 Found 270 hits with Last Name = 'cavender' and Initial = 'd'
Found 270 hits with Last Name = 'cavender' and Initial = 'd' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
C-C chemokine receptor type 2
(Homo sapiens (Human)) | BDBM50377038
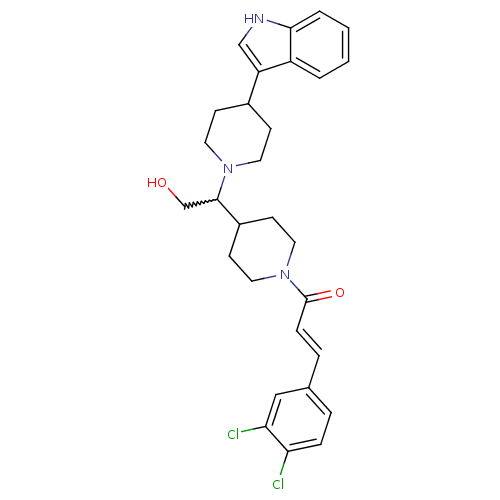
(CHEMBL258205)Show SMILES OCC(C1CCN(CC1)C(=O)\C=C\c1ccc(Cl)c(Cl)c1)N1CCC(CC1)c1c[nH]c2ccccc12 |w:2.1| Show InChI InChI=1S/C29H33Cl2N3O2/c30-25-7-5-20(17-26(25)31)6-8-29(36)34-15-11-22(12-16-34)28(19-35)33-13-9-21(10-14-33)24-18-32-27-4-2-1-3-23(24)27/h1-8,17-18,21-22,28,32,35H,9-16,19H2/b8-6+ | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research and Development
Curated by ChEMBL
| Assay Description
Binding affinity at human CCR2 receptor |
Bioorg Med Chem Lett 18: 3562-4 (2008)
Article DOI: 10.1016/j.bmcl.2008.05.010
BindingDB Entry DOI: 10.7270/Q2Q52QGK |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 11/12/13/14
(Homo sapiens (Human)) | BDBM50122995

((S)-3-(2-(1-methoxypropan-2-ylamino)pyridin-4-yl)-...)Show SMILES COC[C@H](C)Nc1cc(ccn1)-c1c(nc2nc(N)ccn12)-c1ccccc1 |r| Show InChI InChI=1S/C21H22N6O/c1-14(13-28-2)24-18-12-16(8-10-23-18)20-19(15-6-4-3-5-7-15)26-21-25-17(22)9-11-27(20)21/h3-12,14H,13H2,1-2H3,(H,23,24)(H2,22,25,26)/t14-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of LPS-induced p38-related TNF-alpha production in human peripheral blood mononuclear cells |
Bioorg Med Chem Lett 13: 347-50 (2003)
BindingDB Entry DOI: 10.7270/Q2G73D2C |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 11/12/13/14
(Homo sapiens (Human)) | BDBM50122996

(2-Phenyl-3-[2-((S)-1-phenyl-ethylamino)-pyrimidin-...)Show SMILES C[C@H](Nc1nccc(n1)-c1c(nc2nc(N)ccn12)-c1ccccc1)c1ccccc1 Show InChI InChI=1S/C24H21N7/c1-16(17-8-4-2-5-9-17)27-23-26-14-12-19(28-23)22-21(18-10-6-3-7-11-18)30-24-29-20(25)13-15-31(22)24/h2-16H,1H3,(H2,25,29,30)(H,26,27,28)/t16-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of LPS-induced p38-related TNF-alpha production in human peripheral blood mononuclear cells |
Bioorg Med Chem Lett 13: 347-50 (2003)
BindingDB Entry DOI: 10.7270/Q2G73D2C |
More data for this
Ligand-Target Pair | |
C-C chemokine receptor type 2
(Homo sapiens (Human)) | BDBM50377034
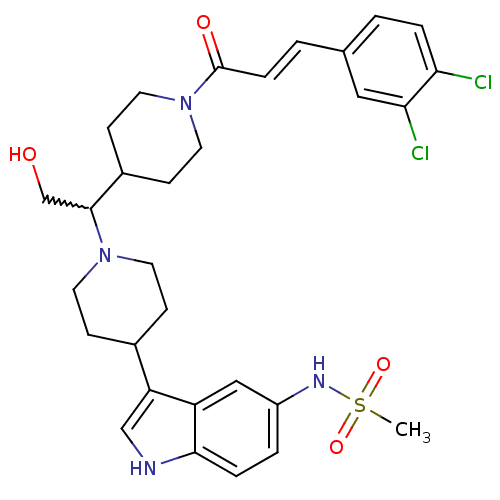
(CHEMBL256301)Show SMILES CS(=O)(=O)Nc1ccc2[nH]cc(C3CCN(CC3)C(CO)C3CCN(CC3)C(=O)\C=C\c3ccc(Cl)c(Cl)c3)c2c1 |w:18.19| Show InChI InChI=1S/C30H36Cl2N4O4S/c1-41(39,40)34-23-4-6-28-24(17-23)25(18-33-28)21-8-12-35(13-9-21)29(19-37)22-10-14-36(15-11-22)30(38)7-3-20-2-5-26(31)27(32)16-20/h2-7,16-18,21-22,29,33-34,37H,8-15,19H2,1H3/b7-3+ | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research and Development
Curated by ChEMBL
| Assay Description
Binding affinity at human CCR2 receptor |
Bioorg Med Chem Lett 18: 3562-4 (2008)
Article DOI: 10.1016/j.bmcl.2008.05.010
BindingDB Entry DOI: 10.7270/Q2Q52QGK |
More data for this
Ligand-Target Pair | |
C-C chemokine receptor type 2
(Homo sapiens (Human)) | BDBM50377039
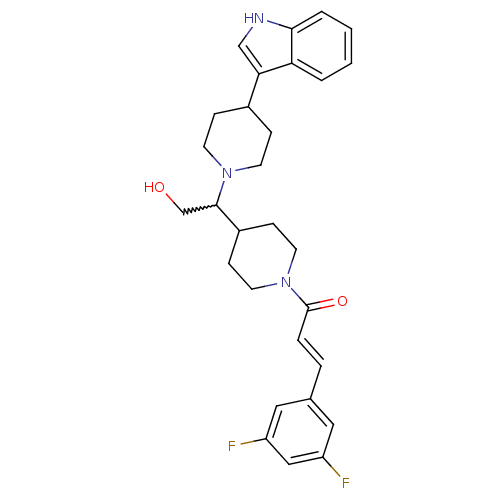
(CHEMBL257191)Show SMILES OCC(C1CCN(CC1)C(=O)\C=C\c1cc(F)cc(F)c1)N1CCC(CC1)c1c[nH]c2ccccc12 |w:2.1| Show InChI InChI=1S/C29H33F2N3O2/c30-23-15-20(16-24(31)17-23)5-6-29(36)34-13-9-22(10-14-34)28(19-35)33-11-7-21(8-12-33)26-18-32-27-4-2-1-3-25(26)27/h1-6,15-18,21-22,28,32,35H,7-14,19H2/b6-5+ | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research and Development
Curated by ChEMBL
| Assay Description
Binding affinity at human CCR2 receptor |
Bioorg Med Chem Lett 18: 3562-4 (2008)
Article DOI: 10.1016/j.bmcl.2008.05.010
BindingDB Entry DOI: 10.7270/Q2Q52QGK |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 11/12/13/14
(Homo sapiens (Human)) | BDBM50215268
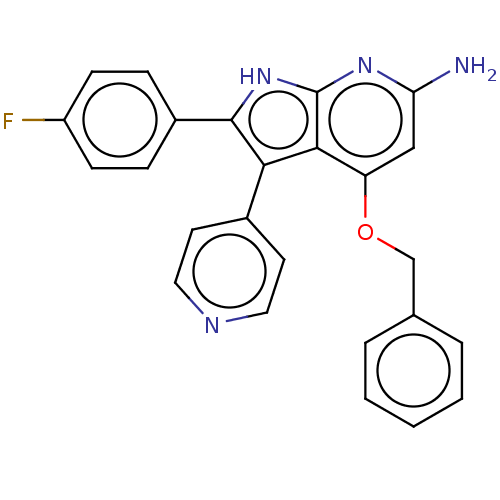
(CHEMBL325211)Show SMILES Nc1cc(OCc2ccccc2)c2c(c([nH]c2n1)-c1ccc(F)cc1)-c1ccncc1 Show InChI InChI=1S/C25H19FN4O/c26-19-8-6-18(7-9-19)24-22(17-10-12-28-13-11-17)23-20(14-21(27)29-25(23)30-24)31-15-16-4-2-1-3-5-16/h1-14H,15H2,(H3,27,29,30) | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 0.910 | n/a | n/a | n/a | n/a | n/a | n/a |
R. W. Johnson Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of TNF-alpha production by lipopolysaccharide-stimulated human peripheral blood mononuclear cells |
Bioorg Med Chem Lett 8: 3335-40 (1998)
BindingDB Entry DOI: 10.7270/Q25T3NNZ |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 14
(Mus musculus (mouse)) | BDBM50122997

