 Found 190 hits with Last Name = 'burford' and Initial = 'nt'
Found 190 hits with Last Name = 'burford' and Initial = 'nt' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Motilin receptor
(Homo sapiens (Human)) | BDBM50143028

(8'N-[1-[1-carbamoyl-3-phenyl-(1S)-propylcarbamoyl]...)Show SMILES NC(=O)[C@H](CCc1ccccc1)NC(=O)[C@H](CCc1ccccc1)NC(=O)C1CCC2(CCNCC2)n2n1c(=O)n(Cc1ccc3OCOc3c1)c2=O Show InChI InChI=1S/C39H45N7O7/c40-34(47)29(14-11-26-7-3-1-4-8-26)42-35(48)30(15-12-27-9-5-2-6-10-27)43-36(49)31-17-18-39(19-21-41-22-20-39)46-38(51)44(37(50)45(31)46)24-28-13-16-32-33(23-28)53-25-52-32/h1-10,13,16,23,29-31,41H,11-12,14-15,17-22,24-25H2,(H2,40,47)(H,42,48)(H,43,49)/t29-,30-,31?/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
In vitro binding affinity towards human motilin receptor |
J Med Chem 47: 1704-8 (2004)
Article DOI: 10.1021/jm0304865
BindingDB Entry DOI: 10.7270/Q2571CRC |
More data for this
Ligand-Target Pair | |
Motilin receptor
(Homo sapiens (Human)) | BDBM50143032
(8'N-[1-(1-carbamoyl-3-phenylpropylcarbamoyl)-3-phe...)Show SMILES NC(=O)C(CCc1ccccc1)NC(=O)C(CCc1ccccc1)NC(=O)C1CCC2(CCNCC2)n2n1c(=O)n(Cc1ccc3OCOc3c1)c2=O Show InChI InChI=1S/C39H45N7O7/c40-34(47)29(14-11-26-7-3-1-4-8-26)42-35(48)30(15-12-27-9-5-2-6-10-27)43-36(49)31-17-18-39(19-21-41-22-20-39)46-38(51)44(37(50)45(31)46)24-28-13-16-32-33(23-28)53-25-52-32/h1-10,13,16,23,29-31,41H,11-12,14-15,17-22,24-25H2,(H2,40,47)(H,42,48)(H,43,49) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
In vitro binding affinity towards human motilin receptor |
J Med Chem 47: 1704-8 (2004)
Article DOI: 10.1021/jm0304865
BindingDB Entry DOI: 10.7270/Q2571CRC |
More data for this
Ligand-Target Pair | |
Motilin receptor
(Homo sapiens (Human)) | BDBM50143038
(8'N-[1-[1-carbamoyl-3-phenyl-(1S)-propylcarbamoyl]...)Show SMILES NC(=O)[C@H](CCc1ccccc1)NC(=O)[C@H](CCc1ccccc1)NC(=O)C1C=CC2(CCNCC2)n2n1c(=O)n(Cc1ccc3OCOc3c1)c2=O |c:30| Show InChI InChI=1S/C39H43N7O7/c40-34(47)29(14-11-26-7-3-1-4-8-26)42-35(48)30(15-12-27-9-5-2-6-10-27)43-36(49)31-17-18-39(19-21-41-22-20-39)46-38(51)44(37(50)45(31)46)24-28-13-16-32-33(23-28)53-25-52-32/h1-10,13,16-18,23,29-31,41H,11-12,14-15,19-22,24-25H2,(H2,40,47)(H,42,48)(H,43,49)/t29-,30-,31?/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
In vitro binding affinity towards human motilin receptor |
J Med Chem 47: 1704-8 (2004)
Article DOI: 10.1021/jm0304865
BindingDB Entry DOI: 10.7270/Q2571CRC |
More data for this
Ligand-Target Pair | |
Motilin receptor
(Homo sapiens (Human)) | BDBM50143039
(8'N-[1-[1-carbamoyl-3-phenyl-(1R)-propylcarbamoyl]...)Show SMILES NC(=O)[C@@H](CCc1ccccc1)NC(=O)[C@@H](CCc1ccccc1)NC(=O)C1CCC2(CCNCC2)n2n1c(=O)n(Cc1ccc3OCOc3c1)c2=O Show InChI InChI=1S/C39H45N7O7/c40-34(47)29(14-11-26-7-3-1-4-8-26)42-35(48)30(15-12-27-9-5-2-6-10-27)43-36(49)31-17-18-39(19-21-41-22-20-39)46-38(51)44(37(50)45(31)46)24-28-13-16-32-33(23-28)53-25-52-32/h1-10,13,16,23,29-31,41H,11-12,14-15,17-22,24-25H2,(H2,40,47)(H,42,48)(H,43,49)/t29-,30-,31?/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
In vitro binding affinity towards human motilin receptor |
J Med Chem 47: 1704-8 (2004)
Article DOI: 10.1021/jm0304865
BindingDB Entry DOI: 10.7270/Q2571CRC |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM21279
(1-(2,4-dichlorophenyl)-5-(4-iodophenyl)-4-methyl-N...)Show SMILES Cc1c(nn(c1-c1ccc(I)cc1)-c1ccc(Cl)cc1Cl)C(=O)NN1CCCCC1 Show InChI InChI=1S/C22H21Cl2IN4O/c1-14-20(22(30)27-28-11-3-2-4-12-28)26-29(19-10-7-16(23)13-18(19)24)21(14)15-5-8-17(25)9-6-15/h5-10,13H,2-4,11-12H2,1H3,(H,27,30) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Binding affinity to human CB1 receptor |
Bioorg Med Chem Lett 17: 3978-82 (2007)
Article DOI: 10.1016/j.bmcl.2007.04.087
BindingDB Entry DOI: 10.7270/Q2FN15XR |
More data for this
Ligand-Target Pair | |
Delta-type opioid receptor
(Homo sapiens (Human)) | BDBM50087826
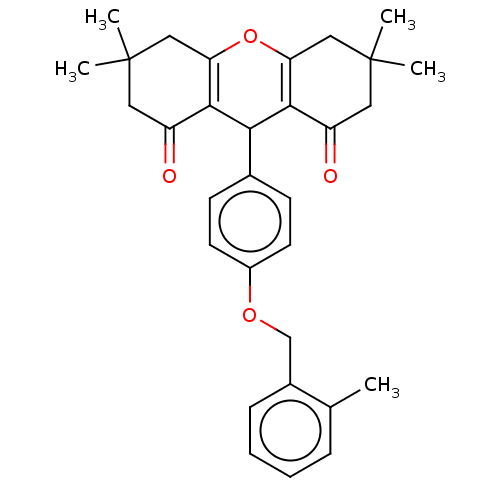
(CHEMBL3426789)Show SMILES Cc1ccccc1COc1ccc(cc1)C1C2=C(CC(C)(C)CC2=O)OC2=C1C(=O)CC(C)(C)C2 |c:29,t:18| Show InChI InChI=1S/C31H34O4/c1-19-8-6-7-9-21(19)18-34-22-12-10-20(11-13-22)27-28-23(32)14-30(2,3)16-25(28)35-26-17-31(4,5)15-24(33)29(26)27/h6-13,27H,14-18H2,1-5H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company
Curated by ChEMBL
| Assay Description
Positive allosteric modulation of human delta opioid receptor expressed in CHO cell membranes assessed as TAN67 Ki at 10 uM after 90 mins by [3H]-dip... |
J Med Chem 58: 4220-9 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00007
