 Found 100 hits with Last Name = 'chang' and Initial = 'z'
Found 100 hits with Last Name = 'chang' and Initial = 'z' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Heat shock protein HSP 90-alpha
(Homo sapiens (Human)) | BDBM81917
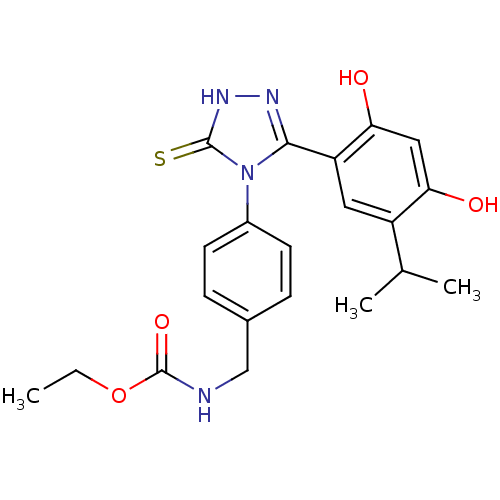
(BX-2819)Show SMILES CCOC(=O)NCc1ccc(cc1)-n1c(n[nH]c1=S)-c1cc(C(C)C)c(O)cc1O Show InChI InChI=1S/C21H24N4O4S/c1-4-29-21(28)22-11-13-5-7-14(8-6-13)25-19(23-24-20(25)30)16-9-15(12(2)3)17(26)10-18(16)27/h5-10,12,26-27H,4,11H2,1-3H3,(H,22,28)(H,24,30) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.0400 | n/a | n/a | n/a | n/a | 7.4 | n/a |
Bayer Healthcare,
| Assay Description
To assess the affinity of compounds binding to Hsp90, we measured their ability to compete with the binding of a fluorescent analog of GA (GM-Bodipy)... |
Chem Biol Drug Des 74: 43-50 (2009)
Article DOI: 10.1111/j.1747-0285.2009.00833.x
BindingDB Entry DOI: 10.7270/Q2KW5DJW |
More data for this
Ligand-Target Pair | |
Heat shock protein HSP 90-alpha
(Homo sapiens (Human)) | BDBM81914
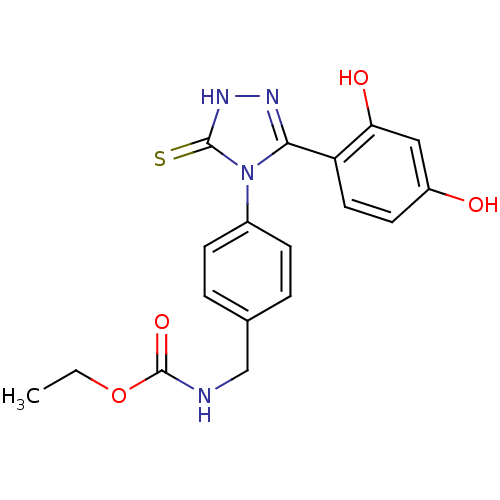
(Ethyl carbamate analog, 3)Show SMILES CCOC(=O)NCc1ccc(cc1)-n1c(n[nH]c1=S)-c1ccc(O)cc1O Show InChI InChI=1S/C18H18N4O4S/c1-2-26-18(25)19-10-11-3-5-12(6-4-11)22-16(20-21-17(22)27)14-8-7-13(23)9-15(14)24/h3-9,23-24H,2,10H2,1H3,(H,19,25)(H,21,27) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.100 | n/a | n/a | n/a | n/a | 7.4 | n/a |
Bayer Healthcare,
| Assay Description
To assess the affinity of compounds binding to Hsp90, we measured their ability to compete with the binding of a fluorescent analog of GA (GM-Bodipy)... |
Chem Biol Drug Des 74: 43-50 (2009)
Article DOI: 10.1111/j.1747-0285.2009.00833.x
BindingDB Entry DOI: 10.7270/Q2KW5DJW |
More data for this
Ligand-Target Pair | |
Heat shock protein HSP 90-alpha
(Homo sapiens (Human)) | BDBM81916
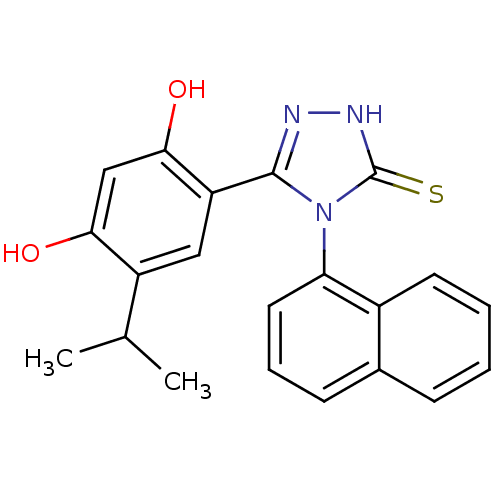
(lspropyl analog, 5)Show SMILES CC(C)c1cc(-c2n[nH]c(=S)n2-c2cccc3ccccc23)c(O)cc1O |(-.66,2.94,;.68,3.71,;.68,5.25,;2.01,2.94,;3.35,3.71,;4.68,2.94,;5.88,3.91,;7.36,3.51,;8.2,4.8,;7.23,6,;7.63,7.48,;5.8,5.45,;4.46,6.22,;3.13,5.44,;1.79,6.21,;1.79,7.76,;3.13,8.53,;3.13,10.07,;4.46,10.84,;5.8,10.07,;5.8,8.53,;4.46,7.76,;4.68,1.4,;6.01,.63,;3.35,.63,;2.01,1.4,;.68,.63,)| Show InChI InChI=1S/C21H19N3O2S/c1-12(2)15-10-16(19(26)11-18(15)25)20-22-23-21(27)24(20)17-9-5-7-13-6-3-4-8-14(13)17/h3-12,25-26H,1-2H3,(H,23,27) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.100 | n/a | n/a | n/a | n/a | 7.4 | n/a |
Bayer Healthcare,
| Assay Description
To assess the affinity of compounds binding to Hsp90, we measured their ability to compete with the binding of a fluorescent analog of GA (GM-Bodipy)... |
Chem Biol Drug Des 74: 43-50 (2009)
Article DOI: 10.1111/j.1747-0285.2009.00833.x
BindingDB Entry DOI: 10.7270/Q2KW5DJW |
More data for this
Ligand-Target Pair | |
Heat shock protein HSP 90-alpha
(Homo sapiens (Human)) | BDBM81915
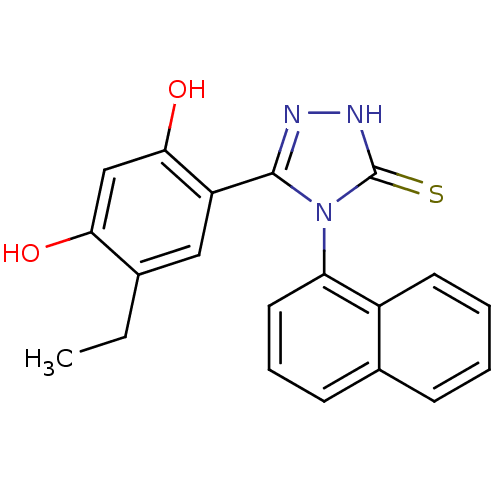
(Ethyl analog, 4)Show SMILES CCc1cc(-c2n[nH]c(=S)n2-c2cccc3ccccc23)c(O)cc1O |(-.66,2.94,;.68,3.71,;2.01,2.94,;3.35,3.71,;4.68,2.94,;5.88,3.91,;7.36,3.51,;8.2,4.8,;7.23,6,;7.63,7.48,;5.8,5.45,;4.46,6.22,;3.13,5.44,;1.79,6.21,;1.79,7.76,;3.13,8.53,;3.13,10.07,;4.46,10.84,;5.8,10.07,;5.8,8.53,;4.46,7.76,;4.68,1.4,;6.01,.63,;3.35,.63,;2.01,1.4,;.68,.63,)| Show InChI InChI=1S/C20H17N3O2S/c1-2-12-10-15(18(25)11-17(12)24)19-21-22-20(26)23(19)16-9-5-7-13-6-3-4-8-14(13)16/h3-11,24-25H,2H2,1H3,(H,22,26) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | 7.4 | n/a |
Bayer Healthcare,
| Assay Description
To assess the affinity of compounds binding to Hsp90, we measured their ability to compete with the binding of a fluorescent analog of GA (GM-Bodipy)... |
Chem Biol Drug Des 74: 43-50 (2009)
Article DOI: 10.1111/j.1747-0285.2009.00833.x
BindingDB Entry DOI: 10.7270/Q2KW5DJW |
More data for this
Ligand-Target Pair | |
Heat shock protein HSP 90-alpha
(Homo sapiens (Human)) | BDBM81912
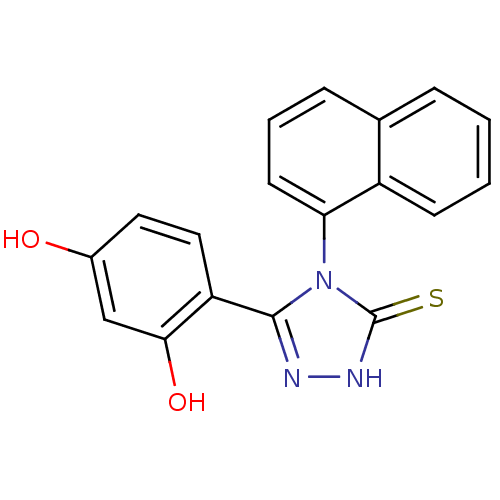
(DC23 | Resorcinol analog, 1)Show SMILES Oc1ccc(-c2n[nH]c(=S)n2-c2cccc3ccccc23)c(O)c1 |(.68,.63,;2.01,1.4,;2.01,2.94,;3.35,3.71,;4.68,2.94,;5.88,3.91,;7.36,3.51,;8.2,4.8,;7.23,6,;7.63,7.48,;5.8,5.45,;4.46,6.22,;3.13,5.44,;1.79,6.21,;1.79,7.76,;3.13,8.53,;3.13,10.06,;4.46,10.84,;5.8,10.07,;5.8,8.52,;4.46,7.76,;4.68,1.4,;6.01,.63,;3.35,.63,)| Show InChI InChI=1S/C18H13N3O2S/c22-12-8-9-14(16(23)10-12)17-19-20-18(24)21(17)15-7-3-5-11-4-1-2-6-13(11)15/h1-10,22-23H,(H,20,24) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | 7.4 | n/a |
Bayer Healthcare,
| Assay Description
To assess the affinity of compounds binding to Hsp90, we measured their ability to compete with the binding of a fluorescent analog of GA (GM-Bodipy)... |
Chem Biol Drug Des 74: 43-50 (2009)
Article DOI: 10.1111/j.1747-0285.2009.00833.x
BindingDB Entry DOI: 10.7270/Q2KW5DJW |
More data for this
Ligand-Target Pair | |
Heat shock protein HSP 90-alpha
(Homo sapiens (Human)) | BDBM81913
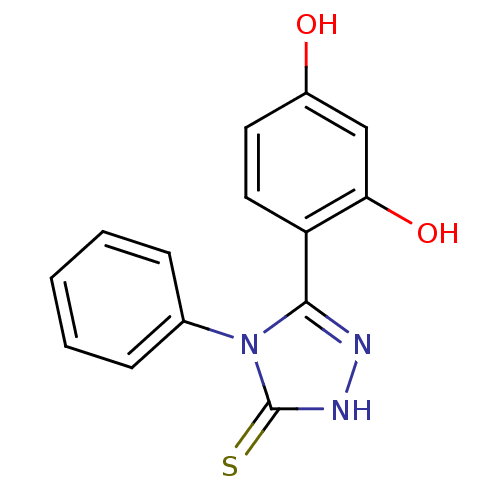
(Unsubstituted phenyl ring analog, 2)Show InChI InChI=1S/C14H11N3O2S/c18-10-6-7-11(12(19)8-10)13-15-16-14(20)17(13)9-4-2-1-3-5-9/h1-8,18-19H,(H,16,20) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | 7.4 | n/a |
Bayer Healthcare,
| Assay Description
To assess the affinity of compounds binding to Hsp90, we measured their ability to compete with the binding of a fluorescent analog of GA (GM-Bodipy)... |
Chem Biol Drug Des 74: 43-50 (2009)
Article DOI: 10.1111/j.1747-0285.2009.00833.x
BindingDB Entry DOI: 10.7270/Q2KW5DJW |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM50312614
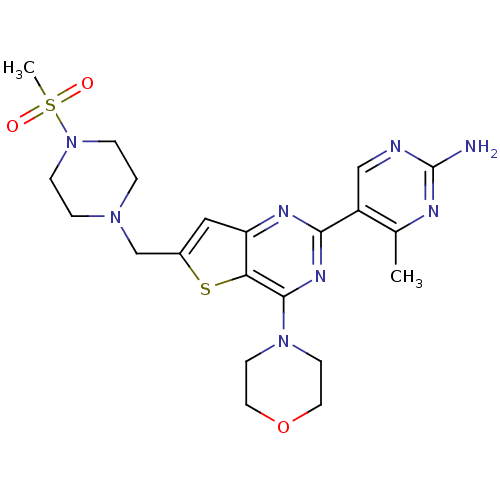
(4-methyl-5-(6-{[4-(methylsulfonyl)piperazin-1-yl]m...)Show SMILES Cc1nc(N)ncc1-c1nc(N2CCOCC2)c2sc(CN3CCN(CC3)S(C)(=O)=O)cc2n1 Show InChI InChI=1S/C21H28N8O3S2/c1-14-16(12-23-21(22)24-14)19-25-17-11-15(13-27-3-5-29(6-4-27)34(2,30)31)33-18(17)20(26-19)28-7-9-32-10-8-28/h11-12H,3-10,13H2,1-2H3,(H2,22,23,24) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Inhibition of recombinant PI3Kdelta assessed as PIP3 product formation by fluorescence polarization assay |
J Med Chem 53: 1086-97 (2010)
Article DOI: 10.1021/jm901284w
BindingDB Entry DOI: 10.7270/Q2FJ2GXT |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM50312612
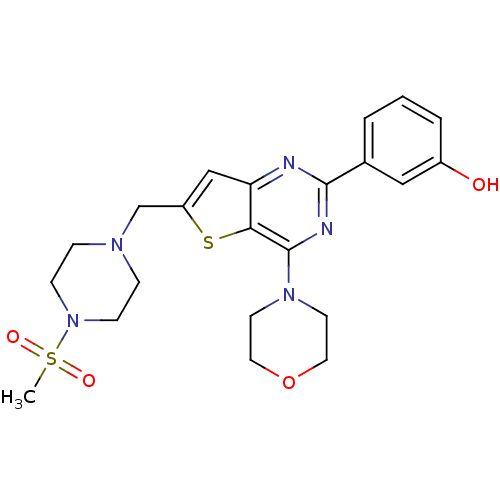
(3-[6-(4-Methanesulfonyl-piperazin-1-ylmethyl)-4-mo...)Show SMILES CS(=O)(=O)N1CCN(Cc2cc3nc(nc(N4CCOCC4)c3s2)-c2cccc(O)c2)CC1 Show InChI InChI=1S/C22H27N5O4S2/c1-33(29,30)27-7-5-25(6-8-27)15-18-14-19-20(32-18)22(26-9-11-31-12-10-26)24-21(23-19)16-3-2-4-17(28)13-16/h2-4,13-14,28H,5-12,15H2,1H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Inhibition of recombinant PI3Kdelta assessed as PIP3 product formation by fluorescence polarization assay |
J Med Chem 53: 1086-97 (2010)
Article DOI: 10.1021/jm901284w
BindingDB Entry DOI: 10.7270/Q2FJ2GXT |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50312614