(2-Phenyl-3-[2-((S)-1-phenyl-ethylamino)-pyridin-4-...)Show SMILES C[C@H](Nc1cc(ccn1)-c1c(nc2nc(N)ccn12)-c1ccccc1)c1ccccc1 Show InChI InChI=1S/C25H22N6/c1-17(18-8-4-2-5-9-18)28-22-16-20(12-14-27-22)24-23(19-10-6-3-7-11-19)30-25-29-21(26)13-15-31(24)25/h2-17H,1H3,(H,27,28)(H2,26,29,30)/t17-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of murine p38 alpha kinase |
Bioorg Med Chem Lett 13: 347-50 (2003)
BindingDB Entry DOI: 10.7270/Q2G73D2C |
More data for this
Ligand-Target Pair | |
C-C chemokine receptor type 2
(Homo sapiens (Human)) | BDBM50377025

(CHEMBL403889)Show SMILES Nc1ccc2[nH]cc(C3CCN(CC3)C(CO)C3CCN(CC3)C(=O)\C=C\c3cc(F)c(F)c(F)c3)c2c1 |w:14.15| Show InChI InChI=1S/C29H33F3N4O2/c30-24-13-18(14-25(31)29(24)32)1-4-28(38)36-11-7-20(8-12-36)27(17-37)35-9-5-19(6-10-35)23-16-34-26-3-2-21(33)15-22(23)26/h1-4,13-16,19-20,27,34,37H,5-12,17,33H2/b4-1+ | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research and Development
Curated by ChEMBL
| Assay Description
Binding affinity at human CCR2 receptor |
Bioorg Med Chem Lett 18: 3562-4 (2008)
Article DOI: 10.1016/j.bmcl.2008.05.010
BindingDB Entry DOI: 10.7270/Q2Q52QGK |
More data for this
Ligand-Target Pair | |
C-C chemokine receptor type 2
(Homo sapiens (Human)) | BDBM50377026
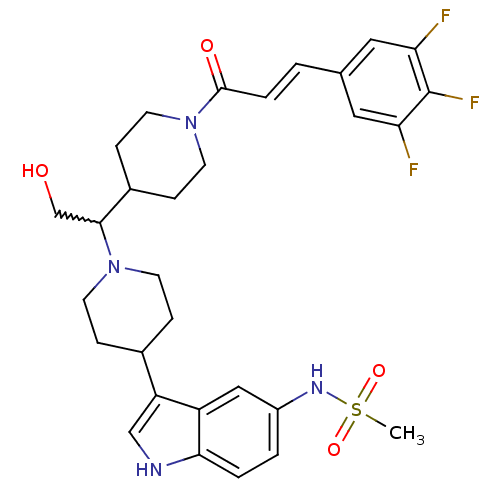
(CHEMBL254772)Show SMILES CS(=O)(=O)Nc1ccc2[nH]cc(C3CCN(CC3)C(CO)C3CCN(CC3)C(=O)\C=C\c3cc(F)c(F)c(F)c3)c2c1 |w:18.19| Show InChI InChI=1S/C30H35F3N4O4S/c1-42(40,41)35-22-3-4-27-23(16-22)24(17-34-27)20-6-10-36(11-7-20)28(18-38)21-8-12-37(13-9-21)29(39)5-2-19-14-25(31)30(33)26(32)15-19/h2-5,14-17,20-21,28,34-35,38H,6-13,18H2,1H3/b5-2+ | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research and Development
Curated by ChEMBL
| Assay Description
Binding affinity at human CCR2 receptor |
Bioorg Med Chem Lett 18: 3562-4 (2008)
Article DOI: 10.1016/j.bmcl.2008.05.010
BindingDB Entry DOI: 10.7270/Q2Q52QGK |
More data for this
Ligand-Target Pair | |
C-C chemokine receptor type 2
(Homo sapiens (Human)) | BDBM50377027
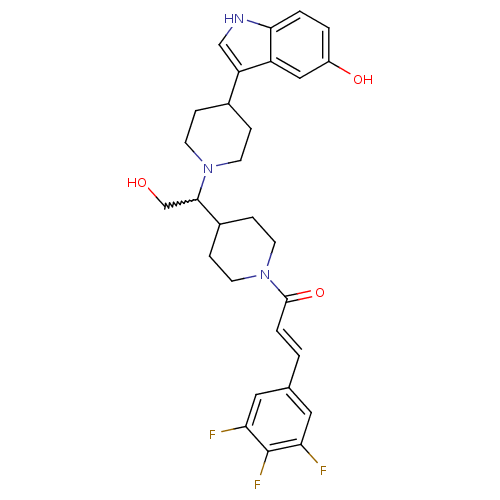
(CHEMBL404904)Show SMILES OCC(C1CCN(CC1)C(=O)\C=C\c1cc(F)c(F)c(F)c1)N1CCC(CC1)c1c[nH]c2ccc(O)cc12 |w:2.1| Show InChI InChI=1S/C29H32F3N3O3/c30-24-13-18(14-25(31)29(24)32)1-4-28(38)35-11-7-20(8-12-35)27(17-36)34-9-5-19(6-10-34)23-16-33-26-3-2-21(37)15-22(23)26/h1-4,13-16,19-20,27,33,36-37H,5-12,17H2/b4-1+ | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research and Development
Curated by ChEMBL
| Assay Description
Binding affinity at human CCR2 receptor |
Bioorg Med Chem Lett 18: 3562-4 (2008)
Article DOI: 10.1016/j.bmcl.2008.05.010
BindingDB Entry DOI: 10.7270/Q2Q52QGK |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase JAK3
(Homo sapiens (Human)) | BDBM50295811
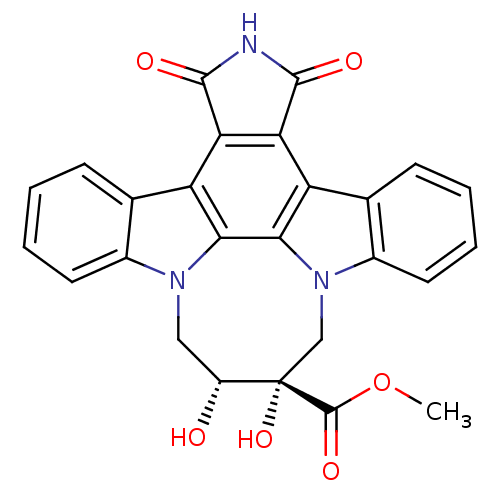
(12,13-[2-carboxymethyl-cis-2,3-dihydroxy)-1,4-buty...)Show SMILES COC(=O)[C@]1(O)Cn2c3ccccc3c3c4C(=O)NC(=O)c4c4c5ccccc5n(C[C@H]1O)c4c23 |r| Show InChI InChI=1S/C26H19N3O6/c1-35-25(33)26(34)11-29-15-9-5-3-7-13(15)18-20-19(23(31)27-24(20)32)17-12-6-2-4-8-14(12)28(10-16(26)30)21(17)22(18)29/h2-9,16,30,34H,10-11H2,1H3,(H,27,31,32)/t16-,26+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development L.L.C. 920 Route 202
Curated by ChEMBL
| Assay Description
Inhibition of JAK3 expressed in insect Sf21 cells assessed as inhibition of biotinylated substrate phosphorylation |
Bioorg Med Chem Lett 19: 3333-8 (2009)
Article DOI: 10.1016/j.bmcl.2009.04.039
BindingDB Entry DOI: 10.7270/Q2ZK5GQ8 |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 11/12/13/14
(Homo sapiens (Human)) | BDBM50067495

(2-(4-Fluoro-phenyl)-4-(3-methoxy-benzyloxy)-3-pyri...)Show SMILES COc1cccc(COc2cc(N)nc3[nH]c(c(-c4ccncc4)c23)-c2ccc(F)cc2)c1 Show InChI InChI=1S/C26H21FN4O2/c1-32-20-4-2-3-16(13-20)15-33-21-14-22(28)30-26-24(21)23(17-9-11-29-12-10-17)25(31-26)18-5-7-19(27)8-6-18/h2-14H,15H2,1H3,(H3,28,30,31) | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a |
R. W. Johnson Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of TNF-alpha production by lipopolysaccharide-stimulated human peripheral blood mononuclear cells |
Bioorg Med Chem Lett 8: 3335-40 (1998)
BindingDB Entry DOI: 10.7270/Q25T3NNZ |
More data for this
Ligand-Target Pair | |
C-C chemokine receptor type 2
(Homo sapiens (Human)) | BDBM50377040
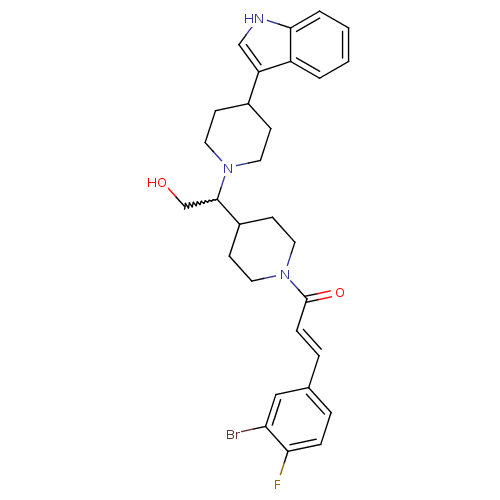
(CHEMBL402442)Show SMILES OCC(C1CCN(CC1)C(=O)\C=C\c1ccc(F)c(Br)c1)N1CCC(CC1)c1c[nH]c2ccccc12 |w:2.1| Show InChI InChI=1S/C29H33BrFN3O2/c30-25-17-20(5-7-26(25)31)6-8-29(36)34-15-11-22(12-16-34)28(19-35)33-13-9-21(10-14-33)24-18-32-27-4-2-1-3-23(24)27/h1-8,17-18,21-22,28,32,35H,9-16,19H2/b8-6+ | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research and Development
Curated by ChEMBL
| Assay Description
Binding affinity at human CCR2 receptor |
Bioorg Med Chem Lett 18: 3562-4 (2008)
Article DOI: 10.1016/j.bmcl.2008.05.010
BindingDB Entry DOI: 10.7270/Q2Q52QGK |
More data for this
Ligand-Target Pair | |
C-C chemokine receptor type 2
(Homo sapiens (Human)) | BDBM50377028