BindingDB Entry DOI: 10.7270/Q27P9149 |
More data for this
Ligand-Target Pair | |
Motilin receptor
(Homo sapiens (Human)) | BDBM50143037
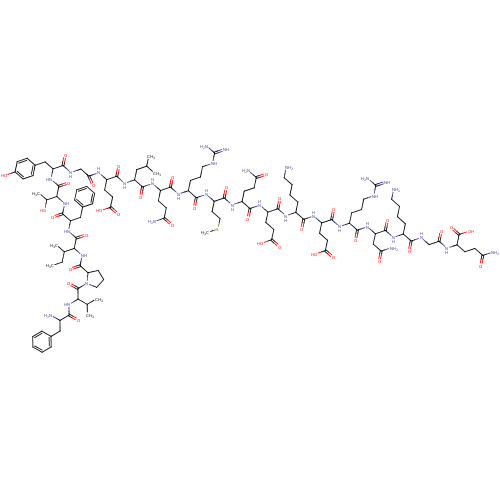
(CHEMBL411576 | MOTILIN)Show SMILES CCC(C)C(NC(=O)C1CCCN1C(=O)C(NC(=O)C(N)Cc1ccccc1)C(C)C)C(=O)NC(Cc1ccccc1)C(=O)NC(C(C)O)C(=O)NC(Cc1ccc(O)cc1)C(=O)NCC(=O)NC(CCC(O)=O)C(=O)NC(CC(C)C)C(=O)NC(CCC(N)=O)C(=O)NC(CCCNC(N)=N)C(=O)NC(CCSC)C(=O)NC(CCC(N)=O)C(=O)NC(CCC(O)=O)C(=O)NC(CCCCN)C(=O)NC(CCC(O)=O)C(=O)NC(CCCNC(N)=N)C(=O)NC(CC(N)=O)C(=O)NC(CCCCN)C(=O)NCC(=O)NC(CCC(N)=O)C(O)=O Show InChI InChI=1S/C120H188N34O35S/c1-9-64(6)97(152-114(184)86-31-22-53-154(86)117(187)96(63(4)5)151-99(169)70(123)56-66-23-12-10-13-24-66)115(185)150-84(57-67-25-14-11-15-26-67)113(183)153-98(65(7)155)116(186)149-83(58-68-32-34-69(156)35-33-68)101(171)135-60-91(161)136-75(39-45-93(163)164)105(175)147-82(55-62(2)3)111(181)145-77(37-43-88(125)158)106(176)140-73(29-20-51-132-119(128)129)103(173)146-80(48-54-190-8)110(180)142-76(36-42-87(124)157)107(177)144-79(41-47-95(167)168)108(178)139-72(28-17-19-50-122)102(172)143-78(40-46-94(165)166)109(179)141-74(30-21-52-133-120(130)131)104(174)148-85(59-90(127)160)112(182)138-71(27-16-18-49-121)100(170)134-61-92(162)137-81(118(188)189)38-44-89(126)159/h10-15,23-26,32-35,62-65,70-86,96-98,155-156H,9,16-22,27-31,36-61,121-123H2,1-8H3,(H2,124,157)(H2,125,158)(H2,126,159)(H2,127,160)(H,134,170)(H,135,171)(H,136,161)(H,137,162)(H,138,182)(H,139,178)(H,140,176)(H,141,179)(H,142,180)(H,143,172)(H,144,177)(H,145,181)(H,146,173)(H,147,175)(H,148,174)(H,149,186)(H,150,185)(H,151,169)(H,152,184)(H,153,183)(H,163,164)(H,165,166)(H,167,168)(H,188,189)(H4,128,129,132)(H4,130,131,133) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
In vitro binding affinity towards human motilin receptor |
J Med Chem 47: 1704-8 (2004)
Article DOI: 10.1021/jm0304865
BindingDB Entry DOI: 10.7270/Q2571CRC |
More data for this
Ligand-Target Pair | |
Motilin receptor
(Homo sapiens (Human)) | BDBM50344952
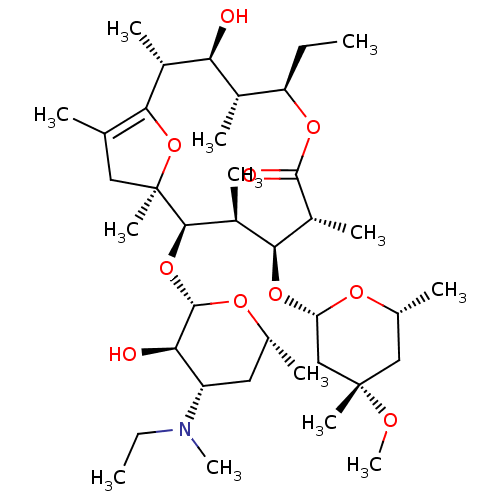
((2R,3S,4R,5R,8R,9S,10S,11R,12S)-5-ethyl-11-((2S,3R...)Show SMILES CC[C@H]1OC(=O)[C@H](C)[C@@H](O[C@H]2C[C@](C)(C[C@@H](C)O2)OC)[C@H](C)[C@@H](O[C@@H]2O[C@H](C)C[C@@H]([C@H]2O)N(C)CC)[C@@]2(C)CC(C)=C(O2)[C@H](C)[C@@H](O)[C@H]1C |wU:24.24,8.8,29.33,36.38,6.6,20.21,2.1,12.12,26.27,wD:22.23,10.9,30.32,45.48,12.18,43.46,47.51,15.15,c:42,(6.35,-6.68,;6.37,-5.14,;7.71,-4.39,;7.7,-5.93,;9.03,-6.7,;9.01,-8.24,;10.37,-5.93,;11.7,-6.7,;10.37,-4.39,;11.7,-5.16,;13.03,-5.93,;13.03,-7.47,;14.35,-8.24,;13.56,-9.57,;15.69,-7.47,;15.69,-5.95,;17.02,-5.16,;14.36,-5.16,;15.1,-9.57,;16.64,-9.57,;11.71,-3.64,;13.04,-4.42,;11.72,-2.1,;13.05,-1.34,;14.38,-.56,;15.71,-1.31,;17.04,-.56,;18.37,-1.33,;17.04,.98,;15.71,1.77,;14.38,.98,;13.05,1.75,;15.71,3.31,;17.04,4.08,;14.38,4.08,;14.38,5.62,;10.39,-1.31,;11.72,-.54,;10.39,.23,;9.06,1,;9.08,2.54,;7.73,.23,;8.82,-.85,;7.72,-1.31,;6.38,-.54,;6.39,-2.08,;5.06,-1.29,;6.38,-3.62,;5.04,-4.35,)| Show InChI InChI=1S/C38H67NO10/c1-14-28-23(6)30(40)24(7)32-20(3)17-38(11,49-32)34(48-36-31(41)27(39(12)15-2)16-21(4)45-36)25(8)33(26(9)35(42)46-28)47-29-19-37(10,43-13)18-22(5)44-29/h21-31,33-34,36,40-41H,14-19H2,1-13H3/t21-,22-,23+,24-,25+,26-,27+,28-,29+,30+,31-,33+,34-,36+,37+,38-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
In vitro binding affinity towards human motilin receptor |
J Med Chem 47: 1704-8 (2004)
Article DOI: 10.1021/jm0304865
BindingDB Entry DOI: 10.7270/Q2571CRC |
More data for this
Ligand-Target Pair | |
Delta-type opioid receptor
(Homo sapiens (Human)) | BDBM50087826

(CHEMBL3426789)Show SMILES Cc1ccccc1COc1ccc(cc1)C1C2=C(CC(C)(C)CC2=O)OC2=C1C(=O)CC(C)(C)C2 |c:29,t:18| Show InChI InChI=1S/C31H34O4/c1-19-8-6-7-9-21(19)18-34-22-12-10-20(11-13-22)27-28-23(32)14-30(2,3)16-25(28)35-26-17-31(4,5)15-24(33)29(26)27/h6-13,27H,14-18H2,1-5H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company
Curated by ChEMBL
| Assay Description
Positive allosteric modulation of human delta opioid receptor expressed in CHO cell membranes assessed as SNC80 Ki at 10 uM after 90 mins by [3H]-dip... |
J Med Chem 58: 4220-9 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00007
BindingDB Entry DOI: 10.7270/Q27P9149 |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM50213486
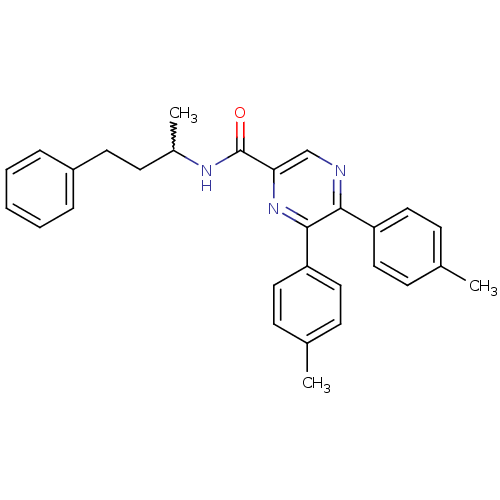
(CHEMBL247515 | N-(4-phenylbutan-2-yl)-5,6-dip-toly...)Show SMILES CC(CCc1ccccc1)NC(=O)c1cnc(-c2ccc(C)cc2)c(n1)-c1ccc(C)cc1 |w:1.0| Show InChI InChI=1S/C29H29N3O/c1-20-9-15-24(16-10-20)27-28(25-17-11-21(2)12-18-25)32-26(19-30-27)29(33)31-22(3)13-14-23-7-5-4-6-8-23/h4-12,15-19,22H,13-14H2,1-3H3,(H,31,33) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Binding affinity to human CB1 receptor |