(4-methyl-5-(6-{[4-(methylsulfonyl)piperazin-1-yl]m...)Show SMILES Cc1nc(N)ncc1-c1nc(N2CCOCC2)c2sc(CN3CCN(CC3)S(C)(=O)=O)cc2n1 Show InChI InChI=1S/C21H28N8O3S2/c1-14-16(12-23-21(22)24-14)19-25-17-11-15(13-27-3-5-29(6-4-27)34(2,30)31)33-18(17)20(26-19)28-7-9-32-10-8-28/h11-12H,3-10,13H2,1-2H3,(H2,22,23,24) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Inhibition of recombinant PI3Kalpha assessed as PIP3 product formation by fluorescence polarization assay |
J Med Chem 53: 1086-97 (2010)
Article DOI: 10.1021/jm901284w
BindingDB Entry DOI: 10.7270/Q2FJ2GXT |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM50312609
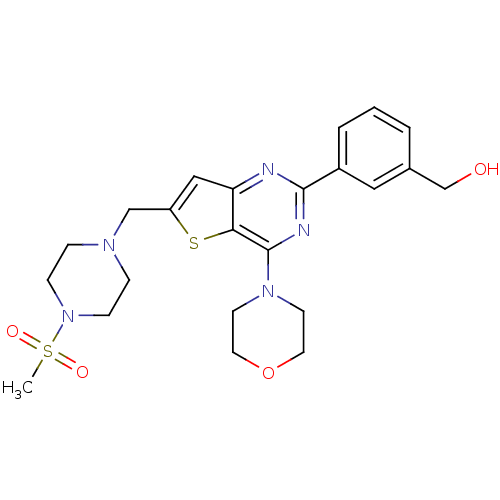
((3-(6-((4-(Methylsulfonyl)piperazin-1-yl)methyl)-4...)Show SMILES CS(=O)(=O)N1CCN(Cc2cc3nc(nc(N4CCOCC4)c3s2)-c2cccc(CO)c2)CC1 Show InChI InChI=1S/C23H29N5O4S2/c1-34(30,31)28-7-5-26(6-8-28)15-19-14-20-21(33-19)23(27-9-11-32-12-10-27)25-22(24-20)18-4-2-3-17(13-18)16-29/h2-4,13-14,29H,5-12,15-16H2,1H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Inhibition of recombinant PI3Kdelta assessed as PIP3 product formation by fluorescence polarization assay |
J Med Chem 53: 1086-97 (2010)
Article DOI: 10.1021/jm901284w
BindingDB Entry DOI: 10.7270/Q2FJ2GXT |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50312612

