChEMBL
| Assay Description
Inhibition of [3H]SCH-23,390 binding to Dopamine receptor D1 at 0.25 nM |
J Med Chem 31: 1941-6 (1988)
BindingDB Entry DOI: 10.7270/Q20002PD |
More data for this
Ligand-Target Pair | |
D(1A) dopamine receptor
(Homo sapiens (Human)) | BDBM50038349

((S)-6-Chloro-2-methyl-1-phenyl-1,2,3,4-tetrahydro-...)Show InChI InChI=1S/C16H16ClNO/c1-18-8-7-12-9-14(17)15(19)10-13(12)16(18)11-5-3-2-4-6-11/h2-6,9-10,16,19H,7-8H2,1H3/t16-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 6.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of North Carolina
Curated by ChEMBL
| Assay Description
In vitro inhibition of rat liver dihydrofolate reductase. |
J Med Chem 32: 2050-8 (1989)
BindingDB Entry DOI: 10.7270/Q2RV0QXV |
More data for this
Ligand-Target Pair | |
D(1A) dopamine receptor
(RAT) | BDBM50022051
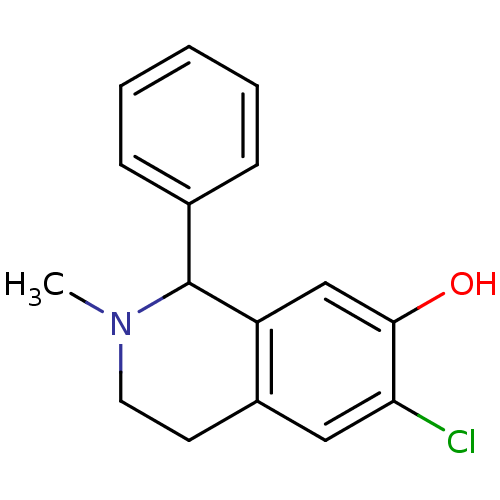
(1-Phenyl-6-chloro-7-hydroxy-N-methyl-1,2,3,4-tetra...)Show InChI InChI=1S/C16H16ClNO/c1-18-8-7-12-9-14(17)15(19)10-13(12)16(18)11-5-3-2-4-6-11/h2-6,9-10,16,19H,7-8H2,1H3 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 13 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of North Carolina
Curated by ChEMBL
| Assay Description
Inhibition of [3H]SCH-23,390 binding to Dopamine receptor D1 at 0.25 nM |
J Med Chem 31: 1941-6 (1988)
BindingDB Entry DOI: 10.7270/Q20002PD |
More data for this
Ligand-Target Pair | |
D(1A) dopamine receptor
(Homo sapiens (Human)) | BDBM50228322
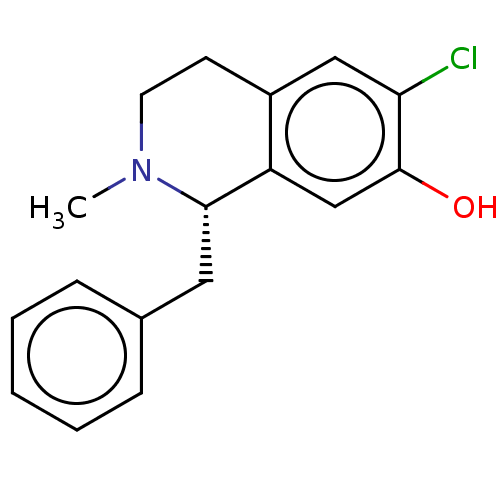
(CHEMBL64117)Show InChI InChI=1S/C17H18ClNO/c1-19-8-7-13-10-15(18)17(20)11-14(13)16(19)9-12-5-3-2-4-6-12/h2-6,10-11,16,20H,7-9H2,1H3/t16-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 27 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of North Carolina
Curated by ChEMBL
| Assay Description
In vitro inhibition of Escherichia coli dihydrofolate reductase. |
J Med Chem 32: 2050-8 (1989)
BindingDB Entry DOI: 10.7270/Q2RV0QXV |
More data for this
Ligand-Target Pair | |
D(1A) dopamine receptor
(RAT) | BDBM50001888

((chloropromazine) [3-(2-Chloro-phenothiazin-10-yl)...)Show InChI InChI=1S/C17H19ClN2S/c1-19(2)10-5-11-20-14-6-3-4-7-16(14)21-17-9-8-13(18)12-15(17)20/h3-4,6-9,12H,5,10-11H2,1-2H3 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PubMed
| 50 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of North Carolina
Curated by ChEMBL
| Assay Description
Inhibition of [3H]SCH-23,390 binding to Dopamine receptor D1 at 0.25 nM |
J Med Chem 31: 1941-6 (1988)
BindingDB Entry DOI: 10.7270/Q20002PD |
More data for this
Ligand-Target Pair | |
D(1A) dopamine receptor
(RAT) | BDBM50022052
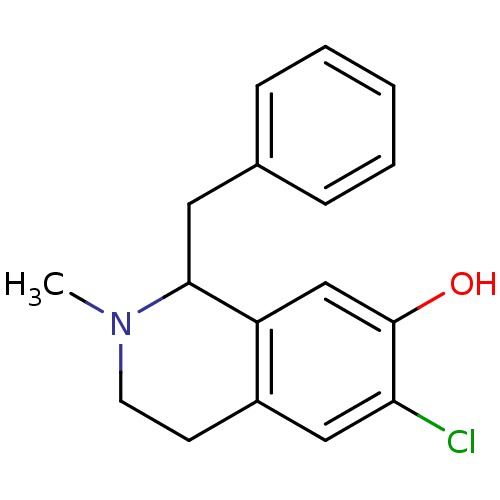
(1-Benzyl-6-chloro-2-methyl-1,2,3,4-tetrahydro-isoq...)Show InChI InChI=1S/C17H18ClNO/c1-19-8-7-13-10-15(18)17(20)11-14(13)16(19)9-12-5-3-2-4-6-12/h2-6,10-11,16,20H,7-9H2,1H3 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 53 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of North Carolina
Curated by ChEMBL
| Assay Description
Inhibition of [3H]SCH-23,390 binding to Dopamine receptor D1 at 0.25 nM |
J Med Chem 31: 1941-6 (1988)
BindingDB Entry DOI: 10.7270/Q20002PD |
More data for this
Ligand-Target Pair | |
D(1A) dopamine receptor
(Homo sapiens (Human)) | BDBM50228321