(CHEMBL255302)Show SMILES CC(=O)Nc1ccc2[nH]cc(C3CCN(CC3)C(CO)C3CCN(CC3)C(=O)\C=C\c3cc(F)c(F)c(F)c3)c2c1 |w:17.18| Show InChI InChI=1S/C31H35F3N4O3/c1-19(40)36-23-3-4-28-24(16-23)25(17-35-28)21-6-10-37(11-7-21)29(18-39)22-8-12-38(13-9-22)30(41)5-2-20-14-26(32)31(34)27(33)15-20/h2-5,14-17,21-22,29,35,39H,6-13,18H2,1H3,(H,36,40)/b5-2+ | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research and Development
Curated by ChEMBL
| Assay Description
Binding affinity at human CCR2 receptor |
Bioorg Med Chem Lett 18: 3562-4 (2008)
Article DOI: 10.1016/j.bmcl.2008.05.010
BindingDB Entry DOI: 10.7270/Q2Q52QGK |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 11/12/13/14
(Homo sapiens (Human)) | BDBM50122997

(2-Phenyl-3-[2-((S)-1-phenyl-ethylamino)-pyridin-4-...)Show SMILES C[C@H](Nc1cc(ccn1)-c1c(nc2nc(N)ccn12)-c1ccccc1)c1ccccc1 Show InChI InChI=1S/C25H22N6/c1-17(18-8-4-2-5-9-18)28-22-16-20(12-14-27-22)24-23(19-10-6-3-7-11-19)30-25-29-21(26)13-15-31(24)25/h2-17H,1H3,(H,27,28)(H2,26,29,30)/t17-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of LPS-induced p38-related TNF-alpha production in human peripheral blood mononuclear cells |
Bioorg Med Chem Lett 13: 347-50 (2003)
BindingDB Entry DOI: 10.7270/Q2G73D2C |
More data for this
Ligand-Target Pair | |
C-C chemokine receptor type 2
(Homo sapiens (Human)) | BDBM50224496
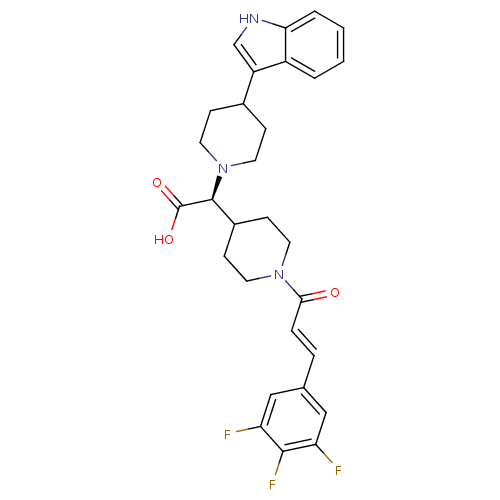
((S,E)-2-(4-(1H-indol-3-yl)piperidin-1-yl)-2-(1-(3-...)Show SMILES OC(=O)[C@H](C1CCN(CC1)C(=O)\C=C\c1cc(F)c(F)c(F)c1)N1CCC(CC1)c1c[nH]c2ccccc12 Show InChI InChI=1S/C29H30F3N3O3/c30-23-15-18(16-24(31)27(23)32)5-6-26(36)34-11-9-20(10-12-34)28(29(37)38)35-13-7-19(8-14-35)22-17-33-25-4-2-1-3-21(22)25/h1-6,15-17,19-20,28,33H,7-14H2,(H,37,38)/b6-5+/t28-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research and Development
Curated by ChEMBL
| Assay Description
Antagonist activity at CCR2 expressed in THP1 cells assessed as MCP1-induced calcium flux |
J Med Chem 50: 5561-3 (2007)
Article DOI: 10.1021/jm070902b
BindingDB Entry DOI: 10.7270/Q24M948Q |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 14
(Mus musculus (mouse)) | BDBM50122995

((S)-3-(2-(1-methoxypropan-2-ylamino)pyridin-4-yl)-...)Show SMILES COC[C@H](C)Nc1cc(ccn1)-c1c(nc2nc(N)ccn12)-c1ccccc1 |r| Show InChI InChI=1S/C21H22N6O/c1-14(13-28-2)24-18-12-16(8-10-23-18)20-19(15-6-4-3-5-7-15)26-21-25-17(22)9-11-27(20)21/h3-12,14H,13H2,1-2H3,(H,23,24)(H2,22,25,26)/t14-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of murine p38 alpha kinase |
Bioorg Med Chem Lett 13: 347-50 (2003)
BindingDB Entry DOI: 10.7270/Q2G73D2C |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 11/12/13/14
(Homo sapiens (Human)) | BDBM50215161
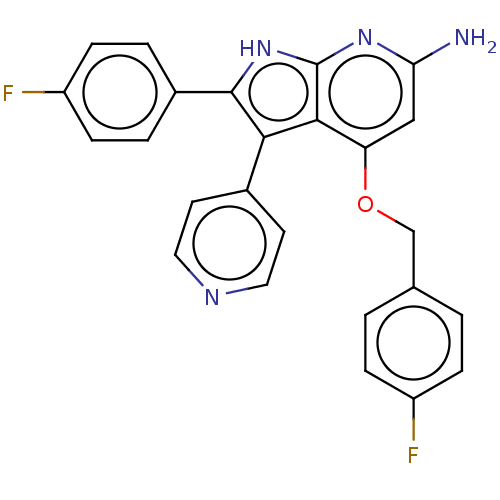
(CHEMBL324468)Show SMILES Nc1cc(OCc2ccc(F)cc2)c2c(c([nH]c2n1)-c1ccc(F)cc1)-c1ccncc1 Show InChI InChI=1S/C25H18F2N4O/c26-18-5-1-15(2-6-18)14-32-20-13-21(28)30-25-23(20)22(16-9-11-29-12-10-16)24(31-25)17-3-7-19(27)8-4-17/h1-13H,14H2,(H3,28,30,31) | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
R. W. Johnson Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of TNF-alpha production by lipopolysaccharide-stimulated human peripheral blood mononuclear cells |
Bioorg Med Chem Lett 8: 3335-40 (1998)
BindingDB Entry DOI: 10.7270/Q25T3NNZ |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 11/12/13/14
(Homo sapiens (Human)) | BDBM50123004
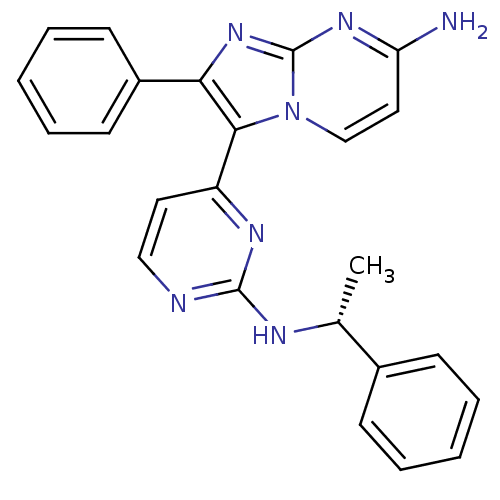
(2-Phenyl-3-[2-((R)-1-phenyl-ethylamino)-pyrimidin-...)Show SMILES C[C@@H](Nc1nccc(n1)-c1c(nc2nc(N)ccn12)-c1ccccc1)c1ccccc1 Show InChI InChI=1S/C24H21N7/c1-16(17-8-4-2-5-9-17)27-23-26-14-12-19(28-23)22-21(18-10-6-3-7-11-18)30-24-29-20(25)13-15-31(22)24/h2-16H,1H3,(H2,25,29,30)(H,26,27,28)/t16-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 2.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of LPS-induced p38-related TNF-alpha production in human peripheral blood mononuclear cells |
Bioorg Med Chem Lett 13: 347-50 (2003)
BindingDB Entry DOI: 10.7270/Q2G73D2C |
More data for this
Ligand-Target Pair | |
C-C chemokine receptor type 2
(Homo sapiens (Human)) | BDBM50239941