(3-[6-(4-Methanesulfonyl-piperazin-1-ylmethyl)-4-mo...)Show SMILES CS(=O)(=O)N1CCN(Cc2cc3nc(nc(N4CCOCC4)c3s2)-c2cccc(O)c2)CC1 Show InChI InChI=1S/C22H27N5O4S2/c1-33(29,30)27-7-5-25(6-8-27)15-18-14-19-20(32-18)22(26-9-11-31-12-10-26)24-21(23-19)16-3-2-4-17(28)13-16/h2-4,13-14,28H,5-12,15H2,1H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Inhibition of recombinant PI3Kalpha assessed as PIP3 product formation by fluorescence polarization assay |
J Med Chem 53: 1086-97 (2010)
Article DOI: 10.1021/jm901284w
BindingDB Entry DOI: 10.7270/Q2FJ2GXT |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50312609

((3-(6-((4-(Methylsulfonyl)piperazin-1-yl)methyl)-4...)Show SMILES CS(=O)(=O)N1CCN(Cc2cc3nc(nc(N4CCOCC4)c3s2)-c2cccc(CO)c2)CC1 Show InChI InChI=1S/C23H29N5O4S2/c1-34(30,31)28-7-5-26(6-8-28)15-19-14-20-21(33-19)23(27-9-11-32-12-10-27)25-22(24-20)18-4-2-3-17(13-18)16-29/h2-4,13-14,29H,5-12,15-16H2,1H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Inhibition of recombinant PI3Kalpha assessed as PIP3 product formation by fluorescence polarization assay |
J Med Chem 53: 1086-97 (2010)
Article DOI: 10.1021/jm901284w
BindingDB Entry DOI: 10.7270/Q2FJ2GXT |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50312606
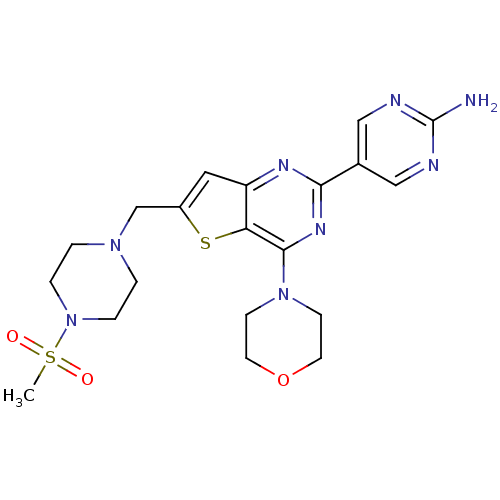
(5-(6-((4-(methylsulfonyl)piperazin-1-yl)methyl)-4-...)Show SMILES CS(=O)(=O)N1CCN(Cc2cc3nc(nc(N4CCOCC4)c3s2)-c2cnc(N)nc2)CC1 Show InChI InChI=1S/C20H26N8O3S2/c1-33(29,30)28-4-2-26(3-5-28)13-15-10-16-17(32-15)19(27-6-8-31-9-7-27)25-18(24-16)14-11-22-20(21)23-12-14/h10-12H,2-9,13H2,1H3,(H2,21,22,23) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Inhibition of recombinant PI3Kalpha assessed as PIP3 product formation by fluorescence polarization assay |
J Med Chem 53: 1086-97 (2010)
Article DOI: 10.1021/jm901284w
BindingDB Entry DOI: 10.7270/Q2FJ2GXT |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM50312606

(5-(6-((4-(methylsulfonyl)piperazin-1-yl)methyl)-4-...)Show SMILES CS(=O)(=O)N1CCN(Cc2cc3nc(nc(N4CCOCC4)c3s2)-c2cnc(N)nc2)CC1 Show InChI InChI=1S/C20H26N8O3S2/c1-33(29,30)28-4-2-26(3-5-28)13-15-10-16-17(32-15)19(27-6-8-31-9-7-27)25-18(24-16)14-11-22-20(21)23-12-14/h10-12H,2-9,13H2,1H3,(H2,21,22,23) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Inhibition of recombinant PI3Kdelta assessed as PIP3 product formation by fluorescence polarization assay |
J Med Chem 53: 1086-97 (2010)
Article DOI: 10.1021/jm901284w
BindingDB Entry DOI: 10.7270/Q2FJ2GXT |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM25028

(4-[2-(1H-indazol-4-yl)-6-[(4-methanesulfonylpipera...)Show SMILES CS(=O)(=O)N1CCN(Cc2cc3nc(nc(N4CCOCC4)c3s2)-c2cccc3[nH]ncc23)CC1 Show InChI InChI=1S/C23H27N7O3S2/c1-35(31,32)30-7-5-28(6-8-30)15-16-13-20-21(34-16)23(29-9-11-33-12-10-29)26-22(25-20)17-3-2-4-19-18(17)14-24-27-19/h2-4,13-14H,5-12,15H2,1H3,(H,24,27) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Inhibition of recombinant PI3Kdelta assessed as PIP3 product formation by fluorescence polarization assay |
J Med Chem 53: 1086-97 (2010)
Article DOI: 10.1021/jm901284w
BindingDB Entry DOI: 10.7270/Q2FJ2GXT |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM25028

