

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Hydroxycarboxylic acid receptor 2 (Homo sapiens (Human)) | BDBM50313984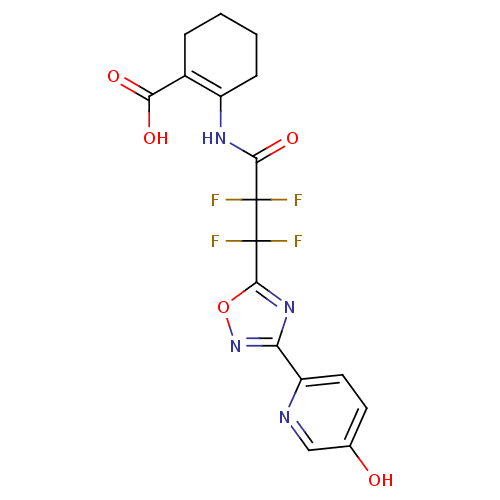 (2-(2,2,3,3-tetrafluoro-3-(3-(5-hydroxypyridin-2-yl...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Displacement of [3H]NA from cloned human GPR109A receptor expressed in CHO-K1 cells by spectrophotometry | J Med Chem 53: 2666-70 (2010) Article DOI: 10.1021/jm100022r BindingDB Entry DOI: 10.7270/Q2NS0V2P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hydroxycarboxylic acid receptor 2 (Homo sapiens (Human)) | BDBM50313977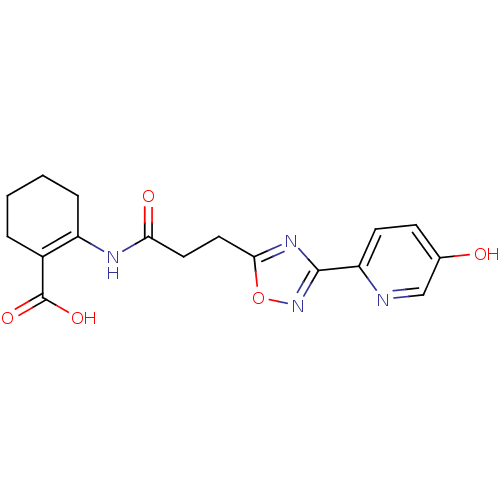 (2-(3-(3-(5-hydroxypyridin-2-yl)-1,2,4-oxadiazol-5-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Displacement of [3H]NA from cloned human GPR109A receptor expressed in CHO-K1 cells by spectrophotometry | J Med Chem 53: 2666-70 (2010) Article DOI: 10.1021/jm100022r BindingDB Entry DOI: 10.7270/Q2NS0V2P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hydroxycarboxylic acid receptor 2 (Homo sapiens (Human)) | BDBM50313976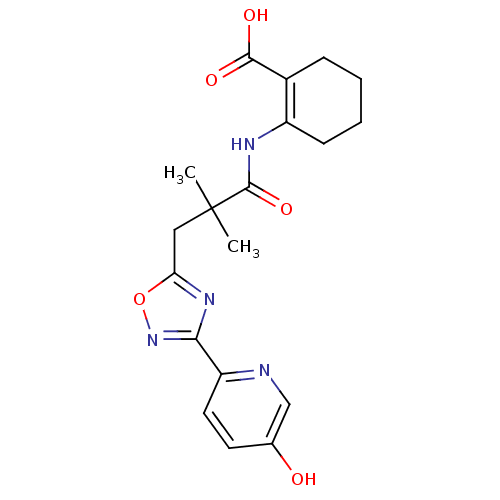 (2-({3-[3-(5-hydroxypyridin-2-yl)-1,2,4-oxadiazol-5...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | 4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Displacement of [3H]NA from cloned human GPR109A receptor expressed in CHO-K1 cells by spectrophotometry | J Med Chem 53: 2666-70 (2010) Article DOI: 10.1021/jm100022r BindingDB Entry DOI: 10.7270/Q2NS0V2P | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Hydroxycarboxylic acid receptor 2 (Homo sapiens (Human)) | BDBM23533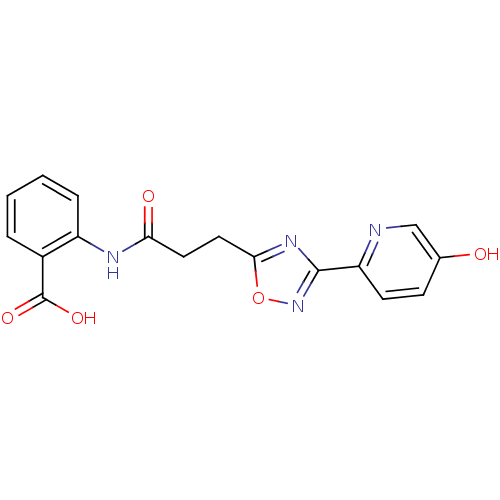 (2-{3-[3-(5-hydroxypyridin-2-yl)-1,2,4-oxadiazol-5-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Displacement of [3H]NA from cloned human GPR109A receptor expressed in CHO-K1 cells by spectrophotometry | J Med Chem 53: 2666-70 (2010) Article DOI: 10.1021/jm100022r BindingDB Entry DOI: 10.7270/Q2NS0V2P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hydroxycarboxylic acid receptor 2 (Homo sapiens (Human)) | BDBM50313978 (2-(3-(3-(5-hydroxypyridin-2-yl)-1,2,4-oxadiazol-5-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 8 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Displacement of [3H]NA from cloned human GPR109A receptor expressed in CHO-K1 cells by spectrophotometry | J Med Chem 53: 2666-70 (2010) Article DOI: 10.1021/jm100022r BindingDB Entry DOI: 10.7270/Q2NS0V2P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hydroxycarboxylic acid receptor 2 (Homo sapiens (Human)) | BDBM50313978 (2-(3-(3-(5-hydroxypyridin-2-yl)-1,2,4-oxadiazol-5-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 8 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Displacement of [3H]NA from cloned human GPR109A receptor expressed in CHO-K1 cells by spectrophotometry | J Med Chem 53: 2666-70 (2010) Article DOI: 10.1021/jm100022r BindingDB Entry DOI: 10.7270/Q2NS0V2P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hydroxycarboxylic acid receptor 2 (Homo sapiens (Human)) | BDBM50313979 (2-(1-((3-(5-hydroxypyridin-2-yl)-1,2,4-oxadiazol-5...