Bioorg Med Chem Lett 17: 3978-82 (2007)
Article DOI: 10.1016/j.bmcl.2007.04.087
BindingDB Entry DOI: 10.7270/Q2FN15XR |
More data for this
Ligand-Target Pair | |
Delta-type opioid receptor
(Homo sapiens (Human)) | BDBM50087826

(CHEMBL3426789)Show SMILES Cc1ccccc1COc1ccc(cc1)C1C2=C(CC(C)(C)CC2=O)OC2=C1C(=O)CC(C)(C)C2 |c:29,t:18| Show InChI InChI=1S/C31H34O4/c1-19-8-6-7-9-21(19)18-34-22-12-10-20(11-13-22)27-28-23(32)14-30(2,3)16-25(28)35-26-17-31(4,5)15-24(33)29(26)27/h6-13,27H,14-18H2,1-5H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 7 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company
Curated by ChEMBL
| Assay Description
Positive allosteric modulation of human delta opioid receptor expressed in CHO cell membranes assessed as leu-enkephalin Ki at 10 uM after 90 mins by... |
J Med Chem 58: 4220-9 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00007
BindingDB Entry DOI: 10.7270/Q27P9149 |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM50213468
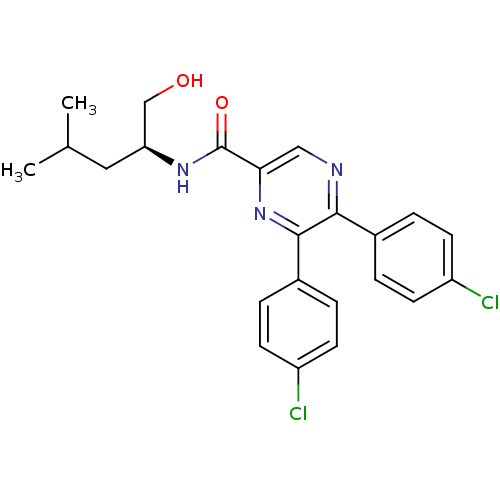
((S)-5,6-bis(4-chlorophenyl)-N-(1-hydroxy-4-methylp...)Show SMILES CC(C)C[C@@H](CO)NC(=O)c1cnc(-c2ccc(Cl)cc2)c(n1)-c1ccc(Cl)cc1 Show InChI InChI=1S/C23H23Cl2N3O2/c1-14(2)11-19(13-29)27-23(30)20-12-26-21(15-3-7-17(24)8-4-15)22(28-20)16-5-9-18(25)10-6-16/h3-10,12,14,19,29H,11,13H2,1-2H3,(H,27,30)/t19-/m0/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 14 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Binding affinity to human CB1 receptor |
Bioorg Med Chem Lett 17: 3978-82 (2007)
Article DOI: 10.1016/j.bmcl.2007.04.087
BindingDB Entry DOI: 10.7270/Q2FN15XR |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM50213467
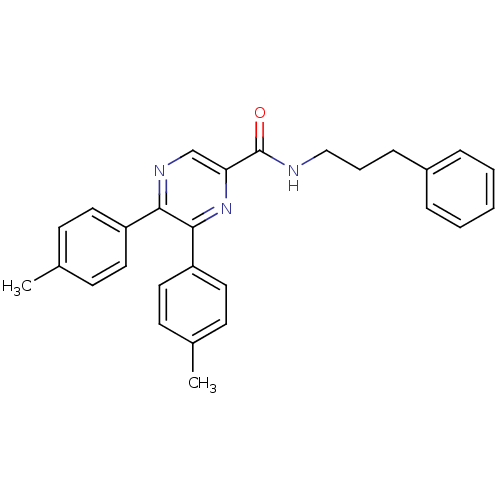
(CHEMBL246063 | N-(3-phenylpropyl)-5,6-dip-tolylpyr...)Show SMILES Cc1ccc(cc1)-c1ncc(nc1-c1ccc(C)cc1)C(=O)NCCCc1ccccc1 Show InChI InChI=1S/C28H27N3O/c1-20-10-14-23(15-11-20)26-27(24-16-12-21(2)13-17-24)31-25(19-30-26)28(32)29-18-6-9-22-7-4-3-5-8-22/h3-5,7-8,10-17,19H,6,9,18H2,1-2H3,(H,29,32) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 18 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Binding affinity to human CB1 receptor |
Bioorg Med Chem Lett 17: 3978-82 (2007)
Article DOI: 10.1016/j.bmcl.2007.04.087
BindingDB Entry DOI: 10.7270/Q2FN15XR |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM50213462
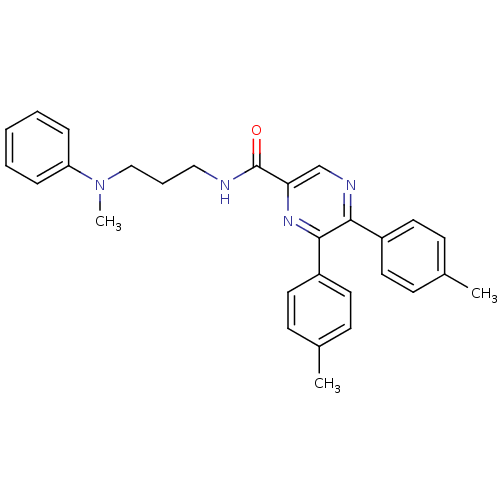
(CHEMBL396695 | N-(3-(methyl(phenyl)amino)propyl)-5...)Show SMILES CN(CCCNC(=O)c1cnc(-c2ccc(C)cc2)c(n1)-c1ccc(C)cc1)c1ccccc1 Show InChI InChI=1S/C29H30N4O/c1-21-10-14-23(15-11-21)27-28(24-16-12-22(2)13-17-24)32-26(20-31-27)29(34)30-18-7-19-33(3)25-8-5-4-6-9-25/h4-6,8-17,20H,7,18-19H2,1-3H3,(H,30,34) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 25 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Binding affinity to human CB1 receptor |
Bioorg Med Chem Lett 17: 3978-82 (2007)
Article DOI: 10.1016/j.bmcl.2007.04.087
BindingDB Entry DOI: 10.7270/Q2FN15XR |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Rattus norvegicus (rat)) | BDBM50213468

((S)-5,6-bis(4-chlorophenyl)-N-(1-hydroxy-4-methylp...)Show SMILES CC(C)C[C@@H](CO)NC(=O)c1cnc(-c2ccc(Cl)cc2)c(n1)-c1ccc(Cl)cc1 Show InChI InChI=1S/C23H23Cl2N3O2/c1-14(2)11-19(13-29)27-23(30)20-12-26-21(15-3-7-17(24)8-4-15)22(28-20)16-5-9-18(25)10-6-16/h3-10,12,14,19,29H,11,13H2,1-2H3,(H,27,30)/t19-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Binding affinity to rat CB1 receptor |
Bioorg Med Chem Lett 17: 3978-82 (2007)
Article DOI: 10.1016/j.bmcl.2007.04.087
BindingDB Entry DOI: 10.7270/Q2FN15XR |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM50213478

(CHEMBL245467 | N-(1-hydroxy-2,3-dihydro-1H-inden-2...)Show SMILES Cc1ccc(cc1)-c1ncc(nc1-c1ccc(C)cc1)C(=O)NC1Cc2ccccc2C1O |w:23.25,31.36| Show InChI InChI=1S/C28H25N3O2/c1-17-7-11-19(12-8-17)25-26(20-13-9-18(2)10-14-20)30-24(16-29-25)28(33)31-23-15-21-5-3-4-6-22(21)27(23)32/h3-14,16,23,27,32H,15H2,1-2H3,(H,31,33) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 33 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Binding affinity to human CB1 receptor |
Bioorg Med Chem Lett 17: 3978-82 (2007)
Article DOI: 10.1016/j.bmcl.2007.04.087
BindingDB Entry DOI: 10.7270/Q2FN15XR |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Rattus norvegicus (rat)) | BDBM50213465
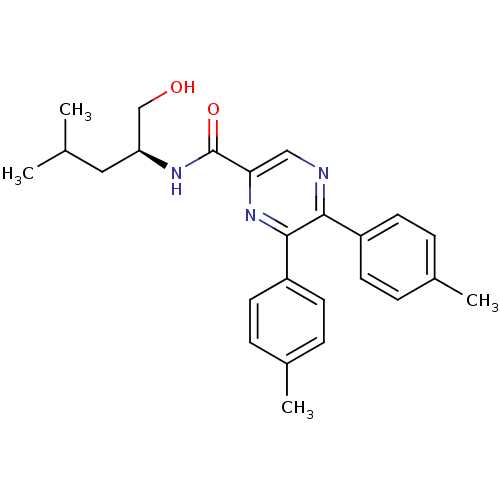