(4-[2-(1H-indazol-4-yl)-6-[(4-methanesulfonylpipera...)Show SMILES CS(=O)(=O)N1CCN(Cc2cc3nc(nc(N4CCOCC4)c3s2)-c2cccc3[nH]ncc23)CC1 Show InChI InChI=1S/C23H27N7O3S2/c1-35(31,32)30-7-5-28(6-8-30)15-16-13-20-21(34-16)23(29-9-11-33-12-10-29)26-22(25-20)17-3-2-4-19-18(17)14-24-27-19/h2-4,13-14H,5-12,15H2,1H3,(H,24,27) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Inhibition of recombinant PI3Kalpha assessed as PIP3 product formation by fluorescence polarization assay |
J Med Chem 53: 1086-97 (2010)
Article DOI: 10.1021/jm901284w
BindingDB Entry DOI: 10.7270/Q2FJ2GXT |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50312613
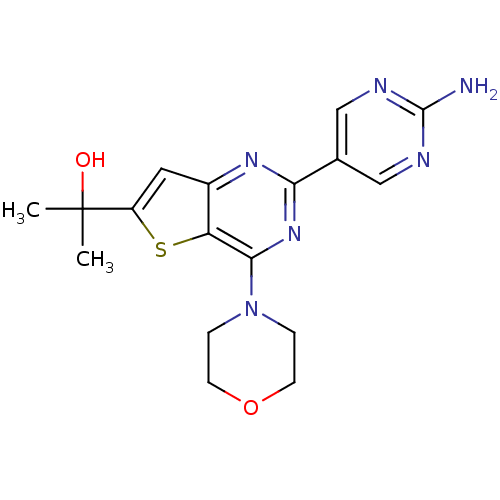
(2-(2-(2-Aminopyrimidin-5-yl)-4-morpholinothieno[3,...)Show SMILES CC(C)(O)c1cc2nc(nc(N3CCOCC3)c2s1)-c1cnc(N)nc1 Show InChI InChI=1S/C17H20N6O2S/c1-17(2,24)12-7-11-13(26-12)15(23-3-5-25-6-4-23)22-14(21-11)10-8-19-16(18)20-9-10/h7-9,24H,3-6H2,1-2H3,(H2,18,19,20) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 3.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Inhibition of recombinant PI3Kalpha assessed as PIP3 product formation by fluorescence polarization assay |
J Med Chem 53: 1086-97 (2010)
Article DOI: 10.1021/jm901284w
BindingDB Entry DOI: 10.7270/Q2FJ2GXT |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50312605
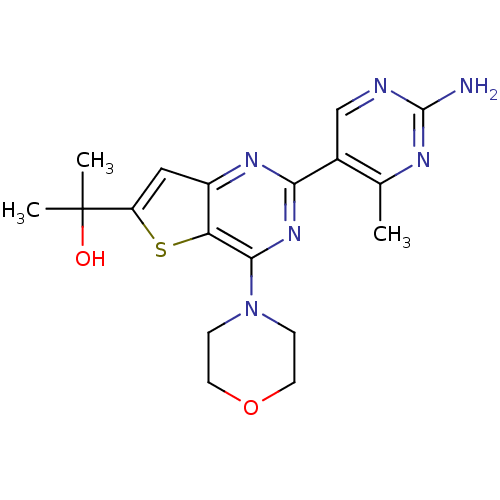
(2-(2-(2-amino-4-methylpyrimidin-5-yl)-4-morpholino...)Show SMILES Cc1nc(N)ncc1-c1nc(N2CCOCC2)c2sc(cc2n1)C(C)(C)O Show InChI InChI=1S/C18H22N6O2S/c1-10-11(9-20-17(19)21-10)15-22-12-8-13(18(2,3)25)27-14(12)16(23-15)24-4-6-26-7-5-24/h8-9,25H,4-7H2,1-3H3,(H2,19,20,21) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 3.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Inhibition of recombinant PI3Kalpha assessed as PIP3 product formation by fluorescence polarization assay |
J Med Chem 53: 1086-97 (2010)
Article DOI: 10.1021/jm901284w
BindingDB Entry DOI: 10.7270/Q2FJ2GXT |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform
(Homo sapiens (Human)) | BDBM50312614

(4-methyl-5-(6-{[4-(methylsulfonyl)piperazin-1-yl]m...)Show SMILES Cc1nc(N)ncc1-c1nc(N2CCOCC2)c2sc(CN3CCN(CC3)S(C)(=O)=O)cc2n1 Show InChI InChI=1S/C21H28N8O3S2/c1-14-16(12-23-21(22)24-14)19-25-17-11-15(13-27-3-5-29(6-4-27)34(2,30)31)33-18(17)20(26-19)28-7-9-32-10-8-28/h11-12H,3-10,13H2,1-2H3,(H2,22,23,24) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| MMDB
PDB
Article
PubMed
| n/a | n/a | 5.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Inhibition of recombinant PI3Kgamma assessed as PIP3 product formation by fluorescence polarization assay |
J Med Chem 53: 1086-97 (2010)
Article DOI: 10.1021/jm901284w
BindingDB Entry DOI: 10.7270/Q2FJ2GXT |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM50312605

(2-(2-(2-amino-4-methylpyrimidin-5-yl)-4-morpholino...)Show SMILES Cc1nc(N)ncc1-c1nc(N2CCOCC2)c2sc(cc2n1)C(C)(C)O Show InChI InChI=1S/C18H22N6O2S/c1-10-11(9-20-17(19)21-10)15-22-12-8-13(18(2,3)25)27-14(12)16(23-15)24-4-6-26-7-5-24/h8-9,25H,4-7H2,1-3H3,(H2,19,20,21) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 5.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Inhibition of recombinant PI3Kdelta assessed as PIP3 product formation by fluorescence polarization assay |
J Med Chem 53: 1086-97 (2010)
Article DOI: 10.1021/jm901284w
BindingDB Entry DOI: 10.7270/Q2FJ2GXT |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50312607
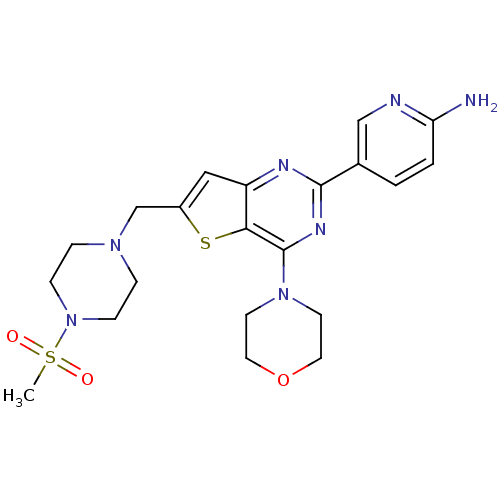
(5-(6-{[4-(methylsulfonyl)piperazin-1-yl]methyl}-4-...)Show SMILES CS(=O)(=O)N1CCN(Cc2cc3nc(nc(N4CCOCC4)c3s2)-c2ccc(N)nc2)CC1 Show InChI InChI=1S/C21H27N7O3S2/c1-33(29,30)28-6-4-26(5-7-28)14-16-12-17-19(32-16)21(27-8-10-31-11-9-27)25-20(24-17)15-2-3-18(22)23-13-15/h2-3,12-13H,4-11,14H2,1H3,(H2,22,23) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 5.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Inhibition of recombinant PI3Kalpha assessed as PIP3 product formation by fluorescence polarization assay |
J Med Chem 53: 1086-97 (2010)
Article DOI: 10.1021/jm901284w
BindingDB Entry DOI: 10.7270/Q2FJ2GXT |
More data for this
Ligand-Target Pair | |
3-phosphoinositide-dependent protein kinase 1
(Homo sapiens (Human)) | BDBM17051