(CHEMBL302393)Show InChI InChI=1S/C16H16ClNO/c1-18-9-12-7-15(17)16(19)8-13(12)14(10-18)11-5-3-2-4-6-11/h2-8,14,19H,9-10H2,1H3/t14-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 87 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of North Carolina
Curated by ChEMBL
| Assay Description
In vitro inhibition of rat liver dihydrofolate reductase. |
J Med Chem 32: 2050-8 (1989)
BindingDB Entry DOI: 10.7270/Q2RV0QXV |
More data for this
Ligand-Target Pair | |
D(1A) dopamine receptor
(RAT) | BDBM50367602

(CHEMBL65397)Show InChI InChI=1S/C15H14ClNO/c16-13-8-11-6-7-17-15(12(11)9-14(13)18)10-4-2-1-3-5-10/h1-5,8-9,15,17-18H,6-7H2/t15-/m0/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 140 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of North Carolina
Curated by ChEMBL
| Assay Description
Inhibition of [3H]SCH-23,390 binding to Dopamine receptor D1 at 0.25 nM |
J Med Chem 31: 1941-6 (1988)
BindingDB Entry DOI: 10.7270/Q20002PD |
More data for this
Ligand-Target Pair | |
D(1A) dopamine receptor
(Homo sapiens (Human)) | BDBM50367602

(CHEMBL65397)Show InChI InChI=1S/C15H14ClNO/c16-13-8-11-6-7-17-15(12(11)9-14(13)18)10-4-2-1-3-5-10/h1-5,8-9,15,17-18H,6-7H2/t15-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 141 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of North Carolina
Curated by ChEMBL
| Assay Description
In vitro inhibition of rat liver dihydrofolate reductase. |
J Med Chem 32: 2050-8 (1989)
BindingDB Entry DOI: 10.7270/Q2RV0QXV |
More data for this
Ligand-Target Pair | |
D(2) dopamine receptor
(Homo sapiens (Human)) | BDBM50228322

(CHEMBL64117)Show InChI InChI=1S/C17H18ClNO/c1-19-8-7-13-10-15(18)17(20)11-14(13)16(19)9-12-5-3-2-4-6-12/h2-6,10-11,16,20H,7-9H2,1H3/t16-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 145 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of North Carolina
Curated by ChEMBL
| Assay Description
In vitro inhibition of Escherichia coli dihydrofolate reductase. |
J Med Chem 32: 2050-8 (1989)
BindingDB Entry DOI: 10.7270/Q2RV0QXV |
More data for this
Ligand-Target Pair | |
D(1A) dopamine receptor
(RAT) | BDBM50022053
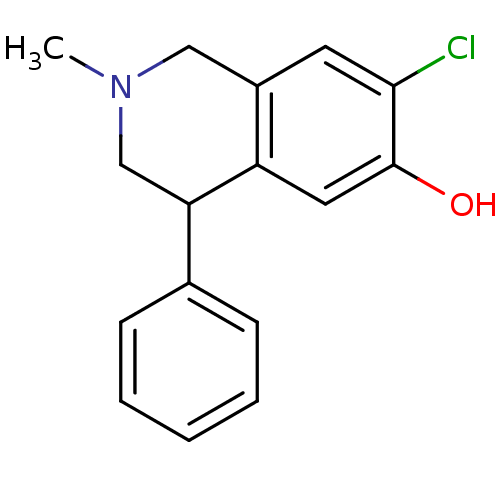
(1-Benzyl-6-chloro-2-methyl-1,2,3,4-tetrahydro-isoq...)Show InChI InChI=1S/C16H16ClNO/c1-18-9-12-7-15(17)16(19)8-13(12)14(10-18)11-5-3-2-4-6-11/h2-8,14,19H,9-10H2,1H3 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 174 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of North Carolina
Curated by ChEMBL
| Assay Description
Inhibition of [3H]SCH-23,390 binding to Dopamine receptor D1 at 0.25 nM |
J Med Chem 31: 1941-6 (1988)
BindingDB Entry DOI: 10.7270/Q20002PD |
More data for this
Ligand-Target Pair | |
D(1A) dopamine receptor
(Homo sapiens (Human)) | BDBM50367601
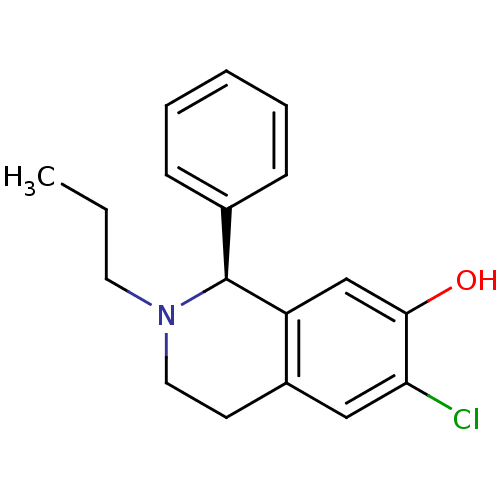
(CHEMBL293828)Show InChI InChI=1S/C18H20ClNO/c1-2-9-20-10-8-14-11-16(19)17(21)12-15(14)18(20)13-6-4-3-5-7-13/h3-7,11-12,18,21H,2,8-10H2,1H3/t18-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 178 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of North Carolina
Curated by ChEMBL
| Assay Description
Antibacterial activity against Escherichia coli |
J Med Chem 32: 2050-8 (1989)
BindingDB Entry DOI: 10.7270/Q2RV0QXV |
More data for this
Ligand-Target Pair | |
D(1A) dopamine receptor
(RAT) | BDBM50367601