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 11 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Displacement of [3H]NA from cloned human GPR109A receptor expressed in CHO-K1 cells by spectrophotometry | J Med Chem 53: 2666-70 (2010) Article DOI: 10.1021/jm100022r BindingDB Entry DOI: 10.7270/Q2NS0V2P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hydroxycarboxylic acid receptor 2 (Homo sapiens (Human)) | BDBM50313981 (CHEMBL1088213 | rac-2-(2-hydroxy-3-(3-(5-hydroxypy...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 22 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Displacement of [3H]NA from cloned human GPR109A receptor expressed in CHO-K1 cells by spectrophotometry | J Med Chem 53: 2666-70 (2010) Article DOI: 10.1021/jm100022r BindingDB Entry DOI: 10.7270/Q2NS0V2P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hydroxycarboxylic acid receptor 2 (Homo sapiens (Human)) | BDBM50313982 (CHEMBL1084393 | rac-2-(3-(3-(5-hydroxypyridin-2-yl...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 29 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Displacement of [3H]NA from cloned human GPR109A receptor expressed in CHO-K1 cells by spectrophotometry | J Med Chem 53: 2666-70 (2010) Article DOI: 10.1021/jm100022r BindingDB Entry DOI: 10.7270/Q2NS0V2P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hydroxycarboxylic acid receptor 2 (Homo sapiens (Human)) | BDBM23515 (CHEMBL573 | Niacin | Nicotinic Acid | [5, 6-3H]-ni...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | DrugBank PDB Article PubMed | 50 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Arena Pharmaceuticals Curated by ChEMBL | Assay Description Displacement of [3H]nicotinic acid from human GPR109a receptor expressed in CHO cells | J Med Chem 51: 5101-8 (2008) Article DOI: 10.1021/jm800258p BindingDB Entry DOI: 10.7270/Q2CF9PWV | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Hydroxycarboxylic acid receptor 2 (Homo sapiens (Human)) | BDBM23515 (CHEMBL573 | Niacin | Nicotinic Acid | [5, 6-3H]-ni...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | DrugBank PDB Article PubMed | 82 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Displacement of [3H]NA from cloned human GPR109A receptor expressed in CHO-K1 cells by spectrophotometry | J Med Chem 53: 2666-70 (2010) Article DOI: 10.1021/jm100022r BindingDB Entry DOI: 10.7270/Q2NS0V2P | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Hydroxycarboxylic acid receptor 2 (Homo sapiens (Human)) | BDBM23515 (CHEMBL573 | Niacin | Nicotinic Acid | [5, 6-3H]-ni...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | DrugBank PDB Article PubMed | 104 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Displacement of [3H]NA from cloned human GPR109A receptor expressed in CHO-K1 cells by spectrophotometry in presence of 4% human serum albumin | J Med Chem 53: 2666-70 (2010) Article DOI: 10.1021/jm100022r BindingDB Entry DOI: 10.7270/Q2NS0V2P | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Hydroxycarboxylic acid receptor 2 (Homo sapiens (Human)) | BDBM23533 (2-{3-[3-(5-hydroxypyridin-2-yl)-1,2,4-oxadiazol-5-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 120 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Displacement of [3H]NA from cloned human GPR109A receptor expressed in CHO-K1 cells by spectrophotometry in presence of 4% human serum albumin | J Med Chem 53: 2666-70 (2010) Article DOI: 10.1021/jm100022r BindingDB Entry DOI: 10.7270/Q2NS0V2P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hydroxycarboxylic acid receptor 2 (Homo sapiens (Human)) | BDBM50313983 (2-(3-(3-(5-hydroxypyridin-2-yl)-1,2,4-oxadiazol-5-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 130 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Displacement of [3H]NA from cloned human GPR109A receptor expressed in CHO-K1 cells by spectrophotometry | J Med Chem 53: 2666-70 (2010) Article DOI: 10.1021/jm100022r BindingDB Entry DOI: 10.7270/Q2NS0V2P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hydroxycarboxylic acid receptor 2 (Homo sapiens (Human)) | BDBM50313977 (2-(3-(3-(5-hydroxypyridin-2-yl)-1,2,4-oxadiazol-5-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 150 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Displacement of [3H]NA from cloned human GPR109A receptor expressed in CHO-K1 cells by spectrophotometry in presence of 4% human serum albumin | J Med Chem 53: 2666-70 (2010) Article DOI: 10.1021/jm100022r BindingDB Entry DOI: 10.7270/Q2NS0V2P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hydroxycarboxylic acid receptor 2 (Homo sapiens (Human)) | BDBM50313978 (2-(3-(3-(5-hydroxypyridin-2-yl)-1,2,4-oxadiazol-5-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 230 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Displacement of [3H]NA from cloned human GPR109A receptor expressed in CHO-K1 cells by spectrophotometry in presence of 4% human serum albumin | J Med Chem 53: 2666-70 (2010) Article DOI: 10.1021/jm100022r BindingDB Entry DOI: 10.7270/Q2NS0V2P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hydroxycarboxylic acid receptor 2 (Homo sapiens (Human)) | BDBM50313978 (2-(3-(3-(5-hydroxypyridin-2-yl)-1,2,4-oxadiazol-5-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 240 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Displacement of [3H]NA from cloned human GPR109A receptor expressed in CHO-K1 cells by spectrophotometry in presence of 4% human serum albumin | J Med Chem 53: 2666-70 (2010) Article DOI: 10.1021/jm100022r BindingDB Entry DOI: 10.7270/Q2NS0V2P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hydroxycarboxylic acid receptor 2 (Homo sapiens (Human)) | BDBM50313980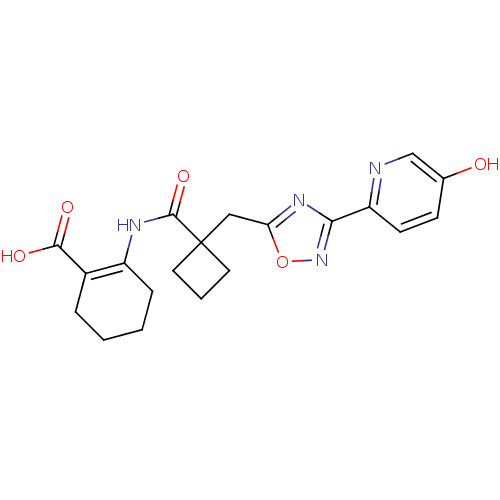 (2-(1-((3-(5-hydroxypyridin-2-yl)-1,2,4-oxadiazol-5...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 360 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Displacement of [3H]NA from cloned human GPR109A receptor expressed in CHO-K1 cells by spectrophotometry | J Med Chem 53: 2666-70 (2010) Article DOI: 10.1021/jm100022r BindingDB Entry DOI: 10.7270/Q2NS0V2P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hydroxycarboxylic acid receptor 2 (Homo sapiens (Human)) | BDBM50273099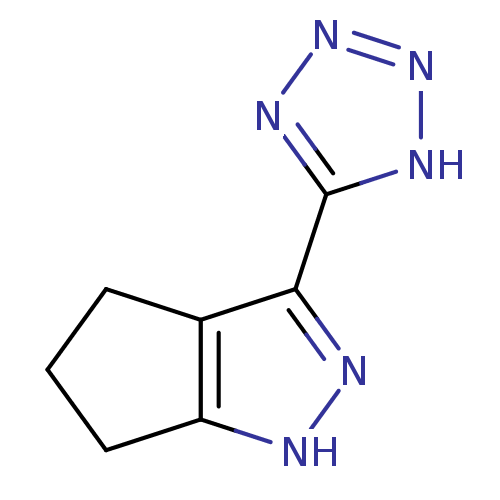 (3-(1H-tetrazol-5-yl)-1,4,5,6-tetrahydrocyclopenta[...