(BX-795 | BX-795, 3 | N-(3-{[5-iodo-4-({3-[(thiophe...)Show SMILES Ic1cnc(Nc2cccc(NC(=O)N3CCCC3)c2)nc1NCCCNC(=O)c1cccs1 Show InChI InChI=1S/C23H26IN7O2S/c24-18-15-27-22(30-20(18)25-9-5-10-26-21(32)19-8-4-13-34-19)28-16-6-3-7-17(14-16)29-23(33)31-11-1-2-12-31/h3-4,6-8,13-15H,1-2,5,9-12H2,(H,26,32)(H,29,33)(H2,25,27,28,30) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 6 | n/a | n/a | n/a | n/a | 7.2 | 22 |
Berlex Biosciences
| Assay Description
The coupled assay can detect inhibitors of AKT2 activation, as well as direct inhibitors of PDK1 or AKT2. Inactive AKT2 is activated in situ by incub... |
J Biol Chem 280: 19867-74 (2005)
Article DOI: 10.1074/jbc.M501367200
BindingDB Entry DOI: 10.7270/Q21Z42PW |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit beta isoform
(Homo sapiens (Human)) | BDBM50312614

(4-methyl-5-(6-{[4-(methylsulfonyl)piperazin-1-yl]m...)Show SMILES Cc1nc(N)ncc1-c1nc(N2CCOCC2)c2sc(CN3CCN(CC3)S(C)(=O)=O)cc2n1 Show InChI InChI=1S/C21H28N8O3S2/c1-14-16(12-23-21(22)24-14)19-25-17-11-15(13-27-3-5-29(6-4-27)34(2,30)31)33-18(17)20(26-19)28-7-9-32-10-8-28/h11-12H,3-10,13H2,1-2H3,(H2,22,23,24) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 6.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Inhibition of recombinant PI3Kbeta assessed as PIP3 product formation by fluorescence polarization assay |
J Med Chem 53: 1086-97 (2010)
Article DOI: 10.1021/jm901284w
BindingDB Entry DOI: 10.7270/Q2FJ2GXT |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM50312607

(5-(6-{[4-(methylsulfonyl)piperazin-1-yl]methyl}-4-...)Show SMILES CS(=O)(=O)N1CCN(Cc2cc3nc(nc(N4CCOCC4)c3s2)-c2ccc(N)nc2)CC1 Show InChI InChI=1S/C21H27N7O3S2/c1-33(29,30)28-6-4-26(5-7-28)14-16-12-17-19(32-16)21(27-8-10-31-11-9-27)25-20(24-17)15-2-3-18(22)23-13-15/h2-3,12-13H,4-11,14H2,1H3,(H2,22,23) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 6.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Inhibition of recombinant PI3Kdelta assessed as PIP3 product formation by fluorescence polarization assay |
J Med Chem 53: 1086-97 (2010)
Article DOI: 10.1021/jm901284w
BindingDB Entry DOI: 10.7270/Q2FJ2GXT |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform
(Homo sapiens (Human)) | BDBM50312606

(5-(6-((4-(methylsulfonyl)piperazin-1-yl)methyl)-4-...)Show SMILES CS(=O)(=O)N1CCN(Cc2cc3nc(nc(N4CCOCC4)c3s2)-c2cnc(N)nc2)CC1 Show InChI InChI=1S/C20H26N8O3S2/c1-33(29,30)28-4-2-26(3-5-28)13-15-10-16-17(32-15)19(27-6-8-31-9-7-27)25-18(24-16)14-11-22-20(21)23-12-14/h10-12H,2-9,13H2,1H3,(H2,21,22,23) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 8.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Inhibition of recombinant PI3Kgamma assessed as PIP3 product formation by fluorescence polarization assay |
J Med Chem 53: 1086-97 (2010)
Article DOI: 10.1021/jm901284w
BindingDB Entry DOI: 10.7270/Q2FJ2GXT |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit beta isoform
(Homo sapiens (Human)) | BDBM50312612

(3-[6-(4-Methanesulfonyl-piperazin-1-ylmethyl)-4-mo...)Show SMILES CS(=O)(=O)N1CCN(Cc2cc3nc(nc(N4CCOCC4)c3s2)-c2cccc(O)c2)CC1 Show InChI InChI=1S/C22H27N5O4S2/c1-33(29,30)27-7-5-25(6-8-27)15-18-14-19-20(32-18)22(26-9-11-31-12-10-26)24-21(23-19)16-3-2-4-17(28)13-16/h2-4,13-14,28H,5-12,15H2,1H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Inhibition of recombinant PI3Kbeta assessed as PIP3 product formation by fluorescence polarization assay |
J Med Chem 53: 1086-97 (2010)
Article DOI: 10.1021/jm901284w
BindingDB Entry DOI: 10.7270/Q2FJ2GXT |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit beta isoform
(Homo sapiens (Human)) | BDBM50312613

(2-(2-(2-Aminopyrimidin-5-yl)-4-morpholinothieno[3,...)Show SMILES CC(C)(O)c1cc2nc(nc(N3CCOCC3)c2s1)-c1cnc(N)nc1 Show InChI InChI=1S/C17H20N6O2S/c1-17(2,24)12-7-11-13(26-12)15(23-3-5-25-6-4-23)22-14(21-11)10-8-19-16(18)20-9-10/h7-9,24H,3-6H2,1-2H3,(H2,18,19,20) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Inhibition of recombinant PI3Kbeta assessed as PIP3 product formation by fluorescence polarization assay |
J Med Chem 53: 1086-97 (2010)
Article DOI: 10.1021/jm901284w
BindingDB Entry DOI: 10.7270/Q2FJ2GXT |
More data for this
Ligand-Target Pair | |
3-phosphoinositide-dependent protein kinase 1
(Homo sapiens (Human)) | BDBM17052
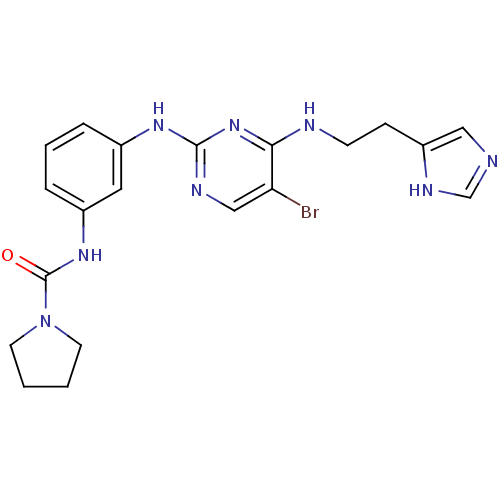
(BX-912 | N-{3-[(5-bromo-4-{[2-(1H-imidazol-4-yl)et...)Show SMILES Brc1cnc(Nc2cccc(NC(=O)N3CCCC3)c2)nc1NCCc1cnc[nH]1 Show InChI InChI=1S/C20H23BrN8O/c21-17-12-24-19(28-18(17)23-7-6-16-11-22-13-25-16)26-14-4-3-5-15(10-14)27-20(30)29-8-1-2-9-29/h3-5,10-13H,1-2,6-9H2,(H,22,25)(H,27,30)(H2,23,24,26,28) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| n/a | n/a | 12 | n/a | n/a | n/a | n/a | 7.2 | 22 |
Berlex Biosciences
| Assay Description
The coupled assay can detect inhibitors of AKT2 activation, as well as direct inhibitors of PDK1 or AKT2. Inactive AKT2 is activated in situ by incub... |
J Biol Chem 280: 19867-74 (2005)
Article DOI: 10.1074/jbc.M501367200
BindingDB Entry DOI: 10.7270/Q21Z42PW |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM50312611
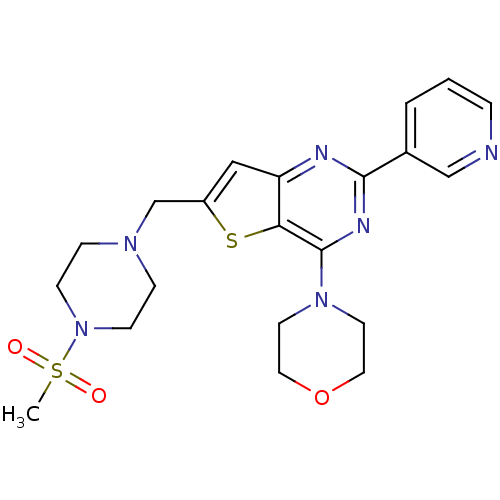
(6-(4-Methanesulfonyl-piperazin-1-ylmethyl)-4-morph...)Show SMILES CS(=O)(=O)N1CCN(Cc2cc3nc(nc(N4CCOCC4)c3s2)-c2cccnc2)CC1 Show InChI InChI=1S/C21H26N6O3S2/c1-32(28,29)27-7-5-25(6-8-27)15-17-13-18-19(31-17)21(26-9-11-30-12-10-26)24-20(23-18)16-3-2-4-22-14-16/h2-4,13-14H,5-12,15H2,1H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Inhibition of recombinant PI3Kdelta assessed as PIP3 product formation by fluorescence polarization assay |
J Med Chem 53: 1086-97 (2010)
Article DOI: 10.1021/jm901284w
BindingDB Entry DOI: 10.7270/Q2FJ2GXT |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit beta isoform
(Homo sapiens (Human)) | BDBM50312611