(CHEMBL293828)Show InChI InChI=1S/C18H20ClNO/c1-2-9-20-10-8-14-11-16(19)17(21)12-15(14)18(20)13-6-4-3-5-7-13/h3-7,11-12,18,21H,2,8-10H2,1H3/t18-/m0/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 179 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of North Carolina
Curated by ChEMBL
| Assay Description
Inhibition of [3H]SCH-23,390 binding to Dopamine receptor D1 at 0.25 nM |
J Med Chem 31: 1941-6 (1988)
BindingDB Entry DOI: 10.7270/Q20002PD |
More data for this
Ligand-Target Pair | |
D(2) dopamine receptor
(Homo sapiens (Human)) | BDBM50228321

(CHEMBL302393)Show InChI InChI=1S/C16H16ClNO/c1-18-9-12-7-15(17)16(19)8-13(12)14(10-18)11-5-3-2-4-6-11/h2-8,14,19H,9-10H2,1H3/t14-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 263 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of North Carolina
Curated by ChEMBL
| Assay Description
In vitro inhibition of rat liver dihydrofolate reductase. |
J Med Chem 32: 2050-8 (1989)
BindingDB Entry DOI: 10.7270/Q2RV0QXV |
More data for this
Ligand-Target Pair | |
D(1A) dopamine receptor
(Homo sapiens (Human)) | BDBM50228323
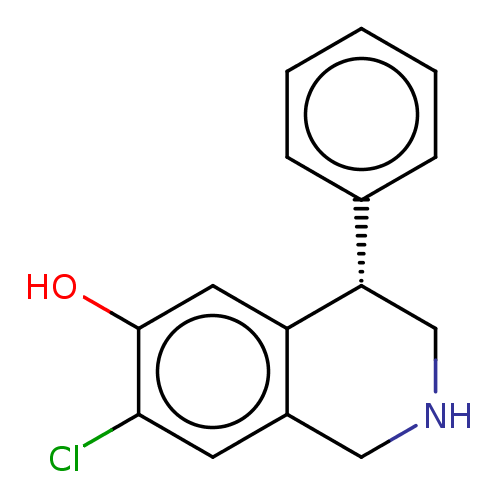
(CHEMBL59603)Show InChI InChI=1S/C15H14ClNO/c16-14-6-11-8-17-9-13(12(11)7-15(14)18)10-4-2-1-3-5-10/h1-7,13,17-18H,8-9H2/t13-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 282 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of North Carolina
Curated by ChEMBL
| Assay Description
In vitro inhibition of Escherichia coli dihydrofolate reductase. |
J Med Chem 32: 2050-8 (1989)
BindingDB Entry DOI: 10.7270/Q2RV0QXV |
More data for this
Ligand-Target Pair | |
D(2) dopamine receptor
(Rattus norvegicus (rat)) | BDBM50022052

(1-Benzyl-6-chloro-2-methyl-1,2,3,4-tetrahydro-isoq...)Show InChI InChI=1S/C17H18ClNO/c1-19-8-7-13-10-15(18)17(20)11-14(13)16(19)9-12-5-3-2-4-6-12/h2-6,10-11,16,20H,7-9H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 287 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of North Carolina
Curated by ChEMBL
| Assay Description
Inhibition of [3H]spiperone binding to Dopamine receptor D2 at 0.02 nM |
J Med Chem 31: 1941-6 (1988)
BindingDB Entry DOI: 10.7270/Q20002PD |
More data for this
Ligand-Target Pair | |
D(1A) dopamine receptor
(RAT) | BDBM50038350
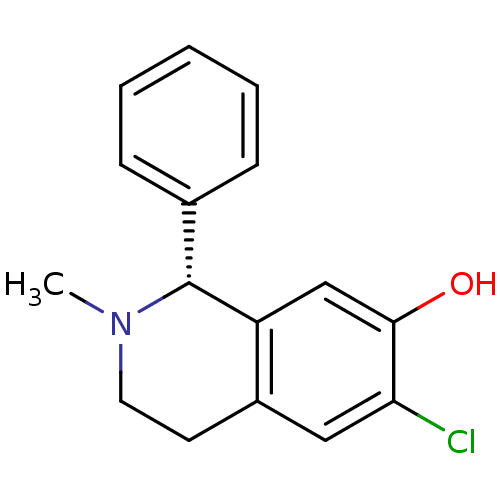
((R)-6-Chloro-2-methyl-1-phenyl-1,2,3,4-tetrahydro-...)Show InChI InChI=1S/C16H16ClNO/c1-18-8-7-12-9-14(17)15(19)10-13(12)16(18)11-5-3-2-4-6-11/h2-6,9-10,16,19H,7-8H2,1H3/t16-/m1/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 442 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of North Carolina
Curated by ChEMBL
| Assay Description
Inhibition of [3H]SCH-23,390 binding to Dopamine receptor D1 at 0.25 nM |
J Med Chem 31: 1941-6 (1988)
BindingDB Entry DOI: 10.7270/Q20002PD |
More data for this
Ligand-Target Pair | |
D(1A) dopamine receptor
(Homo sapiens (Human)) | BDBM50038350

((R)-6-Chloro-2-methyl-1-phenyl-1,2,3,4-tetrahydro-...)Show InChI InChI=1S/C16H16ClNO/c1-18-8-7-12-9-14(17)15(19)10-13(12)16(18)11-5-3-2-4-6-11/h2-6,9-10,16,19H,7-8H2,1H3/t16-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 447 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of North Carolina
Curated by ChEMBL
| Assay Description
In vitro inhibition of Escherichia coli dihydrofolate reductase. |
J Med Chem 32: 2050-8 (1989)
BindingDB Entry DOI: 10.7270/Q2RV0QXV |
More data for this
Ligand-Target Pair | |
D(2) dopamine receptor
(Rattus norvegicus (rat)) | BDBM50022053