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | 505 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Arena Pharmaceuticals Curated by ChEMBL | Assay Description Displacement of [3H]nicotinic acid from human GPR109a receptor expressed in CHO cells | J Med Chem 51: 5101-8 (2008) Article DOI: 10.1021/jm800258p BindingDB Entry DOI: 10.7270/Q2CF9PWV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hydroxycarboxylic acid receptor 2 (Homo sapiens (Human)) | BDBM50313976 (2-({3-[3-(5-hydroxypyridin-2-yl)-1,2,4-oxadiazol-5...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | 595 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Displacement of [3H]NA from cloned human GPR109A receptor expressed in CHO-K1 cells by spectrophotometry in presence of 4% human serum albumin | J Med Chem 53: 2666-70 (2010) Article DOI: 10.1021/jm100022r BindingDB Entry DOI: 10.7270/Q2NS0V2P | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Hydroxycarboxylic acid receptor 2 (Homo sapiens (Human)) | BDBM50313981 (CHEMBL1088213 | rac-2-(2-hydroxy-3-(3-(5-hydroxypy...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 660 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Displacement of [3H]NA from cloned human GPR109A receptor expressed in CHO-K1 cells by spectrophotometry in presence of 4% human serum albumin | J Med Chem 53: 2666-70 (2010) Article DOI: 10.1021/jm100022r BindingDB Entry DOI: 10.7270/Q2NS0V2P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hydroxycarboxylic acid receptor 2 (Homo sapiens (Human)) | BDBM50313984 (2-(2,2,3,3-tetrafluoro-3-(3-(5-hydroxypyridin-2-yl...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 840 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Displacement of [3H]NA from cloned human GPR109A receptor expressed in CHO-K1 cells by spectrophotometry in presence of 4% human serum albumin | J Med Chem 53: 2666-70 (2010) Article DOI: 10.1021/jm100022r BindingDB Entry DOI: 10.7270/Q2NS0V2P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hydroxycarboxylic acid receptor 2 (Homo sapiens (Human)) | BDBM50313980 (2-(1-((3-(5-hydroxypyridin-2-yl)-1,2,4-oxadiazol-5...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.08E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Displacement of [3H]NA from cloned human GPR109A receptor expressed in CHO-K1 cells by spectrophotometry in presence of 4% human serum albumin | J Med Chem 53: 2666-70 (2010) Article DOI: 10.1021/jm100022r BindingDB Entry DOI: 10.7270/Q2NS0V2P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hydroxycarboxylic acid receptor 2 (Homo sapiens (Human)) | BDBM50313982 (CHEMBL1084393 | rac-2-(3-(3-(5-hydroxypyridin-2-yl...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 1.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Displacement of [3H]NA from cloned human GPR109A receptor expressed in CHO-K1 cells by spectrophotometry in presence of 4% human serum albumin | J Med Chem 53: 2666-70 (2010) Article DOI: 10.1021/jm100022r BindingDB Entry DOI: 10.7270/Q2NS0V2P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hydroxycarboxylic acid receptor 2 (Homo sapiens (Human)) | BDBM50313979 (2-(1-((3-(5-hydroxypyridin-2-yl)-1,2,4-oxadiazol-5...