(6-(4-Methanesulfonyl-piperazin-1-ylmethyl)-4-morph...)Show SMILES CS(=O)(=O)N1CCN(Cc2cc3nc(nc(N4CCOCC4)c3s2)-c2cccnc2)CC1 Show InChI InChI=1S/C21H26N6O3S2/c1-32(28,29)27-7-5-25(6-8-27)15-17-13-18-19(31-17)21(26-9-11-30-12-10-26)24-20(23-18)16-3-2-4-22-14-16/h2-4,13-14H,5-12,15H2,1H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Inhibition of recombinant PI3Kbeta assessed as PIP3 product formation by fluorescence polarization assay |
J Med Chem 53: 1086-97 (2010)
Article DOI: 10.1021/jm901284w
BindingDB Entry DOI: 10.7270/Q2FJ2GXT |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit beta isoform
(Homo sapiens (Human)) | BDBM50312609

((3-(6-((4-(Methylsulfonyl)piperazin-1-yl)methyl)-4...)Show SMILES CS(=O)(=O)N1CCN(Cc2cc3nc(nc(N4CCOCC4)c3s2)-c2cccc(CO)c2)CC1 Show InChI InChI=1S/C23H29N5O4S2/c1-34(30,31)28-7-5-26(6-8-28)15-19-14-20-21(33-19)23(27-9-11-32-12-10-27)25-22(24-20)18-4-2-3-17(13-18)16-29/h2-4,13-14,29H,5-12,15-16H2,1H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Inhibition of recombinant PI3Kbeta assessed as PIP3 product formation by fluorescence polarization assay |
J Med Chem 53: 1086-97 (2010)
Article DOI: 10.1021/jm901284w
BindingDB Entry DOI: 10.7270/Q2FJ2GXT |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform
(Homo sapiens (Human)) | BDBM50312605

(2-(2-(2-amino-4-methylpyrimidin-5-yl)-4-morpholino...)Show SMILES Cc1nc(N)ncc1-c1nc(N2CCOCC2)c2sc(cc2n1)C(C)(C)O Show InChI InChI=1S/C18H22N6O2S/c1-10-11(9-20-17(19)21-10)15-22-12-8-13(18(2,3)25)27-14(12)16(23-15)24-4-6-26-7-5-24/h8-9,25H,4-7H2,1-3H3,(H2,19,20,21) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Inhibition of recombinant PI3Kgamma assessed as PIP3 product formation by fluorescence polarization assay |
J Med Chem 53: 1086-97 (2010)
Article DOI: 10.1021/jm901284w
BindingDB Entry DOI: 10.7270/Q2FJ2GXT |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform
(Homo sapiens (Human)) | BDBM50312609

((3-(6-((4-(Methylsulfonyl)piperazin-1-yl)methyl)-4...)Show SMILES CS(=O)(=O)N1CCN(Cc2cc3nc(nc(N4CCOCC4)c3s2)-c2cccc(CO)c2)CC1 Show InChI InChI=1S/C23H29N5O4S2/c1-34(30,31)28-7-5-26(6-8-28)15-19-14-20-21(33-19)23(27-9-11-32-12-10-27)25-22(24-20)18-4-2-3-17(13-18)16-29/h2-4,13-14,29H,5-12,15-16H2,1H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| MMDB
PDB
Article
PubMed
| n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Inhibition of recombinant PI3Kgamma assessed as PIP3 product formation by fluorescence polarization assay |
J Med Chem 53: 1086-97 (2010)
Article DOI: 10.1021/jm901284w
BindingDB Entry DOI: 10.7270/Q2FJ2GXT |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM50312613

(2-(2-(2-Aminopyrimidin-5-yl)-4-morpholinothieno[3,...)Show SMILES CC(C)(O)c1cc2nc(nc(N3CCOCC3)c2s1)-c1cnc(N)nc1 Show InChI InChI=1S/C17H20N6O2S/c1-17(2,24)12-7-11-13(26-12)15(23-3-5-25-6-4-23)22-14(21-11)10-8-19-16(18)20-9-10/h7-9,24H,3-6H2,1-2H3,(H2,18,19,20) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 16 | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Inhibition of recombinant PI3Kdelta assessed as PIP3 product formation by fluorescence polarization assay |
J Med Chem 53: 1086-97 (2010)
Article DOI: 10.1021/jm901284w
BindingDB Entry DOI: 10.7270/Q2FJ2GXT |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50312616
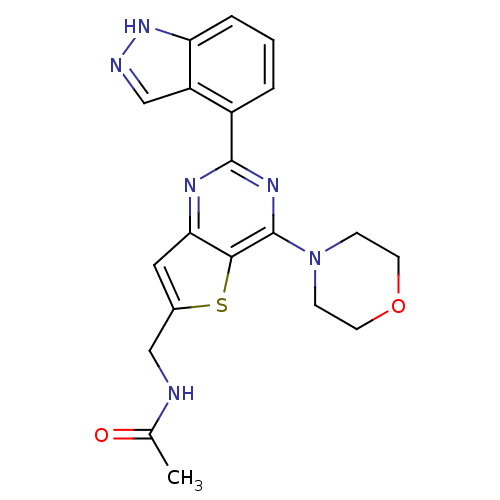
(2-[2-(2-Amino-4-methyl-pyrimidin-5-yl)-4-morpholin...)Show SMILES CC(=O)NCc1cc2nc(nc(N3CCOCC3)c2s1)-c1cccc2[nH]ncc12 Show InChI InChI=1S/C20H20N6O2S/c1-12(27)21-10-13-9-17-18(29-13)20(26-5-7-28-8-6-26)24-19(23-17)14-3-2-4-16-15(14)11-22-25-16/h2-4,9,11H,5-8,10H2,1H3,(H,21,27)(H,22,25) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 16 | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Inhibition of recombinant PI3Kalpha assessed as PIP3 product formation by fluorescence polarization assay |
J Med Chem 53: 1086-97 (2010)
Article DOI: 10.1021/jm901284w
BindingDB Entry DOI: 10.7270/Q2FJ2GXT |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform
(Homo sapiens (Human)) | BDBM50312613