(1-Benzyl-6-chloro-2-methyl-1,2,3,4-tetrahydro-isoq...)Show InChI InChI=1S/C16H16ClNO/c1-18-9-12-7-15(17)16(19)8-13(12)14(10-18)11-5-3-2-4-6-11/h2-8,14,19H,9-10H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 522 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of North Carolina
Curated by ChEMBL
| Assay Description
Inhibition of [3H]spiperone binding to Dopamine receptor D2 at 0.02 nM |
J Med Chem 31: 1941-6 (1988)
BindingDB Entry DOI: 10.7270/Q20002PD |
More data for this
Ligand-Target Pair | |
D(1A) dopamine receptor
(RAT) | BDBM50022054
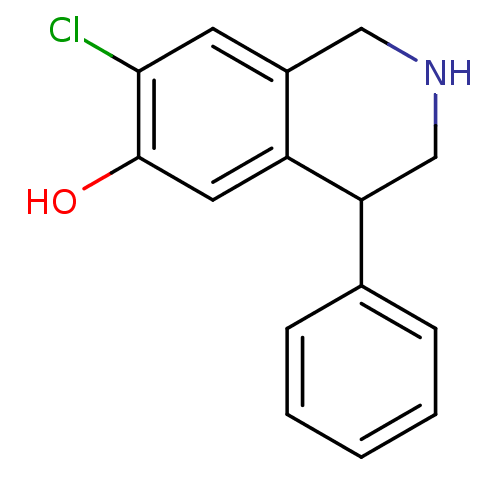
(7-Chloro-4-phenyl-1,2,3,4-tetrahydro-isoquinolin-6...)Show InChI InChI=1S/C15H14ClNO/c16-14-6-11-8-17-9-13(12(11)7-15(14)18)10-4-2-1-3-5-10/h1-7,13,17-18H,8-9H2 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 565 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of North Carolina
Curated by ChEMBL
| Assay Description
Inhibition of [3H]SCH-23,390 binding to Dopamine receptor D1 at 0.25 nM |
J Med Chem 31: 1941-6 (1988)
BindingDB Entry DOI: 10.7270/Q20002PD |
More data for this
Ligand-Target Pair | |
D(2) dopamine receptor
(Rattus norvegicus (rat)) | BDBM50004796

(8-Chloro-5-phenyl-2,3,4,5-tetrahydro-1H-benzo[d]az...)Show InChI InChI=1S/C16H16ClNO/c17-15-8-12-6-7-18-10-14(13(12)9-16(15)19)11-4-2-1-3-5-11/h1-5,8-9,14,18-19H,6-7,10H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 676 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of North Carolina
Curated by ChEMBL
| Assay Description
Inhibition of [3H]spiperone binding to Dopamine receptor D2 at 0.02 nM |
J Med Chem 31: 1941-6 (1988)
BindingDB Entry DOI: 10.7270/Q20002PD |
More data for this
Ligand-Target Pair | |
D(1A) dopamine receptor
(RAT) | BDBM50367600
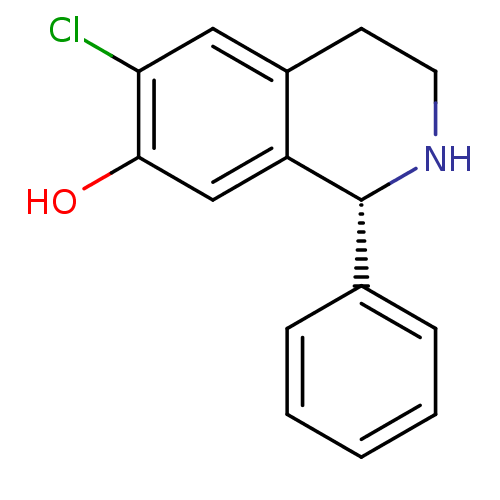
(CHEMBL1788322)Show InChI InChI=1S/C15H14ClNO/c16-13-8-11-6-7-17-15(12(11)9-14(13)18)10-4-2-1-3-5-10/h1-5,8-9,15,17-18H,6-7H2/t15-/m1/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 743 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of North Carolina
Curated by ChEMBL
| Assay Description
Inhibition of [3H]SCH-23,390 binding to Dopamine receptor D1 at 0.25 nM |
J Med Chem 31: 1941-6 (1988)
BindingDB Entry DOI: 10.7270/Q20002PD |
More data for this
Ligand-Target Pair | |
D(2) dopamine receptor
(Homo sapiens (Human)) | BDBM82247

(8-Chloro-3-methyl-5-phenyl-2,3,4,5-tetrahydro-1H-b...)Show InChI InChI=1S/C17H18ClNO/c1-19-8-7-13-9-16(18)17(20)10-14(13)15(11-19)12-5-3-2-4-6-12/h2-6,9-10,15,20H,7-8,11H2,1H3/t15-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| PubMed
| 891 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of North Carolina
Curated by ChEMBL
| Assay Description
In vitro inhibition of Escherichia coli dihydrofolate reductase. |
J Med Chem 32: 2050-8 (1989)
BindingDB Entry DOI: 10.7270/Q2RV0QXV |
More data for this
Ligand-Target Pair | |
D(2) dopamine receptor
(Rattus norvegicus (rat)) | BDBM82247

(8-Chloro-3-methyl-5-phenyl-2,3,4,5-tetrahydro-1H-b...)Show InChI InChI=1S/C17H18ClNO/c1-19-8-7-13-9-16(18)17(20)10-14(13)15(11-19)12-5-3-2-4-6-12/h2-6,9-10,15,20H,7-8,11H2,1H3/t15-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| PubMed
| 900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of North Carolina
Curated by ChEMBL
| Assay Description
Inhibition of [3H]spiperone binding to Dopamine receptor D2 at 0.02 nM |
J Med Chem 31: 1941-6 (1988)
BindingDB Entry DOI: 10.7270/Q20002PD |
More data for this
Ligand-Target Pair | |
D(2) dopamine receptor
(Rattus norvegicus (rat)) | BDBM50022051