((S)-1-(4-(1-(4-(1H-indol-3-yl)piperidin-1-yl)-2-hy...)Show SMILES OC[C@H](C1CCN(CC1)C(=O)\C=C\c1cc(F)c(F)c(F)c1)N1CCC(CC1)c1c[nH]c2ccccc12 Show InChI InChI=1S/C29H32F3N3O2/c30-24-15-19(16-25(31)29(24)32)5-6-28(37)35-13-9-21(10-14-35)27(18-36)34-11-7-20(8-12-34)23-17-33-26-4-2-1-3-22(23)26/h1-6,15-17,20-21,27,33,36H,7-14,18H2/b6-5+/t27-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research and Development
Curated by ChEMBL
| Assay Description
Binding affinity at human CCR2 receptor |
Bioorg Med Chem Lett 18: 3562-4 (2008)
Article DOI: 10.1016/j.bmcl.2008.05.010
BindingDB Entry DOI: 10.7270/Q2Q52QGK |
More data for this
Ligand-Target Pair | |
C-C chemokine receptor type 2
(Homo sapiens (Human)) | BDBM50224501
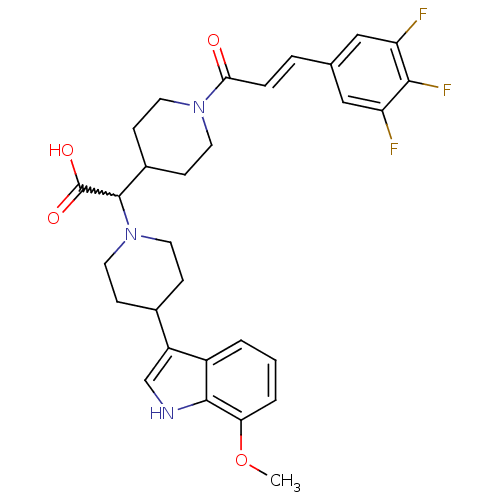
((E)-2-(4-(7-methoxy-1H-indol-3-yl)piperidin-1-yl)-...)Show SMILES COc1cccc2c(c[nH]c12)C1CCN(CC1)C(C1CCN(CC1)C(=O)\C=C\c1cc(F)c(F)c(F)c1)C(O)=O |w:17.41| Show InChI InChI=1S/C30H32F3N3O4/c1-40-25-4-2-3-21-22(17-34-28(21)25)19-7-13-36(14-8-19)29(30(38)39)20-9-11-35(12-10-20)26(37)6-5-18-15-23(31)27(33)24(32)16-18/h2-6,15-17,19-20,29,34H,7-14H2,1H3,(H,38,39)/b6-5+ | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of human CCR2 |
J Med Chem 50: 5561-3 (2007)
Article DOI: 10.1021/jm070902b
BindingDB Entry DOI: 10.7270/Q24M948Q |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 11/12/13/14
(Homo sapiens (Human)) | BDBM50123000
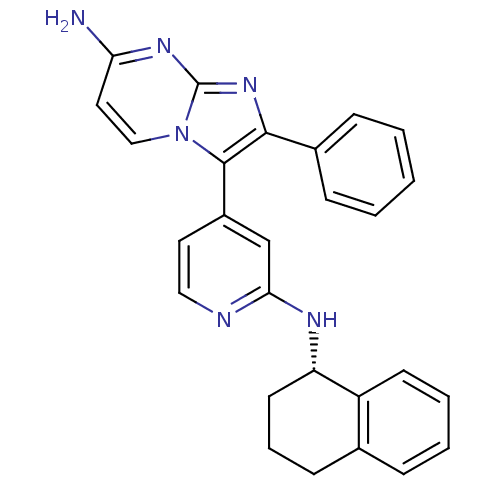
(2-Phenyl-3-{2-[(S)-(1,2,3,4-tetrahydro-naphthalen-...)Show SMILES Nc1ccn2c(c(nc2n1)-c1ccccc1)-c1ccnc(N[C@H]2CCCc3ccccc23)c1 Show InChI InChI=1S/C27H24N6/c28-23-14-16-33-26(25(32-27(33)31-23)19-8-2-1-3-9-19)20-13-15-29-24(17-20)30-22-12-6-10-18-7-4-5-11-21(18)22/h1-5,7-9,11,13-17,22H,6,10,12H2,(H,29,30)(H2,28,31,32)/t22-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of LPS-induced p38-related TNF-alpha production in human peripheral blood mononuclear cells |
Bioorg Med Chem Lett 13: 347-50 (2003)
BindingDB Entry DOI: 10.7270/Q2G73D2C |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase JAK3
(Homo sapiens (Human)) | BDBM50295819
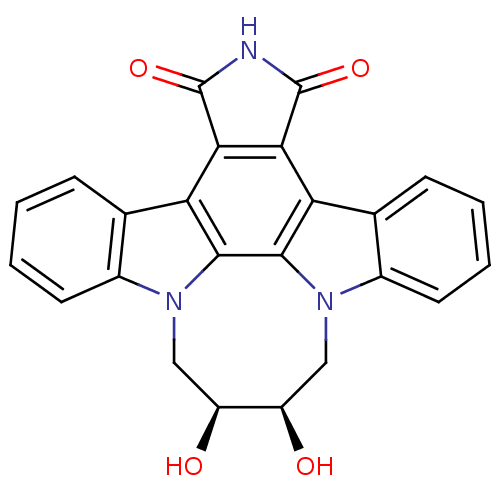
(12,13-(2,3-cis-dihydroxy-butan-1,4-yl)-12,13-dihyd...)Show SMILES O[C@H]1Cn2c3ccccc3c3c4C(=O)NC(=O)c4c4c5ccccc5n(C[C@H]1O)c4c23 |r| Show InChI InChI=1S/C24H17N3O4/c28-15-9-26-13-7-3-1-5-11(13)17-19-20(24(31)25-23(19)30)18-12-6-2-4-8-14(12)27(10-16(15)29)22(18)21(17)26/h1-8,15-16,28-29H,9-10H2,(H,25,30,31)/t15-,16+ | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development L.L.C. 920 Route 202
Curated by ChEMBL
| Assay Description
Inhibition of JAK3 expressed in insect Sf21 cells assessed as inhibition of biotinylated substrate phosphorylation |
Bioorg Med Chem Lett 19: 3333-8 (2009)
Article DOI: 10.1016/j.bmcl.2009.04.039
BindingDB Entry DOI: 10.7270/Q2ZK5GQ8 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase JAK3
(Homo sapiens (Human)) | BDBM50198005

(10,16-bis(hydroxymethyl)-22-[(1E)-3-(1H-imidazol-1...)Show SMILES OCC1Cn2c3ccc(CO)cc3c3c4CNC(=O)c4c4c5cc(\C=C\Cn6ccnc6)ccc5n(C1)c4c23 |w:2.1| Show InChI InChI=1S/C31H27N5O3/c37-15-19-4-6-24-21(11-19)26-23-12-33-31(39)28(23)27-22-10-18(2-1-8-34-9-7-32-17-34)3-5-25(22)36-14-20(16-38)13-35(24)29(26)30(27)36/h1-7,9-11,17,20,37-38H,8,12-16H2,(H,33,39)/b2-1+ | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of JAK3 expressed in Sf21 cells |
Bioorg Med Chem Lett 17: 326-31 (2007)
Article DOI: 10.1016/j.bmcl.2006.10.062
BindingDB Entry DOI: 10.7270/Q2JH3KTP |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase JAK3
(Homo sapiens (Human)) | BDBM50198018
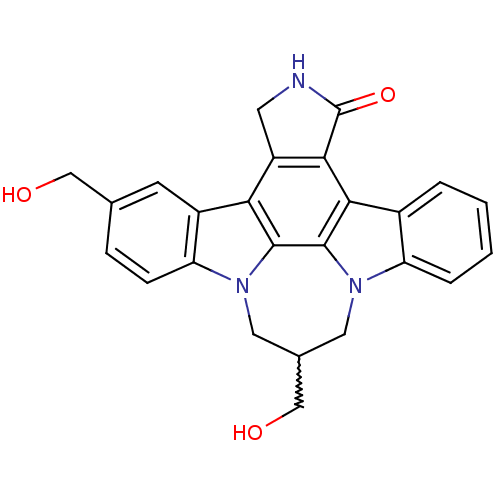
(10,16-bis(hydroxymethyl)-4,14,18-triazaheptacyclo[...)Show SMILES OCC1Cn2c3ccccc3c3c4C(=O)NCc4c4c5cc(CO)ccc5n(C1)c4c23 |w:2.1| Show InChI InChI=1S/C25H21N3O3/c29-11-13-5-6-19-16(7-13)20-17-8-26-25(31)22(17)21-15-3-1-2-4-18(15)27-9-14(12-30)10-28(19)23(20)24(21)27/h1-7,14,29-30H,8-12H2,(H,26,31) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of JAK3 expressed in Sf21 cells |
Bioorg Med Chem Lett 17: 326-31 (2007)
Article DOI: 10.1016/j.bmcl.2006.10.062
BindingDB Entry DOI: 10.7270/Q2JH3KTP |
More data for this
Ligand-Target Pair | |
C-C chemokine receptor type 2
(Homo sapiens (Human)) | BDBM50239941