((S)-N-(1-hydroxy-4-methylpentan-2-yl)-5,6-dip-toly...)Show SMILES CC(C)C[C@@H](CO)NC(=O)c1cnc(-c2ccc(C)cc2)c(n1)-c1ccc(C)cc1 Show InChI InChI=1S/C25H29N3O2/c1-16(2)13-21(15-29)27-25(30)22-14-26-23(19-9-5-17(3)6-10-19)24(28-22)20-11-7-18(4)8-12-20/h5-12,14,16,21,29H,13,15H2,1-4H3,(H,27,30)/t21-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 35 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Binding affinity to rat CB1 receptor |
Bioorg Med Chem Lett 17: 3978-82 (2007)
Article DOI: 10.1016/j.bmcl.2007.04.087
BindingDB Entry DOI: 10.7270/Q2FN15XR |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM50213477
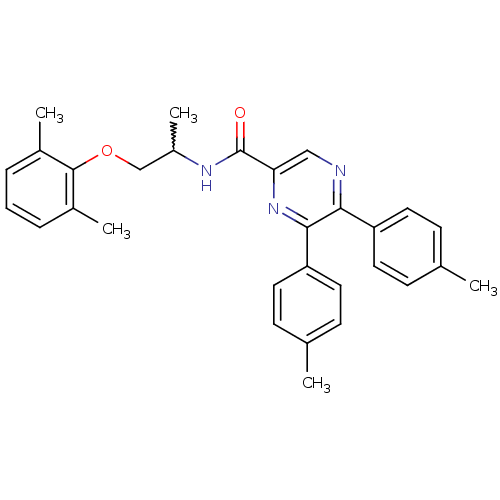
(CHEMBL247516 | N-(1-(2,6-dimethylphenoxy)propan-2-...)Show SMILES CC(COc1c(C)cccc1C)NC(=O)c1cnc(-c2ccc(C)cc2)c(n1)-c1ccc(C)cc1 |w:1.0| Show InChI InChI=1S/C30H31N3O2/c1-19-9-13-24(14-10-19)27-28(25-15-11-20(2)12-16-25)33-26(17-31-27)30(34)32-23(5)18-35-29-21(3)7-6-8-22(29)4/h6-17,23H,18H2,1-5H3,(H,32,34) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 39 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Binding affinity to human CB1 receptor |
Bioorg Med Chem Lett 17: 3978-82 (2007)
Article DOI: 10.1016/j.bmcl.2007.04.087
BindingDB Entry DOI: 10.7270/Q2FN15XR |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM50213465

((S)-N-(1-hydroxy-4-methylpentan-2-yl)-5,6-dip-toly...)Show SMILES CC(C)C[C@@H](CO)NC(=O)c1cnc(-c2ccc(C)cc2)c(n1)-c1ccc(C)cc1 Show InChI InChI=1S/C25H29N3O2/c1-16(2)13-21(15-29)27-25(30)22-14-26-23(19-9-5-17(3)6-10-19)24(28-22)20-11-7-18(4)8-12-20/h5-12,14,16,21,29H,13,15H2,1-4H3,(H,27,30)/t21-/m0/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 45 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Binding affinity to human CB1 receptor |
Bioorg Med Chem Lett 17: 3978-82 (2007)
Article DOI: 10.1016/j.bmcl.2007.04.087
BindingDB Entry DOI: 10.7270/Q2FN15XR |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM50213479
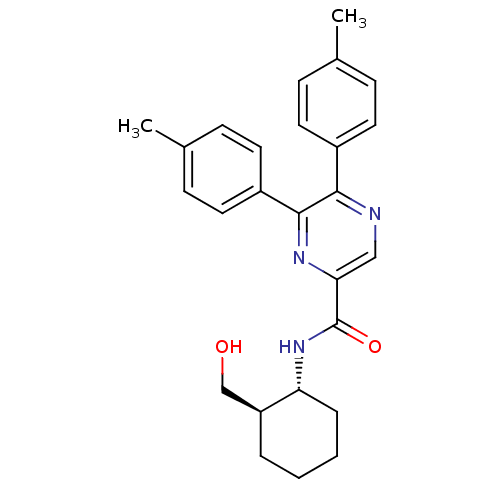
(CHEMBL394295 | N-((1R,2R)-2-(hydroxymethyl)cyclohe...)Show SMILES Cc1ccc(cc1)-c1ncc(nc1-c1ccc(C)cc1)C(=O)N[C@@H]1CCCC[C@H]1CO Show InChI InChI=1S/C26H29N3O2/c1-17-7-11-19(12-8-17)24-25(20-13-9-18(2)10-14-20)28-23(15-27-24)26(31)29-22-6-4-3-5-21(22)16-30/h7-15,21-22,30H,3-6,16H2,1-2H3,(H,29,31)/t21-,22+/m0/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 50 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Binding affinity to human CB1 receptor |
Bioorg Med Chem Lett 17: 3978-82 (2007)
Article DOI: 10.1016/j.bmcl.2007.04.087
BindingDB Entry DOI: 10.7270/Q2FN15XR |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM35871
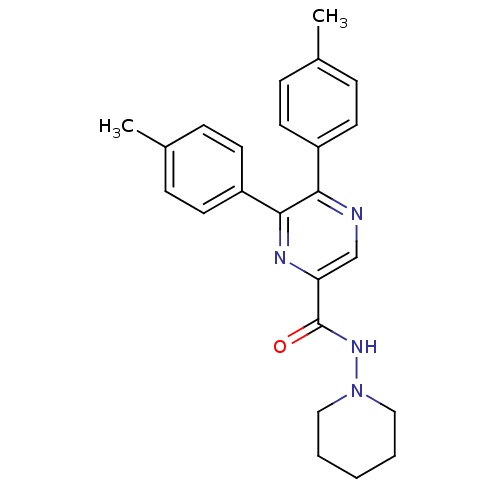
(5,6-di-p-tolyl-pyrazine-2-carboxylic acid piperidi...)Show SMILES Cc1ccc(cc1)-c1ncc(nc1-c1ccc(C)cc1)C(=O)NN1CCCCC1 Show InChI InChI=1S/C24H26N4O/c1-17-6-10-19(11-7-17)22-23(20-12-8-18(2)9-13-20)26-21(16-25-22)24(29)27-28-14-4-3-5-15-28/h6-13,16H,3-5,14-15H2,1-2H3,(H,27,29) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 53 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Binding affinity to human CB1 receptor |
Bioorg Med Chem Lett 17: 3978-82 (2007)
Article DOI: 10.1016/j.bmcl.2007.04.087
BindingDB Entry DOI: 10.7270/Q2FN15XR |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM50213476
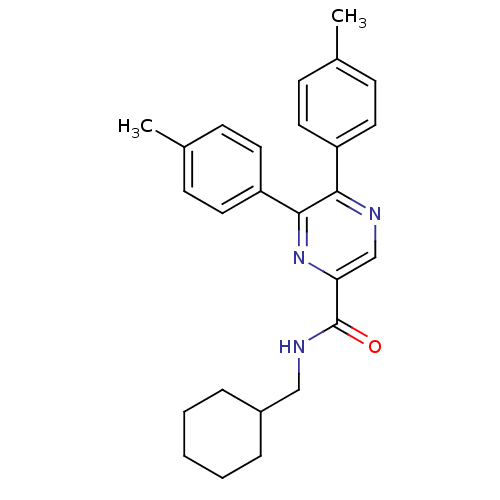
(CHEMBL395923 | N-(cyclohexylmethyl)-5,6-dip-tolylp...)Show SMILES Cc1ccc(cc1)-c1ncc(nc1-c1ccc(C)cc1)C(=O)NCC1CCCCC1 Show InChI InChI=1S/C26H29N3O/c1-18-8-12-21(13-9-18)24-25(22-14-10-19(2)11-15-22)29-23(17-27-24)26(30)28-16-20-6-4-3-5-7-20/h8-15,17,20H,3-7,16H2,1-2H3,(H,28,30) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 59 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Binding affinity to human CB1 receptor |
Bioorg Med Chem Lett 17: 3978-82 (2007)
Article DOI: 10.1016/j.bmcl.2007.04.087
BindingDB Entry DOI: 10.7270/Q2FN15XR |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Rattus norvegicus (rat)) | BDBM50213467

(CHEMBL246063 | N-(3-phenylpropyl)-5,6-dip-tolylpyr...)Show SMILES Cc1ccc(cc1)-c1ncc(nc1-c1ccc(C)cc1)C(=O)NCCCc1ccccc1 Show InChI InChI=1S/C28H27N3O/c1-20-10-14-23(15-11-20)26-27(24-16-12-21(2)13-17-24)31-25(19-30-26)28(32)29-18-6-9-22-7-4-3-5-8-22/h3-5,7-8,10-17,19H,6,9,18H2,1-2H3,(H,29,32) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Binding affinity to rat CB1 receptor |
Bioorg Med Chem Lett 17: 3978-82 (2007)
Article DOI: 10.1016/j.bmcl.2007.04.087
BindingDB Entry DOI: 10.7270/Q2FN15XR |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM50213471
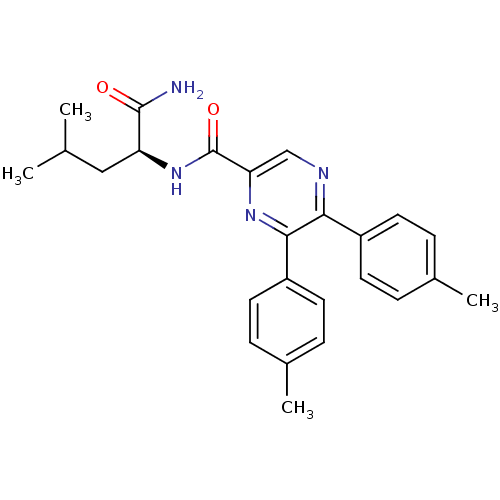