(1-Phenyl-6-chloro-7-hydroxy-N-methyl-1,2,3,4-tetra...)Show InChI InChI=1S/C16H16ClNO/c1-18-8-7-12-9-14(17)15(19)10-13(12)16(18)11-5-3-2-4-6-11/h2-6,9-10,16,19H,7-8H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 915 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of North Carolina
Curated by ChEMBL
| Assay Description
Inhibition of [3H]spiperone binding to Dopamine receptor D2 at 0.02 nM |
J Med Chem 31: 1941-6 (1988)
BindingDB Entry DOI: 10.7270/Q20002PD |
More data for this
Ligand-Target Pair | |
D(2) dopamine receptor
(Homo sapiens (Human)) | BDBM50228323

(CHEMBL59603)Show InChI InChI=1S/C15H14ClNO/c16-14-6-11-8-17-9-13(12(11)7-15(14)18)10-4-2-1-3-5-10/h1-7,13,17-18H,8-9H2/t13-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 1.82E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of North Carolina
Curated by ChEMBL
| Assay Description
In vitro inhibition of rat liver dihydrofolate reductase. |
J Med Chem 32: 2050-8 (1989)
BindingDB Entry DOI: 10.7270/Q2RV0QXV |
More data for this
Ligand-Target Pair | |
D(2) dopamine receptor
(Rattus norvegicus (rat)) | BDBM50038349

((S)-6-Chloro-2-methyl-1-phenyl-1,2,3,4-tetrahydro-...)Show InChI InChI=1S/C16H16ClNO/c1-18-8-7-12-9-14(17)15(19)10-13(12)16(18)11-5-3-2-4-6-11/h2-6,9-10,16,19H,7-8H2,1H3/t16-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 1.85E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of North Carolina
Curated by ChEMBL
| Assay Description
Inhibition of [3H]spiperone binding to Dopamine receptor D2 at 0.02 nM |
J Med Chem 31: 1941-6 (1988)
BindingDB Entry DOI: 10.7270/Q20002PD |
More data for this
Ligand-Target Pair | |
D(2) dopamine receptor
(Homo sapiens (Human)) | BDBM50038349

((S)-6-Chloro-2-methyl-1-phenyl-1,2,3,4-tetrahydro-...)Show InChI InChI=1S/C16H16ClNO/c1-18-8-7-12-9-14(17)15(19)10-13(12)16(18)11-5-3-2-4-6-11/h2-6,9-10,16,19H,7-8H2,1H3/t16-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 1.86E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of North Carolina
Curated by ChEMBL
| Assay Description
Antibacterial activity against Escherichia coli |
J Med Chem 32: 2050-8 (1989)
BindingDB Entry DOI: 10.7270/Q2RV0QXV |
More data for this
Ligand-Target Pair | |
D(2) dopamine receptor
(Rattus norvegicus (rat)) | BDBM50367601

(CHEMBL293828)Show InChI InChI=1S/C18H20ClNO/c1-2-9-20-10-8-14-11-16(19)17(21)12-15(14)18(20)13-6-4-3-5-7-13/h3-7,11-12,18,21H,2,8-10H2,1H3/t18-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 1.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of North Carolina
Curated by ChEMBL
| Assay Description
Inhibition of [3H]spiperone binding to Dopamine receptor D2 at 0.02 nM |
J Med Chem 31: 1941-6 (1988)
BindingDB Entry DOI: 10.7270/Q20002PD |
More data for this
Ligand-Target Pair | |
D(2) dopamine receptor
(Homo sapiens (Human)) | BDBM50367601

(CHEMBL293828)Show InChI InChI=1S/C18H20ClNO/c1-2-9-20-10-8-14-11-16(19)17(21)12-15(14)18(20)13-6-4-3-5-7-13/h3-7,11-12,18,21H,2,8-10H2,1H3/t18-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 1.91E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of North Carolina
Curated by ChEMBL
| Assay Description
In vitro inhibition of dihydrofolate reductase of Escherichia coli |
J Med Chem 32: 2050-8 (1989)
BindingDB Entry DOI: 10.7270/Q2RV0QXV |
More data for this
Ligand-Target Pair | |
D(2) dopamine receptor
(Rattus norvegicus (rat)) | BDBM50022054

(7-Chloro-4-phenyl-1,2,3,4-tetrahydro-isoquinolin-6...)Show InChI InChI=1S/C15H14ClNO/c16-14-6-11-8-17-9-13(12(11)7-15(14)18)10-4-2-1-3-5-10/h1-7,13,17-18H,8-9H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 3.62E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of North Carolina
Curated by ChEMBL
| Assay Description
Inhibition of [3H]spiperone binding to Dopamine receptor D2 at 0.02 nM |
J Med Chem 31: 1941-6 (1988)
BindingDB Entry DOI: 10.7270/Q20002PD |
More data for this
Ligand-Target Pair | |
D(2) dopamine receptor
(Homo sapiens (Human)) | BDBM50367602

(CHEMBL65397)Show InChI InChI=1S/C15H14ClNO/c16-13-8-11-6-7-17-15(12(11)9-14(13)18)10-4-2-1-3-5-10/h1-5,8-9,15,17-18H,6-7H2/t15-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 3.72E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of North Carolina
Curated by ChEMBL
| Assay Description
In vitro inhibition of rat liver dihydrofolate reductase. |
J Med Chem 32: 2050-8 (1989)
BindingDB Entry DOI: 10.7270/Q2RV0QXV |
More data for this
Ligand-Target Pair | |
D(2) dopamine receptor
(Rattus norvegicus (rat)) | BDBM50367602

(CHEMBL65397)Show InChI InChI=1S/C15H14ClNO/c16-13-8-11-6-7-17-15(12(11)9-14(13)18)10-4-2-1-3-5-10/h1-5,8-9,15,17-18H,6-7H2/t15-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 3.75E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of North Carolina
Curated by ChEMBL
| Assay Description
Inhibition of [3H]spiperone binding to Dopamine receptor D2 at 0.02 nM |
J Med Chem 31: 1941-6 (1988)
BindingDB Entry DOI: 10.7270/Q20002PD |
More data for this
Ligand-Target Pair | |
D(2) dopamine receptor
(Homo sapiens (Human)) | BDBM50038350