((S)-1-(4-(1-(4-(1H-indol-3-yl)piperidin-1-yl)-2-hy...)Show SMILES OC[C@H](C1CCN(CC1)C(=O)\C=C\c1cc(F)c(F)c(F)c1)N1CCC(CC1)c1c[nH]c2ccccc12 Show InChI InChI=1S/C29H32F3N3O2/c30-24-15-19(16-25(31)29(24)32)5-6-28(37)35-13-9-21(10-14-35)27(18-36)34-11-7-20(8-12-34)23-17-33-26-4-2-1-3-22(23)26/h1-6,15-17,20-21,27,33,36H,7-14,18H2/b6-5+/t27-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 3.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research and Development
Curated by ChEMBL
| Assay Description
Antagonist activity at CCR2 receptor expressed in THP1 cells assessed as MCP-1-induced calcium flux by chemotoxis assay |
Bioorg Med Chem Lett 18: 3562-4 (2008)
Article DOI: 10.1016/j.bmcl.2008.05.010
BindingDB Entry DOI: 10.7270/Q2Q52QGK |
More data for this
Ligand-Target Pair | |
C-C chemokine receptor type 2
(Homo sapiens (Human)) | BDBM50224496

((S,E)-2-(4-(1H-indol-3-yl)piperidin-1-yl)-2-(1-(3-...)Show SMILES OC(=O)[C@H](C1CCN(CC1)C(=O)\C=C\c1cc(F)c(F)c(F)c1)N1CCC(CC1)c1c[nH]c2ccccc12 Show InChI InChI=1S/C29H30F3N3O3/c30-23-15-18(16-24(31)27(23)32)5-6-26(36)34-11-9-20(10-12-34)28(29(37)38)35-13-7-19(8-14-35)22-17-33-25-4-2-1-3-21(22)25/h1-6,15-17,19-20,28,33H,7-14H2,(H,37,38)/b6-5+/t28-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of human CCR2 |
J Med Chem 50: 5561-3 (2007)
Article DOI: 10.1021/jm070902b
BindingDB Entry DOI: 10.7270/Q24M948Q |
More data for this
Ligand-Target Pair | |
C-C chemokine receptor type 2
(Homo sapiens (Human)) | BDBM50224502
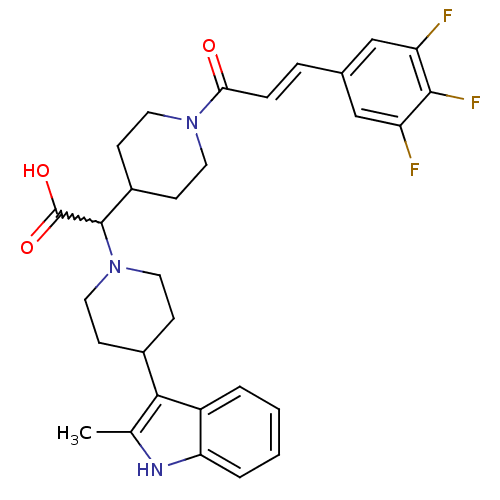
((E)-2-(4-(2-methyl-1H-indol-3-yl)piperidin-1-yl)-2...)Show SMILES Cc1[nH]c2ccccc2c1C1CCN(CC1)C(C1CCN(CC1)C(=O)\C=C\c1cc(F)c(F)c(F)c1)C(O)=O |w:16.40| Show InChI InChI=1S/C30H32F3N3O3/c1-18-27(22-4-2-3-5-25(22)34-18)20-8-14-36(15-9-20)29(30(38)39)21-10-12-35(13-11-21)26(37)7-6-19-16-23(31)28(33)24(32)17-19/h2-7,16-17,20-21,29,34H,8-15H2,1H3,(H,38,39)/b7-6+ | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of human CCR2 |
J Med Chem 50: 5561-3 (2007)
Article DOI: 10.1021/jm070902b
BindingDB Entry DOI: 10.7270/Q24M948Q |
More data for this
Ligand-Target Pair | |
C-C chemokine receptor type 2
(Homo sapiens (Human)) | BDBM50224500

((E)-2-(4-(5-amino-1H-indol-3-yl)piperidin-1-yl)-2-...)Show SMILES Nc1ccc2[nH]cc(C3CCN(CC3)C(C3CCN(CC3)C(=O)\C=C\c3cc(F)c(F)c(F)c3)C(O)=O)c2c1 |w:14.36| Show InChI InChI=1S/C29H31F3N4O3/c30-23-13-17(14-24(31)27(23)32)1-4-26(37)35-9-7-19(8-10-35)28(29(38)39)36-11-5-18(6-12-36)22-16-34-25-3-2-20(33)15-21(22)25/h1-4,13-16,18-19,28,34H,5-12,33H2,(H,38,39)/b4-1+ | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of human CCR2 |
J Med Chem 50: 5561-3 (2007)
Article DOI: 10.1021/jm070902b
BindingDB Entry DOI: 10.7270/Q24M948Q |
More data for this
Ligand-Target Pair | |
C-C chemokine receptor type 2
(Homo sapiens (Human)) | BDBM50246354
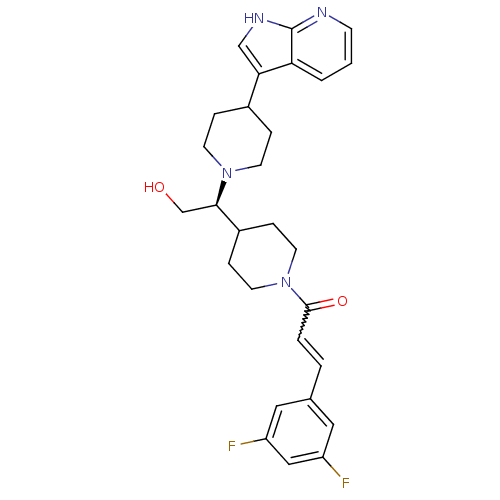
((S)-1-(4-(1-(4-(1H-pyrrolo[2,3-b]pyridin-3-yl)pipe...)Show SMILES OC[C@H](C1CCN(CC1)C(=O)C=Cc1cc(F)cc(F)c1)N1CCC(CC1)c1c[nH]c2ncccc12 |r,w:11.11| Show InChI InChI=1S/C28H32F2N4O2/c29-22-14-19(15-23(30)16-22)3-4-27(36)34-12-7-21(8-13-34)26(18-35)33-10-5-20(6-11-33)25-17-32-28-24(25)2-1-9-31-28/h1-4,9,14-17,20-21,26,35H,5-8,10-13,18H2,(H,31,32)/t26-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research and Development
Curated by ChEMBL
| Assay Description
Antagonist activity at human CCR2 |
Bioorg Med Chem Lett 18: 6468-70 (2008)
Article DOI: 10.1016/j.bmcl.2008.10.061
BindingDB Entry DOI: 10.7270/Q2V40V1J |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 11/12/13/14
(Homo sapiens (Human)) | BDBM50215299

(CHEMBL263536)Show SMILES CCCCOc1cc(N)[nH]c2nc(c(-c3ccncc3)c12)-c1ccc(F)cc1 Show InChI InChI=1S/C22H21FN4O/c1-2-3-12-28-17-13-18(24)26-22-20(17)19(14-8-10-25-11-9-14)21(27-22)15-4-6-16(23)7-5-15/h4-11,13H,2-3,12H2,1H3,(H3,24,26,27) | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
R. W. Johnson Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of TNF-alpha production by lipopolysaccharide-stimulated human peripheral blood mononuclear cells |
Bioorg Med Chem Lett 8: 3335-40 (1998)
BindingDB Entry DOI: 10.7270/Q25T3NNZ |
More data for this
Ligand-Target Pair | |
C-C chemokine receptor type 2
(Homo sapiens (Human)) | BDBM50239941