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 9.13E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Displacement of [3H]NA from cloned human GPR109A receptor expressed in CHO-K1 cells by spectrophotometry in presence of 4% human serum albumin | J Med Chem 53: 2666-70 (2010) Article DOI: 10.1021/jm100022r BindingDB Entry DOI: 10.7270/Q2NS0V2P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hydroxycarboxylic acid receptor 2 (Homo sapiens (Human)) | BDBM50313983 (2-(3-(3-(5-hydroxypyridin-2-yl)-1,2,4-oxadiazol-5-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.47E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Displacement of [3H]NA from cloned human GPR109A receptor expressed in CHO-K1 cells by spectrophotometry in presence of 4% human serum albumin | J Med Chem 53: 2666-70 (2010) Article DOI: 10.1021/jm100022r BindingDB Entry DOI: 10.7270/Q2NS0V2P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mitogen-activated protein kinase 14 (Homo sapiens (Human)) | BDBM50175745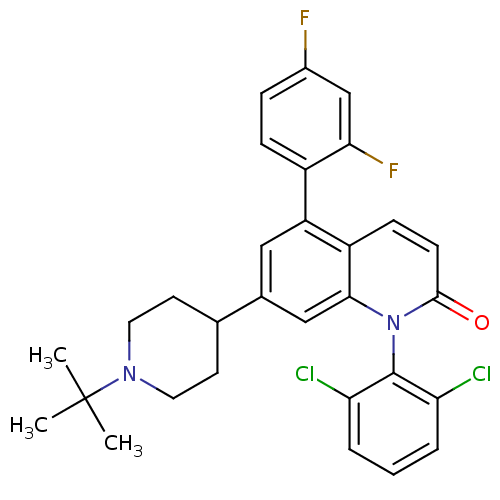 (7-(1-tert-butylpiperidin-4-yl)-1-(2,6-dichlorophen...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.0800 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of wild type p38alpha (unknown origin) | J Biol Chem 282: 34663-71 (2007) Article DOI: 10.1074/jbc.M704236200 BindingDB Entry DOI: 10.7270/Q2G44Q2F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mitogen-activated protein kinase 14 (Homo sapiens (Human)) | BDBM15459 (4-[1-methyl-2-(piperidin-4-yl)-4-[3-(trifluorometh...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of wild type p38alpha (unknown origin) | J Biol Chem 282: 34663-71 (2007) Article DOI: 10.1074/jbc.M704236200 BindingDB Entry DOI: 10.7270/Q2G44Q2F | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (docked) | ||||||||||||
| Mitogen-activated protein kinase 11 (Homo sapiens (Human)) | BDBM15459 (4-[1-methyl-2-(piperidin-4-yl)-4-[3-(trifluorometh...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of wild type p38beta (unknown origin) | J Biol Chem 282: 34663-71 (2007) Article DOI: 10.1074/jbc.M704236200 BindingDB Entry DOI: 10.7270/Q2G44Q2F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mitogen-activated protein kinase 11 (Homo sapiens (Human)) | BDBM50175745 (7-(1-tert-butylpiperidin-4-yl)-1-(2,6-dichlorophen...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.25 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of wild type p38beta (unknown origin) | J Biol Chem 282: 34663-71 (2007) Article DOI: 10.1074/jbc.M704236200 BindingDB Entry DOI: 10.7270/Q2G44Q2F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glucocorticoid receptor (Homo sapiens (Human)) | BDBM50272468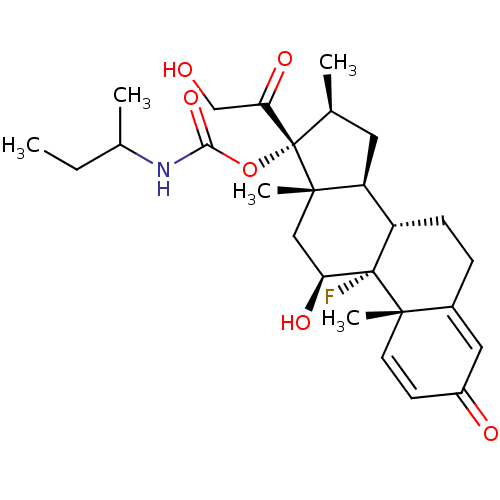 ((8S,9R,10S,11S,13S,14S,16S,17R)-9-fluoro-11-hydrox...