((S)-N-(1-amino-4-methyl-1-oxopentan-2-yl)-5,6-dip-...)Show SMILES CC(C)C[C@H](NC(=O)c1cnc(-c2ccc(C)cc2)c(n1)-c1ccc(C)cc1)C(N)=O Show InChI InChI=1S/C25H28N4O2/c1-15(2)13-20(24(26)30)29-25(31)21-14-27-22(18-9-5-16(3)6-10-18)23(28-21)19-11-7-17(4)8-12-19/h5-12,14-15,20H,13H2,1-4H3,(H2,26,30)(H,29,31)/t20-/m0/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 105 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Binding affinity to human CB1 receptor |
Bioorg Med Chem Lett 17: 3978-82 (2007)
Article DOI: 10.1016/j.bmcl.2007.04.087
BindingDB Entry DOI: 10.7270/Q2FN15XR |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM50213472

(CHEMBL397311 | N-benzyl-N-methyl-5,6-dip-tolylpyra...)Show SMILES CN(Cc1ccccc1)C(=O)c1cnc(-c2ccc(C)cc2)c(n1)-c1ccc(C)cc1 Show InChI InChI=1S/C27H25N3O/c1-19-9-13-22(14-10-19)25-26(23-15-11-20(2)12-16-23)29-24(17-28-25)27(31)30(3)18-21-7-5-4-6-8-21/h4-17H,18H2,1-3H3 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 110 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Binding affinity to human CB1 receptor |
Bioorg Med Chem Lett 17: 3978-82 (2007)
Article DOI: 10.1016/j.bmcl.2007.04.087
BindingDB Entry DOI: 10.7270/Q2FN15XR |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM50213470
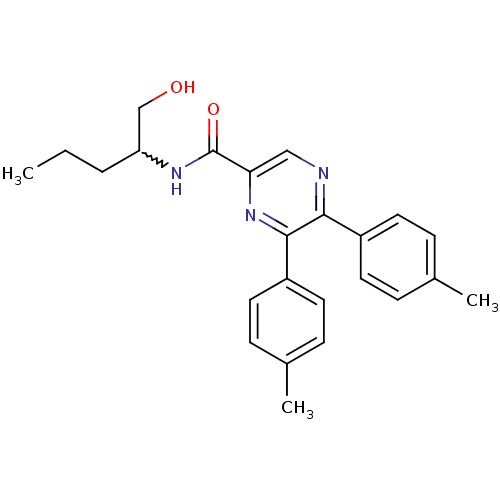
(CHEMBL248125 | N-(1-hydroxypentan-2-yl)-5,6-dip-to...)Show SMILES CCCC(CO)NC(=O)c1cnc(-c2ccc(C)cc2)c(n1)-c1ccc(C)cc1 |w:3.5| Show InChI InChI=1S/C24H27N3O2/c1-4-5-20(15-28)26-24(29)21-14-25-22(18-10-6-16(2)7-11-18)23(27-21)19-12-8-17(3)9-13-19/h6-14,20,28H,4-5,15H2,1-3H3,(H,26,29) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 158 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Binding affinity to human CB1 receptor |
Bioorg Med Chem Lett 17: 3978-82 (2007)
Article DOI: 10.1016/j.bmcl.2007.04.087
BindingDB Entry DOI: 10.7270/Q2FN15XR |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM50213480
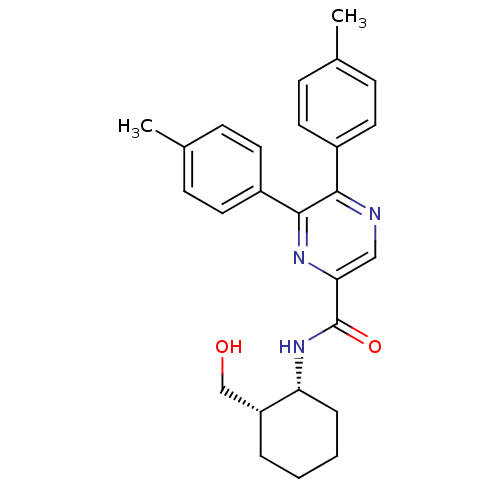
(CHEMBL247552 | N-((1R,2S)-2-(hydroxymethyl)cyclohe...)Show SMILES Cc1ccc(cc1)-c1ncc(nc1-c1ccc(C)cc1)C(=O)N[C@@H]1CCCC[C@@H]1CO Show InChI InChI=1S/C26H29N3O2/c1-17-7-11-19(12-8-17)24-25(20-13-9-18(2)10-14-20)28-23(15-27-24)26(31)29-22-6-4-3-5-21(22)16-30/h7-15,21-22,30H,3-6,16H2,1-2H3,(H,29,31)/t21-,22-/m1/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 167 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Binding affinity to human CB1 receptor |
Bioorg Med Chem Lett 17: 3978-82 (2007)
Article DOI: 10.1016/j.bmcl.2007.04.087
BindingDB Entry DOI: 10.7270/Q2FN15XR |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM50213466
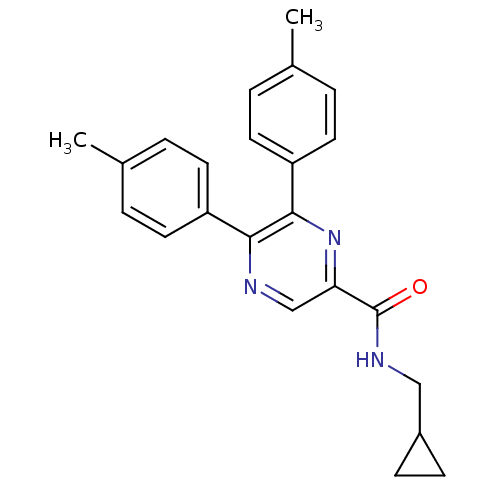
(CHEMBL245860 | N-(cyclopropylmethyl)-5,6-dip-tolyl...)Show SMILES Cc1ccc(cc1)-c1ncc(nc1-c1ccc(C)cc1)C(=O)NCC1CC1 Show InChI InChI=1S/C23H23N3O/c1-15-3-9-18(10-4-15)21-22(19-11-5-16(2)6-12-19)26-20(14-24-21)23(27)25-13-17-7-8-17/h3-6,9-12,14,17H,7-8,13H2,1-2H3,(H,25,27) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 178 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Binding affinity to human CB1 receptor |
Bioorg Med Chem Lett 17: 3978-82 (2007)
Article DOI: 10.1016/j.bmcl.2007.04.087
BindingDB Entry DOI: 10.7270/Q2FN15XR |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM50213475
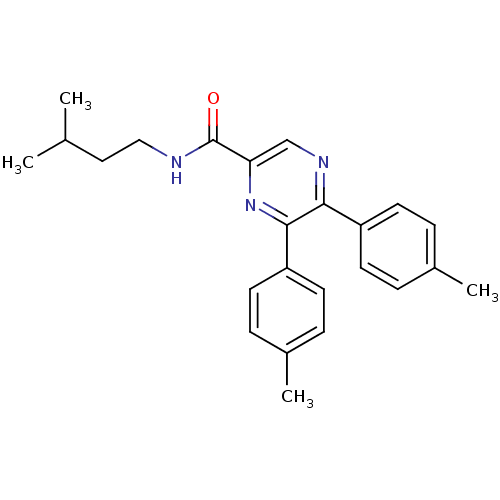
(CHEMBL246478 | N-isopentyl-5,6-dip-tolylpyrazine-2...)Show SMILES CC(C)CCNC(=O)c1cnc(-c2ccc(C)cc2)c(n1)-c1ccc(C)cc1 Show InChI InChI=1S/C24H27N3O/c1-16(2)13-14-25-24(28)21-15-26-22(19-9-5-17(3)6-10-19)23(27-21)20-11-7-18(4)8-12-20/h5-12,15-16H,13-14H2,1-4H3,(H,25,28) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 178 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Binding affinity to human CB1 receptor |
Bioorg Med Chem Lett 17: 3978-82 (2007)
Article DOI: 10.1016/j.bmcl.2007.04.087
BindingDB Entry DOI: 10.7270/Q2FN15XR |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM50213464

(CHEMBL247517 | N-(1-(benzyloxy)butan-2-yl)-5,6-dip...)Show SMILES CCC(COCc1ccccc1)NC(=O)c1cnc(-c2ccc(C)cc2)c(n1)-c1ccc(C)cc1 |w:2.12| Show InChI InChI=1S/C30H31N3O2/c1-4-26(20-35-19-23-8-6-5-7-9-23)32-30(34)27-18-31-28(24-14-10-21(2)11-15-24)29(33-27)25-16-12-22(3)13-17-25/h5-18,26H,4,19-20H2,1-3H3,(H,32,34) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 203 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Binding affinity to human CB1 receptor |
Bioorg Med Chem Lett 17: 3978-82 (2007)
Article DOI: 10.1016/j.bmcl.2007.04.087
BindingDB Entry DOI: 10.7270/Q2FN15XR |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM50213484