((S)-1-(4-(1-(4-(1H-indol-3-yl)piperidin-1-yl)-2-hy...)Show SMILES OC[C@H](C1CCN(CC1)C(=O)\C=C\c1cc(F)c(F)c(F)c1)N1CCC(CC1)c1c[nH]c2ccccc12 Show InChI InChI=1S/C29H32F3N3O2/c30-24-15-19(16-25(31)29(24)32)5-6-28(37)35-13-9-21(10-14-35)27(18-36)34-11-7-20(8-12-34)23-17-33-26-4-2-1-3-22(23)26/h1-6,15-17,20-21,27,33,36H,7-14,18H2/b6-5+/t27-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research and Development
Curated by ChEMBL
| Assay Description
Binding affinity at human CCR2 receptor |
Bioorg Med Chem Lett 18: 3562-4 (2008)
Article DOI: 10.1016/j.bmcl.2008.05.010
BindingDB Entry DOI: 10.7270/Q2Q52QGK |
More data for this
Ligand-Target Pair | |
C-C chemokine receptor type 2
(Homo sapiens (Human)) | BDBM50224523
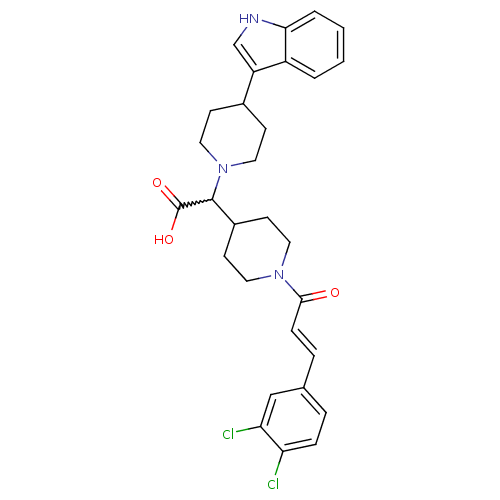
((E)-2-(4-(1H-indol-3-yl)piperidin-1-yl)-2-(1-(3-(3...)Show SMILES OC(=O)C(C1CCN(CC1)C(=O)\C=C\c1ccc(Cl)c(Cl)c1)N1CCC(CC1)c1c[nH]c2ccccc12 |w:3.2| Show InChI InChI=1S/C29H31Cl2N3O3/c30-24-7-5-19(17-25(24)31)6-8-27(35)33-13-11-21(12-14-33)28(29(36)37)34-15-9-20(10-16-34)23-18-32-26-4-2-1-3-22(23)26/h1-8,17-18,20-21,28,32H,9-16H2,(H,36,37)/b8-6+ | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of human CCR2 |
J Med Chem 50: 5561-3 (2007)
Article DOI: 10.1021/jm070902b
BindingDB Entry DOI: 10.7270/Q24M948Q |
More data for this
Ligand-Target Pair | |
C-C chemokine receptor type 2
(Homo sapiens (Human)) | BDBM50224511
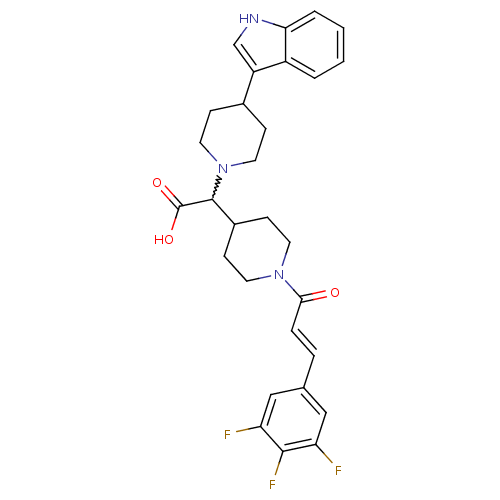
((E)-2-(4-(1H-indol-3-yl)piperidin-1-yl)-2-(1-(3-(3...)Show SMILES OC(=O)C(C1CCN(CC1)C(=O)\C=C\c1cc(F)c(F)c(F)c1)N1CCC(CC1)c1c[nH]c2ccccc12 |w:3.24| Show InChI InChI=1S/C29H30F3N3O3/c30-23-15-18(16-24(31)27(23)32)5-6-26(36)34-11-9-20(10-12-34)28(29(37)38)35-13-7-19(8-14-35)22-17-33-25-4-2-1-3-21(22)25/h1-6,15-17,19-20,28,33H,7-14H2,(H,37,38)/b6-5+ | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research and Development
Curated by ChEMBL
| Assay Description
Binding affinity at human CCR2 receptor |
Bioorg Med Chem Lett 18: 3562-4 (2008)
Article DOI: 10.1016/j.bmcl.2008.05.010
BindingDB Entry DOI: 10.7270/Q2Q52QGK |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase JAK3
(Homo sapiens (Human)) | BDBM50295821
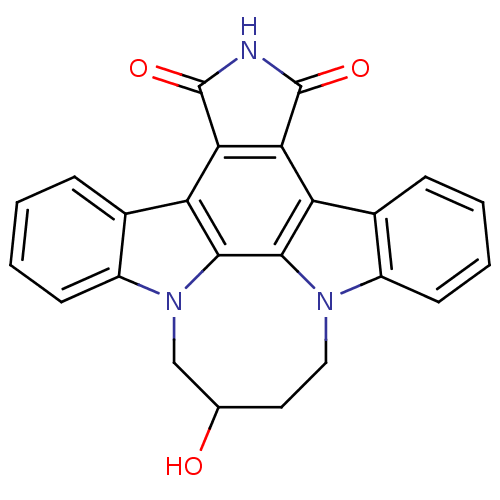
(12,13-(2-hydroxy-butan-1,4-yl)-12,13-dihydro-5,7-d...)Show SMILES OC1CCn2c3ccccc3c3c4C(=O)NC(=O)c4c4c5ccccc5n(C1)c4c23 Show InChI InChI=1S/C24H17N3O3/c28-12-9-10-26-15-7-3-1-5-13(15)17-19-20(24(30)25-23(19)29)18-14-6-2-4-8-16(14)27(11-12)22(18)21(17)26/h1-8,12,28H,9-11H2,(H,25,29,30) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development L.L.C. 920 Route 202
Curated by ChEMBL
| Assay Description
Inhibition of JAK3 expressed in insect Sf21 cells assessed as inhibition of biotinylated substrate phosphorylation |
Bioorg Med Chem Lett 19: 3333-8 (2009)
Article DOI: 10.1016/j.bmcl.2009.04.039
BindingDB Entry DOI: 10.7270/Q2ZK5GQ8 |
More data for this
Ligand-Target Pair | |
C-C chemokine receptor type 2
(Homo sapiens (Human)) | BDBM50224511

((E)-2-(4-(1H-indol-3-yl)piperidin-1-yl)-2-(1-(3-(3...)Show SMILES OC(=O)C(C1CCN(CC1)C(=O)\C=C\c1cc(F)c(F)c(F)c1)N1CCC(CC1)c1c[nH]c2ccccc12 |w:3.24| Show InChI InChI=1S/C29H30F3N3O3/c30-23-15-18(16-24(31)27(23)32)5-6-26(36)34-11-9-20(10-12-34)28(29(37)38)35-13-7-19(8-14-35)22-17-33-25-4-2-1-3-21(22)25/h1-6,15-17,19-20,28,33H,7-14H2,(H,37,38)/b6-5+ | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of human CCR2 |
J Med Chem 50: 5561-3 (2007)
Article DOI: 10.1021/jm070902b
BindingDB Entry DOI: 10.7270/Q24M948Q |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase JAK3
(Homo sapiens (Human)) | BDBM50059889

((staurosporine)3-methoxy-2-methyl-4-methylamino-(2...)Show SMILES CN[C@@H]1CC2O[C@@](C)([C@@H]1OC)n1c3ccccc3c3c4CNC(=O)c4c4c5ccccc5n2c4c13 Show InChI InChI=1S/C28H26N4O3/c1-28-26(34-3)17(29-2)12-20(35-28)31-18-10-6-4-8-14(18)22-23-16(13-30-27(23)33)21-15-9-5-7-11-19(15)32(28)25(21)24(22)31/h4-11,17,20,26,29H,12-13H2,1-3H3,(H,30,33)/t17-,20?,26-,28+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development L.L.C. 920 Route 202
Curated by ChEMBL
| Assay Description
Inhibition of JAK3 expressed in insect Sf21 cells assessed as inhibition of biotinylated substrate phosphorylation |
Bioorg Med Chem Lett 19: 3333-8 (2009)
Article DOI: 10.1016/j.bmcl.2009.04.039
BindingDB Entry DOI: 10.7270/Q2ZK5GQ8 |
More data for this
Ligand-Target Pair | |
C-C chemokine receptor type 2
(Homo sapiens (Human)) | BDBM50377029

(CHEMBL255499)Show SMILES COc1ccc2[nH]cc(C3CCN(CC3)C(CO)C3CCN(CC3)C(=O)\C=C\c3cc(F)c(F)c(F)c3)c2c1 |w:15.16| Show InChI InChI=1S/C30H34F3N3O3/c1-39-22-3-4-27-23(16-22)24(17-34-27)20-6-10-35(11-7-20)28(18-37)21-8-12-36(13-9-21)29(38)5-2-19-14-25(31)30(33)26(32)15-19/h2-5,14-17,20-21,28,34,37H,6-13,18H2,1H3/b5-2+ | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research and Development
Curated by ChEMBL
| Assay Description
Binding affinity at human CCR2 receptor |
Bioorg Med Chem Lett 18: 3562-4 (2008)
Article DOI: 10.1016/j.bmcl.2008.05.010
BindingDB Entry DOI: 10.7270/Q2Q52QGK |
More data for this
Ligand-Target Pair | |
C-C chemokine receptor type 2
(Homo sapiens (Human)) | BDBM50377041