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of human glucocorticoid receptor | Bioorg Med Chem 16: 7535-42 (2008) Article DOI: 10.1016/j.bmc.2008.07.037 BindingDB Entry DOI: 10.7270/Q2CC10HX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mitogen-activated protein kinase 11/12/13/14 (Homo sapiens (Human)) | BDBM50476040 (CHEMBL219796) | PDB KEGG UniProtKB/SwissProt antibodypedia antibodypedia antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.510 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of p38 | Bioorg Med Chem Lett 16: 5468-71 (2006) Article DOI: 10.1016/j.bmcl.2006.06.084 BindingDB Entry DOI: 10.7270/Q2HT2S35 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mitogen-activated protein kinase 11/12/13/14 (Homo sapiens (Human)) | BDBM50194461 (7-amino-5-(2,4-difluorophenyl)-1-(2,6-difluorophen...) | PDB KEGG UniProtKB/SwissProt antibodypedia antibodypedia antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.690 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of p38 | Bioorg Med Chem Lett 16: 5468-71 (2006) Article DOI: 10.1016/j.bmcl.2006.06.084 BindingDB Entry DOI: 10.7270/Q2HT2S35 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glucocorticoid receptor (Homo sapiens (Human)) | BDBM19202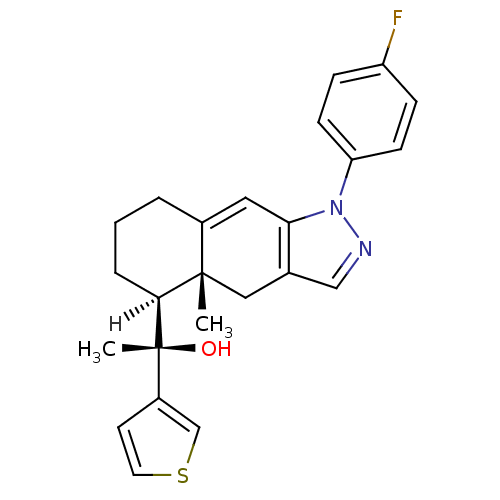 ((1S)-1-[(4aR,5S)-1-(4-fluorophenyl)-4a-methyl-1H,4...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.800 | n/a | 1 | n/a | n/a | 7.5 | 4 |
Merck Research Laboratories | Assay Description The IC50s were determined by incubating the receptors with radiolabeled dexamethasone in the presence of full log scale concentrations (10-11 M to 10... | J Med Chem 47: 2441-52 (2004) Article DOI: 10.1021/jm030585i BindingDB Entry DOI: 10.7270/Q28W3BKZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glucocorticoid receptor (Homo sapiens (Human)) | BDBM19202 ((1S)-1-[(4aR,5S)-1-(4-fluorophenyl)-4a-methyl-1H,4...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of human glucocorticoid receptor alpha by displacement of [3H]-dexamethasone | Bioorg Med Chem Lett 15: 2163-7 (2005) Article DOI: 10.1016/j.bmcl.2005.02.009 BindingDB Entry DOI: 10.7270/Q2WM1CXX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hydroxycarboxylic acid receptor 2 (Homo sapiens (Human)) | BDBM23525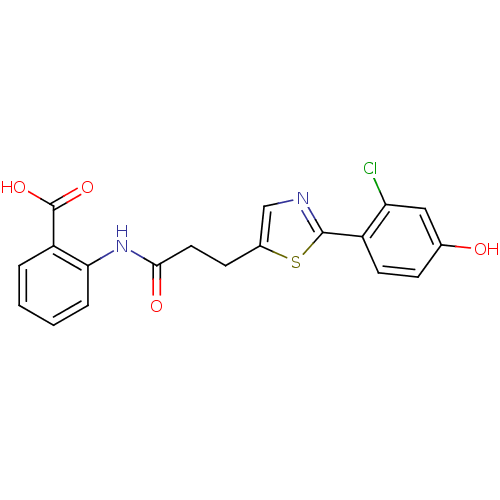 (2-{3-[2-(2-chloro-4-hydroxyphenyl)-1,3-thiazol-5-y...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1 | n/a | 6 | n/a | n/a | 7.4 | 23 |
Merck Research Laboratories | Assay Description Membranes were incubated in binding buffer with [5, 6-3H]-niacin in the presence of test compound. After 4 hours at room temperature, reactions were ... | J Med Chem 50: 6303-6 (2007) Article DOI: 10.1021/jm700942d BindingDB Entry DOI: 10.7270/Q29S1PBG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hydroxycarboxylic acid receptor 2 (Homo sapiens (Human)) | BDBM50277718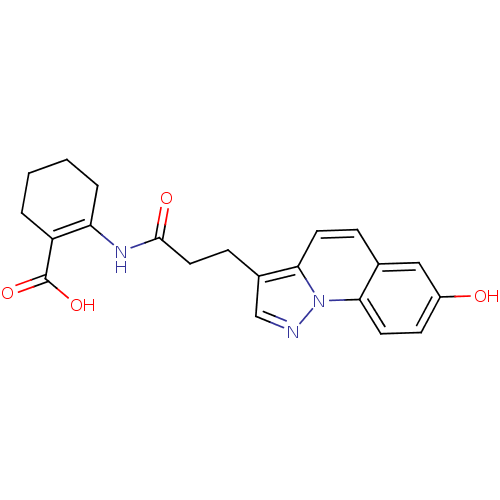 (2-(3-(7-hydroxypyrazolo[1,5-a]quinolin-3-yl)propan...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Displacement of [3H]niacin from human GPR109A expressed in CHO cells | J Med Chem 52: 2587-602 (2009) Article DOI: 10.1021/jm900151e BindingDB Entry DOI: 10.7270/Q2RJ4JDG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hydroxycarboxylic acid receptor 2 (Homo sapiens (Human)) | BDBM50277717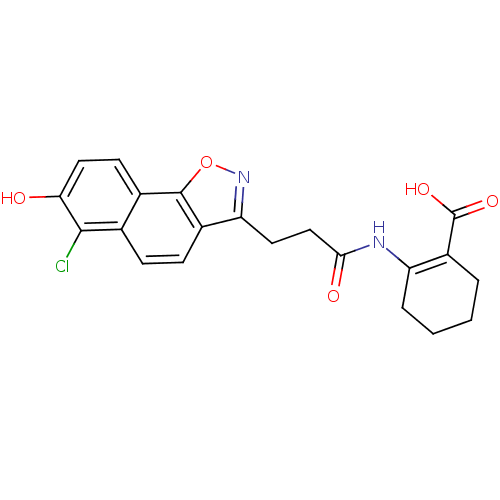 (2-(3-(6-chloro-7-hydroxynaphtho[2,1-d]isoxazol-3-y...