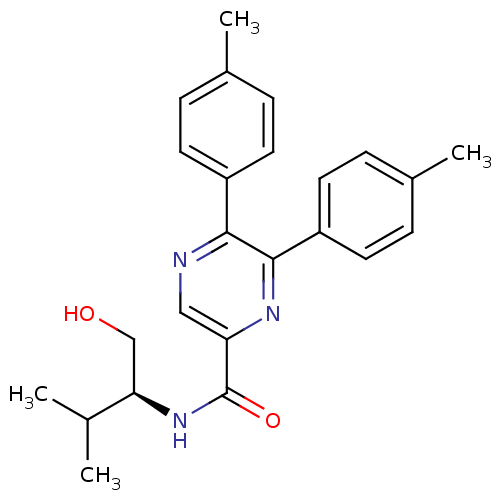
((S)-N-(1-hydroxy-3-methylbutan-2-yl)-5,6-dip-tolyl...)Show SMILES CC(C)[C@@H](CO)NC(=O)c1cnc(-c2ccc(C)cc2)c(n1)-c1ccc(C)cc1 Show InChI InChI=1S/C24H27N3O2/c1-15(2)21(14-28)27-24(29)20-13-25-22(18-9-5-16(3)6-10-18)23(26-20)19-11-7-17(4)8-12-19/h5-13,15,21,28H,14H2,1-4H3,(H,27,29)/t21-/m1/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 237 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Binding affinity to human CB1 receptor |
Bioorg Med Chem Lett 17: 3978-82 (2007)
Article DOI: 10.1016/j.bmcl.2007.04.087
BindingDB Entry DOI: 10.7270/Q2FN15XR |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM50213463

((R)-N-(2-amino-2-oxo-1-phenylethyl)-5,6-dip-tolylp...)Show SMILES Cc1ccc(cc1)-c1ncc(nc1-c1ccc(C)cc1)C(=O)N[C@@H](C(N)=O)c1ccccc1 Show InChI InChI=1S/C27H24N4O2/c1-17-8-12-20(13-9-17)23-24(21-14-10-18(2)11-15-21)30-22(16-29-23)27(33)31-25(26(28)32)19-6-4-3-5-7-19/h3-16,25H,1-2H3,(H2,28,32)(H,31,33)/t25-/m1/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 238 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Binding affinity to human CB1 receptor |
Bioorg Med Chem Lett 17: 3978-82 (2007)
Article DOI: 10.1016/j.bmcl.2007.04.087
BindingDB Entry DOI: 10.7270/Q2FN15XR |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM50213473
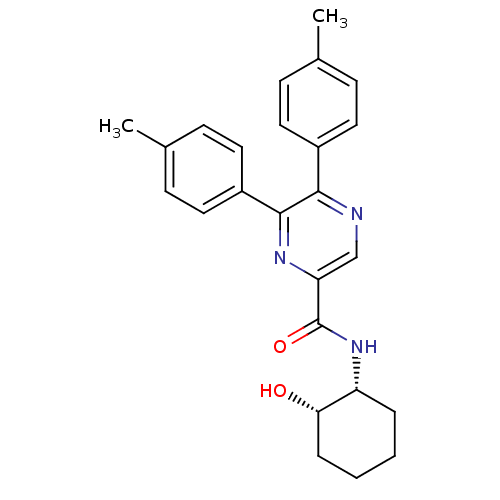
(CHEMBL397387 | N-((1R,2S)-2-hydroxycyclohexyl)-5,6...)Show SMILES Cc1ccc(cc1)-c1ncc(nc1-c1ccc(C)cc1)C(=O)N[C@@H]1CCCC[C@@H]1O Show InChI InChI=1S/C25H27N3O2/c1-16-7-11-18(12-8-16)23-24(19-13-9-17(2)10-14-19)27-21(15-26-23)25(30)28-20-5-3-4-6-22(20)29/h7-15,20,22,29H,3-6H2,1-2H3,(H,28,30)/t20-,22+/m1/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Binding affinity to human CB1 receptor |
Bioorg Med Chem Lett 17: 3978-82 (2007)
Article DOI: 10.1016/j.bmcl.2007.04.087
BindingDB Entry DOI: 10.7270/Q2FN15XR |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM50213483

((R)-N-(1-hydroxy-3-methylbutan-2-yl)-5,6-dip-tolyl...)Show SMILES CC(C)[C@H](CO)NC(=O)c1cnc(-c2ccc(C)cc2)c(n1)-c1ccc(C)cc1 Show InChI InChI=1S/C24H27N3O2/c1-15(2)21(14-28)27-24(29)20-13-25-22(18-9-5-16(3)6-10-18)23(26-20)19-11-7-17(4)8-12-19/h5-13,15,21,28H,14H2,1-4H3,(H,27,29)/t21-/m0/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 316 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Binding affinity to human CB1 receptor |
Bioorg Med Chem Lett 17: 3978-82 (2007)
Article DOI: 10.1016/j.bmcl.2007.04.087
BindingDB Entry DOI: 10.7270/Q2FN15XR |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM50213485
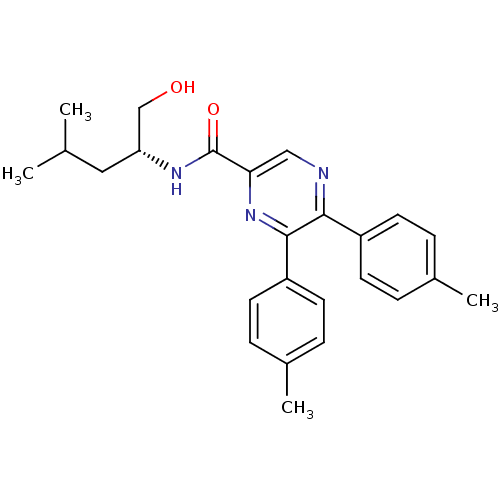
((R)-N-(1-hydroxy-4-methylpentan-2-yl)-5,6-dip-toly...)Show SMILES CC(C)C[C@H](CO)NC(=O)c1cnc(-c2ccc(C)cc2)c(n1)-c1ccc(C)cc1 Show InChI InChI=1S/C25H29N3O2/c1-16(2)13-21(15-29)27-25(30)22-14-26-23(19-9-5-17(3)6-10-19)24(28-22)20-11-7-18(4)8-12-20/h5-12,14,16,21,29H,13,15H2,1-4H3,(H,27,30)/t21-/m1/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 460 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Binding affinity to human CB1 receptor |
Bioorg Med Chem Lett 17: 3978-82 (2007)
Article DOI: 10.1016/j.bmcl.2007.04.087
BindingDB Entry DOI: 10.7270/Q2FN15XR |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM35881
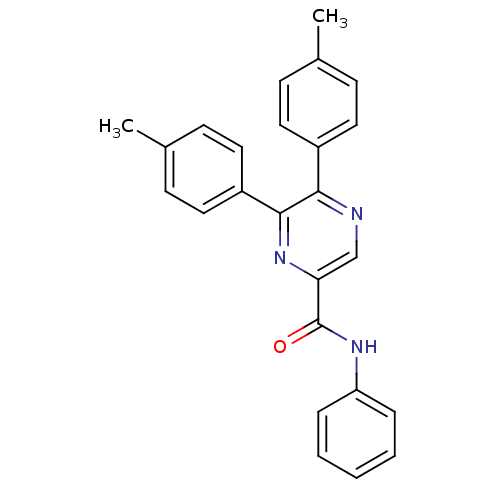
(5,6-di-p-tolyl-pyrazine-2-carboxylic acid phenylam...)Show SMILES Cc1ccc(cc1)-c1ncc(nc1-c1ccc(C)cc1)C(=O)Nc1ccccc1 Show InChI InChI=1S/C25H21N3O/c1-17-8-12-19(13-9-17)23-24(20-14-10-18(2)11-15-20)28-22(16-26-23)25(29)27-21-6-4-3-5-7-21/h3-16H,1-2H3,(H,27,29) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 509 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Binding affinity to human CB1 receptor |
Bioorg Med Chem Lett 17: 3978-82 (2007)
Article DOI: 10.1016/j.bmcl.2007.04.087
BindingDB Entry DOI: 10.7270/Q2FN15XR |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM50213482
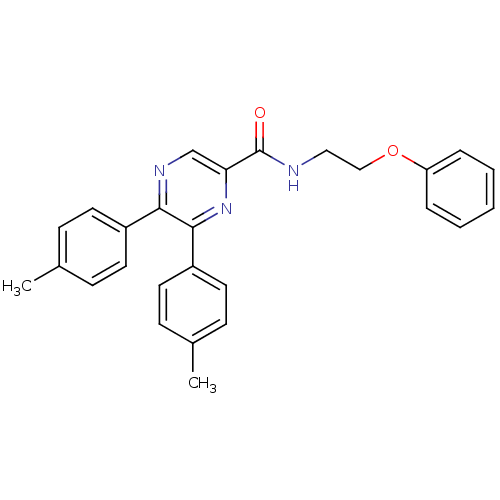
(CHEMBL398114 | N-(2-phenoxyethyl)-5,6-dip-tolylpyr...)Show SMILES Cc1ccc(cc1)-c1ncc(nc1-c1ccc(C)cc1)C(=O)NCCOc1ccccc1 Show InChI InChI=1S/C27H25N3O2/c1-19-8-12-21(13-9-19)25-26(22-14-10-20(2)11-15-22)30-24(18-29-25)27(31)28-16-17-32-23-6-4-3-5-7-23/h3-15,18H,16-17H2,1-2H3,(H,28,31) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 631 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Binding affinity to human CB1 receptor |
Bioorg Med Chem Lett 17: 3978-82 (2007)
Article DOI: 10.1016/j.bmcl.2007.04.087
BindingDB Entry DOI: 10.7270/Q2FN15XR |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM50213487
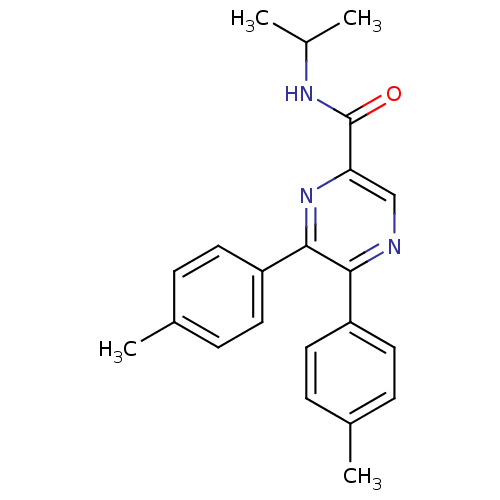