(2-(2-(2-Aminopyrimidin-5-yl)-4-morpholinothieno[3,...)Show SMILES CC(C)(O)c1cc2nc(nc(N3CCOCC3)c2s1)-c1cnc(N)nc1 Show InChI InChI=1S/C17H20N6O2S/c1-17(2,24)12-7-11-13(26-12)15(23-3-5-25-6-4-23)22-14(21-11)10-8-19-16(18)20-9-10/h7-9,24H,3-6H2,1-2H3,(H2,18,19,20) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 16 | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Inhibition of recombinant PI3Kgamma assessed as PIP3 product formation by fluorescence polarization assay |
J Med Chem 53: 1086-97 (2010)
Article DOI: 10.1021/jm901284w
BindingDB Entry DOI: 10.7270/Q2FJ2GXT |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform
(Homo sapiens (Human)) | BDBM50312611

(6-(4-Methanesulfonyl-piperazin-1-ylmethyl)-4-morph...)Show SMILES CS(=O)(=O)N1CCN(Cc2cc3nc(nc(N4CCOCC4)c3s2)-c2cccnc2)CC1 Show InChI InChI=1S/C21H26N6O3S2/c1-32(28,29)27-7-5-25(6-8-27)15-17-13-18-19(31-17)21(26-9-11-30-12-10-26)24-20(23-18)16-3-2-4-22-14-16/h2-4,13-14H,5-12,15H2,1H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 19 | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Inhibition of recombinant PI3Kgamma assessed as PIP3 product formation by fluorescence polarization assay |
J Med Chem 53: 1086-97 (2010)
Article DOI: 10.1021/jm901284w
BindingDB Entry DOI: 10.7270/Q2FJ2GXT |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform
(Homo sapiens (Human)) | BDBM50312612

(3-[6-(4-Methanesulfonyl-piperazin-1-ylmethyl)-4-mo...)Show SMILES CS(=O)(=O)N1CCN(Cc2cc3nc(nc(N4CCOCC4)c3s2)-c2cccc(O)c2)CC1 Show InChI InChI=1S/C22H27N5O4S2/c1-33(29,30)27-7-5-25(6-8-27)15-18-14-19-20(32-18)22(26-9-11-31-12-10-26)24-21(23-19)16-3-2-4-17(28)13-16/h2-4,13-14,28H,5-12,15H2,1H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Inhibition of recombinant PI3Kgamma assessed as PIP3 product formation by fluorescence polarization assay |
J Med Chem 53: 1086-97 (2010)
Article DOI: 10.1021/jm901284w
BindingDB Entry DOI: 10.7270/Q2FJ2GXT |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50312611

(6-(4-Methanesulfonyl-piperazin-1-ylmethyl)-4-morph...)Show SMILES CS(=O)(=O)N1CCN(Cc2cc3nc(nc(N4CCOCC4)c3s2)-c2cccnc2)CC1 Show InChI InChI=1S/C21H26N6O3S2/c1-32(28,29)27-7-5-25(6-8-27)15-17-13-18-19(31-17)21(26-9-11-30-12-10-26)24-20(23-18)16-3-2-4-22-14-16/h2-4,13-14H,5-12,15H2,1H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 21 | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Inhibition of recombinant PI3Kalpha assessed as PIP3 product formation by fluorescence polarization assay |
J Med Chem 53: 1086-97 (2010)
Article DOI: 10.1021/jm901284w
BindingDB Entry DOI: 10.7270/Q2FJ2GXT |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50312615

(2-(2-(1H-indazol-4-yl)-4-morpholinothieno[3,2-d]py...)Show SMILES CC(C)(O)c1cc2nc(nc(N3CCOCC3)c2s1)-c1cccc2[nH]ncc12 Show InChI InChI=1S/C20H21N5O2S/c1-20(2,26)16-10-15-17(28-16)19(25-6-8-27-9-7-25)23-18(22-15)12-4-3-5-14-13(12)11-21-24-14/h3-5,10-11,26H,6-9H2,1-2H3,(H,21,24) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 22 | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Inhibition of recombinant PI3Kalpha assessed as PIP3 product formation by fluorescence polarization assay |
J Med Chem 53: 1086-97 (2010)
Article DOI: 10.1021/jm901284w
BindingDB Entry DOI: 10.7270/Q2FJ2GXT |
More data for this
Ligand-Target Pair | |
Urokinase-type plasminogen activator
(Homo sapiens (Human)) | BDBM50467209
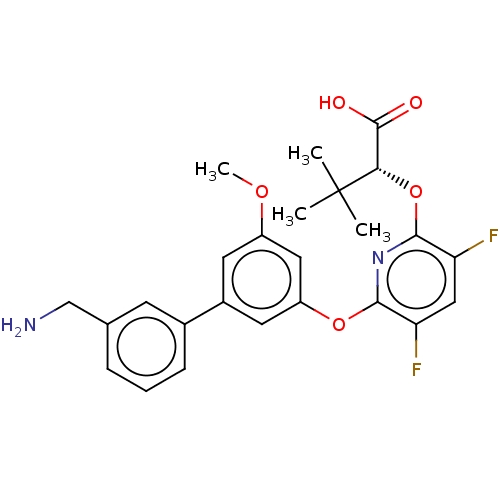
(CHEMBL4291446)Show SMILES COc1cc(Oc2nc(O[C@@H](C(O)=O)C(C)(C)C)c(F)cc2F)cc(c1)-c1cccc(CN)c1 |r| Show InChI InChI=1S/C25H26F2N2O5/c1-25(2,3)21(24(30)31)34-23-20(27)12-19(26)22(29-23)33-18-10-16(9-17(11-18)32-4)15-7-5-6-14(8-15)13-28/h5-12,21H,13,28H2,1-4H3,(H,30,31)/t21-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 23 | n/a | n/a | n/a | n/a | n/a | n/a |
Abdulaziz University for Health Sciences
Curated by ChEMBL
| Assay Description
Inhibition of human uPA |
Bioorg Med Chem Lett 28: 3372-3375 (2018)
Article DOI: 10.1016/j.bmcl.2018.09.001
BindingDB Entry DOI: 10.7270/Q24Q7XNF |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit beta isoform
(Homo sapiens (Human)) | BDBM50312605