((R)-6-Chloro-2-methyl-1-phenyl-1,2,3,4-tetrahydro-...)Show InChI InChI=1S/C16H16ClNO/c1-18-8-7-12-9-14(17)15(19)10-13(12)16(18)11-5-3-2-4-6-11/h2-6,9-10,16,19H,7-8H2,1H3/t16-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 1.91E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of North Carolina
Curated by ChEMBL
| Assay Description
In vitro inhibition of Escherichia coli dihydrofolate reductase. |
J Med Chem 32: 2050-8 (1989)
BindingDB Entry DOI: 10.7270/Q2RV0QXV |
More data for this
Ligand-Target Pair | |
D(2) dopamine receptor
(Rattus norvegicus (rat)) | BDBM50038350

((R)-6-Chloro-2-methyl-1-phenyl-1,2,3,4-tetrahydro-...)Show InChI InChI=1S/C16H16ClNO/c1-18-8-7-12-9-14(17)15(19)10-13(12)16(18)11-5-3-2-4-6-11/h2-6,9-10,16,19H,7-8H2,1H3/t16-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 1.92E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of North Carolina
Curated by ChEMBL
| Assay Description
Inhibition of [3H]spiperone binding to Dopamine receptor D2 at 0.02 nM |
J Med Chem 31: 1941-6 (1988)
BindingDB Entry DOI: 10.7270/Q20002PD |
More data for this
Ligand-Target Pair | |
D(2) dopamine receptor
(Rattus norvegicus (rat)) | BDBM50367600

(CHEMBL1788322)Show InChI InChI=1S/C15H14ClNO/c16-13-8-11-6-7-17-15(12(11)9-14(13)18)10-4-2-1-3-5-10/h1-5,8-9,15,17-18H,6-7H2/t15-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 2.63E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of North Carolina
Curated by ChEMBL
| Assay Description
Inhibition of [3H]spiperone binding to Dopamine receptor D2 at 0.02 nM |
J Med Chem 31: 1941-6 (1988)
BindingDB Entry DOI: 10.7270/Q20002PD |
More data for this
Ligand-Target Pair | |
Matrix metalloproteinase-9
(Homo sapiens (Human)) | BDBM50108151