(CHEMBL246477 | N-isopropyl-5,6-dip-tolylpyrazine-2...)Show SMILES CC(C)NC(=O)c1cnc(-c2ccc(C)cc2)c(n1)-c1ccc(C)cc1 Show InChI InChI=1S/C22H23N3O/c1-14(2)24-22(26)19-13-23-20(17-9-5-15(3)6-10-17)21(25-19)18-11-7-16(4)8-12-18/h5-14H,1-4H3,(H,24,26) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 656 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Binding affinity to human CB1 receptor |
Bioorg Med Chem Lett 17: 3978-82 (2007)
Article DOI: 10.1016/j.bmcl.2007.04.087
BindingDB Entry DOI: 10.7270/Q2FN15XR |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM50213481
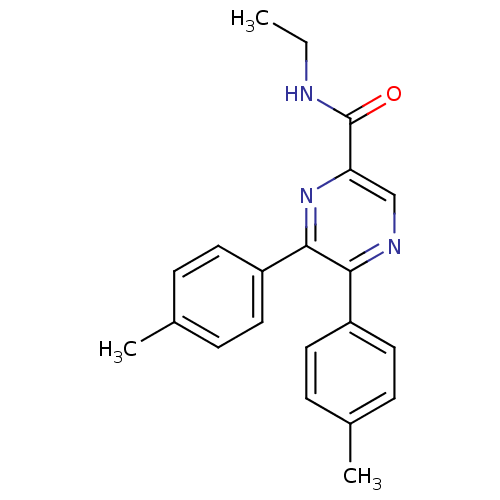
(CHEMBL246476 | N-ethyl-5,6-dip-tolylpyrazine-2-car...)Show SMILES CCNC(=O)c1cnc(-c2ccc(C)cc2)c(n1)-c1ccc(C)cc1 Show InChI InChI=1S/C21H21N3O/c1-4-22-21(25)18-13-23-19(16-9-5-14(2)6-10-16)20(24-18)17-11-7-15(3)8-12-17/h5-13H,4H2,1-3H3,(H,22,25) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.38E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Binding affinity to human CB1 receptor |
Bioorg Med Chem Lett 17: 3978-82 (2007)
Article DOI: 10.1016/j.bmcl.2007.04.087
BindingDB Entry DOI: 10.7270/Q2FN15XR |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM50213469
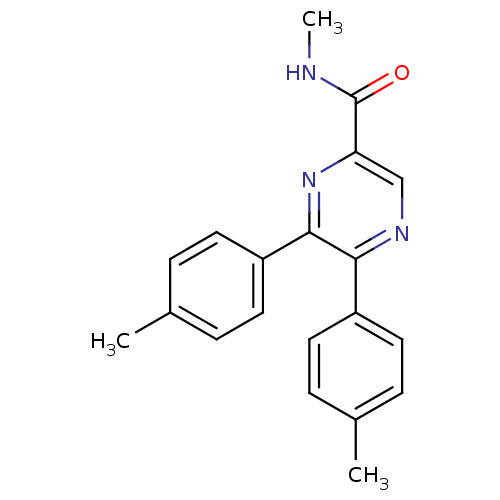
(CHEMBL246273 | N-methyl-5,6-dip-tolylpyrazine-2-ca...)Show SMILES CNC(=O)c1cnc(-c2ccc(C)cc2)c(n1)-c1ccc(C)cc1 Show InChI InChI=1S/C20H19N3O/c1-13-4-8-15(9-5-13)18-19(16-10-6-14(2)7-11-16)23-17(12-22-18)20(24)21-3/h4-12H,1-3H3,(H,21,24) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3.85E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Binding affinity to human CB1 receptor |
Bioorg Med Chem Lett 17: 3978-82 (2007)
Article DOI: 10.1016/j.bmcl.2007.04.087
BindingDB Entry DOI: 10.7270/Q2FN15XR |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM50213474
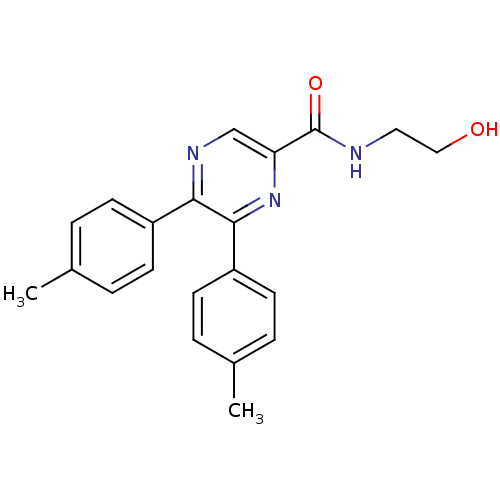
(CHEMBL247712 | N-(2-hydroxyethyl)-5,6-dip-tolylpyr...)Show SMILES Cc1ccc(cc1)-c1ncc(nc1-c1ccc(C)cc1)C(=O)NCCO Show InChI InChI=1S/C21H21N3O2/c1-14-3-7-16(8-4-14)19-20(17-9-5-15(2)6-10-17)24-18(13-23-19)21(26)22-11-12-25/h3-10,13,25H,11-12H2,1-2H3,(H,22,26) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 8.43E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Binding affinity to human CB1 receptor |
Bioorg Med Chem Lett 17: 3978-82 (2007)
Article DOI: 10.1016/j.bmcl.2007.04.087
BindingDB Entry DOI: 10.7270/Q2FN15XR |
More data for this
Ligand-Target Pair | |
Motilin receptor
(Homo sapiens (Human)) | BDBM50143034
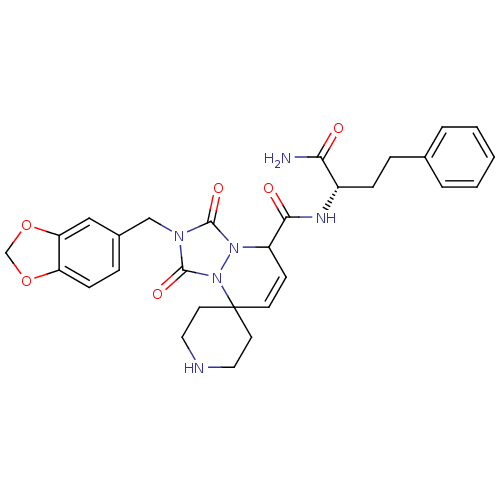
(8'N-[1-carbamoyl-3-phenyl-(1S)-propyl]-2'-benzo[d]...)Show SMILES NC(=O)[C@H](CCc1ccccc1)NC(=O)C1C=CC2(CCNCC2)n2n1c(=O)n(Cc1ccc3OCOc3c1)c2=O |c:17| Show InChI InChI=1S/C29H32N6O6/c30-25(36)21(8-6-19-4-2-1-3-5-19)32-26(37)22-10-11-29(12-14-31-15-13-29)35-28(39)33(27(38)34(22)35)17-20-7-9-23-24(16-20)41-18-40-23/h1-5,7,9-11,16,21-22,31H,6,8,12-15,17-18H2,(H2,30,36)(H,32,37)/t21-,22?/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
In vitro binding affinity towards human motilin receptor |
J Med Chem 47: 1704-8 (2004)
Article DOI: 10.1021/jm0304865
BindingDB Entry DOI: 10.7270/Q2571CRC |
More data for this
Ligand-Target Pair | |
Motilin receptor
(Homo sapiens (Human)) | BDBM50143034

(8'N-[1-carbamoyl-3-phenyl-(1S)-propyl]-2'-benzo[d]...)Show SMILES NC(=O)[C@H](CCc1ccccc1)NC(=O)C1C=CC2(CCNCC2)n2n1c(=O)n(Cc1ccc3OCOc3c1)c2=O |c:17| Show InChI InChI=1S/C29H32N6O6/c30-25(36)21(8-6-19-4-2-1-3-5-19)32-26(37)22-10-11-29(12-14-31-15-13-29)35-28(39)33(27(38)34(22)35)17-20-7-9-23-24(16-20)41-18-40-23/h1-5,7,9-11,16,21-22,31H,6,8,12-15,17-18H2,(H2,30,36)(H,32,37)/t21-,22?/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
In vitro binding affinity towards human motilin receptor |
J Med Chem 47: 1704-8 (2004)
Article DOI: 10.1021/jm0304865
BindingDB Entry DOI: 10.7270/Q2571CRC |
More data for this
Ligand-Target Pair | |
Motilin receptor
(Homo sapiens (Human)) | BDBM50143033
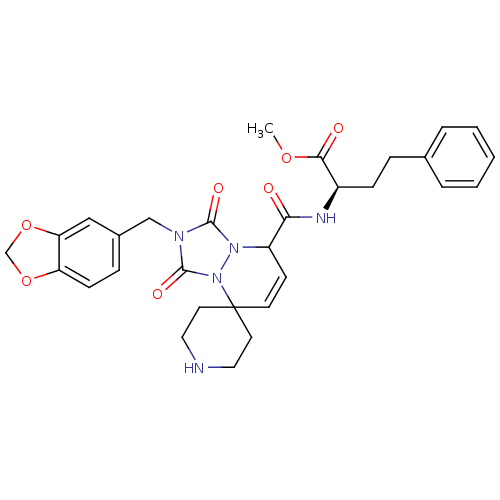
(CHEMBL46272 | methyl 2-[2'-benzo[d][1,3]dioxol-5-y...)Show SMILES COC(=O)[C@@H](CCc1ccccc1)NC(=O)C1C=CC2(CCNCC2)n2n1c(=O)n(Cc1ccc3OCOc3c1)c2=O |c:18| Show InChI InChI=1S/C30H33N5O7/c1-40-27(37)22(9-7-20-5-3-2-4-6-20)32-26(36)23-11-12-30(13-15-31-16-14-30)35-29(39)33(28(38)34(23)35)18-21-8-10-24-25(17-21)42-19-41-24/h2-6,8,10-12,17,22-23,31H,7,9,13-16,18-19H2,1H3,(H,32,36)/t22-,23?/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
In vitro binding affinity towards human motilin receptor |
J Med Chem 47: 1704-8 (2004)
Article DOI: 10.1021/jm0304865