(2-(2-(2-amino-4-methylpyrimidin-5-yl)-4-morpholino...)Show SMILES Cc1nc(N)ncc1-c1nc(N2CCOCC2)c2sc(cc2n1)C(C)(C)O Show InChI InChI=1S/C18H22N6O2S/c1-10-11(9-20-17(19)21-10)15-22-12-8-13(18(2,3)25)27-14(12)16(23-15)24-4-6-26-7-5-24/h8-9,25H,4-7H2,1-3H3,(H2,19,20,21) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 25 | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Inhibition of recombinant PI3Kbeta assessed as PIP3 product formation by fluorescence polarization assay |
J Med Chem 53: 1086-97 (2010)
Article DOI: 10.1021/jm901284w
BindingDB Entry DOI: 10.7270/Q2FJ2GXT |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50312617
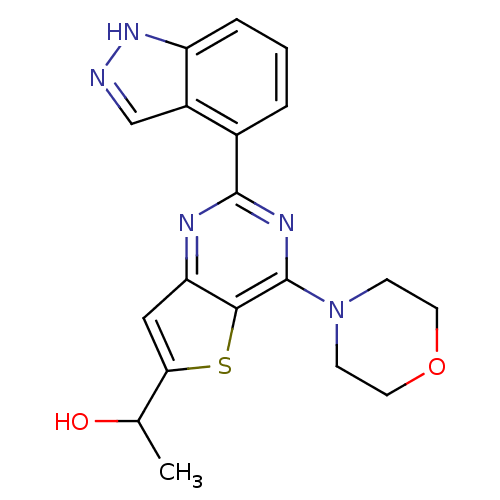
(1-(2-(1H-Indazol-4-yl)-4-morpholinothieno[3,2-d]py...)Show SMILES CC(O)c1cc2nc(nc(N3CCOCC3)c2s1)-c1cccc2[nH]ncc12 Show InChI InChI=1S/C19H19N5O2S/c1-11(25)16-9-15-17(27-16)19(24-5-7-26-8-6-24)22-18(21-15)12-3-2-4-14-13(12)10-20-23-14/h2-4,9-11,25H,5-8H2,1H3,(H,20,23) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 28 | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Inhibition of recombinant PI3Kalpha assessed as PIP3 product formation by fluorescence polarization assay |
J Med Chem 53: 1086-97 (2010)
Article DOI: 10.1021/jm901284w
BindingDB Entry DOI: 10.7270/Q2FJ2GXT |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50312618
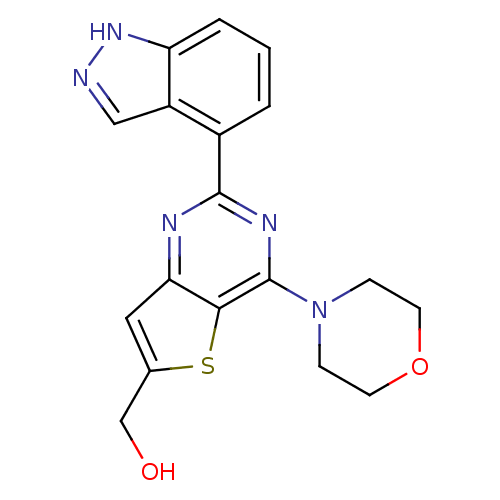
((2-(1H-indazol-4-yl)-4-morpholinothieno[3,2-d]pyri...)Show SMILES OCc1cc2nc(nc(N3CCOCC3)c2s1)-c1cccc2[nH]ncc12 Show InChI InChI=1S/C18H17N5O2S/c24-10-11-8-15-16(26-11)18(23-4-6-25-7-5-23)21-17(20-15)12-2-1-3-14-13(12)9-19-22-14/h1-3,8-9,24H,4-7,10H2,(H,19,22) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 29 | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Inhibition of recombinant PI3Kalpha assessed as PIP3 product formation by fluorescence polarization assay |
J Med Chem 53: 1086-97 (2010)
Article DOI: 10.1021/jm901284w
BindingDB Entry DOI: 10.7270/Q2FJ2GXT |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase mTOR
(Homo sapiens (Human)) | BDBM50312606

(5-(6-((4-(methylsulfonyl)piperazin-1-yl)methyl)-4-...)Show SMILES CS(=O)(=O)N1CCN(Cc2cc3nc(nc(N4CCOCC4)c3s2)-c2cnc(N)nc2)CC1 Show InChI InChI=1S/C20H26N8O3S2/c1-33(29,30)28-4-2-26(3-5-28)13-15-10-16-17(32-15)19(27-6-8-31-9-7-27)25-18(24-16)14-11-22-20(21)23-12-14/h10-12H,2-9,13H2,1H3,(H2,21,22,23) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 29 | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant mTOR by FRET |
J Med Chem 53: 1086-97 (2010)
Article DOI: 10.1021/jm901284w
BindingDB Entry DOI: 10.7270/Q2FJ2GXT |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase mTOR
(Homo sapiens (Human)) | BDBM50312613

(2-(2-(2-Aminopyrimidin-5-yl)-4-morpholinothieno[3,...)Show SMILES CC(C)(O)c1cc2nc(nc(N3CCOCC3)c2s1)-c1cnc(N)nc1 Show InChI InChI=1S/C17H20N6O2S/c1-17(2,24)12-7-11-13(26-12)15(23-3-5-25-6-4-23)22-14(21-11)10-8-19-16(18)20-9-10/h7-9,24H,3-6H2,1-2H3,(H2,18,19,20) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant mTOR by FRET |
J Med Chem 53: 1086-97 (2010)
Article DOI: 10.1021/jm901284w
BindingDB Entry DOI: 10.7270/Q2FJ2GXT |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit beta isoform
(Homo sapiens (Human)) | BDBM25028

(4-[2-(1H-indazol-4-yl)-6-[(4-methanesulfonylpipera...)Show SMILES CS(=O)(=O)N1CCN(Cc2cc3nc(nc(N4CCOCC4)c3s2)-c2cccc3[nH]ncc23)CC1 Show InChI InChI=1S/C23H27N7O3S2/c1-35(31,32)30-7-5-28(6-8-30)15-16-13-20-21(34-16)23(29-9-11-33-12-10-29)26-22(25-20)17-3-2-4-19-18(17)14-24-27-19/h2-4,13-14H,5-12,15H2,1H3,(H,24,27) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 32 | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Inhibition of recombinant PI3Kbeta assessed as PIP3 product formation by fluorescence polarization assay |
J Med Chem 53: 1086-97 (2010)
Article DOI: 10.1021/jm901284w
BindingDB Entry DOI: 10.7270/Q2FJ2GXT |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform
(Homo sapiens (Human)) | BDBM50312607

(5-(6-{[4-(methylsulfonyl)piperazin-1-yl]methyl}-4-...)Show SMILES CS(=O)(=O)N1CCN(Cc2cc3nc(nc(N4CCOCC4)c3s2)-c2ccc(N)nc2)CC1 Show InChI InChI=1S/C21H27N7O3S2/c1-33(29,30)28-6-4-26(5-7-28)14-16-12-17-19(32-16)21(27-8-10-31-11-9-27)25-20(24-17)15-2-3-18(22)23-13-15/h2-3,12-13H,4-11,14H2,1H3,(H2,22,23) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| MMDB
PDB
Article
PubMed
| n/a | n/a | 33 | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Inhibition of recombinant PI3Kgamma assessed as PIP3 product formation by fluorescence polarization assay |
J Med Chem 53: 1086-97 (2010)
Article DOI: 10.1021/jm901284w
BindingDB Entry DOI: 10.7270/Q2FJ2GXT |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Urokinase-type plasminogen activator
(Homo sapiens (Human)) | BDBM50467207

(CHEMBL4288828)Show SMILES CC(C)(C)[C@@H](Oc1nc(Oc2cc(cc(c2)-c2cccc(CN)c2)C#N)c(F)cc1F)C(O)=O |r| Show InChI InChI=1S/C25H23F2N3O4/c1-25(2,3)21(24(31)32)34-23-20(27)11-19(26)22(30-23)33-18-9-15(13-29)8-17(10-18)16-6-4-5-14(7-16)12-28/h4-11,21H,12,28H2,1-3H3,(H,31,32)/t21-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 34 | n/a | n/a | n/a | n/a | n/a | n/a |
Abdulaziz University for Health Sciences
Curated by ChEMBL
| Assay Description
Inhibition of human uPA |
Bioorg Med Chem Lett 28: 3372-3375 (2018)
Article DOI: 10.1016/j.bmcl.2018.09.001
BindingDB Entry DOI: 10.7270/Q24Q7XNF |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit beta isoform
(Homo sapiens (Human)) | BDBM50312606

(5-(6-((4-(methylsulfonyl)piperazin-1-yl)methyl)-4-...)Show SMILES CS(=O)(=O)N1CCN(Cc2cc3nc(nc(N4CCOCC4)c3s2)-c2cnc(N)nc2)CC1 Show InChI InChI=1S/C20H26N8O3S2/c1-33(29,30)28-4-2-26(3-5-28)13-15-10-16-17(32-15)19(27-6-8-31-9-7-27)25-18(24-16)14-11-22-20(21)23-12-14/h10-12H,2-9,13H2,1H3,(H2,21,22,23) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 38 | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Inhibition of recombinant PI3Kbeta assessed as PIP3 product formation by fluorescence polarization assay |
J Med Chem 53: 1086-97 (2010)
Article DOI: 10.1021/jm901284w
BindingDB Entry DOI: 10.7270/Q2FJ2GXT |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data