(CHEMBL257173)Show SMILES OCC(C1CCN(CC1)C(=O)\C=C\c1ccc(F)c(F)c1)N1CCC(CC1)c1c[nH]c2ccccc12 |w:2.1| Show InChI InChI=1S/C29H33F2N3O2/c30-25-7-5-20(17-26(25)31)6-8-29(36)34-15-11-22(12-16-34)28(19-35)33-13-9-21(10-14-33)24-18-32-27-4-2-1-3-23(24)27/h1-8,17-18,21-22,28,32,35H,9-16,19H2/b8-6+ | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research and Development
Curated by ChEMBL
| Assay Description
Binding affinity at human CCR2 receptor |
Bioorg Med Chem Lett 18: 3562-4 (2008)
Article DOI: 10.1016/j.bmcl.2008.05.010
BindingDB Entry DOI: 10.7270/Q2Q52QGK |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 11/12/13/14
(Homo sapiens (Human)) | BDBM50123003
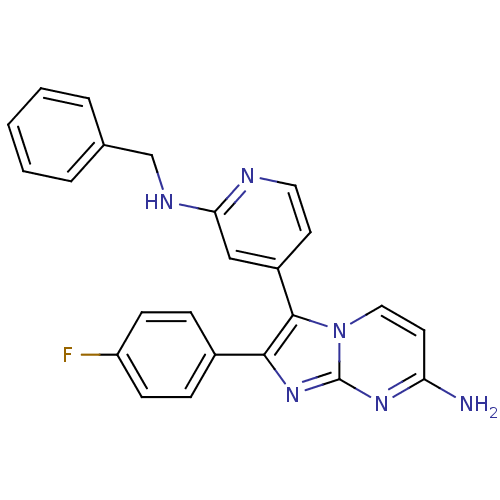
(3-(2-(benzylamino)pyridin-4-yl)-2-(4-fluorophenyl)...)Show SMILES Nc1ccn2c(c(nc2n1)-c1ccc(F)cc1)-c1ccnc(NCc2ccccc2)c1 Show InChI InChI=1S/C24H19FN6/c25-19-8-6-17(7-9-19)22-23(31-13-11-20(26)29-24(31)30-22)18-10-12-27-21(14-18)28-15-16-4-2-1-3-5-16/h1-14H,15H2,(H,27,28)(H2,26,29,30) | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of LPS-induced p38-related TNF-alpha production in human peripheral blood mononuclear cells |
Bioorg Med Chem Lett 13: 347-50 (2003)
BindingDB Entry DOI: 10.7270/Q2G73D2C |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 11/12/13/14
(Homo sapiens (Human)) | BDBM50215274
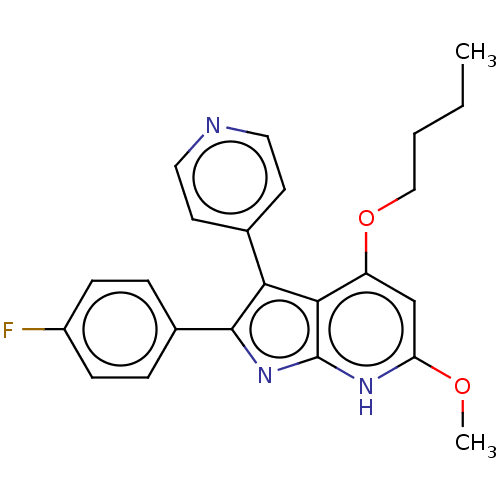
(CHEMBL332917)Show SMILES CCCCOc1cc(OC)[nH]c2nc(c(-c3ccncc3)c12)-c1ccc(F)cc1 Show InChI InChI=1S/C23H22FN3O2/c1-3-4-13-29-18-14-19(28-2)26-23-21(18)20(15-9-11-25-12-10-15)22(27-23)16-5-7-17(24)8-6-16/h5-12,14H,3-4,13H2,1-2H3,(H,26,27) | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
R. W. Johnson Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of TNF-alpha production by lipopolysaccharide-stimulated human peripheral blood mononuclear cells |
Bioorg Med Chem Lett 8: 3335-40 (1998)
BindingDB Entry DOI: 10.7270/Q25T3NNZ |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 14
(Mus musculus (mouse)) | BDBM50123003

(3-(2-(benzylamino)pyridin-4-yl)-2-(4-fluorophenyl)...)Show SMILES Nc1ccn2c(c(nc2n1)-c1ccc(F)cc1)-c1ccnc(NCc2ccccc2)c1 Show InChI InChI=1S/C24H19FN6/c25-19-8-6-17(7-9-19)22-23(31-13-11-20(26)29-24(31)30-22)18-10-12-27-21(14-18)28-15-16-4-2-1-3-5-16/h1-14H,15H2,(H,27,28)(H2,26,29,30) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of murine p38 alpha kinase |
Bioorg Med Chem Lett 13: 347-50 (2003)
BindingDB Entry DOI: 10.7270/Q2G73D2C |
More data for this
Ligand-Target Pair | |
C-C chemokine receptor type 2
(Homo sapiens (Human)) | BDBM50246352
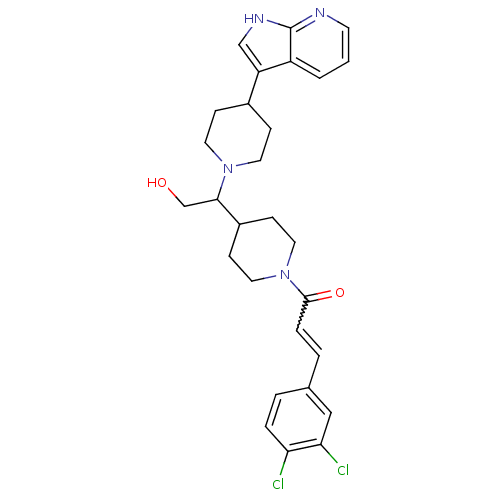
((+/-)-1-(4-(1-(4-(1H-pyrrolo[2,3-b]pyridin-3-yl)pi...)Show SMILES OCC(C1CCN(CC1)C(=O)C=Cc1ccc(Cl)c(Cl)c1)N1CCC(CC1)c1c[nH]c2ncccc12 |w:11.11| Show InChI InChI=1S/C28H32Cl2N4O2/c29-24-5-3-19(16-25(24)30)4-6-27(36)34-14-9-21(10-15-34)26(18-35)33-12-7-20(8-13-33)23-17-32-28-22(23)2-1-11-31-28/h1-6,11,16-17,20-21,26,35H,7-10,12-15,18H2,(H,31,32) | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research and Development
Curated by ChEMBL
| Assay Description
Antagonist activity at human CCR2 |
Bioorg Med Chem Lett 18: 6468-70 (2008)
Article DOI: 10.1016/j.bmcl.2008.10.061
BindingDB Entry DOI: 10.7270/Q2V40V1J |
More data for this
Ligand-Target Pair | |
C-C chemokine receptor type 2
(Homo sapiens (Human)) | BDBM50246351
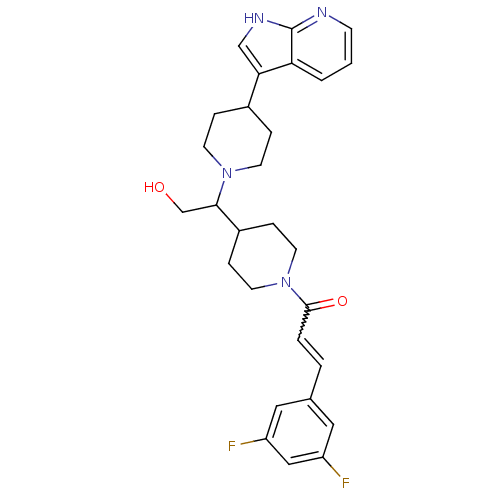
((+/-)-1-(4-(1-(4-(1H-pyrrolo[2,3-b]pyridin-3-yl)pi...)Show SMILES OCC(C1CCN(CC1)C(=O)C=Cc1cc(F)cc(F)c1)N1CCC(CC1)c1c[nH]c2ncccc12 |w:11.11| Show InChI InChI=1S/C28H32F2N4O2/c29-22-14-19(15-23(30)16-22)3-4-27(36)34-12-7-21(8-13-34)26(18-35)33-10-5-20(6-11-33)25-17-32-28-24(25)2-1-9-31-28/h1-4,9,14-17,20-21,26,35H,5-8,10-13,18H2,(H,31,32) | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research and Development
Curated by ChEMBL
| Assay Description
Antagonist activity at human CCR2 |
Bioorg Med Chem Lett 18: 6468-70 (2008)
Article DOI: 10.1016/j.bmcl.2008.10.061
BindingDB Entry DOI: 10.7270/Q2V40V1J |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase JAK3
(Homo sapiens (Human)) | BDBM50059889