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Displacement of [3H]niacin from human GPR109A expressed in CHO cells | J Med Chem 52: 2587-602 (2009) Article DOI: 10.1021/jm900151e BindingDB Entry DOI: 10.7270/Q2RJ4JDG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glucocorticoid receptor (Homo sapiens (Human)) | BDBM19207 ((1S)-1-[(4aR,5S)-1-(4-fluorophenyl)-4a-methyl-1H,4...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.10 | n/a | 4.40 | n/a | n/a | 7.5 | 4 |
Merck Research Laboratories | Assay Description The IC50s were determined by incubating the receptors with radiolabeled dexamethasone in the presence of full log scale concentrations (10-11 M to 10... | J Med Chem 47: 2441-52 (2004) Article DOI: 10.1021/jm030585i BindingDB Entry DOI: 10.7270/Q28W3BKZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glucocorticoid receptor (Homo sapiens (Human)) | BDBM50167799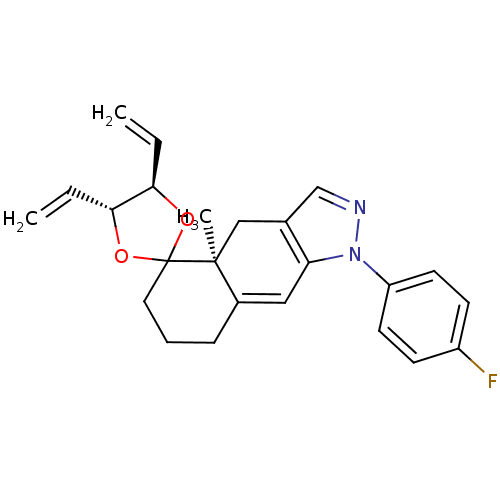 (1-(4-fluorophenyl)-4a-methyl-4',5'-divinyl-(4'R,4a...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Binding affinity for human glucocorticoid receptor | Bioorg Med Chem Lett 15: 2926-31 (2005) Article DOI: 10.1016/j.bmcl.2005.03.027 BindingDB Entry DOI: 10.7270/Q2X34X0Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glucocorticoid receptor (Homo sapiens (Human)) | BDBM50167799 (1-(4-fluorophenyl)-4a-methyl-4',5'-divinyl-(4'R,4a...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of human glucocorticoid receptor alpha isoform | Bioorg Med Chem Lett 15: 2926-31 (2005) Article DOI: 10.1016/j.bmcl.2005.03.027 BindingDB Entry DOI: 10.7270/Q2X34X0Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glucocorticoid receptor (Homo sapiens (Human)) | BDBM50272523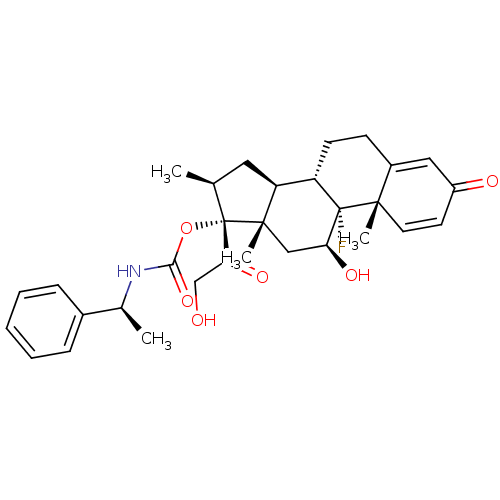 ((11beta,16beta)-9-Fluoro-11,21-dihydroxy-16-methyl...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of human glucocorticoid receptor | Bioorg Med Chem 16: 7535-42 (2008) Article DOI: 10.1016/j.bmc.2008.07.037 BindingDB Entry DOI: 10.7270/Q2CC10HX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mitogen-activated protein kinase 11/12/13/14 (Homo sapiens (Human)) | BDBM50476039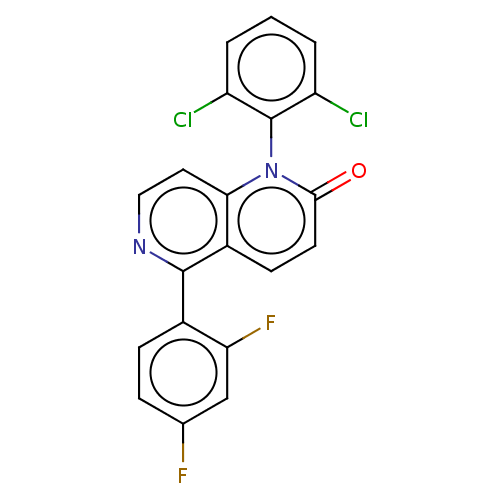 (CHEMBL219869) | PDB KEGG UniProtKB/SwissProt antibodypedia antibodypedia antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of p38 | Bioorg Med Chem Lett 16: 5468-71 (2006) Article DOI: 10.1016/j.bmcl.2006.06.084 BindingDB Entry DOI: 10.7270/Q2HT2S35 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glucocorticoid receptor (Homo sapiens (Human)) | BDBM19224 ((S)-[(4aR,5S)-1-(4-fluorophenyl)-4a-methyl-1H,4H,4...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of human glucocorticoid receptor alpha by displacement of [3H]-dexamethasone | Bioorg Med Chem Lett 15: 2163-7 (2005) Article DOI: 10.1016/j.bmcl.2005.02.009 BindingDB Entry DOI: 10.7270/Q2WM1CXX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glucocorticoid receptor (Homo sapiens (Human)) | BDBM19224 ((S)-[(4aR,5S)-1-(4-fluorophenyl)-4a-methyl-1H,4H,4...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.5 | n/a | 6 | n/a | n/a | 7.5 | 4 |