(CHEMBL75164 | N-{2,3-Dihydroxy-1-[4-(4-trifluorome...)Show SMILES OC[C@@H](O)C(CS(=O)(=O)c1ccc(Oc2ccc(OC(F)(F)F)cc2)cc1)N(O)C=O Show InChI InChI=1S/C18H18F3NO8S/c19-18(20,21)30-14-3-1-12(2-4-14)29-13-5-7-15(8-6-13)31(27,28)10-16(17(25)9-23)22(26)11-24/h1-8,11,16-17,23,25-26H,9-10H2/t16?,17-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.0920 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of MMP9 |
ACS Med Chem Lett 2: 455-460 (2011)
Article DOI: 10.1021/ml200031m
BindingDB Entry DOI: 10.7270/Q2HQ40ZK |
More data for this
Ligand-Target Pair | |
Matrix metalloproteinase-9
(Homo sapiens (Human)) | BDBM50108131
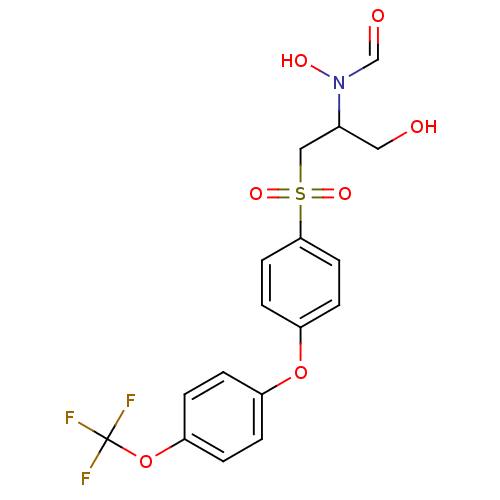
(CHEMBL307413 | N-Hydroxy-N-{1-hydroxymethyl-2-[4-(...)Show SMILES OCC(CS(=O)(=O)c1ccc(Oc2ccc(OC(F)(F)F)cc2)cc1)N(O)C=O Show InChI InChI=1S/C17H16F3NO7S/c18-17(19,20)28-15-3-1-13(2-4-15)27-14-5-7-16(8-6-14)29(25,26)10-12(9-22)21(24)11-23/h1-8,11-12,22,24H,9-10H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.260 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of MMP9 |
ACS Med Chem Lett 2: 455-460 (2011)
Article DOI: 10.1021/ml200031m
BindingDB Entry DOI: 10.7270/Q2HQ40ZK |
More data for this
Ligand-Target Pair | |
Matrix metalloproteinase-9
(Homo sapiens (Human)) | BDBM50108163
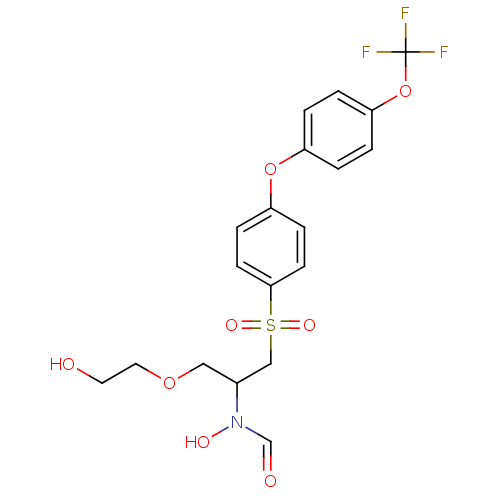
(CHEMBL309329 | N-Hydroxy-N-{1-(2-hydroxy-ethoxymet...)Show SMILES OCCOCC(CS(=O)(=O)c1ccc(Oc2ccc(OC(F)(F)F)cc2)cc1)N(O)C=O Show InChI InChI=1S/C19H20F3NO8S/c20-19(21,22)31-17-3-1-15(2-4-17)30-16-5-7-18(8-6-16)32(27,28)12-14(23(26)13-25)11-29-10-9-24/h1-8,13-14,24,26H,9-12H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of MMP9 |
ACS Med Chem Lett 2: 455-460 (2011)
Article DOI: 10.1021/ml200031m
BindingDB Entry DOI: 10.7270/Q2HQ40ZK |
More data for this
Ligand-Target Pair | |
Matrix metalloproteinase-9
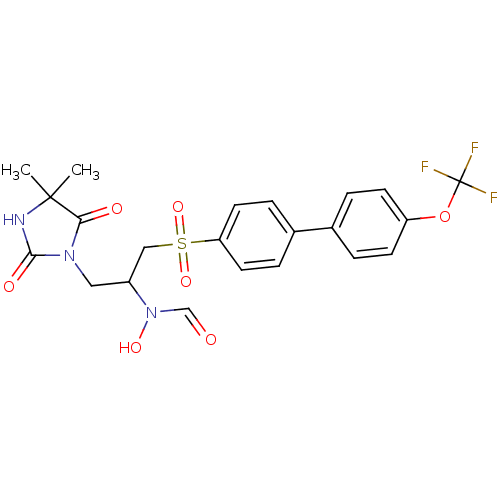
(Homo sapiens (Human)) | BDBM50385603
(CHEMBL2041308)Show SMILES CC1(C)NC(=O)N(CC(CS(=O)(=O)c2ccc(cc2)-c2ccc(OC(F)(F)F)cc2)N(O)C=O)C1=O Show InChI InChI=1S/C22H22F3N3O7S/c1-21(2)19(30)27(20(31)26-21)11-16(28(32)13-29)12-36(33,34)18-9-5-15(6-10-18)14-3-7-17(8-4-14)35-22(23,24)25/h3-10,13,16,32H,11-12H2,1-2H3,(H,26,31) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.340 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of MMP9 |
ACS Med Chem Lett 2: 455-460 (2011)
Article DOI: 10.1021/ml200031m
BindingDB Entry DOI: 10.7270/Q2HQ40ZK |
More data for this
Ligand-Target Pair | |
Matrix metalloproteinase-9
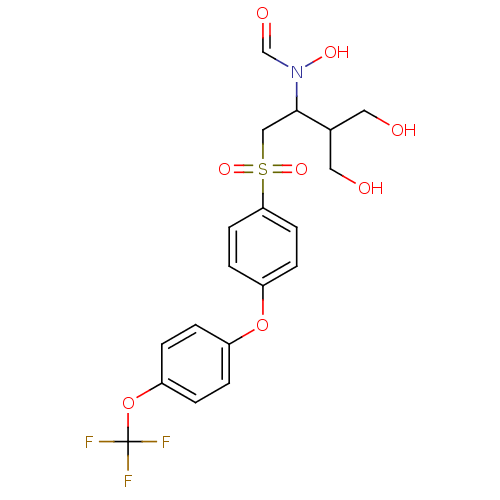
(Homo sapiens (Human)) | BDBM50108171
(CHEMBL77874 | N-Hydroxy-N-{3-hydroxy-2-hydroxymeth...)Show SMILES OCC(CO)C(CS(=O)(=O)c1ccc(Oc2ccc(OC(F)(F)F)cc2)cc1)N(O)C=O Show InChI InChI=1S/C19H20F3NO8S/c20-19(21,22)31-16-3-1-14(2-4-16)30-15-5-7-17(8-6-15)32(28,29)11-18(23(27)12-26)13(9-24)10-25/h1-8,12-13,18,24-25,27H,9-11H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.490 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of MMP9 |
ACS Med Chem Lett 2: 455-460 (2011)
Article DOI: 10.1021/ml200031m
BindingDB Entry DOI: 10.7270/Q2HQ40ZK |
More data for this
Ligand-Target Pair | |
Matrix metalloproteinase-9
(Homo sapiens (Human)) | BDBM50128610