BindingDB Entry DOI: 10.7270/Q2571CRC |
More data for this
Ligand-Target Pair | |
Motilin receptor
(Homo sapiens (Human)) | BDBM50143031
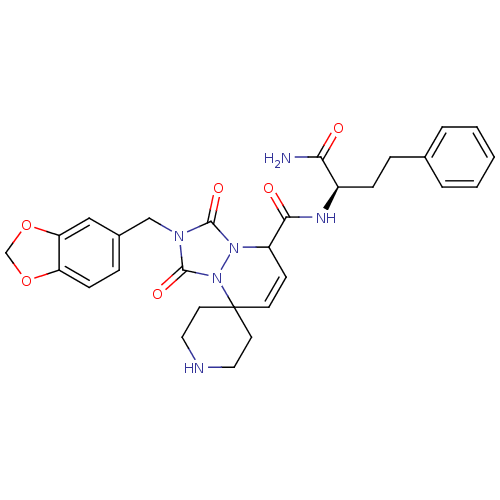
(8'N-[1-carbamoyl-3-phenyl-(1R)-propyl]-2'-benzo[d]...)Show SMILES NC(=O)[C@@H](CCc1ccccc1)NC(=O)C1C=CC2(CCNCC2)n2n1c(=O)n(Cc1ccc3OCOc3c1)c2=O |c:17| Show InChI InChI=1S/C29H32N6O6/c30-25(36)21(8-6-19-4-2-1-3-5-19)32-26(37)22-10-11-29(12-14-31-15-13-29)35-28(39)33(27(38)34(22)35)17-20-7-9-23-24(16-20)41-18-40-23/h1-5,7,9-11,16,21-22,31H,6,8,12-15,17-18H2,(H2,30,36)(H,32,37)/t21-,22?/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
In vitro binding affinity towards human motilin receptor |
J Med Chem 47: 1704-8 (2004)
Article DOI: 10.1021/jm0304865
BindingDB Entry DOI: 10.7270/Q2571CRC |
More data for this
Ligand-Target Pair | |
Motilin receptor
(Homo sapiens (Human)) | BDBM50344942
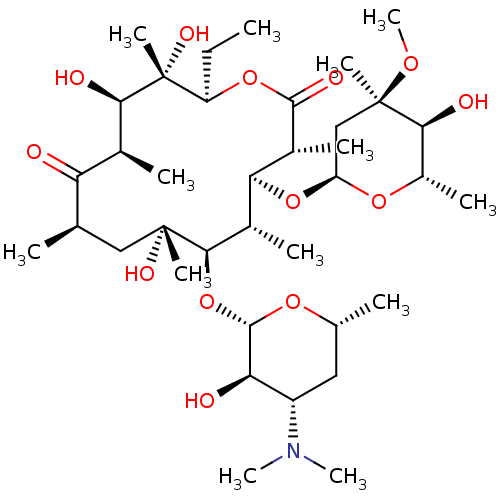
(CHEMBL532 | E-MYCIN E | ERYTHROMYCIN | ERYTHROMYCI...)Show SMILES CC[C@H]1OC(=O)[C@H](C)[C@@H](O[C@H]2C[C@@](C)(OC)[C@@H](O)[C@H](C)O2)[C@H](C)[C@@H](O[C@@H]2O[C@H](C)C[C@@H]([C@H]2O)N(C)C)[C@](C)(O)C[C@@H](C)C(=O)[C@H](C)[C@@H](O)[C@]1(C)O |r| Show InChI InChI=1S/C37H67NO13/c1-14-25-37(10,45)30(41)20(4)27(39)18(2)16-35(8,44)32(51-34-28(40)24(38(11)12)15-19(3)47-34)21(5)29(22(6)33(43)49-25)50-26-17-36(9,46-13)31(42)23(7)48-26/h18-26,28-32,34,40-42,44-45H,14-17H2,1-13H3/t18-,19-,20+,21+,22-,23+,24+,25-,26+,28-,29+,30-,31+,32-,34+,35-,36-,37-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
PDB
Article
PubMed
| 3.70E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
In vitro binding affinity towards human motilin receptor |
J Med Chem 47: 1704-8 (2004)
Article DOI: 10.1021/jm0304865
BindingDB Entry DOI: 10.7270/Q2571CRC |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM93463

(hCB1 Inhibitor, 2{1,1,8})Show SMILES Cc1cccc(c1C)-n1ncc(C(=O)NCCCc2ccccc2)c1-c1ccc(cc1)-c1ccccc1 Show InChI InChI=1S/C33H31N3O/c1-24-11-9-17-31(25(24)2)36-32(29-20-18-28(19-21-29)27-15-7-4-8-16-27)30(23-35-36)33(37)34-22-10-14-26-12-5-3-6-13-26/h3-9,11-13,15-21,23H,10,14,22H2,1-2H3,(H,34,37) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 40 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development
| Assay Description
Inhibition assay using hCB1. |
ACS Comb Sci 14: 197-204 (2012)
Article DOI: 10.1021/co200147y
BindingDB Entry DOI: 10.7270/Q22J69G1 |
More data for this
Ligand-Target Pair | |
Vasoactive intestinal polypeptide receptor 1
(Rattus norvegicus) | BDBM50365986
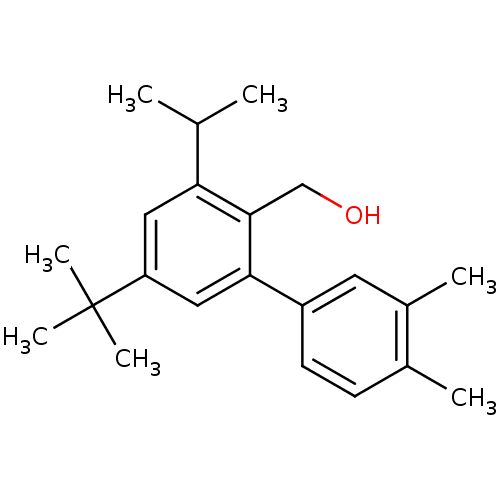
(CHEMBL1956427)Show InChI InChI=1S/C22H30O/c1-14(2)19-11-18(22(5,6)7)12-20(21(19)13-23)17-9-8-15(3)16(4)10-17/h8-12,14,23H,13H2,1-7H3 | KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 81 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Co.
Curated by ChEMBL
| Assay Description
Antagonist activity at vasoactive intestinal peptide receptor 1 in rat RKE cells assessed as inhibition of VIP-induced intracellular cAMP accumulatio... |
Bioorg Med Chem Lett 22: 2287-90 (2012)
Article DOI: 10.1016/j.bmcl.2012.01.082
BindingDB Entry DOI: 10.7270/Q2Q240QR |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM93459

(hCB1 Inhibitor, 2{1,1,3})Show SMILES O=C(NCCCc1ccccc1)c1cnn(c1-c1ccc(cc1)-c1ccccc1)-c1cccc2ccccc12 Show InChI InChI=1S/C35H29N3O/c39-35(36-24-10-13-26-11-3-1-4-12-26)32-25-37-38(33-19-9-17-29-16-7-8-18-31(29)33)34(32)30-22-20-28(21-23-30)27-14-5-2-6-15-27/h1-9,11-12,14-23,25H,10,13,24H2,(H,36,39) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 90 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development
| Assay Description
Inhibition assay using hCB1. |
ACS Comb Sci 14: 197-204 (2012)
Article DOI: 10.1021/co200147y
BindingDB Entry DOI: 10.7270/Q22J69G1 |
More data for this
Ligand-Target Pair | |
Vasoactive intestinal polypeptide receptor 1
(Rattus norvegicus) | BDBM50365985
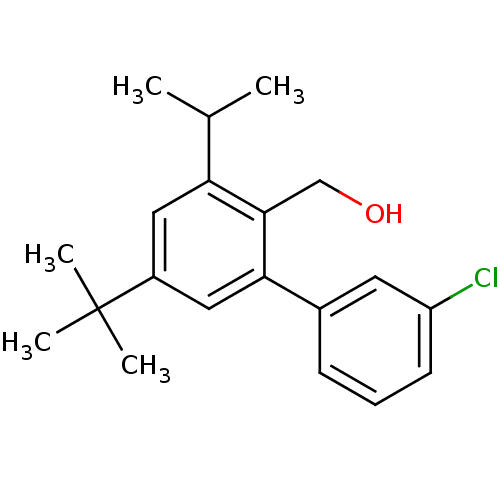
(CHEMBL1956426)Show InChI InChI=1S/C20H25ClO/c1-13(2)17-10-15(20(3,4)5)11-18(19(17)12-22)14-7-6-8-16(21)9-14/h6-11,13,22H,12H2,1-5H3 | KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 100 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Co.
Curated by ChEMBL
| Assay Description
Antagonist activity at vasoactive intestinal peptide receptor 1 in rat RKE cells assessed as inhibition of VIP-induced intracellular cAMP accumulatio... |
Bioorg Med Chem Lett 22: 2287-90 (2012)
Article DOI: 10.1016/j.bmcl.2012.01.082
BindingDB Entry DOI: 10.7270/Q2Q240QR |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data