((staurosporine)3-methoxy-2-methyl-4-methylamino-(2...)Show SMILES CN[C@@H]1CC2O[C@@](C)([C@@H]1OC)n1c3ccccc3c3c4CNC(=O)c4c4c5ccccc5n2c4c13 Show InChI InChI=1S/C28H26N4O3/c1-28-26(34-3)17(29-2)12-20(35-28)31-18-10-6-4-8-14(18)22-23-16(13-30-27(23)33)21-15-9-5-7-11-19(15)32(28)25(21)24(22)31/h4-11,17,20,26,29H,12-13H2,1-3H3,(H,30,33)/t17-,20?,26-,28+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of JAK3 expressed in Sf21 cells |
Bioorg Med Chem Lett 17: 326-31 (2007)
Article DOI: 10.1016/j.bmcl.2006.10.062
BindingDB Entry DOI: 10.7270/Q2JH3KTP |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase JAK3
(Homo sapiens (Human)) | BDBM50295809
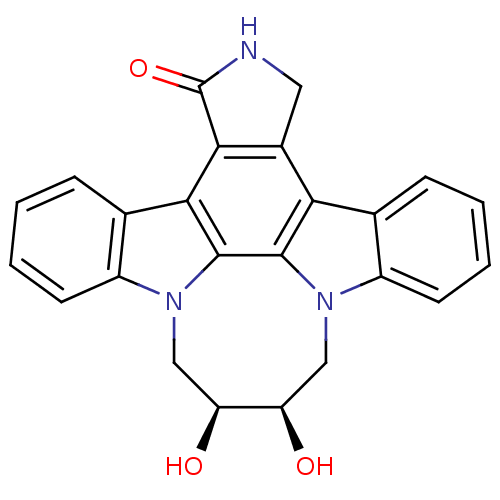
(12,13-(2,3-Cis-dihyroxy-1,4-butyl)-6,7,12,13-tetra...)Show SMILES O[C@@H]1Cn2c3ccccc3c3c4CNC(=O)c4c4c5ccccc5n(C[C@@H]1O)c4c23 |r| Show InChI InChI=1S/C24H19N3O3/c28-17-10-26-15-7-3-1-5-12(15)19-14-9-25-24(30)21(14)20-13-6-2-4-8-16(13)27(11-18(17)29)23(20)22(19)26/h1-8,17-18,28-29H,9-11H2,(H,25,30)/t17-,18+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development L.L.C. 920 Route 202
Curated by ChEMBL
| Assay Description
Inhibition of JAK3 expressed in insect Sf21 cells assessed as inhibition of biotinylated substrate phosphorylation |
Bioorg Med Chem Lett 19: 3333-8 (2009)
Article DOI: 10.1016/j.bmcl.2009.04.039
BindingDB Entry DOI: 10.7270/Q2ZK5GQ8 |
More data for this
Ligand-Target Pair | |
C-C chemokine receptor type 2
(Homo sapiens (Human)) | BDBM50224524
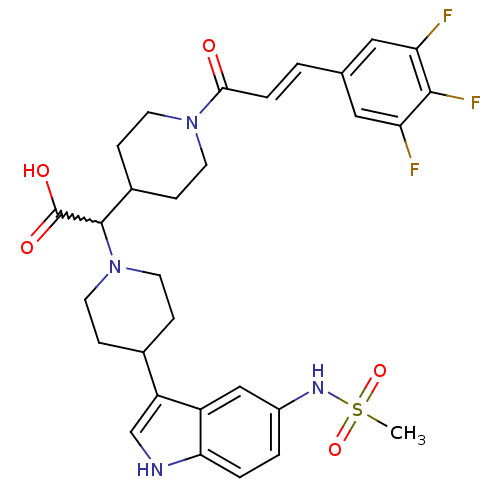
((E)-2-(4-(5-(methylsulfonamido)-1H-indol-3-yl)pipe...)Show SMILES CS(=O)(=O)Nc1ccc2[nH]cc(C3CCN(CC3)C(C3CCN(CC3)C(=O)\C=C\c3cc(F)c(F)c(F)c3)C(O)=O)c2c1 |w:18.40| Show InChI InChI=1S/C30H33F3N4O5S/c1-43(41,42)35-21-3-4-26-22(16-21)23(17-34-26)19-6-12-37(13-7-19)29(30(39)40)20-8-10-36(11-9-20)27(38)5-2-18-14-24(31)28(33)25(32)15-18/h2-5,14-17,19-20,29,34-35H,6-13H2,1H3,(H,39,40)/b5-2+ | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 6.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of human CCR2 |
J Med Chem 50: 5561-3 (2007)
Article DOI: 10.1021/jm070902b
BindingDB Entry DOI: 10.7270/Q24M948Q |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 11/12/13/14
(Homo sapiens (Human)) | BDBM50215267
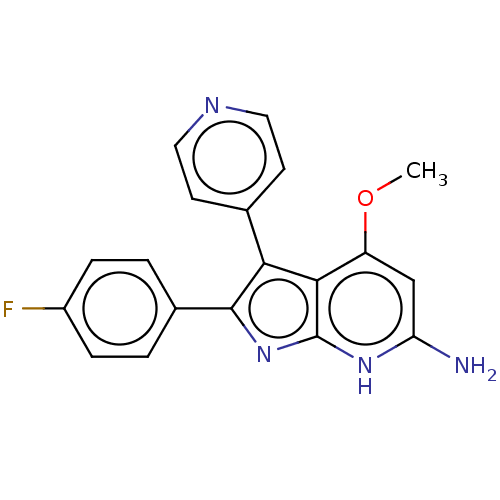
(CHEMBL115769 | RWJ-68354)Show SMILES COc1cc(N)[nH]c2nc(c(-c3ccncc3)c12)-c1ccc(F)cc1 Show InChI InChI=1S/C19H15FN4O/c1-25-14-10-15(21)23-19-17(14)16(11-6-8-22-9-7-11)18(24-19)12-2-4-13(20)5-3-12/h2-10H,1H3,(H3,21,23,24) | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 6.30 | n/a | n/a | n/a | n/a | n/a | n/a |
R. W. Johnson Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of TNF-alpha production by lipopolysaccharide-stimulated human peripheral blood mononuclear cells |
Bioorg Med Chem Lett 8: 3335-40 (1998)
BindingDB Entry DOI: 10.7270/Q25T3NNZ |
More data for this
Ligand-Target Pair | |
C-C chemokine receptor type 2
(Homo sapiens (Human)) | BDBM50377035
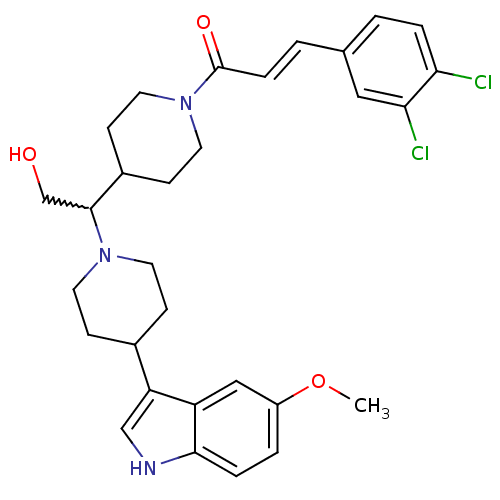
(CHEMBL257628)Show SMILES COc1ccc2[nH]cc(C3CCN(CC3)C(CO)C3CCN(CC3)C(=O)\C=C\c3ccc(Cl)c(Cl)c3)c2c1 |w:15.16| Show InChI InChI=1S/C30H35Cl2N3O3/c1-38-23-4-6-28-24(17-23)25(18-33-28)21-8-12-34(13-9-21)29(19-36)22-10-14-35(15-11-22)30(37)7-3-20-2-5-26(31)27(32)16-20/h2-7,16-18,21-22,29,33,36H,8-15,19H2,1H3/b7-3+ | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research and Development
Curated by ChEMBL
| Assay Description
Binding affinity at human CCR2 receptor |
Bioorg Med Chem Lett 18: 3562-4 (2008)
Article DOI: 10.1016/j.bmcl.2008.05.010
BindingDB Entry DOI: 10.7270/Q2Q52QGK |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 11/12/13/14
(Homo sapiens (Human)) | BDBM15457
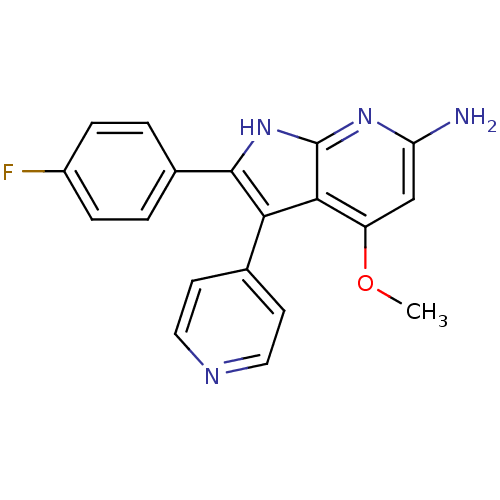
(2-(4-fluorophenyl)-4-methoxy-3-(pyridin-4-yl)-1H-p...)Show SMILES COc1cc(N)nc2[nH]c(c(-c3ccncc3)c12)-c1ccc(F)cc1 Show InChI InChI=1S/C19H15FN4O/c1-25-14-10-15(21)23-19-17(14)16(11-6-8-22-9-7-11)18(24-19)12-2-4-13(20)5-3-12/h2-10H,1H3,(H3,21,23,24) | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of LPS-induced p38-related TNF-alpha production in human peripheral blood mononuclear cells |
Bioorg Med Chem Lett 13: 347-50 (2003)
BindingDB Entry DOI: 10.7270/Q2G73D2C |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data