(2-(4-Methoxy-benzenesulfonyl)-4-methylene-2-(2-met...)Show SMILES [#6]-[#8]-c1ccc(cc1)S(=O)(=O)C([#6]\[#6]=[#6](\[#6])-[#6])([#6]\[#6]=[#6](/[#6])-[#6])[#6](=O)-[#7]-[#8] Show InChI InChI=1S/C19H27NO5S/c1-14(2)10-12-19(18(21)20-22,13-11-15(3)4)26(23,24)17-8-6-16(25-5)7-9-17/h6-11,22H,12-13H2,1-5H3,(H,20,21) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of MMP9 |
ACS Med Chem Lett 2: 455-460 (2011)
Article DOI: 10.1021/ml200031m
BindingDB Entry DOI: 10.7270/Q2HQ40ZK |
More data for this
Ligand-Target Pair | |
Matrix metalloproteinase-9
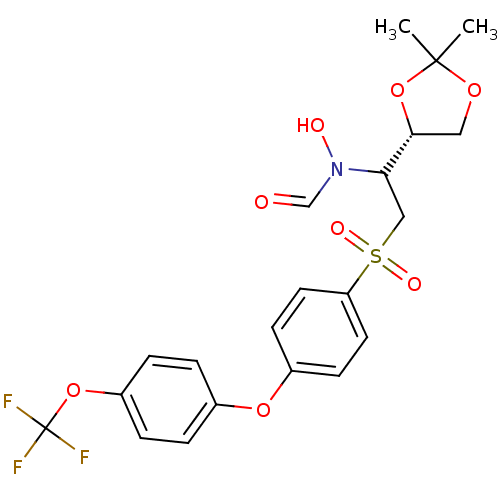
(Homo sapiens (Human)) | BDBM50385604
(CHEMBL2041307)Show SMILES CC1(C)OC[C@@H](O1)C(CS(=O)(=O)c1ccc(Oc2ccc(OC(F)(F)F)cc2)cc1)N(O)C=O |r| Show InChI InChI=1S/C21H22F3NO8S/c1-20(2)30-11-19(33-20)18(25(27)13-26)12-34(28,29)17-9-7-15(8-10-17)31-14-3-5-16(6-4-14)32-21(22,23)24/h3-10,13,18-19,27H,11-12H2,1-2H3/t18?,19-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of MMP9 |
ACS Med Chem Lett 2: 455-460 (2011)
Article DOI: 10.1021/ml200031m
BindingDB Entry DOI: 10.7270/Q2HQ40ZK |
More data for this
Ligand-Target Pair | |
Matrix metalloproteinase-9
(Homo sapiens (Human)) | BDBM50108143
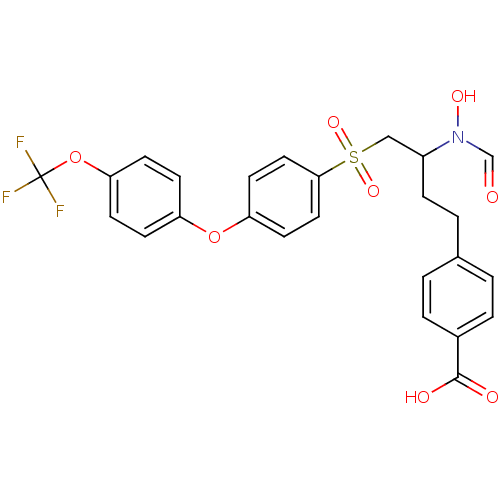
(4-{3-(Formyl-hydroxy-amino)-4-[4-(4-trifluorometho...)Show SMILES ON(C=O)C(CCc1ccc(cc1)C(O)=O)CS(=O)(=O)c1ccc(Oc2ccc(OC(F)(F)F)cc2)cc1 Show InChI InChI=1S/C25H22F3NO8S/c26-25(27,28)37-22-9-7-20(8-10-22)36-21-11-13-23(14-12-21)38(34,35)15-19(29(33)16-30)6-3-17-1-4-18(5-2-17)24(31)32/h1-2,4-5,7-14,16,19,33H,3,6,15H2,(H,31,32) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.530 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of MMP9 |
ACS Med Chem Lett 2: 455-460 (2011)
Article DOI: 10.1021/ml200031m
BindingDB Entry DOI: 10.7270/Q2HQ40ZK |
More data for this
Ligand-Target Pair | |
Matrix metalloproteinase-9
(Homo sapiens (Human)) | BDBM50108138
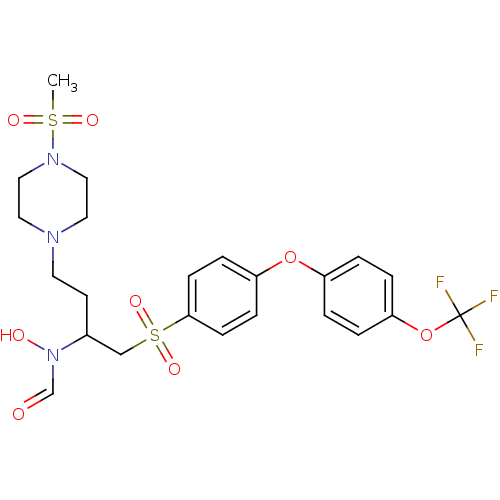
(CHEMBL78052 | N-Hydroxy-N-{3-(4-methanesulfonyl-pi...)Show SMILES CS(=O)(=O)N1CCN(CCC(CS(=O)(=O)c2ccc(Oc3ccc(OC(F)(F)F)cc3)cc2)N(O)C=O)CC1 Show InChI InChI=1S/C23H28F3N3O8S2/c1-38(32,33)28-14-12-27(13-15-28)11-10-18(29(31)17-30)16-39(34,35)22-8-6-20(7-9-22)36-19-2-4-21(5-3-19)37-23(24,25)26/h2-9,17-18,31H,10-16H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.790 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of MMP9 |
ACS Med Chem Lett 2: 455-460 (2011)
Article DOI: 10.1021/ml200031m
BindingDB Entry DOI: 10.7270/Q2HQ40ZK |
More data for this
Ligand-Target Pair | |
Matrix metalloproteinase-9
(Homo sapiens (Human)) | BDBM50108150
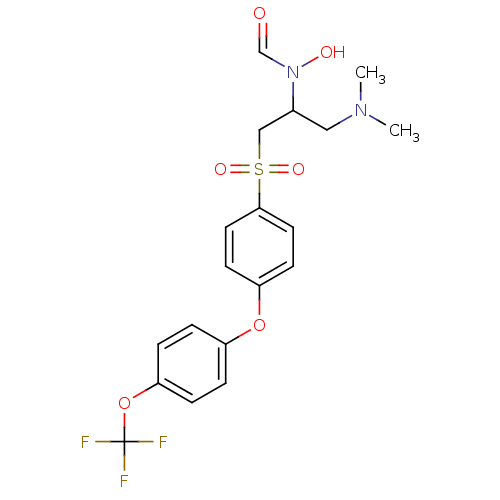
(CHEMBL75567 | N-{1-Dimethylaminomethyl-2-[4-(4-tri...)Show SMILES CN(C)CC(CS(=O)(=O)c1ccc(Oc2ccc(OC(F)(F)F)cc2)cc1)N(O)C=O Show InChI InChI=1S/C19H21F3N2O6S/c1-23(2)11-14(24(26)13-25)12-31(27,28)18-9-7-16(8-10-18)29-15-3-5-17(6-4-15)30-19(20,21)22/h3-10,13-14,26H,11-12H2,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of MMP9 |
ACS Med Chem Lett 2: 455-460 (2011)
Article DOI: 10.1021/ml200031m
BindingDB Entry DOI: 10.7270/Q2HQ40ZK |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data