Merck Research Laboratories | Assay Description The IC50s were determined by incubating the receptors with radiolabeled dexamethasone in the presence of full log scale concentrations (10-11 M to 10... | Bioorg Med Chem Lett 17: 3354-61 (2007) Article DOI: 10.1016/j.bmcl.2007.03.103 BindingDB Entry DOI: 10.7270/Q2542KVJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glucocorticoid receptor (Homo sapiens (Human)) | BDBM19208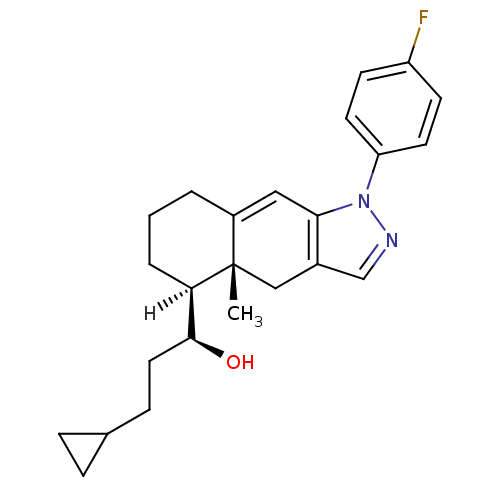 ((1S)-1-[(4aR,5S)-1-(4-fluorophenyl)-4a-methyl-1H,4...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.5 | n/a | 8.60 | n/a | n/a | n/a | n/a |
Merck Research Laboratories | Assay Description The IC50s were determined by incubating the receptors with radiolabeled dexamethasone in the presence of full log scale concentrations (10-11 M to 10... | J Med Chem 47: 2441-52 (2004) Article DOI: 10.1021/jm030585i BindingDB Entry DOI: 10.7270/Q28W3BKZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mitogen-activated protein kinase 11/12/13/14 (Homo sapiens (Human)) | BDBM50476037 (CHEMBL218968) | PDB KEGG UniProtKB/SwissProt antibodypedia antibodypedia antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of p38 | Bioorg Med Chem Lett 16: 5468-71 (2006) Article DOI: 10.1016/j.bmcl.2006.06.084 BindingDB Entry DOI: 10.7270/Q2HT2S35 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glucocorticoid receptor (Homo sapiens (Human)) | BDBM19199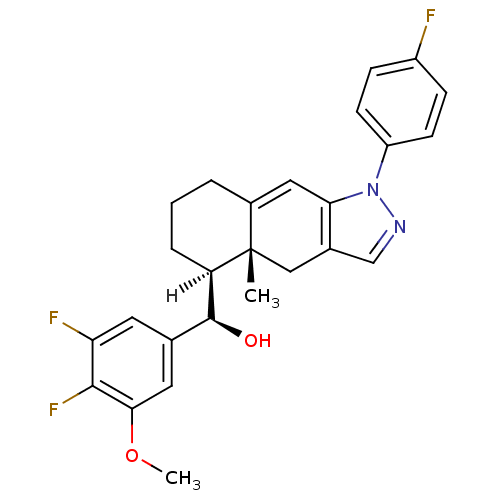 ((R)-[(4aR,5S)-1-(4-fluorophenyl)-4a-methyl-1H,4H,4...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of human glucocorticoid receptor alpha by displacement of [3H]-dexamethasone | Bioorg Med Chem Lett 15: 2163-7 (2005) Article DOI: 10.1016/j.bmcl.2005.02.009 BindingDB Entry DOI: 10.7270/Q2WM1CXX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glucocorticoid receptor (Homo sapiens (Human)) | BDBM19199 ((R)-[(4aR,5S)-1-(4-fluorophenyl)-4a-methyl-1H,4H,4...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.90 | n/a | 14.4 | n/a | n/a | 7.5 | 4 |
Merck Research Laboratories | Assay Description The IC50s were determined by incubating the receptors with radiolabeled dexamethasone in the presence of full log scale concentrations (10-11 M to 10... | J Med Chem 47: 2441-52 (2004) Article DOI: 10.1021/jm030585i BindingDB Entry DOI: 10.7270/Q28W3BKZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glucocorticoid receptor (Homo sapiens (Human)) | BDBM19204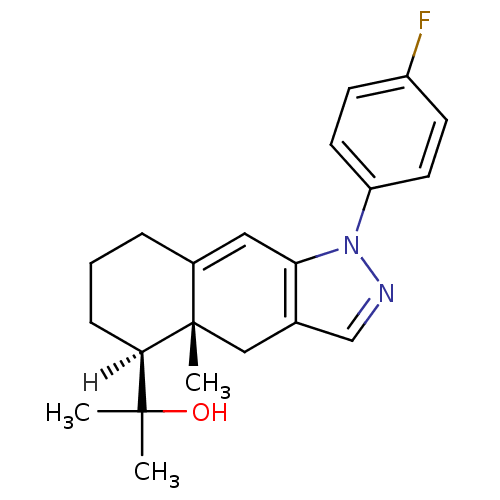 (2-[(4aR,5S)-1-(4-fluorophenyl)-4a-methyl-1H,4H,4aH...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.90 | n/a | 3 | n/a | n/a | 7.5 | 4 |
Merck Research Laboratories | Assay Description The IC50s were determined by incubating the receptors with radiolabeled dexamethasone in the presence of full log scale concentrations (10-11 M to 10... | J Med Chem 47: 2441-52 (2004) Article DOI: 10.1021/jm030585i BindingDB Entry DOI: 10.7270/Q28W3BKZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 823 total ) | Next | Last >> |